Abstract
Multiple myeloma (MM) is a neoplastic clonal proliferation of plasma cells in the bone marrow microenvironment, characterized by overproduction of heavy- and light-chain monoclonal proteins (M-protein). These proteins are mainly found in the serum and/or urine. Reduction in normal gammaglobulins (immunoparesis) leads to an increased risk of infection. The primary site of origin is the bone marrow for nearly all patients affected by MM with disseminated marrow involvement in most cases. MM is known to involve bones and result in myeloma bone disease. Osteolytic lesions are seen in 80% of patients with MM which are complicated frequently by skeletal-related events (SRE) such as hypercalcemia, bone pain, pathological fractures, vertebral collapse, and spinal cord compression. These deteriorate the patient’s quality of life and affect the overall survival of the patient. The underlying pathogenesis of myeloma bone disease involves uncoupling of the bone remodeling processes. Interaction of myeloma cells with the bone marrow microenvironment promotes the release of many biochemical markers including osteoclast activating factors and osteoblast inhibitory factors. Elevated levels of osteoclast activating factors such as RANK/RANKL/OPG, MIP-1-α., TNF-α, IL-3, IL-6, and IL-11 increase bone resorption by osteoclast stimulation, differentiation, and maturation, whereas osteoblast inhibitory factors such as the Wnt/DKK1 pathway, secreted frizzle related protein–2, and runt-related transcription factor 2 inhibit osteoblast differentiation and formation leading to decreased bone formation. These biochemical factors also help in development and utilization of appropriate anti-myeloma treatments in myeloma patients. This review article summarizes the pathophysiology and the recent developments of abnormal bone remodeling in MM, while reviewing various approved and potential treatments for myeloma bone disease.
Keywords: multiple myeloma, bone disease, therapies
1. Introduction
Multiple myeloma (MM) is a neoplastic clonal proliferation of plasma cells in the bone marrow microenvironment, characterized by overproduction of heavy- and light-chain monoclonal proteins (M-protein) [1,2]. These proteins are mainly found in the serum and/or urine. Reduction in normal gammaglobulins (immunoparesis) leads to an increased risk of infection [3]. MM is a relatively uncommon cancer, but it is the second most common hematologic malignancy. MM comprises approximately 2% of all cancers in the US and about 15% of lymphohematopoietic cancers (LHC). In the United States, the lifetime risk of getting MM is 1 in 132 (0.76%). The American Cancer Society estimates that about 34,920 new cases of MM will be diagnosed in the United States for 2021 (19,320 in men and 15,600 in women). About 12,410 deaths are expected to occur (6840 in men and 5570 in women) [4]. This malignancy is seen more commonly in men over the age of 40 years, especially in men who belong to the African American ethnicity. Globally, approximately 86,000 new cases are seen annually, which accounts for 0.8% of all new cancer cases, and there are 63,000 deaths from this disease annually, accounting for 0.9% of all cancer deaths. Globally predominant areas of MM with the highest incidence include the industrialized regions of Australia/New Zealand, Europe, North America, and Asia [5].
We present a systematic review of clinical trials and various preclinical studies, identified through a comprehensive search in using PubMed. Our main goal is to discuss in depth the pathogenesis of bone disease in MM and investigate and critically examine the effects of various treatments that have recently been approved by the US Food and Drug Administration over the last two decades in the management of myeloma bone disease or therapies that are being investigated.
2. Pathogenesis
Patients with MM usually present with hypercalcemia, anemia, renal damage, increased risk for infections, and pathological fracture secondary to osteolytic bone destruction [1,2]. The osteolytic bone disease results from the disruption between the osteoclast, osteoblasts, bone marrow stromal cells, and osteocytes [6,7,8]. Gradual worsening of myeloma bone disease (MBD) presents with excruciating pain, pathological fractures, and symptomatic hypercalcemia [1]. MBD resulting in pathological fractures ultimately leads to poor quality of life secondary to the pain and hypercalcemia consequences. The etiology for the excessive bone mass loss seen in MM is multifactorial. Approximately 80% of patients with MM initially present with abnormal bone structure at the time of diagnosis [1,2]. In a 2003 study by Kyle et al., 67% of patients had osteolytic bone disease and 20% had osteoporosis with pathological fractures at diagnosis. Approximately 60% of MM patients develop a fracture during their disease course [9]. The severity of the bone disease is proportionate with the tumor burden. There is an inverse relationship between the number of osteolytic bone lesions and prognosis [10].
2.1. Normal Bone Remodeling
Bone remodeling occurs on the bone surface where the osteoclasts and osteoblasts are covered by the BRC canopy. The BRC is the space between the bone surfaces which is undergoing remodeling in the canopy of flattened cells. In adult bone, osteocytes comprise 90–95% of cells, whereas osteoclast and osteoblastic cells account for fewer than 10% of them [11]. Osteocytes function as the main regulators of bone homeostasis between osteoclast and osteoblasts [3]. Osteocytes secrete cytokines including sclerostin, DKK1/Wnt pathway inhibitor, RANKL, and OPG. These osteocytes contribute to the activation of bone remodeling process. They respond to mechanical stimulation and initiate bone resorption. Osteocyte death also results in the recruitment of osteoclasts.
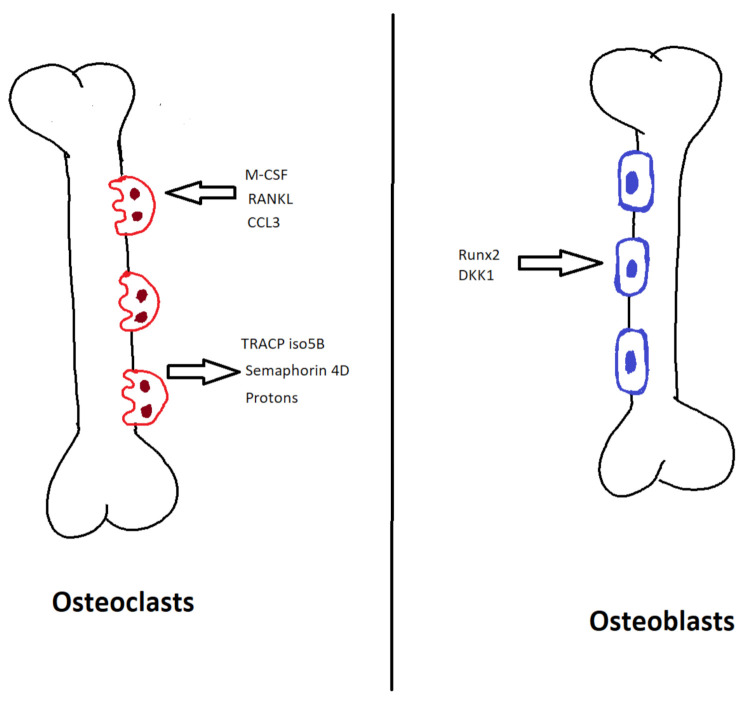
Osteoclasts are multinucleated cells that originate from the monocyte-macrophage cell lineage and cause bone resorption. Monocyte/macrophage colony stimulating factor (M–CSF) and RANKL are responsible for the differentiation of the precursor cells into mature osteoclasts [12]. M-CSF causes osteoclastogenesis, whereas RANKL helps the differentiation and activation into mature osteoclast [13]. During osteoclast development, tartrate-resistant acid phosphatase 5b (TRACP isotype 5B) is formed, a biomarker specific to osteoclasts. During osteoclastogenesis, large multinucleated cell formation occurs, causing bone degradation by active secretion of protons into the resorption pits, which decreases the pH leading to decalcification of the bone matrix [14]. Furthermore, the collagen fibers are degraded by the proteolytic enzyme cathepsin K and various matrix metalloproteinases [15]. Osteoclasts also express semaphorin 4D (SEMA4D), which inhibits osteoblastic differentiation [16] (Figure 1).
Figure 1.
Normal bone remodeling. M-CSF, monocyte/macrophage colony stimulating factor; RANKL, receptor-activated nuclear factor-kappa B ligand; CCL3, C–C motif chemokine ligand 3; TRACP iso5B, tartrate-resistant acid phosphatase 5b; Runx2, runt-related transcription factor 2; DKK1, dickkopf WNT signaling pathway inhibitor 1.
Osteoblasts originate from the mesenchymal stem cell. They are responsible for the new bone formation following bone resorption by the osteoclast. Runt-related transcription factor 2 (Runx2) is responsible for the differentiation of mesenchymal stem cells into osteoblasts [17]. This is followed by the Wnt pathway, which increases β-catenin but helps the differentiation and maturation of osteoblasts. The Wnt pathway is mediated by a complex formation that inhibits the degradation of β-catenin. Wnt pathway is inhibited by Dickkopf 1(DKK1) leading to bone formation [18]. These mature osteoblasts are located along the newly resorbed bone and generate the bone matrix, mainly collagen type I, which is followed by calcification to complete the process of bone formation [19]. During bone formation, some osteoblasts are incorporated into the bone matrix and convert into osteocytes. Osteocytes cause osteoblast differentiation via sclerostin and DKK1 which inhibit the Wnt pathway by binding to LDL receptor-related proteins 5 and 6 (Wnt receptors) on the surface of osteoblasts [11] (Figure 1).
2.2. Abnormal Bone Remodeling in MM
Normal bone remodeling is attained by the homeostasis between several factors balancing bone formation versus bone degradation. In MM, the osteocyte–osteoclast–osteoblast axis is disrupted, leading to the formation of pathognomonic osteolytic lesions [3]. One of the early events in MM is increased bone degradation. Increases in the number and the activity of osteoclast along with inadequate compensation by osteoblasts in patients with MM have been found, resulting in osteolytic lesions when bone formation.
2.3. Bone Marrow Microenvironment, Myeloma Cells, T Lymphocytes and Bone Marrow Stromal Cells (BMSC)
The main pathophysiology behind the occurrence of MBD is increased bone degradation along with impaired bone formation. In monoclonal gammopathy of undetermined significance (MGUS) and early stages of MM, bone architecture is preserved. Occasionally, increased bone formation is seen. With the advanced staging of MM, impaired bone formation along with osteolytic lesions are observed.
The start of pathologic process involves the interaction between the bone marrow microenvironment and the myeloma cells. The bone remodeling compartment (BRC) canopy is a layer of flat cells belonging to osteoblastic lineage that separates the bone from the bone surface during bone remodeling, and it plays an important role in the remodeling process [20,21]. Destruction of the BRC canopy allows the myeloma cells to interact with the bone remodeling cells, causing a discrepancy in the bone homeostasis [22].
Interaction between myeloma cells, lymphocytes, and BMSC within the bone marrow microenvironment plays a role in the development of MBD [23]. Myeloma cells bind to BMSC via very late antigen 4 (VLA-4) and vascular cell addition molecule 1 (VCAM-1). This leads to the secretion of cytokines promoting osteoclast differentiation and activation [3]. The imbalanced ratio of RANKL and OPG (31) and chemokines such as MIP-1-α, IL-5, IL-3, IL-6, IL-7, SDF-1-α, and VEGF are responsible for increased osteoclastogenesis [7]. MM cells also stimulate CCL3 and IL-11 in osteocytes stimulating, osteoclast differentiation and activation [24].
Myeloma cells produce decoy receptor 3 (DCR 3) and are responsible for osteoclast differentiation and activation [25]. DCR3, which belongs to the TNF receptor support family, is overexpressed on myeloma cells and lymphocytes [26]. Myeloma cells also stimulate BMSC and osteoblasts to activate the RANKL/OPG system [27]. Myeloma cells interact with osteoclasts and can alter themselves into multinucleated cells with bone resorptive properties [28].
Myeloma cells adhere to BMSC, which regulates the production of RANKL, IL-6, B-cell activating factor (BAFF), and activin A [29,30]. BAFF belongs to the TNF superfamily, which plays a role in the development of B cells as well as promotes osteoclastogenesis and myeloma cell survival. Activin A belongs to the transforming growth factor (TGF) family and acts by activating osteoclast and inhibiting osteoblasts. High levels of activin A are noticed in an advanced stage of MM [31].
T-lymphocytes also play a role in regulating osteoclast/osteoblast activity, survival, and function [25]. Prabhala et al. demonstrated a subset of T helper cells called Th17-1 cells which secreted IL-17 that not only mediated myeloma bone disease but also increased myeloma cell survival [32,33]. Activated T cells and MM produce osteoclastogenic cytokines such as IL-3, RANKL, DCR 3, and TNF, enhancing osteolysis [26].
2.4. Increased Osteoclastogenesis
Increases of osteoclast activity and bone resorption markers play a major role in myeloma bone disease [34,35]. Important biochemical markers involved in osteoclast activity and differentiation include RANKL/OPG [36,37,38,39] and decoy receptor 3(DcR3) [26,40,41]. Other chemokines include C–C motif chemokine ligand 3 (CCL3, also referred to as MIP-1-α), MIP-1-β [42,43,44], TNF-α [45,46], IL-3, IL-6, IL-11, stromal cell-derived factor 1 alpha (SDF-1-α), B-cell activating factor (BAFF), activin A, and vascular endothelial growth factor (VEGF) [3] (Figure 2).
Figure 2.
Mechanisms of myeloma-related bone disease. CFU-GM, colony forming unit—granulocyte/macrophage; RANKL, receptor-activated nuclear factor-kappa B ligand; TNF-α, tumor necrosis factor alpha; IL-6, interleukin-6; IL-3, interleukin-3; MIP-1-α (CCL3), macrophage inflammatory protein-1 alpha (C–C motif chemokine ligand 3); VEGF, vascular endothelial growth factor; DKK1, dickkopf WNT signaling pathway inhibitor 1; SFRP3, secreted frizzle related protein 3; HGF, hepatocyte growth factor; TGF-β, transforming growth factor beta; BMSC, bone marrow stromal cells.
