Abstract
Tendons are dense connective tissues that transmit muscle forces to the skeleton. After adult injury, healing potential is generally poor and dominated by scar formation. Although the immune response is a key feature of healing, the specific immune cells and signals that drive tendon healing have not been fully defined. In particular, the immune regulators underlying tendon regeneration are almost completely unknown due to a paucity of tendon regeneration models. Using a mouse model of neonatal tendon regeneration, we screened for immune-related markers and identified upregulation of several genes associated with inflammation, macrophage chemotaxis, and TGFβ signaling after injury. Depletion of macrophages using AP20187 treatment of MaFIA mice resulted in impaired functional healing, reduced cell proliferation, reduced ScxGFP+ neo-tendon formation, and altered tendon gene expression. Collectively, these results show that inflammation is a key component of neonatal tendon regeneration and demonstrate a requirement for macrophages in effective functional healing.
Keywords: tendon healing, macrophages, regeneration, mouse
INTRODUCTION
Tendons are dense, connective tissues that connect muscle to the skeleton (1). Injury to tendons is extremely common and results in pain and sustained loss of mechanical function (2–5). This is largely due to the poor healing capacity of tendons and inability restore native matrix architecture since tendons heal by disorganized scar formation (5, 6). Currently, treatment options remain few and are limited to physical therapy or surgical repair, which do not regenerate tendon structure or function. Identifying novel biological mechanisms that improve functional tendon regeneration is therefore an important unmet need.
Toward that end, we previously established a neonatal mouse model of functional tendon regeneration after Achilles tendon transection without repair (7). We found that neonatal tendon regeneration was characterized by early proliferation of tendon cells, followed by their recruitment into the injury site to form a tenogenic neo-tendon. In the absence of tendon cell recruitment, functional properties were not restored (8). Using a similar injury model in adult mice, we also found that adult tendon healing was characterized by fibrotic scar formation, minimal tendon cell proliferation, and persistently reduced functional properties (7), consistent with studies from other groups (9–11).
Although the immune response is a key aspect of tendon healing, the specific immune cells and signals underlying tendon healing have yet to be fully defined. It is generally accepted that tendon healing is initiated by infiltration of pro-inflammatory macrophages (M1-like macrophages) followed by resolution of inflammation by alternatively activated macrophages (M2-like macrophages) (12–15). However, the role of these macrophage populations in promoting or inhibiting tendon healing remains unclear (16). While therapeutic strategies that enhance the presence of M2-like macrophages appear to promote functional tendon healing (17, 18), other studies suggest that increased M2-like macrophage polarization can also drive fibrotic scar formation (19). Most of these studies are correlative however, and there are still very few studies that directly test the role of macrophages in tendon healing via ablation or depletion. One well-established method for macrophage depletion is delivery of liposomal clodronate (20). In adult mouse models of Achilles tendon transection and repair, macrophage depletion by clodronate resulted in reduced cell proliferation and improved mechanical properties (21, 22). Similarly, clodronate depletion in an ACL reconstruction rat model showed improved mechanical properties at the tendon-bone interface (23). Despite these results suggesting that macrophages impair adult functional healing, there is also evidence indicating that increased macrophage recruitment can also improve tensile properties after adult tendon injury (24). To date, the role of neonatal macrophages in tendon healing have not been investigated.
To address these open questions, we determined the immune response during neonatal tendon healing by screening immune-related gene expression and tested the requirement for macrophages using an established genetic model of macrophage depletion to inducibly ablate macrophages. We hypothesized that neonatal tendons would exhibit an anti-inflammatory immune response after injury and that macrophage depletion would impair functional regeneration.
MATERIALS AND METHODS
Experimental procedures
For experiments, the transgenic Macrophage-Fas-Induced Apoptosis (MaFIA) mouse line (25) and ScxGFP tendon reporter line (26) were used. Although the MaFIA line allows GFP-detection of Csfr1-expressing monocytes and macrophages, we were only able detect MaFIAGFP by flow cytometry and not by fluorescence microscopy of either spleen or tendon. Flow cytometry was therefore used to quantify macrophage depletion in mice containing only the MaFIA allele. For other experiments, ScxGFP was incorporated into the MaFIA background to enable ScxGFP cell visualization with macrophage depletion (MaFIA/ScxGFP). EdU was given at 0.05 mg 2 hours prior to harvest to label proliferating cells. Global macrophage depletion was carried out using the homodimerizer AP20187 at 10 mg/kg in carrier solution by subcutaneous injection (Cat. # 635058, Clontech) with carrier-treated controls (4% EtOH, 10% PEG-400, 1.7% Tween20) injected in parallel. Littermates from the same litter were evenly split between AP20187-treatment or carrier-treatment. Full Achilles tendon transection without repair was carried out in neonates at postnatal day 5 after birth (P5), with male and female mice distributed evenly between groups. Transection injuries were induced in the mid-substance region. All procedures were approved by the Institutional Animal Care and Use Committee at Mount Sinai. Mice were sacrificed at days 3 and 14 post-injury (D3, D14) for immunofluorescence and at days 28 and 56 post-injury (D28, D56) for functional analyses.
Flow cytometry quantification
Single cell suspensions were generated from Achilles tendons of MaFIA mice by enzymatic digestion with a solution containing 1 mg/mL collagenase I (Cat. # LS004196, Worthington Biochemical) and 5 mg/mL collagenase IV (Cat. # LS004188, Worthington Biochemical) for 2.5 hrs at 37C. Cells were then stained 1:1000 with DAPI (Cat. # D1306, ThermoFisher) in 2% FBS in PBS to detect live cells. Flow cytometry was carried out on an LSRIIA machine (BD Sciences) using FACSDiva software and analyses using FCS Express 7. Gating for MaFIAGFP was performed using control cells isolated in parallel from a wild type mouse with no GFP.
Immunofluorescence, EdU detection, and fluorescence microscopy
Limbs were immediately fixed in 4% PFA after harvest for 24 hours at 4°C and then decalcified in 50 mM EDTA for 1–2 weeks at 4°C. To embed, limbs were incubated in 5% sucrose (1 hour) and 30% sucrose (overnight) at 4°C and then embedded in OCT medium (Cat. # 23-730, Fisher Scientific). Transverse cryosections (12 μm) were collected in alternating slides. Immunostaining for macrophages was carried out using an antibody against the global macrophage marker F4/80 (Cat. # 14-4801, Affymetrix) or the M2 macrophage marker CD206 (Cat. # 141711, Biolegend) and secondary detection by Cy5 (Cat. # 712-175-150, Jackson ImmunoResearch). Immunostaining for αSMA cells was carried out using anti-αSMA conjugated to Cy3 (Cat. # C6198, Sigma). Immunostaining for laminin was carried out using anti-Laminin (L9393, Sigma) and secondary detection by Cy3 (Cat. # 111-165-003, Jackson Immunoresearch). EdU labeling was detected with the Click it EdU kit in accordance with manufacturer’s instructions (Cat. # C10340, Life Technologies). Fluorescence images of EdU and ScxGFP were acquired using the Zeiss Axio Imager with optical sectioning by Apotome. Cell quantification was performed in Image J software using the CellCounter plugin. Immunostaining intensity was also performed in Image J by quantifying mean intensity within selected regions of interest. All images for quantifications were taken at the same exposure and image manipulations applied equally across samples.
RNA isolation, reverse transcription, and qRT-PCR
RNA was extracted from Achilles tendons using Trizol/chloroform. cDNA was synthesized by reverse transcription using the SuperScript VILO master mix (Cat. # 11755050, Invitrogen). The TaqMan Array Mouse Immune Panel (Cat. # 4414079, Thermo Fisher) with Taqman Fast Advanced Master Mix (Cat. # 4444556, Thermo Fisher) was used to screen 92 genes related to immune response. Gene expression was normalized to Gapdh and analysed using the 2−ΔΔCt method relative to uninjured control tendons. qPCR was carried out by Mount Sinai’s core facilities using SYBR PCR Master Mix (Cat. # 4309155, Thermo Fisher) and gene expression calculated using the 2−ΔΔCt method relative to Gapdh and carrier-treated control tendons at D3. Primers for Il1β (FWD: AGTTGACGGACCCCAAAAGAT; REV: GTTGATGTGCTGCTGCGAGA) and Il10 (FWD: ATTTGAATTCCCTGGGTGAGAAG; REV: CACAGGGGAGAAATCGATGACA) were used. Tendon gene primers were previously described (7).
Gait analysis
On D28, mice were gaited at 10 cm/s for 3–4 s using the DigiGait Imaging System (Mouse Specifics Inc.). A high-speed digital camera was used to capture forelimb paw positions and parameters previously validated for mouse Achilles tendon injury (Swing, Brake, and Propel) were then calculated (7). All parameters were normalized to Stride length to account for differences in animal size since male and female mice were used.
Biomechanical testing
For biomechanical testing, Achilles tendons were dissected at D56, wrapped in PBS-soaked gauze and frozen at −20C until time of testing. Tensile testing was performed in PBS at room temperature using custom 3D printed grips to secure the calcaneus bone and Achilles tendon (27, 28). Tendons were preloaded to 0.05N for ~1 min followed by ramp to failure at 1%/s.
Statistical analysis
Quantitative results are presented as mean±standard deviation. For multi-group comparisons, two way ANOVA was used with independent variables of treatment (carrier vs AP20187) or injury with Tukey’s post-hoc testing (Graphpad Prism). All other analyses were analyzed using Students t-tests. Significant outliers were detected using Grubb’s test (Graphpad Prism). Significance was determined at p<0.05.
