Abstract
For various studies in the clinic as well as the environment, it is essential to be able to selectively isolate Aspergillus fumigatus from samples containing bacteria as well as various other fungi (mainly Mucorales). Six agar media were compared for effectiveness in selectively isolating Aspergillus fumigatus from agricultural plant waste, woodchip waste, green waste, soil, grass and air samples collected in The Netherlands at a 48 °C incubation. The Flamingo Medium incubated at 48 °C, provided the most effective condition for the isolation of A. fumigatus from environmental samples, since it effectively inhibited the growth of competing fungi (mainly Mucorales) present in the environmental samples. Flamingo Medium reduced the number of colonies of Mucorales species by 95% and recovered an average of 20−30% more A. fumigatus colonies compared to the other media. We further confirmed that Flamingo Medium can inhibit the growth of clinical Mucorales, which occasionally present in patient’s tissue and can also be used for clinical applications. We suggest the use of Flamingo Medium as an efficient method for the study of A. fumigatus from important environmental niches for which there is increasing interest. Additionally, it can also be used in the clinic to isolate A. fumigatus especially from tissue contaminated with Mucorales.
Keywords: Aspergillus fumigatus, environmental samples, selective medium, Mucorales
1. Introduction
Aspergillus fumigatus is a common plant-waste-degrading fungus of which spores are abundantly present in the air. When inhaled, these spores can cause diseases in animals and humans ranging from allergic syndromes to acute invasive aspergillosis depending on the host immune system [1,2]. The number of drug classes that are available for the treatment of Aspergillus diseases remains very limited, with the azoles representing the major class. Azole resistance is an emerging concern in A. fumigatus, complicating the treatment of patients [3,4]. Up to 90% of A. fumigatus isolates recovered from patients with azole-resistant invasive aspergillosis exhibit resistance mutations that are associated with resistance selection in the environment [2,5,6,7,8]. The health importance of A. fumigatus and safety of azole fungicide application in agriculture have led to the increased interest in the study of A. fumigatus from environmental samples [9,10]. In previous research we have gathered evidence that azole-resistant A. fumigatus can accumulate and thrive in plant waste that contains agricultural azole residues [6,11]. The concept of a hotspot for azole-resistant A. fumigatus was postulated, which is characterized by an environment that supports the growth and reproduction of A. fumigatus and where azole fungicides with anti-Aspergillus activity are present [6]. In an initial survey, three hotspots were identified in The Netherlands: decaying flower bulb waste from farms, industrial wood-chip waste and industrial green-waste storage [6]. It is likely that additional hotspots will be identified over time, where both conditions are present.
For the identification of hotspots an efficient method for unbiased and quantitative isolation of A. fumigatus from environmental samples is crucial. This isolation of A. fumigatus has commonly been performed in several studies by using selective and non-selective media, including Malt Extract Agar (MEA) supplemented with Chloramphenicol and Streptomycin (MEA+C+S), Sabouraud Detrose Agar supplemented with Chloramphenicol (SDA+C) and Dichloran-Glycerol (DG18) [11,12,13,14]. On these and other media growth of Mucorales species (~103−6 Colony Forming Units/mL (CFU/mL) compromises the usefulness of MEA+C+S and DG18 [14,15,16]. Culturing at 48 °C is a generally applied condition to isolate A. fumigatus, since it restricts the growth of many fungi. However, environmental samples may contain a large number of thermophilic Mucorales species isolates hampering the isolation of A. fumigatus [17,18]. A good example of a mucoraceous thermophile is Rhizomucor pusillus with a maximum growth temperature of 54–58 °C. Another example of thermophilic Mucorales is Lichtheimia corymbifera, which can grow up to 45–50 °C [19]. We previously found that approximately 50% of the plant-waste samples from which A. fumigatus was isolated, also contained Mucorales [6,20]. Thus, an efficient selective growth medium to facilitate selective isolation of A. fumigatus from environmental samples is lacking.
In this present study, we aimed to develop a selective medium for the isolation of A. fumigatus from environmental samples. We have investigated and compared six types of media for their properties of selectively isolating A. fumigatus from samples that also contain high levels of spores or mycelia from Mucorales species. Of these six, three media (MEA+C+S, SDA+C and DG18) are commonly used for isolating A. fumigatus. We further included Modified Rose Bengal Agar (M-RB), which has originally been developed for the isolation of A. flavus and two modified media that have not been evaluated before (Flamingo Medium and MEA-Rose Bengal). In addition, we tested the best of these 6 media for its properties for selective isolation of A. fumigatus from clinical samples that contain both A. fumigatus and Mucorales species.
2. Materials and Methods
2.1. Media Preparation
MEA+C+S: Suspend 30 g of Malt Extra Agar (MEA) (Sigma-Aldrich, Steinheim, Germany) and 15 g of agar in 1 L of distilled water, autoclave at 121 °C for 15 min. Supplement with 1 mL of 50 mg/mL of Chloramphenicol and Streptomycin (Sigma-Aldrich, Steinheim, Germany) before use.