2.5. RANKL/OPG System
In MM, disruption of the BRC canopy by the myeloma cells impairs the process of bone remodeling [22]. This is regulated by several factors but mainly by RANKL, RANK, and decoy receptor osteoprotegerin (OPG), which help maintain bone remodeling homeostasis. RANK is a transmembrane receptor that belongs to the TNF superfamily. It is produced by BMSC, osteoblasts, and activated T lymphocytes [47]. RANKL is a cytokine expressed as a membrane-bound protein by BMSCs of osteoblastic lineage and activated T lymphocytes. The RANK/RANKL/OPG system plays a major role in the development of myeloma bone disease. In normal individuals, the bone homeostasis is well-maintained by the RANKL/OPG system. However, in MM, the RANKL/OPG ratio is increased by an increase in RANKL and decrease in OPG, ultimately resulting in increased bone resorption [38]. The severity of the ratio is directly proportional to the overall survival/prognosis of the disease. An increase in the RANKL:OPG ratio can cause bone loss or increased resorption in various malignancies and non-malignant inflammatory disorders, such as rheumatoid arthritis [48,49,50]. In MM, RANKL levels are increased, whereas OPG levels are decreased compared to normal individuals and patients with MGUS [39]. RANK is expressed on osteoclast precursor cells which are stimulated by RANKL [51]. RANKL is expressed by osteoblast and bone marrow stromal cells (55). OPG inhibits RANKL and also has a high affinity for RANKL [52]. Since osteoblasts express RANKL and secrete OPG, osteoblasts play an integral role in managing both bone formation and degradation. However, the higher is the ratio of RANKL to OPG, the worse is the prognosis [2]. Treatment with OPG or OPG-like molecules prevented both MM growth and bone destruction [38,53]. Recombinant OPG constructs, soluble RANK, OPG peptidomimetics [54,55], and, more recently, anti-RANKL antibodies such as denosumab have been developed to modulate the RANKL–OPG axis and reduce osteoclastic activity and myeloma [56,57,58].
Disruption of the BRC canopy impairs bone remodeling by allowing direct contact between the myeloma cells and the osteoclasts and osteoblasts [22]. Histological studies of iliac crest biopsies showed a direct correlation between the extents of the BRC canopy disruption with the magnitude of osteolytic lesions in patients with MM. A co-culture system with direct contact between myeloma cells and bone marrow stromal cells/pre-osteoblasts showed a significant decrease in OPG production, leading to the increased RANKL/OPG ratio, which results in increased bone degradation [39]. The direct contact between the stromal cells and the myeloma cells demonstrated increased secretion of IL-6 by the stromal cells [59]. IL-6 is known to activate osteoclast formation as well as increased myeloma cell proliferation [60]. Myeloma cells sometimes fuse with osteoclasts, forming myeloma–osteoclast hybrid cells that are more aggressive at eroding bone when compared to non-hybrid osteoclasts [61].
2.6. Decreased Osteoblastogenesis
In MBD, reduced bone formation secondary to decreased osteoblastic activity, leading to extensive bone loss and no repair, also plays a key role in the severity of the disease [62]. In early myeloma disease, the interaction of myeloma cells with the bone marrow microenvironment initiates the production of IL-1 and TNF-α. These cytokines recruit osteoblasts, leading to increased cell activity, which produces IL-6, a potent myeloma cell growth factor and bone resorption factor [63]. Osteoblasts also produce the growth factors IL-3 and GM–CSF, which further stimulate early myeloma cell growth and bone resorption. However, as the disease advances, BRC canopy is disrupted, leading to impaired synchrony between bone resorption and bone formation [22]. If the patient continues to have a high osteoblastic function, they do not develop MBD. Factors involved in the downregulation of osteoblastic activity mainly include Wnt/DKK1 pathway, secreted frizzle related protein–2 (SFRP-2), and Runx2. Other chemokines involved in decreasing osteoblastogenesis include hepatocyte growth factor (HGF), IL-7, sclerostin, and transforming growth factor-beta (TGF-β).
2.7. Wingless (Wnt) Signaling Pathway
The Wnt signaling pathway influences osteoblastogenesis and has significant involvement in bone formation and remodeling [64]. The Wnt signaling pathway is involved in embryogenesis, organ development after birth, and human tissue regeneration [65]. It also helps regulate stem cell production and CNS patterning. Studies have shown that the Wnt signaling pathway regulates cancer cell involvement and epidermal, intestinal, and hematopoietic systems [66,67]
Wnt genes encode Wnt family glycoproteins that transduce signals through frizzled (FZD) family receptors with extracellular Wnt-binding and cytoplasmic dishevelled-binding domains [68]. These Wnt glycoproteins are responsible for cell surface receptor activation, gene expression, cell proliferation, differentiation, and migration [69].
Wnts are classified as canonical if β-catenin dependent and noncanonical if β-catenin levels remain unaltered [70]. β-catenin is a major factor for OPG expression from osteoblasts [64]. Wnt signaling impacts osteoblastogenesis through a canonical pathway involving both intracellular and extracellular interactions, which is initiated by Wnt proteins binding to cell surface receptors made from a complex of lipoprotein related (LRP) 5/6 and FZD transmembrane proteins [71]. This complex induces an intracellular cascade involving dishevelled (DSH), Axin, and GSK-3, which prevents the forceful dilation of β-catenin, thus preventing its breakdown. Elevated β-catenin levels upregulate the transcription of genes involved in osteoblastic development. Of note, inactivation of the gene for LRP5 results in osteoporosis–pseudo-glioma syndrome, while gain-of-function mutation in LRP5 leads to a syndrome of hereditary high bone density [72,73]. These findings suggest that activation of this pathway causes increased osteoblastic activity and inhibition will decrease osteoblastogenesis [74]. Natural inhibitors of this pathway mainly include DKK1 and SFRP. Other regulators of the Wnt signaling pathway include Wnt inhibitory factor–1 (Wif-1), sclerostin, and sclerostin domain containing 1 (SOSTDC-1) [71].
2.8. Dickkopf-1 (DKK-1)
DKK1 is expressed by osteoblast and BMSC [75]. DKK1 has been shown to inhibit osteoblastogenesis via the Wnt pathway by inhibiting osteoblasts’ maturation and new bone formation. Studies have shown that DKK1 inhibits the Wnt signaling pathway by preventing the intracellular interaction, which protects β-catenin from opsonization and subsequent breakdown [76]. Mao et al. demonstrated that the mechanism of inhibition is through competitive binding to LRP6 and removal of trans-membrane protein receptors Kremens 1 and 2 [71,77]. This trimeric complex is then endocytosed, internalizing the receptor for Wnt proteins that are inhibiting the initiation of the Wnt signaling cascade. Ya-Wei Qiang et al. demonstrated that DKK1 inhibition of Wnt signaling indirectly stimulates osteoclastogenesis by inhibiting the maturation of osteoblasts which produce OPG (an inhibitor of osteoclastogenesis), resulting in decreased osteoclast inhibition [75]. In addition, they demonstrated that an increase in immature osteoblasts that produce RANKL stimulating osteoclast differentiation will no longer differentiate, causing a net increase in RANKL expression and subsequent osteoclast differentiation [78,79].
Studies such as those conducted by Tian et al. have shown that human plasma cells purified from bone marrow aspirates of myeloma patients expressed the gene for DKK1, and blood serum levels of DKK 1 were elevated in patients with MBD [80]. Expression of DKK 1 correlates with the stage of the disease, showing an increased level of DKK1 at more advanced stages. Other studies suggest that the DKK1 levels also correlate with the extent of lytic bone disease present [81].
2.9. Secreted Frizzled-Related Proteins (SFRP)
SFRP are other Wnt pathway antagonists that inhibit the binding of Wnt to the membrane-bound receptor, FZD, resulting in the downregulation of osteoblastic activity [62]. SFRP are a family of cysteine-rich glycoproteins which in combination with LRP 5/6 make up the cell membrane surface complex [82]. This complex inhibits Wnt signaling through interception and binding of Wnt proteins, preventing their interaction with the LRP 5/6 and FZD transmembrane proteins, which could initiate the canonical Wnt signaling cascade [71]. There are reports that they are expressed by several cells involved in bone formation and regulation, including myeloma cells and primary human osteoblasts [83,84].
SFRP-1 is consistently highly expressed by osteoblasts and suppress Wnt by 70% (91). SFRP-1 accumulates in pre-osteoblasts and declines upon maturation of the pre-osteoblast population. Thus, an increased number of osteoblastic precursors and reduced differentiation to mature osteoblasts lead to increased production of SFRP-1 [85]. This increased level of SFRP-1 reduces bone mineral density, trabecular volume, and biomechanical properties and increases osteochondral apoptosis [86]. Studies have shown that SFRP-2 is overexpressed specifically in myeloma cells derived from patients with advanced bone disease. Overexpression of SFRP-3 has been noticed with the progression of MGUS to myeloma [87]. Studies have shown that overexpression of SFRP-4 by osteoblast decreases the proliferation, resulting in decreased bone formation and viability [88].
2.10. Runt-Related Transcription Factor 2 (Runx2)/Core Binding Factor Runt Domain α Subunit 1 (CBFA1)
Runx2/CBFA1 is part of the non-canonical Wnt signaling pathway and constitutes a critical regulator of osteoblastogenesis, but this may also be affected by myeloma cells [89]. Runx2/CBFA1 plays an important role in the formation and differentiation of osteoblasts from mesenchymal cells and BMSC [23]. MM cells inhibit Runx2 activity in BMSC and osteoblast precursor cells, thereby impeding osteoblast differentiation [89]. Activation of Runx2/CBFA1 in human BMSC and preosteoblastic cells induce high expression of osteoblastic markers such as alkaline phosphatase and osteocalcin.
Co-cultures of human myeloma cells with mesenchymal cells showed an inhibitory effect on osteoblast formation and reduced expression of Runx2/CBFA1 by mesenchymal cells has been found after coming in direct contact with myeloma cells. In the same study, bone marrow biopsy specimens of myeloma patients with osteolytic lesions showed a markedly reduced number of Runx2/CBFA1 positive cells compared to those without myeloma bone disease [90,91].
Growth factor independence-1 (GFI-1), IL-7, and HGF are some factors that decrease Runx2/CBFA1 activity [92]. GFI-1 is a transcriptional depressor that binds to Runx2 and decreases its expression. IL-7 has a dual effect, increasing osteoclastic activity as well as inhibiting both early and late osteoblastic stimulation, differentiation, and maturation [89,93]. High levels of IL-7 have been demonstrated in the bone marrow of MM patients. IL-7 is also involved in the Runx2–mediated osteoblast suppression by inducing GFI-1 [92]. Overall, targeting Runx2, GFI-1, and IL-7 seems to have an encouraging result in overcoming MM-induced bone destruction. HGF is produced by myeloma cells and an elevated level of HGF has been demonstrated in the serum of MM patients compared to healthy individuals [94]. Besides, high levels of HGF are associated with poor prognosis. TGF-β released from the bone matrix during bone resorption inhibits osteoblastic differentiation and formation [59,95].
In recent studies, cysteine-rich 61 (CYR61/CCN1) protein, which is secreted in the bone marrow microenvironment, has been identified to stimulate osteoblastic differentiation by upregulating Runx2 in MM patients [96]. Studies have also shown that MM cells also overexpress Runx2, and higher levels of Runx2 in advanced stages of the disease are associated with poor prognosis [97]. Runx2 is also known to induce the AKT/β-catenin/survivin pathway along with the transcriptional activation of a gene panel that facilitates the homing of MM cells into the bone niche.
2.11. Extracellular Vesicles (EV) and Non-Coding RNA (ncRNA)
ncRNA includes ribonucleic acids that are not translated into proteins but participate in the translation process involving transfer RNAs and ribosomal RNAs, as well as splicing of small nuclear RNAs. These are considered the housekeeping ncRNAs. The other category includes inducible ncRNAs that are involved in the annealing process of complementary sequences in DNAs or RNAs and control gene expression. Various studies demonstrate that plasma cell neoplasms are regulated by several classes of ncRNAs [98,99]. The ncRNAs involved in the pathogenesis of MM bone disease are noted to be transported between cells by EVs [100]. Evidence has been building up in recent years that shows EVs and their ncRNAs are responsible for the onset of bone disease since they can promote osteoclast activation and inhibit osteogenesis by impacting the differentiation of mesenchymal stem cells [101,102,103,104,105].
3. Management of Multiple Myeloma Bone Disease
3.1. Radiotherapy
Malignant plasma cells exhibit increased sensitivity to radiation. For this reason, radiotherapy alone, without systemic chemotherapy, is considered the primary treatment modality for solitary osseous plasmacytomas. Radiotherapy has demonstrated excellent local control of osseous and extraosseous solitary plasmacytoma [106,107,108,109,110,111]. In MM patients, focal radiotherapy provides an effective modality of palliation for refractory bone pain, impending pathologic fractures, and to treat spinal cord compression. Active bone marrow containing areas, such as pelvic bones, should receive radiation in a judicious fashion if there is a need or plan for stem cell collection in the future.
3.2. Vertebroplasty or Kyphoplasty
These are minimal invasive procedures that include percutaneous injection of bone cement into fractured vertebral body for stabilization. Both these procedures are safe and effective in controlling pain and improving mobility in patients with compression fractures of vertebra from myeloma involvement [112,113].
4. Antiresorptive Therapies
4.1. Bisphosphonates
Bisphosphonates along with denosumab are the approved modalities of bone resorptive therapies for management of myeloma bone disease [114]. Commonly used bisphosphonates include zoledronic acid, pamidronate. and clodronate. Bisphosphonates, especially second-generation compounds which contain nitrogen moieties such as pamidronate or zoledronic acid, are several times more potent than non-nitrogen containing bisphosphonates (clodronate) [115]. These agents bind to hydroxyapatite and then cause osteoclast apoptosis by inhibiting mevalonate pathway via inhibition of farnesyl diphosphate (FPP) synthase, thereby disrupting prenylation of small intracellular guanine triphosphatases, which are essential for osteoclast function and survival [116,117,118].
In the United States, zoledronic acid is approved for treating myeloma bone disease at 4 mg intravenous dosing given every 3–4 weeks, while pamidronate is approved for 90 mg doses administered intravenously every 3–4 weeks. Both these agents have similar efficacy [119,120], and the selection is primarily based on administration time and additional benefits besides bone resorptive properties. Zoledronic acid is administered over 15 min while pamidronate is infused over 2 h. Zoledronic acid is not recommended for usage in severe renal impairment, whereas pamidronate can be used in patients with severe renal impairment by increasing infusion duration up to 4 h and using a reduced dose of 30 mg [121]. Zoledronic acid is preferred for management of patients with concomitant hypercalcemia. It is more effective in hypercalcemia reversal compared to pamidronate [122]. Uncommon but serious and important toxicities associated with bisphosphonate therapy include renal insufficiency and osteonecrosis of jaw [123]. Incidence of osteonecrosis of jaw is higher with zoledronic acid compared to pamidronate (10% vs. 4%) [124]. Major risk factors for osteonecrosis of jaw on bisphosphonate treatment include poor oral hygiene, invasive dental procedures, and local infections [125,126]. Other non-serious toxicities include flu-like symptoms due to an acute phase reaction.
