Abstract
Immune-targeted approaches are rapidly changing the therapeutic landscape for cancer. In spite of that, most patients show resistance or acquire resistance to these therapies. Increasing work describing the tumor microenvironment (TME) has highlighted this space as one of the key determinants in tumor immune response and immunotherapeutic success. Frequently overlooked within this space, cancer-associated fibroblasts (CAFs) within the TME have surfaced as an important dictator of the tumor immune response. Herein, we review recent advances in defining the role of CAF-immune cell interactions in solid tumors and prospects for targeting stroma to overcome resistance to immunotherapy.
Introduction
Cancer immunotherapies have shown marked therapeutic success as of late and generally fall into two broad categories. The first category are designed to enhance endogenous anti-tumor immunity and include vaccines [1], immune agonists like anti-CD40 [2], and inhibitors of immune checkpoints such as anti-CTLA-4 [3] and anti-PD-1/PD-L1 [4]. However, increasing clinical experience indicates that in spite of the remarkable success of these approaches across multiple tumor types, the majority of patients are either resistant or acquire resistance [5]. The second category involves adaptive cell therapies (ACT) such as chimeric antigen receptor T-Cell (CAR-T) therapy [6,7]. Remarkable successes have also been achieved with ACT; however, these have been limited to hematologic tumors, while proof of their utility in solid tumors remains elusive. Therapeutic resistance is also a major barrier in the context of chemotherapy and targeted therapies. It is therefore imperative that the mechanistic basis of resistance be determined to facilitate the rational design of approaches to avoid or overcome therapeutic resistance. Tumor stroma, comprises mesenchyme-derived stromal cells such as cancer-associated fibroblasts (CAFs) and extracellular matrix (ECM), is complicit in tumor initiation, progression and metastasis. Moreover, stroma represents a major barrier to therapeutic efficacy and has recently been identified as a critical mediator of immune suppression in the tumor microenvironment (TME) [8].
Although fibroblasts were historically largely overlooked in the context of cancer, together with ECM, they are now understood to provide biochemical and biomechanical signals critical to malignant cell behavior. CAFs also impact inflammatory and immune cell infiltration and intra-tumoral migration and contribute to the immune suppressive milieu that typically dominates the microenvironment of advanced solid tumors. In this review, we outline recent advances in the understanding of how fibroblasts influence tumor immunity and how this in turn influences the success of immunotherapies. We also discuss current attempts at therapeutically targeting CAFs.
The ‘Stromagenic switch’
Fibroblast activation
Fibroblasts represent a heterogeneous population of mesenchyme-derived cells prominent in all connective tissues. In the broadest sense, fibroblasts can be divided into two primary states, quiescent and activated, although they exhibit significant context-dependent phenotypical and functional diversity.
Fibroblasts under homeostatic conditions exist in most tissues in a relatively quiescent state, referring to their low proliferative capacity and metabolic state. Fibroblast activation is an early response to disruptions in homeostasis, characterized by increased proliferative capacity, increased synthetic activity including production of a provisional matrix, and increased metabolic activity, all designed to restore homeostasis [9].
In the TME, cancer cells can drive fibroblast activation. A number of tumor cell secreted factors can activate fibroblasts including TGFβ, PDGF, EGF, CTGF, and FGF [10,11]. Fibroblasts are also responsive to substratum composition and stiffness with matrix stiffening being associated with fibroblast activation [12•]. Stiffness within compliant normal tissues typically ranges from ~.05—5 kPa [13], while progressive stiffening of tumor tissue can reach up to ~20 kPa [14] in the most desmoplastic tumors such as pancreatic cancer. Together, these signals can also drive dedifferentiation of other mesenchymal cell types, such as pericytes and adipocytes, to a CAF-like state [15,16].
Heterogeneity in fibroblasts
The heterogeneity of CAFs is just beginning to be defined on a molecular level and recent work has been vital in untangling confounding results from earlier studies. Because of initial reports establishing correlations between the prevalence of CAFs and poor prognosis [17,18], a simple paradigm emerged that CAFs are pro-tumorigenic. However, early studies targeting myofibroblasts in the context of pancreatic cancer unexpectedly enhanced tumor progression [19,20]. These seemingly paradoxical observations highlighted a need to better understand the functional diversity of CAFs.
Activated fibroblasts within TME have traditionally been identified based on their expression of alpha smooth muscle actin (α-SMA), and generically referred to as myofibroblasts [21]. More recent studies, however, highlight that α-SMA+ cells represent only a subset of all stromal cells within the TME and that CAFs are in fact heterogeneous based on cell surface markers, gene expression profiling, and functionality. To date, neither a unifying approach to defining, nor a standardized nomenclature for CAF subpopulations has yet emerged but promises to be complex based on the evidence that the state of fibroblast activation is both context-dependent, plastic and likely fall along a continuum rather than into discrete subsets. Nonetheless, multiple markers including fibroblast activation protein (FAP) [22], podoplanin (PDPN) [23], fibroblast-specific protein 1 (FSP-1) [24], meflin [25], and platelet-derived growth factor receptor (PDGFR) [24] have surfaced to describe CAF populations with key functional differences within the TME.
Independently, two subpopulations referred to as myCAFs and iCAF have been described in pancreatic and recently other cancer types [26••]. MyCAFs (like myofibroblasts) are the traditional α-SMA expressing population and inflammatory fibroblasts (iCAFs) are defined by expression of inflammatory cytokines such as IL-6 and CXCL12 [26••,27]. These subpopulations also segregate spatially within TME of pancreatic cancer, with myCAFs primarily tumor adjacent, and iCAFs more distal from the edge of tumor nests [27]. These subpopulations overlap significantly with many markers described above. Moreover, single cell sequencing analyses of various tumor types indicate that fibroblasts segregate into anywhere from three to seven clusters based on transcriptome [26••,28,29•]. Such analyses are proving useful in highlighting the primary characteristics of each subpopulation and may hopefully lead to more exclusive markers for each functional type.
