Abstract
Cardiovascular disorders are leading mortality causes worldwide, often with a latent evolution. Vascular health depends on endothelial function, arterial stiffness, and the presence of atherosclerotic plaques. Preventive medicine deserves special attention, focusing on modifiable cardiovascular risk factors, including diet. A diet rich in fruits and vegetables has well-known health benefits, especially due to its polyphenolic components. Anthocyanins, water-soluble flavonoid species, responsible for the red-blue color in plants and commonly found in berries, exert favorable effects on the endothelial function, oxidative stress, inhibit COX-1, and COX-2 enzymes, exert antiatherogenic, antihypertensive, antiglycation, antithrombotic, and anti-inflammatory activity, ameliorate dyslipidemia and arterial stiffness. The present review aims to give a current overview of the mechanisms involved in the vascular protective effect of anthocyanins from the human diet, considering epidemiological data, in vitro and in vivo preclinical research, clinical observational, retrospective, intervention and randomized studies, dietary and biomarker studies, and discussing preventive benefits of anthocyanins and future research directions.
Keywords: anthocyanins, arterial stiffness, endothelial function, cardiovascular risk, berries, antioxidants
1. Introduction
Cardiovascular disorders are leading mortality causes worldwide, often with a latent evolution. Vascular health depends on endothelial function, arterial stiffness, and the presence of atherosclerotic plaques, and it predicts, if impaired, future major cardiovascular events [1,2,3,4]. Preventive medicine deserves special attention, focusing on modifiable cardiovascular risk factors, including diet. The type and amount of food can influence other cardiovascular controllable risk factors: serum cholesterol, blood pressure, diabetes mellitus, and obesity. A diet rich in fruits and vegetables has well-known health benefits, especially due to its polyphenolic components [5,6,7,8,9,10,11]. 2021 is the International Year of Fruits and Vegetables, according to the General Assembly of the United Nations, which emphasizes the importance of the present topic. The biological and pharmacological effects of dietary natural products have been intensively studied, and the research might have been also fueled by the growing industry related to natural products utilization [12].
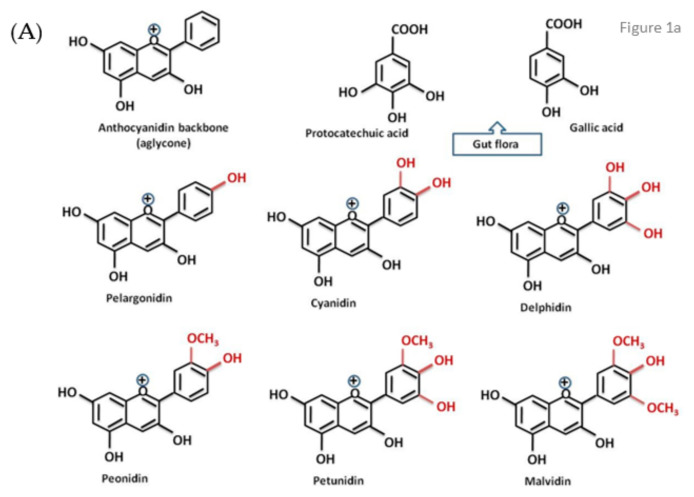
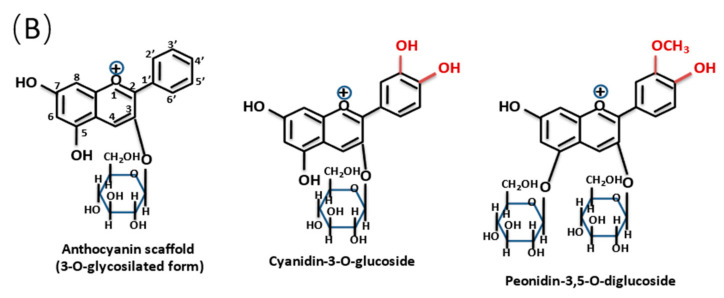
Anthocyanins (ACYs), water-soluble flavonoids, are responsible for the orange or red-blue color in flowers, seeds, fruits, and vegetables [13]. Chemically, anthocyanins include the sugar-free anthocyanidine aglycons and the anthocyanin glycosides [13] (Figure 1). The glycosylated structures are known as anthocyanins, while the non-glycosylated are the anthocyanidins, the precursors of anthocyanins [14]. The most common glycosylation site is position 3 where glucose is found usually as single sugar, especially for cyanidin. In some cases, an additional glucose may be attached in position 5, more often seen for peonidin, pelargonidin and delphinidin. Other sugars bound in position 3 were described in some studies, such as galactose and arabinose, as well as complex oligosaccharides such as rutinose, sophorose and sambubiose [14,15]. They are commonly found in berries, especially strawberries and blueberries, red grapes, apples and pears, blackcurrants, chokeberry, plums, cherries, nectarines, peaches, pomegranate, avocados, bananas, dates, nuts (almonds, hazelnuts, pistachio, pecan nuts), black rice, purple corn, cauliflower, red radishes, beans, cabbage, beets and onions, red and black carrots, purple sweet potato, beans, pepper, eggplant, black olives, and red lettuce, and fruit-derived products like red wine, juices, jam, and marmalade (Table 1) [8,16,17,18,19,20,21,22,23,24,25,26]. During berry ripening, anthocyanin content rises and is responsible for its health benefits [27]. Many anthocyanin-rich fruits are included in the Mediterranean diet and lifestyle, with favorable health effects. ACYs may also represent a source of natural food colorants [28].
Figure 1.
Chemical structures of the most common anthocyanidins (A) and their metabolites, and anthocyanins (B).
Table 1.
Anthocyanin content of the fruits and vegetables mentioned in the manuscript.
| Fruits and Vegetables | Anthocyanin Content | Administrated as | References |
|---|---|---|---|
| Blackberry (Rubus fruticosus) | 820–1800 mg/kg | Fresh fruit | [39] |
| Black mulberry (Morus nigra) | 42.4 mg/100 g | Fresh fruit | [40] |
| Bilberry (Vaccinium myrtillus) | 1610–5963 mg/L | Juice 100% | [23] |
| Black carrots (Daucus carota ssp. sativus var. atrorubens) | 1750 mg/kg | Fresh vegetable | [41] |
| Black chokeberries (Aronia melanocarpa) | 1480 mg/100 g | Fresh fruit | [42] |
| Black soybean (Glycine max) | 0.1–23.04 mg/g | Seed coat | [43] |
| Black currant (Ribes nigrum) | 176–1298 mg/L | Juice 100% | [23] |
| Blood orange (Citrus sinensis) | 4.6 ± 0.7; 72.4 ± 0.6 mg/L | Fresh fruit | [44] |
| Blueberry (Vaccinium virgatum and Vaccinium corymbosum) | 134 mg/kg | Fresh fruit | [45] |
| Cherry (Prunus cerasus) | 22 mg/100 g | Fresh fruit | [23] |
| Cornelian cherry (Cornus mas) | 128.45 ± 5.14 mg/L C3G 226.78 ± 8.61 mg/L |
[46] | |
| Cowpea (Vigna unguiculata) | 1.7–3.9 mg/g | Seeds | [43] |
| Cranberry (Vaccinium macrocarpon) | 460–2000 mg/kg | Fresh fruit | [39] |
| Eggplant (Solanum melongena L.) | 11.53 g/100 g DW delphinidin, 0.55 g/100 g DW of petunidin | Fruit | [25] |
| Grape (Vitis vinifera) | 300–7500 mg/kg | Fresh fruit | [39] |
| Kiwi (Actinidia melanandra) | 478 μg/g in skin, 81 μg/g in flesh |
Fresh fruit | [47] |
| Mahaleb cherries (Prunus mahaleb) (g/kg DW) | 7.80 ± 1.10; 15.60 ± 3.10; 17.70 ± 3.50; 18.90 ± 0.90 | Fresh fruit | [48] |
| Pepper (Capsicum annuum L.) | 0.96 mg anthocyanin/100 g fresh weight | [26] | |
| Pomegranate (Punica granatum) | 43 mg/L | Juice | [23] |
| Purple maize (Zea mays indurate) | 4.3 to 117-mg C3G/g | dark-colored purple corncob | [49] |
| Purple sweet potato (Ipomoea batatas L.) | 0.94- 1.75 g/kg | Fresh weight | [24] |
| Strawberry (Fragaria × ananassa) | 232 mg/100 g | Fresh fruit | [23] |
C3G = cyanidin 3-glucoside; P3OG = pelargonidin-3-O-glucoside; DW = dry weight.
Ingested ACYs undergo oral transformation due to salivary amylase. In the stomach ionic forms of ACYs were identified, which are hydrolyzed by several enzymes in the small intestine resulting in conjugated products, or simpler phenolic compounds, which are hardly absorbed and processed by the gut microbiota as free anthocyanidins and protocatechuic acid [27,29,30]. ACYs are the least well-absorbed polyphenols [31]. It has been reported that more anthocyanin can be absorbed with increasing dose [32]. Some biological activities of ACYs are due to the synergetic effect of their colonic catabolites [30]. Low-molecular-weight catabolites produced by the large intestine can be excreted in the feces within 2–8 h or absorbed again [30]. ACYs undergo dehydroxylation by colonic bacteria, to form hydroxybenzoic acid, followed by conjugation with glycine to form hippuric acid [33]. Anthocyanins and their catabolites also undergo phase 2 enzymatic metabolism, generating the glucuronidated, sulphated, and methylated forms, which persist in the urine for a long time after their intake, related, probably, to their transport in the bile [27]. On the other hand, colonic fermentation of ACYs increases beneficial bacteria, such as Bifidobacterium, Actinobacteria, Bacteroidetes and Lactobacillus [30,34]. The daily intake of anthocyanins can be estimated according to food databases [30]. Single doses of 150 mg to 2 g total anthocyanins given to volunteers, generally in the form of berries, resulted in extremely low plasma concentrations (10–50 nmol/l), and the maximal concentration was reached after 1.5 h in the plasma [31]. The most important factors related to the level of anthocyanins and their metabolites in our organism include: the ability to cross membranes, pH, enzymes of the digestive tract, microbiota, biliary acids, and food matrix [30].
The most important ACYs are, as follows: delphinidin, cyanidin, malvidin, pelargonidin, peonidin, and petunidin (Figure 1a) [13]. Several beneficial effects of ACYs were attributed to protocatechuic acid, a bioactive compound, synthesized by the gut microbiota from ACYs [35,36]. ACYs conjugated with sugar residues might be accompanied by anthocyanidins, the sugar-free parts of ACYs, with lower stability due to missing sugars [37]. One-half cup of blueberries provides about 121 mg of ACYs and consuming 1–2 portions daily can reduce cardiovascular risk [19,27]. Ponzo et al. also demonstrated the relationship between high ACYs intake and low incidence of cardiovascular events and all-cause mortality [38].
Considering that many people prefer natural therapies, the present paper aims to give a current overview of the vascular protective mechanisms attributed to anthocyanins in the diet, considering epidemiological data, in vitro and in vivo preclinical research, clinical observational, retrospective, intervention, and randomized studies, dietary and biomarker studies, and discussing preventive benefits of anthocyanins and future research directions.
2. Preclinical and Clinical Research and Molecular Mechanisms
2.1. Metabolic Effects of Anthocyanins
2.1.1. Clinical Research
Acylated anthocyanins of black carrots (Daucus carota ssp. sativus var. atrorubens) have an anti-oxidative effect (cyanidin-3-O-glucoside), can improve plasma lipid profile, by lowering the LDL-cholesterol levels and serum triglycerides and increasing the HDL-cholesterol levels, and may improve glucose tolerance and insulin resistance (dephinidin-3-O-rutinoside) [50,51,52,53,54]. Dohadwala et al. reported a modest reduction of HDL-cholesterol after 4 weeks of cranberry (Vaccinium macrocarpon) juice consumption in patients with coronary heart disease, related, probably, to the characteristics of the study population and short treatment period, because Ruel et al. revealed an increase of HDL-cholesterol in obese patients after 12 weeks supplementation with cranberry juice [55,56]. Nonesterified fatty acids also decreased after ACYs supplementation in a rat model of metabolic syndrome [36].