Bisphosphonates have been employed in the management of MM-related bone disease since the early 1980s, with increased acceptance and usage over the decades, to being considered now as an essential component of MM management. Intravenous pamidronate at 90 mg every month demonstrated efficacy compared to placebo, and it was also confirmed to be effective in relapsed or refractory patients [116,127]. A study published in 2010 showed that pamidronate at 30 mg/month was as effective as a 90 mg/month dose in reducing time to skeletal-related events and skeletal-related event-free survival [128]. Zoledronic acid established superiority over clodronate in a large randomized controlled trial (MRC Myeloma IX) [129,130]. This trial and later the secondary analysis established the superiority of zoledronic acid over clodronate in all patient subsets including transplant or nontransplant candidates, patients receiving thalidomide maintenance or not, and most importantly in patients presenting with or without bone lesions. The secondary analysis highlights the importance of adding bisphosphonates upfront along with myeloma therapy in all newly diagnosed patients. The results from MRC Myeloma IX trial show a significant increase in progression-free survival and overall survival for patients receiving zoledronic acid compared to clodronate, regardless of whether they had bone lesions. This resulted in ASCO putting forward guidelines recommending initiation of bisphosphonate therapy in any newly diagnosed myeloma patient who is on active myeloma therapy [131].
Studies have been conducted to assess the optimal dosing interval of zoledronic acid, including extending from monthly to every 3 months dosing while maintaining efficacy. The Z-MARK study evaluated efficacy and safety of every 12 weeks dosing of zoledronic acid compared to every 4 weeks dosing in patients who had received 1–2 years of prior monthly bisphosphonate therapy [132]. The overall incidence of skeletal-related events in the second year was low at 4.9% and the 2-year incidence of osteonecrosis of jaw was 3.3%. Another large randomized controlled trial comparing every 12 weeks to a 4-week regimen of zoledronic acid in 1544 patients with MM or bone metastasis from solid malignancies analyzed 278 patients with MM [133]. There was no difference in skeletal-related events, osteonecrosis of jaw, or renal dysfunction among patients in either treatment groups. However, a high study dropout rate was noted in this study. Both these studies suggest a possible feasibility of less frequent zoledronic acid administration, and the final decision is still based on physician discretion and patient preference.
According to the International Myeloma Working Group (IMWG), bisphosphonates can be given until disease progression in patients who do not achieve a complete response (CR) or very good partial response (VGPR) [134]. For those patients with CR or VGPR, IMWG currently recommends 12–24 months of bisphosphonate treatment and further continuation based on physician discretion. The MRC Myeloma IX trial reported a small number of patients who received bisphosphonate therapy for up to 5 years, who showed continued benefit in preventing incidence of skeletal-related events [129,130]. However, incidence of osteonecrosis of jaw also continued to increase in these patients over this time period. In MM patients with at least VGPR, the optimal duration of bisphosphonates is an ongoing area of active research.
According to a meta-analysis conducted by Cochrane database, bisphosphonates have to be administered to 6–15 myeloma patients to prevent one skeletal-related event [135].
4.2. Denosumab
Denosumab is a fully humanized monoclonal antibody that targets RANK-ligand (RANKL), which was approved in 2018 by the FDA for treatment of myeloma bone disease. The RANK–RANKL system is an important pathway in regulation of normal as well as pathologic bone remodeling [136]. RANK–RANKL interaction mediates osteoclast precursors, thereby promoting differentiation into osteoclasts as well as activating mature osteoclasts to resorb bone. Denosumab binds to RANKL with high affinity, thereby preventing activation of RANK and thus inhibiting formation, activation, and survival of osteoclasts, which results in reduction of bone resorption as well as bone destruction in MM.
A randomized trial conducted by Henry et al., comparing denosumab with zoledronic acid in patients with bone metastasis from advanced cancers (excluding breast and prostate cancer) or MM, included 180 patients with MM [56]. Subgroup analysis of this population demonstrated favorable survival with zoledronic acid. In another larger randomized controlled trial aimed at comparing denosumab to zoledronic acid in patients with MM, denosumab was found to be non-inferior to zoledronic acid in delaying skeletal-related events [137]. Overall survival was found to be similar in both arms, but median progression-free survival was 10.7 months longer for patients receiving denosumab. The denosumab arm was associated with higher incidence of hypocalcemia (17% vs. 12%), lower rates of renal dysfunction, and higher rates of doubling of creatinine from baseline (3% vs. 7%), compared to zoledronic acid. Rates of osteonecrosis of jaw were low in both arms and not statistically different (3% vs. 2%).
For patients receiving denosumab or bisphosphonates, regular monitoring of kidney function and dental hygiene along with vitamin D and calcium supplementation is recommended.
5. Systemic Anti-Myeloma Treatments
5.1. Proteasome Inhibitors
Proteasome pathway inhibition has been shown to be involved in regulation of bone remodeling. Proteasome-dependent inhibition of NF-ƙB leads to a reduction in RANKL-mediated osteoclast differentiation [138,139]. The proteasome pathway also plays an important role in osteoblast differentiation and inhibiting it induces new bone formation. In MM mouse models, proteasome inhibitors induced new bone formation by increasing expression of BMP2, a potent osteoblast differentiation inducing agent [140,141,142].
Bortezomib is a highly effective proteasome inhibitor which is currently the mainstay of anti-myeloma treatment regimens [143,144,145,146,147]. Several preclinical [148,149,150,151,152] and clinical studies [153,154,155,156,157,158] have investigated the effect of bortezomib on bone remodeling. Based on evidence from preclinical studies, clinical effects of bortezomib on bone remodeling could be analyzed by direct measurements of bone characteristics. For example, bortezomib-based treatment was shown to increase serum alkaline phosphatase (ALP), bone ALP, and serum parathyroid hormone (PTH) levels, as well as reduce levels of DKK-1 (marker of bone resorption). Some of these clinical studies have also indicated that changes in these bone remodeling markers were noted in both responders and non-responders to bortezomib-based therapy. In a randomized phase III trial (VISTA) that compared bortezomib, melphalan, and prednisone (VMP) regimen to melphalan and prednisone (MP), the rates of bisphosphonate usage, progression of bone disease, and needs for palliative radiation therapy were all lower in the VMP arm compared to the MP arm [154]. In another study evaluating bortezomib, thalidomide, and dexamethasone (VTD) regimen as consolidation in patients with newly diagnosed MM, it was demonstrated that bortezomib-based therapy was associated with RANKL/OPG ratio normalization and achievement of a very low incidence rate of skeletal-related events (2%), even without concomitant use of bisphosphonates [156].
Carfilzomib is a second-generation proteasome inhibitor, which is currently FDA approved for use in relapsed/refractory MM as part of triplet regimen or doublet regimen [159,160,161,162,163,164]. It is also used off-label in newly diagnosed MM patients (in both transplant and non-transplant candidates) and is included in the National Comprehensive Cancer Network (NCCN) guidelines for the same indications [165,166,167,168]. In pre-clinical studies, carfilzomib demonstrated activity against bone resorption and promoted new bone formation [142,169,170]. It was also observed that carfilzomib was more effective in enhancing osteoblastic activity compared to bortezomib, suggesting that carfilzomib may be a more potent promoter of new bone formation. A small phase II study by Suvannasankha et al. explored the effect of single agent carfilzomib on bone metabolism in patients with relapsed MM [171]. Only 10 patients were enrolled in this study, which demonstrated bone anabolic effects along with inhibition of bone resorption. A retrospective analysis of two phase II trials using carfilzomib as a single agent in relapsed/refractory myeloma (PX-171-003 and PX-171-004) indicated that an early elevation in alkaline phosphatase is associated with subsequent myeloma response [172].
5.2. Immunomodulatory Drugs (IMiDs)
IMiDs form an important group of drugs in MM therapy arsenal. They are thalidomide analogs, which induce apoptosis of myeloma cells and enhance antimyeloma T-cell and NK-cell immunity [173,174]. IMiDs also inhibit angiogenesis, thereby making the bone marrow microenvironment inconducive for myeloma cell growth and survival [175]. Bone directed effects of IMiDs have been studied with conflicting results. In a pre-clinical study, pomalidomide (previously known as CC-4047) inhibited osteoclastogenesis through downregulation of the hematopoietic transcriptional factor PU.1 [176]. Another in vitro study demonstrated inhibition of osteoblastic activity by IMiDs, as shown by reduced mineralization and alkaline phosphatase activity [177]. In a clinical setting, treatment of MM with thalidomide and dexamethasone in relapsed/refractory setting resulted in normalization of RANKL/OPG ratio [178]. Similarly, lenalidomide also demonstrated reduced osteoclastic bone resorption by inhibiting osteoclast-activating factors APRIL (a proliferation inducing ligand) and BAFF (B-cell activating factor) [179].
6. Novel Approaches and Future Directions
6.1. Bruton Tyrosine Kinase Inhibitors (BTKi)
Bruton tyrosine kinase (BTK) is a non-receptor tyrosine kinase, which is expressed in many hematopoietic cells, including maturing B-cells, and plays an important role in B-cell maturation and function [180,181]. BTK inhibition has proven to be a highly successful therapeutic target in management of various B-cell malignancies such as chronic lymphocytic leukemia and mantle cell lymphoma [182,183]. BTK has been shown to be strongly expressed in myeloma cells and selectively expressed in osteoclasts but not in osteoblasts [184,185]. Based on this physiologic principle, investigators demonstrated that BTKi such as ibrutinib reduced MM tumor burden while limiting osteoclastogenesis and bone resorption. This principle needs to be tested further in clinical studies.
6.2. Anti-DKK1
DKK1 is expressed by osteoblasts, and it acts as an antagonist of Wnt pathway, which results in inhibition of osteoblast maturation and new bone formation [75]. High expression of DKK1 is noted in patients with MM and extensive bone involvement. In a pre-clinical study using SCID-rab mice, it was demonstrated that anti-DKK1 decreased osteoclastogenesis and promoted new bone formation by stimulating osteoblast activity, in both myeloma-involved and uninvolved bones [186].
6.3. OPG Agonists
Osteoprotogerin (OPG) is secreted by osteoblasts, bone marrow stromal cells, and endothelial cells. OPG blocks the interaction between RANK and RANKL, thereby blocking activation and differentiation of osteoclasts [51,187]. OPG agonists mimic this activity by acting as a decoy receptor for RANKL. Safety and efficacy of OPG agonists (AMGN 0007) was evaluated in a phase I study. In this study, patients with MM and breast cancer patients with bone lesions were treated with a single dose of AMGN 0007 or pamidronate at 90 mg. The efficacy was assessed by measuring levels of bone resorption marker NTX. AMGN 0007 results in decreased levels of NTX comparable to pamidronate, and it was tolerated well [55].
6.4. Anti-Sclerostin
Sclerostin is a cysteine knot-containing protein, produced by osteocytes. It induces apoptosis of mature osteoblasts by activating the caspase pathway and inhibits osteoblast-driven bone formation [188]. Romosozumab, a humanized anti-sclerostin antibody, was found to be effective in management of benign bone disorders [189]. This rationale is further being tested in MM models, currently in preclinical stages, especially in combination with antineoplastic agents such as proteasome inhibitors [190,191].
6.5. TGF-β
TGF-β has been implicated in tumor-induced bone disease in various cancers [192]. The exact mechanism of TGF-β-induced bone disease is unknown. In a preclinical model, a TGF-β inhibitor, SRI31277, was administered to mice with multiple osteolytic lesions and was shown to decrease the tumor burden and decrease phosphorylated SMAD2, which was associated with decrease in osteoclasts and increase in osteoblasts [193]. This would be a useful approach in myeloma bone disease management, as well as skeletal disease management in various other cancers, if proven to be effective in humans.
6.6. Activin A and Sotatercept
Activin A is a member of the TGF-β superfamily, which is released from osteoblast and osteoclast precursors and has been shown to be elevated in patients with myeloma bone disease. Activin A antagonizes bone morphogenetic proteins (BMPs) by competing for their receptors and therefore inhibits BMP-induced apoptosis of malignant plasma cells [194,195]. Sotatercept, a soluble recombinant activin receptor type IIA (ActRIIA) ligand fused to human Fc-Ig fragment, binds activin A/B as well as members of the TGF-β superfamily and disrupts downstream cascades. In a phase I trial, Yee et al. demonstrated that sotatercept in combination with lenalidomide and dexamethasone was well tolerated; preliminary data suggest that sotatercept leads to early increases in both hemoglobin and bone mineral density, and it was noted to be the first agent that may address both these significant morbidity issues in MM [196]. In a phase II trial, sotatercept in addition to melphalan, prednisolone, and thalidomide resulted in an increase in bone alkaline phosphatase, indicating improved bone turnover [195].
6.7. Radionuclides
Radionuclides or radiopharmaceuticals demonstrate affinity for bone undergoing active remodeling and therefore can deliver localized therapeutic effect. This has been studied well in the management of metastatic prostate cancers with bone disease, using radium-223 and samarium-153 [197,198]. Samarium-153 has been evaluated in patients with myeloma bone disease and bone pain, with significant improvement in bone pain [199].
6.8. Recombinant Parathyroid Hormone (rPTH)
The role of rPTH, a teriparatide, in increasing bone mineral density is controversial because of conflicting evidence that rPTH can also stimulate osteoclastogenesis [200]. In addition, there have been reports of malignancies occurring in patients on rPTH, including emergence of myeloma in a patient with osteoporosis treated with rPTH. These concerns have for now halted further extrapolation of rPTH in the management of MBD [201,202].
