Abstract
Pistachio nuts are an important economic tree nut crop which is used directly or processed for many food-related activities. They can become colonized by mycotoxigenic spoilage fungi, especially Aspergillus flavus, mainly resulting in contamination with aflatoxins (AFs), especially aflatoxin B1 (AFB1). The prevailing climate in which these crops are grown changes as temperature and atmospheric CO2 levels increase, and episodes of extreme wet/dry cycles occur due to human industrial activity. The objectives of this study were to evaluate the effect of interacting Climate Change (CC)-related abiotic factors of temperature (35 vs. 37 °C), CO2 (400 vs. 1000 ppm), and water stress (0.98–0.93 water activity, aw) on (a) growth (b) aflD and aflR biosynthetic gene expression and (c) AFB1 production by two strains A. flavus (AB3, AB10) in vitro on milled pistachio-based media and when colonizing layers of shelled raw pistachio nuts. The A. flavus strains were resilient in terms of growth on pistachio-based media and the colonisation of pistachio nuts with no significant difference when exposed to the interacting three-way climate-related abiotic factors. However, in vitro studies showed that AFB1 production was significantly stimulated (p < 0.05), especially when exposed to 1000 ppm CO2 at 0.98–0.95 aw and 35 °C, and sometimes in the 37 °C treatment group at 0.98 aw. The relative expression of the structural aflD gene involved in AFB1 biosynthesis was decreased or only slightly increased, relative to the control conditions at elevated CO, regardless of the aw level examined. For the regulatory aflR gene expression, there was a significant (p < 0.05) increase in 1000 ppm CO2 and 37 °C for both strains, especially at 0.95 aw. The in situ colonization of pistachio nuts resulted in a significant (p < 0.05) stimulation of AFB1 production at 35 °C and 1000 ppm CO2 for both strains, especially at 0.98 aw. At 37 °C, AFB1 production was either decreased, in strain AB3, or remained similar, as in strain AB10, when exposed to 1000 ppm CO2. This suggests that CC factors may have a differential effect, depending on the interacting conditions of temperature, exposure to CO2 and the level of water stress on AFB1 production.
Keywords: climate change, interacting abiotic factors, aflD, aflR, aflatoxins, Aspergillus flavus, pistachios
1. Introduction
Pistachio nuts (Pistacia vera L.) are one of the most economically important tree nut crops in countries such as the USA, Iran, Southern Europe and China. Of these, Iran and the USA are probably the major producers and exporters of pistachio nuts for direct consumption and for processing [1]. The quality and safety of pistachio nuts for human consumption have been compromised by the infection of the ripening nuts, especially pre-harvest, when early splitting of the shells can allow colonization by members of the Aspergillus section Flavi group, predominantly A. flavus. In addition, poor drying and storage practices can also exacerbate infection by A. flavus post-harvest [2,3]. Such infection results in significant contamination with aflatoxin B1 (AFB1), a class 1a carcinogen [4]. This has resulted in many countries importing pistachio nuts, including the EU, to have strict legislative maximum allowable contamination levels with AFB1 or total aflatoxins (AFs). Thus, Iranian pistachio nuts were excluded from importation into the EU, because batches consistently exceeded the prevailing legislative limits for AFs [5]. This required the development of an effective Hazard Analysis Critical Control Plan (HACCP) with appropriate Critical, Control Points in this chain focused on more efficient harvesting and post-harvest management of pistachios from Iran to meet the necessary legislative limits. However, because they are rich in lipids, they can reabsorb moisture from the atmosphere during the post-harvest phase, allowing for further contamination [3].
There has been interest in the implications of climate-related abiotic conditions and how these may affect the production and processing phases of tree nuts, especially pistachios and what impact such changes may have on infection by A. flavus and AFs contamination. However, very few, if any, studies have focused on addressing these issues. Most previous studies have focused on cereals, especially maize and wheat [6,7].
For example, the exposure of stored maize kernels with A. flavus to existing climate related conditions (30 °C, 400 ppm CO2, no drought stress) and to future climate-related abiotic factors (34 °C, 1000 ppm CO2, drought stress) showed that, while colonization was unaffected, AFB1 contamination was stimulated [6,7]. This was supported by increased expression of biosynthetic genes involved in AFs production, including an early structural gene (aflD) and a regulatory gene (aflR) in biosynthetic pathway. Indeed, a subsequent kinetic study also showed that this stimulation of AFB1 occurred when A. flavus was exposed on maize-based media to climate change scenarios [8].
A recent study of the water and temperature relationship of A. flavus strains isolated from pistachio nuts in Saudi Arabia showed that optimum growth on both pistachio nut-based media and raw pistachios was optimal at 35 °C in contrast to maize, where this is usually at 28–30 °C [3,9]. This certainly suggests that strains from pistachio nuts may have evolved to have better tolerance to high temperatures in production areas such as Iran and perhaps the USA from where the strains originated [3]. Indeed, optimal conditions for the growth of four strains of A. flavus from pistachios were found to be at 0.98–0.95 water activity (aw) and 30–35 °C. AFB1 production was also optimal at 30–35 °C and 0.98 aw. Interestingly, while less AFB1 was directly produced on raw shelled pistachio nuts the optimum and marginal boundary conditions were similar [3].
Drought stress interactions with elevated temperature and exposure to climate-related CO2 concentrations may be important. These factors were shown to influence and increased the colonization of maize cobs pre-harvest by Fusarium verticillioides and contamination with fumonisins [10]. Colonization of other commodities, such as stored green coffee by Aspergillus westerdijkiae observed optimal ochratoxin A (OTA) production under the existing climate-related condition of 400 ppm CO2 and intermediate drought stress/temperatures of 0.95–0.97 aw/30 °C. However, when coffee was stored and exposed to elevated CO2 (1000 ppm) and 35 °C, OTA production was stimulated, but only under increased water stress (0.95–0.90 aw) [11].
Interacting climate change related conditions will become more important over the next few decades and is important to understand the potential implications for food security and food safety. The actual estimated atmospheric CO2 concentration is, at present, approximately 417 ppm [12] and is predicted to double to 800 ppm (2×) or to 1000–1200 ppm (3×) in the future. This is suggestive of a temperature increase of 2–4 °C. Furthermore, rainfall patterns are also expected to change, with more extreme wet/dry episodes occurring [8,13]. Other important factors include the effect of light and also fluctuating day/night temperatures which can also impact colonization and toxin contamination of food commodities [14,15,16]. Previous studies have suggested that interactions between these critical abiotic factors may have a significant impact on fungal diseases of staple food crops and perhaps also on the contamination of food and feed with mycotoxins [17,18].
