Abstract
In this study, a platinum(II) complex ([Pt(H2L)(PPh3)] complex) containing a thiocarbohydrazone as the ligand was tested as an anti-proliferative agent against ovarian adenocarcinoma (Caov-3) and human colorectal adenocarcinoma (HT-29) through MTT assays. Apoptotic markers were tested by the AO/PI double staining assay and DNA fragmentation test. Flow cytometry was conducted to measure cell cycle distribution, while the p53 and caspase-8 pathways were tested via immunofluorescence assay. Results demonstrated that the cytotoxic effect of the Pt(II)-thiocarbohydrazone complexes against Caov-3 and HT-29 cells was highly significant, and this effect triggered the activation of the p53 and caspase-8 pathways. Besides, apoptosis stimulated by the Pt(II)-thiocarbohydrazone complex was associated with cell cycle arrest at the G0/G1 phase. These findings suggest that the target complex inhibited the proliferation of Caov-3 and HT-29 cells, resulting in the arrest of the cell cycle and induction of apoptosis via the stimulation of the p53 and caspase-8 pathways. The present data suggests that the Pt(II)-thiocarbohydrazone complex could also be a promising chemotherapeutic agent for other types of cancer cells.
Keywords: Pt(II) complex, anticancer activity, apoptosis, DNA fragmentation, p53, caspase-8
1. Introduction
Cancer is a critical disease of interest to scientists due to its long history of being among the leading causes of death [1]. There is no single disorder representing cancer; rather, it is a collection of disorders marked by the uncontrollable outgrowth of cells. Tumors are a serious risk of lethal disease with no geographic location or organ limits; they induce an annual worldwide mortality exceeding 12.7 million individuals. Mutated genes that regulate growth and are involved in DNA repair, cell division, and death typically causes tumor diseases [2].
Cancer can occur accidentally when a portion of the genetic code is miscopied, whereas DNA damage by chemical, viral, or radiation exposure may lead to cancer induced by the environment. Previous studies on the development of anticancer strategies include various instances related to the interactions of metal ions or metal-containing compounds with biological targets. Selecting the ligand in a metal complex is as essential as selecting the metal due to its effectiveness on biological systems [3,4,5,6]. In pharmacology, heterocyclic compounds play a significant role in the composition of the drug formula.
Purine, thiosemicarbazone, imidazole, and benzohydroxamic ligands containing different donor atoms that can chelate metal ions can exert different anti-cancer functions. In vitro and in vivo investigations of several anticancer drugs containing metal ions showed more potent antineoplastic actions than that of cisplatin [7,8,9,10]. Anticancer drugs based on platinum (Pt) still constitute an essential part of vast regimens of chemotherapy [4]. Although they cause toxicity as a part of their side effects, they also lead to drug resistance [11,12,13]. Meanwhile, the mechanism of action of Pt against cancer cell lines remains unclear. The three foremost steps related to antitumor action are as follows: cell uptake of drugs; the interplay of the drug with DNA, including intervention with the activities of transcription and/or replication; and the platinum-DNA lesion that evokes cell death, which depends on the activation of the signal transduction pathways [14].
The cytotoxicity of platinum-based drugs induced by the activated platinum species binding to intracellular targets is predominantly dependent on DNA. Following the recognition of the DNA adducts by the cellular systems, three concurrent potential pathways are involved. In the first pathway, DNA can be repaired. In the second, the damaged DNA fragment can be bypassed. In the third, apoptosis can take place. Inhibition of transcription assumes the common way to evoke cell apoptosis after treatment with Pt compounds [15]. Based on the potency of platinum complexes as anticancer agents to obtain new more effective and less toxic drug candidates than cisplatin, this work explores the activity of a Pt(II)-thiocarbohydrazone complex as a potential anticancer agent. The attractiveness of thiocarbohydrazones for developing new metal-based drugs relies on their structural diversity providing interesting coordination chemistry and promising biological implications [16,17].
The Caov-3 cell line is an epithelial morphology main line of ovarian cancer cells. They are susceptible to adriamycin, cisplatin, and vinblastine. Improvements in ovarian cancer outcomes are expected, particularly for ovarian clear cell carcinomas (OCCA), from a clear understanding of molecular pathology that may drive early detection approaches and effective treatment [18]. Thus, the present study was performed to evaluate the anti-proliferative and pro-apoptotic effects of the Pt(II)-thiocarbohydrazone complex on Caov-3 and HT-29 cell lines. Our results explained the possible mechanisms involved in the effect of this complex on the used cell lines, namely, mitochondrial damage and the p53 and caspase-8 pathways.
2. Results and Discussion
2.1. Pt(II) Complex as Anti-Proliferative Agent against Caov-3 and HT-29 Cells
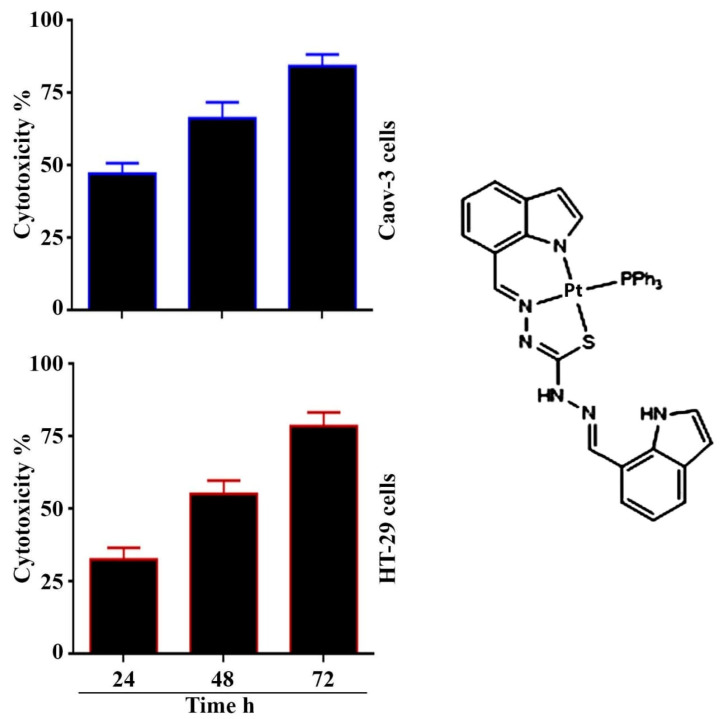
In this study, we measured the cytotoxic effect of the Pt(II) complex at different times as shown in Figure 1. The IC50 values of the Pt(II) complex following 24 h exposure of the cancer cell line was 3.34 μg mL−1 for Caov-3 and 10.32 μg mL−1 for HT-29. Whereas, the IC50 values for 48 h exposure to the complex were 2.41 μg mL−1 for Caov-3 and 4.25 μg mL−1 for HT-29 cancer cell lines. The IC50 values for 72 h exposure were 1.74 μg mL−1 for Caov-3 and 2.5 μg mL−1 for HT-29 cancer cell lines. These results indicated that prolonged treatment significantly reduced the IC50. These results agree with the previous study of Jain et al. [19].
