Abstract
Hereditary transthyretin amyloidosis (ATTRv, v for variant) prevalence in Italy, a non-endemic region, has been established by ATTRv amyloidosis Italian Registry. However, values of prevalence were extremely heterogeneous, considering different regions. To properly establish the prevalence of the disease in the Lazio region, a survey was sent to university regional hospitals and to main regional hospitals, in order to collect all affected patients regularly followed. We identified 100 ATTRv patients and, considering a Lazio population of 5.8/million, we estimated a ATTRv prevalence of 17.2/million. The ATTRv amyloidosis Italian Registry reported a prevalence of 8.0/million in Lazio, while our survey showed a value of double this. Our survey documented a high-prevalence for a non-endemic country. The increased awareness of the disease among general practitioners and medical specialists is a fundamental step to reduce the diagnostic delay and start an effective treatment of this disease.
Keywords: ATTRv, prevalence, amyloid
1. Introduction
Hereditary transthyretin amyloidosis (ATTRv, v for variant) is a severe, heterogeneous multisystem condition with prevalent peripheral nervous system impairment, due to mutations in the transthyretin (TTR) gene [1,2]. The condition, presenting as an adult-onset, autosomal-dominant disease with variable penetrance, is characterized by extracellular deposition of amyloid fibrils in different organs [1,2]. Besides the peripheral nerves, the heart, kidney, gastro-intestinal system, and eyes may also be involved, leading to a life-threatening, multisystem disease with huge clinical variability and course, and death within 10 years on average [1,2].
Depending on the geographic distribution, a wide variability in age at onset and clinical presentation of ATTRv is described [1,3]. Generally, patients from endemic areas, such as Portugal, have an early-onset (<50 years) disease with initial involvement of small nerve fibers, while in non-endemic areas, patients present with a late-onset (>50 years) progressive axonal polyneuropathy [1,3]. Recently, the prevalence in Italy, a non-endemic region, has been established by the ATTRv amyloidosis Italian Registry [4]. However prevalence varies significantly in different regions [4].
2. Materials and Methods
To properly establish the prevalence of ATTRv in the Lazio region a survey was sent to University regional hospitals and to several regional hospitals (Fondazione Policlinico A. Gemelli-IRCCS, Umberto I Hospital, Sant’Andrea Hospital, Fatebenefratelli Hospital, Tor Vergata Hospital, San Camillo Forlanini Hospital, San Filippo Neri Hospital, Campus Biomedico Hospital, and ICOT Hospital), including all referral Centres for ATTRv, in order to collect all affected patients who are in regular follow-up. Gender, current age, mutation, type of onset (early vs. late), presence of familial history, phenotype (neurological; cardiologic; or mixed), and geographical origin of the family were collected. We also requested the number of pre-symptomatic carriers followed in each Centre.
3. Results
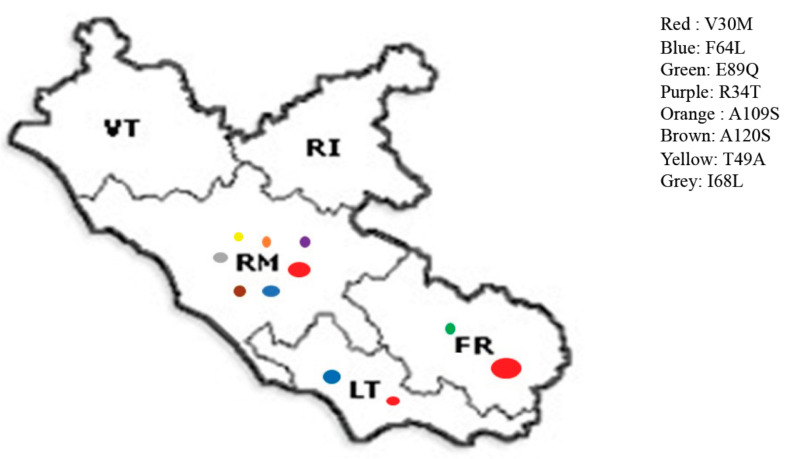
The survey results are summarized in Table 1. All Centres replied to survey. We identified 100 ATTRv patients and, considering the Lazio population of 5.8/million, an ATTRv prevalence of 17.2/million was estimated. The most common clinical phenotype was neurological or mixed. A positive familial history was retrieved in only half of the patients. Three mutations were more frequently observed in the region (Figure 1). Half of the patients carried the V30M, and the majority of these cases came from Lazio. A quarter of the patients had the F64L mutation and all of these had families coming from southern Italy. The third most common mutation was E89Q (14%), all with ancestry from Sicily. We also identified 73 pre-symptomatic carriers.
Table 1.
