Abstract
An estimated 8–16% of the world’s population has chronic kidney disease, defined by low glomerular filtration rate or albuminuria. Progression of chronic kidney disease is associated with adverse outcomes, including incident kidney failure with replacement therapy, accelerated cardiovascular disease, disability, and mortality. Therefore, slowing kidney function decline is paramount in the management of a patient with chronic kidney disease. Ascertaining the cause of kidney disease is an important first step and may compel specific therapies. Effective approaches that apply to the vast majority of patients with chronic kidney disease include the optimization of blood pressure and blockade of the renin-angiotensin aldosterone system, particularly if albuminuria is present. Recent studies suggest that sodium/glucose cotransporter 2 inhibitors are highly effective treatments in patients with diabetes and/or albuminuria. For patients with type 2 diabetes, glycemic control is important in preventing the development of microvascular complications, and glucagon-like peptide 1 receptor agonists may help reduce albuminuria levels. Other strategies include correction of metabolic acidosis, maintaining ideal body weight, following diets that are low in sodium and animal protein, and avoidance of potential nephrotoxins such as nonsteroidal anti-inflammatories, proton-pump inhibitors, and iodinated contrast.
Keywords: chronic kidney disease (CKD), blood pressure (BP), albuminuria, diabetes, CKD progression, renal function, estimated glomerular filtration rate (eGFR), proteinuria, sodium/glucose cotransporter 2 inhibitor (SGLT2i), glucagon-like peptide 1 receptor agonist (GLP1-RA), hypertension management, glycemic control, lifestyle modification, uric acid, review
Introduction
Chronic kidney disease (CKD) affects more than 697 million individuals worldwide and is associated with increased morbidity and mortality. In 2017, 1.2 million deaths and 35.8 million disability-adjusted life-years were attributed to CKD. Among Medicare beneficiaries in the United States, annual spending for kidney failure with replacement therapy (KFRT) and earlier stages of CKD exceeded $120 billion. Different causes of kidney disease may require specific treatments, such as immunosuppressive therapy. However, some strategies to delay the progression of CKD to KFRT are applicable to most patients. Early detection and treatment to slow kidney function decline are paramount to improving outcomes in patients with CKD. Hallmarks of CKD management include control of hypertension and hyperglycemia, inhibition of the renin-angiotensin-aldosterone (RAAS) system, correction of metabolic acidosis, lifestyle modification, and avoidance of nephrotoxins. Two new classes of medications, sodium/glucose cotransporter 2 (SGLT2) inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists, also improve kidney outcomes among individuals with diabetes and/or albuminuria.
Additional Readings
GBD Chronic Kidney Disease Collaboration; Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733.
Saran R, Robinson B, Abbott KC, et al. US Renal Data System 2019 Annual Data Report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2020;75(1)(suppl 1):S1-S64.
Blood pressure control
[ISP]Case 1: A 60 year-old man with CKD glomerular filtration rate category 3b (G3b) and albuminuria category 2 (A2; corresponding to an urinary albumin-creatinine ratio [UACR] of 30–300 mg/g), hypertension, and stable angina returns for follow-up. His estimated GFR (eGFR) has declined from 57 to 44 ml/min/1.73 m2 over the past 13 years. His blood pressure (BP) averages 135/72 mm Hg on a regimen of valsartan 320 mg daily, amlodipine 5 mg daily, and indapamide 1.25 mg daily.
Question 1: Based on the results of SPRINT, which one of the following statements is most accurate regarding a systolic BP goal of <120 vs. <140 mmHg?
All-cause mortality is reduced
CKD progresses more slowly at the lower BP goal
Incidence of KFRT is higher at the lower BP goal
Incidence of kidney transplantation is lower at the lower BP goal
Question 2: Which one of the following patients would be most appropriate for a lower BP goal to help slow progression of CKD?
CKD G3aA1 with UACR 10 mg/g
CKD G4A1 with critical bilateral renal artery stenosis
CKD G3bA3 with UACR 3,000 mg/g
CKD G3bA3 with UACR 1,200 mg/g and history of repeated falls
For the answer to the questions, see the following text.
The AHA/ACC recommend a goal BP <130/80 mmHg for all patients with CKD, whereas the KDIGO guidelines recommend a target of ≤140/90 mmHg when UACR is <30 mg/d and ≤130/80 mmHg when UACR is ≥30 mg/d (Table 1). The KDIGO recommendations are based, in part, on two landmark randomized controlled trials. AASK randomized participants without diabetes to a mean arterial pressure (MAP) goal of ≤92 versus 102–107 mmHg. Although there was no difference in rate of eGFR decline or a composite clinical outcome (eGFR decline, KFRT, or death) overall, participants with a baseline urinary protein-creatinine ratio (UPCR) >0.22 g/g were 27% less likely to develop a doubling of serum creatinine, KFRT, or death when randomized to intensive versus standard BP control in the extended cohort phase. The MDRD Study randomized participants to a MAP goal of 92 versus 107 mmHg. Again, there were no differences overall, but participants with proteinuria ≥3 g/d had lesser GFR decline in the intensive BP control group. These and other trials of BP control are summarized in Table 2.
Table 1:
Summary of guidelines for slowing kidney function decline in patients with CKD.
| Guidelines | BP Control | RAAS Inhibition | Glycemic Control | Diet |
|---|---|---|---|---|
| KDIGO 2012 | Goal BP: ≤140/90 mm Hg (if UACR <30 mg/g) or ≤130/80 mm Hg (if UACR ≥30 mg/g) | Start ACEI or ARB if diabetes and UACR 30–300 mg/g; start ACEI or ARB if UACR ≥300 mg/g | Goal HbA1c ~7.0%a; avoid HbA1c <7.0% if at risk of hypoglycemia; allow HbA1c >7.0% if comorbidities, limited life expectancy, or at risk of hypoglycemia | Goal <2 g/d of sodium; protein intake <1.3 g/kg/d if at risk for CKD progression; protein intake 0.8 g/kg/d if GFR<30 ml/min/1.73 m2 |
| AHA/ACC 2017 | Goal BP: <130/80 mmHg | Start ACEI if HTN and eGFR <60 ml/min/1.73 m2 or UACR ≥300 mg/g; use ARB if above indications and ACEI not tolerated | n/a | Sodium reduction if HTN |
| ADA and EASD 2018 (for patients with type 2 diabetes) | n/a | n/a | Goal HbA1c ≤7.0% but should be individualized; consider SGLT2i if at risk for CKD progression; consider GLP-1RA if SGLT2i not tolerated or contraindicated | n/a |
| ERBP 2015 (for patients with CKD G3b+) | Goal SBP: <140 mmHg | Start ACEI if diabetes and cardiovascular indication | Goal HbA1c ≤8.5% if comorbidities, limited life expectancy, or at risk of hypoglycemia; goal HbA1c ≤7.0–8.0% otherwise | n/a |
None of the guidelines included recommendations relating to uric acid.
The 2020 KDIGO Diabetes in CKD guideline recommends an individualized HbA1c target of <6.5% to <8.0%.
Abbreviations: AHA, American Heart Association; ACC, American College of Cardiology; BP=blood pressure; SBP=systolic blood pressure; ACEI=angiotensin-converting-enzyme inhibitor; ARB=angiotensin-receptor blocker; eGFR=estimated glomerular filtration rate; UACR=urinary albumin-creatinine ratio; HbA1c=hemoglobin A1c; SGLT2i =sodium/glucose cotransporter 2 inhibitor; GLP-1RA =glucagon-like peptide 1 receptor agonist; NA=not available; KDIGO=Kidney Disease: Improving Global Outcomes; ADA=American Diabetes Association; EASD=European Association for the Study of Diabetes; ERBP=European Renal Best Practice; HTN, hypertension
Based on KDIGO CKD Work Group 2013 (Kidney Int Suppl, https://doi.org/10.1038/kisup.2012.77); KDIGO Diabetes Work Group 2020(Kidney Int, https://doi.org/10.1016/j.kint.2020.06.019); Whelton et al 2018 (Hypertension, https://doi.org/10.1161/hyp.0000000000000065); Davies et al 2018 (Diabetologia, https://doi.org/10.1007/s00125-018-4729-5); Bilo et al 2015 (Nephrol Dial Transplant, https://doi.org/10.1093/ndt/gfv100).
Table 2:
Summary of major clinical trials on intensive versus standard BP control and kidney function decline.
| AASK trial and cohort (n=1,094) | MDRD Study (n=840) | REIN-II (n=335) | SPRINT (n=9,361) | |||||
|---|---|---|---|---|---|---|---|---|
| Kidney-related inclusion criteria | GFR 20–65 ml/min/1.73 m2; UPCR ≤2.5 g/d | Scr 1.2 (F) or 1.4 (M) to 7.0 mg/dL or CLcr <70 ml/min/1.73 m2; Proteinuria <10 g/d | Proteinuria 1–3 g/d & CLcr <45 ml/min/1.73 m2 or Proteinuria ≥3 g/d & CLcr <70 ml/min/1.73 m2 | eGFR 20–60 ml/min/1.73 m2; Proteinuria <1 g/d | ||||
| Follow-up | Range: 8.8–12.2 y | Mean: 2.2 y | Median: 1.6 y | Median: 3.3 y | ||||
| % with diabetes | 0% | 0% with “diabetes mellitus requiring insulin therapy” | 0% with “type 1 diabetes mellitus” | 0% | ||||
| Intervention | MAP ≤92 vs. 102–107 mm Hg | MAP 92 vs. 107 mm Hg | BP <130/80 vs. DBP <90 mm Hg | SBP <120 vs. <140 mm Hg | ||||
| Subgroups | UPCR ≤0.22 g/g | UPCR >0.22 g/g | GFR 25–55 ml/min/1.73 m2 | GFR 13–24 ml/min/1.73 m2 | Proteinuria 1–3 g/d | Proteinuria ≥3 g/d | eGFR 20–59 ml/min/1.73 m2 | eGFR >60 ml/min/1.73 m2 |
| Mean baseline eGFR, ml/min/1.73 m2 | 51.1 | 40.0 | 38.6a | 18.5a | ~32.9–35.9 | ~31.1–41.7 | ~47.8–47.9 | ~81.1–81.3 |
| Baseline UPCR or UPE | Median: 0.04 g/g | Median: 0.68 g/g | Median: 0.15 g/g | Median: 0.63 g/g | Mean: ~1.7–1.8 g/d | Mean: ~4.9 g/d | n/a | n/a |
| Baseline UACR | n/a | n/a | n/a | n/a | n/a | n/a | Mean; ~41.1–44.1 mg/g | |
| Kidney function declinec | HR: 1.18 (0.93–1.50) | HR: 0.73 (0.58–0.93) | −1.6 (−0.8, 3.9) ml/min/3 y | −0.5 (−0.4, 1.4) ml/min/y | HR: 1.06 (0.51–2.20) | HR: 1.09 (0.55–2.19) | HR: 0.89 (0.42–1.87) | HR: 3.49 (2.44–5.10) |
| p-interaction | 0.02 | 0.02b | 0.01b | n/a | n/a | |||
denotes GFR;
for interaction with baseline proteinuria (<1 g/d vs. 1-<3 g/d vs. ≥3 g/d);
defined in each study as follows: AASK (HR for doubling of serum creatinine, KFRT, or death); MDRD Study (mean difference in rate of GFR decline); REIN-II (HR for KFRT); SPRINT, baseline eGFR 20–59 ml/min/1.73 m2 (HR for first occurrence of ≥50% reduction in eGFR, maintenance dialysis, or kidney transplantation); SPRINT, eGFR ≥60 ml/min/1.73 m2 (HR for ≥30% reduction in eGFR to a level <60 ml/min/1.73 m2). Values in parentheses are 95% CI. Abbreviations: AASK, African American Study of Kidney Disease and Hypertension; GFR= glomerular filtration rate; eGFR=estimated glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; UPCR=urinary protein-creatinine ratio; UPE=urine protein excretion; UACR=urinary albumin-creatinine ratio; HR=hazard ratio; REIN, Ramipril Efficacy in Nephropathy; SPRINT, Systolic Blood Pressure Intervention Trial; n/a=not available.