RESULTS
Neonatal tendon injury activates an early pro-inflammatory immune response
To define the immune response associated with neonatal tendon regeneration, we carried out a gene expression screen using the Taqman Array Mouse Immune Response panel comprising 96 genes. Neonatal injured and contralateral uninjured Achilles tendons were screened at 3 days post-injury (D3), prior to recruitment of tenogenic cells in the gap space. While we initially expected neonatal injured tendons to exhibit a minimal immune response, we found 27 genes that were upregulated after injury (≥2-fold, p<0.05, Table 1) with 4 genes that were downregulated (≤−2-fold, p<0.05, Table 1). Upregulated markers included genes associated with macrophages and monocyte/macrophage chemotaxis (Ccl2, Ccl3, Ccr2, Ccr7, Cd68) and T cells (Tbx21, Cd3e, Ctla4, Cxcr3). Surprisingly, there was a robust inflammatory response, indicated by the upregulation of several pro-inflammatory cytokines (Il6, Il1β, Il12β, Il18); however, at this timepoint, the anti-inflammatory cytokine Il10 was also upregulated. In addition to upregulation of Tgfβ1, we detected significant downregulation of Ski, a known negative regulator of TGFβ signaling. Collectively, our qPCR screen indicates that neonates mount a robust pro-inflammatory immune response after injury that may be driven by recruitment of macrophages and T cells.
Table 1:
Differentially expressed genes detected by Taqman Array Mouse Immune Response panel in response to neonatal tendon injury at D3 post-injury.
| Gene | Fold Change (Up-regulated) | p value |
|---|---|---|
| Gzmb | 183.2675 | 0.045003 |
| Ccl5 | 38.95 | 0.000215 |
| Il6 | 23.5654 | 0.026617 |
| Prf1 | 23.4663 | 0.006136 |
| Tbx21 | 22.367 | 0.003557 |
| Sele | 18.1162 | 0.028513 |
| Tnfrsf18 | 14.0241 | 0.009632 |
| Cd3e | 13.907 | 0.006046 |
| Ctla4 | 13.7629 | 0.00171 |
| Vcam1 | 13.5854 | 0.013771 |
| Il1β | 11.77 | 0.000642 |
| Il10 | 11.3756 | 0.002664 |
| Ccl3 | 10.1302 | 0.017634 |
| Ccl2 | 10.0745 | 0.024852 |
| Ccr7 | 9.366 | 0.000269 |
| Fasl | 7.1758 | 0.001069 |
| Il12β | 6.952 | 0.02003 |
| H2-Eb1 | 6.9387 | 0.000538 |
| Cd80 | 5.7704 | 0.030404 |
| Ccr2 | 5.7292 | 0.005281 |
| Cxcr3 | 4.0058 | 0.010695 |
| Csf2 | 3.737 | 0.009016 |
| Stat1 | 3.5504 | 0.040912 |
| Il18 | 3.2386 | 0.045934 |
| Cd68 | 3.1542 | 0.003324 |
| Hmox1 | 2.4951 | 0.011452 |
| Tgfb1 | 2.1161 | 0.011681 |
| Genes | Fold Change (Down-regulated) | p value |
| Nos2 | −2.3753 | 0.005565 |
| Ski | −2.23 | 0.000631 |
| Vegfa | −2.1495 | 0.009757 |
| Ace | −2.0735 | 0.040496 |
Increased macrophages and macrophage localization with tenocytes after neonatal tendon injury
Analysis of the pan-macrophage marker F4/80 in transverse cryosections from uninjured tendon (P8 post-birth) showed tendon-resident macrophages are normally sparsely detected in the tendon periphery as well as in fascia surrounding the tendon (Figure 1A, B). By D3 post-injury, we observed a dramatic increase in F4/80+ cells surrounding the injured tendon. Comparison of sections taken from the tendon stub far from the cut site and sections close to the cut site showed that F4/80+ macrophages were only located in close proximity to ScxGFP+ tendon cells in sections near the injury site (Figure 1C).
Figure 1: Macrophages are recruited after neonatal tendon injury and localize to cut site tendon cells.
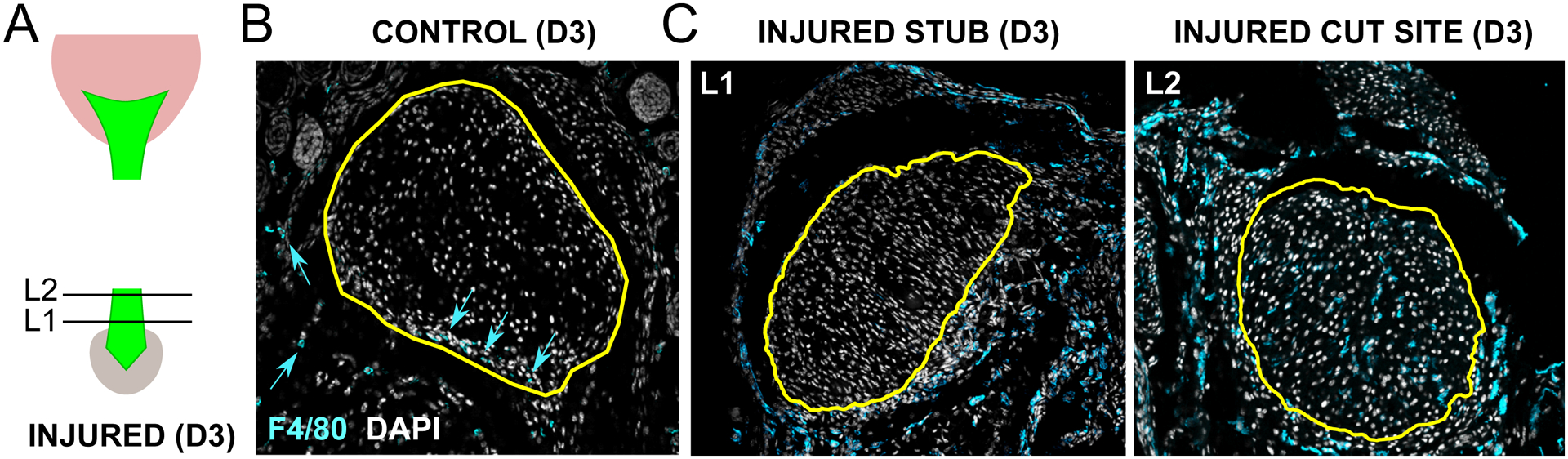
(A) Transverse cryosections were collected from tendon stub (L1) and cut site (L2) levels shown in schematic. Immunostaining for pan-macrophage marker F4/80 of (B) uninjured control and (C) injured tendon at D3 post-injury. Yellow outline indicates tendon region. Blue arrows point to sparse F4/80+ tendon-resident macrophages residing in tendon periphery. Scale bar: 100 μm.
Repeated depletion of macrophages during postnatal growth resulted in adverse systemic effects unrelated to tendon healing
The close proximity of macrophages to tenocytes suggested potential interactions between these cells. We therefore tested the functional role of macrophages on neonatal tendon regeneration using the Macrophage Fas-Induced Apoptosis transgenic mutant (MaFIA) in which monocytes and macrophages express a mutant human FK506 binding protein. Macrophages could therefore be inducibly and reversibly depleted by delivery of the homodimerizer AP20187. For all experiments, AP20187 or carrier was injected for three consecutive days (P2, P3, P4) prior to Achilles tendon transection at P5 to deplete monocytes and macrophages prior to injury. We then tested three different regimens of macrophage depletion after P5 injury, including twice a week, once a week, or a single booster dose at D7 post-injury. When AP20187 was delivered twice a week following injury, we observed swelling of both injured and uninjured hindlimbs of treated mice and impaired growth (Figure 2A–C). Once a week treatment improved survival of mice out to nearly D56, however restricted growth was observed beginning at d35 (Figure 2D–F). Swelling was also observed in both injured and uninjured hindlimbs of surviving mice at d56. Finally, we treated mice with a single booster injection at d7 and observed no difference in growth and no swelling of hindlimbs (Figure 3A–C). Since the single booster treatment showed minimal systemic effects and has also been reported in other studies (29), we carried out subsequent analyses using this treatment regimen. Analysis of ablation efficiency by flow cytometry of MaFIAGFP+ showed 86% reduction in macrophages at d3 in the injured tendon and 93% reduction in the spleen following AP20187 treatment (Figure 3D).
Figure 2: Prolonged and repeated depletion of macrophages results in adverse systemic effects.