CAF – immune cell interactions
A major impact of CAFs on the TME is through their immunomodulatory capacity. Fibroblasts are able to direct and coordinate immune cell infiltration either directly—via secreted cytokines and surface proteins— or indirectly—through deposition of various ECM substrates and remodeling of matrix. It should be noted that some tumor-associated macrophages (TAMs) can also contribute significantly to matrix remodeling in the TME [30]. Understanding these interactions is vital considering the recent explosion of immunotherapies, as CAFs not only influence de novo immune responses, but also dictate the success of immunotherapies through these mechanisms.
CAF influence on myeloid cells
Clues that CAFs play a critical role in immunosuppression came from clinical data showing correlations in expression of stromal markers with infiltration of immunosuppressive cell types such as TAMs and myeloid-derived suppressor cells (MDSC) [31]. MDSC correlate not only with poorer overall survival across a variety of cancers, but also with disease resistance to immunotherapy [17,32].
Myeloid cells in TME are known to drive immunosuppression including suppression of cytotoxic T-cell activity [33]. CAFs secrete many signaling molecules known to influence both recruitment and activation state of myeloid cells including: CXCL1, CXCL2, CXCL5, CXCL6/GCP-2, CXCL8, CXCL9, CXCL10, CXCL12/SDF1, CCL2/MCP-1, CCL3, CCL5/Rantes, CCL7, CCL20, CCL26, IL-1β, IL-6, IL-10, VEGF, TGF-β, indoleamine-2,3-dioxygenase (IDO), prostaglandin (PG) E2 (PGE2), tumor necrosis factor (TNF) or nitric oxide (NO) [34,35].
Two of these pathways in particular are well studied in this context: CXCL12/CXCR4 and IL-6/STAT3 (Figure 1). CXCL12 in the TME is largely derived from CAFs and plays an important role in recruiting myeloid cells and promoting an immunosuppressive phenotype. Inhibiting either CXCL12 or its receptor CXCR4, has been shown to decrease intra-tumoral MDSCs [18,36–40]. Further, PGE2 and TGF-β regulate CXCL12/CXCR4 expression and have been proposed as another potential target [38,41,42]. Likewise, myeloid STAT3 is activated in response to CAF-derived IL-6 and is an important regulator of myeloid state that can drive differentiation to regulatory dendritic cells (DCs) [43]. Blocking either STAT3 or IL-6 can disrupt this signaling and reprogram the immunosuppressive milieu of the TME [40,44,45].
Figure 1.
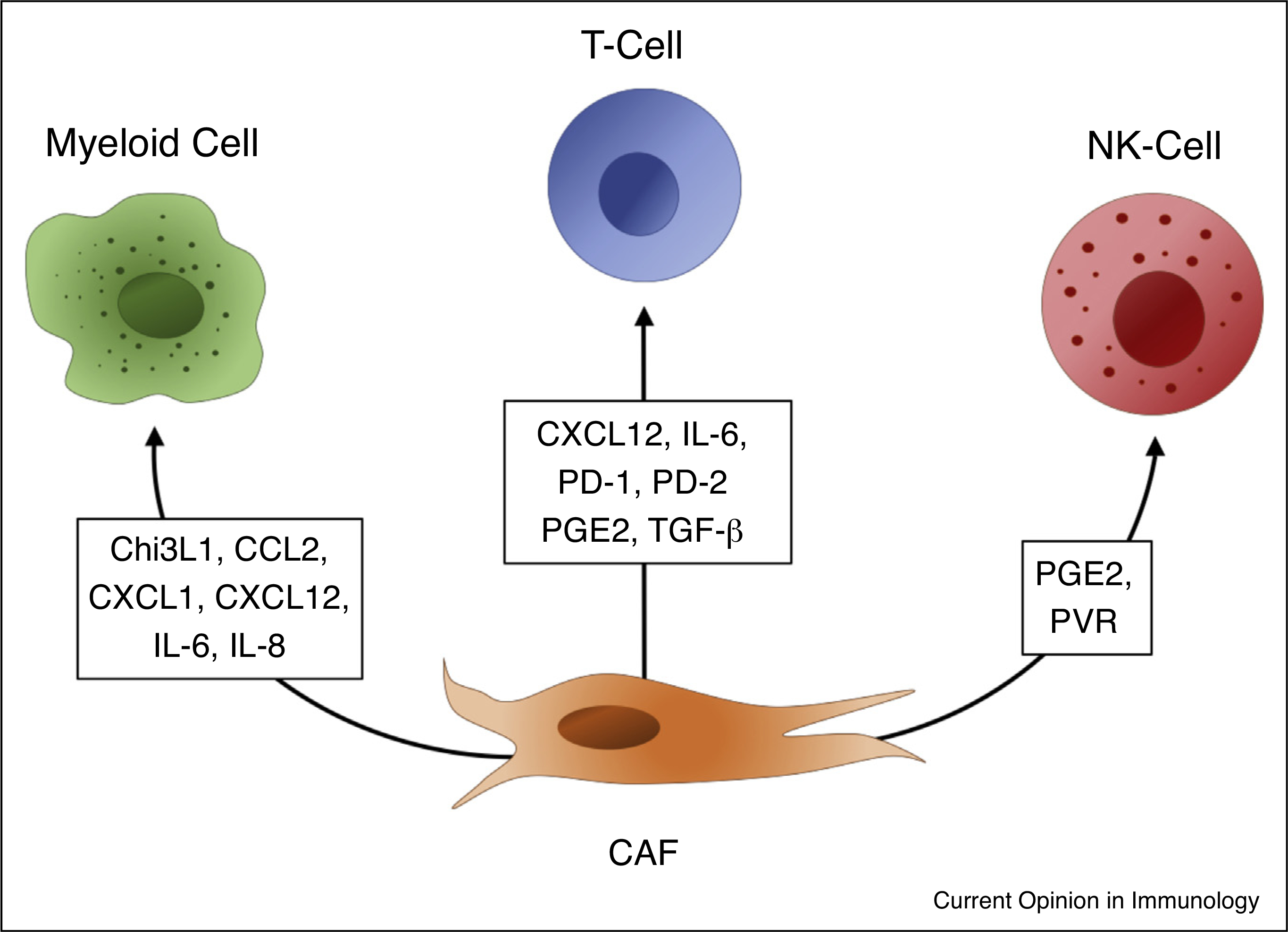
Chitinase 3-like 1 (Chi3L1), chemokine (C-C motif) ligand 2, chemokine (C-X-C motif) ligand 1 (CXCL1), chemokine (C-X-C motif) ligand 12 (CXCL12), Interleukin 6 (IL-6), Interleukin 8 (IL-8), Programmed cell death protein 1 (PD-1), Programmed cell death protein 2 (PD-2), Prostaglandin E2 (PGE2), Transforming growth factor beta (TGFbeta), Polio Virus Receptor (PVR), cancer-associated fibroblast (CAF).
