Abstract
Small ruminant mastitis is a serious problem, mainly caused by Staphylococcus spp. Different virulence factors affect mastitis pathogenesis. The aim of this study was to investigate virulence factors genes for biofilm production and antimicrobial resistance to β-lactams and tetracyclines in 137 staphylococcal isolates from goats (86) and sheep (51). The presence of coa, nuc, bap, icaA, icaD, blaZ, mecA, mecC, tetK, and tetM genes was investigated. The nuc gene was detected in all S. aureus isolates and in some coagulase-negative staphylococci (CNS). None of the S. aureus isolates carried the bap gene, while 8 out of 18 CNS harbored this gene. The icaA gene was detected in S. aureus and S. warneri, while icaD only in S. aureus. None of the isolates carrying the bap gene harbored the ica genes. None of the biofilm-associated genes were detected in 14 isolates (six S. aureus and eight CNS). An association was found between Staphylococcus species and resistance to some antibiotics and between antimicrobial resistance and animal species. Nine penicillin-susceptible isolates exhibited the blaZ gene, questioning the reliability of susceptibility testing. Most S. aureus isolates were susceptible to tetracycline, and no cefazolin or gentamycin resistance was detected. These should replace other currently used antimicrobials.
Keywords: mastitis, staphylococci, virulence factors, genes, biofilm, antimicrobial resistance
1. Introduction
Mastitis is the inflammation of the mammary gland, mainly due to intramammary infection (IMI). In small ruminants, this disease is considered a serious economic issue due to the mortality of lactating females, cost of treatment, reduced milk yield and quality [1,2], as well as a public health concern associated with risk of consumer food poisoning [3,4]. Several pathogens can cause mastitis in small ruminants; however, species of staphylococci are the most frequently isolated microorganisms from goat and sheep milk [2,5,6,7,8].
Staphylococcus aureus is one of the main pathogens associated with mastitis in small ruminants [9]. Incidence of clinical mastitis in sheep due to this bacterium may reach 20% with a mortality rate between 25% and 50%, and the affected mammary halves in surviving animals are frequently destroyed. Chronic mastitis may cause a 25 to 30% reduction in milk yield from the affected udder [10].
Coagulase negative staphylococci (CNS), although not as virulent as S. aureus, often cause subclinical mastitis in small ruminants [5,11,12,13]. This type of infection, most times not detected by the farmer, clearly reduces milk production, also changing milk composition, indirectly impairing the milk product’s properties [14]. CNS are the most prevalent pathogens of the mammary gland in goats and sheep with subclinical mastitis, affecting 60% to 80.7% in goats and 45% to 48% in sheep [1]. Other authors have reported as much as 70.1% of subclinical mastitis in sheep is caused by CNS [5].
Virulence factors are bacterial molecules that enhance their capacity to establish and to survive within the host and, thus, contribute to bring damage to the host. Staphylococci possess a wide array of virulence factors [15].
Coagulase enzyme acts on plasma fibrinogen to form fibrin clots that protect the microorganisms from phagocytosis and shelter them from other cellular and soluble host defence mechanisms. This enzyme, encoded by the coa gene, is commonly used to distinguish coagulase positive staphylococci (CPS), namely S. aureus, S. intermedius, and S. pseudintermedius, from CNS species [16]. Nevertheless, this gene has been found also in species known as CNS such as S. epidermidis, S. chromogenes, and S. hominis [17]. The coa gene has also recently been associated with biofilm production [18].
The staphylococcal nuclease is a thermostable nuclease encoded by the nuc gene [19], which hydrolyzes DNA and RNA in host cells, causing tissue destruction and spreading of staphylococci [20], also promoting the escape of microorganisms when retained by neutrophil extracellular traps (NETs), allowing the bacteria to evade this host defence mechanism [21,22]. For decades, the nuc gene has been considered the golden standard for Staphylococcus aureus identification and is still used presently [23,24,25]. However, the nuc gene has been detected in staphylococci of animal origin other than S. aureus [26]. Moreover, the nuc encoded staphylococcal thermonuclease is a biofilm inhibitor that degrades the environmental DNA (eDNA) associated with biofilm [27,28].
The production of biofilm is considered a major virulence factor that, besides protecting from host defence mechanisms, also shields bacteria against antimicrobial agents [29]. Furthermore, the persistence of biofilm-producing isolates in the dairy environment enhances the dispersal of virulence factors though the transfer of genetic material to other bacteria [30]. Biofilm major components are an exopolysaccharide matrix, proteins, and eDNA, along with the bacterial cells [31]. The exopolysaccharide, polysaccharide intercellular adhesin (PIA), is also a non-protein adhesin [32] assisting in bacterial adhesion to different surfaces, comprising the first critical event in the establishment of an infection [33]. Staphylococcal PIA is encoded by the ica operon [34], and biofilm-associated protein (Bap) is a surface protein connected to the cell wall encoded by the bap gene [35].
Antimicrobial resistance (AMR) is a major problem hampering the treatment of an ever increasing range of infections caused by bacteria [36]. Staphylococci resistance has been reported for different antimicrobials used for mastitis control in small ruminants [7,36,37,38]. Genes often described in Staphylococcus spp. isolated from the milk of small ruminants are blaZ and mecA, responsible for β-lactam resistance and tetK and tetM, accounting for tetracycline resistance [39,40,41]. The presence of resistant bacteria in contaminated food products may lead to the transfer of resistance genes to the indigenous microbiota in the human gut [42].
The aim of this study was to identify Staphylococcus species isolated from small ruminants’ milk samples and investigate the presence of genes encoding virulence factors associated with both biofilm (coa, nuc, bap, icaA, and icaD) and antimicrobial resistance to β-lactams (blaZ, mecA, and mecC) and tetracyclines (tetK and tetM).
2. Results and Discussion
2.1. Bacteriological Results
From the 646 milk samples collected from goats (508) and sheep (138), bacteriological cultures resulted positive in 191 samples: 131 goat milk and 60 sheep milk. A total of 137 staphylococcal isolates were recovered, of which 86 were isolated from goat and 51 from sheep milk samples.
2.2. Staphylococci Identification
Excellent (96 to 99% probability) and very good (93 to 95% probability) identification was observed for most Staphylococcus. Unidentified isolates and isolates with low discrimination results were confirmed by 16S rRNA gene sequencing.
Concerning goat milk samples, four S. aureus, one Staphylococcus sp., and 12 different CNS species were found: S. caprae (25), S. chromogenes (10), S. epidermidis (11), S. simulans (8), S. warneri (7), S. capitis (4), S. lentus (4), S. hominis (4), S. hyicus (3), S. auricularis (2), S. haemolyticus (2), and S. equorum (1).
On the other hand, 31 S. aureus and seven different CNS species were recovered from sheep milk samples: S. chromogenes (9), S. epidermidis (3), S. auricularis (2), S. haemolyticus (2), S. simulans (2), S. lentus (1), and S. rostri (1). Staphylococcus rostri has only been seldom isolated from the milk of a sheep with subclinical mastitis [43,44].
In the CNS group, S. caprae was the most found species and was isolated only from goat’s milk samples. It is a commensal organism that prevails in the skin of the goat udder [19] This species is most commonly found in cases of goat mastitis [37,45,46,47], but it was also isolated from sheep [5,48], buffalo [17], and cow’s milk [49].
In this study, other Staphylococcus species were only isolated from goats: S. warneri, S. capitis, S. hominis, S hyicus, and S. equorum. This was probably because the sheep sampling was smaller, since all these species have been isolated before from sheep milk by several other authors [44].
2.3. Biofilm Production
Of the 137 Staphylococcus isolates analyzed, 103 were biofilm producers (75%). Biofilm-forming isolates belong to the following species: S. aureus (29/35), S. caprae (22/25), S. chromogenes (12/19), S. epidermidis (11/14), S. warneri (7/7), S. simulans (6/10), S. auricularis (4/4), S. capitis (3/4), S. lentus (3/5), S. haemolyticus (2/4), S. hominis (2/4), S. equorum (1/1), and Staphylococcus sp. (1/1). All S. epidermidis goat isolates were found to produce biofilm in the present study, in accordance with the findings of others authors that reported S. epidermidis as the most commonly found species in biofilm-associated human infections [50]. However, none of the sheep S. epidermidis isolates were biofilm producers. In fact, other studies had already reported only 8% of biofilm-producing isolates among sheep mastitis S. epidermidis [51].
2.4. Genes Associated to Biofilm
We investigated the presence of coa and nuc genes in all 137 staphylococcal isolates, mainly for identification purposes and due to historical reasons. In fact, the ability of a strain to produce coagulase, encoded by the coa gene, is the basis of the primary classification of staphylococci in coagulase-positive or coagulase-negative [16].
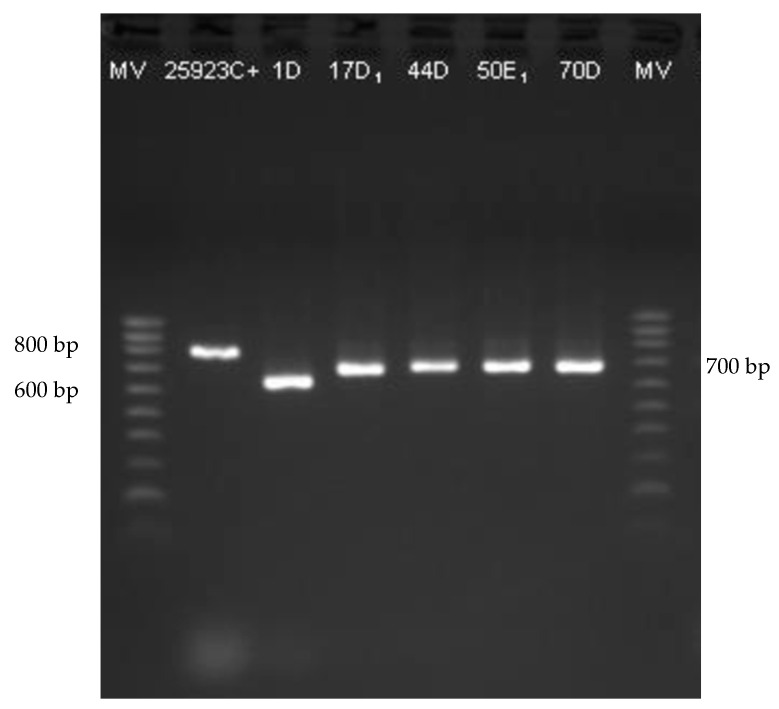
All S. aureus isolates (35) harbored the coa gene, as well as isolate B200E1, not identified to the species level. Based on this result, this isolate was probable also S. aureus. Therefore, the 101 Staphylococcus isolates not carrying the coa gene were confirmed as CNS. Furthermore, in the present study, different amplicons of the coa gene with band sizes ranging from 400 to 900 bp were detected (Figure 1), as already reported by others [52,53,54,55]. In fact, the coa gene also has a discriminatory power between isolates because of the heterogeneity of its 3’ variable region containing 81-bp tandem short sequence repeats (SSR) [56,57,58].