Based on information in Appel et al 2010 (NEJM, https://doi.org/10.1056/nejmoa0910975); Klahr et al 1994 (NEJM, https://doi.org/10.1056/nejm199403313301301); Ruggenenti et al 2005 (Lancet, https://doi.org/10.1016/s0140-6736(05)71082-5); The SPRINT Research Group 2015 (NEJM, https://doi.org/10.1056/nejmoa1511939).
More recently, SPRINT randomized adults without diabetes but at increased risk for cardiovascular events to a systolic BP <120 versus <140 mmHg. Intensive BP control was associated with lower risk of myocardial infarction, acute coronary syndrome, stroke, heart failure, and cardiovascular death (hazard ratio [HR], 0.75 [95% CI, 0.64–0.89]) and all-cause mortality (HR, 0.73 [95% CI, 0.60–0.90]). Results were consistent among participants with baseline CKD (n=2,646). Intensive BP control did not prevent adverse kidney outcomes (≥50% eGFR decline or KFRT). Among participants without baseline CKD (n=6,677), intensive BP control resulted in 3.5-fold higher risk of ≥30% reduction in eGFR to <60 ml/min/1.73 m2, a finding which may reflect hemodynamic changes rather than true kidney injury.
For Question 1, (a) reduced all-cause mortality is the correct answer. Lower BP goal did not slow progression of CKD, and SPRINT was not powered to assess KFRT and kidney transplantation events. For Question 2, (c) the patient with CKD G3bA3 and UACR 3,000 mg/g would most likely benefit from a lower BP goal based on subgroup analysis from clinical trials. Patients with A1 albuminuria, critical bilateral renal artery stenosis, or repeated falls are less likely to benefit from a lower BP goal or may be at higher risk of treatment-related complications.
Additional Readings
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3(1):1–150.*ESSENTIAL READING
Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71(6):e13-e115.
Wright JT, Bakris G, Greene T, et al; Effect of Blood Pressure Lowering and Antihypertensive Drug Class on Progression of Hypertensive Kidney Disease: Results from the AASK trial. JAMA. 2002;288(19):2421–2431.
Klahr S, Levey AS, Beck GJ, et al; The Effects of Dietary Protein Restriction and Blood-Pressure Control on the Progression of Chronic Renal Disease. N Engl J Med. 1994;330(13):877–884.*ESSENTIAL READING
Appel LJ, Wright JT, Greene T, et al; Intensive Blood-Pressure Control in Hypertensive Chronic Kidney Disease. N Engl J Med 2010;363(10):918–929.*ESSENTIAL READING
Ruggenenti P, Perna A, Loriga G, et al; Blood-pressure control for renoprotection in patients with non-diabetic chronic renal disease (REIN-2): multicenter, randomized controlled trial. Lancet.2005;365(9463):939–946.
The SPRINT Research Group. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373(22):2103–2116.*ESSENTIAL READING
Cheung AK, Rahman M, Reboussin DM, et al; Effects of Intensive BP Control in CKD. J Am Soc Nephrol. 2017;28(9):2812–2823.
RAAS inhibition
Case 2: A 46 year-old woman with type 2 diabetes returns for her 2nd appointment. History is notable for retinopathy and CKD G3aA3 attributed to diabetic kidney disease. She denies orthostatic symptoms and chest discomfort. Automated office BP is 118/75 mmHg on atenolol and chlorthalidone. Laboratory testing reveals stable eGFR (at 55 ml/min/1.73 m2) with UACR 1,200 mg/g.
Question 3: Which one of the following would be the most appropriate anti-hypertensive therapy to help slow CKD progression?
No change in therapy since BP is controlled to goal
Change atenolol to an angiotensin-receptor blocker (ARB)
Change chlorthalidone to an ARB
Add an ARB to the current 2 drug regimen
Case 3: A 56 year-old woman with CKD G3aA3 due to biopsy-proven diabetic kidney disease has an average out-of-office BP of 144/83 mmHg on a regimen of lisinopril 20 mg daily, chlorthalidone 50 mg daily, and amlodipine 10 mg daily. UACR is 800 mg/g.
Question 4: Which one of the following interventions would be most appropriate to reduce the risk of CKD progression?
Add an ARB to current regimen
Change lisinopril to a mineralocorticoid receptor antagonist (MRA)
Increase lisinopril dose
Change chlorthalidone to indapamide
For the answers to the questions, see the following text.
The cornerstone of albuminuria management is RAAS inhibition. The KDIGO guidelines recommend that all adults with CKD, hypertension, and UACR >300 mg/g be treated with an angiotensin-converting-enzyme inhibitor (ACEI) or ARB. Among those with diabetes and UACR > 30 mg/g, ACEI or ARB use should be considered. RAAS inhibition in patients with CKD and hypertension is also supported by all major hypertension guidelines (Table 1). Multiple trials have demonstrated that ACEI or ARB therapy delays CKD progression among individuals with albuminuria (Table 3). The REIN trial, which randomized patients with CKD to ramipril versus placebo, showed that mean GFR decline was significantly slower in the ramipril group among participants with proteinuria ≥3 g/d. In the RENAAL study, patients with type 2 diabetes and CKD randomized to losartan had a 16% lower risk of developing a doubling of serum creatinine, KFRT, or death compared to placebo. Similarly, IDNT reported that irbesartan was associated with lower risk of a doubling of serum creatinine, serum creatinine ≥6.0 mg/dL, KFRT, or death compared to amlodipine or placebo among patients with hypertension and CKD attributed to type 2 diabetes. Finally, in AASK, use of ramipril was independently associated with 22% and 38% lower risks of the clinical composite outcome (GFR decline ≥50% or ≥25 ml/min/1.73 m2 from baseline, KFRT, or death) compared to metoprolol and amlodipine, respectively.
Table 3:
Summary of major clinical trials on ACEI and ARB therapy on kidney function decline.
| REIN, Stratum 2 (n=166) | RENAAL (n=1,513) | AASK (n=1,094) | IDNT (n=1,715) | VA NEPHRON-D (n=1,448) | |
|---|---|---|---|---|---|
| Kidney-related inclusion criteria | CLcr 20–70 ml/min/1.73 m2; proteinuria ≥3 g/d | Scr 1.3–3.0 mg/dL; UACR ≥300 mg/g | GFR 20–65 ml/min/1.73 m2; UPCR ≤2.5 g/d | Scr 1.0 (F) or 1.2 (M) 3.0 mg/dL; proteinuria ≥900 mg/d | eGFR 30-<90 ml/min/1.73 m2; UACR ≥300 mg/g |
| Follow-up | Mean: ~1.3 y | Mean: 3.4 y | Range: 3.0–6.4 y | Mean: 2.6 y | Median: 2.2 y |
| % with diabetes | 0%* | 100% | 0% | 100% | 100% |
| % with HTN | 87% | 93%c | 100% | 100% | n/a |
| Intervention | Ramipril vs Placebo | Losartan vs Placebo | Ramipril vs Metoprolol vs Amlodipine | Irbesartan vs Placebo vs Amlodipine | Losartan + Lisinopril vs Losartan + Placebo |
| Mean baseline eGFR, GFR, or Scr | GFR 37.4–40.2 ml/min/1.73 m2 | Scr ~1.9b mg/dL | GFR 45.6 ml/min/1.73 m2 | Scr ~1.7b mg/dL | eGFR ~53.6–53.7 ml/min/1.73 m2 |
| Baseline UPCR or UPE | Mean: 5.1–5.6 g/d | n/a | Median: 0.08 g/g | Median: ~2.9 g/d | Median: ~1.6–2.1 g/g |
| Baseline UACR or UAE | n/a | Median: ~1,237–1,261 mg/g | n/a | Median: ~1.9 g/d | Median: 847 mg/g |
| Kidney function decline** | 0.53 vs 0.88 ml/min/mo; p=0.03a | HR: 0.84 (0.72–0.98) | Risk Reduction for ramipril: 22% (1%−38%) vs. Metoprolol, 38% (14%−56%) vs. Amlodipine | RR for Irbesartan: 0.81 (0.67–0.99) vs. Placebo, 0.76 (0.63–0.92) vs. Amlodipine | HR: 0.88 (0.70–1.12) |
Analysis among 117 participants with at least 3 GFR measurements;
eGFR or GFR not available;
Percent receiving anti-HTN drugs at baseline;
None with “insulin-dependent diabetes mellitus”
Abbreviations: ACEI=angiotensin-converting-enzyme inhibitor; ARB= angiotensin-receptor blocker; GFR=glomerular filtration rate; eGFR=estimated glomerular filtration rate; UPCR=urinary protein-creatinine ratio; UPE=urine protein excretion; UACR=urinary albumin-creatinine ratio; UAE=urine albumin excretion; RR=relative risk; Scr= serum creatinine; RENAAL, Reduction in End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan; VA NEPHRON-D, Veterans Affairs Nephropathy in Diabetes Study; CLcr, creatinine clearance; IDNT, Irbesartan Diabetic Nephropathy Trial; HTN, hypertension; REIN, Ramipril Efficacy in Nephropathy; F, female; M, male; n/a=not available.
defined in each study as follows: RENAAL (HR for SCr doubling, KFRT, or death); IDNT (RR for SCr doubling, SCr≥6.0 mg/dL, KFRT, or death); AASK (GFR decline ≥50% or ≥25 ml/min/1.73 m2 from baseline, KFRT, or death); VA NEPHRON-D (HR first occurrence of absolute decline in eGFR ≥30 ml/min/1.73 m2 if eGFR at randomization ≥60 ml/min/1.73 m2, relative decline in eGFR ≥50% if eGFR at randomization <60 ml/min/1.73 m2; eGFR <15 ml/min/1.73 m2, KFRT; or death).
