Abstract
Introduction:
We sought to determine whether electrical impedance myography (EIM) could serve as a diagnostic procedure for evaluation of radiculopathy.
Methods:
Twenty-seven patients with clinically and radiologically diagnosed cervical or lumbosacral radiculopathy who met a “gold standard” definition underwent EIM and standard needle electromyography (EMG) of multiple upper or lower extremity muscles.
Results:
EIM reactance values revealed consistent reductions in the radiculopathy-affected myotomal muscles as compared with those on the unaffected side; the degree of asymmetry was associated strongly with the degree of EMG abnormality (P < 0.001). EIM had a sensitivity of 64.5% and a specificity of 77.0%; in comparison, EMG had a sensitivity of 79.7% but a specificity of 69.7%.
Conclusions:
These findings support the potential for EIM to serve as a new non-invasive tool to assist in diagnosis of radiculopathy; however, further refinement of the technique is needed for this specific application.
Keywords: cervical radiculopathy, electrical impedance, electromyography, lumbosacral radiculopathy, muscle, reactance
Low back and neck pain remains one of the more common complaints in clinical practice, accounting for approximately 4–5% of all medical encounters.1 Whereas initial evaluation and therapy choice continue to be based predominantly on clinical history and physical examination, many patients are referred for additional testing, including magnetic resonance imaging (MRI) and needle electromyography (EMG). For patients with a clear clinical picture, MRI can be helpful in establishing the etiology and level of nerve root compression. However, radiologic abnormalities can be very non-specific, because degenerative disk disease is seen on imaging in up to 50% of the normal population and disk herniation in 20%.1–4 For patients in whom the diagnosis or localization remains unclear, concentric needle EMG/nerve conduction study (NCS) is the test of choice, because it provides physiologic data that can help to identify precisely the time course and level of the lesion and assist in determining the clinical relevance of the MRI data. Nonetheless, standard concentric needle EMG is limited in 3 major respects. First, the sensitivity of NCS/EMG in radiculopathy is relatively low, ranging from 51% to 86%, depending on the study.4–11 Second, the EMG examination itself is highly subjective. Indeed, the interrater reliability of the technique is only fair. In the single study that addressed this issue, there was only a 60% concordance in diagnoses among experienced electromyographers.12 Even agreement on the presence or absence of abnormal insertional activity was only 57.5%. Finally, although various approaches have been suggested to help reduce the pain associated with the examination,13–15 EMG remains an uncomfortable procedure for many patients.
One technique that could prove useful in the diagnosis of radiculopathy is electrical impedance myography (EIM). EIM is a non-invasive technique for assessment of muscle in which a high-frequency, low-intensity electrical current is applied to the skin overlying a muscle or muscle group, and the consequent surface voltage patterns are measured.16 Data have shown that EIM can serve as both a diagnostic tool and a measure of disease severity in neurogenic and myopathic diseases, including amyotrophic lateral sclerosis and inflammatory myopathy.17,18 In addition, a previous study identified asymmetries in impedance data in patients with cervical and lumbosacral radiculopathy.19 Side-to-side differences in the major EIM parameter, the phase, were found to be greater in radiculopathy patients than in normal subjects, with the phase on the affected side being consistently lower. A specific effort to localize the level of the radiculopathy using EIM abnormalities, however, was not attempted in that study.
The present study builds on our earlier observations in EIM and radiculopathy. Using a more convenient hand-held array in which electrode sizes and interelectrode distances are fixed,20 we now test whether side-to-side changes in EIM signal can assist with specific localization of radiculopathy and assess how EIM values compare with EMG data.
METHODS
Subject Recruitment.
Approval was obtained from the Committee for Clinical Investigations at Beth Israel Deaconess Medical Center (BIDMC), and all subjects provided written informed consent. We recruited subjects with cervical and lumbosacral radiculopathies. Inclusion criteria were: (1) age 18–85 years and (2) radiculopathy diagnosed by a clinical “gold standard” defined as 2 or more of the following: (a) neck/lower back pain; (b) radicular pain and/or sensory symptoms, including numbness and tingling, in a clear radicular distribution; and (c) abnormality on examination, including radicular sensory loss, myotomal weakness, or reflex change. MRI data were considered supportive but were not required. A clinically affected muscle was defined as a muscle innervated by the involved root based on the above criteria.