Figure 1.
Agarose gel electrophoresis of S. aureus coa gene PCR products. NZYDNA Ladder V (200–1000 bp) (NZYTech, Lisbon, Portugal).
The nuc gene was detected in 67 out of 137 isolates (48.9%), of which only 35 were S. aureus. The other nuc positive isolates included: S. chromogenes (8), S. warneri (4), S. auricularis (3), S. caprae (3), S. hyicus (3), S. lentus (3), S. epidermidis (2), S. simulans (2), S. capitis (1), S. haemolyticus (1), S. hominis (1), and Staphylococcus sp. (1). Furthermore, an association was found between the Staphylococcus species and the presence of the nuc gene (χ2 = 70.968, df = 14, p < 0.001). In fact, all S. aureus harbor the nuc gene, while most CNS (70/101) do not. However, the nuc gene was also detected in more than 50% of the isolates in some CNS species: S. warneri (4/7), S. lentus (3/5), S. auricularis (3/4), and S. hyicus (3/3).
The presence of the nuc gene was used in the past to identify S. aureus [23,25]. The nuc gene is present in most S. aureus isolates; however, some isolates not carrying this gene have been described [59,60]. Moreover, the nuc gene has also been detected in other species of Staphylococcus, both CPS and CNS [61,62].
For the detection of the biofilm production genes, bap, icaA, and icaD, the 44 nuc-positive biofilm-producing isolates were selected. nuc-positive biofilm-producing staphylococci and biofilm-associated genes are shown in Table 1.
Table 1.
nuc-positive biofilm-producing staphylococcal isolates and biofilm-associated genes.
| Isolate | Origin | Animal | Bacterial Species |
coa | nuc | bap | icaA | icaD |
|---|---|---|---|---|---|---|---|---|
| 1D | PT | goat | S. aureus | + | + | − | + | + |
| 13D1 | PT | goat | S. warneri | − | + | − | − | − |
| 17D1 | PT | goat | S. aureus | + | + | − | − | + |
| 44D | PT | goat | S. aureus | + | + | − | + | + |
| 47D2 | PT | goat | S. chromogenes | − | + | + | − | − |
| 50E1 | PT | goat | S. aureus | + | + | − | + | + |
| 54E1 | PT | goat | S. warneri | − | + | + | − | − |
| 54E2 | PT | goat | S. warneri | − | + | − | + | − |
| 55D1 | PT | goat | S. capitis | − | + | − | − | − |
| 60D2 | PT | goat | S. chromogenes | − | + | + | − | − |
| 65D | PT | goat | S. caprae | − | + | − | − | − |
| 70D | PT | sheep | S. aureus | + | + | − | − | + |
| 71E | PT | sheep | S. aureus | + | + | − | − | − |
| 72D | PT | sheep | S. aureus | + | + | − | − | + |
| 72E | PT | sheep | S. aureus | + | + | − | − | + |
| 83D | PT | sheep | S. aureus | + | + | − | − | − |
| B51E | BR | goat | S. chromogenes | − | + | − | − | − |
| B64 | BR | goat | S. chromogenes | − | + | − | − | − |
| B76E | BR | goat | S. chromogenes | − | + | + | − | − |
| B101 | BR | goat | S. warneri | − | + | − | + | − |
| B159D | BR | goat | S. chromogenes | − | + | + | − | − |
| B159E | BR | goat | S. chromogenes | − | + | + | − | − |
| B190D | BR | goat | S. auricularis | − | + | − | − | − |
| B209D2 | BR | goat | S. simulans | − | + | + | − | − |
| B209E | BR | goat | S. simulans | − | + | − | − | − |
| B219D3 | BR | sheep | S. auricularis | − | + | − | − | − |
| B219D5 | BR | sheep | S. aureus | + | + | − | − | − |
| B223D | BR | sheep | S. aureus | + | + | − | − | − |
| B250D | BR | sheep | S. auricularis | − | + | + | − | − |
| CQ152E1 | PT | sheep | S. aureus | + | + | − | + | + |
| CQ185D1 | PT | sheep | S. aureus | + | + | − | + | + |
| CQ196E | PT | sheep | S. aureus | + | + | − | − | + |
| CQ201E | PT | sheep | S. aureus | + | + | − | − | + |
| CQ268D1 | PT | sheep | S. aureus | + | + | − | − | + |
| CQ270E1 | PT | sheep | S. aureus | + | + | − | − | − |
| CQ285D | PT | sheep | S. aureus | + | + | − | − | + |
| CQ286D | PT | sheep | S. aureus | + | + | − | − | + |
| CQ290D1 | PT | sheep | S. aureus | + | + | − | − | + |
| CQ290D2 | PT | sheep | S. aureus | + | + | − | − | + |
| CQ296D | PT | sheep | S. aureus | + | + | − | − | + |
| CQ335E | PT | sheep | S. aureus | + | + | − | − | − |
| CQ336E2 | PT | sheep | S. aureus | + | + | − | − | + |
| CQ349D | PT | sheep | S. aureus | + | + | − | − | − |
| CQ354D | PT | sheep | S. aureus | + | + | − | − | + |
PT-Portugal; BR-Brazil.
The bap gene was amplified in eight isolates: S. chromogenes (5), S. auricularis (1), S. simulans (1), and S. warneri (1). None of the S. aureus nuc-positive biofilm-producing isolates carries the bap gene. In fact, the bap gene has been reported mainly in S. aureus strains isolated from cattle [24,63,64]. However, Martins et al. [65] have detected the bap gene in four sheep milk S. aureus isolates. In our study, 8 out of 18 CNS nuc-positive biofilm-producing isolates harbored the bap gene. The bap gene encodes a cell wall associated protein named Bap (for biofilm associated protein), which enhances biofilm formation as it mediates bacterial primary attachment to abiotic surfaces and intercellular adherence [35]. Other studies have reported the presence of the bap gene in several CNS isolates [66].
The presence of the icaA gene was detected in seven isolates: S. aureus (5) and S. warneri (2). On the other hand, the icaD gene was present in 19 S. aureus isolates. Furthermore, five S. aureus isolates carried both icaA and icaD genes simultaneously. Xu, Tan, Zhang, Xia, and Sun [59] detected the icaD gene in 20 out of 28 S. aureus bovine mastitis isolates, while it was not detected in any of the 76 CNS analyzed. The same authors reported the absence of the icaA gene in all analyzed staphylococcal isolates [59].
No isolate carrying the bap gene harbored the ica operon genes, as reported before by other authors [67]. However, Marques et al. [68] found one single bovine mastitis S. aureus isolate (out of 20) that simultaneously carried bap, icaA, and icaD.
None of the three biofilm-associated genes were detected in 14 of the nuc-positive biofilm-producing isolates: S. aureus (6) and CNS (8). Other authors have also reported the absence of bap, icaA, and icaD genes in biofilm-producing S. aureus [24,69,70]. Despite no association being found between the presence of the nuc gene and biofilm production, most biofilm-producing isolates harbored the nuc gene (53.4%), while it was only detected in about 35% of the non-producers. Nevertheless, Kiedrowski, Kavanaugh, Malone, Mootz, Voyich, Smeltzer, Bayles, and Horswill [28] described an inverse correlation between Nuc thermonuclease activity and biofilm formation and confirmed the important role for eDNA in the S. aureus biofilm matrix.
Apparently, CNS produce biofilm mainly via Bap, as already suggested by Zuniga et al. [71], who found the bap gene to be more frequently present in CNS when compared to CPS.
Meanwhile, most S. aureus seem to form biofilm through PIA since they harbor the icaA and icaD genes. Other authors have reported that a low prevalence of the bap gene in S. aureus indicates that the ica operon-dependent mechanism may be the main responsible for the adhesion and biofilm formation in this species [68]. Notwithstanding, it has been reported that biofilm synthesis in S. aureus can also be encoded by the bap gene [72].
Other biofilm formation mechanisms in staphylococci not harboring the classical biofilm-production genes, bap, icaA, and icaD, need to be explored. Furthermore, some of the isolates not carrying bap, icaA, and icaD also did not harbor the coa gene, which has been reported as associated with biofilm formation [18]. However, the nuc gene might be an important factor to consider since all 44 isolates were biofilm producers and harbored the nuc gene, although Nuc has been referred to as a biofilm inhibitor [27,28].
2.5. Antimicrobial Resistance
Out of 137 staphylococcal isolates analyzed for antimicrobial susceptibility, 15 were multidrug resistant, 36 were non-susceptible to two antimicrobial categories, and 61 to one antimicrobial category, according to the classification proposed by Magiorakos et al. [73]. Moreover, no antimicrobial resistances were detected in 24 staphylococcal isolates.
Staphylococci isolated from milk from small ruminants with mastitis are known for their multiresistance [74]. In this work, the multidrug resistant (MDR) isolates belonged to the following species: S. aureus (8), S. lentus (3), S. chromogenes (2), S. caprae (1), and S. warneri (1). Contrarily, Taponen and Pyorala [75] reported that multiresistance was more common in CNS than in S. aureus from bovine mastitis.