Based on information in The GISEN Group 1997 (Lancet, https://doi.org/10.1016/S0140-6736(96)11445-8), Brenner et al 2001 (NEJM, https://doi.org/10.1056/nejmoa011161), Wright et al 2002 (JAMA, https://doi.org/10.1001/jama.288.19.2421), Lewis et al 2001 (NEJM, https://doi.org/10.1056/nejmoa011303), Fried et al 2013 (NEJM, https://doi.org/10.1056/nejmoa1303154).
Current literature does not support the use of dual blockade with an ACE-I and ARB in diabetic kidney disease. VA NEPHRON-D, which randomized veterans with type 2 diabetes and CKD G2-G3bA3 to losartan plus lisinopril or losartan alone, was terminated early due to safety concerns with the combination therapy group having a markedly higher risk of hyperkalemia (HR, 2.8 [95% CI, 1.8–4.3]) and acute kidney injury (AKI) (HR, 1.7 [95% CI, 1.3–2.2]) compared to the monotherapy group. Additionally, there was no significant difference in risk of kidney function decline between the two treatment groups, though follow-up was short (Table 3).
Decreased sodium intake may enhance the renoprotective effects of RAAS inhibitors. A meta-analysis of 11 studies (23 cohorts with 516 participants) reported that dietary sodium restriction (average decrease of 92 mmol/d) was associated with a 32% lower urine albumin excretion (UAE). The reduction in UAE was greater in cohorts with concomitant RAAS blockade therapy than in cohorts without (pooled mean differences of −41.9% and −17.2%, respectively; p = 0.01 for interaction), suggesting a synergistic effect of low sodium intake with RAAS inhibition. In a post-hoc analysis of 500 participants in REIN and REIN II trials receiving ramipril therapy, a diet with >14 g/d of salt was associated with 3.3-fold and 2.4-fold greater risks of kidney failure compared to diets of <7 g/d and 7–14 g/d of salt, respectively. Importantly, the proteinuria-reducing effects of ramipril were greatest in the low sodium diet group. In another post-hoc analysis of the RENAAL study and IDNT (n=1,177), ARB therapy was associated with a 43% lower risk of a renal event, defined as a doubling of serum creatinine or KFRT, compared to non-RAAS inhibitor therapy among participants in the lowest tertile of 24-hour urinary sodium-creatinine ratio with no significant difference in risk between the two treatment groups for higher tertiles of sodium intake (P < 0.001 for interaction; Figure 1). Given these findings, patients on RAAS inhibitors for treatment of albuminuria should be encouraged to follow a low sodium diet.
Figure 1:
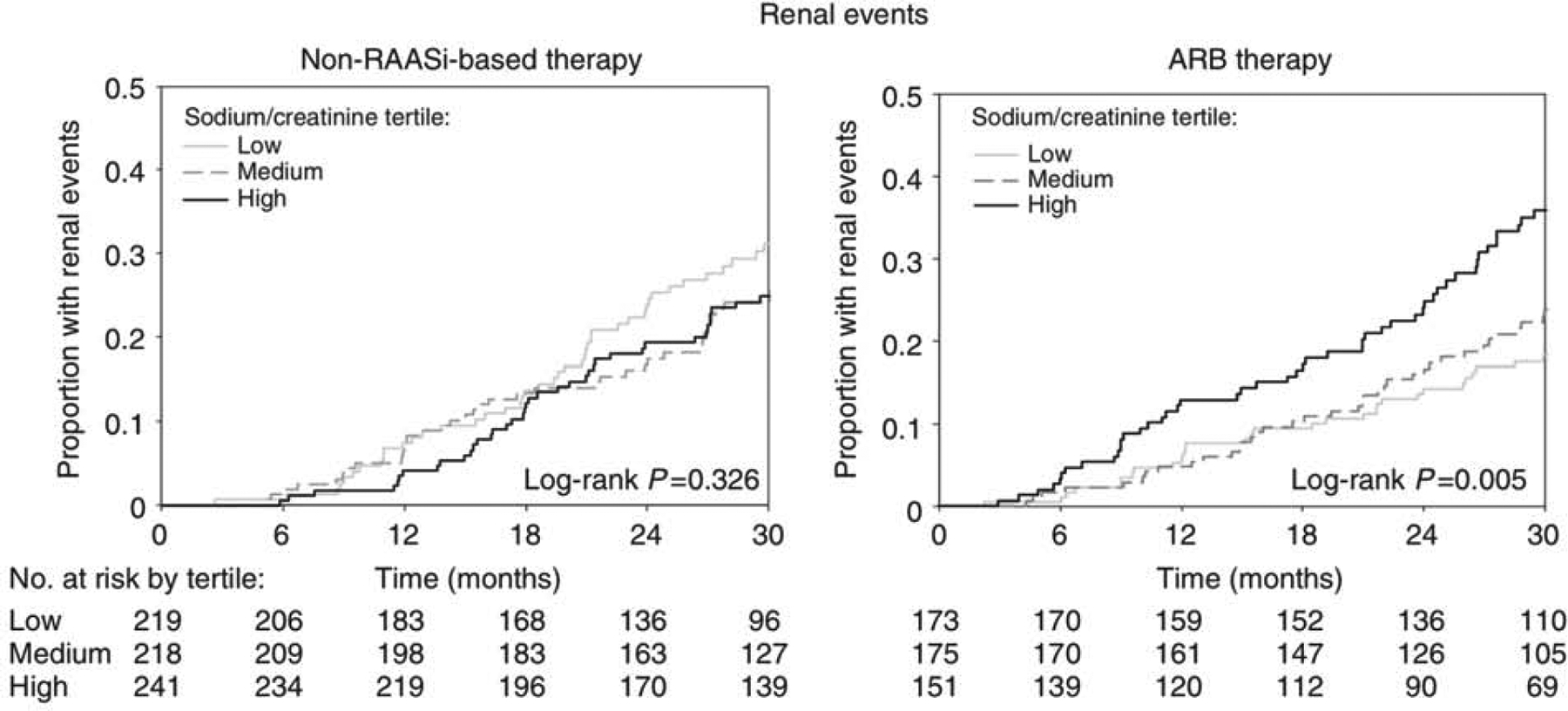
Kaplan-Meier curves for renal events by tertiles of 24-hour urinary sodium-creatinine ratio (<121 mmol/g; 121 to <153 mmol/g; ≥153 mmol/g) among RENAAL and IDNT trial participants on non-RAASi-based therapy and ARB therapy. Renal event defined as a doubling of serum creatinine from baseline or KFRT (RENAAL and IDNT) or serum creatinine ≥6.0 mg/dL (IDNT only). Adapted from Heerspink et al 2012 (Kidney Int. https://doi.org/10.1038/ki.2012.74) with permission of the copyright holder. Original graphic © 2012 International Society of Nephrology. Abbreviations: Non-RAASi=non-renin-angiotensin-aldosterone system inhibitor; ARB=angiotensin receptor blocker.
For patients intolerant of ACEI/ARB therapy, a MRA can be considered. A recent meta-analysis of 31 randomized controlled trials evaluated the efficacy and safety of MRAs (spironolactone, eplerenone, canrenone, or finerenone) compared to active control or placebo in reducing albuminuria. In the 18 trials (n=2,036) that examined UACR as an outcome, proportional change in UACR from baseline to end of treatment was 22% lower in MRAs compared to active control and placebo. The effect persisted when comparing MRAs to placebo (n=1,436 in 11 trials) in patients on ACE-I/ARB therapy. When comparing MRAs to renin-angiotensin blockers, there was no significant difference in change in albuminuria (n=201 in 2 trials) but risk of incident hyperkalemia was 70% higher (n=855 in 5 trials). Although reduction in albuminuria is not a universally accepted surrogate endpoint for KFRT, the FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) trial of patients with type 2 diabetes and CKD (>98% on concomitant ACE-I or ARB therapy) reported that finerenone conferred an 18% lower risk of composite kidney outcome (sustained decline in eGFR by ≥40% or to <15 ml/min/1.73 m2, KFRT, or death from kidney causes) compared to placebo. Thus, MRAs reduce albuminuria and may also slow CKD progression. These benefits, however, must be balanced against the potential risk of hyperkalemia.
In Question 3, (b) changing atenolol to an ARB is the correct answer. ACE-I and ARB have been shown to slow progression of CKD in patients with diabetes while beta-blockers have not. In the management of hypertension, beta-blockers are add-on therapy after the use of 1st-line agents such as ACE-I or ARB and thiazide diuretics. Adding an ARB to the current regimen is less desirable, as this may result in hypotension in a patient with BP already controlled to goal. In Question 4, (c) increasing the lisinopril dose is the best answer. Combination ACE-I and ARB therapy is associated with an increased risk of adverse outcomes. Although MRA may reduce albuminuria when combined with an ACE-I or ARB, no randomized controlled trials have been performed to support changing an ACE-I to an MRA with the intent of slowing progression to KFRT. Exchanging chlorthalidone for indapamide is not anticipated to slow this progression.
Additional Readings
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3(1):1–150.*ESSENTIAL READING
The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia). Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuria, non-diabetic nephropathy. Lancet. 1997;349(9069):1857–1863.
Brenner BM, Cooper ME, De Zeeuw D, et al; Effects of Losartan on Renal and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Nephropathy. N Engl J Med. 2001;345(12):861–869.*ESSENTIAL READING
Wright JT, Bakris G, Greene T, et al; Effect of Blood Pressure Lowering and Antihypertensive Drug Class on Progression of Hypertensive Kidney Disease: Results from the AASK trial. JAMA. 2002;288(19):2421–2431.*ESSENTIAL READING
Lewis EJ, Hunsicker LG, Clark WR, et al; Renoprotective Effect of the Angiotensin-Receptor Antagonist Irbesartan in Patients with Nephropathy due to Type 2 Diabetes. N Engl J Med. 2001;345(12):851–860.
Fried LF, Emanuele N, Zhang JH, et al; Combined Angiotensin Inhibition for the Treatment of Diabetic Nephropathy. N Engl J Med. 2013;369(20):1892–1903.*ESSENTIAL READING
D’Elia L, Rossi G, Schiano di Cola M, et al; Meta-Analysis of the Effect of Dietary Sodium Restriction with or without Concomitant Renin-Angiotensin-Aldosterone System-Inhibiting Treatment on Albuminuria. Clin J Am Soc Nephrol. 2015;10(9):1542–1552.
Vegter S, Perna A, Postma MJ, et al; Sodium Intake, ACE Inhibition, and Progression to ESRD. J Am Soc Nephrol.2012;23(1):165–173.*ESSENTIAL READING
Lambers Heerspink HJ, Holtkamp FA, Parving HH, et al; Moderation of dietary sodium potentiates the renal and cardiovascular protective effects of angiotensin receptor blockers. Kidney Int. 2012;82(3):330–337.