SDA+C: Suspend 10 g of Sabouraud Glucose Agar (Sigma-Aldrich, Steinheim, Germany) in 1 L of distilled water, autoclave at 121 °C for 15 min. Supplement with 1 mL of 50 mg/mL of Chloramphenicol (Sigma-Aldrich, Steinheim, Germany) before use.
DG18 (Sigma-Aldrich, Steinheim, Germany): Suspend 31.6 g of medium and 220 g of glycerol in 1 L of distilled water, autoclave at 121 °C for 15 min.
M-RB: Suspend 3 g of sucrose and NaNO3; 0.3 g of KH2PO4; 0.5 g of MgSO4·7H2O and KCl; 0.7 g of K2HPO4; 10 g of NaCl and 1 mL of Adye & Mateles Reagent stock X1000/mL dH2O(0.7 mg Na2B4O7·10H2O; 0.5 mg of (NH4)6MO7O24·4H2O; 10 mg of Fe2(SO4)3·6H2O; 0.3 mg CuSO4·5H2O; 0.11 mg of MnSO4·H2O; 17.5 mg of ZnSO4·7H2O) into 1 L of distilled water; autoclave at 121 °C for 15 min. Supplement with 1 mL of 50 mg/mL of Chloramphenicol and Streptomycin (Sigma-Aldrich, Steinheim, Germany), and 5 mL 5 mg/mL Rose Bengal and 10 mL of 1 mg/mL of Dichloran before use [21].
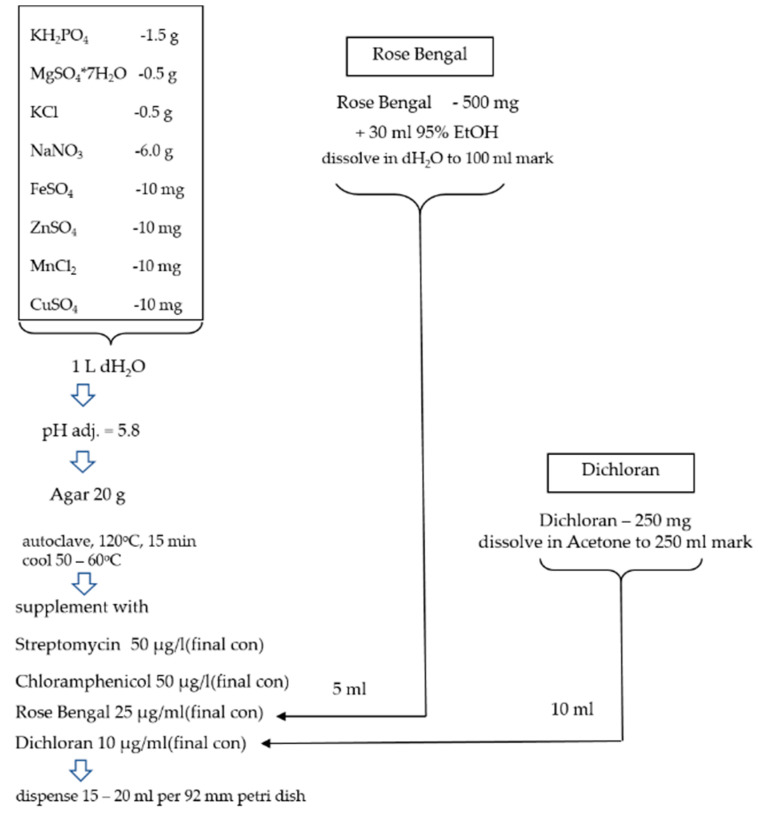
Flamingo Medium: Suspend 6.0 g NaNO3, 1.5 g KH2PO4, 0.5 g MgSO4. 7H2O, 0.5 g KCl, 10 mg of FeSO4, ZnSO4, MnCl2 and CuSO4 and agar 15 g in 1 L of distilled water (adjust pH to 5.8), autoclave at 121 °C for 15 min. Supplement with 5 mL 5 mg/mL Rose Bengal 10 mL of 1 mg/mL of Dichloran and 1 mL of 50 mg/mL of Chloramphenicol and Streptomycin (Sigma-Aldrich, Steinheim, Germany) before use (see Appendix A).
MEA-RB: Suspend 30 g Malt Extra Agar (Sigma-Aldrich, Steinheim, Germany) and 15 g of agar in 1 L of distilled water, autoclave at 121 °C for 15 min. Supplement with 5 mL 5 mg/mL Rose Bengal, 10 mL of 1 mg/mL of Dichloran, 1 mL of 50 mg/mL of Chloramphenicol and Streptomycin (Sigma-Aldrich, Steinheim, Germany) before use.
Collection of environmental samples: Soil samples, wood chips and green-waste samples were obtained from a previous study [22]. Plant-waste samples (decaying flower bulb waste) were collected from a farm in the province of North-Holland, The Netherlands. Samples were taken at least 100 m apart.