7. Conclusions
Over the years, and most notably within the last decade, treatment of MM has become more effective with the incorporation of various novel therapies such as proteasome inhibitors, immunomodulatory drugs, monoclonal antibodies, histone deacetylase inhibitors, selective inhibitors of nuclear export, and the latest anti-BCMA therapy. Therefore, it is imperative to develop better supportive strategies, including more effective management of myeloma bone disease, to match the effectiveness of newer and more effective anti-myeloma therapies. Bisphosphonates and more recently denosumab have been the mainstay of current MBD management. With a better understanding of the complex biology of myeloma bone disease, development of treatments aimed at targeting the bone and marrow microenvironment will be able to treat myeloma effectively while preserving bone health and potentially improving overall disease outcomes.
Funding
This research received no external funding.
Conflicts of Interest
The authors do not have any conflicts of interest to disclose.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kyle R.A., Gertz M.A., Witzig T.E., Lust J.A., Lacy M.Q., Dispenzieri A., Fonseca R., Rajkumar S.V., Offord J.R., Larson D.R., et al. Review of 1027 Patients with Newly Diagnosed Multiple Myeloma. Mayo Clin. Proc. 2003;78:21–33. doi: 10.4065/78.1.21. [DOI] [PubMed] [Google Scholar]
- 2.Kyle R.A., Therneau T.M., Rajkumar S.V., Larson D.R., Plevak M.F., Melton L.J. Incidence of multiple myeloma in Olmsted County, Minnesota: Trend over 6 decades. Cancer. 2004;101:2667–2674. doi: 10.1002/cncr.20652. [DOI] [PubMed] [Google Scholar]
- 3.O’Donnell E.K., Raje N.S. Myeloma bone disease: Pathogenesis and treatment. Clin. Adv. Hematol. Oncol. 2017;15:285–295. [PubMed] [Google Scholar]
- 4.American Cancer Society . American Cancer Society: Cancer Facts and Figures 2021. American Cancer Society; Atlanta, GA, USA: 2021. [Google Scholar]
- 5.Alexander D.D., Mink P.J., Adami H.-O., Cole P., Mandel J.S., Oken M.M., Trichopoulos D. Multiple myeloma: A review of the epidemiologic literature. Int. J. Cancer. 2007;120(Suppl. S12):40–61. doi: 10.1002/ijc.22718. [DOI] [PubMed] [Google Scholar]
- 6.Edwards C.M., Zhuang J., Mundy G.R. The pathogenesis of the bone disease of multiple myeloma. Bone. 2008;42:1007–1013. doi: 10.1016/j.bone.2008.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vallet S., Mukherjee S., Vaghela N., Hideshima T., Fulciniti M., Pozzi S., Santo L., Cirstea D., Patel K., Sohani A.R., et al. Activin A promotes multiple myeloma-induced osteolysis and is a promising target for myeloma bone disease. Proc. Natl. Acad. Sci. USA. 2010;107:5124–5129. doi: 10.1073/pnas.0911929107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Podar K., Chauhan D., Anderson K.C. Bone marrow microenvironment and the identification of new targets for myeloma therapy. Leukemia. 2008;23:10–24. doi: 10.1038/leu.2008.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terpos E., Berenson J., Cook R.J., Lipton A., Coleman R.E. Prognostic variables for survival and skeletal complications in patients with multiple myeloma osteolytic bone disease. Leukemia. 2010;24:1043–1049. doi: 10.1038/leu.2010.62. [DOI] [PubMed] [Google Scholar]
- 10.Walker R., Barlogie B., Haessler J., Tricot G., Anaissie E., Shaughnessy J.D., Jr., Epstein J., Van Hemert R., Erdem E., Hoering A., et al. Magnetic Resonance Imaging in Multiple Myeloma: Diagnostic and Clinical Implications. J. Clin. Oncol. 2007;25:1121–1128. doi: 10.1200/JCO.2006.08.5803. [DOI] [PubMed] [Google Scholar]
- 11.Bonewald L.F. The amazing osteocyte. J. Bone Miner. Res. 2011;26:229–238. doi: 10.1002/jbmr.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roodman G. Cell biology of the osteoclast. Exp. Hematol. 1999;27:1229–1241. doi: 10.1016/S0301-472X(99)00061-2. [DOI] [PubMed] [Google Scholar]
- 13.Wada T., Nakashima T., Hiroshi N., Penninger J.M. RANKL–RANK signaling in osteoclastogenesis and bone disease. Trends Mol. Med. 2006;12:17–25. doi: 10.1016/j.molmed.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Baron R., Neff L., Louvard D., Courtoy P.J. Cell-mediated extracellular acidification and bone resorption: Evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J. Cell Biol. 1985;101:2210–2222. doi: 10.1083/jcb.101.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delaissé J.-M., Andersen T.L., Engsig M.T., Henriksen K., Troen T., Blavier L. Matrix metalloproteinases (MMP) and cathepsin K contribute differently to osteoclastic activities. Microsc. Res. Tech. 2003;61:504–513. doi: 10.1002/jemt.10374. [DOI] [PubMed] [Google Scholar]
- 16.Negishi-Koga T., Shinohara M., Komatsu N., Bito H., Kodama T., Friedel R.H., Takayanagi H. Suppression of bone formation by osteoclastic expression of semaphorin 4D. Nat. Med. 2011;17:1473–1480. doi: 10.1038/nm.2489. [DOI] [PubMed] [Google Scholar]
- 17.Datta H.K., Ng W.F., Walker J.A., Tuck S.P., Varanasi S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008;61:577–587. doi: 10.1136/jcp.2007.048868. [DOI] [PubMed] [Google Scholar]
- 18.Gavriatopoulou M., Dimopoulos M., Christoulas D., Migkou M., Iakovaki M., Gkotzamanidou M., Terpos E. Dickkopf-1: A suitable target for the management of myeloma bone disease. Expert Opin. Ther. Targets. 2009;13:839–848. doi: 10.1517/14728220903025770. [DOI] [PubMed] [Google Scholar]
- 19.Khosla S., Westendorf J.J., Oursler M.J. Building bone to reverse osteoporosis and repair fractures. J. Clin. Investig. 2008;118:421–428. doi: 10.1172/JCI33612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauge E.M., Qvesel D., Eriksen E.F., Mosekilde L., Melsen F. Cancellous Bone Remodeling Occurs in Specialized Compartments Lined by Cells Expressing Osteoblastic Markers. J. Bone Miner. Res. 2001;16:1575–1582. doi: 10.1359/jbmr.2001.16.9.1575. [DOI] [PubMed] [Google Scholar]
- 21.Andersen T.L., Sondergaard T.E., Skórzyńska-Dziduszko K., Dagnaes-Hansen F., Plesner T.L., Hauge E.M., Plesner T., Delaisse J.-M. A Physical Mechanism for Coupling Bone Resorption and Formation in Adult Human Bone. Am. J. Pathol. 2009;174:239–247. doi: 10.2353/ajpath.2009.080627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen T.L., Søe K., Sondergaard T.E., Plesner T., Delaisse J.-M. Myeloma cell-induced disruption of bone remodelling compartments leads to osteolytic lesions and generation of osteoclast-myeloma hybrid cells. Br. J. Haematol. 2010;148:551–561. doi: 10.1111/j.1365-2141.2009.07980.x. [DOI] [PubMed] [Google Scholar]
- 23.Hameed A., Brady J.J., Dowling P., Clynes M., O’Gorman P. Bone Disease in Multiple Myeloma: Pathophysiology and Management. Cancer Growth Metastasis. 2014;7:CGM.S16817–CGM.S16842. doi: 10.4137/CGM.S16817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giuliani N., Ferretti M., Bolzoni M., Storti P., Lazzaretti M., Palma B.D., Bonomini S., Martella E., Agnelli L., Neri A., et al. Increased osteocyte death in multiple myeloma patients: Role in myeloma-induced osteoclast formation. Leukemia. 2012;26:1391–1401. doi: 10.1038/leu.2011.381. [DOI] [PubMed] [Google Scholar]
- 25.Oranger A., Carbone C., Izzo M., Grano M. Cellular Mechanisms of Multiple Myeloma Bone Disease. Clin. Dev. Immunol. 2013;2013:1–11. doi: 10.1155/2013/289458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Colucci S., Brunetti G., Mori G., Oranger A., Centonze M., Mori C., Cantatore F.P., Tamma R., Rizzi R., Liso V., et al. Soluble decoy receptor 3 modulates the survival and formation of osteoclasts from multiple myeloma bone disease patients. Leukemia. 2009;23:2139–2146. doi: 10.1038/leu.2009.136. [DOI] [PubMed] [Google Scholar]
- 27.Giuliani N., Rizzoli V., Roodman G.D. Multiple myeloma bone disease: Pathophysiology of osteoblast inhibition. Blood. 2006;108:3992–3996. doi: 10.1182/blood-2006-05-026112. [DOI] [PubMed] [Google Scholar]
- 28.Calvani N., Cafforio P., Silvestris F., Dammacco F., Silvestris F. Functional osteoclast-like transformation of cultured human myeloma cell lines. Br. J. Haematol. 2005;130:926–938. doi: 10.1111/j.1365-2141.2005.05710.x. [DOI] [PubMed] [Google Scholar]
- 29.Tai Y.-T., Li X.-F., Breitkreutz I., Song W., Neri P., Catley L., Podar K., Hideshima T., Chauhan D., Raje N., et al. Role of B-Cell–Activating Factor in Adhesion and Growth of Human Multiple Myeloma Cells in the Bone Marrow Microenvironment. Cancer Res. 2006;66:6675–6682. doi: 10.1158/0008-5472.CAN-06-0190. [DOI] [PubMed] [Google Scholar]
- 30.Hideshima T., Bergsagel P.L., Kuehl W.M., Anderson K.C. Advances in biology of multiple myeloma: Clinical applications. Blood. 2004;104:607–618. doi: 10.1182/blood-2004-01-0037. [DOI] [PubMed] [Google Scholar]
- 31.Terpos E., Kastritis E., Christoulas D., Gkotzamanidou M., Eleutherakis-Papaiakovou E., Kanellias N., Papatheodorou A., Dimopoulos M. Circulating activin-A is elevated in patients with advanced multiple myeloma and correlates with extensive bone involvement and inferior survival; no alterations post-lenalidomide and dexamethasone therapy. Ann. Oncol. 2012;23:2681–2686. doi: 10.1093/annonc/mds068. [DOI] [PubMed] [Google Scholar]
- 32.Noonan K., Marchionni L., Anderson J., Pardoll D., Roodman G.D., Borrello I. A novel role of IL-17–producing lymphocytes in mediating lytic bone disease in multiple myeloma. Blood. 2010;116:3554–3563. doi: 10.1182/blood-2010-05-283895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prabhala R.H., Pelluru D., Fulciniti M., Prabhala H.K., Nanjappa P., Song W., Pai C., Amin S., Tai Y.-T., Richardson P.G., et al. Elevated IL-17 produced by Th17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115:5385–5392. doi: 10.1182/blood-2009-10-246660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Terpos E., Dimopoulos M. Myeloma bone disease: Pathophysiology and management. Ann. Oncol. 2005;16:1223–1231. doi: 10.1093/annonc/mdi235. [DOI] [PubMed] [Google Scholar]
- 35.Bataille R., Chappard D., Marcelli C., Dessauw P., Sany J., Baldet P., Alexandre C. Mechanisms of bone destruction in multiple myeloma: The importance of an unbalanced process in determining the severity of lytic bone disease. J. Clin. Oncol. 1989;7:1909–1914. doi: 10.1200/JCO.1989.7.12.1909. [DOI] [PubMed] [Google Scholar]
- 36.Colucci S., Brunetti G., Rizzi R., Zonno A., Mori G., Colaianni G., Del Prete D., Faccio R., Liso A., Capalbo S., et al. T cells support osteoclastogenesis in an in vitro model derived from human multiple myeloma bone disease: The role of the OPG/TRAIL interaction. Blood. 2004;104:3722–3730. doi: 10.1182/blood-2004-02-0474. [DOI] [PubMed] [Google Scholar]
- 37.Roux S., Amazit L., Meduri G., Guiochon-Mantel A., Milgrom E., Mariette X. RANK (Receptor Activator of Nuclear Factor kappa B) and RANK Ligand Are Expressed in Giant Cell Tumors of Bone. Am. J. Clin. Pathol. 2002;117:210–216. doi: 10.1309/BPET-F2PE-P2BD-J3P3. [DOI] [PubMed] [Google Scholar]
- 38.Pearse R.N., Sordillo E.M., Yaccoby S., Wong B.R., Liau D.F., Colman N., Michaeli J., Epstein J., Choi Y. Multiple myeloma disrupts the TRANCE/ osteoprotegerin cytokine axis to trigger bone destruction and promote tumor progression. Proc. Natl. Acad. Sci. USA. 2001;98:11581–11586. doi: 10.1073/pnas.201394498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Giuliani N., Bataille R., Mancini C., Lazzaretti M., Barillé S. Myeloma cells induce imbalance in the osteoprotegerin/osteoprotegerin ligand system in the human bone marrow environment. Blood. 2001;98:3527–3533. doi: 10.1182/blood.V98.13.3527. [DOI] [PubMed] [Google Scholar]
- 40.Brunetti G., Oranger A., Mori G., Centonze M., Colaianni G., Rizzi R., Liso V., Zallone A., Grano M., Colucci S. The formation of osteoclasts in multiple myeloma bone disease patients involves the secretion of soluble decoy receptor 3. Ann. N. Y. Acad. Sci. 2010;1192:298–302. doi: 10.1111/j.1749-6632.2009.05304.x. [DOI] [PubMed] [Google Scholar]
- 41.Giuliani N., Colla S., Rizzoli V. New insight in the mechanism of osteoclast activation and formation in multiple myeloma: Focus on the receptor activator of NF-κB ligand (RANKL) Exp. Hematol. 2004;32:685–691. doi: 10.1016/j.exphem.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 42.Vallet S., Pozzi S., Patel K., Vaghela N., Fulciniti M., Veiby P., Hideshima T., Santo L., Cirstea D., Scadden D.T., et al. A novel role for CCL3 (MIP-1α) in myeloma-induced bone disease via osteocalcin downregulation and inhibition of osteoblast function. Leukemia. 2011;25:1174–1181. doi: 10.1038/leu.2011.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Uneda S., Hata H., Matsuno F., Harada N., Mitsuya Y., Kawano F., Mitsuya H. Macrophage inflammatory protein-1 alpha is produced by human multiple myeloma (MM) cells and its expression correlates with bone lesions in patients with MM. Br. J. Haematol. 2003;120:53–55. doi: 10.1046/j.1365-2141.2003.04040.x. [DOI] [PubMed] [Google Scholar]