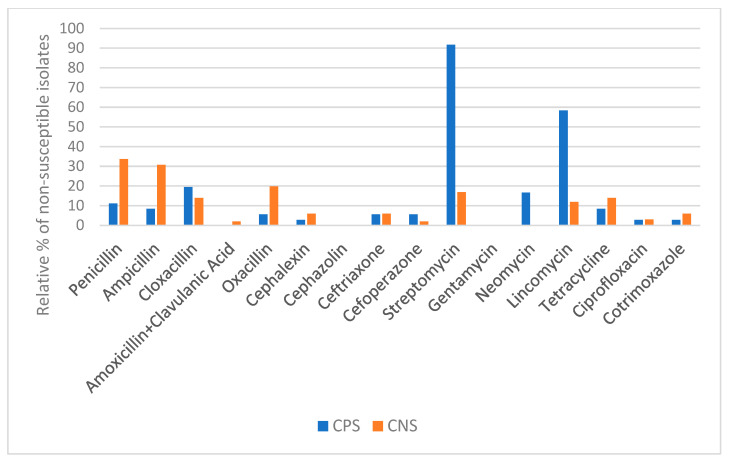
Susceptibility patterns of CPS and CNS isolates are shown in Figure 2. For most antimicrobials tested, a higher percentage of resistant isolates was observed among CNS when compared to CPS. Vasileiou et al. [76] also reported more resistant CNS isolates than S. aureus. However, mastitis caused by CNS responds much better to antimicrobial treatment than S. aureus mastitis [75].
Figure 2.
Susceptibility patterns of CPS (n = 36) and CNS (n = 101) isolates to antimicrobials.
Staphylococcal isolates were mainly non-susceptible to streptomycin (50/137), penicillin (38/137), ampicillin (34/137), lincomycin (33/137), oxacillin (22/137), cloxacillin (21/137), and tetracycline (17/137), as previously reported [77] (Figure 2). Moreover, most CPS isolates were non-susceptible to streptomycin and lincomycin. On the other hand, CNS isolates were mostly non-susceptible to the β-lactams and tetracyclines.
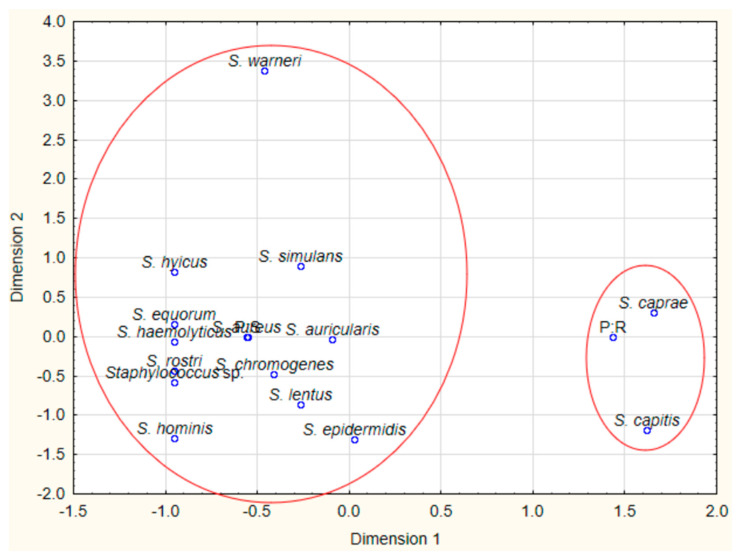
In addition, an association was found between Staphylococcus species and antimicrobial resistance to penicillin (χ2 = 45.981, df = 14, p < 0.001), ampicillin (χ2 = 48.327, df = 14, p < 0.001), streptomycin (χ2 = 137.705, df = 28, p < 0.001), lincomycin (χ2 = 156.536, df = 28, p < 0.001), cephalexin (χ2 = 57.219, df = 28, p < 0.05), and tetracycline (χ2 = 51.626, df = 28, p < 0.05). Regarding the results shown by the correspondence analysis, most S. caprae and S. capitis isolates were resistant to penicillin and ampicillin, while all other staphylococci were mostly susceptible to these antimicrobials (Figure 3). Most S. aureus isolates exhibited an intermediate susceptibility pattern to streptomycin and lincomycin [78]. Additionally, all S. hyicus isolates were resistant to streptomycin, while S. lentus and S. rostri were resistant to lincomycin (Figure 3).
Figure 3.
CA biplots of the relationship between bacterial species and tolerance to the antimicrobials penicillin (P), ampicillin (AMP), streptomycin (S), and lincomycin (MY).
No staphylococci resistant to cefazolin and gentamycin were identified. Moreover, no non-susceptible S. aureus isolates were found to amoxicillin + clavulanic acid. A number of CNS isolates, although resistant to penicillinase-labile penicillins, were susceptible to amoxicillin + clavulanic acid, which was expected due to the inhibitory action of clavulanic acid against β-lactamases [79]. Regarding CNS isolates, none were found to be resistant to neomycin.
One S. aureus and one CPS Staphylococcus sp. were found to be resistant to oxacillin, while CNS oxacillin resistant isolates belonged to eight species: S. chromogenes (5), S. caprae (4), S. lentus (3), S. simulans (3), S. epidermidis (2), S. auricularis (1), S. hominis (1), and S. warneri (1). Other authors previously reported the presence of methicillin resistant coagulase-negative staphylococci (MR-CNS) [80,81].
Regarding tetracycline, most S. aureus isolates (32/35) were susceptible, while non-susceptible isolates belonged to the following CNS species: S. caprae (4), S. haemolyticus (3), S. lentus (2), S. capitis (1), S. hominis (2), S. rostri (1), and S. warneri (1). Tetracycline has been widely used in veterinary medicine, and other studies have reported a higher percentage of resistant isolates: 42.8% [82] and 28.9% [45]. On the contrary, our results show a relatively low percentage of non-susceptible isolates (12.4%). In recent years, there has been an abusive use of more recent antimicrobial molecules, such as cephalosporins and quinolones, that may justify the observed reversal in the patterns of resistance to tetracyclines. To avoid the use of critically important antimicrobials for human medicine, tetracyclines, gentamycin, or cefazolin, a first-generation cephalosporin, may be an option for the control of mastitis in small ruminants. However, there should be a tight control over the development of antimicrobial resistances.
Interestingly, an association between resistance to some antibiotics and animal species was found: penicillin (χ2 = 26.931, df = 1, p < 0.001), ampicillin (χ2 = 26.818, df = 1, p < 0.001), oxacillin (χ2 = 6.241, df = 1, p < 0.05), streptomycin (χ2 = 26.231, df = 2, p < 0.001), and lincomycin (χ2 = 20.831, df = 2, p < 0.001). For example, isolates from goats (G) were more resistant than sheep (S) isolates to β-lactams, penicillin (G-43%; S-2%), ampicillin (G-39%; S- = 0%), and oxacillin (G-22%; S-6%). These differences might be due to different management systems, as suggested by Barrero-Domínguez et al. [45], who reported sheep and goat staphylococcal isolates with the same pulsotypes to exhibit distinct resistance patterns.
2.6. Antimicrobial Resistance Genes
The 44 biofilm producing isolates were selected for the detection of antimicrobial resistance genes involved in the resistance to β-lactams and tetracyclines, namely, blaZ, mecA, mecC, tetK, and tetM. Table 2 shows the antimicrobial genes detected in each isolate, along with its antimicrobial resistance profile.
Table 2.
nuc-positive biofilm-producing staphylococcal isolates, phenotypic resistance to selected antimicrobials and their associated antimicrobial resistance genes.
| Isolate | Origin | Animal | Bacterial Species | P | AMP | OB | AMC | OXA | TET | blaZ | mecA | mecC | tetK | tetM |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1D | PT | goat | S. aureus | R | R | R | S | S | S | + | - | - | - | |
| 13D1 | PT | goat | S. warneri | S | S | S | S | S | S | - | - | - | - | |
| 17D1 | PT | goat | S. aureus | R | R | R | S | S | R | + | - | - | + | |
| 44D | PT | goat | S. aureus | R | R | S | S | S | S | + | - | - | - | |
| 47D2 | PT | goat | S. chromogenes | R | R | R | R | R | S | + | - | - | - | - |
| 50E1 | PT | goat | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| 54E1 | PT | goat | S. warneri | S | S | R | S | S | S | + | - | - | - | |
| 54E2 | PT | goat | S. warneri | S | S | R | S | R | S | - | - | - | - | - |
| 55D1 | PT | goat | S. capitis | S | S | S | S | S | S | - | - | - | - | |
| 60D2 | PT | goat | S. chromogenes | R | R | S | S | S | S | + | - | - | - | |
| 65D | PT | goat | S. caprae | R | R | S | S | S | S | + | - | - | - | |
| 70D | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| 71E | PT | sheep | S. aureus | S | S | R | S | S | S | - | - | - | - | |
| 72D | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| 72E | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| 83D | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| B51E | BR | goat | S. chromogenes | S | S | S | S | S | S | + | - | - | - | |
| B64 | BR | goat | S. chromogenes | S | S | S | S | S | S | + | - | - | - | |
| B76E | BR | goat | S. chromogenes | S | S | S | S | S | S | + | - | - | - | |
| B101 | BR | goat | S. warneri | S | S | S | S | S | R | + | - | - | - | |
| B159D | BR | goat | S. chromogenes | S | S | S | S | S | S | + | - | - | - | |
| B159E | BR | goat | S. chromogenes | S | S | S | S | S | S | + | - | - | - | |
| B190D | BR | goat | S. auricularis | R | S | S | S | S | S | - | - | - | - | |
| B209D2 | BR | goat | S. simulans | S | S | S | S | S | S | - | - | - | - | |
| B209E | BR | goat | S. simulans | S | S | S | S | S | S | + | - | - | - | |
| B219D3 | BR | sheep | S. auricularis | S | S | S | S | S | S | - | - | - | - | |
| B219D5 | BR | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| B223D | BR | sheep | S. aureus | S | S | S | S | R | S | - | - | - | - | - |
| B250D | BR | sheep | S. auricularis | S | S | S | S | S | S | + | - | - | - | |
| CQ152E1 | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| CQ185D1 | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| CQ196E | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| CQ201E | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| CQ268D1 | PT | sheep | S. aureus | S | S | R | S | S | S | - | - | - | - | |
| CQ270E1 | PT | sheep | S. aureus | S | S | R | S | S | S | - | - | - | - | |
| CQ285D | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| CQ286D | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| CQ290D1 | PT | sheep | S. aureus | S | S | R | S | S | S | - | - | - | - | |
| CQ290D2 | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| CQ296D | PT | sheep | S. aureus | S | S | S | S | S | R | - | - | + | - | |
| CQ335E | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| CQ336E2 | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| CQ349D | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - | |
| CQ354D | PT | sheep | S. aureus | S | S | S | S | S | S | - | - | - | - |
Penicillin (P), ampicillin (AMP), cloxacillin (OB), amoxicillin + clavulanic acid (AMC), oxacillin (OXA), tetracyclines-tetracycline (TET).
