Abstract
Multiple myeloma (MM) is characterized by an accumulation of malignant plasma cells (PCs) in the bone marrow (BM). The amplification of 1q21 is one of the most common cytogenetic abnormalities occurring in around 40% of de novo patients and 70% of relapsed/refractory MM. Patients with this unfavorable cytogenetic abnormality are considered to be high risk with a poor response to standard therapies. The gene(s) driving amplification of the 1q21 amplicon has not been fully studied. A number of clear candidates are under investigation, and some of them (IL6R, ILF2, MCL-1, CKS1B and BCL9) have been recently proposed to be potential drivers of this region. However, much remains to be learned about the biology of the genes driving the disease progression in MM patients with 1q21 amp. Understanding the mechanisms of these genes is important for the development of effective targeted therapeutic approaches to treat these patients for whom effective therapies are currently lacking. In this paper, we review the current knowledge about the pathological features, the mechanism of 1q21 amplification, and the signal pathway of the most relevant candidate genes that have been suggested as possible therapeutic targets for the 1q21 amplicon.
Keywords: multiple myeloma, 1q21, chromosome aberrations, amplification, IL6R, ILF2, MCL-1, CKS1B, BCL9
1. Introduction
Multiple myeloma (MM) is characterized by an abnormal proliferation of plasma cells (PCs) in the bone marrow (BM). Despite the significant improvement in overall survival (OS), most MM patients eventually relapse and develop refractory disease [1]. MM is a genetically complex disease characterized by a distinct clinical heterogeneity in the response rate and survival outcomes. Nevertheless, the progression of the disease demonstrates a wide range of heterogeneity, with the OS time ranging from less than 12 months to more than 10 years [2,3]. The heterogeneity in MM can be explained either by the intrinsic genetic heterogeneity of PCs or at least in part by the BM microenvironment, which generates a high-risk environment that facilitates cancer cell survival [4,5,6].
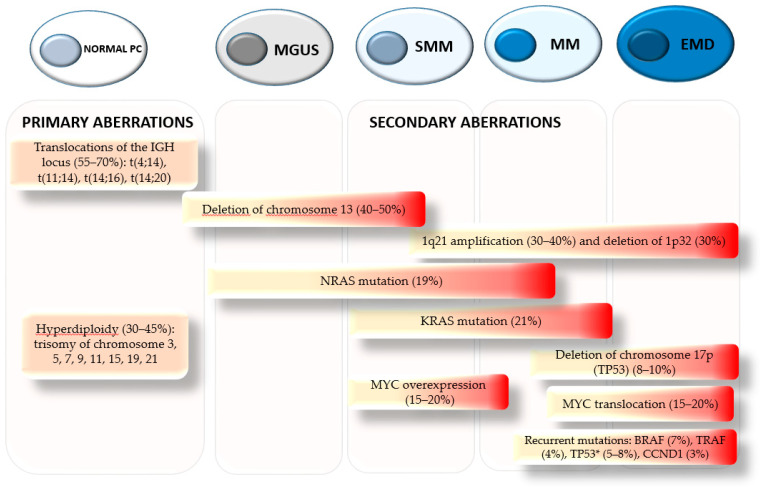
The presence or absence of recurrent chromosomal abnormalities is considered one of the major prognostic landmarks for MM patients. Primary immunoglobulin translocations (t) involving immunoglobulin heavy chain (IGH) at the 14q32 region including t(4;14), t(14;16), t(14;20), and the hyperdiploid including trisomies of the odd-numbered chromosomes, occur as initiating events during tumor pathogenesis (Figure 1). Secondary abnormalities occur later during disease progression. The most frequent secondary abnormalities can be classified as translocations, mutations, deletions, and amplifications (Figure 1). MYC translocation is found in about 15% of MM at diagnosis and 50% of more advanced stages [7]. Approximately one-third of MYC translocations involve rearrangements with the IGH locus [8,9]. In addition, copy number change at the MYC locus is found in around 20% of NDMM patients [9]. The overexpression of MYC is typically associated with a poor prognosis in patients with MM. The RAS gene family (NRAS and KRAS) is one of the most frequently mutated groups of genes in MM [10]. The prevalence of both NRAS and KRAS mutations in MM patients is between 20 and 35%. It has been suggested that KRAS plays a more influential role in the pathogenesis of MM. RAS mutations are usually associated with less favorable disease outcomes, greater tumor burden, and shortened survival time [10]. The tumor suppressor gene TP53 is located on chromosome 17 (17p). Deletions of the 17p region can lead to monoallelic inactivation of TP53 [11]. Recently, bi-allelic inactivation of TP53 has been associated with high risk, poor OS, and resistance to conventional MM treatments [12,13]. In MM, TP53 mutations are uncommon at diagnosis and represent late events in disease progression, suggested to play an essential role in MM pathogenesis [13,14]. In a multivariate analysis, D’Agostino et al. showed that mutation of P53 was correlated with early relapse in MM patients. In addition, the study showed that patients with both, deletion of the 17p and P53 mutations, have a shorter OS compared to patients with only one chromosomal aberration [15]. An increased risk of acquired P53 mutation was also observed in patients carrying a deletion of 17p [15]. Along with TP53 mutation, deletion of chromosome 13 has also been associated with adverse disease outcome [16].
Figure 1.
Genetic events from initiation to progression in multiple myeloma pathogenesis. Chromosomal aberrations involving immunoglobulin heavy chain (IGH) at the 14q32 region and the hyperdiploid are considered primary translocations as they are mutually exclusive and present in asymptomatic stages. Secondary aberrations follow primary events contributing to tumor progression and relapse. Secondary events cooperate with primary events to produce the malignant PC phenotype. The progression from MGUS-SMM to MM is associated with RAS mutation, MYC overexpression, and amplification of 1q21. Incidence of MYC translocation, deletion of 17p, and recurrent mutations increased as disease progressed. The initiation of aberrant clones at the onset of the disease is indicated in yellow. Red indicates the accumulation of malignant clones during disease progression. Percentage is indicated at new diagnosis. Asterisk indicates P53 mutation.
Amplification of chromosome 1q (1q21 amp) is one of the most common secondary cytogenetic abnormalities in patients with MM. 1q21 amp is often associated with poor prognosis, drug resistance, and disease progression [17]. The 1q21 region is known to contain several oncogenes and genes that may show simultaneous amplification and/or deregulated expression. To date, the relevant gene(s) driving the high-risk disease progression associated with 1q21 amp has not been fully identified, suggesting that more than one candidate gene may be responsible for the poor outcome in this group of patients. In this review, we provide an overview of the pathological features, prognostic values, and the signal pathway of several target genes located on the 1q21 region. This review can shed light on alternative therapeutic approaches to treat MM patients carrying 1q21 amp that usually do not benefit from currently available treatments.
2. Pathogenesis and Clinical Implications of 1q21 Amplification
The 1q21 region is gained (three copies) or amplified (≥ four copies) in about 40% of de novo cases and 70% of the relapsed–refractory (RR) MM patients (Figure 1) [18,19]. Patients with this unfavorable cytogenetic abnormality are considered to be high risk with a poor response to standard therapies [17,20]. The amplification of 1q21 commonly occurs as arm-level aberrations or focal amplifications, and both cases are the result of the genomic instability from the 1q12 region [21,22]. In several studies, Sawyer et al. underlined the mechanism for the amplification of 1q21 in MM [21,22,23,24]. He suggested that the amplification of the 1q region occurs when duplication of the 1q12 pericentriomeric region translocates, leading to jumping segmental duplications of the chromosome 1q band, resulting from de-condensation of the pericentromeric heterochromatin region [21,22,23,24]. A second type of 1q arm-level copy number alteration commonly seen is the formation of isochromosomes, which results in the duplication of one arm and the loss of the other, resulting in an amplification of the 1q21 region [23]. The 1q21 amplified region can be detected by fluorescent in situ hybridization (FISH) [18]. This method has become a standard clinical feature for the identification of patients with 1q21 amp [18].
Studies have shown that gain and/or amplification of 1q21 can be found at any stages of MM disease, from monoclonal gammopathy of uncertain significance (MGUS) (15–20%), to smoldering (SSM) (30%), while in highly proliferative disease such as MM and extramedullary disease (EMD) the accumulation of additional copies of 1q21 results in as much as 70% of patients having more than three copies (Figure 1) [18,25,26]. In fact, the heterogeneous genetic background of patients with 1q21 amp has a profound impact on survival when compared to those with 1q21 gain. A study by Hanamura et al. showed that patients with amplification of 1q21 at diagnosis tended to have a more aggressive clinical course than those with 1q21 gain [18]. The OS was similar in both groups; however, RRMM patients carrying 1q21 amp showed a shorter PFS and OS survival due to the more aggressive tumor behavior when compared with those with 1q21 gain [18].
