Abstract
Pharmacokinetic boosting of antiretroviral (ARV) therapies with either ritonavir or cobicistat is used to achieve target drug exposure, lower pill burden, and provide simplified dosing schedules. Several ARVs require boosting, including the integrase inhibitor elvitegravir as well as protease inhibitors such as darunavir, atazanavir, and lopinavir. The use of boosted regimens in pregnant women living with HIV has been studied for a variety of ARVs; however, a recent recommendation by the US Food and Drug Administration advised against cobicistat-boosted regimens in pregnancy due to substantially lower drug exposures observed in clinical pharmacokinetic studies. The objectives of this article are to review pharmacokinetic enhancement of ARVs with ritonavir and cobicistat during pregnancy and postpartum, describe clinical implications, and provide recommendations for future research.
Keywords: cobicistat, drug metabolism, HIV, pregnancy, pharmacokinetics, ritonavir
The US Perinatal HIV Guidelines recommend that all pregnant women with HIV receive antiretroviral therapy (ART) throughout pregnancy.1 The main goals of therapy are to maximize virologic suppression, prevent perinatal HIV transmission, and minimize maternal and fetal adverse effects. The perinatal use of ART has reduced rates of HIV mother-to-child transmission from 15% to 30% to <2% in the United States and Europe.1 Pharmacokinetic studies of antiretrovirals (ARVs) in pregnant women are necessary to determine safe and effective doses for both mother and fetus.
Physiological changes during pregnancy impact drug disposition in a variety of ways.2 Temporal changes in drug disposition occur during pregnancy for several reasons, including alterations in gastrointestinal absorption, drug distribution, expression and activity of drug-metabolizing enzymes, and renal excretion.2 For example, the activity of hepatic cytochrome P450 (CYP) 3A is increased by between 50% and 100% during pregnancy.3 ARVs that are primarily eliminated by CYP3A-mediated metabolism, such as protease inhibitors (PIs), typically show decreased exposure during pregnancy. Pregnancy also leads to reduced concentrations of albumin and α1-acid glycoprotein, which results in decreased plasma protein binding for some drugs.3 Many ARVs bind extensively to plasma proteins, and only the unbound drug can cross cell membranes and reach the infected cellular reservoir of HIV. Decreased protein binding in pregnancy may lead to higher unbound concentrations, but the overall effect of pregnancy can affect total and unbound ARV concentrations differently. For example, total concentration of an ARV may decrease while unbound concentrations remain consistent, suggesting no dose change is necessary; in other situations, pregnancy may decrease both the total and unbound concentrations of an ARV, leading to a dose adjustment or avoidance of the drug in pregnancy.
Pharmacokinetic boosting of ARVs is used to increase drug exposure, lower pill burden, and provide simplified dosing schedules (ie, once-daily dosing).4 The use of pharmacokinetic (PK) boosting to increase exposure of other HIV medications began as early as 1997 with the discovery that ritonavir increased levels of saquinavir.5 Ritonavir, a PI and strong CYP3A inhibitor, inhibits the metabolism of other concomitantly administered PIs that are CYP3A substrates. Ritonavir is commonly used at subtherapeutic doses to boost other PIs such as darunavir, lopinavir, and atazanavir.6
Cobicistat is a second-generation PK enhancer first approved by the US Food and Drug Administration (FDA) in 2012 as part of a single-tablet fixed-dose combination product to increase systemic exposure of elvitegravir and is currently also used to boost the PIs darunavir and atazanavir. Unlike ritonavir, cobicistat has no anti-HIV activity.7 Cobicistat has several advantages over ritonavir, including more selective inhibition of CYP3A4.7 In November 2018, the FDA released a statement that cobicistat-containing ART is not recommended during pregnancy.8 The product labels for cobicistat with atazanavir or darunavir, as well as for elvitegravir/cobicistat/emtricitabine/tenofovir, were updated to indicate that these products are not recommended for initiation or continued use during pregnancy. These labeling changes were made due to substantially lower exposures of cobicistat, elvitegravir, darunavir, and atazanavir observed in PK studies during pregnancy.8,9 The FDA recommendation also explicitly states that darunavir/ritonavir and atazanavir/ritonavir remain viable treatment options for pregnant women.
The objectives of this article are to review PK enhancement of ARVs with cobicistat and ritonavir during pregnancy and postpartum, describe clinical implications, and provide recommendations for future research.
Ritonavir
Ritonavir was the third PI to be licensed, receiving FDA approval in 1996. Ritonavir was initially used as an antiviral agent before later pharmacologic analyses demonstrated its advantage in combination therapy as a low-dose booster for other PIs.10 However, the use of full-dose ritonavir as an antiviral is no longer recommended because of its low potency and poor tolerance due to the side effect profile and associated toxicities, including nausea, headache, diarrhea, elevated hepatic enzymes, and elevated serum cholesterol and triglycerides.10,11
The concomitant administration of ritonavir at subtherapeutic doses (100–400 mg daily) affects the bioavailability and elimination of many other ARVs.12 Ritonavir has a complex drug interaction profile. Ritonavir is a strong inhibitor of CYP3A enzyme present in both the intestinal tract and liver, thus inhibiting the metabolism of other drugs that are substrates of this enzyme. Ritonavir also exhibits a biphasic, time-dependent effect on the efflux transporter P-glycoprotein (P-gp) of inhibition followed by induction.13 Along with inhibiting CYP3A, ritonavir also inhibits CYP2D6 and induces CYP1A2, CYP2C9, CYP2C19, and CYP2B6.13,14 Thus, ritonavir coadministered with drugs metabolized by CYP3A and CYP2D6 leads to increased plasma concentrations, whereas coadministration with drugs metabolized by CYP1A2, CYP2C9, CYP2C19, and CYP2B6 leads to decreased plasma concentrations and potential therapeutic failure. Drugs that are metabolized by a combination of these enzymes may be subject to increased or decreased exposure when coadministered with ritonavir, depending on the relative contribution of each individual pathway and the degree to which it is affected by ritonavir.
Ritonavir-boosted PIs, specifically darunavir/ritonavir or atazanavir/ritonavir, are recommended in nonpregnant adults in certain clinical situations.15 While boosted PIs generally carry the disadvantage of greater drug interaction potential, darunavir/ritonavir may be appropriate for patients who urgently need to start ART before resistance results are available due to boosted darunavir’s high barrier to resistance and low rate of treatment-emergent resistance. Ritonavir-boosted atazanavir, darunavir, or lopinavir can also be used as a second-line regimen for nonpregnant adults.15 Atazanavir/ritonavir and darunavir/ritonavir are the preferred PIs for use in ARV-naïve pregnant women, with lopinavir/ritonavir as the alternative PI.1 While other boosted PIs, including fosamprenavir, indinavir, saquinavir, and tipranavir may be commercially available, they are no longer commonly used in clinical practice in the United States due to the availability of more potent and safer alternatives within the PI class.
Pharmacokinetics of Ritonavir-Boosted Regimens in Pregnancy
Darunavir/Ritonavir
Darunavir/ritonavir is typically dosed as 800 mg/100 mg once daily in nonpregnant treatment-naïve adults or treatment-experienced adults with no prior darunavir resistance-associated mutations or 600 mg/100 mg twice daily for treatment-experienced adults with darunavir resistance-associated mutations.16,17 For once-daily dosing in treatment-naïve nonpregnant adults (800 mg/10 mg), the median area under the plasma concentration–time curve from time zero to 24 hours after dosing at steady state (AUC0-24,ss) for darunavir was 69.35 μg • h/mL at week 4, 63.49 μg • h/mL at week 24, and 76.85 μg • h/mL at week 48.18 In a study analyzing once-daily darunavir/ritonavir dosing in 16 pregnant women, the mean AUC0-24,ss was 61.30 μg • h/mL during the second trimester, 60.44 μg • h/mL during the third trimester and 94.53 μg • h/mL postpartum.19 The AUC0-24,ss for total darunavir was 34% to 35% lower during the third trimester of pregnancy vs postpartum, but unbound darunavir AUC at 24 hours (AUC24) was 20% to 24% lower.19 Several studies have assessed darunavir protein binding during pregnancy, with 1 study reporting no change in darunavir protein binding during the third trimester20 and 2 studies reporting decreased unbound darunavir concentrations during the third trimester,19,21 both of which considered the change clinically insignificant. Another study summarizing the results from 5 available PK studies examining darunavir/ritonavir in pregnant women with HIV revealed reductions in total darunavir plasma concentrations between 20% and 50% during the third trimester of pregnancy.22 A study assessing the use of an increased dose of darunavir/ritonavir (800 mg/100 mg twice daily) during pregnancy failed to show an increase in exposure compared to the standard 600 mg/10 mg twice daily, and AUC from time zero to 12 hours after dosing (AUC0-12) remained lower and apparent clearance higher in the second and third trimesters compared to postpartum with use of the increased dose during pregnancy (Table 1).23 Prior studies of ritonavir-boosted darunavir during pregnancy have demonstrated minimum plasma concentrations (Cmin) well above the mean protein binding adjusted darunavir half maximal effective concentration for wild-type HIV-1 (55 ng/mL) (Table 1).24 Twice-daily dosing of darunavir/ritonavir is favored in pregnancy over once-daily dosing due to decreased AUC and trough levels with once-daily dosing.1
Table 1.
