Abstract
Breast cancer (BC) is the most frequent cancer among women and represents the second leading cause of cancer-specific death. A subset of patients with metastatic breast cancer (MBC) presents limited disease, termed ‘oligometastatic’ breast cancer (OMBC). The oligometastatic disease can be managed with different treatment strategies to achieve long-term remission and eventually cure. Several approaches are possible to cure the oligometastatic disease: locoregional treatments of the primary tumor and of all the metastatic sites, such as surgery and radiotherapy; systemic treatment, including target-therapy or immunotherapy, according to the biological status of the primary tumor and/or of the metastases; or the combination of these approaches. Encouraging results involve local ablative options, but these trials are limited by being retrospective and affected by selection bias. Systemic therapy, e.g., the use of CDK4/6 inhibitors for hormone receptor-positive (HR+)/HER-2 negative BC, leads to an increase of progression-free survival (PFS) and overall survival (OS) in all the subgroups, with favorable toxicity. Regardless of the lack of substantial data, this subset of patients could be treated with curative intent; the appropriate candidates could be mostly young women, for whom a multidisciplinary aggressive approach appears suitable. We provide a global perspective on the current treatment paradigms of OMBC.
Keywords: oligometastatic breast cancer, locoregional therapy, CDK4/6 inhibitors, multidisciplinary
1. Introduction
Breast cancer is the most frequent cancer among women and represents the second leading cause of cancer-specific death [1]. Metastatic breast cancer (MBC) includes about 6% of cases of de novo disease, and about 20–30% of early-stage cancers recurred at distant sites [2]. The behavior of stage IV breast cancer may differ, depending on the biology of the tumor, the likelihood of spreading to certain sites (e.g., bone in hormone receptor-positive disease), and the disease burden. A subset of patients with MBC presents limited disease, termed ‘oligometastatic’ breast cancer (OMBC) [3].
The concept of oligometastases represents a condition midway between locoregionally confined cancer and disseminated disease, in which tumor burden is low and the number of affected organs is limited, typically with 1 to 5 secundarisms [4,5,6,7,8].
Even though the incidence of OMBC is not clearly defined (1–10%), it seems that a considerable amount of all new MBC presents as oligometastatic. A tri-institutional retrospective analysis of 2249 patients with stage I–III disease who had first treatment failure showed that 21.9% were characterized by oligometastatic disease [9]. This boundary between oligo- and polymetastatic disease is increasingly recognized because of treatment and survival implications [3].
Given the likelihood of limited spread, it is possible to achieve longer survival, and, in 2–3% of cases, cure, with aggressive metastasis-directed therapy [5,8].
Moreover, OMBC is characterized by its chronicity and evolvement: primary cancer may present synchronous limited metastases, or the primitive tumor over time can develop a few metachronous metastases. We define oligorecurrence as the development of metachronous oligometastases with a controlled primary site [10], whereas oligoprogression represents a condition where a limited number of metastases progress, while all other sites of the disease remain stable, commonly during systemic treatment [11,12]. This distinction is representative of different scenarios and related prognosis, and it has a clinical implication in terms of survival [4].
For example, the patients with oligometastatic disease included in the previously cited study present a significantly longer overall survival (OS) as compared to polymetastatic patients with a follow-up of more than three years.
Prior reviews on oligometastatic disease investigated the effect of local techniques, namely surgical and radiotherapy. Recently, new techniques directed to disease biology provide information about next-generation treatment strategies, leading to a deeper biological understanding of OMBC and related treatment options. We provide a global perspective on the current treatment paradigms of OMBC [3].
2. Options for Treatment of Oligometastatic Breast Cancer
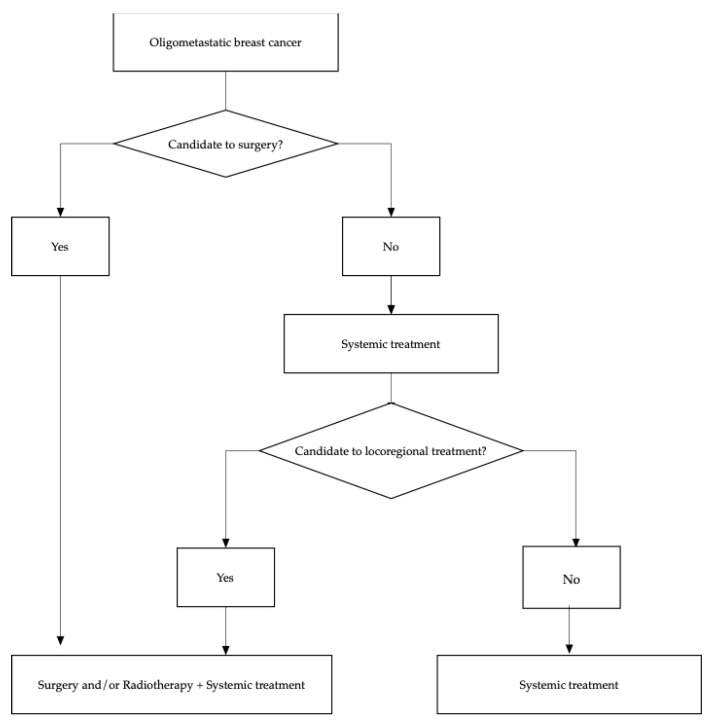
The oligometastatic disease can be managed with different treatment strategies to achieve long-term remission and eventually cure. In Figure 1 a flow chart of treatment options is presented.
Figure 1.
Diagram flow of therapeutic options in oligometastatic breast cancer.
Several approaches are possible to cure the metastatic disease: locoregional treatment of the primary tumor and the metastases; systemic treatment, including target-therapy or immunotherapy, according to the biological status of the primary tumor and/or of the metastases; or the combination of these approaches [13].
Locoregional options both of the primitive tumor and of the metastases lead to long-lasting remissions reported in several case series; however, unlike other tumor entities, prospective data are lacking [13].
2.1. Surgery
In oligometastatic cancer, several trials involve surgery [14] (Table 1). The role of surgery in metastatic disease is unknown in terms of prognosis. Retrospective analyses demonstrate that patients who underwent surgery on the primitive tumor show a better prognosis compared to those who received only systemic therapy [15,16].
Table 1.