Immunomodulatory drugs IMMDs and proteasome inhibitors (PI) have become an essential part of the MM treatment regimen due to their well-established clinical efficacy. Both drugs have been successfully used to treat MM high-risk patients, in particular patients with t(4:14) and del(17p). In a randomized study, Cavo et al. showed that patients with t(4:14) and/or del(17p) who received a combination of PI + IMMDs + dexamethasone (VDT) after autologous stem cell transplantation (ASCT) have a longer PFS and OS than those patients who only received IMMDs + dexamethasone (TD) as a base treatment [27]. A study by Shaughnessy et al. demonstrated that patients carrying 1q21 amp treated with bortezomib showed an upregulation of proteasomes genes including PSMD4, a gene commonly upregulated and associated with adverse disease outcome in patients with 1q21 amp [28]. The results from this study suggest that additional copies of 1q21 may mediate the resistance to bortezomib in these patients, whom often have an early progression and a shorter OS [28]. A more recent retrospective study has shown that patients with 1q21 gain have a better response to VDT induction therapy and accomplish a very good response (VGR) and/or complete response (CR) after ASCT when compared to patients without 1q21 gain [29]. In addition to bortezomib, it has been proposed that carfilzomib, a novel second-generation PI, may be capable to overcome bortezomib resistance in high-risk MM patients [30]. Early-phase clinical studies have demonstrated that NDMM high-risk patients treated with carfilzomib-based induction achieved impressively high rates of MRD negativity and longer PFS. However, the results regarding the outcome of patients with 1q21 amp have not been published yet [30]. On the other hand, encouraging results regarding patients with 1q21 gain have come from the FORTE trial [30]. The results from this study showed that NDMM patients who received carfilzomib in combination with cyclophosphamide and dexamethasone (KRd) prior to and after ASCT have a longer PFS while patients with 1q21 amplification showed a very poor response to the treatment [30].
The coexistence of 1q21 amp with other cytogenetic aberrations has a negative impact on MM outcome. Recently, Abdallah et al. evaluated the impact of 1q21 amp on clinical characteristics and OS in more than 1300 NDMM patients. The results from a multivariate analysis show that 1q21 amp was associated with a decreased OS when other cytogenetic abnormalities, such as the loss of the short arm of chromosome 17 or translocations t(4;14), t(14;16), and t(16;20), were included [31]. This prognostic value was retained when advanced ISS stage and older age was considered [31]. In another study, Walker et al. characterized a double-hit subgroup in NDMM patients. This cohort of patients was described to have either the bi-allelic inactivation of TP53 or ISS stage III with more than four copies of 1q21. The double-hit was rare, present in only 6.1% of the patients in the study [32]. In addition, a study performed by Jin et al. found that patients with concomitant 1q21 amp and MYC rearrangement presented a higher frequency of EMD and hypercalcemia than patients with 1q21 amp or MYC rearrangement alone [33]. The rate of CR and VGR was relatively lower in patients with a combination of 1q21 amp and MYC rearrangement when compared to patients carrying only one aberration [33].
3. 1q21 Amplification and Potential Druggable Targets Genes
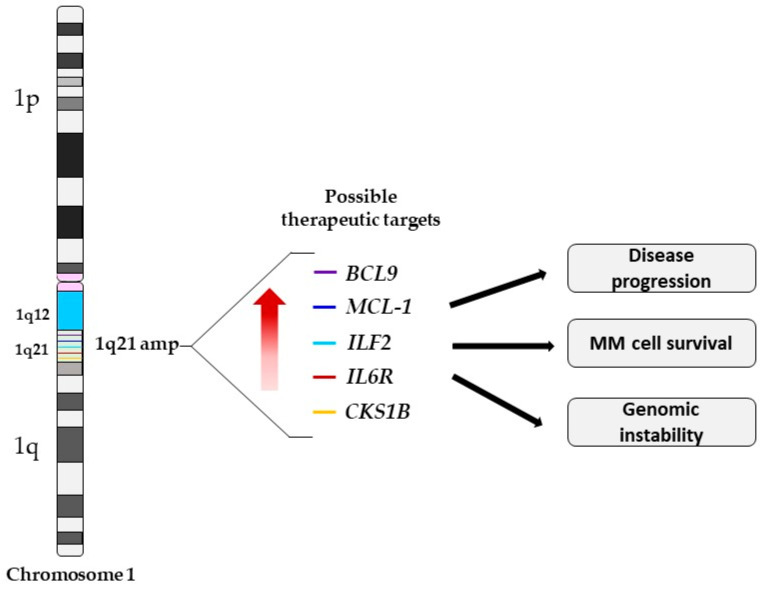
Amplification of 1q21 is an important prognostic marker in MM. This region contains a large number of genes including IL6R, MCL-1, ILF2, BCL9 and CKS1B that have been reported to drive disease aggressiveness in 1q21 amp cases (Table 1). The amplification of the 1q21 region often results in the simultaneous upregulation and/or deregulated expression of these genes, which frequently correlates with poor prognosis and drug resistance (Figure 2) [28,34,35,36,37]. The exact gene(s) driving the amplification of 1q21 have not been fully characterized. Several candidate genes have been proposed as potential 1q21 targets. In this part of the review, we provide a summary of the mechanisms of the most relevant MM targetable genes located on the 1q21 region.
Table 1.
Biological function and implications of candidate genes in MM cells with 1q21 amp.
| Candidate Genes | Chromosomal Location | Biological Function | Function of Overexpressed Gene in MM Cells with 1q21 | References |
|---|---|---|---|---|
| MCL-1 | 1q21.2 | Anti-apoptotic | Apoptosis resistance, disease progression, and shorter OS | [35,38] |
| CSK1B | 1q21.3 | Cell division | Regulation of the p27 proteasome degradation | [39,40] |
| IL6R | 1q21.3 | IL6 regulator | Increased IL6 sensitivity and hyper-activation of the STAT3 pathway | [34,41] |
| ILF2 | 1q21.3 | mRNA stability, mRNA post-transcriptional regulation, and in mitotic control. | Promotes tolerance of genomic instability, enhancing MM cell survival and drug resistance | [36,42,43] |
| BCL9 | 1q21.2 | T ranscriptional co-activator of the Wnt/β-catenin pathway | Promotes cell proliferation? | [44,45] |
Figure 2.
Schematic representation of chromosome 1 indicating the location of the possible genetic driver genes in this region, denoted by colored lines. The amplification of the 1q21 region results in the simultaneous overexpression of several genes leading to disease progression, MM cell survival, and an increase in genomic instability. BCL9, B cell lymphoma 9; MCL-1, myeloid cell leukemia-1; ILF2, interleukin enhancer binding factor 2; IL6R, interleukin-6 receptor; and CKS1B, CDC28 protein kinase regulatory subunit 1B.
3.1. MCL-1
The myeloid cell leukemia-1 (MCL-1) belongs to the BCL-2 family [46]. Both MCL-1 and BCL-2 have functional similarities and share the ability to promote cell survival [47]. The RNA expression of both genes is independently regulated and the tissue distribution shows significant differences, suggesting that both proteins may have different roles in controlling apoptotic pathways [48]. High expression levels of MCL-1 are commonly seen in hematological cancers [49]. MCL-1 is known to act as a gene inducing tumor progression [38]. The MCL-1 transcriptional regulation is cell type-dependent, modulated by several extracellular stimuli such as growth factors, endothelial growth factors, interferons, and cytokines such as interleukin 6 (IL6), which plays a critical role in MM progression [38]. In addition, several cytoplasmic pathways (e.g., PI3K/Akt, JAK/STAT, and MEK/ERK) have been shown to be activated by extracellular stimuli affecting the downstream expression of MCL-1 [38]. The expression of MCL-1 can be regulated in a paracrine fashion by IL6. IL6 binds its receptor and activates Janus family kinase-2 (JAK2), thereby inducing the signal transducer activator of the transcription 3 (STAT3) homodimerization [50]. Once homodimerized, STAT3 translocates to the nucleus, increasing MCL-1 transcription [50]. MCL-1 expression can also be independently regulated from other survival signals from the BM microenvironment, such as interferon α (IFNα) which induces MCL-1 in a STAT3-dependent manner [50]. In many cancers, overexpression of MCL-1 contributes to the development of malignancies and it is often associated with resistance to conventional therapies. The role of MCL-1 in MM has been evaluated in several studies looking at the effect of MCL-1 expression levels on cell proliferation [51]. Studies have demonstrated that expression of MCL-1 is important for B lymphocyte development and PC survival [52]. Wuilleme-Toumi et al. evaluated the expression of MCL-1 in BM PCs from MM patients at diagnosis and after relapse [35]. They found that overexpression of MCL-1 leads to apoptosis resistance associated with disease progression and shorter OS [35]. MCL-1 is located in the 1q21 amplicon. Notably, patients carrying 1q21 amp have an increase in MCL-1 expression which correlates with a poor OS [53]. MCL-1 overexpression is frequently observed and appears to be a key factor in resistance. Therefore, targeting MCL-1 may be an effective approach to treat this group of MM patients that do not benefit from current therapies. The BCL2 inhibitor venetoclax has been approved to treat MM patients in 2016 [54,55,56]. Studies showed that therapy combining bortezomib and dexamethasone obtained a better response only in RRMM patients with high expression levels of BCL2 [55].