Alexandrou ME, Papagianni A, Tsapas A, et al; Effects of mineralocorticoid receptor antagonists in proteinuric kidney disease: a systematic review and meta-analysis of randomized controlled trials. J Hypertens. 2019;37(12):2307–2324.*ESSENTIAL READING
Bakris GL, Agarwal R, Anker SD, et al; Effect of Finerenone on Chronic Kidney Disease Outcomes in Type 2 Diabetes. N Engl J Med. 2020;383(23):2219–2229.
Glycemic control
Case 4: A 48 year-old man with type 2 diabetes is seen for routine follow-up. He has stable CKD (G3aA2 for 3 years) in addition to retinopathy, hypertension, and hyperlipidemia. He is currently taking losartan 50 mg daily, metoprolol 75 mg twice daily, metformin 500 mg twice daily, and atorvastatin 20 mg daily. His BP is 127/68 mm Hg with heart rate of 64 bpm. The remainder of the physical examination is unremarkable. Laboratory evaluation demonstrates eGFR 52 ml/min/1.73 m2, UACR 35 mg/g, and hemoglobin A1c (HbA1c) 7.9%.
Question 5: Which one of the following interventions would be most likely to slow progression of his CKD?
Increase losartan to reduce BP to <120/80 mm Hg
Change metoprolol to a dihydropyridine calcium channel blocker
Increase metformin to target HbA1c <7%
No changes, as management of hypertension and glycemic control are at goal
For the answer to the question, see the following text.
The 2020 KDIGO guidelines on diabetes in CKD recommend that HbA1c goals be individualized based on CKD severity, comorbidities, and hypoglycemia risk, among other factors (Table 4). Dosing adjustments or discontinuation of glucose-lowering agents are often necessary as CKD progresses. In particular, insulins, sulfonylureas, and meglitinides are more likely to cause hypoglycemia in the patient with reduced kidney function.
Table 4:
Factors to consider when determining hemoglobin A1c targets in patients with diabetes and chronic kidney disease.
| Factors | Goal hemoglobin A1c | |
|---|---|---|
| <6.5% | <8.0% | |
| CKD severity | Lower (e.g., G1) | Higher (e.g., G5) |
| Macrovascular complications | None or mild | Severe |
| Comorbidities | None or few | Many |
| Life expectancy | Long | Short |
| Hypoglycemia awareness | Good | Diminished |
| Resources for hypoglycemia management | Available | Limited |
| Likelihood of treatments causing hypoglycemia | Low | High |
Based on information in KDIGO Diabetes Work Group 2020 (Kidney Int, https://doi.org/10.1016/j.kint.2020.06.019). Abbreviations: CKD, chronic kidney disease; G, glomerular filtration rate category
Most major randomized controlled trials suggest that intensive glycemic control reduces albuminuria and possibly also kidney function decline in patients with diabetes (Table 5). In the ADVANCE-ON and DCCT/EDIC studies, intensive glycemic control was associated with a 46% lower risk of KFRT or death from kidney disease and 50% lower risk of incident eGFR <60 ml/min/1.73 m2, respectively. A large meta-analysis of the ADVANCE, ACCORD, UKPDS, and VADT trials showed that more intensive glycemic control was associated with a 20% lower risk of developing a primary kidney event (i.e., incident eGFR <30 ml/min/1.73 m2, UACR >300 mg/g, KFRT, or death from kidney disease), mostly due to a reduction in risk of albuminuria. Participants with more intensive control were also more likely to have regression of UACR from >300 to 30–300 mg/g (HR, 1.23 [95% CI, 1.03–1.48]), regression of UACR from 30–300 to < 30 mg/g (HR, 1.15 [95% CI, 1.03–1.28]), and maintenance of UACR < 30 mg/g (HR, 1.16 [95% CI, 1.08–1.25]).
Table 5:
Summary of major clinical trials on intensive versus standard glycemic control on kidney outcomes.
| ADVANCE (n=11,140) | ACCORD (n=10,251) | UKPDS (n=3,867) | VADT (n=1,791) | DCCT (n=1,441) | EDIC (n=1,349) | |
|---|---|---|---|---|---|---|
| Kidney-related inclusion criteria | Not specified | Scr ≤132.6 μmol/L | Scr ≤175 μmol/L | Scr ≤1.6 mg/dL | Scr <1.2 mg/dL or CLcr >100 ml/min/1.73 m2; UAE <40 mg/d | Scr <1.2 mg/dL or CLcr >100 ml/min/1.73 m2; UAE <40 mg/d |
| Follow-up | Median: 5 y | Mean: ~3.5 y | Median: ~10 y | Median: 5.6 y | Mean: 6.5 y | Mean: ~8 y |
| Diabetes type | Type 2 | Type 2 | Type 2 | Type 2 | Type 1 | Type 1 |
| Diabetes duration | Median: 7 y | Median: ~10 y | “newly diagnosed” | Mean: ~11.5 y | Mean: ~2.6a & ~8.6–8.9b y | Mean: ~12 y |
| Intervention | HbA1c ≤6.5% vs. standard* | HbA1c <6.0% vs. 7.0%−7.9% | Median HbA1c ~7.0% vs. ~7.9% | Median HbA1c ~6.9% vs. 8.4% | HbA1c <6.05% vs. standard | None (obs follow-up of DCCT) |
| Mean baseline HbA1c | ~7.5% | ~8.1% | 7.08% | ~9.4% | ~8.8%a & ~8.9–9.0b | ~7.4d & 9.1e |
| Baseline eGFR, CLcr, or Scr | Mean eGFR ~78.0–78.3 ml/min/1.73 m2 | Median eGFR ~90 ml/min/1.73 m2 | n/a | Mean Scr ~1.0 mg/dL | Mean CLcr ~127–128a & ~128–130b mL/min | Mean CLcr ~122 mL/min |
| Baseline UACR or UAE* | Median UACR ~14.9–15.0 μg/mg | Median UACR ~1.54 mg/mmol | n/a | n/a | Mean UAE ~12a & ~19–21b mg/d | Median UAE 8.6d & 10.1e mg/d |
| Kidney outcomec | UACR ≥30 μg/mg: HR, 0.91 (0.85–0.98); UACR >300 μg/mg: HR, 0.70 (0.57–0.85); KFRT: HR, 0.35 (0.15–0.83) | UACR ≥30 mg/g: HR, 0.79 (0.69–0.90); UACR ≥300 mg/g: HR, 0.69 (0.55–0.85); KFRT or SCr >291.72 μmol/L: HR, 0.95 (0.73–1.24) | UACR >30 mg/g: RR, 0.70 (0.46–1.07); “Proteinuria”: RR, 0.58 (0.23–1.43); Pcr doubling: RR, 1.25 (0.16–9.55) | Any ↑ in albuminuria: 9.1% vs. 13.8% (p=0.01); From normal to UACR ≥30 mg/g: 10.0% vs. 14.7% (p=0.03); Scr doubling: 8.8% vs. 8.8% (p=0.99) | Risk reduction: 39% (21%−52%) for UAE ≥40 mg/d; 54% (19%−74%) for UAE ≥300 mg/d | Risk reduction: 59% (39%−73%) for UAE ≥40 mg/d; 84% (67%−92%) for UAE >300 mg/d |
| Risk reduction: 50% (18%, 69%)f for sustained eGFR <60 ml/min/1.73 m2 | ||||||
primary prevention cohort and
secondary intervention cohort of the DCCT;
for UKPDS, from 0–15 years of follow-up with outcome assessed every 3 years;
intensive and
conventional groups of original DCCT;
over median follow-up of 22 years (includes DCCT and EDIC years 1–16);
based on local guidelines;
Baseline UPCR information not available.
Abbreviations: eGFR=estimated glomerular filtration rate; UPCR=urinary protein-creatinine ratio; UACR=urinary albumin-creatinine ratio; UAE=urine albumin excretion; CLcr=creatinine clearance; HbA1c=hemoglobin A1c; RR, relative risk; obs, observational; ADVANCE, Action in Diabetes and Cardiovascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation; ACCORD, Action to Control Cardiovascular Risk in Diabetes; DCC, Diabetes Control and Complications Trial; EDIC, Epidemiology of Diabetes Interventions and Complications; UKPDS, UK Prospective Diabetes Study; VADT, Veterans Affairs Diabetes Trial; n/a=not available.
Based on information in Perkovic et al 2013 (Kidney Int, https://doi.org/10.1038/ki.2012.401), Ismail-Beigi 2010 (Lancet. https://doi.org/10.1016/s0140-6736(10)60576-4); UKPDS Group 1998 (Lancet. https://doi.org/10.1016/S0140-6736(98)07019-6); Duckworth et al 2009 (NEJM, https://doi.org/10.1056/nejmoa0808431), DCCT group 1993 (NEJM, https://doi.org/10.1056/nejm199309303291401); EDIC group 2003 (JAMA. https://doi.org/10.1001/jama.290.16.2159), DCCT/EDIC group 2011 (NEJM, https://doi.org/10.1056/nejmoa1111732).
For Question 5, (c) targeting a HbA1c of 7% or less is the best option among those listed to help slow CKD progression. In patients with CKD G4-G5 or significant competing comorbidities where risk of hypoglycemia is higher and benefits of intense control less well-established, the HbA1c target should be individualized. The patient’s BP is currently controlled to goal, and further reduction or substitution of a dihydropyridine calcium blocker for a beta-blocker has not been shown to slow CKD progression.
Additional Readings
Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020;98(4S):S1-S115.*ESSENTIAL READING
Bilo H, Coentrão L, Couchoud C, et al. for the Guideline Development Group; Clinical Practice Guideline on Management of Patients With Diabetes and Chronic Kidney Disease Stage 3b or Higher (eGFR<45 ml/min). Nephrol Dial Transplant. 2015;30(Suppl 2):ii1-ii142.
Ismail-Beigi F, Craven T, Banerji MA, et al; Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomized trial. Lancet. 2010;376(9739):419–430.
Perkovic V, Heerspink HL, Chalmers J, et al; Intensive glucose control improves kidney outcomes in patients with type 2 diabetes. Kidney Int. 2013;83(3):517–523.
Wong MG, Perkovic V, Chalmers J, et al. for the ADVANCE-ON Collaborative Group; Long-term Benefits of Intensive Glucose Control for Preventing End-Stage Kidney Disease: ADVANCE-ON. Diabetes Care. 2016;39(5):694–700.
The Diabetes Control and Complications Trial Research Group; The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N Engl J Med. 1993;329(14):977–986.
Epidemiology of Diabetes Interventions and Complications Research Group; Sustained Effect of Intensive Treatment of Type 1 Diabetes Mellitus on Development and Progression of Diabetic Nephropathy. JAMA. 2003;290(16):2159–2167.
The DCCT/EDIC Research Group. Intensive Diabetes Therapy and Glomerular Filtration Rate in Type 1 Diabetes. N Engl J Med. 2011;365(25):2366–2376.
UK Prospective Diabetes Study Group; Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998;352(9131):837–853.