Steady-State Pharmacokinetics of Ritonavir-Boosted Darunavir, Atazanavir, Lopinavir, and Fosamprenavir in Pregnancy, Postpartum, and in Nonpregnant Adults
| Parameter | Second Trimester | Third Trimester | Postpartum | Nonpregnant Adults | Levels in Pregnancy vs Postpartum |
|---|---|---|---|---|---|
| Darunavir (600/100 mg twice daily) 21,24 | N = 11a | N = 11a | N = 11a | N = 285a | ↓ |
| Cmin, ng/mL | 1980 ± 840 | 2498 ± 1193 | 2711 ± 2268 | 58 398 ± 26 797 | |
| Cmax, ng/mL | 4601 ± 1125 | 5111 ± 1517 | 2499 ± 2411 | ||
| AUC0-12, ng • h/mL | 38 950 ± 10 010 | 43 700 ± 16 400 | 55 300 ± 27 020 | ||
| Darunavir (800/100 mg daily) 24,85 | N = 15b | N = 30b | N = 23b | N = 335b | ↓ |
| Cmax, ng/mL | 6770 (4350-7840) | 5780 (4310-7290) | 8110 (6930-10 300) | 87 854 (45 000-219 240) | |
| AUC0-24, ng • h/mL | 64 600 (35 900-72 300) | 63 500 (46 000-75 200) | 103 900 (85 900-135 700) | ||
| Darunavir (800/100 mg twice daily) 23 | N = 9b | N = 24b | N = 24b | ↓ | |
| Cmin, ng/mL | 2840 (1210-3230) | 2390 (1720-2820) | 1120 (520-5010) | ||
| Cmax, ng/mL | 6220 (5300-8420) | 6550 (5380-7430) | 8960 (7930-10 850) | ||
| AUC0-12, ng • h/mL | 55 100 (46 400-57 700) | 51 800 (41 200-57 700) | 79 600 (66 600-103 000) | ||
| Atazanavir (300/100 mg daily) 25,86 | N = 1 | N = 18b | N = 13b | N = 28a | ↓ |
| Cmin, ng/mL | 2000 | 700 (400-900) | 1200 (1100-1800) | 1441 ± 757 | |
| Cmax, ng/mL | 9100 | 3600 (2800-5100) | 4100 (3000-5800) | 6450 ± 2031 | |
| AUC0-24, ng • hr/mL | 88 200 | 41 900 (27 400-60 800) | 57 900 (47 100-64 800) | 61 435 ± 22 911 | |
| Lopinavir (400/100 mg twice daily) 33,39 | N = 6c | N = 11c | N = 5c | N = 19a | ↓ |
| Cmin, ng/mL | 4590 (4090-5258) | 2505 (2008-3488) | 4654 (3054–6786) | 5500 ± 2700 | |
| Cmax, ng/mL | 9228 (7939-11 026) | 7533 (6749-8735) | 9961 (8420-11 750) | 9800 ± 3700 | |
| AUC0-12, ng • h/mL | 80 940 (70 712-94 656) | 58 023 (50 449-70 723) | 89 446 (74 042-107 941) | 92 600 ± 36 700 | |
| Fosamprenavir (700/100 mg twice daily) 48,87 | N = 8b | N = 28b | N = 22b | N = 24c | ↓ |
| Cmin, ng/mL | 1910 (340-2390) | 1480 (860-1800) | 2420 (1360-3080) | 2120 (1770–2540) | |
| Cmax, ng/mL | 5610 (4470-6640) | 5120 (3600-6260) | 6750 (4310-9240) | 6080 (5380-6860) | |
| AUC0-12, ng • h/mL | 43 500 (38 500-50 400) | 32 150 (21 450-39 700) | 51 600 (45 200-59 600) | 79 200 (69 000-90 600) |
AUC0-12, area under the plasma concentration–time curve from time zero to 12 hours after dosing; AUC0-24, area under the plasma concentration–time curve from time zero to 24 hours after dosing; Cmax, maximum plasma concentration; Cmin, minimum plasma concentration.
Results presented as mean ± standard deviation.
Results presented as median (interquartile range).
Results presented as geometric mean (95% confidence interval).
Atazanavir/Ritonavir
Atazanavir/ritonavir is FDA-approved for treatment-experienced and treatment-naïve adult patients living with HIV at a dose of 300 mg/100 mg once daily. In 38 pregnant women (18 without tenofovir, 20 with tenofovir), median atazanavir AUC0-24,ss was reduced during the third trimester compared to postpartum for subjects not receiving tenofovir (41.9 vs 57.9 μg • h/mL; P = .02; Table 1) and for subjects receiving tenofovir (28.8 vs 39.6 μg• h/mL; P = .04).25 During the third trimester, AUC0-24,ss was below the PK target—defined as the 10th percentile atazanavir AUC (29.4 μg • h/mL) in nonpregnant historical controls taking atazanavir/ritonavir 300 mg/100 mg once daily—in 33% (6/18) of women not receiving tenofovir and 55% (11/20) of women receiving tenofovir. Despite these lower exposures during pregnancy, intracellular/plasma ratios are similar during pregnancy and postpartum. For example, an observational study in 25 pregnant women with HIV treated with atazanavir/ritonavir showed atazanavir intracellular/plasma ratios of 1.32 in the first trimester, 1.34 in the second trimester, 1.38 in the third trimester, and 1.07 postpartum.26 A separate study in 17 women comparing atazanavir/ritonavir 300 mg/100 mg daily exposure during the third trimester to postpartum demonstrated concentrations above the wild-type HIV 90% inhibitory concentration.27 Due to these lower exposures of atazanavir/ritonavir 300 mg/100 mg daily during pregnancy, a study assessing the PK in pregnancy of an increased dose of atazanavir/ritonavir 400 mg/100 mg demonstrated adequate atazanavir exposure during the second and third trimester of pregnancy.28 Another study showed that lower atazanavir exposure during pregnancy was not associated with a lack of virologic suppression at delivery.29 Therefore, the standard dose of 300 mg/100 mg is recommended for some patients (including treatment-naïve women), but careful monitoring is warranted. A dose adjustment of atazanavir to 400 mg should be considered for treatment-experienced patients, especially those taking tenofovir or medications that alter atazanavir bioavailability such as H2 receptor blockers (eg, famotidine, ranitidine).29
Lopinavir/Ritonavir
Lopinavir/ritonavir has been evaluated in multiple PK studies in nonpregnant adults.30,31 Population PK models have been developed to quantitatively describe the boosting effect of ritonavir on lopinavir. Ritonavir decreases the apparent oral clearance (CL/F) of lopinavir by 39%.32 Lopinavir/ritonavir has also been studied extensively in pregnancy. Lopinavir/ritonavir was originally approved as a soft-gel capsule, which was later replaced by a heat-stable tablet formulation with improved bioavailability.33,34 A systematic review including 9 studies and 2675 lopinavir/ritonavir-treated pregnant women supports the safety and efficacy of the standard dose of 400 mg/100 mg twice daily, suggesting no unique safety or efficacy concerns with the standard dosing.35 An intensive PK study comparing the capsule formulation to the tablet formulation in 19 pregnant women (8 in capsule group, 11 in tablet group) demonstrated total lopinavir exposures in the third trimester were lower than those in the second trimester (35% and 28% for capsule and tablet group, respectively) and postpartum (35% for tablet group) (Table 1 shows parameters for the tablet formulation).33 Notably, the AUC0-12 was 15% higher in the third trimester and Cmax was 25% higher in the tablet formulation group.33 Due to the reduced lopinavir concentrations of both the capsule and tablet formulations during pregnancy, studies assessing an increased dose of 533 mg/133 mg of the capsule formulation and 600 mg/150 mg of the tablet formulation demonstrate lopinavir concentrations similar to those seen in nonpregnant adults receiving standard doses (400 mg/100 mg twice daily).36,37 While these studies suggest that higher lopinavir/ritonavir doses could be used during pregnancy (especially in patients who are PI experienced), other studies have found that while lower lopinavir/ritonavir plasma levels are observed with 400 mg/100 mg dosing, the levels were not decreased below the threshold associated with efficacy in nonpregnant treatment-naïve populations (trough <1000 ng/mL).38 Additionally, Cmin for lopinavir given at the standard dose in pregnant women was well above mean half maximal effective concentration values of 0.04 to 0.018 μg/mL for HIV-1 laboratory strains in 50% human serum.39
Other factors must be considered when determining whether higher doses of lopinavir/ritonavir are warranted. A population PK study of 154 pregnant women taking lopinavir/ritonavir 400 mg/100 mg (standard) or 600 mg/150 mg (increased) twice daily demonstrated that body weight is related to lopinavir clearance and volume of distribution.40 The analysis revealed that while standard dosing provides adequate lopinavir trough concentrations for the majority of pregnant women, those with body weights > 100 kg and/or those with poor adherence on the standard dose were at a higher risk of subtherapeutic trough concentrations.40 In contrast, a separate population PK analysis found that body weight was not related to lopinavir volume of distribution in pregnant women,41 and increased doses in patients with high body weight are not currently recommended. Lopinavir also extensively binds to both α1-acid glycoprotein and albumin, which are reduced during pregnancy; however, the resulting increase in the unbound concentration did not overcome the overall reduction in total lopinavir concentrations during pregnancy.42 A separate study reported that unbound lopinavir concentrations remained unchanged during pregnancy despite dose increases.43 To further elucidate the differences between standard and increased doses of lopinavir in pregnancy, a separate trial randomized 32 pregnant women to the standard dose and 31 women to the increased dose at gestational ages between 14 and 33 weeks.44 The frequency of adverse events was comparable between the groups. Among women with baseline viral load >50 copies/mL assigned to the standard dose group, 45% had an antepartum (within the last 4 weeks of pregnancy) viral load >50 copies/mL; for those who were assigned to the increased dose group, only 10.5% had an antepartum viral load >50 copies/mL.44 Thus, while the standard dose of lopinavir/ritonavir in pregnant women generally demonstrates comparable virologic response rates to nonpregnant adults, increased doses may be warranted in pregnant women with HIV during the second and third trimesters for PI-experienced women, women who started treatment during pregnancy with a baseline viral load of >50 copies/mL, women with body weights >100 kg during pregnancy, or those with adherence issues.35,40,44
Uncommonly Used Protease Inhibitors
Some of the other PIs that are no longer used in clinical practice have been studied to some extent in pregnancy. Saquinavir/ritonavir has been evaluated in multiple PK studies in pregnant women, the first of which demonstrated adequate median Cmin (0.91 mg/L in the third trimester and 0.86 mg/L at delivery)45 and a second study showed similar PK parameters for weeks 24 and postpartum.46 A third analysis of PK, efficacy, and safety of saquinavir/ritonavir in 13 pregnant women compared to 15 nonpregnant women demonstrated that saquinavir plasma concentrations were significantly lower in pregnant women compared to nonpregnant women; however, all the pregnant women displayed saquinavir AUC0-12 >10,000 ng • h/mL and 92.3% had viral load <400 copies/mL at birth.47 Thus, saquinavir exposures are lower during pregnancy with standard doses of saquinavir/ritonavir, though not low enough to necessitate a dose change.