Figure 1.
(left lane) Cytotoxicity of [Pt(H2L)(PPh3)] against Caov-3 and HT-29 cells at different time (24, 48 and 72 h). IC50 for Caov-3 cells were 3.34, 2.41, 1.74 μg mL−1, respectively. While, for HT-29 cells were 10.32, 4.25, 2.5 μg mL−1, respectively. (right lane) Structure of Pt(II) complex.
The activity of the here-presented Pt complex was compared with that of cisplatin (a clinically-used anticancer drug) in analogy to other works on platinum complexes [20,21]; the IC50 values of [Pt(H2L) (PPh3)], presented in Figure 1, resulted lower demonstrating greater cytotoxic effects than cisplatin towards the same cancer cell lines. While other study of Paschke et al. verified that the CholCOO(CH2 )n CH(CH2 NH2 ) Pt2 CBDC (carbo-ChAPt) as well as the CholCOO(CH2)n CH(CH2 NH2 ) PtCl2 (cis- ChAPt) showed similar cytotoxicity against testicular cancer cells as their partner compounds carboplatin and cisplatin [11].
2.2. Pt(II) Complex Induces Apoptosis in Caov-3 and HT-29 Cells
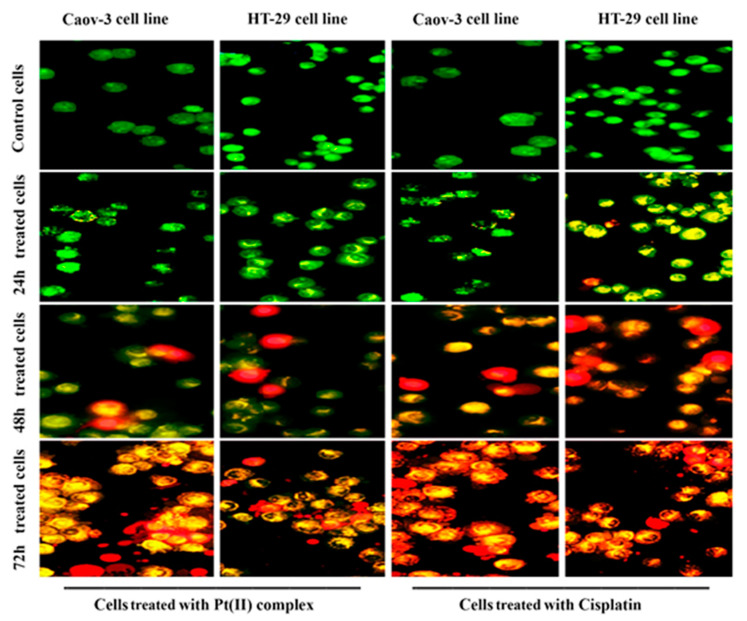
The (AO/PI) dual stain is a fluorescent combination stain that enables to detection of morphological modification in the nucleus by producing distinctive fluorescent colors. Apoptotic cells have increased fluorescence stain permeability to the plasma membrane. After treating the cell lines with the IC50 concentrations of the tested compound for 24, 48, and 72 h, dual cell staining and fluorescence microscope visualization were performed to recognize changes in nuclear morphology [21]. The treatment of cells with IC50 concentrations of the [Pt(H2L)(PPh3)] complex led to disruption of the membrane and vacuoles of the lysosomes compared with untreated cells with effects increased with the incubation time (Figure 2). The same results were observed after treated the Caov-3 and HT-29 cells with cisplatin. The results showed the high potential of the platinum complex to cause cell death due to the ability of the molecules to penetrate through the membrane [22,23]. A mixture of AO/PI dyes was used to further examine the potential of the tested compound to induce cancer cell death. In this process, the structure of the nuclei of the cells was observed to be intact, with a stable bright green color. Compared with the membranes of untreated cells, the membranes of cancer cells treated with the test compounds exhibited less integrity apoptotic cells typically display nuclei that are distinguished by their red to green color, while their chromatin condensation differs. The morphological changes in the treated cells indicated that apoptosis rather than necrosis caused the observed cell death. Through the combination of the AO stain into DNA, cells suffering from early cell death or apoptosis were identified, leading to the emission of bright green fluorescence after 24 h. Mild apoptosis was observed during therapy in the form of condensed chromatin and membrane blebbing, which are apoptosis-related time-dependent morphological features. Additionally, the same dose of the Pt(II) complex caused late apoptosis events after 48 and 72 h that were recognized through many changes that included binding between two stains and damaged DNA, leading to the emission of a reddish-orange color. In the present work, we measured the possibility that inducing apoptosis was related to the anti-proliferation events in the tested cancer cell line Caov-3 and HT-29 after treatment with the Pt(II) complex.