The blaZ gene was detected in 15 staphylococcal isolates belonging to the following species: S. chromogenes (7), S. aureus (3), S. warneri (2), S. auricularis (1), S. caprae (1), and S. simulans (1). Unexpectedly, nine penicillin-susceptible isolates harbor the blaZ gene, namely S. chromogenes (5), S. warneri (2), S. auricularis (1), and S. simulans (1). El Feghaly et al. [83] also reported penicillin-susceptible isolates harboring the blaZ gene and concluded that conventional methods for susceptibility testing such as Kirby-Bauer penicillin disk diffusion may not be reliable. According to CLSI [78], there may be rare isolates of staphylococci containing β-lactamase genes, which may result negative in phenotypic β-lactamase detection. Additionally, all isolates resistant to penicillin must be considered resistant to all penicillinase-labile penicillins [78].
No staphylococcal isolates harboring the mecA or mecC genes were detected, although two isolates were found to be non-susceptible to oxacillin and cloxacillin simultaneously, one only to oxacillin and seven to cloxacillin alone. According to the CLSI (2016), oxacillin disk diffusion testing is not reliable for detecting methicillin resistance, at least in S. aureus, and cefoxitin should be used for disk diffusion testing. However, Barrero-Domínguez, Luque, Galán-Relaño, Vega-Pla, Huerta, Román, and Astorga [45] also did not detect the mecA gene in a cefoxitin-resistant MRSA strain. Thus, other resistance mechanisms cannot be excluded, namely, overproduction of β-lactamase, modified penicillin-binding proteins, distinct SCCmec elements, as well as putative mecA mutations [84,85]. Furthermore, Becker et al. [86] have recently reported the presence of a mecB gene in a MRSA strain, negative for both mecA and mecC genes. However, concerning mecC detection in our study, we cannot conclude that the isolates with a negative PCR result did not harbor the mecC gene, since no positive control strain was available.
An association was found between the resistance to penicillin (χ2 = 11.650, df = 1, p < 0.05) and ampicillin (χ2 = 15.828, df = 1, p < 0.001) and the presence of the antimicrobial resistance gene blaZ. The association between resistance to penicillin and ampicillin and the presence of the antimicrobial resistance gene blaZ has been reported before by other authors [87,88]. However, no association was detected between the resistance to oxacillin and cloxacillin and the presence of the antimicrobial resistance gene mecA for this subgroup of 44 isolates.
Only one S. aureus isolate carrying the tetK and another one carrying the tetM gene were identified. Both showed resistance to tetracycline. A S. warneri tetracycline-resistant isolate did not harbor either tetK or tetM (Table 3). El-Razik, Arafa, Hedia, and Ibrahim [82] found a S. intermedius isolate showing intermediate resistance to tetracycline, not harboring tetK, tetL, tetM, and tetO genes.
3. Materials and Methods
3.1. Milk Samples Collection and Bacteriological Analyses
A total of 328 small ruminants (258 goats and 70 sheep), belonging to 23 both traditional and industrial dairy farms in Portugal and Brazil, were used to collect 646 half-udder milk samples (508 from goats and 138 from sheep).
Milk samples were aseptically collected in a sterile bottle after the teat was carefully disinfected with 70% ethanol and the first flush was rejected. The samples were kept refrigerated and transported to the laboratory. Ten microliters of each milk sample were plated onto MacConkey agar (Oxoid, Hampshire, UK, CM0007) and onto blood agar (BA) (Oxoid, Hampshire, UK; CM0271 with 5% sheep blood) and incubated at 37 °C for 24 h to 48 h.
Colonies from BA were transferred to brain heart infusion agar (BHI) (Oxoid, Hampshire, UK, CM1136) and again incubated at 37 °C for 24h for primary identification of the Staphylococcus genus through morphological and biochemical characteristics, namely, colony morphology, Gram staining, and catalase reaction, according to Markey et al. [89].
Identification of the species level of all isolates was performed by automated compact system VITEK 2 (bioMérieux, Marcy l’Etoile, France) using GP ID cards following the manufacturer’s instructions. Biochemical identification was confirmed by 16S rRNA gene sequencing whenever necessary, using the primers described previously [90].
3.2. Phenotypic Characterisation of Staphylococcal Isolates
3.2.1. Biofilm Detection
Biofilm production was evaluated following the procedures described by Merino et al. [91] with some modifications. In brief, isolates were grown overnight in trypticase soy broth (TSB) at 37 °C. This overnight culture was diluted 1:40 in TSB supplemented with 0.25% glucose, and 200 mL of this cell suspension was used to inoculate microplates. After 24 h of incubation at 37 °C, the microplates were washed three times with 200 μL H2O, dried in an inverted position, and stained with 100 μL of 0.25% crystal violet for 2 to 3 min at room temperature. Afterwards, the microplates were rinsed again three times with H2O, dried, the dye dissolved in 200 μL ethanol-acetone (80:20), and the absorbance measured at 620 nm. Each assay was performed in triplicate and repeated three times. Staphylococcus epidermidis ATCC 12,228 and ATCC 35,984 were used as negative and positive controls, respectively. A blank control was also used.
3.2.2. Antimicrobial Sensitivity Test
The antimicrobial sensitivity test (AST) was performed as described before [77] following the performance standard M02-A11 [92]. Resistance to 16 antimicrobials, belonging to six antimicrobial categories, according to Magiorakos et al. [73], was evaluated: (1) β-lactams-penicillin (P), ampicillin (AMP), cloxacillin (OB), amoxicillin + clavulanic acid (AMC), oxacillin (OXA), cephalexin (CL), cefazolin (KZ), ceftriaxone (CRO), cefoperazone (CFP); (2) aminoglycosides-streptomycin (S), gentamycin (CN), neomycin (N); (3) lincosamides-lincomycin (MY); (4) tetracyclines-tetracycline (TET); (5) fluoroquinolones-ciprofloxacin (CIP); and (6) folate pathway inhibitors-cotrimoxazole (sulfamides + trimethoprim) (STX).
For the interpretation of AST results, the CLSI clinical breakpoints M100-S25 were used [78]. Isolates showing intermediate resistance, now called “susceptible increased exposure” [93], were considered non-susceptible. Moreover, isolates resistant to three or more antimicrobial categories were considered multidrug resistant [73].
3.3. Molecular Characterisation of Staphylococcal Isolates
The presence of coa and nuc genes was investigated in all staphylococcal isolates. nuc-positive biofilm-producing isolates were selected for the detection of the biofilm production genes, bap, icaA, and icaD, and the antimicrobial resistance genes blaZ, mecA, tetK, and tetM. The presence of the mecC gene was investigated only for nuc-positive biofilm-producing isolates, which were simultaneously resistant to oxacillin and cloxacillin and did not harbor the mecA gene.
3.3.1. Rapid DNA Extraction
Total DNA was extracted as described previously [94]. Bacterial cultures were grown for 24 h in BHI (Oxoid, Hampshire, UK, CM1136). After this period, they were transferred to microtubes with 200 μL of ultrapure water and centrifuged at 12,000× g for two minutes. Two hundred microliters of sterile saline solution (8.5%) were added to the pellet and centrifuged again at 12,000× g for two minutes. Subsequently, 100 μL of 0.05 M NaOH was added to the pellet and boiled for four minutes, then placed immediately on ice. Afterwards, 600 μL of ultrapure water was added to the microtubes and centrifuged at 4000× g for three minutes. Subsequently, 400 μL were transferred to a new microtube and stored at −20 °C until use.
3.3.2. PCR Amplification
All amplifications were done in a PTC1148C-MJ Mini thermocycler (BioRad, Hercules, CA, USA).
Amplified DNA fragments were stained with 1X Red Gel (Biotium, Fremont, CA, USA) and run on 1.5% (w/v) agarose gels with 0.5X TBE (Tris-borate-EDTA) buffer. Different NZYDNA Ladders (NZYtech, Lisbon, Portugal) were used as molecular weight markers, depending on the size of the PCR products.
Agarose gels were photographed under ultraviolet light using the Gel Doc XR+ Gel Documentation System (BioRad Universal Hood II, Philadelphia, PA, USA).
For all PCR amplifications, 50 μL PCR reactions were prepared with 5 μL of DNA template, 1 U GoTaq DNA polymerase (Promega, Madison, WI, USA), 1X Green Go Taq Flexi buffer (Promega, WI, USA), 1.5 mM MgCl2 (Promega, WI, USA), 0.2 mM each dNTP (VWR, part of Avantor, Radnor, PA, USA), and 15 pmol each primer (STAB VIDA, Caparica, Portugal). Specific and individual modifications or optimizations were done whenever necessary.
The primers used for amplification of the different genes are listed in Table 3.
Table 3.
