We read with great interest the paper entitled “Impact of physical activity of cognitive functions: a new field for research and management of Cystic Fibrosis” by Elce et al. [1]. Physical activity (PA) was described as a non-pharmacological intervention found to be associated not only with important clinical outcomes, such as survival, but also with cognitive abilities, thus suggesting this field of investigation as a potential leverage to optimize the management of cystic fibrosis (CF). They provided a comprehensive summary of the evidence regarding the effects of PA on neurocognitive functions expected in patients with CF.
Considering the broad expression of the Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) protein in the adult nervous system [2,3,4,5], and the available findings from neuroimaging studies on chronic obstructive pulmonary disease (COPD), which is characterized by specific patterns of moderate-to-severe deficits in higher neuronal and complex visual-motor processes [6,7], in 2016, our multidisciplinary team started investigating the functional connectivity of patients with CF, combining neuropsychological assessment and resting-state functional magnetic resonance imaging (rs-fMRI) with the results of exercise testing.
The notion of rest as a passive state has been challenged by functional neuroimaging studies showing that sets of brain regions display coherent activity at rest [8]. One such network, comprising the medial pre-frontal cortex (MPFC), posterior cingulate cortex (PCC), precuneus, inferior parietal and lateral temporal cortices, has a topographical consistency across subjects that has led some authors to posit the existence of an active, organized, baseline mode of brain function that would constitute a fundamental default mode network (DMN) of brain function [9,10]. The widely distributed brain areas that compose the DMN include regions associated with both cognitive and affective functions, though it is increasingly recognized that traditionally defined areas often overlap the boundaries of brain architectonic areas [11].
The functional connectivity of DMN sub-regions via a seed-based correlation analysis of rs-fMRI data was investigated in 11 adult subjects with a confirmed diagnosis of CF homozygous for the Phe508del mutation. Balancing administration time and the need for a sensitive evaluation in a population known as cognitively unaffected, neuropsychological evaluation (NPE) covered six higher-function cognitive-domains: attention, memory, executive functions, language, perceptive and praxis. Furthermore, to evaluate exercise capacity, an incremental cycling protocol to volitional fatigue was performed without metabolic measurement [12], as described elsewhere [13].
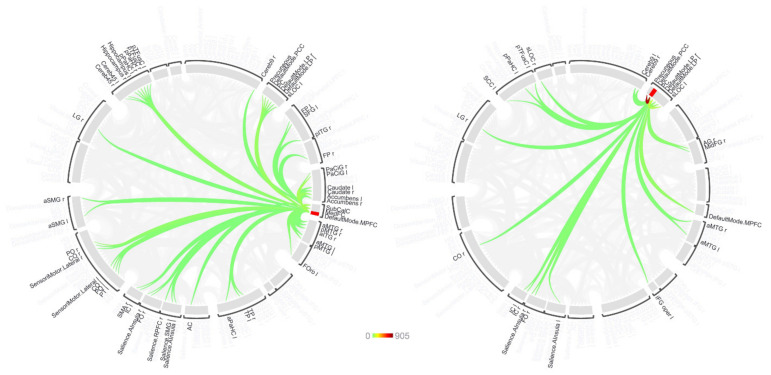
The functional connectivity between each seed region and other brain areas showed significant dependencies on exercise capacity and NPE components in the second-level rs-fMRI analysis after FDR-correction, suggesting that modulation of these connections is associated with exercise, the neuropsychological components, or both. Using the MPFC as the seed (Figure 1), functional connectivity with the frontal medial cortex, the left paracingulate gyrus and the subcallosal cortex showed the largest difference between normal and abnormal exercise responders, while accounting for the effects of two neuropsychological components, summarized as working memory and depression and/or anxiety, and the subjective perception of health and the visuo-spatial functions.
Figure 1.
Results of second-level rs-fMRI analysis for seeds in the MPFC and PCC (left and right, respectively) displayed as a connectome ring. Around the ring, 165 brain regions are indicated that were evaluated for connectivity with the seed in the first-level analysis. The coloured traces represent connections that were seen in second-level analysis to be significantly associated with a combination of exercise performance and first principal component of the neuropsychological evaluation results after corrected for multiple comparison (FDR, p < 0.05). The rings were plotted with a common scale (coloured bar) based on the largest F-statistic encountered in this cohort (PCC-precuneus).
Similarly, the precuneus, the posterior cingulated gyrus, and DMN regions parietal lobe (PL)-right and PL-left showed the largest differences in functional connectivity with the PCC seed. The F-values for connectivity differences to the PCC tended to be greater than those seen for the other seeds, with the differences in connectivity between the PCC and the precuneus having the largest F-values encountered. For PL-left seeding, the lateral occipital left and other components of the DMN (specifically PL-right and PCC) showed the largest effect, while for PL-right seeding the largest differences were seen in the DMN regions PL-left and PCC, the right lateral occipital cortex, the precuneus and the left lateral occipital.