Randomized trials that evaluate the efficacy of surgery in MBC.
| Trial | Number of Patients | Site of Metastases | Biological Subtype | Site of Surgery |
Outcome |
|---|---|---|---|---|---|
| Tata Memorial, NCT00193778 |
350 | Bone and/or visceral | HR+ /HER2− HR+/HER2+ |
|
1. No differences in OS 2. Better locoregional PFS FOR surgery 3. Worse DPFS for surgery |
| MF0701, NCT00557986 |
274 | Bone and/or lung and/or liver | HR+ 85.5% HER2+ 30.4% TN 7.3% |
|
1. Increase in median survival for surgery upfront 2. Superior survival for locoregional treatment in women with luminal tumors, age < 55 years, and solitary bone metastases |
| ECOG-ACRIN E 2108, NCT01242800 |
258 | Bone and/or any organ system, including CNS |
HR+/HER2− 60% HER2+ 26% TN 15% |
|
1. No difference in OS and PFS 2. Possible detrimental effect of locoregional treatment in TN mBC 3. Increase of 2.5x risk of locoregional progression in patients who received systemic therapy without locoregional treatment |
| TBCRC 013, NCT00941759 |
127 | Bone and/or any organ system, including CNS |
HR+/HER2– HR+/HER2+ HR−/HER2+ HR−/HER2− |
|
1. No improvement of PFS and OS for surgery in patients who have responded to first-line treatment |
To corroborate a possible role of local treatments for the prognosis at the beginning of the metastatic disease, there is evidence that a multidisciplinary approach (surgery + radiotherapy, axillary dissection) is better for locoregional control of the disease, despite it being only surgery of the mammary node/mastectomy [17]. However, the findings of these studies are weakened due to selection bias: for example, patients with a less extended metastatic disease and/or who are responsive to medical treatments have more opportunities to undergo surgery on the primitive tumor than those who present a more advanced disease and/or who are less responsive to medical treatments.
In the literature, three randomized trials evaluated the efficacy of surgery in MBC at the beginning of the disease.
In Tata Memorial Trial [18], among 350 women who enrolled, 173 underwent surgery and medical treatment and 177 received only medical treatment. This trial demonstrates that there are no differences in OS between the two groups. Surgical treatment is related to a better locoregional PFS, but also a worse DPFS (distant progression-free survival).
In the MF0701 [19] study, of 274 women who were enrolled, 138 underwent surgery and systemic treatment, while 136 were administered only systemic treatment. Patients with HR+ could receive hormone therapy. The protocol permitted upfront randomization (before the beginning of medical treatment) and the option of surgery on the primitive tumor during the local progression in the systemic treatment group. This trial showed a significant increase in median survival in those patients who underwent surgery upfront (46 vs. 37 months, HR 0.66 p < 0.005). An analysis of the subgroups showed that the survival was superior to locoregional treatment in women with luminal tumors, age < 55 years, and solitary bone metastases.
ECOG-ACRIN E 2108 [20] studied 258 patients with de novo MBC with no progression after 4–8 months of systemic treatment that were randomized to continue systemic treatment or to receive radical locoregional treatment (surgery with free margins and subsequent radiotherapy, if indicated). About 60% presented an HR+/HER-2 negative tumor, 26% HER-2+, 15% were triple negative. In addition, 37% of these patients presented only bone metastases. The survival analysis showed no difference in OS and PSF in the two cohorts in the general population. The subgroup analysis suggests a possible detrimental effect of locoregional treatment in the subgroup of patients with triple-negative BC. Thus, even though we observed an increase of 2.5 times in the risk of locoregional progression in patients who received systemic therapy without locoregional treatment, there is no benefit in terms of quality of life from locoregional treatment.
Moreover, a prospective cohort trial [21] shows that in patients who have responded to first-line treatment, surgery on the primitive tumor does not improve PFS and OS, so that the predominant prognostic role is given by medical treatments, histopathologic features, and tumor burden. Conclusively, in patients with de novo MBC, the surgery approach has a palliative role (e.g., ulcerative lesions). In the absence of results of the effectiveness in OS, this procedure is considered in selected cases and after discussion with the patient.
There are three other randomized trials, one of which has finished the accrual, and it could furnish other elements to the argument.
In clinical practice, surgery is reserved for vertebral metastases with medullary compression, pathological fractures, pleural or pericardial effusion, and single visceral metastasis (e.g., liver, lung, brain).
In this regard, the resection of liver metastases in MBC is little explored, although in other tumors such as colorectal cancer it is widely recognized [6].
Different case series [6,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36] show different survival rates (22–61 months) for liver metastases resection. A monocentric experience with 51 patients reported a 16% increase of 10-year OS rate [26]; 8.9% of these patients never presented any recurrence after surgery. However, this result is affected by a selection bias of the sample: the resection, but also the indolent course of the disease, the specific genetic profile of the tumor, and the ability of subclones to metastasize to a certain organ likely play a crucial prognostic role. Therefore, these reports need confirmation with prospective randomized trials [37].
A prospective data collection of 41 patients, who underwent liver metastases resection, revealed that positive resection margins and a short disease-free interval until the detection of liver metastases may lead to poor long-term survival [38]. Comparable results can be assumed for pulmonary lesions metastasectomy [39,40]: a short disease-free interval, the presence of several metastases, incomplete resection of them, and a non-luminal subtype are considered negative prognostic factors [41].
In summary: in OMBC, surgery on the metastases is still experimental because there are no data from prospective randomized trials with large samples. In addition, OMBC, even the indolent behavior, is a widespread disease, where local treatments alone could not be sufficient. However, these preliminary results may identify subgroups of patients with more favorable outcomes and for whom the surgery could lead to long-term survival [13].
2.2. Radiotherapy
Patients with oligometastatic disease or with oligorecurrence in a single area could be treated with local therapy such as stereotactic body radiotherapy (SBRT), even associated with chemotherapy. Possible target lesions include brain, lung, liver, and lymph nodes.
Oligorecurrent metastases in the brain, lung, and liver can be definitively treated with SBRT. Instead, there are some controversies regarding lymph node oligometastases, thus further phase III trials are needed [42].
The use of stereotactic ablative radiotherapy (SABR) produces favorable outcomes, since it presents high accuracy to the target lesion, very conformal dose distributions, and delivers a highly ablative dose over a treatment duration of 1–5 treatments maximum.
Several works strengthen the use of SABR in OM disease, mostly randomized controlled trials (RCTs) [4].