Targeting MCL-1 itself will be a more promising therapeutic approach for MM patients carrying 1q21 amp. MCL-1 inhibitor S63845 has shown promising results for MM patients with 1q21 amp [57]. A study conducted by Slomp et al. showed that primary BM MM cells from patients carrying 1q21 amp were significantly more sensitive to treatment with MCL-1 inhibitor S63845 than MM cells from patients without this chromosomal aberration, suggesting that S63845 may be more effective in MM patients with 1q21 amp [53]. The MCL-1 inhibitor S64315/MIK665 is currently being tested for MM in phase I trial. Overall, the identification of MCL-1 inhibitors will offer a great advance for the generation of effective treatment that could benefit MM patients with 1q21 amp.
3.2. CKS1B
The CDC28 protein kinase regulatory subunit 1B (CKS1B) belongs to the cyclin kinase subunit 1 protein family. It is known to play a pivotal role in cell proliferation [39]. CKS1B was identified as a protein component of the SCFSKP2-CKS1 ubiquitin ligase complex [39,58]. In this complex, CKS1B enhances the interaction between p27Kip1 (a CDK inhibitor) and SKP2, leading to cell proliferation [59]. The amplification of CKS1B results in the regulation of the p27Kip1 proteasome degradation in the cell cycle, and increased mitotic activity [40]. CKS1B is one of the most commonly overexpressed genes in MM located at the 1q21.3 region [39]. Studies by Shaughnessy et al. showed that upregulation of CKS1B is positively associated with an increased copy number in the 1q21 region [17]. In MM patients overexpression of CKS1B results in a shorter PFS and decreased OS [17,60]. In addition, CKS1B expression is strongly correlated with increased copy number in bortezomib RRMM patients [61]. Evidence suggests that MM cells with more than four copies of 1q21 are potentially associated with a more adverse phenotype and drug resistance than those with three copies. Studies by Shi et al. demonstrated that CKS1B mediates MM cell proliferation and chemoresistance through the activation of STAT3 and MEK/ERK/BCL2 signaling pathways [62]. The results from this study show that upregulation of CKS1B triggers STAT3 and MEK/ERK signaling pathways, showing that these pathways are the downstream signaling pathways of CKS1B [62]. CKS1B overexpression is frequently observed in patients with 1q21 amp conferring resistance to MM chemotherapeutic agents. Considerable evidence indicates that targeting CKS1B is beneficial for the treatment of patients with 1q21 amp. Recent studies have provided preliminary insights about the different ways to target this novel gene. Huang et al. investigated the effect of MLN4924, a novel ubiquitin-like inhibitor involved in the process of ubiquitin-like modification [63]. MLN4924 selectively inhibits NAE and prevents the formation of the SCFSkp2 complex. The results from this study show MM cells overexpressing CKS1B are more sensitive to MLN4924 treatment. Treatment of CSK1B overexpressing MM cells with MLN4924 results in a decrease in cell proliferation and the induction of cellular senescence [63].
In another study, Malek et al. identified DT204, a novel compound able to inhibit degradation of p27 mediated by SCFSkp2, preventing Skp2 incorporation into the SCFSkp2 complex [64]. DT204 has been shown to reduce myeloma viability by inducing cell cycle arrest and enhancing the anti-myeloma effect of BTZ in MM cell lines and patient samples [64]. Recently, using the COMPARE algorithm to identify the correlation between CKS1B gene expression and drug activity, Tian et al. identified 9-dimethyl amino-ethoxy ellipticine (EPED3), a highly stable compound derivative of the plant alkaloid ellipticine [65]. Studies using MM cells showed that treatment with EPED3 induced cell death. Similar results were obtained using cells with acquired resistance to anti-myeloma drugs [65]. Therapeutic strategies targeting CKS1B may hold promise for the treatment of MM patients with 1q21 amp. Targeting CKS1B will allow the design of better therapeutic strategies to overcome adverse prognosis and drug resistance observed in patients with 1q21 amplification. However, further clarification on the mechanism underlying CKS1B function and its regulation will provide additional insights on the best way to target this gene.
3.3. ILF2
The interleukin enhancer binding factor 2 (ILF2) encodes nuclear factor (NF) 45, the regulatory subunit of NF90/NF110 complexes that have been reported to play essential roles in mRNA stability, post-transcriptional regulation, translation, and in mitotic control [42,43]. The potential role of ILF2 as a therapeutic target in 1q21 region has been demonstrated by Marchesini et al. [36]. The authors showed that ILF2 is a key modulator of the homologous recombination (HR) DNA repair pathway in myeloma cells [36]. Overexpression of ILF2 promotes tolerance of genomic instability, thereby enhancing MM cell survival and drug resistance [32].
At a mechanistic level, Marchesini et al. demonstrated that increased ILF2 expression drives resistance to genotoxic stress agents by regulating the nuclear localization of the Y box-binding protein 1 (YB-1) and its interplay with the splicing factor U2AF65, promoting mRNA splicing of transcripts involved in HD DNA repair [36]. In line with these findings, clinical observations from the same research group also demonstrated that nuclear expression of ILF2 strongly correlates with the expression of YB-1 in MM patients with 1q21 amp [36]. Notably, the work by Marchesini et al. supports the possible clinical use of ILF2 as a potential therapeutic target for 1q21 [36]. Blocking expression of ILF2 in MM patients with 1q21 amp could enhance the efficacy and overcome resistance to DNA-damaging drugs such as melphalan and cyclophosphamide [36].
3.4. IL6R
The interleukin-6 receptor (IL6R) is a critical gene located within the amplified 1q21 region [66]. IL6R has been documented to be a predictor of poor outcome in MM patients [66,67,68,69]. IL6R is part of the ligand-binding domain of IL6, a pleiotropic cytokine required for the terminal differentiation of B cells [70]. IL6 has been shown to be a potent driver for MM disease progression [71]. Elevated levels of IL6 in MM patients are often associated with prevention of drug-induced-apoptosis and disease progression [66,71,72,73]. IL6R is a complex associated with gp130. The association of this complex activates tyrosine phosphorylation of JAK and others downstream pathways such as STAT3 and the RAS/mitogen-activated protein kinase (MAPK) [71,74,75,76,77]. Once phosphorylated by JAK, STAT3 dimerizes and translocates to the nucleus, leading to the transcription of various pro-survival and anti-apoptotic genes, such as MCL-1 and BCL-2 [78,79].
STAT3 was found to be constitutively active in primary MM tumors and cell lines [80]. Activation of IL6/STAT3 pathway was correlated with drug resistance and adverse outcome [80,81,82]. Studies using MM cells have revealed that the STAT3 pathway is constitutively active in around 48% of patients. The over-activation of STAT3 resulted in poor survival and drug resistance [82]. In a study using CRISPR-associated protein-9 knockout screening in MM cell lines, Ogiya et al. identified that the JAK–STAT3 pathway mediates CD38 downregulation [83]. Pharmacological inhibition of the JAK–STAT3 pathway results in STAT3 phosphorylation in MM cell lines, an upregulation of CD38 expression in primary MM patients, and an increase in daratumumab antibody-mediated cellular cytotoxicity against MM cell lines [83]. Several studies have demonstrated that inhibition of the IL6R/STAT3 pathway with anti-IL6, IL6R antagonists, or JAK-inhibitors induced apoptosis of human myeloma cell lines in vitro and enhanced the antitumor activity of other anti-myeloma drugs in vivo, attenuating chemoresistance [84,85,86,87]. However, the outcome in clinical trials using anti IL6 antibodies was less significant, probably due to the lack of understanding of the IL6/STAT3 pathway [3].
Recently, using mRNA from patients’ BM PCs, it was demonstrated that the expression level of IL6R was associated with the 1q21 copy number [88,89]. Teoh et al. reported that amplification of the 1q21 region results in an increased expression of IL6R and ADAR1 (an RNA-editing enzyme); both critical genes are located on the 1q21 region [34]. The concomitant gain of IL6R and ADAR1 resulted in MM cell proliferation through the hyper-activation of the STAT3 pathway, conferring hypersensitivity of IL6, and as a consequence, driving the over-activation of the STAT3 pathway in cells with 1q21 amp [34]. These results explain why targeting IL6 may not be sufficient for these patients. Targeting IL6R along with other components of the complex, such as STAT3, might be more beneficial. However, further studies focusing on the understanding of the mechanism of action of the IL6R, ADAR1, and STAT3 pathways will provide better insights for the development of effective treatments for high-risk patients with 1q21 amp.