Figure 2.
Fluorescence microscopy images of cell lines stained with AO and PI. Cells were treated with IC50 concentrations of [Pt(H2L)(PPh3)] complex at different times.
2.3. Pt(II) Complex Causes DNA Fragmentation in Caov-3 and HT-29 Cells
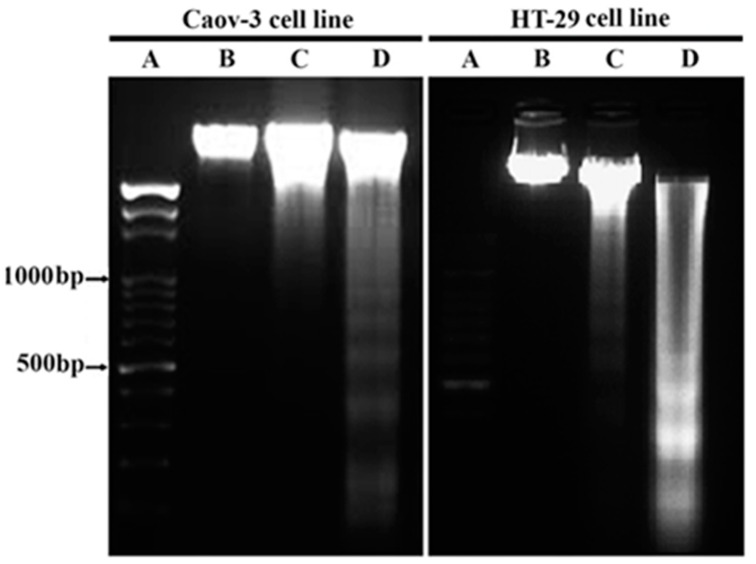
The formation of nucleosome units from the breakdown of DNA is one of the main characteristics of apoptosis. To identify the fragmentation of the nucleic acid inside the cancer cells, the ovarian adenocarcinoma and colorectal adenocarcinoma cell lines (Caov-3 and HT-29, respectively) were incubated with the corresponding IC50 values of the Pt- complex for 48 and 72 h. After lysing and extracting the nucleic acid of the cell lines, gel electrophoresis was used to identify the changes in the nucleic acid. As shown in Figure 3, a comparison of nucleic acid fragmentation at 72 h for both cell lines showed a ladder range from 500 bp to 1000 bp. These results confirmed the formation of nucleosome units as compared with the control cell line, indicating the death of the cells via apoptosis [24]. No ladder was observed in the control cells. Thus, the cytotoxic effect of the treatment with the Pt complex allowed the crumbling of apoptotic DNA, and cell death was possibly mediated via an apoptotic mechanism. The replication of DNA molecule can be inhibited through a breakdown in its molecule, which is caused by inter-nucleosome cleavage accompanied with apoptosis, proposing that linalool-Gold nanoparticles GNP (LG) and linalool-GNP-CALNN peptide (LGC) can evoke fragmentation and damage in nucleic acid via different free radicals [25]. The reason behind this kind of DNA cleavage is the cleavage among the exposed linker regions of nucleosomes in response to the stimulation of endogenous endonucleases [26].
Figure 3.
DNA fragmentation assay. Lane A: 1000 bp molecular weight marker, Lane B: untreated cells, Lane C: treated cells for 48 h, Lane D: treated cells for 72 h.
2.4. Effect of the Pt(II) Complex in Mitochondrial Membrane Potential of Caov-3 and HT-29 Cells
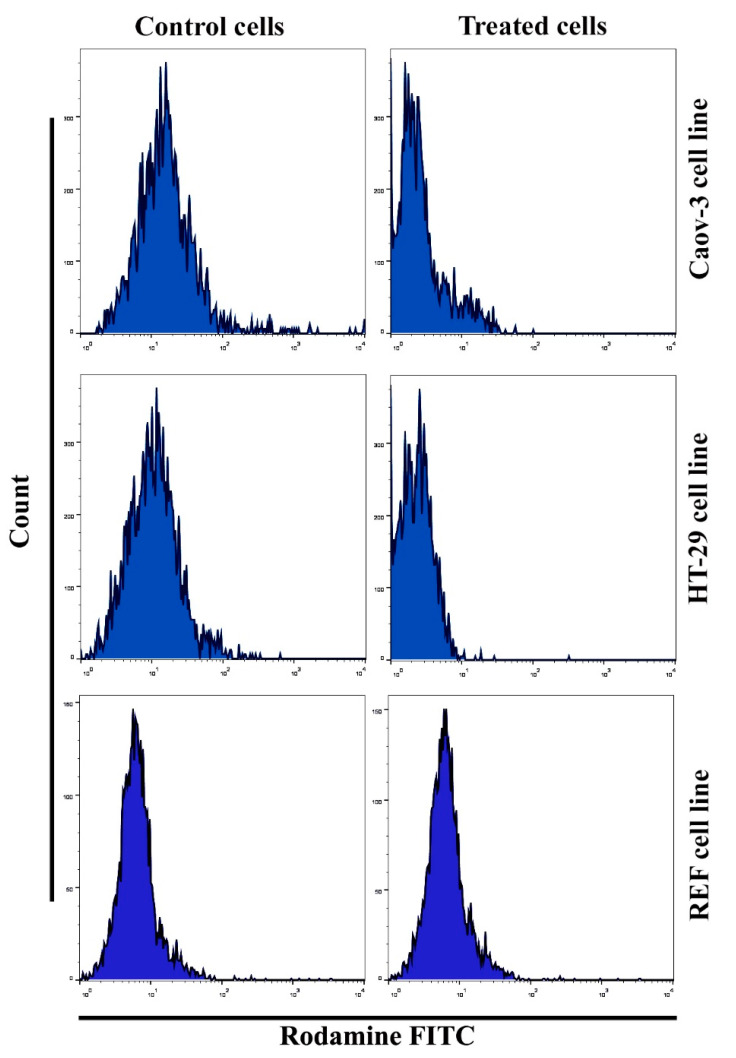
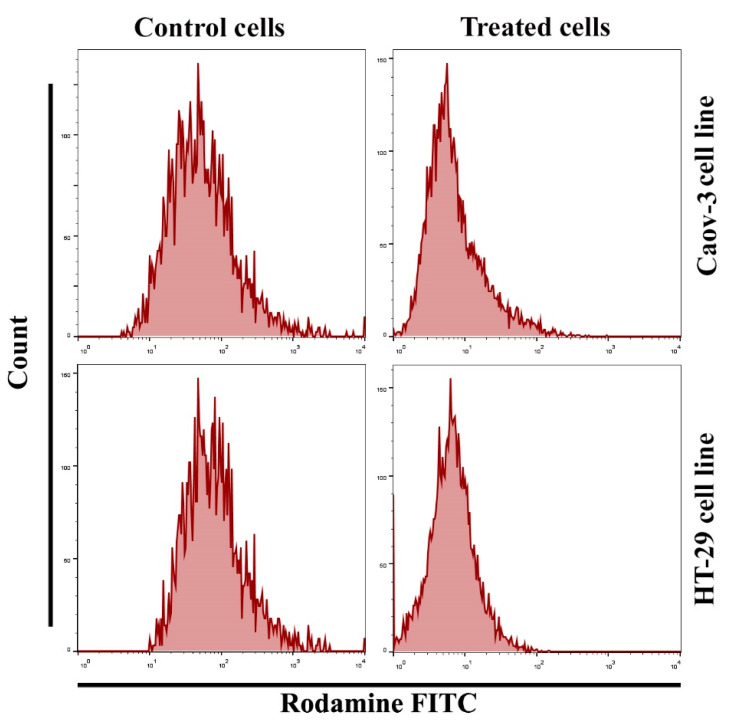