Demographic and clinical characteristics of hATTR Lazio patients.
| All Patients | V30M | F64L | E89Q | R34T | I68L | A120S | A109S | T49A | |
|---|---|---|---|---|---|---|---|---|---|
| Number of patients | 100 | 53 (53%) | 23 (23%) | 14 (14%) | 3 (3%) | 3 (3%) | 2 (2%) | 1 (1%) | 1 (1%) |
| M/F | 63/37 (1.7) | 37/16 (2.3) | 1/22 (0.04) | 5/9 (0.55) | 0/3 (0) | 2/1 (2) | 0/2 (0) | M | F |
| Age (mean; | 69.42; | 69.7; | 71.0; | 69.4; | 51.7; | 74.3; | 70; | 78 | 54 |
| median; | 70.5; | 70; | 72; | 70.5; | 58; | 73; | 70; | ||
| standard deviation; | 10.9; | 10.9; | 8.2; | 13.4; | 10.9; | 9.1; | 5.8; | ||
| range) | 39–87 | 47–87 | 51–83 | 43–84 | 39–58 | 73–84 | 66–74 | ||
| Early onset | 17 (17%) | 8 (15%) | 3 (13%) | 3 (21.4%) | 2 (66.7%) | 1 (33.3%) | 0 | Late onset | Late onset |
| vs. | vs. | vs. | vs. | vs. | vs. | vs. | vs. | ||
| Late onset | 83 (83%) | 45 (85%) | 20 (87%) | 11 (78.6%) | 1 (33.3%) | 2 (66.7%) | 2 (100%) | ||
| Family history | 58 (58%) | 35 (66%) | 11 (47.8%) | 4 (28.6%) | 2 (66.7%) | 3 (100%) | 2 (100%) | yes | not available |
| Phenotype | Cardiologic: | Cardiologic: | Cardiologic: | Cardiologic: | Cardiologic: | Cardiologic: | Cardiologic: | Neuropathic | Neuropathic |
| 7 (7%) | 1 (1.9%) | 0 | 1 (7.1%) | 2 (66.7%) | 3 (100%) | 0 | |||
| Neuropathic: | Neuropathic: | Neuropathic: | Neuropathic: | Neuropathic: | Neuropathic: | Neuropathic: | |||
| 45 (45%) | 28 (52.8%) | 12 (52.17%) | 2 (14.3%) | 0 | 0 | 1 (50.0%) | |||
| Mixed: | Mixed: | Mixed: | Mixed: | Mixed: | Mixed: | Mixed: | |||
| 48 (48%) | 24 (45.3%) | 11 (47.8%) | 11 (78.6%) | 1 (33.3%) | 0 | 1 (50.0%) | |||
|
Familial origin
from Italy (Northern vs. Centre vs. Southern) |
Northern: 0 | Northern: 0 | Northern: 0 | Northern: 0 | Northern: 0 | Northern: 0 | |||
| Centre: | Centre: | Centre: | Centre: | Centre: | Centre: | ||||
| 47 (47%) | 42 (79.2%) | 2 (8.7%) | 0 | 1 (33.3%) | 2 (100%) | ||||
| (Lazio: 46%) | (Lazio: 77.4%) | (Lazio: 8.7%) | (Lazio: 33.3%) | (Lazio: 100%) | |||||
| Southern: | Southern: | Southern: | Southern: | Southern: | Southern: | Southern | |||
| 36 (36%) | 4 (7.5%) | 17 (74%) | 12 (85.7%) | 2 (66.7%) | 0 | (Sicily) | |||
| (Sicily: 19%; Campania: | (Campania: 5.6%) | (Calabria: 21.7%; | (Sicily: 85.7%) | (Sicily: 66.7%) | |||||
| 6%; Calabria: 6%; | Apulia: 21.7%; Sicily: | ||||||||
| Puglia: 5%) | 17.4%; | ||||||||
| Campania: 13.0%) | |||||||||
| Abroad: 1 (1%) | Abroad: 1 (1.9%) | ||||||||
| Not available: | Not available: | Not available: | Not available: | Not available | Not available | ||||
| 16 (16%) | 6 (11.3%) | 4 (17.39%) | 2 (24.3%) |
Legend to the Table: Northern Italy includes Lombardy, Piedmont, Veneto, Alto-Adige, Liguria, Emilia-Romagna, and Tuscany; central Italy includes Lazio, Abruzzo, and Molise; southern Italy includes Campania, Sicily, Apulia, and Calabria. Most frequent regions of familial origin are specified.
Figure 1.
Distribution of TTR mutations in Lazio region among different provinces. Diameter of circles is proportional to number of patients.
4. Discussion
Prevalence of ATTRv is extremely variable around the world [4]. High prevalence was reported in endemic countries (such as Portugal or Sweden) with the highest prevalence reported in northern Portugal (1631.2/million) and northern Sweden (1040/million) [1,5]. Considering non-endemic countries, a prevalence of 7.52/million was reported in France, while a prevalence of 1.48/million was found in Germany. [5,6] In Italy, the prevalence estimated by the ATTRv amyloidosis Italian Registry is 4.33/million, with considerable differences among regions, varying from 2.5/million in Piedmont to 9.3/million in Sicily [4].