The main mechanisms explaining the reduction of total cholesterol levels include increasing fecal excretion of sterols, down-regulation of the gene expression of the hepatic HMG-CoA reductase, reduction in serum apo B- and apo-CIII-containing triglyceride-rich particles, inhibition of cholesteryl-ester transfer protein, slowing intestinal lipid absorption and increase of the expression of LDL-receptor [51,53]. Dietary fibers, the main components of fruits and vegetables, can also reduce LDL and short-chain fatty acids, as well as liver cholesterol synthesis [52].
Strawberry (Fragaria × ananassa) supplementation in healthy volunteers, 500 g daily for 1 month, reduced total and LDL cholesterol and triglycerides levels, without any influence on HDL values [57]. Placebo-controlled studies demonstrated an increase of HDL- cholesterol level and functionality in patients with dyslipidemia after ACYs supplementation [58].
Anthocyanin intake had a stronger association with weight control, enabling weight maintenance, in a study including more than 120,000 patients, followed for up to 24 years, included in the Health Professionals Follow-up Study, Nurses’ Health Study, and Nurses’ Health Study II, compared to flavonols, flavan-3-ols and flavonoid polymers [59]. Preventing even small amounts of weight gain can reduce cardiovascular risk [59]. ACY intake was associated not only with a lower fat mass, but may also influence central adiposity, independent of genetic and environmental factors [20].
2.1.2. Preclinical Research
ACYs from cornelian cherry (Cornus mas) increased expression of peroxisome proliferator-activated receptor (PPAR) alpha and gamma in the liver, contributing to the antiatherosclerotic effect [7]. Inhibition of PPARγ2 expression, an adipogenic transcription factor, impairs hepatic lipid accumulation and lipogenesis as effects of black chokeberries (Aronia melanocarpa), known to be rich in ACYs [60]. Black chokeberries prevent lipotoxicity and hepatocellular injury, delaying the progression of nonalcoholic fatty liver disease (NAFL) to nonalcoholic steatohepatitis (NASH) and liver cirrhosis [60]. ACYs down- regulate key enzymes required for cholesterol and fatty acid synthesis and activates free fatty oxidation [24]. Cyanidin-3-O-glucoside was reported to up-regulate the lipase gene and enhance the lipolytic activity of rat adipocytes [61].
Several animal studies demonstrated the benefits of ACYs on reverse-cholesterol transport and HDL formation, via regulation of lipids transporters and increase of paraoxonase 1 activity [58]. Anthocyanidin-3-glucoside promotes reverse cholesterol transport mediated by its gut microbiota metabolite, protocatechuic acid [29]. ACYs from purple maize (Zea mays indurate) and chokeberries were able to reverse or attenuate metabolic syndrome, due to their anti-inflammatory effect, preventing inflammatory cell infiltration into the tissues, in male Wistar rats [36].
ACYs also improve the carbohydrate metabolism, impairing intestinal absorption of glucose by inhibiting alpha-glucosidase and alpha-amylase, protecting pancreatic beta-cells from oxidative stress, and normalizing cardiac NADPH oxidase expression [53,62,63].
Anthocyanins reduce the expression of neuropeptide Y, modulating appetite and food intake, and increase gamma-aminobutyric acid receptor, reducing protein kinase A-alpha and phosphorylated cAMP-response element-binding protein in the hypothalamus, controlling body weight and adipose tissue size [50,64]. Additionally, fibers that often co-occur with ACYs in food can decrease postprandial glucose by reducing gastric emptying times [28]. Pelargonidin 3-glucoside-enriched strawberries reduced abdominal fat and body weight gain in rats with metabolic syndrome induced by a diet rich in carbohydrates and fats [65]. Obesity includes, besides excess adipose tissue, also macrophages infiltration, and inflammation, which lead to insulin-resistance [66]. The anti-inflammatory effect of ACY and the effect on insulin sensitivity also enable weight loss. The anti-obesity effects of ACYs are also related to changes in adipocytokine expression (up-regulation of adiponectin and down-regulation of plasminogen activator inhibitor-1 and interleukin-6), and upregulation of gene expression in adipocytes [61,67].
Concluding, anthocyanins exert hypolipidemic and anti-obesity effects, also improving glucose metabolism and insulin sensitivity (Table 2).
Table 2.
Key-mechanisms of cardiovascular benefits of anthocyanins (ACYs).
| Effects | Mechanisms of Action of ACYs |
|---|---|
Lipid metabolism:
|
|
|
|
Carbohydrate metabolism:
|
|
| Anti-obesity: lower body weight, adipose tissue size, central adiposity, and food intake | |
| Endothelial function |
|
| Vessel wall | |
| Anti-inflammatory |
|
|
Antioxidant (73.53 ± 0.13 mg per 100 g fresh weight basis sample: Cichorium intybus) [81] |
|
| Anti-atherosclerotic |
|
| Platelet function | |
| Antihypertensive |
in brackets: the concentration or dose of anthocyanins responsible of the effect observed; CD = cluster of differentiation; C3G = cyanidin 3-glucoside; Cy = cyanidin; Pr = A protocatechuic acid, GA = gallic acid; IC50 = the half maximal inhibitory concentration.
2.2. Effects of Anthocyanins on Endothelial Function
Endothelial dysfunction is a key mechanism in the development of atherosclerotic plaque, related to the loss of the barrier function, proinflammatory and prothrombotic effects, and reduction of nitric oxide (NO) [72]. Many cardiovascular risk factors can cause endothelial dysfunction, including hypertension, smoking, dyslipidemia, diabetes mellitus [72]. Endothelial dysfunction is a hallmark of several cardiovascular disorders, including hypertension and coronary heart disease [87]. The endothelial function may be assessed using flow-mediated vasodilation, on the brachial artery, or ultrasonography, defined as a change in brachial artery diameter in response to hyperemia [88,89].
Anthocyanin-rich foods and beverages can activate endothelial NO synthase and improve endothelial function in vitro and in vivo [71,72] (Table 2). Gallic acid, a microbiota anthocyanin metabolite, can increase NO levels by increasing phosphorylation of endothelial NO synthase [29]. The vasodilator effect of NO is related to its action on the smooth muscle cells and production of cGMP [72]. Considering that the half-life of NO is short, cGMP can be used as an index of NO activity and endothelial function [72]. ACYs protect the endothelial cells due to the activation of the nuclear factor 2 pathway (Nrf2), which regulates the NO synthase and production of NO [75]. Daily blueberry (Vaccinium virgatum and Vaccinium corymbosum) consumption reduced blood pressure and arterial stiffness, related probably to increase of NO production [90]. NO synthase inhibitors abolish the effect of ACYs on endothelium-dependent vasodilation in human subjects and rats [72]. Tabart et al. demonstrated that amplitude of vascular relaxation after blackcurrant (Ribes nigrum) juice supplementation on isolated porcine coronary artery rings was correlated to the total ACYs content and concentration and not their antioxidant capacity [91]. Anthocyianidins also improve endothelial function by impairing the expression of endothelin-1 [73]. Estradiol and ACYs with phytoestrogenic properties activate NO synthase via interaction with estrogen receptors [92]. Blackcurrant anthocyanins increased endothelial NO synthase mRNA expression and NO production in human endothelial cells and an ovariectomized rat model [92].
The endothelial protective effects of ACYs are also related to their antioxidant activity [74]. Luteolinidin is an anthocyanidin that can improve endothelial function due to its antioxidant properties [86].
The low plasma concentration and oral bioavailability of flavonoids question their involvement in improving endothelial function, but there is a membrane transporter bilitranslocase, a bilirubin-specific transporter, able to rapidly mediate the uptake of several flavonoids, including cyanidin-3 glucoside into the endothelial cells [74]. Moreover, Bharat et al. indicated that the vascular benefits of blueberry ACYs are due to their metabolites, hippuric, hydroxyhippuric, benzoic, vanillic, and isovanillic acid in a study on human aortic endothelial cells [93]. However, intact ACYs are also responsible for the improvement of flow-mediated vasodilation [72]. Vascular benefits include improvement of endothelial function and NO production, anti-inflammatory and antioxidant effect [93].
It was suggested that ACYs can be incorporated into the membrane and into the cytosol of the endothelial cells, preventing the occurrence of endothelial dysfunction and protecting against oxidative stressors [57,70]. Increased levels of nonesterified fatty acids (NEFAs) inhibited aortic endothelial nitric oxide synthase, causing hypertension [36]. ACYs can reduce NEFAs, thus improving endothelial NO synthesis [36].
Thandapilly et al. demonstrated an improved arterial relaxation and a significant reduction in blood pressure and attenuated cardiac hypertrophy in spontaneously hypertensive rats after freeze-dried grape (Vitis vinifera) powder administrated for 10 weeks [94]. Red grapes contain a variety of polyphenols, including ACYs [94].
Findings, whether the effects of ACYs are just acute or also chronic on endothelial function, are conflicting. Cranberry juice, a mix of polyphenols, especially ACYs, showed just an acute and no chronic benefit on flow-mediated vasodilation (FMD) of the brachial artery, in patients with coronary heart disease, probably due to severely impaired endothelial function in those patients, but improved arterial stiffness [56]. Several other clinical studies reported just an acute and no chronic benefit on endothelial function, related to the kinetics of ACYs [56,95]. Most of the chronic intervention studies reported improvements in vascular function, especially FMD [96]. It was suggested that ACYs may influence the composition of the arterial wall, and the effect could persist for a longer time, but additional studies are required to confirm such mechanism [56]. Anyway, such effects would represent no surprise, since ACYs have been shown to increase skin levels of collagen and elastin in ovariectomized rats [97]. Anthocyanin plasma metabolites from blueberries caused both acute and chronic flow-mediated dilation improvements in mice [98].
2.3. Anti-Inflammatory Effects of Anthocyanins
Dietary ACYs have been shown to reduce systemic and vascular inflammation in several studies [99]. Atherosclerosis is a chronic inflammatory disease, and the anti-inflammatory effect of ACYs can slow down the atherosclerotic process [99]. ACYs were already mentioned to reverse or attenuate metabolic syndrome in rats [36]. Anthocyanins exert their anti-inflammatory effects by activating the nuclear factor 2 pathway (Nrf2), impairing overproduction of inflammatory cytokines in response to oxidative stress and of chemokines in response to inflammation, also limiting NF-k beta activation and inhibiting the expression of vascular smooth muscle cell adhesion molecule and COX-2 expression in vascular smooth muscle cells [75]. Suppression of anti-arthritic effects of black soybean (Glycine max) seed coats was also mediated via suppression of NF-k beta signaling [100]. ACYs can suppress the adhesion of monocytes to the endothelium [101,102,103]. The anti-inflammatory and antioxidant effects of ACYs depend on their structure. Non-acylated ACYs, from mahaleb cherries (Prunus mahaleb) or blackcurrant, had higher anti-inflammatory and antioxidant activity compared to ACYs acylated with cinnamic acid (black carrot and “Sun Black” tomato) [103]. The explanation is related to a more important inhibitory effect of non-acylated anthocyanins on TNF-α-induced expression of adhesion molecules in endothelial cells than acylated anthocyanins [103]. Berries with higher cyanidin content, especially blackberries (Rubus), chokeberries, and bilberries (Vaccinium myrtillus), are more likely related to the anti-inflammatory effect [18].
Anthocyanins significantly decreased the levels of inflammatory markers such as high sensitivity C-reactive protein, soluble vascular cell adhesion molecule-1, and plasma interleukin-1β in hypercholesterolemic patients [99]. ACYs supplementation for 4 weeks also decreased high sensitivity C-reactive protein levels in patients with metabolic syndrome [104]. In cell culture assays, an anthocyanin mixture inhibited interleukin 6 and C reactive protein production and vascular cell adhesion molecule 1 (VCAM-1) secretion, in a dose-dependent manner [99]. The anti-inflammatory effect of the ACYs mixture was stronger when compared with the effects of delphinidin and cyanidin separately, and different anthocyanin compounds had additive or synergistic effects in mediating the anti-inflammatory activity [99]. The modulation of vascular inflammation due to ACYs is related to gene expression changes, affecting cell adhesion, migration, immune response, and cell differentiation [98]. On the other hand, fibrinogen increased after two months with black chokeberry extract in patients with metabolic syndrome, despite benefits for blood pressure, serum level of endothelin-1, lipids, and oxidative status [105].
