Abstract
Adipose tissue and skeletal muscle is associated with non-alcoholic fatty liver disease (NAFLD). This study evaluates the association between body composition and histologic severity in patients with NAFLD. Using the cross-sectional CT images at the level of L3 vertebra and the histologic findings of 178 patients with biopsy-proven NAFLD, we analyzed the correlation of the histologic findings to the skeletal muscle index (SMI), subcutaneous adipose tissue index (SATI), and visceral adipose tissue index (VATI), which is defined as the body composition area (cm2) by height squared (m2). The clinical and laboratory features with body composition were analyzed to determine the risk factors for advanced fibrosis. The VATI significantly increased in severe non-alcoholic steatohepatitis (NASH) or advanced fibrosis. In addition, the VATI was correlated with the NAFLD activity score (NAS) and the fibrosis stage. In multivariate analyses, age (odds ratio (OR), 1.09; 95% confidence interval (CI), 1.02–1.19; p = 0.025), severe NASH (OR, 8.66; 95% CI, 2.13–46.40; p = 0.005), and visceral adiposity (OR, 6.77; 95% CI, 1.81–29.90; p = 0.007) were independently associated with advanced fibrosis in patients with NAFLD. Visceral adiposity is correlated with the histologic severity of NAFLD, which is independently associated with advanced fibrosis.
Keywords: non-alcoholic fatty liver disease, non-alcoholic steatohepatitis, sarcopenia, adipose tissue, skeletal muscle, body composition
1. Introduction
The incidence of non-alcoholic fatty liver disease (NAFLD), one of the most important chronic liver diseases, has been increasing in obese people over the past three decades [1]. In Western countries, approximately one-fourth of the population is affected by NAFLD, which has led to an increase in healthcare costs [2]. The spectrum of NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH), which leads to cirrhosis and hepatocellular carcinoma [3]. Several epidemiological studies have reported associations between NAFLD and metabolic syndrome (MetS), type 2 diabetes, obesity, and cardiovascular diseases [4,5]. Recently, as a variable clinical manifestation, fat accumulation in the visceral organs harboring genetic polymorphism was considered as a subtype of NAFLD without obesity [6]. Regardless of obesity, visceral adipose tissue (VAT) is associated with MetS and T2D [7]. Specifically, VAT is associated with the degree of hepatic fat infiltration, even in non-obese adults [8]. A positive energy balance induces fat accumulation in the subcutaneous adipose tissue (SAT), which has a relatively lesser effect on insulin resistance (IR) at the initial stage of NAFLD [9]. However, when adipose tissue dysfunction with intolerance of energy excess develops, fat is accumulated in the visceral organs, including the liver, heart, skeletal muscle, and VAT [10]. Considering that blood flows from VAT and drains into the liver through the portal vein, high concentrations of portal free fatty acids and cytokines secreted from VAT adipocytes may contribute to NAFLD and IR [11]. Additionally, crosstalk between the liver, adipose tissue, and pro-inflammatory molecules, such as interleukin-6 (IL-6) and tumor necrosis factor-α, released from activated macrophages and adipokines plays a pivotal role in NAFLD progression [12].
The skeletal muscle is the primary site of glucose disposal, which is stimulated by exercise and crosstalk between insulin and the pancreas [13]. With the development of sarcopenia, defined as the age-dependent loss of skeletal muscle, the risk of MetS, NAFLD, and cardiovascular diseases increases [14,15,16]. Specifically, sarcopenia is an independent risk factor for the progression of NAFLD, including NASH and advanced fibrosis, regardless of obesity and IR [17]. Therefore, the development of visceral obesity and sarcopenia could be independent pathogenic mechanisms of NAFLD. Although several studies have reported on the association of these two risk factors with NAFLD, most of these studies evaluated this association as an indirect measurement of body composition or using noninvasive methods for NAFLD diagnosis, leading to potential confounders.
This cross-sectional study investigated the association between the histologic features, skeletal muscle mass, and adipose tissue distribution using computed tomography (CT) scans in patients with NAFLD.
2. Methods
2.1. Patients
Data of patients who underwent an ultrasound-guided percutaneous liver biopsy for suspected NAFLD were collected consecutively at two tertiary hospitals retrospectively between January 2013 and December 2019. The exclusion criteria were as follows: chronic viral hepatitis, significant alcohol consumption (male > 140 g/week; female > 70 g/week), evidence of drug-induced liver injury, secondary fatty liver caused by drugs or hereditary diseases, and the unavailability of a CT scan within 1 month based on medical records. This study was reviewed and approved by the Institutional Review Board of Yeungnam University Hospital (IRB No. 2020-03-009) and the requirement of obtaining written informed consent from the patients was waived due to the retrospective nature. This study was conducted in accordance with the principles of the Declaration of Helsinki, and it was not possible to involve patients or the public in the design, conduct, reporting, or dissemination plans.
2.2. Histopathological Evaluation
A single experienced pathologist at each hospital independently reviewed all of the specimens at each hospital. NAFLD was defined as excessive hepatic fat infiltration of greater than 5%, determined by the histology, without evidence of other liver diseases [18]. NASH was defined as a combination of the presence of steatosis, lobular inflammation, and ballooning degeneration according to the current guidelines [18,19]. The NAFLD activity score (NAS) was evaluated according to the system devised by the Pathology Committee of the NASH Clinical Research Network [20]. Steatosis, lobular inflammation, and ballooning degeneration were scored on 1–3, 0–3, and 0–2 scales, respectively. A NAS > 4 was defined as severe NAFLD. The fibrosis stage was estimated using a 5-point scale according to the Kleiner scoring system as F0–F4, and advanced fibrosis was defined as stages F3 and F4 [20].
