Abstract
Recent cohort studies indicate a potential role of the antioxidant α-tocopherol in reducing bone loss and risk of fractures, especially hip fractures. We performed a Mendelian randomization investigation of the associations of circulating α-tocopherol with estimated bone mineral density (eBMD) using heel ultrasound and fractures, identified from hospital records or by self-reports and excluding minor fractures. Circulating α-tocopherol was instrumented by three genetic variants associated with α-tocopherol levels at p < 5 × 10−8 in a genome-wide association meta-analysis of 7781 participants of European ancestry. Summary-level data for the genetic associations with eBMD in 426,824 individuals and with fracture (53,184 cases and 373,611 non-cases) were acquired from the UK Biobank. Two of the three genetic variants were strongly associated with eBMD. In inverse-variance weighted analysis, a genetically predicted one-standard-deviation increase of circulating α-tocopherol was associated with 0.07 (95% confidence interval, 0.05 to 0.09) g/cm2 increase in BMD, which corresponds to a >10% higher BMD. Genetically predicted circulating α-tocopherol was not associated with odds of any fracture (odds ratio 0.97, 95% confidence interval, 0.91 to 1.05). In conclusion, our results strongly strengthen a causal link between increased circulating α-tocopherol and greater BMD. Both an intervention study in those with a low dietary intake of α-tocopherol is warranted and a Mendelian randomization study with fragility fractures as an outcome.
Keywords: alpha-tocopherol, bone mineral density, fracture, Mendelian randomization, vitamin E
1. Introduction
Worldwide, 1.6 million hip fractures are estimated to occur each year [1] at an average of 80 years of age. Hip fractures and other types of fragility fractures have, besides heavy costs for the society, a profound impact on quality of life; only one-third of these fracture patients regain their pre-fracture level of function [1]. There is also a higher risk of death after the fracture event [2], and especially vulnerable are older men [2]. Scandinavia has one of the highest incidences of fragility fractures in the world, with a lifetime cumulative incidence of 50% in women and 25% in men [3,4]. The risk of hip fracture, the most devastating fragility fracture, increases 44-fold in Swedish women from 55 to 85 years of age, so that the lifetime risk of hip fracture is 25% in women and 12% in men [4]. Bone mineral density (BMD) is a strong determinant of future risk of hip fracture. Hip fracture rates are more than doubled for each standard-deviation lower BMD at the hip [5], whereas the association between other BMD and fractures sites is generally less strong [5,6].
Fracture risk and bone loss are determined both by genotype and by environmental factors, where the importance of lifestyle factors increases with advancing age [7,8]. Of importance, there are now strong indications that oxidative stress is a central biological mechanism for bone cell senescence, skeletal aging, and loss of BMD [9,10,11], an important determinant contributing to fracture risk [5,12].
By decreasing the generation of free radicals leading to lower oxidative stress, the risk of low BMD in the elderly might be reduced [9,13,14]. The antioxidant α-tocopherol is the most potent form of vitamin E that has the potential to scavenge free radicals and has been proposed to favorably affect BMD [9,13,14,15,16]. However, some experimental evidence indicates that excessive intakes of α-tocopherol decrease bone mass in mice [17], a finding not confirmed by more recent evidence [18,19]. Studies in humans on α-tocopherol in relation to bone health are limited and include only observational study designs. A majority of [14,19,20,21,22] but not all [23,24] previous observational studies indicate that low dietary intakes and low serum levels of α-tocopherol are associated with lower BMD and an increased risk of fracture, especially hip fractures. Of importance, in many European countries, the mean α-tocopherol intake is below the recommended levels [25].
Genetic variants that specifically affect a biomarker can be used as instrumental variables (proxies) for the biomarker to determine whether the biomarker is causally associated with the outcome. This approach, recognized as Mendelian randomization, has previously been applied to assess the associations of lifelong circulating metabolite concentrations with various diseases [26,27,28] but has not been used to examine serum concentrations of α-tocopherol and bone phenotypes.
Thus, the Mendelian randomization (MR) design can overcome residual confounding and other biases in observational studies, thereby strengthening causal inference for an exposure–outcome association by leveraging genetic variants as proxy indicators for an exposure [29]. In this study, we used the MR design to examine the associations of genetically predicted circulating α-tocopherol levels with BMD and risk of any type of fracture.
