Abstract
Globally, fungal inocula are being explored as agents for the optimization of composting processes. This research primarily evaluates the effects of inoculating organic vegetable heaps with the entomopathogenic fungus Clonostachys rosea f. catenula (Hypocreales) on the biophysicochemical properties of the end-product of composting. Six heaps of fresh cabbage (Brassica oleracea var. capitata) waste were inoculated with C. rosea f. catenula conidia and another six were not exposed to the fungus. The composted materials from the fungus- and control-treated heaps were subsequently used as a medium to cultivate tomatoes (Solanum lycopersicum). The biophysicochemical characteristics of the composted materials were also assessed after composting. In addition, the protective effect of the fungal inoculum against red spider mite (Tetranychus urticae) infestations in the tomatoes was evaluated through the determination of conidial colonization of the plant tissue and the number of plants infested by the insect. Furthermore, phytotoxicity tests were carried out post experiment. There were few significant variations (p < 0.05) in heap temperature or moisture level between treatments based on the weekly data. We found no significant differences in the levels of compost macronutrient and micronutrient constituents. Remarkably, the composted materials, when incorporated into a growth medium from fungus-treated heaps, induced a 100% endophytic tissue colonization in cultivated tomato plants. While fewer red spider mite infestations were observed in tomato plants grown in composted materials from fungus-treated heaps, the difference was not significant (χ2 = 0.96 and p = 0.32). The fungal treatment yielded composted materials that significantly (p < 0.05) enhanced tomato seed germination, and based on the phytotoxicity test, the composted samples from the heaps exposed to the C. rosea f. catenula inoculum were not toxic to tomato seeds and seedlings. In conclusion, this study showed that C. rosea f. catenula improved the quality of composted materials in terms of fungal endophytism and seed germination.
Keywords: composting, organic vegetable wastes, inoculation, Clonostachys rosea f. catenula, tissue nutrient content, toxicity
1. Introduction
Numerous drawbacks, including farming practices, transportation, storage of perishable food crops, inefficient disposal, and pollution [1], are associated with the poor management of organic wastes. It is, therefore, not surprising that it has led to an increased exploration into alternative solutions to the management and beneficiation of organic wastes. Compost is an eco-friendly natural material obtained when organic plant residues are reduced to organic fertilizer and soil conditioners through a biological process known as composting [2,3]. Since fungi and bacteria are integral components of composting, it is possible to optimize the quality, biophysicochemical constituents, and yield of the end-product of composting through manipulations of fungal content in composting materials.
Reports over the years on the potential enhancement effects of entomopathogenic microbes on composting processes, though few, have opened new opportunities for managing solid organic wastes through the use of fungal inocula. Fungal species are present during the mesophilic and thermophilic stages of composting [4]. Dead organic materials serve as their host and provide energy to the fungi responsible for the breakdown of organic matter [5,6], and in the process, the fungal saprophytes enhance the biodegradation of the recalcitrant lignocellulose of plant-based organic matter [7]. Hence, certain saprophytic fungi play a vital role in the decomposition and conversion process during composting [5,8,9,10]. Interestingly, entomopathogenic fungi have also been reported to produce lignocellulolytic enzymes, such as endoproteases, aminopeptidases, esterases, and chitinases [11], all of which play crucial roles during composting [12,13]. It is therefore not surprising that entomopathogenic fungal species have been grouped among microorganisms that biodegrade or mineralize organic materials [6].
A literature survey showed that there is very little information available on fungal inoculants and their ability to optimize the biodegradation of solid raw materials to humus. For example, four mesophilic fungi, Aspergillus niger, Aspergillus sp. (R.), Thrichoderma viride, and Penicillium sp., that were inoculated into compost materials consisting of jowar (Sorghum bicolor) stalk, wheat (Triticum aestivum) straw (5:3), and jamun (Syzygium cumini) leaves, reduced the composting duration by one month and improved the quality of the composted end-product [14]. Three bacterial isolates have also been reported to improve the chemical characteristic of end-products obtained from composting [15]. Inspiring findings, such as the aforementioned, have motivated researchers to further investigate the mechanisms through which saprophytic fungi influence the decomposition of raw organic wastes, as well as the quality of the end-products of composting. Evidently, this knowledge will improve our understanding and utilization of the fungus–plant residue relationship for sustainable waste management. Some fungal entomopathogens have been reported to enhance plant growth and plant resistance to phytopathogens and herbivores [16]. Moreover, these fungi can persist in soils and growth media, while offering protection to plants and improving plant nutrition [17,18]. They also produce hormones, signaling factors [19], and nutrients [20] that can enhance plant growth.
Solid organic vegetable waste and spoilage is a serious challenge faced by many developing countries [15]. These types of residues are recalcitrant and difficult to decompose. The addition of conidia of entomopathogenic fungal endophytes to raw composting materials could potentially enhance the composting process, resulting in improved biophysicochemical characteristics, such as a high nutrient content and compost maturity. These fungi can also contribute to the production of high quality compost materials that can improve plant growth and health, and protect plants against arthropod pests; thus, contributing to the sustainable management and utilization of organic wastes. One well-known entomopathogenic fungal endophyte is Clonostachys rosea, a soil-borne saprophyte that belongs to the phylum Ascomycota and order Hypocreales. Clonostachys rosea has some interesting traits that can affect the biophysicochemical properties of composts, as well as the composting of organic wastes. For example, the fungus can biotransform bioactive compounds, breakdown starch and plastics, and produce extracellular enzymes, secondary metabolites, and hydrocarbon [21,22]. Clonostachys rosea f. catenula is a potentially ligninolytic fungus [23] and a versatile antagonist against fungal pathogens of plants [24]. The objective of this study was to evaluate the effect of C. rosea f. catenula inoculation on the biophysicochemical properties of compost, endophytic activity, phytotoxicity of composted materials, and the protective effect of the composted material containing the fungus against the insect infestation of potted tomatoes (Solanum lycopersicum L.) by Tetranychus urticae (Acari).
2. Materials and Methods
2.1. Collection of Solid Waste Materials and Preparation of Compost Heaps
Disposed fresh cabbage (Brassica oleracea var. capitate) wastes were collected from two farms (Groente Verpakkers and Schultz Vars Produkte Mark) situated in the Philippi horticultural area, Cape Town, South Africa. The collection of waste was done in the mornings (between 08:00 and 12:00), and sanitary hand gloves were used to avoid contamination. The collected wastes were placed in black polythene bags. The wastes were chopped (approx. size 2–4 cm2), and then thoroughly mixed to form a single big heap. Subsequently, the big heap was sub-divided into 12 equal heaps of 1 m diameter and 50 cm height.
2.2. Fungal Isolate
An indigenous C. rosea f. catenula strain (SM4A), which was originally isolated from a soil sample collected from a vineyard in the Cape Winelands region [25], was used for this study. Cultures of the fungus are being maintained at Cape Peninsula University of Technology in Bellville, South Africa. Pure sub-cultures of the fungus were cultivated in half-strength Potato Dextrose Agar (PDA) containing 0.02 g/L of ampicillin (Sigma-Aldrich) and 0.04 g/L of streptomycin (Sigma-Aldrich (Pty) Ltd., Johannesburg, South Africa) in 9 and 14 cm diameter Petri dishes and incubated in the laboratory at 25 °C for 12: 12 h L:D. Four-week-old (28 days) C. rosea f. catenula conidia obtained from the PDA plates were used for inoculation of the composting materials. Mature conidia were harvested by gently scraping them off the surface of an agar plate with a spatula and transferring them onto a sterile plastic container. The conidia were suspended in 2 L glass bottles containing sterile 0.01% (v/v) Tween 80 in distilled water. The bottles were capped and mixed by agitating for five minutes by shaking and using a magnetic stirrer (at 20 °C and 300 rpm for 30 min) to form a homogenous conidial suspension. The conidial suspension was enumerated using a haemocytometer (Neubauer, Merck, South Africa) and observed with a light microscope at 40× magnification. In order to obtain the desired concentration (1 × 104 conidia mL−1), the volume of 0.01% (v/v) Tween 80 was increased or conidia was added to the glass bottle. A conidial germination test to determine conidial viability was carried out, according to the method described by Inglis et al. [26], and a high spore germination of more than 90% was obtained.