Many other CAF-secreted factors are also directly linked to myeloid modulation. CCL2 is produced by a FAP+ subset of CAFs and has been shown to attract and to activate STAT3 in myeloid cells [46]. Chitinase 3-like 1 (Chi3L1) secreted by CAFs drives M2 polarization in macrophages [47], and CXCL1 has been implicated as a mediator of CAF-dependent accumulation of MDSCs [48] (Figure 1). It is important to note however, that the contributions of these pathways are context dependent. For example, IL-8/CXCR2 can mediate recruitment of myeloid cells independent of CXCL12/CXCR4 [49,50••]. As is often the case, one could theorize these discrepancies arise from varying abundance of heterogeneous CAFs across different models.
CAF influence on T-Cells
CAF markers correlate with an immuno-tolerant T-cell landscape in the TME, as defined by an increased ratio of FoxP3+ to CD8+ T-cells, which is also associated with poor clinical outcome [51]. While CAFs can influence adaptive immune cells indirectly through their effect on myeloid cells, CAFs can also exert direct effects on regulatory and cytotoxic T-cells [34].
Subcutaneous tumors developed in mice co-injected with fibroblasts showed a greater ratio of FoxP3+/ CD8+ T-cells than those in mice injected with tumor cells alone. This effect was attenuated by treatment with anti-IL-6 antibodies consistent with a potential role for CAF-derived IL-6 [51]. Further, fibroblast IL-6 has been shown to drive differentiation of interleukin-17-producing T helper (TH17) cells [52] which can be pro-tumorigenic or anti-tumorigenic depending on the tumor type and CAF-mediated signals [53].
Activated fibroblasts can suppress cytotoxic T-cell responses through PD-1 and PD-2 signaling by either expressing PD-L1/2 themselves [54,55], or driving expression of PD-L1/2 on tumor cells via CXCL5 (Figure 1) [56•]. TGF-β-associated ECM genes in fibroblasts was reported to be a strong predictor of immunotherapeutic failure and TGF-β blocking antibodies co-administered with anti-PD-L1 therapy significantly improved response to therapy in mouse models [57••,58]. As with myeloid cells, inhibition of CXCL12 also relieved immunosuppression of T-cells and promoted infiltration of cytotoxic T-cells during anti-PD-1 therapy [37,59].
CAFs also have the capacity for MHC Class I restricted antigen presentation to T-cells. However, rather than activating T-cells, engagement of PD-L2 and FASL on the CAF cell surface can result in killing of antigen-specific cytotoxic CD8 T-cells [60•]. A population of MHC-II expressing fibroblasts have recently been highlighted that can present antigen to naïve CD4+ T cells. However, CAF secretion of PGE2 (Figure 1) and a lack of expression of costimulatory molecules by these CAFs appeared to preferentially promote expansion of CD4+, CD25high, Foxp3+ regulatory T-cells (Tregs) [26••,61].
It is important to note that not all fibroblast-mediated influences on T-cells are immunosuppressive. A limited number of studies provide evidence for T cell-stimulating signaling from fibroblasts. For instance, in some settings IL-6 produced by fibroblasts in response to T-cell exposure enhanced T-cell stimulation [62]. These disparities are likely explained by the heterogeneous nature of CAFs in vivo with certain populations performing opposing functions [63]. It also important to consider that these immune-stimulating populations may be misrepresented in past literature as common in vitro methods of cultivating fibroblasts can quickly drive a more immunosuppressive state, masking certain populations [62,64].