Primer sequences for amplification of the different genes.
| Gene | Primer | Sequence | Reference |
|---|---|---|---|
| coa | coa-F | 5′ ATA GAG ATG CTG GTA CAG G 3′ | [55] |
| coa-R | 5′ GCT TCC GAT TGT TCG ATG C 3′ | ||
| coa | coa2-F | 5′ TA CTC AAC CGA CGA CAC CG 3′ | [54] |
| coa2-R | 5′ GAT TTT GGA TGA AGC GGA TT 3′ | ||
| nuc | nuc-F | 5′ GCG ATT GAT GGT GAT ACG GTT 3′ | [95] |
| nuc-R | 5′ AGC CAA GCC TTG ACG AAC TAA AGC 3′ | ||
| bap | bap-F | 5′ CCC TAT ATC GAA GGT GTA GAA TTG CAC 3′ | [35] |
| bap-R | 5′ GCT GTT GAA GTT AAT ACT GTA CCT GC 3′ | ||
| icaA | icaA-F | 5′ CCT AAC TAA CGA AAG GTA G 3′ | [96] |
| icaA-R | 5′ AAG ATA TAG CGA TAA GTG C 3′ | ||
| icaD | icaD-F | 5′ AAA CGT AAG AGA GGT GG 3′ | [96] |
| icaD-R | 5′ GGC AAT ATG ATC AAG ATA C 3′ | ||
| blaZ | blaZ-F | 5′ AAG AGA TTT GCC TAT GCT TC 3′ | [97] |
| blaZ-R | 5′ GCT TGA CCA CTT TTA TCA GC 3′ | ||
| mecA | mecA-F | 5′ AAA ATC GAT GGT AAA GGT TGG C 3′ | [98] |
| mecA-R | 5′ AGT TCT GCA GTA CCG GAT TTG C 3′ | ||
| mecC | mecC-F | 5′ GAA AAA AAG GCT TAG AAC GCC TC 3′ | [99] |
| mecC-R | 5′ GAA GAT CTT TTC CGT TTT CAG C 3′ | ||
| tetK | tetK-F | 5′ GTA GCG ACA ATA GGT AAT AGT 3′ | [59] |
| tetK-R | 5′ TAG TGA CAA TAA ACC TCC TA 3′ | ||
| tetM | tetM-F | 5′ AGT GGA GCG ATT ACA GAA 3′ | [59] |
| tetM-R | 5′ CAT ATG TCC TGG CGT GTC TA 3′ |
For the detection of the coa gene, different primer sequences were used. Staphylococcus aureus ATCC 25,923 was used as positive control. The first pair of primers, coa-F and coa-R, amplified a 676 bp fragment [55]. The amplification program was as follows: 3 min at 95 °C, and 35 cycles of 30 s at 94 °C, 30 s at 55 °C, 30 s at 72 °C, and finally, 5 min at 72 °C. The second pair of primers, coa2-F and coa2-R, amplified a fragment of 1517 bp [54]. The amplification program comprised an initial denaturation of 45 s at 94 °C, followed by 29 cycles at 94 °C for 20 s, 55 °C for 1 min, and 72 °C for 90 s, and a final extension step of 2 min at 72 °C.
For the amplification of the nuc gene, primers nuc-F and nuc-R, amplifying a 267 bp DNA fragment, were used [95]. S. aureus ATCC 25,923 was used as positive control and S. epidermidis ATCC 12,228 as negative control. The amplification program was the following: 5 min at 94 °C, followed by 37 cycles, consisting of 94 °C for 1 min, 55 °C for 30 s, and 72 °C for 30 s, ending with a final extension step at 72 °C for 7 min.
For detecting the bap gene, primers bap-F and bap-R were used for the amplification of a 971 bp fragment [35]. No positive control strain was available. The amplification program was as follows: 94 °C for 2 min, followed by 35 cycles of 94 °C for 45 s, 56.5 °C for 45 s, and 72 °C for 50 s, and finally, 72 °C for 5 min.
Primers icaA-F and icaA-R were used for the amplification of a 1315 bp fragment of the icaA gene [96]. S. epidermidis ATCC 35,984 was used as positive control. The following amplification program was used: 92 °C for 5 min, followed by 30 cycles of 92 °C for 45 s, 49 °C for 45 s, and 72 °C for 1 min, and a final extension step of 7 min at 72 °C.
For the icaD gene, primers icaD-F and icaD-R were used to amplify a 381 bp fragment [96]. S. epidermidis ATCC 35,984 was used as positive control. The same amplification program as for icaA was used, except for the extension step within the cycles, which was 72 °C for 30 s.
The presence of the blaZ gene was detected using primers blaZ-F and blaZ-R, which amplified a 517 bp fragment [97]. Staphylococcus aureus ATCC 29,213 was used as positive control and S. aureus ATCC 25,923 as negative control [100]. The amplification program was as follows: 94 °C for 4 min, followed by 37 cycles of 94 °C for 1 min, 50.5 °C for 30 s, and 72 °C for 30 s, and finally, 72 °C for 5 min [97].
To detect the mecA gene, primers mecA-F and mecA-R were used to amplify a fragment of 532 bp [98]. Staphylococcus epidermidis ATCC 35,984 was used as positive control [101] and S. aureus ATCC 25,923 as negative control [102]. The following amplification program was used: 94 °C for 2 min, followed by 29 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, and a final extension of 5 min at 72 °C.
Primers mecC-F and mecC-R were used to amplify a 138 bp fragment [99]. No positive control strain was available. The following amplification program was used: 95 °C for 2 min, followed by 30 cycles of 94 °C for 30 s, 50 °C for 30 s, and 72 °C for 30 s, and a final extension of 10 min at 72 °C.
Primers tetK-F and tetK-R were used to amplify a 360 bp fragment of the tetK gene [59]. No positive control strain was available. For the amplification of the tetM gene, tetM-F and tetM-R were used to amplify a fragment of 158 bp [59]. No positive control strain was available. The amplification program for both tet genes was: 94 °C for 2 min, followed by 29 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s, with a final step of 5 min at 72 °C.
3.4. Data Analysis
The chi-square test of association was used: to assess the relationship between the presence of the nuc gene with Staphylococcus species; to investigate if the presence of the nuc gene was associated with biofilm production; to check if the resistance to antimicrobials was associated with bacterial species and with the animal species from which these were isolated. For the abovementioned analyses, all 137 isolates were considered.
For the subgroup of 44 nuc-positive biofilm-producing isolates, the chi-square test of association was performed to evaluate the putative relationship between phenotypic resistance to antimicrobials and the presence of four resistance genes.
Multiple correspondence analysis (MCA) was used as an exploratory data analysis technique to detect a structure in the relationships between bacterial species and resistance to selected antimicrobials, divided either into two (susceptible and resistant) or three classes (susceptible, intermediate, and resistant), depending on the antimicrobial.
All statistical analyses were performed using the software STATISTICA Version 12 (StatSoft, Inc., Tulsa, OK, USA).
4. Conclusions
Mastitis aetiology showed to be diverse in the two small ruminant species studied. The most abundant species was S. caprae, which, however, was only present in goats.
The nuc gene was detected in 67 isolates, of which only 35 were S. aureus. Most CNS did not harbor this gene; however, it was detected in more than 50% of S. warneri, S. lentus, S. auricularis, and S. hyicus. Although many studies still consider the nuc gene as the sole character to identify S. aureus, our results have clearly demonstrated that this gene is insufficient, because it is present in numerous staphylococcal isolates other than S. aureus.
Most staphylococci were biofilm producers. The bap gene was only detected in CNS, while ica operon genes were mainly detected in S. aureus isolates, suggesting that CNS produce biofilm mainly via Bap, and most S. aureus form biofilm through PIA. Furthermore, biofilm-producing staphylococcal isolates not harboring the classical biofilm-production genes bap, icaA, and icaD carry the nuc gene. Therefore, the role of the Nuc thermonuclease in staphylococci biofilm formation needs to be further investigated.
Antimicrobial resistance seems to be a growing concern in the treatment of sheep and goat mastitis, with only a low number of isolates (18%) not showing any antimicrobial resistances. Furthermore, CNS were generally more resistant to antimicrobials than CPS. Additionally, an association between animal species and resistance to some antimicrobials was found, suggesting different managing systems for the two species.
All staphylococcal isolates were susceptible to cefazolin and gentamycin. Furthermore, all S. aureus isolates were shown to be susceptible to amoxicillin + clavulanic acid and most (32/35) to tetracycline. The use of these antimicrobials to control mastitis may be encouraged to avoid the use of others critically important for human medicine that are currently being used, such as third generation cephalosporins and quinolones. Nevertheless, antimicrobial susceptibility tests cannot be neglected, as the development of resistant strains is always a problem.
Regarding antimicrobial resistance genes, nine penicillin-susceptible isolates exhibited the blaZ gene, highlighting the poor reliability of conventional methods for susceptibility testing. Moreover, no staphylococcal isolates harboring the mecA or mecC genes were detected among those found to be non-susceptible to oxacillin. Hence, other methicillin resistance mechanisms need to be explored.
Acknowledgments
The authors acknowledge Guilhermina Pias for her technical support.