Fosamprenavir/ritonavir and indinavir/ritonavir pharmacokinetics have limited data during pregnancy. An intensive PK study of amprenavir (the active moiety of fosamprenavir) was performed during the second and third trimesters of pregnancy and postpartum in 29 women. The AUC0-12 of amprenavir was 40% lower and apparent oral clearance was 68% higher in the third trimester compared to postpartum (Table 1).48 Although amprenavir plasma concentrations were lower during pregnancy and postpartum, the reduced amprenavir concentrations were still above the exposures needed for viral suppression. For indinavir/ritonavir, a study conducted in 26 pregnant Thai women taking indinavir/ritonavir 400 mg/100 mg twice daily demonstrated a 42% decrease in AUC0-12 of indinavir and a 27% decrease in Cmax in the second trimester compared to postpartum; there was a 40% decrease in AUC0-12 and 37% decrease in Cmax in the third trimester compared to postpartum.49 Importantly, ~30% of women failed to achieve target trough concentrations of >0.10 μg/mL, with indinavir exposure during the second and third trimesters significantly reduced compared to postpartum. A second study in 32 pregnant women receiving indinavir/ritonavir 400 mg/100 mg twice daily demonstrated 18% of subjects had trough concentrations below 0.12 μg/mL and 93% of subjects had HIV RNA levels <200 copies/mL at delivery.50 The available data are insufficient to provide definitive dosing recommendations for the use of indinavir/ritonavir during pregnancy.
All 3 of these regimens—saquinavir/ritonavir, fosamprenavir/ritonavir, and indinavir/ritonavir—are no longer used in clinical practice and are not recommended for use during pregnancy.1 Tipranavir/ritonavir is a fourth boosted PI regimen that has no data available in pregnancy and is also not recommended.
Cobicistat
Cobicistat, approved by the FDA as Tybost in 2014, is a PK booster developed to overcome known issues with ritonavir. Cobicistat was developed as an analog to ritonavir, yet unlike ritonavir, does not have any intrinsic anti-HIV activity.51 One of the advantages of cobicistat is its ease of coformulation with other antiretrovirals due to its solubility.51 Ritonavir often requires a separate capsule or tablet, which increases pill burden on patients, whereas cobicistat can be coformulated with other ARVs into single-tablet fixed-dose combination products. Some recently approved coformulated cobicistat products are single-tablet regimens, including Stribild (2012, elvitegravir/cobicistat/emtricitabine/tenofovir disoproxil fumarate), Genvoya (2015, elvitegravir/cobicistat/emtricitabine, tenofovir alafenamide [TAF]), and Symtuza (2018, darunavir/cobicistat/emtricitabine/TAF). Other coformulated cobicistat products, such as the combination tablets Prezcobix (2015, darunavir/cobicistat) and Evotaz (2015, atazanavir/cobicistat) provide only part of a complete ART regimen (Table 2). Another distinct advantage of cobicistat is its tolerability for patients, with improved taste and a significantly lower risk of nausea and vomiting compared to ritonavir (incidence of nausea 2% for cobicistat compared to 60% for ritonavir).52,53 Ritonavir’s issues with tolerability can be especially problematic during the early stages of pregnancy.
Table 2.
Currently Available Ritonavir (RTV) and Cobicistat (COBI) Fixed-Dose Drug Combinations and Their Use During Pregnancy
| Drug Name | Formulation | Company | FDA Approval Date | Relevant Clinical PK Studies in Pregnancy | Use in Pregnancy |
|---|---|---|---|---|---|
| TDF/FTC/EVG/COBI (Stribild) | TDF 300 mg FTC 200 mg EVG 25 mg COBI 150 mg |
Gilead Sciences | August 2012 | PANNAa (Colbers et al)92 | Not currently recommended for use during pregnancy due to low cobicistat exposures during the second and third trimesters of pregnancy8,55,66 |
| TAF/FTC/EVG/COBI (Genvoya) | TAF 10 mg FTC 200 mg EVG 25 mg COBI 150 mg |
Gilead Sciences | November 2015 | IMPAACT P1026sb (Momper et al)61 PANNA (Schalkwijk et al)88 |
Not currently recommended for use during pregnancy due to low cobicistat exposures during the second and third trimesters of pregnancy8,55,66 |
| TAF/FTC/DRV/COBI (Symtuza) | TAF 10 mg FTC 200 mg DRV 800 mg COBI 150 mg |
Janssen | July 2018 | IMPAACT P1026 (Momper et al)64 | Not currently recommended for use during pregnancy due to low cobicistat exposures during the second and third trimesters of pregnancy8,55,66 |
| ATV/COBI (Evotaz) | ATV 300 mg COBI 150 mg |
Bristol-Myers Squibb | January 2015 | IMPAACT P1026s (Momper et al)9 | Not currently recommended for use during pregnancy due to low cobicistat exposures during the second and third trimester of pregnancy8,55,66 |
| DRV/COBI (Prezcobix) | DRV 800 mg COBI 150 mg |
Janssen | January 2015 | IMPAACT P1026s (Momper et al)64 | Not currently recommended for use during pregnancy due to low cobicistat exposures during the second and third trimesters of pregnancy 8,55,66 |
| LPV/RTV (Kaletra) | LPV 100 mg RTV 25 mg or LPV 200 mg RTV 50 mg |
Abbvie | September 2000 | PACTG (IMPAACT) 1026s (Stek et al)89 (Mirochnick et al)37 (Best et al)36 | With standard 400 mg/100 mg twice daily dosing, reduction of LPV plasma concentrations during pregnancy of ~30% compared to nonpregnant adults. 89–91 May increase LPV/r to 600 mg/150 mg twice daily.36,37 |
ATV, atazanavir; DRV, darunavir; EVG, elvitegravir; FTC, emtricitabine; LPV, lopinavir; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
Pharmacokinetics of Newly Developed Antiretroviral Agents in HIV-Positive Pregnant Women (PANNA), an ongoing, nonrandomized, open-label, multicenter study of antiretroviral pharmacokinetics in pregnant women with HIV in Europe.
International Maternal Pediatric Adolescent AIDS Clinical Trials (formerly Pediatric AIDS Clinical Trials Group) (IMPAACT P1026s), an ongoing, nonrandomized, open-label, multicenter study of antiretroviral pharmacokinetics in pregnant women with HIV in the United States, Brazil, Thailand, and Africa.
