Abstract
This systematic review and meta-analysis evaluated antimicrobial photodynamic therapy (aPDT) efficacy in periodontitis. The review protocol was conducted in accordance with PRISMA statements, Cochrane Collaboration recommendations and is registered in PROSPERO (CRD 42020161516). Electronic and hand search strategies were undertaken to gather data on in vivo human RCTs followed by qualitative analysis. Differences in probing pocket depth (PPD) and clinical attachment level (CAL) were calculated with 95% confidence intervals and pooled in random effects model at three and six months. Heterogeneity was analyzed, using Q and I2 tests. Publication bias was assessed by visual examination of the funnel plot symmetry. Sixty percent of 31 eligible studies showed a high risk of bias. Meta-analysis on 18 studies showed no additional benefit in split mouth studies in terms of PPD reduction (SMD 0.166; 95% CI −0.278 to 0.611; P = 0.463) and CAL gain (SMD 0.092; 95% CI −0.013 to 0.198; P = 0.088). Similar findings noted for parallel group studies; PPD reduction (SMD 0.076; 95% CI −0.420 to 0.573; P = 0.763) and CAL gain (SMD 0.056; 95% CI −0.408 to 0.552; P = 0.745). Sensitivity analysis minimized heterogeneity for both outcome variables; however, intergroup differences were not statistically significant. Future research should aim for well-designed RCTs in order to determine the effectiveness of aPDT.
Keywords: antimicrobial photodynamic therapy, periodontitis, scaling and root planing, systematic review, meta-analysis
Highlights
Limitations of scaling and root planing (SRP) have directed the research to assess alternative comprehensive treatment strategies.
Antimicrobial Photodynamic therapy (aPDT) involves photo-excitation of photosensitizer dye upon illumination by a light of a matched wavelength.
This systematic review and meta-analysis evaluated the effectiveness of aPDT in the treatment of periodontitis.
In spite of the inconsistencies in their findings and methodological bias, the majority of the studies have demonstrated aPDT effectiveness.
The efficacy of aPDT in improving treatment outcomes when it is utilized in the non-surgical management of periodontitis remains debatable.
1. Introduction
Antimicrobial Photodynamic therapy (aPDT) involves photo-excitation, which occurs when a photosensitizer (PS) dye is illuminated by a light of a matched wavelength, resulting in its activation and stimulation of a phototoxic response in the presence of ambient oxygen [1]. It has been persistently observed that bacterial recolonizations of Aggregatibacter actinomycetemcomitans (A.a) occur in periodontal pockets even after scaling and root planing (SRP) [2]. Aggressive periodontitis (AgP) is frequently associated with fewer local etiologic factors; therefore, it is believed that the affected patients are more likely to benefit from the antimicrobial effect of aPDT [3]. In contrast, chronic periodontitis (CP) patients usually have complex and thick deposits of polymicrobial communities on the affected root surfaces [4]. This may hamper penetration of PS, thereby reducing its effect and leading to an increase in the ‘red complex’ bacterial counts within a short period of time, resulting in a disease relapse [5]. Hence, the concept of replacing conventional SRP with aPDT is a controversial one with several imperative demerits, as enlisted above.
Utilization of adjunctive aPDT and its comparison with the gold standard SRP is a concept that has been studied extensively in both CP and AgP patients [6,7,8,9,10,11,12,13,14,15,16]. While SRP can quantitively lower the biomass of bacteria, aPDT has a more qualitative approach of a non-invasive nature, by creating alterations in cell membranes or Deoxyribonucleic Acid (DNA) damage [5]. Hence, a combination of these two therapies can be vouched for, since their mechanisms of action on microbiota and role in the periodontal repair process is distinct from the other and thus might have synergistic effects [17].
Distant sites of infection such as tonsils or base of tongue, which are affected due to the spread of tissue penetrating periopathogens, can be successfully reduced with local or systemic antibiotics (AB) [18]. Nonetheless, many clinicians often conduct NSPT without adjunctive AB, which is only used when initial treatment has failed [19]. In AgP, evidence suggests that SRP+AB therapy does not show satisfactory long-term results, unless re-instrumentation of affected sites is performed, as an additional step in the maintenance phase [20]. Furthermore, owing to the development of antibiotic resistant strains, it has been suggested that AB usage should be restricted to those with a highly active disease or a specific microbiological profile [21]. In order to maintain an adequate mean inhibitory concentration (MIC) of any antimicrobial drug, either a sustained-release carrier medium is required or, conversely, a prompt bactericidal approach is needed to overcome the problem of physical displacement from the sulcus [22]. The aPDT falls into the latter category, demonstrating a 4--6-fold logarithmic bacterial reduction within a time frame of 60 s along with repeated applications [23]. A comprehensive assessment to evaluate the impact of these new trials on the role of aPDT in the treatment of periodontitis is unresolved, owing to the diversity in the methodology and results of existing scientific evidence [6,7,8,9,10,11,12,13,14,15,16].
In lieu of the prevailing pertinent literature, the present systematic review and meta-analysis aimed to provide a systematic evaluation of available scientific evidence to determine the efficacy of aPDT in the treatment of periodontitis. The objectives of this critical review were to evaluate the outcomes of this treatment strategy through various PS-laser wavelength combinations, as well as the laser parameters, in order to deduce an ideal PS-laser wavelength combination and treatment protocol for future scientific research.
2. Materials and Methods
2.1. Protocol and Registration
The present systematic review was reported based on the guidelines of Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Statement and Cochrane Collaboration recommendations (Supplementary file 1) [24,25]. The review protocol is published in the Prospective Register of Systematic Reviews (PROSPERO); ref CRD 42020161516.
2.2. Population (P), Intervention (I), Comparison (C) and Outcomes (O)—PICO
Population: Patients diagnosed with Periodontitis (CP or AgP) [26]
Intervention: Utilisation of aPDT as a monotherapy or as an adjunct to SRP
Comparison: Utilisation of SRP alone or SRP with adjunctive AB therapy
Outcome: Evaluation of clinical and/or microbiological and/or immunological profiles
2.3. Focused Research Question
Is aPDT effective as a primary mode of treatment or as an adjunct to SRP compared to SRP alone or in combination with local or systemic antibiotics (AB), in terms of clinical or microbiological or immunological profiles, in patients with Periodontitis?
2.4. Search Strategy
The search strategy only included terms relating to or describing the study’s domain and intervention. The use of relevant free text keywords and medical subject heading (Mesh) terms, which were logically connected with the help of Cochrane MEDLINE filters for controlled trials of interventions, was implemented. Individual search algorithms were developed for the following databases: MEDLINE (NCBI PubMed and PMC), Cochrane Central Register of Controlled Trials (CCRCT), Scopus, ScienceDirect, Google Scholar, EMBASE and EBSCO. Electronic search databases were searched thoroughly from their earliest records until 31 December 2019. The following journals were manually searched: Journal of Periodontology, Photomedicine and Laser Surgery, Clinical Oral Investigation, Journal of Clinical Periodontology, Journal of Dental Research, Lasers in Medical Science, Journal of Photochemistry and Photobiology and Photodiagnosis and Photodynamic Therapy. Related review articles and reference lists of all identified articles were searched through for further studies. Abstracts of the American Academy of Periodontology (AAP) and the European Federation of Periodontology (EFP) as well as sources for grey literature were screened to detect unpublished studies. In some instances, an attempt was made to establish a communication with the corresponding author in an attempt to obtain additional information related to the study; however, the attempts were unsuccessful. Search strategy was performed by two blinded, independent reviewers (S.D. and R.H.). In order to assess inter-reviewer reliability analysis, Kappa (κ) statistics were performed and a minimum value of 0.8 was considered acceptable [27]. In case of any disagreements, reviewers would discuss the discrepancies with a third author (S.B.), if necessary.
2.5. Search Algorithms
“Photodynamic therapy” OR “photochemotherapy”
AND
“Scaling” OR “Root planing” OR “non-surgical periodontal therapy”
AND
“Periodontitis” OR “Chronic Periodontitis” OR “Aggressive Periodontitis” OR “Early Onset Periodontitis”
2.6. Eligibility Criteria
2.6.1. Inclusion Criteria
Subjects diagnosed with CP or AgP according to 1999 AAP Classification of Periodontal diseases and conditions [26].
Studies included: In vivo human RCT’s comparing the efficacy of aPDT in CP or AgP as monotherapy or adjunctive to SRP compared to SRP alone or in combination with AB.
Parallel group (PG) and split-mouth (SM) studies.
Age group >18 years, fit and healthy subjects.
No language restrictions for search strategy.
Studies that have utilized any PS dye (regardless dose and incubation period) and laser wavelength combination.
Studies reporting at least one of the following parameters as an outcome variable: probing pocket depth (PPD), loss of clinical attachment level (CAL), bleeding on probing (BOP), plaque index (PI), gingival index (GI), microbiological profile, or immunological profile.
Studies with a minimum follow-up period of at least one month after treatment.
2.6.2. Exclusion Criteria
Subjects with systemic diseases or on medications that can influence the outcome variables.
Subjects who have undergone any periodontal therapy and/or antibiotic therapy in the last six months prior to RCT enrolment.
Studies utilizing low level laser therapy or laser therapy alone, as one of the intervention groups as compared to aPDT.
Studies involving utilization of aPDT for residual pockets or in supportive periodontal therapy (SPT).
Studies that have utilized light emitting diodes (LEDs) as a light source.
No outcome variable of interest.
Pregnancy.
Smoking.
Narrative and systematic reviews, in vitro studies, in vivo animal studies, commentaries, interviews, updates, case series and case reports.
2.7. Systematic Review Outcomes
2.7.1. Primary Outcome Measures
Changes in PPD and CAL from baseline up to the end of follow-up.
2.7.2. Secondary Outcome Measures
Changes in GR, BOP, PI, GI, microbiological and immunological profile from baseline up to the end of follow-up.
2.8. Data Extraction
Two reviewers independently (S.D. and R.H.) selected eligible studies from the search. They performed the review, assessment and data extraction for each eligible study. Each study received an identification with the name of the first author, year of publication and origin. A tabular representation of additional relevant information such as impact factor of journal, study design, sample size, demographics of the participants, baseline characteristics, intervention and comparator groups, type of photosensitizer used and dosage, laser parameters utilized, number of aPDT sessions performed, follow-up duration, statistical tests performed and results and conclusions, were gathered from each eligible study.
2.9. Qualitative Analysis
A qualitative assessment for each study was performed using the Revised Cochrane Risk-of-Bias (RoB) tool for Randomized trials, Version 2.0 (RoB 2) by two independent reviewers (S.D. and R.H.) [28,29,30]. Detailed assessment under the following headings was performed: 1. Bias arising from the randomization process; 2. Bias due to deviations from intended interventions; 3. Bias due to missing outcome data; 4. Bias in measurement of the outcome; 5. Bias in selection of the reported result. Depending upon fulfilment of above-mentioned criteria, the studies were determined as low, moderate or high RoB. Disagreements between the reviewers were resolved by discussion with a third author (S.B.) as well as use of ‘discrepancy check’ feature in RoB 2, in order to obtain consensus.
2.10. Statistical Analysis of Data
When appropriate and quantifiable data of interest were extracted from the eligible studies and combined for meta-analyses, using Stata version 15.1 software (StataCorp, Pyrmont, Australia), random effects meta-analyses were conducted to reflect the expected heterogeneity. As continuous outcomes were expected, overall treatment effects were calculated through pooled standardized mean differences (SMDs) with associated 95% confidence intervals (95% CIs) for PPD and CAL. When information was presented in median and inter-quartile ranges, means and SDs were estimated [31]. Results from SM and PG studies were pooled separately at 3 and 6 months, respectively. A pooled overall effect was considered statistically significant when p < 0.05. Consequently, statistical heterogeneity to identify outlier studies was performed by visual inspection of forest plots. Additionally, the Cochran Q test was conducted to assess statistical heterogeneity (p < 0.10) [32]. I2 statistics for homogeneity was expressed in a range of 0–100%, with the following interpretation; 0% = no evidence of heterogeneity; 30–60% = moderate heterogeneity; 75–100% = high heterogeneity [33]. Sensitivity analysis was conducted to negate the effect of heterogeneity in between included studies by identifying the outlier studies by visual inspection of forest plots [34]. Publication bias was evaluated by visual assessment of funnel plot symmetry.
3. Results
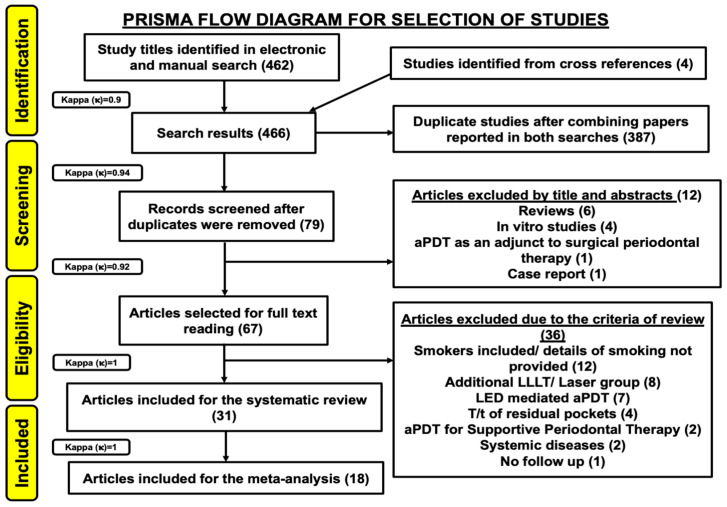
3.1. Study Selection
Four hundred and sixty-two study titles were obtained from a combined electronic and manual search. Four study titles were obtained from cross-references. Therefore, a total of 466 study titles were included from all databases in the preliminary screening (inter-reviewer agreement, κ = 0.9). Three hundred and eighty-seven articles were excluded, due to duplication and the remaining 79 records were further evaluated (inter-reviewer agreement, κ = 0.94). Twelve articles were excluded based on their titles and abstracts, mainly due to an inappropriate study design (inter-reviewer agreement, κ = 0.92). Thus, 67 articles were assessed for their eligibility. These articles were evaluated based on eligibility criteria. Additionally, 36 studies were excluded due to following reasons: Smokers were included or smoking details were not provided in 12 studies [23,35,36,37,38,39,40,41,42,43,44,45]; Laser or LLLT was utilized, as an adjunct to SRP in eight studies [46,47,48,49,50,51,52,53]; LED-aPDT was performed in seven studies [54,55,56,57,58,59,60]; aPDT was used in management of residual pockets in four studies [61,62,63,64] and as an adjunct to supportive periodontal therapy in two studies [65,66]; patients with systemic diseases were included in two studies [67,68], whereas one study did not perform a follow-up assessment [69] (inter-reviewer agreement, κ = 1). Hence, out of 67 full text articles, 31 articles were included and analyzed in the present systematic review [2,3,5,17,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96]. All included articles were in vivo human studies. A meta-analysis on 18 out of 31 studies which assessed efficacy of SRP+aPDT was conducted [17,71,73,74,75,76,80,81,84,85,86,88,89,90,91,92,93,96] (inter-reviewer agreement, κ = 1). Figure 1 depicts the PRISMA flow diagram for search strategy utilized in the present systematic review and meta-analysis.
Figure 1.
PRISMA flow diagram of the study selection criteria.
3.2. Study Characteristics
3.2.1. Country of Origin
A substantial diversity in the country of origin was noted amongst included papers (Table 1). Distribution of studies was as follows: 11 in Brazil [2,3,5,17,71,82,83,87,92,95,96], 6 in India [78,81,88,89,91,93], 4 in Germany [76,77,80,94], 4 in Iran [70,85,86,90], 3 in Poland [72,73,74], whereas there is 1 study each, in the following countries; Spain [75], Japan [79], Thailand [84].
Table 1.
