Abstract
Death, as a biological phenomenon, is well understood and a commonly employed endpoint for clinical trials. However, death identification and adjudication may be difficult for pragmatic clinical trials (PCT) that rely upon electronic health record and patient reported data. We propose a novel death identification and verification approach that is being used in the ToRsemide compArisoN with furoSemide FOR Management of Heart Failure (TRANSFORM-HF) PCT. We describe our hybrid approach that includes gathering information from clinical trial sites, a centralized call center, and National Death Index searches. Our methods detail how a possible death is triggered from each of these components and the types of information we require to verify a triggered death. Our different trigger / verification elements collectively define the TRANSFORM-HF PCT’s definition of a death event. .
Keywords: clinical trial, death, heart failure, national death index
1. Introduction
Delaying death by administering therapeutic interventions is a major clinical research objective. Clinical trial investigators select death as both a safety and efficacy endpoint because it is objectively measurable and serves as the ultimate test for new therapies.[1–3] However, death identification and adjudication processes can be quite different for explanatory trials (conducted in an idealized setting that gives the intervention the best chance to demonstrate benefit) and pragmatic trials (conducted in real-world settings to determine whether the intervention will have benefit in actual practice).[4] In traditional explanatory trials, sites typically are responsible for identifying study subject deaths, and a centralized Clinical Events Committee adjudicates the types of death. However, death identification and adjudication may be more complicated for pragmatic clinical trials (PCTs) that rely upon patient data collected for other purposes (e.g., from the electronic health record [EHR] as part of routine care or claims processing) or data collected from patients (e.g., directly by patient report or indirectly from patient devices). If these are the only PCT data sources, unless a patient dies during a health care encounter, their death data are not routinely available to a PCT.
Explanatory clinical trial investigators typically learn about a patient death when the patient cannot be contacted and a study coordinator contacts a proxy to schedule a study visit or when the study coordinator searches the internet to determine the patient’s current location. In addition, there is no timely and comprehensive national death database in the United States (US) that easily can be linked with EHR records. We propose a novel death identification and verification approach that is being used in the ToRsemide compArisoN with furoSemide FOR Management of Heart Failure (TRANSFORM-HF) PCT: funded by the National Heart, Lung, and Blood Institute (U01HL125511-01A1), registered with ClinicalTrials.gov (NCT03296813), and approved by the Duke University Medical Center Institutional Review Board (Pro00080595).
2. TRANSFORM-HF – Study Description
TRANSFORM-HF is a randomized, unblinded, two-arm, multi-center PCT of patients hospitalized for new or worsening heart failure. Approximately 6000 patients will be enrolled at approximately 50 US Study Sites and we expect to observe more than 721 death events. Enrolled patients are randomized in a 1:1 allocation ratio to either oral torsemide OR oral furosemide and receive a prescription for one of these oral loop diuretics prior to index hospitalization discharge. Post-discharge data collection is performed by the Duke Clinical Research Institute’s (DCRI) Call Center using a centralized follow-up procedure. There are no in-person study-specific patient follow-up visits. The full details on disease background and eligibility are described in the clinical study protocol.[5]
3. Death Event Ascertainment
All-cause mortality (defined as death after randomization) is the TRANSFORM-HF primary endpoint. Enrolling Study Sites are responsible for ascertaining patient deaths occurring during the index hospitalization period, and the DCRI Call Center is responsible for patient death ascertainment during the follow-up period. Because patient death ascertainment may be imprecise using these methods alone, TRANSFORM-HF also will rely upon National Death Index searches as a secondary source for patient death information.
3.1. Site Ascertainment
If a TRANSFORM-HF patient dies during their index admission, the Study Site is responsible for triggering and verifying the death event. This occurs by entering patient death information into the trial’s electronic case report form (eCRF). The Site then sends a copy of the patient’s discharge summary to the DCRI Call Center. The discharge summary will note the patient’s discharge status as expired (see Figure 1).
