Abstract

Sexually transmitted infections, including the human immunodeficiency virus (HIV) and the human papillomavirus (HPV), disproportionally impact those in low-resource settings. Early diagnosis is essential for managing HIV. Similarly, HPV causes nearly all cases of cervical cancer, the majority (90%) of which occur in low-resource settings. Importantly, infection with HPV is six times more likely to progress to cervical cancer in women who are HIV-positive. An inexpensive, adaptable point-of-care test for viral infections would make screening for these viruses more accessible to a broader set of the population. Here, we report a novel, cost-effective electrochemical platform using gold leaf electrodes to detect clinically relevant viral loads. We have combined this platform with loop-mediated isothermal amplification and a CRISPR-based recognition assay to detect HPV. Lower limits of detection were demonstrated down to 104 total copies of input nucleic acids, which is a clinically relevant viral load for HPV DNA. Further, proof-of-concept experiments with cervical swab samples, extracted using standard extraction protocols, demonstrated that the strategy is extendable to complex human samples. This adaptable technology could be applied to detect any viral infection rapidly and cost-effectively.
Short abstract
Gold leaf electrodes were fabricated and functionalized with methylene blue DNA. These electrodes were used to detect target-activated Cas12a endonuclease activity.
Introduction
Low-resource settings (LRS) disproportionately carry the burden of infectious disease.1 Sexually transmitted infections (STIs) affect millions of people per year and constitute a global health crisis.2 80% of new STI cases take place in low-resource settings (LRS), which lack the facilities, trained personnel, and money to carry out many laboratory-based STI tests.2 Point-of-care (POC) tests that can be used with minimal equipment and personnel have been shown to mitigate disease outbreaks in LRS.3 However, wide adaptation of POC tests in LRS is prohibitive due to the tests’ relatively high cost.2,4,5 Here, we report a novel, affordable, gold leaf electrode platform with a retail cost of materials of $0.50/test. We combine these electrodes with loop-mediated-isothermal amplification (LAMP) and CRISPR-based detection to detect multiple diseases at a total retail cost of ∼$2.30/test. As a proof of concept, this novel platform was tested with clinical samples that were extracted using traditional laboratory extraction techniques. While this adds costs, this novel platform can be paired with existing paperfluidic technologies for low-cost clinical sample preparation.6−8 We focus here on human immunodeficiency virus (HIV) and human papillomavirus (HPV) as model systems, but this platform could be used to detect any viral disease, including SARS-CoV-2.
HIV is an RNA virus that, when left untreated, progresses to the lethal acquired immune deficiency syndrome (AIDS).9 Early diagnosis and treatment are crucial to manage HIV and prevent AIDS. While there are a number of lateral flow−based rapid antigen/antibody tests for HIV, one limitation is that there is a longer window period for these tests (18–90 days postexposure) compared to nucleic acid tests (10–33 days postexposure).10 Because of the high costs of conventional methods, nucleic acid tests are not employed in LRS, which can lead to undetected cases of HIV and further spread of the virus.11 Our technology demonstrates a proof-of-concept platform that, when paired with other microfluidic technology, has the potential to be more cost-effective than traditional nucleic acid tests and has the potential to detect early acute HIV infections that are missed by current rapid tests. Our technology could also be modified at the sequence level and be applied to detect any infectious agent with minor modifications. In this work, we first use plasmid DNA containing the HIV gag gene as a model system to demonstrate our CRISPR-based detection of pathogenic DNA.
Infection with HPV causes nearly all cases of cervical cancer,12 which is the fourth-most common cancer in women and trans men globally.13 Anyone who has a cervix should be screened for HPV. Two strains, HPV 16 and 18, cause over 70% of cervical cancer cases.12 This form of cancer can be easily cured if diagnosed and treated early;12 cervical cancer diagnostics via HPV DNA testing offers high sensitivity (>96–100%) and specificity (>90–100%).6 HPV DNA testing is part of gold-standard cervical cancer diagnostics in high-resource settings due to its high sensitivity and specificity.14 LRS lack the funds, infrastructure, and personnel needed to carry out such laboratory tests.15 Thus, over 90% of deaths due to cervical cancer occur in LRS.12 Therefore, it is imperative to develop low-cost, easy-to-use point-of-care tests for HPV to enable preventative screening for cervical cancer in such LRS. Importantly, infection with HPV is six times more likely to progress to cervical cancer in women who are HIV-positive,16 making the rapid diagnosis of these viruses essential for the health and well-being of the global population.
There are currently five FDA-approved tests for detecting high risk HPV (hrHPV) DNA.17 These are laboratory-based tests that cost between $30 and $75/test.17 The HC2 Assay (Qiagen, Germantown, MD) uses antibodies to bind to DNA–RNA hybrids but is too costly to be widely implemented in LRS. The Cervista HPV HR and HPV 16/18 (Hologic, Marlborough, MA) require amplification to detect nucleic acids. The Cobas HPV (Roche, Indianapolis, IN) and Aptima (Hologic, Marlborough, MA) tests use PCR and transcription-mediated amplification, respectively. These tests all require specialized laboratory equipment and trained personnel and are unusable in many LRS. Additional POC tests for HPV include careHPV (Qiagen, Germantown, MD) and GeneXpert (Cepheid, Sunnyvale, CA). The careHPV test costs $5–42/test, while the GeneXpert costs $20/test.18 However, the instrumentation cost for the careHPV test is $20,000, while the instrumentation cost for the GeneXpert tests ranges from $11,530 to 71,500.18 The retail cost of a hand-held potentiostat needed to run our electrochemical tests is $2000 (Palmsens).
Here, we present a universal biosensor for the detection of any genetic material, with demonstrations for both HIV and HPV sensing. Our sensor uses a novel integrated, three-electrode platform fabricated using pure 24K gold leaf. Gold leaf is a 0.1 μm thick sheet of gold often sold for craft or decoration purposes19 that results in a lower fabrication cost due to the small amount of gold required. Each device cost ∼$0.50 to fabricate and uses only $0.16 worth of gold. This cost is nearly an order of magnitude less expensive than commercially available screen-printed gold electrodes, which are sold for a retail cost of ∼$4/electrode. Although pure gold leaf has been reported for use in electrodes,19,20 these electrodes have not been surface-modified for biosensing purposes. Alloyed gold leaf that contains a blend of gold and silver has been used to make functionalized nanoporous gold electrodes.21−23 However, the fabrication of these electrodes requires chemical etching of the silver, necessitating a harsh chemical treatment that must be performed in a fume hood and specialized chemical disposal and electrical equipment. To our knowledge, this is the first report of an integrated, three-electrode device constructed from pure gold leaf and the first report of pure gold leaf being used for biosensing purposes. Furthermore, as our device fabrication does not require any specialized equipment, harsh chemicals, or cleanroom space, these electrodes can easily be produced in LRS without the cost or equipment requirements of commonly used methods such as physical vapor deposition24 or screen printing.25 Our fabrication method only requires a sheet of gold leaf, adhesives, a stencil, and a razor blade for assembly, making it suitable for production in LRS. Additionally, these devices can be read using a hand-held potentiostat.