Tabular representation of eligible in vivo human RCTs in terms of demography, study design, intervention groups, methods of assessment, evaluation period and outcomes. Refer to Supplementary file 2 for list of abbreviations.
| Study, Year, Origin and Citation | Journal Name/ Impact Factor (IF) |
Study Design | Type of Periodontitis | Sample Size (n) | Gender M/F |
Age (Years) (Mean ± SD) | Intervention Groups | Evaluation Period | Parameters Assessed | Conclusions | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De Oliveira et al., 2009 (Brazil) [2] | Journal of Periodontology IF 2020: 3.742 IF 2009: 2.580 |
SM-RCT | AgP (A minimum of 20 teeth (mean, 26 teeth) with at least one tooth in each posterior sextant and at least one posterior sextant with a minimum of three natural teeth; ≥5 mm of attachment loss around at least seven teeth involved, excluding first molars and central incisors) |
10 | 2/8 | 18–35 Mean: 31.01 ± 4.43 |
SRP (Hand instruments) (10 teeth) |
aPDT (10 teeth) |
−7 (baseline), 0 (immediately after interventions), +1, +7, +30 and +90 days. |
TNF-α and RANKL assessment | NSPT with PDT or SRP led to statistically significant reductions in TNF-a level 30 days following treatment (p < 0.05) with no statistically significant intergroup differences (p > 0.5). | ||
| De Oliveira et al., 2007 (Brazil) [3] | Journal of Periodontology IF 2020: 3.742 IF 2007: 2.426 |
SM-RCT | AgP (A minimum of 20 teeth (mean, 26 teeth) with at least one tooth in each posterior sextant and at least one posterior sextant with a minimum of three natural teeth; ≥5 mm of attachment loss around at least seven teeth involved, excluding first molars and central incisors) |
10 | 2/8 | 18–35 Mean: 31.01 ± 4.43 |
SRP (Hand instruments) (10 teeth) |
aPDT (10 teeth) |
Baseline, 3 months | PD, RCAL, GR, PI, GI, BOP | PDT and SRP showed statistically significant clinical results (p < 0.05) in the non-surgical treatment of aggressive periodontitis with no statistically significant differences (p > 0.5) in intergroup comparison. | ||
| Novaes et al., 2012 (Brazil) [5] | Lasers in Medical Science IF 2019: 2.574 IF 2012: 2.645 |
SM-RCT | AgP (A minimum of 20 teeth (mean, 26 teeth) with at least one tooth in each posterior sextant, and at least one posterior sextant with a minimum of three natural teeth; ≥5 mm of attachment loss around at least seven teeth involved, excluding first molars and central incisors) |
10 | 2/8 | 18–35 Mean: 31 |
SRP (Hand instruments) |
aPDT | −7, 0 (Baseline), and 3 months | Plaque sample analysis for estimation of 40 subgingival species using DNA-DNA hybridization. | aPDT was more effective in reducing the counts of A.a (p = 0.00) whereas, SRP reduced red complex bacteria. Combination of both treatment methods would be beneficial for the non-surgical treatment of AgP | ||
| Franco et al., 2014 (Brazil) [17] | Photodiagnosis and Photodynamic Therapy IF 2020: 2.894 IF 2014: 2.359 |
SM-RCT | CP (At least 20 teeth with at least one posterior tooth in each quadrant, and periodontal pockets ≥ 5 mm on at least seven teeth) |
15 | NI | 39.5 | SRP (Hand instruments) |
SRP+aPDT | Baseline and 90 days | BOP, PI, PD, CAL, qPCR gene expression analysis. | Significant improvement in BOP was noted with aPDT group (p = 0.03). PDT increased the expression of RANK and OPG, which could indicate a reduction in osteoclastogenesis. Furthermore, the use of PDT in conjunction with conventional treatment significantly increased the expression of FGF2, which has an important role in the periodontal repair process. | ||
| Pourabbas et al., 2014 (Iran) [70] | Journal of Periodontology IF 2020: 3.742 IF 2014: 2.900 |
SM-RCT | CP (≥12 natural teeth with a minimum of three in each quadrant; ≥3 mm attachment loss in about a minimum of 30% of the existing teeth; ≥1 site per quadrant with PPD of ≥4 mm and BOP) |
24 | 10/14 | 46 ± 8 | SRP (Sonic and hand instruments) |
SRP+aPDT | Baseline and 3 months | PD, BOP, CAL, GR, IL-1β, TNF-α, MMP-8 and MMP-9 analysis | Intragroup comparison showed significant improvements (p < 0.001) for all variables in 3-month follow-up compared with baseline. TNF-α was significantly improved in the SRP+aPDT versus SRP group (p < 0.001). Total levels of PMNs were reduced for all patients compared with baseline levels (p < 0.001). | ||
| Moreira et al., 2015 (Brazil) [71] | Journal of Periodontology IF 2020: 3.742 IF 2015: 3.159 |
SM-RCT | AgP (A minimum of 20 teeth and two pairs of single rooted contralateral teeth with proximal sites presenting PD and CAL ≥ 5 mm) |
20 | 2/18 | 18–35 30.6 ± 4.25 |
SRP + sham procedure (Hand and ultrasonic instruments) 40 teeth/128 sites |
SRP+aPDT 40 teeth/135 sites |
Baseline,3 months | PD, CAL, GR, PI, BOP Microbiological analysis for counts of 40 bacterial species using DNA- DNA Hybridization Immunological evaluation for GCF levels of IL-1β, IL- 10 and TNF-α. |
In deep periodontal pockets analysis (PD ≥ 7 mm at baseline), Test Group presented a decrease in PD and a clinical attachment gain significantly higher than Control Group at 90 days (p < 0.05). Test Group also demonstrated significantly less periodontal pathogens of red and orange complexes and a lower ratio IL-1β/IL-10 than Control Group (p < 0.05). Four adjunctive sessions of aPDT after SRP have clinical, microbiological and immunological benefits over SRP alone in management of AgP. | ||
| Skurska et al., 2015 (Poland) [72] | BMC Oral Health IF 2019: 1.911 IF 2015: 1.605 |
PG-RCT | AgP (At least 3 sites with PD ≥ 6 mm) |
35 SRP+AB: 17 SRP+aPDT:18 |
12/24 SRP+aPDT: 7/10 SRP+AB: 5/13 |
23–55 SRP+aPDT: 37.3 ± 8.0 SRP+AB: 34.7 ± 9.0 |
SRP+ AB 141 sites AB: 375 mg of amoxicillin + 250 mg of metronidazole TDS for 7 days, starting on the day of SRP (Hand and ultrasonic instruments) |
SRP+aPDT 137 sites |
Baseline, 3 and 6 months | MMP-8 and MMP-9 assessment | In the AB group, patients showed a statistically significant (p = 0.01) decrease of MMP-8 GCF level at both 3- and 6-months post treatment. In the PDT group, the change of MMP-8 GCF level was not statistically significant. Both groups showed at 3 and 6 months a decrease in MMP-9 levels. However, this change did not reach statistical significance. SRP+AB is more effective in reducing GCF MMP-8 levels compared to SRP+aPDT. | ||
| Arweiler et al., 2014 (Poland) [73] | Clinical Oral Investigations IF 2019: 2.903 IF 2014: 2.704 |
PG-RCT | AgP (At least 3 sites with PD ≥ 6 mm) |
35 SRP+aPDT: 17 SRP+AB: 18 |
12/24 SRP+aPDT: 7/10 SRP+AB: 5/13 |
23–55 SRP+aPDT: 37.3 ± 8.0 SRP+AB: 34.7 ± 9.0 |
SRP+AB 141 sites AB: 375 mg Amoxicillin + 250 mg Metronidazole TDS for 7 days (starting from day of SRP) (Hand and ultrasonic instruments) |
SRP+aPDT 137 sites |
Baseline, 6 months | PD, CAL, GR, PI, BOP, FMPI, FMBOP | Intragroup comparison revealed statistically significant PD reduction from baseline (p < 0.001). SRP+AB showed significant differences in PD reduction and lower number of deep pockets ≥ 7 mm (p < 0.001) as compared to SRP+aPDT (p = 0.03). | ||
| Arweiler et al., 2013 (Poland) [74] | Schweiz Monatsschr Zahnmed IF 2020: NA IF 2013: NA |
PG-RCT | AgP (At least 3 sites with PD ≥ 6 mm) |
35 SRP+aPDT: 17 SRP+AB: 18 |
12/24 SRP+aPDT: 7/10 SRP+AB: 5/13 |
23–55 SRP+aPDT: 37.3 ± 8.0 SRP+AB: 34.7 ± 9.0 |
SRP+AB 141 sites AB: 375mg Amoxicillin+250 mg MTZ TDS for 7 days (starting from day of SRP) (Hand and ultrasonic instruments) |
SRP+aPDT 137 sites |
Baseline, 3 months | PD, CAL, GR, PI, BOP, FMPI, FMBOP | SRP+AB showed significant differences in PD reduction, CAL gain and lower number of deep pockets ≥ 7 mm as compared to SRP+aPDT (p < 0.001). | ||
| Vidal et al., 2017 (Spain) [75] | Journal of Clinical Periodontology IF 2020: 5.241 IF 2017: 4.165 |
PG-RCT | CP (Four or more periodontal pockets with a PPD ≥ 5 mm and BOP) |
37 | 11/26 | 55 ± 2 | SRP (Hand and ultrasonic instruments) |
SRP+aPDT | Baseline, 5, 13 and 25 weeks | PI, PD, GR, CAL, BOP, GCF volume, microbiological and biochemical parameters | RANKL and abundance of A.a was significantly decreased in the SRP+aPDT group compared with the SRP group (p < 0.05). Except of a reduction in A.a, SRP+ aPDT resulted in no additional improvement compared with SRP alone. | ||
| Braun et al., 2008 (Germany) [76] | Journal of Clinical Periodontology IF 2020: 5.241 IF 2008: 3.525 |
SM-RCT | CP (At least one premolar and one molar in every quadrant with a minimum of four teeth each; at least one tooth with an attachment loss of >3 mm in every quadrant) |
20 | 9/11 | 46.6 ± 6.1 | SRP (Hand and piezo- electric ultrasonic instruments) |
SRP+aPDT | Baseline, 1 week, 3 months | SFFR, BOP, RAL PD, GR | Values for RAL, PD, SFFR and BOP decreased significantly 3 months after treatment in the control group with a higher impact on the sites treated with adjunctive aPDT (p < 0.05). GR increased 3 months after treatment with and without adjunctive aPDT, with no difference between the groups (p > 0.05). In patients with CP, clinical outcomes can be improved by adjunctive aPDT. | ||
| Berakdar et al., 2012 (Germany) [77] | Head and Face Medicine IF 2020: 1.492 IF 2012: 1.519 |
SM-RCT | CP (At least four teeth with a PPD of ≥5 mm) |
22 | 12/10 | 59.3 ± 11.7 | SRP (Hand instruments) |
SRP+aPDT | Baseline, 1, 3 and 6 months | BOP, PI, PD, CAL | At 1, 3 and 6 months after both types of treatment, an improvement in BOP and CAL was observed. The greater reduction of the PD, achieved by a combination of SRP/PDT, was statistically significant after 6 months (p = 0.007). | ||
| Raut et al., 2018 (India) [78] | Journal of Indian Society of Periodontology IF 2020: 0.460 IF 2018: 0.44 |
PG-RCT | CP (PPD > 5 mm and CAL > 4 mm) |
50 | SRP group: 12/13 SRP+aPDT group: 16/9 |
SRP group: 46.90 ± 4.32 SRP+aPDT group: 51 ± 2.83 |
SRP+ sham procedure (Hand and ultrasonic instruments) |
SRP+aPDT | Baseline and 6 months | PI, BOP, CAL, PD, microbiological analysis | Significant reduction was seen in PD, CAL and BOP in the test group as compared to control group after 6 months (p < 0.05). However, intergroup comparison of PI showed nonsignificant results (p > 0.05). Anaerobic culture of plaque samples of test group also revealed a significant reduction of microorganisms in comparison with control group. | ||
| Hokari et al., 2018 (Japan) [79] | International Journal of Dentistry IF 2019: 0.58 IF 2018: 0.58 |
PG-RCT | CP (Moderate: 3–4 mm clinical attachment loss, severe: ≥5 mm loss, generalized: >30% of sites affected) |
30 | aPDT group: 7/8 MO group: 6/9 |
aPDT group: 61.4 ± 10.2 MO group: 66.7 ± 9.5 |
SRP+ Minocycline ointment (MO) (Ultrasonic instruments) |
SRP+aPDT |
Baseline, 1 and 4 weeks | BOP, PD, CAL, PI, GI, microbiological and inflammatory marker analysis | Local MO administration exhibited a significant decrease in scores for clinical parameters (p < 0.01) and a significant reduction in bacterial counts (p < 0.01) and IL-1β and IF-γ levels at 1 and 4 weeks after treatment (p < 0.01). No significant changes were observed in the aPDT group, except in clinical parameters. | ||
| Hill et al., 2019 (Germany) [80] | Photodiagnosis and Photodynamic Therapy IF 2020: 2.894 IF 2019: 2.821 |
SM-RCT | CP (At least one single and one multi-rooted tooth with at least 4 mm PPD in each quadrant) |
20 | 3/17 | 61.1 | SRP (Hand and piezo- electric ultrasonic instruments) |
SRP+aPDT | Baseline, 2 week, 3 and 6 months | BOP, SFFR, PD, GR, RAL, Microbiological analysis | Median values for BOP, RAL, PD, decreased significantly in both groups (p < 0.05) after three months of treatment without significant difference between the groups (p > 0.05). Two weeks after treatment, the SFFR showed significantly lower mean values in the test group (aPDT). With the applied parameters, this study does not conclusively support ICG-based aPDT, though it is promising because no adverse effects occurred. |
||
| Ahad et al., 2016 (India) [81] | Journal of Lasers in Medical Sciences IF 2020: 1.570 IF 2016: 0.68 |
SM-RCT | CP (At least 2 teeth in different quadrants with PD ≥ 6 mm, and BOP) |
30 | 21/9 | 38.67 ± 10.52 | SRP (Hand and ultrasonic instruments) |
SRP+aPDT | Baseline, 1 and 3 months | PI, mSBI, PD, CAL | At 1 month follow-up, intergroup difference in mean change was statistically significant in terms of mSBI and PD for the adjunctive aPDT group (p < 0.05), at 3 months interval, no statistically significant difference was observed between test and control groups except in terms of mSBI (p > 0.05), thus proving that aPDT improved the gingival status in the nonsurgical management of CP. | ||
| Balata et al., 2013 (Brazil) [82] | Journal of Applied Oral Science IF 2019: 2.005 IF 2013: 1.153 |
SM-RCT | CP (Periodontal pockets with CAL ≥ 5 mm, BOP and radiographic bone loss; minimum of 2 teeth with PD ≥ 7 mm and 2 other teeth with a PD ≥ 5 mm, all with BOP and located on opposite sides of the mouth; and ≥16 teeth in both jaws) |
22 | 8/14 | 43.18 | SRP (Ultrasonic instruments) |
SRP+aPDT | Baseline, 1, 3 and 6 months | PI, GI, BOP, GR, CAL | Both groups revealed statistically significant improvement in the clinical parameters (p < 0.05) with no statistically significant differences upon intergroup comparison (p > 0.05). aPDT did not provide any additional benefit to those obtained with full-mouth ultrasonic debridement used alone. | ||
| Bechara et al., 2018 (Brazil) [83] | Photodiagnosis and Photodynamic Therapy IF 2020: 2.894 IF 2018: 2.624 |
PG-RCT | AgP (Single-rooted teeth in multiple quadrants, with both PPD and CAL ≥ 5 mm, and with BOP) |
36 patients (72 sites) | CLM group: 1/17 Placebo group: 1/17 |
<35 years CLM group: 33.11 ± 4.26 Placebo group: 31.26 ± 4.73 |
CLM group (n = 18) Clarithromycin 500 mg BD for 3 days |
Placebo group (n = 18) | Baseline, 3 months and 6 months | PD, CAL, BOP, GR | At 3 months, UPD+aPDT, UPD+CLM and UPD + CLM + aPDT groups all exhibited reduced PD relative to the UPD group (p < 0.05). However, at 6 months, the mean PD reduction was greater in the antibiotic groups (UPD+CLM and UPD+CLM+aPDT) than in the UPD and UPD+aPDT groups (p < 0.05). Regarding clinical attachment level, only the UPD+CLM+aPDT group presented a significant gain relative to the UPD and UPD+aPDT groups (p < 0.05). | ||
| UPD + CLM (18 sites) | UPD+ CLM+ aPDT (18 sites) |
UPD (18 sites) | UPD+ aPDT (18 sites) | ||||||||||
| Bundidpun et al., 2017 (Thailand) [84] | Laser Therapy IF 2020: 0.43 IF 2017: 0.53 |
SM-RCT | CP (Generalized moderate to severe chronic periodontitis, presence of at least 20 teeth, at least one molar tooth in each quadrant with a minimum of four teeth, at least two teeth and one molar tooth presented with PD > 6 mm in each quadrant) |
20 | 7/13 | 47.25 ± 8.91 | SRP (Piezo-electric ultrasonic instruments) |
SRP+aPDT | Baseline, 1, 3 and 6 months | PD, CAL, PI, GBI, GI | All parameters in test group were better than that control group, with statistically significant differences of GBI and GI (p < 0.05) at 3 and 6 months after treatment but no statistically significant differences of PD, CAL and PI. | ||
| Chitsazi et al., 2014 (Iran) [85] | Journal of Dental Research, Dental Clinics, Dental Prospects IF 2020: 0.69 IF 2014: 1.30 |
SM-RCT | AgP (Minimum of 12 teeth with at least 3 teeth in each quadrant with ≥4 mm of probing depth) |
24 | 9/15 | 29 | SRP (Piezo-electric ultrasonic instruments) |
SRP+aPDT | Baseline, 3 months | PD, CAL, GR, PI, GI, BOP, Microbiological analysis for A.a | Intragroup comparison showed an improvement in all the clinical parameters and a significant reduction in the counts of A.a at 90 days compared to baseline (p < 0.05). None of the periodontal parameters exhibited significant differences between the two groups (p > 0.05). | ||
| Chitsazi et al., 2014 (Iran) [86] | Journal of Advanced Periodontology and Implant Dentistry IF 2020: NA IF 2014: NA |
SM-RCT | CP (At least one site per quadrant exhibiting pocket depth of ≥4 mm with bleeding on probing) |
22 | 10/12 | 46.1 | SRP (Sonic instruments) |
SRP+aPDT | Baseline, 1 and 3 months | PD, CAL, BOP, GR, microbiological analysis | PD values decreased significantly in both groups after 1 month (p = 0.001) and 3 months (p = 0.001) in the SRP and (p = 0.001) in the PDT groups the inter-group differences were not significant after 1 (p = 0.25) and 3 months (p = 0.51). Clinical measurements showed significant decreases after 1 and 3 months at both sites, without inter-group differences, except for BOP after 1 (p = 0.004) and 3 months (p = 0.0001). | ||
| Garcia et al., 2011 (Brazil) [87] | Revista Periodontia IF 2020: NA IF 2011: NA |
SM-RCT | AgP (Bone loss first molars and incisors, and other teeth adjacent, with PPD ≥ 5 mm and loss of CAL ≥ 2 mm) |
10 | 4/6 | 39.3 ± 5.84 | SRP (Hand and ultrasonic instruments) |