The pharmacology of cobicistat also confers a significant benefit over ritonavir in that its inhibition and induction of drug metabolism enzymes is more targeted. While both ritonavir and cobicistat strongly inhibit intestinal and hepatic CYP3A, which is the primary mechanism of their PK boosting effects, cobicistat is more targeted to CYP3A with weak inhibition of CYP2D6 as well as minimal induction potential.54 In contrast, ritonavir inhibits CYP3A, CYP2C8, CYP2C9, CYP2D6, and P-gp while also inducing CYP2B6, CYP2C9, CYP2C19, UGT1A4, and P-gp.13,14 Ritonavir’s nonspecific enzyme inhibition and induction leads to a multitude of drug-drug interactions that can be avoided with cobicistat’s more targeted enzyme inhibition profile. Cobicistat also inhibits the intestinal efflux transporter P-gp, which increases absorption of P-gp substrates such as TAF.55,56 In terms of laboratory monitoring, cobicistat increases serum creatinine without affecting renal function due to altered proximal tubular secretion of creatinine through inhibition of drug transporters.57
Cobicistat is used as a PK booster for several ARVs, including elvitegravir, darunavir, and atazanavir. Elvitegravir is a potent second-generation integrase inhibitor with a short half-life that would normally require multiple doses per day. Notably, elvitegravir is only available coformulated with cobicistat. Since elvitegravir is primarily metabolized by CYP3A, several studies have leveraged boosting with ritonavir and cobicistat to improve its bioavailability and PK. One such study comparing ritonavir vs cobicistat-boosted elvitegravir revealed that cobicistat 150 mg resulted in bioequivalent levels of elvitegravir and ritonavir 100 mg with significantly higher AUC and Cmax values (P < 0.03) but similar trough concentration (Ctrough) values.58 Several PK studies have also been conducted to compare the effects of cobicistat and ritonavir on the PIs atazanavir and darunavir. Both atazanavir and darunavir are available coformulated as single tablets with cobicistat (Table 2). A phase I crossover study of 42 healthy subjects demonstrated that atazanavir/ritonavir 300 mg/100 mg and atazanavir/cobicistat 300 mg/150 mg resulted in bioequivalent atazanavir exposure in terms of AUC, Cmax, and Ctrough; in contrast, atazanavir/cobicistat 300 mg/100 mg resulted in lower AUC, Cmax, and Ctrough.59 Another phase I crossover study of 33 healthy subjects compared the boosting effects of ritonavir and cobicistat on darunavir, comparing darunavir 800 mg/ritonavir 100 mg to darunavir 800 mg/cobicistat 150 mg.60 The study confirmed that cobicistat 150 mg and ritonavir 100 mg led to bioequivalent darunavir AUC, Cmax, and Ctrough values.
Pharmacokinetics of Cobicistat-Boosted Regimens in Pregnancy
Elvitegravir/Cobicistat
A study assessing elvitegravir/cobicistat PK in 30 pregnant women compared second trimester, third trimester, and postpartum parameters.61 Elvitegravir AUC0-24 was 24% lower in the second trimester compared to postpartum and 44% lower in the third trimester compared to postpartum; cobicistat AUC0-24 was 44% lower in the second trimester compared to postpartum and 59% lower in the third trimester compared to postpartum.61 The Cmin of elvitegravir was 82% lower in second trimester compared to postpartum and 86% lower in third trimester compared to postpartum; cobicistat Cmin was similarly lowered by 61% in second trimester compared to postpartum and 67% lower in third trimester compared to postpartum. This study demonstrates that standard elvitegravir and cobicistat dosing during pregnancy results in significantly lower exposure, which may increase the risk of virologic failure and mother-to-child transmission. (Table 3) Several case studies have also shown significantly decreased elvitegravir exposure in pregnant women.62,63 Importantly, elvitegravir Cmin values observed during pregnancy (16.8 and 18.3 ng/mL) are well below the concentration that inhibits 95% of viral replication of 45 ng/mL. Due to the current evidence demonstrating significantly reduced and likely subtherapeutic plasma concentrations of elvitegravir/cobicistat, the FDA currently discourages the use of elvitegravir/cobicistat during pregnancy.8
Table 3.
Steady-State Pharmacokinetic Parameters of Cobicistat-Boosted Elvitegravir, Darunavir, and Atazanavir During Pregnancy and Postpartum, and in Nonpregnant HIV-Infected Adults
| Parameter | Second Trimester | Third Trimester | Postpartum | Nonpregnant Adults | Levels in Pregnancy vs Postpartum |
|---|---|---|---|---|---|
| Elvitegravir (150/150 mg daily)61 | N = 17a | N = 26a | N = 25a | 450 (260)b | ↓ |
| Cmin, ng/mL | 16.8 (10.9-21.8) | 18.3 (5.0-61.3) | 185.6 (48.5-377.1) | 1700 (400) | |
| Cmax, ng/mL | 1447.1 (1133.6-1579.0) | 1432.8 (705.7-1570.4) | 1713.1 (955.7-2284.6) | 23 000 (7500) | |
| AUC0-24, ng • h/mL | 15 283 (11 939-19 038) | 14 4004 (9119-18 798) | 21 039 (13 532-32 788) | ||
| Darunavir (800/150 mg daily)64 | N = 16a | N = 25a | N = 18a | N = 298c | ↓ |
| Cmin, ng/mL | 440 (50-1550) | 490 (50-3470) | 1450 (50-5530) | 2043 ± 1257 | |
| Cmax, ng/mL | 4610 (1820-9700) | 4140 (1980-7010) | 7060 (930-12 390) | 100 152 ± 32 042 | |
| AUC0-24, ng • h/mL | 47 220 (13 500-93 600) | 43 620 (12 700-89 300) | 96 030 (4500-231 780) | ||
| Atazanavir (300/150 mg daily)9 | N = 3a | N = 5a | N = 5a | N = 22c | ↓ |
| Cmin, ng/mL | 160 (140-220) | 120 (90-190) | 450 (420-610) | 800 ± 720 | |
| Cmax, ng/mL | 2700 (2000-3200) | 1780 (900-2780) | 3210 (1930-3900) | 3910 ± 1940 | |
| AUC0-24, ng • h/mL | 21 200 (20 600-22 500) | 17 000 (8900-27 500) | 27 400 (24 000-36 400) | 46 130 ± 26 180 |
AUC0-24, area under the plasma concentration–time curve from time zero to 24 hours after dosing; Cmax, maximum plasma concentration; Cmin, minimum plasma concentration.
Results presented as median (interquartile range).
Results presented as geometric mean (standard deviation [SD]).
Results presented as mean ± SD.
Darunavir/Cobicistat
A study examining the PK of darunavir/cobicistat during pregnancy and postpartum in 25 women during their third trimester of pregnancy (second trimester data available for 16 and postpartum data available for 18) demonstrated significantly lower darunavir exposures in pregnancy compared to postpartum. Comparing second trimester to postpartum darunavir levels demonstrated a 33% lower AUC24, a 26% lower Cmax, a 71% lower Cmin, and a 98% higher CL/F. In the third trimester compared to postpartum, darunavir AUC24 was 48% lower, Cmax was 36% lower, and Cmin was 75% lower, while CL/F was 155% higher.64 In a second open-label study on 6 pregnant women (second and third trimester vs 6-12 week postpartum), total darunavir exposure was lower during pregnancy compared to postpartum.65 During pregnancy compared to postpartum, the darunavir AUC24 was 50% to 56% lower, Cmax was 37% to 49% lower, and Cmin was 89% to 92% lower; unbound darunavir exposure was also reduced with AUC24 40% to 45% lower, Cmax 32% to 41% lower, and Cmin 88% to 92% lower.65 The cobicistat exposure was also lower in pregnancy compared to postpartum, which may explain the significantly reduced darunavir concentrations: cobicistat AUC24 was 49% to 63% lower, Cmax was 27% to 50% lower, and Cmin was 83% lower during pregnancy65 (Table 3). The data from these studies provided the basis for the FDA’s recommendation not to use darunavir/cobicistat during pregnancy. In contrast, darunavir/ritonavir remains a viable treatment option in pregnant women.8
Atazanavir/Cobicistat
In a small study of 6 pregnant women with HIV receiving atazanavir in fixed-dose combination with cobicistat as part of clinical care, atazanavir exposure was lower in pregnancy compared to postpartum.9 The AUC24 was 23% lower in the second trimester compared to postpartum and 38% lower in the third trimester compared to postpartum; peak and trough concentrations demonstrated a similar trend, with 16% lower Cmax and 65% lower Cmin in the second trimester compared to postpartum and 45% lower Cmax and 73% lower Cmin in the third trimester compared to postpartum. CL/F was 15% higher in the second trimester compared to postpartum and 61% higher in the third trimester compared to postpartum.9 This study demonstrates the effect of the second and third trimesters of pregnancy in lowering AUC24, Cmax, and Cmin while increasing CL/F, with the third trimester having a greater impact on the values than the second trimester (Table 3). Atazanavir/ritonavir remains a recommended treatment in pregnancy.8
Discussion
Cobicistat fixed-dose combinations are not currently recommended for use during pregnancy due to low plasma concentrations of these regimens during the second and third trimesters of pregnancy.8,55,66 Darunavir and cobicistat trough concentrations were reduced by approximately 80% to 90% in the second and third trimesters of pregnancy compared to postpartum.65 Comparably, second- and third-trimester concentrations of elvitegravir/cobicistat were also lower by approximately 50% to 60% compared to postpartum.61 Reduced boosting by cobicistat may be associated with a higher risk of virologic failure, loss of virologic suppression, inadequate therapeutic effect, and possible development of drug resistance during pregnancy and postpartum.65 The reproductive health policy implications of these findings for pregnant women with HIV are significant.66
Cobicistat Adequately Boosts Tenofovir Alafenamide During Pregnancy but Not Protease Inhibitors/Integrase Inhibitors
Several studies have been conducted to characterize the plasma pharmacokinetics of TAF during pregnancy versus postpartum, one of which was conducted to evaluate TAF 10 mg with 150 mg of cobicistat (n = 27 women). The second trimester showed a 21% lower AUC24 of TAF compared to postpartum, and the third trimester showed a 14% lower AUC24 compared to postpartum; however, there was no statistically significant difference in AUC24 between second and third trimesters of pregnancy compared to postpartum.67 A second study that evaluated TAF 25 mg boosted with either ritonavir or cobicistat in 17 women showed only a 6% decrease in third-trimester TAF AUC24 compared to postpartum, and a 38% decrease in Cmax in the third trimester compared to postpartum.68 Like the first study, no statistically significant difference was found between the third trimester TAF PK parameters (including AUC24, Cmax, CL/F, apparent volume of distribution [V/F], and elimination half-life) and postpartum. These data demonstrate that plasma TAF exposures with cobicistat during pregnancy and postpartum are within the range of those typically observed in nonpregnant adults.67 Thus, based on preliminary data, TAF can safely be used with concomitant cobicistat during pregnancy. While plasma TAF levels were characterized in these studies, there are currently no studies of intracellular tenofovir concentrations for pregnant women using TAF.