In addition, we developed three Cas12a assays to detect DNA targets containing the HIV gag gene, HPV 16 E7 gene, and HPV 18 E7 gene. We coupled these CRISPR-based detection assays with loop-mediated isothermal amplification (LAMP), a low-cost amplification method suitable for use in LRS,26 to detect clinically relevant viral loads of HPV DNA. For this, we use HPV 18 as a model system. Finally, we demonstrate that we can extract HPV 18 DNA from clinical samples, amplify them using LAMP, and use our gold leaf electrodes for the electrochemical, CRISPR-based detection of HPV 18 LAMP amplicons.
Scheme 1.
Cas12a is engineered to bind to HPV 18 DNA. This binding results in endonuclease activity that causes the Cas12a to cleave MB (methylene blue)-labeled oligonucleotides immobilized on gold leaf electrodes, resulting in a signal decrease from the methylene blue (top). Cas12a is not activated by non-HPV 18 genetic material; in this case, Cas12a does not exhibit endonuclease activity and does not cleave oligonucleotides immobilized on gold leaf electrodes, causing the methylene blue signal to stay the same (bottom).
Results and Discussion
We used CRISPR Cas12a, an enzyme that exhibits endonuclease activity upon binding to a specific sequence of DNA,27 to detect viral DNA from clinical samples or cDNA. We chose an electrochemical28−33 readout to detect Cas12a trans-cleavage activity rather than a fluorescent27,34−39 or colorimetric34−37 readout; electrochemical readout is favorable for point-of-care applications in LRS due to its sensitivity, low cost, and direct transduction to easily quantifiable electric signals via portable and easy-to-use electrochemical hardware.40,41
Specific Detection of HIV, HPV 16, and HPV 18 via Electrochemical Detection of Cas12a Endonuclease Activity
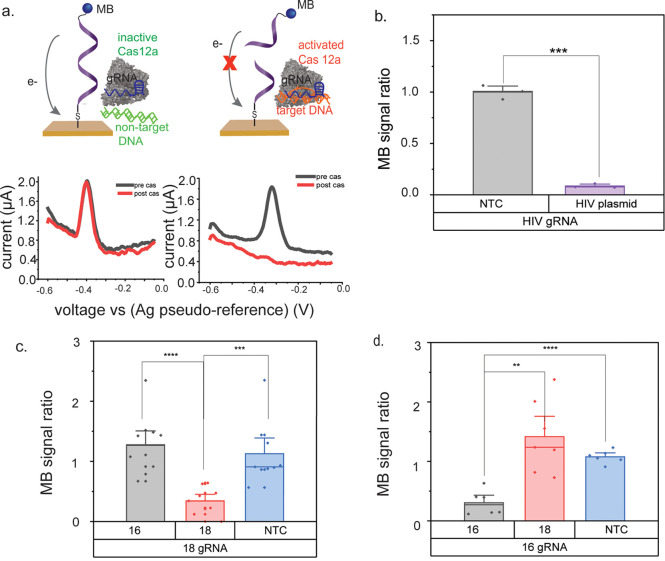
Gold leaf electrodes were fabricated (Figure 1), and their surface topography was characterized using optical profilometry (Figure S9a) and cyclic voltammetry (Figure S9b,c). Electrodes that were used for biosensing purposes were pretreated with 0.5 M H2SO4 (Figure S6) and functionalized with methylene blue-tagged oligos (Figures S7 and S8). Methylene blue is a well-established redox mediator in electrochemical biosensors; it generates a characteristic, reversible peak and is stable under biologically relevant conditions.42,43 Additionally, methylene blue is available as a modification to commercial oligonucleotides, minimizing the preparation necessary for DNA-modified electrodes. To monitor the cleavage of the methylene blue-tagged oligos (and therefore endonuclease activity at the electrode surface), oligo-functionalized electrodes were scanned by square wave voltammetry (SWV) both before and after treatment with either activated or inactivated Cas12a. The ratio of the electrochemical signal from methylene blue after Cas12a treatment to the signal before treatment was determined for each electrode. This ratio is small (significantly <1) in the presence of activated Cas12a and ∼1 in the absence of the endonuclease (Figure 2a).
Figure 1.
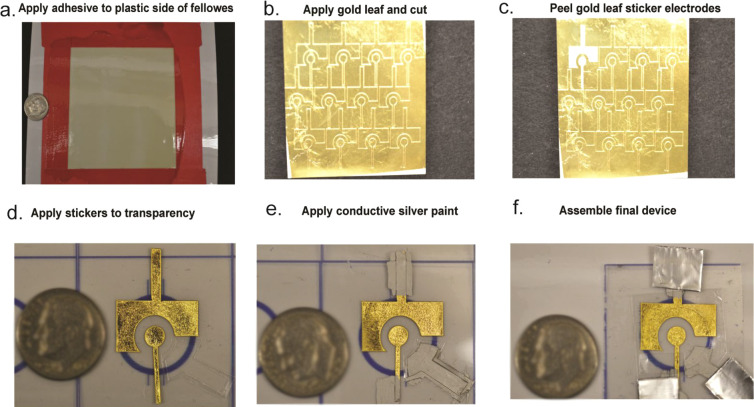
Electrode fabrication. (a) A gold leaf adhesive is applied to one side of a sheet of Fellowes. (b) Gold leaf is applied to the adhesive and cut. (c) Gold leaf stickers are peeled off. (d) Gold leaf stickers are placed on transparency film, and a sticker outlining the reference electrode is placed on the transparency film as well. (e) Conductive silver paint is applied to the reference electrode and the leads of the counter and working electrodes. (f) Aluminum foil contacts are placed at the leads of all three electrodes, and a sample well is placed over all three electrodes.
Figure 2.
Electrochemical detection of HPV 18-activated Cas12a using gold leaf electrodes. (a) Square wave voltammograms of gold leaf electrodes modified with methylene blue (MB)-labeled oligonucleotides. Target-activated Cas12a cleaves MB-oligos and results in a signal decrease from the MB. Inactive Cas12a does not cleave MB-oligos, causing the MB signal to stay the same. (b) Cas12a is engineered to detect HPV 16 DNA. The MB signal decreases only in the presence of HPV 16 DNA. (c) Cas12a is engineered to detect HPV 18 DNA. The MB signal decreases only in the presence of HPV 18 DNA. (d) Cas12a is engineered to detect HPV 16 DNA. The MB signal decreases only in the presence of HPV 16 DNA. The asterisks represent statistical significance according to a t-test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
To test the detection scheme, we designed Cas12a assays for HIV, HPV 16, and HPV 18. As shown in Figure 2b, we used DNA-modified gold leaf electrodes to monitor HIV DNA-activated Cas12a endonuclease activity. HIV is an RNA virus that needs to be reverse transcribed to DNA for detection. In this report, we used Cas12a to detect a plasmid containing the HIV genetic sequence. In our assay, the guide RNA (gRNA) of the Cas12a enzyme is engineered to recognize the p24 locus of the HIV gag gene. The ratio of signal after treatment with the Cas12a enzyme to signal before treatment with the Cas12a enzyme is ∼1 for the no-template control but less than 0.25 in the presence of HIV DNA, demonstrating that the methylene blue signal only decreases in the presence of the HIV sequence.