CAF influence on natural killer cells
CAFs are able to influence NK cells through both contact-dependent and independent mechanisms. CAFs can block upregulation of NKp44, NKp30, and DNAM-1 triggering receptors as well as the acquisition of cytolytic granules in NK cells stimulated by IL-2, both are important steps in NK cell cytotoxicity [65]. CAF PGE2 was identified as one of the main signaling molecules driving NK cell dysfunction across a variety of cancer types (Figure 1) and inhibitors of either PGE2 or IDO ablated this effect in culture [65–68]. However, as with many other CAF effects, heterogeneity in these responses is observed and studies using CAFs from endometrial cancer promoted NK dysfunction not through PGE2, but through contact dependent mechanisms involving downregulation of cell-surface poliovirus receptor (PVR/CD155) (Figure 1), an important NK cell ligand, on CAFs [69]. Better understanding of the mechanisms through which CAFs manipulate NK cell activation will be imperative moving forward as NK cells become an attractive new target for off-the-shelf adoptive cell immunotherapy [70].
Strategies to target CAFs to enhance therapeutic efficacy
Targeting CAFs within the TME is a fairly new concept, but given that ECM dysregulation is one of the strongest predictors of failure in immunotherapies such as PD-L1 blockade [58], it has gained considerable interest as of late. Several approaches are being taken to target fibroblasts: 1) taking advantage of the heterogeneity of the population in order to shift the preponderance of pro-tumorigenic populations, including immunosuppressive populations, versus anti-tumorigenic subpopulations 2) targeting pathways that drive differentiation and reprogramming of CAFs, and 3) targeting pathways by which activated fibroblasts negatively influence the TME. (Table 1)
Table 1.
Description of current therapy development underway for stromal targeting in cancer
| Target | Name | Drug/biological | Mechanism | Current status |
|---|---|---|---|---|
|
| ||||
| FAK [80] | Defactinib (VS-6063,PF-04554878) | Small molecule | Downstream of integrin signalling | Clinical trials ongoing |
| Angiotensin receptor [87] | Losartan | Small molecule | Reduces fibroblast contractility | Clinical trials ongoing |
| Hedgehog [81] | IPI-926 (Saridegib) and Vismodegib | Small molecule | Prevents activation of CAFs | Clinical trials ongoing, some reports lack efficacy |
| ROCK [88] | AT13148 | Small molecule | Contractility | Phase I Clinical trial completed |
| LOXL2 [89] | Simtuzumab (GS 6624) | Blocking Ab | Anti-crosslinking | Pre-clinical |
| CTGF [83] | FG-3019 | Blocking Ab | Blocks receptor binding | Early phase clinical trials ongoing |
| Vitamin D receptor [90] | Paricalcitol | Small molecule agonist | ‘Normalizes’ stellate cells | Clinical trial started |
| TGF-β [84] | Multiple | Blocking Ab and small molecule receptor inhibitors | Prevents activation of CAFs and immune-suppression | Phase I, II, and III trials underway |
| FAP [91] | Multiple antibodies and RO6874281 | Blocking Ab or antibody-IL2 fusion | Block FAP+ CAF function, promoting T-cell function | Phase I and II trials underway |
| FGFR [92] | JNJ-42756493 | Small molecule | Prevent activation of CAFs | Phase I and II trials underway |
| Hyaluronan [93] | PEGPH20 | PEGylated recombinant human hyaluronidase | Degrades hyaluronan | Phase III: Enhanced chemotherapy response, did not prolong survival |
Targeting CAF heterogeneity
While early attempts to therapeutically target CAFs within the TME failed [19,20], more recent attempts based off our improved understanding of fibroblast heterogeneity have proved more successful. One of the most successful approaches has been in targeting FAP+ fibroblasts. The cancer supporting role of FAP-expressing fibroblasts has been known for some time [71]. FAP+ cells can both promote tumor progression and present a barrier to immunotherapies through both their production of ECM and direct signaling pathways [72,73]. Multiple different approaches to depleting this population have shown therapeutic promise in preclinical models, with early verification coming from genetic depletion [37,74] and progressing to more translatable approaches like vaccines [75], drug delivering nanoparticles activated by FAP cleavage [76], and CAR-T cells directed at FAP+ cells [77,78]. Such treatments in isolation have shown efficacy against cancer and also enhance the activity of conventional chemotherapies and immunotherapies [79]. So far, FAP+ populations have been the primary focus in stromal depletion therapies but as more populations become better defined, we will likely see other targets exploited.
Targeting CAF-specific pathways
Targeted therapies have also been developed and can be classified into two main groups: those that target drivers and those that target effectors. Drugs targeting CAF development and maintenance are aimed upstream and influence factors that drive the phenotypic switch. These include FAK inhibitors [80], Hedgehog inhibitors [81], fibroblast growth factor receptor (FGFR) inhibitors [82], connective tissue growth factor (CTFG) antagonists [83], and TGF-β inhibitors [84], all of which target tumor cells’ ability to activate neighboring fibroblasts. (Table 1)
Effector therapies target pathways already active in CAFs in order to limit their tumor protective abilities. These include vitamin D ligands [85], which reprogram CAFs to a more quiescent-like state, and angiotensin inhibitors [86], which influence CAF matrix deposition, decompressing the tumor, improving its vasculature, and thus making it more susceptible to chemo and immunotherapy. Again in FAP+ populations, disrupting pathways that mediate immunosuppression have been successful in tumor models, for example disruption of CXCL12 signaling [37].
Overall, our understanding of how fibroblasts orchestrate and behave across various TMEs is just beginning. Preclinical and clinical studies are showing that fibroblasts are feasible targets for improving immunotherapy response as well as many other therapies. The success of this field will depend upon the discoveries of this coming decade as key gaps are filled so that more targeted therapeutic approaches can be delivered.