Exclusion criteria were: (1) presence of a known neuromuscular disorder affecting the limbs to be studied (except for mild axonal polyneuropathy or mild compression neuropathies); (2) obesity (body mass index ≥40 kg/m2); (3) presence of an implanted electrical device such as a cardiac pacemaker; and (4) clinical symptoms suggestive of bilateral radiculopathy.
Clinical Evaluation.
A brief medical history was obtained, and a neurologic examination was performed on all subjects. The level and side of radiculopathy were then diagnosed clinically based on the history and examination and standard anatomic principles, relying on dermatomal and myotomal findings to localize the lesion. MRI was not considered necessary for the diagnosis of radiculopathy. However, in subjects who had spine MRI performed, data considered supportive for a diagnosis of radiculopathy included foraminal stenosis of at least moderate severity or herniated nucleus pulposus with root impingement at the clinically appropriate root level. Importantly, all subjects received a clinical diagnosis that included the side, level(s), and severity prior to any electrophysiologic testing. A determination of “clinically affected” and “clinically unaffected” muscles was then made for the purposes of analysis using accepted myotomal innervation patterns. For example, in a subject with symptoms suggestive of C7 radiculopathy, the triceps and flexor carpi radialis would be considered clinically affected and deltoid and biceps clinically unaffected.
Standard Electromyography.
Concentric needle EMG was performed in all subjects using a TECA EMG machine (TECA Synergy T2; Carefusion, San Diego, California). To ensure consistency of testing between both EIM and EMG, specific sets of upper and lower extremity muscles were developed for testing across all subjects. This was especially needed, because EIM could not be performed with confidence on deeper muscles. In the upper extremities, 8 muscles were assessed, including abductor digiti minimi, extensor indicis, extensor digitorum communis, flexor carpi radialis, brachioradialis, triceps, biceps, and deltoid. In the lower extremities, 7 muscles were assessed, including extensor hallucis longus, tibialis anterior, medial gastrocnemius, vastus medialis, vastus lateralis, biceps femoris (long head), and tensor fascia lata. These muscle choices represented the C5–T1 and L2–S1 myotomes, respectively, in each patient. Abnormal spontaneous activity, motor unit potential amplitude, phases, duration, and recruitment patterns for each muscle were assessed semiquantitatively. A standard ordinal scale was used to grade fibrillation potentials from 0 to 4+.21 Motor unit potential duration, amplitude, phases, and recruitment were graded as: 0 = normal; 1 = mildly abnormal; 2 = mildly to moderately abnormal; 3 = moderately abnormal; 4 = severely abnormal. For an individual muscle to be considered abnormal, it was required to have either evidence for definite ongoing denervation (i.e., clear, sustained fibrillation potentials of ≥1+) or chronic reinnervation (i.e., as measured by having at least 3 of the 4 motor unit potential characteristics being ≥1).
EIM Testing.
All subjects underwent EIM testing using a custom-constructed handheld electrode array (Proxy Manufacturing, Inc., Methuen, Massachusetts) (Fig. 1). Impedance data were obtained using the ImpSFB7 bioimpedance spectroscopy device (Impedimed, Inc., San Diego, California), which collects 3-kHz to 1-MHz impedance data. Resistance (R) and reactance (X) were output directly from the device; the phase (θ) was then computed via the relationship [θ = arctan (X/R)], and each of the 3 parameters was plotted against frequency. EIM was performed on the same muscles as EMG.
FIGURE 1.
Hand-held array placed over the wrist extensor compartment (impedance-measuring device not shown).
Data Analysis.
Resistance, reactance, and phase data at 50 kHz were extracted from the multifrequency data set for each muscle. In all the analyses that follow, significance was determined at the 95% confidence level (2-tailed). For each of the 3 EIM parameters, the following comparisons were made: (1) a side-to-side comparison of the averaged raw impedance values for individual unaffected muscles (i.e., muscles not clinically involved by the radiculopathy) according to Wilcoxon signed rank test; and (2) a side-to-side comparison of the averaged raw impedance values for individual clinically affected muscles according to Wilcoxon signed rank test. After this initial set of analyses, the difference in values between the 2 sides (unaffected side – affected side) was then computed for each muscle pair (i.e., bilateral deltoid, bilateral biceps, etc.). The percentage differences were then calculated for each muscle pair by taking the difference and dividing by the mean of the 2 muscles. Unaffected muscle average values were then calculated by averaging the clinically unaffected muscle pair percentages; affected muscle average values were calculated by averaging the clinically affected muscles. Thus, for each patient we obtained separate average percent differences for both unaffected and affected muscles. Muscles from upper and lower limbs were analyzed separately.