We designed Cas12a assays for both HPV 16 and HPV 18 using a similar approach. We used the HPV 18 and HPV 16 assays as a model system to further develop the platform. As shown in Figure 2d, we designed a Cas12a assay to target the E7 gene of HPV 16. In this case, the methylene blue signal decreases only when the Cas12a/gRNA complex is incubated with HPV 16 DNA. The methylene blue signal does not decrease in the presence of HPV 18 DNA. Figure 2c shows an assay designed to target the E7 gene of HPV 18. Here, the methylene blue signal decreases only in the presence of HPV 18 DNA and not in the presence of HPV 16 DNA, demonstrating the specificity of these assays.
Detection of 1.2 × 104 Total Copies of HPV 18 DNA via Combined LAMP and CRISPR-Based Electrochemical Detection
In order to increase the sensitivity of our test, we first amplified the target DNA through LAMP amplification (Figures 3 and 4). LAMP is an inexpensive, sensitive isothermal amplification technique suitable for use in LRS due to its sensitivity and ability to be integrated into portable devices.44 Despite the specific nature of the LAMP primers, one detriment of LAMP assays is that they can suffer from a high false-positive rate due to self-priming of at least two of the six primers involved.6 This limitation is problematic for assays that rely on turbidity or labeled primers for detection. There have been many efforts to reduce LAMP false-positives caused by nonspecific amplification. These include using fluorescently tagged primers to minimize false-positives,45,46 mathematical modeling to predict desired LAMP amplicon sizes,47 and the inclusion of dimethyl sulfoxide (DMSO) in the amplification.48,49 DMSO is a hazardous chemical, and its application in a LAMP reaction requires significant optimization.48,49 Because the CRISPR-Cas12a reaction occurs after the LAMP reaction, it can be used to detect LAMP amplicons without requiring additional LAMP reaction optimization. One key advantage of this detection scheme is that the detection of HPV 18 LAMP amplicons is sequence-specific due to the Cas12a gRNA; therefore, our assay does not detect spurious amplification products with off-target sequences (Figure 3).
Figure 3.
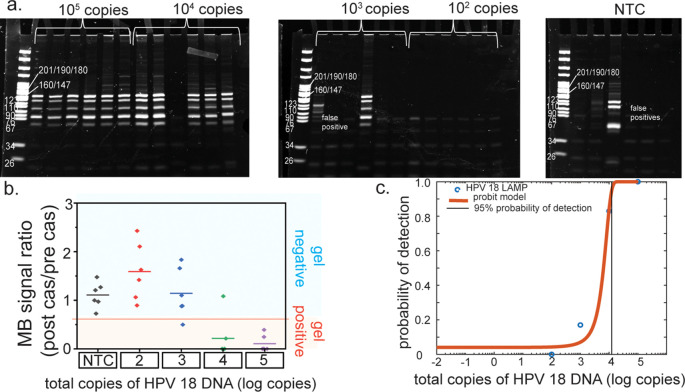
Cas12a assay increases the specificity of loop-mediated isothermal amplification (LAMP) assay. (a) The HPV 18 LAMP assay shows amplification of HPV 18 DNA and false-positives, as indicated by the banding pattern on a 12% acrylamide gel. (b) Only the HPV 18 amplicons result in a signal decrease from the methylene blue (MB). The asterisks represent statistical significance according to a t-test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
Figure 4.
Loop-mediated isothermal amplification (LAMP) lower limit of detection study. (a) 12% acrylamide gel of digested HPV 18 LAMP amplicons; stochastic amplification occurs below 104 cp/μL of input HPV 18 plasmid DNA. (b) Only samples that contained positive HPV 18 LAMP amplicons, as determined by gel electrophoresis, resulted in a signal decrease in methylene blue on the gold leaf electrodes below a given threshold. (c) Probit analysis of the electrochemical data indicates that the lower limit of detection for the electrochemical detection of LAMP amplicons is 1.2 × 104 total copies of HPV 18 DNA.
To determine the limit of detection and detection range for our LAMP assay, the concentration of HPV 18 plasmid that was input to the LAMP reaction was varied from 107 total copies to 0 total copies, and the results were monitored with an acrylamide gel readout. As seen in Figures 4 and S4, 100% amplification was achieved at concentrations ranging from 107 to 105 total copies. At 103 and 104 total copies, stochastic amplification is observed. We see no amplification of HPV 18 DNA at or below 102 copies. In the NTCs, false-positive amplification bands are observed, which can be identified and differentiated from HPV 18 amplicons because their banding patterns differ, and they do not activate the Cas12a enzyme. Twenty minutes of LAMP amplification resulted in sufficient amplicon synthesis.