The mitochondria play a crucial and essential role in inducing apoptotic activities through cell death stimulation. Changes in this organ are characterized by the loss of its membrane potential (∆ψm) and the release of cytochrome c protein into the cytoplasm, leading to caspase-3 via the caspase-9 pathway. In this study, apoptosis was detected using flow cytometry assay according to the manufacturer’s protocol. An important and relevant marker for the apoptotic cell death process is the reduction in mitochondrial membrane potential. The mitochondrial membrane potential level was examined after staining the cells with the Rh123 probe via flow cytometry. The number of apoptotic cancer cells after Pt(II) complex treatment was calculated. A significant increase in apoptosis due to Pt(II) complex treatment was observed in Caov3 and HT-29 cells (Figure 4), while no change observed after treated the normal cell line Rat embryonic fibroblast (REF) cells. The same results were seen after treated cancer cells with cisplatin as shown in (Figure 5). A significant decrease in cancer cells treated with the Pt(II) complex at IC50 for 24 h was noted in the Rh123 stain, which corresponded to the depletion of mitochondrial membrane potential compared with the untreated control cell classes.
Figure 4.
Effect of [Pt(H2L)(PPh3)] complex on the loss of mitochondrial membrane potential.
Figure 5.
Effect of cisplatin on the loss of mitochondrial membrane potential.
2.5. Cell Cycle Analysis
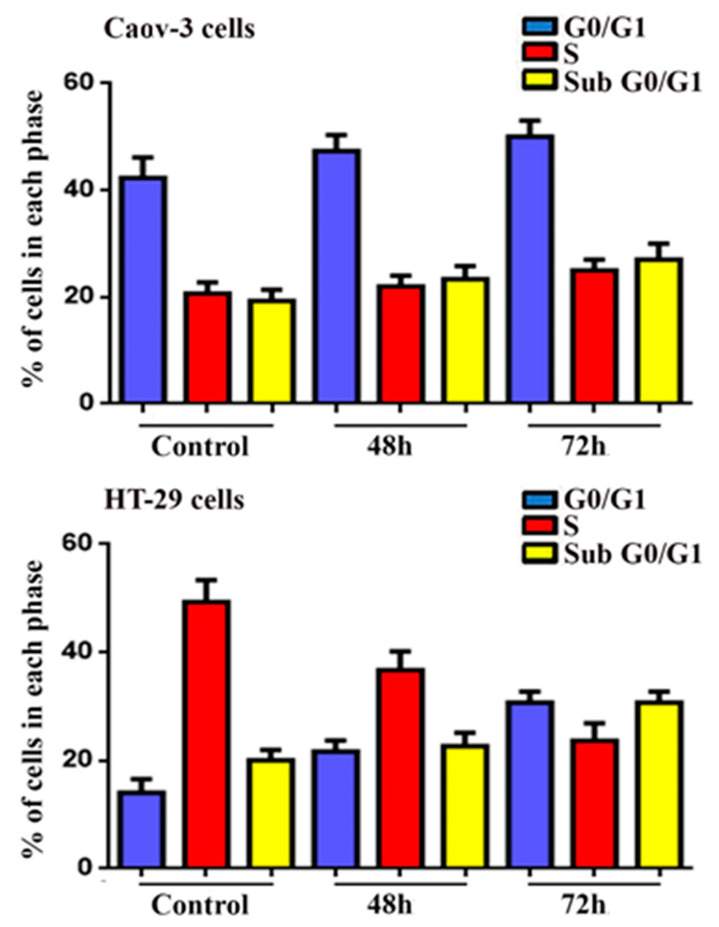
As shown in Figure 6, the DNA content of cells was replicated during the synthesis phase (S phase), whereas the cells in the Gap0 (G0) and Gap1 (G1) phases had unreplicated nucleic acid. Meanwhile, the Gap2 (G2) and mitosis (M) phases showed replicated nucleic acid. In the flow cytometry analysis, the sub-G0/G1 peak was observed in histograms due to the degradation of nucleic acid and the formation of hypodiploid. Sub-G0/G1 is a selected marker of cellular apoptosis [27]. Analysis by flow cytometry of the ovarian adenocarcinoma and colorectal adenocarcinoma was carried out after respective IC50 treatments of the Pt complex for 48 and 72 h. The histogram showed a gradual increase in the sub-G0/G1 phase during the incubation period. The results showed the capacity of the Pt complex to induce the apoptosis pathway of Caov-3 and HT-29 cancer cells via the G0/G1 phase [28].