3.5. BCL9
The B cell lymphoma 9 (BCL9) gene also resides on chromosome 1q21. BCL9 is one of the nuclear Wnt pathway components and plays an important role in the transcriptional activity of β-catenin [44]. The dysregulation of the canonical Wnt/β-catenin pathway and the accumulation of nuclear β-catenin have been implicated in numerous human malignancies, including MM [45]. Gene-expression analysis revealed that BCL9 is overexpressed in approximately 60% of late-stage MM patients and in MM cell lines [90]. Mani et al. showed that BCL9 knockdown in MM cell lines reduced cell proliferation and colony-forming activity, probably due to the decreased cell cycle progression or increased apoptosis, whereas overexpression of BCL9 in MM1S cells significantly increased the colony-forming ability. Therefore, studies in NOD/SCID mice transplanted with MM1S BCL9 shRNA cells showed an increased in OS, fewer GFP tumor nodules, and smaller foci of cells infiltrating the bone marrow [45]. Takada et al. investigated the pharmacologic blockade of the β-catenin–BCL9 complex by the use of a stabilized alpha-helix of BCL9 (SAH-BCL9) [91]. He showed that SAH-BCL9 was able to target β-catenin, dissociates native BCL-9/β-catenin complexes, selectively suppresses Wnt transcription, and exhibits mechanism-based antitumor effects [91]. These data indicate that targeting BCL9 may reduce tumor formation and resistance to therapy, resulting as a consequence of BCL9 upregulation, emphasizing the importance of BCL9 as a target for the 1q21 region.
MicroRNAs (miRNA) are able to regulate RNA by modulating the expression of a particular gene. Several studies have identified miRNAs to play a role during MM development [92,93,94,95]. Recently, Zhao et al. identified a novel therapeutic tool to target BCL9 using miRNA. The study showed that miR-30 was able to regulate BCL9 expression in vitro [96]. Upregulation of miR-30s in MM cell lines leads to a decrease in cell proliferation and survival, in addition to the downregulation of BCL9 and Wnt transcriptional activity [96]. These findings demonstrated the potential use of BCL9 as a novel therapeutic gene to target the 1q21 region in MM.
4. Possible 1q21 Targets Genes Outside of Chromosome 1q
The amplification of 1q21 can drive the dysregulation of several genes outside of chromosome 1q. The dysregulation of these genes can result as a direct or indirect consequence of 1q21 amplification. Several studies have demonstrated that overexpression of CKS1B, which results from the amplification of the 1q21 region, leads to low expression levels and incorrect degradation of p27Kip1, which regulates Cdk2-E activity and the late stage of G1/S transition of the cell cycle [17,97,98]. Lower levels of p27Kip1 have been associated with poor prognosis in MM [99]. Protein and mRNA purified from BM PCs of 351 NDMM patients showed an inverse correlation between CKS1B and p27Kip1 expression levels [17]. Furthermore, knockdown of CKS1B in MM cell lines resulted in MM cell death and stabilization of p27Kip1 [97]. Correlation analysis of 94 NDMM showed that patients with lower expression of p27Kip1 have a shorter OS compared to patients with high-expression p27Kip1 protein [98]. These results show an alternative mechanism that influences MM cell growth and survival in patients with 1q21 amp. Therefore, p27Kip1 may be a novel therapeutic target for the 1q21 region in MM.
The anti-apoptotic proteins BCL2, MCL-1, and BCL-XL (BCL2L1) are expressed in MM cells [100]. Increased expression, particularly of MCL-1 and BCL-XL, is associated with worse patient outcome. Recently, Slomp et al. found that MCL-1 was significantly upregulated in patients with 1q21 amplification and the MCL-1/BCL2, MCL1/BCL-XL ratios were higher in patients with 1q21 amp when compared with control patients [53]. In addition, amplified PCs from 1q21 showed an increase in sensitivity when treated with the combination of MCL-1 plus BCL2 inhibitors and an increase in cell death after treatment with MCL-1 plus BCL-XL inhibitors [53]. These results suggest that combination therapies with possible target genes outside of the 1q region could greatly benefit patients with 1q21 amplification. A more recent study identified that the expression levels of EPB41L4A (erythrocyte protein band 4.1-like 4a), a target gene for the Wnt/β-catenin pathway, were downregulated in a copy number manner in patients with 1q21 amp. Correlation analysis of over 500 MM expression profiles showed that patients with relapsed MM had lower expression of EPB41L4A than patients without recurrence disease [101]. At the moment, there are no other studies investigating the direct or indirect role of 1q21 amplification on the downregulation of EPB41L4A. More research investigating the role and the biological implications of EPB41L4A in patients with 1q21 amp will provide a better understanding of this possible target gene in MM.
5. Conclusions
Despite advances in MM therapy in recent years, MM remains an incurable disease with a highly heterogeneous genetic background [102,103]. Cytogenetic abnormalities are one of the most important prognostic factors for NDMM patients. Amplification of 1q21 is one of the most acquired genetic lesions associated with high risk and adverse prognostic factors. The incidence of 1q21 copy number is correlated with clinical outcome. MM patients harboring more than four copies of 1q21, in particular RRMM patients, have more adverse clinical outcomes than those with three 1q21 copies [103,104,105] The amplification of the 1q21 region can occur as isochromosomes, duplications, or jumping translocations, leading to the chromosomal instability of MM cells which may account for the genetic complexity and heterogeneity that characterize the patients carrying multiple copies of 1q21 [21,22,23]. Along with the amplification of the 1q region, increased expression and/or deregulation of several known MM related genes are shown to contribute to the disease pathogenesis. Many studies have suggested that amplification of the 1q21 region could be an independent detrimental prognostic factor in MM patients; however, amplification of 1q21 has not been uniformly accepted as part of the MM ISS high-risk stratification system. As part of the ongoing effort to further improve treatment outcomes of patients with 1q21 gain/amplification, the European Myeloma Network in collaboration with the HARMONY project is trying to revise the R-ISS risk stratification model to include 1q21 copy number alterations as part of the general prognosis of NDMM patients [106]. Therefore, it is necessary to understand the molecular mechanism of the genes upregulated at the 1q21 region in order to allow the development of individualized patient treatment. The relevant driver genes present in the 1q21 region have not been fully explored. Among these genes, candidate oncogenes such as IL6R, MCL-1, BCL9, CKS1B and ILF2 deserve particular interest because each of these genes has been proposed as a candidate participant in myelomagenesis [34,36,46,63]. The identification of potential therapeutic targets, along with the proper risk stratification for patients with 1q21 amp, will not only provide the rationale for individualized therapies according to clinical risk, but also will also provide effective first-line treatments with a durable response for 1q21 MM patients who do not benefit from current therapies.
Acknowledgments
We would like to thank the Associazione Italiana contro Leucemie, Linfomi e Mielomi ONLUS, ParmAIL for the support.
Author Contributions
J.B.G. and R.A.E. wrote the manuscript; J.B.G., R.A.E. and P.S. designed the figures; N.G., G.S., G.T., L.C., D.T. and V.M. reviewed the manuscript. All the authors approved the final version of manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from Ricerca Finalizzata del Ministero della Salute Italiana PE-2016-02361261.