- 44.Abe M., Hiura K., Wilde J., Moriyama K., Hashimoto T., Ozaki S., Wakatsuki S., Kosaka M., Kido S., Inoue D., et al. Role for macrophage inflammatory protein (MIP)-1α and MIP-1β in the development of osteolytic lesions in multiple myeloma. Blood. 2002;100:2195–2202. doi: 10.1182/blood.V100.6.2195. [DOI] [PubMed] [Google Scholar]
- 45.Kitaura H., Sands M.S., Aya K., Zhou P., Hirayama T., Uthgenannt B., Wei S., Takeshita S., Veis D., Silva M.J., et al. Marrow Stromal Cells and Osteoclast Precursors Differentially Contribute to TNF-α-Induced Osteoclastogenesis In Vivo. J. Immunol. 2004;173:4838–4846. doi: 10.4049/jimmunol.173.8.4838. [DOI] [PubMed] [Google Scholar]
- 46.Nanes M.S. Tumor necrosis factor-α: Molecular and cellular mechanisms in skeletal pathology. Gene. 2003;321:1–15. doi: 10.1016/S0378-1119(03)00841-2. [DOI] [PubMed] [Google Scholar]
- 47.Feige U. Osteoprotegerin. Ann. Rheum. Dis. 2001;60:iii81–iii84. doi: 10.1136/ard.60.90003.iii81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Romas E., Gillespie M., Martin T. Involvement of receptor activator of NFκB ligand and tumor necrosis factor-α in bone destruction in rheumatoid arthritis. Bone. 2002;30:340–346. doi: 10.1016/S8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 49.Moschen A.R., Kaser A., Enrich B., Ludwiczek O., Gabriel M., Obrist P., Wolf A.M., Tilg H. The RANKL/OPG system is activated in inflammatory bowel disease and relates to the state of bone loss. Gut. 2005;54:479–487. doi: 10.1136/gut.2004.044370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mancino A.T., Klimberg V., Yamamoto M., Manolagas S.C., Abe E. Breast Cancer Increases Osteoclastogenesis by Secreting M-CSF and Upregulating RANKL in Stromal Cells. J. Surg. Res. 2001;100:18–24. doi: 10.1006/jsre.2001.6204. [DOI] [PubMed] [Google Scholar]
- 51.Boyle W.J., Simonet W.S., Lacey D.L. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 52.Hofbauer L.C., Schoppet M. Clinical Implications of the Osteoprotegerin/RANKL/RANK System for Bone. J. Am. Med. Assoc. 2015 doi: 10.1145/2713168.2713186. [DOI] [Google Scholar]
- 53.Croucher P., Shipman C.M., Lippitt J., Perry M., Asosingh K., Hijzen A., Brabbs A.C., Van Beek E.J.R., Holen I., Skerry T.M., et al. Osteoprotegerin inhibits the development of osteolytic bone disease in multiple myeloma. Blood. 2001;98:3534–3540. doi: 10.1182/blood.V98.13.3534. [DOI] [PubMed] [Google Scholar]
- 54.Heath D.J., Vanderkerken K., Cheng X., Gallagher O., Prideaux M., Murali R., Croucher P.I. An Osteoprotegerin-like Peptidomimetic Inhibits Osteoclastic Bone Resorption and Osteolytic Bone Disease in Myeloma. Cancer Res. 2007;67:202–208. doi: 10.1158/0008-5472.CAN-06-1287. [DOI] [PubMed] [Google Scholar]
- 55.Body J.-J., Greipp P., Coleman R.E., Facon T., Geurs F., Fermand J.-P., Harousseau J.-L., Lipton A., Mariette X., Williams C.D., et al. A Phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97:887–892. doi: 10.1002/cncr.11138. [DOI] [PubMed] [Google Scholar]
- 56.Henry D.H., Costa L., Goldwasser F., Hirsh V., Hungria V., Prausova J., Scagliotti G.V., Sleeboom H., Spencer A., Vadhan-Raj S., et al. Randomized, Double-Blind Study of Denosumab Versus Zoledronic Acid in the Treatment of Bone Metastases in Patients with Advanced Cancer (Excluding Breast and Prostate Cancer) or Multiple Myeloma. J. Clin. Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- 57.Vij R., Horvath N., Spencer A., Taylor K., Vadhan-Raj S., Vescio R., Smith J., Qian Y., Yeh H., Jun S. An open-label, phase 2 trial of denosumab in the treatment of relapsed or plateau-phase multiple myeloma. Am. J. Hematol. 2009;84:650–656. doi: 10.1002/ajh.21509. [DOI] [PubMed] [Google Scholar]
- 58.Raje N.S., Yee A.J. Denosumab, a RANK ligand inhibitor, for the management of bone loss in cancer patients. Clin. Interv. Aging. 2012;7:331–338. doi: 10.2147/CIA.S14566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanaka Y., Abe M., Hiasa M., Oda A., Amou H., Nakano A., Takeuchi K., Kitazoe K., Kido S., Inoue D., et al. Myeloma Cell-Osteoclast Interaction Enhances Angiogenesis Together with Bone Resorption: A Role for Vascular Endothelial Cell Growth Factor and Osteopontin. Clin. Cancer Res. 2007;13:816–823. doi: 10.1158/1078-0432.CCR-06-2258. [DOI] [PubMed] [Google Scholar]
- 60.Cheung W.-C., Van Ness B. Distinct IL-6 signal transduction leads to growth arrest and death in B cells or growth promotion and cell survival in myeloma cells. Leukemia. 2002;16:1182–1188. doi: 10.1038/sj.leu.2402481. [DOI] [PubMed] [Google Scholar]
- 61.Andersen T.L., Boissy P., Sondergaard T.E., Kupisiewicz K., Plesner T., Rasmussen T., Haaber J., Kølvraa S., Delaissé J.-M. Osteoclast nuclei of myeloma patients show chromosome translocations specific for the myeloma cell clone: A new type of cancer–host partnership? J. Pathol. 2006;211:10–17. doi: 10.1002/path.2078. [DOI] [PubMed] [Google Scholar]
- 62.Roodman G.D. Novel targets for myeloma bone disease. Expert Opin. Ther. Targets. 2008;12:1377–1387. doi: 10.1517/14728222.12.11.1377. [DOI] [PubMed] [Google Scholar]
- 63.Bataille R., Chappard D., Marcelli C., Dessauw P., Baldet P., Sany J., Alexandre C. Recruitment of new osteoblasts and osteoclasts is the earliest critical event in the pathogenesis of human multiple myeloma. J. Clin. Investig. 1991;88:62–66. doi: 10.1172/JCI115305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Albers J., Keller J., Baranowsky A., Beil F.T., Catala-Lehnen P., Schulze J., Amling M., Schinke T. Canonical Wnt signaling inhibits osteoclastogenesis independent of osteoprotegerin. J. Cell Biol. 2013;200:537–549. doi: 10.1083/jcb.201207142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wan Y., Lu C., Cao J., Zhou R., Yao Y., Yu J., Zhang L., Zhao H., Li H., Zhao J., et al. Osteoblastic Wnts differentially regulate bone remodeling and the maintenance of bone marrow mesenchymal stem cells. Bone. 2013;55:258–267. doi: 10.1016/j.bone.2012.12.052. [DOI] [PubMed] [Google Scholar]
- 66.Reya T., Clevers H. Wnt signalling in stem cells and cancer. Nat. Cell Biol. 2005;434:843–850. doi: 10.1038/nature03319. [DOI] [PubMed] [Google Scholar]
- 67.Willert K., Brown J.D., Danenberg E., Duncan A.W., Weissman I.L., Reya T., Yates J.R., Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nat. Cell Biol. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 68.Katoh Y., Katoh M. Identification and characterization of rat Wnt1 and Wnt10b genes in silico. Int. J. Oncol. 2005;26:841–845. doi: 10.3892/ijo.26.3.841. [DOI] [PubMed] [Google Scholar]
- 69.van Amerongen R., Bowman A.N., Nusse R. Developmental Stage and Time Dictate the Fate of Wnt/β-Catenin-Responsive Stem Cells in the Mammary Gland. Cell Stem Cell. 2012;11:387–400. doi: 10.1016/j.stem.2012.05.023. [DOI] [PubMed] [Google Scholar]
- 70.Bartis D., Csongei V., Weich A., Kiss E., Barkó S., Kovács T., Avdicevic M., D’Souza V.K., Rapp J., Kvell K., et al. Down-Regulation of Canonical and Up-Regulation of Non-Canonical Wnt Signalling in the Carcinogenic Process of Squamous Cell Lung Carcinoma. PLoS ONE. 2013;8:e57393. doi: 10.1371/journal.pone.0057393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Westendorf J.J., Kahler R.A., Schroeder T.M. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 72.Boyden L.M., Mao J., Belsky J., Mitzner L., Farhi A., Mitnick M.A., Wu D., Insogna K., Lifton R.P. High Bone Density Due to a Mutation in LDL-Receptor–Related Protein 5. N. Engl. J. Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- 73.Gong Y., Slee R.B., Fukai N., Rawadi G., Roman-Roman S., Reginato A.M., Wang H., Cundy T., Glorieux F.H., Lev D., et al. LDL Receptor-Related Protein 5 (LRP5) Affects Bone Accrual and Eye Development. Cell. 2001;107:513–523. doi: 10.1016/S0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- 74.Diarra D., Stolina M., Polzer K., Zwerina J., Ominsky M.S., Dwyer D., Korb A., Smolen J., Hoffmann M., Scheinecker C., et al. Dickkopf-1 is a master regulator of joint remodeling. Nat. Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 75.Qiang Y.-W., Chen Y., Stephens O., Brown N., Chen B., Epstein J., Barlogie B., Shaughnessy J.D. Myeloma-derived Dickkopf-1 disrupts Wnt-regulated osteoprotegerin and RANKL production by osteoblasts: A potential mechanism underlying osteolytic bone lesions in multiple myeloma. Blood. 2008;112:196–207. doi: 10.1182/blood-2008-01-132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Krupnik V.E., Sharp J.D., Jiang C., Robison K., Chickering T.W., Amaravadi L., Brown D.E., Guyot D., Mays G., Leiby K., et al. Functional and structural diversity of the human Dickkopf gene family. Gene. 1999;238:301–313. doi: 10.1016/S0378-1119(99)00365-0. [DOI] [PubMed] [Google Scholar]
- 77.Mao B., Wu W., Davidson G., Marhold J., Li M., Mechler B.M., Delius H., Hoppe D., Stannek P., Walter C., et al. Kremen proteins are Dickkopf receptors that regulate Wnt/β-catenin signalling. Nat. Cell Biol. 2002;417:664–667. doi: 10.1038/nature756. [DOI] [PubMed] [Google Scholar]
- 78.Gunn W.G., Conley A., Deininger L., Olson S.D., Prockop D.J., Gregory C.A. A Crosstalk Between Myeloma Cells and Marrow Stromal Cells Stimulates Production of DKK1 and Interleukin-6: A Potential Role in the Development of Lytic Bone Disease and Tumor Progression in Multiple Myeloma. Stem Cells. 2006;24:986–991. doi: 10.1634/stemcells.2005-0220. [DOI] [PubMed] [Google Scholar]
- 79.Glass D.A., Bialek P., Ahn J.D., Starbuck M., Patel M.S., Clevers H., Taketo M.M., Long F., McMahon A.P., Lang R.A., et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation blasts to inhibit osteoclast differentiation; thus, they broaden our knowledge of the functions Wnt proteins. Dev. Cell. 2005;8:751–764. doi: 10.1016/j.devcel.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 80.Tian E., Zhan F., Walker R., Rasmussen E., Ma Y., Barlogie B., Shaughnessy J.D., Jr. The Role of the Wnt-Signaling Antagonist DKK1 in the Development of Osteolytic Lesions in Multiple Myeloma. N. Engl. J. Med. 2003;349:2483–2494. doi: 10.1056/NEJMoa030847. [DOI] [PubMed] [Google Scholar]
- 81.Kaiser M., Mieth M., Liebisch P., Oberländer R., Rademacher J., Jakob C., Kleeberg L., Fleissner C., Braendle E., Peters M., et al. Serum concentrations of DKK-1 correlate with the extent of bone disease in patients with multiple myeloma. Eur. J. Haematol. 2008;80:490–494. doi: 10.1111/j.1600-0609.2008.01065.x. [DOI] [PubMed] [Google Scholar]
- 82.Kawano Y., Kypta R. Secreted antagonists of the Wnt signalling pathway. J. Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 83.Fulciniti M., Tassone P., Hideshima T., Vallet S., Nanjappa P., Ettenberg S.A., Shen Z., Patel N., Tai Y.-T., Chauhan D., et al. Anti-DKK1 mAb (BHQ880) as a potential therapeutic agent for multiple myeloma. Blood. 2009;114:371–379. doi: 10.1182/blood-2008-11-191577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.James I., Kumar S., Barnes M., Gress C., Hand A., Dodds R., Connor J., Bradley B., Campbell D., Grabill S., et al. FrzB-2: A human secreted frizzled-related protein with a potential role in chondrocyte apoptosis. Osteoarthr. Cartil. 2000;8:452–463. doi: 10.1053/joca.1999.0321. [DOI] [PubMed] [Google Scholar]
- 85.Bodine P.V., Billiard J., Moran R.A., Ponce-De-Leon H., McLarney S., Mangine A., Scrimo M.J., Bhat R.A., Stauffer B., Green J., et al. The Wnt antagonist secreted frizzled-related protein-1 controls osteoblast and osteocyte apoptosis. J. Cell. Biochem. 2005;96:1212–1230. doi: 10.1002/jcb.20599. [DOI] [PubMed] [Google Scholar]
- 86.Wang F.-S., Lin C.-L., Chen Y.-J., Wang C.-J., Yang K.D., Huang Y.-T., Sun Y.-C., Huang H.-C. Secreted Frizzled-Related Protein 1 Modulates Glucocorticoid Attenuation of Osteogenic Activities and Bone Mass. Endocrinology. 2005;146:2415–2423. doi: 10.1210/en.2004-1050. [DOI] [PubMed] [Google Scholar]
- 87.DE Vos J., Couderc G., Tarte K., Jourdan M., Requirand G., Delteil M.-C., Rossi J.-F., Mechti N., Klein B. Identifying intercellular signaling genes expressed in malignant plasma cells by using complementary DNA arrays. Blood. 2001;98:771–780. doi: 10.1182/blood.V98.3.771. [DOI] [PubMed] [Google Scholar]
- 88.Nakanishi R., Akiyama H., Kimura H., Otsuki B., Shimizu M., Tsuboyama T., Nakamura T. Osteoblast-Targeted Expression of Sfrp4 in Mice Results in Low Bone Mass. J. Bone Miner. Res. 2007;23:271–277. doi: 10.1359/jbmr.071007. [DOI] [PubMed] [Google Scholar]
- 89.Giuliani N., Colla S., Morandi F., Lazzaretti M., Sala R., Bonomini S., Grano M., Colucci S., Svaldi M., Rizzoli V. Myeloma cells block RUNX2/CBFA1 activity in human bone marrow osteoblast progenitors and inhibit osteoblast formation and differentiation. Blood. 2005;106:2472–2483. doi: 10.1182/blood-2004-12-4986. [DOI] [PubMed] [Google Scholar]