The objectives of this study were to evaluate the effects of three way interactions between climate change-related factors of temperature (35 vs. 37 °C) × water availability (0.98, 0.95, 0.93 aw) × exposure to CO2 (400 vs. 1000 ppm) on (a) growth, (b) AFB1 production, and (c) expression of biosynthetic genes (aflR, aflD) involved in AFs production in vitro on a pistachio nut-based medium for two strains of A. flavus isolated from pistachio nuts. This was complimented with similar studies in situ on stored raw pistachio nuts on growth and AFB1 contamination.
2. Results
2.1. In Vitro Effect of Climate Change Factors on Growth of Aspergillus flavus Strains on Pistachio Nut-Based Media
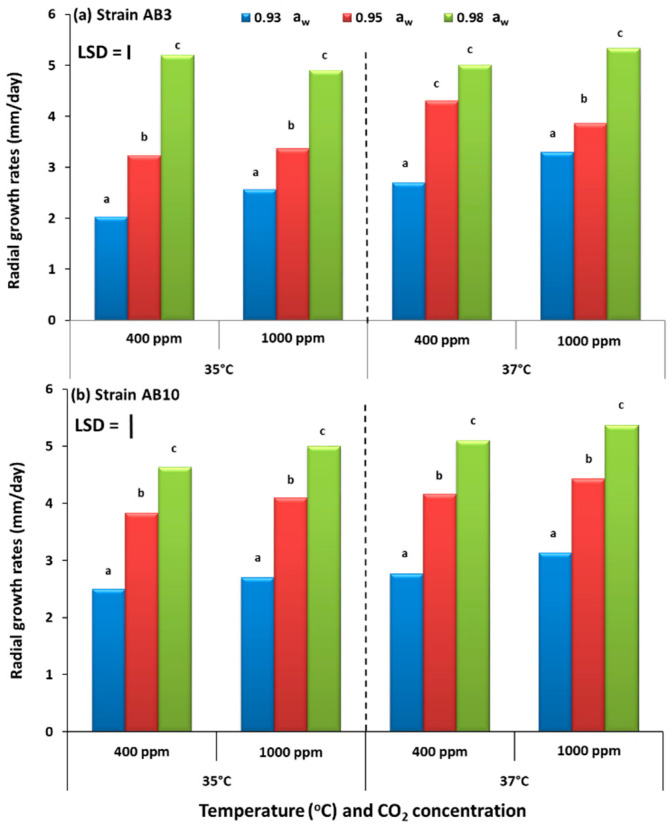
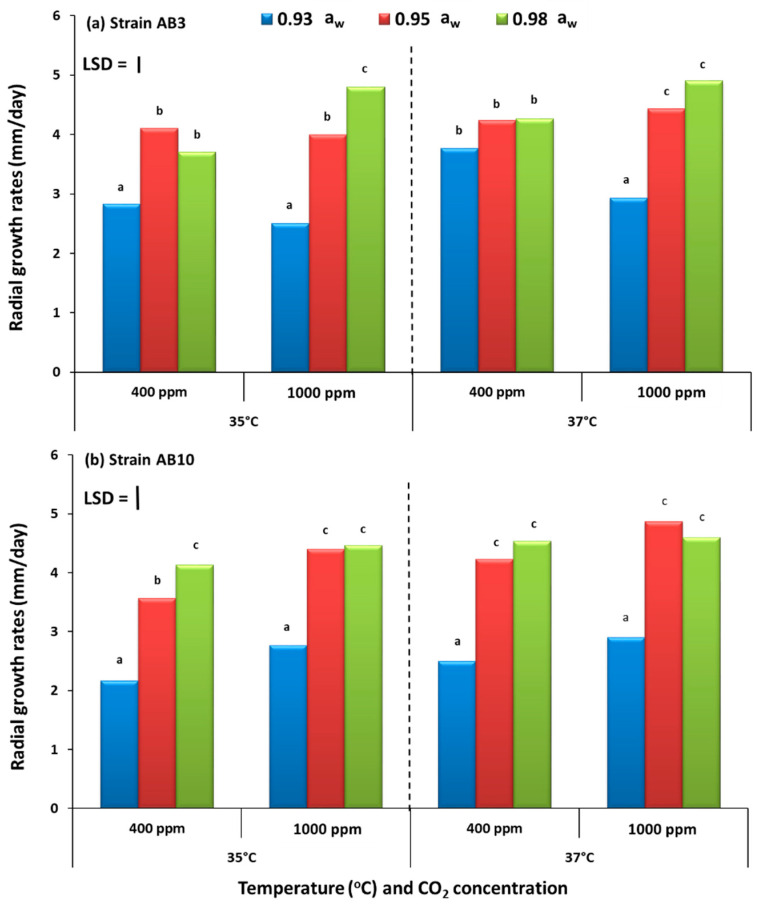
The effect of changing the three-way interacting climate-related abiotic factors on the growth of the two strains of A. flavus on the pistachio nut agar (PNA) nutritional media is shown in Figure 1. For both strains, growth on the pistachio-based medium was relatively similar with no significant differences in relation to the three interacting abiotic conditions. Colonization increased with more available water (0.98 aw) than under drier conditions at both 35 and 37 °C, regardless of CO2 exposure treatments.
Figure 1.
Comparison of growth rates of (mm/day) of A. flavus strain AB3 (a) and strain AB10 (b) grown on pistachio nut-based media and incubated at 35 and 37 °C under different concentrations of CO2 (400 vs. 1000 ppm) at 0.93–0.98 water activity (aw). Bars indicate Fisher’s Least Significant Difference (LSD, p ≤ 0.05). Different letters indicate significant differences.