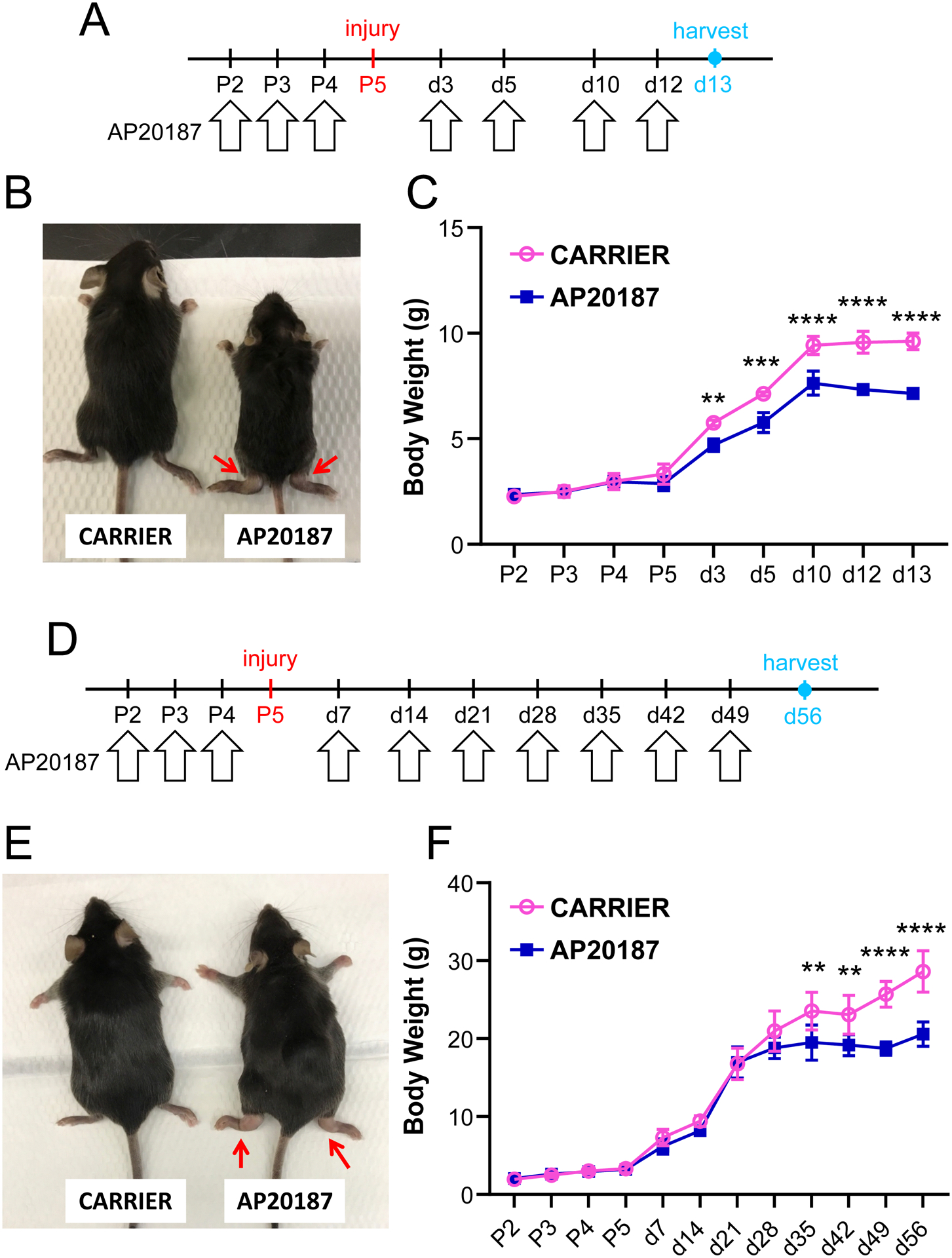
(A) Schematic showing twice a week dosing regimen of AP20187 after postnatal day P5 Achilles tendon injury. (B) AP20187-treated mice were smaller and showed abnormal swelling of tarsal regions at D13 post-injury (red arrows) with (C) impaired weight gain (n=3–5). (D) Schematic showing once a week dosing regimen of AP20187 after postnatal day P5 Achilles tendon injury. (E) AP20187-treated mice also developed abnormal swelling of tarsal regions at D56 post-injury (red arrows) and (F) impaired weight gain at later timepoints (n=3–5). Data reported as mean±stdev and analyzed by 2-way ANOVA with Tukey’s post-hoc test. ** p<0.01; *** p<0.001 **** p<0.0001.
Figure 3: Validation of macrophage depletion by AP20187.
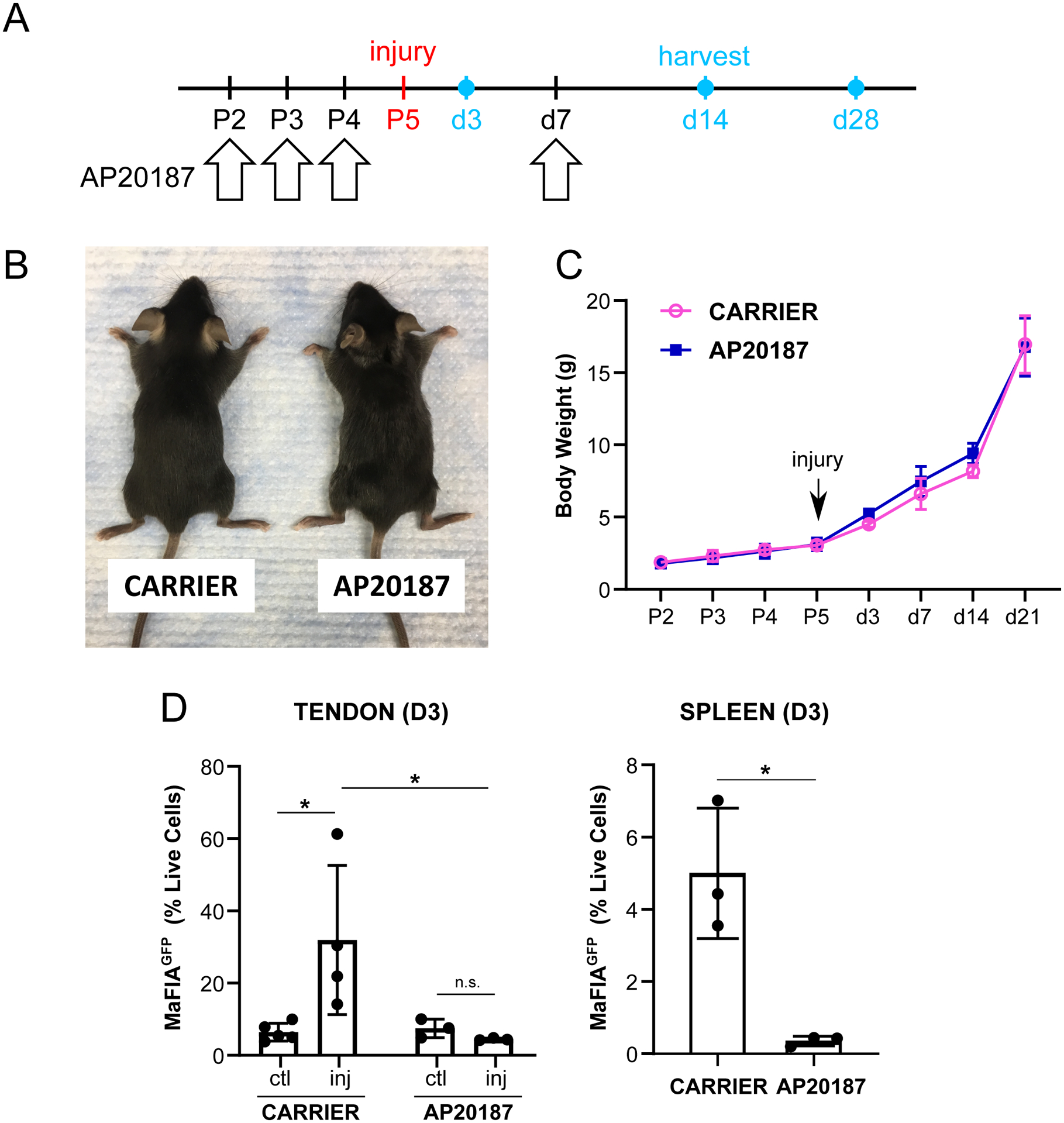
(A) Schematic showing a single booster dose of AP20187 at D7 post-injury after postnatal day P5 Achilles tendon injury. (B) AP20187-treated mice did not demonstrate swelling of hindlimb tarsal regions compared to carrier-treated at D28 post-injury. (C) Weight gain was comparable at all timepoints (n=4–9). (D) Flow cytometry quantification of MaFIAGFP cells at D3 post-injury in tendon and spleen (n=3–4). Data reported as mean±stdev. Body weight and tendon MaFIAGFP data analyzed by 2-way ANOVA with Tukey’s posthoc test. Spleen MaFIAGFP data analyzed by Student’s t-test. * p<0.05. n.s. indicates p>0.1.
Macrophages rebound by D14 in AP21087-treated tendons
To determine the dynamics of macrophage recruitment over time, we immunostained for all macrophages using an antibody against F4/80. Consistent with flow cytometry quantification, F4/80 staining at D3 was higher for carrier-treated injured tendons compared to AP20187-treated injured tendons (Figure 4). By D14 post-injury, macrophage levels were significantly decreased in carrier-treated tendons but were higher in AP20187-treated tendons. Since the final dose of AP20187 was given at D7 post-injury, this suggested a potential rebounding effect with the cessation of drug. To determine macrophage type, we next immunostained for M2-like macrophages using anti-CD206. Qualitative analyses indicated more M2-like macrophages in carrier-treated tendons at D3 with minimal differences at D14 (Supplemental Figure 1). Since F4/80 staining also showed enhanced presence of macrophages in AP20187-treated tendons at D14, our data suggest that there may be more pro-inflammatory, M1-like macrophages in these tendons. Collectively, this data indicate that macrophage recruitment to the injury site is dysregulated with ablation during the first two weeks of healing.
Figure 4: Dynamics of macrophage recruitment were dysregulated with AP20187 treatment.