Our preliminary data document a significant association between the posterior cingulate and MPFC. There is a consensus in the literature concerning the existence of an anterior and a posterior part of the DMN, each having an area that may be termed its main hub. The MPFC is the anterior hub, and the posterior cingulate cortex—precuneus (PCC/Precuneus) in the posterior DMN [14]. Anterior regions of the network such as the medial prefrontal cortex have been associated with self-referential processing [15], and participate in close functional coupling between MPFC and PCC [16].
The connectivity of the MPFC correlated with the exercise capacity and NPE components and included frontal medial cortex, left paracingulate gyrus and subcallosal cortex, all of which belong to the executive control network [17]. The human inferior PL-left plays a pivotal role in many cognitive functions [18].
The present findings also suggest a particularly strong effect of exercise and NPE domains on the connectivity of the PCC and precuneus, with the PCC showing a greater association with exercise response and NPE components than we found for the other seed regions. The PCC and precuneus are major nodes within the DMN and have high metabolic activity and dense structural connectivity to widespread brain regions, which suggests that it has a role as a cortical hub [19]. Previous studies demonstrated the involvement of the precuneus in the manipulation of mental images and in internally guided attention derived from visuo-spatial imagery studies; these findings implicate its unique capacity in mental representation of the internal self [20]. These results are consistent with this notion, considering the involvement of the precuneus in the altered functional connectivity of both the PCC and PL-right. Although we cannot presently describe the mechanisms determining the observed modulation of connectivity, our findings suggest a relation between exercise and neuropsychological functions in patients with CF at the level of brain connectivity that is worth further exploration. If confirmed by larger and more heterogenous studies, these findings could provide interesting food for thought concerning daily clinical practice. For example, the existence of a linkage between emotions and the higher functions, both necessary to carry out a complex daily care plan such as that for the treatment of CF, might suggest the importance of ensuring that patients have enough time to memorize long lists of information and therapies, because it will likely influence adherence. Also, a higher level of exercise capacity may translate not only into a life-prolonging effect, but also into a neurocognitive enhancement advantage. In CF, very few studies have focused on neurocognitive or neuropsychological aspects combined with PA. The recent paper by Gold et al. [21] reported interesting results involving a mixed cohort of children with and without CF undergoing lung transplantation, whereas our sample included adults in a very narrow range of age, linking together PA, neuropsychological data and brain connectivity.
To the best of our knowledge, this report on a small cohort is the first to illustrate characteristics of rs-fMRI connectivity in individuals with CF. The observed modulation of brain networks under resting-state conditions may pave the way for future investigations aimed to support the relevance of physical activity for the well-being of persons with CF and the need for a through-targeted neuropsychological evaluation. As Elce et al. concluded in their review, the effects of exercise on mental health represent a promising and unexplored research field in CF. As a low-cost intervention, physical activity would be important not only in relation to lung health, but also to the cognitive functions that drive the choices we make in everyday life.
Acknowledgments
We are grateful to the patients who bravely embraced this research project, dedicating their valuable spare time to the advancement of knowledge of the CF disease. Special thanks go to Federica Carta (PT, Cystic Fibrosis Centre, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico) for her support. Additional thanks to the entire multidisciplinary team of Carla Colombo.