Air samples were collected in Wageningen using a Coriolis air sampler (Bertin, France) with a setting of 200 L/min during 2 min. Samples were taken at least 100 m apart. The airborne particles were dissolved into 15 mL of saline with 0.05% Tween 80 in a plastic cone. After collection, the liquid was used for plating on the various growth media. For the samples without visible colonies, the liquid was concentrated with 5000 rpm centrifuging (Germany) for 1 min, 10 mL supernatant was removed, and the sediment was suspended in 5 mL liquid, which was used for replating.
2.2. Isolating A. fumigatus Using Various Media
For each sample of plant waste, wood chips, green waste, and soil, 5 g was added to 10 mL sterile saline (0.8 g/L NaCl in water) with 0.05% Tween 80 and diluted. As stated above, air samples were collected in 5 mL saline. After vortexing for 2 min, 50 µL of a diluted suspension (100 to10−3) was plated on the six test media (MEA+C+S, SDA+C, DG18 and Flamingo, M-RB, and MEA-RB). Three replicates were applied for each medium. Cultures were incubated at 48 °C, which is generally used for selective growth of A. fumigatus [20] After three days of incubation, colonies of A. fumigatus and surface area covered by Mucorales species on MEA+C+S, SDA+C; MEA-RB and Flamingo medium were recorded. DG18 and M-RB plates were recorded after five days because A. fumigatus colonies were not easily recognized after three days on DG18 medium, and A. fumigatus colonies were too small and did not yet sporulate on M-RB medium. The colonies that showed Aspergillus morphology were selected and verified for A. fumigatus molecular characteristics by amplifying (PCR) and sequencing part of the ß-tubulin and carboxypeptidase-5 genes [8,12]. The genes encoding β-tubulin and carboxypeptidase-5 were amplified with the primer sets benA (forward, 5′-AATTGGTGCCGCTTTCTGG-3′; reverse, 5′-AGTTGTCGGGACGGAATAG-3′) and cxp (forward, 5′-GAACATTAGCCCCAGTTGAG-3′; reverse primer, 5′-CACTTCTTCTTGCACGTAGTC-3′), respectively. The amplified DNA fragments were purified with ExoSAP-IT™ PCR Product Cleanup Reagent (Thermo Fisher (Waltham, MA, USA)). DNA sequencing of the forward strand of each fragment was performed at the Eurofins Genomics (Ebersberg, Germany). The resulting sequences were aligned in CLUSTALW46 using the program BioEdit47.
2.3. Validation of Flamingo Medium
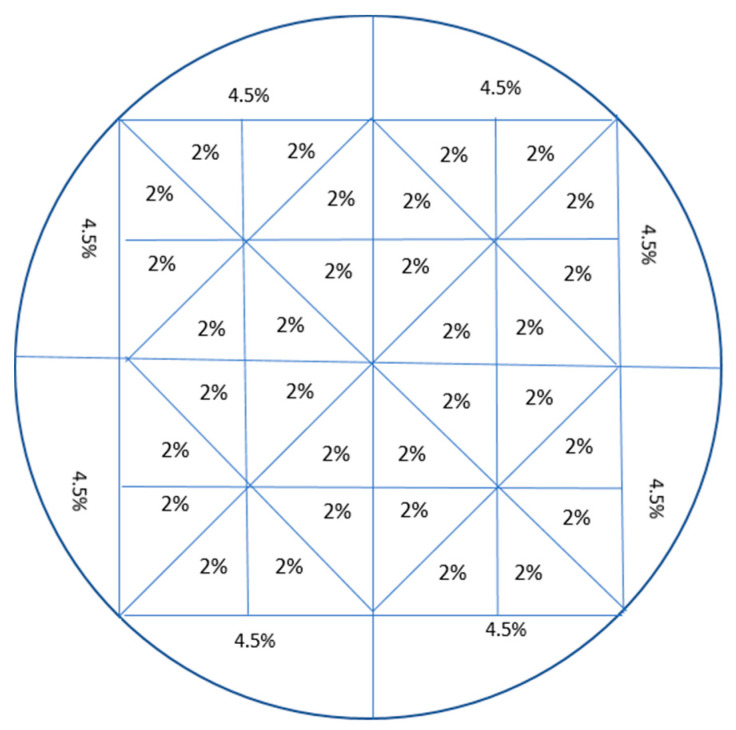
We tested whether the Flamingo Medium allows for quantitative isolation of A. fumigatus unbiased for known specific genotypes by plating different genotypes (three replicates) of A. fumigatus, either in mixed population or as single culture on both MEA and Flamingo Medium, after three days of growth at 48 °C, the total number of colonies were counted. Additionally, the selectivity of Flamingo Medium for A. fumigatus was further confirmed by testing artificial mixtures of clinical A. fumigatus and clinical Mucorales. The clinical Mucorales fungi included Rhizomucor pusillus (V103-44); Rhizopus arhieus (V204-34); Rhizopus microsporus (V154-27); Lichtheimia corymbifera (V250-74) and were cultured from patients and stored in the fungus culture collection at the Radboud University Medical Centre. If patient samples contain both Mucorales species and A. fumigatus, the latter is commonly overgrown by the Mucorales fungus, which may preclude species identification and in vitro susceptibility testing. As both fungi are thermophilic, separation by incubation at high temperature is not possible. Of each Mucorales species 50 µL of a spore suspension (concentration of 1000 CFU/g) was mixed with 50 µL of a spore suspension of clinical isolate A. fumigatus V30-40 (concentration of 1000 CFU/g) and plated on the DG18 and Flamingo Medium. After three-day incubation at 48 °C, the number of A. fumigatus was recorded. The areas covered by fungi from the Mucorales group was measured using a transparent plastic format as shown in Appendix B. Each area has defined surface size. By placing this transparent plastic format under bottom, the plates with fungal colonies, the area covered by non- A. fumigatus (fungi of Mucorales group) was estimated by adding up all grids covered by these colonies.