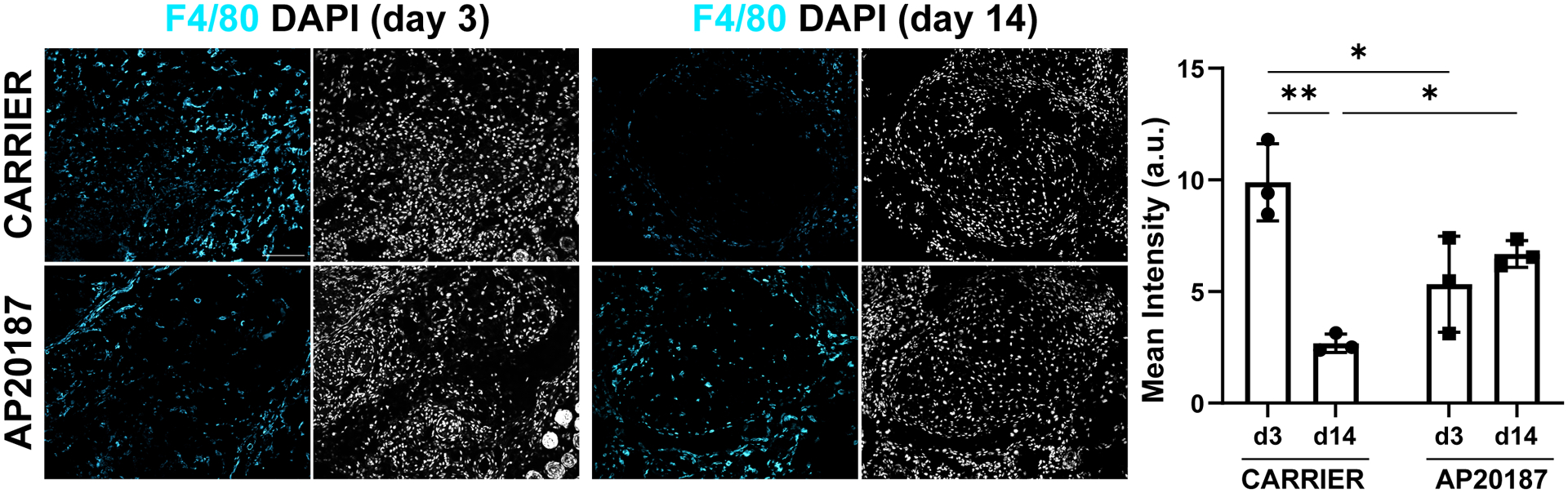
Quantification of F4/80 immunostaining for macrophages showed disrupted presence of macrophages with AP20187 at D3 and D14 post-injury. Data reported as mean±stdev and analyzed by 2-way ANOVA with Tukey’s post-hoc tests. * p<0.05 ** p<0.01. n=3. Scalebars: 100 μm.
Tendon mechanical properties are not restored after macrophage depletion
To determine the functional consequence of dysregulated macrophage recruitment, we carried out gait analysis and tensile testing. In previous studies, we found that swing, brake, and propel parameters (normalized to stride length) were affected by Achilles tendon injury (7, 8). Analysis of gait at D28 showed no differences with AP20187-treatment, either compared to contralateral uninjured control or compared to carrier treatment for any of these three parameters (Figure 5). However, direct tensile testing of tendons at D56 showed impaired functional restoration with macrophage ablation after injury. Stiffness was reduced in AP20187-treated injured tendons compared to the contralateral control tendon, and max force was reduced compared to both contralateral control and carrier injured tendons (Figure 6). There was no difference in mechanical properties between control and injured limbs with carrier treatment. These results indicate that macrophage dynamics play a critical role in functional neonatal tendon regeneration.
Figure 5: Recovery of gait after tendon injury was not affected by macrophage depletion.
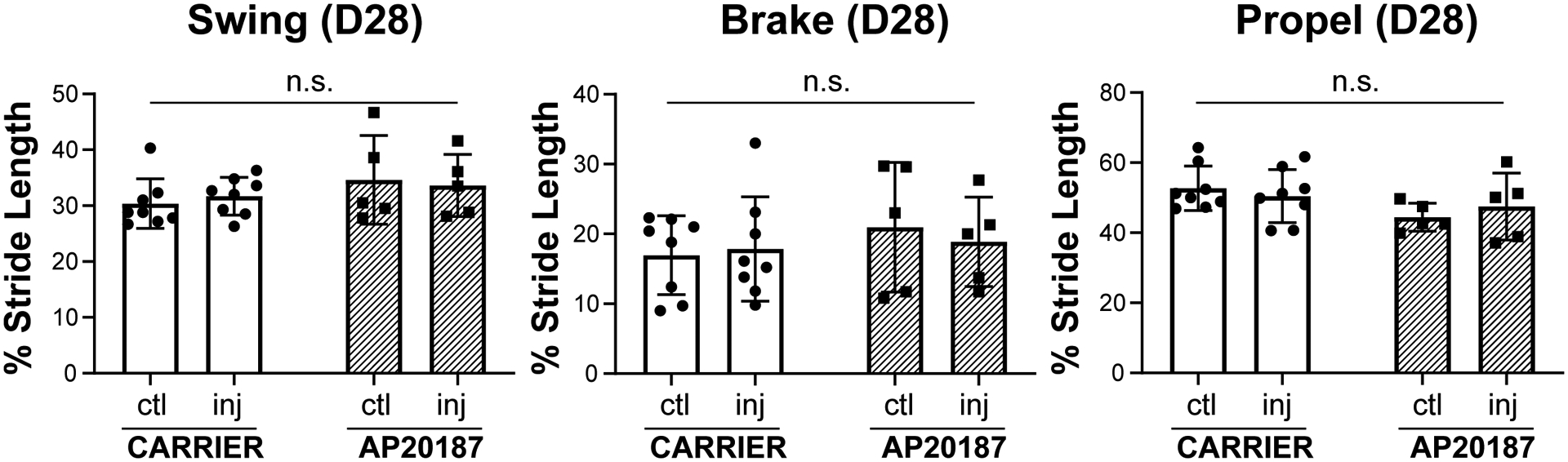
Gait analysis showed functional recovery of all gait parameters (Swing, Brake, and Propel) at D28 post-injury regardless of treatment. Data reported as mean±stdev and analyzed by 2-way ANOVA with Tukey’s post-hoc tests. n.s. indicates p>0.1. n=5–8.
Figure 6: Mechanical properties are impaired after tendon injury with macrophage depletion.
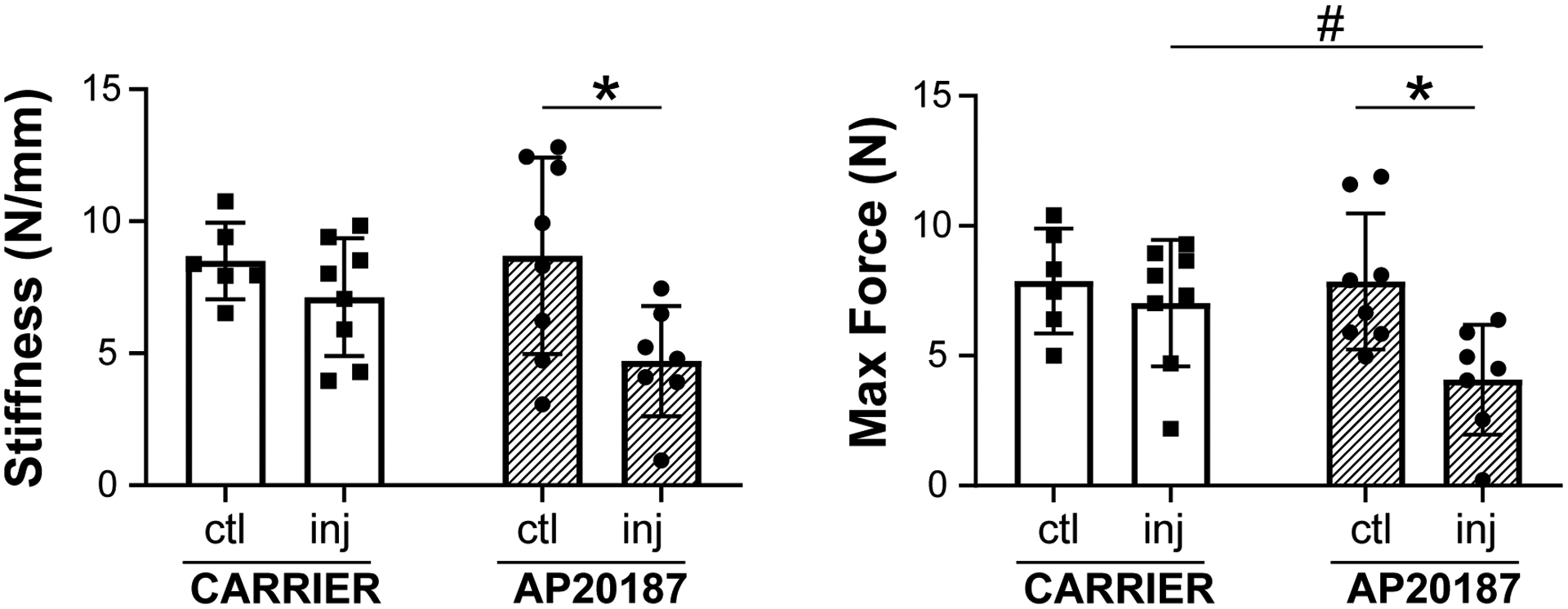
Tensile testing of Achilles tendons at D56 post-injury showed AP20187-treated tendons did not fully restore stiffness and max force after injury compared to contralateral controls. Data reported as mean±stdev and analyzed by 2-way ANOVA with Tukey’s post-hoc tests. * p<0.05 # p<0.1. n=6–8.
Early ScxGFP+ cell proliferation and subsequent ScxGFP+ neo-tendon formation is impaired with macrophage depletion
To understand the cellular deficits that might be associated with functional loss, we analyzed proliferation at D3 by EdU incorporation (2 hrs) and ScxGFP neo-tendon formation at D14 (Figure 7A). With carrier treatment, intense proliferation of both ScxGFP+ and ScxGFP− cells was observed at D3 in the tendon cut site (Figure 7B). In contrast, minimal proliferation occurred with AP20187 treatment, and the number of proliferating ScxGFP+ cells was reduced. By D14, a robust ScxGFP+ neo-tendon was formed in carrier-treated mice, whereas macrophage depletion resulted in significantly reduced ScxGFP+ area (Figure 7C) with no significant difference in DAPI+ area (Supplemental Figure 2, p>0.1). Taken together, the data suggest that macrophage depletion results in defects in tendon cell proliferation leading to reduced ScxGFP cell recruitment and neo-tendon formation.
Figure 7: Reduced proliferation and ScxGFP neo-tendon formation after tendon injury with macrophage depletion.