Patients with a limited number of brain metastases and controlled extracranial disease may benefit from locoregional treatment combined with systemic therapy, which crosses the blood–brain barrier. Currently, stereotactic radiosurgery (SRS) is the recommended option for resected cavity and non-resected brain metastases [43] and achieves longer overall survival (OS) compared to whole-brain palliative irradiation [44,45].
Concerning lung OM disease, stereotactic techniques demonstrate a 2-year local control rate of 77.9% and a 2-year OS of 53.7%, according to a systematic review [46].
At the same time, a regional nodal recurrence after conservative breast treatment affects about 1% to 5.4% of patients with early-stage breast cancer [47,48,49]. A phase II study with SBRT or intensity-modulated radiation therapy for OMBC showed encouraging results [50]. Even though the principal site of metastases was the bone, several cases of lymph node metastases were treated with SBRT or intensity-modulated radiation therapy, without reporting severe toxicity. Furthermore, 90% of patients with oligorecurrence had an objective response to salvaging radiotherapy and the 3-year treated tumor control rate was 93% [51]. However, despite the lack of reports about SBRT for oligorecurrent lymph node metastases of breast cancer, this subgroup of patients seems to be well suited for SBRT, especially those who did not receive previous irradiation, because of the indolent behavior of the disease. Nonetheless, patients should be carefully monitored over time, because of the risk of late toxicities.
The research is moving forward, with an ongoing randomized phase II/III trial (NRG-BR002), which evaluates the role of these techniques in OMBC [42,52].
2.3. Systemic Treatments
Systemic treatment remains a milestone in the management of metastatic breast cancer.
Considering hormone receptor (HR) positive, HER2-negative metastatic breast cancers, certainly CDK4/6 inhibitors in combination with endocrine therapy have changed the paradigm of the treatment [53].
Concerns about the difference among the three CDK4/6 inhibitors involve the significant OS improvement, demonstrated from MONALEESA-3, MONALEESA-7, and MONARCH-2 trials, but not reported in PALOMA-1, PALOMA-3, and MONALEESA-2 trials [54,55,56,57,58,59].
As a result, a meta-analysis of all these randomized controlled trials evaluated the OS improvement among Palbociclib, Ribociclib and Abemaciclib, and focused on the efficacy of these compounds in some relevant subgroups of patients.
Of 5862 patients from MONALEESA-2, MONALEESA-3, and MONALEESA-7 trials, 2429 presented visceral (lung or liver) disease, 929 had bone-only disease, and 2504 had visceral and bone disease. Of 2845 patients, grouped by the number of metastases, 782 had only one metastatic site, 635 two, and 1428 three or more. The pooled results of the meta-analysis showed no heterogeneity for all these subgroups, with a statistically significant improvement in PFS with a similar hazard ratio [60].
Therefore, this meta-analysis demonstrates that CDK4/6 inhibitors plus endocrine therapy are beneficial in terms of PFS, regardless of the presence of visceral metastases, the number of metastatic sites, and the length of the treatment-free interval. Consequently, the pooled estimate for the overall population is also feasible for OMBC patients [60].
However, in luminal breast cancer, even after a first-line systemic treatment, OM disease could be persistent; therefore, due to the introduction and approval from FDA and EMA of Alpelisib, it is advisable to test the presence of PIK3CA mutation. Patients with PIK3CA mutation may benefit from Alpelisib plus Fulvestrant association, both with bone metastases and visceral metastases, as shown by the subgroup analysis of the SOLAR-1 study [61]. Instead, patients without the expression of PIK3CA mutation should receive a further line of hormonal treatment; this can be Everolimus plus Exemestane or Fulvestrant alone or, in selected patients, chemotherapy; confirmed data about the use of CDK4-6 inhibitors beyond progression are still unknown, and to date there are ongoing phase III studies comparing Alpelisib plus Fulvestrant versus Fulvestrant alone (CBYL719C2303 study-EPIK-B5).
In summary, a key role in the OMBC treatment is maintaining hormonal target therapy, reserving chemotherapy in cases of visceral crisis or widespread disease.
Unlike the luminal BC, often HER-2-like and triple-negative tumors have a different presentation since they have more aggressive behavior. Therefore, in these subtypes the strategy overlaps with a polymetastatic disease: in case of an HER-2 like OMBC, the use of anti-HER-2 molecules remains the first goal; instead, the current targets for triple-negative tumors are PD-L1 and BRCA mutations, and the use of Atezolizumab plus Nab-paclitaxel and Olaparib, respectively, showed better outcomes in terms of PFS and quality of life [62,63].
2.4. Combination of Radiotherapy and Systemic Treatment
Although CDK4/6 inhibitors are largely involved in the treatment of MBC, preliminary findings suggest a possible synergic effect of these compounds when combined with radiotherapy, especially in OM disease [64].
CDK4/6 inhibitors can act as a DNA double-strand break repair inhibitor, thus amplifying the anticancer effect of RT [65].
Therefore, the simultaneous administration of a radio-sensitizing drug could significantly improve symptoms and disease control. Despite the potential benefit of this combination, there is little literature on this topic, and clinicians could be frightened, since the radio-sensitizing effect may also increase the toxicity, involving healthy tissues as well [66,67]. The consequence might lead to, on one hand, improperly interrupting the systemic treatment or the radiotherapy.
Table 2 shows the preliminary results from small patient samples with the combination of CDK4/6 inhibitors with RT.
Table 2.
Trials that evaluate the efficacy of concomitant RT and CDK4/6-i in MBC.
| Trial | Number of Patients | CDK4/6-I | Outcome |
|---|---|---|---|
| Hans et al. | 5 | Palbociclib | 5 pain relief 1 stable disease |
| Meattini et al. | 5 | Ribociclib | 3 stable disease 2 partial response |
| Chowdary et al. | 16 | Palbociclib | 16 pain relief 0 local failures |
| Ippolito et al. | 16 | Palbociclib Ribociclib |
16 pain relief 2 complete responses 2 partial responses 1 stable disease |
| Mudit et al. | 16 | Palbociclib | 16 pain relief 0 local failures |
| Guerini et al. | 18 | Palbociclib Ribociclib Abemaciclib |
16 pain relief 0 pain recurrence 17 local control 1 local recurrence |
Hans et al. described five patients treated with Palbociclib and concurrent palliative RT without severe toxicity [68]: all patients experienced pain relief, but follow-up time and local control were not reported.