- 90.Prince M., Banerjee C., Javed A., Green J., Lian J.B., Stein G.S., Bodine P.V.N., Komm B.S. Expression and regulation of RUNX2/Cbfa1 and osteoblast phenotypic mark-ers during the growth and differentiation of human osteoblasts. J. Cell Biochem. 2001 doi: 10.1002/1097-4644(20010301)80:3<424::AID-JCB160>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 91.Silvestris F., Cafforio P., De Matteo M., Calvani N., Frassanito M.A., Dammacco F. Negative Regulation of the Osteoblast Function in Multiple Myeloma through the Repressor Gene E4BP4 Activated by Malignant Plasma Cells. Clin. Cancer Res. 2008;14:6081–6091. doi: 10.1158/1078-0432.CCR-08-0219. [DOI] [PubMed] [Google Scholar]
- 92.D’Souza S., Del Prete D., Jin S., Sun Q., Huston A.J., Kostov F.E., Sammut B., Hong C.-S., Anderson J.L., Patrene K.D., et al. Gfi1 expressed in bone marrow stromal cells is a novel osteoblast suppressor in patients with multiple myeloma bone disease. Blood. 2011;118:6871–6880. doi: 10.1182/blood-2011-04-346775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Giuliani N., Rizzoli V. Myeloma cells and bone marrow osteoblast interactions: Role in the development of osteolytic lesions in multiple myeloma. Leuk. Lymphoma. 2007;48:2323–2329. doi: 10.1080/10428190701648281. [DOI] [PubMed] [Google Scholar]
- 94.Standal T., Hjorth-Hansen H., Rasmussen T., Dahl I.M.S., Lenhoff S., Brenne A.-T., Seidel C., Baykov V., Waage A., Børset M., et al. Osteopontin is an adhesive factor for myeloma cells and is found in increased levels in plasma from patients with multiple myeloma. Haematologica. 2004;89:174–182. [PubMed] [Google Scholar]
- 95.Takeuchi K., Abe M., Hiasa M., Oda A., Amou H., Kido S., Harada T., Tanaka O., Miki H., Nakamura S., et al. TGF-β Inhibition Restores Terminal Osteoblast Differentiation to Suppress Myeloma Growth. PLoS ONE. 2010;5:e9870. doi: 10.1371/journal.pone.0009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu H., Peng F., Liu Z., Jiang F., Li L., Gao S., Wang G., Song J., Ruan E., Shao Z., et al. CYR61/CCN1 stimulates proliferation and differentiation of osteoblasts in vitro and contributes to bone remodelling in vivo in myeloma bone disease. Int. J. Oncol. 2016;50:631–639. doi: 10.3892/ijo.2016.3815. [DOI] [PubMed] [Google Scholar]
- 97.Trotter T.N., Li M., Pan Q., Peker D., Rowan P.D., Li J., Zhan F., Suva L.J., Javed A., Yang Y. Myeloma cell–derived Runx2 promotes myeloma progression in bone. Blood. 2015;125:3598–3608. doi: 10.1182/blood-2014-12-613968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amodio N., D’Aquila P., Passarino G., Tassone P., Bellizzi D. Epigenetic modifications in multiple myeloma: Recent advances on the role of DNA and histone methylation. Expert Opin. Ther. Targets. 2016;21:91–101. doi: 10.1080/14728222.2016.1266339. [DOI] [PubMed] [Google Scholar]
- 99.Morelli E., Gullà A., Rocca R., Federico C., Raimondi L., Malvestiti S., Agosti V., Rossi M., Costa G., Giavaresi G., et al. The Non-Coding RNA Landscape of Plasma Cell Dyscrasias. Cancers. 2020;12:320. doi: 10.3390/cancers12020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang Y., Liu J., Ma J., Sun T., Zhou Q., Wang W., Wang G., Wu P., Wang H., Jiang L., et al. Exosomal circRNAs: Biogenesis, effect and application in human diseases. Mol. Cancer. 2019;18:1–10. doi: 10.1186/s12943-019-1041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raimondi L., De Luca A., Amodio N., Manno M., Raccosta S., Taverna S., Bellavia D., Naselli F., Fontana S., Schillaci O., et al. Involvement of multiple myeloma cell-derived exosomes in osteoclast differentiation. Oncotarget. 2015;6:13772–13789. doi: 10.18632/oncotarget.3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Raimondo S., Saieva L., Vicario E., Pucci M., Toscani D., Manno M., Raccosta S., Giuliani N., Alessandro R., Raccosta S. Multiple myeloma-derived exosomes are enriched of amphiregulin (AREG) and activate the epidermal growth factor pathway in the bone microenvironment leading to osteoclastogenesis. J. Hematol. Oncol. 2019;12:2. doi: 10.1186/s13045-018-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li B., Xu H., Han H., Song S., Zhang X., Ouyang L., Qian C., Hong Y., Qiu Y., Zhou W., et al. Exosome-mediated transfer of lncRUNX2-AS1 from multiple myeloma cells to MSCs contributes to osteogenesis. Oncogene. 2018;37:5508–5519. doi: 10.1038/s41388-018-0359-0. [DOI] [PubMed] [Google Scholar]
- 104.Zhang L., Lei Q., Wang H., Xu C., Liu T., Kong F., Yang C., Yan G., Sun L., Zhao A., et al. Tumor-derived extracellular vesicles inhibit osteogenesis and exacerbate myeloma bone disease. Theranostics. 2019;9:196–209. doi: 10.7150/thno.27550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Raimondo S., Urzì O., Conigliaro A., Bosco G.L., Parisi S., Carlisi M., Siragusa S., Raimondi L., De Luca A., Giavaresi G., et al. Extracellular Vesicle microRNAs Contribute to the Osteogenic Inhibition of Mesenchymal Stem Cells in Multiple Myeloma. Cancers. 2020;12:449. doi: 10.3390/cancers12020449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Liebross R.H., Ha C.S., Cox J.D., Weber D.M., Delasalle K., Alexanian R. Solitary bone plasmacytoma: Outcome and prognostic factors following radiotherapy. Int. J. Radiat. Oncol. 1998;41:1063–1067. doi: 10.1016/S0360-3016(98)00186-2. [DOI] [PubMed] [Google Scholar]
- 107.Ozsahin M., Tsang R.W., Poortmans P., Belkacémi Y., Bolla M., Dincbas H.F., Landmann C., Castelain B., Buijsen J., Curschmann J., et al. Outcomes and patterns of failure in solitary plasmacytoma: A multicenter Rare Cancer Network study of 258 patients. Int. J. Radiat. Oncol. 2006;64:210–217. doi: 10.1016/j.ijrobp.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 108.Frassica D.A., Frassica F.J., Schray M.F., Sim F.H., Kyle R.A. Solitary plasmacytoma of bone: Mayo clinic experience. Int. J. Radiat. Oncol. Biol. Phys. 1989;16:43–48. doi: 10.1016/0360-3016(89)90008-4. [DOI] [PubMed] [Google Scholar]
- 109.Hu K., Yahalom J. Radiotherapy in the management of plasma cell tumors. Oncology. 2000;14:101–108. [PubMed] [Google Scholar]
- 110.Dimopoulos M., Goldstein J., Fuller L., Delasalle K., Alexanian R. Curability of solitary bone plasmacytoma. J. Clin. Oncol. 1992;10:587–590. doi: 10.1200/JCO.1992.10.4.587. [DOI] [PubMed] [Google Scholar]
- 111.Creach K.M., Foote R.L., Neben-Wittich M.A., Kyle R.A. Radiotherapy for Extramedullary Plasmacytoma of the Head and Neck. Int. J. Radiat. Oncol. 2009;73:789–794. doi: 10.1016/j.ijrobp.2008.04.077. [DOI] [PubMed] [Google Scholar]
- 112.Garland P., Gishen P., Rahemtulla A. Percutaneous vertebroplasty to treat painful myelomatous vertebral deposits—long-term efficacy outcomes. Ann. Hematol. 2010;90:95–100. doi: 10.1007/s00277-010-1021-2. [DOI] [PubMed] [Google Scholar]
- 113.Dudeney S., Lieberman I., Reinhardt M.-K., Hussein M. Kyphoplasty in the Treatment of Osteolytic Vertebral Compression Fractures as a Result of Multiple Myeloma. J. Clin. Oncol. 2002;20:2382–2387. doi: 10.1200/JCO.2002.09.097. [DOI] [PubMed] [Google Scholar]
- 114.Raje N., Roodman G.D. Advances in the Biology and Treatment of Bone Disease in Multiple Myeloma. Clin. Cancer Res. 2011;17:1278–1286. doi: 10.1158/1078-0432.CCR-10-1804. [DOI] [PubMed] [Google Scholar]
- 115.Dunford J.E., Thompson K., Coxon F.P., Luckman S.P., Hahn F.M., Poulter C.D., Ebetino F.H., Rogers M.J. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J. Pharmacol. Exp. Ther. 2001;296:235–242. [PubMed] [Google Scholar]
- 116.Berenson J.R., Lichtenstein A., Porter L., Dimopoulos M., Bordoni R., George S., Lipton A., Keller A., Ballester O., Kovacs M., et al. Long-term pamidronate treatment of advanced multiple myeloma patients reduces skeletal events. Myeloma Aredia Study Group. J. Clin. Oncol. 1998;16:593–602. doi: 10.1200/JCO.1998.16.2.593. [DOI] [PubMed] [Google Scholar]
- 117.Rogers M.J., Gordon S., Benford H.L., Coxon F.P., Luckman S.P., Monkkonen J., Frith J.C. Cellular and molecular mechanisms of action of bisphosphonates. Cancer. 2000 doi: 10.1002/1097-0142(20000615)88:12+<2961::AID-CNCR12>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 118.Luckman S.P., Hughes D.E., Coxon F.P., Russell R.G.G., Rogers M. Nitrogen-Containing Bisphosphonates Inhibit the Mevalonate Pathway and Prevent Post-Translational Prenylation of GTP-Binding Proteins, Including Ras. J. Bone Miner. Res. 1998;13:581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 119.Rosen L.S., Gordon D., Kaminski M., Howell A., Belch A., Mackey J., Apffelstaedt J., Hussein M., Coleman R.E., Reitsma D.J., et al. Zoledronic acid versus pamidronate in the treatment of skeletal metastases in patients with breast cancer or osteolytic lesions of multiple myeloma: A phase III, double-blind, comparative trial. Cancer J. 2001;7:377–387. [PubMed] [Google Scholar]
- 120.Mhaskar R., Redzepovic J., Wheatley K., Clark O.A.C., Miladinovic B., Glasmacher A., Kumar A., Djulbegovic B. Bisphosphonates in multiple myeloma: A network meta-analysis. Cochrane Database Syst. Rev. 2012:CD003188. doi: 10.1002/14651858.CD003188.pub3. [DOI] [PubMed] [Google Scholar]
- 121.Bird J.M., Owen R.G., D’Sa S., Snowden J.A., Pratt G., Ashcroft J., Yong K., Cook G., Feyler S., Davies F., et al. Guidelines for the diagnosis and management of multiple myeloma 2011. Br. J. Haematol. 2011;154:32–75. doi: 10.1111/j.1365-2141.2011.08573.x. [DOI] [PubMed] [Google Scholar]
- 122.Major P., Lortholary A., Hon J., Abdi E., Mills G., Menssen H.D., Yunus F., Bell R., Body J., Quebe-Fehling E., et al. Zoledronic Acid Is Superior to Pamidronate in the Treatment of Hypercalcemia of Malignancy: A Pooled Analysis of Two Randomized, Controlled Clinical Trials. J. Clin. Oncol. 2001;19:558–567. doi: 10.1200/JCO.2001.19.2.558. [DOI] [PubMed] [Google Scholar]
- 123.Wyngaert T.V.D., Huizing M.T., Vermorken J.B. Osteonecrosis of the jaw related to the use of bisphosphonates. Curr. Opin. Oncol. 2007;19:315–322. doi: 10.1097/CCO.0b013e32819f820b. [DOI] [PubMed] [Google Scholar]
- 124.Durie B.G. Mayo Clinic Proceedings. Elsevier; Amsterdam, The Netherlands: 2007. Use of Bisphosphonates in Multiple Myeloma: IMWG Response to Mayo Clinic Consensus Statement; pp. 516–517. [DOI] [PubMed] [Google Scholar]
- 125.Tosi P., Zamagni E., Cangini D., Tacchetti P., Di Raimondo F., Catalano L., D’Arco A., Ronconi S., Cellini C., Offidani M., et al. Osteonecrosis of the jaws in newly diagnosed multiple myeloma patients treated with zoledronic acid and thalidomide-dexamethasone. Blood. 2006;108:3951–3952. doi: 10.1182/blood-2006-07-033571. [DOI] [PubMed] [Google Scholar]
- 126.Bamias A., Kastritis E., Bamia C., Moulopoulos L., Melakopoulos I., Bozas G., Koutsoukou V., Gika D., Anagnostopoulos A., Papadimitriou C., et al. Osteonecrosis of the Jaw in Cancer After Treatment With Bisphosphonates: Incidence and Risk Factors. J. Clin. Oncol. 2005;23:8580–8587. doi: 10.1200/JCO.2005.02.8670. [DOI] [PubMed] [Google Scholar]
- 127.Berenson J.R., Lichtenstein A., Porter L., Dimopoulos M., Bordoni R., George S., Lipton A., Keller A., Ballester O., Kovacs M.J., et al. Efficacy of Pamidronate in Reducing Skeletal Events in Patients with Advanced Multiple Myeloma. N. Engl. J. Med. 1996;334:488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 128.Gimsing P., Carlson K., Turesson I., Fayers P., Waage A., Vangsted A.J., Mylin A., Gluud C., Juliusson G., Gregersen H., et al. Effect of pamidronate 30 mg versus 90 mg on physical function in patients with newly diagnosed multiple myeloma (Nordic Myeloma Study Group): A double-blind, randomised controlled trial. Lancet Oncol. 2010;11:973–982. doi: 10.1016/S1470-2045(10)70198-4. [DOI] [PubMed] [Google Scholar]
- 129.Morgan G.J., Child J.A., Gregory W.M., Szubert A.J., Cocks K., Bell S.E., Navarro-Coy N., Drayson M.T., Owen R.G., Feyler S., et al. Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): Secondary outcomes from a randomised controlled trial. Lancet Oncol. 2011;12:743–752. doi: 10.1016/S1470-2045(11)70157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Morgan G.J., Davies F., Gregory W.M., Cocks K., Bell S.E., Szubert A.J., Navarro-Coy N., Drayson M.T., Owen R.G., Feyler S., et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): A randomised controlled trial. Lancet. 2010;376:1989–1999. doi: 10.1016/S0140-6736(10)62051-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Anderson K., Ismaila N., Flynn P.J., Halabi S., Jagannath S., Ogaily M.S., Omel J., Raje N., Roodman G.D., Yee G.C., et al. Role of Bone-Modifying Agents in Multiple Myeloma: American Society of Clinical Oncology Clinical Practice Guideline Update. J. Clin. Oncol. 2018;36:812–818. doi: 10.1200/JCO.2017.76.6402. [DOI] [PubMed] [Google Scholar]