There are fundamental differences between cobicistat’s boosting effect on protease and integrase inhibitors compared to its boosting effect on TAF.55,69 Data from TAF use during pregnancy suggest that cobicistat effectively boosts plasma concentrations of TAF (when used as 10 mg or 25 mg),67 but does not optimally boost plasma concentrations of atazanavir, darunavir, or elvitegravir.9,61 This is biologically plausible because cobicistat boosts plasma concentrations of TAF by inhibiting efflux transporters (P-gp and breast cancer resistance protein) in the gastrointestinal tract, thereby increasing TAF’s bioavailability (Figure 1). Unlike elvitegravir and PIs, TAF is not a substrate for hepatic CYP3A4 metabolism. Contrarily, the reduced boosting effect of cobicistat during pregnancy for PIs and integrase inhibitors is likely due to reduced systemic exposure of cobicistat secondary to increased CYP3A4 and CYP2D6 activity.70 Cobicistat boosts TAF exposures by enhancing gut TAF absorption (Figure 1), but the low plasma concentrations of cobicistat in pregnant women explain its failure to effectively boost darunavir or atazanavir during pregnancy. This difference in site and mechanism of action likely explains the differences in effectiveness of cobicistat boosting of TAF compared to protease and integrase inhibitors during pregnancy.
Figure 1.
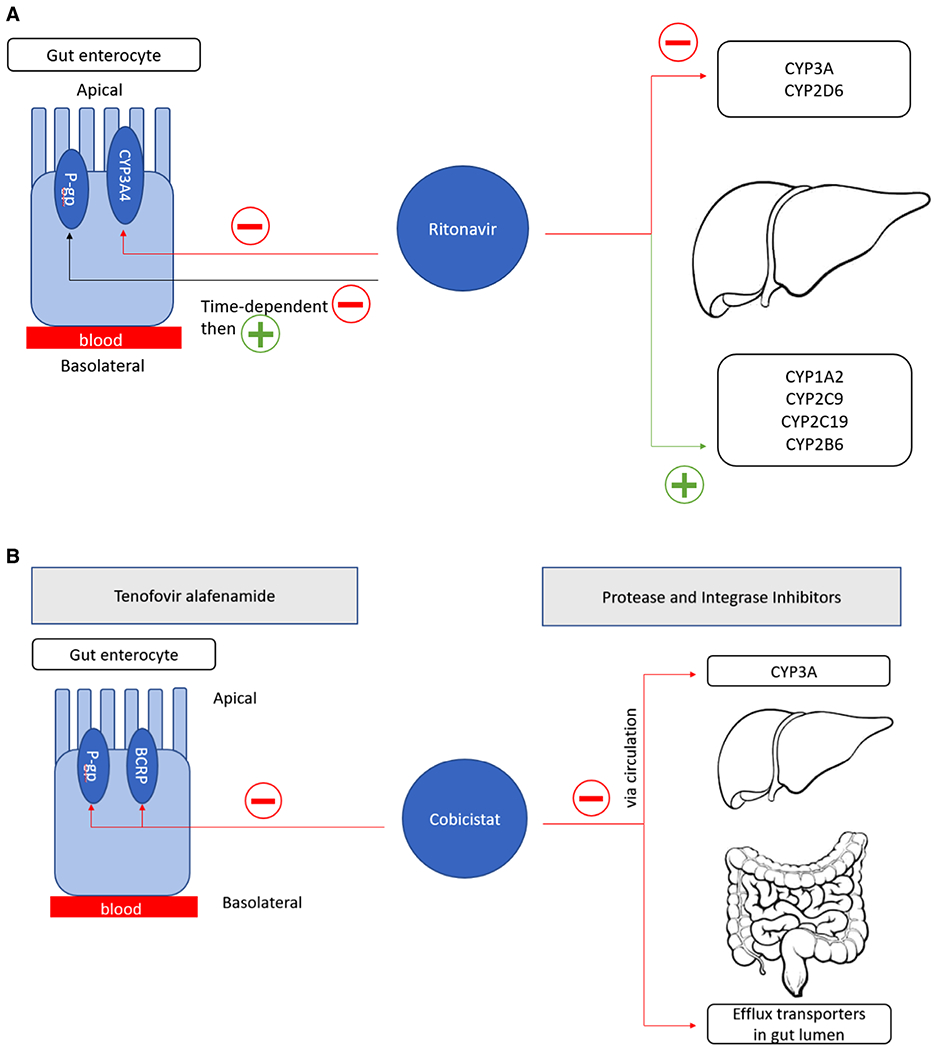
Mechanisms of ritonavir boosting and cobicistat boosting. (A) Ritonavir inhibits CYP3A in both the liver and gut and demonstrates time-dependent inhibition followed by induction of P-gp in the gut. Ritonavir also inhibits CYP2D6 in the liver while inducing CYP1A2, CYP2C9, CYP2C19, and CYP2B6. (B) Cobicistat boosts TAF plasma exposures by inhibiting efflux transporters (P-gp and BCRP) in gut enterocytes, enhancing oral bioavailability. Cobicistat boosts protease and integrase inhibitors by selectively inhibiting CYP3A metabolism in the liver and intestinal tract and by inhibiting efflux transporters (to a smaller extent). BRCP, breast cancer resistance protein; CYP, cytochrome P450; P-gp, P-glycoprotein; TAF, tenofovir alafenamide.
Implications for Practice
When selecting an ARV regimen for pregnant women living with HIV, patient preference, pill burden, patient adherence, efficacy, and potential drug-drug interactions are important considerations.71 Following the FDA’s guidance on cobicistat, there will be regimen modification from cobicistat-containing ARVs to other Department of Health and Human Services HIV guideline-designated preferred (ritonavir-boosted atazanavir, ritonavir-boosted darunavir, dolutegravir, raltegravir, tenofovir disoproxil fumarate/emtricitabine, abacavir/lamivudine) or alternative (rilpivirine, efavirenz, ritonavir-boosted lopinavir, zidovudine) regimens before or during pregnancy. We recommend careful case-by-case consideration of risks and benefits of possible regimen switches in women on well-tolerated ARVs with suppressed viral load in pregnancy from cobicistat regimens to recommended or alternative regimens, as several studies have demonstrated loss of virologic suppression at the time of delivery in women whose antiretroviral therapies were switched during pregnancy. For example, a 2010 prospective cohort study demonstrated that switching of antiretroviral therapy in pregnant women with HIV was associated with an increased risk of unsuppressed viral load (HIV RNA levels >400 copies/mL) at the time of delivery and postpartum.72 Discontinuing and altering therapy in these pregnant women led to increased viral load, enhanced disease progression, and an increased risk of perinatal HIV transmission. In a different multicenter collaborative cohort of women with HIV in the United Kingdom whose antiretroviral regimens were changed during pregnancy (62% during the first trimester, 29% during the second trimester, and 9% during the third trimester), more than 25% of women had unsuppressed viral load at the time of delivery, likely attributable to switching therapy during pregnancy.73 The US Perinatal Guidelines recommend that in cases where women are already on a well-tolerated HIV regimen that suppresses viral load to undetectable levels, women may either switch or continue on the same regimen throughout their pregnancies with more frequent virologic monitoring.1 Therefore, caution must be exercised when switching from cobicistat-containing antiretrovirals to other first- or second-line medications.
Lessons Learned
The November 2018 FDA revised drug labeling for cobicistat containing antiretroviral agents that recommended against use of cobicistat fixed-dose combinations in pregnancy was made several years after these cobicistat-containing regimens were first approved for use in adults in the United States.55,66 The persistent gap between initial licensure of drugs and availability of pertinent pregnancy-specific PK and safety information has remained a long-standing failure of standard drug development programs. Pregnancy-specific PK and pharmacodynamic information is not required for approval of most new drugs, and drug development programs in the United States routinely exclude pregnant women in their phase II and III protocols. Consequently, these cobicistat-containing fixed-dose combinations, which provide favorable and well-tolerated alternatives to ritonavir containing antiretroviral regimens in nonpregnant adults, were widely prescribed to pregnant women with HIV for over 6 years before pregnancy-specific PK data describing the dangerously reduced drug concentrations during the second and third trimesters of pregnancy became available. The cobicistat-pregnancy experience has provided 2 key lessons: (1) Because boosters act on different drugs via different mechanisms, pregnancy may alter the boosting effect on some drugs but not others, as cobicistat boosting differed based on the antiretroviral drug class (as previously discussed); and (2) it emphasized the need to encourage the recruitment and involvement of pregnant women in early phases of drug trials (phases IIb and III), and the need to continue to find innovative ways to study drug disposition in pregnant women.74 Drug development programs should include pregnant women so that pregnancy-specific PK and safety data are available at time of initial approval to help guide clinicians and patients on drug selection and dosing.