Statistical analyses: Data distribution analyses were performed via SPSS-Analyze-Descriptive Statistics-Explore Plots-Histogram. Significance tests for differences in detected colonies among media in detection of A. fumigatus and surface area covered by non-A. fumigatus (Mucorales) were performed with a Kruskal–Wallis test. Differences between the total A. fumigatus CFUs on the Flamingo Medium and MEA were tested for statistically significant differences with a pairwise t-test.
3. Results
3.1. Flamingo Medium Is Highly Effective for the Isolation of A. fumigatus
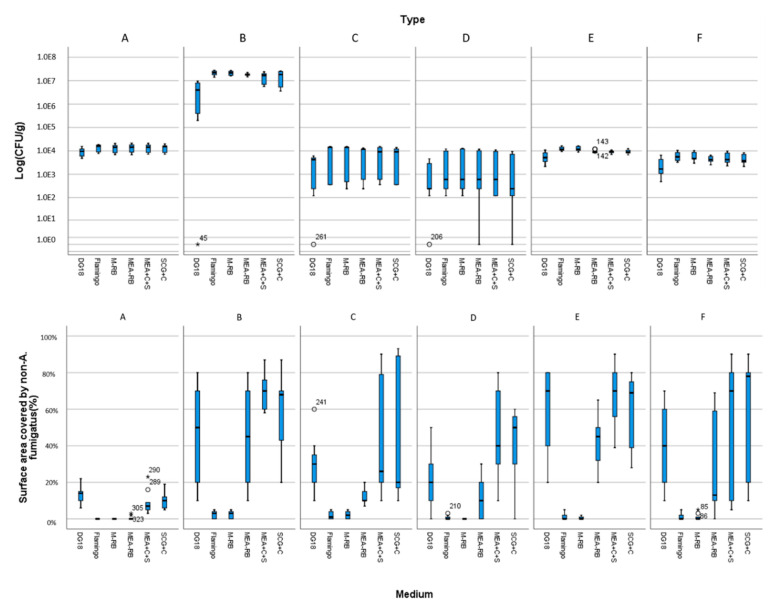
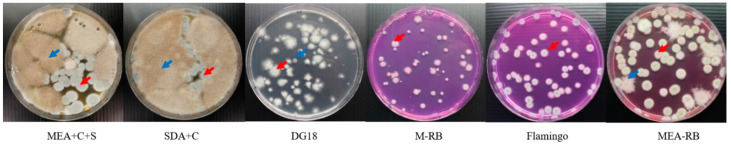
Figure 1 shows counts of A. fumigatus (above) and surface area covered by non-A. fumigatus maily Mucorales (below, Appendix C) of various samples plated on the six media that we compared for selectivity of A. fumigatus isolation. Of six media compared, on average Flamingo Medium was the most efficient isolation medium. It reduced the number of Mucorales colonies by 95% and isolated 20−30% more A. fumigatus colonies compared to the other media in a short incubation time of three days (Figure 1, Supplementary Table S1 with detailed information). MEA+C+S, SDA+C; MEA-RB and Flamingo Medium allowed for fast germination and growth of A. fumigatus after three days of incubation, Flamingo Medium best limited growth of most Mucorales species. M-RB has the same limitation of Mucorales species growth as Flamingo Medium, however, it required 5–7 days for A. fumigatus colonies to fully grow. Moreover, all colonies on the Flamingo Medium, but not all colonies on M-RB, were able to sufficiently sporulate for further culturing and testing. Carboxypeptidase-5 genes sequencing of colonies with different morphology on Flamingo Medium confirmed that all isolates are A. fumigatus. Figure 2 shows the isolation plates of A. fumigatus from one of plant-waste samples (ID: S127) on six different media.
Figure 1.
The total count of A. fumigatus detected in various samples (above) and the surface area of agar plates covered with Mucorales (below) using six different isolation media for each sample. Three biological and three technical replicates were used for each measurement. (A) Air; (B) plant waste; (C) ditch water; (D) grass/root; (E) soil; and (F) wood.
Figure 2.
The cultures grown from plant-waste sample 127 on six growth media. I MEA+C+S, SDA+C, DG18, flamingo medium (after three days of incubation at 48 °C), DG18, and M-RB (after five days of incubation at 48 °C). The red arrow points to a typical A. fumigatus colony; the blue arrow points to a typical colony of a Mucorales species.