2.3. Experimental Setup and Treatments
A single factor experimental design was used to test the main effect of one factor (fungus inoculum of C. rosea f. catenula) with one conidial concentration on composting materials consisting of solid raw organic cabbage wastes. This experiment was conducted in open-air conditions at the Department of Horticultural Sciences, Cape Peninsula University of Technology (Bellville Campus), South Africa. The compost heaps consisting of chopped cabbage wastes were placed two meters apart on a flat concrete surface with a black plastic lining. Six compost heaps were inoculated with a 1000 mL suspension of C. rosea f. catenula at a concentration of 1 × 104 conidia mL−1. Each of the six control heaps received 1000 mL of sterile distilled water containing 0.01% (v/v) Tween 80 (without the fungal conidia). All the heaps were thoroughly mixed and physically turned twice a week using clean and sterile spades to increase aeration and oxygen to the microorganisms. The spades used were dipped into 2% (v/v) sodium hypochlorite solution, and then rinsed with sterile water before reuse. Each heap was randomly allocated to test and control. The experiment ran for three months. The compost heaps where uncovered and exposed to natural environmental conditions throughout the composting process. Water was equally supplied to the compost heaps using a watering can. All the heaps were watered once every two weeks and watering was stopped from nine to 12 weeks, and each of the composting mixtures received 1000 mL, with a total of 9 L of sterile water added in total. Throughout the experiment, heap temperature was monitored weekly and twice each day in the mornings and afternoons using a 1 m long thermometer probe (Major Tech). A 2-in-1 soil pH-Moisture Meter (Major Tech (Cape Agricultural Products) Cape Town, South Africa) with a humidity reference scale of 0–10 (equivalent to 0–100% humidity) was used to monitor pH and moisture content. The humidity reference scale was further classified as follows: 0–3 (low humidity), 4–7 (moderate humidity), and 8–10 (high humidity). All heap measurements were taken in the center, avoiding the side walls as these could be influenced by wind and direct sunlight. The ambient environmental conditions, such as temperature and humidity data, were recorded. The average daily data was subsequently pooled to obtain weekly means, which were eventually analyzed statistically and presented as results.
2.4. Heap Color Assessment
The color of the compost was observed and classified using the Munsell Book of color [27] as a reference at the end of the experiment. The color of compost heaps is an important physical characteristic that can be used to assess the rate of composting and maturity of the compost.
2.5. Chemical Analyses of Composted Materials and Plant Tissue Nutrient Content
Chemical analyses of the composted materials were carried out by Bemlab (Pty) Ltd. Somerset West, Cape Town, South Africa. The micronutrients (B, Fe, Zn, Cu, Mn) and macronutrient (N, P, K, Ca, Mg, and C) contents were determined at the end of the trial using the methods described by Campbell and Plank [28], Miller [29], Walkley [30], and the Non-affiliated Soil Analyses Work Committee [31], as described in Mtikhulu et al. [32].
Tomato leaf samples were analyzed for tissue micro- and macro-nutrients at Bemlab (Somerset, Cape Town, South Africa). Leaves were washed with a Teepol solution, rinsed with de-ionized water, and dried at 70 °C overnight in an oven. The dried leaves were then milled and ashed at 480 ˚C, and shaken up in a 50:50 HCl (50%) solution for extraction through filter paper [28,29]. The K, P, C, Ca, Mg, Na, Mn, Fe, Cu, Zn, and B contents of the extracts were measured using an ICP-AES analysis method [24]. The total nitrogen (N) content of the leaves was determined through combustion in a Leco N-analyzer (Leco Corporation, St Joseph, MI, USA).
2.6. End-Product Toxicity Assessments: Germination and Seedling Tests
Germination and seedling toxicity tests were carried out to assess composting end-product toxicity on tomatoes. Composted materials obtained from the control and test treatments following three months of composting were mixed with coarse river sand obtained from Stanler Farms Nursery Pty. Ltd., Cape Town, as follows: 25% fungus treated compost end-product and 75% (by volume) course river sand as test sets, and 25% untreated compost end-product and 75% (by volume) course river sand as control sets. The samples were each placed in 15 cm plastic potting containers. Sixty oxheart heirloom tomato seeds were sown in separate pots for the germination test. For the seedling toxicity test, sixty oxheart heirloom tomato seedlings were also transplanted into separate pots. Composted samples were obtained from three randomly selected heaps belonging to test and control treatments. The samples were watered once each day and maintained for eight weeks in the greenhouse under the following conditions: an average day temperature of 25 ± 5 °C and relative humidity (RH) of 65 ± 5%.
2.7. Evaluating Endophytic Activity and Red Spider Mite Infestations on Tomato Plants Following Treatment of Raw Compost Materials with C. rosea f. catenula
Twenty potted plants were randomly allocated to a substrate mix containing composted materials from heaps that were pre-treated with C. rosea f. catenula or composted materials from heaps that were not pre-treated with the fungus. The soil mix consisted of 25% composting end-product and 75% coarse river sand by volume (25:75). The plants were irrigated with 250 mL of sterile distilled water by drenching each potted plant and were maintained in a greenhouse that had residual red spider mite (Tetranychus urticae) infestations, which were allowed to infest the plant naturally. The experiment was conducted in an environmentally controlled greenhouse (an average day temperature of 25 ± 5 °C and relative humidity (RH) of 65 ± 5%) over two months.
Red spider mite infestation levels on tomatoes were assessed in the environmentally controlled greenhouse by counting the number of plants that were infested on control and fungal-exposed treatments at the end of the experiment (two months post-treatment).
2.8. Re-Isolation of C. rosea f. catenula
Endophytic colonization of C. rosea f. catenula of the tomato plant leaves was assessed at 21 days by re-isolation. One leaf was carefully excised from each plant and transferred to a laminar flow cabinet in the laboratory. From each leaf, rectangular leaf sections of 1–2 mm2 were cut. These sections were individually surface-sterilized with 1% (v/v) sodium hypochlorite for 1 min, followed by 1 min in 70% (v/v) ethanol and rinsed twice in sterile distilled water, and then transferred onto the selective medium (half-strength PDA at 19.5 g/L containing 0.02 g/L ampicillin (Sigma-Aldrich), and streptomycin 0.04 g/L (Sigma-Aldrich, Johannesburg, South Africa)), and incubated at 25 °C. The leaf sections were visually examined on a daily basis for any presence of fungal growth: C. rosea f. catenula produces white dense mycelia that become creamy at the edge. Growth from the tissues of sterilized leaf sections was used as evidence of endophytic fungal growth. A total of 18 (9 control and 9 treatment) plants were randomly sampled and 54 leaf sections were plated equating to three leaf sections per plant. The presence of C. rosea f. catenula in at least one of the leaf sections was considered as an indication of successful plant colonization. The data was expressed in percentage colonization (number of plant replicates colonized ÷ number of plant replicates excised) × 100.
2.9. Statistical Analyses
The experimental data (heap temperature, pH, moisture, compost nutrient contents, number of seeds germinated, number of live seedlings and endophytic test) were analyzed using one-way analysis of variance (ANOVA) for comparison of the control and treatment groups, while the Tukey HSD test was used to separate means at a level of significance, p = 0.05. Pearson Chi-square (χ2) test was used to compare significant difference of end-product toxicity on tomato seed germination and seedling growth in the fungus and control treatments, and the number of plants that were infested with the red spider mites in fungus and control composted materials. All computations were performed using PAST version 3.20 (Øyvind Hammer, Oslo, Norway) [33]. The values reported in this paper are mean ±SE (standard error).
3. Results
3.1. Effect of Fungal Treatment on Compost Heap Temperature and Heap Humidity
Heap temperature showed significant differences between treatments at weeks 1, 7, 8, and 9 in the morning (DF; degree of freedom = 1, 10; p = 0.05), and weeks 7 and 9 in the afternoon (Table 1).
Table 1.
Variation in heap temperature (mean ± SE) following exposure to Clonostachys rosea f. catenula inoculum and control treatment (no fungus) during the composting of raw vegetable waste over a 12-week period of composting.
| Fungal Treatment | Control Treatment | |||
|---|---|---|---|---|
| Weeks | Morning | Afternoon | Morning | Afternoon |
| 1 | 35.12 ± 0.6 b | 36.58 ± 0.9 a | 39.35 ± 0.2 a | 36.28 ± 0.3 a |
| 2 | 26.92 ± 1.5 a | 25.88 ± 0.2 a | 28.68 ± 0.2 a | 26.83 ± 0.3 a |
| 3 | 23.77 ± 0.5 a | 25.62 ± 0.5 a | 24.08 ± 0.1 a | 29.53 ± 2.0 a |
| 4 | 28.98 ± 1.7 a | 30.53 ± 0.6 a | 27.9 ± 0.2 a | 31.98 ± 0.6 a |
| 5 | 29.12 ± 1.6 a | 31.62 ± 0.7 a | 29.63 ± 1.5 a | 33.2 ± 0.4 a |
| 6 | 28.53 ± 0.4 a | 34.48 ± 0.9 a | 28.72 ± 0.3 a | 34.17 ± 0.3 a |
| 7 | 24.37 ± 0.3 b | 27.76 ± 0.4 b | 26.8 ± 0.3 a | 31.22 ± 0.8 a |
| 8 | 26.9 ± 0.8 b | 29.62 ± 2.3 a | 29.15 ± 0.5 a | 29.55 ± 0.8 a |
| 9 | 27.37 ± 0.3 b | 31.05 ± 0.4 b | 29.45 ± 0.2 a | 34.23 ± 0.8 a |
| 10 | 28.82 ± 1.0 a | 32.58 ± 0.9 a | 30.68 ± 1.0 a | 33.00 ± 0.8 a |
| 11 | 27.23 ± 0.9 a | 31.62 ± 0.9 a | 28.65 ± 0.6 a | 34.33 ± 0.9 a |
| 12 | 29.03 ± 2.0 a | 33.22 ± 0.9 a | 33.37 ± 1.0 a | 34.55 ± 0.9 a |
Values are means ± SE (standard error). Values followed by the same lowercase letters in the same row do not show significant difference at p > 0.05 following comparison using the Tukey test. Means with the same letters are not significantly different.