- 132.Raje N., Vescio R., Montgomery C.W., Badros A., Munshi N., Orlowski R., Hadala J.T., Warsi G., Argonza-Aviles E., Ericson S.G., et al. Bone Marker–Directed Dosing of Zoledronic Acid for the Prevention of Skeletal Complications in Patients with Multiple Myeloma: Results of the Z-MARK Study. Clin. Cancer Res. 2015;22:1378–1384. doi: 10.1158/1078-0432.CCR-15-1864. [DOI] [PubMed] [Google Scholar]
- 133.Himelstein A.L., Foster J.C., Khatcheressian J.L., Roberts J.D., Seisler D.K., Novotny P.J., Qin R., Go R.S., Grubbs S.S., O’Connor T., et al. Effect of Longer-Interval vs Standard Dosing of Zoledronic Acid on Skeletal Events in Patients with Bone Metastases: A randomized clinical trial. JAMA. 2017;317:48–58. doi: 10.1001/jama.2016.19425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Terpos E., Morgan G., Dimopoulos M., Drake M.T., Lentzsch S., Raje N., Sezer O., García-Sanz R., Shimizu K., Turesson I., et al. International Myeloma Working Group Recommendations for the Treatment of Multiple Myeloma–Related Bone Disease. J. Clin. Oncol. 2013;31:2347–2357. doi: 10.1200/JCO.2012.47.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mhaskar R., Kumar A., Miladinovic B., Djulbegovic B. Bisphosphonates in multiple myeloma: An updated network meta-analysis. Cochrane Database Syst. Rev. 2017;12:CD003188. doi: 10.1002/14651858.CD003188.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Teitelbaum S.L., Ross F.P. Genetic regulation of osteoclast development and function. Nat. Rev. Genet. 2003;4:638–649. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 137.Raje N., Terpos E., Willenbacher W., Shimizu K., García-Sanz R., Durie B., Legieć W., Krejčí M., Laribi K., Zhu L., et al. Denosumab versus zoledronic acid in bone disease treatment of newly diagnosed multiple myeloma: An international, double-blind, double-dummy, randomised, controlled, phase 3 study. Lancet Oncol. 2018;19:370–381. doi: 10.1016/S1470-2045(18)30072-X. [DOI] [PubMed] [Google Scholar]
- 138.Zavrski I., Krebbel H., Wildemann B., Heider U., Kaiser M., Possinger K., Sezer O. Proteasome inhibitors abrogate osteoclast differentiation and osteoclast function. Biochem. Biophys. Res. Commun. 2005;333:200–205. doi: 10.1016/j.bbrc.2005.05.098. [DOI] [PubMed] [Google Scholar]
- 139.Von Metzler I., Krebbel H., Hecht M., Manz R.A., Fleissner C., Mieth M., Kaiser M., Jakob C., Sterz J., Kleeberg L., et al. Bortezomib inhibits human osteoclastogenesis. Leukemia. 2007;21:2025–2034. doi: 10.1038/sj.leu.2404806. [DOI] [PubMed] [Google Scholar]
- 140.Garrett I., Chen D., Gutierrez G., Zhao M., Escobedo A., Rossini G., Harris S., Gallwitz W., Kim K., Hu S., et al. Selective inhibitors of the osteoblast proteasome stimulate bone formation in vivo and in vitro. J. Clin. Investig. 2003;111:1771–1782. doi: 10.1172/JCI16198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pennisi A., Li X., Ling W., Khan S., Zangari M., Yaccoby S. The proteasome inhibitor, bortezomib suppresses primary myeloma and stimulates bone formation in myelomatous and nonmyelomatous bones in vivo. Am. J. Hematol. 2009;84:6–14. doi: 10.1002/ajh.21310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hurchla M.A., Garcia A., Hornick M.C., Ocio E.M., Li A., Blanco J.F., Collins L., Kirk C.J., Piwnica-Worms D., Vij R., et al. The epoxyketone-based proteasome inhibitors carfilzomib and orally bioavailable oprozomib have anti-resorptive and bone-anabolic activity in addition to anti-myeloma effects. Leukemia. 2012;27:430–440. doi: 10.1038/leu.2012.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Harousseau J.-L., Palumbo A., Richardson P.G., Schlag R., Dimopoulos M., Shpilberg O., Kropff M., Kentos A., Cavo M., Golenkov A., et al. Superior outcomes associated with complete response in newly diagnosed multiple myeloma patients treated with nonintensive therapy: Analysis of the phase 3 VISTA study of bortezomib plus melphalan-prednisone versus melphalan-prednisone. Blood. 2010;116:3743–3750. doi: 10.1182/blood-2010-03-275800. [DOI] [PubMed] [Google Scholar]
- 144.Dimopoulos M., Richardson P.G., Schlag R., Khuageva N.K., Shpilberg O., Kastritis E., Kropff M., Petrucci M.T., Delforge M., Alexeeva J., et al. VMP (Bortezomib, Melphalan, and Prednisone) Is Active and Well Tolerated in Newly Diagnosed Patients with Multiple Myeloma with Moderately Impaired Renal Function, and Results in Reversal of Renal Impairment: Cohort Analysis of the Phase III VISTA Study. J. Clin. Oncol. 2009;27:6086–6093. doi: 10.1200/JCO.2009.22.2232. [DOI] [PubMed] [Google Scholar]
- 145.Kumar S., Flinn I., Richardson P.G., Hari P., Callander N., Noga S.J., Stewart A.K., Turturro F., Rifkin R., Wolf J., et al. Randomized, multicenter, phase 2 study (EVOLUTION) of combinations of bortezomib, dexamethasone, cyclophosphamide, and lenalidomide in previously untreated multiple myeloma. Blood. 2012;119:4375–4382. doi: 10.1182/blood-2011-11-395749. [DOI] [PubMed] [Google Scholar]
- 146.Richardson P.G., Xie W., Jagannath S., Jakubowiak A., Lonial S., Raje N.S., Alsina M., Ghobrial I.M., Schlossman R.L., Munshi N.C., et al. A phase 2 trial of lenalidomide, bortezomib, and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood. 2014;123:1461–1469. doi: 10.1182/blood-2013-07-517276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Jagannath S., Richardson P.G., Barlogie B., Berenson J.R., Singhal S., Irwin D., Srkalovic G., Schenkein D.P., Esseltine D.L., Anderson K.C. Bortezomib in combination with dexamethasone for the treatment of patients with relapsed and/or refractory multiple myeloma with less than optimal response to bortezomib alone. Haematologica. 2006;91:929–934. [PubMed] [Google Scholar]
- 148.Hongming H., Jian H. Bortezomib inhibits maturation and function of osteoclasts from PBMCs of patients with multiple myeloma by downregulating TRAF6. Leuk. Res. 2009;33:115–122. doi: 10.1016/j.leukres.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 149.Deleu S., Lemaire M., Arts J., Menu E., Van Valckenborgh E., Broek I.V., De Raeve H., Coulton L., Van Camp B., Croucher P., et al. Bortezomib Alone or in Combination with the Histone Deacetylase Inhibitor JNJ-26481585: Effect on Myeloma Bone Disease in the 5T2MM Murine Model of Myeloma. Cancer Res. 2009;69:5307–5311. doi: 10.1158/0008-5472.CAN-08-4472. [DOI] [PubMed] [Google Scholar]
- 150.Giuliani N., Morandi F., Tagliaferri S., Lazzaretti M., Bonomini S., Crugnola M., Mancini C., Martella E., Ferrari L., Tabilio A., et al. The proteasome inhibitor bortezomib affects osteoblast differentiation in vitro and in vivo in multiple myeloma patients. Blood. 2007;110:334–338. doi: 10.1182/blood-2006-11-059188. [DOI] [PubMed] [Google Scholar]
- 151.Qiang Y.-W., Hu B., Chen Y., Zhong Y., Shi B., Barlogie B., Shaughnessy J.J.D. Bortezomib induces osteoblast differentiation via Wnt-independent activation of β-catenin/TCF signaling. Blood. 2009;113:4319–4330. doi: 10.1182/blood-2008-08-174300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sanvoranart T., Supokawej A., Kheolamai P., U-Pratya Y., Klincumhom N., Manochantr S., Wattanapanitch M., Issaragrisil S. Bortezomib enhances the osteogenic differentiation capacity of human mesenchymal stromal cells derived from bone marrow and placental tissues. Biochem. Biophys. Res. Commun. 2014;447:580–585. doi: 10.1016/j.bbrc.2014.04.044. [DOI] [PubMed] [Google Scholar]
- 153.Zangari M., Yaccoby S., Pappas L., Cavallo F., Kumar N.S., Ranganathan S., Suva L.J., Gruenwald J.M., Kern S., Zhan F., et al. A prospective evaluation of the biochemical, metabolic, hormonal and structural bone changes associated with bortezomib response in multiple myeloma patients. Haematologica. 2010;96:333–336. doi: 10.3324/haematol.2010.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Delforge M., Terpos E., Richardson P.G., Shpilberg O., Khuageva N.K., Schlag R., Dimopoulos M., Kropff M., Spicka I., Petrucci M.T., et al. Fewer bone disease events, improvement in bone remodeling, and evidence of bone healing with bortezomib plus melphalan-prednisone vs. melphalan-prednisone in the phase III VISTA trial in multiple myeloma. Eur. J. Haematol. 2011;86:372–384. doi: 10.1111/j.1600-0609.2011.01599.x. [DOI] [PubMed] [Google Scholar]
- 155.Heider U., Kaiser M., Müller C., Jakob C., Zavrski I., Schulz C.-O., Fleissner C., Hecht M., Sezer O. Bortezomib increases osteoblast activity in myeloma patients irrespective of response to treatment. Eur. J. Haematol. 2006;77:233–238. doi: 10.1111/j.1600-0609.2006.00692.x. [DOI] [PubMed] [Google Scholar]
- 156.Terpos E., Christoulas D., Kastritis E., Roussou M., Migkou M., Eleutherakis-Papaiakovou E., Gavriatopoulou M., Gkotzamanidou M., Kanellias N., Manios E., et al. VTD consolidation, without bisphosphonates, reduces bone resorption and is associated with a very low incidence of skeletal-related events in myeloma patients post ASCT. Leukemia. 2013;28:928–934. doi: 10.1038/leu.2013.267. [DOI] [PubMed] [Google Scholar]
- 157.Zangari M., Esseltine D., Lee C.-K., Barlogie B., Elice F., Burns M.J., Kang S.-H., Yaccoby S., Najarian K., Richardson P., et al. Response to bortezomib is associated to osteoblastic activation in patients with multiple myeloma. Br. J. Haematol. 2005;131:71–73. doi: 10.1111/j.1365-2141.2005.05733.x. [DOI] [PubMed] [Google Scholar]
- 158.T’Seyen S., Pans S., Laenen A., Devos T., Dierickx D., Schoemans H., Delforge M. Bone healing with bortezomib-based regimens in multiple myeloma: A retrospective imaging study. Int. J. Hematol. Oncol. 2014;3:387–394. doi: 10.2217/ijh.14.38. [DOI] [Google Scholar]
- 159.Moreau P., Mateos M.-V., Berenson J.R., Weisel K., Lazzaro A., Song K., Dimopoulos M.A., Huang M., Zahlten-Kumeli A., Stewart A.K. Once weekly versus twice weekly carfilzomib dosing in patients with relapsed and refractory multiple myeloma (A.R.R.O.W.): Interim analysis results of a randomised, phase 3 study. Lancet Oncol. 2018;19:953–964. doi: 10.1016/S1470-2045(18)30354-1. [DOI] [PubMed] [Google Scholar]
- 160.Berenson J.R., Cartmell A., Bessudo A., Lyons R.M., Harb W., Tzachanis D., Agajanian R., Boccia R., Coleman M., Moss R.A., et al. CHAMPION-1: A phase 1/2 study of once-weekly carfilzomib and dexamethasone for relapsed or refractory multiple myeloma. Blood. 2016;127:3360–3368. doi: 10.1182/blood-2015-11-683854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dimopoulos M.A., Moreau P., Palumbo A., Joshua D., Pour L., Hajek R., Facon T., Ludwig H., Oriol A., Goldschmidt H., et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): A randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 162.Stewart A.K., Rajkumar S.V., Dimopoulos M., Masszi T., Spicka I., Oriol A., Hajek R., Rosiñol L., Siegel D.S., Mihaylov G.G., et al. Carfilzomib, Lenalidomide, and Dexamethasone for Relapsed Multiple Myeloma. N. Engl. J. Med. 2015;372:142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 163.Berdeja J.G., Hart L.L., Mace J.R., Arrowsmith E.R., Essell J.H., Owera R.S., Hainsworth J.D., Flinn I.W. Phase I/II study of the combination of panobinostat and carfilzomib in patients with relapsed/refractory multiple myeloma. Haematologica. 2015;100:670–676. doi: 10.3324/haematol.2014.119735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Shah J.J., Stadtmauer E.A., Abonour R., Cohen A.D., Bensinger W.I., Gasparetto C., Kaufman J.L., Lentzsch S., Vogl D.T., Gomes C.L., et al. Carfilzomib, pomalidomide, and dexamethasone for relapsed or refractory myeloma. Blood. 2015;126:2284–2290. doi: 10.1182/blood-2015-05-643320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bringhen S., Petrucci M.T., Larocca A., Conticello C., Rossi D., Magarotto V., Musto P., Boccadifuoco L., Offidani M., Omedé P., et al. Carfilzomib, cyclophosphamide, and dexamethasone in patients with newly diagnosed multiple myeloma: A multicenter, phase 2 study. Blood. 2014;124:63–69. doi: 10.1182/blood-2014-03-563759. [DOI] [PubMed] [Google Scholar]
- 166.Korde N., Roschewski M., Zingone A., Kwok M., Manasanch E.E., Bhutani M., Tageja N., Kazandjian D., Mailankody S., Wu P., et al. Treatment with Carfilzomib-Lenalidomide-Dexamethasone With Lenalidomide Extension in Patients With Smoldering or Newly Diagnosed Multiple Myeloma. JAMA Oncol. 2015;1:746–754. doi: 10.1001/jamaoncol.2015.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jakubowiak A.J., Dytfeld D., Griffith K.A., Lebovic D., Vesole D.H., Jagannath S., Al-Zoubi A., Anderson T., Nordgren B., Detweiler-Short K., et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–1809. doi: 10.1182/blood-2012-04-422683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.National Comprehensive Cancer Network Multiple Myeloma (Version 4.2020) [(accessed on 19 July 2020)]; Available online: https://www.nccn.org/professionals/physician_gls/pdf/myeloma.pdf.