2.3. Assessment of Skeletal Muscle Mass and Adipose Tissue Distribution
The area of skeletal muscle mass and adipose tissue distribution were evaluated based on the cross-sectional area at the level of the third lumbar (L3) vertebra, which shows a high correlation with whole-body skeletal muscle mass and adipose tissue distribution, on abdominal CT images using a Picture Archiving and Communications System (Centricity, GE Healthcare) [21,22]. Abdominal CT images for body composition were analyzed using MATLAB version R2014a (MathWorks Inc., Natick, MA, USA). The cross-sectional areas of each body composition at L3 were calculated using an open-source software program (BMI_CT, https://sourceforge.net/projects/muscle-fat-area-measurement/) (accessed on 27 November 2017) with standard Hounsfield unit thresholds of −29 to 150 for the skeletal muscle and −190 to −30 for the visceral fat [23]. The area of the skeletal muscle mass was defined as a combination of the areas of the psoas, paraspinal, transversus abdominis, rectus abdominis, and external and internal oblique muscles. The area of the SAT was estimated as the boundaries of the skeletal muscle and line of the abdominal skin (Figure S1). The three-body composition indexes (cm2/m2), including the skeletal muscle index (SMI), SAT index (SATI), and VAT index (VATI), were defined as the body composition area (cm2) by height squared (m2). Sarcopenia was defined as an SMI < 50 cm2/m2 in men and an SMI < 39 cm2/m2 in women [24].
2.4. Assessment of Clinical and Laboratory Variables
Anthropometric measurements, including height, weight, and seated blood pressure (BP), were measured and recorded by trained hospital staff. Obesity was defined by a body mass index (BMI) ≥ 25 kg/m2, according to the Asia Pacific region criteria [25]. Diabetes mellitus was defined as a fasting plasma glucose (FPG) level ≥ 126 mg/dL and antidiabetic medication use [26]. Hypertension was defined as (i) a systolic BP level ≥ 140 mmHg, (ii) a diastolic BP level ≥ 90 mmHg, or (iii) antihypertensive medication use. The following routine laboratory variables were assessed within 1 week of liver biopsy: FPG, serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyltransferase, blood urea nitrogen, creatinine, high-sensitivity C-reactive protein, and lipid profile.
2.5. Statistical Analyses
Quantitative data are expressed as medians with interquartile ranges, unless otherwise indicated. Statistically significant differences between patients were determined using the Mann–Whitney U test for continuous data and Fisher’s exact test for categorical data. The association between the histologic findings and body composition indices was analyzed using the Spearman’s correlation coefficient. The association between advanced liver fibrosis and the body composition indices was analyzed using a logistic regression model. A receiver-operating characteristic (ROC) analysis for the body composition indices was performed to estimate the best cutoff values (COVs), calculated based on Youden’s index to predict advanced fibrosis. Any p-values < 0.05 were considered to be statistically significant. Statistical analyses were performed using R version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Baseline Characteristics
Of the 222 patients, 44 were excluded due to unavailability of CT scan images or histologic evidence of NAFLD (Figure S2). The baseline characteristics of the remaining 178 patients with biopsy-proven NAFLD are summarized in Table 1. The median age of the patients was 53.5 years (interquartile range (IQR), 38.0–64.0 years), and 86 patients (48.3%) were male. On the histologic profiles, the median NAS was 4.0 (IQR, 3.0–5.0). Among the 131 (73.6%) patients diagnosed with NASH, 61 (34.3%) had severe NASH. Furthermore, 47 (26.4%) and 32 (18.0%) patients had advanced liver fibrosis and cirrhosis, respectively. The median SMI, SATI, and VATI were 51.0 cm2/m2 (IQR, 45.8–58.2 cm2/m2), 64.4 cm2/m2 (IQR, 48.1–86.1 cm2/m2), and 66.7 cm2/m2 (IQR, 50.2–86.1 cm2/m2), respectively.
Table 1.
Baseline characteristics.
| Variable | Biopsy-Proven NAFLD Patients n = 178 |
|---|---|
| Age, year | 53.5 [38.0–64.0] |
| Male, n (%) | 86 (48.3) |
| Body mass index, kg/m2 | 26.8 [24.4–29.5] |
| Comorbidities, n (%) | |
| Obesity | 123 (69.1) |
| Diabetes mellitus | 54 (30.7) |
| Hypertension | 60 (34.1) |
| Liver function profiles | |
| Aspartate aminotransferase, IU/L | 62.0 [43.0–96.0] |
| Alanine aminotransferase, IU/L | 78.5 [43.0–96.0] |
| Platelet count, ×109/uL | 216.5 [179.0–274.0] |
| Gamma-glutamyl transferase, IU/L | 62.5 [36.0–100.0] |
| Serum albumin, g/dL | 4.4 [4.1–4.7] |
| Prothrombin time, INR | 1.1 [1.0–1.1] |
| Metabolic profiles | |
| Fasting plasma glucose, mg/dL | 108.5 [96.0–123.5] |
| Total cholesterol, mg/dL | 177.5 [158.0–210.0] |
| Triglyceride, mg/dL | 146.0 [106.5–223.5] |
| High-density lipoprotein, mg/dL | 45.8 [35.0–53.2] |
| Low-density lipoprotein, mg/dL | 99.7 [78.5–133.0] |
| C-reactive protein, mg/dL | 0.2 [0.1–0.3] |
| Biopsy profiles | |
| NAFLD activity score | 4.0 [3.0–5.0] |
| NAFLD activity score ≥ 5, n (%) | 61 (34.3) |
| NASH, n (%) | 131 (73.6) |
| Severe NASH, n (%) | 61 (34.3) |
| Fibrosis stage, 0/1/2/3/4, n (%) | 44/53/34/15/32 (24.7/29.8/19.1/8.4/18.0) |
| Advanced liver fibrosis, n (%) | 47 (26.4) |
| Cirrhosis, n (%) | 32 (18.0) |
| Muscle and fat distribution based on CT | |
| Skeletal muscle mass cm2/m2 | 51.0 [45.8–58.2] |
| Subcutaneous adipose tissue index cm2/m2 | 64.4 [48.1–86.1] |
| Visceral adipose tissue index cm2/m2 | 66.7 [50.2–86.1] |
Values are expressed as median (interquartile range (IQR)) or n (%). NAFLD, non-alcoholic fatty liver disease; INR, international normalized ratio; NASH, non-alcoholic steatohepatitis; CT, computed tomography.