3.2. Flamingo Medium Allows Rapid Growth for a Range of Genotypes of A. fumigatus
A series of common previously isolated environmental A. fumigatus genotypes (see Appendix D) were plated on MEA and Flamingo Medium in three replicates. We tested whether total CFUs on the Flamingo Medium were significantly different from the total counts on MEA by using a pairwise t-test after three days of incubation at 48 °C. The total number of A. fumigatus detected did not differ between the Flamingo and MEA media, both when plating a natural mixed population, and different single genotypes with different Cytochrome P450 14-alpha sterol demethylase (cyp51A) mutations.
3.3. RB and Dichloran Are Key Components in Flamingo Medium
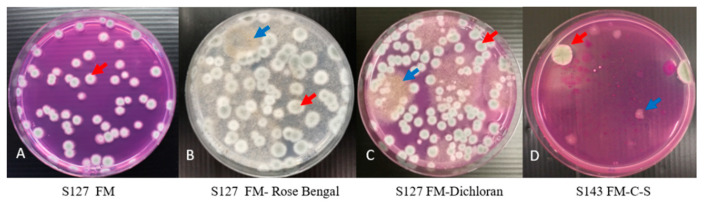
Flamingo Medium can efficiently and selectively isolate A. fumigatus from environmental samples and this medium effectively inhibited growth of 95% Mucorales from agricultural samples when these were plated on the Flamingo Medium, illustrating that the Falmingo Medium is selective. To investigate which element is critical for inhibiting the growth of Mucorales in Flamingo Medium, RB and Dichloran were removed step by step. As shown in Figure 3B,C, Mucorales were able to grow on the medium when either RB or Dichloran was omitted, suggesting that both RB and Dichloran are essential components of the Flamingo Medium to achieve the inhibitory effect.
Figure 3.
Culturing of A. fumigatus from plant-waste sample 127 on Flamingo Medium (A), and Flamingo Medium without Rose Bengal (B) and Flamingo Medium without Dichloran (C). The culture of A. fumigatus from grass/root sample 143 on the Flamingo Medium without Chloramphenicol and Streptomycin is shown in panel (D). All plates were incubated for three days at 48 °C. The red arrow points to a typical A. fumigatus colony; the blue arrow points to a typical colony of a Mucorales species. FM: Flamingo Medium.
While on Flamingo Medium the growth of Mucorales fungi is greatly reduced, on 50% of the environmental samples (mainly soil, grass, wood chips) growth of Mucorales is still faintly visible, such as grass/root sample 143 (blue arrow Figure 3D). After introducing the antibiotics of Chloramphenicol and Streptomycin into Flamingo Medium, for 22% out of 50% of environmental samples, growth of Mucorales was completely inhibited when plated. Therefore, we conclude that the combination of RB, Dichloran, Chloramphenicol and Streptomycin effectively inhibits growth of Mucorales species from the environmental samples and makes the Flamingo Medium completely selective.
3.4. The Validation of Flamingo Medium for Isolating Clinical A. fumigatus from an Artificial Mixture of Clinical Isolates of A. fumigatus and Mucorales
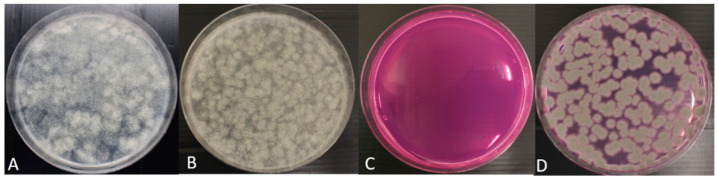
We prepared mixtures of Mucolares fungi with and without an A. fumigatus strain. These mixtures were plated on both DG18 and on Flamingo Medium. After incubation for three days at 48 °C, results showed that Mucorales fungi grew on DG18 and not on Flamingo Medium, while A. fumigatus grew on both media. Results are illustrated in Figure 4.
Figure 4.
The validation of Flamingo Medium for isolating clinical A. fumigatus by artificially mixing clinical A. fumigatus and clinical Mucorales species. (A) mixed clinical Mucorales species on DG18; (B) mixed Mucorales species and A. fumigatus (V30-40) on DG18. (C) mixed Mucorales species on Flamingo Medium. (D) mixed Mucorales species and A. fumigatus (V30-40) on Flamingo Medium. Mixture of Mucorales species consisted of Rhizomucor pusillus (V103-44), Rhizopus arhieus (V204-34), Rhizopus microsporus (V154-27), and Lichtheimia corymbifera (V250-74).
4. Discussion
This study compared six growth media for their effectiveness in isolating A. fumigatus from environmental samples in which other thermophilic fungi were present, including Mucorales species. We developed a new medium, called Flamingo Medium. Flamingo Medium produced the best results for the quantitative isolation of A. fumigatus isolates from environmental samples irrespective of their genotype, while suppressing the growth of Mucorales species tested. Growth reduction of Mucorales species was on average 95% lower on Flamingo Medium than on MEA, SDA and DG18.