Figure 6.
Cell cycle analysis of Caov-3 and HT-29 cell line after treatment with platinum(II) complex at different times as indicated. Bars show mean ± SEM.
2.6. Pt(II) Complex Induces the P53 and Caspase-8 Pathways
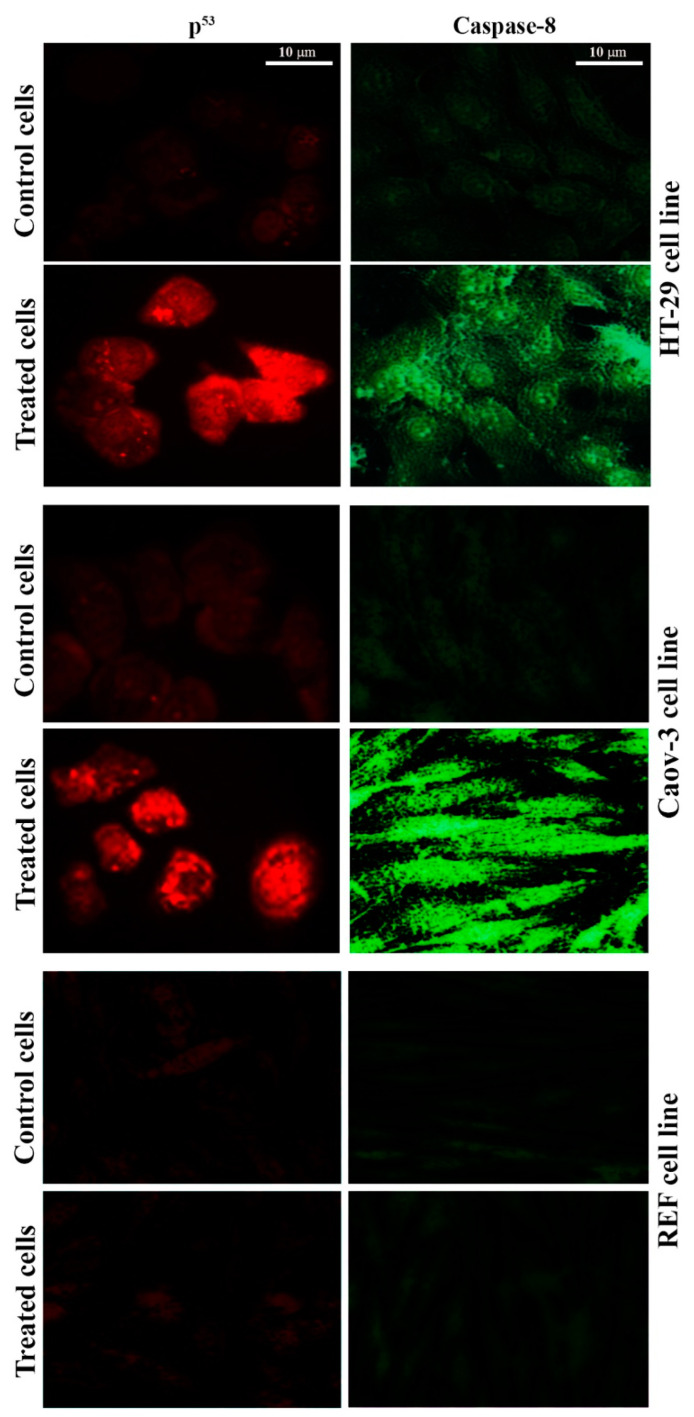
The tumor suppressor p53 gene and caspase enzymes are helpful in the regular monitoring of cells, and they prevent them from transforming into cancerous cells. When a cell shows any pattern of malignancy, an activation process of the mechanisms responsible for DNA repair is initiated for the restoration of the altered DNA [29,30]. To confirm that the Pt(II) complex caused the induction of p53 and caspase-8, an immunofluorescent test was performed. Figure 7 demonstrates p53 and caspase-8 induction in the cell lines following treatment with the Pt(II) complex. The fluorescence of p53 and caspase-8 was very low in the control cells, but p53 and caspase-8 immunofluorescent signaling were very clear in the treated Coav-3 and HT-29 cells. The signal was disappeared when the normal cell line REF was treated with the same complex. While the results demonstrated that cisplatin induces apoptosis in Caov-3 and HT-29 cell lines through the p53 and caspase-8 pathway as shown in Figure 8. These results suggested that the Pt(II) complex can induce apoptosis via p53 and caspase-8.
Figure 7.
[Pt(H2L)(PPh3)] complex inducing apoptosis through p53 and Caspase-8 pathway. Immunofluorescence results of p53 and Caspase-8. Cells were untreated (control), or treated with [Pt(H2L)(PPh3)] complex as indicated. Cells were fixed, permeabilized, and then stained with primary and secondary antibodies. Scale bar 10 µm.
Figure 8.
Cisplatin induces apoptosis through p53 and Caspase-8 pathway. Immunofluorescence results of p53 and Caspase-8. Cells were untreated (control), or treated with cisplatin as indicated. Cells were fixed, permeabilized, and then stained with primary and secondary antibodies. Scale bar 10 µm.
3. Materials and Methods
3.1. Preparation and Characterization of the Pt Complex
The Pt(II) complex preparation and characterization methods were identical to those previously described [17].