3.2. Skeletal Muscle and Adipose Tissue Distribution According to Severe Non-Alcoholic Steatohepatitis and Advanced Fibrosis
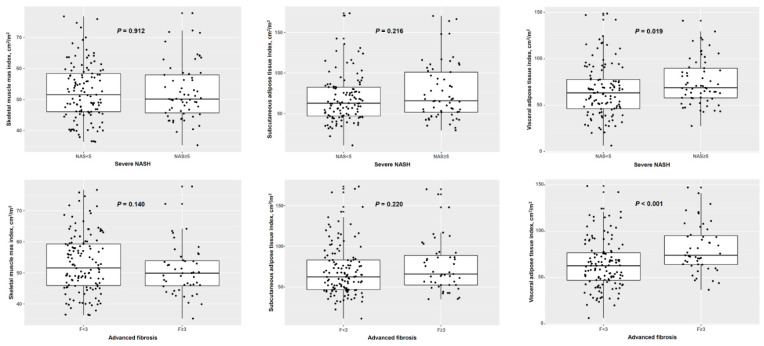
A comparison among the SMI, SATI, and VATI according to severe NASH or advanced fibrosis is shown in Figure 1. A comparative analysis of severe NASH revealed that the SMI (51.5 vs. 50.2 cm2/m2, p = 0.912) and SATI (62.8 vs. 65.9 cm2/m2, p = 0.216) were not significantly different between severe and non-severe NASH. However, the VATI was significantly greater in patients with severe NASH than in those without severe NASH (63.2 vs. 68.8 cm2/m2, p = 0.019). A comparative analysis of advanced fibrosis revealed that the SMI (51.6 vs. 49.9 cm2/m2, p = 0.140) and SATI (62.3 vs. 65.9 cm2/m2, p = 0.220) were not significantly different between advanced and non-advanced fibrosis. However, the VATI was significantly greater in patients with severe NASH than in those without severe NASH (62.8 vs. 74.1 cm2/m2, p < 0.001).
Figure 1.
Adipose tissue distribution and skeletal muscle mass according to severe non-alcoholic steatohepatitis and advanced fibrosis. NASH, non-alcoholic steatohepatitis.
3.3. Correlation of Histologic Findings with Skeletal Muscle and Adipose Tissue Distribution
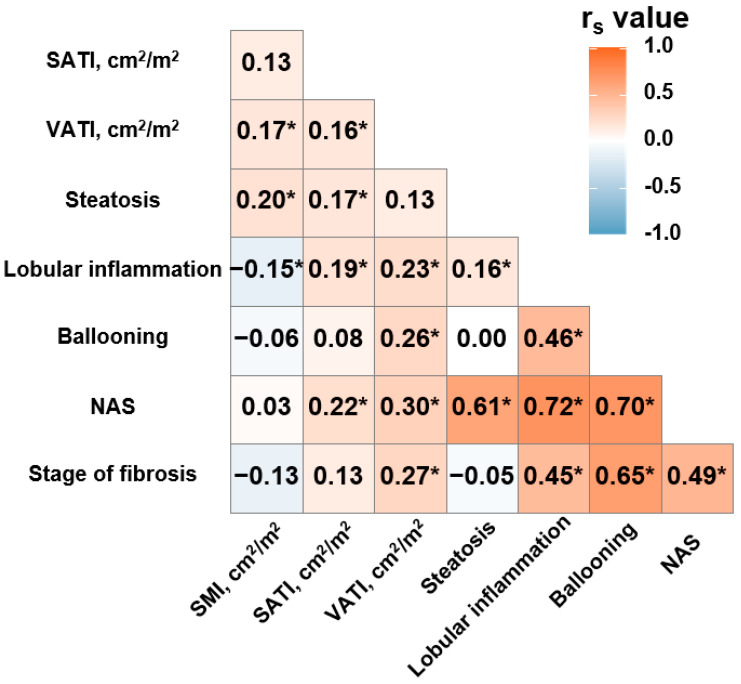
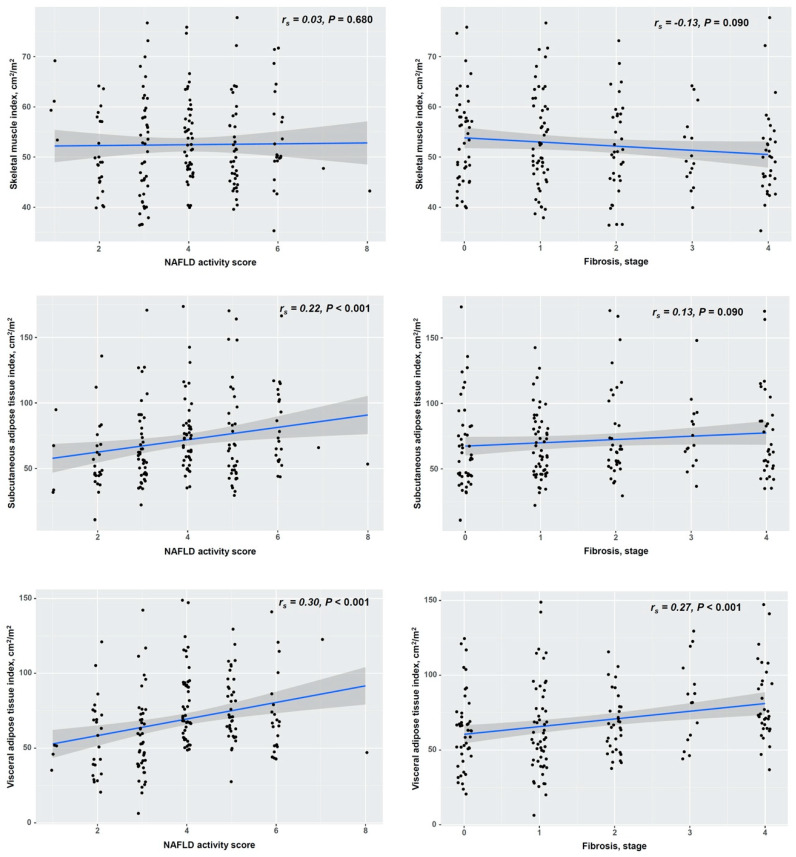
Next, we analyzed the correlation of the SMI, SATI, and VATI with the histologic grades of NAFLD, including steatosis, lobular inflammation, and ballooning degeneration (Figure 2 and Figure S3). The SMI was positively correlated with hepatic steatosis (rs = 0.20, p = 0.01) and negatively correlated with lobular inflammation (rs = −0.15, p = 0.04), but it was not with ballooning degeneration (rs = −0.06, p = 0.40). The SATI was positively correlated with hepatic steatosis (rs = 0.17, p = 0.02) and lobular inflammation (rs = 0.19, p = 0.01), but it was not with ballooning degeneration (rs = 0.08, p = 0.300). Although the VATI was not correlated with hepatic steatosis (rs = 0.13, p = 0.090), it was positively correlated with lobular inflammation (rs = 0.23, p < 0.001) and ballooning degeneration (rs = 0.26, p < 0.001). Additionally, we analyzed the correlation among the SMI, SATI, and VATI, the NAS, and the fibrosis stage (Figure 