Concluding, ACYs, especially non-acylated ACYs, modulate vascular inflammation, preventing over-infiltration with immune cells, which is a vessel protecting mechanism, exerting also anti-aging effects. The anti-inflammatory effects of ACYs were revealed monitoring C reactive protein and high sensitivity C-reactive protein level, as well as adhesion molecules. Data are missing related to other inflammatory markers, associated with atherosclerosis and plaque instability, such as serum amyloid A, pentraxin, apolipoprotein-associated phospholipase A2, and soluble CD40 ligand [106]. ACYs deserve special attention when assessing the inflammatory potential of the diet [107].
2.4. Anthocyanins as Antioxidants
Oxidative stress is involved in the pathophysiology of arterial stiffness, impairs endothelial function due to uncoupling of endothelial NO synthase, and damages endothelial proteins, lipids, and DNA [3]. Reactive oxygen species (ROS) cause oxidative changes of tetrahydrobiopterin and cysteins, resulting in superoxide and not NO production [87,108].
ACYs have higher antioxidant activity compared to other flavonoids. Their ability to limit oxidative stress has been extensively studied in vitro and in vivo [13,37,78,81]. Due to the fast metabolism of ACYs, their maximal plasmatic antioxidant value is reached between 15 and 30 min after ingestion [53]. In vivo studies revealed higher radical scavenging activity and decreased free-radical production due to ACYs [37,82].
As already mentioned, anthocyanins protect pancreatic beta-cells from oxidative stress induced by glucose, reduce lipid peroxidation, and the negative effects of ROS [62,75]. Activation of the nuclear factor Nrf2 pathway regulates the expression of antioxidant proteins, able to protect against injury and inflammation [75]. The antioxidant effect was also attributed to protocatechuic acid, a metabolite of ACYs, and was also demonstrated in deoxycorticosterone acetate-salt hypertensive rats [35,36,109]. Luteolinidin, a potent antioxidant, and radical scavenger inhibits CD38, increasing the myocardial and endothelial NAD(P) pool and facilitating NO production [87]. Ischemia/reperfusion of the heart causes CD38 activation, and luteolinidin, as a CD38 inhibitor, preserves cardiac function and reduces myocardial infarction size after reperfusion [87].
Several clinical studies confirmed the antioxidant effect of ACYs. Strawberry supplementation, in 23 healthy volunteers, resulted, after 1 month, in a decrease of oxidative stress biomarkers such as serum malondialdehyde, urinary 8-OHdG, and isoprostanes, and increased total plasma antioxidant capacity [57]. Lynn et al. found an increased antioxidant status in healthy adults, measured as the ferric reducing ability of plasma, after intake of cherry (Prunus cerasus) juice concentrate, rich in ACYs [17].
Cyanidin-3-O-glucoside, the most widely distributed anthocyanin, is a good scavenger of superoxide, but not hydroxyl radicals, with a pH-dependent oxidative potential [53]. Cyanidin, from grape seeds extract, protects DNA against oxidation, better than catechin [83]. Despite the antioxidant effect, activation of the Nrf2 increases cholesterol in the plasma and liver, promoting the atherosclerotic process [110].
The antioxidant effects of anthocyanins contribute to the improvement of endothelial function and arterial elasticity. Antioxidant effects were reported especially for delphinidins, protocatechuic acid and luteolinidin, and they all should be considered when assessing dietary antioxidant properties.
2.5. Other Vascular Effects of Anthocyanins
Nrf2 activated by ACYs exerts several anti-atherosclerotic effects, inhibiting the proliferation of vascular smooth muscle cells, reducing the level of oxidized LDL by activating CD 36 scavenger receptor [75]. Jiang et al. reported a reduced intima-media thickness and reduced atherosclerotic injuries, besides an improved lipid profile and atherogenic index, as well as decreased malondialdehyde content and increased anti-oxidative activity in atherosclerotic rats after black mulberry (Morus nigra) extract, rich in ACYs [40]. Extracts from boiled cowpea, a widely produced pulse grain, as well as whole seeds of cowpea, containing high amounts of anthocyanidins, inhibited LDL oxidation in humans [28]. The beneficial effect of some ACYs on atherosclerosis is mediated by gut microbiota metabolites, considering that ingested dietary ACYs are partly absorbed, while large amounts enter the colon and are degraded by gut microbiota as free anthocyanidins and protocatechuic acid [29].
Normal platelet function is important for cardiovascular health [85]. ACYs improve platelet function, impairing platelet aggregability [73,85]. Strawberries, rich in ACYs, significantly reduced the number of activated platelets in healthy controls, after 1 month of supplementation [57]. ACYs supplements also significantly decreased ADP-induced platelet activation in patients with metabolic syndrome [104]. In other words, ACYs prevent thrombosis.
Inhibition of angiotensin-converting enzyme (ACE) activity by delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa, widely used in Mexico, explains probably the antihypertensive effect of the mentioned ACYs [86]. The antihypertensive effect was also attributed to protocatechuic acid in deoxycorticosterone acetate-salt hypertensive rats [109] and was confirmed by several clinical studies [111]. ACYs have been shown to prevent not just hypertension, but also cardiac hypertrophy [62]. Gallic acid, a microbiota anthocyanin metabolite, inhibited ACE, reducing blood pressure in spontaneously hypertensive rats (SHR), an effect comparable to captopril [29].
ACYs are also effective in metabolic syndrome by controlling serum lipids, glucose tolerance, insulin resistance, and blood pressure [36,52]. ACYs interact with the microbiota, acting as a prebiotic agent, decreasing the access of bacterial components into the body, and producing several metabolites able to reduce systemic inflammation and impair lipid uptake by adipocytes and release of adipokines [112].
Nanashima et al. compared the effects of an anthocyanin-rich blackcurrant extract and 4 blackcurrant ACYs, revealing phytoestrogenic activity of ACYs, related to the regulation of the metabolism of extracellular matrix components in the skin [97]. Further studies should provide insights related to the influence on the composition of the arterial wall.
2.6. Anthocyanins and Gene Expression
Genetic effects are involved in the regulation of ACYs biosynthesis in several fruits, such as grapes and red-fleshed kiwi (Actinidia melanandra) and vegetables [13,113,114]. In human subjects, besides down-regulation of the gene expression of the hepatic HMG-CoA reductase, which was already mentioned, the cardiovascular effect of ACYs is related to their gene expression modulating effect [115]. Supplementation with a bilberry anthocyanin-rich extract attenuated atherosclerotic injuries in apolipoprotein E-deficient mice [115]. The explanation relies on impairing mRNA levels of genes related to atherosclerosis in cultured macrophages and endothelial cells [115]. The nutrigenomic analysis identified 1261 genes modulated by the ACY-rich extract in the aorta, including down-regulation of genes involved in oxidative stress, coding for adhesion molecules or angiogenesis, and the up-regulation of genes associated with increased cell adhesion and decreased paracellular permeability [115]. Wild blueberry (Cyanococcus) consumption influenced the expression of more than 600 genes and 3 microRNAs, related also to an increase in peripheral blood mononuclear cells [98].
Genetic influences of ACYs were also mentioned related to their anti-inflammatory effect, reducing pro-inflammatory genes (NFKB1, PTGS2) [53]. ACYs upregulate also gene expression in adipocytes, especially lipid metabolism and signal-transduction genes [61].
3. Population-Based Studies
3.1. Observational and Intervention Studies
A few observational and intervention studies focused on the vascular effects of ACYs, revealing a reduction of arterial stiffness [116], endothelial function, and serum lipids [117,118] (Table 3). ACYs were administrated as chokeberry or pomegranate (Punica granatum) juice [117,118].
Table 3.
Vascular effects of anthocyanins. Observational and intervention studies.
| Study Population | Anthocyanin Source | Methods | Findings, Conclusions | Ref. |
|---|---|---|---|---|
| 1898 women, 18–75 years old, from the TwinsUK registry | Validated food-frequency questionnaire | PWV, AI, central blood pressure, MAP, IMT | Consumption of 1–2 portions of berries daily reduced arterial stiffness and cardiovascular disease risk | [116] |
| 35 men with mild hypercholesterolemia | Chockeberry juice, 6 weeks regular drinking | NO, FMD, serum lipids | Regular drinking of chockeberry juice improves endothelial function and serum lipids (total and LDL cholesterol and triglycerides) in men with hypercholesterolemia. | [118] |
| 10 patients with carotid atherosclerosis | Pomegranate juice up to 3 years/control group | Common carotid IMT, blood samples | Significant IMT and SBP reduction, serum paraoxonase activity increased, LDL oxidation impaired, decreased antibodies against oxidized LDL, serum antioxidant status increased | [117] |
PWV = pulse wave velocity, AI = augmentation index, MAP= mean arterial pressure, IMT = intima-media thickness, NO = nitric oxide, FMD = flow-mediated dilation, SBP = systolic blood pressure, Ref. = references.
3.2. Randomized Controlled Trials (RCT)
Related to the acute arterial effects of ACYs, they can increase exercise performance. Blackcurrant extract increased femoral artery diameter during a submaximal sustained isometric contraction of the quadriceps muscle, emphasizing the ergogenic effects of this polyphenols [21]. Vasodilation of the femoral artery was accompanied by a hemodynamic response with a decrease of systolic, diastolic, mean arterial pressure and peripheral vascular resistance, increased cardiac output and stroke volume, and increased hemoglobin content in the vastus medialis [21].
Matsumoto et al. used near-infrared spectroscopy (NIRS) in 9 healthy men to measure left forearm blood flow (FBF) after venous occlusion and muscle oxygen consumption after arterial occlusion, before and hourly, for 4 h, after ingestion of blackcurrant anthocyanin [119]. Left forearm blood flow increased for 2–5 h after ACYs administration, with no significant difference in muscle oxygen consumption between ACYs and placebo intake [119]. The same article demonstrated increased peripheral blood flow and reduced muscle fatigue, improving shoulder stiffness after typing for 30 min if ACYs were previously administrated, but no improvement in typing performance [119].
Zhu et al. combined a short-term crossover study and a long-term interventional trial (12 weeks) in patients with hypercholesterolemia [72]. The maximal plasma concentration of delphinidins and cyanidins was obtained 1 h after dietary ACYs and was associated with the highest flow-mediated dilation (FMD) and plasma cGMP [72]. Long-term ACYs supplementation significantly increased FMD, cGMP, and HDL-cholesterol, and decreased vascular adhesion molecule-1 and LDL cholesterol [72]. Endothelial dysfunction in patients with hypercholesterolemia is related to dyslipidemia and inflammation, which were ameliorated by ACYs [72]. Zhu et al. also reported positive correlations between the changes in cGMP and HDL cholesterol concentrations and FMD in the ACYs group, and the disappearance of the endothelial effects of ACYs in the presence of NO-cGMP inhibitors [72].
FMD was also improved in a study including overweight or obese healthy participants of European origin, after 2 weeks of blood orange juice intake [120]. Blood pressure, lipid profile, high-sensitivity C-reactive protein, and endothelin-1 were not affected by the intervention. The authors concluded that the endothelial function was improved, and NO bioavailability increased due to the combined actions of ACYs and flavanone metabolites [120].
The longest RCT, including 115 patients with metabolic syndrome, revealed that 150 g blueberries/day for 6 months, resulted in improvement of endothelial function, arterial stiffness, and lipid profile, with no effect on insulin sensitivity [121]. There is a synergy between endothelial dysfunction and arterial stiffness, which might explain the vascular benefits of ACYs [121].
Smoking impairs endothelial function [122]. Blackcurrants administrated before smoking can attenuate the decrease in FMD in young smokers [123]. In other words, inadequate dietary intake of ACYs can contribute to cardiovascular disease in smokers, related, probably, to oxidative stress, which impairs nitric oxide production [122,123]. Adding vitamin E to ACYs increased endothelial functions in healthy nonsmokers as well as smokers [123]. Not all studies revealed the antioxidant effects of ACYs as a benefit for the endothelial function [124]. Balestra et al., revealed an independent antioxidant effect of ACYs, probably involving cellular signaling modulation [124].