3.2. Maintenance of Cell Cultures
Caov-3 and HT-29 cancer cell lines were used. These cell lines were kept in RPMI-1640 in the presence of fetal bovine serum (10%), penicillin (100 units mL−1), and streptomycin (100 µg mL−1). Cells were passaged in trypsin-EDTA, reseeding was at 80% confluence (twice a week), and incubation was at 37 °C [31].
3.3. MTT Assay
The cytotoxicity test was carried following prior methods [32]. Cells were seeded at 1 × 105 cells mL−1 in wells of microtiter plates supplied with RPMI and permitted to adhere overnight. Dilutions of the platinum complex (0.39–50 μg mL−1) were prepared. The tested compound’s solutions were added in triplicate and incubated for 24, 48 and 72 h. Thereafter, the MTT solution was added to the cells. The medium was aspirated following the incubation period, and the DMSO solution was added to the wells. Absorption was calculated on a microplate reader for each well under a wavelength of 492 nm. Regression analysis was conducted to extract the concentration for 50% inhibition of cell growth (IC50).
3.4. Double Staining with Acridine Orange (AO)–Propidium Iodide (PI)
The ability of the platinum complex to induce apoptosis was investigated using AO/PI. In brief, after 24 h of seeding cells on 12-well glass slides, they were exposed to the IC50 preparations of the tested compound for 24, 48, and 72 h. Dual fluorescent dyes were inserted into each well after washing twice with PBS. To observe the cells, a fluorescent microscope was used [33].
3.5. Nucleic Acid Fragmentation Induction
DNA fragmentation analysis was performed based on the method in the kit guide of magnesium tissue culture for DNA extraction. Cell lines were exposed to IC50 concentrations of the compounds for 48 and 72 h. Cells with a density of 1.5 × 106 cell mL−1 were used for this experiment. After reaching the confluence, the cells were detached and suspended in PBS. Centrifugation (1200 rpm, 4 °C, and 10 min) was performed to remove the media, followed by dissolving with DNA loading buffer and enforcement to agarose gel. Electrophoresis was run following staining with ethidium bromide. Nucleic acid fragmentation was observed by using a UV illuminator device [34].
3.6. Cell Cycle Investigation via Flow Cytometry
Flow cytometry was used to investigate the cell cycle in exposed ovarian adenocarcinoma and colorectal adenocarcinoma (Caov-3 and HT-29) cell lines treated with the Pt complex. In brief, cells (5 × 104 cells mL−1) were exposed to the IC50 concentration of the Pt complex for the three-time duration (24, 48, and 72 h). After cell fixation, washing with PBS was conducted to remove excess ethanol. Propidium iodide (PI; 10 mg mL−1) was used to stain the exposed cells for 1 h at 37 °C. RNase A (10 mg mL−1) was employed to prevent the PI stain from binding with DNA molecules. The DNA content of the treated cells was investigated by flow cytometry [35].
3.7. Potential Assay of the Mitochondrial Membrane
Rh123, a fluorescent dye repossessed by lively mitochondria without cytotoxicity, determines the critical apoptotic events in cells treated with the Pt complex. This dye was used before and after treatment with the Pt complex to study the membrane potential of mitochondrial cells. In brief, 24 h seeding of cells in 96-well plates was followed by treatment with the IC50 dose of the extracted active compound and staining with Rh123 dye at 2.5 M for 1 h at 37 °C. The cells were detached by 0.5% trypsin-EDTA and then centrifuged at 300 rpm for 5 min. Cells were suspended in FACS buffer and calculated by flow cytometry, and histograms were created [36].
3.8. Immunofluorescence Assay
In brief, cells were first processed by washing with PBS, fixation with PFA (4%, 30 min, RT), permeabilization with Triton-X (0.5%, 30 min, RT), and blocking with normal goat serum (10%, 30 min). Subsequently, cells were treated with 1 µg mL−1 of each of the primary antibodies of anti-p53 and anti-caspase-8 (24 h at 4 °C) and washed with PBS three times. Cells were treated with 1 mg mL−1 of each of the secondary antibodies (Alexa Fluor 488-conjugated goat anti-rabbit IgG or Alexa Fluor 568-conjugated goat anti-mouse IgG) for 2 h at RT. After additional washing of the cells three times using PBS, they were examined under a fluorescent microscope [37].
3.9. Statistical Analysis
Unpaired t-test (GraphPad Prism 6, San Diego, CA, USA) was employed to statistically process the data, which were shown as the mean ± SEM of the three replicates per experiment [38].
4. Conclusions
This work provides a model of a Pt complex with thiocarbohydrazone ligand playing a dianionic role in chelating platinum. The ligand provides two tridentate binding pockets that could be of the NNN or NNS sort based on the geometry of the ligand, due to the existence of two indolic rings in the framework. The platinum(II) complex had extraordinary cytotoxic effects on ovarian adenocarcinoma and colorectal adenocarcinoma and provided a confirmation to a role of intrinsic pathways in the induction of apoptosis in these cells. Additionally, further in vitro and in vivo experiments are needed to fully evaluate the potential of this compound in terms of the optimal dose, route of administration, and usefulness as an efficient chemotherapeutic drug in treating different types of cancers.
Acknowledgments
The authors extend their appreciation to “Research Center of the Female Scientific and Medical Colleges”, Deanship of Scientific Research, King Saud University for Supporting this project. Also, the authors extend their appreciation to the Sulaimani Polytechnic University, University of Babylon, University of Baghdad, University of Misan, and the University of Technology for their technical support.