3). The SMI was not correlated with either the NAS (rs = 0.03, p = 0.680) or the fibrosis stage (rs = −0.13, p = 0.090). The SATI was positively correlated with the NAS (rs = 0.22, p < 0.001) but not with the fibrosis stage (rs = 0.13, p = 0.090). However, the VATI was positively correlated with both the NAS (rs = 0.30, p < 0.001) and the fibrosis stage (rs = 0.27, p < 0.001), which reflects the histologic severity of NAFLD.
Figure 2.
Correlation heatmap of pathologic findings, adipose tissue distribution and skeletal muscle mass. SMI, skeletal muscle index; SATI, subcutaneous adipose tissue index; VATI, visceral adipose tissue index; NAS, NAFLD activity score. Asterisk means p < 0.05. The heatmap data were analyzed using Spearman’s correlation.
Figure 3.
Correlation of adipose tissue distribution and skeletal muscle mass with the non-alcoholic fatty liver disease activity score and fibrosis stage. NAFLD, non-alcoholic fatty liver disease. The data were analyzed using Spearman’s correlation.
3.4. Association of Skeletal Muscle and Adipose Tissue Distribution with Advanced Fibrosis
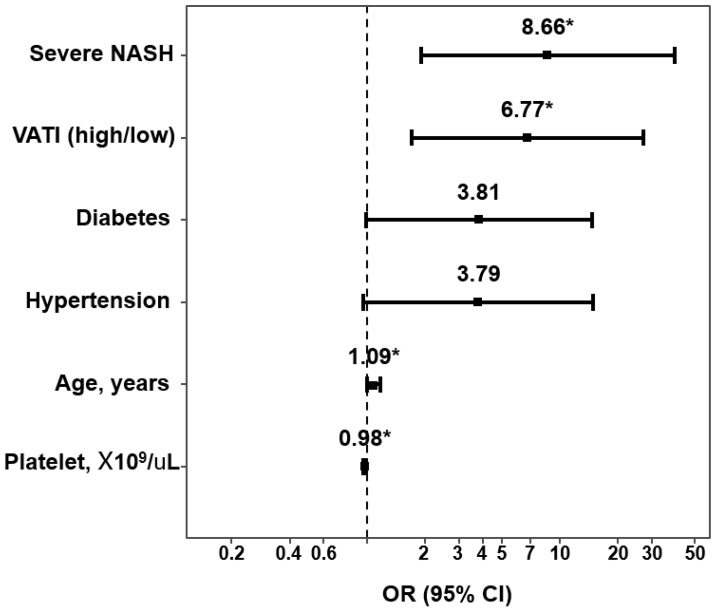
Before the multivariate analysis, we performed an ROC analysis of the VATI for advanced fibrosis to establish the optimal COVs (Figure S4). The areas under the ROC curves (AUCs) of the VATI for advanced fibrosis in male and female patients were 0.822 and 0.658, respectively. The optimal COVs of the VATI for male and female patients were 93.96 and 64.02 cm2/m2, respectively. All of the AUCs of the SMI and SATI were <0.6, suggesting poor discrimination in interpreting the AUC value, and validated COVs for the SATI were not observed. Therefore, we adjusted the mean COVs for the SATI, which was 65.5 cm2/m2 in male and 77.1 cm2/m2 in female patients. The COVs of the SMI were adjusted with the previously described values [24]. After converting the SMI, SATI, and VATI with categorical variables, age (OR, 1.09; 95% CI, 1.02–1.19; p = 0.025), platelet counts (OR, 0.98; 95% CI, 0.96–0.99; p < 0.001), severe NASH (OR, 8.66; 95% CI, 2.13–46.40; p = 0.005), and a high VATI (OR, 6.77; 95% CI, 1.81–29.90; p = 0.007) were found to be independent risk factors for advanced fibrosis in a multivariate analysis (Figure 4).
Figure 4.