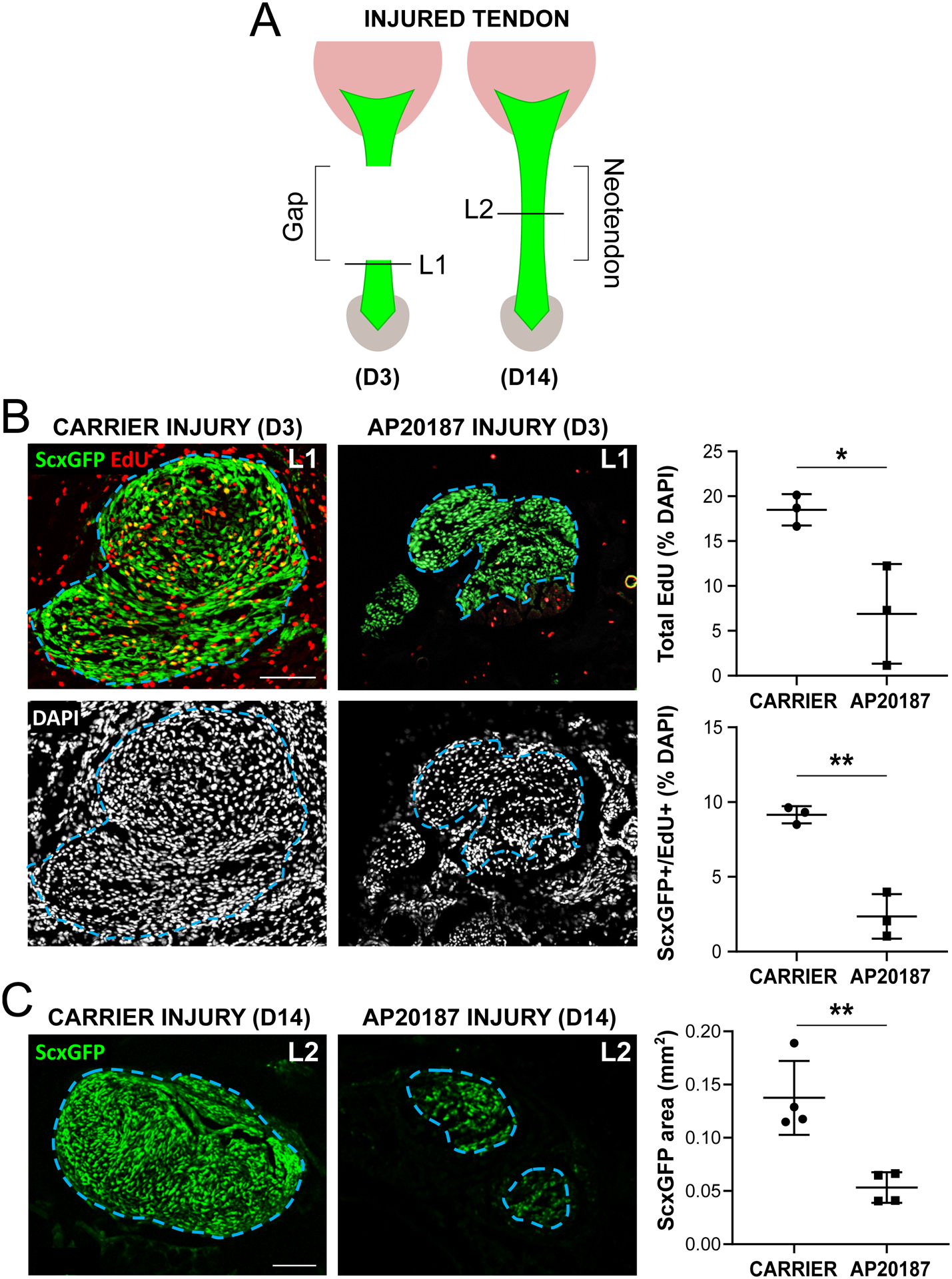
(A) Transverse cryosections were collected from tendon cut site at D3 (L1) and neo-tendon region at D14 (L2) post-injury. (B) EdU and ScxGFP imaging and cell quantification at D3 post-injury (n=3). (C) ScxGFP area quantification at D14 post-injury (n=4). Blue dashed outlines show tendon region of interest based on ScxGFP expression. Data reported as mean±stdev and analyzed by Student’s t-tests. * p<0.05 ** p<0.01. Scalebars: 100 μm.
Recruitment of αSMA cells is not affected by macrophage ablation
In previous studies, we found transient recruitment of αSMA cells after neonatal tendon injury that persisted after adult injury (7). Since ScxGFP area was significantly reduced but DAPI area was not affected in AP20187-treated tendons, we considered the possibility that the non-ScxGFP cells in the neo-tendon may be αSMA cells. However, quantification of αSMA cells in transverse cryosections did not show any differences between carrier-treated and AP20187-treated tendons (Figure 8). For both groups, there was intense staining for αSMA cells in the gap space at D3, with minimal staining by D14.
Figure 8: Recruitment of αSMA myofibroblasts is not affected by macrophage depletion.
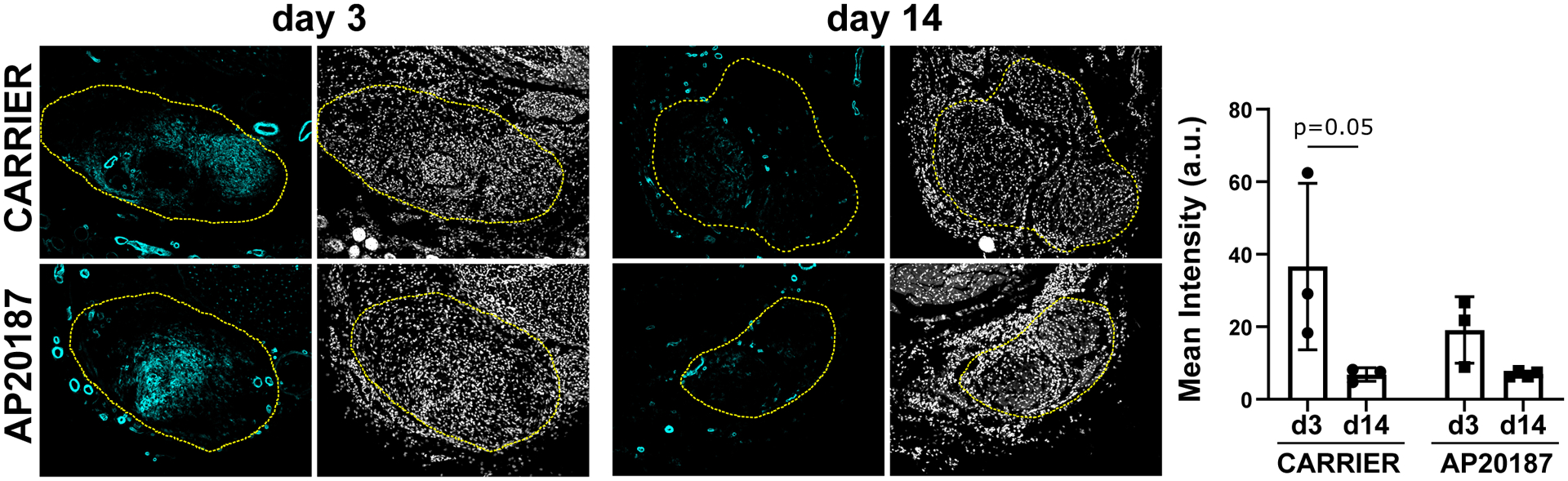
Quantification for αSMA immunostaining in the gap region at D3 and in the neo-tendon at D14 post-injury in transverse sections (n=3). Yellow dashed outlines show region of interest selected based on DAPI. Data reported as mean±stdev and analyzed by 2-way ANOVA with Tukey’s post-hoc tests. Scalebar: 100 μm.
AP20187 treatment results in altered gene expression of the contralateral control tendon
Although the neo-tendon area was reduced with macrophage depletion, we still observed a population of ScxGFP+ cells recruited at D14, indicating a positive tenogenic phenotype. To better define the tenogenic phenotype of these cells, we carried out gene expression analysis at D3 and D14 by qPCR. Expression of known markers Tnmd, and Mkx was reduced in carrier treated injured limbs relative to contralateral controls at D3 (Figure 9A), with no difference in Scx expression, which may be due to high variability in the data. At D14, a reduction in Tnmd expression was observed only in AP20187-treated control tendons relative to carrier-treated controls with no differences in Mkx expression (Figure 9B). Unexpectedly, Scx expression in AP20187-treated control tendons was dramatically increased at D14 relative to all other groups (Figure 9A, B). There was no difference in tendon gene expression between carrier-treated and AP-treated injured limbs at any timepoint. Since gene expression results suggested alterations in the uninjured tendon with macrophage depletion, we analyzed contralateral control tendons from carrier- and AP20187-treated mice in transverse sections using laminin immunostaining to visualized epitenon and ScxGFP expression for tendon. Qualitatively, we did not detect noticeable differences in tendon or epitenon morphology or ScxGFP expression (Supplemental Figure 3).
Figure 9: Tendon gene expression is altered with macrophage depletion.