SRP+aPDT | Baseline, 3 months | PD, RCAL, furcation involvement, tooth mobility | Both groups showed improved clinical results in the nonsurgical treatment of AgP with no statistically significant intergroup differences (p > 0.05). | ||
| Joseph et al., 2014 (India) [88] | Journal of Clinical Periodontology IF 2020: 5.241 IF 2014: 4.641 |
PG-RCT | CP (A minimum of 20 teeth; PPD 4–6 mm at least in two different quadrants of the mouth) |
90 | 39/51 | 39.6 ± 8.7 | SRP (Hand and ultrasonic instruments) |
SRP+aPDT | Baseline, 2 weeks, 1, 3 and 6 months | PPD, CAL, GI, GBI, PI, halitosis. | PD and CAL showed statistically significant reduction in the test group on evaluation at 3 months and 6 months as compared to the control group (p < 0.05). A statistically significant improvement in GI and GBI was seen for the test group after 2 weeks and 1 month of aPDT (p < 0.01), whereas the improvement in GI and GBI at 3 months and in plaque index at 2 weeks after aPDT was less (p < 0.05). In addition, a significant difference was detected for the test group at 1 month in terms of halitosis, which did not persist for long (p < 0.05). | ||
| Malgikar et al., 2015 (India) [89] | Journal of Dental Lasers IF 2020: 0.696 IF 2015: NA |
SM-RCT | CP (At least one site in each quadrant of the mouth having deep PPD ≥ 5 mm and radiographic signs of alveolar bone loss) |
24 | 15/9 | M: 36.73 ± 8.46 F: 34.33 ± 6.80 |
SRP (Hand and piezo- electric ultrasonic instruments) |
SRP+aPDT | Baseline, 1, 3 and 6 months. | PI, GI, mSBI, PD, CAL. | A statistically significant decrease in PD, CAL, PI, GI, mSBI scores was seen in SRP+aPDT at the end of 6 months (p < 0.001). | ||
| Monzavi et al., 2016 (Iran) [90] | Photodiagnosis and Photodynamic Therapy IF 2020: 2.894 IF 2016: 2.503 |
SM-RCT | CP (At least three teeth exhibiting residual pocket depth of ≥ 5 mm with bleeding on probing) |
50 | 25/25 | 49.6 ± 8.5 | SRP (Hand and ultrasonic instruments) |
SRP+aPDT | Baseline, 1 and 3 months | BOP, PI, CAL, PPD, FMPS, FMBS | There were no significant differences between two groups at baseline. BOP, PPD and FMBS showed significant improvements in the test group (p ≤ 0.001). In terms of PI, FMPS and CAL, no significant differences were observed between both groups (p ≥ 0.05). | ||
| Raj et al., 2016 (India) [91] | Indian Journal of Dental Research IF 2020: 0.37 IF 2016: 0.08 |
PG-RCT | CP (More than 16 natural teeth; PPD ≥ 5 mm) |
20 | 8/12 | NI | SRP (Type of instruments utilized-NI) |
SRP+aPDT | Baseline and 3 months | PI, GI, PD, CAL and microbiological analysis | There was a significant reduction in PI, GI, PD, CAL and microbiologic parameters in test group, following SRP and PDT, when compared with SRP alone in control group (p < 0.001). SRP+aPDT has shown additional improvement in periodontal parameters when compared to SRP alone and has a beneficial effect in chronic periodontitis patients. | ||
| Sena et al., 2019 (Brazil) [92] | Photobiomodulation, Photomedicine and Laser Surgery IF 2019: 1.913 |
SM-RCT | CP (At least six sites with PD 5–9 mm; and BOP) |
9 (6 sites/ patient: total-54 sites) | NI | NI | SRP+ placebo procedure (Hand and ultrasonic instruments) |
SRP+aPDT | Baseline and 3 months | BOP, PD, CAL, VPI | There was a statistically significant decrease in BOP for test group (p = 0.003) and control group (p = 0.001). Intragroup comparison for PD and CAL showed statistically significant differences from baseline (p < 0.05) with no intergroup differences (p > 0.05). Hence, SRP+aPDT did not show any additional benefits over SRP alone. | ||
| Shingnapurkar et al., 2016 (India) [93] | Indian Journal of Dental Research IF 2020: 0.37 IF 2016: 0.08 |
SM-RCT | CP (PD > 5 mm) |
60 sites | NI | NI | SRP+ sham procedure (Hand and ultrasonic instruments) |
SRP+aPDT | Baseline, 1 and 3 months | PI, GI, PD, RAL | Mean baseline values for PI, GI, PPD and RAL were not different in the test group and control group. Statistically significant difference in PPD and RAL, 3 months after treatment was seen in test group as compared to the control group (p < 0.05). | ||
| Sigusch et al., 2010 (Germany) [94] | Journal of Periodontology IF 2020: 3.742 IF 2010: 2.946 |
PG-RCT | CP (<30% of sites with PPD >3.5 mm) |
24 (12 in each group) | PDT group: 4/8 Control group: 3/9 |
PDT group F: 39.75 M: 45 Control group: F: 44.22 M:42.67 |
SRP+ sham procedure (Type of instruments utilized- NI) |
SRP+aPDT | Baseline, 1, 4 and 12 weeks. | PI, reddening, PD, BOP, CAL, GR Quantitative analysis for F.n. |
In patients with localized CP who received aPDT treatment, significant reductions in reddening, BOP, and mean PD and CAL were observed during the observation period and with respect to controls (p < 0.001). Four and 12 weeks after aPDT, the mean PD and CAL showed significant differences from baseline values and from those of the control group. In the aPDT group, 12 weeks after treatment, the F.n. DNA concentration was found to be significantly reduced compared to the baseline level (p < 0.001) compared to control group. | ||
| Theodoro et al., 2012 (Brazil) [95] | Lasers in Medical Science IF 2019: 2.574 IF 2012: 2.645 |
SM-RCT | CP (At least three non-adjacent sites with BOP and a PD of 5–9 mm at least 20 teeth in the oral cavity) |
33 | 12/21 | 43.12 ± 8.2 | SRP (Hand instruments) |
SRP+ PS (TBO) only | SRP+aPDT | Baseline, 60, 90 and 180 days | VPI, GI, BOP, PD, CAL, GR, microbiological analysis | All treatment groups showed an improvement in all clinical parameters, and a significant reduction in the proportion of sites positive for periodontopathogens at 60, 90 and 180 days compared to baseline (p < 0.05). None of the periodontal parameters showed a significant difference among the groups (p > 0.05). At 180 days, PDT treatment led to a significant reduction in the percentage of sites positive for all bacteria compared to SRP alone (p < 0.05). | |
| Theodoro et al., 2017 (Brazil) [96] | Journal of Photochemistry and Photobiology B IF 2020: 4.383 IF 2017: 3.438 |
PG-RCT | CP (Severe generalized CP in at least 6 teeth and with one or several sites with PD ≥ 5 mm; a loss of CAL ≥ 5 mm; a minimum of 30% of the sites with PD and CAL ≥ 4 mm and BOP; and the presence of at least 15 teeth) |
34 | AB group: 7/7 aPDT group: 9/5 |
AB group: 46.3 ± 6.8 aPDT group: 48.8 ± 8.3 |
SRP+ (MTZ+ AMX) MTZ dose: 400mg TDS-7 days AMX dose: 500mg TDS-7 days (Type of instruments utilized for SRP-NI) |
SRP +aPDT+ placebo pills | Baseline and 90 days | BOP, PD, CAL | There was a significant improvement in CAL only in the intermediate pocket in the aPDT group com- pared to the MTZ + AMX group between baseline and 90 days post-treatment (p = 0.01). There was a reduction of both BOP and the percentage of residual pockets at 90 days after treatment compared with baseline in both groups (p < 0.05). | ||
3.2.2. Study Design
Twenty studies were conducted using a SM study design [2,3,5,17,70,71,77,80,81,82,84,85,86,87,89,90,92,93,94,95], whereas a PG study design was utilized in the remaining 11 studies [72,73,74,75,76,78,79,83,88,91,96] (Table 1).
3.2.3. Selection Criteria
Several inconsistencies were observed amongst the included studies [2,3,5,17,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96], which have been outlined in Table 1, in which 21 out of 31 studies included patients with CP [17,70,75,76,77,78,79,80,81,82,84,86,88,89,90,91,92,93,94,95,96], whereas the remaining 10 studies included patients with AgP [2,3,5,71,72,73,74,83,85,87].
3.2.4. Documentation of Laser Parameters
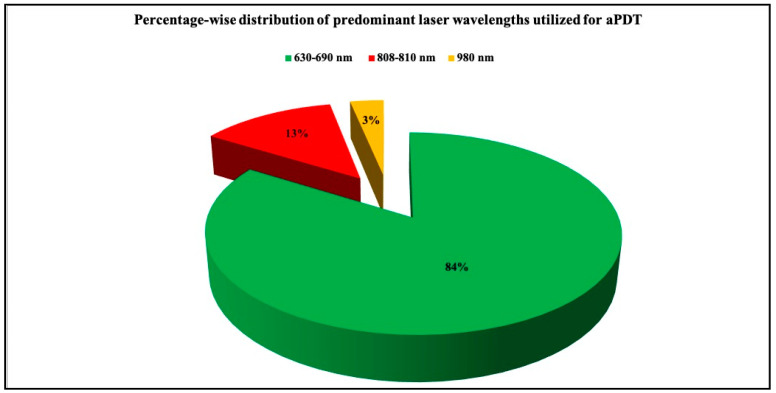
Table 2 describes various dye laser combinations, as well as laser dosimetry that was utilized to perform aPDT in all eligible studies. Twenty-six out of 31 studies utilized a laser wavelength in the range of 630–690 nm [2,3,5,17,70,71,72,73,74,75,76,77,79,81,82,83,84,85,86,87,88,91,92,94,95,96] to perform aPDT. While four studies utilized a laser wavelength in the range of 808–810 nm [78,80,90,93], one of the included studies utilized a 980 nm diode laser wavelength to perform aPDT [89] (Table 2) (Figure 2). Emission mode was reported only in five studies [78,88,90,92,93], in which four of them utilized a continuous wave emission mode [78,88,90,92], whilst the remaining one study utilized a gated continuous wave emission mode [93]. Eighteen out of the 31 eligible studies used the laser fibre tip in ‘contact mode’ with the periodontal pocket in order to perform aPDT [2,3,5,78,81,82,84,85,86,88,89,90,91,92,93,94,95,96]. Only 5 studies reported total energy, and it ranged from 1.5–9 J [82,90,92,93,95,96]. Only 19 studies reported power output in the range of 30 mW–1 W [71,75,76,77,78,79,80,82,83,84,85,87,89,90,91,92,93,94,95,96], whereas the use of a power meter to measure the therapeutic power output, reaching the target tissues was not performed in any of the included studies. Spot size was reported in only four studies [5,85,92,96] ranging from 0.02–0.07 cm2. Ten out of the 31 studies reported the diameter of fibre tip, [2,3,5,80,81,82,88,89,93,94] ranging from 200–600 μm. The energy density (fluence) was calculated in 18 out of 31 studies [5,17,70,71,72,73,74,78,79,80,82,83,86,87,92,93,95,96], and its value ranged from 0.01–2829 J/cm2, whereas the power density (irradiance) values ranged from 60 mW–4 W/cm2 and were calculated in 13 studies [2,3,5,17,71,72,73,74,81,88,92,94,95]. Finally, the exposure time for laser irradiation was mentioned in all included studies except one study [80], and the values ranged from 10–120 s/site amongst included studies.
Table 2.
Tabular representation of PS dye and laser parameters utilized for aPDT in the selected eligible in vivo human studies. Refer to Supplementary file 2 for list of abbreviations.
| Study, Year, Origin and Citation | Photosensitizer (PS) Used and Its Concentration | Pre-Irradiation Exposure Time to PS (min) |
Laser Wavelength Utilized | Emission Mode Contact/No Contact Tip Initiation |
Energy (J) |
Power Output (W) |
Pulse Length (Duration), Pulse Interval | Use of Power Meter | Distance from Target | Spot Size/Fibre-Tip Diameter/Spot Diameter | Energy Density [Fluence] (J/cm2) |
Power Density [Irradiance] (W/cm2) | Exposure Time to Laser Irradiation [Minute (min)/Second (s)] |
No. of aPDT Applications |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| De Oliveira et al., 2009 (Brazil) [2] | Phenothiazine chloride (10 mg/mL) | 1 min | 660 nm | Contact mode, fibre tip was place at the entrance of the gingival sulcus | NI | NI | NI | NI | NA | Tip diameter: 600 µm | NI | 60 mW/cm2 | 10 s/site (6 sites = 1 min/tooth) | 1 |
| De Oliveira et al., 2007 (Brazil) [3] | Phenothiazine chloride (10 mg/mL) | 1 min | 660 nm | Contact mode, fibre tip was place at the entrance of the gingival sulcus | NI | NI | NI | NI | NI | Tip diameter: 600 µm | NI | 60 mW/cm2 | 10 s/site (6 sites = 1 min/tooth) | 1 |
| Novaes et al., 2012 (Brazil) [5] | Phenothiazine chloride | NI | 660 nm | Contact mode, fibre tip was place at the entrance of the gingival sulcus | NI | NI | NI | NI | NI | Tip diameter: 600 µm [8.5 cm long optic fibre with 60° angulated tip] Spot size: 0.06 |
212.23 J/cm2 | 60 mW/cm2 | 10 s/site (6 sites/tooth) 60 s/tooth | 1 |
| Franco et al., 2014 (Brazil) [17] | Methylene blue (0.01%) | 5 min | 660 nm | NI | NI | NI | NI | NI | NI | NI | 5.4 J/cm2 | 60 mW/cm2 | 5 s/site (6 sites/tooth) 90 s/tooth |
4 |
| Pourabbas et al., 2014 (Iran) [70] | Toluidine blue | 60 s | 638 nm | NI | NI | NI | NI | NI | NI | NI | 8–10 J/cm2 | NI | 120 s | 1 |
| Moreira et al., 2015 (Brazil) [71] | Phenothiazine chloride (10 mg/mL) | 1 min | 670 nm | NI | NI | 75 mW | NI | NI | NI | Tip diameter: 600 µm | Fluence/site: 2.49 J/cm2 Fluence/tooth: 14.94 J/cm2 |
0.25 W/cm2 | 10 s /site | 4 (0, 2nd, 7th and 14th day) |
| Skurska et al., 2015 (Poland) [72] | Phenothiazine chloride | 3 min | 660 nm | NI | NI | NI | NI | NI | NI | NI | 120 J/cm2 | 60 mw/cm2 | 60 s/site | 2 (0 and 7th day) |
| Arweiler et al., 2014 (Poland) [73] | Phenothiazine chloride | 3 min | 660 nm | NI | NI | NI | NI | NI | NI | NI | 120 J/cm2 | 60 mw/cm2 | 60 s/site | 2 (0 and 7th day) |
| Arweiler et al., 2013 (Poland) [74] | Phenothiazine chloride | 3 min | 660 nm | NI | NI | NI | NI | NI | NI | NI | 120 J/cm2 | 60 mw/cm2 | 60 s/site | 2 (0 and 7th day) |
| Vidal et al., 2017 (Spain) [75] | Methylene blue (0.005%) | NI | 670 nm | NI | NI | 150 mW | NI | NI | NI | NI | NI | NI | 60 s/pocket | 3 (1, 5 and 13 weeks) |
| Braun et al., 2008 (Germany) [76] | Phenothiazine chloride | 3 min | 660 nm | NI | NI | 100 mW | NI | NI | NI | NI | NI | NI | 10 s/site (6 sites = 1 min /tooth) | 1 |
| Berakdar et al., 2012 (Germany) [77] | Methylene blue 0.005% |
NI | 670 nm | NI | NI | 150 mW | NI | NI | NI | NI | NI | NI | 1 min | 1 |
| Raut et al., 2018 (India) [78] | Indocyanine green (5 mg/mL) | 60 s | 810 nm | CW, contact mode | NI | 80 mW | NI | NI | NA | NI | 5.4 J/cm2 | NI | 60 s | 1 |
| Hokari et al., 2018 (Japan) [79] | Methylene blue dye 0.01% |
1 min | 670 nm | NI, contact mode | NI | 140 mW | NI | NI | NA | NI | 21 J/cm2 | NI | 60 s | 2 (0 and 7th day) |
| Hill et al., 2019 (Germany) [80] | Indocyanine green (0.1 mg/mL) | 60 s | 808 nm | NI | NI | 100 mW | NI | NI | NI | Tip diameter: 300 µm | 2829 J/cm2 | NI | NI | 1 |
| Ahad et al., 2016 (India) [81] | Phenothiazine chloride | 3 min | 660 nm | Contact mode | NI | NI | NI | NI | NA | Tip diameter: 0.6 µm | NI | 100 mW/cm2 | 10 s/site (6 sites, 1 min/tooth) | 1 |
| Balata et al., 2013 (Brazil) [82] | Methylene blue 0.005% |
2 min | 660 nm | 90° angle with the gingival surface and with no contact with the tissues | 9 J | 100 mW | NI | NI | NI | Tip diameter: 600 µm tip | 320 J/cm2 | NI | 90 s/site | 1 |
| Bechara et al., 2018 (Brazil) [83] | Methylene Blue (10 mg/mL) |
1 min | 660 nm | NI | NI | 60 mW | NI | NI | NI | NI | 129 J/cm2 | NI | 60 s/tooth (2 sites/tooth) | 1 |
| Bundidpun et al., 2017 (Thailand) [84] | Phenothiazine chloride | 1 min | 660 nm | Contact mode | NI | 100 mW | NI | NI | NA | NI | NI | NI | 10 s/site (6 sites) 1 min/tooth |
1 |
| Chitsazi et al., 2014 (Iran) [85] | Toluidine Blue | 1 min | 670–690 nm | Contact mode | NI | 75 mW | NI | NI | NA | NI | NI | NI | 120 s/site | 1 |
| Chitsazi et al., 2014 (Iran) [86] | Tolonium chloride (Toluidine Blue O) |
60 s | 638 nm | Contact mode | NI | NI | NI | NI | NA | NI | 8–10 J/cm2 | NI | 120 s | 1 |
| Garcia et al., 2011 (Brazil) [87] | Methylene blue (0.005%) | 5 min | 660 nm | NI | NI | 40 mW | NI | NI | NI | NI | 120 J/cm2 | NI | 120 s/site | 1 |
| Joseph et al., 2014 (India) [88] | Methylene blue (10 mg/mL) | 3 min | 655 nm | CW, contact mode, tip was inserted into the gingival sulcus | NI | NI | NI | NI | NA | Tip diameter: 200 µm Probe tip diameter: 0.5 mm |
NI | 60 mW/ cm2 | 60 s/site (4 sites/ tooth) | 1 |
| Malgikar et al., 2015 (India) [89] | Methylene blue 1% |
3 min | 980 nm | Contact mode, tip was initiated | NI | Peak Power: 5 W Average power 1 W |
Pulse length: 200 µs, Pulse interval: 200 |
NI | NA | Tip diameter: 400 µm | NI | NI | 30–45 s/site | 1 |
| Monzavi et al., 2016 (Iran) [90] | Indocyanine green (1 mg/mL) | NI | 810 nm | CW, contact mode | PBM tip: 6 J Bulb tip: 4 J |
200 mW | NI | NI | NA | Use of two types of tips: PBM tip was placed on papilla and then the bulb tip was inserted inside the pocket from each buccal or lingual/palatal side, moving from the bottom of the pocket to the coronal aspect. | NI | NI | PBM tip: 30 s Bulb tip: 10 s |
4 (0, 7th, 17th and 27th days) |
| Raj et al., 2016 (India) [91] | Toluidine blue | 1 min | 635 nm | Contact mode | NI | 500 W | NI | NI | NA | NI | NI | NI | 60 s | 1 |
| Sena et al., 2019 (Brazil) [92] | Chloro-aluminum pthalocyanine (AlClFc) 5 µM | 5 min | 660 nm | CW, laser optical fiber tip was positioned parallel to the tooth axis in contact with the gingival margin (without penetrating the pocket) | 1.5 J | 100 mW | NI | NI | NA | Spot size: 0.028 cm2 | 54 J/cm2 | 4 W/cm2 | 15 s | 1 |
| Shingnapurkar et al., 2016 (India) [93] | Indocyanine green (1 mg/mL) |
3 min | 810 nm | Gated CW, Contact mode | 3 J | 200 mW | Pulse duration: 25 µm Duty cycle 50% |
NI | NA | Tip diameter: 400 µm | 0.0125 J/cm2 | NI | 30 s/site | 1 |
| Sigusch et al., 2010 (Germany) [94] | Phenothiazine chloride | 1 min | 660 nm | Contact mode | NI | NI | NI | NI | NA | Tip diameter: 600 µm tip | NI | 60 mW/cm2 | 10 s/site (6 sites =1 min /tooth) | 1 |
| Theodoro et al., 2012 (Brazil) [95] | Toluidine blue O 100 µg/mL |
1 min | 660 nm | The laser optical fiber tip was positioned parallel to and in contact with the selected site | 4.5 J | 30 mW | NI | NI | NA | Spot size: 0.07 cm2 | 64.28 J/cm2 | 0.4 W/cm2 | 150 s | 1 |
| Theodoro et al., 2017 (Brazil) [96] | Methylene blue (10 mg/mL) | 1 min | 660 nm | Contact mode | 4.8 J | 100 mW | NI | NI | NA | Spot size 0.03 cm2 | 160 J/cm2 | NI | 48 s | 3 (0, 48 h, 96 h) |
Figure 2.