Next, we compared the sensitivity and specificity of EMG and EIM in the clinical diagnosis of radiculopathy. EMG was considered to be positive if there were abnormalities in at least 2 muscles innervated by the clinically affected nerve root. The study was considered negative if a subject had no abnormalities, had EMG abnormalities in just 1 muscle, or had EMG abnormalities in 2 muscles in a clinically unaffected myotome. The resulting EMG data (positive or negative) were compared with the clinical diagnosis of radiculopathy to obtain specificity and sensitivity of EMG. For EIM, the average clinically affected muscle side-to-side reactance asymmetry values were compared with the clinically unaffected group average. Because EIM provided numerical values (in contrast to the categorical EMG data), a receiver operating characteristic (ROC) curve was generated to assist with this analysis. The single point closest to 100% sensitivity and 100% specificity of the test was selected as the maximized sensitivity and specificity for the test.
We then evaluated whether the EMG data and EIM asymmetries could detect differences in radiculopathy severity based on the presence of muscle weakness and if there was concordance between EIM and EMG abnormalities. We divided the subjects into 2 clinical groups based on the presence or absence of weakness: none–mild and moderate–severe. We also dichotomized the EMG data into normal–mild (average of <2 on the 4-point ordinal scales for fibrillation potential activity or degree of chronic reinnervation) and moderate–severe (average of ≥2 for fibrillation potential activity or degree of chronic reinnervation). We compared the 2 clinical groups separately to the degree of EIM asymmetry (Mann–Whitney test for continuous data) and EMG severity groups (chi-square; categorical data). We then compared EIM severity with EMG severity (Mann–Whitney test).
RESULTS
Subject Demographics.
A total of 27 radiculopathy patients were studied, including 11 with cervical radiculopathy and 16 with lumbosacral radiculopathy. There were 9 men and 2 women in the cervical radiculopathy group (mean age 54 years, range 29–71 years). In the lumbosacral radiculopathy group there were 11 men and 5 women (mean age 61.5 years, range 27–78 years). The 11 cervical radiculopathy subjects included: 1 C5; 1 C5–6; 2 C5–7; 2 C6; 1 C6–7; 1 C7–8; 1 C7/T1; 1 C7–8/T1; and 1 C8. The 16 lumbosacral radiculopathy subjects included: 1 L2–4/S1; 8 L5; 2 L5/S1; and 5 S1. No subject underwent testing of both upper and lower extremities.
EIM Asymmetry in Clinically Unaffected and Affected Muscles.
As a group, no significant asymmetries were detected in the clinically unaffected muscles for any of the EIM parameters (Table 1 and Fig. 2). However, the changes in reactance trended toward significance for the upper limbs (mean difference ± standard error of 3.7 ± 2.1%, P = 0.059), although no trend was apparent in the lower limbs (4.3 ± 2.2%, P = 0.13).
Table 1.
Summary of side-to-side differences for EIM.