The combination of ACYs with bromelain, a protein-digesting enzyme derived from pineapples, is beneficial for vascular health in humans, improving endothelial function, BP, antioxidant effect and oxygen utility capacity [125].
The majority of RCTs included in this review reported significant improvements in arterial stiffness following acute and chronic consumption of anthocyanin-rich foods, in patients with diverse disorders, body mass index, and age (Table 4).
Table 4.
Vascular effects of anthocyanins (ACYs). Randomized controlled trials (RCT).
| Study Population | ACY/ Placebo |
Methods | Findings, Conclusions | Reference |
|---|---|---|---|---|
| 18 healthy adults | combined ACYs and bromelain supplement (BE) | randomised crossover design; FMD, BP, TAC, resting heart rate, oxygen utility capacity and fatigability measured pre- and post-BE and placebo intake |
BE intake is effective for improving endothelial function, BP, TAC and oxygen utility capacity | [125] |
| 14 older adults | 7-days 2X 300 mg capsule with 35% blackcurrant extract/ placebo |
double-blind, placebo-controlled, crossover design study with a washout period of 28 days | ACY intake reduces carotid femoral PWV and central BP in older adults; no effects on blood lipids |
[126] |
| 19 patients, 20 to 60 years old, with metabolic syndrome (MetS) | 240 mL of tart cherry juice (rich in ACYs) or an isocaloric placebo-control drink, twice daily for 12 weeks | single-blind, placebo-controlled, parallel-arm pilot clinical trial PWV, brachial and aortic BP, AI, and biomarkers of cardiovascular and metabolic health, assessed at baseline and 6 and 12 weeks |
no significant changes in hemodynamics and arterial stiffness lower oxidized low-density lipoprotein, soluble vascular cell adhesion molecule-1 and total cholesterol after tart cherry juice than control |
[127] |
| 15 healthy overweight and obese men and women | 200 mL blood orange juice twice daily) for 2 weeks with a washout period of 1 week | primary outcome: FMD |
favorable effects on endothelial function | [120] |
| 115 participants, age 63 ± 7 years; 68% male | daily intake of 1 cup (150 g) of blueberries for 6 months |
double-blind, parallel RCT; insulin resistance, FMD, AI, lipoprotein status, and NO | improvements in vascular function, lipid status, and NO bioactivity | [121] |
| 41 participants, aged 25–84 years | 500 mL blood orange juice providing 50 mg ACYs/ 500 mL blonde orange juice without ACYs for 28 days |
open label, two-arm cross-over trial; total, HDL- and LDL-cholesterol, glucose, fructosamine, NO, CRP, aortic SBP and DBP or carotid-femoral and brachial-ankle PWV |
No significant differences were observed between the variables measured at the start and end of each treatment period. The lack of effect may be due to the modest concentration of ACYs in the blood orange juice | [128] |
| 14 healthy male and female adults | Participants consumed 200 g/day of cooked purple potato containing 288 mg ACYs, or a white potato containing negligible ACYs for 14 days, separated by a 7-day washout period. | PWV, SBP, DBP, HDL, LDL, TG, glucose, insulin, and CRP. | PWV was significantly reduced following purple potato consumption for 14-days | [22] |
| 60 postmenopausal women with pre- and stage 1-hypertension | 8 weeks, 25 g or 50 g freeze-dried strawberry powder (FDSP) | double-blind, placebo-controlled, parallel arm clinical trial BP, arterial stiffness, superoxide dismutase (SOD) at baseline, 4 and 8 weeks |
BP and arterial stiffness improved in the 25 g FDSP group | [129] |
| 13 healthy men, age: 25 ± 4 years | New Zealand blackcurrant (NZBC) extract (600 mg/day)/ placebo for 7-days separated by 14-days washout | double-blind, crossover design, Participants produced isometric maximal voluntary contractions (iMVC) and a 120-s 30%iMVC of the quadriceps: electromyography, near-infrared spectroscopy, hemodynamic and ultrasound recordings |
Intake of NZBC extract impaired cardiovascular responses, muscle oxygen saturation, muscle activity and femoral artery diameter of the quadriceps and may increase exercise performance | [21] |
| 16 volunteers performing a single standard dive | 2 groups: one of them received 2x 200 mg of an ACYs-rich extract from red oranges, 12 and 4 h before diving | FMD | ACYs administration reduces the harmful endothelial effects of a recreational single dive | [124] |
| 48 postmenopausal women with pre- and stage 1 hypertension | 8-week, 22 g freeze-dried blueberry (BB) powder/control daily | double-blind, placebo-controlled clinical trial BP, PWV, CRP, NO and SOD at baseline, 4 and 8 weeks |
Daily BB reduces BP and arterial stiffness, related to increase of NO production | [90] |
| 25 men and postmenopausal women, 18–50 years old | 6 weeks, 250 g BB powder/ placebo daily |
BP, vascular performance testing, blood samples at baseline and after 6 weeks | BB ingestion for 6 weeks increases natural killer cells and reduces AI, SBP, DBP in sedentary males and females | [111] |
| 47 healthy adults, 30–50 years | 6 weeks, 30 mL tart cherry juice concentrate diluted with water/energy matched control drink | BP, arterial stiffness, CRP, total cholesterol, LDL, ferric reducing ability of plasma at baseline and after 6 weeks | Tart cherry juice concentrate has no effect on arterial stiffness, CRP, and cardiovascular risk markers, but increases antioxidant status | [17] |
| 21 healthy men | 766, 1278 and 1791 mg blueberry polyphenols (BBPP)/Control 319, 637, 766, 1278, 1791 mg total blueberry/ control |
Double-blind, controlled, crossover trial; FMD Intake-dependence study, from baseline to 1 h |
FMD increased significantly at 1–2 and 6 h after consumption of BBPP. At 1 h after consumption, FMD increased dose-dependently to up to 766 mg BBPP. The vascular benefits are linked to the circulating phenolic metabolites and activity of the neutrophil NADPH oxidase | [130] |
| 11 young, healthy male nonsmokers and 13 smokers | supplement A (50 mg of blackcurrant ACY) and supplement B (50 mg of blackcurrant anthocyanin plus vitamin E | Double-blind trial; FMD and skin temperature |
Oral ACYs and Vitamin E supplementation can attenuate the smoking-induced acute endothelial dysfunction and peripheral blood flow in smokers | [123] |
| 44 patients with coronary artery disease | 480 mL of cranberry juice/placebo for 4 weeks | BP, PWV, brachial artery flow-mediated dilation, digital pulse amplitude | Chronic cranberry juice consumption reduced arterial stiffness, with only an acute benefit on endothelial vasodilator function | [56] |
| 12 patients with hypercholesterolemia 150 hypercholesterol-emic individuals |
320 mg ACYs/ placebo 320 mg ACYs/ placebo |
FMD before and after the intervention FMD, cGMP |
ACYs supplementation improves endothelium-dependent vasodilation in patients with hypercholesterolemia, related to activation of the NO-cGMP signaling pathway, improvement of serum lipids and an anti-inflammatory effect | [72] |
| Subjects at moderate risk for coronary heart disease |
240 mL pomegranate juice/day (n- = 146)/control bevarage (n = 143) up to 18 months | IMT | No significant effect of pomegranate juice was noticed on IMT progression rate. A slowed IMT progression was noticed in patients with increased oxidative stress and impaired TG/HDL profile | [131] |
| 9 healthy men | 17 mg kg(-1) BCA or placebo |
double-blind, placebo-controlled, crossover study NIRS, improvement in shoulder stiffness plasma ACYs measured prior to ingestion and 1, 2, and 4 h later |
FBF increased significantly after BCA ingestion | [119] |
PWV = pulse wave velocity, FMD = flow-mediated dilation, AI = augmentation index, NO = nitric oxide, BP = blood pressure; SBP = systolic blood pressure, DBP = diastolic blood pressure, HDL = high-density lipoproteins, LDL = low-density lipoproteins, TG = triglycerides, CRP = C-reactive protein; FDSP = freeze-dried strawberry powder; iMVC = isometric maximal voluntary contractions; NZBC = New Zealand blackcurrant extract; BB = freeze-dried blueberry; SOD = superoxide dismutase; BBPP = blueberry polyphenols; BCA = blackcurrant anthocyanin; NIRS = near-infrared spectroscopy; FBF = left forearm blood flow; TAC = total antioxidant capacity.
4. Study Limitations
Contradictory results may be explained by low bioavailability, variable concentrations and doses, instability and different pharmakokinetics depending on the source, destabilization and storage of anthocyanins, different experimental setups, methodologies, follow-up periods, differences between in vivo and in vitro studies, concomitant medication which may influence vascular function or other polyphenol-containing foods and beverages throughout the study period.
The concentration of ACYs varies among cultivars [52]. Methods of processing and their duration may also influence ACYs content and effects, certain components are more affected than others by cooking [22,53]. As an example, boiled cowpea caused a more pronounced decrease of glycemic index than fried or mashed cowpea [132]. Heat, light, pH, structure, oxygen, solvents, metal ions, enzymes, other flavonoids, proteins, co-pigments, and storage may destabilize and degrade ACYs [52,53,96]. On the other hand, high light intensity, blue and red light and UV-A irradiation stimulate ACYs production in several plants due to the influence on biosynthetic genes [13]. Low temperatures also induced ACYs accumulation in several vegetables [13]. Heating enables anthocyanin degradation, but its stability depends on the source of the flavonoid. ACYs from black carrot have higher heat stability compared to those from sour cherry, grape or citrus juices, elderberry, and strawberry [52]. Gerardi et al. reported preservation of the phenolic content of tomato puree enriched with several anthocyanin-rich food colorants after pasteurization, as well as a higher antioxidant capacity [133]. ACYs from black carrot and blood orange include acylated anthocyanins, with a higher stability in neutral or acidic media [52]. Results also showed a significant decrease in anthocyanin stability at pH above 5 [52]. Cooking increases non-acylated anthocyanins, with a higher bioavailability, but not acylated anthocyanins, with a shorter half-life [52]. Non-thermal technologies may reduce ACYs loses from prepared foods [53].
Increasing storage time was associated with degradation of ACYs by many physicochemical factors, explaining why ACYs-based products are not widely used as pigments [53]. ACYs are stable in potatoes, but in pepper and eggplant, concentration decreases upon ripening [13].
Absorption, gastrointestinal transit, and plasma concentration of ACYs depend on their structure, especially on the presence of carbohydrates [52,53]. ACYs are exposed along the gastrointestinal tract, to pH and ions, which affect their bioavailability and bioactivity [53]. An important limitation in most of the studies is lack of control of absorption and metabolism of ACYs [134]. As an example, cranberry anthocyanins are poorly absorbed and rapidly removed from the plasma, with maximal concentrations detectable in plasma between 1 and 3 h, with important differences between study participants [95]. Phytic acid enhances gastrointestinal absorption of ACYs, but is also a strong chelator of minerals, especially iron, and may cause mineral deficiencies, requiring safety tests when used in foods [135]. Most studies do not consider in vivo degradation of ACYs or colonic metabolites [16]. ACYs composition and bioactivity were strongly affected by in vitro gastrointestinal digestion, but antioxidant activity was preserved [136]. Only small amounts of ACYs are excreted in the urine [37].
Bioactive compounds from the same food may act synergistically. Usually, it is impossible to consider all the components of fruits and vegetables: diverse polyphenols, proteins, carbohydrates, fibers, vitamins, and minerals. Food frequency questionnaires might not include all sources of ACY intake. For the moment, there are no biomarkers for ACYs because their metabolites are not well known [16]. It is possible that individuals with a high ACYs intake have a healthier lifestyle, while those with a poor intake have a diet with less fiber, antioxidant vitamins and more saturated fats [38]. Use of extracts from fruits, containing just anthocyanins, might be more effective in assessing their biological actions [37].