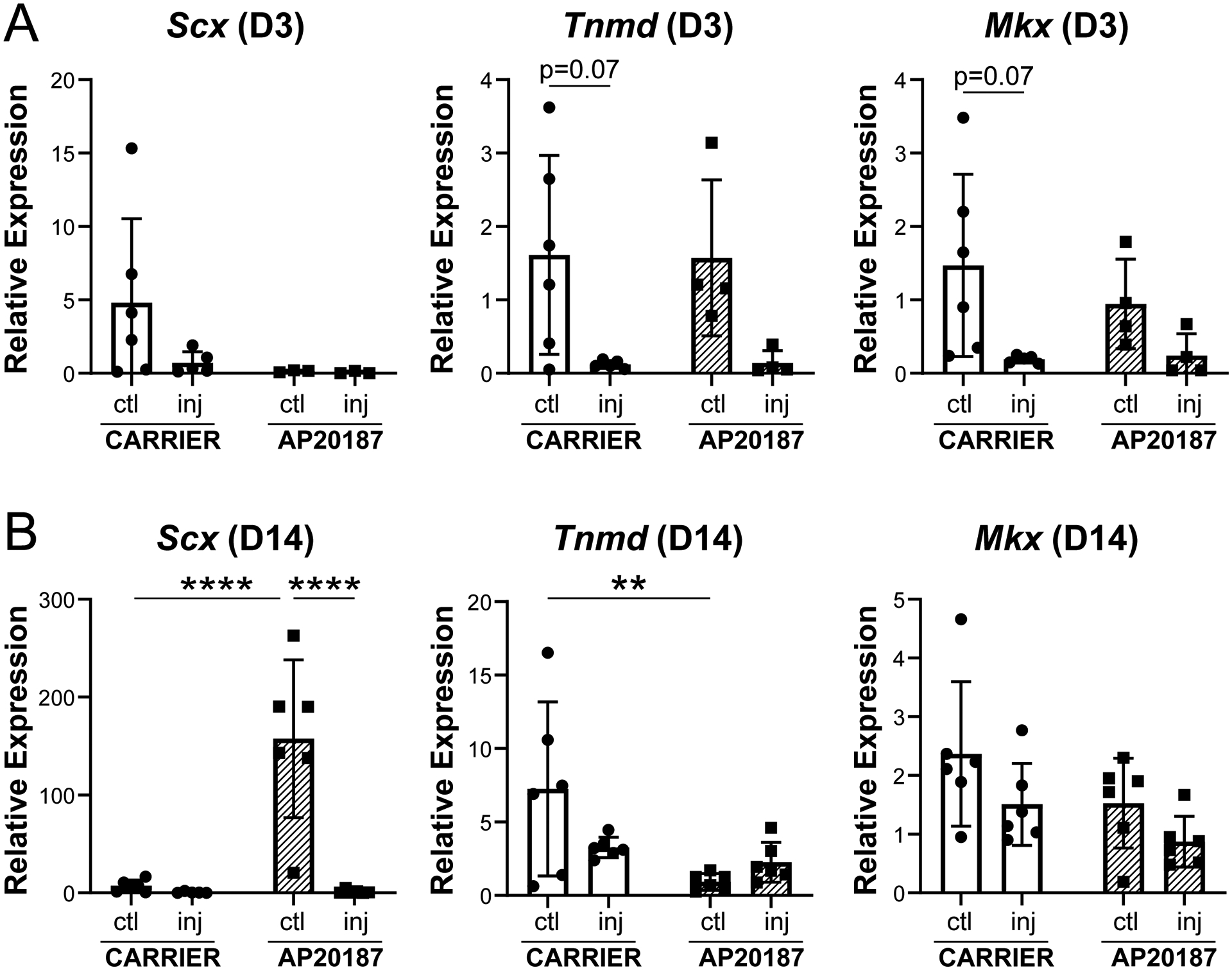
Expression of tendon genes Scx, Tnmd, and Mkx at (A) D3 and (B) D14 post-injury. Data reported as mean±stdev and analyzed by 2-way ANOVA with Tukey’s post-hoc tests. ** p<0.01 **** p<0.0001. n=4–6.
Macrophage depletion may enhance early inflammation
To determine the effects of macrophage depletion on local inflammation, Il1β and Il10 expression was determined by qPCR at D3 and D14. At D3, Il1β expression was increased with injury compared to controls with AP20187 treatment (Figure 10A). At D14, there was no difference across groups (Figure 10B). There was also no difference in Il10 expression at any timepoint for either treatment or injury. Overall, this data suggest that there may be early elevation of inflammation with macrophage depletion.
Figure 10: Expression of cytokines with macrophage depletion.
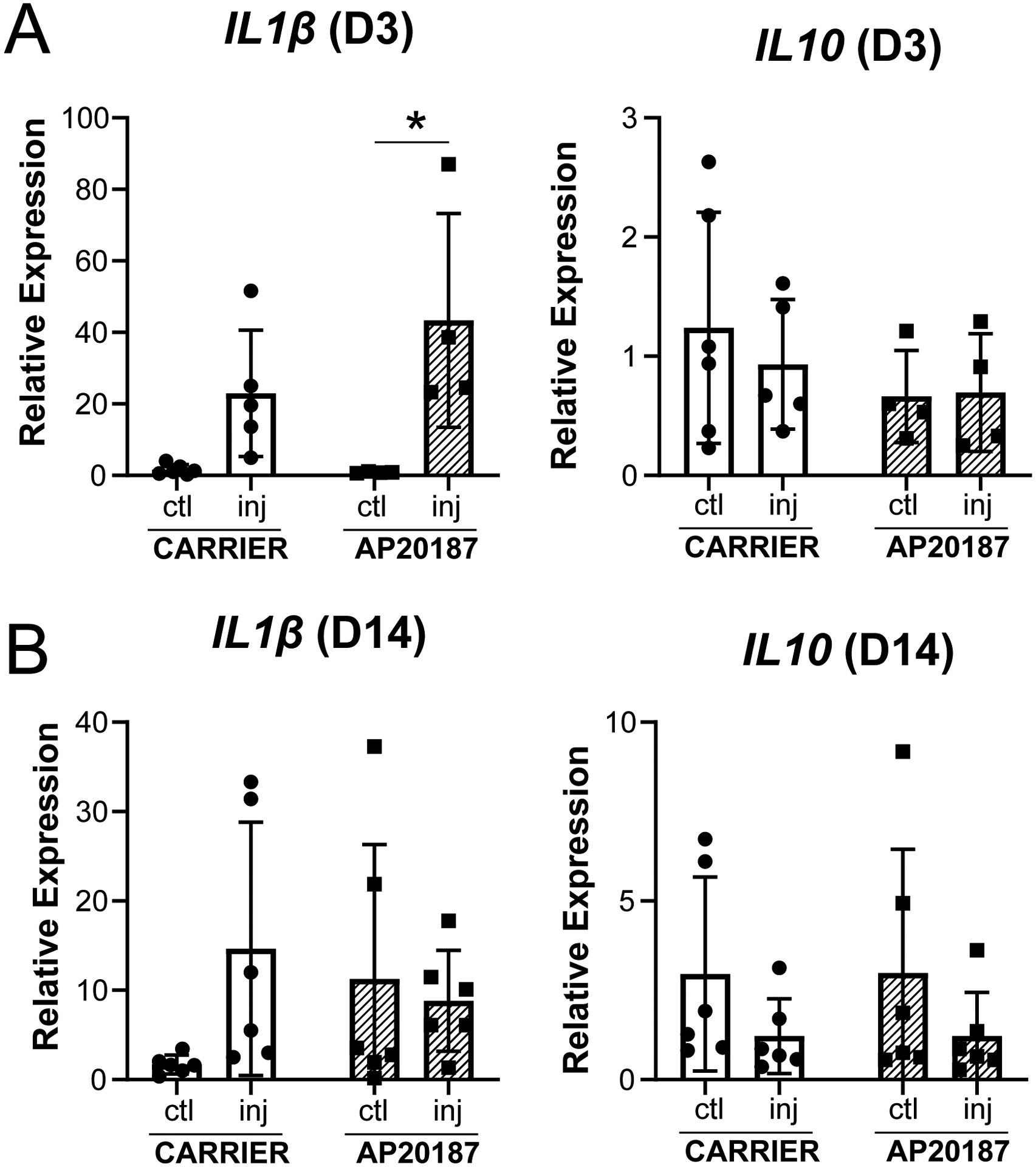
Expression of Il1β and Il10 at (A) D3 and (B) D14 post-injury. Data reported as mean±stdev and analyzed by 2-way ANOVA with Tukey’s post-hoc tests. * p<0.05. n=4–6.
DISCUSSION
In this study, we screened for immune gene expression markers after acute tendon injury and found upregulation of several pro-inflammatory markers, suggesting that early inflammation is a key feature of neonatal tendon regeneration. This was contrary to our initial hypothesis, as it was previously suggested that scar-less fetal regeneration occurred in a permissive immune environment characterized by minimal inflammation (30–32). Increased inflammation in the fetal environment resulted in failed regeneration of fetal skin and tendons (30, 33); this could be rescued by dampening inflammation via IL-10 over-expression (30). It is important to note, however, that direct comparisons to adult tendon injury was not performed in this study; it may be that despite upregulation of inflammatory markers, neonatal inflammation may still be substantially lower compared to adult. Interestingly, there is also evidence that adult tissues are unable to regenerate when placed in the fetal environment, suggesting that a permissive immune environment may be necessary, but not sufficient, to fully transform adult scar-mediated healing (34). Although fetal tendons transplanted into the adult environment were still able to regenerate (35), immunodeficient hosts were used in these studies, and the fetal tendons therefore did not experience a normal adult immune response to injury. To date, there are still few studies focused on the immune regulation of neonatal regeneration. However, it is well appreciated that the neonatal immune system is less mature compared to adult and is biased toward type 2 immunity in response to infections (36, 37). Many categories of neonatal immune cells (including neutrophils, natural killer cells, monocytes, and others) also show substantial functional differences and numbers compared to adults (37), reflecting the unique immune environment of the neonate. While we observed enhanced expression of pro-inflammatory markers, analyses were limited to a single early timepoint. Ongoing studies will define the temporal dynamics of inflammation and its resolution (or persistence) in neonates in direct comparison with adult counterparts after tendon injury. It may be that neonatal inflammation is reduced compared to adults or is more rapidly resolved.