| Resistance (Ω) |
Reactance (Ω) |
Phase (°) |
||||
|---|---|---|---|---|---|---|
| Upper limbs | Lower limbs | Upper limbs | Lower limbs | Upper limbs | Lower limbs | |
| Clinically unaffected muscles | ||||||
| Symptomatic side | 73.4 | 80.7 | 12.0 | 10.4 | 10.4 | 8.53 |
| Asymptomatic side | 73.0 | 79.9 | 12.5 | 10.9 | 10.7 | 8.80 |
| Side-to-side difference | 0.03 ± 1.6% | −0.26 ± 1.4% | 3.7 ± 2.1% | 4.3 ± 2.2% | 3.6 ± 2.4% | 4.5 ± 2.4% |
| P-value | 0.51 | 0.17 | 0.059 | 0.13 | 0.30 | 0.22 |
| Clinically affected muscles | ||||||
| Symptomatic side | 78.2 | 74.9 | 13.4 | 8.66 | 11.0 | 7.56 |
| Asymptomatic side | 79.2 | 77.8 | 14.2 | 10.0 | 11.4 | 8.11 |
| Side-to-side difference | 2.0 ± 1.8% | 5.8 ± 2.8% | 8.2 ± 3.1% | 12.0 ± 3.3% | 6.1 ± 3.3% | 6.0 ± 3.9% |
| P-value | 0.69 | 0.17 | 0.005 | 0.002 | 0.07 | 0.21 |
Values given as mean ± standard error. P-values assess the significance of the difference between the 2 sides; P < 0.05 considered statistically significant (bold).
FIGURE 2.
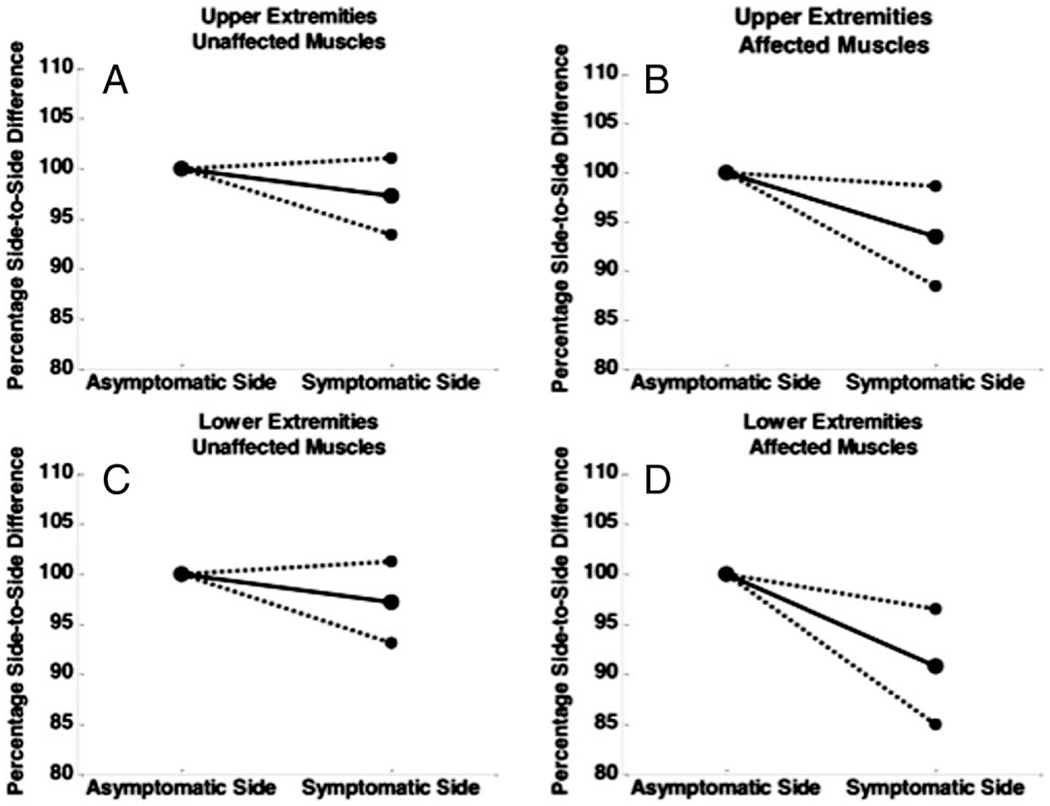
Side-to-side differences in 50-kHZ reactance of the symptomatic side (expressed as a percentage of asymptomatic side) in radiculopathy patients. For unaffected muscles, there is no significant difference from side to side. (A, C) Results for unaffected muscles. (B, D) Results for affected muscles. The dashed lines represent the 95% confidence intervals.
In contrast, the average reactance was consistently lower in the clinically affected upper extremity muscles than in the opposite unaffected extremity (mean difference ± standard error of 8.2 ± 3.1%, P = 0.005). This finding was also seen in the lower extremities, with an average 12.0 ± 3.3% difference in reactance; the affected side muscles had lower values (P = 0.002).