In order to determine the lower limit of detection (LLOD) for the electrochemical detection, we tested Cas12a activation by LAMP reaction products from inputs of 0 copies, 102 copies, 103 copies, 104 copies, and 105 copies of DNA. Using receiver operating characteristics, a threshold value was chosen as the diagnostic cutoff for our sensor (Figure S2). Any ratio above this threshold is designated as being a “signal off”, while any ratio below this threshold is designated as being a “signal on”. All of the concentrations of HPV 18 LAMP amplicons turned on the sensor, while the blank wells and false acrylamide gel positive did not. A probit analysis was conducted and yielded a LLOD of 1.2 × 104 total copies, a clinically relevant viral load.50 Many studies have implicated higher viral loads of HPV 16 and 18 in progression to cervical cancer.51 While there are conflicting results regarding a clinical threshold for HPV 18,52 viral loads of at least 104 total copies have been correlated with progression to cervical cancer for HPV 16.6
Detection of HPV 18 DNA Extracted from Cervical Swabs
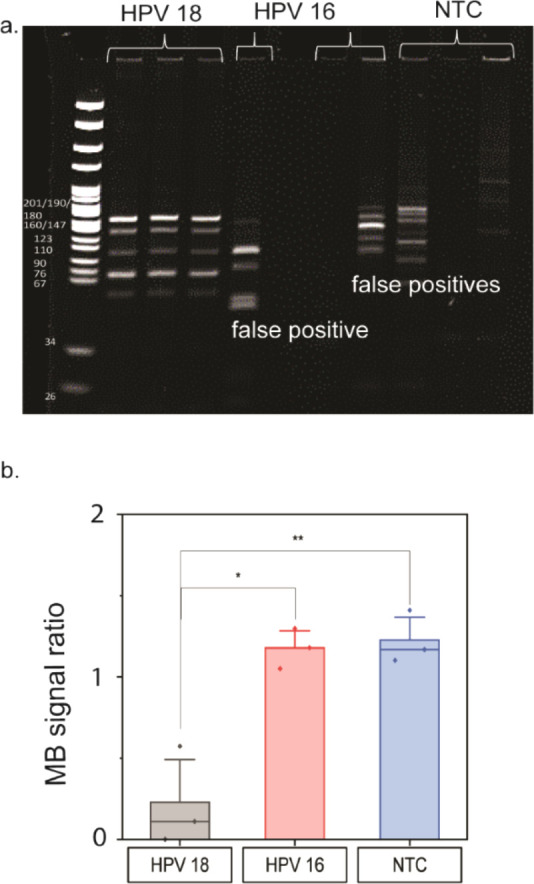
Finally, to demonstrate the viability of our LAMP/CRISPR/electrochemical assay as a clinical diagnostic tool, especially for LRS, we demonstrated the detection of HPV 18 DNA extracted from clinical samples. As a proof of concept, we tested these electrodes with DNA extracted from clinical samples using traditional laboratory techniques that require centrifuges and incubation periods, leading to a sample processing time of 2–3 h. However, this new platform could be paired with paperfluidic sample processing6−8 to reduce the time, cost, and infrastructure needed for clinical sample extraction. After extraction, the samples were amplified using LAMP. While we performed LAMP reactions using a thermal cycler, they can also be performed with wireless resistive heating elements53 which are more affordable and practical for LRS. A total of three HPV 18 PCR-negative and five HPV 18 PCR-positive clinical samples were tested. All five PCR-positive clinical samples were detected as positive using the gold leaf electrodes (Figure 5). All of these samples exhibited a decrease in methylene blue signal that was below the calculated threshold, determined using a receiver operating characteristic (ROC) curve, an established method to assess the diagnostic performance of a test that has a binary output (Figure S4). None of the PCR negative sample signals were statistically different than the NTCs (p > 0.05 when performing a t test). Only one biological replicate of one of the negative samples (sample B) showed a decrease in MB signal below the designated threshold; this sample corresponded to a positive HPV 18 gel band due to one instance of stochastic amplification of the negative samples (Figure S5). While we acknowledge that this is highly unlikely, given that our PCR sensitivity was ∼102 total copies, it is possible that this PCR negative sample did not contain a PCR-detectable amount of HPV 18 DNA, resulting in the stochastic LAMP amplification. We have previously observed LAMP amplification of PCR-negative clinical samples.46
Figure 5.
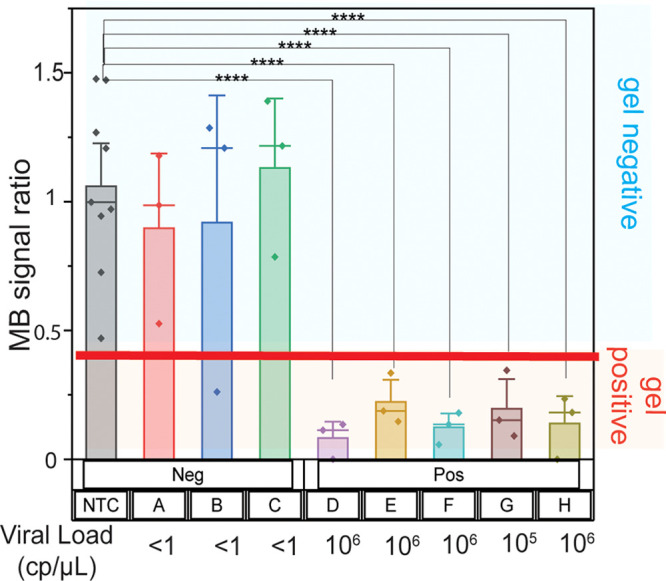
Detection of HPV 18 DNA extracted from clinical samples. Samples that tested positive for HPV 18 by qPCR activated the Cas12a enzyme, resulting in a methylene blue (MB) signal decrease below a given threshold. Samples that were deemed negative by qPCR did not result in a MB signal decrease below a given threshold, with the exception of one technical replicate for sample B. This replicate did contain amplified HPV 18 DNA, as determined by gel electrophoresis; stochastic amplification of HPV 18 was observed for that sample. The asterisks represent statistical significance according to a t-test. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001.
Conclusions
Here, we report a novel electrochemical test that combines inexpensive, easy-to-fabricate gold leaf electrodes, LAMP, and CRISPR-based detection for the successful identification of HIV plasmid DNA and HPV DNA from clinical samples using an electrochemical “signal off” sensor. For a disease like HPV, diagnosis is made if DNA is present above a given viral load, and there is less need to quantify the viral load. Our sensor has a binary output and is able to detect HPV 18 DNA above a clinically relevant viral load.
The electrochemical detection platform has 100% sensitivity and 100% specificity, as it successfully detects all HPV-18 LAMP amplicons while failing to detect samples that do not contain HPV-18 amplicons. When combined with LAMP, the full assay maintains 100% sensitivity and specificity analytically, with 100% sensitivity and 89% specificity from clinical samples. The detection of HPV-18 positive clinical sample extracts are statistically significant, with p values of <0.0001 according to a t-test. More details concerning sensor variability are included in the Supporting Information.
To our knowledge, our work is the first demonstration of the compatibility of gold leaf with LAMP and CRISPR-based detection for clinical samples. Furthermore, our integration of LAMP with a CRISPR-based readout improves the specificity of LAMP, which can otherwise suffer from high false-positive results. We successfully implemented a Cas12a assay for HIV, HPV 16, and HPV 18, demonstrating a Cas12a-based sensor platform technology that can easily be engineered to target a variety of diseases.
Materials and Methods
Fabrication of Gold Leaf Electrodes
As shown in Figure 1, an oil-based gold leaf adhesive (DUX, DUX-QUIC) was applied to the glossy side of a self-adhesive sheet (Fellowes, FEL5221502) and left to dry for 2.5 h. 24K Gold leaf (L.A. Gold Leaf, DBL2400-BK25T) was transferred to the dried adhesive by manually applying pressure. The working (4 mm diameter) and counter electrodes were cut using a flatbed cutter plotter (Graphtec, FCX2000) to make peelable gold leaf stickers. A wax printer (ColorQube, 8870) was used to print wells on transparency sheets (Apollo, VCG7060E). Inspiration for this design was obtained from Adkins et al., who used similar transparency wells and silver conductive paint in their flexible microwire devices.54 Gold leaf sticker electrodes were applied to the transparency sheet. A reference electrode was painted on using silver conductive paint (SPI, 05001-AB). Silver paint was painted on the leads of the working and counter electrodes and used to glue folded aluminum foil (Fisherbrand, 01-213-101) contacts to the ends of the electrodes. A well made of Fellowes adhesive was applied to the top of the electrode to create a sample barrier. A rectangular piece of Fellowes adhesive was used to cover any silver paint on the lead of the working electrode that was within the sample well so that only the gold metal on the working electrode was exposed to assay solution.