2. Methods
2.1. Selection of Genetic Variants
We used three uncorrelated single-nucleotide polymorphisms (SNPs) related to circulating α-tocopherol concentrations at the level of genome-wide significance (p < 5 × 10−8) in a meta-analysis of three genome-wide association studies comprising 7781 individuals of European ancestry [30]. The association estimates were adjusted for age, cancer status, and body mass index and, because it is well recognized that vitamin E levels are influenced by blood lipids, additional adjustment was made for total and high-density lipoprotein cholesterol. The three SNPs included rs964184 on 11q23.3 close to BUD13, ZNF259, and APOA1/C3/A4/A5 (p = 7.8 × 10−12), rs2108622 on 19pter-p13.11 close to CYP4F2 (p = 1.4 × 10−10), and rs11057830 on 12q24.31 close to SCARB1 (p = 8.2 × 10−9). These genetic variants explained around 1.7% of the variation in log serum α-tocopherol levels [30]. Mean (±standard deviation) α-tocopherol levels ranged from 11.9 (±3.4) mg/L to 19.1 (±9.7) mg/L in the included GWASs [30].
2.2. Summary-Level Data for Outcomes
Summary-level data for the associations between the α-tocopherol-related SNPs and estimated BMD (eBMD), using heel quantitative ultrasound in 426,824 participants and fracture (53,184 cases and 373,611 non-cases) were taken from GWASs based on data from UK Biobank [31]. The heel quantitative ultrasound method can measure BMD to a similar degree as dual-energy X-ray absorptiometry and is an inexpensive, easy to implement, and radiation-free technique [32]. Mean (±standard deviation [SD]) eBMD levels were 0.56 ± 0.12 g/cm2 in men and 0.51 ± 0.11 g/cm2 in women. Fractures were identified by hospital Episodes Statistics using ICD-10 codes (n = 20,122) and questionnaire-based self-reported data (n = 48,818). Omitted were fractures of the face and skull, hands and feet, atypical femoral fractures, periprosthetic fractures, restored fractures, and pathological fractures caused by malignancy [31]. The genetic estimates were adjusted for ancestry-informative genetic principal components 1 to 20, genotyping array, assessment center, sex, and age.
2.3. Two-Sample Summary-Level MR Analysis
A ratio estimate for each of the three SNPs was computed by dividing the beta coefficient for the SNP–eBMD or the SNP–fracture association by the beta coefficient for the SNP–α-tocopherol association. These ratio estimates were pooled in a fixed-effects inverse-variance weighted model to obtain the MR estimates per one SD increment of the association of serum α-tocopherol with BMD and fracture risk. The SD was estimated from a population-based Swedish cohort of men (n = 2047; https://www.pubcare.uu.se/ulsam/, (accessed on 1 June 2021)). Mean (±SD) serum α-tocopherol levels were 13.1 (±3.5) mg/L and 2.5 (±0.25) mg/L on the normal and log-transformed scale, respectively. Stata software (version 14.0) was used for the analyses.
2.4. Pleiotropy Assessment
We searched the PhenoScanner database [33] to assess whether the α-tocopherol-associated SNPs were associated with known risk factors for low BMD or fracture risk. We considered the following factors: body mass index, fat-free soft tissue body mass, height, type 2 diabetes, smoking, alcohol consumption, and steroid hormones, including estrogens, testosterone, and cortisol.
3. Results
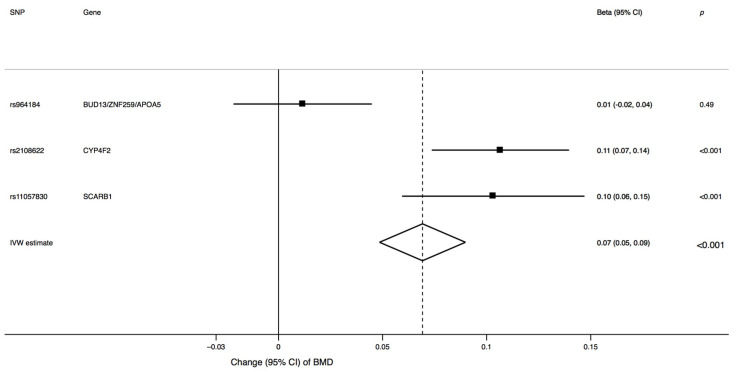
The characteristics of the three SNPs associated with α-tocopherol and their associations with eBMD and fracture risk are shown in Supplementary Table S1. Two of the three α-tocopherol-associated SNPs were strongly associated with eBMD (Figure 1). Genetically predicted one-SD increment in circulating α-tocopherol levels was associated with 0.07 (95% confidence interval, 0.05 to 0.09) g/cm2 higher eBMD (p < 0.001), which corresponds to >10% higher eBMD.