Risk factors for advanced fibrosis in patients with biopsy-proven non-alcoholic fatty liver disease in multivariate analysis. NASH, non-alcoholic fatty liver disease; VATI, visceral adipose tissue index. Asterisk means p < 0.05.
The presence of diabetes mellitus and hypertension tended to be independent risk factors for advanced fibrosis but were not statistically significant. (Table 2) In addition, these results were consistent in multivariate analysis with multiple adjusted models (Table S1).
Table 2.
Univariate and multivariate analysis using categorical body composition for advanced liver fibrosis in patients with non-alcoholic fatty liver disease.
| Parameter | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Age, years | 1.12 | 1.08–1.16 | <0.001 | 1.09 | 1.02–1.19 | 0.025 |
| Male (yes/no) | 3.79 | 1.80–7.94 | <0.001 | |||
| Obesity (yes/no) | 1.23 | 0.59–2.58 | 0.576 | |||
| Diabetes mellitus (yes/no) |
4.40 | 2.14–9.02 | <0.001 | 3.81 | 1.04–15.98 | 0.051 |
| Hypertension (yes/no) | 5.85 | 2.81–12.15 | <0.001 | 3.79 | 0.99–15.89 | 0.056 |
| ALT, U/L | 0.98 | 0.98–0.99 | 0.002 | |||
| GGT, U/L | 1.00 | 1.00–1.00 | 0.753 | |||
| PLT, ×109/L | 0.97 | 0.97–0.98 | <0.001 | 0.98 | 0.96–0.99 | <0.001 |
| Albumin, g/dL | 1.06 | 0.94–1.19 | 0.360 | |||
| PT INR | 0.91 | 0.59–1.41 | 0.669 | |||
| CRP, mg/dL | 0.76 | 0.37–1.56 | 0.458 | |||
| HDL, mg/dL | 0.98 | 0.95–1.01 | 0.187 | |||
| LDL, mg/dL | 0.98 | 0.97–1.00 | 0.005 | |||
| TG, mg/dL | 0.99 | 0.99–1.00 | 0.020 | |||
| Severe NASH (yes/no) | 3.85 | 1.92–7.74 | <0.001 | 8.66 | 2.13–46.40 | 0.005 |
| SMI (high/low) | 0.23 | 0.05–1.03 | 0.055 | |||
| SATI (high/low) | 1.13 | 0.57–2.21 | 0.732 | |||
| VATI (high/low) | 6.90 | 3.28–14.54 | <0.001 | 6.77 | 1.81–29.90 | 0.007 |
OR, odds ratio; CI, confidence interval; ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; PLT, platelet counts; PT INR, prothrombin time international normalized ratio; CRP, c-reactive protein; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; NASH, non-alcoholic steatohepatitis; SMI, skeletal muscle index; SATI, subcutaneous adipose tissue index; VATI, visceral adipose tissue index.
4. Discussion
In this study, cross-sectional images of CT scans were analyzed to determine the association between adipose tissue and skeletal muscle distribution and the histologic severity of NAFLD. Unlike the SMI and SATI, the VATI significantly increased in patients with severe NASH or advanced fibrosis. Moreover, the VATI is associated with both the NAS and the fibrosis stage, which is independently associated with advanced fibrosis.
The mechanisms regarding the association between sarcopenia and NAFLD are related to IR, chronic inflammation, oxidative stress, vitamin D deficiency, low physical activity, central obesity, and crosstalk with hepatokines (fibroblast growth factor 21, leukocyte cell-derived chemotaxin-2, and hepassocin) and myokines (irisin and myostatin) [27]. Although several studies have reported on the association between sarcopenia and NAFLD, most of these studies determined this association using noninvasive methods such as ultrasounds, the fatty liver index or the hepatic steatosis index for NAFLD, and bioimpedance analysis (BIA) for sarcopenia [28,29]. Specifically, all of the studies on patients with biopsy-proven NAFLD have evaluated sarcopenia using BIA, which leads to a possible discordance of the actual skeletal muscle mass [30]. Particularly, the discordance between CT and BIA can be frequently observed in adults aged > 65 years, female patients, and individuals with a BMI < 25 kg/m2 [30]. Therefore, according to this study, it would be appropriate to determine the skeletal muscle mass using CT when a high proportion of cases with advanced NAFLD are enrolled. Here, a low SMI was not associated with NAFLD severity and was negatively associated with lobular inflammation, a result inconsistent with the results of previous studies [17]. However, when analysis was performed in obese patients, the SMI was negatively associated with the fibrosis stage (rs = −0.18, p = 0.050, Figure S5). When sarcopenia and obesity are combined, the so-called sarcopenic obesity, sarcopenia-related factors may aggravate obesity-related diseases. An excessive fat accumulation induces the accumulation of ectopic fat in the skeletal muscle, which causes mitochondrial dysfunction and IR and creates a pro-inflammatory environment [31]. Thus, we believe that skeletal muscle loss was associated with lobular inflammation of the liver and commitment obesity acts synergistically, subsequently affecting NAFLD progression.
Activated adipocytes with hypertrophy and hyperplasia beyond their intrinsic limits induce the accumulation of pro-inflammatory macrophages and dysregulated immune cells, which produce various adipokines, immune cell-released cytokines, and chemokines [31]. Recently, the crosstalk between adipose tissue IR and liver macrophage has been considered to be a possible mechanism of NAFLD progression [32]. Unlike subcutaneous adiposity, visceral adiposity is more closely associated with metabolic diseases and adverse outcomes [9]. In this study, the SATI was significantly associated with steatosis grade, but the VATI was not. However, the SATI was not associated with ballooning degeneration and the fibrosis stage, but the VATI was. Adipose tissue distribution is dependent on sex in NAFLD. In a study of adolescent NAFLD patients, only visceral adiposity was associated with the severity of steatosis in male patients [33]. VAT adipocytes have a greater IR and a higher expression of androgen receptors, adiponectin, and IL-6 release than SAT [34]. However, unlike in a previous study on adolescents, the VATI was associated with the severity of steatosis in women with NAFLD only in our subgroup analysis (Figure S6). Here, the female patients were older than the male patients (44.5 ± 18.7 vs. 56.5 ± 11.8 years, Table S2). We believe that age and sex affect fat accumulation in the liver. Thus, the discrepancy observed in the previous study is probably due to age and the postmenopausal status of woman.