2.2. In Vitro Effect of Climate Change Interacting Factors on Aflatoxin B1 Production from Aspergillus flavus Strains on Pistachio-Based Media
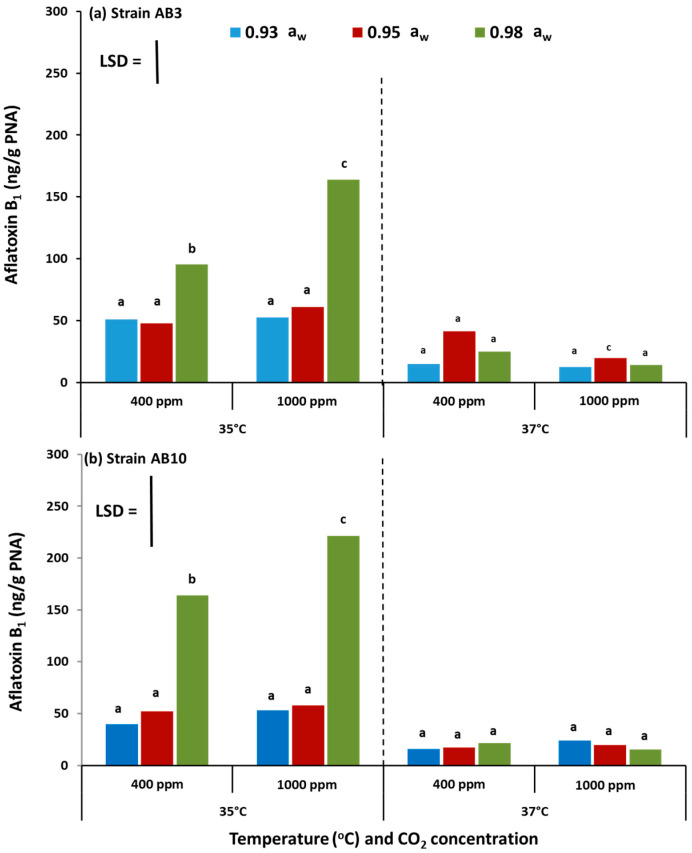
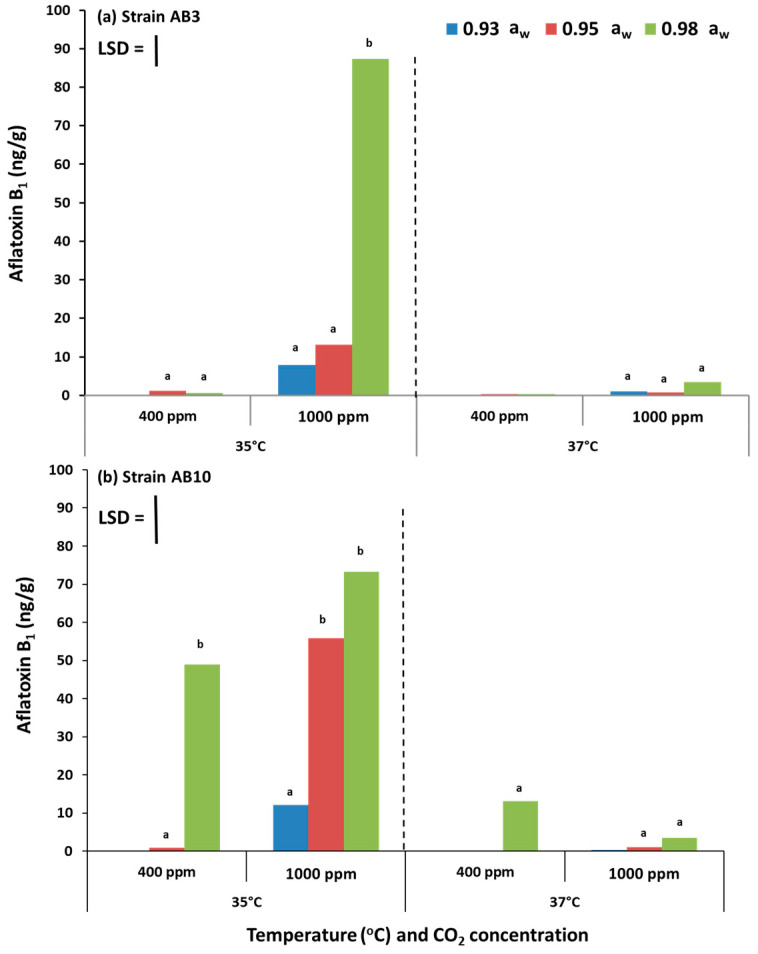
Figure 2 shows the effect of the interaction between climate change-related abiotic factors on AFB1 production by the two strains of A. flavus (AB3 and AB10) examined on the milled pistachio-based media as the only nutritional source. Generally, AFB1 production was higher at 35 °C at the two CO2 levels (400 and 1000 ppm CO2) for both stains. At this temperature, the production was significantly increased at 1000 ppm CO2 and 0.98 aw when compared with atmospheric air at 400 ppm CO2. At 37 °C, AFB1 production by the A. flavus strains when exposed to 1000 ppm CO2 was either decreased (strain AB3) or remained similar in the other strain.
Figure 2.
Impact of climate change-related interacting abiotic factors on aflatoxin B1 production by A. flavus strain AB3 (a) and strain AB10 (b) grown on pistachio nut-based media for a period of 10 days. Bars indicate Fisher’s Least Significant Difference (LSD, p ≤ 0.05). Different letters indicate significant differences.