Figure 1.
Association between genetically predicted one-standard-deviation increase in circulating α-tocopherol and BMD. CI, confidence interval; BMD, bone mineral density; IVW, inverse, variance weighted; SNP, single-nucleotide polymorphism.
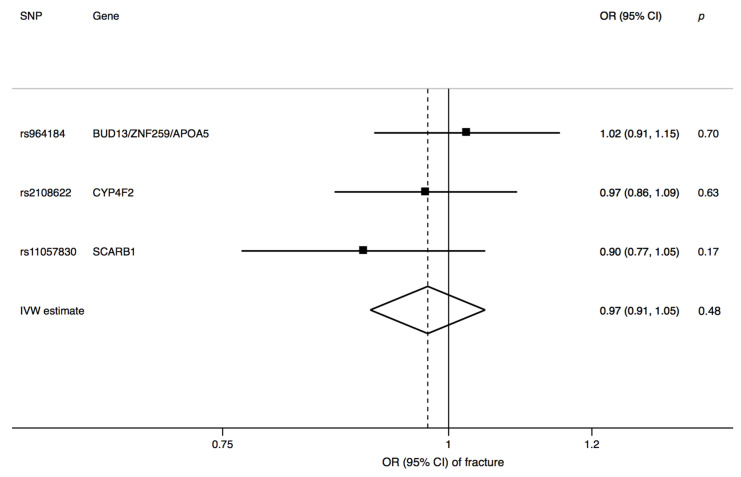
Genetically predicted circulating α-tocopherol was not associated with odds of fracture (odds ratio 0.97, 95% confidence interval, 0.91 to 1.05; p = 0.48) (Figure 2). None of the three SNPs was associated with known risk factors for low BMD and fracture at p <0.01.
Figure 2.
Association between genetically predicted one-standard-deviation increase in circulating α-tocopherol and fracture risk. CI, confidence interval; IVW, inverse, variance weighted; OR, odds ratio; SNP, single-nucleotide polymorphism.
4. Discussion
This MR investigation showed that genetically proxied higher circulating α-tocopherol levels were clearly and independently associated with greater eBMD. Our results are consistent with several observational studies that found a positive correlation between serum α-tocopherol and higher BMD [14,19,20,21,22]. We were unable to find a significant association between genetically predicted α-tocopherol levels and fracture risk. In cohort studies, strongest associations have been found with hip fracture as an outcome, not yet available for MR study designs.
Previous cohort studies reveal that both higher blood levels of α-tocopherol and higher dietary α-tocopherol intake are related to higher BMD and reduced risk of osteoporosis and fractures, including hip fractures [14,19,20,21]. At the time of the fracture event, hip fracture patients have been shown to have lower serum α-tocopherol concentrations compared with controls [22], and serum α-tocopherol concentrations after the hip fracture event are associated with lower levels of inflammatory markers [14,34]. In addition, higher circulating α-tocopherol concentrations are related to improved physical performance after a hip fracture event [14,35]. Interestingly, no association was found between bone mineral density and serum vitamin E concentrations in the Women’s Health Initiative Study [14,24]. Of notice, however [14], the women in that study had a mean total intake of vitamin E (including supplements) of about 30 mg/day [24], which is three times higher than the recommended intake.