A threshold of intake should be defined to obtain cardiovascular benefits. The discrepancies between in vivo and in vitro results may be explained by the high amounts of ACYs, often not physiologically relevant, with low bioavailability in the human body, in most in vitro studies [85]. It is possible that the amount required to achieve a specific biological action is much larger than the one obtained from the diet [53]. Malto/cyclodextrins, liposomes or concentrated sources of ACYs (purees or freeze-dried fruits) are solutions to preserve the bioavailability of ACYs, considering the limited splanchnic metabolism [53]. ACYs are non-toxic molecules within a normal, physiological consumption [53]. No toxicity has been reported for luteolinidin at doses 4-fold above the minimally effective dose, and liposomal delivery enabled rapid cardiac uptake [87].
Dietary choices and the habitual intake of ACYs depend on several factors in different populations, regions, seasons, and individuals with different socio-cultural, ethnic and financial characteristics, as well as technical advances in agricultural and food industry [38,53].
5. Future Research Directions
Further large sample size randomized controlled trials need to confirm the effectiveness of anthocyanin supplementation in improving vascular function, structure, and platelet activation. Further in vitro and in vivo studies may identify new chemical and biological aspects of ACYs and provide additional mechanistic knowledge. Further research considering absorption and dose-response effects is warranted. However, solutions must be found to maintain an adequate number of metabolites in plasma and target tissues, considering the nutraceutical effects of ACYs in living systems. The future belongs to foodomic studies, functional food research, phytopharmaceuticals containing ACYs and components with synergistic action, and exploratory epigenetic studies.
A dietary score would be required to compare the anti-inflammatory, antioxidant, anti-atherosclerotic, antihypertensive, antiglycation and antithrombotic properties of ACYs-rich foods, their effect on arterial stiffness, gene expression, phytoestrogenic and ergogenic effects, considering also other components with synergistic action.
6. Conclusions
Current positive scientific evidence from epidemiological, observational and intervention studies, randomized controlled trials and mechanistic research, is promising, revealing that anthocyanins represent an inexpensive, accessible, and effective approach, in control of atherosclerosis, cardiovascular risk and cardiovascular aging. The cardiovascular health promoting effects of ACY are possible through multiple mechanisms. Anthocyanins exert favorable effects on the endothelial function, oxidative stress, inhibit COX-1 and COX-2 enzymes, exert antiatherogenic, antihypertensive, antiglycation, antithrombotic and anti-inflammatory activities, ameliorate dyslipidemia and arterial stiffness. Anthocyanins exert also ergogenic effects, probably by influencing vasodilation and relaxation during exercise.
The present review supports the recommendations of the European Society of Cardiology on cardiovascular disease prevention, that cardiovascular risk may be reduced by a diet rich in fruit and vegetables. The role of anthocyanins in the global food chain should increase, and physicians of different specialties and people worldwide should be aware about their health effects in dietary choices. The presented data may help to refine previous dietary recommendations for the slowing of cardiovascular ageing, increasing health- and lifespan and prevention of cardiovascular disorders.
Acknowledgments
In this section, you can acknowledge any support given which is not covered by the author contribution or funding sections. This may include administrative and technical support, or donations in kind (e.g., materials used for experiments).
Author Contributions
I.M. wrote the first draft of the manuscript; C.F., D.C.V., C.G., C.M., D.S., C.T.L., J.O.H., O.K.H. and A.G.A. substantively revised it and approved the submitted version. All authors have read and agreed to the published version of the manuscript.
Funding
Authors Jarosław O. Horbańczuk and Atanas G. Atanasov acknowledge the financial support from: The National Centre for Research and Development (NCBR) of Poland (project number POIR.01.01.01-00-0593/18).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vlachopoulos C., Aznaoridis K., Stefanidis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness. A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2010;55:1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 2.Mozos I., Malainer C., Horbańczuk J., Gug C., Stoian D., Luca C.T., Atanasov A.G. Inflammatory markers for arterial stiffness in cardiovascular diseases. Front. Immunol. 2017;8:1058. doi: 10.3389/fimmu.2017.01058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mozos I., Luca C.T. Crosstalk between Oxidative and Nitrosative Stress and Arterial Stiffness. Curr. Vasc. Pharmacol. 2017;15:446–456. doi: 10.2174/1570161115666170201115428. [DOI] [PubMed] [Google Scholar]
- 4.Iurciuc S., Cimpean A.M., Mitu F., Heredea R., Iurciuc M. Vascular aging and subclinical atherosclerosis: Why such a never ending and challenging story in cardiology? Clin. Interv. Aging. 2017;12:1339–1345. doi: 10.2147/CIA.S141265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Böhm V. Lycopene and heart health. Mol. Nutr. Food. Res. 2012;56:296–303. doi: 10.1002/mnfr.201100281. [DOI] [PubMed] [Google Scholar]
- 6.Piepoli M.F., Hoes A.W., Agewall S., Albus C., Brotons C., Catapano A.L., Cooney M.T., Corrà U., Cosyns B., Deaton C., et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice. The Sixth Joint Task Force of the Eu-ropean Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts). Developed with the special contribution of the European Association for Cardiovascular Prevention and Rehabilitation (EACPR) Eur. Hearth J. 2016;37:2315–2381. doi: 10.1093/eurheartj/ehw106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sozanski T., Kucharska A.Z., Rapak A., Szumny D., Trocha M., Merwid-Ląd A., Dzimira S., Piasecki T., Piórecki N., Magdalan J., et al. Iridoid-loganic acid versus anthocyanins from the Cornusmas fruits (cornelian cherry): Common and different effects on diet-induced atherosclerosis, PPARs expression and inflammation. Atherosclerosis. 2016;254:151–160. doi: 10.1016/j.atherosclerosis.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Wang D., Özen C., Abu-Reidah I.M., Chigurupati S., Patra J.K., Horbanczuk J.O., Jóźwik A., Tzvetkov N.T., Uhrin P., Atanasov A.G. Vasculoprotective Effects of Pomegranate (Punica granatum L.) Front. Pharmacol. 2018;9:544. doi: 10.3389/fphar.2018.00544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnett D.K., Blumenthal R.S., Albert M.A., Buroker A.B., Goldberger Z.D., Hahn E.J., Himmelfarb C.D., Khera A., Lloyd-Jones D., McEvoy J.W., et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140:e596–e646. doi: 10.1161/CIR.0000000000000678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeung A.W.K., Aggarwal B.B., Orhan I.E., Horbanczuk O.K., Barreca D., Battino M., Belwal T., Bishayee A., Daglia M., Devkota H.P., et al. Resveratrol, a popular dietary supplement for human and animal health: Quantitative research literature analysis-A review. Anim. Sci. Pap. Rep. 2019;37:103–118. [Google Scholar]
- 11.Yeung A.W.K., Orhan I.E., Aggarwal B.B., Battino M., Belwal T., Bishayee A., Daglia M., Devkota H.P., El-Demerdash A., Balacheva A.A., et al. Berberine, a popular dietary supplement for human and animal health: Quantitative research literature analysis—A review. Anim. Sci. Pap. Rep. 2020;38:5–19. [Google Scholar]
- 12.Yeung A.W.K., Aggarwal B.B., Barreca D., Battino M., Belwal T., Horbańczuk O.K., Berindan-Neagoe I., Bishayee A., Daglia M., Devkota H.P., et al. Dietary natural products and their potential to influence health and disease including animal model studies. Anim. Sci. Pap. Rep. 2018;36:345–358. [Google Scholar]
- 13.Liu Y., Tikunov Y., Schouten R., Marcelis L.F.M., Visser R.G.F., Bovy A. Anthocyanin biosynthesis and degradation mechanisms in Solanaceous Vegetables: A review. Front. Chem. 2018;6:52. doi: 10.3389/fchem.2018.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira H., Correia P., Pereira A.R., Araújo P., Mateus N., de Freitas V., Oliveira J., Fernandes I. Exploring the Applica-tions of the Photoprotective Properties of Anthocyanins in Biological Systems. Int. J. Mol. Sci. 2020;21:7464. doi: 10.3390/ijms21207464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61:1361779. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cassidy A., Mukamal K.J., Liu L., Franz M., Eliassen A.H., Rimm E.B. High anthocyanin intake is associated with a reduced risk of myocardial infarction in young and middle-aged women. Circulation. 2013;127:188–196. doi: 10.1161/CIRCULATIONAHA.112.122408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynn A., Mathew S., Moore C.T., Russell J., Robinson E., Soumpasi V., Barker M.E. Effect of tart cherry juice supplement on arterial stiffness and inflammation in healthy adults: A randomised controlled trial. Plant Foods Hum. Nutr. 2014;69:122–127. doi: 10.1007/s11130-014-0409-x. [DOI] [PubMed] [Google Scholar]
- 18.Fang J. Classification of fruits based on anthocyanin types and relevance to their health effects. Nutrition. 2015:1301–1306. doi: 10.1016/j.nut.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 19.Cassidy A. Berry anthocyanin intake and cardiovascular health. Mol. Asp. Med. 2017 doi: 10.1016/j.mam.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Jennings A., MacGregor A., Spector T., Cassidy A. Higher dietary flavonoid intakes are associated with lower objectively measured body composition in women: Evidence from discordant monozygotic twins. Am. J. Clin. Nutr. 2017;105:626–634. doi: 10.3945/ajcn.116.144394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cook M.D., Myers S.D., Gault M.L., Willems M.E.T. Blackcurrant Alters Physiological Responses and Femoral Artery Diameter during Sustained Isometric Contraction. Nutrients. 2017;9:556. doi: 10.3390/nu9060556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsang C., Smail N.F., Almoosawi S., McDougall G.J.M., Al-Dujaili E.A.S. Antioxidant Rich Potato Improves Arterial Stiffness in Healthy Adults. Plant Foods Hum. Nutr. 2018;73:203–208. doi: 10.1007/s11130-018-0673-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wallace T.C., Giusti M.M. Anthocyanins in Health and Disease. CRC Press Taylor & Francis Group; Boca Raton, FL, USA: 2014. pp. 49–52. [Google Scholar]
- 24.Wegener C.B., Jansen G., Jürgens H.U., Schütze W. Special quality traits of coloured potato breeding clones: Anthocyanins, soluble phenols and antioxidant capacity. J. Sci. Food Agric. 2009;89:206–215. doi: 10.1002/jsfa.3426. [DOI] [Google Scholar]