Electrochemical Measurements
Electrochemical measurements were carried out with a Gamry Reference 600+ potentiostat. Cyclic voltammetry was carried out for pretreatment of the electrodes in 0.5 M H2SO4. The potential was swept from −0.1 to 1.2 V with a scan rate of 100 mV/s and a step size of 2 mV for 10 cycles. Square wave voltammetry was used to monitor the signal from the methylene blue-tagged oligos in 10 mM Tris, 0.25 mM MgCl2, and 0.05 mM CaCl2. The potential was swept from −0.6 V to −0.05 V with a step size of 4 mV, a frequency of 15 Hz, and a pulse size of 25 mV. A plastic backing was used to stabilize the device during all electrochemical measurements.
Electrode Pretreatment
Electrodes underwent potential cycling using cyclic voltammetry in 200 μL of 0.5 M H2SO4 (LabChem, LC257701). The potential was swept from −0.1 to 1.2 V with a scan rate of 100 mV/s and a step size of 2 mV for 10 cycles. The electrodes were washed with 20 mL of DI water and scanned from −0.1 to 1.2 V for 1 cycle in 200 μL of DI water to ensure that all the sulfuric acid had been washed away. The electrodes were left to air-dry in a clean hood until oligo deposition.
Oligo Reduction
Prior to immobilization, oligonucleotides that were thiolated at the 5′ end and labeled with methylene blue at the 3′ end (LGC Biosearch Technologies) were reduced with Tris(2-carboxyethyl)phosphine hydrochloride (TCEP) (Goldbio, TCEP10). Specifically, 0.08 μM oligonucleotides were reduced with 100× TCEP for at least 1 h. The oligo sequence can be found in Table S3.
Oligo Immobilization
Immediately prior to immobilization, the oligonucleotides were diluted in a 1:1 ratio with 2× immobilization buffer (1.5 M NaCl, 0.25 M Tris) for a final oligo concentration of 0.04 μM oligo in 0.75 M NaCl, 0.125 M Tris. Eighteen microliters of this solution was deposited on the working electrode. Each electrode was in its own sealable Petri dish (Cole-Parmer, UX-14005-26). The Petri dishes were sealed to prevent evaporation and were left under aluminum foil for 2 h. After 2 h, the electrodes were rinsed twice with 200 μL of DI water. Immediately following, 18 μL of 1 mM 6-mercapto-1-hexanol (MCH) was deposited on the working electrode. The Petri dishes were sealed and left under aluminum foil for 1 h. After 1 h, the electrodes were rinsed twice with 200 μL of DI water. The electrodes were then scanned using square wave voltammetry in 200 μL of 10 mM Tris, 0.25 mM MgCl2, and 0.05 mM CaCl2.
LAMP Assay
LAMP amplicons were generated using a standard LAMP reaction mix using Bst DNA polymerase (NEB, M0275S). The reaction consisted of a 25 μL volume containing 1× isothermal amplification buffer (NEB, M0275S), 1 mM dNTPs, 0.5 M betaine, 0.2 μM F3 and B3 primers, 1.6 μM FIP and BIP primers, 0.8 μM LF and LB primers, and 320 units/mL of Bst 2.0 polymerase. The LAMP reaction used to generate Figure 3 was carried out at 63 °C for 45 min. The LAMP reactions in Figures 4 and 5 were carried out at 63 °C for 20 min. The LAMP primers are listed in Table S2. All primers except for the B3 primer are from Saetiew et al.55
LAMP Digest
LAMP amplicons were digested using Blpl (NEB, R-585S) to differentiate between HPV 18 LAMP amplicons and any spurious amplification. Digested amplicons were imaged on a 12% acrylamide gel. The digest reaction was performed using NEB’s standard digest protocol using 1 μL of LAMP products in a 50 μL volume reaction.
Cas12a Assay
The Cas12a reaction was carried out in a 30 μL reaction volume containing 33.3 nM Cas12a (NEB, M0653S), 24.3 nM gRNA (IDT), 1× NEB buffer 2.1 (NEB, B7202), and variable concentrations of DNA. For the plasmid data generated in Figure 2, the final reaction volumes contained 100 ng of plasmid DNA. LAMP reaction products were diluted by a factor of 100 before inputting 3 μL of the LAMP amplicon into the Cas12a reaction mix. Eighteen microliters of the Cas12a reaction was deposited on the functionalized electrodes. The electrodes were sealed in Petri dishes and placed in a 37 °C oven for 1 h. After 1 h, the electrodes were rinsed twice with 200 μL of buffer and measured with 200 μL of buffer using square wave voltammetry. The peak current of the methylene blue square wave voltammograms before and after treatment with Cas12a was calculated, and the ratio of the peak currents after treatment with Cas12a to before treatment with Cas12a was computed.
HIV, HPV 16, and HPV 18 gRNA Preparation
HPV 16 and 18 gRNA were purchased from Integrated DNA Technologies (IDT). Their sequences can be found in Table S4.
HIV p24 sgRNA Synthesis
Starting from a commercially available plasmid (Addgene #47912) with an exchangeable SP6 transcription locus flanked by EcoRI/KpnI restriction sites on either side, we replaced the entire SP6 locus with the following HIV p24/Cas12a sgRNA sequence ordered from IDT: 5′-GAATTCATTTAGGTGACACTATAGtaatttctactaagtgtagatagcattatcagaaggagccacGGTACC-3′ (capital letters represent the restriction enzyme, the SP6 promoter, and the TATA binding site; GAATTC = 5′ EcoRI site, ATTTAGGTGACACTATAG = SP6 promoter with TATA box, taatttctactaagtgtagat = Lb Cas12a crRNA, GGTACC= 3′ Kpnl/Acc65i site). Then, the plasmid was linearized with Acc65i digestion (to prevent 3′ overhangs at the KpnI site) and gel purified prior to performing in vitro transcription using the Promega Ribomax SP6 kit. RNA products were purified twice with DNase, acid–phenol–chloroform extractions, and isopropanol precipitations prior to use.
HPV and HIV Plasmid Preparation
HPV 16 plasmids were generated according to a previously established protocol.6,46 HPV 18 plasmids were custom ordered from GENEWIZ. pUC57 plasmids containing an EcoRV sites flanking the HPV 18 E7 sequence were linearized with ScaI-HF (NEB, R3122S) and AatII (NEB, R0117S). HIV plasmid preparation can be found in the Materials and Methods section of the Supporting Information.