Sensitivity of EMG and EIM in Radiculopathy.
Table 2 shows the sensitivities and specificities of EIM and EMG obtained by comparing data from clinically unaffected muscles with clinically affected muscles. As can be seen, EMG performed somewhat better than EIM overall, achieving 79.7% sensitivity and 69.7% specificity, whereas EIM had 64.5% sensitivity and 77.0% specificity.
Table 2.
Sensitivity and specificities of EMG and EIM.
| EMG upper | EMG lower | Mean EMG | EIM upper | EIM lower | Mean EIM | |
|---|---|---|---|---|---|---|
| Sensitivity (%) | 72.7 | 86.7 | 79.7 | 72.7 | 56.3 | 64.5 |
| Specificity (%) | 72.7 | 66.7 | 69.7 | 72.7 | 81.3 | 77.0 |
Clinical Disease Severity as Assessed by EMG and EIM.
There were 12 individuals in the mild category and 15 in the moderate–severe category. A non-significant relationship was identified between EMG severity and the presence/severity of weakness in clinically affected muscles (P = 0.65). Similarly, no significant relationship was identified between EIM asymmetry changes and weakness [50-kHz reactance (mean ± standard error): severe group, 1.41 ± 0.13 Ω; mild group, 0.65 ± 0.08 Ω (P = 0.16)]. However, EMG and EIM severity appeared to be associated. EIM reactance asymmetry was significantly greater in muscles considered moderate–severe on EMG as compared with muscles judged normal–mild [50-kHz reactance asymmetry (mean ± syandard error): moderate–severe group, 2.02 ± 0.25 Ω; mild group, 0.30 ± 0.24 Ω (P < 0.001)].
DISCUSSION
The main purpose of this study was to determine whether EIM has the potential to serve as a test for use by neurologists and physiatrists in the diagnosis and localization of radiculopathy. The data show that there are, in fact, relative reductions in the reactance of clinically affected muscles as compared with clinically unaffected muscles and that EIM has an overall sensitivity of 64.5% and specificity of 77%.
We chose to evaluate side-to-side differences to determine asymmetries between healthy and affected muscles. Although it would be ideal to use the actual impedance values rather than side-to-side differences to obtain a diagnosis, establishing a set of normal values for each muscle is challenging because impedance values were also impacted by 2 major physical factors: muscle size and subcutaneous fat thickness. By using side-to-side comparisons, this inherent variability in the measurement is reduced. This may be considered similar to the standard practice of performing side-to-side comparisons of nerve conduction values rather than relying solely on population normal values. Although previous normal values have been described for EIM,22 these values were established only for a limited number of muscles and used a different electrode set-up. Therefore, they could not be applied in our study. Nonetheless, we are collecting normative data using this hand-held array and will seek to determine whether an approach to radiculopathy diagnosis can be achieved that does not require side-to-side comparisons.
EIM parameters, unlike EMG data, can be altered substantially by muscle disuse23; however, as compared with frank neurogenic injury, animal studies have suggested that disuse changes are subtler (unpublished results). The findings in this study hint at the possibility of disuse impacting muscles that should be unaffected by clinical radiculopathy directly. Specifically, the clinically unaffected muscles had, on average, lower reactance values on the radiculopathy side as compared with the unaffected side, almost reaching significance for the upper limbs (P = 0.059). Still, differences in reactance were considerably more pronounced in muscles innervated by the injured nerve root.
In our 2005 study, the 50-kHZ phase showed significant alterations in radiculopathy, with consistently lower values in radiculopathy-affected limbs; reactance was not reported in that study.19 In the present work, phase values showed no difference. The main reason for this inconsistency is that a different electrode approach was used in the earlier study, in which the current-emitting electrodes were placed far from the voltage-measuring ones (the current-emitting electrodes were placed on either both hands or both feet, and voltage-measuring electrodes on the skin overlay the muscle being tested). This approach has the advantage of helping to avoid the impact of subcutaneous fat on the data, but it is considerably clumsier to apply and requires adhesive electrodes and detailed length measurements to assist in proper electrode placement. Of the 3 major EIM parameters, reactance tends to be least impacted by subcutaneous fat thickness when a hand-held array is used, such as the one employed in this study, and is thus the preferred variable to study.24,25 Other work has also shown major alterations in the reactance values with neurogenic injury, which are likely related to reductions in muscle fiber size secondary to axon loss.23 In contrast, resistance, and to a lesser extent the phase, are more impacted by subcutaneous fat,25 and thus the slight alterations in those values caused by radiculopathy may be more difficult to detect.