In several studies, visceral adiposity was associated with the development [35,36] and severity [37,38,39] of NAFLD. In most of those studies, NAFLD diagnosis was established using a radiologic examination, such as ultrasound or CT, which has low sensitivity for detecting mild hepatic steatosis [35,36]. In some studies, the assessment of adipose tissue is established with the visceral adipose index by using several anthropometric and metabolic parameters, which have limited validation [35,37]. Although a few studies have been conducted on patients with biopsy-proven NAFLD, they had an inadequate sample size to reach conclusive outcomes [37,38,39]. Based on these findings, alteration of body composition is believed to be an important factor for NAFLD progression. A few studies have suggested that the skeletal muscle mass to visceral fat ratio, which is assessed to estimate sarcopenic obesity, is closely associated with MetS and cardiovascular diseases [40,41]. This simple index, used to assess sarcopenic obesity, was evaluated to determine NAFLD progression using transient elastography and controlled attenuation parameters in a few studies [41,42]. Although this index has not been analyzed in this study due to the small number of patients with sarcopenic obesity (n = 8, data not shown), it would be a potential risk factor when determining NAFLD progression based on our subgroup analysis (Figure S5).
This study has some limitations. First, there may be selection bias due to the enrollment of patients with possible severe disease who underwent a liver biopsy. Second, the laboratory tests regarding IR, such as homeostasis model assessment-IR and adipose tissue IR, were not analyzed due to the retrospective design of this study, with significant missing values. Third, the prognosis of NAFLD was not analyzed due to the cross-sectional design of this study. However, the liver fibrosis stage, which is closely associated with NAFLD mortality, was analyzed to estimate the prognosis of NAFLD [43]. Fourth, because all of the patients in this study are Asian, these results cannot be generalized in a Western population. Thus, further evaluation is required in a Western population. However, this study has the following strengths: both skeletal muscle mass and adipose tissue distribution were evaluated in biopsy-proven NAFLD with an adequate sample size and a comprehensive analysis of the histologic review.
In conclusion, the histologic severity of NAFLD is correlated with visceral adiposity. Visceral adiposity is independently associated with advanced fibrosis in patients with NAFLD. The measurement of visceral adiposity may be helpful to assess the risk of advanced liver disease in patients with NAFLD.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/diagnostics11061061/s1. Table S1: Adjusted odds ratio of high visceral adipose tissue index for fibrosis in patients with biopsy-proven non-alcoholic fatty liver disease. Table S2: Sex differences in baseline characteristics. Figure S1: (a) Cross-sectional computed tomography image at the level of the third vertebra to measure skeletal muscle and fat distribution. (b) Red and blue areas represent subcutaneous and visceral adipose tissues, respectively, and green area represents skeletal muscle. Figure S2: Flow diagram of the study. Figure S3: Association of adipose tissue distribution and skeletal muscle mass with hepatic steatosis, lobular inflammation, and ballooning degeneration. Figure S4: Receiver-operating characteristic curve of visceral adipose tissue index for advanced fibrosis, a. male; b. female. Figure S5: Correlation of skeletal muscle mass according to the fibrosis stage in obese patients with NAFLD. Figure S6: Correlation of visceral adipose tissue according to hepatic steatosis, a. male; b. female
Author Contributions
Conceptualization, J.-G.P. and S.-Y.P.; methodology, M.-K.K., J.-G.P. and S.-Y.P.; software, M.-K.K. and J.-G.P.; formal analysis, M.-K.K. and J.-G.P.; investigation, J.-G.P., M.-K.K., J.-H.B., Y.-O.K., W.-Y.T., S.-Y.J., Y.-R.L., K.H., G.K., H.-W.L., M.-H.H., J.-H.C. and S.-Y.P.; resources, M.-K.K.; data curation, M.-K.K. and J.-H.B.; writing—original draft, J.-G.P. and M.-K.K.; writing—review and editing, J.-G.P. and S.-Y.P.; visualization, J.-G.P.; supervision, J.-G.P. and S.-Y.P.; project administration, J.-G.P.; funding acquisition, J.-G.P. and M.-K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Science, ICT and Future Planning (2017R1C1B5076851), and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) and funded by the Korean government (2019M3E5D1A02068089).
Institutional Review Board Statement
This study was reviewed and approved by the Institutional Review Board of Yeungnam University Hospital (IRB No. 2020-03-009).
Informed Consent Statement
Informed consent from participants was waived because of the retrospective nature of this study.