Recommendations for Future Research
The research agenda for 2020 and beyond for pharmacoenhancers during pregnancy must focus on rapidly defining optimal ARV regimens in pregnant women with HIV. To achieve this goal, we must first encourage the recruitment, involvement, and retention of pregnant women in early phases of drug trials (phases IIb and III),74 and continue to find innovative ways to study drug disposition in pregnant women. There is a critical need and clear obligation to conduct well-designed drug trials in pregnant women before drug approval. Inclusion of pregnant women into clinical trials as part of drug development programs is critical to obtain the pregnancy-specific PK and safety information necessary for these medications to be safely and effectively used in pregnant women. The experience with cobicistat demonstrates the risks to the well-being of pregnant women and their infants when understanding the effects of pregnancy on drug disposition and pharmacodynamics is treated as an afterthought left out of the pathway to drug licensure.74 Use of cobicistat was common in US pregnant women living with HIV from its initial licensure in 2012 through 2018, when data from post-marketing PK and safety studies in pregnant women became available showing that cobicistat use in pregnancy provided suboptimal antiretroviral therapy. At that time, drug labels and treatment guidelines were rapidly changed to recommend that cobicistat regimens not be used in pregnant women. There is a growing recognition that pregnant women are not protected by being left out of drug development programs. The 21st Century Cures Act established a Task Force on Research Specific to Pregnant Women and Lactating Women, whose report includes as its first recommendation inclusion and integration of pregnant women and lactating women in the clinical research agenda.75 The FDA has released a draft guidance addressing the scientific and ethical considerations for the inclusion of pregnant women in clinical trials, specifically mentioning the need to include pregnant women to establish safe and effective treatment during pregnancy.76 The guidance acknowledges that the inclusion of pregnant women should be guided by human subject protection and risk-benefit assessment, as there is no easy way to address the ethical considerations of including pregnant women in clinical trials. Nonetheless, the fair inclusion of pregnant women in clinical trials necessitates that pregnant women should be included in the earliest phases of the research process when feasible in order to further develop the evidence base for drug use in this population.77
Second, additional data are needed to understand whether increased cobicistat (Tybost) dosing during pregnancy will result in adequate boosting of CYP3A-metabolized antiretrovirals in future studies. Although cobicistat exposure is considerably decreased in pregnancy, higher-than-standard dosing (ie, > 150 mg once daily) has not been studied during pregnancy.66 While several studies have shown that cobicistat may increase serum creatinine levels, this effect appears be due to inhibition of renal tubular secretion of creatinine and not a reduction in the actual glomerular filtration rate.57,78,79 In addition, cobicistat does not have the gastrointestinal adverse effects of ritonavir and was generally well tolerated in postmarketing studies in pregnant women.9,61 Therefore, increased doses of cobicistat are likely going to be well tolerated during pregnancy as a result of physiologic changes in pregnancy that favor drug disposition. There are no studies to investigate increased doses of cobicistat in pregnancy; however, approaches such as physiologically based PK modeling may be used to predict how increased doses of cobicistat may lead to increased exposures of cobicistat and, in turn, the agents it is boosting during pregnancy.
Third, there is need to continue to encourage the development of newer (third-generation) pharmacoenhancers for HIV management. Several promising pharmacoenhancer compounds, for example, Sequoia Pharmaceutical’s SPI-452 and SPI-251 (developed for boosting darunavir and atazanavir),80 Pfizer/ViiV’s PF-3716539,81 and Tibotec’s TMC-55844582 all failed phase 1 trials for use as stand-alone boosters and for use in fixed-dose combinations.83 Presently, there are no new pharmacoenhancer compounds in development in the 2020 antiretroviral therapy new-drugs-in-development pipeline summary.84
In conclusion, pregnancy decreases exposure to the PK enhancers ritonavir and cobicistat and to their boosted antiretrovirals metabolized by CYP3A, but to a greater extent with cobicistat. Clinically, this means that the theoretical risks of treatment failure and perinatal transmission due to low exposures of these medications during pregnancy need to be discussed with pregnant women. The use of cobicistat boosted atazanavir, darunavir, and elvitegravir regimens are not recommended during pregnancy.1 Yet the benefits and risks of switching to alternative regimens must be carefully weighed, as several studies have demonstrated loss of virologic suppression at the time of delivery in women whose antiretroviral therapies were switched during pregnancy.72,73 Women who continue on a cobicistat-containing regimen during pregnancy should have absorption optimized and have more frequent virologic monitoring.1 Future research should focus on encouraging the recruitment of pregnant women in earlier phases of drug development to enable the timely acquisition of pregnancy-related data, studying increased doses of cobicistat in pregnant women, and encouraging the development of newer boosters for management of pregnant women with HIV.
Footnotes
Conflicts of Interest
Drs Salama, Eke, and Best declare no conflicts of interest. Dr Mirochnick has received research support from ViiV Healthcare, Merck, and Gilead Sciences. Dr Momper has received research grant support from Gilead Sciences and Veloxis Pharmaceuticals.
References
- 1.Panel on Treatment of Pregnant Women with HIV Infection and Prevention of Perinatal Transmission. Recommendations for the use of antiretroviral drugs in pregnant women with HIV infection and interventions to reduce perinatal HIV transmission in the United States. https://files.aidsinfo.nih.gov/contentfiles/lvguidelines/PerinatalGL.pdf. Accessed July 14, 2020.
- 2.Mirochnick M, Capparelli E. Pharmacokinetics of antiretrovirals in pregnant women. Clin Pharmacokinet. 2004;43(15):1071–1087. [DOI] [PubMed] [Google Scholar]
- 3.Feghali M, Venkataramanan R, Caritis S. Pharmacokinetics of drugs in pregnancy. Semin Perinatol. 2015;39(7):512–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larson KB, Wang K, Delille C, Otofokun I, Acosta EP. Pharmacokinetic enhancers in HIV therapeutics. Clin Pharmacokinet. 2014;53(10):865–872. [DOI] [PubMed] [Google Scholar]
- 5.Merry C, Barry MG, Mulcahy F, et al. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS. 1997;11(4):F29–F33. [DOI] [PubMed] [Google Scholar]
- 6.Zeldin RK, Petruschke RA. Pharmacological and therapeutic properties of ritonavir-boosted protease inhibitor therapy in HIV-infected patients. J Antimicrob Chemother. 2004;53(1):4–9. [DOI] [PubMed] [Google Scholar]
- 7.Shah BM, Schafer JJ, Priano J, Squires KE. Cobicistat: a new boost for the treatment of human immunodeficiency virus infection. Pharmacotherapy. 2013;33(10):1107–1116. [DOI] [PubMed] [Google Scholar]
- 8.Boyd SD, Sampson MR, Viswanathan P, Struble KA, Arya V, Sherwat AI. Cobicistat-containing antiretroviral regimens are not recommended during pregnancy: viewpoint. AIDS. 2019;33(6):1089–1093. [DOI] [PubMed] [Google Scholar]
- 9.Momper JD, Stek A, Wang J, et al. Pharmacokinetics of atazanavir boosted with cobicistat during pregnancy and postpartum. Presented at: 20th International Workshop on Clinical Pharmacology of HIV, Hepatitis, and Other Antiviral Drugs; May 16, 2019; Noordwijk, the Netherlands. [Google Scholar]
- 10.Hull MW, Montaner JS. Ritonavir-boosted protease inhibitors in HIV therapy. Ann Med. 2011;43(5):375–388. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan AK, Nelson MR. Marked hyperlipidaemia on ritonavir therapy. AIDS. 1997;11(7):938–939. [PubMed] [Google Scholar]
- 12.Acosta EP. Pharmacokinetic enhancement of protease inhibitors. J Acquir Immune Defic Syndr. 2002;29(Suppl 1):S11–S18. [DOI] [PubMed] [Google Scholar]
- 13.Foisy MM, Yakiwchuk EM, Hughes CA. Induction effects of ritonavir: implications for drug interactions. Ann Pharmacother. 2008;42(7):1048–1059. [DOI] [PubMed] [Google Scholar]
- 14.von Moltke LL, Durol AL, Duan SX, Greenblatt DJ. Potent mechanism-based inhibition of human CYP3A in vitro by amprenavir and ritonavir: comparison with ketoconazole. Eur J Clin Pharmacol. 2000;56(3):259–261. [DOI] [PubMed] [Google Scholar]
- 15.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed July 14, 2020.