Studies in animals have shown that supplementation with α-tocopherol improves fracture healing and may also improve osseointegration of metallic implants [14,15,36,37,38,39] These findings contrast with results in a Japanese study [17], which reported that high α-tocopherol intake was harmful for bone by stimulating bone resorption followed by a decrease in bone mass. The investigators supplemented young rodents (mice and rats) with vitamin E equivalent to a 30-fold higher dose than the normal intake recommended for these species [14,40]. Continued high-dose administration of α-tocopherol might be toxic [41] and results in appetite loss, in turn leading to impaired weight and skeletal growth. A high vitamin E intake may also adversely influence the use of vitamin D, leading to decreased bone mass [42]. In contrast, other experimental evidence indicates that a high vitamin E dose had positive effects on bone health in rodents [15,18,19], and thus, the findings presented in the study by Fujita and colleagues [17] displaying an adverse effect on bone health by high dosing of vitamin E could not later be replicated. Indeed, anti-osteoporotic properties of vitamin E have been demonstrated in different animal models [43]. Vitamin E modulates the levels of inflammatory mediators and reactive oxygen species, acting systemically and locally, with a potent regulatory role in bone metabolism [43]. Specifically, vitamin E plays an essential role in oxidative stress signaling, with effects on the receptor activator of nuclear factor kappa-B (RANK)/receptor activator of nuclear factor kappa-B ligand (RANKL)/osteoprotegerin (OPG) and Wnt/β-catenin systems, affecting osteoclast and osteoblast activity [43]. There are strong indications of an effect of oxidative stress on bone senescence [9,10,11,44,45,46]. Supporting the view that vitamin E dose is of importance also in humans, a meta-analysis of placebo-controlled randomized trials revealed that low-dosage vitamin E supplements can reduce all-cause mortality [47], whereas high-dosage vitamin E supplements, with a mean dose of 400 mg/day corresponding to 40 times the recommended intake [47], may in contrast lead to higher death rates [14,47].
A strength of our study is the MR design that reduced potential confounding and reverse causation bias and thus strengthened the causal inference in the association between circulating α-tocopherol and BMD. The current study was confined to participants of European origin. Thus, it is not likely that our findings were affected by a population stratification bias. The genetic instrument was not developed in the UK Biobank, while this large cohort was used for instrument–outcome association analyses to estimate a causal effect of blood levels of α-tocopherol on the outcomes BMD and fractures. An overlap in participants between the instrument-development and outcome samples can cause bias towards the risk factor–outcome association [48].
A limitation of our study is that few SNPs were available as genetic instruments for α-tocopherol, which limited the possibility to assess possible pleiotropy (i.e., where one genetic variant influences multiple phenotypes) through robust MR methods such as MR-Egger regression. Although none of the SNPs was associated with known risk factors for low BMD and fracture risk, the variants in the BUD13/ZNF259/APOA5 and CYP4F2 gene regions were associated with circulating phylloquinone (at p = 6 × 10−8 and p = 8.8 × 10−7, respectively) [49]. Nevertheless, there is little evidence that phylloquinone affects BMD [50,51,52]. A major limitation for the fracture analysis is the hitherto relatively young age of fracture cases in the UK Biobank, inclusion of self-reported fractures [53], and a mixture of different types of fractures. An MR study focusing on fragility fractures, especially hip fractures, would be of interest, but such GWAS data are not available.
5. Conclusions
In conclusion, our results strengthen the view that increasing circulating α-tocopherol is associated with higher BMD. A randomized clinical trial to investigate the effect on BMD and fracture risk of moderate doses corresponding to the daily recommended intake of vitamin E is warranted.
Acknowledgments
The authors acknowledge the participants and investigators of the UK Biobank study and thank the investigators of the genome-wide association studies providing summary-level data for α-tocopherol, BMD, and fracture.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13061940/s1, Table S1: Summary statistics data for the associations of the single-nucleotide polymorphisms with α-tocopherol and their associations with bone mineral density and any fracture.