Surprisingly, the relationship between clinical severity and both EMG and EIM severity was relatively weak. This may be due, in part, to the relatively small number of subjects in each group. However, the data still show a strong concordance between EIM asymmetry and the severity of abnormalities on EMG, supporting the idea that EIM and EMG are sensitive to similar pathologies.
This study had several limitations. First, the design of the hand-held electrode array was not ideal. Specifically, recent work has shown that the spacing of the electrodes was not optimized, resulting in the measurements being excessively affected by the subcutaneous fat layer thickness (unpublished results). Also, the array did not assess electrical anisotropy (the directional dependence of electrical current flow through muscle). This is a potentially sensitive measure for the presence of neurogenic disease.24 Second, the Imp SFB7® is not designed specifically to measure the small impedances encountered during these localized measurements over discrete areas of muscle and thus may provide limited accuracy. Third, the number of subjects included in this study was relatively small, and all the data were obtained from only a single site. Fourth, we limited our EIM and EMG assessments to specific sets of muscles in both upper and lower extremities. This was necessary to complete the study in a consistent fashion, in part given limitations on which muscles EIM could be performed. However, it does limit the accuracy of the study to some extent because, the most affected muscles in a given subject may not have been evaluated. Finally, this research is mainly hypothesis-generating, because we did not specifically delineate a primary EIM outcome measure a priori. Our findings will need to be duplicated with reactance asymmetry as a predetermined value before we can show convincingly that EIM could serve as a useful test for radiculopathy. As a next step, a blinded “inverse” protocol could be performed, in which EIM reactance asymmetry values may be measured on symptomatic subjects without knowledge of clinical diagnosis, MRI findings, or EMG data in an attempt to localize the disease by EIM and then assess its overall accuracy.
In conclusion, EIM shows promise to serve as a non-invasive tool for identification of muscles affected by cervical or lumbosacral radiculopathy. However, it is clear that further development and study will be required before neuromuscular specialists could use it as a complementary technology to standard needle electromyography.
Acknowledgments
This study was supported by the National Institutes of Health (K24NS060951) and the National Institute of Neurological Disorders and Stroke.
Abbreviations:
- BIDMC
Beth Israel Deaconess Medical Center
- EIM
electrical impedance; myography
- EMG
electromyography
- LL
lower limbs
- MUP
motor unit potential
- R
resistance
- UL
upper limbs
- X
reactance
- θ
phase
REFERENCES
- 1.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990;72:403–408. [PubMed] [Google Scholar]
- 2.Boden SD, McCowin PR, Davis DO, Dina TS, Mark AS, Wiesel S. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am 1990;72:1178–1184. [PubMed] [Google Scholar]
- 3.Greenberg JO, Schnell RG. Magnetic resonance imaging of the lumbar spine in asymptomatic adults. Cooperative study—American Society of Neuroimaging. J Neuroimaging 1991;1:2–7. [DOI] [PubMed] [Google Scholar]
- 4.Weinreb JC, Wolbarsht LB, Cohen JM, Brown CE, Maravilla KR. Prevalence of lumbosacral intervertebral disk abnormalities on MR images in pregnant and asymptomatic nonpregnant women. Radiology 1989;170:125–128. [DOI] [PubMed] [Google Scholar]
- 5.Aminoff MJ, Goodin DS, Parry GJ, Barbaro NM, Weinstein PR, Rosenblum ML. Electrophysiologic evaluation of lumbosacral radiculopathies: electromyography, late responses, and somatosensory evoked potentials. Neurology 1985;35:1514–1518. [DOI] [PubMed] [Google Scholar]