Meattini et al. described five patients treated with Ribociclib and concurrent palliative RT for bone metastases [69]: two patients developed grade 3–4 toxicity (one neutropenia and one vomit and diarrhea) and two needed temporary suspension of Ribociclib; radiotherapy was never suspended. At a 3-month assessment, three stable diseases and two partial responses were observed.
Chowdary et al. evaluated 16 patients treated with Palbociclib and RT for symptomatic metastases [64]. No side effect differences were found compared to the use of Palbociclib alone; all patients experienced prolonged pain control, and no local failures were described. However, only 31.3% of patients did not interrupt Palbociclib during the RT, while the other patients suspended the CDK4/6 inhibitor 14 days before or after RT, with a median interval of 5 days.
Ippolito et al. analyzed 16 patients treated with Palbociclib or Ribociclib concomitant to RT [70]. First, 68.7% of patients received palliative RT for bone metastases with a median dose of 30 Gy, while the remaining with OM disease were treated with higher doses (median 50 Gy). At 6.3 months follow-up, the only toxicity reported was neutropenia, apparently not worsened by radiotherapy, because it had already existed during the previous cycles of systemic treatment. Patients with bone metastases experienced all pain relief; the other subgroup developed complete responses (two patients with visceral and/or soft tissue), partial responses (two patients with bone disease), and stable disease (one patient with bone involvement) [71,72,73,74,75,76].
Two other retrospective analyses evaluated risks and benefits from the concomitant therapy with CDK4/6 inhibitors and RT. In one experience 16 patients under treatment with Palbociclib and radiotherapy were studied. At a follow-up of 14.7 months, none reported relevant acute or late toxicities: all reported that side effects were mild. All the patients achieved pain relief, and no local failures were developed [64]. The second study analyzed 18 patients treated with radiotherapy and concomitant CDK4/6 inhibitors for bone involvement. The hematologic toxicity was mild during the end of RT and the subsequent cycles of systemic treatment (grade 3–4 neutropenia) [72]; the other relevant side effect was grade 1 gastrointestinal toxicity. Three months after the end of RT, 88.9% of patients experienced pain relief, with no pain recurrence. With a median follow-up of 13.7 months, only one patient developed local recurrence. This study involves the largest cohort with concomitant CDK 4/6 inhibitors and RT published, but numbers are still limited.
These preliminary works suggest that the combination of CDK4/6 inhibitors and RT, particularly on bone metastases, is safe, with limited toxicities in terms of time and grade. The hematologic toxicity is comparable between the combination of these approaches and the medical treatment alone, while the gastrointestinal side effects could be more relevant; therefore, clinicians should be careful in case of RT of the abdominal or pelvic area.
Although the results of these trials are limited by the small number of the sample, the clinical and radiological outcomes are promising. Future studies with a larger population and a longer follow-up will validate these results [64,77].
3. Conclusions
Even though metastatic breast cancer is considered incurable, OMBC presents a better prognosis [78].
Regardless of the lack of substantial data, this subset of patients could be treated with curative intent, mostly young women for whom a multidisciplinary aggressive approach appears suitable [3,78].
For these patients with a favorable nature for their disease, a multidisciplinary aggressive approach might improve survival [78].
Specifically, a combination of local and systemic treatment can achieve such long-term effects [13].
Local ablative options (radiotherapy/surgery) play a key role in this setting, as can be assumed from retrospective trials, but these encouraging results need confirmation by prospective randomized studies [78].
Moreover, preliminary data suggest an increase of disease-free survival after surgery on distant metastases; however, the selection of the appropriate candidates concerns the biology of the disease, and unfortunately, valuable comparative data are still missing. For this reason, surgery on breast cancer metastases remains an experimental approach.
Systemic therapy, e.g., the use of CDK4/6 inhibitors for HR+/HER2 negative BC, leads to an increase of PFS and OS in all the subgroups, with favorable toxicity.
Therefore, combined strategies increase the probability of producing results such as tumor-size reduction, long-lasting responses, and, eventually, cure [79].
All of these treatment strategies present a higher rate of success when the metastatic disease is detected early, so it is crucial to involve modern imaging equipment and liquid biopsies to model a personalized and multidisciplinary treatment [13].
4. Future Directions
The lack of strong data concerning the management of OMBC clearly emerges, due to the quality and heterogeneity of the systematic reviews and meta-analyses.
However, the increasing interest in the OM phenotype is emerging, and several prospective phase II/III randomized controlled trials involving new strategies for OMBC are ongoing (Table 3). A phase III study in the Netherlands (NCT01646034) is evaluating the role of high-dose chemotherapy with carboplatin, thiotepa, and cyclophosphamide in homologous recombination-deficient OMBC, since it seems that these tumors are particularly sensitive to alkylating agents which disrupt double-stranded DNA. Several trials are assessing the use of SABR and/or traditional surgery associated with systemic therapy in the first-line setting for newly diagnosed OMBC (e.g., CLEAR, NCT03750396; STEREO-SEIN, NCT02089100; NCT02364557). For instance, a pilot phase I study in Australia is evaluating the role of SABR followed by 6 months of anti-PD1 therapy with pembrolizumab, intending to show both safety and enhanced immune activation (BOSTON-II, NCT02303366).
Table 3.
Ongoing trials in oligometastatic BC.
| Trial | Objective | Site of Metastases |
|---|---|---|
| NCT01646034 | Role of high-dose polychemotherapy in HRD OMBC | 1 to 3 distant metastatic lesions, with or without primary tumor, local recurrence, or locoregional lymph node metastases, including the ipsilateral axillary, parasternal, and periclavicular regions |
| CLEAR, NCT03750396 |
Local treatment (including surgical resection, stereotactic body radiotherapy, palliative radiotherapy, and radiofrequency ablation) in addition to endocrine treatment as 1st line for HR+/HER2- OMBC | ≤2 lesions in single organ or site (lung, bone, liver, adrenal glands, distant LNs) |
| STEREO-SEIN, NCT02089100 | Role of metastases SBRT with curative intent in de novo oligometastatic disease | ≤5 metastatic lesions (measurable or not) No brain metastases |
| NCT02364557 | Use of SABR and/or traditional surgery in addition to standard of care systemic therapy in the first-line setting for newly diagnosed OMBC | ≤4 metastases in lung, bone, spine, abdominal-pelvic (lymph node/adrenal gland), liver, mediastinal/cervical lymph node |
| BOSTON-II, NCT02303366 |
Role of SABR followed by 6 months of anti-PD1 therapy with pembrolizumab, intending to show both safety and enhanced immune activation | 1 to 5 metastases No evidence of visceral metastases in liver or brain |
The comparison of these trial results is weakened by the different definition of ‘oligometastatic disease’, which could include from two to five distant lesions. For further future studies, it would be reasonable to employ a universal definition of ‘oligometastatic’ within the breast cancer investigative community [3].