Author Contributions
Conceptualization, A.A.I., M.M.K. and T.H.A.-N.; methodology, T.A.-M., A.A.A. and S.A.; software, M.J., Z.J.T. and U.I.S.; validation, T.A.-M., A.A.A. and S.A.; formal analysis, A.A.I., M.M.K., T.H.A.-N. and G.M.S.; investigation, S.A., M.J., Z.J.T. and U.I.S.; resources, T.A.-M., A.A.A. and S.A.; data curation, A.A.I., M.M.K. and T.H.A.-N.; writing—original draft preparation, A.A.I., M.M.K. and T.H.A.-N.; writing—review and editing, T.A.-M., A.A.A., S.A. and G.M.S.; visualization, M.J., Z.J.T. and U.I.S.; supervision, A.A.I., G.M.S. and M.J.; project administration, G.M.S. and M.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO . Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2019. Volume 21. World Health Organization; Geneva, Switzerland: 2020. pp. 1–80. [Google Scholar]
- 2.Thomas R.K., Baker A.C., DeBiasi R.M., Winckler W., LaFramboise T., Lin W.M., Wang M., Feng W., Zander T., MacConaill L.E., et al. High-throughput oncogene mutation profiling in human cancer. Nat. Genet. 2007;39:347–351. doi: 10.1038/ng1975. [DOI] [PubMed] [Google Scholar]
- 3.Barnard C. Platinum anti-cancer agents. Platin. Met. Rev. 1989;33:162–167. [Google Scholar]
- 4.Bruijnincx P.C., Sadler P.J. Controlling platinum, ruthenium, and osmium reactivity for anticancer drug design. Adv. Inorg. Chem. 2009;61:1–62. doi: 10.1016/S0898-8838(09)00201-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Warad I., Eftaiha A.A.F., Al-Nuri M.A., Husein A.I., Assal M., Abu-Obaid A., Al-Zaqri N., Hadda T.B., Hammouti B. Metal ions as antitumor complexes—Review. J. Mater. Environ. Sci. 2013;4:542–557. [Google Scholar]
- 6.Barnes J.L., Zubair M., John K., Poirier M.C., Martin F.L. Carcinogens and DNA damage. Biochem. Soc. Trans. 2018;46:1213–1224. doi: 10.1042/BST20180519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Florea A.-M., Büsselberg D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apps M.G., Choi E., Wheate N.J. The state-of-play and future of platinum drugs. Endocr Relat Cancer. 2015;22:R219–R233. doi: 10.1530/ERC-15-0237. [DOI] [PubMed] [Google Scholar]
- 9.Ellahioui Y., Prashar S., Gomez-Ruiz S. Anticancer applications and recent investigations of metallodrugs based on gallium, tin and titanium. Inorganics. 2017;5:4. doi: 10.3390/inorganics5010004. [DOI] [Google Scholar]
- 10.Ndagi U., Mhlongo N., Soliman M.E. Metal complexes in cancer therapy–an update from drug design perspective. Drug des. Devel. Ther. 2017;11:599–616. doi: 10.2147/DDDT.S119488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paschke R., Kalbitz J., Paetz C., Luckner M., Mueller T., Schmoll H.-J., Mueller H., Sorkau E., Sinn E. Cholic acid–carboplatin compounds (CarboChAPt) as models for specific drug delivery: Synthesis of novel carboplatin analogous derivatives and comparison of the cytotoxic properties with corresponding cisplatin compounds. J. Inorg. Biochem. 2003;94:335–342. doi: 10.1016/S0162-0134(03)00024-2. [DOI] [PubMed] [Google Scholar]
- 12.Wheate N.J., Walker S., Craig G.E., Oun R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010;39:8113–8127. doi: 10.1039/c0dt00292e. [DOI] [PubMed] [Google Scholar]
- 13.AlSaady T.A., Kaiser N.M., Hamid N.O. Effect of antioxidants on cisplatin-induced cytotoxicity and oxidative stress in colon cancer cells. EurAsian J. BioSci. 2020;14:5375–5382. [Google Scholar]
- 14.Boulikas T. Clinical overview on Lipoplatin™: A successful liposomal formulation of cisplatin. Expert Opin. Investig. Drugs. 2009;18:1197–1218. doi: 10.1517/13543780903114168. [DOI] [PubMed] [Google Scholar]
- 15.Gomez-Ruiz S., Maksimović-Ivanić D., Mijatović S., Kaluđerović G.N. On the discovery, biological effects, and use of cisplatin and metallocenes in anticancer chemotherapy. Bioinorg. Chem. Appl. 2012;2012:140284. doi: 10.1155/2012/140284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonaccorso C., Marzo T., La Mendola D. Biological applications of thiocarbohydrazones and their metal complexes: A perspective review. Pharmaceuticals. 2020;13:4. doi: 10.3390/ph13010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ibrahim A.A., Khaledi H., Ali H.M. A multiprotic indole-based thiocarbohydrazone in the formation of mono-, di-and hexa-nuclear metal complexes. Polyhedron. 2014;81:457–464. doi: 10.1016/j.poly.2014.06.057. [DOI] [Google Scholar]
- 18.Van Simaeys D., López-Colón D., Sefah K., Sutphen R., Jimenez E., Tan W. Study of the molecular recognition of aptamers selected through ovarian cancer cell-SELEX. PLoS ONE. 2010;5:e13770. doi: 10.1371/journal.pone.0013770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jain D., Patel N., Shelton M., Basu A., Roque R., Siede W. Enhancement of cisplatin sensitivity by NSC109268 in budding yeast and human cancer cells is associated with inhibition of S-phase progression. Cancer Chemother. Pharmacol. 2010;66:945–952. doi: 10.1007/s00280-010-1246-8. [DOI] [PubMed] [Google Scholar]
- 20.Riccardi C., Capasso D., Rozza G.M., Platella C., Montesarchio D., Di Gaetano S., Marzo T., Pratesi A., Messori L., Roviello G.N., et al. Synthesis, DNA binding studies, and antiproliferative activity of novel Pt(II)-complexes with an L-alanyl-based ligand. J. Inorg. Biochem. 2020;203:110868. doi: 10.1016/j.jinorgbio.2019.110868. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim A.A., Khaledi H., Hassandarvish P., Ali H.M., Karimian H. Indole-7-carbaldehyde thiosemicarbazone as a flexidentate ligand toward Zn II, Cd II, Pd II and Pt II ions: Cytotoxic and apoptosis-inducing properties of the Pt II complex. Dalton Trans. 2014;43:3850–3860. doi: 10.1039/c3dt53032a. [DOI] [PubMed] [Google Scholar]