3.2. Variation in Environmental Conditions (Relative Humidity and Ambient Temperature)
The data for environmental conditions were collected in the mornings and afternoons and are shown in Appendix A; Table A1.
3.3. Heap Moisture
The fungal inoculum did not have any significant effect (p > 0.05) on heap moisture compared to the control at weeks 1, 3–4, 6–7, and 10–12 in both the morning and afternoon. However, there were significant differences (DF = 1, 10; p < 0.05) recorded in heap moisture in the morning at weeks 5 and 9, and afternoon at weeks 2 and 8 post-treatment (Table 2).
Table 2.
Changes in heap moisture reading (mean ± SE) following exposure to Clonostachys rosea f. catenula inoculum and control treatment (no fungus) during composting of raw cabbage waste.
| Fungal Treatment | Control Treatment | |||
|---|---|---|---|---|
| Weeks | Morning | Afternoon | Morning | Afternoon |
| 1 | 52.97 ± 22.2 a | 46.65 ± 1.8 a | 51.92 ± 1.7 a | 40.98 ± 7.5 a |
| 2 | 43.53 ± 3.3 a | 48.5 ± 2.6 a | 39.92 ± 1.9 a | 40.96 ± 1.5 b |
| 3 | 44.23 ± 4.9 a | 46.97 ± 3.9 a | 39.33 ± 2.7 a | 40.3 ± 3.9 a |
| 4 | 44.87 ± 2.9 a | 40.58 ± 4.9 a | 42.23 ± 3.0 a | 47.23 ± 3.4 a |
| 5 | 43.2 ± 0.8 a | 49.23 ± 2.6 a | 52.1 ± 3.7 b | 52.23 ± 1.2 a |
| 6 | 44.45 ± 1.7 a | 43.42 ± 1.5 a | 40.1 ± 15.5 a | 45.45 ± 1.0 a |
| 7 | 37.9 ± 1.2 a | 38.42 ± 3.1 a | 42.52 ± 2.2 a | 37.85 ± 1.6 a |
| 8 | 44.72 ± 1.1 a | 49.47 ± 1.2 a | 38.87 ± 3.9 a | 44.45 ± 2.1 b |
| 9 | 22.15 ± 1.6 a | 19.48 ± 0.9 a | 17.9 ± 0.7 b | 21.07 ± 1.3 a |
| 10 | 0.00 ± 0.0 a | 0.00 ± 0.0 a | 0.00 ± 0.0 a | 0.00 ± 0.0 a |
| 11 | 12.07 ± 1.4 a | 0.00 ±0.0 a | 11.37 ± 0.9 a | 0.00 ± 0.0 a |
| 12 | 0.00 ± 0.0 a | 0.00 ± 0.0 a | 0.00 ± 0.0 a | 0.00 ± 0.0 a |
Values are means% ± SE (standard error). Values followed by the same lowercase letters in the same row do not show a significant difference (p > 0.05) following a comparison using the Tukey test. Means with the same letters are not significantly different.
3.4. pH Variations
There were no significant differences (p > 0.05) in pH of compost heaps with fungal-treated materials and the control at week one to 12 weeks for both the morning and afternoon readings, including the final end-product after composting (Table 3). An interesting similarity was observed in the end-product of composting, where the pH values were alkaline for both treatments, ranging from 7.5 to 7.6 (Table 4).
Table 3.
pH variation (mean ± SE) following exposure to Clonostachys rosea f. catenula inoculum and control treatment (no fungus) during the composting of raw cabbage waste over a 12-week period of composting.
| Fungal Treatment | Control Treatment | |||
|---|---|---|---|---|
| Weeks | Morning | Afternoon | Morning | Afternoon |
| 1 | 4.50 ± 0.1 a | 4.70 ± 0.1 a | 4.60 ± 0.1 a | 4.80 ± 0.1 a |
| 2 | 5.30 ± 0.2 a | 5.00 ± 0.2 a | 5.20 ± 0.0 a | 5.60 ± 0.4 a |
| 3 | 5.20 ± 0.2 a | 4.90 ± 0.2 a | 5.40 ± 0.2 a | 5.30 ± 0.1 a |
| 4 | 5.00 ± 0.1 a | 5.20 ± 0.3 a | 5.00 ± 0.1 a | 4.80 ± 0.2 a |
| 5 | 5.10 ± 0.0 a | 4.60 ± 0.1 a | 4.50 ± 0.2 a | 4.50 ± 0.1 a |
| 6 | 5.40 ± 0.3 a | 5.00 ± 0.1 a | 5.20 ± 0.1 a | 4.90 ± 0.0 a |
| 7 | 5.30 ± 0.1 a | 5.3 ± 0.2 a | 5.00 ± 0.5 b | 5.30 ± 0.1 a |
| 8 | 5.00 ± 0.1 a | 4.7 ± 0.1 a | 5.30 ± 0.3 a | 5.00 ± 0.1 a |
| 9 | 6.10 ± 0.1 a | 6.2 ± 0.0 a | 6.30 ± 0.0 a | 6.10 ± 0.1 a |
| 10 | 7.00 ± 0.0 a | 7.00 ± 0.0 a | 7.00 ± 0.0 a | 7.00 ± 0.0 a |
| 11 | 6.40 ± 0.1 a | 7.00 ± 0.1 a | 6.50 ± 0.0 a | 7.00 ± 0.0 a |
| 12 | 7.00 ± 0.0 a | 7.00 ± 0.0 a | 7.00 ± 0.0 a | 7.00 ± 0.0 a |
Values are means ± SE (standard error). Values followed by the same lowercase letters in the same row do not show a significant difference at (p > 0.05) following comparison by using the Tukey test. Means with the same letters are not significantly different.
Table 4.
The effect of treatment with Clonostachys rosea f. catenula on the chemical composition of raw cabbage compost over the 12-week period of composting.
| Parameters | Fungus Treatment | Control Treatment |
|---|---|---|
| pH | 7.60 ± 0.0 a | 7.50 ± 0.0 a |
| N mg/kg | 22,540.00 ± 1111.1 a | 24,660.00 ± 2182.1 a |
| P mg/kg | 6400.00 ± 158.1 a | 5860.00 ± 254.2 a |
| K mg/kg | 6740.00 ± 958.4 a | 7400.00 ± 931.1 a |
| Ca mg/kg | 39,880.00 ± 446.5 a | 38,220.00 ± 950.5 a |
| Mg mg/kg | 2960.00 ± 67.8 a | 3180.00 ± 345.5 a |
| Na mg/kg | 2099.60 ± 571 a | 1680.90 ± 217.6 a |
| Mn mg/kg | 34.90 ± 1.5 a | 36.20 ± 0.9 a |
| Fe mg/kg | 199.92 ± 14.7 a | 210.30 ± 6.6 a |
| Cu mg/kg | 6.90 ± 1.1 a | 8.32 ± 1.3 a |
| Zn mg/kg | 75.04 ± 6.8 a | 68.54 ± 8.6 a |
| B mg/kg | 26.83 ± 1.9 a | 27.90 ± 2.7 a |
| C mg/kg | 221.34 ± 23.3 a | 182.56 ± 12.8 a |
| C/N ratio | 8:1 ± 0.4 a | 8:1 ± 0.2 a |
Values are means ± SE. (standard error). Values followed by the same lowercase letters in the same row do not show a significant difference at p > 0.05) following a comparison by using the Tukey test. Means with same the letters are not significantly different.
3.5. Changes in the Color of the Compost during Composting
Variations in color from green raw materials in the initial stage of the experiment to brown (control) and dark brown (fungus-treated heaps) color at twelve weeks post treatment were observed between treatments. The Munsell Book of Colors [27] guideline classified the fungus-treated compost as 5 YR 3/3 (dark-brown color) and the control compost as 5 YR 4/4 (brown in color).
3.6. Chemical Analysis of Compost: Macro-and Micro-Nutrients
Generally, there was no significant difference (p > 0.05) in the macro-and micro-nutrient levels in composted materials between treatments (Table 4).
3.7. Re-Isolation of the Fungus from the Compost End-Product
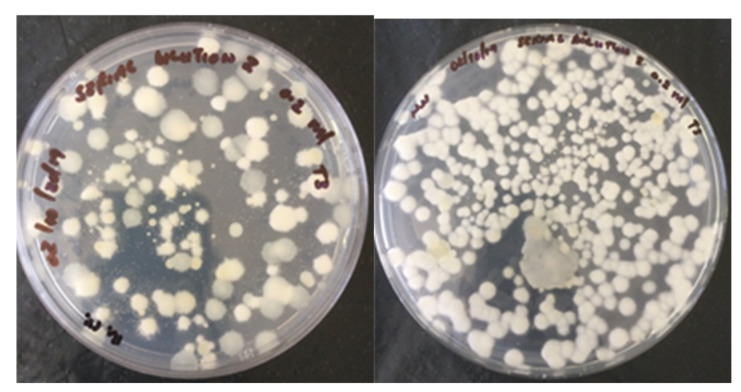
Clonostachys rosea f. catenula was successfully re-isolated from the composted materials that were exposed to fungal inoculum (Figure 1), but not from the control-treated heaps.
Figure 1.
Re-isolation of Clonostachys rosea f. catenula isolate from the composted end-product materials obtained from fungus-treated heaps.