2.3. In Vitro Effect of Climate Change Interacting Factors on Relative Genes Expression of the aflD and aflR Genes Involved in the Biosynthetic Pathway for Aflatoxin B1 Production
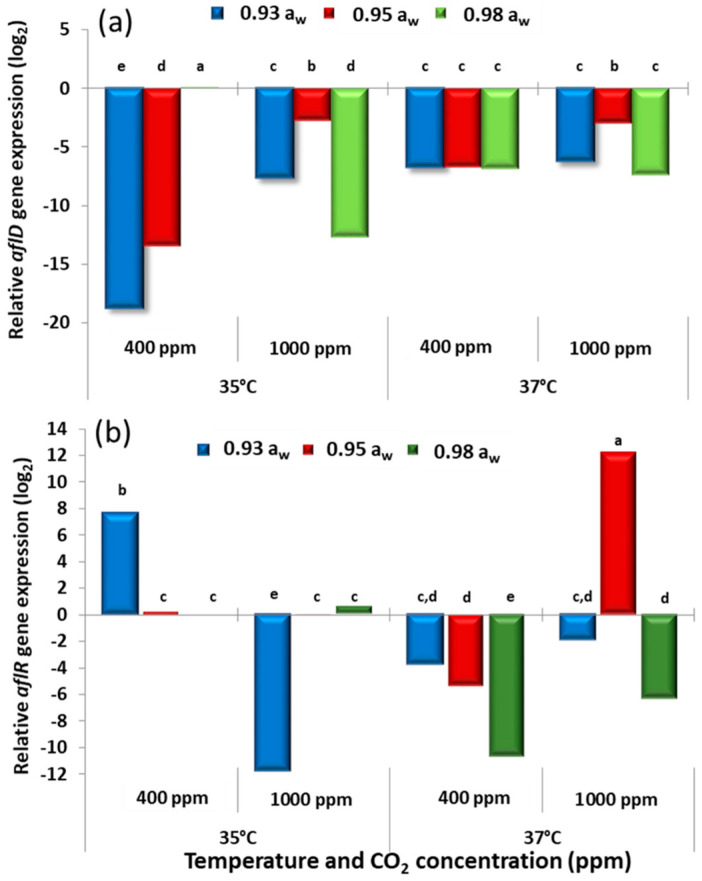
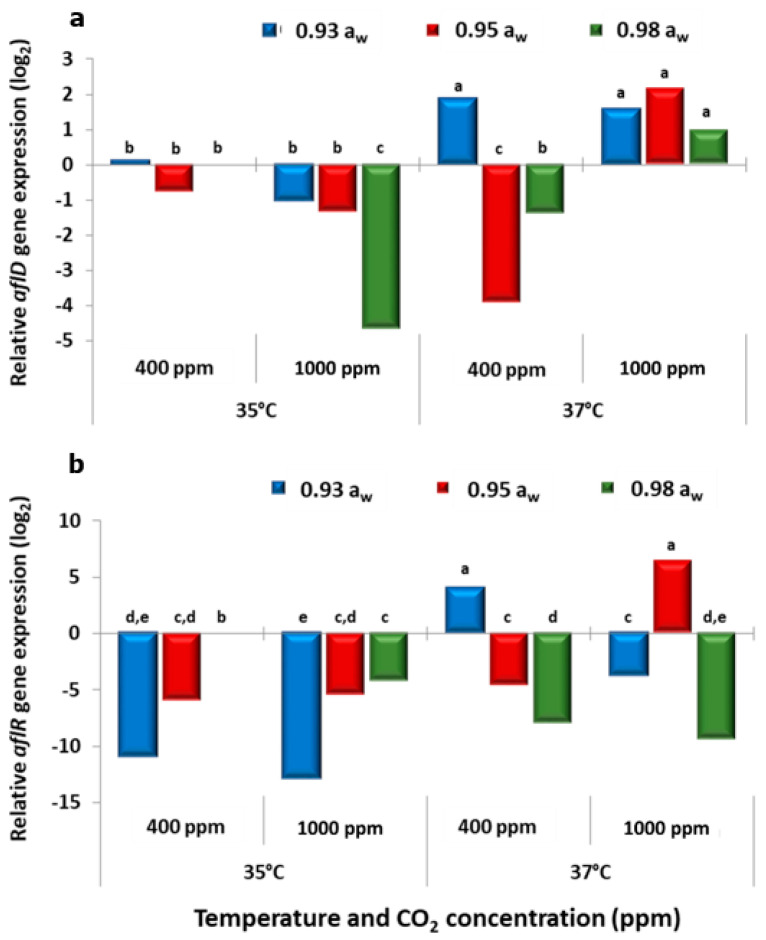
Figure 3 and Figure 4 show the effect of the climate change-related abiotic factors on the relative gene expression of the structural aflD and the regulatory gene aflR for the two strains. The control conditions (calibrator) used for comparisons between treatments was the 35 °C, 400 ppm CO2, 0.98 aw. For the aflD gene at 35 °C, the relative expression was higher at 400 ppm CO2 for both strains. However, for strain AB3, the expression was higher at 1000 ppm CO2 and 0.95 aw. However, at 37 °C, the expression was generally increased at 1000 ppm CO2 when compared with existing atmospheric CO2 levels. With regard to the regulatory aflR gene, in some aw treatments the expression was higher in the 1000 ppm CO2 treatment at 37 °C for both strains. This suggests that the interaction between the three-climate change-related abiotic factors stimulated the expression of this gene which paralleled the effects on production of AFB1 under some conditions.
Figure 3.
Effect of climate change-related interacting abiotic factors on relative expression of (a) aflD structural gene and (b) aflR regulatory gene of strain AB3 of A. flavus after 10 days incubation on a milled pistachio nut agar. The calibrator corresponded to A. flavus strains grown at 35 °C and 0.98 aw in atmospheric air (400 ppm CO2), and has a value equal to 0. Different letters indicate significant differences (p ≤ 0.05).
Figure 4.
Effect of climate change-related interacting abiotic factors on relative expression of (a) aflD structural gene and (b) aflR regulatory gene of strain AB10 of A. flavus after 10 days incubation on a milled pistachio nut agar. The calibrator corresponded to A. flavus strains grown at 35 °C and 0.98 aw in atmospheric air (400 ppm CO2) and has a value equal to 0. Different letters indicate significant differences (p ≤ 0.05).
2.4. In Situ Effect of Climate Change Factors on Growth and Aflatoxin B1 Production by Strains of Aspergillus flavus Strains on Pistachio Nuts
2.4.1. Effects on Colonization of Raw Pistachio Nuts
The relative colonization rates of raw pistachio nuts suggest that regardless of temperature or CO2 exposure, there were practically no significant differences at 0.98 and 0.95 aw treatments for both strains examined (Figure 5). Thus, exposure to 1000 ppm CO2 had little effect on colonization rates at both 35 and 37 °C suggesting good resilience to the imposed climate-related abiotic conditions. However, the growth of the AB3 strain was slightly decreased at 1000 ppm CO2 under the lowest aw tested (0.93 aw) in the two incubation temperatures.
Figure 5.
Effect of the three-way interacting climate-related abiotic factors on colonisation rates (mm/day) of single layers of raw pistachio nuts by A. flavus strain AB3 (a) and strain AB10 (b) over a period of 10 days. Bars indicate Fisher’s Least Significant Difference (LSD, p ≤ 0.05). Different letters indicate significant differences.
2.4.2. In Situ Effect of Climate Change Interacting Factors on Aflatoxin B1 Production from Aspergillus flavus Strains on Pistachio Nuts
Figure 6 shows the effect of the three-way interacting climate-related abiotic factors on AFB1 production by the two strains of A. flavus after 10 days colonization of the layers of shelled raw pistachio nuts. There was an increase in AFB1 production when they were exposed to 1000 ppm CO2 for both strains, especially at 35 °C and 0.98 aw (strain AB3) and 0.95 aw (strain AB10). Overall, at 37 °C, there was significantly less AFB1 produced, regardless of the interacting abiotic conditions. There was also some indication that when exposed to 1000 ppm CO2 and 37 °C more AFB1 was produced than at 400 ppm CO2 exposure and the same temperature for strain AB3.
Figure 6.
Comparison of the aflatoxin B1 production by A. flavus strain AB3 (a) and strain AB10 (b) when grown on single layers of raw shelled pistachio nuts and incubated under interacting climate-related abiotic factors. Bars indicate Fisher’s Least Significant Difference (LSD, p ≤ 0.05). Different letters indicate significant differences.