- 22.Baharara J., Ramezani T., Hosseini N., Mousavi M. Silver nanoparticles synthesized coating with Zataria multiflora leaves extract induced apoptosis in hela cells through p53 activation. Iran. J. Pharm. Res. IJPR. 2018;17:627. [PMC free article] [PubMed] [Google Scholar]
- 23.Alarifi S., Ali H., Saad Alkahtani M.S.A. Regulation of apoptosis through bcl-2/bax proteins expression and DNA damage by nano-sized gadolinium oxide. Int. J. Nanomed. 2017;12:4541. doi: 10.2147/IJN.S139326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lobana T.S., Pannu A., Hundal G., Butcher R.J., Castineiras A. Synthesis and structures of monomeric [chloro (isatin-3-thiosemicarbazone) bis (triphenylphosphine)] copper (I) and dimeric [dichlorobis (thiophene-2-carbaldehyde thiosemicarbazone) bis (triphenylphosphine)] dicopper (I)] complexes. Polyhedron. 2007;26:2621–2628. doi: 10.1016/j.poly.2006.12.044. [DOI] [Google Scholar]
- 25.Phaniendra A., Jestadi D.B., Periyasamy L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015;30:11–26. doi: 10.1007/s12291-014-0446-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clark D.J. Nucleosome positioning, nucleosome spacing and the nucleosome code. J. Biomol. Struct. Dyn. 2010;27:781–793. doi: 10.1080/073911010010524945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukherjee S., Mitra I., Fouzder C., Mukherjee S., Ghosh S., Chatterji U., Moi S.C. Effect of Pt (II) complexes on cancer and normal cells compared to clinically used anticancer drugs: Cell cycle analysis, apoptosis and DNA/BSA binding study. J. Mol. Liq. 2017;247:126–140. doi: 10.1016/j.molliq.2017.09.104. [DOI] [Google Scholar]
- 28.Galluzzi L., Vitale I., Abrams J., Alnemri E., Baehrecke E., Blagosklonny M., Dawson T.M., Dawson V., El-Deiry W., Fulda S. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–120. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhawan D., Chadha V.D. Zinc: A promising agent in dietary chemoprevention of cancer. Indian J. Med. Res. 2010;132:676. [PMC free article] [PubMed] [Google Scholar]
- 30.Erasimus H., Gobin M., Niclou S., Van Dyck E. DNA repair mechanisms and their clinical impact in glioblastoma. Mutat. Res. Rev. Mutat. Res. 2016;769:19–35. doi: 10.1016/j.mrrev.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Jabir M.S., Saleh Y.M., Sulaiman G.M., Yaseen N.Y., Sahib U.I., Dewir Y.H., Alwahibi M.S., Soliman D.A. Green synthesis of silver nanoparticles using Annona muricata extract as an inducer of apoptosis in cancer cells and inhibitor for NLRP3 inflammasome via enhanced autophagy. Nanomaterials. 2021;11:384. doi: 10.3390/nano11020384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jabir M., Sahib U.I., Taqi Z., Taha A., Sulaiman G.M., Albukhaty S., Al-Shammari A., Alwahibi M., Soliman D., Dewir Y.H. Linalool-Loaded Glutathione-Modified Gold Nanoparticles Conjugated with CALNN Peptide as Apoptosis Inducer and NF-κB Translocation Inhibitor in SKOV-3 Cell Line. Int. J. Nanomed. 2020;15:9025. doi: 10.2147/IJN.S276714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sulaiman G.M., Waheeb H.M., Jabir M.S., Khazaal S.H., Dewir Y.H., Naidoo Y. Hesperidin loaded on gold nanoparticles as a drug delivery system for a successful biocompatible, anti-cancer, anti-inflammatory and phagocytosis inducer model. Sci. Rep. 2020;10:9362. doi: 10.1038/s41598-020-66419-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jabir M.S., Nayef U.M., Abdulkadhim W.K., Sulaiman G.M. Supermagnetic Fe3O4-PEG nanoparticles combined with NIR laser and alternating magnetic field as potent anti-cancer agent against human ovarian cancer cells. Mater. Res. Express. 2019;6:115412. doi: 10.1088/2053-1591/ab50a0. [DOI] [Google Scholar]
- 35.Sulaiman G.M. In vitro study of molecular structure and cytotoxicity effect of luteolin in the human colon carcinoma cells. Eur. Food Res. Technol. 2015;241:83–90. doi: 10.1007/s00217-015-2436-8. [DOI] [Google Scholar]
- 36.Alsaedi I.I., Taqi Z.J., Hussien A.M.A., Sulaiman G.M., Jabir M.S. Graphene nanoparticles induces apoptosis in MCF-7 cells through mitochondrial damage and NF-KB pathway. Mater. Res. Express. 2019;6:095413. doi: 10.1088/2053-1591/ab33af. [DOI] [Google Scholar]
- 37.Jabir M.S., Hussien A.A., Sulaiman G.M., Yaseen N.Y., Dewir Y.H., Alwahibi M.S., Soliman D.A., Rizwana H. Green synthesis of silver nanoparticles from Eriobotrya japonica extract: A promising approach against cancer cells proliferation, inflammation, allergic disorders and phagocytosis induction. Artif. Cells Nanomed. Biotechnol. 2021;49:48–60. doi: 10.1080/21691401.2020.1867152. [DOI] [PubMed] [Google Scholar]
- 38.Al-Musawi S., Albukhaty S., Al-Karagoly H., Sulaiman G.M., Jabir M.S., Naderi-Manesh H. Dextran-coated superparamagnetic nanoparticles modified with folate for targeted drug delivery of camptothecin. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020;11:045009. doi: 10.1088/2043-6254/abc75b. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.