Author Contributions
Conceptualization, A.F.; methodology, A.P., G.F., F.M., I.P.; resources, A.F. and V.B.; writing—review and editing V.B., A.F.; supervision, R.M., F.C. and G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Henry N.L., Shah P.D., Haider I., Freer P.E., Jagsi R., Sabel M.S. Cancer of the Breast. Abeloff’s Clin. Oncol. 2020;12:1560–1603. [Google Scholar]
- 2.O’Shaughnessy J. Extending Survival with Chemotherapy in Metastatic Breast Cancer. Oncology. 2005;10:20–29. doi: 10.1634/theoncologist.10-90003-20. [DOI] [PubMed] [Google Scholar]
- 3.Makhlin I., Fox K. Oligometastatic Breast Cancer: Is This a Curable Entity? A Contemporary Review of the Literature. Curr. Oncol. Rep. 2020;22:1–10. doi: 10.1007/s11912-020-0867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Shafa F., Arifin A.J., Rodrigues G.B., Palma D.A., Louie A.V. A Review of Ongoing Trials of Stereotactic Ablative Radiotherapy for Oligometastatic Cancers: Where Will the Evidence Lead? Front. Oncol. 2019;9:543. doi: 10.3389/fonc.2019.00543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palma D.A., Louie A.V., Rodrigues G.B. New Strategies in Stereotactic Radiotherapy for Oligometastases. Clin. Cancer Res. 2015;21:5198–5204. doi: 10.1158/1078-0432.CCR-15-0822. [DOI] [PubMed] [Google Scholar]
- 6.Huang F., Wu G., Yang K. Oligometastasis and oligo-recurrence. Radiat. Oncol. 2014;9:230. doi: 10.1186/s13014-014-0230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pagani O., Senkus E., Wood W., Colleoni M., Cufer T., Kyriakides S., Costa A., Winer E.P., Cardoso F., Force E.-M.T. International guidelines for management of metastatic breast cancer: Can metastatic breast cancer be cured? J. Natl. Cancer Inst. 2010;102:456–463. doi: 10.1093/jnci/djq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cardoso F., Costa A., Senkus E., Aapro M., Andre F., Barrios C.H., Bergh J., Bhattacharyya G., Biganzoli L., Cardoso M.J., et al. 3rd ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 3) Ann. Oncol. 2017;28:16–33. doi: 10.1093/annonc/mdw544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jain S.K., Dorn P.L., Chmura S.J., Weichselbaum R.R., Hasan Y. Incidence and implications of oligometastatic breast cancer. J. Clin. Oncol. 2012;30:e11512. doi: 10.1200/jco.2012.30.15_suppl.e11512. [DOI] [Google Scholar]
- 10.Niibe Y., Hayakawa K. Oligometastases and Oligo-recurrence: The New Era of Cancer Therapy. Jpn. J. Clin. Oncol. 2010;40:107–111. doi: 10.1093/jjco/hyp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Correa R.J.M., Salama J.K., Milano M.T., Palma D.A. Stereotactic body radiotherapy for oligometastasis opportunities for biology to guide clinical management. Cancer J. 2016;22:247–256. doi: 10.1097/PPO.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 12.Reyes D.K., Pienta K.J. The biology and treatment of oligometastatic cancer. Oncotarget. 2015;6:8491–8524. doi: 10.18632/oncotarget.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westphal T., Gampenrieder S.P., Rinnerthaler G., Greil R. Cure in metastatic breast cancer. Memo Mag. Eur. Med. Oncol. 2018;11:172–179. doi: 10.1007/s12254-018-0426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Divisi D., Barone M., Zaccagna G., Gabriele F., Crisci R. Surgical approach in the oligometastatic patient. Ann. Transl. Med. 2018;6:94. doi: 10.21037/atm.2018.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Criscitiello C., Giuliano M., Curigliano G., Laurentiis M.D., Arpino G., Carlomagno N., Placido S.D., Golshan M., Santangelo M. Surgery of the primary tumor in de novo metastatic breast cancer: To do or not to do? Eur. J. Surg. Oncol. 2015;41:1288–1292. doi: 10.1016/j.ejso.2015.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Harris E., Barry M., Kell M.R. Meta-analysis to determine if surgical resection of the primary tumour in the setting of stage IV breast cancer impacts on survival. Ann. Surg. Oncol. 2013;20:2828–2834. doi: 10.1245/s10434-013-2998-2. [DOI] [PubMed] [Google Scholar]
- 17.Warschkow R., Güller U., Tarantino I., Cerny T., Schmied B.M., Thuerlimann B., Joerger M. Improved Survival after Primary Tumor Surgery in Metastatic Breast Cancer: A Propensity-adjusted, Population-based SEER Trend Analysis. Ann. Surg. 2016;263:1188–1198. doi: 10.1097/SLA.0000000000001302. [DOI] [PubMed] [Google Scholar]
- 18.Badwe R., Hawaldar R., Nair N., Kaushik R., Parmar V., Siddique S., Budrukkar A., Mittra I., Gupta S. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: An open-label randomised controlled trial. Lancet Oncol. 2015;16:1380–1388. doi: 10.1016/S1470-2045(15)00135-7. [DOI] [PubMed] [Google Scholar]
- 19.Soran A., Ozmen V., Ozbas S., Karanlik H., Muslumanoglu M., Igci A., Canturk Z., Utkan Z., Ozaslan C., Evrensel T. Randomized Trial Comparing Resection of Primary Tu-mor with No Surgery in Stage IV Breast Cancer at Presentation: Protocol MF07-01. Ann. Surg. Oncol. 2018;25:3141–3149. doi: 10.1245/s10434-018-6494-6. [DOI] [PubMed] [Google Scholar]
- 20.Khan S.A., Zhao F., Solin L.J., Goldstein L.J., Cella D., Basik M., Golshan M., Julian T.B., Pockaj B.A., Lee C.A. A randomized phase III trial of systemic therapy plus early local therapy versus systemic therapy alone in women with de novo stage IV breast cancer: A trial of the ECOG-ACRIN Research Group (E2108) J. Clin. Oncol. 2020;38:LBA2. doi: 10.1200/JCO.2020.38.18_suppl.LBA2. [DOI] [Google Scholar]
- 21.King T.A., Lyman J., Gonen M., Reyes S., Hwang E.-S.S., Rugo H.S., Liu M.C., Boughey J.C., Jacobs L.K., McGuire K.P., et al. A prospective analysis of surgery and survival in stage IV breast cancer (TBCRC 013) J. Clin. Oncol. 2016;34:1006. doi: 10.1200/JCO.2016.34.15_suppl.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raab R., Nussbaum K.T., Behrend M., Weimann A. Liver metastases of breast cancer: Results of liver resection. Anticancer Res. 1998;18:2231–2233. [PubMed] [Google Scholar]