3. Discussion
The study has examined, for the first time, the potential effects of interacting climate change-related abiotic factors and the relative resilience of strains of A. flavus in terms of growth and AFB1 production on pistachio-based matrices and on raw pistachio nuts. The in vitro studies also examined the effects on structural and regulatory genes involved in the biosynthesis of aflatoxin-related secondary metabolites. Overall, these studies showed that there were no significant differences in relative growth rates of the two A. flavus strains in relation to existing and future climate-related environmental stresses, both in vitro on the PNA medium or in situ when colonizing raw pistachio nuts. Interestingly, on the milled pistachio nut-based nutritional media AFB1 production were stimulated by exposure to 1000 ppm CO2 but only at 35 °C. Colonization of raw pistachio nuts resulted in some stimulation of AFB1 at 37 °C but only with exposure to existing CO2 concentrations.
The present study focused on 35 °C and 37 °C as the two temperatures to compare. This is because the water and temperature relations of four strains, including the two in this study, of A. flavus isolated from pistachio nuts under prevailing CO2 conditions (400 ppm), showed that colonization was optimal at 35 °C and 0.98 aw, regardless of whether the pistachio-based medium was modified with ionic (NaCl) or non-ionic (glycerol) solutes [3]. Thus, an increase of 2 °C was chosen to represent climate-related increases in the next 10–15 years. Previous studies with maize, wheat and coffee have all used +4 °C and double or triple the existing CO2 levels and in some cases drought stress [11,13,19,20,21].
Most of the previous work on the resilience of A. flavus strains to climate change-related abiotic factors has focused on those originating from maize [8,22]. On a conducive defined nutritional medium for toxin production, it was found that growth was unaffected by 650 or 800 ppm CO2 exposure at 35 °C under freely available or water stress conditions. In addition, both aflD and aflR gene expression and phenotypic AFB1 production were significantly stimulated when compared to 30 °C + 400 ppm CO2 and 0.93–0.98 aw [16,22]. Subsequent studies on stored maize grain confirmed this stimulation, although the stimulation was not as profound as that observed in vitro [7,16]. Work on other mycotoxigenic fungi in grapes with strains of A. carbonarius examined fluctuating extreme day/night temperature cycles and elevated CO2 (1000 ppm) and found an overall stimulation of growth and OTA production [23]. This contrasted with studies on coffee which showed stimulatory effects on OTA production by A. westerdijkiae strains but not strains of A. carbonarius [20].
The results obtained for the two strains of A. flavus in terms of relative expression of mycotoxin-related biosynthesis were different. They were both molecularly identified and compared with a type strain of A. flavus originating from maize. It is thus difficult to explain the differences found between strains AB3 and AB10. The relative expression of both aflD and aflR observed could be due to the molecular analyses after 10 days incubation. The structural aflD gene is early in the biosynthetic pathway and usually expressed earlier than the aflR. The results for the regulatory gene thus may be of more interest [20]. Previous studies have suggested that the aflD gene, on maize-based matrices is expressed after 4–5 days [24,25]. However, the aflR gene expression appeared to parallel the AFB1 production by both A. flavus strains. This was especially so at 35 °C + 1000 ppm CO2 at 0.98 aw when compared to the control.
Medina at el. [22] found that the maximum relative expression of aflD gene was at 34 °C + 400 ppm CO2 with a decrease in expression with elevated CO2 and water stress. In contrast, aflR gene expression was found to significantly increase, only under drier conditions (0.92 aw + 650 ppm CO2). In contrast, the expression of both aflD and aflR genes increased significantly at 37 °C under treatment conditions of 0.95/0.92 aw and 650 and 1000 ppm CO2. These increases were associated with an increase in AFB1 production. More recent studies of the impact of two way aw × temperature interactions and three-way climate-related interacting factors showed that there were significant changes in the transcriptome of A. flavus both in vitro and in stored maize. Secondary metabolite pathways, sugar transporters and other related gene clusters were found to significantly change in RNAseq studies [7,16]. In the studies performed with A. carbonarius on grape-based media, fluctuating day/night temperatures and 1000 ppm CO2 resulted in an up-regulation of both structural (AcOTApks, AcOTAnrps, AcOTAhal, AcOTAp450, AcOTAbZIP) and regulatory genes of the velvet complex (laeA/veA/velB, “velvet complex”) involved in OTA biosynthesis [23]. Studies with Fusarium langsethiae, a non-xerophilic species, which grows under cooler conditions on oats was also found to responded to three-way climate-related abiotic factors. These showed that the Tri5 gene expression was reduced in all conditions except at elevated temperature, 30 °C, when compared to 25 °C, and exposure to 1000 ppm CO2 with a 5.3-fold significant increase in expression. Other biosynthetic genes (Tri6, Tri16) were upregulated, in elevated CO2 conditions. In addition, mycotoxin production was higher at 25 °C than at 30 °C in vitro. In stored oats, at 0.98 aw, elevated CO2 led to a significant increase (73-fold) in T2/HT-2 toxin, especially at 30 °C [26].
Overall, the effects of three-way interacting climate-related abiotic factors on mycotoxin production by different toxigenic species have found differential effects. Thus, colonization and ochratoxin A (OTA) production by strains of A. westerdijkiae and A. carbonarius on coffee-based media and in stored coffee showed some differences [11,19,20]. Akbar et al. [20] showed that for A. westerdijkiae, while growth was relative unaffected, OTA production was stimulated by climate change-related interacting factors, both in vitro and in situ. However, for A. carbonarius there was no effect on growth or OTA production. Thus, differential effects of these interacting factors may occur. Vaughan et al. [10] found effects on fungal biomass during infection of maize by F. verticillioides in ripening maize, but no effect on fumonisins production when exposed to 650 ppm CO2. However, when drought stress was included, there was a stimulation of fumonisins production in ripening maize cobs [24].
Another important factor may be the relative changes in pest damage. It has been suggested that pest reproduction rates increase significantly under climate-related scenarios and more damage to ripening crops may also influence toxin production and perhaps the ratio of related toxins [7,13]. In addition, the impact of acclimatisation needs to be addressed. Studies by Vary et al. [27] showed that growing F. graminearum for 20 generation in elevated CO2 resulted in higher infection of ripening wheat, with increased head blight symptoms. Studies of acclimatization of A. flavus strains from pistachios showed that after five generations of sub-culturing at 1000 ppm CO2, some strains produced more AFB1 than the same strains which were non- acclimatized [13,28]. This suggests that more definitive studies are necessary on the acclimatisation of fungal pathogens and their effect on crops under climate-related scenarios to better understand their resilience and infection rates of different economically important commodities [13].