3D pie diagram illustrating the percentage-wise distribution of predominant laser wavelengths utilized for aPDT in the included studies.
3.2.5. PS Utilized
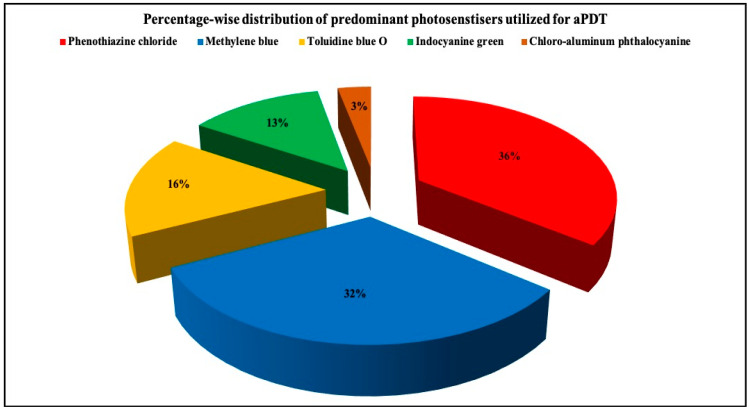
Type of PS varied amongst eligible clinical trials. Eleven studies utilized phenothiazine chloride [2,3,5,71,72,73,74,76,81,84,94] while 10 employed methylene blue [17,75,77,79,82,87,88,89,96]. Five studies utilized toluidine blue O [70,85,86,91,95], four studies used indocyanine green [78,80,90,93], whereas chloro-aluminum phthalocyanine was utilized in one study [92] (Figure 3). Interestingly, 18 out of 31 studies specified the concentration of the PS [2,3,17,71,75,77,78,79,80,82,83,87,88,89,90,92,95,96], while 13 studies failed to report the same [5,70,72,73,74,76,81,84,85,86,91,93,94] (Table 2).
Figure 3.
3D pie diagram illustrating the percentage-wise distribution of predominant photosensitizers utilized for aPDT in the included studies.
3.2.6. Utilization of aPDT as a Mono-Therapeutic or an Adjunctive Therapeutic Agent
While 28 out of the 31 eligible studies utilized SRP+aPDT, aPDT monotherapy was performed in three studies [2,3,5] (Table 1).
3.2.7. Comparison in between SRP+ aPDT versus SRP+AB
Six out of the 31 eligible studies compared efficacy of SRP+aPDT versus SRP+AB [72,73,74,79,83,96] (Table 1).
3.2.8. Number of aPDT Sessions
While a single session of aPDT was applied in 22 out of the 31 included studies [2,3,5,70,76,77,78,80,81,82,83,84,85,86,87,88,89,91,92,93,94,95], multiple aPDT sessions were performed in nine studies [17,71,72,73,74,75,79,90,96]. None of the eligible studies compared single versus multiple sessions of aPDT (Table 2).
3.2.9. Follow-Up Assessment
A follow-up assessment at three months from the baseline visit was performed in 18 out of the 31 eligible studies [2,3,5,17,70,71,74,76,81,85,86,87,90,91,92,93,94,96], whereas 12 studies conducted a longer follow-up assessment at six months [72,73,75,77,78,80,82,83,84,88,89,95]. Only one study performed a follow-up assessment at one month from the baseline visit [79]. A long-term follow-up of a minimum one year from baseline visit lacked in all eligible studies.
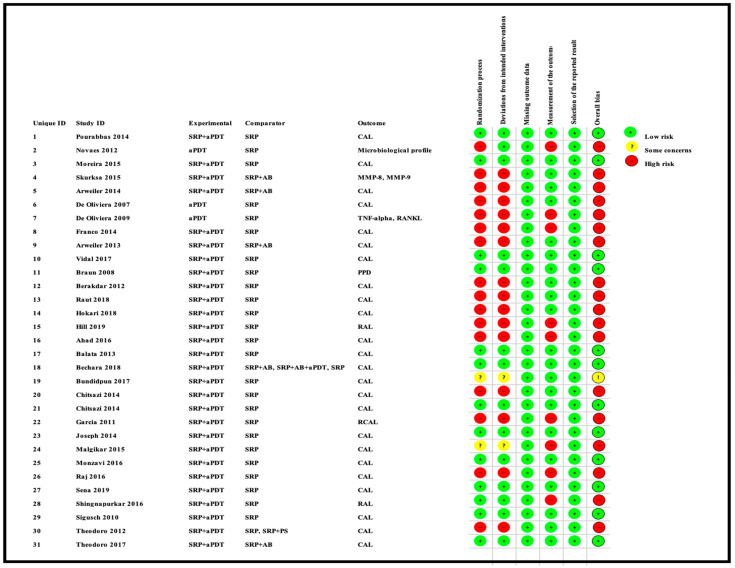
3.3. Qualitative Assessment
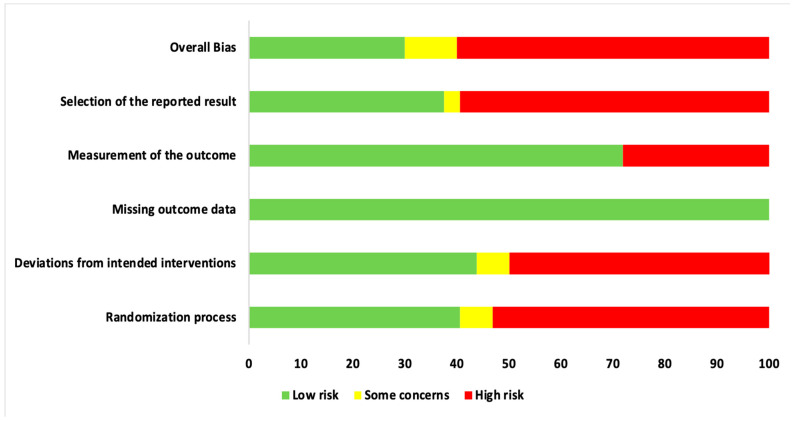
Qualitative assessment was performed using the RoB 2 tool, designed for in vivo human RCTs, as depicted in Figure 4 and Figure 5. The most recent version of this tool was utilized to perform a qualitative assessment for both randomized PG and SM human RCTs [29,30]. Figure 4 represents a risk of bias assessment summary of all eligible studies. Figure 5 is a graphical representation of percentage RoB score for each risk domain, which has been evaluated, using the abovementioned tool. Furthermore, 53.1% of included trials were at a high risk of inadequate randomization, whereas 40.6% and 6.3% of included trials were at a low risk or had some concerns, respectively. In addition, 50% of included studies were at a high risk of deviations from intended interventions, whereas 43.7% and 6.3% of them were at a low risk or had some concerns, respectively. All included papers reported substantial evidence (100%) for reporting missing outcome data and, hence, were at a low risk. Although a majority of studies were free of bias arising from reporting outcome measurement (71.9%), 28.1% were at a high risk. In terms of selective reporting of the results, inferences are as follows: 59.4% studies were at a high risk, 37.5% studies were at a low risk, and 3.1% studies had some concerns. Overall, 60% studies reported a high risk of bias, while 35% studies had a low risk of bias, and the final 5% studies had some concerns. It should be noted that information provided in these figures represents the consensual answers verified using the ‘Discrepancy check’ feature of RoB 2 tool, across two independent reviewers (S.D. and R.H.) (inter-reviewer agreement, κ = 0.94), and, in case of any disagreements, a third author (S.B.) was consulted.
Figure 4.
Risk of Bias assessment summary of the included studies based on the consensual answers across two individual assessors (S.D. and R.H.).
Figure 5.
Risk of Bias assessment graph of the included studies expressed as percentages, based on the consensual answers across two individual assessors (S.D. and R.H.).
3.4. Quantitative Assessment
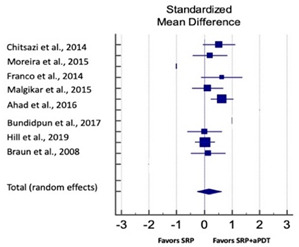
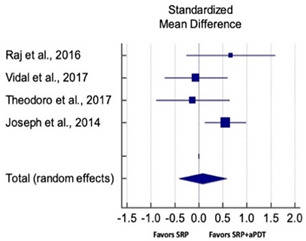
3.4.1. Outcome Variables
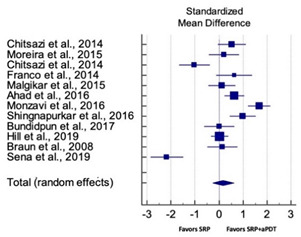
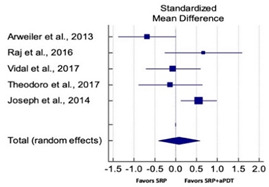
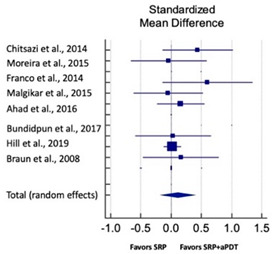
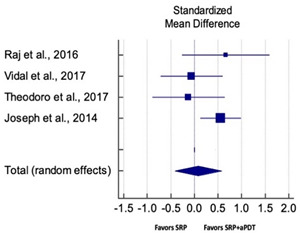
Primary outcomes of 18 out of 31 studies, which have assessed efficacy of SRP+aPDT in the management of periodontitis, contributed to this meta-analysis [17,71,73,74,75,76,80,81,84,85,86,88,89,90,91,92,93,96]. Data were pooled separately for SM and PG studies for differences in PPD and CAL respectively at three and six months, respectively. At three months, the mean difference in PPD reduction was not statistically significant for SM studies (SMD 0.166; 95% CI −0.278 to 0.611; P = 0.463) and PG studies (SMD 0.076; 95% CI −0.420 to 0.573; P = 0.763) along with a high heterogeneity for SM studies (Q = 15.81; P = 0.0001; I2 = 91.21%) and moderate heterogeneity (Q = 11.87; P = 0.018; I2 = 66.31%) for PG studies (Table 3). The mean difference in PPD reduction at six months did not show a statistically significant difference for SM studies (SMD 0.005; 95% CI –0.126 to 0.136; P = 0.935) as well as PG studies (SMD 0.141; 95% CI −1.007 to 1.288; P = 0.809) although contrasting findings were noted in terms of level of heterogeneity which was not evident for SM studies (Q = 0.06; P = 0.99; I2 = 0.00%) and high for PG studies (Q = 18.71; P = 0.0001; I2 = 89.31%) (Table 4). CAL gain at three months was not statistically significant in SM studies (SMD 0.092; 95% CI −0.013 to 0.198; P = 0.088) with no evident heterogeneity (Q = 8.74; P = 0.655; I2 = 0.00%) as well as in PG studies (SMD 0.056; 95% CI −0.408 to 0.552; P = 0.745) with moderate heterogeneity (Q = 8.95; P = 0.028; I2 = 70.31%) (Table 3). At six months, results for SM studies were not statistically significant (SMD −0.013; 95% CI −0.148 to 0.121; P = 0.846) with no evident heterogeneity (Q = 0.03; P = 0.984; I2 = 0.00%), whereas, for PG studies, the findings were statistically significant (SMD -0.441; 95% CI −0.805 to −0.075; P = 0.018) with no evidence of heterogeneity (Q = 1.70; P = 0.42; I2 = 0.00%) but favoring control group (Table 4).
Table 3.
Forest plots illustrating the overall PPD reduction and CAL gain at three months. Refer to Supplementary file 2 for a list of abbreviations.