Cervical Clinical Samples
Deidentified cervical swab samples were obtained from the Biospecimen Archive Research Core (BARC) at Boston University Medical Center (Boston, MA). The BD SurePath (BD, Franklin Lakes, NJ) cervical swabs were designated as being HPV 16 positive, HPV 18 positive, or “Other HPV” by the pathology lab according to a Cobas 4800 HPV Test (Roche Diagnostics, Basel, Switzerland). The BD SurePath samples were prepared as previously reported.46 In short, clinical samples were taken from the container and aliquoted into 1.7-mL tubes. In order to pellet the sample, all tubes were spun down at 13 000 rpm. After disposing of the supernatant, the pellets were washed in 300 μL of 1× PBS two times, requiring a 15 min spin down at 4 °C, 13 000 rpm after each wash; the supernatant was discarded each time. A DNeasy Blood and Tissue kit (QIAGEN, Valencia, CA) was used to extract DNA from the tissue samples; DNA was eluted with 25 μL of buffer AE twice. Three samples were Cobas-negative, and five were Cobas-positive for the L1 HPV 18 loci.
The cost of cervical sample processing was $23.84 per sample, and the extraction process took 2–3 h. To reduce the time and cost associated with the clinical sample processing, our electrochemical platform can be paired with existing paperfluidic platforms6−8 for the low-cost extraction of nucleic acids from clinical samples.
PCR Characterization of Clinical Samples
Quantitative PCR was performed on all clinical sample extracts on a QuantStudio 5 (Applied Biosystems, Foster City, CA) with a multiplexed HPV 18, HPV 16, and RNase P assay (RNase P serves as an internal control for human DNA to ensure the clinical sample contained human cells) to quantify the DNA concentration in copies/μL. We used previously published primer and probe sets for the HPV 18 E7 Gene, HPV 16 E7 Gene and RNase P.6,46 The RNase P reference plasmid was cloned in-house, while plasmids were synthesized by GENEWIZ (Cambridge, MA). Twenty-five microliter reactions containing 5 μL of sample or plasmid DNA were prepared with 1× TaqMan buffer, 3.5 mM MgCl2, 200 μM dNTPS, 0.1× ROX reference dye, 0.025 U/μL Taq DNA polymerase, and 50 nM primers and 50 nM probes for HPV 18, HPV 16, and RNase P. Reactions were heated for an initial denaturing at 95 °C for 10 min, followed by 45 PCR cycles of 95 °C for 30 s, 55 °C for 30 s, 60 °C for 1.5 min, and a final extension of 60 °C for 5 min. The HPV 18 E7 gene, HPV 16 E7 gene, and RNase P plasmid DNA stocks were titrated to create standard curves using the QuantStudio Design and Analysis Software (Applied Biosystems, Foster City, CA). The standard curve and clinical samples were each run in triplicate. We ruled the samples PCR-positive if amplified before 40 cycles, and PCR-negative if they amplified after 40 cycles.
Cleaning Protocols
Recent findings show that LAMP amplicons can easily contaminate their surroundings.56 In order to mitigate this risk, all PCR and LAMP reactions were set up in a designated cleanroom where no amplification takes place. All materials were cleaned with DNA Away and RNase Away prior to sample handling. In addition, we ensured that our no-template controls (NTCs) did not contain HPV 18 LAMP amplicons by gel electrophoresis and the Cas12a assay.
Acknowledgments
This work was funded by the Dorf-Ebner Distinguished Faculty Fellow award (C.M.K.) and the Boston University Precision Diagnostics Center (M.B., J.R.) and DARPA W911NF-16-849 C-0044 (C.M.K., M.Z.) We thank the Biospecimen Archive Research Core at Boston University (BARC) for the clinical samples. This work was performed in part at the Center for Nanoscale Systems (CNS), a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI), which is supported by the National Science Foundation under NSF Award No. 1541959. CNS is part of Harvard University.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acscentsci.1c00186.
Fluorescent detection of target-activated Cas12a endonuclease activity (Figure S1); qPCR primers (Table S1); HPV LAMP primers (Table S2); methylene-blue-modified oligonucleotide sequence (Table S3); gRNA sequences for HPV 16 and 18 (Table S4); coefficient of variation data (Table S5); threshold determination for electrochemical detection of LAMP amplicons in buffer (Figure S2); threshold determination for electrochemical detection of LAMP amplicons of clinical sample extracts (Figure S3); LAMP lower limit of detection study for HPV 18 (Figure S4); blpl-digested LAMP amplicons of clinical samples (Figure S5); pretreatment of gold leaf electrodes using cyclic voltammetry in 0.5 M H2SO4 (Figure S6); detection of methylene-blue-tagged DNA on gold leaf electrodes (Figure S7); impedance analysis of DNA on gold leaf electrodes (Figure S8); gold leaf surface characterization (Figure S9); materials and methods; references (PDF)
Author Contributions
The manuscript was written through contributions of all authors. M.Z. and J.R. performed the experiments, and M.Z., C.K., A.L.F., A.F., and M.B. contributed to experimental design, writing, and data analysis.
The authors declare no competing financial interest.
Supplementary Material
References
- Atkinson K.; Mabey D.; Atkinson K.; Mabey D.. The Burden of Communicable Diseases in Low- and Middle-Income Countries. In Revolutionizing Tropical Medicine; John Wiley & Sons, Inc., 2019; pp 1–36. 10.1002/9781119282686.ch1. [DOI] [Google Scholar]
- Cristillo A. D.; Bristow C. C.; Peeling R., et al. Point-of-care sexually transmitted infection diagnostics: Proceedings of the STAR sexually transmitted infection-clinical trial group programmatic meeting. In Sexually Transmitted Diseases; Lippincott Williams and Wilkins, 2017; Vol 44, pp 211–218. 10.1097/OLQ.0000000000000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney A. L.; Rubin J. S.. Evaluating the Impact of Point-of-Care Diagnostics on Disease Outbreaks in Low Resource Settings; Massachusetts Institute of Technology, 2017. Accessed February 5, 2021. https://dspace.mit.edu/handle/1721.1/112069. [Google Scholar]
- Simeon K.; Sharma M.; Dorward J.; Naidoo J.; Dlamini N.; Moodley P.; Samsunder N.; Barnabas R. V.; Garrett N.; Drain P. K. Comparative cost analysis of point-of-care versus laboratory-based testing to initiate and monitor HIV treatment in South Africa. PLoS One 2019, 14 (10), e0223669 10.1371/journal.pone.0223669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos N. G.; Castle P. E.; Wright T. C.; Kim J. J. Cervical cancer screening in low-resource settings: A cost-effectiveness framework for valuing tradeoffs between test performance and program coverage. Int. J. Cancer 2015, 137 (9), 2208–2219. 10.1002/ijc.29594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez N. M.; Wong W. S.; Liu L.; Dewar R.; Klapperich C. M. A fully integrated paperfluidic molecular diagnostic chip for the extraction, amplification, and detection of nucleic acids from clinical samples. Lab Chip 2016, 16 (4), 753–763. 10.1039/C5LC01392E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolluri N.; Albarran N.; Fan A.; et al. SNAPflex: A paper-and-plastic device for instrument-free RNA and DNA extraction from whole blood. Lab Chip 2020, 20 (18), 3386–3398. 10.1039/D0LC00277A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horst A. L.; Rosenbohm J. M.; Kolluri N.; et al. A paperfluidic platform to detect Neisseria gonorrhoeae in clinical samples. Biomed. Microdevices 2018, 20 (2), 35. 10.1007/s10544-018-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoto S. L.; Lugongolo M.; Govender U.; Mthunzi-Kufa P. Point of care diagnostics for HIV in resource limited settings: An overview. Medicina 2018, 54 (1), 3. 10.3390/medicina54010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Types of HIV Tests | Testing | HIV Basics | HIV/AIDS | CDC. Accessed March 18, 2021. https://www.cdc.gov/hiv/basics/hiv-testing/test-types.html.