- 16.Geretti AM, Moeketsi M, Demasi R, van Delft Y, Mohammed P. Efficacy of once daily darunavir/ritonavir in PI-naive, NNRTI-experienced patients in the ODIN Trial. AIDS Res Treat. 2015;2015:962574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orkin C, DeJesus E, Khanlou H, et al. Final 192-week efficacy and safety of once-daily darunavir/ritonavir compared with lopinavir/ritonavir in HIV-1-infected treatment-naive patients in the ARTEMIS trial. HIV Med. 2013;14(1):49–59. [DOI] [PubMed] [Google Scholar]
- 18.Sekar V, Vanden Abeele C, Van Baelen B, et al. Pharmacokinetic-pharmacodynamic analyses of once-daily darunavir-ritonavir in the ARTEMIS study. Poster session presented at: 15th Conference on Retroviruses and Opportunistic Infections; February 3-6, 2008; Boston, MA. [Google Scholar]
- 19.Crauwels HM, Kakuda TN, Ryan B, et al. Pharmacokinetics of once-daily darunavir/ritonavir in HIV-1-infected pregnant women. HIV Med. 2016;17(9):643–652. [DOI] [PubMed] [Google Scholar]
- 20.Colbers A, Molto J, Ivanovic J, et al. Pharmacokinetics of total and unbound darunavir in HIV-1-infected pregnant women. J Antimicrob Chemother. 2015;70(2):534–542. [DOI] [PubMed] [Google Scholar]
- 21.Zorrilla CD, Wright R, Osiyemi OO, et al. Total and unbound darunavir pharmacokinetics in pregnant women infected with HIV-1: results of a study of darunavir/ritonavir 600/100 mg administered twice daily. HIV Med. 2014;15(1):50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoo S, Peytavin G, Burger D, et al. Pharmacokinetics and safety of darunavir/ritonavir in HIV-infected pregnant women. AIDS Rev. 2017;19(1):16–23. [PubMed] [Google Scholar]
- 23.Eke AC, Stek AM, Wang J, et al. Darunavir pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2020;83(4):373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prezista (darunavir) [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2019. [Google Scholar]
- 25.Mirochnick M, Best BM, Stek AM, et al. Atazanavir pharmacokinetics with and without tenofovir during pregnancy. J Acquir Immune Defic Syndr. 2011;56(5):412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foca E, Calcagno A, Bonito A, et al. Atazanavir intracellular concentrations remain stable during pregnancy in HIV-infected patients. J Antimicrob Chemother. 2017;72(11):3163–3166. [DOI] [PubMed] [Google Scholar]
- 27.Ripamonti D, Cattaneo D, Maggiolo F, et al. Atazanavir plus low-dose ritonavir in pregnancy: pharmacokinetics and placental transfer. AIDS. 2007;21(18):2409–2415. [DOI] [PubMed] [Google Scholar]
- 28.Kreitchmann R, Best BM, Wang J, et al. Pharmacokinetics of an increased atazanavir dose with and without tenofovir during the third trimester of pregnancy. J Acquir Immune Defic Syndr. 2013;63(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Else LJ, Jackson V, Brennan M, et al. Therapeutic drug monitoring of atazanavir/ritonavir in pregnancy. HIV Med. 2014;15(10):604–610. [DOI] [PubMed] [Google Scholar]
- 30.Crommentuyn KM, Mulder JW, Mairuhu AT, et al. The plasma and intracellular steady-state pharmacokinetics of lopinavir/ritonavir in HIV-1-infected patients. Antivir Ther. 2004;9(5):779–785. [PubMed] [Google Scholar]
- 31.Zhu L, Liao S, Child M, et al. Pharmacokinetics and inhibitory quotient of atazanavir/ritonavir versus lopinavir/ritonavir in HIV-infected, treatment-naive patients who participated in the CASTLE Study. J Antimicrob Chemother. 2012;67(2):465–468. [DOI] [PubMed] [Google Scholar]
- 32.Crommentuyn KM, Kappelhoff BS, Mulder JW, et al. Population pharmacokinetics of lopinavir in combination with ritonavir in HIV-1-infected patients. Br J Clin Pharmacol. 2005;60(4):378–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Else LJ, Douglas M, Dickinson L, Back DJ, Khoo SH, Taylor GP. Improved oral bioavailability of lopinavir in melt-extruded tablet formulation reduces impact of third trimester on lopinavir plasma concentrations. Antimicrob Agents Chemother. 2012;56(2):816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khuong-Josses MA, Azerad D, Boussairi A, Ekoukou D. Comparison of lopinavir level between the two formulations (soft-gel capsule and tablet) in HIV-infected pregnant women. HIV Clin Trials. 2007;8(4):254–255. [DOI] [PubMed] [Google Scholar]
- 35.Pasley MV, Martinez M, Hermes A, d’Amico R, Nilius A. Safety and efficacy of lopinavir/ritonavir during pregnancy: a systematic review. AIDS Rev. 2013;15(1):38–48. [PubMed] [Google Scholar]
- 36.Best BM, Stek AM, Mirochnick M, et al. Lopinavir tablet pharmacokinetics with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2010;54(4):381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirochnick M, Best BM, Stek AM, et al. Lopinavir exposure with an increased dose during pregnancy. J Acquir Immune Defic Syndr. 2008;49(5):485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lambert JS, Else LJ, Jackson V, et al. Therapeutic drug monitoring of lopinavir/ritonavir in pregnancy. HIV Med. 2011;12(3):166–173. [DOI] [PubMed] [Google Scholar]
- 39.Kaletra (lopinavir/ritonavir) [package insert]. North Chicago, IL: AbbVie Inc; 2019. [Google Scholar]
- 40.Cressey TR, Urien S, Capparelli EV, et al. Impact of body weight and missed doses on lopinavir concentrations with standard and increased lopinavir/ritonavir doses during late pregnancy. J Antimicrob Chemother. 2015;70(1):217–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salem AH, Jones AK, Santini-Oliveira M, et al. No need for lopinavir dose adjustment during pregnancy: a population pharmacokinetic and exposure-response analysis in pregnant and nonpregnant HIV-infected subjects. Antimicrob Agents Chemother. 2016;60(1):400–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aweeka FT, Stek A, Best BM, et al. Lopinavir protein binding in HIV-1-infected pregnant women. HIV Med. 2010;11(4):232–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patterson KB, Dumond JB, Prince HA, et al. Protein binding of lopinavir and ritonavir during 4 phases of pregnancy: implications for treatment guidelines. J Acquir Immune Defic Syndr. 2013;63(1):51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonafe SM, Costa DA, Vaz MJ, et al. A randomized controlled trial to assess safety, tolerability, and antepartum viral load with increased lopinavir/ritonavir dosage in pregnancy. AIDS Patient Care STDS. 2013;27(11):589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunet C, Reliquet V, Jovelin T, et al. Effectiveness and safety of saquinavir/ritonavir in HIV-infected pregnant women: INEMA cohort. Med Mal Infect. 2012;42(9):421–428. [DOI] [PubMed] [Google Scholar]
- 46.Martinez-Rebollar M, Lonca M, Perez I, et al. Pharmacokinetic study of saquinavir 500 mg plus ritonavir (1000/100 mg twice a day) in HIV-positive pregnant women. Ther Drug Monit. 2011;33(6):772–777. [DOI] [PubMed] [Google Scholar]
- 47.von Hentig N, Nisius G, Lennemann T, et al. Pharmacokinetics, safety and efficacy of saquinavir/ ritonavir 1,000/100 mg twice daily as HIV type-1 therapy and transmission prophylaxis in pregnancy. Antivir Ther. 2008;13(8):1039–1046. [PubMed] [Google Scholar]
- 48.Eke AC, Wang J, Amin K, et al. Fosamprenavir with ritonavir pharmacokinetics during pregnancy. Antimicrob Agents Chemother. 2020;64(4):e02260–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cressey TR, Best BM, Achalapong J, et al. Reduced indinavir exposure during pregnancy. Br J Clin Pharmacol. 2013;76(3):475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghosn J, De Montgolfier I, Cornelie C, et al. Antiretroviral therapy with a twice-daily regimen containing 400 milligrams of indinavir and 100 milligrams of ritonavir in human immunodeficiency virus type 1-infected women during pregnancy. Antimicrob Agents Chemother. 2008;52(4):1542–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mathias AA, German P, Murray BP, et al. Pharmacokinetics and pharmacodynamics of GS-9350: a novel pharmacokinetic enhancer without anti-HIV activity. Clin Pharmacol Ther. 2010;87(3):322–329. [DOI] [PubMed] [Google Scholar]
- 52.Tybost (cobicistat) [package insert]. Foster City, CA: Gilead; 2019. [Google Scholar]
- 53.Norvir (ritonavir) [package insert]. North Chicago, IL: AbbVie Inc; 2019. [Google Scholar]
- 54.Xu L, Liu H, Murray BP, et al. Cobicistat (GS-9350): A potent and selective inhibitor of human CYP3A as a novel pharmacoenhancer. ACS Med Chem Lett. 2010;1(5): 209–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eke AC, Mirochnick M. Ritonavir and cobicistat as pharmacokinetic enhancers in pregnant women. Expert Opin Drug Metab Toxicol. 2019;15(7):523–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lepist EI, Phan TK, Roy A, et al. Cobicistat boosts the intestinal absorption of transport substrates, including HIV protease inhibitors and GS-7340, in vitro. Antimicrob Agents Chemother. 2012;56(10):5409–5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.German P, Liu HC, Szwarcberg J, et al. Effect of cobicistat on glomerular filtration rate in subjects with normal and impaired renal function. J Acquir Immune Defic Syndr. 2012;61(1): 32–40. [DOI] [PubMed] [Google Scholar]