Figure 1.
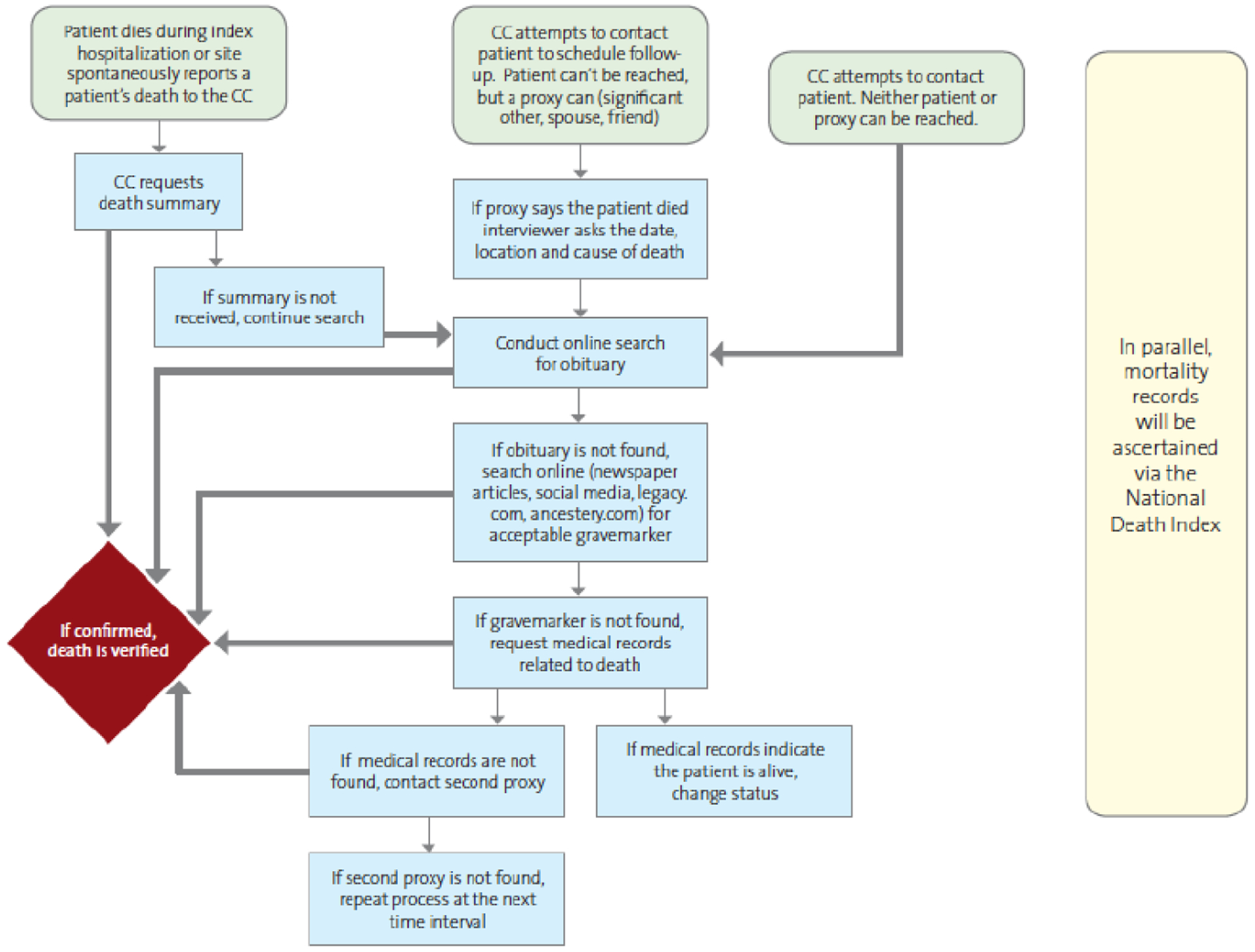
Study Site and Call Center Death Ascertainment.