Duckworth W, Abraira C, Mortiz T, et al. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N Engl J Med. 2009;360(2):129–139.
Zoungas S, Arima H, Gerstein HC, et al; Effects of intensive glucose control on microvascular outcomes in patients with type 2 diabetes: a meta-analysis of individual participant data from randomized controlled trials. Lancet Diabetes Endocrinol. 2017;5(6):431–437.*ESSENTIAL READING
SGLT-2 inhibitors
Case 4, cont: The patient returns for follow-up. His CKD has steadily worsened over the past 12 months. Laboratory studies reveal:
| Parameter | Present | 6 mo prior | 12 mo prior |
|---|---|---|---|
| Sodium, mEq/L | 132 | 134 | 131 |
| Potassium, mEq/L | 5.3 | 5.2 | 5.4 |
| Glucose, mg/dL | 315 | 280 | 210 |
| eGFR, mL/min/1.73 m2 | 46 | 51 | 52 |
| UACR, mg/g | 1,000 | 400 | 35 |
| HbA1c | 9.4% | -- | 7.9% |
Question 6: Which one of the following interventions would be the next best step?
Start SGLT2 inhibitor to reduce albuminuria
Start SGLT2 inhibitor to achieve an HbA1c <7%
Do not start SGLT2 inhibitor because of risk for worsening hyperkalemia
Do not start SGLT2 inhibitor because of risk for worsening hyponatremia
Case 5: A 70 year-old woman with moderate obesity (BMI 32 kg/m2) and uncontrolled diabetes mellitus (HbA1c 8.2%) returns to discuss management of her CKD G3aA2. Her history includes coronary artery disease and recurrent furunculosis requiring antibiotics several times per year.
Question 7: In counseling her on the potential benefits and risks of therapy with an SGLT2 inhibitor, for which one of the following adverse effects is she at greatest risk?
Genitourinary fungal infections
Lower extremity amputation
Severe hypoglycemia
Acute kidney injury
For the answers to the questions, see the following text.
In recent years, SGLT2 inhibitors have emerged as new and exciting therapies for delaying CKD progression, particularly among patients with type 2 diabetes and/or albuminuria. Current ADA and EASD guidelines recommend that SGLT2 inhibitors be considered in all patients with type 2 diabetes at risk of CKD progression, regardless of cardiovascular disease history (Table 1).
Initial reports of the potential kidney protective effects of SGLT2 inhibitors came from cardiovascular outcome trials. These studies, however, primarily included individuals with mild or no CKD and were limited by small numbers of kidney events. In 2019, the results of the landmark CREDENCE trial were published. This trial, the first to examine the association of a SGLT2 inhibitor with a primary kidney outcome, reported that among patients with type 2 diabetes and CKD G2-G3bA3, randomization to canagliflozin was associated with a 30% lower risk (95% CI, 18%−41%) of developing a composite outcome (KFRT, sustained eGFR <15 ml/min/1.73 m2, doubling of serum creatinine from baseline, death from kidney disease, or death from cardiovascular disease) compared to placebo. Similar conclusions were obtained when considering individual components of the composite outcome. Importantly, all participants were on an ACE-I or ARB, thus suggesting that the benefits of canagliflozin extended beyond standard pharmacologic therapy (i.e., RAAS inhibition).
Neuen et al. performed a meta-analysis of four randomized-controlled trials that investigated the effect of SGLT2 inhibitors on major kidney outcomes among patients with type 2 diabetes and eGFR ≥30 ml/min/1.73 m2: CREDENCE, CANVAS Program, EMPA-REG OUTCOME, and DECLARE-TIMI 58. The majority of patients in the latter 3 trials did not have baseline CKD (Table 6). Among 38,723 participants, use of SGLT2 inhibitors was associated with a 33% lower risk of a composite outcome of dialysis, transplantation, or death from kidney disease compared to placebo. Importantly, the benefits of SGLT2 inhibitors were statistically significant in all subgroups of baseline eGFR (30-<45; 45-<60; 60-<90; and ≥90 ml/min/1.73 m2). Estimated GFR decline was also slower in the SGLT2 inhibitor group versus placebo in CREDENCE (absolute difference, 2.74 [95% CI, 2.37–3.11] ml/min/1.73 m2 per year), CANVAS Program (absolute difference, 1.18 [95% CI, 1.02–1.35] ml/min/1.73 m2 per year), and EMPA-REG OUTCOME (absolute difference, 1.68 [95% CI, 1.02–1.35] ml/min/1.73 m2 per year).
Table 6:
Summary of trials on SGLT2 inhibitors with kidney outcomes in patients with and without type 2 diabetes.
| Trial | CREDENCE (n=4,401) | CANVAS Program (n=10,142) | EMPA-REG OUTCOME (n=7,020) | DECLARE-TIMI 58 (n=17,160) | DAPA-CKD (n=4,304) |
|---|---|---|---|---|---|
| Study or Participant Characteristics | |||||
| Inclusion criteria | eGFR 30–<90 ml/min/1.73 m2; UACR >300–5000 mg/g | eGFR ≥30 ml/min/1.73 m2 | eGFR ≥30 ml/min/1.73 m2 | CLcr ≥60 ml/min | eGFR 25–75 ml/min/1.73 m2; UACR 200–5,000 mg/g |
| SGLT2i | Canagliflozin | Canagliflozin | Empagliflozin | Dapagliflozin | Dapagliflozin |
| Median follow-up, y | 2.6 | 2.4 | 3.1 | 4.2 | 2.4 |
| Baseline ACEI or ARB use | 4,395 (99.9%) | 8,116 (80%) | 5,666 (81%) | 13,950 (81%) | 1354 (31%)a; 2,870 (67%)b |
| eGFR | |||||
| ≥90 ml/min/1.73 m2 | 0 (0%) | 2,476 (24%) | 1,538 (22%) | 8,162 (48%) | 0 (0%) |
| 60–<90 ml/min/1.73 m2 | 1,809 (41%) | 5,625 (55%) | 3,661 (52%) | 7,732 (45%) | 454 (11%) |
| 45–<60 ml/min/1.73 m2 | 1,279 (29%) | 1,485 (15%) | 1,249 (18%) | 1,265 (7%) | 1,328 (31%) |
| 30–<45 ml/min/1.73 m2 | 1,313 (30%) | 554 (5%) | 570 (8%) | N/A | 1,898 (44%) |
| <30 ml/min/1.73 m2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 624 (14%) |
| Missing | 0 (0%) | 2 (<0.1%) | 2 (<0.1%) | 1 (<0.1%) | 0 (0%) |
| UACR | |||||
| <30 mg/g | 0 (0%) | 7,007 (69%) | 4,171 (59%) | 11,644 (68%) | n/ac |
| 30–300 mg/g | 0 (0%) | 2,266 (22%) | 2,013 (29%) | 4,030 (24%) | n/a |
| >300 mg/g | 4,401 (100%) | 760 (7%) | 769 (11%) | 1,169 (7%) | n/a |
| Missing | 0 (0%) | 109 (1%) | 67 (1%) | 317 (2%) | n/a |
| Relative Risk or Hazard Ratio comparing SGLT2i to placebo | |||||
| Dialysis, Tx, or death from kidney disease | 0.72 (0.54–0.97) | 0.56 (0.23–1.32) | 0.90 (0.30–2.67) | 0.42 (0.20–0.87) | N/A |
| Dialysis, Tx, or sustained eGFR < 15 mL/min/1.73 m2 ** | 0.68 (0.54–0.86) | 0.77 (0.30–1.97) | 0.60 (0.18–1.98) | 0.31 (0.13–0.79) | 0.64 (0.50–0.82) |
| Substantial loss of kidney function*; Dialysis, Tx, or sustained eGFR < 15 mL/min/1.73 m2 **; or death from kidney disease | 0.66 (0.53–0.81) | 0.53 (0.33–0.84) | 0.54 (0.40–0.75) | 0.53 (0.43–0.66) | 0.56 (0.45–0.68) |
on ACEI;
on ARB;
n=2079 (48%) with UACR>1,000 mg.
All participants in CREDENCE, CANVAS Program, EMPA-REG OUTCOME, and DECLARE-TIMI 58 were with type 2 diabetes. In DAPA-CKD, 67.5% of participants were with type 2 diabetes.
defined as a doubling of serum creatinine with the exception of DECLARE-TIMI 58 (sustained 40% decline in eGFR) and DAPA-CKD (sustained 50% decline in eGFR).
except for the EMPA-REG OUTCOME trial, which did not include sustained eGFR < 15 mL/min/1.73 m2.
Abbreviations: SGLT2i =sodium/glucose cotransporter 2 inhibitor; ACEI=angiotensin-converting-enzyme inhibitor; ARB=angiotensin-receptor blocker; eGFR=estimated glomerular filtration rate; UACR=urinary albumin-creatinine ratio; Tx, transplantation; CREDENCE, Canagliflozin and Renal Events in Diabetes with Established Nephropathy Clinical Evaluation; CANVAS, Canagliflozin Cardiovascular Assessment Study; EMPA-REG OUTCOME, BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients; DECLARE-TIMI 58, Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58; DAPA-CKD, Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease; n/a=not available. Based on information in Neun et al 2019 (Lancet Diabetes Endocrinol, https://doi.org/10.1016/s2213-8587(19)30256-6) and Heerspink et al 2020 (NEJM, https://doi.org/10.1056/nejmoa2024816).
More recently, the DAPA-CKD trial demonstrated that the renoprotective effects of SGLT2 inhibitors likely extend beyond patients with type 2 diabetes. This trial, which enrolled individuals with CKD (32.5% without type 2 diabetes), was stopped early because of clear efficacy of dapagliflozin over placebo for the primary outcome (sustained eGFR decline ≥50% from baseline, KFRT, sustained eGFR <15 ml/min/1.73 m2, or death from kidney or cardiovascular cause), with an HR of 0.61 [95% CI, 0.51–0.72]. The benefits of dapagliflozin were consistent in participants with and without type 2 diabetes. Safety profiles were similar between the two treatment arms with the exception of volume depletion (more common with dapagliflozin) and major hypoglycemia (more common with placebo).
Several mechanisms have been proposed for how SGLT2 inhibitors improve kidney outcomes. Blockade of SGLT-2, responsible for ~90% of glucose reabsorption that occurs in the proximal tubule, promotes urinary excretion of glucose. However, SGLT-2 inhibitors are associated with only modest HbA1c reductions, suggesting that the kidney effects are not driven by improved glycemic control. Rather, increased distal delivery of sodium to the macula densa (in tandem with glucose excretion) activates tubuloglomerular feedback, leading to vasoconstriction of the afferent arteriole and ultimately a reduction in intraglomerular pressure. SGLT-2 inhibitors also reduce systolic and diastolic BP, likely due to osmotic diuresis (from glucosuria), natriuresis, and possibly inhibition of sympathetic nervous system activity. Other potential mechanisms for the renoprotective effects of SGLT2 inhibitors include weight loss, lowering of serum uric acid levels, and reduction of albuminuria.