| Overall PPD Reduction for SM Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.463 |
|
| Chitsazi et al., 2014 | 0.525 | 0.289 | −0.056 to 1.106 | 8.28 | ||
| Moreira et al., 2015 | 0.205 | 0.311 | −0.425 to 0.834 | 8.11 | ||
| Chitsazi et al., 2014 | −1.023 | 0.316 | −1.659 to −0.386 | 8.07 | ||
| Franco et al., 2014 | 0.631 | 0.364 | −0.115 to 1.378 | 7.67 | ||
| Malgikar et al., 2015 | 0.119 | 0.284 | −0.453 to 0.691 | 8.31 | ||
| Ahad et al., 2016 | 0.639 | 0.204 | 0.235 to 1.043 | 8.88 | ||
| Monzavi et al., 2016 | 1.669 | 0.231 | 1.211 to 2.127 | 8.70 | ||
| Shingnapurkar et al., 2016 | 0.995 | 0.271 | 0.454 to 1.537 | 8.42 | ||
| Bundidpun et al., 2017 | 0.007 | 0.310 | −0.620 to 0.635 | 8.11 | ||
| Hill et al., 2019 | 0.039 | 0.072 | −0.103 to 0.181 | 9.47 | ||
| Braun et al., 2008 | 0.139 | 0.310 | −0.490 to 0.767 | 8.11 | ||
| Sena et al., 2019 | −2.169 | 0.340 | −2.851 to −1.487 | 7.87 | ||
| Total (random effects) | 0.166 | 0.227 | −0.278 to 0.611 | 100.00 | ||
| Heterogeneity: Q = 15.81; DF = 11; P = 0.0001; I2 = 91.21% | ||||||
| Overall PPD Reduction for PG Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.763 |
|
| Arweiler et al., 2013 | −0.681 | 0.340 | −1.374 to 0.011 | 19.80 | ||
| Raj et al., 2016 | 0.669 | 0.441 | −0.258 to 1.595 | 15.89 | ||
| Vidal et al., 2017 | −0.060 | 0.322 | −0.714 to 0.593 | 20.59 | ||
| Theodoro et al., 2017 | −0.127 | 0.374 | −0.897 to 0.643 | 18.43 | ||
| Joseph et al., 2014 | 0.556 | 0.215 | 0.127 to 0.984 | 25.28 | ||
| Total (random effects) | 0.076 | 0.252 | −0.420 to 0.573 | 100.00 | ||
| Heterogeneity: Q = 11.87; DF = 4; P = 0.018; I2 = 66.31% | ||||||
| Overall CAL Gain for SM Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.088 |
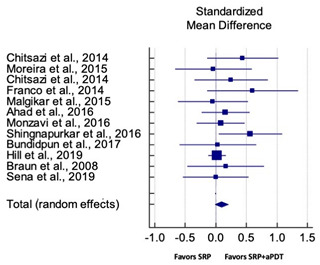
|
| Chitsazi et al., 2014 | 0.439 | 0.287 | −0.140 to 1.017 | 3.52 | ||
| Moreira et al., 2015 | −0.040 | 0.310 | −0.667 to 0.588 | 3.02 | ||
| Chitsazi et al., 2014 | 0.249 | 0.297 | −0.351 to 0.849 | 3.29 | ||
| Franco et al., 2014 | 0.601 | 0.364 | −0.144 to 1.346 | 2.20 | ||
| Malgikar et al., 2015 | −0.048 | 0.284 | −0.620 to 0.523 | 3.60 | ||
| Ahad et al., 2016 | 0.158 | 0.199 | −0.237 to 0.552 | 7.35 | ||
| Monzavi et al., 2016 | 0.080 | 0.199 | −0.314 to 0.474 | 7.37 | ||
| Shingnapurkar et al., 2016 | 0.564 | 0.260 | 0.043 to 1.084 | 4.30 | ||
| Bundidpun et al., 2017 | 0.032 | 0.310 | −0.595 to 0.660 | 3.02 | ||
| Hill et al., 2019 | 0.019 | 0.072 | −0.123 to 0.161 | 55.29 | ||
| Braun et al., 2008 | 0.161 | 0.310 | −0.468 to 0.789 | 3.01 | ||
| Sena et al., 2019 | 0.000 | 0.268 | −0.538 to 0.538 | 4.04 | ||
| Total (random effects) | 0.092 | 0.233 | −0.013 to 0.198 | 100.00 | ||
| Heterogeneity: Q = 8.74; DF = 11; P = 0.655; I2 = 0.00% | ||||||
| Overall CAL Gain for PG Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.745 |
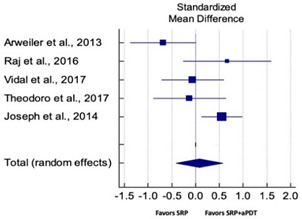
|
| Arweiler et al., 2013 | −0.662 | 0.340 | −1.356 to 0.010 | 19.80 | ||
| Raj et al., 2016 | 0.669 | 0.441 | −0.258 to 1.595 | 15.89 | ||
| Vidal et al., 2017 | −0.102 | 0.372 | −0.514 to 0.793 | 20.59 | ||
| Theodoro et al., 2017 | −0.106 | 0.374 | −0.997 to 0.743 | 18.43 | ||
| Joseph et al., 2014 | 0.456 | 0.255 | −0.120 to 0.673 | 25.28 | ||
| Total (random effects) | 0.056 | 0.358 | −0.408 to 0.552 | 100.00 | ||
| Heterogeneity: Q = 8.95; DF = 4; P = 0.028; I2 = 70.31% | ||||||
Table 4.
Forest plots illustrating the overall PPD reduction and CAL gain at 6 months. Refer to Supplementary file 2 for a list of abbreviations.
| Overall PPD Reduction for SM Studies at 6 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.935 |
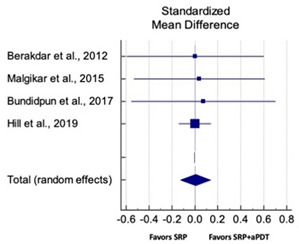
|
| Berakdar et al., 2012 | 0.040 | 0.296 | −0.598 to 0.598 | 5.08 | ||
| Malgikar et al., 2015 | 0.037 | 0.284 | −0.535 to 0.609 | 5.52 | ||
| Bundidpun et al., 2017 | 0.072 | 0.310 | −0.555 to 0.701 | 4.63 | ||
| Hill et al., 2019 | 0.060 | 0.072 | −0.142 to 0.142 | 84.77 | ||
| Total (random effects) | 0.005 | 0.066 | −0.126 to 0.136 | 100.00 | ||
| Heterogeneity: Q = 0.06; DF = 3; P = 0.99; I2 = 0.00% | ||||||
| Overall PPD Reduction for PG Studies at 6 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.809 |
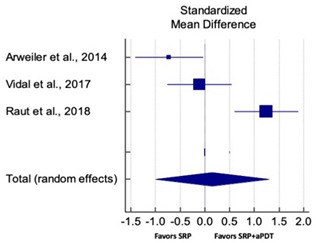
|
| Arweiler et al., 2014 | −0.722 | 0.342 | −1.417 to −0.027 | 33.04 | ||
| Vidal et al., 2017 | −0.109 | 0.322 | −0.763 to 0.545 | 33.47 | ||
| Raut et al., 2018 | 1.241 | 0.321 | 0.594 to 1.888 | 33.49 | ||
| Total (random effects) | 0.141 | 0.579 | −1.007 to 1.288 | 100.00 | ||
| Heterogeneity: Q = 18.71; DF = 2; P = 0.0001; I2 = 89.31% | ||||||
| Overall CAL Gain for SM Studies at 6 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.846 |
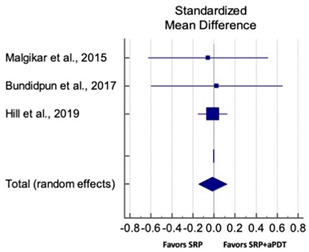
|
| Malgikar et al., 2015 | −0.057 | 0.284 | −0.629 to 0.515 | 5.82 | ||
| Bundidpun et al., 2017 | 0.025 | 0.310 | −0.602 to 0.653 | 4.88 | ||
| Hill et al., 2019 | −0.012 | 0.072 | −0.155 to 0.130 | 89.30 | ||
| Total (random effects) | −0.013 | 0.068 | −0.148 to 0.121 | 100.00 | ||
| Heterogeneity: Q = 0.03; DF = 2; P = 0.984; I2 = 0.00% | ||||||
| Overall CAL Gain for PG Studies at 6 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.018 |
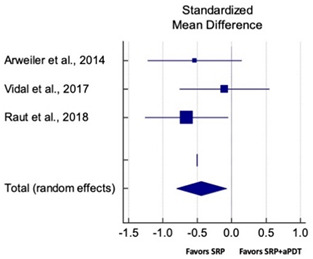
|
| Arweiler et al., 2014 | −0.539 | 0.337 | −1.224 to 0.146 | 29.91 | ||
| Vidal et al., 2017 | −0.103 | 0.322 | −0.756 to 0.551 | 32.69 | ||
| Raut et al., 2018 | −0.658 | 0.301 | −1.265 to −0.050 | 37.40 | ||
| Total (random effects) | −0.441 | 0.184 | −0.805 to −0.075 | 100.00 | ||
| Heterogeneity: Q = 1.70; DF = 2; P = 0.42; I2 = 0.00% | ||||||
Assessment of secondary outcome variables was conducted in the majority of included studies, which are as follows: Changes in GR, BOP, PI and GI in 28 studies [3,17,70,71,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96], microbiological analysis in 11 studies [5,71,75,78,79,80,85,86,91,94,95], and immuno-histological in seven studies [2,17,70,71,72,75,79]. Table 5 provides an overview of clinical parameters which have been assessed in 28 of 31 included studies along with corresponding level of significance, in accordance to data provided in Table 1. Eleven studies performed a microbiological analysis [5,71,75,78,79,80,85,86,91,94,95], out of which five studies reported that aPDT therapy could significantly reduce periopathogenic burden [71,75,78,91,94] and six studies failed to achieve this outcome [5,79,80,85,86,95]. In terms of immune-histological analysis, seven studies [2,17,70,71,72,75,79] assessed various pro-inflammatory cytokines and growth factors such as; IL-1β, IL-10, IF-γ, TNF-α, MMP-8, MMP-9, RANK, RANK-L, OPG and FGF-2 (Table 1). Biomarkers for assessment of bone resorption (RANK, RANK-L, OPG) were assessed in three studies [2,17,75]. Two studies [17,75] assessed efficacy of SRP+aPDT in comparison to conventional SRP alone, and showed that SRP+aPDT successfully suppressed the bone resorption process. Levels of IL-1β, IL-10, IF-γ, and TNF-α were assessed in three studies [70,71,79], of which two studies have confirmed immunological benefits of aPDT [70,71], whereas one study [79] failed to show any advantage of aPDT for the same. It should, however, be noted that, while the former two studies [70,71] have compared the efficacy of SRP+aPDT to conventional SRP alone, the latter study [79] has compared SRP+aPDT to SRP+AB and demonstrated the advantages of AB over aPDT. Additionally, SRP+aPDT showed an increased expression of FGF-2, which plays a role in tissue repair as compared to SRP alone, and was assessed in only one study [17]. A meta-analysis on secondary outcomes was not possible due to disparity in scoring methodology, incomplete, or incomparable data.
Table 5.
Tabular representation describing the assessment of clinical parameters used for the selected eligible in vivo human studies. Refer to Supplementary file 2 for a list of abbreviations.
| Study, Year, Origin and Citation | PPD | CAL | BOP/SBI | PI | GI | GR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Statistically Significant Y/N/NI/NS |
Not Statistically Significant Y/N/NI/NS |
Statistically Significant Y/N/NI/NS |
Not Statistically Significant Y/N/NI/NS |
Statistically Significant Y/N/NI/NS |
Not Statistically Significant Y/N/NI/NS |
Statistically Significant Y/N/NI/NS |
Not Statistically Significant Y/N/NI/NS |
Statistically Significant Y/N/NI/NS |
Not Statistically Significant Y/N/NI/NS |
Statistically Significant Y/N/NI/NS |
Not Statistically Significant Y/N/NI/NS |
|
| De Oliveira et al., 2007 (Brazil) [3] | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y |
| Franco et al., 2014 (Brazil) [17] | N | Y | N | Y | Y | N | N | Y | NS | NS | NS | NS |
| Pourabbas et al., 2014 (Iran) [70] | N | Y | N | Y | N | Y | NS | NS | NS | NS | N | Y |
| Moreira et al., 2015 (Brazil) [71] | Y | N | Y | N | Y | N | Y | N | NS | NS | Y | N |
| Arweiler et al., 2014 (Poland) [73] | N | Y | N | Y | N | Y | N | Y | NS | NS | N | Y |
| Arweiler et al., 2013 (Poland) [74] | N | Y | N | Y | N | Y | N | Y | NS | NS | N | Y |
| Vidal et al., 2017 (Spain) [75] | N | Y | N | Y | N | Y | N | Y | NS | NS | N | Y |
| Braun et al., 2008 (Germany) [76] | Y | N | Y | N | Y | N | NS | NS | NS | NS | N | Y |
| Berakdar et al., 2012 (Germany) [77] | Y | N | Y | N | N | Y | N | Y | NS | NS | NS | NS |
| Raut et al., 2018 (India) [78] | Y | N | Y | N | Y | N | N | Y | NS | NS | NS | NS |
| Hokari et al., 2018 (Japan) [79] | N | Y | N | Y | N | Y | N | Y | N | Y | NS | NS |
| Hill et al., 2019 (Germany) [80] | N | Y | N | Y | N | Y | NS | NS | NS | NS | N | Y |
| Ahad et al., 2016 (India) [81] | N | Y | N | Y | Y | N | N | Y | NS | NS | NS | NS |
| Balata et al., 2013 (Brazil) [82] | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y |
| Bechara et al., 2018 (Brazil) [83] | Y | N | Y | N | Y | N | NS | NS | NS | NS | Y | N |
| Bundidpun et al., 2017 (Thailand) [84] | N | Y | N | Y | Y | N | N | Y | Y | N | NS | NS |
| Chitsazi et al., 2014 (Iran) [85] | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y |
| Chitsazi et al., 2014 (Iran) [86] | N | Y | N | Y | N | Y | NS | NS | NS | NS | N | Y |
| Garcia et al., 2011 (Brazil) [87] | N | Y | N | Y | NS | NS | NS | NS | NS | NS | NS | NS |
| Joseph et al., 2014 (India) [88] | Y | N | Y | N | N | Y | N | Y | N | Y | NS | NS |
| Malgikar et al., 2015 (India) [89] | Y | N | Y | N | Y | N | Y | N | Y | N | NS | NS |
| Monzavi et al., 2016 (Iran) [90] | Y | N | N | Y | Y | N | N | Y | NS | NS | NS | NS |
| Raj et al., 2016 (India) [91] | Y | N | N | Y | NS | NS | Y | N | Y | N | NS | NS |
| Sena et al., 2019 (Brazil) [92] | N | Y | N | Y | N | Y | N | Y | NS | NS | NS | NS |
| Shingnapurkar et al., 2016 (India) [93] | Y | N | Y | N | NS | NS | N | Y | N | Y | NS | NS |
| Sigusch et al., 2010 (Germany) [94] | Y | N | Y | N | Y | N | Y | N | NS | NS | Y | N |
| Theodoro et al., 2012 (Brazil) [95] | N | Y | N | Y | N | Y | N | Y | N | Y | N | Y |
| Theodoro et al., 2017 (Brazil) [96] | N | Y | N | Y | N | Y | NS | NS | NS | NS | NS | NS |
3.4.2. Sensitivity Analysis
A sensitivity analysis was conducted due to the noteworthy heterogeneity arising from outlier studies which were detected upon visual inspection of Forest plots [74,86,90,92,93] (Table 3 and Table 4). This analysis was conducted only for the three-month follow-up due to unavailability of data in included studies (Table 6). In terms of PPD reduction, SM studies (SMD 0.282; 95% CI −0.286 to 0.624; P = 0.153) as well as PG studies (SMD 0.257; 95% CI −0.230 to 0.683; P = 0.361) did not report a statistically significant improvement. No evident heterogeneity (Q = 9.14; P = 0.7; I2 = 0.00%) in SM studies and in PG studies (Q = 8.87; P = 0.22; I2 = 0.00%) was noted (Table 6). Although improvement in CAL gain was noted after omitting outlier studies, this difference was statistically not significant in both SM (SMD 0.162; 95% CI −0.326 to 0.406; P = 0.166) and PG studies (SMD 0.227; 95% CI −0.420 to 0.673; P = 0.234) with no evident heterogeneity (Q = 8.40; P = 0.625; I2 = 0.00%) in SM studies as well as in PG studies (Q = 9.7; P = 0.22; I2 = 0.00%) (Table 6).
Table 6.
Forest plots based on sensitivity analysis illustrating the overall PPD reduction and CAL gain at 3 months without outlier studies. Refer to Supplementary file 2 for a list of abbreviations.
| Overall PPD Reduction for SM Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.153 |
|
| Chitsazi et al., 2014 | 0.525 | 0.289 | −0.056 to 1.106 | 12.28 | ||
| Moreira et al., 2015 | 0.205 | 0.311 | −0.425 to 0.834 | 12.11 | ||
| Franco et al., 2014 | 0.631 | 0.364 | −0.115 to 1.378 | 11.67 | ||
| Malgikar et al., 2015 | 0.119 | 0.284 | −0.453 to 0.691 | 12.31 | ||
| Ahad et al., 2016 | 0.639 | 0.204 | 0.235 to 1.043 | 12.88 | ||
| Bundidpun et al., 2017 | 0.007 | 0.310 | −0.620 to 0.635 | 12.11 | ||
| Hill et al., 2019 | 0.039 | 0.072 | −0.103 to 0.181 | 14.47 | ||
| Braun et al., 2008 | 0.139 | 0.310 | −0.490 to 0.767 | 12.11 | ||
| Total (random effects) | 0.282 | 0.234 | −0.286 to 0.624 | 100.00 | ||
| Heterogeneity: Q = 9.14; DF = 7; P = 0.71; I2 = 0.00% | ||||||
| Overall PPD Reduction for PG Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.361 |
|
| Raj et al., 2016 | 0.669 | 0.441 | −0.258 to 1.595 | 18.89 | ||
| Vidal et al., 2017 | −0.060 | 0.322 | −0.714 to 0.593 | 25.59 | ||
| Theodoro et al., 2017 | −0.127 | 0.374 | −0.897 to 0.643 | 22.43 | ||
| Joseph et al., 2014 | 0.556 | 0.215 | 0.127 to 0.984 | 33.19 | ||
| Total (random effects) | 0.257 | 0.278 | −0.230 to 0.683 | 100.00 | ||
| Heterogeneity: Q = 8.87; DF = 3; P = 0.22; I2 = 0.00% | ||||||
| Overall CAL Gain for SM Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.166 |
|
| Chitsazi et al., 2014 | 0.439 | 0.287 | −0.140 to 1.017 | 5.52 | ||
| Moreira et al., 2015 | −0.040 | 0.310 | −0.667 to 0.588 | 5.02 | ||
| Franco et al., 2014 | 0.601 | 0.364 | −0.144 to 1.346 | 4.20 | ||
| Malgikar et al., 2015 | −0.048 | 0.284 | −0.620 to 0.523 | 5.60 | ||
| Ahad et al., 2016 | 0.158 | 0.199 | −0.237 to 0.552 | 10.35 | ||
| Bundidpun et al., 2017 | 0.032 | 0.310 | −0.595 to 0.660 | 5.02 | ||
| Hill et al., 2019 | 0.019 | 0.072 | −0.123 to 0.161 | 59.29 | ||
| Braun et al., 2008 | 0.161 | 0.310 | −0.468 to 0.789 | 5.01 | ||
| Total (random effects) | 0.162 | 0.253 | −0.326 to 0.406 | 100.00 | ||
| Heterogeneity: Q = 8.40; DF = 7; P = 0.625; I2 = 0.00% | ||||||
| Overall CAL Gain for PG Studies at 3 Months | ||||||
| Study | SMD | SE | 95% CI | Weight (%) | P = 0.234 |
|
| Raj et al., 2016 | 0.669 | 0.441 | −0.258 to 1.595 | 18.89 | ||
| Vidal et al., 2017 | −0.102 | 0.372 | −0.514 to 0.793 | 25.59 | ||
| Theodoro et al., 2017 | −0.106 | 0.374 | −0.997 to 0.743 | 22.43 | ||
| Joseph et al., 2014 | 0.456 | 0.255 | −0.120 to 0.673 | 33.19 | ||
| Total (random effects) | 0.227 | 0.352 | −0.420 to 0.673 | 100.00 | ||
| Heterogeneity: Q = 9.7; DF = 3; P = 0.22; I2 = 0.00% | ||||||
3.4.3. Publication Bias
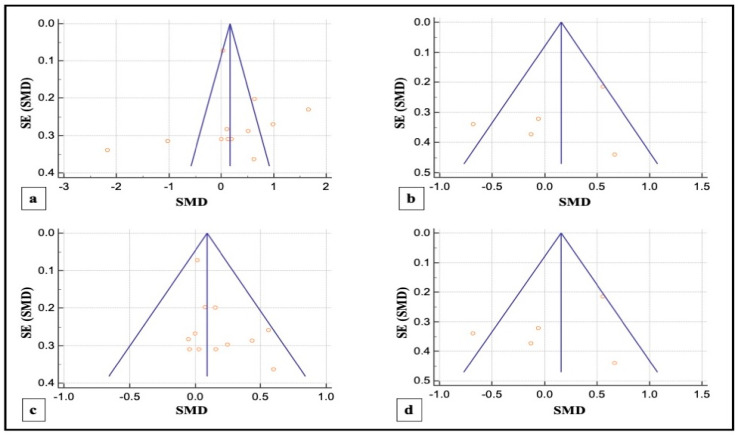
Visual inspection of funnel plots revealed noticeable asymmetry in SM-study analysis for PPD reduction indicating a probable risk of publication bias in SM studies included in this meta-analysis. However, slight asymmetries for PG studies were noted in corresponding funnel plots suggestive of a low risk of publication bias in the same (Figure 6).