Author Contributions
Conceptualization, M.L. and M.C.Q.; methodology, M.L. and M.C.Q.; validation, M.L. and M.C.Q.; formal analysis, M.L. and M.C.Q.; investigation, N.C.A.; resources, M.L. and M.C.Q.; writing—original draft preparation, N.C.A.; writing—review and editing, M.L. and M.C.Q.; visualization, N.C.A., M.L. and M.C.Q.; supervision, M.L., M.M.C. and M.C.Q.; project administration, M.L. and M.C.Q.; funding acquisition, M.L. and M.C.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by National Funds through FCT-Foundation for Science and Technology under Project UIDB/05183/2020. Nara Andrade was funded by CNPq through a PhD grant (249398/2013-3-GDE), from Brazil.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Olechnowicz J., Jaśkowski J.M. Mastitis in small ruminants. Med. Weter. 2014;70:67–72. [Google Scholar]
- 2.Gelasakis A.I., Mavrogianni V.S., Petridis I.G., Vasileiou N.G.C., Fthenakis G.C. Mastitis in sheep—The last 10 years and the future of research. Vet. Microbiol. 2015;181:136–146. doi: 10.1016/j.vetmic.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 3.De Buyser M.-L., Dufour B., Maire M., Lafarge V. Implication of milk and milk products in food-borne diseases in France and in different industrialised countries. Int. J. Food Microbiol. 2001;67:1–17. doi: 10.1016/S0168-1605(01)00443-3. [DOI] [PubMed] [Google Scholar]
- 4.Argudín M.Á., Mendoza M.C., Rodicio M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins. 2010;2:1751–1773. doi: 10.3390/toxins2071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Queiroga M.C. Prevalence and aetiology of sheep mastitis in Alentejo region of Portugal. Small Rumin. Res. 2017;153:123–130. doi: 10.1016/j.smallrumres.2017.06.003. [DOI] [Google Scholar]
- 6.Abdalhamed A.M., Zeedan G.S.G., Abou Zeina H.A.A. Isolation and identification of bacteria causing mastitis in small ruminants and their susceptibility to antibiotics, honey, essential oils, and plant extracts. Vet. World. 2018;11:355–362. doi: 10.14202/vetworld.2018.355-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayis A.A.E., Fadlalla E. Antibiotic Susceptibility of Major Bacteria Cause Ovine Mastitis in River Nile State, Sudan. Imp. J. Interdiscip. Res. 2017;3:908–916. [Google Scholar]
- 8.Salaberry S.R.S., Saidenberg A.B.S., Zuniga E., Gonsales F.F., Melville P.A., Benites N.R. Microbiological analysis and sensitivity profile of Staphylococcus spp. in subclinical mastitis of dairy goats. Arq. Bras. Med. Veterinária Zootec. 2016;68:336–344. doi: 10.1590/1678-4162-8205. [DOI] [Google Scholar]
- 9.Bergonier D., Sobral D., Feßler A.T., Jacquet E., Gilbert F.B., Schwarz S., Treilles M., Bouloc P., Pourcel C., Vergnaud G. Staphylococcus aureus from 152 cases of bovine, ovine and caprine mastitis investigated by Multiple-locus variable number of tandem repeat analysis (MLVA) Vet. Res. 2014;45:97. doi: 10.1186/s13567-014-0097-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Constable P.D., Hinchcliff K.W., Done S.H., Grünberg W. Veterinary Medicine: A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats. 11th ed. Elsevier; St. Louis, MO, USA: 2017. pp. 1904–2001. [Google Scholar]
- 11.Dore S., Liciardi M., Amatiste S., Bergagna S., Bolzoni G., Caligiuri V., Cerrone A., Farina G., Montagna C.O., Saletti M.A., et al. Survey on small ruminant bacterial mastitis in Italy, 2013–2014. Small Rumin. Res. 2016;141:91–93. doi: 10.1016/j.smallrumres.2016.07.010. [DOI] [Google Scholar]
- 12.Mishra A.K., Sharma N., Singh D.D., Gururaj K., Abhishek, Kumar V., Sharma D.K. Prevalence and bacterial etiology of subclinical mastitis in goats reared in organized farms. Vet. World. 2018;11:20–24. doi: 10.14202/vetworld.2018.20-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ruiz Romero R.A. Identification of and antimicrobial resistance in bacteria causing caprine mastitis in three states and a city in Central Mexico under manual and mechanical milking conditions. J. Dairyvet. Anim. Res. 2018;7 doi: 10.15406/jdvar.2018.07.00201. [DOI] [Google Scholar]
- 14.Leitner G., Rovai M., Merin U. Clinical and subclinical intrammamay infection caused by coagulase negative staphylococci negatively affect milk yield and its quality in dairy sheep. Small Rumin. Res. 2019;180:74–78. doi: 10.1016/j.smallrumres.2019.07.012. [DOI] [Google Scholar]
- 15.Madigan M.T., Stahl D.A., Sattley W.M., Buckley D.H., Bender K.S. Brock Biology Of Microorganisms, Global Edition. Pearson Education Limited; London, UK: 2018. [Google Scholar]
- 16.Murray P.R., Rosenthal K.S., Pfaller M.A. Medical Microbiology. 9th ed. Elsevier; Amsterdam, The Netherlands: 2020. [Google Scholar]
- 17.Pizauro L.J.L., de Almeida C.C., Soltes G.A., Slavic D., de Ávila F.A., Zafalon L.F., MacInnes J.I. Short communication: Detection of antibiotic resistance, mecA, and virulence genes in coagulase-negative Staphylococcus spp. from buffalo milk and the milking environment. J. Dairy Sci. 2019;102:11459–11464. doi: 10.3168/jds.2018-15920. [DOI] [PubMed] [Google Scholar]
- 18.Zapotoczna M., McCarthy H., Rudkin J.K., O’Gara J.P., O’Neill E. An Essential Role for Coagulase in Staphylococcus aureus Biofilm Development Reveals New Therapeutic Possibilities for Device-Related Infections. J. Infect. Dis. 2015;212:1883–1893. doi: 10.1093/infdis/jiv319. [DOI] [PubMed] [Google Scholar]
- 19.Schleifer K.-H., Bell J.A. Family VIII-Staphylococcaceae fam. nov. In: Vos P.D., Garrity G.M., Jones D., Krieg N.R., Ludwig W., Rainey F.A., Schleifer K.-H., Whitman W.B., editors. Bergey’s Manual of Systematic Bacteriology. 2nd ed. Volume 3. Springer; New York, NY, USA: 2009. pp. 392–421. [Google Scholar]
- 20.Hu Y., Meng J., Shi C., Hervin K., Fratamico P.M., Shi X. Characterization and comparative analysis of a second thermonuclease from Staphylococcus aureus. Microbiol. Res. 2013;168:174–182. doi: 10.1016/j.micres.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 21.Berends E.T.M., Horswill A.R., Haste N.M., Monestier M., Nizet V., Von Köckritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J. Innate Immun. 2010;2:576–586. doi: 10.1159/000319909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenny E.F., Herzig A., Krüger R., Muth A., Mondal S., Thompson P.R., Brinkmann V., Bernuth H.V., Zychlinsky A. Diverse stimuli engage different neutrophil extracellular trap pathways. eLife. 2017;6 doi: 10.7554/eLife.24437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kateete D.P., Kimani C.N., Katabazi F.A., Okeng A., Okee M.S., Nanteza A., Joloba M.L., Najjuka F.C. Identification of Staphylococcus aureus: DNase and Mannitol salt agar improve the efficiency of the tube coagulase test. Ann. Clin. Microbiol. Antimicrob. 2010;9:23. doi: 10.1186/1476-0711-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres G., Vargas K., Sánchez-Jiménez M., Reyes-Velez J., Olivera-Angel M. Genotypic and phenotypic characterization of biofilm production by Staphylococcus aureus strains isolated from bovine intramammary infections in Colombian dairy farms. Heliyon. 2019;5:e02535. doi: 10.1016/j.heliyon.2019.e02535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McClure J.A., Zaal DeLongchamp J., Conly J.M., Zhang K. Novel Multiplex PCR Assay for Detection of Chlorhexidine-Quaternary Ammonium, Mupirocin, and Methicillin Resistance Genes, with Simultaneous Discrimination of Staphylococcus aureus from Coagulase-Negative Staphylococci. J. Clin. Microbiol. 2017;55:1857–1864. doi: 10.1128/JCM.02488-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gudding R. Differentiation of staphylococci on the basis of nuclease properties. J. Clin. Microbiol. 1983;18:1098–1101. doi: 10.1128/JCM.18.5.1098-1101.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mann E.E., Rice K.C., Boles B.R., Endres J.L., Ranjit D., Chandramohan L., Tsang L.H., Smeltzer M.S., Horswill A.R., Bayles K.W. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS ONE. 2009;4:e5822. doi: 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kiedrowski M.R., Kavanaugh J.S., Malone C.L., Mootz J.M., Voyich J.M., Smeltzer M.S., Bayles K.W., Horswill A.R. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS ONE. 2011;6:e26714. doi: 10.1371/journal.pone.0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Flemming H.-C., Wingender J., Szewzyk U., Steinberg P., Rice S.A., Kjelleberg S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 30.Turchi B., Bertelloni F., Marzoli F., Cerri D., Tola S., Azara E., Longheu C.M., Tassi R., Schiavo M., Cilia G., et al. Coagulase negative staphylococci from ovine milk: Genotypic and phenotypic characterization of susceptibility to antibiotics, disinfectants and biofilm production. Small Rumin. Res. 2020;183:106030. doi: 10.1016/j.smallrumres.2019.106030. [DOI] [Google Scholar]
- 31.Buttner H., Dietrich M., Rohde H. Structural basis of Staphylococcus epidermidis biofilm formation: Mechanisms and molecular interactions. Front. Cell. Infect. Microbiol. 2015;5:14. doi: 10.3389/fcimb.2015.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arciola C.R., Campoccia D., Ravaioli S., Montanaro L. Polysaccharide intercellular adhesin in biofilm: Structural and regulatory aspects. Front. Cell. Infect. Microbiol. 2015;5:7. doi: 10.3389/fcimb.2015.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heilmann C. Adhesion Mechanisms of Staphylococci. Adv. Exp. Med. Biol. 2011;715:105–123. doi: 10.1007/978-94-007-0940-9_7. [DOI] [PubMed] [Google Scholar]