In terms of impacts of climate change scenarios, more studies are necessary to examine whether A. flavus strains may switch to the production of other mycotoxins, such as cyclopiazonic acid, or whether there could be a change in the ration of these mycotoxins and other secondary metabolites. This type of information together with ecophysiological data on optimal and marginal conditions for growth and AFB1 production [3] could be beneficial in the development of accurate predictive models on the relative risks of AFB1 toxin contamination of pistachios under future climate-related abiotic conditions.
4. Conclusions
This study has shown that A. flavus strains are very resilient when exposed to three-way interacting climate change-related abiotic factors. These was no impact on growth and colonization of both pistachio nut-based media and raw pistachio nuts. However, there were some effects in vitro on the relative expression of a structural and regulatory genes (aflD, aflR) and on AFB1 production. Exposure to elevated atmospheric CO2 affected AFB1 production with an increase in toxin production at 35 °C + 1000 ppm CO2. Significantly less AFB1 was produced at 37 °C + 1000 ppm CO2, regardless of the aw level examined, when compared to the control (400 ppm CO2).
5. Materials and Methods
5.1. Aspergillus flavus Strains
Two strains of A. flavus isolated from pistachio nut samples were molecularly identified using two sets of primers (ITS 1/2, ITS 3/4, Table S1). They were coded as AB3 and AB10 and identification was further confirmed by comparison with a characterized type strain of A. flavus (NRRL 3357) from the Agricultural Research Service Culture Collection (USA), isolated from maize grain (Table S1). They were all aflatoxin B1 producers [3].
5.2. Preparation of In Vitro Growth Media and Inoculation
The medium used was a 3% milled pistachio nut agar (PNA). To prepare this medium, raw unsalted pistachio nut were milled to a powder in a homogeniser. The milled pistachio powder was then sieved to obtain a uniform size. Thirty grams (30 g) of the pistachio powder and 20 g technical agar (Thermo Fisher Scientific Oxoid Ltd, Basingstoke, Hampshire, UK) and 0.05 g chloramphenicol (antibacterial agent) was added to 1 L distilled water for the basal medium (0.99 aw). The aw was modified by initially making up mixtures of water/glycerol solutions by adding 122.5, 245.0 and 355 g/1 L glycerol to water. These were shaken vigorously and then measured and added, in a similar manner to water, to the pistachio nut flour and agar. This gave the target water availability conditions of 0.93, 0.95 and 0.98, respectively. The treatments were then autoclaved at 121 °C for 15 min. After autoclaving, the PNA treatments were cooled, mixed thoroughly, poured into 9 cm sterile Petri plates (17.5–20 mL per plate) and allowed to completely cool and solidify. The aw was measured using an Aqualab 4TE (Decagon Instruments) and found to be within 0.003 aw of the target values. The media aw treatments were enclosed in separate closed polyethylene bags and stored at 4 °C until use.
The PNA plates were subsequently equilibrated at 25 °C and then centrally inoculated with the strains of A. flavus (AB3, AB10). Inoculum consisted of a conidial spore suspension of each strain made from fresh 5–7 days old growing cultures on PNA at 25 °C. The culture surface was gently scraped with sterile loop and conidia were transferred into sterile 25 ml Universal glass tubes containing 10 ml sterile water + 0.1% Tween 80 solution (Tween 80, ACROS organics, Fisher Scientific, Loughborough, UK). The concentration of the spore suspension was determined using a haemocytometer (Olympus BX40 microscope, Microoptical Co.; slide Marienfeld superior, Germany; microscope glass cover slips, No. 3, 18 × 18 mm, Chance Propper Ltd, Smethwick, Warley, UK) and adjusted by dilution with sterile water to 106 spores/mL. The treatments and replicates were centrally inoculated with 10 µL of the spore suspension. The different aw treatments and replicates were then centrally inoculated with the individual A. flavus strains (AB3, AB10). Three replicates of each strain were placed in the CC environmental chambers and the Equilibrium Relative Humidity (ERH) was maintained with glycerol/water solutions (2 × 500 mL) in beakers to maintain the target aw levels. The experiments were carried out twice.
5.3. Climate Change Experimental System
The environmental chambers used included an inlet and outlet valve on either side and could be sealed during exposure and storage [8]. The in vitro and in situ treatments and replicates were placed into individual separate chambers. They were flushed with either air (400 ppm) or 1000 ppm CO2 (speciality gases; British Oxygen Company, Guildford, Surrey, UK). The chambers were flushed with the required CO2 concentrations at a rate of 2 L/min to replace 3× chamber volume. This process was repeated every two days immediately after growth measurements were recorded and the chambers then sealed again. The treatments were incubated at 35 and 37 °C. Trial measurements showed that the CO2 build up during incubation, because of the chamber volume was only increased slightly over the two days period by between 125–150 ppm when measured using Gas Chromatography.
5.4. Effect of Interacting Climate Change Abiotic Factors on In Situ Colonisation of Pistachio Nuts by Aspergillus flavus
5.4.1. Moisture Adsorption Curve
The pistachio nuts used in this study were gamma irradiated at 12–15 kGys (Synergy Health Sterilisation UK Ltd., Swindon, Wiltshire, UK) to remove any resident microbiota present. To accurately modify the aw of the raw pistachio nuts, a water adsorption curve was developed. The relationship between added water and aw was obtained by adding known amounts of water to 5 g sub-samples of raw pistachio nuts in 25 mL Universal glass bottles. These were shaken, sealed and left at 4 °C overnight. After thorough mixing and equilibration at 25 °C, the aw was determined for each sub-sample using the Aqualab 4TE water activity meter (Aqualab 4TE; Decagon Devices, Inc., Pullman, WA, USA). The data for aw vs. amounts of added water were plotted. This was used to accurately add known quantities of sterile water to the pistachio nuts to accurately obtain the target aw treatment values. We used the following modified aw levels of the raw pistachio nuts: 0.93, 0.95 and 0.98 (=13–14, 18–19, 26–27% moisture content, m.c.). The pistachio nuts were placed in sterile Duran flasks with the added sterile water and equilibrated at 4 °C overnight with thorough periodic mixing. The pistachio nuts were equilibrated in the laboratory and the aw values confirmed.