Figure 6.
(a,b) Funnel plots illustrating the publication bias in overall PPD reduction in SM and PG studies, respectively; (c,d) funnel plots illustrating the publication bias in overall CAL gain in SM and PG studies, respectively. Each circle represents a single included study, the y-axis and x-axis represent the standard error of the effect estimate and the results of the study respectively and the graphical plot resembles an inverted funnel with scatter due to sampling variations.
4. Discussion
Based on the hypothesis that aPDT monotherapy or as an adjunct to NSPT can enhance the clinical or microbiological or immunological profile in comparison to conventional SRP, or SRP+AB, a critical appraisal of the available scientific evidence was conducted. After meticulous scrutiny, 31 studies were included in the present systematic review [2,3,5,17,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96]. Owing to the methodological discrepancies, only 18 out 31 studies were eligible for a meta-analysis [17,71,73,74,75,76,80,81,84,85,86,88,89,90,91,92,93,96]. This report is the first to evaluate the role of SRP+aPDT compared to SRP alone or SRP+AB in SM and PG studies in AgP as well as in CP patients. The results of this meta-analysis indicated that, in comparison to SRP alone or SRP+AB, SRP+aPDT failed to show any additional benefit in the management of periodontitis up to six months. A significant heterogeneity was reported, arising from confounders in aPDT protocols. Subsequently, after omitting outlier studies [74,86,90,92,93], sensitivity analysis was able to eliminate heterogeneity completely but failed to report statistically significant improvements in primary outcome variables. Furthermore, risk of publication bias was reported indicating a possible selective outcome reporting in eligible published studies. In some instances, missing outcomes could not be detected by comparing the published report with the respective study protocol due to unavailability of the latter. Until now, seven meta-analyses have been reported to assess the role of aPDT in periodontitis [6,7,8,9,10,11,12]. The present review protocol is in accordance with the existing reviews [8,10,11,12]. Azaripour et al. is the only other systematic review and meta-analysis that has assessed the efficacy of SRP+aPDT as compared to SRP alone for SM and PG studies [8]. While three reviews report short-term benefits of aPDT up to 6 months [7,8,9], four have reported otherwise [6,10,11,12]. Our findings are in accordance with findings of the latter scientific reports. In order to gain an insight on merits and inadequacies of each included study, a comprehensive and systematic investigation was performed as follows:
4.1. Role of Baseline Characteristics
A key feature of RCTs is the application of balanced baseline characteristics in treatment arms of the trial in order to achieve unbiased treatment outcomes [97]. Most often, researchers provide a tabular representation of relevant variables to confirm an impartial baseline evaluation. In case of missing information on baseline characteristics, a ‘selection bias’ can be suspected [98]. All eligible studies have provided this vital information and were free from any kind of ‘selection bias’. Additionally, evidence-based studies have suggested the potential harmful effects of smoking on the onset and progression of periodontitis, for which smokers were excluded [99,100]. Likewise, the inter-relationship of periodontitis and its systemic manifestations are well-established, resulting in the exclusion of patients with systemic diseases [101,102]. Utilization of ‘placebo/sham’ procedures to enhance clinical outcomes of the trial is an evidence-based verified concept [103]. In the present systematic review, only six out of 31 studies [71,78,92,93,94,96] have utilized a ‘placebo/sham’ procedure as an adjunct to conventional SRP. Furthermore, the role of SRP+aPDT+AB, compared to SRP+AB and SRP+aPDT as well the efficacy of SRP+PS compared to the conventional SRP and SRP+ aPDT have been assessed in this review [83,95]. Differences in study designs were apparent and the majority of clinicians have utilized the SM study design in oral health research [104]. Hence, this meta-analysis included both SM and PG studies in order to assess whether the estimated intervention effect has differed between them.
4.2. Assessment Methods for Various Parameters and Their Inferences to Determine aPDT Efficacy
A decrease in periodontal inflammation is directly proportional to a decrease in the incidence of BOP and detectable plaque levels [105]. Furthermore, the endpoints of a comprehensive periodontal therapy include PPD reduction and CAL gain, both of which are crucial for determining treatment success [106]. Clinical evidence has proven that there is a direct correlation between initial severity of PPD and CAL values and the amount of post-operative differences [107,108,109]. In terms of disease severity at baseline evaluation, a lack of homogeneity in pre-treatment values of PPD and CAL in the data extracted from various studies was observed (Table 1). Therefore, variations in level of significance across clinical parameters were noted, thus making it difficult to provide a cumulative result (Table 5). Utilization of a narrow laser optic fibre tip in deep periodontal pockets facilitates easy and atraumatic periodontal pocket probing ultimately resulting in the PPD and GR reduction post-operatively [3,88]. Furthermore, evidence-based research has proven that clinical outcome remains unaffected by the type of instrumentation utilized in SRP [76,110], which was observed to vary across all eligible studies (Table 1).
An imbalance amongst the local etiological factors such as dental plaque and calculus, inflammation, and a host defense system can have a great impact on the disease severity and progression [17]. Hence, it is essential to monitor the microbiological and the molecular changes of various growth factors and proinflammatory cytokines. De Oliveira and co-workers have demonstrated, through their studies, the importance of SRP in reducing bacterial load from tooth surface and the failure of aPDT monotherapy in reducing the bacterial counts of A.a. periodontal disease activity as well as bone resorption [2,5].
4.3. Representation of the Treatment Outcomes
Positive outcomes of any treatment strategy are governed by several factors such as evaluation of disease status at carefully planned follow-up visits, signs of uneventful healing, role of supportive periodontal therapy (SPT) and patient compliance with treatment [111,112,113]. The majority of included studies performed follow-up assessment for up to 3 or 6 months, whereas results of a longer follow-up ranging up to 1–2 years was lacking. Collective data obtained from longitudinal studies have confirmed the role of long-term follow up visits in greater reduction of clinical parameters. These findings have been confirmed by three studies included in the present review [73,77,95]. With regard to healing outcomes, 24 out of 31 studies reported no uneventful healing associated with the absence of any postoperative complications, after application of aPDT [2,3,5,70,71,72,73,74,75,77,78,79,80,81,83,84,85,86,87,88,89,90,92,95]. While six studies have failed to provide any information on healing outcomes [17,76,82,91,93,94], one study [96] has reported a gastrointestinal complication in one patient of the aPDT group. This happens to be unique evidence that has not been registered elsewhere. The presence of residual plaque and calculus resulting in a relapse of periodontal disease severity being inevitable with aPDT monotherapy. Hence, the role of SPT is quintessential. In the present systematic review, apart from the three sequential studies conducted by De Oliveira and co-workers [2,3,5] which utilized aPDT monotherapy, all of the other studies have utilized SRP+aPDT. In the former group of studies, a supragingival professional tooth cleaning was performed 14 days prior to the application of aPDT monotherapy in the treatment sites, which have extensively improved the post-operative clinical findings. Likewise, amongst the studies that have assessed the efficacy of SRP+aPDT, only seven studies [71,75,82,84,90,92,95] have mentioned a planned SPT protocol, which was implemented throughout their study period. Furthermore, information on oral hygiene instructions tailored according to respective study criteria has been specified in all included studies. Co-relation of patient’s compliance in adhering to hygiene instructions cannot be overlooked, since it is vital for treatment success and prevention of disease recurrence [114]. Quantitative measurement of plaque by means various indices can aid in monitoring compliance towards therapy. In connection to this, the assessment of plaque levels was performed in 21 studies included in this review [3,17,71,73,74,75,77,78,79,81,82,84,85,88,89,90,91,92,93,94]. Therefore, an inconsistency in the representation of treatment outcomes in this review is evident.
4.4. Role of Laser Parameters
Apart from study methodology, laser parameters are crucial in determining treatment outcome. Calibration of therapeutic power output with a power-meter can aid in regulating low output power for achieving the desired aPDT effect at treatment sites [115]. This technique can also prevent any inadvertent damage caused by utilization of high output power, resulting in a photothermal effect. However, none of the included studies have utilized a power-meter to calibrate the power output. Furthermore, some other parameters that have been overlooked were: emission mode, contact/non-contact mode, energy/treated site, power output, spot size/fibre diameter, fluence and irradiance. Diode lasers have a high affinity to pigment, which, in the case of aPDT, is the PS. However, inflamed periodontal tissues are rich in blood and high levels of proteins, which are also rich in pigments. Traumatic instrumentation can lacerate the sulcus lining and elicit bleeding [23]. Consequently, the overall aPDT effect, which could be achieved by an effective PS dye-laser wavelength combination, will be compromised [23,116]. Additionally, placement of the fibre tip inside the gingival sulcus needs to be performed judiciously, in order to avoid further trauma to inflamed gingival sulcus, serving as a niche for plaque accumulation and favoring disease relapse [3,88].
Bacterial re-colonization after SRP has been proven to occur after three weeks [117] and, hence, multiple aPDT sessions can help to delay this pathological process. Annaji et al., 2016 [46] have compared the efficacy of three sessions (0, 7th and 21st day) versus a single session (0 day) of aPDT in the management of CP, and concluded that the group receiving multiple sessions demonstrated superior treatment outcomes. Nine studies included in this review conducted multiple aPDT sessions [17,71,72,73,74,75,79,90,96], and have unanimously concluded that the utilization of multiple aPDT sessions has positive healing effects. However, the frequency of aPDT application varies in all these studies (Table 2). As a result, a conclusion on the ideal number of sessions and frequency of application of aPDT cannot be drawn.
Additionally, PS concentration and pre-irradiation time (wash-out or PS incubation time) are governed by the binding capacity of PS to target cells [118]. It was observed in 13 out of 31 studies, whereby the PS concentration was not reported [5,70,72,73,74,76,81,84,85,86,91,93,94], whereas a range of PS concentrations have been utilized in the remaining eligible studies. Furthermore, four studies have failed to mention PS pre-irradiation time [5,75,77,90], whereas the remaining 27 studies have reported this time, which ranged from 1–5 min for different PS. An in vitro study by Fumes et al. [119] evaluated the effect of different PS pre-irradiation time periods (1, 2 and 5 min) in aPDT on the biofilms formed by Streptococcus mutans and Candida albicans, by monitoring the microbial load and have successfully demonstrated that the efficacy of all pre-irradiation times was equal. They emphasized patient discomfort associated with longer pre-irradiation times and thus have advised the use of shorter pre-irradiation times (1 min) in future studies. Up until now, there have been no studies that have determined the minimal duration of PS incubation as well as its role against periopathogens. Hence, further studies on this subject should be sought after. Moreover, discrepancies in the reported data, in terms of the number of sites receiving aPDT application per tooth as well the irradiation time, were noticed amongst the eligible studies (Table 2). These voids have raised concerns regarding the reliability of existing literature, which lacks a reproducible methodology and ultimately hampers the rational use of aPDT in non-surgical management of periodontitis.
4.5. Role of RoB Assessment
A vast majority of bias were raised from inadequate randomization, deviations from intended interventions and selective reporting of results (Figure 4 and Figure 5). Another key finding of this systematic review was the presence of a potential conflict of interest in 10 out of the 31 studies [2,3,5,72,73,74,76,79,80,94]. Therefore, the results of the included studies are questionable, and their biased methodology cannot be relied upon.
4.6. Limitations of the Present Systematic Review
The majority of the included studies have assessed the efficacy of SRP+aPDT in comparison to aPDT monotherapy resulting in a lack of meta-analysis on the latter. Utilization of a limited number of teeth (mostly anterior teeth) or on specific sites (interproximal sites in case of deep pockets) or on any two quadrants of the dental arch that could facilitate cross contamination from the untreated sites and overshadow the putative benefits of aPDT was noted. In this review, efficacy of aPDT was monitored in systemically healthy non-smokers only, and its benefits in their immunocompromised counterparts were not established. A lack of long-term follow-up in order to determine the stability in healing after aPDT was also observed. The number of studies included in the meta-analysis was nearly half of the studies eligible for a systematic review due to paucity and inconsistency in available literature. Owing to the aforesaid drawbacks, the objective of this review could not be accomplished.
4.7. Future Scope
Future investigations should compare aPDT monotherapy versus SRP+aPDT and provide details of all appropriate laser and PS parameters. Efficacy of aPDT in smokers with various systemic diseases in CP and AgP should be established. The role of patient related outcomes and SPT should be emphasized upon. Nevertheless, future RCTs must have a robust methodology with balanced baseline characteristics, performed by experienced, masked and calibrated clinicians, which will reduce bias. Owing to the evident benefits of a PG-study design, clinicians should prefer its utilization in order to minimize potential ‘carry-over’ effects. Additionally, researchers should conduct a full mouth study protocol with a long-term follow-up assessment of minimum 6 months duration, consisting of, but not limited to, a vast range of clinical, microbiological, radiographic as well as, immune-histological profiles.
5. Conclusions
Within the limits of the present systematic review and meta-analysis, it can be concluded that the efficacy of aPDT in improving treatment outcomes, when it is utilized in the non-surgical management of periodontitis, remains debatable. However, the results of a majority of included studies have demonstrated the effectiveness of aPDT, and this role is more pronounced for SRP+aPDT rather than aPDT monotherapy. A careful and critical appraisal was performed which helped to obtain a qualitative assessment of eligible studies, thus highlighting the substantial flaws that prevent a reproducible methodology. Data on standardized aPDT study protocol, ideal PS dye-laser combination, optimal laser and PS parameters remain inconsistent and inconclusive amongst the prevalent literature owing to a highly inferior RoB in many studies. Finally, future research should aim for well-designed, robust and preferably PG-RCTs that will overcome the abovementioned limitations and confounders, in order to achieve palpable progress in this field of research while ensuring the use of an appropriate local laser safety protocol.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pharmaceutics13060836/s1, PRISMA Checklist (Supplementary file 1), List of abbreviations (Supplementary file 2).