- 23.Pocard M., Pouillart P., Asselain B., Salmon R.-J. Hepatic resection in metastatic breast cancer: Results and prognostic factors. Eur. J. Surg. Oncol. 2000;26:155–159. doi: 10.1053/ejso.1999.0761. [DOI] [PubMed] [Google Scholar]
- 24.Yoshimoto M., Tada T., Saito M., Takahashi K., Makita M., Uchida Y., Kasumi F. Surgical treatment of hepatic metastases from breast cancer. Breast Cancer Res. Treat. 2000;59:177–184. doi: 10.1023/A:1006398401352. [DOI] [PubMed] [Google Scholar]
- 25.Pocard M., Pouillart P., Asselain B., Falcou M.C., Salmon R.J. Hepatic resection for breast cancer metastases: Results and prognosis (65cases) Ann. Chir. 2001;126:413–420. doi: 10.1016/S0003-3944(01)00526-0. [DOI] [PubMed] [Google Scholar]
- 26.Ercolani G., Zanello M., Serenari M., Cescon M., Cucchetti A., Ravaioli M., Gaudio M.D., D’Errico A., Brandi G., Pinna A.D. Ten-year survival after liver resection for breast metastases: A single-center experience. Dig. Surg. 2018;4:372–380. doi: 10.1159/000486523. [DOI] [PubMed] [Google Scholar]
- 27.Elias D., Maisonnette F., Druet-Cabanac M., Ouellet J.F., Guinebretiere J.M., Spielmann M., Delaloge S. An attempt to clarify indications for hepatectomy for liver metastases from breast cancer. Am. J. Surg. 2003;185:158–164. doi: 10.1016/S0002-9610(02)01204-7. [DOI] [PubMed] [Google Scholar]
- 28.Weinrich M., Weiß C., Schuld J., Rau B.M. Liver Resections of Isolated Liver Metastasis in Breast Cancer: Results and Possible Prognostic Factors. HPB Surg. 2014;2014:893829. doi: 10.1155/2014/893829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ercolani G., Grazi G.L., Ravaioli M., Ramacciato G., Cescon M., Varotti G., Del Gaudio M., Vetrone G., Pinna A.D. The role of liver resections for noncolorectal, nonneuroendocrine metastases: Experience with 142 observed cases. Ann. Surg. Oncol. 2005;12:459–466. doi: 10.1245/ASO.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 30.Vlastos G., Smith D.L., Singletary S.E., Mirza N.Q., Tuttle T.M., Popat R.J., Curley S.A., Ellis L.M., Roh M.S., Vauthey J.N. Long-term survival after an aggressive surgical approach in patients with breast cancer hepatic metastases. Ann. Surg. Oncol. 2004;11:869–874. doi: 10.1245/ASO.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Sakamoto Y., Yamamoto J., Yoshimoto M., Kasumi F., Kosuge T., Kokudo N., Makuuchi M. Hepatic resection formetastatic breast cancer: Prognostic analysis of 34 patients. World J. Surg. 2005;29:524–547. doi: 10.1007/s00268-004-7688-6. [DOI] [PubMed] [Google Scholar]
- 32.Adam R., Aloia T., Krissat J., Bralet M.P., Paule B., Giacchetti S., Delvart V., Azoulay D., Bismuth H., Castaing D. Is liver re-section justified for patients with hepatic metastases from breast cancer? Ann. Surg. 2006;244:897–907. doi: 10.1097/01.sla.0000246847.02058.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Margonis G.A., Buettner S., Sasaki K., Kim Y., Ratti F., Russolillo N., Ferrero A., Berger N., Gamblin T.C., Poultsides G., et al. The role of liver directed surgery in patients with hepatic metastasis from primary breast cancer: A multi-institutional analysis. HPB. 2016;18:700–705. doi: 10.1016/j.hpb.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye T., Yang B., Tong H., Zhang Y., Xia J. Long-term outcomes of surgical resection for liver metastasis from breast cancer. Hepatogastroenterology. 2015;62:688–692. [PubMed] [Google Scholar]
- 35.Kobryn E., Kobryn K., Wroblewski T., Kobryn K., Pietrzak R., Rykowski P., Ziarkiewicz-Wroblewska B., Lamparski K., Zieniewicz K., Patkowski W. Is there a rationale for aggressive breast cancer liver metas-tases resections in Polish female patients? Analysis of overall survival following hepatic resection at a single centre in Poland. Ann. Agric. Environ. Med. 2016;23:683–687. doi: 10.5604/12321966.1226866. [DOI] [PubMed] [Google Scholar]
- 36.Zegarac M., Nikolic S., Gavrilovic D., Jevric M., Kolarevic D., Nikolic-Tomasevic Z., Kocic M., Djurisic I., Inic Z., Ilic V., et al. Prognostic factors for longer disease free survival and overall survival after surgical resection of isolated liver metastasis from breast cancer. J. BUON. 2013;18:859–865. [PubMed] [Google Scholar]
- 37.D’Angelica M. Hepatic resection for metastatic breast cancer: An exercise in selection bias. HPB. 2016;18:631–632. doi: 10.1016/j.hpb.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoffmann K., Franz C., Hinz U., Schirmacher P., Herfarth C., Eichbaum M., Büchler M.W., Schemmer P. Liver Resection for Multimodal Treatment of Breast Cancer Metastases: Identification of Prognostic Factors. Ann. Surg. Oncol. 2010;17:1546–1554. doi: 10.1245/s10434-010-0931-5. [DOI] [PubMed] [Google Scholar]
- 39.Friedel G., Pastorino U., Ginsberg R.J., Goldstraw P., Johnston M., Pass H., Putnam J.B., Toomes H. International Registry of Lung Metastases L. Results of lung metastasectomy from breast cancer: Prognostic criteria on the basis of 467 cases of the International Registry of Lung Metastases. Eur. J. Cardiothorac. Surg. 2002;22:335–344. doi: 10.1016/S1010-7940(02)00331-7. [DOI] [PubMed] [Google Scholar]
- 40.Livartowski A., Chapelier A., Beuzeboc P., Dierick A., Asselain B., Dartevelle P., Pouillart P. Surgical excision of pulmonary metastasis of cancer of the breast: Apropos of 40 patients. Bull. Cancer. 1998;85:799–802. [PubMed] [Google Scholar]
- 41.Fan J., Chen D., Du H., Shen C., Che G. Prognostic factors for resection of isolated pulmonary metastases in breast cancer patients: A systematic review and meta-analysis. J. Thorac. Dis. 2015;7:1441–1451. doi: 10.3978/j.issn.2072-1439.2015.08.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsushita H., Jingu K., Umezawa R., Yamamoto T., Ishikawa Y., Takahashi N., Katagiri Y., Kadoya N. Stereotactic Radiotherapy for Oligometastases in Lymph Nodes—A Review. Technol. Cancer Res. Treat. 2018;17:1–8. doi: 10.1177/1533033818803597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Comprehensive Cancer Network Central Nervous System Cancers (Version 2.2018) [(accessed on 21 February 2019)]; Available online: https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.