Conflicts of Interest
N.G. received research funding and honoraria from Amgen, Bristol Mayers Squibb, Celgene, Millenium Pharmaceutical, and Janssen Pharmaceutical. The other authors declare no competing financial interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cavo M., Tacchetti P., Patriarca F., Petrucci M.T., Pantani L., Galli M., Di Raimondo F., Crippa C., Zamagni E., Palumbo A., et al. Bortezomib with thalidomide plus dexamethasone compared with thalidomide plus dexamethasone as induction therapy before, and consolidation therapy after, double autologous stem-cell transplantation in newly diagnosed multiple myeloma: A randomised phase 3 study. Lancet. 2010;376:2075–2085. doi: 10.1016/S0140-6736(10)61424-9. [DOI] [PubMed] [Google Scholar]
- 2.Chng W.J., Dispenzieri A., Chim C.S., Fonseca R., Goldschmidt H., Lentzsch S., Munshi N., Palumbo A., Miguel J.S., Sonneveld P., et al. IMWG consensus on risk stratification in multiple myeloma. Leukemia. 2014;28:269–277. doi: 10.1038/leu.2013.247. [DOI] [PubMed] [Google Scholar]
- 3.Hillengass J., Usmani S., Rajkumar S.V., Durie B.G.M., Mateos M.V., Lonial S., Joao C., Anderson K.C., Garcia-Sanz R., Riva E., et al. International myeloma working group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol. 2019;20:e302–e312. doi: 10.1016/S1470-2045(19)30309-2. [DOI] [PubMed] [Google Scholar]
- 4.Avet-Loiseau H., Attal M., Campion L., Caillot D., Hulin C., Marit G., Stoppa A.M., Voillat L., Wetterwald M., Pegourie B., et al. Long-term analysis of the IFM 99 trials for myeloma: Cytogenetic abnormalities [t(4;14), del(17p), 1q gains] play a major role in defining long-term survival. J. Clin. Oncol. 2012;30:1949–1952. doi: 10.1200/JCO.2011.36.5726. [DOI] [PubMed] [Google Scholar]
- 5.Perrot A., Lauwers-Cances V., Tournay E., Hulin C., Chretien M.L., Royer B., Dib M., Decaux O., Jaccard A., Belhadj K., et al. Development and Validation of a Cytogenetic Prognostic Index Predicting Survival in Multiple Myeloma. J. Clin. Oncol. 2019;37:1657–1665. doi: 10.1200/JCO.18.00776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pawlyn C., Cairns D., Kaiser M., Striha A., Jones J., Shah V., Jenner M., Drayson M., Owen R., Gregory W., et al. The relative importance of factors predicting outcome for myeloma patients at different ages: Results from 3894 patients in the Myeloma XI trial. Leukemia. 2020;34:604–612. doi: 10.1038/s41375-019-0595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avet-Loiseau H., Gerson F., Magrangeas F., Minvielle S., Harousseau J.L., Bataille R., Intergroupe Francophone du M. Rearrangements of the c-myc oncogene are present in 15% of primary human multiple myeloma tumors. Blood. 2001;98:3082–3086. doi: 10.1182/blood.V98.10.3082. [DOI] [PubMed] [Google Scholar]
- 8.Walker B.A., Wardell C.P., Brioli A., Boyle E., Kaiser M.F., Begum D.B., Dahir N.B., Johnson D.C., Ross F.M., Davies F.E., et al. Translocations at 8q24 juxtapose MYC with genes that harbor superenhancers resulting in overexpression and poor prognosis in myeloma patients. Blood Cancer J. 2014;4:e191. doi: 10.1038/bcj.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker B.A., Wardell C.P., Murison A., Boyle E.M., Begum D.B., Dahir N.M., Proszek P.Z., Melchor L., Pawlyn C., Kaiser M.F., et al. APOBEC family mutational signatures are associated with poor prognosis translocations in multiple myeloma. Nat. Commun. 2015;6:6997. doi: 10.1038/ncomms7997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chng W.J., Gonzalez-Paz N., Price-Troska T., Jacobus S., Rajkumar S.V., Oken M.M., Kyle R.A., Henderson K.J., Van Wier S., Greipp P., et al. Clinical and biological significance of RAS mutations in multiple myeloma. Leukemia. 2008;22:2280–2284. doi: 10.1038/leu.2008.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lionetti M., Barbieri M., Manzoni M., Fabris S., Bandini C., Todoerti K., Nozza F., Rossi D., Musto P., Baldini L., et al. Molecular spectrum of TP53 mutations in plasma cell dyscrasias by next generation sequencing: An Italian cohort study and overview of the literature. Oncotarget. 2016;7:21353–21361. doi: 10.18632/oncotarget.7241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weinhold N., Ashby C., Rasche L., Chavan S.S., Stein C., Stephens O.W., Tytarenko R., Bauer M.A., Meissner T., Deshpande S., et al. Clonal selection and double-hit events involving tumor suppressor genes underlie relapse in myeloma. Blood. 2016;128:1735–1744. doi: 10.1182/blood-2016-06-723007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavan S.S., He J., Tytarenko R., Deshpande S., Patel P., Bailey M., Stein C.K., Stephens O., Weinhold N., Petty N., et al. Bi-allelic inactivation is more prevalent at relapse in multiple myeloma, identifying RB1 as an independent prognostic marker. Blood Cancer J. 2017;7:e535. doi: 10.1038/bcj.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neri A., Baldini L., Trecca D., Cro L., Polli E., Maiolo A.T. p53 gene mutations in multiple myeloma are associated with advanced forms of malignancy. Blood. 1993;81:128–135. doi: 10.1182/blood.V81.1.128.128. [DOI] [PubMed] [Google Scholar]
- 15.D’Agostino M., Zaccaria G.M., Ziccheddu B., Rustad E.H., Genuardi E., Capra A., Oliva S., Auclair D., Yesil J., Colucci P., et al. Early Relapse Risk in Patients with Newly Diagnosed Multiple Myeloma Characterized by Next-generation Sequencing. Clin. Cancer Res. 2020;26:4832–4841. doi: 10.1158/1078-0432.CCR-20-0951. [DOI] [PubMed] [Google Scholar]
- 16.Agnelli L., Bicciato S., Fabris S., Baldini L., Morabito F., Intini D., Verdelli D., Callegaro A., Bertoni F., Lambertenghi-Deliliers G., et al. Integrative genomic analysis reveals distinct transcriptional and genetic features associated with chromosome 13 deletion in multiple myeloma. Haematologica. 2007;92:56–65. doi: 10.3324/haematol.10414. [DOI] [PubMed] [Google Scholar]
- 17.Shaughnessy J. Amplification and overexpression of CKS1B at chromosome band 1q21 is associated with reduced levels of p27Kip1 and an aggressive clinical course in multiple myeloma. Hematology. 2005;10(Suppl. 1):117–126. doi: 10.1080/10245330512331390140. [DOI] [PubMed] [Google Scholar]
- 18.Hanamura I., Stewart J.P., Huang Y., Zhan F., Santra M., Sawyer J.R., Hollmig K., Zangarri M., Pineda-Roman M., van Rhee F., et al. Frequent gain of chromosome band 1q21 in plasma-cell dyscrasias detected by fluorescence in situ hybridization: Incidence increases from MGUS to relapsed myeloma and is related to prognosis and disease progression following tandem stem-cell transplantation. Blood. 2006;108:1724–1732. doi: 10.1182/blood-2006-03-009910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu K.L., Beverloo B., Lokhorst H.M., Segeren C.M., van der Holt B., Steijaert M.M., Westveer P.H., Poddighe P.J., Verhoef G.E., Sonneveld P., et al. Abnormalities of chromosome 1p/q are highly associated with chromosome 13/13q deletions and are an adverse prognostic factor for the outcome of high-dose chemotherapy in patients with multiple myeloma. Br. J. Haematol. 2007;136:615–623. doi: 10.1111/j.1365-2141.2006.06481.x. [DOI] [PubMed] [Google Scholar]
- 20.Barlogie B., Tricot G., Anaissie E., Shaughnessy J., Rasmussen E., van Rhee F., Fassas A., Zangari M., Hollmig K., Pineda-Roman M., et al. Thalidomide and hematopoietic-cell transplantation for multiple myeloma. N. Engl. J. Med. 2006;354:1021–1030. doi: 10.1056/NEJMoa053583. [DOI] [PubMed] [Google Scholar]
- 21.Sawyer J.R., Tricot G., Lukacs J.L., Binz R.L., Tian E., Barlogie B., Shaughnessy J., Jr. Genomic instability in multiple myeloma: Evidence for jumping segmental duplications of chromosome arm 1q. Genes Chromosomes Cancer. 2005;42:95–106. doi: 10.1002/gcc.20109. [DOI] [PubMed] [Google Scholar]
- 22.Sawyer J.R., Tricot G., Mattox S., Jagannath S., Barlogie B. Jumping translocations of chromosome 1q in multiple myeloma: Evidence for a mechanism involving decondensation of pericentromeric heterochromatin. Blood. 1998;91:1732–1741. doi: 10.1182/blood.V91.5.1732. [DOI] [PubMed] [Google Scholar]