3.8. End-Product Toxicity—Germination and Seedling Test
When tested at a 25%:75% compost:sand ratio mix, composted materials from fungus-treated heaps were more favorable to the germination of tomato seeds, where a significant difference (p < 0.05) in seed germination (χ2 = 12.102; p = 0.0005) was obtained. We obtained 93% successful seed germination in the fungus inoculated compost compared with 68% in the control treatment. In the seedling test, no seedling was affected by the fungus or control treatment: 100% of seedlings were alive. Furthermore, no symptoms of yellowing and burning of leaf edges of the plants were observed on the tested seedlings (Table 5).
Table 5.
Seed germination and seedling test of Solanum lycopersicum plants cultivated in growth medium made up of the end-products of composting that were obtained from Clonostachys rosea f. catenula-containing compost heaps and control compost heaps (no fungus). The growth medium was composed of 25% composted materials and 75% sand.
| Percentage Seed Germination | Percentage Seedling Growth | ||
|---|---|---|---|
| Treatment | Control | Treatment | Control |
| 93 a | 68 b | 100 a | 100 a |
Means with the same lowercase letters in the same row in either seed germination or seedling growth are not significantly different following Pearson Chi-square test (χ2) at a p = 0.05 level of significance for seed germination and seedling growth. Means with the same letters are not significantly different.
3.9. Re-Isolation of Fungus from Tomato Tissues
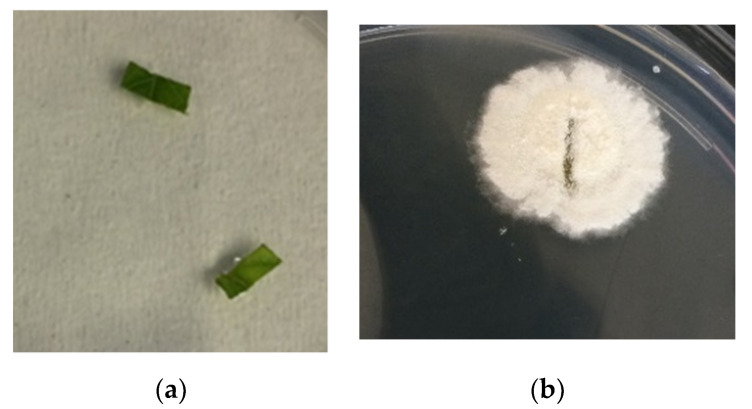
At 21 days after inoculation, C. rosea f. catenula was successfully re-isolated from 9 of 18 leaf sections placed onto PDA plates, representing 9 of 9 plants (100%) cultivated on composted end-product materials obtained from fungus-treated raw compost heaps (Figure 2). No fungal outgrowth of C. rosea f. catenula was observed in the plants grown in the control treatment compost and sand substrate.
Figure 2.
Re-isolation of Clonostachys rosea f. catenula isolated from leaf sections of tomato plants obtained from compost materials inoculated with Clonostachys rosea f. catenula and control treatment (no fungus) plated on PDA selective media. These samples were surface sterilized: (a) The petri dish corresponding to control samples, (b) and C. rosea f. catenula treated samples.
3.10. Protection of Tomato Plants against Red Spider Mite Infestation
A lower T. urticae infestation level was observed among plants cultivated on composted materials from fungus treatment. However, overall, the C. rosea f. catenula isolate had a limited effect on T. urticae infestation occurrence on tomato plants grown in compost end-product substrate originating from control and fungus-treated heaps (χ2 = 0.96; p = 0.32). Of the 20 potted plants, cases of red spider mite infestations ranged between 6 and 9 for fungus and control treatments, respectively (Table 6).
Table 6.
Infestation occurrence of Tetranychus urticae on tomato seedlings cultivated in substrate mix containing composted materials from either organic waste heaps that were inoculated with Clonostachys rosea f. catenula or control treated heaps.
| Plants Cultivated on Fungus-Treated Heaps | Plants Cultivated on Control-Treated Heaps | |
|---|---|---|
| Number of plants infested by T. urticae | 6/20 | 9/20 |
Samples of plants that were infested with red spider mites from the total number of planted tomato seedlings.
3.11. Effect of Fungus on Plant Tissue Nutrient Content
Generally, compost end-product from heaps inoculated with fungus had no significant effect (p > 0.05) on the tissue nutrient levels of plants compared with the control compost samples. However, K (32,750 mg/kg), Ca (19,250), and Na (2887.5 mg/kg) were higher in the leaf tissues of fungus-treated plants (DF = 1, 6; p < 0.05) compared to the control plants, which were 26,000 mg/kg K, 17425 mg/kg Ca, and 1950.5 mg/kg Na (Table 7). However, the Fe concentration in plants exposed to fungus-treated materials was in general lower compared to the control treatment compost (p > 0.05).
Table 7.
Tissue nutrient contents (mean ± SE) of leaves of Solanum lycopersicum plants grown for eight weeks under greenhouse conditions and that were exposed to compost originating from control compost heaps and Clonostachys rosea f. catenula inocula exposed compost heaps.
| Parameters | Treatment | Control |
|---|---|---|
| Macronutrients | ||
| N mg/kg | 16,575.00 ± 1527.2 a | 17,600.00 ± 1987.0 a |
| P mg/kg | 1500.00 ± 91.3 a | 2200.00 ± 385.1 a |
| K mg/kg | 32,750.00 ± 3637.2 a | 26,000.00 ± 4830.5 a |
| Ca mg/kg | 19,250.00 ± 1600.8 a | 17,425.00 ± 2917.9 a |
| Mg mg/kg | 5000.00 ± 430.1 a | 5650.00 ± 1278.3 a |
| Na mg/kg | 2887.50 ± 617.2 a | 1950.50 ± 355 a |
| Micronutrients | ||
| Mn mg/kg | 59.10 ± 9.1 a | 49.70 ± 11.9 a |
| Fe mg/kg | 120.50 ± 14.9 a | 153.85 ± 65.4 a |
| Cu mg/kg | 4.20 ± 0.5 a | 4.35 ± 0.9 a |
| Zn mg/kg | 50.10 ± 1.5 a | 53.80 ± 10.6 a |
| B mg/kg | 56.37 ± 6.9 a | 46.58 ± 4.8 a |
| C mg/kg | 413.30 ± 1.3 a | 414.10 ± 6.5 a |
| C/N ratio | 25:1 ± 2.6 a | 24:1 ± 2.6 a |
Values are means ± SE (standard error). Values followed by the same letter in the same row do not show a significant difference at p > 0.05 following a comparison by using the Tukey test. Means with the same letters are not significantly different.
4. Discussion
The inoculation of C. rosea f. catenula on composting heaps did not show varied effects on the physical parameter of heap temperature over time in this study. The highest weekly average temperature recorded in this study was 39.35 °C, and this was relatively low when compared to similar studies [34,35]; however, the heaps in the current study were small and in open air conditions. Gaur et al. [14] observed maximum temperatures of 45 °C and 51 °C during composting of a mixture of jowar stalk and wheat straw, and jamun leaves inoculated with Aspergillus niger and Trichoderma viride, respectively. However, the study by Sakar et al. [36] differed from the present study. For example, the study by Sakar et al. [36] did not show an increase in temperature in pits and earthen pots of compost wastes containing dung and market waste, and vegetable waste and dung, but when the ratio of 1:5 chopped rice straw was added to the waste, a heap temperature of 65.9 °C was observed after 24 h. The influence of the experimental conditions, such as heap size, ambient temperature and humidity, and turning frequencies on the composting process and the outcomes cannot be underestimated [32]. Possible reasons for the low temperature of composting in the present study could have been due to the high water content of the solid vegetable wastes and frequent turning of the heaps in open air [32]. Temperature is a key parameter in composting, as it indicates the state of decomposition and guarantees sanitation of the end-product [37]. The rise and drop in temperature suggests that the composting process might have gone through three phases: the mesophilic, thermophilic, and maturation phases.
In this study, the data show that the effect of fungus on moisture content was not significant. However, the heap moisture content decreased in both treatments over time, from weeks 1 to 12. Moisture is considered as one of the important parameters affecting the composting process [15,38]. However, the moderate moisture content of 40–60% obtained in this study falls within the range of optimum moisture content for composting [39]. Ribeiro et al. [34] also expressed that values of 50 to 60% moisture content are ideal for efficient composting. Higher moisture content during composting causes waterlogging that might delay the composting process [36,40,41,42]. It is worth noting that the inoculation of composting materials with beneficial microorganisms has the potential to sustain the water content of heaps.
We also assessed the nutrient contents of the cabbage wastes in this study and found that N, P, K, Na, C, and B were not significantly influenced by exposing composting materials to a C. rosea f. catenula inoculum. These findings contradict those of Jusoh et al. [43], who reported higher levels of P and K content (p < 0.05) in a mixture of rice straw with a solution of effective microorganisms in comparison to those without microorganisms. This study recorded the same C/N ratio of 8:1 for both treatments in the end-product of composting (Table 4). Nevertheless, Kumar et al. [44] and Petric et al. [45] reported results with essential C/N values between 20 and 50. Previously, Mtimkulu [32] reported improved C/N ratio in treatments with spent wine filter material content (10:1; 13:1, and 10:1). A low C/N ratio suggests higher available nitrogen per carbon, and inert nitrogen [46]. Yang et al. [47], Wang et al. [48], and Rastogi et al. [49] observed decreased C/N ratios in the process of composting, which can be attributed to an elevated waste biodegradation (carbon) to mineralization (nitrogen) ratio.