5.4.2. Inoculation and Growth Assessment of Effects of Climate Change-Related Abiotic Factors on Colonization of Pistachio Nuts
Single layers of treatment pistachio nuts were spread into 9 cm Petri plates in a sterile flow bench. These were centrally inoculated with an individual A. flavus strain (AB3, AB10). Inoculum was obtained by spread plating 0.2 mL of a 106 mL conidial suspension onto 9 cm PNA Petri plates. These were incubated at 25 °C overnight. A 4 mm diameter surface-sterilised cork-borer was used to obtain agar discs containing germlings to centrally inoculate the layers of raw pistachio nut treatments using a surface-sterilised inoculum needle. The experiment was carried out with three replicates per treatment at 35 and 37 °C and repeated once. Inoculated treatment and replicate Petri plates for each strain were immediately placed into the environmental chambers, closed and flushed with CO2 as described previously. The colonization rates were measured in two directions at right angles to each other, every two days for ten days.
5.5. Gene Expression Studies
Sampling was carried out following ten days incubation in triplicate for gene expression studies. The biomass was aseptically harvested, immediately frozen in liquid nitrogen, and stored at −80 °C for subsequent RNA extraction.
5.5.1. RNA Isolation from Pistachio Nuts
Using the bead-beating method published by Leite et al. [29], RNA was extracted with some modifications. Frozen biomass (150 mg) was transferred into an autoclaved 2 mL extraction tube containing 0.5 mm sized glass beads. Then, 1 mL of RLT buffer (provided by the RNeasy® Plant Mini Kit) (Qiagen, Hilden, Germany) supplemented with 10 µL of β-mercaptoethanol was added. The tubes were immediately frozen in liquid nitrogen. After a quick vortex to help disrupt the mycelium, samples then were placed on ice for thawing. The tubes were agitated for 25 s followed by a 5 s interval and another 25 s of agitation. This mixture was centrifuged at 9820 g for 5 min at 4 °C in a temperature-controlled centrifuge system. A pre-frozen 2 mL Safe-Lock tube (Eppendorf, Hamburg, Germany) was used to place the collected supernatant. According to instructions of the RNeasy® Plant Mini Kit (Qiagen, Germany), the RNA purification was carried out. RNA obtained was eluted in 50 µL of RNase free water and kept at −80 °C until used for reverse transcription. The RNA concentration and purity (A260/A280 ratio) were determined spectrophotometrically using a 2.5 µL aliquot on the PicodropTM (Spectra Services Inc., Phoenix, AZ, USA).
5.5.2. Primers and Probes
Nucleotide sequences of primers and probes used in this study are included in Table 1 [18]. The design of the primer pairs of nortaq-1/nortaq2 and aflRtaq1/aflRTaq2 and the hydrolysis probe norprobe and AflRprobe were, respectively, designed based on the aflD and aflR genes involved in the aflatoxin biosynthetic pathway. The primer pair bentaq1/bentaq2 and the hydrolysis probe benprobe were designed and relied on the β-tubulin gene. The norprobe and AflRprobe were labelled at the 5′ end with the reporter molecule 6-carboxyfluorescein (FAM) and at the 3′ end with the quencher Black Hole Quencher 2 (BHQ2). However, the reporter cyanine-5 (CY5) was used for the benprobe and labelled at the 5′ end with the quencher BHQ2 at the 3′ end.
Table 1.
Nucleotide sequences of primers for RT-qPCR assay designed on the basis of AflD (nor-1), AflR and β-tubulin genes.
| Gene | Primers and Probes | Primer Sequence | Position |
|---|---|---|---|
| AflD | nortaq1 | GTCCAAGCAACAGGCCAAGT | 516 a |
| nortaq2 | TCGTGCATGTTGGTGATGGT | 562 a | |
| norprobe | [FAM]TGTCTTGATCGCGCCCG[BHQ2] | 537 a | |
| AflR | aflRTaq1 | TCGTCCTTATCGTTCTCAAGG | 1.646 b |
| aflRTaq2 | ACTGTTGCTACAGCTGCCACT | 1.735 b | |
| aflRprobe | [FAM]AGCAGGCACCCAGTGTACCTCAAC[BHQ2] | 1.6889 b | |
| β-tubulin | Bentaq1 | CTTGTTGACCAGGTTGTCCAT | 65 c |
| Bentaq2 | GTCGCAGCCCTCAGCCT | 99 c | |
| benprobe | [CY5]CGATGTTGTCCGTCGCGAGGCT[BHQ2] | 82 c |
a Positions are in accordance with the published sequence of the aflD gene of Aspergillus flavus (GeneBank accession no. XM_002379908.1). b Positions are in accordance with the published sequence of aflR gene of Aspergillus flavus (GeneBank accession no. AF441435.2). c Positions are in accordance with the published sequence of ß-tubulin gene of Aspergillus flavus (GeneBank accession no. AF036803.1).
5.5.3. Reverse Transcription to Convert cDNA from mRNA
Five μL of total RNA (≈500 ng) was used to synthesise cDNA from mRNA. For this, the Omniscript RT kit (Qiagen) was used, and the protocol described by the manufacturer was followed. The reverse transcription component mix consisted of 2 µL 10× Buffer RT, 2 µL dNTP Mix (5 mM each dNTP), 2 µL Oligo-dT primer (10 µM), 1 µL RNase inhibitor (10 units/µL), 1 µL Omniscript Reverse Transcriptase, 7 µL RNase-free water, and 5 µL total RNA. Once the mix was ready, it was incubated for 60 min at 37 °C. cDNA was stored at −20 ℃ for long-term storage.
5.5.4. Amplification of aflD and aflR Genes Through Real-Time Quantitative PCR (RT-qPCR)
To amplify the structural gene aflD (nor-1) and the regulatory gene aflR of the aflatoxin biosynthetic pathway as the target genes, a quantitative RT-qPCR assay was used. The β-tubulin gene was used as a control gene [9,18]. The Bio-Rad CFX96 Real Time PCR Detection System (Bio-Rad, Watford, UK) was used to perform two RT-qPCR assays to amplify the aflD gene and the housekeeping β-tubulin gene in the first one, and the other one to quantify the aflR gene expression using the β-tubulin gene as control [16]. They were prepared in triplicates of 12.5μL reaction mixture in MicroAmp optical 96-well reaction plates and sealed with optical adhesive covers (Bio-Rad). Three replicates of an RNA control sample together with a template-free negative control were also included in the runs. The TaqMan system with primers and probes were used in all cases. The reaction mixtures consisted of 6.25 μL Premix Ex TaqTM (Takara Bio Inc., Otsu, Shiga, Japan), 830 nM of each primer, 330 nM of each probe, and 1.5 μL of cDNA template in a final volume of 12.5 μL. The optimal thermal cycling conditions included an initial step of 10 min at 95 °C and all 45 cycles at 95 °C for 15 s, 55 °C for 20 s and 72 °C for 30 s. Ct determinations were automatically performed by the instrument using default parameters and obtained from the BIO-RAD detection system.