- 25.Li H., Deng Z., Zhu H., Hu C., Liu R., Young J.C., Tsao R. Highly pigmented vegetables: Anthocyanin compositions and their role in antioxidant activities. Food Res. Int. 2012;46:250–259. doi: 10.1016/j.foodres.2011.12.014. [DOI] [Google Scholar]
- 26.Wang G., Chen B., Du H., Zhang F., Zhang H., Wang Y., He H., Geng S., Zhang X. Genetic mapping of anthocyanin ac-cumulation-related genes in pepper fruits using a combination of SLAF-seq and BSA. PLoS ONE. 2018;13:e0204690. doi: 10.1371/journal.pone.0204690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalt W., Cassidy A., Howard L.R., Krikorian R., Stull A.J., Tremblay F., Zamora-Ros R. Recent Research on the Health Benefits of Blueberries and Their Anthocyanins. Adv. Nutr. 2020;11:224–236. doi: 10.1093/advances/nmz065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Awika J.M., Duodu K.G. Bioactive polyphenols and peptides in cowpea (Vigna unguiculata) and their health promoting properties: A review. J. Funct. Foods. 2017;38:686–697. doi: 10.1016/j.jff.2016.12.002. [DOI] [Google Scholar]
- 29.Wang Z., Zhao Y. Gut microbiota derived metabolites in cardiovascular health and disease. Protein Cell. 2018;9:416–431. doi: 10.1007/s13238-018-0549-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tena N., Martín J., Asuero A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants. 2020;9:451. doi: 10.3390/antiox9050451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81(Suppl. S1):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 32.Carkeet C., Clevidence B.A., Novotny J.A. Anthocyanin excretion by humans increases linearly with increasing strawberry dose. J. Nutr. 2008;138:897–902. doi: 10.1093/jn/138.5.897. [DOI] [PubMed] [Google Scholar]
- 33.De Ferrars R.M., Czank C., Zhang Q., Botting N.P., Kroon P.A., Cassidy A., Kay C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014;171:3268–3282. doi: 10.1111/bph.12676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu Y., Sun H., He S., Lou Q., Yu M., Tang M., Tu L. Metabolism and prebiotics activity of anthocyanins from black rice (Oryza sativa L.) in vitro. PLoS ONE. 2018;13:e0195754. doi: 10.1371/journal.pone.0195754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vitaglione P., Donnarumma G., Napolitano A., Galvano F., Gallo A., Scalfi L., Fogliano V. Protocatechuic acid is the major human metabolite of cyanidin-glucosides. J. Nutr. 2007;137:204–206. doi: 10.1093/jn/137.9.2043. [DOI] [PubMed] [Google Scholar]
- 36.Bhaswant M., Raihanah S., Mathai M.L., Mouatt P., Brown L. Anthocyanins in chockeberry and purple maize attenuate diet-induced metabolic syndrome in rats. Nutrition. 2017;41:24–31. doi: 10.1016/j.nut.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Kruger M.J., Davies N., Myburgh K.H., Lecour S. Proanthocyanidins, anthocyanins and cardiovascular diseases. Food Res. Int. 2014;59:41–52. doi: 10.1016/j.foodres.2014.01.046. [DOI] [Google Scholar]
- 38.Ponzo V., Goitre I., Fadda M., Gambino R., De Francesco A., Soldati L., Gentile L., Magistroni P., Cassander M., Bo S. Dietary flavonoid intake and cardiovascular risk: A population-based cohort study. J. Transl. Med. 2015;13:218. doi: 10.1186/s12967-015-0573-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horbowicz M., Kosson R., Grzesiuk A., Debski H. Anthocyanins of fruits and vegetables their occurrence, analysis, and role in human nutrition. Veg. Crop. Res. Bull. 2018;68:5–22. doi: 10.2478/v10032-008-0001-8. [DOI] [Google Scholar]
- 40.Jiang Y., Dai M., Nie W.J., Yang X.R., Zeng X.C. Effects of the ethanol extract of black mulberry (Morus nigra L.) fruit on experimental atherosclerosis in rats. J. Ethnopharmacol. 2017;200:228–235. doi: 10.1016/j.jep.2017.02.037. [DOI] [PubMed] [Google Scholar]
- 41.Goyal M.R., Suleria H.A.R. Human Health Benefits of Plant Bioactive Compounds. Potentials and Prospects. Apple Academic Press; Oakville, ON, Canada: 2020. p. 75. [Google Scholar]
- 42.Wu X., Beecher G.R., Holden J.M., Haytowitz D.B., Gebhardt S.E., Prior R.L. Concentrations of anthocyanins in common foods in the United States and estimation of normal consumption. J. Agric. Food Chem. 2006;54:4069–4075. doi: 10.1021/jf060300l. [DOI] [PubMed] [Google Scholar]
- 43.Watson R.R., Preedy V.R., Zibadi S. Polyphenols: Mechanisms of Action in Human Health and Disease. 2nd ed. Elsevier Academic Press; London, UK: 2018. pp. 84–86. [Google Scholar]
- 44.Carmona L., Alquézar B., Tárraga S., Peña L. Protein analysis of moro blood orange pulp during storage at low temperatures. Food Chem. 2019;277:75–83. doi: 10.1016/j.foodchem.2018.10.108. [DOI] [PubMed] [Google Scholar]
- 45.Miller K., Feucht W., Schmid M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients. 2019;11:1510. doi: 10.3390/nu11071510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.David L., Danciu V., Moldovan B., Filip A. Effects of In Vitro Gastrointestinal Digestion on the Antioxidant Capacity and Anthocyanin Content of Cornelian Cherry Fruit Extract. Antioxidants. 2019;8:114. doi: 10.3390/antiox8050114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peng Y., Lin-Wang K., Cooney J.M., Wang T., Espley R.V., Allan A.C. Differential regulation of the anthocyanin profile in purple kiwifruit (Actinidia species) Hortic. Res. 2019;6:3. doi: 10.1038/s41438-018-0076-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blando F., Albano C., Liu Y., Nicoletti I., Corradini D., Tommasi N., Gerardi C., Mita G., Kitts D.D. Polyphenolic com-position and antioxidant activity of the under-utilised Prunus mahaleb L. fruit. J. Sci. Food Agric. 2016;96:2641–2649. doi: 10.1002/jsfa.7381. [DOI] [PubMed] [Google Scholar]
- 49.Lao F., Giusti M.M. Quantification of Purple Corn (Zea mays L.) Anthocyanins Using Spectrophotometric and HPLC Ap-proaches: Method Comparison and Correlation. Quantification of Purple Corn (Zea mays L.) Anthocyanins Using Spectro-photometric and HPLC Approaches: Method Comparison and Correlation. Food Anal. Methods. 2016;9:1367–1380. [Google Scholar]
- 50.Kwon S.H., Ahn I.S., Kim S.O., Kong C.S., Chung H.Y., Do M.S., Park K.Y. Anti-obesity and hypolipidemic effects of black soybean anthocyanins. J. Med. Food. 2007;10:552–556. doi: 10.1089/jmf.2006.147. [DOI] [PubMed] [Google Scholar]
- 51.Liu C., Sun J., Lu Y., Bo Y. Effects of Anthocyanin on Serum Lipids in Dyslipidemia Patients: A Systematic Review and Meta-Analysis. PLoS ONE. 2016;11:e0162089. doi: 10.1371/journal.pone.0162089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Akhtar S., Rauf A., Imran M., Qamar M., Riaz M., Mubarak M.S. Black carrot (Daucus corota L.), dietary and health promoting perspectives of its polyphenols: A review. Trends. Food Sci. Technol. 2017;66:36–47. doi: 10.1016/j.tifs.2017.05.004. [DOI] [Google Scholar]
- 53.Olivas-Aguirre F.J., Rodrigo-Garcia J., Martinez-Ruiz N.D., Cárdenas-Robles A.I., Mendoza-Díaz S.O., Álvarez-Parrilla E., González-Aguilar G.A., de la Rosa L.A., Ramos-Jiménez A., Wall-Medrano A., et al. Cyanidin-3-O-glucoside: Physi-cal-Chemistry, Foodomics and Health Effects. Molecules. 2016;21:1264. doi: 10.3390/molecules21091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kato M., Tani T., Terahara N., Tsuda T. The Anthocyanin Delphinidin 3-Rutinoside Stimulates Glucagon-Like Peptide-1 Secretion in Murine GLUTag Cell Line via the Ca2+/Calmodulin-Dependent Kinase II Pathway. PLoS ONE. 2015;10:e0126157. doi: 10.1371/journal.pone.0126157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ruel G., Pomerleau S., Couture P., Lemieux S., Lamarche B., Couillard C. Favourable impact of low-calorie cranberry juice consumption on plasma HDL-cholesterol concentrations in men. Br. J. Nutr. 2006;96:357–364. doi: 10.1079/BJN20061814. [DOI] [PubMed] [Google Scholar]
- 56.Dohadwala M.M., Holbrook M., Hamburg N.M., Shenouda S.M., Chung W.B., Titas M., Kluge M.A., Wang N., Palmisano J., Milbury P.E., et al. Effects of cranberry juice consumption on vascular function in patients with coronary artery disease. Am. J. Clin. Nutr. 2011;93:934–940. doi: 10.3945/ajcn.110.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alvarez-Suarez J.M., Giampieri F., Tulipani S., Casoli T., Di Stefano G., González-Paramás A.M., Santos-Buelga C., Busco F., Quiles J.L., Cordero M.D., et al. One-month strawberry-rich anthocyanin supplementation ameliorates cardiovascular risk, oxidative stress markers and platelet activation in humans. J. Nutr. Biochem. 2014;25:289–294. doi: 10.1016/j.jnutbio.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Millar C.L., Duclos Q., Blesso C.N. Effects of Dietary Flavonoids on Reverse Cholesterol Transport, HDL Metabolism, and HDL Function. Adv. Nutr. 2017;8:226–239. doi: 10.3945/an.116.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertoia M.L., Rimm E.B., Mukamal K.J., Hu F.B., Willett W.C., Cassidy A. Dietary flavonoid intake and weight maintenance: Three prospective cohorts of 124,086 US men and women followed for up to 24 years. BMJ. 2016;352:17. doi: 10.1136/bmj.i17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park C.H., Kim J.H., Lee E.B., Hur W., Kwon O.J., Yoon S.K., Park H.J. Aronia melanocarpa Extract Ameliorates Hepatic Lipid Metabolism through PPARγ2 Downregulation. PLoS ONE. 2017;12:e0169685. doi: 10.1371/journal.pone.0169685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tsuda T., Ueno Y., Kojo H., Yoshikawa T., Osawa T. Gene expression profile of isolated rat adipocytes treated with an-thocyanins. BiochimBiophys Acta. 2005;1733:137–147. doi: 10.1016/j.bbalip.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 62.Al-Awwadi N.A., Araiz C., Bornet A., Delbosc S., Cristol J.P., Linck N., Azay J., Teissedre P.L., Cros G. Extracts enriched in different polyphenolic families normalize increased cardiac NADPH oxidase expression while having differential effects on insulin resistance, hypertension, and cardiac hypertrophy in high-fructose-fed rats. J. Agric. Food Chem. 2005;53:151–157. doi: 10.1021/jf048919f. [DOI] [PubMed] [Google Scholar]
- 63.Akkarachiyasit S., Charoenlertkul P., Yibchok-anun S., Adisakwattana S. Inhibitory activities of cyanidin and its glycosides and synergistic effect with acarbose against intestinal α-glucosidase and pancreatic α-amylase. Int. J. Mol. Sci. 2010;11:3387–3396. doi: 10.3390/ijms11093387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Badshah H., Ullah I., Kim S.E., Kim T.H., Lee H.Y., Kim M.O. Anthocyanins attenuate body weight gain via modulating neuropeptide Y and GABAB1 receptor in rats hypothalamus. Neuropeptides. 2013;47:347–353. doi: 10.1016/j.npep.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 65.Ghattamaneni N.K., Sharma A., Panchal S.K., Brown L. Pelargonidin 3-glucoside-enriched strawberry attenuates symptoms of DSS-induced inflammatory bowel disease and diet-induced metabolic syndrome in rats. Eur. J. Nutr. 2020;59:2905–2918. doi: 10.1007/s00394-019-02130-1. [DOI] [PubMed] [Google Scholar]
- 66.Molonia M.S., Occhiuto C., Muscarà C., Speciale A., Bashllari R., Villarroya F., Saija A., Cimino F., Cristani M. Cya-nidin-3-O-glucoside restores insulinsignaling and reduces inflammation in hypertrophic adipocytes. Arch. Biochem. Biophys. 2020;691:108488. doi: 10.1016/j.abb.2020.108488. [DOI] [PubMed] [Google Scholar]