- 23.Sawyer J.R., Tian E., Heuck C.J., Epstein J., Johann D.J., Swanson C.M., Lukacs J.L., Johnson M., Binz R., Boast A., et al. Jumping translocations of 1q12 in multiple myeloma: A novel mechanism for deletion of 17p in cytogenetically defined high-risk disease. Blood. 2014;123:2504–2512. doi: 10.1182/blood-2013-12-546077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sawyer J.R., Tian E., Thomas E., Koller M., Stangeby C., Sammartino G., Goosen L., Swanson C., Binz R.L., Barlogie B., et al. Evidence for a novel mechanism for gene amplification in multiple myeloma: 1q12 pericentromeric heterochromatin mediates breakage-fusion-bridge cycles of a 1q12 approximately 23 amplicon. Br. J. Haematol. 2009;147:484–494. doi: 10.1111/j.1365-2141.2009.07869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.An G., Li Z., Tai Y.T., Acharya C., Li Q., Qin X., Yi S., Xu Y., Feng X., Li C., et al. The impact of clone size on the prognostic value of chromosome aberrations by fluorescence in situ hybridization in multiple myeloma. Clin. Cancer Res. 2015;21:2148–2156. doi: 10.1158/1078-0432.CCR-14-2576. [DOI] [PubMed] [Google Scholar]
- 26.Merz M., Hielscher T., Hoffmann K., Seckinger A., Hose D., Raab M.S., Hillengass J., Jauch A., Goldschmidt H. Cytogenetic abnormalities in monoclonal gammopathy of undetermined significance. Leukemia. 2018;32:2717–2719. doi: 10.1038/s41375-018-0202-1. [DOI] [PubMed] [Google Scholar]
- 27.Cavo M., Pantani L., Petrucci M.T., Patriarca F., Zamagni E., Donnarumma D., Crippa C., Boccadoro M., Perrone G., Falcone A., et al. Bortezomib-thalidomide-dexamethasone is superior to thalidomide-dexamethasone as consolidation therapy after autologous hematopoietic stem cell transplantation in patients with newly diagnosed multiple myeloma. Blood. 2012;120:9–19. doi: 10.1182/blood-2012-02-408898. [DOI] [PubMed] [Google Scholar]
- 28.Shaughnessy J.D., Jr., Qu P., Usmani S., Heuck C.J., Zhang Q., Zhou Y., Tian E., Hanamura I., van Rhee F., Anaissie E., et al. Pharmacogenomics of bortezomib test-dosing identifies hyperexpression of proteasome genes, especially PSMD4, as novel high-risk feature in myeloma treated with Total Therapy 3. Blood. 2011;118:3512–3524. doi: 10.1182/blood-2010-12-328252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmidt T.M., Barwick B.G., Joseph N., Heffner L.T., Hofmeister C.C., Bernal L., Dhodapkar M.V., Gupta V.A., Jaye D.L., Wu J., et al. Gain of Chromosome 1q is associated with early progression in multiple myeloma patients treated with lenalidomide, bortezomib, and dexamethasone. Blood Cancer J. 2019;9:94. doi: 10.1038/s41408-019-0254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.D’Agostino M., Ruggeri M., Aquino S., Giuliani N., Arigoni M., Gentile M., Olivero M., Vincelli I.D., Capra A., Mussatto C. Impact of Gain and Amplification of 1q in Newly Diagnosed Multiple Myeloma Patients Receiving Carfilzomib Based Treatment in the Forte Trial. Blood. 2020;136:38–40. doi: 10.1182/blood-2020-137060. [DOI] [Google Scholar]
- 31.Abdallah N., Greipp P., Kapoor P., Gertz M.A., Dispenzieri A., Baughn L.B., Lacy M.Q., Hayman S.R., Buadi F.K., Dingli D., et al. Clinical characteristics and treatment outcomes of newly diagnosed multiple myeloma with chromosome 1q abnormalities. Blood Adv. 2020;4:3509–3519. doi: 10.1182/bloodadvances.2020002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker B.A., Mavrommatis K., Wardell C.P., Ashby T.C., Bauer M., Davies F., Rosenthal A., Wang H., Qu P., Hoering A., et al. A high-risk, Double-Hit, group of newly diagnosed myeloma identified by genomic analysis. Leukemia. 2019;33:159–170. doi: 10.1038/s41375-018-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin Y., Yu X., Du J., Li H., Tang W., Jia C., Zan Y., Chen M., Zhang Y., Yu M., et al. The combination of C-Myc rearrangement and 1q21 gain is associated with poor prognosis in multiple myeloma. Ann. Hematol. 2021;100:1251–1260. doi: 10.1007/s00277-021-04475-2. [DOI] [PubMed] [Google Scholar]
- 34.Teoh P.J., Chung T.H., Chng P.Y.Z., Toh S.H.M., Chng W.J. IL6R-STAT3-ADAR1 (P150) interplay promotes oncogenicity in multiple myeloma with 1q21 amplification. Haematologica. 2020;105:1391–1404. doi: 10.3324/haematol.2019.221176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wuilleme-Toumi S., Robillard N., Gomez P., Moreau P., Le Gouill S., Avet-Loiseau H., Harousseau J.L., Amiot M., Bataille R. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia. 2005;19:1248–1252. doi: 10.1038/sj.leu.2403784. [DOI] [PubMed] [Google Scholar]
- 36.Marchesini M., Ogoti Y., Fiorini E., Aktas Samur A., Nezi L., D’Anca M., Storti P., Samur M.K., Ganan-Gomez I., Fulciniti M.T., et al. ILF2 Is a Regulator of RNA Splicing and DNA Damage Response in 1q21-Amplified Multiple Myeloma. Cancer Cell. 2017;32:88–100. doi: 10.1016/j.ccell.2017.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fonseca R., Van Wier S.A., Chng W.J., Ketterling R., Lacy M.Q., Dispenzieri A., Bergsagel P.L., Rajkumar S.V., Greipp P.R., Litzow M.R., et al. Prognostic value of chromosome 1q21 gain by fluorescent in situ hybridization and increase CKS1B expression in myeloma. Leukemia. 2006;20:2034–2040. doi: 10.1038/sj.leu.2404403. [DOI] [PubMed] [Google Scholar]
- 38.Le Gouill S., Podar K., Harousseau J.L., Anderson K.C. Mcl-1 regulation and its role in multiple myeloma. Cell Cycle. 2004;3:1259–1262. doi: 10.4161/cc.3.10.1196. [DOI] [PubMed] [Google Scholar]
- 39.Ganoth D., Bornstein G., Ko T.K., Larsen B., Tyers M., Pagano M., Hershko A. The cell-cycle regulatory protein Cks1 is required for SCF(Skp2)-mediated ubiquitinylation of p27. Nat. Cell Biol. 2001;3:321–324. doi: 10.1038/35060126. [DOI] [PubMed] [Google Scholar]
- 40.Spruck C., Strohmaier H., Watson M., Smith A.P., Ryan A., Krek T.W., Reed S.I. A CDK-independent function of mammalian Cks1: Targeting of SCF(Skp2) to the CDK inhibitor p27Kip1. Mol. Cell. 2001;7:639–650. doi: 10.1016/S1097-2765(01)00210-6. [DOI] [PubMed] [Google Scholar]
- 41.Jones S.A., Horiuchi S., Topley N., Yamamoto N., Fuller G.M. The soluble interleukin 6 receptor: Mechanisms of production and implications in disease. FASEB J. 2001;15:43–58. doi: 10.1096/fj.99-1003rev. [DOI] [PubMed] [Google Scholar]
- 42.Nourreddine S., Lavoie G., Paradis J., Ben El Kadhi K., Meant A., Aubert L., Grondin B., Gendron P., Chabot B., Bouvier M., et al. NF45 and NF90 Regulate Mitotic Gene Expression by Competing with Staufen-Mediated mRNA Decay. Cell Rep. 2020;31:107660. doi: 10.1016/j.celrep.2020.107660. [DOI] [PubMed] [Google Scholar]
- 43.Guan D., Altan-Bonnet N., Parrott A.M., Arrigo C.J., Li Q., Khaleduzzaman M., Li H., Lee C.G., Pe’ery T., Mathews M.B. Nuclear factor 45 (NF45) is a regulatory subunit of complexes with NF90/110 involved in mitotic control. Mol. Cell. Biol. 2008;28:4629–4641. doi: 10.1128/MCB.00120-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sustmann C., Flach H., Ebert H., Eastman Q., Grosschedl R. Cell-type-specific function of BCL9 involves a transcriptional activation domain that synergizes with beta-catenin. Mol. Cell. Biol. 2008;28:3526–3537. doi: 10.1128/MCB.01986-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mani M., Carrasco D.E., Zhang Y., Takada K., Gatt M.E., Dutta-Simmons J., Ikeda H., Diaz-Griffero F., Pena-Cruz V., Bertagnolli M., et al. BCL9 promotes tumor progression by conferring enhanced proliferative, metastatic, and angiogenic properties to cancer cells. Cancer Res. 2009;69:7577–7586. doi: 10.1158/0008-5472.CAN-09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krajewski S., Bodrug S., Krajewska M., Shabaik A., Gascoyne R., Berean K., Reed J.C. Immunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1 in control of programmed cell death in vivo. Am. J. Pathol. 1995;146:1309–1319. [PMC free article] [PubMed] [Google Scholar]
- 47.Zhuang L., Lee C.S., Scolyer R.A., McCarthy S.W., Zhang X.D., Thompson J.F., Hersey P. Mcl-1, Bcl-XL and Stat3 expression are associated with progression of melanoma whereas Bcl-2, AP-2 and MITF levels decrease during progression of melanoma. Modern Pathol. 2007;20:416–426. doi: 10.1038/modpathol.3800750. [DOI] [PubMed] [Google Scholar]