Depletion of macrophages during early healing resulted in impaired tensile properties but not gait. This difference may be due to lower sensitivity of gait analysis as a measurement of tendon function, relative to direct mechanical testing of the tissue itself. We also used a relatively low speed (10 cm/s, walking) which may not sufficiently challenge the mice. Future studies will determine the effects of higher speeds (30 cm/s, running). In addition to reduced functional properties, we also identified reduced ScxGFP tendon cell proliferation and recruitment with macrophage depletion. This is consistent with studies in adult mice showing reduced proliferation with clodronate-induced macrophage depletion (21). While these studies in adult mice also showed improved tensile properties with macrophage depletion, we found impaired tensile properties, suggesting that macrophage dynamics and requirements may differ between neonates and adults after injury. This is supported by several studies in naturally regenerative organisms such as axolotl and zebrafish. In these organisms, macrophage depletion results in failed regeneration of tissues such as the heart, limb, and tail fins (38–40). Most remarkably, in axolotl limbs, regeneration can be rescued by re-amputating the stump once macrophage populations are replenished (38). Macrophage sub-populations also play different roles in regeneration in these models, both in contributing to initial scar formation and in subsequent resolution of scar tissue (41). Future studies will test whether neonatal mouse macrophages are more similar to macrophages in these organisms and are uniquely capable of directing a regenerative response compared to adult macrophages.
The close proximity of macrophages to tendon cells at D3 suggests that secreted factors from macrophages may stimulate tendon cell proliferation. While TGFβ is an attractive candidate based on our gene expression array screen and has been implicated in other macrophage depletion models of tendon healing (21, 23) as well as fetal tendon healing (35, 42), our previous studies inhibiting TGFβ signaling showed no effect on tendon cell proliferation at D3 (8). Interestingly, Vcam1 was also upregulated in the Taqman array at D3 suggesting activation of a fibroblastic phenotype. While this activation may reflect the presence of αSMA myofibroblasts that are recruited into the gap at this time, recent research now show that macrophages can also contribute directly to fibrosis via collagen synthesis (43). Since other fibroblast markers such as THY1, CD248, and PDPN are activated in human adult tendon disease (44, 45), future studies will screen these and other fibroblast markers to determine whether a similar signature is observed in neonatal tendon healing and whether these are expressed by macrophages. Since molecular regulators of macrophage-tendon cross-talk are still unidentified, transcriptional profiling by RNA sequencing of isolated MaFIAGFP macrophages or single cell RNA sequencing will be highly informative.
One major limitation of this study includes the timing of AP20187 delivery and macrophage depletion. The goal of this study was to determine broadly the role of macrophages; we therefore depleted macrophages prior to injury with an additional booster injection at D7. While previous research in adult tendons suggest that M2-like macrophages do not emerge until D28 in the healing timeline (12), immunostaining for M2 marker CD206 showed the presence of M2-like macrophages, therefore these were likely depleted as well. In our study, we observed reduced ScxGFP+ cell proliferation at D3, during the time when M1-like macrophages are expected to be dominant. This suggests that M1-like macrophages are important for stimulating tendon cell proliferation. While other studies also showed a requirement for macrophages in cell proliferation (21, 22), this is the first study to distinguish tendon cells (ScxGFP+) from exogenous or dedifferentiated (ScxGFP− and αSMA+) cells. Our finding that inflammation may be crucial for the early healing response is consistent with other research showing that inhibition of inflammation too early in tendon healing can have detrimental outcomes on functional properties (46). Future studies will determine the temporal dynamics and functions of distinct macrophage sub-populations recruited during neonatal tendon healing and test depletion strategies targeting early (D0–D3), middle (D5–D9), and late (D20–24) phases. Temporal depletion of macrophages will also determine whether impaired neo-tendon formation at D14 is due solely to reduced proliferation of tendon cells or whether there is an additional contribution of tendon cell migration.
Another limitation includes interpretation of impaired tensile properties. Due to the small size of mouse tendons, we were unable to reliably measure cross-sectional area and therefore only report structural properties. Although DAPI area was not significantly different at D14, it is possible that there is reduction in neo-tendon size at D56 with AP20187 treatment. In this case, our interpretation that functional restoration is impaired may be flawed, since material properties may not be different or may even be improved. Future studies will analyze cross-sectional area histologically at D56.
Although carrier-treated mice restored gait and mechanical function after injury, gene expression analyses indicated a potential alteration or delay in healing at early timepoints. Our previous studies generally showed restored or upregulation of tendon markers by D14, however carrier-treated injured tendons in this study maintained decreased expression of Tnmd and Mkx. This may be due to an effect of the carrier solution on tendon healing. Similarly, Il1β was also elevated in carrier-treated injured tendons at D14; in unpublished data from the lab using untreated mice, we generally observe resolution of neonatal inflammation at this timepoint (not shown). Interestingly, there was also an effect of AP20187-treatment on the contralateral uninjured tendon, in terms of tendon gene expression. While flow cytometry did not show a difference in macrophage number in control tendons with AP20187-treatment, we cannot rule out the possibility that there may be some degree of tendon-resident macrophage apoptosis leading to low-level local inflammation independent of injury. We also observed nearly complete macrophage depletion in the spleen, which may alter systemic circulating factors that may affect the contralateral control limb. In future studies, the impact of local vs systemic effects will be distinguished by local injection of AP20187 directly to the injured or control limb.
Finally, although macrophages are key players in the healing process, they are not the only immune cells that may regulate regeneration. During muscle regeneration, specific T cell subpopulations (Th1, Th2 and Tregs) have been shown to induce or impede regenerative healing (47–50). Our qPCR array screen also identified markers associated with T cells, consistent with previous studies showing their recruitment during tendon healing and in human tendinopathy (51–53). Ongoing work will test the role of T cells in tendon regeneration and scar formation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by NIH/NIAMS (R01AR069537, R56AR076984) to AHH and F31AR073626 to DK. We gratefully thank Dr. Ronen Schweitzer for providing ScxGFP mice and the qPCR Core and Flow Cytometry Core at Mount Sinai for their assistance with this project.
NON-STANDARD ABBREVIATIONS LIST
- MaFIA
Macrophage Fas-Induced Apoptosis
- ScxGFP
Scleraxis-Green Fluorescent Protein
- TGFβ
Transforming growth factor β
Footnotes
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest to disclose.
REFERENCES
- 1.Benjamin M, Kaiser E, and Milz S (2008) Structure-function relationships in tendons: a review. J Anat 212, 211–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beason DP, Kuntz AF, Hsu JE, Miller KS, and Soslowsky LJ (2012) Development and evaluation of multiple tendon injury models in the mouse. J Biomech 45, 1550–1553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(2011) United States Bone and Joint Initiative: The Burden of Musculoskeletal Disease in the United States, Second Edition, American Academy of Orthopaedic Surgeons, Rosemont, IL [Google Scholar]
- 4.Sereysky JB, Flatow EL, and Andarawis-Puri N (2013) Musculoskeletal regeneration and its implications for the treatment of tendinopathy. Int J Exp Pathol 94, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharma P, and Maffulli N (2005) Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am 87, 187–202 [DOI] [PubMed] [Google Scholar]
- 6.Magnusson SP, and Kjaer M (2019) The impact of loading, unloading, ageing and injury on the human tendon. J Physiol 597, 1283–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howell K, Chien C, Bell R, Laudier D, Tufa SF, Keene DR, Andarawis-Puri N, and Huang AH (2017) Novel Model of Tendon Regeneration Reveals Distinct Cell Mechanisms Underlying Regenerative and Fibrotic Tendon Healing. Sci Rep 7, 45238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaji DA, Howell KL, Balic Z, Hubmacher D, and Huang AH (2020) TGFbeta signaling is required for tenocyte recruitment and functional neonatal tendon regeneration. eLife 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ackerman JE, Nichols AE, Studentsova V, Best KT, Knapp E, and Loiselle AE (2019) Cell non-autonomous functions of S100a4 drive fibrotic tendon healing. eLife 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang K, Asai S, Hast MW, Liu M, Usami Y, Iwamoto M, Soslowsky LJ, and Enomoto-Iwamoto M (2016) Tendon mineralization is progressive and associated with deterioration of tendon biomechanical properties, and requires BMP-Smad signaling in the mouse Achilles tendon injury model. Matrix Biol 52–54, 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arble JR, Lalley AL, Dyment NA, Joshi P, Shin DG, Gooch C, Grawe B, Rowe D, and Shearn JT (2016) The LG/J Murine Strain Exhibits Near-Normal Tendon Biomechanical Properties Following a Full-Length Central Patellar Tendon Defect. Connect Tissue Res [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunwoo JY, Eliasberg CD, Carballo CB, and Rodeo. (2020) The role of the macrophage in tendinopathy and tendon healing. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 38 [DOI] [PubMed] [Google Scholar]