- 67.Tsuda T., Ueno Y., Yoshikawa T., Kojo H., Osawa T. Micoarray profiling of gene expression in human adipocytes in response to anthocyanins. Biochem. Pharmacol. 2006;71:1184–1197. doi: 10.1016/j.bcp.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 68.Zhu Y., Huang X., Zhang Y., Wang Y., Liu Y., Sun R., Xia M. Anthocyanin supplementation improves HDL-associated paraoxonase 1 activity and enhances cholesterol efflux capacity in subjects with hypercholesterolemia. J. Clin. Endocrinol. Metab. 2014;99:561–569. doi: 10.1210/jc.2013-2845. [DOI] [PubMed] [Google Scholar]
- 69.Fallah A.A., Sarmast E., Fatehi P., Jafari T. Impact of dietary anthocyanins on systemic and vascular inflammation: Sys-tematic review and meta-analysis on randomised clinical trials. Food Chem. Toxicol. 2020;135:110922. doi: 10.1016/j.fct.2019.110922. [DOI] [PubMed] [Google Scholar]
- 70.Youdim K.A., Martin A., Joseph J.A. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radic. Biol. Med. 2000;29:51–60. doi: 10.1016/S0891-5849(00)00329-4. [DOI] [PubMed] [Google Scholar]
- 71.Iwasaki-Kurashige K., Loyaga-Rendon R.Y., Matsumoto H., Tokunaga T., Azuma H. Possible mediators involved in de-creasing peripheral vascular resistance with blackcurrant concentrate (BC) in hind-limb perfusion model of the rat. Vascul. Pharmacol. 2006;44:215–223. doi: 10.1016/j.vph.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 72.Zhu Y., Xia M., Yang Y., Liu F., Li Z., Hao Y., Mi M., Jinm T., Ling W. Purified anthocyanin supplementation improves endothelial function via NO-cGMP activation in hypercholesterolemic individuals. Clin. Chem. 2011;57:1524–1533. doi: 10.1373/clinchem.2011.167361. [DOI] [PubMed] [Google Scholar]
- 73.Watson R.R., Schönlau F. Nutraceutical and antioxidant effects of a delphinidin-rich maqui berry extract Delphinol®: A review. Minerva Cardioangiol. 2015;63(Suppl. S1):1–12. [PubMed] [Google Scholar]
- 74.Ziberna L., Tramer F., Moze S., Vrhovsek U., Mattivi F., Passamonti S. Transport and bioactivity of cyanidin 3-glucoside into the vascular endothelium. Free Radic. Biol. Med. 2012;52:1750–1759. doi: 10.1016/j.freeradbiomed.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 75.Aboonabi A., Singh I. Chemopreventive role of anthocyanins in atherosclerosis via activation of Nrf2-ARE as an indicator and modulator of redox. Biomed. Pharmacother. 2015;72:30–36. doi: 10.1016/j.biopha.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Karlsen A., Retterstøl L., Laake P., Paur I., Bøhn S.K., Sandvik L., Blomhoff R. Anthocyanins inhibit nuclear factor-kappaB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. J. Nutr. 2007;137:1951–1954. doi: 10.1093/jn/137.8.1951. [DOI] [PubMed] [Google Scholar]
- 77.Del Bo’ C., Marino M., Riso P., Møller P., Porrini M. Anthocyanins and metabolites resolve TNF-α-mediated production of E-selectin and adhesion of monocytes to endothelial cells. Chem. Biol. Interact. 2019;300:49–55. doi: 10.1016/j.cbi.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 78.Garcia-Alonso M., Minihane A.M., Rimbach G., Rivas-Gonzalo J.C., de Pascual-Teresa S. Red wine anthocyanins are rapidly absorbed in humans and affect monocyte chemoattractant protein 1 levels and antioxidant capacity of plasma. J. Nutr. Biochem. 2009;20:521–529. doi: 10.1016/j.jnutbio.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 79.Adhikari D.P., Francis J.A., Schutzki R.E., Chandra A., Nair M.G. Quantification and characterisation of cyclo-oxygenase and lipid peroxidation inhibitory anthocyanins in fruits of Amelanchier. Phytochem. Anal. 2005;16:175–180. doi: 10.1002/pca.840. [DOI] [PubMed] [Google Scholar]
- 80.Valenza A., Bonfanti C., Pasini M.E., Bellosta P. Anthocyanins Function as Anti-Inflammatory Agents in a Drosophila Model for Adipose Tissue Macrophage Infiltration. Biomed. Res. Int. 2018;2018:6413172. doi: 10.1155/2018/6413172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Migliorini A.A., Piroski C.S., Daniel T.G., Cruz T.M., Escher G.B., Vieira do Carmo M.A., Azevedo L., Marques M.B., Granato D., Rosso N.D. Red Chicory (Cichorium intybus) Extract Rich in Anthocyanins: Chemical Stability, Antioxidant Activity, and Antiproliferative Activity In Vitro. J. Food Sci. 2019;84:990–1001. doi: 10.1111/1750-3841.14506. [DOI] [PubMed] [Google Scholar]
- 82.Chiang A., Wu H., Yeh H., Chu C.S., Lin H.C., Lee W.C. Antioxidant effects of black rice extract through the induction of superoxide dismutase and catalase activities. Lipids. 2006;41:797–803. doi: 10.1007/s11745-006-5033-6. [DOI] [PubMed] [Google Scholar]
- 83.Aybastier O., Dawbaa S., Demir C. Investigation of antioxidant ability of grape seeds extract to prevent oxidatively induced DNA damage by gas cromatography-tandem mass spectrometry. J. Chroamtogr. B Anal. Technol. Biomed. Life. Sci. 2018;1072:328–335. doi: 10.1016/j.jchromb.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 84.Pantan R., Tocharus J., Suksamrarn A., Tocharus C. Synergistic effect of atorvastatin and Cyanidin-3-glucoside on angio-tensin II-induced inflammation in vascular smooth muscle cells. Exp. Cell Res. 2016;342:104–112. doi: 10.1016/j.yexcr.2016.02.017. [DOI] [PubMed] [Google Scholar]
- 85.Bachmair E.M., Ostertag L.M., Zhang X., de Roos B. Dietary manipulation of platelet function. Pharmacol. Ther. 2014;144:97–113. doi: 10.1016/j.pharmthera.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 86.Ojeda D., Jiménez-Ferrer E., Zamilpa A., Herrera-Arellano A., Tortoriello J., Alvarez L. Inhibition of angiotensin con-vertin enzyme (ACE) activity by the anthocyanins delphinidin- and cyanidin-3-O-sambubiosides from Hibiscus sabdariffa. J. Ethnopharmacol. 2010;127:7–10. doi: 10.1016/j.jep.2009.09.059. [DOI] [PubMed] [Google Scholar]
- 87.Boslett J., Hermann C., Zhao Y.J., Lee H.C., Zweier J.L. Luteolinidin protects the postischemic heart through CD38 inhibition with preservation of NAD(P)(H) J. Pharmacol. Exp. Ther. 2017;361:99–108. doi: 10.1124/jpet.116.239459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Caraba A., Crişan V., Romoşan I., Mozoş I., Murariu M. Vitamin D Status, Disease Activity, and Endothelial Dysfunction in Early Rheumatoid Arthritis Patients. Dis. Markers. 2017;2017:5241012. doi: 10.1155/2017/5241012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tibaut M., Caprnda M., Kubatka P., Sinkovič A., Valentova V., Filipova S., Gazdikova K., Gaspar L., Mozos I., Egom E.E., et al. Markers of Atherosclerosis: Part 2—Genetic and Imaging Markers. Heart Lung Circ. 2019;28:678–689. doi: 10.1016/j.hlc.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 90.Johnson S.A., Figueroa A., Navaei N., Wong A., Kalfon R., Ormsbee L.T., Feresin R.G., Elam M.L., Hooshmand S., Payton M.E., et al. Daily blueberry consumption improves blood pressure and arterial stiffness in postmenopausal women with pre- and stage 1-hypertension: A randomized, double-blind, placebo-controlled clinical trial. J. Acad. Nutr. Diet. 2015;115:369–377. doi: 10.1016/j.jand.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 91.Tabart J., Auger C., Kevers C., Dommes J., Pollet B., Defraigne J.O., Schini-Kerth V.B., Pincemail J. The potency of commercial blackcurrant juices to induce relaxation in porcine coronary artery rings is not correlated to their antioxidant capacity but to their anthocyanin content. Nutrition. 2018;51–52:53–59. doi: 10.1016/j.nut.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 92.Horie K., Nanashima N., Maeda H. Phytoestrogenic Effects of Blackcurrant Anthocyanins Increased Endothelial Nitric Oxide Synthase (eNOS) Expression in Human Endothelial Cells and Ovariectomized Rats. Molecules. 2019;24:1259. doi: 10.3390/molecules24071259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bharat D., Cavalcanti R.R.M., Petersen C., Begaye N., Cutler B.R., Costa M.M.A., Ramos R.K.L.G., Ferreira M.R., Li Y., Bharath L.P., et al. Blueberry metabolites attenuate lipotoxicity-induced endothelial dysfunction. Mol. Nutr. Food Res. 2018;62 doi: 10.1002/mnfr.201700601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thandapilly S.J., LeMaistre J.L., Louis X.L., Anderson C.M., Netticadan T., Anderson H.D. Vascular and cardiac effects of grape powder in the spontaneously hypertensive rat. Am. J. Hypertens. 2012;25:1070–1076. doi: 10.1038/ajh.2012.98. [DOI] [PubMed] [Google Scholar]
- 95.Milbury P.E., Vita J.A., Blumberg J.B. Anthocyanins are bioavailable in humans following an acute dose of cranberry juice. J. Nutr. 2010;140:1099–1104. doi: 10.3945/jn.109.117168. [DOI] [PubMed] [Google Scholar]
- 96.Fairlie-Jones L., Davison K., Fromentin E., Hill A.M. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients. 2017;9:908. doi: 10.3390/nu9080908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nanashima N., Horie K., Maeda H., Tomisawa T., Kitajima M., Nakamura T. Blackcurrant Anthocyanins Increase the Levels of Collagen, Elastin, and Hyaluronic Acid in Human Skin Fibroblasts and Ovariectomized Rats. Nutrients. 2018;10:495. doi: 10.3390/nu10040495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rodriguez-Mateos A., Istas G., Boschek L., Feliciano R.P., Mills C.E., Boby C., Gomez-Alonso S., Milenkovic D., Heiss C. Circulating Anthocyanin Metabolites Mediate Vascular Benefits of Blueberries: Insights from Randomized Controlled Trials, Metabolomics, and Nutrigenomics. J. Gerontol. A Biol. Sci. Med. Sci. 2019;74:967–976. doi: 10.1093/gerona/glz047. [DOI] [PubMed] [Google Scholar]
- 99.Zhu Y., Ling W., Guo H., Song F., Ye Q., Zou T., Li D., Zhang Y., Li G., Xiao Y., et al. Anti-inflammatory effect of purified dietary anthocyanin in adults with hypercholesterolemia: A randomized controlled trial. Nutr. Metab. Cardiovasc. Dis. 2013;23:843–849. doi: 10.1016/j.numecd.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 100.Min H.K., Kim S.M., Baek S.Y., Woo J.W., Park J.S., Cho M.L., Lee J., Kwok S.K., Kim S.W., Park S.H., et al. Anthocyanin extracted from black soybean seedcoats prevents autoimmune arthritis by suppressing the development of Th17 cells and synthesis of proinflammatory cytokines by such cells, via inhibition of NF-kB. PLoS ONE. 2015;10:e0138201. doi: 10.1371/journal.pone.0138201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuntz S., Asseburg H., Dold S., Rompp A., Frohling B., Kunz C., Rudloff S. Inhibition of low-grade inflammation by anthocyanins from grape extract in an in vitro epithelial-endothelial co-culture model. Food Funct. 2015;6:1136–1149. doi: 10.1039/C4FO00755G. [DOI] [PubMed] [Google Scholar]
- 102.Del Bo C., Roursgaard M., Porrini M., Loft S., Moller P., Riso P. Different effects of anthocyanins and phenolic acids from wild blueberry (Vaccinium angustifolium) on monocytes adhesion to endothelial cells in a TNF-alpha stimulated pro-inflammatory environment. Mol. Nutr. Food Res. 2016;60:2355–2366. doi: 10.1002/mnfr.201600178. [DOI] [PubMed] [Google Scholar]