- Karris M. Y.; Anderson C. M.; Morris S. R.; Smith D. M.; Little S. J. Cost savings associated with testing of antibodies, antigens, and nucleic acids for diagnosis of acute HIV infection. J. Clin. Microbiol. 2012, 50 (6), 1874–1878. 10.1128/JCM.00106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human papillomavirus (HPV) and cervical cancer. Accessed January 1, 2021. https://www.who.int/news-room/fact-sheets/detail/human-papillomavirus-(hpv)-and-cervical-cancer.
- Cervical cancer. Accessed January 1, 2021. https://www.who.int/health-topics/cervical-cancer#tab=tab_1.
- Zhao X.; Wu Q.; Wang X.; et al. The performance of human papillomavirus DNA detection with type 16/18 genotyping by hybrid capture in primary test of cervical cancer screening: a cross-sectional study in 10,669 Chinese women. Clin. Microbiol. Infect. 2018, 24, 1322–1327. 10.1016/j.cmi.2018.02.027. [DOI] [PubMed] [Google Scholar]
- Gupta R.; Gupta S.; Mehrotra R.; Sodhani P. Cervical cancer screening in resource-constrained countries: Current status and future directions. Asian Pacific J. Cancer Prev. 2017, 18 (6), 1461–1467. 10.22034/APJCP.2017.18.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innovative, affordable screening and treatment to prevent cervical cancer - Unitaid. Accessed February 5, 2021. https://unitaid.org/project/innovative-affordable-screening-and-treatment-to-prevent-cervical-cancer/#en.
- Shah S. S.; Senapati S.; Klacsmann F. Current Technologies and Recent Developments for Screening of HPV-Associated Cervical and Oropharyngeal Cancers. Cancers 2016, 8 (9), 85. 10.3390/cancers8090085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kundrod K. A.; Smith C. A.; Hunt B.; Schwarz R. A.; Schmeler K.; Richards-Kortum R. Advances in technologies for cervical cancer detection in low-resource settings. Expert Rev. Mol. Diagn. 2019, 19 (8), 695–714. 10.1080/14737159.2019.1648213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos M. S. F.; Ameku W. A.; Gutz I. G. R.; Paixão T. R. L. C. Gold leaf: From gilding to the fabrication of disposable, wearable and low-cost electrodes. Talanta 2018, 179, 507–511. 10.1016/j.talanta.2017.11.021. [DOI] [PubMed] [Google Scholar]
- Shimada K; Toyoda T. Gold leaf counter electrodes for dye-sensitized solar cells. Jpn. J. Appl. Phys. 2018, 57, 03EJ04. 10.7567/JJAP.57.03EJ04. [DOI] [Google Scholar]
- Collinson M. M.; Chaniotakis N.; Kara D.; Lemos V. A.; Lodyga-Chruscinska E.; Rittich B. Nanoporous Gold Electrodes and Their Applications in Analytical Chemistry. ISRN Anal. Chem. 2013, 2013, 1. 10.1155/2013/692484. [DOI] [Google Scholar]
- Hondred J. A.; Johnson Z. T.; Claussen J. C. Nanoporous gold peel-and-stick biosensors created with etching inkjet maskless lithography for electrochemical pesticide monitoring with microfluidics. J. Mater. Chem. C 2020, 8 (33), 11376–11388. 10.1039/D0TC01423K. [DOI] [Google Scholar]
- Francis N. J.; Knospe C. R. Fabrication and Characterization of Nanoporous Gold Electrodes for Sensor Applications. Adv. Eng. Mater. 2019, 21 (3), 1800857. 10.1002/adem.201800857. [DOI] [Google Scholar]
- Chambers D. L.; Wan C. T.. Physical Vapor Deposition of Gold and Its Alloys. In Precious Metals 1981; Elsevier, 1982; pp 219–238. 10.1016/b978-0-08-025392-3.50030-2. [DOI] [Google Scholar]
- Taleat Z.; Khoshroo A.; Mazloum-Ardakani M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181 (9–10), 865–891. 10.1007/s00604-014-1181-1. [DOI] [Google Scholar]
- Notomi T.; Mori Y.; Tomita N.; Kanda H. J. Microbiol. (Seoul, Repub. Korea) 2015, 53 (1), 1–5. 10.1007/s12275-015-4656-9. [DOI] [PubMed] [Google Scholar]
- Chen J. S.; Ma E.; Harrington L. B.; et al. CRISPR-Cas12a target binding unleashes indiscriminate single-stranded DNase activity. Science (Washington, DC, U. S.) 2018, 360 (6387), 436–439. 10.1126/science.aar6245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y.; Somoza R. A.; Wang L. Exploring the Trans-Cleavage Activity of CRISPR Cas12a (cpf1) for the Development of a Universal Electrochemical Biosensor. Angew. Chem., Int. Ed. 2019, 58, 17399. 10.1002/anie.201910772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruch R.; Johnston M.; Kling A.; et al. Crispr-Powered Electrochemical Microfluidic Multiplexed Biosensor For Target Amplification-Free Mirna Diagnostics. Biosens. Bioelectron. 2021, 177, 112887. 10.1016/j.bios.2020.112887. [DOI] [PubMed] [Google Scholar]
- Bruch R.; Baaske J.; Chatelle C.; Meirich M.; Madlener S.; Weber W.; Dincer C.; Urban G. A. CRISPR/Cas13a-Powered Electrochemical Microfluidic Biosensor for Nucleic Acid Amplification-Free miRNA Diagnostics. Adv. Mater. 2019, 31 (51), 1905311. 10.1002/adma.201905311. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Yan Y.; Que H.; Yang T.; Cheng X.; Ding S.; Zhang X.; Cheng W. CRISPR/Cas12a-mediated Interfacial Cleaving of Hairpin DNA Reporter for Electrochemical Nucleic Acids Sensing. ACS Sensors 2020, 5, 557. 10.1021/acssensors.9b02461. [DOI] [PubMed] [Google Scholar]
- Xu W.; Jin T.; Dai Y.; Liu C. C. Surpassing the detection limit and accuracy of the electrochemical DNA sensor through the application of CRISPR Cas systems. Biosens. Bioelectron. 2020, 155, 112100. 10.1016/j.bios.2020.112100. [DOI] [PubMed] [Google Scholar]
- Li F.; Ye Q.; Chen M.; et al. An ultrasensitive CRISPR/Cas12a based electrochemical biosensor for Listeria monocytogenes detection. Biosens. Bioelectron. 2021, 179, 113073. 10.1016/j.bios.2021.113073. [DOI] [PubMed] [Google Scholar]