Author Contributions
All authors have read and agreed to the published version of the manuscript. Conceptualization, K.M., S.C.L.; methodology, K.M., S.C.L.; formal analysis, S.C.L.; writing, K.M., S.C.L.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Swedish Ethical Review Authority (2019-02793, date of approval 2019-05-27).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data used in this study are publicly available summary statistics data, with relevant data available from cited studies. The summary statistics data analyzed in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Riggs B.L., Melton L.J.D. The prevention and treatment of osteoporosis. N. Engl. J. Med. 1992;327:620–627. doi: 10.1056/NEJM199208273270908. [DOI] [PubMed] [Google Scholar]
- 2.Michaelsson K., Nordstrom P., Nordstrom A., Garmo H., Byberg L., Pedersen N.L., Melhus H. Impact of hip fracture on mortality: A cohort study in hip fracture discordant identical twins. J. Bone Miner. Res. 2014;29:424–431. doi: 10.1002/jbmr.2029. [DOI] [PubMed] [Google Scholar]
- 3.Jarvinen T.L., Michaelsson K., Jokihaara J., Collins G.S., Perry T.L., Mintzes B., Musini V., Erviti J., Gorricho J., Wright J.M., et al. Overdiagnosis of bone fragility in the quest to prevent hip fracture. BMJ. 2015;350:h2088. doi: 10.1136/bmj.h2088. [DOI] [PubMed] [Google Scholar]
- 4.Kanis J.A., Johnell O., Oden A., Jonsson B., De Laet C., Dawson A. Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone. 2000;27:585–590. doi: 10.1016/S8756-3282(00)00381-1. [DOI] [PubMed] [Google Scholar]
- 5.Marshall D., Johnell O., Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stone K.L., Seeley D.G., Lui L.Y., Cauley J.A., Ensrud K., Browner W.S., Nevitt M.C., Cummings S.R. Osteoporotic Fractures Research, G. BMD at multiple sites and risk of fracture of multiple types: Long-term results from the Study of Osteoporotic Fractures. J. Bone Miner. Res. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 7.Michaelsson K., Melhus H., Ferm H., Ahlbom A., Pedersen N.L. Genetic liability to fractures in the elderly. Arch. Intern. Med. 2005;165:1825–1830. doi: 10.1001/archinte.165.16.1825. [DOI] [PubMed] [Google Scholar]
- 8.Moayyeri A., Hammond C.J., Hart D.J., Spector T.D. Effects of age on genetic influence on bone loss over 17 years in women: The Healthy Ageing Twin Study (HATS) J. Bone Miner. Res. 2012;27:2170–2178. doi: 10.1002/jbmr.1659. [DOI] [PubMed] [Google Scholar]
- 9.Manolagas S.C., Parfitt A.M. What old means to bone. Trends Endocrinol. Metab. TEM. 2010;21:369–374. doi: 10.1016/j.tem.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corrado A., Cici D., Rotondo C., Maruotti N., Cantatore F.P. Molecular Basis of Bone Aging. Int. J. Mol. Sci. 2020;21:3679. doi: 10.3390/ijms21103679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marie P.J. Bone cell senescence: Mechanisms and perspectives. J. Bone Miner. Res. 2014;29:1311–1321. doi: 10.1002/jbmr.2190. [DOI] [PubMed] [Google Scholar]
- 12.Trajanoska K., Morris J.A., Oei L., Zheng H.F., Evans D.M., Kiel D.P., Ohlsson C., Richards J.B., Rivadeneira F., Consortium G.G., et al. Assessment of the genetic and clinical determinants of fracture risk: Genome wide association and mendelian randomisation study. BMJ. 2018;362:k3225. doi: 10.1136/bmj.k3225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerullo F., Gambassi G., Cesari M. Rationale for antioxidant supplementation in sarcopenia. J. Aging Res. 2012;2012:316943. doi: 10.1155/2012/316943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michaelsson K., Wolk A., Byberg L., Arnlov J., Melhus H. Intake and serum concentrations of alpha-tocopherol in relation to fractures in elderly women and men: 2 cohort studies. Am. J. Clin. Nutr. 2014;99:107–114. doi: 10.3945/ajcn.113.064691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shuid A.N., Mohamad S., Muhammad N., Fadzilah F.M., Mokhtar S.A., Mohamed N., Soelaiman I.N. Effects of alpha-tocopherol on the early phase of osteoporotic fracture healing. J. Orthop. Res. 2011;29:1732–1738. doi: 10.1002/jor.21452. [DOI] [PubMed] [Google Scholar]