Some special features of the Flamingo Medium may explain its effectiveness. (1) Compared with commonly used growth media such as MEA, SDA, and DG18, Flamingo Medium provides nitrate as sole nitrogen source, which directly limits Mucorales species growth [21]. (2) Another essential component in Flamingo Medium is Rose Bengal. This compound strongly inhibits the fast-growing Mucorales. Further, Rose Bengal is a selective agent that inhibits bacterial growth and restricts the size and height of colonies of the more rapidly growing molds [23]. We demonstrated here that Rose Bengal allows for the growth of A. fumigatus irrespective of its genotype (i.e., of the 11 genotypes tested). (3) Another crucial compound is Dichloran, as this compound affects the colony diameter and enumeration of fungi, leading to tiny colonies on the plates. This can largely suppress the fast-growing fungi such as Mucorales [24].
Antibiotics Chloramphenicol and Streptomycin are generally supplemented in the medium to suppress bacterial growth from environmental samples when isolating fungi [20,21]. These two antibiotics play a critical role especially when isolating fungi at the temperature ranging from 25 to 37 °C. When isolating A. fumigatus at the temperature of 48 °C with Flamingo Medium, Chloramphenicol and Streptomycin were not considered critical elements, which was also confirmed by all environmental samples. Bacterial growth was not observed on Flamingo Medium without Chloramphenicol and Streptomycin. However, Mucorales colonies appeared on the plates in 50% of the samples. By adding these two antibiotics, 10% of the samples became Mucorales-free. Therefore, these two antibiotics in combination with Rose Bengal and Dichloran not only inhibit growth of bacteria, but also that of Mucorales. Furthermore, since the Mucorales group consists of a large variation in species, not all Mucorales species were completely inhibited on the Flamingo Medium (such as Figure 3D), but all were suppressed to small colonies, which does not influence the isolation of A. fumigatus in general.
Separating different fungal species is critical to allow study of individual species in mixed habitats. Composting vegetation represents a habitat selective for thermophilic fungi including A. fumigatus and some Mucorales. Both are considered to play an important role in the decomposition of cellulose, and other more recalcitrant plant material [25]. Although incubation at 48 °C is effective for culturing A. fumigatus, the presence of Mucorales species in environmental samples precludes selection of individual A. fumigatus colonies. As Mucorales are fast-growing thermotolerant fungi A. fumigatus is rapidly overgrown. Flamingo medium provides an effective way to suppress the growth of various Mucorales species, while supporting the growth of A. fumigatus. Flamingo Medium can therefore be widely used for isolating A. fumigatus from all sorts of environmental samples. Directly obtaining pure A. fumigatus isolates is simplified by omitting the extra steps of purification. Additionally, we found that the Flamingo Medium can be used for isolating other fungi at lower temperatures, such as A. niger and A. flavus at 30 °C and 37 °C, respectively (data not shown).
In addition to environmental studies, Flamingo Medium might also be useful in clinical mycology for occasional cases where Aspergillus and Mucorales co-infections are observed in patients with invasive fungal diseases. In addition to the identification of the fungal pathogens, in vitro susceptibility testing is important in A. fumigatus. In-host and environmental resistance selection has been widely published, especially against medical triazoles. As a pure culture is required for in vitro susceptibility testing Flamingo Medium may be useful to select A. fumigatus. Indeed, separating Mucorales from A. fumigatus was successful, when clinical isolates were used.
Developing this method for unbiased quantitative environmental sampling of A. fumigatus is of significant importance. It will allow for more effective monitoring of A. fumigatus in any sort of environmental niches, and further understanding of the origins and spread of A. fumigatus. This will facilitate isolation of environmental A. fumigatus in the context of hot spot research for the development of azole resistance and various other research where the isolation of A. fumigatus from the environment is crucial. Additionally, Flamingo medium exhibited the potential of selectively isolating A. fumigatus from clinical samples where Mucorales species are present that commonly compromise efficient A. fumigatus isolation. This may be of use when A. fumigatus from patient material that also harbors other fungi.
Acknowledgments
We thank Mathijs Nieuwenhuis for relaxed flamingo talks. We further thank Bwalya Katati for inspiring us to develop this new medium especially for Aspergillus fumigatus. We thank Peter C. Leendertse from CLM for collecting and providing the wood chip, soil, and green waste samples.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9061155/s1, Table S1: Full dataset on total counts of A. fumigatus (CFU/g) detected in various samples and the surface area covered by Mucorales (%) using six different isolation media for each sample. Three technical replicates were used for each sample on each medium. Table S2: Supplementary data on the statistics.
Appendix A
Figure A1.
The flow of preparation showing how to prepare flamingo medium.
Appendix B
Figure A2.
The areas covered by fungi from the Mucorales group were measured using a transparent plastic format as shown below. Each area had a defined surface size. By placing this transparent plastic format under the bottom of the plates with fungal colonies, the area covered by non-A. fumigatus (fungi of the Mucorales group) was estimated by adding up all the grids covered by these colonies.
Appendix C
Figure A3.