Author Contributions
Conceptualization, S.G., R.M.N. and A.C.; methodology, S.G., R.M.N. and A.C.; software, R.B. and P.E.S.; formal analysis, S.G. and P.E.S.; investigation, S.G., R.M.N., C.C. and A.C.; resources, R.B., P.E.S., C.C. and A.C.; data curation, R.B. and P.E.S.; writing—original draft preparation, S.G., R.M.N., P.E.S. and A.C.; writing—review and editing, C.C. and P.E.S.; supervision, C.C. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico (ECONECTIVITY 1438/2016 nr. 2297).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elce V., Del Pizzo A., Nigro E., Frisso G., Martiniello L., Daniele A., Elce A. Impact of Physical Activity on Cognitive Functions: A New Field for Research and Management of Cystic Fibrosis. Diagnostics. 2020;10:489. doi: 10.3390/diagnostics10070489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mulberg A.E., Weyler R.T., Altschuler S.M., Hyde T.M. Cystic fibrosis transmembrane conductance regulator expression in human hypothalamus. Neuroreport. 1998;9:141–144. doi: 10.1097/00001756-199801050-00028. [DOI] [PubMed] [Google Scholar]
- 3.Shepherd R.W., Holt T.L., Vasques-Velasquez L., Coward W.A., Prentice A., Lucas A. Increased energy expenditure in young children with cystic fibrosis. Lancet. 1988;1:1300–1303. doi: 10.1016/S0140-6736(88)92119-8. [DOI] [PubMed] [Google Scholar]
- 4.Marcorelles P., Friocourt G., Uguen A., Ledé F., Laquerrière A. Cystic Fibrosis Transmembrane Conductance Regulator Protein ( CFTR ) Expression in the Developing Human Brain: Comparative Immunohistochemical Study between Patients with Normal and Mutated CFTR. J. Histochem. Cytochem. 2014;62:791–801. doi: 10.1369/0022155414546190. [DOI] [PubMed] [Google Scholar]
- 5.Guo Y., Su M., Mcnutt M.A., Gu J. Expression and Distribution of Cystic Fibrosis Transmembrane Conductance Regulator in Neurons of the Human Brain. J. Histochem. Cytochem. 2009;57:1113–1120. doi: 10.1369/jhc.2009.953455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esser R.W., Stoeckel M.C., Kirsten A., Watz H., Taube K., Lehmann K., Petersen S., Magnussen H., Leupoldt A. Von Structural Brain Changes in patients with Chronic Obstructive Pulmonary Disease. Chest. 2016;149:426–434. doi: 10.1378/chest.15-0027. [DOI] [PubMed] [Google Scholar]
- 7.Parshall M.B., Schwartzstein R.M., Adams L., Banzett R.B., Manning H.L., Bourbeau J., Calverley P.M., Gift A.G., Harver A., Lareau S.C., et al. An official American thoracic society statement: Update on the mechanisms, assessment, and management of dyspnea. Am. J. Respir. Crit. Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Papo D. Why should cognitive neuroscientists study the brain’s resting state? Front. Hum. Neurosci. 2013;7:45. doi: 10.3389/fnhum.2013.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole D.M., Smith S.M., Beckmann C.F. Advances and pitfalls in the analysis and interpretation of resting-state FMRI data. Front. Syst. Neurosci. 2010;4:8. doi: 10.3389/fnsys.2010.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buckner R.L., DiNicola L.M. The brain’s default network: Updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 2019;20:593–608. doi: 10.1038/s41583-019-0212-7. [DOI] [PubMed] [Google Scholar]
- 12.Hebestreit H., Arets G.M., Boas S. Statement on Exercise Testing in Cystic Fibrosis. Respiration. 2015;90:332–351. doi: 10.1159/000439057. [DOI] [PubMed] [Google Scholar]
- 13.Gambazza S., Guarise R., Carta F., Ambrogi F., Mirabella M., Brivio A., Colombo C. Exercise capacity and ventilation inhomogeneity in cystic fibrosis: A cross-sectional study. Pediatr. Pulmonol. 2020;55:394–400. doi: 10.1002/ppul.24525. [DOI] [PubMed] [Google Scholar]
- 14.Whitfield-Gabrieli S., Ford J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012;8:49–76. doi: 10.1146/annurev-clinpsy-032511-143049. [DOI] [PubMed] [Google Scholar]
- 15.Gusnard D.A., Akbudak E., Shulman G.L., Raichle M.E. Medial Prefrontal Cortex and Self-Referential Mental Activity: Relation to a Default Mode of Brain Function. Proc. Natl. Acad. Sci. USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brewer J.A., Garrison K.A., Whitfield-Gabrieli S. What about the “ self ” is processed in the posterior cingulate cortex ? Front. Hum. Neurosci. 2013;7:1–7. doi: 10.3389/fnhum.2013.00647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang J., Xie S., Guo X., Becker B., Fox P.T., Eickhoff S.B., Jiang T. Correspondent Functional Topography of the Human Left Inferior Parietal Lobule at Rest and Under Task Revealed Using Resting-State fMRI and Coactivation Based Parcellation. Hum. Brain Mapp. 2017;38:1659–1675. doi: 10.1002/hbm.23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leech R., Braga R., Sharp D.J. Echoes of the Brain within the Posterior Cingulate Cortex. J. Neurosci. 2012;32:215–222. doi: 10.1523/JNEUROSCI.3689-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Utevsky A.V., Smith D.V., Huettel S.A. Precuneus Is a Functional Core of the Default-Mode Network. J. Neurosci. 2014;34:932–940. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold A., Young J.M., Solomon M., Grasemann H. Neuropsychological outcomes following pediatric lung transplantation. Pediatr. Pulmonol. 2020;55:2427–2436. doi: 10.1002/ppul.24915. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.