- 16.Mehat M.Z., Shuid A.N., Mohamed N., Muhammad N., Soelaiman I.N. Beneficial effects of vitamin E isomer supplementation on static and dynamic bone histomorphometry parameters in normal male rats. J. Bone Miner. Metab. 2010;28:503–509. doi: 10.1007/s00774-010-0159-2. [DOI] [PubMed] [Google Scholar]
- 17.Fujita K., Iwasaki M., Ochi H., Fukuda T., Ma C., Miyamoto T., Takitani K., Negishi-Koga T., Sunamura S., Kodama T., et al. Vitamin E decreases bone mass by stimulating osteoclast fusion. Nat. Med. 2012;18:589–594. doi: 10.1038/nm.2659. [DOI] [PubMed] [Google Scholar]
- 18.Kasai S., Ito A., Shindo K., Toyoshi T., Bando M. High-Dose alpha-Tocopherol Supplementation Does Not Induce Bone Loss in Normal Rats. PLoS ONE. 2015;10:e0132059. doi: 10.1371/journal.pone.0132059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chin K.Y., Ima-Nirwana S. The effects of alpha-tocopherol on bone: A double-edged sword? Nutrients. 2014;6:1424–1441. doi: 10.3390/nu6041424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holvik K., Gjesdal C.G., Tell G.S., Grimnes G., Schei B., Apalset E.M., Samuelsen S.O., Blomhoff R., Michaelsson K., Meyer H.E. Low serum concentrations of alpha-tocopherol are associated with increased risk of hip fracture. A NOREPOS study. Osteoporos. Int. 2014;25:2545–2554. doi: 10.1007/s00198-014-2802-6. [DOI] [PubMed] [Google Scholar]
- 21.Mulligan A.A., Hayhoe R.P.G., Luben R.N., Welch A.A. Positive Associations of Dietary Intake and Plasma Concentrations of Vitamin E with Skeletal Muscle Mass, Heel Bone Ultrasound Attenuation and Fracture Risk in the EPIC-Norfolk Cohort. Antioxidants. 2021;10:159. doi: 10.3390/antiox10020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D’Adamo C.R., Shardell M.D., Hicks G.E., Orwig D.L., Hochberg M.C., Semba R.D., Yu-Yahiro J.A., Ferrucci L., Magaziner J.S., Miller R.R. Serum vitamin E concentrations among highly functioning hip fracture patients are higher than in nonfracture controls. Nutr. Res. 2011;31:205–214. doi: 10.1016/j.nutres.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang T.C., Duthie G.G., Aucott L.S., Macdonald H.M. Vitamin E homologues alpha- and gamma-tocopherol are not associated with bone turnover markers or bone mineral density in peri-menopausal and post-menopausal women. Osteoporos. Int. 2016;27:2281–2290. doi: 10.1007/s00198-015-3470-x. [DOI] [PubMed] [Google Scholar]
- 24.Wolf R.L., Cauley J.A., Pettinger M., Jackson R., Lacroix A., Leboff M.S., Lewis C.E., Nevitt M.C., Simon J.A., Stone K.L., et al. Lack of a relation between vitamin and mineral antioxidants and bone mineral density: Results from the Women’s Health Initiative. Am. J. Clin. Nutr. 2005;82:581–588. doi: 10.1093/ajcn/82.3.581. [DOI] [PubMed] [Google Scholar]
- 25.Jenab M., Salvini S., van Gils C.H., Brustad M., Shakya-Shrestha S., Buijsse B., Verhagen H., Touvier M., Biessy C., Wallstrom P., et al. Dietary intakes of retinol, beta-carotene, vitamin D and vitamin E in the European Prospective Investigation into Cancer and Nutrition cohort. Eur. J. Clin. Nutr. 2009;63(Suppl. 4):S150–S178. doi: 10.1038/ejcn.2009.79. [DOI] [PubMed] [Google Scholar]
- 26.Larsson S.C., Michaelsson K., Burgess S. Mendelian randomization in the bone field. Bone. 2019;126:51–58. doi: 10.1016/j.bone.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larsson S.C., Melhus H., Michaelsson K. Circulating Serum 25-Hydroxyvitamin D Levels and Bone Mineral Density: Mendelian Randomization Study. J. Bone Miner. Res. 2018;33:840–844. doi: 10.1002/jbmr.3389. [DOI] [PubMed] [Google Scholar]