3.2. Call Center Ascertainment
The DCRI Call Center will conduct follow-up interviews with patients at 30-days, 6-months, and 12-months following randomization. All patients will be followed for a minimum of 12 months. Patients enrolled early in the course of the trial will have extended follow-up, up to 30 months for the first 500 patients.
If the call center cannot reach a patient to schedule an interview, they will follow the steps outlined in Figure 1 to determine the patient’s vital status. This is a two-phase process during which the call center first obtains information (a trigger) that a patient death may have occurred. During the second phase (verification), the call center obtains source documents to verify that a patient death has occurred. If a source document cannot be obtained, the patient is considered an unconfirmed death.
TRANSFORM-HF patients complete Medical Release and Patient Contact forms during their enrollment. In the Patient Contact form, patients list their personal contact information plus that of spouses and friends or relatives not living with them who may be contacted as a proxy in their absence. If a proxy says the patient has died, this serves to trigger the death verification process for that patient. If proxies cannot be contacted to verify the patient’s vital status, the DCRI Call Center will conduct online searches. Sources for online searches include: ancestry.com, legacy.com, obituaries, newspaper articles, and social media. Lastly, the DCRI Call Center may contact the medical records/release of information (ROI) department at the patient’s enrolling hospital or other hospitals the patient has visited to obtain a death/discharge summary; the billing offices at those hospitals to obtain billing records; or request patient charts from their primary care provider or other healthcare providers. Because the Call Center’s processes may not identify all patient deaths, the study team is using the National Death Index (NDI) as a secondary information source to improve death event data capture.
3.3. National Death Index Ascertainment
The Centers for Disease Control and Prevention National Center for Health Statistics (NCHS) contracts with state vital statistics offices to receive and compile annual death registries in the NDI, a centralized database of all US deaths. The NCHS only allows use of the NDI for public health and health policy research. Unlike the other death data sources (e.g., Social Security Administration’s Death Master File, Medicare Master Beneficiary Summary File, or individual state vital statistics), all US jurisdictions report all deaths to the NCHS, which makes the NDI the most complete death data set available in the US today. The implication is that NDI deaths are actual deaths and the absence of an NDI death means that a patient may be considered alive at the end of the reporting year. This distinction becomes important when determining a study subject’s last known status (i.e., dead or alive).
An early release file is made available when approximately 90% of the (previous) year’s death records have been received and processed. At present time, this file typically is available in late January or early February and is considered preliminary. The final file reflects “all” of the (previous) year’s death records. This file is usually available in late October or early November. The final file is static and is rarely modified. Extensive information regarding user fees, file specifications, and details on the NDI matching methodology are provided in the NDI Users Guide.[6] TRANSFORM-HF plans to conduct annual NDI final file searches in the first two study years and will conduct early release file and final file searches in later years when the total number of death events is greater.
4. Death Event Verification
In TRANSFORM-HF, all deaths must be verified before they are counted as death events for the study. The study team has defined specific procedures for DCRI Call Center, NDI search, and Study Site death verification (see Figures 1 and 2). In order to gain information on the number of mortality events that are yielded from the different methodologies—and a better understanding regarding the timing and agreement between them—the NDI searches will be conducted as a parallel procedure to the DCRI Call Center processes.
Figure 2.