The ATTRv amyloidosis Italian Registry reported a prevalence of 8.0/million in Lazio, while our survey showed a doubled value [4]. It is likely our survey, involving more hospitals (not always included in the Registry), was able to catch almost all diagnosed patients followed in our region. However, the real number of ATTRv in our region could be also higher, considering not only overlooked diagnoses but also pre-symptomatic carriers regularly followed in each Centre.
Considering the different mutations, we found a high proportion of V30M mutation in Lazio; the great majority of the pedigree of these patients came from Lazio region, confirming the existence of an autochthonous cluster of V30M in this region. All V30M were distributed in the south of Lazio (Frosinone or Latina), and in the province of Rome. Interestingly, we did not find any ATTRv patient from the provinces of Rieti and Viterbo, in the north of Lazio region; however we cannot exclude overlooked diagnoses in this area.
We found two additional mutations (F64L and E89Q) widely distributed in Lazio. However, the pedigrees of these patients come from southern Italy, namely Sicily, for E89Q, confirming data reported by the ATTRv amyloidosis Italian Registry [4]. Migration of the southern Italian population to Rome in the recent years may explain these data.
5. Conclusions
ATTRv is rare and disabling disease, but today many therapies are available for this condition. Our survey confirmed the presence of a V30M cluster in Lazio, and reported a high-prevalence for a non-endemic country. The increased awareness of the disease among general practitioners and medical specialists is a fundamental step to reduce the diagnostic delay and start an effective treatment in ATTRv.
Author Contributions
Conceptualization, M.L. and V.G.; methodology, all authors; formal analysis, all authors; investigation, all authors; resources, all authors; data curation, all authors; writing—original draft preparation, M.L. and V.G.; writing—review and editing, all authors.; supervision, M.L. All authors have read and agreed to the published version of the manuscript.
Funding
The APC of this manuscript has been supported by an unrestricted grant by SOBI (Swedish Orphan Biovitrum).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Fondazione Policlinico Gemelli.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Marco Luigetti, received financial grants (honoraria and speaking) from Akcea, Alnylam and Pfizer, and travel grants from Akcea, Alnylam, Pfizer, Kedrion, and Grifols. Giovanni Antonini, received honoraria from Kedrion, Alnylam, Syneos, and Farmitalia; and travel grants from Kedrion, Pfeizer, Alnylam, Sanofi-Genzime, and Akcea. Roberto Massa received honoraria from Akcea, Alnylam, Genzyme, and Lundbeck. Elena Maria Pennisi received travel grants and honoraria from Akcea and Sanofi Genzyme, and research fund from Ultragenyx. Mario Sabatelli received financial grants (honoraria and speaking) from Akcea, and travel grants from Grifols. Other authors have no potential conflicts of interest to be disclosed. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Adams D., Koike H., Slama M., Coelho T. Hereditary transthyretin amyloidosis: A model of medical progress for a fatal disease. Nat. Rev. Neurol. 2019;15:387–404. doi: 10.1038/s41582-019-0210-4. [DOI] [PubMed] [Google Scholar]
- 2.Luigetti M., Romano A., Di Paolantonio A., Bisogni G., Sabatelli M. Diagnosis and treatment of hereditary transthyretin amyloidosis (hATTR) polyneuropathy: Current perspectives on improving patient care. Ther. Clin. Risk Manag. 2020;16:109–123. doi: 10.2147/TCRM.S219979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luigetti M., Conte A., Del Grande A., Bisogni G., Madia F., Lo Monaco M., Laurenti L., Obici L., Merlini G., Sabatelli M. TTR-related amyloid neuropathy: Clinical, electrophysiological and pathological findings in 15 unrelated patients. Neurol. Sci. 2013;34:1057–1063. doi: 10.1007/s10072-012-1105-y. [DOI] [PubMed] [Google Scholar]
- 4.Russo M., Obici L., Bartolomei I., Cappelli F., Luigetti M., Fenu S., Cavallaro T., Chiappini M.G., Gemelli C., Pradotto L.G., et al. ATTRv amyloidosis Italian Registry: Clinical and epidemiological data. Amyloid. 2020;27:259–265. doi: 10.1080/13506129.2020.1794807. [DOI] [PubMed] [Google Scholar]
- 5.Schmidt H.H., Waddington-Cruz M., Botteman M.F., Carter J.A., Chopra A.S., Hopps M., Stewart M., Fallet S., Amass L. Estimating the global prevalence of transthyretin familial amyloid polyneuropathy. Muscle Nerve. 2018;57:829–837. doi: 10.1002/mus.26034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parman Y., Adams D., Obici L., Galán L., Guergueltcheva V., Suhr O.B., Coelho T. European Network for TTR-FAP (ATTReuNET). Sixty years of transthyretin familial amyloid polyneuropathy (TTR-FAP) in Europe: Where are we now? A European network approach to defining the epidemiology and management patterns for TTR-FAP. Curr. Opin. Neurol. 2016;29(Suppl. 1):S3–S13. doi: 10.1097/WCO.0000000000000288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.