Several non-A. fumigatus fungi grew on (non-selective) media when environmental samples were plated. These are mainly fungi from the Mucorales group.
Appendix D
Table A1.
Counts (shown as the average of three replicates ± SEM) of A. fumigatus (various types on cyp51A, mixed population, and single colonies culture) on the MEA medium and the flamingo medium after three days of growth at 48 °C.
| # A. fum Colonies on MEA Medium | # A. fum Colonies on Flamingo Medium |
Significance Test (Pairwise t-Test) |
|
|---|---|---|---|
| Environmental mixed population Mucor-free S56 | 47 ± 5 | 50 ± 6 | p > 0.05 |
| Environmental mixed population Mucor-free S81 | 156 ± 7 | 139 ± 17 | p > 0.05 |
| Environmental mixed population Mucor-free S72 | 86 ± 3 | 81 ± 2 | p > 0.05 |
| Mixture of TR34/L98H, WT, TR46/Y121F/T289A | 75 ± 5 | 79 ± 3 | p > 0.05 |
| Environmental WT (81-4) | 65 ± 3 | 61 ± 3 | p > 0.05 |
| Environmental TR34/L98H (66-3) | 73 ± 4 | 76 ± 6 | p > 0.05 |
| Environmental isolate TR343/L98H (30-40-1) | 80 ± 4 | 84 ± 3 | p > 0.05 |
| Environmental TR46/Y121F/T289A (50-32) | 69 ± 7 | 66 ± 4 | p > 0.05 |
| Environmental isolate TR463/Y121F/T289A (70-28) | 75 ± 6 | 69 ± 5 | p > 0.05 |
| Environmental isolate TR464/Y121F/T289A (39-2) | 86 ± 5 | 83 ± 3 | p > 0.05 |
| Environmental resistant isolate without tandem repeats (72-8) | 96 ± 5 | 92 ± 5 | p > 0.05 |
Author Contributions
Conceptualization, methodology, validation, formal analysis, investigation, data curation, J.Z. and A.J.M.D.; resources, P.E.V.; draft preparation, J.Z., P.E.V., S.E.S. and A.J.M.D.; writing—review & editing, J.Z., P.E.V., A.J.M.D. and S.E.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Netherlands Organization for Scientific Research (NWO) under the Groen III program (GROEN.2019.002): One health consequences of circularity—What lessons to learn from the saprophytic and human pathogenic fungus Aspergillus fumigatus?
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Verweij P.E., Snelders E., Kema G.H., Mellado E., Melchers W.J. Azole resistance in Aspergillus fumigatus: A side-effect of environmental fungicide use? Lancet Infect. Dis. 2009;9:789–795. doi: 10.1016/S1473-3099(09)70265-8. [DOI] [PubMed] [Google Scholar]
- 2.Lestrade P.P.A., Buil J.B., van der Beek M.T., Kuijper E.J., van Dijk K., Kampinga G., Rijnders B.J.A., Vonk A., de Greef S.C., Schoffelen A.F., et al. Paradoxal trends in azole-resistant Aspergillus fumigatus in a national multicenter surveillance program, The Netherlands, 2013–2018. Emerg. Infect. Dis. 2020;26:1447–1455. doi: 10.3201/eid2607.200088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Verweij P.E., Chowdhary A., Melchers W.J.G., Meis J.F. Azole Resistance inAspergillus fumigatus: Can We Retain the Clinical Use of Mold-Active Antifungal Azoles? Clin. Infect. Dis. 2016;62:362–368. doi: 10.1093/cid/civ885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buil J.B., Hare R.K., Zwaan B.J., Arendrup M.C., Melchers W.J.G., Verweij P.E. The fading boundaries between patient and environmental routes of triazole resistance selection in Aspergillus fumigatus. PLoS Pathog. 2019;15:e1007858. doi: 10.1371/journal.ppat.1007858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lestrade P.P., Bentvelsen R.G., Schauwvlieghe A.F.A.D., Schalekamp S., van der Velden W.J., Kuiper E.J., van Paassen J., van der Hoven B., van der Lee H.A., Melchers W.J., et al. Voriconazole resistance and mortality in invasive aspergillosis: A multicenter retrospective cohort study. Clin. Infect. Dis. 2019;68:1463–1471. doi: 10.1093/cid/ciy859. [DOI] [PubMed] [Google Scholar]
- 6.Schoustra S.E., Debets A.J., Rijs A.J., Zhang J., Snelders E., Leendertse P.C., Melchers W.J., Rietveld A.G., Zwaan B.J., Verweij P.E. Environmental hotspots for azole resistance selection of Aspergillus fumigatus, The Netherlands. Emerg. Infect. Dis. 2019;25:1347. doi: 10.3201/eid2507.181625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Snelders E., Camps S.M., Karawajczyk A., Schaftenaar G., Kema G.H., van der Lee H.A., Klaassen C.H., Melchers W.J., Verweij P.E. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS ONE. 2012;7:e31801. doi: 10.1371/journal.pone.0031801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J., Van Den Heuvel J., Debets A.J., Verweij P.E., Melchers W.J., Zwaan B.J., Schoustra S.E. Evolution of cross-resistance to medical triazoles in Aspergillus fumigatus through selection pressure of environmental fungicides. Proc. Biol. Sci. 2017;284:20170635. doi: 10.1098/rspb.2017.0635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowdhary A., Kathuria S., Xu J., Meis J.F. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog. 2013;9:e1003633. doi: 10.1371/annotation/4ffcf1da-b180-4149-834c-9c723c5dbf9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rybak J.M., Fortwendel J.R., Rogers P.D. Emerging threat of triazole-resistant Aspergillus fumigatus. J. Antimicrob. Chemother. 2019;74:835–842. doi: 10.1093/jac/dky517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang J., Snelders E., Zwaan B.J., Schoustra S.E., Meis J.F., Van Dijk K., Hagen F., Van Der Beek M.T., Kampinga G.A., Zoll J. A novel environmental azole resistance mutation in Aspergillus fumigatus and a possible role of sexual reproduction in its emergence. mBio. 2017;8:e00791-17. doi: 10.1128/mBio.00791-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang S.E., Sumabat L.G., Melie T., Mangum B., Momany M., Brewer M.T. Evidence for the agricultural origin of antimicrobial resistance in a fungal pathogen of humans. bioRxiv. 2020 doi: 10.1101/2020.05.24.113787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber A.E., Riedel J., Sae-Ong T., Kang K., Brabetz W., Panagiotou G., Deising H.B., Kurzai O. Effects of agricultural fungicide use on Aspergillus fumigatus abundance, antifungal susceptibility, and population structure. mBio. 2020;11:e02213-20. doi: 10.1128/mBio.02213-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viegas C., Almeida B., Aranha Caetano L., Afanou A., Straumfors A., Veríssimo C., Gonçalves P., Sabino R. Algorithm to assess the presence of Aspergillus fumigatus resistant strains: The case of Norwegian sawmills. Int. J. Environ. Health Res. 2020;8:1–9. doi: 10.1080/09603123.2020.1810210. [DOI] [PubMed] [Google Scholar]
- 15.Siopi M., Rivero-Menendez O., Gkotsis G., Panara A., Thomaidis N.S., Alastruey-Izquierdo A., Pournaras S., Meletiadis J. Nationwide surveillance of azole-resistant Aspergillus fumigatus environmental isolates in Greece: Detection of pan-azole resistance associated with the TR46/Y121F/T289A cyp51A mutation. J. Antimicrob. Chemother. 2020;75:3181–3188. doi: 10.1093/jac/dkaa316. [DOI] [PubMed] [Google Scholar]
- 16.Engel T.G.P., Tehupeiory-Kooreman M., Melchers W.J.G., Reijers M.H., Merkus P., Verweij P.E. Evaluation of a new culture protocol for enhancing fungal detection rates in respiratory samples of cystic fibrosis patients. J. Fungi. 2020;6:82. doi: 10.3390/jof6020082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Hoog G.S., Guarro J., Gené J., Figueras M. Atlas of Clinical Fungi. Centraalbureau voor Schimmelcultures (CBS); Hilversum, The Netherlands: 2000. [Google Scholar]
- 18.Larone D. Part II: Detailed Description. Medically Important Fungi: A Guide to Identification. 3rd ed. ASM Press; Washington, DC, USA: 1995. [Google Scholar]
- 19.Richardson M.D., Rautemaa-Richardson R. Biotic environments supporting the persistence of clinically relevant Mucormycetes. J. Fungi. 2019;6:4. doi: 10.3390/jof6010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J., Lopez Jimenez L., Snelders E., Debets A.J.M., Rietveld A.G., Zwaan B.J., Verweij P.E., Schoustra S.E. Dynamics of aspergillus fumigatus in azole fungicide-containing plant waste in The Netherlands (2016–2017) Appl. Environ. Microbiol. 2021;87 doi: 10.1128/aem.02295-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotty P.J. Comparison of four media for the isolation of Aspergillus flavus group fungi. Mycopathologia. 1994;125:157–162. doi: 10.1007/BF01146521. [DOI] [PubMed] [Google Scholar]
- 22.Leendertse P.C., Gommer R., van Beek Culemborg J. Groen-en houtafval als bron van azolen-resistente schimmel, deel A: Deskstudie. CLM-Rapport 1065. 2021 in press. [Google Scholar]
- 23.Gutarowska B., Piotrowska M. Methods of mycological analysis in buildings. Build. Environ. 2007;42:1843–1850. doi: 10.1016/j.buildenv.2006.02.015. [DOI] [Google Scholar]
- 24.Henson O.E. Dichloran as an inhibitor of mold spreading in fungal plating media: Effects on colony diameter and enumeration. Appl. Environ. Microbiol. 1981;42:656–660. doi: 10.1128/AEM.42.4.656-660.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richardson J.P., Naglik J.R. Special issue: Mucosal fungal infections. J. Fungi. 2018;4:43. doi: 10.3390/jof4020043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.