Data Availability Statement
The data that support the findings of this study are also available from the corresponding authors (J.G.P. and S.Y.P.) upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Williams R., Aspinall R., Bellis M., Camps-Walsh G., Cramp M., Dhawan A., Ferguson J., Forton D., Foster G., Gilmore I., et al. Addressing liver disease in the UK: A blueprint for attaining excellence in health care and reducing premature mortality from lifestyle issues of excess consumption of alcohol, obesity, and viral hepatitis. Lancet. 2014;384:1953–1997. doi: 10.1016/S0140-6736(14)61838-9. [DOI] [PubMed] [Google Scholar]
- 2.NatCen Social Research. University College London Department of Epidemiology and Public Health . Health Survey for England. UK Data Archive; Colchester, UK: 2012. [Google Scholar]
- 3.Brunt E.M., Wong V.W., Nobili V., Day C.P., Sookoian S., Maher J.J., Bugianesi E., Sirlin C.B., Neuschwander-Tetri B.A., Rinella M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Primers. 2015;1:15080. doi: 10.1038/nrdp.2015.80. [DOI] [PubMed] [Google Scholar]
- 4.Mantovani A., Byrne C.D., Bonora E., Targher G. Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-analysis. Diabetes Care. 2018;41:372–382. doi: 10.2337/dc17-1902. [DOI] [PubMed] [Google Scholar]
- 5.Kang M.K., Park J.G., Kim M.C. Association between Atrial Fibrillation and Advanced Liver Fibrosis in Patients with Non-Alcoholic Fatty Liver Disease. Yonsei Med. J. 2020;61:860–867. doi: 10.3349/ymj.2020.61.10.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu Z., Zhang Y., Graham S., Wang X., Cai D., Huang M., Pique-Regi R., Dong X.C., Chen Y.E., Willer C., et al. Causal relationships between NAFLD, T2D and obesity have implications for disease subphenotyping. J. Hepatol. 2020;73:263–276. doi: 10.1016/j.jhep.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reijrink M., de Boer S.A., Spoor D.S., Lefrandt J.D., Lambers Heerspink H.J., Boellaard R., Greuter M.J., Borra R.J.H., Hillebrands J.L., Slart R., et al. Visceral adipose tissue volume is associated with premature atherosclerosis in early type 2 diabetes mellitus independent of traditional risk factors. Atherosclerosis. 2019;290:87–93. doi: 10.1016/j.atherosclerosis.2019.09.016. [DOI] [PubMed] [Google Scholar]
- 8.Yu A.H., Duan-Mu Y.Y., Zhang Y., Wang L., Guo Z., Yu Y.Q., Wang Y.S., Cheng X.G. Correlation between Non-Alcoholic Fatty Liver Disease and Visceral Adipose Tissue in Non-Obese Chinese Adults: A CT Evaluation. Korean J. Radiol. 2018;19:923–929. doi: 10.3348/kjr.2018.19.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Despres J.P., Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 10.Tchernof A., Despres J.P. Pathophysiology of human visceral obesity: An update. Physiol. Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011. [DOI] [PubMed] [Google Scholar]
- 11.Bjorntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–496. doi: 10.1161/01.ATV.10.4.493. [DOI] [PubMed] [Google Scholar]
- 12.Cordeiro A., Costa R., Andrade N., Silva C., Canabrava N., Pena M.J., Rodrigues I., Andrade S., Ramalho A. Does adipose tissue inflammation drive the development of non-alcoholic fatty liver disease in obesity? Clin. Res. Hepatol. Gastroenterol. 2020;44:294–402. doi: 10.1016/j.clinre.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Mizgier M.L., Casas M., Contreras-Ferrat A., Llanos P., Galgani J.E. Potential role of skeletal muscle glucose metabolism on the regulation of insulin secretion. Obes. Rev. 2014;15:587–597. doi: 10.1111/obr.12166. [DOI] [PubMed] [Google Scholar]
- 14.Moon J.S., Yoon J.S., Won K.C., Lee H.W. The role of skeletal muscle in development of nonalcoholic Fatty liver disease. Diabetes Metab. J. 2013;37:278–285. doi: 10.4093/dmj.2013.37.4.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang M.K., Kim K.O., Kim M.C., Park J.G., Jang B.I. Sarcopenia Is a New Risk Factor of Nonalcoholic Fatty Liver Disease in Patients with Inflammatory Bowel Disease. Dig. Dis. 2020;38:507–514. doi: 10.1159/000506938. [DOI] [PubMed] [Google Scholar]
- 16.Kang M.K., Park J.G. Low Skeletal Muscle Mass Is a Risk Factor for Subclinical Atherosclerosis in Patients with Nonalcoholic Fatty Liver Disease. Diagnostics. 2021;11:854. doi: 10.3390/diagnostics11050854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koo B.K., Kim D., Joo S.K., Kim J.H., Chang M.S., Kim B.G., Lee K.L., Kim W. Sarcopenia is an independent risk factor for non-alcoholic steatohepatitis and significant fibrosis. J. Hepatol. 2017;66:123–131. doi: 10.1016/j.jhep.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 18.Chalasani N., Younossi Z., Lavine J.E., Diehl A.M., Brunt E.M., Cusi K., Charlton M., Sanyal A.J. The diagnosis and management of non-alcoholic fatty liver disease: Practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–2023. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 19.Yoo J.J., Kim W., Kim M.Y., Jun D.W., Kim S.G., Yeon J.E., Lee J.W., Cho Y.K., Park S.H., Sohn J.H., et al. Recent research trends and updates on nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2019;25:1–11. doi: 10.3350/cmh.2018.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kleiner D.E., Brunt E.M., Van Natta M., Behling C., Contos M.J., Cummings O.W., Ferrell L.D., Liu Y.C., Torbenson M.S., Unalp-Arida A., et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 21.Kang S.H., Jeong W.K., Baik S.K., Cha S.H., Kim M.Y. Impact of sarcopenia on prognostic value of cirrhosis: Going beyond the hepatic venous pressure gradient and MELD score. J. Cachexia Sarcopenia Muscle. 2018;9:860–870. doi: 10.1002/jcsm.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebadi M., Tandon P., Moctezuma-Velazquez C., Ghosh S., Baracos V.E., Mazurak V.C., Montano-Loza A.J. Low subcutaneous adiposity associates with higher mortality in female patients with cirrhosis. J. Hepatol. 