Footnotes
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Melief CJ, van Hall T, Arens R, Ossendorp F, van der Burg SH: Therapeutic cancer vaccines. J Clin Invest 2015, 125:3401–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty GL, Li Y, Long KB: Cancer immunotherapy: activating innate and adaptive immunity through CD40 agonists. Expert Rev Anticancer Ther 2017, 17:175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leach DR, Krummel MF, Allison JP: Enhancement of antitumor immunity by CTLA-4 blockade. Science 1996, 271:1734–1736. [DOI] [PubMed] [Google Scholar]
- 4.Gong J, Chehrazi-Raffle A, Reddi S, Salgia R: Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: a comprehensive review of registration trials and future considerations. J Immunother Cancer 2018, 6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O’Donnell JS, Teng MWL, Smyth MJ: Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 2019, 16:151–167. [DOI] [PubMed] [Google Scholar]
- 6.June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC: CAR T cell immunotherapy for human cancer. Science 2018, 359:1361–1365. [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME: Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 2008, 8:299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA: The pancreas cancer microenvironment. Clin Cancer Res 2012, 18:4266–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darby IA, Laverdet B, Bonte F, Desmouliere A: Fibroblasts and myofibroblasts in wound healing. Clin Cosmet Investig Dermatol 2014, 7:301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gardner H, Strehlow D, Bradley L, Widom R, Farina A, de Fougerolles A, Peyman J, Koteliansky V, Korn JH: Global expression analysis of the fibroblast transcriptional response to TGFbeta. Clin Exp Rheumatol 2004, 22:S47–57. [PubMed] [Google Scholar]
- 11.Kuzet SE, Gaggioli C: Fibroblast activation in cancer: when seed fertilizes soil. Cell Tissue Res 2016, 365:607–619. [DOI] [PubMed] [Google Scholar]
- 12. Avery D, Govindaraju P, Jacob M, Todd L, Monslow J, Puré E: Extracellular matrix directs phenotypic heterogeneity of activated fibroblasts. Matrix Biol 2018, 67:90–106. • By culturing fibroblasts on matrices of varying stiffness, the authors showed that fibroblast activation can be directed by both ECM composition and elasticity, recreating phenotypes similar to those found in the TME.
- 13.Cox TR, Erler JT: Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 2011, 4:165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalli M, Stylianopoulos T: Defining the role of solid stress and matrix stiffness in cancer cell proliferation and metastasis. Front Oncol 2018, 8:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hosaka K, Yang Y, Seki T, Fischer C, Dubey O, Fredlund E, Hartman J, Religa P, Morikawa H, Ishii Y et al. : Pericyte-fibroblast transition promotes tumor growth and metastasis. Proc Natl Acad Sci U S A 2016, 113:E5618–5627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zoico E, Darra E, Rizzatti V, Budui S, Franceschetti G, Mazzali G, Rossi AP, Fantin F, Menegazzi M, Cinti S et al. : Adipocytes WNT5a mediated dedifferentiation: a possible target in pancreatic cancer microenvironment. Oncotarget 2016, 7:20223–20235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, Sevillano M, Palomo-Ponce S, Tauriello DV, Byrom D et al. : Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat Genet 2015, 47:320–329. [DOI] [PubMed] [Google Scholar]
- 18.Comito G, Giannoni E, Segura CP, Barcellos-de-Souza P, Raspollini MR, Baroni G, Lanciotti M, Serni S, Chiarugi P: Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 2014, 33:2423–2431. [DOI] [PubMed] [Google Scholar]
- 19.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, Laklai H, Sugimoto H, Kahlert C, Novitskiy SV et al. : Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 2015, 28:831–833. [DOI] [PubMed] [Google Scholar]
- 20.Rhim AD, Oberstein PE, Thomas DH, Mirek ET, Palermo CF, Sastra SA, Dekleva EN, Saunders T, Becerra CP, Tattersall IW et al. : Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 2014, 25:735–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Darby I, Skalli O, Gabbiani G: Alpha-smooth muscle actin is transiently expressed by myofibroblasts during experimental wound healing. Lab Invest 1990, 63:21–29. [PubMed] [Google Scholar]
- 22.Park JE, Lenter MC, Zimmermann RN, Garin-Chesa P, Old LJ, Rettig WJ: Fibroblast activation protein, a dual specificity serine protease expressed in reactive human tumor stromal fibroblasts. J Biol Chem 1999, 274:36505–36512. [DOI] [PubMed] [Google Scholar]
- 23.Astarita JL, Acton SE, Turley SJ: Podoplanin: emerging functions in development, the immune system, and cancer. Front Immunol 2012, 3:283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sugimoto H, Mundel TM, Kieran MW, Kalluri R: Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther 2006, 5:1640–1646. [DOI] [PubMed] [Google Scholar]
- 25.Maeda K, Enomoto A, Hara A, Asai N, Kobayashi T, Horinouchi A, Maruyama S, Ishikawa Y, Nishiyama T, Kiyoi H et al. : Identification of meflin as a potential marker for mesenchymal stromal cells. Sci Rep 2016, 6:22288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Elyada E, Bolisetty M, Laise P, Flynn WF, Courtois ET, Burkhart RA, Teinor JA, Belleau P, Biffi G, Lucito MS et al. : Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov 2019, 9:1102–1123. •• Using single-cell sequencing, this group provided the most comprehensive analysis of both human and mouse pancreatic ductal adenocarcinoma and identified a newly defined, immunosuppresive CAF population which expresses MHCII.