- 28.Larsson S.C., Burgess S., Michaelsson K. Association of Genetic Variants Related to Serum Calcium Levels With Coronary Artery Disease and Myocardial Infarction. JAMA. 2017;318:371–380. doi: 10.1001/jama.2017.8981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stephen Burgess S.G.T. Mendelian Randomization: Methods for Using Genetic Variants in Causal Estimation. Chapman and Hall/CRC; Boca Raton, FL, USA: 2015. [Google Scholar]
- 30.Major J.M., Yu K., Wheeler W., Zhang H., Cornelis M.C., Wright M.E., Yeager M., Snyder K., Weinstein S.J., Mondul A., et al. Genome-wide association study identifies common variants associated with circulating vitamin E levels. Hum. Mol. Genet. 2011;20:3876–3883. doi: 10.1093/hmg/ddr296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris J.A., Kemp J.P., Youlten S.E., Laurent L., Logan J.G., Chai R.C., Vulpescu N.A., Forgetta V., Kleinman A., Mohanty S.T., et al. An atlas of genetic influences on osteoporosis in humans and mice. Nat. Genet. 2019;51:258–266. doi: 10.1038/s41588-018-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim S.K. Identification of 613 new loci associated with heel bone mineral density and a polygenic risk score for bone mineral density, osteoporosis and fracture. PLoS ONE. 2018;13:e0200785. doi: 10.1371/journal.pone.0200785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kamat M.A., Blackshaw J.A., Young R., Surendran P., Burgess S., Danesh J., Butterworth A.S., Staley J.R. PhenoScanner V2: An expanded tool for searching human genotype-phenotype associations. Bioinformatics. 2019 doi: 10.1093/bioinformatics/btz469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.D’Adamo C.R., Miller R.R., Shardell M.D., Orwig D.L., Hochberg M.C., Ferrucci L., Semba R.D., Yu-Yahiro J.A., Magaziner J., Hicks G.E. Higher serum concentrations of dietary antioxidants are associated with lower levels of inflammatory biomarkers during the year after hip fracture. Clin. Nutr. 2012 doi: 10.1016/j.clnu.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Adamo C.R., Miller R.R., Hicks G.E., Orwig D.L., Hochberg M.C., Semba R.D., Yu-Yahiro J.A., Ferrucci L., Magaziner J., Shardell M.D. Serum vitamin E concentrations and recovery of physical function during the year after hip fracture. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:784–793. doi: 10.1093/gerona/glr057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Durak K., Sonmez G., Sarisozen B., Ozkan S., Kaya M., Ozturk C. Histological assessment of the effect of alpha-tocopherol on fracture healing in rabbits. J. Int. Med. Res. 2003;31:26–30. doi: 10.1177/147323000303100104. [DOI] [PubMed] [Google Scholar]
- 37.Turk C., Halici M., Guney A., Akgun H., Sahin V., Muhtaroglu S. Promotion of fracture healing by vitamin E in rats. J. Int. Med. Res. 2004;32:507–512. doi: 10.1177/147323000403200508. [DOI] [PubMed] [Google Scholar]
- 38.Kurklu M., Yildiz C., Kose O., Yurttas Y., Karacalioglu O., Serdar M., Deveci S. Effect of alpha-tocopherol on bone formation during distraction osteogenesis: A rabbit model. J. Orthop. Traumatol. 2011;12:153–158. doi: 10.1007/s10195-011-0145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Savvidis M., Papavasiliou K., Taitzoglou I., Giannakopoulou A., Kitridis D., Galanis N., Vrabas I., Tsiridis E. Postoperative Administration of Alpha-tocopherol Enhances Osseointegration of Stainless Steel Implants: An In Vivo Rat Model. Clin. Orthop. Relat. Res. 2020;478:406–419. doi: 10.1097/CORR.0000000000001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.National Research Council (U.S.) Nutrient Requirements of Laboratory Animals. 4th rev. ed. National Academy of Sciences; Washington, DC, USA: 1995. Subcommittee on Laboratory Animal Nutrition. p. xii, 173p. [Google Scholar]