2018;69:608–616. doi: 10.1016/j.jhep.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 23.Kim S.S., Kim J.H., Jeong W.K., Lee J., Kim Y.K., Choi D., Lee W.J. Semiautomatic software for measurement of abdominal muscle and adipose areas using computed tomography: A STROBE-compliant article. Medicine. 2019;98:e15867. doi: 10.1097/MD.0000000000015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carey E.J., Lai J.C., Wang C.W., Dasarathy S., Lobach I., Montano-Loza A.J., Dunn M.A., Fitness L.E. Exercise in Liver Transplantation, C. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transplant. 2017;23:625–633. doi: 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oh S.W. Obesity and metabolic syndrome in Korea. Diabetes Metab. J. 2011;35:561–566. doi: 10.4093/dmj.2011.35.6.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Diabetes Association Standards of medical care in diabetes—2013. Diabetes Care. 2013;36(Suppl. 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim J.A., Choi K.M. Sarcopenia and fatty liver disease. Hepatol. Int. 2019;13:674–687. doi: 10.1007/s12072-019-09996-7. [DOI] [PubMed] [Google Scholar]
- 28.Cai C., Song X., Chen Y., Chen X., Yu C. Relationship between relative skeletal muscle mass and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Hepatol. Int. 2020;14:115–126. doi: 10.1007/s12072-019-09964-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kang M.K., Park J.G., Lee H.J., Kim M.C. Association of low skeletal muscle mass with advanced liver fibrosis in patients with non-alcoholic fatty liver disease. J. Gastroenterol. Hepatol. 2019;34:1633–1640. doi: 10.1111/jgh.14607. [DOI] [PubMed] [Google Scholar]
- 30.Jo M.H., Lim T.S., Jeon M.Y., Lee H.W., Kim B.K., Park J.Y., Kim D.Y., Ahn S.H., Han K.H., Kim S.U. Predictors of Discordance in the Assessment of Skeletal Muscle Mass between Computed Tomography and Bioimpedance Analysis. J. Clin. Med. 2019;8:322. doi: 10.3390/jcm8030322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kalinkovich A., Livshits G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res. Rev. 2017;35:200–221. doi: 10.1016/j.arr.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Rosso C., Kazankov K., Younes R., Esmaili S., Marietti M., Sacco M., Carli F., Gaggini M., Salomone F., Moller H.J., et al. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J. Hepatol. 2019;71:1012–1021. doi: 10.1016/j.jhep.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 33.Ayonrinde O.T., Olynyk J.K., Beilin L.J., Mori T.A., Pennell C.E., de Klerk N., Oddy W.H., Shipman P., Adams L.A. Gender-specific differences in adipose distribution and adipocytokines influence adolescent nonalcoholic fatty liver disease. Hepatology. 2011;53:800–809. doi: 10.1002/hep.24097. [DOI] [PubMed] [Google Scholar]
- 34.Ibrahim M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010;11:11–18. doi: 10.1111/j.1467-789X.2009.00623.x. [DOI] [PubMed] [Google Scholar]
- 35.Xu C., Ma Z., Wang Y., Liu X., Tao L., Zheng D., Guo X., Yang X. Visceral adiposity index as a predictor of NAFLD: A prospective study with 4-year follow-up. Liver Int. 2018;38:2294–2300. doi: 10.1111/liv.13941. [DOI] [PubMed] [Google Scholar]
- 36.Kim D., Chung G.E., Kwak M.S., Seo H.B., Kang J.H., Kim W., Kim Y.J., Yoon J.H., Lee H.S., Kim C.Y. Body Fat Distribution and Risk of Incident and Regressed Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2016;14:132–138.e4. doi: 10.1016/j.cgh.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Petta S., Amato M.C., Di Marco V., Camma C., Pizzolanti G., Barcellona M.R., Cabibi D., Galluzzo A., Sinagra D., Giordano C., et al. Visceral adiposity index is associated with significant fibrosis in patients with non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2011;35:238–247. doi: 10.1111/j.1365-2036.2011.04929.x. [DOI] [PubMed] [Google Scholar]
- 38.Choudhary N.S., Duseja A., Kalra N., Das A., Dhiman R.K., Chawla Y.K. Correlation of adipose tissue with liver histology in Asian Indian patients with nonalcoholic fatty liver disease (NAFLD) Ann. Hepatol. 2012;11:478–486. doi: 10.1016/S1665-2681(19)31461-9. [DOI] [PubMed] [Google Scholar]
- 39.Sobhonslidsuk A., Jongjirasiri S., Thakkinstian A., Wisedopas N., Bunnag P., Puavilai G. Visceral fat and insulin resistance as predictors of non-alcoholic steatohepatitis. World J. Gastroenterol. 2007;13:3614–3618. doi: 10.3748/wjg.v13.i26.3614. [DOI] [PubMed] [Google Scholar]
- 40.Kim T.N., Park M.S., Lim K.I., Yang S.J., Yoo H.J., Kang H.J., Song W., Seo J.A., Kim S.G., Kim N.H., et al. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: The Korean Sarcopenic Obesity Study (KSOS) Diabetes Res. Clin. Pract. 2011;93:285–291. doi: 10.1016/j.diabres.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Shida T., Oshida N., Oh S., Okada K., Shoda J. Progressive reduction in skeletal muscle mass to visceral fat area ratio is associated with a worsening of the hepatic conditions of non-alcoholic fatty liver disease. Diabetes Metab. Syndr. Obes. 2019;12:495–503. doi: 10.2147/DMSO.S185705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shida T., Akiyama K., Oh S., Sawai A., Isobe T., Okamoto Y., Ishige K., Mizokami Y., Yamagata K., Onizawa K., et al. Skeletal muscle mass to visceral fat area ratio is an important determinant affecting hepatic conditions of non-alcoholic fatty liver disease. J. Gastroenterol. 2018;53:535–547. doi: 10.1007/s00535-017-1377-3. [DOI] [PubMed] [Google Scholar]
- 43.Hagstrom H., Nasr P., Ekstedt M., Hammar U., Stal P., Hultcrantz R., Kechagias S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017;67:1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are also available from the corresponding authors (J.G.P. and S.Y.P.) upon reasonable request.