- 27.Ohlund D, Handly-Santana A, Biffi G, Elyada E, Almeida AS, Ponz-Sarvise M, Corbo V, Oni TE, Hearn SA, Lee EJ et al. : Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med 2017, 214:579–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lambrechts D, Wauters E, Boeckx B, Aibar S, Nittner D, Burton O, Bassez A, Decaluwe H, Pircher A, Van den Eynde K et al. : Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med 2018, 24:1277–1289. [DOI] [PubMed] [Google Scholar]
- 29. Dominguez CX, Muller S, Keerthivasan S, Koeppen H, Hung J, Gierke S, Breart B, Foreman O, Bainbridge TW, Castiglioni A et al. : Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov 2020, 10:232–253. • Using single-cell transcriptomics, this group was able to identify a novel marker (LRRC15) on a CAF subpopulation that is significantly correlated with poor response to anti-PD-L1 therapy in cancer patients.
- 30.Zhu Y, Herndon JM, Sojka DK, Kim KW, Knolhoff BL, Zuo C, Cullinan DR, Luo J, Bearden AR, Lavine KJ et al. : Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity 2017, 47:597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi H, Sakakura K, Kudo T, Toyoda M, Kaira K, Oyama T, Chikamatsu K: Cancer-associated fibroblasts promote an immunosuppressive microenvironment through the induction and accumulation of protumoral macrophages. Oncotarget 2017, 8:8633–8647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz-Montero CM, Finke J, Montero AJ: Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Semin Oncol 2014, 41:174–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gabrilovich DI, Nagaraj S: Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009, 9:162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziani L, Chouaib S, Thiery J: Alteration of the antitumor immune response by cancer-associated fibroblasts. Front Immunol 2018, 9:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lazennec G, Richmond A: Chemokines and chemokine receptors: new insights into cancer-related inflammation. Trends Mol Med 2010, 16:133–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benedicto A, Romayor I, Arteta B: CXCR4 receptor blockage reduces the contribution of tumor and stromal cells to the metastatic growth in the liver. Oncol Rep 2018, 39:2022–2030. [DOI] [PubMed] [Google Scholar]
- 37.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL et al. : Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A 2013, 110:20212–20217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P: PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res 2011, 71:7463–7470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gok Yavuz B, Gunaydin G, Gedik ME, Kosemehmetoglu K, Karakoc D, Ozgur F, Guc D: Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1(+) TAMs. Sci Rep 2019, 9:3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng Y, Cheng J, Fu B, Liu W, Chen G, Zhang Q, Yang Y: Hepatic carcinoma-associated fibroblasts enhance immune suppression by facilitating the generation of myeloid-derived suppressor cells. Oncogene 2017, 36:1090–1101. [DOI] [PubMed] [Google Scholar]
- 41.Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA et al. : Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A 2010, 107:20009–20014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Katoh H, Hosono K, Ito Y, Suzuki T, Ogawa Y, Kubo H, Kamata H, Mishima T, Tamaki H, Sakagami H et al. : COX-2 and prostaglandin EP3/EP4 signaling regulate the tumor stromal proangiogenic microenvironment via CXCL12-CXCR4 chemokine systems. Am J Pathol 2010, 176:1469–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng JT, Deng YN, Yi HM, Wang GY, Fu BS, Chen WJ, Liu W, Tai Y, Peng YW, Zhang Q: Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis 2016, 5: e198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chomarat P, Banchereau J, Davoust J, Palucka AK: IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol 2000, 1:510–514. [DOI] [PubMed] [Google Scholar]
- 45.Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, Fuchs JR, Eubank TD, Frankel WL, Bekaii-Saab T et al. : Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res 2013, 73:3007–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W, Dang Y, Chu Y, Fan J, He R: FAP promotes immunosuppression by cancer-associated fibroblasts in the tumor microenvironment via STAT3-CCL2 signaling. Cancer Res 2016, 76:4124–4135. [DOI] [PubMed] [Google Scholar]
- 47.Cohen N, Shani O, Raz Y, Sharon Y, Hoffman D, Abramovitz L, Erez N: Fibroblasts drive an immunosuppressive and growth-promoting microenvironment in breast cancer via secretion of Chitinase 3-like 1. Oncogene 2017, 36:4457–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, Hashimoto A, Vonteddu P, Behera R, Goins MA et al. : Cancer-associated fibroblasts neutralize the anti-tumor effect of CSF1 receptor blockade by inducing PMN-MDSC infiltration of tumors. Cancer Cell 2017, 32:654–668 e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang R, Qi F, Zhao F, Li G, Shao S, Zhang X, Yuan L, Feng Y: Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death Dis 2019, 10:273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen IX, Chauhan VP, Posada J, Ng MR, Wu MW, Adstamongkonkul P, Huang P, Lindeman N, Langer R, Jain RK: Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc Natl Acad Sci U S A 2019, 116:4558–4566. •• Using the FDA approved drug Plerixafor, the authors were able to reduce fibrosis, alleviate immunosuppression, and significantly enhance the efficacy of immune checkpoint blockers in preclinical models thus showing that stromal pathways can be targeted with already available drugs.
- 51.Kato T, Noma K, Ohara T, Kashima H, Katsura Y, Sato H, Komoto S, Katsube R, Ninomiya T, Tazawa H et al. : Cancer-associated fibroblasts affect intratumoral CD8(+) and FoxP3 (+) T cells via IL6 in the tumor microenvironment. Clin Cancer Res 2018, 24:4820–4833. [DOI] [PubMed] [Google Scholar]
- 52.Komatsu N, Okamoto K, Sawa S, Nakashima T, Oh-hora M, Kodama T, Tanaka S, Bluestone JA, Takayanagi H: Pathogenic conversion of Foxp3+ T cells into TH17 cells in autoimmune arthritis. Nat Med 2014, 20:62–68. [DOI] [PubMed] [Google Scholar]
- 53.Guery L, Hugues S: Th17 cell plasticity and functions in cancer immunity. Biomed Res Int 2015, 2015:314620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinchuk IV, Saada JI, Beswick EJ, Boya G, Qiu SM, Mifflin RC, Raju GS, Reyes VE, Powell DW: PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology 2008, 135:1228–1237 1237 e1221–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cho YA, Yoon HJ, Lee JI, Hong SP, Hong SD: Relationship between the expressions of PD-L1 and tumor-infiltrating lymphocytes in oral squamous cell carcinoma. Oral Oncol 2011, 47:1148–1153. [DOI] [PubMed] [Google Scholar]
- 56. Li Z, Zhou J, Zhang J, Li S, Wang H, Du J: Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int J Cancer 2019, 145:1946–1957. • This paper mechanistically connected CAF signaling to PD-L1 expression on tumor cells and showed that inhibition of CXCR2 (the receptor for CXCL5) could inhibit PD-L1 expression in a xenograft model.