Traditionally, C/N ratio is used to indicate the degree of maturity of compost [50,51]. Poincelot [52] suggested that a C/N ratio below 20 is indicative of mature compost. Therefore, based on our results, fungal-treated compost heaps and control-treated heaps were mature and complete at the end of the composting period. Similarly, the N and C in the plant tissue nutrients did not show a significant difference (p > 0.05) in both treatments; a higher C/N ratio (25:1) was obtained (Table 7). Fungus treated heaps appeared to have a dark brown color compared to the control heaps. This effect demonstrates that perhaps the fungal inoculum enhanced the decomposition of organic wastes. This affirmation correlates with the findings of Sakar et al. [36].
The existence of micronutrients, including some heavy metals, in the compost was determined at the end of the composting period, and interestingly, no effect on micro-nutrients was observed in the fungus-treated heaps compared with the control (Table 4). Overall, these results suggest that inoculation of cabbage wastes with the C. rosea f. catenula strain did not change the chemical characteristics of the final product of composting. Previously, a decrease in the status of nutrients had been obtained by Chaturvedi et al. [53], while, the findings of Jusoh et al. [43] indicated that the composting process of rice straw containing effective microorganisms tended to increase heavy metals (Cu and Zn). Previous research by Paré et al. [54] also explored the increased accumulation of heavy metals during the composting of biosolids. These trace metals are required in small quantities by plants, as they can be phytotoxic at high concentrations [52]. Paré et al. [54] confirmed that the extractability and exchangeability of some of these heavy metals could be reduced during the composting of biosolids and municipal solid wastes.
No differences in the pH values of the treated heaps and the control heaps over the period of composting were recorded. The pH in this study ranged from 4.5–7.0. Previous studies showed that pH values ranging from 5.5 to 9.0 are suitable for the composting process, while values between 6.5 to 8.0 are thought to be most effective [55]. According to Chen et al. [41], a pH range from 6.8 to 7.3 is optimum for composting. Zhang and Sun [46] suggested that pH values from 5.5 to 8.0 are ideal for composting, whereas Bernal et al. [39] suggested that a pH value from 6.7 to 9.0 is optimum to promote good microbial activities. The pH of compost has a noticeable effect on the microbial populace, and it increases as a result of the decomposition of acids, which releases ammonium [15]. The pH values in the reports by Miller [29], Christian et al. [55], Sarkar et al. [36], and Ribeiro et al. [34] were closer to those observed in the present study (pH ranging between 7.0 and 7.6). This pH range of 7 to 7.6 is within the optimum range of 5.5 to 8.0 for compost pile materials with fungi [41]; thereby, suggesting that the compost in the current study was subjected to good oxidation.
In this study, C. rosea f. catenula conidia were successfully re-isolated from the composted materials originating from fungus pre-treated heaps and from all tomato plants that were cultivated on the composted materials obtained from the fungus pre-treated heaps. These results suggest that the fungus was certainly persistent in the composted materials; thereby, enhancing endophytic quality of the composted materials. C. rosea f. catenula is potentially pathogenic to insects and antagonistic against phytopathogens. Hence, its presence should add value to organic composts. The re-isolation of C. rosea f. catenula from leaf sections of the cultivated tomato plants clearly demonstrated the C. rosea f. catenula strain used in this study was an efficient endophyte. The colonization of potted tomato plants by endophytic fungi has been reported previously [56]. Xia et al. [57] isolated six endophytic fungal isolates belonging to very diverse orders, such as Eurotiales, Pleosporales, Hypocreales, and Saccharomycetales. The recovery of C. rosea f. catenula from the leaves of the tomato plants, indicates the potential of these strains to become successful endophytic agents. Andrade-Linares et al. [58] also reported endophytic colonization by species belonging to the genera Verticillium, Penicillium, Cladosporium, Fusarium, and Trichoderma, which can also be isolated from stems. It is worth mentioning that successful colonization of fungal endophytes across different types of tissues is influenced by factors such as fungal species, fungal strain, and host [59,60,61], as well as plant species, growth stages, soil types, and environmental conditions [62]. Variability in the colonization efficiency of strawberry leaves [63] and potato tubers [64] with a different strain of C. rosea was previously reported. Nordström [65] reported variable colonization of tomato and Arabidopsis thaliana by a C. rosea f. catenula strain. Sutton et al. [20] also reported that C. rosea is a plant endophyte on different plant species and was isolated from roots and stems of soya beans (Glycine max), which were either inoculated with, or were grown in, soil infested with C. rosea [66]. Nordström [65] suggested that the rhizosphere can affect the establishment of C. rosea. In another study, it was reported that growth media has a greater influence on the level of plant endophytic colonization than the inoculation method [67].
Clonostachys rosea f. catenula was assessed against a tomato plant red spider mite infestation and the results showed that the fungus did not significantly reduce the insect infestation levels despite the high endophytism observed in this study, suggesting that many factors influence the efficacies of endophytic entomopathogens in vivo. Moloinyane and Nchu [68] did not observe a reduction of grapevine mealy bug infestations by the endophyte Beauveria bassiana, and they argued that factors such as host species, fungal strain, and insect species may influence the efficacy of endophytic entomopathogens on both the plant and the insect pest. However, inoculation method and the effect of endophytic colonization can be influenced by biotic and abiotic factors, such as growth substrate, plant species and age, fungal species and inoculum density [59,66,69]. For instance, Tefera and Vidal [59] demonstrated that despite the inoculation method used, B. bassiana colonized the entire parts of sorghum plants. However, a study by Posada et al. [69] showed that plants species, fungal isolate, and the inoculation methods used influenced the endophytic colonization by B. bassiana. Some studies have demonstrated fungal endophytes reduces insect infestations [16,70,71,72,73,74]. For instance, the effect of environmental changes on colonization of corn by the fungal isolate B. bassiana was reported by Bing and Lewis [75] and its effect on targeted pests (European corn borer larvae) Ostrinia nubilalis (Hübner). However, others did not find any benefit of endophytic fungal colonization on insect infestations [68]. It appears many other factors moderated the fungus–plant–herbivore relationship, which research has shown is quite complex. It is worth noting that this fungus is known as an antagonist, and which is widely used as a biocontrol agent for divergent plant pathogens, including plant pathogenic fungi such as Alternaria and Fusarium species [76].
Results of this study showed a superior germination of tomato seeds sown in composted materials from heaps that were inoculated with C. rosea f. catenula during composting, and no phytotoxicity was observed on percentage seedling growth in both treatments. The increased germination in treated compost, as well as the increased seedling growth, may have been due to the presence of the fungus. A recent study showed that endophytic fungi can produce phytohormones, particularly gibberellins, that alleviate the harmful effects of abiotic stresses and enhance crop growth [77]. According to Davies [78], these phytohormones can regulate various developmental and physiological processes in plants such as seed germination, seedling development, stem and leaf growth, and flower and fruit growth. Previous studies reported on plant-growth promoting fungi (PGPF) from various genera including, Thrichoderma, Fusarium, and Penicillium, which can be beneficial in several crop plants [79,80]. A similar study has been reported, wherein Penicillium oxalicum (Pennisetum glaucum (L.) R. Br.) enhanced the seed germination and seedling vigor of Pearl millet [81]. However, the authors indicated that the efficacy of the endophytic fungus may vary significantly among treatments. Similarly, [79] observed enhanced seed germination and seedling vigor where seed priming with a conidial suspension of PGPF T. harzianum was used compared to a non-primed control in sunflower (Helianthus annuus L.). Moreover, Jogaiah et al. [82] demonstrated enhanced seed germination and seedling vigor in seeds of tomato treated with a conidial suspension of different PGPF. Murali et al. [83] also demonstrated the use of these microbes as inoculants to treat seeds for germination and seedling growth. Herrera et al. [84] found that seeds sown on a media composition of white peat mixed with municipal solid waste compost had better quality seedlings than those grown with standard peat mixtures. The compost:river sand ratio (25% treated compost and 75% course river sand) was a suitable treatment for the vegetative growth of seedlings in both treatments (Table 5).
This study suggests that inoculating organic waste materials with endophytic entomopathogenic C. rosea f. catenula conidia could enhance the rate of decomposition of organic waste heaps, help to produce high quality composted materials rich in endophytes, and that composting materials could serve as a substrate for storing entomopathogenic fungi for long periods, and for fungal application in the field. The finished end-product from the fungus-inoculated heaps could improve the physical stability, chemical constituent richness, and promote plant vigor and growth enhancement. This compost end-product contains plant-growth promoting and protection properties and could substitute chemical fertilizers and synthetic pesticides, inhibit toxic chemicals to crop plants, and be recommendable in agricultural application. Future evaluation of enzymes could help to better understand the mechanism through which the fungus influences composting, changes in microbial species and populations over time, and identify the specific microbes involved. Assessment of phytotoxic chemicals of these fungi in composted materials could help understand the potential risk they may have in the cultivation of plants.