- 41.Yasunaga T., Kato H., Ohgaki K., Inamoto T., Hikasa Y. Effect of vitamin E as an immunopotentiation agent for mice at optimal dosage and its toxicity at high dosage. J. Nutr. 1982;112:1075–1084. doi: 10.1093/jn/112.6.1075. [DOI] [PubMed] [Google Scholar]
- 42.Aburto A., Britton W.M. Effects of different levels of vitamins A and E on the utilization of cholecalciferol by broiler chickens. Poult. Sci. 1998;77:570–577. doi: 10.1093/ps/77.4.570. [DOI] [PubMed] [Google Scholar]
- 43.Wong S.K., Mohamad N.V., Ibrahim N., Chin K.Y., Shuid A.N., Ima-Nirwana S. The Molecular Mechanism of Vitamin E as a Bone-Protecting Agent: A Review on Current Evidence. Int. J. Mol. Sci. 2019;20:1453. doi: 10.3390/ijms20061453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melhus H., Michaelsson K., Holmberg L., Wolk A., Ljunghall S. Smoking, antioxidant vitamins, and the risk of hip fracture. J. Bone Miner. Res. 1999;14:129–135. doi: 10.1359/jbmr.1999.14.1.129. [DOI] [PubMed] [Google Scholar]
- 45.Basu S., Michaelsson K., Olofsson H., Johansson S., Melhus H. Association between oxidative stress and bone mineral density. Biochem. Biophys. Res. Commun. 2001;288:275–279. doi: 10.1006/bbrc.2001.5747. [DOI] [PubMed] [Google Scholar]
- 46.Ostman B., Michaelsson K., Helmersson J., Byberg L., Gedeborg R., Melhus H., Basu S. Oxidative stress and bone mineral density in elderly men: Antioxidant activity of alpha-tocopherol. Free Radic. Biol. Med. 2009;47:668–673. doi: 10.1016/j.freeradbiomed.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 47.Miller E.R., 3rd, Pastor-Barriuso R., Dalal D., Riemersma R.A., Appel L.J., Guallar E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 48.Davies N.M., Holmes M.V., Davey Smith G. Reading Mendelian randomisation studies: A guide, glossary, and checklist for clinicians. BMJ. 2018;362:k601. doi: 10.1136/bmj.k601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zwakenberg S.R., Burgess S., Sluijs I., Weiderpass E., Consortium E.-C., Beulens J.W.J., van der Schouw Y.T. Circulating phylloquinone, inactive Matrix Gla protein and coronary heart disease risk: A two-sample Mendelian Randomization study. Clin. Nutr. 2020;39:1131–1136. doi: 10.1016/j.clnu.2019.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bolton-Smith C., McMurdo M.E., Paterson C.R., Mole P.A., Harvey J.M., Fenton S.T., Prynne C.J., Mishra G.D., Shearer M.J. Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J. Bone Miner. Res. 2007;22:509–519. doi: 10.1359/jbmr.070116. [DOI] [PubMed] [Google Scholar]
- 51.Cheung A.M., Tile L., Lee Y., Tomlinson G., Hawker G., Scher J., Hu H., Vieth R., Thompson L., Jamal S., et al. Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): A randomized controlled trial. PLoS Med. 2008;5:e196. doi: 10.1371/journal.pmed.0050196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mott A., Bradley T., Wright K., Cockayne E.S., Shearer M.J., Adamson J., Lanham-New S.A., Torgerson D.J. Effect of vitamin K on bone mineral density and fractures in adults: An updated systematic review and meta-analysis of randomised controlled trials. Osteoporos. Int. 2019;30:1543–1559. doi: 10.1007/s00198-019-04949-0. [DOI] [PubMed] [Google Scholar]
- 53.Siggeirsdottir K., Aspelund T., Sigurdsson G., Mogensen B., Chang M., Jonsdottir B., Eiriksdottir G., Launer L.J., Harris T.B., Jonsson B.Y., et al. Inaccuracy in self-report of fractures may underestimate association with health outcomes when compared with medical record based fracture registry. Eur. J. Epidemiol. 2007;22:631–639. doi: 10.1007/s10654-007-9163-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data used in this study are publicly available summary statistics data, with relevant data available from cited studies. The summary statistics data analyzed in this study are available on request from the corresponding author.