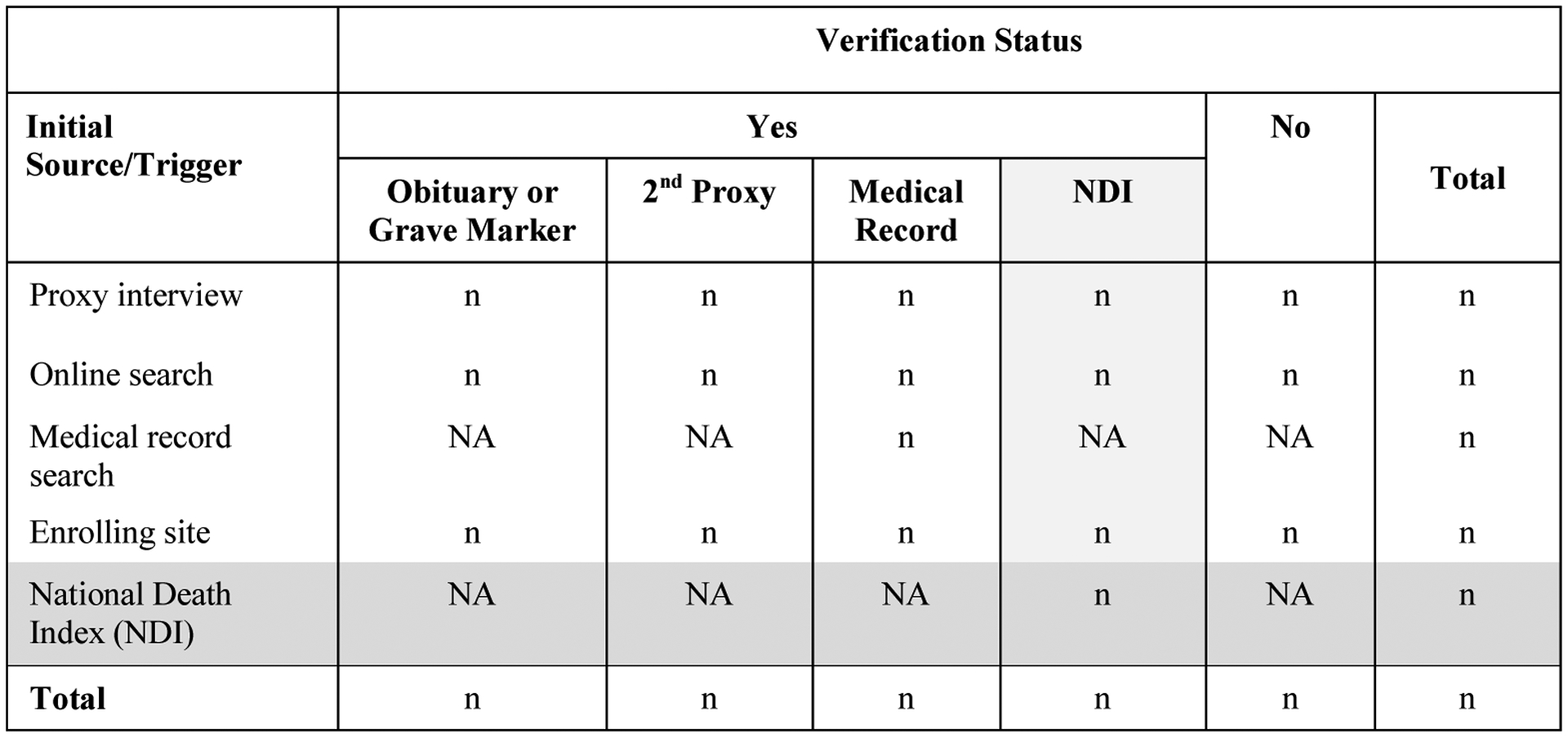
Death Triggers and Verification Methods.
4.1. DCRI Call Center Death Verification
The DCRI Call Center triggers the death verification process through four routes: proxy interviews, online searches, medical record searches, and notifications from enrolling Study Sites. Proxy interviews are the primary means by which the DCRI Call Center first learns about a patient death.
The DCRI Call Center will attempt to obtain verification for all triggered events. Acceptable verification includes: an obituary or grave marker, second proxy confirming the patient’s death, or medical records documenting the death. An acceptable obituary/grave marker must include a first name, last name, middle initial (when applicable), and date of birth that matches information entered for the patient at enrollment. Age may be substituted when the obituary/grave marker does not include the date of birth, but only when the patient’s state of residence matches as an additional criterion. Finally, an NDI search containing the patient’s death information may be used as sole verification of a death event triggered by the Call Center when other verification methods are unsuccessful.