- 13.Chisari E, Rehak L, Khan WS, and Maffulli N (2020) The role of the immune system in tendon healing: a systematic review. British medical bulletin 133 [DOI] [PubMed] [Google Scholar]
- 14.Kawamura S, Ying L, Kim HJ, Dynybil C, and Rodeo SA (2005) Macrophages accumulate in the early phase of tendon-bone healing. J Orthop Res 23, 1425–1432 [DOI] [PubMed] [Google Scholar]
- 15.Sugg KB, Lubardic J, Gumucio JP, and Mendias CL (2014) Changes in macrophage phenotype and induction of epithelial-to-mesenchymal transition genes following acute Achilles tenotomy and repair. J Orthop Res 32, 944–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jomaa G, Kwan CK, Fu SC, Ling SK, Chan KM, Yung PS, and Rolf C (2020) A systematic review of inflammatory cells and markers in human tendinopathy. BMC Musculoskelet Disord 21, 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chamberlain CS, Clements AEB, Kink JA, Choi U, Baer GS, Halanski MA, Hematti P, and Vanderby. (2019) Extracellular Vesicle-Educated Macrophages Promote Early Achilles Tendon Healing. Stem cells (Dayton, Ohio) 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schoenenberger AD, Tempfer H, Lehner C, Egloff J, Mauracher M, Bird A, Widmer J, Maniura-Weber K, Fucentese SF, Traweger A, Silvan U, and Snedeker JG (2020) Macromechanics and polycaprolactone fiber organization drive macrophage polarization and regulate inflammatory activation of tendon in vitro and in vivo. Biomaterials 249 [DOI] [PubMed] [Google Scholar]
- 19.Ackerman JE, Geary MB, Orner CA, Bawany F, and Loiselle AE (2017) Obesity/Type II diabetes alters macrophage polarization resulting in a fibrotic tendon healing response. PLoS One 12, e0181127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Rooijen N, and van Nieuwmegen R (1984) Elimination of phagocytic cells in the spleen after intravenous injection of liposome-encapsulated dichloromethylene diphosphonate. An enzyme-histochemical study. Cell and tissue research 238 [DOI] [PubMed] [Google Scholar]
- 21.de la Durantaye M, Piette AB, van Rooijen N, and Frenette J (2014) Macrophage depletion reduces cell proliferation and extracellular matrix accumulation but increases the ultimate tensile strength of injured Achilles tendons. J Orthop Res 32, 279–285 [DOI] [PubMed] [Google Scholar]
- 22.Godbout C, Bilodeau R, Van Rooijen N, Bouchard P, and Frenette J (2010) Transient neutropenia increases macrophage accumulation and cell proliferation but does not improve repair following intratendinous rupture of Achilles tendon. J Orthop Res 28, 1084–1091 [DOI] [PubMed] [Google Scholar]
- 23.Hays PL, Kawamura S, Deng XH, Dagher E, Mithoefer K, Ying L, and Rodeo SA (2008) The role of macrophages in early healing of a tendon graft in a bone tunnel. J Bone Joint Surg Am 90, 565–579 [DOI] [PubMed] [Google Scholar]
- 24.Best KT, Lee FK, Knapp E, Awad HA, and Loiselle AE (2019) Deletion of NFKB1 enhances canonical NF-kappaB signaling and increases macrophage and myofibroblast content during tendon healing. Sci Rep 9, 10926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burnett SH, Kershen EJ, Zhang J, Zeng L, Straley SC, Kaplan AM, and Cohen DA (2004) Conditional macrophage ablation in transgenic mice expressing a Fas-based suicide gene. J Leukoc Biol 75, 612–623 [DOI] [PubMed] [Google Scholar]
- 26.Pryce B, Brent A, Murchison N, Tabin C, and Schweitzer R (2007) Generation of transgenic tendon reporters, ScxGFP and ScxAP, using regulatory elements of the scleraxis gene. Developmental dynamics : an official publication of the American Association of Anatomists 236, 1677–1682 [DOI] [PubMed] [Google Scholar]
- 27.Abraham AC, Shah SA, Golman M, Song L, Li X, Kurtaliaj I, Akbar M, Millar NL, Abu-Amer Y, Galatz LM, and Thomopoulos S (2019) Targeting the NF-kappaB signaling pathway in chronic tendon disease. Sci Transl Med 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurtaliaj I, Golman M, Abraham AC, and Thomopoulos S (2019) Biomechanical Testing of Murine Tendons. J Vis Exp [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu CL, McNeill J, Goon K, Little D, Kimmerling K, Huebner J, Kraus V, and Guilak F (2017) Conditional Macrophage Depletion Increases Inflammation and Does Not Inhibit the Development of Osteoarthritis in Obese Macrophage Fas-Induced Apoptosis-Transgenic Mice. Arthritis & rheumatology (Hoboken, N.J.) 69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morris MW, Allukian M, Herdrich BJ, Caskey RC, Zgheib C, Xu J, Dorsett-Martin W, Mitchell ME, and Liechty KW (2014) Modulation of the inflammatory response by increasing fetal wound size or interleukin-10 overexpression determines wound phenotype and scar formation. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 22 [DOI] [PubMed] [Google Scholar]
- 31.Walraven M, Talhout W, Beelen RH, van Egmond M, and Ulrich MM (2016) Healthy human second-trimester fetal skin is deficient in leukocytes and associated homing chemokines. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 24 [DOI] [PubMed] [Google Scholar]
- 32.Herdrich BJ, Danzer E, Davey MG, Allukian M, Englefield V, Gorman JH, Gorman RC, and Liechty KW (2010) Regenerative healing following foetal myocardial infarction. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery 38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herdrich BJ, Danzer E, Davey MG, Bermudez DM, Radu A, Zhang L, Zhang Z, Soslowsky LJ, and Liechty KW (2010) Fetal tendon wound size modulates wound gene expression and subsequent wound phenotype. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society 18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longaker MT, Whitby DJ, Ferguson MW, Lorenz HP, Harrison MR, and Adzick. (1994) Adult skin wounds in the fetal environment heal with scar formation. Annals of surgery 219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Favata M, Beredjiklian P, Zgonis M, Beason D, Crombleholme T, Jawad A, and Soslowsky L (2006) Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 24, 2124–2132 [DOI] [PubMed] [Google Scholar]
- 36.Debock I, and Flamand V (2014) Unbalanced Neonatal CD4(+) T-Cell Immunity. Front Immunol 5, 393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basha S, Surendran N, and Pichichero M (2014) Immune responses in neonates. Expert Rev Clin Immunol 10, 1171–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Godwin JW, Pinto AR, and Rosenthal NA (2013) Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci U S A 110, 9415–9420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Godwin JW, Debuque R, Salimova E, and Rosenthal NA (2017) Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen Med 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morales RA, and Allende ML (2019) Peripheral Macrophages Promote Tissue Regeneration in Zebrafish by Fine-Tuning the Inflammatory Response. Front Immunol 10, 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bevan L, Lim ZW, Venkatesh B, Riley PR, Martin P, and Richardson RJ (2020) Specific macrophage populations promote both cardiac scar deposition and subsequent resolution in adult zebrafish. Cardiovasc Res 116, 1357–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beredjiklian P, Favata M, Cartmell J, Flanagan C, Crombleholme T, and Soslowsky L (2003) Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Annals of biomedical engineering 31, 1143–1152 [DOI] [PubMed] [Google Scholar]
- 43.Simoes FC, Cahill TJ, Kenyon A, Gavriouchkina D, Vieira JM, Sun X, Pezzolla D, Ravaud C, Masmanian E, Weinberger M, Mayes S, Lemieux ME, Barnette DN, Gunadasa-Rohling M, Williams RM, Greaves DR, Trinh LA, Fraser SE, Dallas SL, Choudhury RP, Sauka-Spengler T, and Riley PR (2020) Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat Commun 11, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dakin SG, Buckley CD, Hussein Al-Mossawi M, Hedley R, Wheway K, Watkins B, and Carr AJ (2017) Persistent stromal fibroblast activation is present in chronic tendinopathy. Arthritis Research & Therapy 19, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kendal AR, Layton T, Al-Mossawi H, Appleton L, Dakin S, Brown R, Loizou C, Rogers M, Sharp R, and Carr A (2020) Multi-omic single cell analysis resolves novel stromal cell populations in healthy and diseased human tendon. Sci Rep 10, 13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blomgran P, Hammerman M, and Aspenberg P (2017) Systemic corticosteroids improve tendon healing when given after the early inflammatory phase. Scientific reports 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burzyn D, Kuswanto W, Kolodin D, Shadrach JL, Cerletti M, Jang Y, Sefik E, Tan TG, Wagers AJ, Benoist C, and Mathis D (2013) A special population of regulatory T cells potentiates muscle repair. Cell 155, 1282–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuswanto W, Burzyn D, Panduro M, Wang KK, Jang YC, Wagers AJ, Benoist C, and Mathis D (2016) Poor Repair of Skeletal Muscle in Aging Mice Reflects a Defect in Local, Interleukin-33-Dependent Accumulation of Regulatory T Cells. Immunity 44, 355–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sadtler K, Estrellas K, Allen BW, Wolf MT, Fan H, Tam AJ, Patel CH, Luber BS, Wang H, Wagner KR, Powell JD, Housseau F, Pardoll DM, and Elisseeff JH (2016) Developing a pro-regenerative biomaterial scaffold microenvironment requires T helper 2 cells. Science 352, 366–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ito M, Komai K, Mise-Omata S, Iizuka-Koga M, Noguchi Y, Kondo T, Sakai R, Matsuo K, Nakayama T, Yoshie O, Nakatsukasa H, Chikuma S, Shichita T, and Yoshimura A (2019) Brain regulatory T cells suppress astrogliosis and potentiate neurological recovery. Nature 565, 246–250 [DOI] [PubMed] [Google Scholar]
- 51.Blomgran P, Blomgran R, Ernerudh J, and Aspenberg P (2016) A possible link between loading, inflammation and healing: Immune cell populations during tendon healing in the rat. Scientific reports 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Millar NL, Akbar M, Campbell AL, Reilly JH, Kerr SC, McLean M, Frleta-Gilchrist M, Fazzi UG, Leach WJ, Rooney BP, Crowe LA, Murrell GA, and McInnes IB (2016) IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Sci Rep 6, 27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Noah AC, Li TM, Martinez LM, Wada S, Swanson JB, Disser NP, Sugg KB, Rodeo SA, Lu TT, and Mendias CL (2020) Adaptive and Innate Immune Cell Responses in Tendons and Lymph Nodes After Tendon Injury and Repair. Journal of applied physiology [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