5. Conclusions
In conclusion, this is the first report of the direct influence of C. rosea f. catenula on the composting of solid organic vegetable wastes and the tissue colonization of tomato plants. Clonostachys rosea f. catenula had no effect on the chemical composition of the end-product of composting of solid vegetable waste compared to the control treatment over the period of composting. However, the C. rosea f. catenula treatment enhanced the germination of tomato seeds and improved the physical characteristics of the composts. The C. rosea f. catenula strain did not confer protection against T. urticae infestation of tomatoes. Interestingly, the conidia of C. rosea f. catenula persisted in the composted materials for several months and was able to endophytically colonize tomato seedlings. We propose that organic wastes should be used as substrates for long term storage of endophytic entomopathogenic fungi. These findings also contributed to our understanding of the mechanisms involved in the fungus–plant residue–insect relationship. The effects of fungal inoculants on changes in enzyme activities during composting should be considered in the future.
Acknowledgments
We thank the Groente Verpakkers and Schultz Vars Produkte Mark vegetable farms, Philippi, South Africa, for supplying the vegetable wastes materials used in this study. Terrence Hlokane Mabela and Phumlani Roto, of the Department of Horticultural Sciences, Cape Peninsula University of Technology (Bellville), provided support during collection and transportation of the vegetable waste materials.
Appendix A
Table A1.
Changes in relative humidity (mean ± SE %) and ambient temperature readings (mean ± SE °C) during the composting of raw vegetable wastes over a 12-week period of composting.
| Relative Humidity | Ambient Temperature | |||
|---|---|---|---|---|
| Weeks | Morning | Afternoon | Morning | Afternoon |
| 1 | 50.2 ± 3.5 | 52.84 ± 5.1 | 30.52 ± 3.6 | 31.16 ± 4.7 |
| 2 | 54.42 ± 1 | 57.1 ± 1.7 | 25.04 ± 1.0 | 24.18 ± 1.9 |
| 3 | 55.54 ± 4.9 | 53.96 ± 2.7 | 24.66 ± 0.9 | 23.12 ± 0.4 |
| 4 | 60.76 ± 2.7 | 56.16 ± 3.0 | 26.26 ± 1 | 26.32 ± 1.3 |
| 5 | 59.4 ± 3.1 | 56.32 ± 5.7 | 25.68 ± 0.7 | 24.78 ± 0.6 |
| 6 | 63.3 ± 2.6 | 66.04 ± 3.4 | 28.08 ± 1.4 | 26.34 ±1.2 |
| 7 | 53.36 ± 1.5 | 51.6 ± 2.4 | 28.1 ± 0.6 | 29.42 ±1.4 |
| 8 | 55.36 ± 2.0 | 53.74 ± 2.7 | 27.68 ± 0.6 | 26.62 ± 0.8 |
| 9 | 59.18 ± 3.6 | 60.02 ± 2.4 | 28.08 ± 1.6 | 27.68 ± 0.3 |
| 10 | 59.64 ± 0.3 | 57.06 ± 2 | 26.34 ± 0.6 | 27.76 ± 0.5 |
| 11 | 60.76 ± 2.7 | 56.06 ± 1.8 | 27.2 ± 1.0 | 28.26 ± 0.6 |
| 12 | 51.5 ± 1.4 | 47.74 ± 2.6 | 28.66 ± 0.6 | 27.18 ± 0.6 |
Author Contributions
Conceptualizing, N.N. and F.N.; resources, N.N. and F.N.; investigation, N.N.; writing—original draft preparation, N.N; writing—review and editing, N.N., F.N., M.F., and M.L.R.-H.; supervision, F.N. and M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Cape Peninsula University of Technology (University Research Fund; Grant no: R1660) and the student received financial support from National Research Foundation, South Africa; Grant no. MND190618448458. ‘Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and therefore the NRF does not accept any liability in regards thereto’.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aschemann-Witzel J., De Hooge I., Amani P., Bech-Larsen T., Oostindjer M. Consumer-related food waste: Causes and potential for action. Sustainability. 2015;7:6457–6477. doi: 10.3390/su7066457. [DOI] [Google Scholar]
- 2.Abajeeh A.R., Nchu F. Isolation and pathogenicity of some South African entomopathogenic fungi (Ascomycota) against eggs and larvae of Cydia pomonella (Lepidoptera: Tortricidae) Biocontrol. Sci. Technol. 2015;25:828–842. doi: 10.1080/09583157.2015.1019831. [DOI] [Google Scholar]
- 3.Alexander R. Compost markets grow with environmental applications. Biocycle. 1999;40:43–48. [Google Scholar]
- 4.Ntsobi N., Etsassala N.G.E.R., Akinpelu E.A., Nchu F. Assessment of the Effects of Beauveria bassiana (Hypocreales) Inoculum on the Composting of Vegetable Wastes; Proceedings of the 18th International Conference on Agricultural, Chemical and Environmental Sciences; Johannesburg, South Africa. 16–17 November 2020; [DOI] [Google Scholar]
- 5.Baldrian P., Valášková V. Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol. Rev. 2008;32:501–521. doi: 10.1111/j.1574-6976.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- 6.Hou W., Lian B., Dong H., Jiang H., Wu X. Distinguishing ectomycorrhizal and saprophytic fungi using carbon and nitrogen isotopic compositions. Geosci. Front. 2012;3:351–356. doi: 10.1016/j.gsf.2011.12.005. [DOI] [Google Scholar]
- 7.Ferreira J.A., Mahboubi A., Lennartsson P.R., Taherzadeh M.J. Waste biorefineries using filamentous ascomycetes fungi: Present status and future prospects. Bioresour. Technol. 2016;215:334–345. doi: 10.1016/j.biortech.2016.03.018. [DOI] [PubMed] [Google Scholar]
- 8.Partanen P., Hultman J., Paulin L. Bacterial diversity at different stages of the composting process. BMC Microbiol. 2010;10:1–11. doi: 10.1186/1471-2180-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobbie E.A., Macko S.A., Shugart H.H. Insights into nitrogen and carbon dynamics of ectomycorrhizal and saprotrophic fungi from isotopic evidence. Oecologia. 1991;118:353–360. doi: 10.1007/s004420050736. [DOI] [PubMed] [Google Scholar]
- 10.McMillan J.D., Boynton B.L. Arabinose utilization by xylose-fermenting yeasts and fungi. Appl. Biochem. Biotechnol. 1994;45:569–584. doi: 10.1007/BF02941831. [DOI] [PubMed] [Google Scholar]
- 11.Fernandez A.H., Roig A., Serramiá A., Civantos C.G.O., Sánchez M.A.M. Application of compost of two-phase olive mill waste on olive grove: Effects on soil, olive fruit and olive oil quality. Waste Manag. 1994;34:1139–1147. doi: 10.1016/j.wasman.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes E.K.K., Bittencourt V.R.E.P., Roberts D.W. Perspectives on the potential of entomopathogenic fungi in biological control of ticks. Exp. Parasitol. 2012;130:30–305. doi: 10.1016/j.exppara.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 13.St Leger R.J., Cooper R.M., Charnley A.K. Cuticle-degrading enzymes of entomopathogenic fungi: Regulation of production of chitinolytic enzymes. Microbiology. 1986;132:1509–1517. doi: 10.1099/00221287-132-6-1509. [DOI] [Google Scholar]
- 14.Gaur A.C., Sadasivam K.V., Mathur R.S., Magu S.P. Role of mesophilic fungi in composting. Agric. Wastes. 1982;4:453–460. doi: 10.1016/0141-4607(82)90040-3. [DOI] [Google Scholar]
- 15.Pan I., Dam B., Sen S.K. Composting of common organic wastes using microbial inoculants. 3 Biotech. 2012;2:127–134. doi: 10.1007/s13205-011-0033-5. [DOI] [Google Scholar]
- 16.Saikkonen K., Wäli P., Helander M., Faeth S.H. Evolution of endophyte–plant symbioses. Trends Plant Sci. 2004;9:275–280. doi: 10.1016/j.tplants.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Pieterse C.M., Zamioudis C., Berendsen R.L., Weller D.M., Van Wees S.C., Bakker P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014;52:347–375. doi: 10.1146/annurev-phyto-082712-102340. [DOI] [PubMed] [Google Scholar]
- 18.Chandler D. Basic and applied research on entomopathogenic fungi. In: Lacey L.A., editor. Microbial Control of Insect and Mite Pests. 2nd ed. Academic Press; Amsterdam, The Netherlands: 2016. [DOI] [Google Scholar]
- 19.Lahoz E., Contillo R., Porrone F. Induction of systemic resistance to Erysiphe orontii cast in tobacco by application on roots of an isolate of Gliocladium roseum Bainier. J. Phytopathol. 2004;152:465–470. doi: 10.1111/j.1439-0434.2004.00876.x. [DOI] [Google Scholar]
- 20.Sutton J.C., Liu W., Ma J., Brown W.G., Stewart J.F., Walker G.D. Evaluation of the fungal endophyte Clonostachys rosea as an inoculant to enhance growth, fitness and productivity of crop plants. In: Leskovar D.I., editor. IV International Symposium on Seed, Transplant and Stand Establishment of Horticultural Crops; Translating Seed and Seedling. Volume 782. International Society for Horticultural Science; Leuven, Belgium: 2008. pp. 279–286. [DOI] [Google Scholar]