4.2. National Death Index Death Verification
Researchers submit study records for NDI searches in specific record formats using the NCHS’s coding structures. Three combinations of data elements are accepted: (1) Social Security Number (SSN), sex, full date of birth; (2) Last name, first initial, month of birth, year of birth; and (3) Last name, first initial, SSN. Additional data elements may be submitted to increase the likelihood of a true match including the following: middle initial, father’s surname, state of birth, state of residence, marital status, and race (using NCHS race categories).
NDI search results are returned to researchers via output files. The NDI process first searches for possible matches using seven criteria. If any criteria are met, the death record is selected as a possible match. Next, each selected record is scored and classified into one of 5 groups to aid researchers in determining whether any selected record is a true match.
The TRANSFORM-HF study team plans to narrow down the possible matches to the best single match and use the NDI suggested algorithm for determining whether the selected record is a true match with an enrolled patient. There may be cases where the NDI information is the only source for the mortality event, and therefore serves as both the trigger source and verification.
4.3. Study Site Death Verification
As stated above, Study Sites are responsible for identifying and verifying deaths occurring during the index hospitalization. These deaths are verified by sending a copy of the patient’s death/discharge summary to the DCRI Call Center. Although less frequent, Study Sites may also voluntarily notify the TRANSFORM-HF study team that a patient has died after discharge. In these instances the DCRI Call Center will utilize the procedures described above to verify the death.
4.4. Mortality Review Committee
The TRANSFORM-HF Mortality Review Committee is comprised of clinicians and representatives from the DCRI Call Center and statistical team. This committee’s primary purpose is to review cases where there appears to be conflicting data that calls into question the reliability of data from another source (e.g., last known date alive).
5. Limitations
We acknowledge several limitations to our methods. First, we do not adjudicate type of death. Second, the Call Center’s procedures depend upon information supplied on patient contact forms. If that information is incomplete or changes, follow-up may become difficult. Third, we do not know the extent to which missing data will impact our NDI search results. Nonetheless, we believe our procedures improve upon current death identification and verification processes used in explanatory and pragmatic trials.
6. Conclusion
As PCTs work to streamline trial data collection and infrastructure and minimize burden on site investigators and patients, robust and accurate means of capturing death events are needed. We propose a hybrid solution that involves Study Sites, a centralized Call Center procedure, and NDI searches. Through this process, we have described what constitutes a death event for the TRANSFORM-HF PCT. Other studies may use different death event definitions. However, it is important to clearly define this endpoint before patients are enrolled. We recommend that PCT sponsors and investigators convene an international study group to determine how mortality will be defined and measured in PCTs.
References
- [1].Allen LA, Hernandez AF, O’Connor CM, Felker GM. Endpoints for clinical trials in acute heart failure syndreomes. J Am Coll Cardiol. 2009;53(24):2248–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Freemantle N, Calvert M, Wood J, et al. Composite outcomes in randomized trials: greater precision but with greater uncertainty? JAMA 2003;289(19):2554–9. [DOI] [PubMed] [Google Scholar]
- [3].Gottlieb SS. Dead is dead – artificial definitions are no substitute. Lancet 1997;349(9053):662–3. [DOI] [PubMed] [Google Scholar]
- [4].Loudon K, Treweek S, Sullivan F, et al. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ (2015), 350:h2147. [DOI] [PubMed] [Google Scholar]
- [5].TRANSFORM-HF Protocol. Available at clinicaltrials.gov/ct2show/NCT03296813, Accessed Aug 2018.
- [6].National Center for Health Statistics. National Death Index Users Guide. Hyattsville, MD. 2013. Available at https://www.cdc.gov/nchs/data/ndi/NDI_Users_Guide.pdf. Accessed Aug 2018. [Google Scholar]