Although SGLT-2 inhibitors are generally well-tolerated, some safety concerns warrant mentioning. Genitourinary fungal infections, particularly in women, are the most commonly reported adverse effect. Fournier gangrene, which occurs much more rarely, is another potential severe complication. The US Food and Drug Administration (FDA) issued a black box warning on canagliflozin regarding an increased risk of lower limb amputations based on a nearly 2-fold higher risk of amputations in the CANVAS Program. In contrast, there was no heightened amputation risk in CREDENCE. Still, it is prudent to perform regular foot exams in all patients on SGLT-2 inhibitors, particularly those with a history of neuropathy, peripheral vascular disease, and/or diabetic foot ulcers. An increased risk of fracture with SGLT-2 inhibitors was reported in CANVAS Program but not CREDENCE, EMPA-REG OUTCOME, or DECLARE-TIMI 58. The role of SGLT2 inhibitors as a precipitant for AKI remains controversial, with some published studies reporting protective effects.
For Question 6, SGLT-2 inhibitors are associated with (a) a reduction in albuminuria and a 30–40% decreased risk of CKD progression. This class of medications is not commonly associated with hyponatremia or hyperkalemia and results in only a small reduction in HbA1c. For Question 7, while all choices have been reported with the use of SGLT-2 inhibitors, the most common is (a) genitourinary fungal infection.
Additional Readings
Davies MJ, D’Alessio DA, Fradkin J, et al; Management of hyperglycaemia in type 2 diabetes, 2018. A consensus by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2018;61:2461–2498.
Perkovic V, Jardine MJ, Neal B, et al; Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 2019;380(24):2295–2306.*ESSENTIAL READING
Neuen, BL, Young T, Heerspink HJL, et al; SGLT2 inhibitors for the prevention of kidney failure in patients with type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2019;7(11):845–854.*ESSENTIAL READING
van Bommel EJM, Muskiet MHA, Tonneijck L, et al; SGLT2 Inhibition in the Diabetic Kidney – From Mechanisms to Clinical Outcome. Clin J Am Soc Nephrol. 2017;12(4):700–710.
Heerspink HJL, Stefánsson BV, Correa-Rotter R, et al; Dapagliflozin in Patients with Chronic Kidney Disease. N Engl J Med. 2020; 383(15):1436–1446.
Neal B, Perkovic V, Mahaffey KW, et al; Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 2017; 377(7): 644–657.
GLP-1 receptor agonists
Case 5: A 60 year-old man with type 2 diabetes mellitus and CKD G4A3 (eGFR 27 ml/min/1.73 m2) has stable UACR (1,800 mg/g for 1 year). BP is 126/70 mm Hg and HbA1c is 9.0%. Medications include lisinopril 40 mg daily, diltiazem sustained release 180 mg daily, and insulin glargine.
Question 8: Which one of the following interventions would be appropriate to manage risk factors associated with CKD progression?
Addition of a GLP-1 receptor agonist to reduce HbA1c but at an increased risk for hypoglycemia
Addition of metformin rather than a GLP-1 receptor agonist due to the favorable side-effect profile of metformin
Addition of a GLP-1 receptor agonist to slow progression to kidney failure without change in albuminuria
Addition of a GLP-1 receptor agonist to reduce BP to <120/80 mm Hg
For the answer to the question, see the following text.
The GLP-1 receptor agonists are another novel class of diabetes medications that improve kidney outcomes. In the AWARD-7 trial, dulaglutide 0.75 mg or 1.5 mg weekly resulted in slower eGFR decline over 52 weeks compared with daily insulin glargine among participants with type 2 diabetes, CKD G3-G4, and UACR >300 mg/g. Furthermore, UACR reduction occurred with dulaglutide in a dose-dependent manner. Among participants with baseline UACR ≤300 mg/g, no significant differences in eGFR decline were observed between the three treatment arms. In a meta-analysis of five cardiovascular outcome trials (ELIXA; LEADER; SUSTAIN-6; EXSCEL; REWIND), Kristensen et al. reported that GLP-1 receptor agonists were associated with a 17% lower risk of a composite kidney outcome (new-onset UACR >300 mg/g, a doubling of serum creatinine, ≥40% decline in eGFR, KFRT, or death from kidney disease), with an HR of 0.83 [95% CI, 0.78–0.89]. However, when considering the more restrictive outcome of worsening kidney function, defined by a doubling of serum creatinine or ≥40% eGFR decline (except for EXSCEL which also included KFRT or death from kidney disease), there was no statistically significant protective effect of GLP-1 receptor agonists (HR, 0.87 [95% CI, 0.73–1.03]).
GLP-1 receptor agonists act by binding to the GLP-1 receptor, enhancing glucose-dependent insulin secretion, delaying gastric emptying, and decreasing appetite. Modest improvements in body weight, BP, and lipid parameters have also been reported. Prior studies suggest that multiple cell types (e.g., glomerular, tubular, and vascular) within the kidney have GLP-1 receptors but the mechanisms by which GLP-1 receptor agonists improve kidney outcomes are less clear. Altered renal hemodynamics, increased natriuresis, and reductions in inflammation and reactive oxidative species have all been proposed.
The most frequent side effect of GLP-1 receptor agonists is nausea, which usually resolves after 4–8 weeks of continued therapy. Diarrhea, hypoglycemia (particularly if used in combination with insulin therapy), tachycardia, gallbladder disease, pancreatitis, and retinopathy may also occur. Other major safety concerns include increased risks of pancreatic and thyroid cancer. Although Kristensen et al. did not find an association of GLP-1 receptor agonist therapy with severe hypoglycemia, pancreatitis, pancreatic or thyroid cancer, the trials excluded individuals with a personal or family history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2, and the FDA label warns against the use of GLP-1 receptor agonists in these patients.
Although no trial has directly compared SGLT2 inhibitors with GLP-1 receptor agonists, a meta-analysis of 8 cardiovascular trials found a 38% (HR, 0.62 [95% CI, 0.58–0.67) and 18% (HR, 0.82 [95% CI, 0.75–0.89]) lower risk of new-onset UACR >300 mg/g, doubling of serum creatinine, ≥40% decline in eGFR, KFRT, or death from kidney disease for SGLT-2 inhibitors and GLP-1 receptor agonists, respectively. When incident UACR >300 mg/g was not included in the outcome, SGLT2 inhibitors were associated with a 45% lower risk (HR, 0.55 [95% CI, 0.48–0.64]) whereas no association was observed for GLP-1 receptor agonists (HR, 0.92 [95% CI, 0.80–1.06]). Thus, SGLT2 inhibitors appear to be more effective in slowing kidney disease progression and should be considered before GLP-1 receptor agonists (Figure 2).
Figure 2:
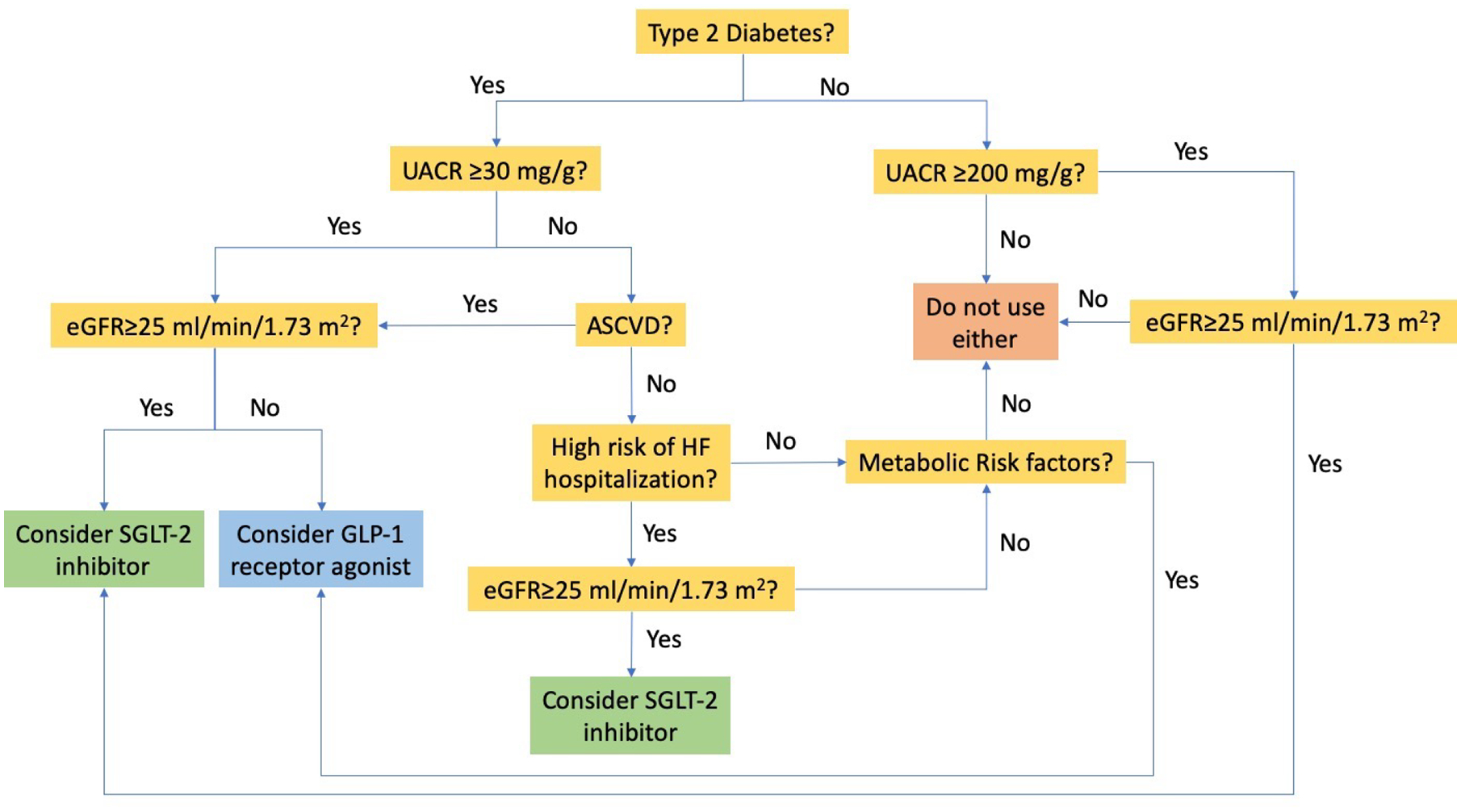
Proposed algorithm for SGLT2 inhibitor and GLP-1 receptor agonist use in chronic kidney disease. Metabolic risks factors include uncontrolled diabetes or obesity/weight gain. Based on information in Li et al 2020 (Clin J Am Soc Nephrol. https://doi.org/10.2215/CJN.02690320). Abbreviations: SGLT2=sodium/glucose cotransporter 2; GLP-1=glucagon-like peptide 1; eGFR=estimated glomerular filtration rate; UACR=urinary albumin-creatinine ratio; ASCVD=atherosclerotic cardiovascular disease; HF=heart failure.