- 48.Itoh T., Itoh A., Pleasure D. Bcl-2-related protein family gene expression during oligodendroglial differentiation. J. Neurochem. 2003;85:1500–1512. doi: 10.1046/j.1471-4159.2003.01795.x. [DOI] [PubMed] [Google Scholar]
- 49.Belmar J., Fesik S.W. Small molecule Mcl-1 inhibitors for the treatment of cancer. Pharmacol. Ther. 2015;145:76–84. doi: 10.1016/j.pharmthera.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jourdan M., De Vos J., Mechti N., Klein B. Regulation of Bcl-2-family proteins in myeloma cells by three myeloma survival factors: Interleukin-6, interferon-alpha and insulin-like growth factor 1. Cell Death Differ. 2000;7:1244–1252. doi: 10.1038/sj.cdd.4400758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Derenne S., Monia B., Dean N.M., Taylor J.K., Rapp M.J., Harousseau J.L., Bataille R., Amiot M. Antisense strategy shows that Mcl-1 rather than Bcl-2 or Bcl-x(L) is an essential survival protein of human myeloma cells. Blood. 2002;100:194–199. doi: 10.1182/blood.V100.1.194. [DOI] [PubMed] [Google Scholar]
- 52.Peperzak V., Vikstrom I., Walker J., Glaser S.P., LePage M., Coquery C.M., Erickson L.D., Fairfax K., Mackay F., Strasser A., et al. Mcl-1 is essential for the survival of plasma cells. Nat. Immunol. 2013;14:290–297. doi: 10.1038/ni.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Slomp A., Moesbergen L.M., Gong J.N., Cuenca M., von dem Borne P.A., Sonneveld P., Huang D.C.S., Minnema M.C., Peperzak V. Multiple myeloma with 1q21 amplification is highly sensitive to MCL-1 targeting. Blood Adv. 2019;3:4202–4214. doi: 10.1182/bloodadvances.2019000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar S., Kaufman J.L., Gasparetto C., Mikhael J., Vij R., Pegourie B., Benboubker L., Facon T., Amiot M., Moreau P., et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017;130:2401–2409. doi: 10.1182/blood-2017-06-788786. [DOI] [PubMed] [Google Scholar]
- 55.Moreau P., Chanan-Khan A., Roberts A.W., Agarwal A.B., Facon T., Kumar S., Touzeau C., Punnoose E.A., Cordero J., Munasinghe W., et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017;130:2392–2400. doi: 10.1182/blood-2017-06-788323. [DOI] [PubMed] [Google Scholar]
- 56.Zhu H., Almasan A. Development of venetoclax for therapy of lymphoid malignancies. Drug Des. Dev. Ther. 2017;11:685–694. doi: 10.2147/DDDT.S109325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kotschy A., Szlavik Z., Murray J., Davidson J., Maragno A.L., Le Toumelin-Braizat G., Chanrion M., Kelly G.L., Gong J.N., Moujalled D.M., et al. The MCL1 inhibitor S63845 is tolerable and effective in diverse cancer models. Nature. 2016;538:477–482. doi: 10.1038/nature19830. [DOI] [PubMed] [Google Scholar]
- 58.Harper J.W. Protein destruction: Adapting roles for Cks proteins. Curr. Biol. 2001;11:R431–R435. doi: 10.1016/S0960-9822(01)00253-6. [DOI] [PubMed] [Google Scholar]
- 59.Cardozo T., Pagano M. The SCF ubiquitin ligase: Insights into a molecular machine. Nat. Rev. Mol. Cell Biol. 2004;5:739–751. doi: 10.1038/nrm1471. [DOI] [PubMed] [Google Scholar]
- 60.Shaughnessy J.D., Jr., Barlogie B. Using genomics to identify high-risk myeloma after autologous stem cell transplantation. Biol. Blood Marrow Transplant. 2006;12:77–80. doi: 10.1016/j.bbmt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 61.Chen M.H., Qi C., Reece D., Chang H. Cyclin kinase subunit 1B nuclear expression predicts an adverse outcome for patients with relapsed/refractory multiple myeloma treated with bortezomib. Hum. Pathol. 2012;43:858–864. doi: 10.1016/j.humpath.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 62.Shi L., Wang S., Zangari M., Xu H., Cao T.M., Xu C., Wu Y., Xiao F., Liu Y., Yang Y., et al. Over-expression of CKS1B activates both MEK/ERK and JAK/STAT3 signaling pathways and promotes myeloma cell drug-resistance. Oncotarget. 2010;1:22–33. doi: 10.18632/oncotarget.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang J., Zhou Y., Thomas G.S., Gu Z., Yang Y., Xu H., Tricot G., Zhan F. NEDD8 Inhibition Overcomes CKS1B-Induced Drug Resistance by Upregulation of p21 in Multiple Myeloma. Clin. Cancer Res. 2015;21:5532–5542. doi: 10.1158/1078-0432.CCR-15-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Malek E., Abdel-Malek M.A., Jagannathan S., Vad N., Karns R., Jegga A.G., Broyl A., van Duin M., Sonneveld P., Cottini F., et al. Pharmacogenomics and chemical library screens reveal a novel SCF(SKP2) inhibitor that overcomes Bortezomib resistance in multiple myeloma. Leukemia. 2017;31:645–653. doi: 10.1038/leu.2016.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tian E., Landowski T.H., Stephens O.W., Yaccoby S., Barlogie B., Shaughnessy J.D., Jr. Ellipticine derivative NSC 338258 represents a potential new antineoplastic agent for the treatment of multiple myeloma. Mol. Cancer Ther. 2008;7:500–509. doi: 10.1158/1535-7163.MCT-07-0524. [DOI] [PubMed] [Google Scholar]
- 66.Barille S., Bataille R., Amiot M. The role of interleukin-6 and interleukin-6/interleukin-6 receptor-alpha complex in the pathogenesis of multiple myeloma. Eur. Cytokine Netw. 2000;11:546–551. [PubMed] [Google Scholar]
- 67.Pulkki K., Pelliniemi T.T., Rajamaki A., Tienhaara A., Laakso M., Lahtinen R. Soluble interleukin-6 receptor as a prognostic factor in multiple myeloma. Finnish Leukaemia Group. Br. J. Haematol. 1996;92:370–374. doi: 10.1046/j.1365-2141.1996.d01-1470.x. [DOI] [PubMed] [Google Scholar]
- 68.Ohtani K., Ninomiya H., Hasegawa Y., Kobayashi T., Kojima H., Nagasawa T., Abe T. Clinical significance of elevated soluble interleukin-6 receptor levels in the sera of patients with plasma cell dyscrasias. Br. J. Haematol. 1995;91:116–120. doi: 10.1111/j.1365-2141.1995.tb05255.x. [DOI] [PubMed] [Google Scholar]
- 69.Kim S.Y., Min H.J., Park H.K., Oh B., Kim T.Y., She C.J., Hwang S.M., Kim M., Kim H.K., Kim I., et al. Increased copy number of the interleukin-6 receptor gene is associated with adverse survival in multiple myeloma patients treated with autologous stem cell transplantation. Biol. Blood Marrow Transplant. 2011;17:810–820. doi: 10.1016/j.bbmt.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 70.Klein B. Cytokine, cytokine receptors, transduction signals, and oncogenes in human multiple myeloma. Semin. Hematol. 1995;32:4–19. [PubMed] [Google Scholar]
- 71.Gado K., Domjan G., Hegyesi H., Falus A. Role of INTERLEUKIN-6 in the pathogenesis of multiple myeloma. Cell Biol. Int. 2000;24:195–209. doi: 10.1006/cbir.2000.0497. [DOI] [PubMed] [Google Scholar]
- 72.Matthes T., Manfroi B., Huard B. Revisiting IL-6 antagonism in multiple myeloma. Crit. Rev. Oncol. Hematol. 2016;105:1–4. doi: 10.1016/j.critrevonc.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 73.Bataille R., Jourdan M., Zhang X.G., Klein B. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J. Clin. Investig. 1989;84:2008–2011. doi: 10.1172/JCI114392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Juge-Morineau N., Francois S., Puthier D., Godard A., Bataille R., Amiot M. The gp 130 family cytokines IL-6, LIF and OSM but not IL-11 can reverse the anti-proliferative effect of dexamethasone on human myeloma cells. Br. J. Haematol. 1995;90:707–710. doi: 10.1111/j.1365-2141.1995.tb05605.x. [DOI] [PubMed] [Google Scholar]
- 75.Urashima M., Teoh G., Chauhan D., Hoshi Y., Ogata A., Treon S.P., Schlossman R.L., Anderson K.C. Interleukin-6 overcomes p21WAF1 upregulation and G1 growth arrest induced by dexamethasone and interferon-gamma in multiple myeloma cells. Blood. 1997;90:279–289. doi: 10.1182/blood.V90.1.279.279_279_289. [DOI] [PubMed] [Google Scholar]
- 76.Chauhan D., Pandey P., Hideshima T., Treon S., Raje N., Davies F.E., Shima Y., Tai Y.T., Rosen S., Avraham S., et al. SHP2 mediates the protective effect of interleukin-6 against dexamethasone-induced apoptosis in multiple myeloma cells. J. Biol. Chem. 2000;275:27845–27850. doi: 10.1074/jbc.M003428200. [DOI] [PubMed] [Google Scholar]
- 77.Song Z., Ren D., Xu X., Wang Y. Molecular cross-talk of IL-6 in tumors and new progress in combined therapy. Thorac. Cancer. 2018;9:669–675. doi: 10.1111/1759-7714.12633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xiong A., Yang Z., Shen Y., Zhou J., Shen Q. Transcription Factor STAT3 as a Novel Molecular Target for Cancer Prevention. Cancers. 2014;6:926–957. doi: 10.3390/cancers6020926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu H., Lee H., Herrmann A., Buettner R., Jove R. Revisiting STAT3 signalling in cancer: New and unexpected biological functions. Nat. Rev. Cancer. 2014;14:736–746. doi: 10.1038/nrc3818. [DOI] [PubMed] [Google Scholar]