Author Contributions
Conceptualization, S.D., R.H.; methodology, S.D., R.H.; software, I.R.B., T.S.; validation, S.D., S.B., R.H., I.R.B.; formal analysis, S.B., I.R.B.; investigation, S.D., R.H.; resources, S.D., R.H., S.B., T.S., I.R.B.; data curation, S.D., R.H.; writing: original draft preparation, S.D., R.H.; writing: review and editing, S.D., R.H.; visualization, S.B., I.R.B., T.S.; supervision, S.D., R.H., S.B., T.S., I.R.B.; project administration, T.S., I.R.B.; funding acquisition, T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rajesh S., Koshi E., Philip K., Mohan A. Antimicrobial photodynamic therapy: An overview. J. Indian Soc. Periodontol. 2011;15:323–327. doi: 10.4103/0972-124X.92563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Oliveira R.R., Schwartz-Filho H.O., Novaes A.B., Jr., de Souza R.F., Taba M., Jr., Scombatti de Souza S.L., Ribeiro F.J. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: Cytokine profile in gingival crevicular fluid, preliminary results. J. Periodontol. 2009;80:98–105. doi: 10.1902/jop.2009.070465. [DOI] [PubMed] [Google Scholar]
- 3.De Oliveira R.R., Schwartz-Filho H.O., Novaes A.B., Jr., Taba M., Jr. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: A preliminary randomized controlled clinical study. J. Periodontol. 2007;78:965–973. doi: 10.1902/jop.2007.060494. [DOI] [PubMed] [Google Scholar]
- 4.Van der Velden U. What exactly distinguishes aggressive from chronic periodontitis: Is it mainly a difference in the degree of bacterial invasiveness? Periodontology 2000. 2017;75:24–44. doi: 10.1111/prd.12202. [DOI] [PubMed] [Google Scholar]
- 5.Novaes A.B., Jr., Schwartz-Filho H.O., de Oliveira R.R., Feres M., Sato S., Figueiredo L.C. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: Microbiological profile. Lasers Med. Sci. 2012;27:389–395. doi: 10.1007/s10103-011-0901-6. [DOI] [PubMed] [Google Scholar]
- 6.Atieh M.A. Photodynamic therapy as an adjunctive treatment for chronic periodontitis: A meta-analysis. Lasers Med. Sci. 2010;4:605–613. doi: 10.1007/s10103-009-0744-6. [DOI] [PubMed] [Google Scholar]
- 7.Sgolastra F., Petrucci A., Severino M., Graziani F., Gatto R., Monaco A. Adjunctive photodynamic therapy to non-surgical treatment of chronic periodontitis: A systemic review and meta-analysis. J. Clin. Periodontol. 2013;40:514–526. doi: 10.1111/jcpe.12094. [DOI] [PubMed] [Google Scholar]
- 8.Azaripour A., Dittrich S., Van Noorden C.J.F., Willershausen C. Efficacy a photodynamic therapy as adjunct treatment of chronic periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2018;33:407–423. doi: 10.1007/s10103-017-2383-7. [DOI] [PubMed] [Google Scholar]
- 9.Sgolastra F., Petrucci A., Gatto R., Mazzo G., Monaco A. Photodynamic therapy in the treatment of chronic periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2013;28:669–682. doi: 10.1007/s10103-011-1002-2. [DOI] [PubMed] [Google Scholar]
- 10.Souza E., Medeiros A.C., Gurgel B.C., Sarmento C. Antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: A systematic review and meta-analysis. Lasers Med. Sci. 2016;31:187–196. doi: 10.1007/s10103-015-1836-0. [DOI] [PubMed] [Google Scholar]
- 11.Azarpazhooh A., Shah P.S., Tenenbaum H.C., Goldberg M.B. The effect of photodynamic therapy for periodontitis: A systematic review and meta-analysis. J. Periodontol. 2010;81:4–14. doi: 10.1902/jop.2009.090285. [DOI] [PubMed] [Google Scholar]
- 12.Akram Z., Hyder T., Al-Hammoudi N., Binshabaib M.S., Alharthi S.S., Hanif A. Efficacy of photodynamic therapy versus antibiotics as an adjunct to scaling and root planing in the treatment of periodontitis: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2017;19:86–92. doi: 10.1016/j.pdpdt.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Vohra F., Akram Z., Safii S.H., Vaithilingam R.D., Ghanem A., Sergis K., Javed F. Role of antimicrobial photodynamic therapy in the treatment of aggressive periodontitis: A systematic review. Photodiagnosis Photodyn. Ther. 2016;13:139–147. doi: 10.1016/j.pdpdt.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 14.Chatzopoulos G.S., Doufexi A.E. Photodynamic therapy in the treatment of aggressive periodontitis: A systematic review. Med. Oral Patol. Oral Cir. Bucal. 2016;21:e192–e200. doi: 10.4317/medoral.21046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy P., Reddy C., Krishna Kumar R.V.S., Sudhir K.M., Srinivasulu G. Effect of photodynamic therapy on aggressive periodontitis—A systematic review. Int. J. Sci. Res. Manag. 2017;5:7177–7185. [Google Scholar]
- 16.Pal A., Paul S., Perry R., Puryer J. Is the use of antimicrobial photodynamic therapy or systemic antibiotics more effective in improving periodontal health when used in conjunction with localised non-surgical periodontal therapy? A systematic review. Dent. J. 2019;7:108. doi: 10.3390/dj7040108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Franco E.J., Pogue R.E., Sakamoto L.H., Cavalcante L.L., Carvalho D.R., de Andrade R.V. Increased expression of genes after periodontal treatment with photodynamic therapy. Photodiagnosis Photodyn. Ther. 2014;11:41–47. doi: 10.1016/j.pdpdt.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Van Assche N., Van Essche M., Pauwels M., Teughels W., Quirynen M. Do periodontopathogens disappear after full-mouth tooth extraction? J. Clin. Periodontol. 2009;36:1043–1047. doi: 10.1111/j.1600-051X.2009.01477.x. [DOI] [PubMed] [Google Scholar]
- 19.Guerrero A., Griffiths G.S., Nibali L., Suvan J., Moles D.R., Laurell L., Tonetti M.S. Adjunctive benefits of systemic amoxicillin and metronidazole in non-surgical treatment of generalized aggressive periodontitis: A randomized placebo-controlled clinical trial. J. Clin. Periodontol. 2005;32:1096–1107. doi: 10.1111/j.1600-051X.2005.00814.x. [DOI] [PubMed] [Google Scholar]
- 20.Sigusch B., Beier M., Klinger G., Pfister W., Glockmann E. A 2-step non-surgical procedure and systemic antibiotics in the treatment of rapidly progressive periodontitis. J. Periodontol. 2001;72:275–283. doi: 10.1902/jop.2001.72.3.275. [DOI] [PubMed] [Google Scholar]
- 21.Herrera D., Sanz M., Jepsen S., Needleman I., Roldán S. A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J. Clin. Periodontol. 2002;29:136–162. doi: 10.1034/j.1600-051X.29.s3.8.x. [DOI] [PubMed] [Google Scholar]
- 22.Pallasch T.J. Antibiotic resistance. Dent. Clin. N. Am. 2003;47:623–639. doi: 10.1016/S0011-8532(03)00039-9. [DOI] [PubMed] [Google Scholar]
- 23.Andersen R.C., Loebel N.G. Photodynamic disinfection in the treatment of chronic adult periodontitis: A multicenter clinical trial. J. Dent. Health Oral Disord. Ther. 2017;8:00289. doi: 10.15406/jdhodt.2017.08.00289. [DOI] [Google Scholar]
- 24.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J.P.T., Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 The Cochrane Collaboration. [(accessed on 12 February 2021)];2011 Available online: http://www.cochrane-handbook.org.
- 26.Armitage G.C. Development of a classification system for periodontal diseases and conditions. Ann. Periodontol. 1999;4:1–6. doi: 10.1902/annals.1999.4.1.1. [DOI] [PubMed] [Google Scholar]
- 27.McHugh M.L. Inter-rate reliability: The kappa statistic. Biochem. Med. 2012;22:276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 29.Altman D.G., Schulz K.F., Moher D., Egger M., Davidoff F., Elbourne D., Gøtzsche P.C., Lang T., CONSORT GROUP (Consolidated Standards of Reporting Trials) The revised CONSORT statement for reporting randomized trials: Explanation and elaboration. Ann. Intern Med. 2001;134:663–694. doi: 10.7326/0003-4819-134-8-200104170-00012. [DOI] [PubMed] [Google Scholar]
- 30.Higgins J.P.T., Eldridge S., Li T. Chapter 23: Including variants on randomized trials. In: Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A., editors. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. Cochrane; Oxford, UK: 2019. [(accessed on 12 February 2021)]. (updated July 2019) Available online: www.training.cochrane.org/handbook. [Google Scholar]
- 31.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lau J., Ioannidis J.P., Schmid C.H. Quantitative synthesis in systematic reviews. Ann. Intern Med. 1997;127:820–826. doi: 10.7326/0003-4819-127-9-199711010-00008. [DOI] [PubMed] [Google Scholar]
- 33.Higgins J.P.T., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 34.Sterne J.A.C., Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001;54:1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 35.Theodoro L.H., Assem N.Z., Longo M., Alves M.L.F., Duque C., Stipp R.N., Vizoto N.L., Garcia V.G. Treatment of periodontitis in smokers with multiple sessions of antimicrobial photodynamic therapy or systemic antibiotics: A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2018;22:217–222. doi: 10.1016/j.pdpdt.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 36.Al-Zahrani M.S., Austah O.N. Photodynamic therapy as an adjunctive to scaling and root planing in treatment of chronic periodontitis in smokers. Saudi Med. J. 2011;32:1183–1188. [PubMed] [Google Scholar]
- 37.Tabenski L., Moder D., Cieplik F., Schenke F., Hiller K.A., Buchalla W., Schmalz G., Christgau M. Antimicrobial photodynamic therapy vs. local minocycline in addition to non-surgical therapy of deep periodontal pockets: A controlled randomized clinical trial. Clin. Oral Investig. 2017;21:2253–2264. doi: 10.1007/s00784-016-2018-6. [DOI] [PubMed] [Google Scholar]
- 38.Ge L., Shu R., Li Y., Li C., Luo L., Song Z., Xie Y., Liu D. Adjunctive effect of photodynamic therapy to scaling and root planing in the treatment of chronic periodontitis. Photomed. Laser Surg. 2011;29:33–37. doi: 10.1089/pho.2009.2727. [DOI] [PubMed] [Google Scholar]
- 39.Loebel N.G., Galler C.C., Andersen R.C. Antimicrobial photodynamic therapy in the treatment of chronic adult periodontitis. Oral Health Dental Sci. 2018;2:1–7. doi: 10.33425/2639-9490.1009. [DOI] [Google Scholar]
- 40.Harmouche L., Courval A., Mathieu A., Peti C., Huck O., Severac F., Davideau J.L. Impact of tooth-related factors on photodynamic therapy effectiveness during active periodontal therapy: A 6-months split-mouth randomized clinical trial. Photodiagnosis Photodyn. Ther. 2019;27:167–172. doi: 10.1016/j.pdpdt.2019.05.022. [DOI] [PubMed] [Google Scholar]
- 41.Christodoulides N., Nikolidakis D., Chondros P., Becker J., Schwarz F., Rossler R., Sculean A. Photodynamic therapy as an adjunct to non-surgical periodontal treatment: A randomized, controlled clinical trial. J. Periodontol. 2008;79:1638–1644. doi: 10.1902/jop.2008.070652. [DOI] [PubMed] [Google Scholar]
- 42.Polansky R., Haas M., Heschl A., Wimmer G. Clinical effectiveness of photodynamic therapy in the treatment of periodontitis. J. Clin. Periodontol. 2009;36:575–580. doi: 10.1111/j.1600-051X.2009.01412.x. [DOI] [PubMed] [Google Scholar]
- 43.Badea M.E., Serbanescu A., Hedesiu M., Badea A.F. Photodynamic Laser therapy in patients with periodontitis. TMJ. 2010;60:18–22. [Google Scholar]
- 44.Andersen R., Loebel N., Hammond D., Wilson M. Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J. Clin. Dent. 2007;18:34–38. [PubMed] [Google Scholar]
- 45.Alwaeli H.A., Al-Khateeb S.N., Al-Sadi A. Long-term clinical effect of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. Lasers Med. Sci. 2015;30:801–807. doi: 10.1007/s10103-013-1426-y. [DOI] [PubMed] [Google Scholar]
- 46.Annaji S., Sarkar I., Rajan P., Pai J., Malagi S., Bharmappa R., Kamath V. Efficacy of photodynamic therapy and lasers as an adjunct to scaling and root planing in the treatment of aggressive periodontitis—A clinical and microbiologic short term study. J. Clin. Diagn. Res. 2016;10:ZC08–ZC12. doi: 10.7860/JCDR/2016/13844.7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dilsiz A., Canakci V., Aydin T. Clinical effects of potassium-titanyl-phosphate laser and photodynamic therapy on outcomes of treatment of chronic periodontitis: A randomized controlled clinical trial. J. Periodontol. 2013;84:278–286. doi: 10.1902/jop.2012.120096. [DOI] [PubMed] [Google Scholar]
- 48.Lui J., Corbet E.F., Jin L. Combined photodynamic and low-level laser therapies as an adjunct to nonsurgical treatment of chronic periodontitis. J. Periodontal Res. 2011;46:89–96. doi: 10.1111/j.1600-0765.2010.01316.x. [DOI] [PubMed] [Google Scholar]
- 49.Mistry A., Pereira R., Kini V., Padhye A. Effect of combined therapy using diode laser and photodynamic therapy on levels of IL-17 in gingival crevicular fluid in patients with chronic periodontitis. J. Lasers Med. Sci. 2016;7:250–255. doi: 10.15171/jlms.2016.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Srikanth K., Chandra R.V., Reddy A.A., Reddy B.H., Reddy C., Naveen A. Effect of a single session of antimicrobial photodynamic therapy using indocyanine green in the treatment of chronic periodontitis: A randomized controlled pilot trial. Quintessence Int. 2015;46:391–400. doi: 10.3290/j.qi.a33532. [DOI] [PubMed] [Google Scholar]
- 51.Grzech-Leśniak K., Matys J., Dominiak M. Comparison of the clinical and microbiological effects of antibiotic therapy in periodontal pockets following laser treatment: An in vivo study. Adv. Clin. Exp. Med. 2018;27:1263–1270. doi: 10.17219/acem/70413. [DOI] [PubMed] [Google Scholar]
- 52.Kamma J.J., Vasdekis V.G., Romanos G.E. The effect of diode laser (980 nm) treatment on aggressive periodontitis: Evaluation of microbial and clinical parameters. Photomed. Laser Surg. 2009;27:11–19. doi: 10.1089/pho.2007.2233. [DOI] [PubMed] [Google Scholar]
- 53.Matarese G., Ramaglia L., Cicciù M., Cordasco G., Isola G. The effects of diode laser therapy as an adjunct to scaling and root planing in the treatment of aggressive periodontitis: A 1-year randomized controlled clinical trial. Photomed. Laser Surg. 2017;35:702–709. doi: 10.1089/pho.2017.4288. [DOI] [PubMed] [Google Scholar]
- 54.Annaji S., Sarkar I., Rajan P., Pai J., Malagi S., Kamath V., Barmappa R. Comparative evaluation of the efficacy of curcumin gel with and without photo activation as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A split mouth clinical and microbiological study. J. Nat. Sci. Biol. Med. 2015;6:S102–S109. doi: 10.4103/0976-9668.166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Borekci T., Meseli S.E., Noyan U., Kuru B.E., Kuru L. Efficacy of adjunctive photodynamic therapy in the treatment of generalized aggressive periodontitis: A randomized controlled clinical trial. Lasers Surg. Med. 2019;51:167–175. doi: 10.1002/lsm.23010. [DOI] [PubMed] [Google Scholar]
- 56.Pulikkotil S.J., Toh C.G., Mohandas K., Leong K. Effect of photodynamic therapy adjunct to scaling and root planing in periodontitis patients: A randomized clinical trial. Aust. Dent. J. 2016;61:440–445. doi: 10.1111/adj.12409. [DOI] [PubMed] [Google Scholar]
- 57.Saitawee D., Teerakapong A., Morales N.P., Jitprasertwong P., Hormdee D. Photodynamic therapy of Curcuma longa extract stimulated with blue light against Aggregatibacter actinomycetemcomitans. Photodiagnosis Photodyn. Ther. 2018;22:101–105. doi: 10.1016/j.pdpdt.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Bassir S.H., Moslemi N., Jamali R., Mashmouly S., Fekrazad R., Chiniforush N., Shamshiri A.R., Nowzari H. Photoactivated disinfection using light-emitting diode as an adjunct in the management of chronic periodontitis: A pilot double-blind split-mouth randomized clinical trial. J. Clin. Periodontol. 2013;40:65–72. doi: 10.1111/jcpe.12024. [DOI] [PubMed] [Google Scholar]
- 59.Husejnagic S., Lettner S., Laky M., Georgopoulos A., Moritz A., Rausch-Fan X. Photoactivated disinfection in periodontal treatment: A randomized controlled clinical split-mouth trial. J. Periodontol. 2019;90:1260–1269. doi: 10.1002/JPER.18-0576. [DOI] [PubMed] [Google Scholar]
- 60.Maria A.M., Ursarescu I.G., Solomon S., Foia L. Evaluation the effects of led photo-activated disinfection on periodontal clinical parameters in patients with chronic periodontitis. Balk. J. Dent. Med. 2016;20:29–32. doi: 10.1515/bjdm-2016-0004. [DOI] [Google Scholar]
- 61.Campos G.N., Pimentel S.P., Ribeiro F.V., Casarin R.C., Cirano F.R., Saraceni C.H., Casati M.Z. The adjunctive effect of photodynamic therapy for residual pockets in single- rooted teeth: A randomized controlled clinical trial. Lasers Med. Sci. 2013;28:317–324. doi: 10.1007/s10103-012-1159-3. [DOI] [PubMed] [Google Scholar]
- 62.Corrêa M.G., Oliveira D.H., Saraceni C.H., Ribeiro F.V., Pimentel S.P., Cirano F.R., Casarin R.C. Short-term microbiological effects of photodynamic therapy in non-surgical periodontal treatment of residual pockets: A split-mouth RCT. Lasers Surg. Med. 2016;48:944–950. doi: 10.1002/lsm.22449. [DOI] [PubMed] [Google Scholar]
- 63.Petelin M., Perkič K., Seme K., Gašpirc B. Effect of repeated adjunctive antimicrobial photodynamic therapy on subgingival periodontal pathogens in the treatment of chronic periodontitis. Lasers Med. Sci. 2015;30:1647–1656. doi: 10.1007/s10103-014-1632-2. [DOI] [PubMed] [Google Scholar]