- 21.Sankhla I.S., Sharma G., Tak A. Fungal degradation of bioplastics: An overview. In: Singh J., Gehlot P., editors. New and Future Developments in Microbial Biotechnology and Bioengineering. Elsevier; Amsterdam, The Netherlands: 2020. pp. 35–47. Chapter 4. [DOI] [Google Scholar]
- 22.Sun Z.B., Li S.D., Ren Q., Xu J.L., Lu X., Sun M.H. Biology and applications of Clonostachys rosea. J. Appl. Microbiol. 2020;129:486–495. doi: 10.1111/jam.14625. [DOI] [PubMed] [Google Scholar]
- 23.Bohacz J., Korniłłowicz-Kowalska T. Modification of post-industrial lignin by fungal strains of the genus Trichoderma isolated from different composting stages. J. Environ. Manag. 2020;266:110573–110583. doi: 10.1016/j.jenvman.2020.110573. [DOI] [PubMed] [Google Scholar]
- 24.Morandi M., Sutton J.C., Maffia L.A. Effects of host and microbial factors on development of Clonostachys rosea and control of Botrytis cinerea in Rose. Eur. J. Plant. Pathol. 2000;106:439–448. doi: 10.1023/A:1008738513748. [DOI] [Google Scholar]
- 25.Moloinyane S. Master’s Thesis. Cape Peninsula University of Technology; Bellville, South Agrica: 2018. Effect of selected Entomopathogenic Fungal Endophytes (Ascomycota) Against Grapevine Mealybug. [Google Scholar]
- 26.Inglis G.D., Enkerli J., Goettel M.S. Laboratory techniques used for entomopathogenic fungi: Hypocreales. In: Lacey L.A., editor. Manual of Techniques in Invertebrate Pathology. 2nd ed. Academic Press; London, UK: 2012. pp. 189–253. Chapter 7. [Google Scholar]
- 27.Munsell A.H. In: Munsell Book of Colour. Baltimore M.D., editor. Macbeth Division of Kollmorgen Corp; Waltham, MA, USA: 1976. [Google Scholar]
- 28.Campbell C.R., Plank C.O. Preparation of plant tissue for laboratory analysis. In: Kalra Y.P., editor. Handbook of Reference Methods for Plant Analysis. CRC Press; Boca Raton, FL, USA: 1998. p. 37. Chapter 3. [Google Scholar]
- 29.Miller R.O. High-temperature oxidation: Dry ashing. In: Kalra Y.P., editor. Handbook of Reference Methods for Plant Analysis. CRC Press; Boca Raton, FL, USA: 1998. pp. 53–56. Chapter 5. [Google Scholar]
- 30.Walkley A., Black I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37:29–38. doi: 10.1097/00010694-193401000-00003. [DOI] [Google Scholar]
- 31.Non-Affiliated Soil Analysis Work Committee . Handbook of Standard Soil Testing Methods for Advisory Purposes. 3rd ed. Soil Science Society of South Africa; Pretoria, South Africa: 1990. p. 160. [Google Scholar]
- 32.Mtimkulu Y., Meyer A.H., Mulidzi A.R., Shange P.L., Nchu F. Assessing and monitoring the effects of filter material amendments on the biophysiochemical properties during composting of solid winery waste under open field and varying climatic conditions. Waste Manag. 2016;59:59–69. doi: 10.1016/j.wasman.2016.10.042. [DOI] [PubMed] [Google Scholar]
- 33.Hammer Ø., Harper D.A.T., Ryan P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. [(accessed on 1 April 2021)];Palaeontol. Electron. 2001 4:9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm. [Google Scholar]
- 34.Ribeiro N.D.Q., Souza T.P., Costa L.M.A.S., Castro C.P.D., Dias E.S. Microbial additives in the composting process. Agric. Sci. 2017;41:159–168. doi: 10.1590/1413-70542017412038216. [DOI] [Google Scholar]
- 35.Sundberg C., Jönsson H. Process inhibition due to organic acids in fed-batch composting of food waste—Influence of starting culture. Biodegradation. 2005;16:205–213. doi: 10.1007/s10532-004-0628-1. [DOI] [PubMed] [Google Scholar]
- 36.Sarkar S., Pal S., Chanda S. Optimization of a vegetable waste composting process with a significant thermophilic phase. Procedia Environ. Sci. 2016;35:435–440. doi: 10.1016/j.proenv.2016.07.026. [DOI] [Google Scholar]
- 37.Paradelo R., Moldes A.B., Barral M.T. Evolution of organic matter during the mesophilic composting of lignocellulosic winery wastes. J. Environ. Manag. 2013;116:18–26. doi: 10.1016/j.jenvman.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 38.Anastasi A., Varese G.C., Filipello Marchisio V. Isolation and identification of fungal communities in compost and vermicompost. Mycologia. 2005;97:33–44. doi: 10.1080/15572536.2006.11832836. [DOI] [PubMed] [Google Scholar]
- 39.Bernal M.P., Alburquerque J.A., Moral R. Composting of animal manures and chemical criteria for compost maturity assessment: A review. Bioresour. Technol. 2009;100:5444–5453. doi: 10.1016/j.biortech.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 40.Saidi N., Cherif M., Jedidi N., Fumio M., Boudabous A., Hassen A. Evolution of biochemical parameters during composting of various waste compost. Am. J. Environ. Sci. 2008;4:332–341. doi: 10.3844/ajessp.2008.332.341. [DOI] [Google Scholar]
- 41.Chen L., Moore A., de Haro-Marti M.D. Dairy Compost Production and Use in Idaho: On-Farm Composting Management. CIS 1190. [(accessed on 1 April 2021)];2012 Available online: http://www.extension.uidaho.edu/nutrient/pdf/OnFarm%20Composting%20Managment.pdfAccessed03/08/2020.
- 42.Makan A., Assobhei O., Mountadar M. Effect of initial moisture content on the in-vessel composting under air pressure of organic fraction of municipal solid waste in Morocco. Iran. J. Environ. Health Sci. Eng. 2013;10:1–9. doi: 10.1186/1735-2746-10-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jusoh M.L.C., Abd Manaf L., Latiff P.A. Composting of rice straw with effective microorganisms (EM) and its influence on compost quality. Iran. J. Environ. Health Sci. Eng. 2013;10:1–9. doi: 10.1186/1735-2746-10-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kumar M., Ou Y.L., Lin J.G. Co-composting of green waste and food waste at low C/N ratio. Waste Manag. 2010;30:602–609. doi: 10.1016/j.wasman.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 45.Petric I., Avdihodžić E., Ibrić N. Numerical simulation of composting process for mixture of organic fraction of municipal solid waste and poultry manure. Ecol. Eng. 2015;75:242–249. doi: 10.1016/j.ecoleng.2014.12.003. [DOI] [Google Scholar]
- 46.Zhang L., Sun X. Influence of bulking agent on physical, chemical and microbiological properties during two-stage composting of green waste. Waste Manag. 2016;48:115–126. doi: 10.1016/j.wasman.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 47.Yang F., Li G., Shi H., Wang Y. Effects of phosphogypsum and superphosphate on compost maturity and gaseous emissions during kitchen waste composting. Waste Manag. 2015;36:70–76. doi: 10.1016/j.wasman.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 48.Wang X., Cui H., Shi J., Zhao X., Zhao Y., Wei Z. Relationship between bacterial diversity and environmental parameters during composting of different raw materials. Bioresour. Technol. 2015;198:395–402. doi: 10.1016/j.biortech.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 49.Rastogi M., Nandal M., Nain L. Additive effect of cow dung slurry and cellulolytic bacterial inoculation on humic fractions during composting of municipal solid waste. Int. J. Recycl. Org. Waste Agric. 2019;8:325–332. doi: 10.1007/s40093-019-0277-3. [DOI] [Google Scholar]
- 50.Zeng G.M., Huang H.L., Huang D.L., Yuan X.Z., Jiang R.Q., Yu M., Yu H.Y., Zhang J.C., Wang R.Y., Liu X.L. Effect of inoculating white-rot fungus during different phases on the compost maturity of agricultural wastes. Process Biochem. 2009;44:396–400. doi: 10.1016/j.procbio.2008.11.012. [DOI] [Google Scholar]
- 51.Guoxue L.i., Zhang F., Sun Y., Wong J.W.C., Fang M. Chemical evaluation of sewage composting as mature indicator for composting process. Water Air Soil Pollut. 2001;132:333–345. doi: 10.1023/A:1013254815976. [DOI] [Google Scholar]
- 52.Poincelot R.P. A scientific examination of the principles and practice of composting. Compos. Sci. 1974;15:24–31. [Google Scholar]
- 53.Chaturvedi S., Singh B., Nain L., Khare S.K., Pandey A.K., Satya S. Evaluation of hydrolytic enzymes in bioaugmented compost of Jatropha cake under aerobic and partial anaerobic conditions. Ann. Microbiol. 2010;60:685–691. doi: 10.1007/s13213-010-0113-5. [DOI] [Google Scholar]
- 54.Paré T., Dinel H., Schnitzer M. Extractability of trace metals during co-composting of biosolids and municipal solid wastes. Biol. Fertil. Soils. 1999;29:31–37. doi: 10.1007/s003740050521. [DOI] [Google Scholar]