- 103.Blando F., Calabriso N., Berland H., Maiorano G., Gerardi C., Carluccio M.A., Andersen Ø.M. Radical Scavenging and Anti-Inflammatory Activities of Representative Anthocyanin Groupings from Pigment-Rich Fruits and Vegetables. Int. J. Mol. Sci. 2018;19:169. doi: 10.3390/ijms19010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Aboonabi A., Meyer R.R., Gaiz A., Singh I. Anthocyanins in berries exhibited anti-atherogenicity and antiplatelet activities in a metabolic syndrome population. Nutr. Res. 2020;76:82–93. doi: 10.1016/j.nutres.2020.02.011. [DOI] [PubMed] [Google Scholar]
- 105.Broncel M., Kozirog M., Duchnowicz P., Koter-Michalak M., Sikora J., Chojnowska-Jezierska J. Aronia melanocarpa extract reduces blood pressure, serum endothelin, lipid, and oxidative stress marker levels in patients with metabolic syndrome. Med. Sci. Monit. 2010;16:CR28–CR34. [PubMed] [Google Scholar]
- 106.Tibaut M., Caprnda M., Kubatka P., Sinkovič A., Valentova V., Filipova S., Gazdikova K., Gaspar L., Mozos I., Egom E.E., et al. Markers of Atherosclerosis: Part 1—Serological Markers. Hearth Lung Circ. 2019;28:667–677. doi: 10.1016/j.hlc.2018.06.1057. [DOI] [PubMed] [Google Scholar]
- 107.Farhangi A.M., Najafi M. Empirically developed dietary inflammatory potential (EDIP) in patients candidate for coronary artery bypass grafting surgery (CABG): Association with metabolic parameters, dietary antioxidant quality score and dietary phytochemical index. PLoS ONE. 2018;13:e0208711. doi: 10.1371/journal.pone.0208711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dumitrescu C., Biondi R., Xia Y., Cardounel A.J., Druhan L.J., Ambrosio G., Zweier J.L. Myocardial ischemia results in tetrahydrobiopterin (BH4) oxidation with impaired endothelial function ameliorated by BHP. Proc. Natl. Acad. Sci. USA. 2007;104:15081–15086. doi: 10.1073/pnas.0702986104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Safaeian L., Emami R., Hajhashemi V., Haghighatian Z. Antihypertensive and antioxidant effects of protocatechuic acid in deoxycorticosterone acetate-salt hypertensive rats. Biomed. Pharmacother. 2018;100:147–155. doi: 10.1016/j.biopha.2018.01.107. [DOI] [PubMed] [Google Scholar]
- 110.Barajas B., Che N., Yin F., Rowshanrad A., Orozco L.D., Gong K.W., Wang X., Castellani L.W., Reue K., Lusis A.J., et al. NF-E2-related factor 2 promotes atherosclerosis by effects on plasma lipoproteins and cholesterol transport that overshadow antioxidant protection. Arterioscler. Thromb. Vasc. Biol. 2011;31:58–66. doi: 10.1161/ATVBAHA.110.210906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.McAnulty L.S., Collier S.R., Landram M.J., Whittaker D.S., Isaacs S.E., Klemka J.M., Cheek S.L., Arms J.C., McAnulty S.R. Six weeks ingestion of whole blueberry powder increases natural killer cell counts and reduces arterial stiffness in sedentary males and females. Nutr. Res. 2014;34:577–584. doi: 10.1016/j.nutres.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 112.Faria A., Fernandes I., Norberto S., Mateus N., Calhau C. Interplay between anthocyanins and gut microbiota. J. Agric. Food. Chem. 2014;62:6898–6902. doi: 10.1021/jf501808a. [DOI] [PubMed] [Google Scholar]
- 113.Basile T., Alba V., Gentilesco G., Savino M., Tarricone L. Anthocyanins pattern variation in relation to thinning and girdling in commercial Sugrathirteen® table grape. Sci. Hortic. 2018;227:202–206. doi: 10.1016/j.scienta.2017.09.045. [DOI] [Google Scholar]
- 114.Li Y., Fang J., Qi X., Lin M., Zhong Y., Sun L. A key structural gene, AaLDOX, is involved in anthocyanin biosynthesis in all red-fleshed kiwifruit (Actinidia arguta) based on transcriptome analysis. Gene. 2018;648:31–41. doi: 10.1016/j.gene.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 115.Mauray A., Felgines C., Morand C., Mazur A., Scalbert A., Milenkovic D. Bilberry anthocyanin-rich extract alters expression of genes related to atherosclerosis development in aorta of apo E-deficient mice. Nutr. Metab. Cardiovasc Dis. 2012;22:72–80. doi: 10.1016/j.numecd.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 116.Jennings A., Welch A.A., Fairweather-Tait S.J., Kay C., Minihane A.M., Chowienczyk P., Jiang B., Cecelja M., Spector T., Macgregor A., et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012;96:781–788. doi: 10.3945/ajcn.112.042036. [DOI] [PubMed] [Google Scholar]
- 117.Aviram M., Rosenblat M., Gaitini D., Nitecki S., Hoffman A., Dornfeld L., Volkova N., Presser D., Attias J., Liker H., et al. Pomegranate juice consumption for 3 years by patients with carotid artery stenosis reduces common carotid intima-media thickness, blood pressure and LDL oxidation. Clin. Nutr. 2004;23:423–433. doi: 10.1016/j.clnu.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 118.Poreba R., Skoczynska A., Gac P., Poreba M., Jedrychowska I., Affelska-Jercha A., Turczyn B., Wojakowska A., Oszmianski J., Andrzejak R. Drinking of chockeberryjuice from the ecological farm Dzieciolowo and distensibility of brahial artery in men with mild hypercholesterolemia. Ann. Agric. Environ. Med. 2009;16:305–308. [PubMed] [Google Scholar]
- 119.Matsumoto H., Takenami E., Iwasaki-Kurashige K., Osada T., Katsumura T., Hamaoka T. Effects of blackcurrant antho-cyanin intake on peripheral muscle circulation during typing work in humans. Eur. J. Appl. Physiol. 2005;94:36–45. doi: 10.1007/s00421-004-1279-y. [DOI] [PubMed] [Google Scholar]
- 120.Li L., Lyall G.K., Martinez-Blazquez J.A., Vallejo F.A., Tomas-Barberan F., Birch K.M., Boesch C. Blood Orange Juice Consumption Increases Flow-Mediated Dilation in Adults with Overweight and Obesity: A Randomized Controlled Trial. J. Nutr. 2020;150:2287–2294. doi: 10.1093/jn/nxaa158. [DOI] [PubMed] [Google Scholar]
- 121.Curtis P.J., van der Velpen V., Berends L., Jennings A., Feelisch M., Umpleby A.M., Evans M., Fernandez B.O., Meiss M.S., Minnion M., et al. Blueberries improve biomarkers of cardiometabolic function in participants with metabolic syn-drome-results from a 6-month, double-blind, randomized controlled trial. Am. J. Clin. Nutr. 2019;109:1535–1545. doi: 10.1093/ajcn/nqy380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mozos I., Maidana J.P., Stoian D., Stehlik M. Gender Differences of Arterial Stiffness and Arterial Age in Smokers. Int. J. Environ. Res. Public Health. 2017;14:565. doi: 10.3390/ijerph14060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tomisawa T., Nanashima N., Kitajima M., Mikami K., Takamagi S., Maeda H., Horie K., Lai F.C., Osanai T. Effects of Blackcurrant Anthocyanin on Endothelial Function and Peripheral Temperature in Young Smokers. Molecules. 2019;24:4295. doi: 10.3390/molecules24234295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Balestra C., Cimino F., Theunissen S., Snoeck T., Provyn S., Canali R., Bonina A., Virgili F. A red orange extract modulates the vascular response to a recreational dive: A pilot study on the effect of anthocyanins on the physiological consequences of scuba diving. Nat. Prod. Res. 2016;30:2101–2106. doi: 10.1080/14786419.2015.1107062. [DOI] [PubMed] [Google Scholar]
- 125.Pekas E.J., Shin J., Headid R.J., Son W.M., Layec G., Yadav S.K., Scott S.D., Park S.Y. Combined anthocyanins and bromelain supplement improves endothelial function and skeletal muscle oxygenation status in adults: A double-blind place-bo-controlled randomised crossover clinical trial. Br. J. Nutr. 2021;125:161–171. doi: 10.1017/S0007114520002548. [DOI] [PubMed] [Google Scholar]
- 126.Okamoto T., Hashimoto Y., Kobayashi R., Nakazato K., Willems M.E.T. Effects of blackcurrant extract on arterial functions in older adults: A randomized, double-blind, placebo-controlled, crossover trial. Clin. Exp. Hypertens. 2020;42:640–647. doi: 10.1080/10641963.2020.1764015. [DOI] [PubMed] [Google Scholar]
- 127.Johnson S.A., Navaei N., Pourafshar S., Jaime S.J., Akhavan N.S., Alvarez-Alvarado S., Proaño G.V., Litwin N.S., Clark E.A., Foley E.M., et al. Effects of Montmorency Tart Cherry Juice Consumption on Cardiometabolic Biomarkers in Adults with Metabolic Syndrome: A Randomized Controlled Pilot Trial. J. Med. Food. 2020;23:1238–1247. doi: 10.1089/jmf.2019.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hollands W.J., Armah C.N., Doleman J.F., Perez-Moral N., Winterbone M.S., Kroon P.A. 4-Week consumption of anthocyanin-rich blood orange juice does not affect LDL-cholesterol or other biomarkers of CVD risk and glycaemia compared with standard orange juice: A randomised con-trolled trial. Br. J. Nutr. 2018;119:415–421. doi: 10.1017/S0007114517003865. [DOI] [PubMed] [Google Scholar]
- 129.Feresin R.G., Johnson S.A., Pourafshar S., Campbell J.C., Jaime S.J., Navaei N., Elam M.L., Akhavan N.S., Alva-rez-Alvarado S., Tenenbaum G., et al. Impact of daily strawberry consumption on blood pressure and arterial stiffness in pre- and stage 1-hypertensive postmenopausal women: A randomized controlled trial. Food Funct. 2017;8:4139–4149. doi: 10.1039/C7FO01183K. [DOI] [PubMed] [Google Scholar]
- 130.Rodriguez-Mateos A., Rendeiro C., Bergillos-Meca T., Tabatabaee S., George T.W., Heiss C., Spencer J.P. Intake and time dependence of blueberry flavonoid-induced improvements in vascular function: A randomized, controlled, double-blind, crossover intervention study with mechanistic insights into biological activity. Am. J. Clin. Nutr. 2013;98:1179–1191. doi: 10.3945/ajcn.113.066639. [DOI] [PubMed] [Google Scholar]
- 131.Davidson M.H., Maki K.C., Dicklin M.R., Feinstein S.B., Witchger M., Bell M., McGuire D.K., Provost J.-C., Liker H., Aviram M. Effects of consumption of pomegranate juice on carotid intima-media thickness in men and women at moderate risk for coronary heart disease. Am. J. Cardiol. 2009;104:936–942. doi: 10.1016/j.amjcard.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 132.Oboh H.A., Agu K. The effects of various traditional processing methods on the glycemic index and glycemic load of cowpeas (Vigna Unguiculata) J. Food Biochem. 2008;32:576–596. doi: 10.1111/j.1745-4514.2010.00423.x. [DOI] [Google Scholar]
- 133.Gerardi C., Albano C., Calabriso N., Carluccio M.A., Durante M., Mita G., Renna M., Serio F., Blando F. Techno-functional properties of tomato puree fortified with anthocyanin pigments. Food Chem. 2018;240:1184–1192. doi: 10.1016/j.foodchem.2017.08.057. [DOI] [PubMed] [Google Scholar]
- 134.Rodriguez-Mateos A., Heiss C., Borges G., Crozier A. Berry (poly) phenols and cardiovascular health. J. Agric. Food Chem. 2014;62:3842–3851. doi: 10.1021/jf403757g. [DOI] [PubMed] [Google Scholar]
- 135.Matsumoto H., Ito K., Yonekura K., Tsuda T., Ichiyanagi T., Hirayama M., Konishi T. Enhanced absorption of anthocyaninsafter oral administration of phyticacid in rats and humans. J. Agric. Food Chem. 2007;55:2489–2496. doi: 10.1021/jf063199t. [DOI] [PubMed] [Google Scholar]
- 136.Burgos-Edwards A., Jiimenez-Aspee F., Thomas-Valdes S., Schmeda-Hirschmann G., Theoduloz C. Qualitative and quan-titative changes in polyphenol composition and bioactivity of Ribes magellanicum and R. punctatum after in vitro gastro-intestinal digestion. Food Chem. 2017;237:1073–1082. doi: 10.1016/j.foodchem.2017.06.060. [DOI] [PubMed] [Google Scholar]