- 58.German P, Warren D, West S, Hui J, Kearney BP. Pharmacokinetics and bioavailability of an integrase and novel pharmacoenhancer-containing single-tablet fixed-dose combination regimen for the treatment of HIV. J Acquir Immune Defic Syndr. 2010;55(3):323–329. [DOI] [PubMed] [Google Scholar]
- 59.Ramanathan S, Warren D, Wei L, Kearney BP. Pharmacokinetic boosting of atazanavir with the pharmacoenhancer GS-9350 versus ritonavir (abstract 614). Poster session presented at: 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; September 12-15, 2009; San Francisco, CA. [Google Scholar]
- 60.Mathias A, Liu H, Warren D, Sekar V, Kearney BP. Relative bioavailability and pharmacokinetics of darunavir when boosted with pharmacoenhancer GS-9350 versus ritonavir (abstract 28). Poster session presented at: 11th International Workshop on Clinical Pharmacology of HIV Therapy; April 7-9, 2010; Sorrento, Italy. [Google Scholar]
- 61.Momper JD, Best BM, Wang J, et al. Elvitegravir/cobicistat pharmacokinetics in pregnant and postpartum women with HIV. AIDS. 2018;32(17):2507–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marzolini C, Decosterd L, Winterfeld U, et al. Free and total plasma concentrations of elvitegravir/cobicistat during pregnancy and postpartum: a case report. Br J Clin Pharmacol. 2017;83(12):2835–2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nguyen B, Foisy MM, Hughes CA. Pharmacokinetics and safety of the integrase inhibitors elvitegravir and dolutegravir in pregnant women with HIV. Ann Pharmacother. 2019;53(8):833–844. [DOI] [PubMed] [Google Scholar]
- 64.Momper JD, Best BM, Wang J, et al. Pharmacokinetics of darunavir boosted with cobicistat during pregnancy and postpartum. Poster session presented at: 22nd International AIDS Conference; July 23-27, 2018; Amsterdam, the Netherlands. [Google Scholar]
- 65.Crauwels HM, Osiyemi O, Zorrilla C, Bicer C, Brown K. Reduced exposure to darunavir and cobicistat in HIV-1-infected pregnant women receiving a darunavir/cobicistat-based regimen. HIV Med. 2019;20(5):337–343. [DOI] [PubMed] [Google Scholar]
- 66.Eke AC, Mirochnick MH. Cobicistat as a pharmacoenhancer in pregnancy and postpartum: progress to date and next steps. J Clin Pharmacol. 2019;59(6):779–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Momper JD, Best BM, Wang J, et al. Tenofovir alafenamide pharmacokinetics with and without cobicistat in pregnancy. Podium presentation at: 22nd International AIDS Conference; July 23-27, 2018; Amsterdam, the Netherlands. [Google Scholar]
- 68.Brooks KM, Pinilla M, Shapiro D, et al. Pharmacokinetics of tenofovir alafenamide 25 mg with PK boosters during pregnancy and postpartum. Podium presentation at: 20th International Workshop on Clinical Pharmacology of HIV, Hepatitis & Other Antiviral Drugs; May 14-16, 2019; Noordwijk, the Netherlands. [Google Scholar]
- 69.Eke AC, Brooks KM, Gebreyohannes RD, Sheffield JS, Dooley KE, Mirochnick M. Tenofovir alafenamide use in pregnant and lactating women living with HIV. Expert Opin Drug Metab Toxicol. 2020;16(4):333–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sheffield JS, Siegel D, Mirochnick M, et al. Designing drug trials: considerations for pregnant women. Clin Infect Dis. 2014;59(Suppl 7):S437–S444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Iacob SA, Iacob DG, Jugulete G. Improving the adherence to antiretroviral therapy, a difficult but essential task for a successful HIV treatment—clinical points of view and practical considerations. Front Pharmacol. 2017;8:831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Floridia M, Ravizza M, Pinnetti C, et al. Treatment change in pregnancy is a significant risk factor for detectable HIV-1 RNA in plasma at end of pregnancy. HIV Clin Trials. 2010;11(6):303–311. [DOI] [PubMed] [Google Scholar]
- 73.Huntington SE, Bansi LK, Thorne C, et al. Treatment switches during pregnancy among HIV-positive women on antiretroviral therapy at conception. AIDS. 2011;25(13):1647–1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Eke AC, Dooley KE, Sheffield JS. Pharmacologic research in pregnant women - time to get it right. N Engl J Med. 2019;380(14):1293–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Task Force on Research Specific to Pregnant Women and Lactating Women. Report to Secretary, Health and Human Services and Congress. 2018; https://www.nichd.nih.gov/sites/default/files/2018-09/PRGLAC_Report.pdf. Accessed July 7, 2020.
- 76.US Food and Drug Administration. Guidance for Industry.Pregnant women: scientific and ethical considerations for inclusion in clinical trials. 2018; https://www.fda.gov/media/112195/download. Accessed July 5, 2020.
- 77.van der Graaf R, van der Zande ISE, den Ruijter HM, et al. Fair inclusion of pregnant women in clinical trials: an integrated scientific and ethical approach. Trials. 2018;19(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eron JJ Jr, Lelievre JD, Kalayjian R, et al. Safety of elvitegravir, cobicistat, emtricitabine, and tenofovir alafenamide in HIV-1-infected adults with end-stage renal disease on chronic haemodialysis: an open-label, single-arm, multicentre, phase 3b trial. Lancet HIV. 2018;6(1);e15–e24. [DOI] [PubMed] [Google Scholar]
- 79.Fisher M, McDonald C, Moyle G, et al. Switching from ritonavir to cobicistat in HIV patients with renal impairment who are virologically suppressed on a protease inhibitor. J Int AIDS Soc. 2014;17(4 Suppl 3):19824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.S Gulnik M, Afonina E. Preclinical and early clinical evaluation of SPI-452, a new pharmacokinetic enhancer [abstract]. Poster session presented at: 16th Conference on Retroviruses and Opportunistic Infections (CROI); February 8-11, 2009; Montreal, Canada. [Google Scholar]
- 81.Pfizer. Glaxo SmithKline (GSK) and Pfizer’s ARV pipeline. https://www.nature.com/articles/nrd2917/tables/1. Accessed March 22, 2020.
- 82.Tibotec. PEPI-TiDP23-C103: First-in-human study to examine the safety, tolerability, and plasma pharmacokinetics of increasing single and repeated oral doses of TMC558445 and of a combined single day dosing of oral TMC558445 and oral TMC310911 and also oral darunavir. https://aidsinfo.nih.gov/clinical-trials/details/NCT00838760. Accessed March 22, 2020.
- 83.Krauss J, Bracher F. Pharmacokinetic enhancers (boosters)-escort for drugs against degrading enzymes and beyond. Sci Pharm. 2018;86(4):E43. [DOI] [PubMed] [Google Scholar]
- 84.Collins S HIV pipeline 2020: new drugs in development – March 2020. http://i-base.info/htb/wp-content/uploads/2020/03/i-Base-HIV-pipeline-March-2020.pdf. Accessed March 22, 2020.
- 85.Stek A, Best BM, Wang J, et al. Pharmacokinetics of once versus twice daily darunavir in pregnant HIV-infected women. J Acquir Immune Defic Syndr. 2015;70(1):33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reyataz (atazanavir) [package insert]. Princeton, NJ: Bristol-Myers Squibb Company; 2019. [Google Scholar]
- 87.Lexiva (fosamprenavir) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2017. [Google Scholar]
- 88.Schalkwijk S, Colbers A, Konopnicki D, Greupink R, Rusel F, Burger D. First reported use of elvitegravir and cobicistat during pregnancy. AIDS. 2016;30(5):807–808. [DOI] [PubMed] [Google Scholar]
- 89.Stek AM, Mirochnick M, Capparelli E, et al. Reduced lopinavir exposure during pregnancy. AIDS. 2006;20(15):1931–1939. [DOI] [PubMed] [Google Scholar]
- 90.Bouillon-Pichault M, Jullien V, Azria E, et al. Population analysis of the pregnancy-related modifications in lopinavir pharmacokinetics and their possible consequences for dose adjustment. J Antimicrob Chemother. 2009;63(6): 1223–1232. [DOI] [PubMed] [Google Scholar]
- 91.Ramautarsing RA, van der Lugt J, Gorowara M, et al. Thai HIV-1-infected women do not require a dose increase of lopinavir/ritonavir during the third trimester of pregnancy. AIDS. 2011;25(10):1299–1303. [DOI] [PubMed] [Google Scholar]
- 92.Colbers A, Schalkwijk S, Konopnicki D, Rockstroh J, Burger D. Elvitegravir pharmacokinetics during pregnancy and postpartum. Presented at: 19th International Workshop on Clinical Pharmacology of Antiviral Therapy; May 22–24, 2018; Baltimore, MD. Abstract 17. http://www.natap.org/2018/Pharm/Pharm_11.htm. Accessed August 4, 2020. [Google Scholar]