- 44.Gondi V., Hermann B.P., Mehta M.P., Tomé W.A. Hippocampal dosimetry predicts neurocognitive function impairment after fractionated stereotactic radiotherapy for benign or low-grade adult brain tumors. Int. J. Radiat. Oncol. Biol. Phys. 2013;85:348–354. doi: 10.1016/j.ijrobp.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 45.Li J., Bentzen S.M., Renschler M., Mehta M.P. Regression after Whole-Brain Radiation Therapy for Brain Metastases Correlates with Survival and Improved Neurocognitive Function. J. Clin. Oncol. 2007;25:1260–1266. doi: 10.1200/JCO.2006.09.2536. [DOI] [PubMed] [Google Scholar]
- 46.Ashworth A., Rodrigues G., Boldt G., Palma D. Is there an oligometastatic state in non-small cell lung cancer? A systematic review of the literature. Lung Cancer. 2013;82:197–203. doi: 10.1016/j.lungcan.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 47.Pejavar S., Wilson L.D., Haffty B.G. Regional nodal recurrence in breast cancer patients treated with conservative surgery and radiation therapy (BCSþRT) Int. J. Radiat. Oncol. Biol. Phys. 2006;66:1320–1327. doi: 10.1016/j.ijrobp.2006.07.1379. [DOI] [PubMed] [Google Scholar]
- 48.Whelan T.J., Olivotto I.A., Parulekar W.R., Ackerman I., Chua B.H., Nabid A., Katherine A., Vallis M.B., White J.R., Rousseau P. Regional nodal irradiation in early-stage breast cancer. N. Engl. J. Med. 2015;373:307–316. doi: 10.1056/NEJMoa1415340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stranzl H., Peintinger F., Ofner P., Prettenhofer U., Mayer R., Hackl A. Regional Nodal Recurrence in the Management of Breast Cancer Patients with One to Three Positive Axillary Lymph Nodes. Strahlenther. Onkol. 2004;180:623–628. doi: 10.1007/s00066-004-1241-2. [DOI] [PubMed] [Google Scholar]
- 50.Trovo M., Furlan C., Polesel J., Fiorica F., Arcangeli S., Giaj-Levra N., Alongi F., Conte A.D., Militello L., Muraro E., et al. Radical radiation therapy for oligometastatic breast cancer: Results of a prospective phase II trial. Radiother. Oncol. 2018;126:177–180. doi: 10.1016/j.radonc.2017.08.032. [DOI] [PubMed] [Google Scholar]
- 51.Miyata M., Ohguri T., Yahara K., Yamaguchi S., Imada H., Korogi Y. Salvage radiotherapy for second oligo-recurrence in patients with breast cancer. J. Radiat. Res. 2017;59:58–66. doi: 10.1093/jrr/rrx066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.NRG Oncology. [(accessed on 11 February 2021)]; Available online: https://www.nrgoncology.org/Clinical-Trials/NRG-BR002.