- 34.Otto M. Staphylococcal Biofilms. Microbiol. Spectr. 2018;6 doi: 10.1128/microbiolspec.GPP3-0023-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cucarella C., Solano C., Valle J., Amorena B., Lasa I.N.I., Penadés J.R. Bap, a Staphylococcus aureus Surface Protein Involved in Biofilm Formation. J. Bacteriol. 2001;183:2888–2896. doi: 10.1128/JB.183.9.2888-2896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceniti C., Britti D., Santoro A.M.L., Musarella R., Ciambrone L., Casalinuovo F., Costanzo N. Phenotypic antimicrobial resistance profile of isolates causing clinical mastitis in dairy animals. Ital. J. Food Saf. 2017;6 doi: 10.4081/ijfs.2017.6612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peixoto R.D.M., França C.A.D., Souza Júnior A.F.D., Veschi J.L.A., Costa M.M.D. Etiologia e perfil de sensibilidade antimicrobiana dos isolados bacterianos da mastite em pequenos ruminantes e concordância de técnicas empregadas no diagnóstico. Pesqui. Veterinária Bras. 2010;30:735–740. doi: 10.1590/S0100-736X2010000900005. [DOI] [Google Scholar]
- 38.Andrade N.P.C., Peixoto R.M., Nogueira D.M., Krewer C.C., Costa M.M. Perfil de sensibilidade aos antimicrobianos de Staphylococcus spp. coagulase negativa de um rebanho leiteiro caprino em Santa Maria da Boa Vista-PE. Med. Veterinária. 2012;6:1–6. [Google Scholar]
- 39.Al-Ashmawy M.A., Sallam K.I., Abd-Elghany S.M., Elhadidy M., Tamura T. Prevalence, Molecular Characterization, and Antimicrobial Susceptibility of Methicillin-Resistant Staphylococcus aureus isolated from Milk and Dairy Products. Foodborne Pathog. Dis. 2016;13:156–162. doi: 10.1089/fpd.2015.2038. [DOI] [PubMed] [Google Scholar]
- 40.Merz A., Stephan R., Johler S. Staphylococcus aureus Isolates from Goat and Sheep Milk Seem to Be Closely Related and Differ from Isolates Detected from Bovine Milk. Front. Microbiol. 2016;7:319. doi: 10.3389/fmicb.2016.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akkou M., Bentayeb L., Ferdji K., Medrouh B., Bachtarzi M.-A., Ziane H., Kaidi R., Tazir M. Phenotypic characterization of Staphylococci causing mastitis in goats and microarray-based genotyping of Staphylococcus aureus isolates. Small Rumin. Res. 2018;169:29–33. doi: 10.1016/j.smallrumres.2018.10.015. [DOI] [Google Scholar]
- 42.Lee J.H. Methicillin (Oxacillin)—Resistant Staphylococcus aureus Strains Isolated from Major Food Animals and Their Potential Transmission to Humans. Appl. Environ. Microbiol. 2003;69:6489–6494. doi: 10.1128/AEM.69.11.6489-6494.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Persson Y., Nyman A.-K., Söderquist L., Tomic N., Waller K.P. Intramammary infections and somatic cell counts in meat and pelt producing ewes with clinically healthy udders. Small Rumin. Res. 2017;156:66–72. doi: 10.1016/j.smallrumres.2017.09.012. [DOI] [Google Scholar]
- 44.Queiroga M.C., Laranjo M., Duarte E.L. Mastitis in Sheep: A disease to be Taken Seriously. In: Papst M., editor. Sheep Diseases. Nova Science Publisher, Inc; New York, NY, USA: 2019. pp. 1–91. [Google Scholar]
- 45.Barrero-Domínguez B., Luque I., Galán-Relaño Á., Vega-Pla J.L., Huerta B., Román F., Astorga R.J. Antimicrobial Resistance and Distribution of Staphylococcus spp. Pulsotypes Isolated from Goat and Sheep Bulk Tank Milk in Southern Spain. Foodborne Pathog. Dis. 2019;16:723–730. doi: 10.1089/fpd.2018.2593. [DOI] [PubMed] [Google Scholar]
- 46.Gosselin V.B., Lovstad J., Dufour S., Adkins P.R.F., Middleton J.R. Use of MALDI-TOF to characterize staphylococcal intramammary infections in dairy goats. J. Dairy Sci. 2018;101:6262–6270. doi: 10.3168/jds.2017-14224. [DOI] [PubMed] [Google Scholar]
- 47.Bergonier D., Berthelot X. New advances in epizootiology and control of ewe mastitis. Livest. Prod. Sci. 2003;79:1–16. doi: 10.1016/S0301-6226(02)00145-8. [DOI] [Google Scholar]
- 48.Martins K.B., Faccioli P.Y., Bonesso M.F., Fernandes S., Oliveira A.A., Dantas A., Zafalon L.F., Cunha M.D.L.R.S. Characteristics of resistance and virulence factors in different species of coagulase-negative staphylococci isolated from milk of healthy sheep and animals with subclinical mastitis. J. Dairy Sci. 2017;100:2184–2195. doi: 10.3168/jds.2016-11583. [DOI] [PubMed] [Google Scholar]
- 49.Park J.Y., Fox L.K., Seo K.S., McGuire M.A., Park Y.H., Rurangirwa F.R., Sischo W.M., Bohach G.A. Comparison of phenotypic and genotypic methods for the species identification of coagulase-negative staphylococcal isolates from bovine intramammary infections. Vet. Microbiol. 2011;147:142–148. doi: 10.1016/j.vetmic.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Becker K., Heilmann C., Peters G. Coagulase-negative staphylococci. Clin. Microbiol. Rev. 2014;27:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Queiroga M.C., Duarte E.L., Laranjo M. Sheep mastitis Staphylococcus epidermidis biofilm effects on cell adhesion and inflammatory changes. Small Rumin. Res. 2018;168:6–11. doi: 10.1016/j.smallrumres.2018.09.009. [DOI] [Google Scholar]
- 52.Aher T., Roy A., Kumar P. Molecular detection of virulence genes associated with pathogenicity of Gram positive isolates obtained from respiratory tract of apparently healthy as well as sick goats. Vet. World. 2012;5:676. doi: 10.5455/vetworld.2012.676-681. [DOI] [Google Scholar]
- 53.Saei H.D. Coa types and antimicrobial resistance profile of Staphylococcus aureus isolates from cases of bovine mastitis. Comp. Clin. Pathol. 2012;21:301–307. doi: 10.1007/s00580-010-1096-0. [DOI] [Google Scholar]
- 54.Aarestrup F.M., Dangler C.A., Sordillo L.M. Prevalence of coagulase gene polymorphism in Staphylococcus aureus isolates causing bovine mastitis. Can. J. Vet. Res. 1995;59:124–128. [PMC free article] [PubMed] [Google Scholar]
- 55.Hookey J.V., Richardson J.F., Cookson B.D. Molecular typing of Staphylococcus aureus based on PCR restriction fragment length polymorphism and DNA sequence analysis of the coagulase gene. J. Clin. Microbiol. 1998;36:1083–1089. doi: 10.1128/JCM.36.4.1083-1089.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Soltan Dallal M.M., Khoramizadeh M.R., Amiri S.A., Saboor Yaraghi A.A., Mazaheri Nezhad Fard R. Coagulase gene polymorphism of Staphylococcus aureus isolates: A study on dairy food products and other foods in Tehran, Iran. Food Sci. Hum. Wellness. 2016;5:186–190. doi: 10.1016/j.fshw.2016.09.004. [DOI] [Google Scholar]
- 57.Shopsin B., Gomez M., Waddington M., Riehman M., Kreiswirth B.N. Use of Coagulase Gene (coa) Repeat Region Nucleotide Sequences for Typing of Methicillin-Resistant Staphylococcus aureus Strains. J. Clin. Microbiol. 2000;38:3453–3456. doi: 10.1128/JCM.38.9.3453-3456.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tiwari H.K., Sapkota D., Gaur A., Mathuria J.P., Singh A., Sen M.R. Molecular typing of clinical Staphylococcus aureus isolates from northern India using coagulase gene PCR-RFLP. Southeast Asian J. Trop. Med. Public Health. 2008;39:467–473. [PubMed] [Google Scholar]
- 59.Xu J., Tan X., Zhang X., Xia X., Sun H. The diversities of staphylococcal species, virulence and antibiotic resistance genes in the subclinical mastitis milk from a single Chinese cow herd. Microb. Pathog. 2015;88:29–38. doi: 10.1016/j.micpath.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Van Leeuwen W., Roorda L., Hendriks W., Francois P., Schrenzel J. A nuc-deficient methicillin-resistant Staphylococcus aureus strain. FEMS Immunol. Med. Microbiol. 2008;54:157. doi: 10.1111/j.1574-695X.2008.00478.x. [DOI] [PubMed] [Google Scholar]
- 61.Hirotaki S., Sasaki T., Kuwahara-Arai K., Hiramatsu K. Rapid and Accurate Identification of Human-Associated Staphylococci by Use of Multiplex PCR. J. Clin. Microbiol. 2011;49:3627–3631. doi: 10.1128/JCM.00488-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Silva W.P.D., Silva J.A., Macedo M.R.P.D., Araújo M.R.D., Mata M.M., Gandra E.A. Identification of Staphylococcus aureus, S. intermedius and S. hyicus by PCR amplification of coa and nuc genes. Braz. J. Microbiol. 2003;34:125–127. doi: 10.1590/S1517-83822003000500043. [DOI] [Google Scholar]
- 63.Cucarella C., Tormo M.A., Úbeda C., Trotonda M.P., Monzón M., Peris C., Amorena B., Lasa I., Penadés J.R. Role of Biofilm-Associated Protein Bap in the Pathogenesis of Bovine Staphylococcus aureus. Infect. Immun. 2004;72:2177–2185. doi: 10.1128/IAI.72.4.2177-2185.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vautor E., Magnone V., Rios G., Le Brigand K., Bergonier D., Lina G., Meugnier H., Barbry P., Thiéry R., Pépin M. Genetic differences among Staphylococcus aureus isolates from dairy ruminant species: A single-dye DNA microarray approach. Vet. Microbiol. 2009;133:105–114. doi: 10.1016/j.vetmic.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 65.Martins K.B., Faccioli-Martins P.Y., Riboli D.F., Pereira V.C., Fernandes S., Oliveira A.A., Dantas A., Zafalon L.F., da Cunha M.E.L. Clonal profile, virulence and resistance of Staphylococcus aureus isolated from sheep milk. Braz. J. Microbiol. 2015;46:535–543. doi: 10.1590/S1517-838246220131164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tormo M.Á., Knecht E., Götz F., Lasa I., Penadés J.R. Bap-dependent biofilm formation by pathogenic species of Staphylococcus: Evidence of horizontal gene transfer? Microbiology. 2005;151:2465–2475. doi: 10.1099/mic.0.27865-0. [DOI] [PubMed] [Google Scholar]
- 67.Szweda P., Schielmann M., Milewski S., Frankowska A., Jakubczak A. Biofilm production and presence of ica and bap genes in Staphylococcus aureus strains isolated from cows with mastitis in the eastern Poland. Pol. J. Microbiol. 2012;61:65–69. doi: 10.33073/pjm-2012-009. [DOI] [PubMed] [Google Scholar]