For Question 8, studies best support (a) a reduction in UACR following addition of a GLP-1 receptor agonist. There is an increased risk of hypoglycemia when used concurrently with insulin, and a reduction in the rate of progression to kidney failure has not been shown. While GLP-1 receptor agonists may result in a small reduction in BP, lowering to <120/80 mm Hg has not been shown to slow CKD progression. Initiating metformin would be inappropriate at this eGFR.
Additional Readings
Tuttle KR, Lakshmanan MC, Rayner B, et al; Dulaglutide versus insulin glargine in patients with type 2 diabetes and moderate-to-severe chronic kidney disease (AWARD-7): a multicenter, open-label, randomized trial. Lancet Diabetes Endocrinol. 2018;6(8):605–617.
Kristensen S, RØrth R, Jhund PS, et al; Cardiovascular, mortality, and kidney outcomes with GLP-1 receptor agonists in patients with type 2 diabetes: a systematic review and meta-analysis of cardiovascular outcome trials. Lancet Diabetes Endocrinol. 2019;7(10):776–785.*ESSENTIAL READING
Muskiet MHA, Tonneijck L, Smits MM, et al; GLP-1 and the kidney: from physiology to pharmacology and outcomes in diabetes. Nat Rev Nephrol. 2017;13:605–628.
Zelniker TA, Wiviott SD, Raz I, et al; Comparison of the Effects of Glucagon-Like Peptide Receptor Agonists and Sodium-Glucose Cotransporter 2 Inhibitors for Prevention of Major Adverse Cardiovascular and Renal Outcomes in Type 2 Diabetes Mellitus. Circulation. 2019;139(17):2022–2031.*ESSENTIAL READING
Li J, Albajrami O, Zhuo et al; Decision Algorithm for Prescribing SGLT2 Inhibitors and GLP-1 Receptor Agonists for Diabetic Kidney Disease. Clin J Am Soc Nephrol. 2020;15(11):1678–1688.
Chronic metabolic acidosis and dietary protein restriction
Case 6: A 63 year-old woman with CKD G4A2 and osteopenia returns for follow-up, having been last seen 4 months ago. She underwent left nephrectomy 30 years ago following trauma. She reports no interval symptoms and weight has been stable. Her eGFR has slowly declined from 33 to 28 ml/min/1.73 m2 over the past 2 years, with her last two total carbon dioxide values in the range of 19 – 21 mmol/L. On physical examination, BP 118/65 mm Hg, lungs are clear, and she has trace pedal edema.
Question 9: Which one of the following is most accurate in treating metabolic acidosis associated with CKD?
Modest dietary protein restriction should decrease urine ammoniagenesis
Dietary supplementation with sodium bicarbonate should decrease bone mineral density
Modest dietary protein restriction should increase skeletal muscle catabolism
Case 7: A 58 year-old man with IgA nephropathy has progressive CKD over the past 18 months. eGFR is 29 ml/min/1.73 m2 with total carbon dioxide ranging 18 – 20 mmol/L.
Question 10: Which one of the following interventions has the greatest efficacy in improving metabolic acidosis?
Increase daily fruit intake to 4 servings
Add sodium bicarbonate 650 mg tablet once daily
Ensure 2 servings of pasta daily
Replace one serving of red meat with one serving of fish daily
For the answers to the questions, see the following text.
Metabolic acidosis is a common complication of CKD due to impairments in the kidney’s ability to excrete acid. Dietary composition also influences acid-base balance, with animal-derived proteins contributing primarily hydrogen ions, and fruits and vegetables contributing alkali. Thus, treatment of metabolic acidosis in patients with CKD typically relies on three strategies: reduction of dietary animal protein, increased consumption of fruits and vegetables, and administration of oral alkali salts. Metabolic acidosis is a risk factor for KFRT, decreased bone mineralization, and sarcopenia. Correction of metabolic acidosis may slow CKD progression. A systematic review of 13 small, primarily open-label clinical trials suggested that both oral alkali supplementation and dietary interventions slow GFR decline, with a meta-analyzed effect on mean GFR decline of >3 ml/min/1.73 m2 per year for both strategies. However, only four of the 13 studies had durations more than 1 year, and four had durations of 6 months or less. The largest randomized controlled trial of dietary protein restriction to date (MDRD Study) suggested a more modest effect size that failed to demonstrate statistical significance. Among participants randomized to usual-protein, low-protein, or very-low-protein diet (1.3 vs. 0.58 vs. 0.28 g/d per kilogram of body weight), the difference in mean GFR decline over a mean follow-up of 2.2 years was 0.8 ml/min per year for very-low-protein vs. low-protein (p=0.07) and 0.4 ml/min per year for the low-protein vs. usual-protein diets (p=0.30).
Despite little clinical trial evidence, the KDIGO guideline suggests consideration of dietary protein restriction to <1.3 g/d per kilogram of body weight for patients with or at risk for CKD G3 and 0.8 g/d per kilogram of body weight per day for patients with CKD G4-G5, given the theoretical benefit. Patients with CKD and bicarbonate <22 mmol/l should also be treated with oral alkali therapy to maintain bicarbonate concentrations in the normal range, recognizing the risks of increased BP and edema. Diets enriched in fruits and vegetables may provide as much or more alkali than bicarbonate supplementation and, in one small study, were similarly effective in slowing eGFR decline (Figure 3).
Figure 3:
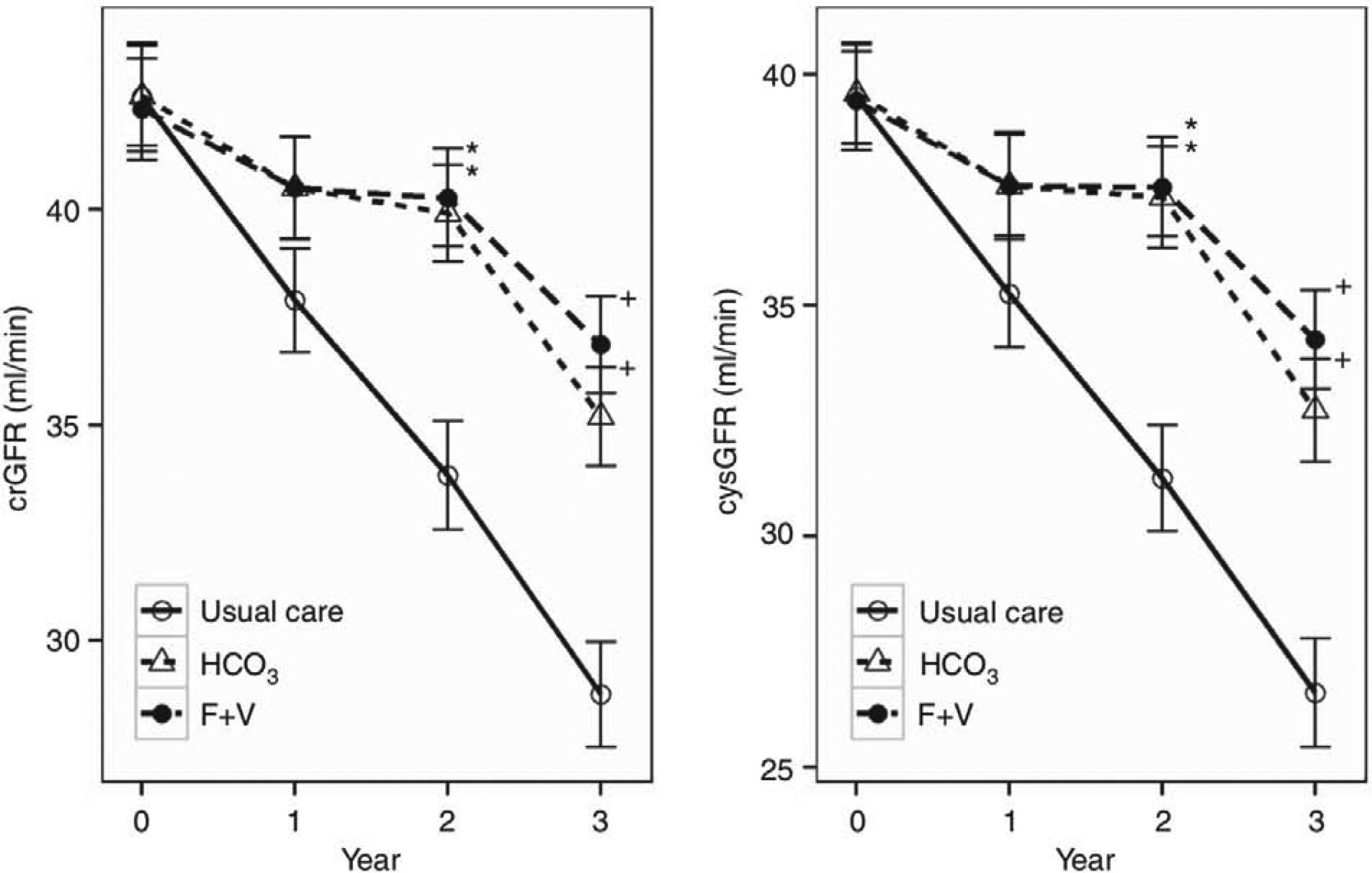
Mean (± standard errors) estimated glomerular filtration rates among patients with CKD G3 randomized to usual care, sodium bicarbonate supplementation, base-producing fruits and vegetables. Reproduced from Goraya et al 2014 (Kidney Int. https://doi.org/10.1038/ki.2014.83) with permission of the copyright holder. Original graphic © 2014 International Society of Nephrology. Abbreviations: crGFR=plasma creatinine-based glomerular filtration rate; cysGFR=plasma cystatin C-based glomerular filtration rate; HCO3=sodium bicarbonate supplementation; F+V=fruits and vegetables.
Returning to Question 9, chronic metabolic acidosis is associated with progression of CKD, stimulates increased renal ammoniagenesis, increases bone resorption, and is associated with the development of sarcopenia. Therefore, the best answer is (a) as reducing protein intake will decrease dietary acid production. For Question 10, the best answer is (a) since 4 servings of fruits and vegetables provide more alkali than low dose sodium bicarbonate. Carbohydrates and animal meats contribute to net acid production, although replacing servings of red meat with fish may have other health benefits.
Additional Readings
Raphael KL; Metabolic Acidosis in CKD: Core Curriculum 2019. Am J Kidney Dis. 2019;74(2):263–275.
Banerjee T, Crews DC, Wesson DE, et al; High Dietary Acid Load Predicts ESRD among Adults with CKD. J Am Soc Nephrol. 2015;26(7):1693–1700.
Navaneethan SD, Shao J, Buysse J, Bushinsky DA; Effects of Treatment of Metabolic Acidosis in CKD: A Systematic Review and Meta-Analysis. Clin J Am Nephrol. 2019;14(7):1011–1020.