- 57. Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, Kadel EE III, Koeppen H, Astarita JL, Cubas R et al. : TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018, 554:544–548. •• This study showed that a signature of TGFβ signalling in fibroblasts was one of the strongest predictors of checkpoint therapy failure in metastatic urothelial cancer and that co-administration of therapy with TGFβ blocking antibodies may improve response.
- 58.Chakravarthy A, Khan L, Bensler NP, Bose P, De Carvalho DD: TGF-beta-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat Commun 2018, 9:4692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zboralski D, Hoehlig K, Eulberg D, Fromming A, Vater A: Increasing tumor-infiltrating T Cells through inhibition of CXCL12 with NOX-A12 synergizes with PD-1 Bblockade. Cancer Immunol Res 2017, 5:950–956. [DOI] [PubMed] [Google Scholar]
- 60. Lakins MA, Ghorani E, Munir H, Martins CP, Shields JD: Cancer-associated fibroblasts induce antigen-specific deletion of CD8 (+) T Cells to protect tumour cells. Nat Commun 2018, 9:948. • Study showed that CAFs are able to directly kill CD8+ T cells in an antigen-specific and antigen-dependent manner via PD-L2 and FASL.
- 61.Pinchuk IV, Beswick EJ, Saada JI, Boya G, Schmitt D, Raju GS, Brenmoehl J, Rogler G, Reyes VE, Powell DW: Human colonic myofibroblasts promote expansion of CD4+ CD25high Foxp3+ regulatory T cells. Gastroenterology 2011, 140:2019–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barnas JL, Simpson-Abelson MR, Brooks SP, Kelleher RJ Jr, Bankert RB: Reciprocal functional modulation of the activation of T lymphocytes and fibroblasts derived from human solid tumors. J Immunol 2010, 185:2681–2692. [DOI] [PubMed] [Google Scholar]
- 63.Nazareth MR, Broderick L, Simpson-Abelson MR, Kelleher RJ Jr, Yokota SJ, Bankert RB: Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J Immunol 2007, 178:5552–5562. [DOI] [PubMed] [Google Scholar]
- 64.Ghebeh H, Dermime S: Comment on “Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells”. J Immunol 2007, 179:732 author reply 733. [DOI] [PubMed] [Google Scholar]
- 65.Balsamo M, Scordamaglia F, Pietra G, Manzini C, Cantoni C, Boitano M, Queirolo P, Vermi W, Facchetti F, Moretta A et al. : Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci U S A 2009, 106:20847–20852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li T, Yang Y, Hua X, Wang G, Liu W, Jia C, Tai Y, Zhang Q, Chen G: Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett 2012, 318:154–161. [DOI] [PubMed] [Google Scholar]
- 67.Li T, Yi S, Liu W, Jia C, Wang G, Hua X, Tai Y, Zhang Q, Chen G: Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med Oncol 2013, 30:663. [DOI] [PubMed] [Google Scholar]
- 68.Bahrami B, Hosseini A, Talei AR, Ghaderi A, Razmkhah M: Adipose derived stem cells exert immunomodulatory effects on natural killer cells in breast cancer. Cell J 2017, 19:137–145. [PMC free article] [PubMed] [Google Scholar]
- 69.Inoue T, Adachi K, Kawana K, Taguchi A, Nagamatsu T, Fujimoto A, Tomio K, Yamashita A, Eguchi S, Nishida H et al. : Cancer-associated fibroblast suppresses killing activity of natural killer cells through downregulation of poliovirus receptor (PVR/CD155), a ligand of activating NK receptor. Int J Oncol 2016, 49:1297–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Saetersmoen ML, Hammer Q, Valamehr B, Kaufman DS, Malmberg KJ: Off-the-shelf cell therapy with induced pluripotent stem cell-derived natural killer cells. Semin Immunopathol 2019, 41:59–68. [DOI] [PubMed] [Google Scholar]
- 71.Fearon DT: The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol Res 2014, 2:187–193. [DOI] [PubMed] [Google Scholar]
- 72.Puré E, Blomberg R: Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics. Oncogene 2018, 37:4343–4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lo A, Li CP, Buza EL, Blomberg R, Govindaraju P, Avery D, Monslow J, Hsiao M, Puré E: Fibroblast activation protein augments progression and metastasis of pancreatic ductal adenocarcinoma. JCI Insight 2017, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT: Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science 2010, 330:827–830. [DOI] [PubMed] [Google Scholar]