- 68.Marques V.F., Motta C.C.D., Soares B.D.S., Melo D.A.D., Coelho S.D.M.D.O., Coelho I.D.S., Barbosa H.S., Souza M.M.S.D. Biofilm production and beta-lactamic resistance in Brazilian Staphylococcus aureus isolates from bovine mastitis. Braz. J. Microbiol. 2017;48:118–124. doi: 10.1016/j.bjm.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Khoramian B., Jabalameli F., Niasari-Naslaji A., Taherikalani M., Emaneini M. Comparison of virulence factors and biofilm formation among Staphylococcus aureus strains isolated from human and bovine infections. Microb. Pathog. 2015;88:73–77. doi: 10.1016/j.micpath.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 70.Vitale M., Galluzzo P., Buffa P.G., Carlino E., Spezia O., Alduina R. Comparison of Antibiotic Resistance Profile and Biofilm Production of Staphylococcus aureus Isolates Derived from Human Specimens and Animal-Derived Samples. Antibiotics. 2019;8:97. doi: 10.3390/antibiotics8030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zuniga E., Melville P.A., Saidenberg A.B.S., Laes M.A., Gonsales F.F., Salaberry S.R.S., Gregori F., Brandão P.E., dos Santos F.G.B., Lincopan N.E., et al. Occurrence of genes coding for MSCRAMM and biofilm-associated protein Bap in Staphylococcus spp. isolated from bovine subclinical mastitis and relationship with somatic cell counts. Microb. Pathog. 2015;89:1–6. doi: 10.1016/j.micpath.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 72.Notcovich S., DeNicolo G., Flint S., Williamson N., Gedye K., Grinberg A., Lopez-Villalobos N. Biofilm-Forming Potential of Staphylococcus aureus Isolated from Bovine Mastitis in New Zealand. Vet. Sci. 2018;5:8. doi: 10.3390/vetsci5010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magiorakos A.P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbarth S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 74.França C.A., Peixoto R.M., Cavalcante M.B., Melo N.F., Oliveira C.J.B., Veschi J.A., Mota R.A., Costa M.M. Antimicrobial resistance of Staphylococcus spp. from small ruminant mastitis in Brazil. Pesqui. Veterinária Bras. 2012;32:747–753. doi: 10.1590/S0100-736X2012000800012. [DOI] [Google Scholar]
- 75.Taponen S., Pyorala S. Coagulase-negative staphylococci as cause of bovine mastitis—Not so different from Staphylococcus aureus? Vet. Microbiol. 2009;134:29–36. doi: 10.1016/j.vetmic.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 76.Vasileiou N.G.C., Sarrou S., Papagiannitsis C., Chatzopoulos D.C., Malli E., Mavrogianni V.S., Petinaki E., Fthenakis G.C. Antimicrobial Agent Susceptibility and Typing of Staphylococcal Isolates from Subclinical Mastitis in Ewes. Microb. Drug Resist. 2019;25:1099–1110. doi: 10.1089/mdr.2019.0009. [DOI] [PubMed] [Google Scholar]
- 77.Queiroga M.C., Andrade N., Laranjo M. Antimicrobial Action of Propolis Extracts Against Staphylococci. In: Torres-Hergueta E., Méndez-Vilas A., editors. Understanding Microbial Pathogens: Current Knowledge and Educational Ideas on Antimicrobial Research. Formatex Research Center; Badajoz, Spain: 2018. pp. 28–35. [Google Scholar]
- 78.CLSI . M100S-Performance Standards for Antimicrobial Susceptibility Testing-Approved Standard. 26th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2016. [Google Scholar]
- 79.Reading C., Cole M. Clavulanic acid: A beta-lactamase-inhiting beta-lactam from Streptomyces clavuligerus. Antimicrob. Agents Chemother. 1977;11:852–857. doi: 10.1128/AAC.11.5.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barbier F., Ruppé E., Hernandez D., Lebeaux D., Francois P., Felix B., Desprez A., Maiga A., Woerther P.L., Gaillard K., et al. Methicillin-Resistant Coagulase-Negative Staphylococci in the Community: High Homology of SCCmec IVa between Staphylococcus epidermidis and Major Clones of Methicillin-Resistant Staphylococcus aureus. J. Infect. Dis. 2010;202:270–281. doi: 10.1086/653483. [DOI] [PubMed] [Google Scholar]
- 81.Yamada K., Namikawa H., Fujimoto H., Nakaie K., Takizawa E., Okada Y., Fujita A., Kawaguchi H., Nakamura Y., Abe J., et al. Clinical Characteristics of Methicillin-resistant Coagulase-negative Staphylococcal Bacteremia in a Tertiary Hospital. Intern. Med. 2017;56:781–785. doi: 10.2169/internalmedicine.56.7715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.El-Razik K.A.A., Arafa A.A., Hedia R.H., Ibrahim E.S. Tetracycline resistance phenotypes and genotypes of coagulase-negative staphylococcal isolates from bubaline mastitis in Egypt. Vet. World. 2017;10:702–710. doi: 10.14202/vetworld.2017.702-710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.El Feghaly R.E., Stamm J.E., Fritz S.A., Burnham C.-A.D. Presence of the blaZ beta-lactamase gene in isolates of Staphylococcus aureus that appear penicillin susceptible by conventional phenotypic methods. Diagn. Microbiol. Infect. Dis. 2012;74:388–393. doi: 10.1016/j.diagmicrobio.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 84.Paterson G.K., Harrison E.M., Holmes M.A. The emergence of mecC methicillin-resistant Staphylococcus aureus. Trends Microbiol. 2014;22:42–47. doi: 10.1016/j.tim.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Z., Shi L., Alam M.J., Li L., Yamasaki S. Integron-bearing methicillin-resistant coagulase-negative staphylococci in South China, 2001–2004. FEMS Microbiol. Lett. 2008;278:223–230. doi: 10.1111/j.1574-6968.2007.00994.x. [DOI] [PubMed] [Google Scholar]
- 86.Becker K., van Alen S., Idelevich E.A., Schleimer N., Seggewiß J., Mellmann A., Kaspar U., Peters G. Plasmid-Encoded Transferable mecB-Mediated Methicillin Resistance in Staphylococcus aureus. Emerg. Infect. Dis. 2018;24:242–248. doi: 10.3201/eid2402.171074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akpaka P.E., Roberts R., Monecke S. Molecular characterization of antimicrobial resistance genes against Staphylococcus aureus isolates from Trinidad and Tobago. J. Infect. Public Health. 2017;10:316–323. doi: 10.1016/j.jiph.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 88.Yılmaz E.Ş., Aslantaş Ö. Antimicrobial resistance and underlying mechanisms in Staphylococcus aureus isolates. Asian Pac. J. Trop. Med. 2017;10:1059–1064. doi: 10.1016/j.apjtm.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 89.Markey B., Leonard F., Archambault M., Cullinane A., Maguire D. Clinical Veterinary Microbiology. 2nd ed. Mosby Ltd.; London, UK: 2013. [Google Scholar]
- 90.Laranjo M., Machado J., Young J.P.W., Oliveira S. High diversity of chickpea Mesorhizobium species isolated in a Portuguese agricultural region. FEMS Microbiol. Ecol. 2004;48:101–107. doi: 10.1016/j.femsec.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 91.Merino N., Toledo-Arana A., Vergara-Irigaray M., Valle J., Solano C., Calvo E., Lopez J.A., Foster T.J., Penadés J.R., Lasa I. Protein A-mediated multicellular behavior in Staphylococcus aureus. J. Bacteriol. 2009;191:832–843. doi: 10.1128/JB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.CLSI . M02-A11-Performance Standards for Antimicrobial Disk Susceptibility Tests-Approved Standard-Eleventh Edition. Clinical and Laboratory Standards Institute (CLSI); Wayne, PA, USA: 2012. [Google Scholar]
- 93.EUCAST . European Committee on Antimicrobial Susceptibility Testing-Breakpoint Tables for Interpretation of MICs and Zone Diameters. UCAST; Växjö, Sweden: 2020. Version 10.0. [Google Scholar]
- 94.Rivas R., Vizcaino N., Buey R.M., Mateos P.F., Martinez-Molina E., Velazquez E. An effective, rapid and simple method for total RNA extraction from bacteria and yeast. J. Microbiol. Methods. 2001;47:59–63. doi: 10.1016/S0167-7012(01)00292-5. [DOI] [PubMed] [Google Scholar]
- 95.Brakstad O.D.D.G., Aasbakk K., Maeland J.A. Detection of Staphylococcus aureus by Polymerase Chain Reaction Amplification of the nuc Gene. J. Clin. Microbiol. 1992;30:1654–1660. doi: 10.1128/JCM.30.7.1654-1660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vasudevan P., Nair M.K., Annamalai T., Venkitanarayanan K.S. Phenotypic and genotypic characterization of bovine mastitis isolates of Staphylococcus aureus for biofilm formation. Vet. Microbiol. 2003;92:179–185. doi: 10.1016/S0378-1135(02)00360-7. [DOI] [PubMed] [Google Scholar]
- 97.Sawant A., Gillespie B., Oliver S. Antimicrobial susceptibility of coagulase-negative Staphylococcus species isolated from bovine milk. Vet. Microbiol. 2009;134:73–81. doi: 10.1016/j.vetmic.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 98.Strommenger B., Kettlitz C., Werner G. Multiplex PCR assay for simultaneous detection of nine clinically relevant antibiotic resistance genes in Staphylococcus aureus. J. Clin. Microbiol. 2003;41:4089–4094. doi: 10.1128/JCM.41.9.4089-4094.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stegger M., Andersen P.S., Kearns A., Pichon B., Holmes M.A., Edwards G., Laurent F., Teale C., Skov R., Larsen A.R. Rapid detection, differentiation and typing of methicillin-resistant Staphylococcus aureus harbouring either mecA or the new mecA homologue mecALGA251. Clin. Microbiol. Infect. 2012;18:395–400. doi: 10.1111/j.1469-0691.2011.03715.x. [DOI] [PubMed] [Google Scholar]
- 100.Ferreira A.M., Martins K.B., Silva V.R.D., Mondelli A.L., Cunha M.D.L.R.D.S.D. Correlation of phenotypic tests with the presence of the blaZ gene for detection of beta-lactamase. Braz. J. Microbiol. 2017;48:159–166. doi: 10.1016/j.bjm.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Horstkotte M.A., Knobloch J.K.M., Rohde H., Mack D. Rapid Detection of Methicillin Resistance in Coagulase-Negative Staphylococci by a Penicillin-Binding Protein 2a-Specific Latex Agglutination Test. J. Clin. Microbiol. 2001;39:3700–3702. doi: 10.1128/JCM.39.10.3700-3702.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Coelho S.D.M.D.O., Moraes R.A.M., Soares L.D.C., Pereira I.A., Gomes L.P., Souza M.M.S.D. Mapeamento do perfil de resistência e detecção do gene mecA em Staphylococcus aureus e Staphylococcus intermedius oxacilina-resistentes isolados de espécies humanas e animais. Ciência Rural. 2007;37:195–200. doi: 10.1590/S0103-84782007000100031. [DOI] [Google Scholar]