- 169.Hu B., Chen Y., Usmani S.Z., Ye S., Qiang W., Papanikolaou X., Heuck C.J., Yaccoby S., Williams B., Van Rhee F., et al. Characterization of the Molecular Mechanism of the Bone-Anabolic Activity of Carfilzomib in Multiple Myeloma. PLoS ONE. 2013;8:e74191. doi: 10.1371/journal.pone.0074191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li Y., Li J., Zhuang W., Wang Q., Ge X., Zhang X., Chen P., Fu J., Li B. Carfilzomib promotes the osteogenic differentiation potential of mesenchymal stem cells derived from myeloma patients by inhibiting notch1 activity in vitro. Leuk. Res. 2014;38:970–976. doi: 10.1016/j.leukres.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 171.Suvannasankha A., Abonour R., Farag S., Silbermann R.W., Wongsaengsak S., Cangany M.H., Rush-Taylor A., Tann M., Althouse S.K., Perkins S.M., et al. Phase 2 study of carfilzomib and bone metabolism in patients with relapsed multiple myeloma. Blood. 2017;130(Suppl. S1):1826. [Google Scholar]
- 172.Zangari M., Aujay M., Zhan F., Hetherington K.L., Berno T., Vij R., Jagannath S., Siegel D., Stewart A.K., Wang L., et al. Alkaline phosphatase variation during carfilzomib treatment is associated with best response in multiple myeloma patients. Eur. J. Haematol. 2011;86:484–487. doi: 10.1111/j.1600-0609.2011.01602.x. [DOI] [PubMed] [Google Scholar]
- 173.Quach H., Ritchie D., Stewart A.K., Neeson P., Harrison S., Smyth M., Prince H.M. Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia. 2009;24:22–32. doi: 10.1038/leu.2009.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Davies F., Raje N., Hideshima T., Lentzsch S., Young G., Tai Y.-T., Lin B., Podar K., Gupta D., Chauhan D., et al. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98:210–216. doi: 10.1182/blood.V98.1.210. [DOI] [PubMed] [Google Scholar]
- 175.Munshi N.C., Anderson K.C. New Strategies in the Treatment of Multiple Myeloma. Clin. Cancer Res. 2013;19:3337–3344. doi: 10.1158/1078-0432.CCR-12-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Anderson G., Gries M., Kurihara N., Honjo T., Anderson J., Donnenberg V., Donnenberg A., Ghobrial I., Mapara M.Y., Stirling D., et al. Thalidomide derivative CC-4047 inhibits osteoclast formation by down-regulation of PU.1. Blood. 2006;107:3098–3105. doi: 10.1182/blood-2005-08-3450. [DOI] [PubMed] [Google Scholar]
- 177.Bolomsky A., Schreder M., Meißner T., Hose D., Ludwig H., Pfeifer S., Zojer N. Immunomodulatory drugs thalidomide and lenalidomide affect osteoblast differentiation of human bone marrow stromal cells in vitro. Exp. Hematol. 2014;42:516–525. doi: 10.1016/j.exphem.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 178.Terpos E., Mihou D., Szydlo R., Tsimirika K., Karkantaris C., Politou M., Voskaridou E., Rahemtulla A., Dimopoulos M., Zervas K. The combination of intermediate doses of thalidomide with dexamethasone is an effective treatment for patients with refractory/relapsed multiple myeloma and normalizes abnormal bone remodeling, through the reduction of sRANKL/osteoprotegerin ratio. Leukemia. 2005;19:1969–1976. doi: 10.1038/sj.leu.2403890. [DOI] [PubMed] [Google Scholar]
- 179.Breitkreutz I., Vallet S., Raab M.S., Tai Y.-T., Raje N., Hideshima T., Chauhan D., Munshi N.C., Richardson P., Anderson K.C. Lenalidomide and Bortezomib Inhibit Osteoclast Differentiation and Activation in Multiple Myeloma: Clinical Implications. Blood. 2006;108:3485. doi: 10.1182/blood.V108.11.3485.3485. [DOI] [Google Scholar]
- 180.Burger J.A., Buggy J.J. Bruton tyrosine kinase inhibitor ibrutinib (PCI-32765) Leuk. Lymphoma. 2013;54:2385–2391. doi: 10.3109/10428194.2013.777837. [DOI] [PubMed] [Google Scholar]
- 181.Aoki Y., Isselbacher K.J., Pillai S. Bruton tyrosine kinase is tyrosine phosphorylated and activated in pre-B lymphocytes and receptor-ligated B cells. Proc. Natl. Acad. Sci. USA. 1994;91:10606–10609. doi: 10.1073/pnas.91.22.10606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Cinar M., Hamedani F., Mo Z., Cinar B., Amin H.M., Alkan S. Bruton tyrosine kinase is commonly overexpressed in mantle cell lymphoma and its attenuation by Ibrutinib induces apoptosis. Leuk. Res. 2013;37:1271–1277. doi: 10.1016/j.leukres.2013.07.028. [DOI] [PubMed] [Google Scholar]
- 183.Jain N., O’Brien S. Ibrutinib (PCI-32765) in Chronic Lymphocytic Leukemia. Hematol. Oncol. Clin. North Am. 2013;27:851–860. doi: 10.1016/j.hoc.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Shinohara M., Koga T., Okamoto K., Sakaguchi S., Arai K., Yasuda H., Takai T., Kodama T., Morio T., Geha R.S., et al. Tyrosine Kinases Btk and Tec Regulate Osteoclast Differentiation by Linking RANK and ITAM Signals. Cell. 2008;132:794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 185.Yoon Y.S., Kim C.W., Lim S.-B., Yu C.S., Kim S.Y., Kim T.W., Kim M.-J., Kim J.C. Palliative surgery in patients with unresectable colorectal liver metastases: A propensity score matching analysis. J. Surg. Oncol. 2013;109:239–244. doi: 10.1002/jso.23480. [DOI] [PubMed] [Google Scholar]
- 186.Yaccoby S., Ling W., Zhan F., Walker R., Barlogie B., Shaughnessy J.D. Antibody-based inhibition of DKK1 suppresses tumor-induced bone resorption and multiple myeloma growth in vivo. Blood. 2006;109:2106–2111. doi: 10.1182/blood-2006-09-047712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Mulcahy L.E., Taylor D., Lee T.C., Duffy G.P. RANKL and OPG activity is regulated by injury size in networks of osteocyte-like cells. Bone. 2011;48:182–188. doi: 10.1016/j.bone.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 188.Sutherland M.K., Geoghegan J.C., Yu C., Turcott E., Skonier J.E., Winkler D.G., Latham J.A. Sclerostin promotes the apoptosis of human osteoblastic cells: A novel regulation of bone formation. Bone. 2004;35:828–835. doi: 10.1016/j.bone.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 189.Gavriatopoulou M., Dimopoulos M., Kastritis E., Terpos E. Emerging treatment approaches for myeloma-related bone disease. Expert Rev. Hematol. 2017;10:217–228. doi: 10.1080/17474086.2017.1283213. [DOI] [PubMed] [Google Scholar]
- 190.McDonald M.M., Reagan M.R., Youlten S.E., Mohanty S.T., Seckinger A., Terry R.L., Pettitt J.A., Simic M.K., Cheng T.L., Morse A., et al. Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood. 2017;129:3452–3464. doi: 10.1182/blood-2017-03-773341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Delgado-Calle J., Anderson J., Cregor M.D., Hiasa M., Chirgwin J.M., Carlesso N., Yoneda T., Mohammad K.S., Plotkin L.I., Roodman G.D., et al. Bidirectional Notch Signaling and Osteocyte-Derived Factors in the Bone Marrow Microenvironment Promote Tumor Cell Proliferation and Bone Destruction in Multiple Myeloma. Cancer Res. 2016;76:1089–1100. doi: 10.1158/0008-5472.CAN-15-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Silbermann R., Roodman G.D. Current Controversies in the Management of Myeloma Bone Disease. J. Cell. Physiol. 2016;231:2374–2379. doi: 10.1002/jcp.25351. [DOI] [PubMed] [Google Scholar]
- 193.Lu A., Pallero M.A., Lei W., Hong H., Yang Y., Suto M.J., Murphy-Ullrich J.E. Inhibition of Transforming Growth Factor-β Activation Diminishes Tumor Progression and Osteolytic Bone Disease in Mouse Models of Multiple Myeloma. Am. J. Pathol. 2016;186:678–690. doi: 10.1016/j.ajpath.2015.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Olsen O.E., Wader K.F., Hella H., Mylin A.K., Turesson I., Nesthus I., Waage A., Sundan A., Holien T. Activin A inhibits BMP-signaling by binding ACVR2A and ACVR2B. Cell Commun. Signal. 2015;13:1–7. doi: 10.1186/s12964-015-0104-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Abdulkadyrov K., Salogub G.N., Khuazheva N.K., Sherman M.L., Laadem A., Barger R., Knight R., Srinivasan S., Terpos E. Sotatercept in patients with osteolytic lesions of multiple myeloma. Br. J. Haematol. 2014;165:814–823. doi: 10.1111/bjh.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Yee A.J., Laubach J.P., Nooka A.K., O’Donnell E.K., Weller E.A., Couture N.R., Wallace E.E., Burke J.N., Harrington C.C., Puccio-Pick M., et al. Phase 1 Dose-Escalation Study of Sotatercept (ACE-011) in Combination with Lenalidomide and Dexamethasone in Patients with Relapsed and/or Refractory Multiple Myeloma. Blood. 2015;126:4241. doi: 10.1182/blood.V126.23.4241.4241. [DOI] [Google Scholar]
- 197.Autio K.A., Pandit-Taskar N., Carrasquillo J.A., Ba R.D.S., Slovin S.F., Rathkopf D.E., Ba C.H., Heller G., Scher H.I., Larson S.M., et al. Repetitively dosed docetaxel and153samarium-EDTMP as an antitumor strategy for metastatic castration-resistant prostate cancer. Cancer. 2013;119:3186–3194. doi: 10.1002/cncr.28103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Parker C., Nilsson D.S., Heinrich S.D., Helle S.I., O’Sullivan J.M., Fosså S.D., Chodacki A., Wiechno P., Logue J., Seke M., et al. Alpha Emitter Radium-223 and Survival in Metastatic Prostate Cancer. N. Engl. J. Med. 2013;369:213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 199.Abruzzese E., Iuliano F., Trawinska M.M., Di Maio M. 153Sm: Its use in multiple myeloma and report of a clinical experience. Expert Opin. Investig. Drugs. 2008;17:1379–1387. doi: 10.1517/13543784.17.9.1379. [DOI] [PubMed] [Google Scholar]
- 200.Löwik C., van der Pluijm G., Bloys H., Hoekman K., Bijvoet O., Aarden L., Papapoulos S. Parathyroid hormone (PTH) and PTH-like protein (PLP) stimulate interleukin-6 production by osteogenic cells: A possible role of interleukin-6 in osteoclastogenesis. Biochem. Biophys. Res. Commun. 1989;162:1546–1552. doi: 10.1016/0006-291X(89)90851-6. [DOI] [PubMed] [Google Scholar]
- 201.Forslund T., Koski A.-M., Koistinen A., Sikiö A. Malignant Myeloma in a Patient After Treatment for Osteoporosis with Teriparatide; a Rare Coincidence. Clin. Med. Insights Case Rep. 2008;1:119–122. doi: 10.4137/CCRep.S1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Koski A.-M., Sikiö A., Forslund T. Teriparatide treatment complicated by malignant myeloma. BMJ Case Rep. 2010;2010 doi: 10.1136/bcr.01.2010.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]