- 75.Chen M, Xiang R, Wen Y, Xu G, Wang C, Luo S, Yin T, Wei X, Shao B, Liu N et al. : A whole-cell tumor vaccine modified to express fibroblast activation protein induces antitumor immunity against both tumor cells and cancer-associated fibroblasts. Sci Rep 2015, 5:14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ji T, Zhao Y, Ding Y, Wang J, Zhao R, Lang J, Qin H, Liu X, Shi J, Tao N et al. : Transformable peptide nanocarriers for expeditious drug release and effective cancer therapy via cancer-associated fibroblast activation. Angew Chem Int Ed Engl 2016, 55:1050–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lo A, Wang LS, Scholler J, Monslow J, Avery D, Newick K, O’Brien S, Evans RA, Bajor DJ, Clendenin C et al. : Tumor-promoting desmoplasia is disrupted by depleting FAP-expressing stromal cells. Cancer Res 2015, 75:2800–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang LC, Lo A, Scholler J, Sun J, Majumdar RS, Kapoor V, Antzis M, Cotner CE, Johnson LA, Durham AC et al. : Targeting fibroblast activation protein in tumor stroma with chimeric antigen receptor T cells can inhibit tumor growth and augment host immunity without severe toxicity. Cancer Immunol Res 2014, 2:154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valkenburg KC, de Groot AE, Pienta KJ: Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol 2018, 15:366–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin HM, Lee BY, Castillo L, Spielman C, Grogan J, Yeung NK, Kench JG, Stricker PD, Haynes AM, Centenera MM et al. : Effect of FAK inhibitor VS-6063 (defactinib) on docetaxel efficacy in prostate cancer. Prostate 2018, 78:308–317. [DOI] [PubMed] [Google Scholar]
- 81.Ko AH, LoConte N, Tempero MA, Walker EJ, Kate Kelley R, Lewis S, Chang WC, Kantoff E, Vannier MW, Catenacci DV et al. : A phase I study of FOLFIRINOX plus IPI-926, a hedgehog pathway inhibitor, for advanced pancreatic adenocarcinoma. Pancreas 2016, 45:370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishina T, Takahashi S, Iwasawa R, Noguchi H, Aoki M, Doi T: Safety, pharmacokinetic, and pharmacodynamics of erdafitinib, a pan-fibroblast growth factor receptor (FGFR) tyrosine kinase inhibitor, in patients with advanced or refractory solid tumors. Invest New Drugs 2018, 36:424–434. [DOI] [PubMed] [Google Scholar]
- 83.Neesse A, Frese KK, Bapiro TE, Nakagawa T, Sternlicht MD, Seeley TW, Pilarsky C, Jodrell DI, Spong SM, Tuveson DA: CTGF antagonism with mAb FG-3019 enhances chemotherapy response without increasing drug delivery in murine ductal pancreas cancer. Proc Natl Acad Sci U S A 2013, 110:12325–12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Gramont A, Faivre S, Raymond E: Novel TGF-beta inhibitors ready for prime time in onco-immunology. Oncoimmunology 2017, 6:e1257453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sherman MH, Yu RT, Engle DD, Ding N, Atkins AR, Tiriac H, Collisson EA, Connor F, Van Dyke T, Kozlov S et al. : Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 2014, 159:80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chauhan VP, Martin JD, Liu H, Lacorre DA, Jain SR, Kozin SV, Stylianopoulos T, Mousa AS, Han X, Adstamongkonkul P et al. : Angiotensin inhibition enhances drug delivery and potentiates chemotherapy by decompressing tumour blood vessels. Nat Commun 2013, 4:2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hu C, Liu X, Ran W, Meng J, Zhai Y, Zhang P, Yin Q, Yu H, Zhang Z, Li Y: Regulating cancer associated fibroblasts with losartan-loaded injectable peptide hydrogel to potentiate chemotherapy in inhibiting growth and lung metastasis of triple negative breast cancer. Biomaterials 2017, 144:60–72. [DOI] [PubMed] [Google Scholar]
- 88.Rath N, Munro J, Cutiongco MF, Jagiello A, Gadegaard N, McGarry L, Unbekandt M, Michalopoulou E, Kamphorst JJ, Sumpton D et al. : Rho kinase inhibition by AT13148 blocks pancreatic ductal adenocarcinoma invasion and tumor growth. Cancer Res 2018, 78:3321–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Benson AB 3rd, Wainberg ZA, Hecht JR, Vyushkov D, Dong H, Bendell J, Kudrik F: A phase II randomized, double-blind, placebo-controlled study of simtuzumab or placebo in combination with gemcitabine for the first-line treatment of pancreatic adenocarcinoma. Oncologist 2017, 22:241–e215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bigelsen S: Evidence-based complementary treatment of pancreatic cancer: a review of adjunct therapies including paricalcitol, hydroxychloroquine, intravenous vitamin C, statins, metformin, curcumin, and aspirin. Cancer Manag Res 2018, 10:2003–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Narra K, Mullins SR, Lee HO, Strzemkowski-Brun B, Magalong K, Christiansen VJ, McKee PA, Egleston B, Cohen SJ, Weiner LM et al. : Phase II trial of single agent Val-boroPro (Talabostat) inhibiting fibroblast activation protein in patients with metastatic colorectal cancer. Cancer Biol Ther 2007, 6:1691–1699. [DOI] [PubMed] [Google Scholar]
- 92.Bahleda R, Italiano A, Hierro C, Mita A, Cervantes A, Chan N, Awad M, Calvo E, Moreno V, Govindan R et al. : Multicenter phase I study of erdafitinib (JNJ-42756493), oral pan-fibroblast growth factor receptor inhibitor, in patients with advanced or refractory solid tumors. Clin Cancer Res 2019, 25:4888–4897. [DOI] [PubMed] [Google Scholar]
- 93.Doherty GJ, Tempero M, Corrie PG: HALO-109–301: a phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol 2018, 14:13–22. [DOI] [PubMed] [Google Scholar]