- 6.Kuruoglu R, Oh SJ, Thompson B. Clinical and electromyographic correlations of lumbosacral radiculopathy. Muscle Nerve 1994;17:250–251. [PubMed] [Google Scholar]
- 7.Leblhuber F, Reisecker F, Boehm-Jurkovic H, Witzmann A, Deisenhammer E. Diagnostic value of different electrophysiologic tests in cervical disk prolapse. Neurology 1988;38:1879–1881. [DOI] [PubMed] [Google Scholar]
- 8.Nardin RA, Patel MR, Gudas TF, Rutkove SB, Raynor EM. Electromyography and magnetic resonance imaging in the evaluation of radiculopathy. Muscle Nerve 1999;22:151–155. [DOI] [PubMed] [Google Scholar]
- 9.Tonzola RF, Ackil AA, Shahani BT, Young RR. Usefulness of electrophysiological studies in the diagnosis of lumbosacral root disease. Ann Neurol 1981;9:305–308. [DOI] [PubMed] [Google Scholar]
- 10.Wu ZA, Tsai CP, Yang DA, Chu FL, Chang T. Electrophysiologic study and computerized tomography in diagnosis of lumbosacral radiculopathy. Chinese Med J Free China Ed [Zhonghua yi xue za zhi] 1987;39:119–125. [PubMed] [Google Scholar]
- 11.Levin KH, Maggiano HJ, Wilbourn AJ. Cervical radiculopathies: comparison of surgical and EMG localization of single-root lesions. Neurology 1996;46:1022–1025. [DOI] [PubMed] [Google Scholar]
- 12.Kendall RW, Werner RA. Interrater reliability of the needle examination in lumbosacral radiculopathy. Muscle Nerve 2006;34:238–241. [DOI] [PubMed] [Google Scholar]
- 13.Annaswamy TM, Morchower AH. Effect of lidocaine iontophoresis on pain during needle electromyography. Am J Phys Med Rehabil 2011;90:961–968. [DOI] [PubMed] [Google Scholar]
- 14.El-Salem K, Shakhatreh M. Prospective double-blind crossover trial of ibuprofen in reducing EMG pain. Muscle Nerve 2008;38:1016–1020. [DOI] [PubMed] [Google Scholar]
- 15.Strommen JA, Daube JR. Determinants of pain in needle electromyography. Clin Neurophysiol 2001;112:1414–1418. [DOI] [PubMed] [Google Scholar]
- 16.Rutkove SB. Electrical impedance myography: background, current state, and future directions. Muscle Nerve 2009;40:936–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutkove SB, Caress JB, Cartwright MS, et al. Electrical impedance myography as a biomarker to assess ALS progression. Amyotroph Latereral Scler 2012;7:e45004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarulli A, Esper GJ, Lee K, Aaron R, Shiffman CA, Rutkove SB. Electrical impedance myography in the bedside assessment of inflammatory myopathy. Neurology 2005;65:451–452. [DOI] [PubMed] [Google Scholar]
- 19.Rutkove SB, Esper GJ, Lee K, Aaron R, Shiffman CA. Electrical impedance myography in the detection of radiculopathy. Muscle Nerve 2005;32:335–341. [DOI] [PubMed] [Google Scholar]
- 20.Narayanaswami P, Spieker AJ, Mongiovi P, Keel JC, Muzin SC, Rutkove SB. Utilizing a handheld electrode array for localized muscle impedance measurements. Muscle Nerve 2012;46:357–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Preston DC, Shaprio BE. Electromyography and neuromuscular disorders. Boston: Butterworth-Heinemann; 2005. [Google Scholar]
- 22.Rutkove SB, Fogerson PM, Garmirian LP, Tarulli AW. Reference values for 50-kHz electrical impedance myography. Muscle Nerve 2008;38:1128–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tarulli AW, Duggal N, Esper GJ, Garmirian LP, Fogerson PM, Lin CH, et al. Electrical impedance myography in the assessment of disuse atrophy. Arch Phys Med Rehabil 2009;90:1806–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sung M, Spieker AJ, Narayanaswami P, Rutkove SB. The effect of subcutaneous fat on electrical impedance myography when using a handheld electrode array: the case for measuring reactance. Clin Neurophysiol 201;124:400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jafarpoor M, Li J, White JK, Rutkove SB. Optimizing electrode configuration for electrical impedance measurements of muscle via the finite element method. Trans Biomed Eng 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