- 80.Catlett-Falcone R., Landowski T.H., Oshiro M.M., Turkson J., Levitzki A., Savino R., Ciliberto G., Moscinski L., Fernandez-Luna J.L., Nunez G., et al. Constitutive activation of Stat3 signaling confers resistance to apoptosis in human U266 myeloma cells. Immunity. 1999;10:105–115. doi: 10.1016/S1074-7613(00)80011-4. [DOI] [PubMed] [Google Scholar]
- 81.Jung Y.Y., Lee J.H., Nam D., Narula A.S., Namjoshi O.A., Blough B.E., Um J.Y., Sethi G., Ahn K.S. Anti-myeloma Effects of Icariin Are Mediated Through the Attenuation of JAK/STAT3-Dependent Signaling Cascade. Front. Pharmacol. 2018;9:531. doi: 10.3389/fphar.2018.00531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Quintanilla-Martinez L., Kremer M., Specht K., Calzada-Wack J., Nathrath M., Schaich R., Hofler H., Fend F. Analysis of signal transducer and activator of transcription 3 (Stat 3) pathway in multiple myeloma: Stat 3 activation and cyclin D1 dysregulation are mutually exclusive events. Am. J. Pathol. 2003;162:1449–1461. doi: 10.1016/S0002-9440(10)64278-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ogiya D., Liu J., Ohguchi H., Kurata K., Samur M.K., Tai Y.T., Adamia S., Ando K., Hideshima T., Anderson K.C. The JAK-STAT pathway regulates CD38 on myeloma cells in the bone marrow microenvironment: Therapeutic implications. Blood. 2020;136:2334–2345. doi: 10.1182/blood.2019004332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Demartis A., Bernassola F., Savino R., Melino G., Ciliberto G. Interleukin 6 receptor superantagonists are potent inducers of human multiple myeloma cell death. Cancer Res. 1996;56:4213–4218. [PubMed] [Google Scholar]
- 85.De Vos J., Jourdan M., Tarte K., Jasmin C., Klein B. JAK2 tyrosine kinase inhibitor tyrphostin AG490 downregulates the mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription (STAT) pathways and induces apoptosis in myeloma cells. Br. J. Haematol. 2000;109:823–828. doi: 10.1046/j.1365-2141.2000.02127.x. [DOI] [PubMed] [Google Scholar]
- 86.Lin L., Benson D.M., Jr., DeAngelis S., Bakan C.E., Li P.K., Li C., Lin J. A small molecule, LLL12 inhibits constitutive STAT3 and IL-6-induced STAT3 signaling and exhibits potent growth suppressive activity in human multiple myeloma cells. Int. J. Cancer. 2012;130:1459–1469. doi: 10.1002/ijc.26152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scuto A., Krejci P., Popplewell L., Wu J., Wang Y., Kujawski M., Kowolik C., Xin H., Chen L., Wang Y., et al. The novel JAK inhibitor AZD1480 blocks STAT3 and FGFR3 signaling, resulting in suppression of human myeloma cell growth and survival. Leukemia. 2011;25:538–550. doi: 10.1038/leu.2010.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lazzari E., Mondala P.K., Santos N.D., Miller A.C., Pineda G., Jiang Q., Leu H., Ali S.A., Ganesan A.P., Wu C.N., et al. Alu-dependent RNA editing of GLI1 promotes malignant regeneration in multiple myeloma. Nat. Commun. 2017;8:1922. doi: 10.1038/s41467-017-01890-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Walker B.A., Leone P.E., Chiecchio L., Dickens N.J., Jenner M.W., Boyd K.D., Johnson D.C., Gonzalez D., Dagrada G.P., Protheroe R.K., et al. A compendium of myeloma-associated chromosomal copy number abnormalities and their prognostic value. Blood. 2010;116:e56–e65. doi: 10.1182/blood-2010-04-279596. [DOI] [PubMed] [Google Scholar]
- 90.Van Andel H., Kocemba K.A., Spaargaren M., Pals S.T. Aberrant Wnt signaling in multiple myeloma: Molecular mechanisms and targeting options. Leukemia. 2019;33:1063–1075. doi: 10.1038/s41375-019-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Takada K., Zhu D., Bird G.H., Sukhdeo K., Zhao J.J., Mani M., Lemieux M., Carrasco D.E., Ryan J., Horst D., et al. Targeted disruption of the BCL9/beta-catenin complex inhibits oncogenic Wnt signaling. Sci. Transl. Med. 2012;4:148ra117. doi: 10.1126/scitranslmed.3003808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bartel D.P. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pichiorri F., Suh S.S., Rocci A., De Luca L., Taccioli C., Santhanam R., Zhou W., Benson D.M., Jr., Hofmainster C., Alder H., et al. Downregulation of p53-inducible microRNAs 192, 194, and 215 impairs the p53/MDM2 autoregulatory loop in multiple myeloma development. Cancer Cell. 2010;18:367–381. doi: 10.1016/j.ccr.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Croce C.M. Oncogenes and cancer. N. Engl. J. Med. 2008;358:502–511. doi: 10.1056/NEJMra072367. [DOI] [PubMed] [Google Scholar]
- 95.Lionetti M., Agnelli L., Lombardi L., Tassone P., Neri A. MicroRNAs in the pathobiology of multiple myeloma. Curr. Cancer Drug Targets. 2012;12:823–837. doi: 10.2174/156800912802429274. [DOI] [PubMed] [Google Scholar]
- 96.Zhao J.J., Lin J., Zhu D., Wang X., Brooks D., Chen M., Chu Z.B., Takada K., Ciccarelli B., Admin S., et al. miR-30-5p functions as a tumor suppressor and novel therapeutic tool by targeting the oncogenic Wnt/beta-catenin/BCL9 pathway. Cancer Res. 2014;74:1801–1813. doi: 10.1158/0008-5472.CAN-13-3311-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhan F., Colla S., Wu X., Chen B., Stewart J.P., Kuehl W.M., Barlogie B., Shaughnessy J.D., Jr. CKS1B, overexpressed in aggressive disease, regulates multiple myeloma growth and survival through SKP2- and p27Kip1-dependent and -independent mechanisms. Blood. 2007;109:4995–5001. doi: 10.1182/blood-2006-07-038703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chang H., Jiang N., Jiang H., Saha M.N., Qi C., Xu W., Reece D. CKS1B nuclear expression is inversely correlated with p27Kip1 expression and is predictive of an adverse survival in patients with multiple myeloma. Haematologica. 2010;95:1542–1547. doi: 10.3324/haematol.2010.022210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sherr C.J. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- 100.Gupta V.A., Ackley J., Kaufman J.L., Boise L.H. BCL2 Family Inhibitors in the Biology and Treatment of Multiple Myeloma. Blood Lymphat. Cancer Targets Ther. 2021;11:11–24. doi: 10.2147/BLCTT.S245191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang W., Lai R., He X., Liu X., Zhang Y., Yang Z., Yang P., Wang J., Hu K., Yuan X., et al. Clinical prognostic implications of EPB41L4A expression in multiple myeloma. J. Cancer. 2020;11:619–629. doi: 10.7150/jca.33805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kumar S.K., Rajkumar S.V. The multiple myelomas—Current concepts in cytogenetic classification and therapy. Nat. Rev. Clin. Oncol. 2018;15:409–421. doi: 10.1038/s41571-018-0018-y. [DOI] [PubMed] [Google Scholar]
- 103.Palumbo A., Anderson K. Multiple myeloma. N. Engl. J. Med. 2011;364:1046–1060. doi: 10.1056/NEJMra1011442. [DOI] [PubMed] [Google Scholar]
- 104.Walker B.A., Boyle E.M., Wardell C.P., Murison A., Begum D.B., Dahir N.M., Proszek P.Z., Johnson D.C., Kaiser M.F., Melchor L., et al. Mutational Spectrum, Copy Number Changes, and Outcome: Results of a Sequencing Study of Patients With Newly Diagnosed Myeloma. J. Clin. Oncol. 2015;33:3911–3920. doi: 10.1200/JCO.2014.59.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mohty B., El-Cheikh J., Yakoub-Agha I., Avet-Loiseau H., Moreau P., Mohty M. Treatment strategies in relapsed and refractory multiple myeloma: A focus on drug sequencing and ‘retreatment’ approaches in the era of novel agents. Leukemia. 2012;26:73–85. doi: 10.1038/leu.2011.310. [DOI] [PubMed] [Google Scholar]
- 106.D’Agostino Mattia M., Lahuerta J.-J., Wester R., Waage A., Bertsch U., Zamagni E., Mateos M.-V., Larocca A., Dall’Olio D., van de Donk N.W.C.J., et al. A New Risk Stratification Model (R2-ISS) in Newly Diagnosed Multiple Myeloma: Analysis of Mature Data from 7077 Patients Collected By European Myeloma Network within Harmony Big Data Platform. Blood. 2020;136:34–37. doi: 10.1182/blood-2020-137021. [DOI] [Google Scholar]