- 55.Christian A.H., Evanylo G.K., Green R. Compost: What Is It and What’s It to You? Virginia Cooperative Extension Service Publication; Blacksburg, VI, USA: 1997. pp. 452-231. [Google Scholar]
- 56.Pappas M.L., Liapoura M., Papantoniou D., Avramidou M., Kavroulakis N., Weinhold A., Broufas G.D., Papadopoulou K.K. The beneficial endophytic fungus fusarium solani strain K alters tomato responses against spider mites to the benefit of the plant. Front. Plant Sci. 2018;9:1603. doi: 10.3389/fpls.2018.01603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xia Y., Sahib M.R., Amna A., Opiyo S.O., Zhao Z., Gao Y.G. Culturable endophytic fungal communities associated with plants in organic and conventional farming systems and their effects on plant growth. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-018-38230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andrade-Linares D.R., Grosch R., Franken P., Rexer K.H., Kost G., Restrepo S., de Garcia M.C.C., Maximova E. Colonization of roots of cultivated Solanum lycopersicum by dark septate and other ascomycetous endophytes. Mycologia. 2011;103:710–721. doi: 10.3852/10-329. [DOI] [PubMed] [Google Scholar]
- 59.Tefera T., Vidal S. Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. BioControl. 2009;54:663–669. doi: 10.1007/s10526-009-9216-y. [DOI] [Google Scholar]
- 60.Arnold A.E., Herre E.A. Canopy cover and leaf age affect colonization by tropical fungal endophytes: Ecological pattern and process in Theobroma cacao (Malvaceae) Mycologia. 2003;95:388–398. doi: 10.1080/15572536.2004.11833083. [DOI] [PubMed] [Google Scholar]
- 61.Gurulingappa P., Sword G.A., Murdoch G., McGee P.A. Colonization of crop plants by fungal entomopathogens and their effects on two insect pests when in planta. Biol. Control. 2010;55:34–41. doi: 10.1016/j.biocontrol.2010.06.011. [DOI] [Google Scholar]
- 62.Yang H., Ye W., Ma J., Zeng D., Rong Z., Xu M., Wang Y., Zheng X. Endophytic fungal communities associated with field-grown soybean roots and seeds in the Huang-Huai region of China. PeerJ. 2018;6:e4713. doi: 10.7717/peerj.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cota L.V., Maffia L.A., Mizubuti E.S.G. Brazilian isolates of Clonostachys rosea: Colonization under different temperature and moisture conditions and temporal dynamics on strawberry leaves. Lett. Appl. Microbiol. 2008;46:312–317. doi: 10.1111/j.1472-765X.2007.02312.x. [DOI] [PubMed] [Google Scholar]
- 64.Assefa Jima T. Master’s Thesis. Swedish University of Agricultural Sciences; Uppsala, Sweden: 2013. Postharvest Biological Control of Fusarium Dry-Rot Diseases in Potato Tubers Using Clonostachys rosea Strain IK726. [Google Scholar]
- 65.Nordström S.A. Master’s Thesis. Swedish University of Agricultural Sciences; Uppsala, Sweden: 2014. Endophytic Growth of Clonostachys Rosea in Tomato and Arabidopsis Thaliana. [Google Scholar]
- 66.Mueller J., Sinclair J. Occurrence and role of Gliocladium roseum in field—Grown soybeans in Illinois. Trans. Br. Mycol. Soc. 1986;86:677–680. doi: 10.1016/S0007-1536(86)80078-X. [DOI] [Google Scholar]
- 67.Bamisile B.S., Dash C.K., Akutse K.S., Keppanan R., Afolabi O.G., Hussain M., Qasim M., Wang L. Prospects of endophytic fungal entomopathogens as biocontrol and plant growth promoting agents: An insight on how artificial inoculation methods affect endophytic colonization of host plants. Microbiol. Res. 2018;217:34–50. doi: 10.1016/j.micres.2018.08.016. [DOI] [PubMed] [Google Scholar]
- 68.Moloinyane S., Nchu F. The effects of endophytic Beauveria bassiana inoculation on infestation level of Planococcus ficus, growth and volatile constituents of potted greenhouse grapevine (Vitis vinifera L.) Toxins. 2019;11:72. doi: 10.3390/toxins11020072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Posada F., Aime M.C., Peterson S.W., Rehner S.A., Vega F.E. Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) Mycol. Res. 2007;111:748–757. doi: 10.1016/j.mycres.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Parsa S., Ortiz V., Vega F.E. Establishing fungal entomopathogens as endophytes: Towards endophytic biological control. J. Vis. Exp. 2013:50360. doi: 10.3791/50360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arnold A.E., Lewis L.C. Ecology and evolution of fungal endophytes and their roles against insects. In: Vega E., Blackwel M., editors. Insect-Fungal Associations: Ecology and Evolution. Oxford University Press; New York, NY, USA: 2005. pp. 74–96. Chapter 4. [Google Scholar]
- 72.Jallow M.F., Dugassa-Gobena D., Vidal S. Influence of an endophytic fungus on host plant selection by a polyphagous moth via volatile spectrum changes. Arthropod-Plant Interact. 2008;2:53–62. doi: 10.1007/s11829-008-9033-8. [DOI] [Google Scholar]
- 73.Reddy N.P., Khan A.P.A., Devi U.K., Sharma H.C., Reineke A. Treatment of millet crop plant (Sorghum bicolor) with the entomopathogenic fungus (Beauveria bassiana) to combat infestation by the stem borer, Chilo partellus Swinhoe (Lepidoptera: Pyralidae) J. Asia Pac. Entomol. 2009;12:221–226. doi: 10.1016/j.aspen.2009.06.001. [DOI] [Google Scholar]
- 74.Vega F.E., Goettel M.S., Blackwell M., Chandler D., Jackson M.A., Keller S., Koike M., Maniania N.K., Monzon A., Ownley B.H., et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecolol. 2009;2:149–159. doi: 10.1016/j.funeco.2009.05.001. [DOI] [Google Scholar]
- 75.Bing L.A., Lewis L.C. Suppression of Ostrinia nubilalis (Hübner) (Lepidoptera: Pyralidae) by endophytic Beauveria bassiana (Balsamo) Vuillemin. Environ. Entomol. 1991;20:1207–1211. doi: 10.1093/ee/20.4.1207. [DOI] [Google Scholar]
- 76.Nygren K., Dubey M., Zapparata A., Iqbal M., Tzelepis G.D., Durling M.B., Jensen D.F., Karlsson M. The mycoparasitic fungus Clonostachys rosea responds with both common and specific gene expression during interspecific interactions with fungal prey. Evol. Appl. 2018;11:931–949. doi: 10.1111/eva.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bilal L., Asaf S., Hamayun M., Gul H., Iqbal A., Ullah I., Lee I.J., Husaain A. Plant growth promoting fungi Aspergillus fumigatus TS1 and Fusarium proliferatum BRL1 produce gibberellins and regulates plant endogenous hormones. Symbiosis. 2018;76:117–127. doi: 10.1007/s13199-018-0545-4. [DOI] [Google Scholar]
- 78.Davies P.J. The plant hormones: Their nature, occurrence, and functions. In: Davies P.J., editor. Plant Hormones. Springer; Dordrecht, NY, USA: 2010. pp. 1–15. Chapter 1. [DOI] [Google Scholar]
- 79.Nagaraju A., Sudisha J., Murthy S.M., Ito S. Seed priming with Trichoderma harzianum isolates enhances plant growth and induces resistance against Plasmopara halstedii, an incitant of sunflower downy mildew disease. Australas. Plant Pathol. 2012;41:609–620. doi: 10.1007/s13313-012-0165-z. [DOI] [Google Scholar]
- 80.Chowdappa P., Mohan Kumar S.P., Lakshmi M.J., Upreti K.K. Growth stimulation and induction of systemic resistance in tomato against early and late blight by Bacillus subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol. Control. 2013;65:109–117. doi: 10.1016/j.biocontrol.2012.11.009. [DOI] [Google Scholar]
- 81.Murali M., Amruthesh K.N. Plant growth-promoting fungus Penicillium oxalicum enhances plant growth and induces resistance in pearl millet against downy mildew disease. J. Phytopathol. 2015;163:743–754. doi: 10.1111/jph.12371. [DOI] [Google Scholar]
- 82.Jogaiah S., Abdelrahman M., Tran L.S., Shinichi I. Characterization of rhizosphere fungi that mediate resistance in tomato against bacterial wilt disease. J. Exp. Bot. 2013;64:3829–3842. doi: 10.1093/jxb/ert212. [DOI] [PubMed] [Google Scholar]
- 83.Murali M., Amruthesh K.N., Sudisha J., Niranjana S.R., Shetty H.S. Screening for plant growth promoting fungi and their ability for growth promotion and induction of resistance in pearl millet against downy mildew disease. J. Phytol. 2012;4:30–36. [Google Scholar]
- 84.Herrera F., Castillo J.E., Chica A.F., Bellido L.L. Use of municipal solid waste compost (MSWC) as a growing medium in the nursery production of tomato plants. Bioresour. Technol. 2008;99:287–296. doi: 10.1016/j.biortech.2006.12.042. [DOI] [PubMed] [Google Scholar]