Klahr S, Levey AS, Beck GJ, et al; The Effects of Dietary Protein Restriction and Blood Pressure Control on the Progression of Chronic Renal Disease. NEJM. 1994;330(13):877–884.*ESSENTIAL READING
Jain N, Reilly RF. Effects of dietary interventions on the Incidence and Progression of CKD. Nature Reviews Nephrology. 2014;10(12):712–724.
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl. 2013;3:1–150.*ESSENTIAL READING
Goraya N, Simoni J, Jo CH, Wesson DE; Treatment of metabolic acidosis in patients with stage 3 chronic kidney disease with fruits and vegetables or oral bicarbonate reduces urine angiotensinogen and preserves glomerular filtration rate. Kidney Int. 2014;86(5):1031–1038.
Avoidance of nephrotoxins
Case 8: A 62 year-old woman with recent diagnosis of ovarian cancer presented to the emergency room with urinary tract infection and atrial fibrillation. Her medical history is notable for CKD G4A1 in the setting of hypertension and chronic hepatitis B. Home medications include lisinopril, tenofovir disoproxil fumarate, and multivitamin. CT of the abdomen/pelvis demonstrates a large ovarian mass with peritoneal carcinomatosis.
Question 11: Which one of the following medications is safest to use in setting of CKD G4?
Gentamicin
Amiodarone
Tenofovir disoproxil fumarate
Cisplatin
Case 9: A 74 year-old man with CKD G4A2 in the setting of diabetes mellitus and IgA nephropathy is hospitalized for a non-healing lower extremity ulceration. His eGFR is currently 23 ml/min/1.73m2, as compared to 26 one month prior. You are consulted prior to planned lower extremity angiography for recommendations to reduce the risk for contrast-associated AKI.
Question 12: Which one of the following interventions is most appropriate prior to administration of intra-arterial iodinated IV contrast in hospitalized patients with diabetes and CKD?
Oral N-acetylcysteine
3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor
Vitamin C
Normal saline hydration
For the answers to the questions, see the following text.
Nephrotoxins can contribute to CKD progression by causing AKI, chronic interstitial nephritis, tubular dysfunction, or glomerular changes. Avoidance of nephrotoxins is not always possible, especially in the hospital or acute care setting; thus, an individualized approach that carefully weighs the risks versus benefits for each patient is necessary. Many chemotherapeutic (e.g., platinum-based agents, gemcitabine, immunotherapies) and antimicrobial agents (e.g., aminoglycosides, colistin, amphotericin B, tenofovir disoproxil fumarate) require special attention in CKD given their potential for harm to the kidney and/or need for dose adjustments. Despite known toxicities, alternative drug options may not be appropriate due to susceptibility patterns or decreased efficacy. In such cases, counseling patients on the potential worsening of CKD, close monitoring of kidney function, and dose adjustments as needed is a reasonable approach. Other potential nephrotoxins include gastrointestinal agents (e.g., phosphate-containing bowel preparations, proton-pump inhibitors), pain relievers (e.g., nonsteroidal anti-inflammatory agents), and herbal supplements or remedies. Although proton-pump inhibitors do not necessarily need to be stopped in patients with CKD, providers should review need and candidacy for alternative therapy (e.g., H2-blockers) regularly.
Contrast-associated AKI remains a concern among patients with CKD, particularly those with eGFR <30 ml/min/1.73 m2 or diabetes. Proposed mechanisms of injury include vasoconstriction leading to renal ischemia, direct tubular toxicity, and oxidative stress from free radical generation. High-osmolar (>1,200 mOsm/kg) ionic contrast agents are more likely to be nephrotoxic than low-osmolar (700–850 mOsm/kg) or iso-osmolar (~290 mOsm/kg) nonionic agents. AKI risk is thought to be higher with arterial compared to venous contrast administration. However, patients with CKD should not be denied necessary tests that require contrast for diagnosis and management. Fundamental risk reduction measures include: 1) use of minimum dose of contrast necessary; 2) use of low- or iso-osmolar agents; 3) expansion of intravascular volume as tolerated with intravenous normal saline before, during, and after the procedure; and 4) avoidance of concurrent nephrotoxins.
Returning to Question 11, the best answer is (b) because amiodarone does not require discontinuation or dose adjustment in CKD G4. For Question 12, while all listed agents have been reported to reduce the risk of contrast associated kidney injury, the best answer is (d) as periprocedural hydration with normal saline is the most widely accepted prophylaxis.
Additional Readings
Chen TK, Knicely DH, Grams ME; Chronic Kidney Disease Diagnosis and Management: A Review. JAMA. 2019;322(13):1294–1304.*ESSENTIAL READING
Perazella MA. Pharmacology behind Common Drug Nephrotoxicities. Clin J Am Soc Nephrol. 2018;13(12):1897–1908.
McCullough PA, Choi JP, Feghali GA, et al; Contrast-Induced Acute Kidney Injury. J Am Coll Cardiol. 2016;68(13):1465–1473.
Uric acid–lowering therapies
Prior studies have reported an association between elevated serum uric acid levels and increased risk of CKD progression. Whether uric acid directly causes CKD progression or is an indirect marker of some other process is unclear. Two recent trials, PERL and CKD-FIX, investigated whether treatment with allopurinol slowed eGFR decline. The PERL study enrolled patients with type 1 diabetes and early diabetic kidney disease (mean GFR 68 ml/min/1.73 m2 and median UAE rate 60 mg/d) and showed no difference in GFR slope over 3 years between allopurinol and placebo groups. The CKD-FIX study, which included individuals with more advanced CKD (mean eGFR 32 ml/min/1.73 m2 and median UACR 717 mg/g), also reported no significant difference in eGFR change between allopurinol and placebo over two years (−3.33 and −3.23 ml/min/1.73 m2 per year; mean difference, −0.10 [95% CI, 1.18–0.97] ml/min/1.73 m2 per year). Of note, baseline uric acid levels in both trials were not markedly elevated (PERL: 6.1 mg/dL; CKD-FIX: 8.2 mg/dL). Another randomized clinical trial of febuxostat in patients with CKD G3 and asymptomatic hyperuricemia (mean uric acid ~7.8 mg/dL) similarly found no difference in eGFR slopes compared to placebo.
Additional Readings
Doria A, Galecki AT, Spino C, et al. for the PERL Study Group; Serum Urate Lowering with Allopurinol and Kidney Function in Type 1 Diabetes. N Engl J Med. 2020;382(26):2493–2503.
Badve SV, Pascoe EM, Tiku A, et al. for the CKD-FIX Study Investigators. Effects of Allopurinol on the Progression of Chronic Kidney Disease. N Engl J Med. 2020;382(26):2504–2513.
Kimura K, Hosoya T, Uchida S, et al; Febuxostat Therapy for Patients With Stage 3 CKD and Asymptomatic Hyperuricemia: A Randomized Trial. Am J Kidney Dis. 2018;72(6):798–810.
Weight loss and bariatric surgery
Case 10: A 54 year-old man has CKD G4A1 in the setting of diabetes mellitus and hypertension. His weight has been stable for the past year after losing 15 pounds. Medications include lisinopril 40 mg daily and a GLP-1 receptor agonist. His BP is 125/70 mm Hg and BMI is 32 kg/m2. Labs demonstrate an eGFR 27 ml/min/1.73 m2, UACR 25 mg/g, HbA1c 7.2%, and serum uric acid 8.1 mg/dL. The kidney failure risk equation (KFRE) predicts a 4.6% risk of kidney failure at 2 years.
Question 13: Which one of the following interventions would be most appropriate to reduce risk of progression to kidney failure?
Referral for bariatric surgery
Start allopurinol
Start a 2nd anti-hypertensive agent to lower BP to <120/80 mm Hg
No additional therapy
For the answer to the question, see the following text.
In observational studies, higher BMI has been associated with substantially greater risk of developing hypertension and diabetes and a more modest risk for CKD. Mechanisms for the latter association may be through the development of hypertension/diabetes, or there may independent effects through inflammation and hemodynamic alterations in the glomerulus. Weight loss through lifestyle modification or bariatric surgery may improve kidney outcomes. A meta-analysis of four small studies suggested a benefit of weight loss achieved through non-surgical interventions on reduction in albuminuria; however, only two of the studies were clinical trials, with 40 and 18 participants each. A post-hoc analysis of the Look AHEAD trial of people with type 2 diabetes who were overweight or had obesity and suggested a 31% reduction in risk of developing very-high-risk CKD (defined as G4, G3bA2-3, or G3aA3) associated with intensive lifestyle intervention, which aimed to reduce caloric consumption and increase physical activity. Interestingly, the effect of the intervention was only partially mediated by weight loss and reductions in HbA1c and systolic BP. A propensity-matched study of 985 patients who underwent bariatric surgery compared with 985 controls with obesity suggested that long-term GFR decline was attenuated in the bariatric surgery group, with a 57% lower risk of doubling of serum creatinine, an eGFR<15 ml/min/1.73 m2, or KFRT over a median follow-up of 4 years. Thus, it appears that weight loss may confer benefits for both GFR decline and worsening albuminuria, although clinical trial evidence is lacking.
In Question 13, of the options shown, response (d), or no additional therapy, would be the most appropriate intervention. Bariatric surgery at this BMI or a BP goal <125/70 mm Hg have not been shown to slow progression to kidney failure. And, as discussed in the previous section, randomized controlled trials have not shown a benefit of uric acid lowering therapy in preserving kidney function.
Additional Readings
Chang AR, Grams ME, Ballew SH, et al; Adiposity and Risk of Decline in Glomerular Filtration Rate: Meta-Analysis of Individual Participant Data in a Global Consortium. BMJ. 2019;364:k5301.
The Look AHEAD Research Group; Effect of a long-term behavioural weight loss intervention on nephropathy in overweight or obese adults with type 2 diabetes: a secondary analysis of the Look AHEAD randomised clinical trial. Lancet Diabetes Endocrinol. 2014;2(10):801–809. *ESSENTIAL READING
Navaneethan SD, Yehnart H, Moustarah F, et al; Weight Loss Interventions in Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Clin J Am Soc Nephrol. 2009;4(10):1565–1574.
Chang AR, Chen Y, Still C, et al. Bariatric Surgery is Associated with Improvement in Kidney Outcomes. Kidney Int. 2016;90(1):164–171.
Support:
TKC is supported by a NIH/NIDDK K08DK117068 and a George M. O’Brien Center for Kidney Research Pilot and Feasibility Grant from Yale University (under Award Number NIH/NIDDK P30DK079310). MEG is supported by NIH/NIDDK R01DK115534.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: CJS is a member of the American Board of Internal Medicine (ABIM) Longitudinal Assessment Committee for Nephrology. No ABIM exam questions are shared or otherwise disclosed in this article. The other authors declare that they have no relevant financial interests.