- Tsou J. H.; Leng Q.; Jiang F. A CRISPR Test for Detection of Circulating Nucleic Acids. Transl Oncol. 2019, 12 (12), 1566–1573. 10.1016/j.tranon.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Ji P.; Fan H.; et al. CRISPR/Cas12a technology combined with immunochromatographic strips for portable detection of African swine fever virus. Commun. Biol. 2020, 3 (1), 62. 10.1038/s42003-020-0796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J.; Lin H.; Li H.; et al. Cas12a-Based On-Site and Rapid Nucleic Acid Detection of African Swine Fever. Front. Microbiol. 2019, 10, 2830. 10.3389/fmicb.2019.02830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broughton J. P.; Deng X.; Yu G. CRISPR–Cas12-based detection of SARS-CoV-2. Nat. Biotechnol. 2020, 38, 870. 10.1038/s41587-020-0513-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X.; Yin K.; Li Z.; et al. Ultrasensitive and visual detection of SARS-CoV-2 using all-in-one dual CRISPR-Cas12a assay. Nat. Commun. 2020, 11 (1), 1–10. 10.1038/s41467-020-18575-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Zhong M.; Liu Y. Rapid and sensitive detection of COVID-19 using CRISPR/Cas12a-based detection with naked eye readout, CRISPR/Cas12a-NER. Sci. Bull. 2020, 65, 1436. 10.1016/j.scib.2020.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bono M. S.; Beasley S.; Hanhauser E.; Hart A. J.; Karnik R.; Vaishnav C. Fieldwork-based determination of design priorities for point-of-use drinking water quality sensors for use in resource-limited environments. Zia A, ed. PLoS One 2020, 15 (1), e0228140 10.1371/journal.pone.0228140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattani A.; Singh S. V.; Agrawal A.; Khan M. H.; Singh P. Recent progress in electrochemical biosensors as point of care diagnostics in livestock health. Anal. Biochem. 2019, 579, 25–34. 10.1016/j.ab.2019.05.014. [DOI] [PubMed] [Google Scholar]
- Kang D.; Ricci F.; White R. J.; Plaxco K. W. Survey of Redox-Active Moieties for Application in Multiplexed Electrochemical Biosensors. Anal. Chem. 2016, 88 (21), 10452–10458. 10.1021/acs.analchem.6b02376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Fernández E.; Avlonitis N.; Murray A. F.; Mount A. R.; Bradley M. Methylene blue not ferrocene: Optimal reporters for electrochemical detection of protease activity. Biosens. Bioelectron. 2016, 84, 82–88. 10.1016/j.bios.2015.11.088. [DOI] [PubMed] [Google Scholar]
- Ganguli A.; Mostafa A.; Berger J.; et al. Rapid isothermal amplification and portable detection system for SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020, 117 (37), 22727–22735. 10.1073/pnas.2014739117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardinge P.; Murray J. A. H. Reduced False Positives and Improved Reporting of Loop-Mediated Isothermal Amplification using Quenched Fluorescent Primers. Sci. Rep. 2019, 9, 7400. 10.1038/s41598-019-43817-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landaverde L.; Wong W.; Hernandez G.; Fan A.; Klapperich C. Method for the elucidation of LAMP products captured on lateral flow strips in a point of care test for HPV 16. Anal. Bioanal. Chem. 2020, 412 (24), 6199–6209. 10.1007/s00216-020-02702-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L.; Blakely H.; Tripathi A. Mathematical model to reduce loop mediated isothermal amplification (LAMP) false-positive diagnosis. Electrophoresis 2019, 40 (20), 2706–2717. 10.1002/elps.201900167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. G.; Brewster J. D.; Paul M.; Tomasula P. M. Two methods for increased specificity and sensitivity in loop-mediated isothermal amplification. Molecules 2015, 20 (4), 6048–6059. 10.3390/molecules20046048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. Evaluation and improvement of LAMP assays for detection of Escherichia coli serogroups O26, O45, O103, O111, O121, O145, and O157. Afr Health Sci. 2018, 17 (4), 1011–1021. 10.4314/ahs.v17i4.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez N. M.; Wong W. S.; Liu L.; Dewar R.; Klapperich C. M. A Fully Integrated Paperfluidic Molecular Diagnostic Chip for the Extraction, Amplification, and Detection of Nucleic Acids from Clinical Samples. Lab Chip 2016, 16 (4), 753–763. 10.1039/C5LC01392E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berggrund M.; Gustavsson I.; Aarnio R.; et al. HPV viral load in self-collected vaginal fluid samples as predictor for presence of cervical intraepithelial neoplasia. Virol. J. 2019, 16 (1), 146. 10.1186/s12985-019-1253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong B.; Sun P.; Ruan G.; et al. Type-specific high-risk human papillomavirus viral load as a viable triage indicator for high-grade squamous intraepithelial lesion: a nested case–control study. Cancer Manage. Res. 2018, 10, 4839–4851. 10.2147/CMAR.S179724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardy T.; Sink H.; Koel A.; Rang T. Development of a low-cost, wireless smart thermostat for isothermal DNA amplification in Lab-On-A-Chip devices. Micromachines 2019, 10 (7), 437. 10.3390/mi10070437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins J. A.; Henry C. S. Electrochemical detection in paper-based analytical devices using microwire electrodes. Anal. Chim. Acta 2015, 891, 247–254. 10.1016/j.aca.2015.07.019. [DOI] [PubMed] [Google Scholar]
- Saetiew C.; Limpaiboon T.; Jearanaikoon P.; et al. Rapid detection of the most common high-risk human papillomaviruses by loop-mediated isothermal amplification. J. Virol. Methods 2011, 178 (1–2), 22–30. 10.1016/j.jviromet.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Robinson-Mccarthy L. R.; Mijalis A. J.; Filsinger G. T.. et al. New recommended policies for pathogen surveillance testing of researchers and improved stewardship of diagnostic DNA. OSF Preprints 2020, 10.31219/osf.io/9svjq. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.