- 64.Cappuyns I., Cionca N., Wick P., Giannopoulou C., Mombelli A. Treatment of residual pockets with photodynamic therapy, diode laser, or deep scaling. A randomized, split-mouth controlled clinical trial. Lasers Med. Sci. 2012;27:979–986. doi: 10.1007/s10103-011-1027-6. [DOI] [PubMed] [Google Scholar]
- 65.Lulic M., Leiggener G.I., Salvi G.E., Ramseier C.A., Mattheos N., Lang N.P. One-year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: A proof-of-principle randomized-controlled clinical trial. J. Clin. Periodontol. 2009;36:661–666. doi: 10.1111/j.1600-051X.2009.01432.x. [DOI] [PubMed] [Google Scholar]
- 66.Rühling A., Fanghänel J., Houshmand M., Kuhr A., Meisel P., Schwahn C., Kocher T. Photodynamic therapy of persistent pockets in maintenance patients-A clinical study. Clin. Oral Investig. 2010;14:637–644. doi: 10.1007/s00784-009-0347-4. [DOI] [PubMed] [Google Scholar]
- 67.Barbosa F.I., Araújo P.V., Machado L.J.C., Magalhães C.S., Guimarães M.M., Moreira A.N. Effect of photodynamic therapy as an adjuvant to non-surgical periodontal therapy: Periodontal and metabolic evaluation in patients with type 2 diabetes mellitus. Photodiagnosis Photodyn. Ther. 2018;22:245–250. doi: 10.1016/j.pdpdt.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 68.Ramos U.D., Ayub L.G., Reino D.M., Grisi M.F., Taba M., Jr., Souza S.L., Palioto D.B., Novaes A.B., Jr. Antimicrobial photodynamic therapy as an alternative to systemic antibiotics: Results from a double-blind, randomized, placebo-controlled, clinical study on type 2 diabetics. J. Clin. Periodontol. 2016;43:147–155. doi: 10.1111/jcpe.12498. [DOI] [PubMed] [Google Scholar]
- 69.Alvarenga L.H., Gomes A.C., Carribeiro P., Godoy-Miranda B., Noschese G., Simões Ribeiro M., Kato I.T., Bussadori S.K., Pavani C., Geraldo Y.G., et al. Parameters for antimicrobial photodynamic therapy on periodontal pocket- Randomized clinical trial. Photodiagnosis Photodyn. Ther. 2019;27:132–136. doi: 10.1016/j.pdpdt.2019.05.035. [DOI] [PubMed] [Google Scholar]
- 70.Pourabbas R., Kashefimehr A., Rahmanpour N., Babaloo Z., Kishen A., Tenenbaum H.C., Azarpazhooh A. Effects of photodynamic therapy on clinical and gingival crevicular fluid inflammatory biomarkers in chronic periodontitis: A split-mouth randomized clinical trial. J. Periodontol. 2014;85:1222–1229. doi: 10.1902/jop.2014.130464. [DOI] [PubMed] [Google Scholar]
- 71.Moreira A.L., Novaes A.B., Jr., Grisi M.F., Taba M., Jr., Souza S.L., Palioto D.B., de Oliveira P.G., Casati M.Z., Casarin R.C., Messora M.R. Antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: A split-mouth randomized controlled trial. J. Periodontol. 2015;86:376–386. doi: 10.1902/jop.2014.140392. [DOI] [PubMed] [Google Scholar]
- 72.Skurska A., Dolinska E., Pietruska M., Pietruski J.K., Dymicka V., Kemona H., Arweiler N.B., Milewsk R., Sculean A. Effect of nonsurgical periodontal treatment in conjunction with either systemic administration of amoxicillin and metronidazole or additional photodynamic therapy on the concentration of matrix metalloproteinases 8 and 9 in gingival crevicular fluid in patients with aggressive periodontitis. BMC Oral Health. 2015;15:63. doi: 10.1186/s12903-015-0048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Arweiler N.B., Pietruska M., Pietruski J., Skurska A., Dolińsk E., Heumann C., Auschill T.M., Sculean A. Six-month results following treatment of aggressive periodontitis with antimicrobial photodynamic therapy or amoxicillin and metronidazole. Clin. Oral Investig. 2014;18:2129–2135. doi: 10.1007/s00784-014-1193-6. [DOI] [PubMed] [Google Scholar]
- 74.Arweiler N.B., Pietruska M., Skurska A., Dolińska E., Pietruski J.K., Bläs M., Auschill T.M., Sculean A. Nonsurgical treatment of aggressive periodontitis with photodynamic therapy or systemic antibiotics. Three-month results of a randomized, prospective, controlled clinical study. Schweiz. Monatsschr. Zahnmed. 2013;123:532–544. [PubMed] [Google Scholar]
- 75.Segarra-Vidal M., Guerra-Ojeda S., Vallés L.S., López-Roldán A., Mauricio M.D., Aldasoro M., Alpiste-Illueca F., Vila J.M. Effects of photodynamic therapy in periodontal treatment: A randomized, controlled clinical trial. J. Clin. Periodontol. 2017;44:915–925. doi: 10.1111/jcpe.12768. [DOI] [PubMed] [Google Scholar]
- 76.Braun A., Dehn C., Krause F., Jepsen S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. J. Clin. Periodontol. 2008;35:877–884. doi: 10.1111/j.1600-051X.2008.01303.x. [DOI] [PubMed] [Google Scholar]
- 77.Berakdar M., Callaway A., Eddin M.F., Ross A., Willershausen B. Comparison between scaling-root-planing (SRP) and SRP/Photodynamic therapy: Six-month study. Head Face Med. 2012;8:12. doi: 10.1186/1746-160X-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raut C.P., Sethi K.S., Kohale B.R., Mamajiwala A., Warang A. Indocyanine green- mediated photothermal therapy in treatment of chronic periodontitis: A clinico- microbiological study. J. Indian Soc. Periodontol. 2018;22:221–227. doi: 10.4103/jisp.jisp_128_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hokari T., Morozumi T., Komatsu Y., Shimizu T., Yoshino T., Tanaka M., Tanaka Y., Nohno K., Kubota T., Yoshie H. Effects of antimicrobial photodynamic therapy and local administration of minocycline on clinical, microbiological, and inflammatory markers of periodontal pockets: A Pilot Study. Int. J. Dent. 2018:1748584. doi: 10.1155/2018/1748584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hill G., Dehn C., Hinze A.V., Frentzen M., Meister J. Indocyanine green-based adjunctive antimicrobial photodynamic therapy for treating chronic periodontitis: A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2019;26:29–35. doi: 10.1016/j.pdpdt.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 81.Ahad A., Lamba A.K., Faraz F., Tandon S., Chawla K., Yadav N. Effect of antimicrobial photodynamic therapy as an adjunct to nonsurgical treatment of deep periodontal pockets: A clinical study. J. Lasers Med. Sci. 2016;7:220–226. doi: 10.15171/jlms.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balata M.L., Andrade L.P., Santos D.B., Cavalcanti A.N., Tunes Uda R., Ribeiro Édel P., Bittencourt S. Photodynamic therapy associated with full-mouth ultrasonic debridement in the treatment of severe chronic periodontitis: A randomized-controlled clinical trial. J. Appl. Oral Sci. 2013;21:208–214. doi: 10.1590/1678-7757201302366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bechara Andere N.M.R., Dos Santos N.C.C., Araujo C.F., Mathias I.F., Rossato A., de Marco A.C., Santamaria M., Jr., Jardini M.A.N., Santamaria M.P. Evaluation of the local effect of nonsurgical periodontal treatment with and without systemic antibiotic and photodynamic therapy in generalized aggressive periodontitis. A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2018;24:115–120. doi: 10.1016/j.pdpdt.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 84.Bundidpun P., Srisuwantha R., Laosrisin N. Clinical effects of photodynamic therapy as an adjunct to full-mouth ultrasonic scaling and root planing in treatment of chronic periodontitis. Laser Ther. 2018;27:33–39. doi: 10.5978/islsm.18-OR-03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chitsazi M.T., Shirmohammadi A., Pourabbas R., Abolfazli N., Farhoudi I., Azar B.D., Farhadi F. Clinical and microbiological effects of photodynamic therapy associated with non- surgical treatment in aggressive periodontitis. J. Dent. Res. Dent. Clin. Dent. Prospects. 2014;8:153–159. doi: 10.5681/joddd.2014.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chitsazi M.T., Kashefimehr A., Pourabbas R., Shirmohammadi A., Ghasemi Barghi V., Daghigh Azar B. Efficacy of subgingival application of xanthan-based chlorhexidine gel adjunctive to full-mouth root planing assessed by real-time PCR: A Microbiologic and Clinical Study. J. Dent. Res. Dent. Clin. Dent. Prospects. 2013;7:95–101. doi: 10.5681/joddd.2013.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garcia F.B., Dias A.T., Tinoco B.E.M., Fischeer R.G. Evaluation of the efficacy of photodynamic therapy as adjunct to periodontal treatment in patients with aggressive periodontitis. Rev. Periodontia. 2011;21:12–19. [Google Scholar]
- 88.Betsy J., Prasanth C.S., Baiju K.V., Prasanthila J., Subhash N. Efficacy of antimicrobial photodynamic therapy in the management of chronic periodontitis: A randomized controlled clinical trial. J. Clin. Periodontol. 2014;4:573–581. doi: 10.1111/jcpe.12249. [DOI] [PubMed] [Google Scholar]
- 89.Malgikar S., Reddy S.H., Babu P.R., Sagar S.V., Kumar P.S., Reddy G.J. A randomized controlled clinical trial on efficacy of photodynamic therapy as an adjunct to nonsurgical treatment of chronic periodontitis. J. Dent. Lasers. 2015;9:75–79. doi: 10.4103/0976-2868.170562. [DOI] [Google Scholar]
- 90.Monzavi A., Chinipardaz Z., Mousavi M., Fekrazad R., Moslemi N., Azaripour A., Bagherpasand O., Chiniforush N. Antimicrobial photodynamic therapy using diode laser activated indocyanine green as an adjunct in the treatment of chronic periodontitis: A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2016;14:93–97. doi: 10.1016/j.pdpdt.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 91.Raj K.R., Musalaiah S., Nagasri M., Kumar P.A., Reddy P.I., Greeshma M. Evaluation of efficacy of photodynamic therapy as an adjunct to nonsurgical periodontal therapy in treatment of chronic periodontitis patients: A clinico-microbiological study. Indian J. Dent. Res. 2016;27:483–487. doi: 10.4103/0970-9290.195622. [DOI] [PubMed] [Google Scholar]
- 92.Sena I.A.A., Silva D.N.A., Azevedo M.L.D.S., da Silva N.T., Longo J.P.F., de Moraes M., de Aquino Martins A.R.L. Antimicrobial photodynamic therapy using a chloro-aluminum phthalocyanine adjuvant to nonsurgical periodontal treatment does not improve clinical parameters in patients with chronic periodontitis. Photobiomodul. Photomed. Laser Surg. 2019;37:729–735. doi: 10.1089/photob.2019.4651. [DOI] [PubMed] [Google Scholar]
- 93.Shingnapurkar S.H., Mitra D.K., Kadav M.S., Shah R.A., Rodrigues S.V., Prithyani S.S. The effect of indocyanine green-mediated photodynamic therapy as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A comparative split-mouth randomized clinical trial. Indian J. Dent. Res. 2016;27:609–617. doi: 10.4103/0970-9290.199598. [DOI] [PubMed] [Google Scholar]
- 94.Sigusch B.W., Engelbrecht M., Völpel A., Holletschke A., Pfister W., Schütze J. Full-mouth antimicrobial photodynamic therapy in Fusobacterium nucleatum-infected periodontitis patients. J. Periodontol. 2010;81:975–981. doi: 10.1902/jop.2010.090246. [DOI] [PubMed] [Google Scholar]
- 95.Theodoro L.H., Silva S.P., Pires J.R., Soares G.H.G., Pontes A.E.F., Zuza E.P., Spolidório D.M., de Toledo B.E., Garcia V.G. Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment: A 6-month follow-up. Lasers Med. Sci. 2012;27:687–693. doi: 10.1007/s10103-011-0942-x. [DOI] [PubMed] [Google Scholar]
- 96.Theodoro L.H., Lopes A.B., Nuernberg M.A.A., Cláudio M.M., Miessi D.M.J., Alves M.L.F., Duque C., Mombelli A., Garcia V.G. Comparison of repeated applications of aPDT with amoxicillin and metronidazole in the treatment of chronic periodontitis: A short-term study. J. Photochem. Photobiol. B. 2017;174:364–369. doi: 10.1016/j.jphotobiol.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 97.Festic E., Rawal B., Gajic O. How to improve assessment of balance in baseline characteristics of clinical trial participants-example from PROSEVA trial data? Ann. Transl. Med. 2016;4:79. doi: 10.3978/j.issn.2305-5839.2016.01.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tripepi G., Jager K.J., Dekker F.W., Zoccali C. Selection bias and information bias in clinical research. Nephron. Clin. Pract. 2010;115:c94–c99. doi: 10.1159/000312871. [DOI] [PubMed] [Google Scholar]
- 99.Johnson G.K., Hill M. Cigarette smoking and the periodontal patient. J. Periodontol. 2004;75:196–209. doi: 10.1902/jop.2004.75.2.196. [DOI] [PubMed] [Google Scholar]
- 100.Leite F.R.M., Nascimento G.G., Scheutz F., López R. Effect of smoking on Periodontitis: A Systematic Review and Meta-regression. Am. J. Prev. Med. 2018;54:831–841. doi: 10.1016/j.amepre.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 101.Arigbede A.O., Babatope B.O., Bamidele M.K. Periodontitis and systemic diseases: A literature review. J. Indian Soc. Periodontol. 2012;16:487–491. doi: 10.4103/0972-124X.106878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Komine-Aizawa S., Aizawa S., Hayakawa S. Periodontal diseases and adverse pregnancy outcomes. J. Obstet. 2019;45:5–12. doi: 10.1111/jog.13782. [DOI] [PubMed] [Google Scholar]
- 103.Blasini M., Movsas S., Colloca L. Placebo hypoalgesic effects in pain: Potential applications in dental and orofacial pain management. Semin. Orthod. 2018;24:259–268. doi: 10.1053/j.sodo.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smaïl-Faugeron V., Fron-Chabouis H., Courson F., Durieux P. Erratum to: Comparison of intervention effects in split-mouth and parallel-arm randomized controlled trials: A meta-epidemiological study. BMC Med. Res. Methodol. 2015;15:72. doi: 10.1186/s12874-015-0056-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lang N.P., Joss A., Orsanic T., Gusberti F.A., Siegrist B.E. Bleeding on probing. A predictor for the progression of periodontal disease? J. Clin. Periodontol. 1986;13:590–596. doi: 10.1111/j.1600-051X.1986.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 106.Greenstein G. Contemporary interpretation of probing depth assessments: Diagnostic and therapeutic implications. A literature review. J. Periodontol. 1997;68:1194–1205. doi: 10.1902/jop.1997.68.12.1194. [DOI] [PubMed] [Google Scholar]
- 107.Ramfjord S.P., Caffesse R.G., Morrison E.C., Hill R.W., Kerry G.J., Appleberry E.A., Nissle R.R., Stults D.L. 4 modalities of periodontal treatment compared over 5 years. J. Clin. Periodontol. 1987;14:445–452. doi: 10.1111/j.1600-051X.1987.tb02249.x. [DOI] [PubMed] [Google Scholar]
- 108.Kaldahl W.B., Kalkwarf K.L., Patil K.D., Molvar M.P., Dyer J.K. Long-term evaluation of periodontal therapy: I. Response to 4 therapeutic modalities. J. Periodontol. 1996;67:93–102. doi: 10.1902/jop.1996.67.2.93. [DOI] [PubMed] [Google Scholar]
- 109.Mailoa J., Lin G.H., Khoshkam V., MacEachern M., Chan H.L., Wang H.L. Long-term effect of four surgical periodontal therapies and one non-surgical therapy: A Systematic Review and Meta-Analysis. J. Periodontol. 2015;86:1150–1158. doi: 10.1902/jop.2015.150159. [DOI] [PubMed] [Google Scholar]
- 110.Singh S., Uppoor A., Nayak D. A comparative evaluation of the efficacy of manual, magnetostrictive and piezoelectric ultrasonic instruments- An in vitro profilometric and SEM study. J. Appl. Oral Sci. 2012;20:21–26. doi: 10.1590/S1678-77572012000100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Loos B.G., Needleman I. Endpoints of active periodontal therapy. J. Clin. Periodontol. 2020;47:61–71. doi: 10.1111/jcpe.13253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Manresa C., Sanz-Miralles E.C., Twigg J., Bravo M. Supportive periodontal therapy (SPT) for maintaining the dentition in adults treated for periodontitis. Cochrane Database Syst. Rev. 2018;1:CD009376. doi: 10.1002/14651858.CD009376.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wu D., Yang H.J., Zhang Y., Li X.E., Jia Y.R., Wang C.M. Prediction of loss to follow-up in long-term supportive periodontal therapy in patients with chronic periodontitis. PLoS ONE. 2018;13:e0192221. doi: 10.1371/journal.pone.0192221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lindhe J., Westfelt E., Nyman S., Socransky S.S., Haffajee A.D. Long-term effect of surgical/non-surgical treatment of periodontal disease. J. Clin. Periodontol. 1984;11:448–458. doi: 10.1111/j.1600-051X.1984.tb01344.x. [DOI] [PubMed] [Google Scholar]
- 115.De Freitas L.F., Hamblin M.R. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J. Sel. Top Quantum Electron. 2016;22:7000417. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Meisel P., Kocher T. Photodynamic therapy for periodontal diseases: State of the art. J. Photochem. Photobiol. B. 2005;79:159–170. doi: 10.1016/j.jphotobiol.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 117.Sbordone L., Ramaglia L., Gulletta E., Iacono V. Recolonization of the subgingival microflora after scaling and root planing in human periodontitis. J. Periodontol. 1990;61:579–584. doi: 10.1902/jop.1990.61.9.579. [DOI] [PubMed] [Google Scholar]
- 118.Carrera E.T., Dias H.B., Corti S.C.T., Marcantonio R.A.C., Bernardi A.C.A., Bagnato V.S. The application of antimicrobial photodynamic therapy (aPDT) in dentistry: A critical review. Laser Phys. 2016;26:1–23. doi: 10.1088/1054-660X/26/12/123001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fumes A.C., Romualdo P.C., Monteiro R.M., Watanabe E., Corona S.A.M., Borsatto M.C. Influence of pre-irradiation time employed in antimicrobial photodynamic therapy with diode laser. Lasers Med. Sci. 2018;33:67–73. doi: 10.1007/s10103-017-2336-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.