- 53.Rugo H.S., Rumble R.B., Macrae E., Barton D.L., Connolly H.K., Dickler M.N., Fallowfield L., Fowble B., Ingle J.N., Jahanzeb M., et al. Endocrine Therapy for Hormone Receptor–Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline. J. Clin. Oncol. 2016;34:3069–3103. doi: 10.1200/JCO.2016.67.1487. [DOI] [PubMed] [Google Scholar]
- 54.Turner N.C., Slamon D.J., Ro J., Bondarenko I., Im S.-A., Masuda N., Colleoni M., DeMichele A., Loi S., Verma S., et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 55.Hortobagyi G.N., Stemmer S.M., Burris H.A., Yap Y.S., Sonke G.S., Paluch-Shimon S., Campone M., Petrakova K., Blackwell K.L., Winer E.P., et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letro-zole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018;29:1541–1547. doi: 10.1093/annonc/mdy155. [DOI] [PubMed] [Google Scholar]
- 56.Im S.-A., Lu Y.-S., Bardia A., Harbeck N., Colleoni M., Franke F., Chow L., Sohn J., Lee K.-S., Campos-Gomez S., et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 57.Slamon D.J., Neven P., Chia S., Fasching P.A., De Laurentiis M., Im S.-A., Petrakova K., Bianchi G.V., Esteva F.J., Martín M., et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 58.Sledge G.W., Toi M., Neven P., Sohn J., Inoue K., Pivot X., Burdaeva O., Okera M., Masuda N., Kaufman P.A., et al. The E_ect of Abemaciclib plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2019;6:116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Finn R.S., Boer K., Bondarenko I., Patel R., Pinter T., Schmidt M., Shparyk Y.V., Thummala A., Voitko N., Bananis E., et al. Overall survival results from the randomized phase 2 study of palbociclib in combination with letrozole versus letrozole alone for first-line treatment of ER+/HER2− advanced breast cancer (PALOMA-1, TRIO-18) Breast Cancer Res. Treat. 2020;183:419–428. doi: 10.1007/s10549-020-05755-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rossi V., Berchialla P., Giannarelli D., Nisticò C., Ferretti G., Gasparro S., Russillo M., Catania G., Vigna L., Mancusi R.L., et al. Should All Patients with HR-Positive HER2-Negative Metastatic Breast Cancer Receive CDK 4/6 Inhibitor as First-Line Based Therapy? A Network Meta-Analysis of Data from the PALOMA 2, MONALEESA 2, MONALEESA 7, MONARCH 3, FALCON, SWOG and FACT Trials. Cancers. 2019;26:1661. doi: 10.3390/cancers11111661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.André F., Ciruelos E., Rubovszky G., Campone M., Loibl S., Rugo H.S., Iwata H., Conte P., Mayer I.A., Kaufman B., et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 62.Schmid P., Adams S., Rugo H.S., Schneeweiss A., Barrios C.H., Iwata H., Diéras V., Hegg R., Im S.A., Shaw Wright G., et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018;379:2108–2121. doi: 10.1056/NEJMoa1809615. [DOI] [PubMed] [Google Scholar]
- 63.Robson M., Im S.-A., Senkus E., Xu B., Domchek S.M., Masuda N., Delaloge S., Li W., Tung N., Armstrong A., et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 64.Chowdhary M., Sen N., Chowdhary A., Usha L., Cobleigh M.A., Wang D., Patel K.R., Barry P.N., Rao R.D. Safety and Efficacy of Palbociclib and Radiation Therapy in Patients with Metastatic Breast Cancer: Initial Results of a Novel Combination. Adv. Radiat. Oncol. 2019;4:453–457. doi: 10.1016/j.adro.2019.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang C.-Y., Hsieh F.-S., Wang C.-Y., Chen L.-J., Chang S.-S., Tsai M.-H., Hung M.-H., Kuo C.-W., Shih C.-T., Chao T.-I., et al. Palbociclib enhances radiosensitivity of hepatocellular carcinoma and cholangiocarcinoma via inhibiting ataxia telangiectasia–mutated kinase–mediated DNA damage response. Eur. J. Cancer. 2018;102:10–22. doi: 10.1016/j.ejca.2018.07.010. [DOI] [PubMed] [Google Scholar]
- 66.Kawamoto T., Shikama N., Sasai K. Severe acute radiation-induced enterocolitis after combined palbociclib and palliative radiotherapy treatment. Radiother. Oncol. 2019;131:240–241. doi: 10.1016/j.radonc.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 67.Messer J.A., Ekinci E., Patel T.A., Teh B.S. Enhanced dermatologic toxicity following concurrent treatment with palbociclib and radiation therapy: A case report. Rep. Pract. Oncol. Radiother. 2019;24:276–280. doi: 10.1016/j.rpor.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hans S., Cottu P., Kirova Y.M. Preliminary results of the association of Palbociclib and radiotherapy in metastatic breast cancer patients. Radiother. Oncol. 2018;126:181. doi: 10.1016/j.radonc.2017.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Meattini I., Desideri I., Scotti V., Simontacchi G., Livi L. Ribociclib plus letrozole and concomitant palliative radiotherapy for metastatic breast cancer. Breast. 2018;42:1–2. doi: 10.1016/j.breast.2018.08.096. [DOI] [PubMed] [Google Scholar]
- 70.Ippolito E., Greco C., Silipigni S., Dell’Aquila E., Petrianni G.M., Tonini G., Fiore M., D’Angelillo R.M., Ramella S. Concurrent radiotherapy with palbociclib or ribociclib for metastatic breast cancer patients: Preliminary assessment of toxicity. Breast. 2019;46:70–74. doi: 10.1016/j.breast.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 71.Finn R.S., Martin M., Rugo H.S., Jones S., Im S.-A., Gelmon K., Harbeck N., Lipatov O.N., Walshe J.M., Moulder S., et al. Palbociclib and Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 72.Cristofanilli M., Turner N.C., Bondarenko I., Ro J., Im S.-A., Masuda N., Colleoni M., DeMichele A., Loi S., Verma S., et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016;17:425–439. doi: 10.1016/s1470-2045(15)00613-0. [DOI] [PubMed] [Google Scholar]
- 73.O’Shaughnessy J., Petrakova K., Sonke G.S., Conte P., Arteaga C.L., Cameron D.A., Hart L.L., Villanueva C., Jakobsen E., Beck J.T., et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2− advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res. Treat. 2018;168:127–134. doi: 10.1007/s10549-017-4518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tripathy D., Im S.-A., Colleoni M., Franke F., Bardia A., Harbeck N., Hurvitz S.A., Chow L., Sohn J., Lee K.S., et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): A randomised phase 3 trial. Lancet Oncol. 2018;19:904–915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 75.Sledge G.W., Jr., Toi M., Neven P., Sohn J., Inoue K., Pivot X., Burdaeva O., Okera M., Masuda N., Kaufman P.A., et al. MONARCH 2: Abemaciclib in Combination with Fulvestrant in Women with HR+/HER2− Advanced Breast Cancer Who Had Progressed while Receiving Endocrine Therapy. J. Clin. Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 76.Goetz M.P., Toi M., Campone M., Sohn J., Paluch-Shimon S., Huober J., Park I.H., Trédan O., Chen S.-C., Manso L., et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 77.Guerini A.E., Pedretti S., Salah E., Simoncini E.L., Maddalo M., Pegurri L., Pedersini R., Vassalli L., Pasinetti N., Peretto G., et al. A single-center retrospective safety analysis of cyclin-dependent kinase 4/6 inhibitors concurrent with radiation therapy in metastatic breast cancer patients. Sci. Rep. 2020;10:1–8. doi: 10.1038/s41598-020-70430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kwapisz D. Oligometastatic breast cancer. Breast Cancer. 2018;26:138–146. doi: 10.1007/s12282-018-0921-1. [DOI] [PubMed] [Google Scholar]
- 79.Kent C.L., McDuff S.G.R., Salama J.K. Oligometastatic breast cancer: Where are we now and where are we headed?—A narrative review. Ann. Palliat. Med. 2021;10:5954–5968. doi: 10.21037/apm-20-1128. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.