Data analysis was carried out using the software CFX ManagerTM Software (Bio-Rad). Relative quantification of the expression of aflD and aflR genes were carried out using the housekeeping gene β-tubulin as an endogenous control to normalise the quantification of the mRNA target for differences in the amount of total cDNA added to the reaction in the relative quantification assays and used for all treatments. The expression ratio was calculated as previously described by Livak and Schmittgen [30]. Prior to the analysis, we found that the experimental treatments did not influence the expression of the internal control gene, and the amplification efficiencies of the target and reference genes were practically equal (93.1% for aflR and 95.2% for β-tubulin genes). This method allows for the calculation of the expression ratio of a target gene between a tested sample and its relative calibrator (“control” sample). In this work, the calibrator corresponded to A. flavus strains grown at 35 °C and 0.98 aw at atmospheric air (400 ppm CO2). Log2 values of the relative expression of the aflD and aflR genes were graphically represented. The statistical design was factorial CRD, 2 factors and the statistical analysis obtained using SPSS® software.
5.6. Quantification of Aflatoxin B1 Production
Aflatoxin B1 quantification: Preparation of aflatoxin standards: A 200 μL stock solution of aflatoxins (B1, B2, G1, G2) standard in methanol containing 250 ng AFB1 was prepared and pipetted into 2 mL Eppendorf tubes for overnight evaporation until dryness in a fume hood similar to the samples.
In vitro aflatoxin B1 analyses: Colony Extraction: Initially agar plugs were cut out across the diameter of colonies using a surface sterilised 4 mm diameter cork borer (approx. 4–6). The agar plugs were placed in pre-weighed 2 mL Eppendorf tube and weighed again. Five-hundred millilitres of HPLC-grade chloroform was added to the tubes and shaken for 30 min using a KS 501 digital orbital shaker (125 rpm; IKA (R) Werke GmbH & Co. KG, Germany). The chloroform extract was transferred to a new Eppendorf tube, dried gently under air for derivatisation.
Derivatisation of aflatoxin B1 extract: Derivatisation of the AFB1 extract was performed according to the AOC method (Kok, 1994). First, 200 μL hexane was added to the tube followed by 50 μL of triflouroacetic acid. The mixture was vortexed for 30 s and left for 5 min. A mixture of water:acetonitrile (9:1) was then added to the tube, and vortexed for 30 s and left for 10 min to allow for separation of the layers. Then, the aqueous layer was filtered using a syringe nylon filter (13 mm × 0.22 μm; Jaytee Biosciences Ltd., Herne Bay, UK) into amber salinized 2 mL HPLC vials (Agilent, Santa Clara, CA, USA) before HPLC analysis. All analytical reagents used were HPLC-grade.
Quantification of aflatoxin B1 with High Performance Liquid Chromatography HPLC: A reverse-phase HPLC with fluorescence detection was used to confirm the identity and quantify AFB1. An Agilent 1200 series HPLC system was used for the analysis. It consisted of an in-line degasser, auto sampler, binary pump and a fluorescence detector (excitation and emission wavelengths of 360 and 440 nm, respectively). Separation was achieved using a C18 column (Phenomenex Gemini; 150 × 4.6, 3 μm particle size; Phenomenex, Torrance, CA, USA) with a Phenomenex Gemini C18 3 mm, 3 μm guard cartridge. Isocratic elution with methanol:water:acetonitrile (30:60:10, v/v/v) as the mobile phase was performed at a flow rate of 1.0 mL/min. The injection volume was 20 µL. A set of standards was injected (1 to 5 ng AFB1, AFB2, AFG1 and AFG2 per injection) and standard curves were generated by plotting the area underneath the peaks against the amounts of AFB1 standard injected.
Isolation and Quantification of Aflatoxin B1 in Pistachio Nuts
The pistachio nut samples were all dried in a drying oven at 50 °C in the dark. They were subsequently ground (Waring blender, Merck Ltd., Feltham, UK) and weighed (25 g). The background aflatoxin B1 levels in the nuts used in the experiments was 0.015 ng/g. This was taken into account as a correction factor in the final quantification of the results. Acetonitrile/water 60/40 (100 mL) was used as an extraction solvent. The mixture was blended for 3 min and the extract filtered into a smaller sample container. PBS buffer (pH 7.4, Thermo Fisher Scientific) was used for sample dilution, then the diluted extract was passed through an Immunoaffinity Column (IAC; AflaStar™; Romer Labs, Tulln an der Donau, Austria) with a flow rate between 1–3 mL/min. The column was rinsed with 2 × 10 mL sterile distilled water. HPLC-grade methanol (1.5–3 mL) was then applied to the column and the eluent was collected in a new amber glass vial and left to dry overnight at room temperature before the derivatisation step as detailed previously.
5.7. Statistical Analysis
Three replicates per treatment were used in all experimental studies and carried out twice. Means were obtained by taking the average of each of the three measurements with the standard error of the means (±SE). Datasets were tested for normality and homoscedasticity using the Shapiro–Wilk and Levene test, respectively. Analysis of Variance (ANOVA) was applied to analyse the variation of means with 95% confidence interval. Normal distribution of data were checked by the normality test Kolmogorov–Smirnov using Minitab statistical software. Fisher’s Least Significant Difference (LSD) was used to identify differences between the means with p ≤ 0.05 as significant difference using the same statistical software.
Acknowledgments
The authors are grateful to Taif University Researchers Supporting Project Number (TURSP-2020/295), Taif University, Taif, Saudi Arabia for funding this research which was carried out in the Applied Mycology Group, Cranfield University, United Kingdom.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/toxins13060385/s1, Table S1. Sequencing results of isolated strains and type strain using ITS1 and 2 and ITS3 and 4 primer pairs for molecular identification of strains AB3 and AB10.
Author Contributions
Conceptualization, N.M.; Data curation, A.B. and A.R.; Investigation, A.B.; Methodology, A.R., A.M. and N.M.; Supervision, A.R., A.M. and N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the Results and Conclusions of this article are deposited and available via the corresponding author at Cranfield University.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This is the first study to examine the potential impacts that three-way interacting climate change-related abiotic factors affect growth, biosynthetic genes involved in aflatoxin production and phenotypic AFB1, both in vitro on milled raw pistachio nut-based media and in situ on shelled raw pistachio nuts.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAOSTAT . Top Production—Pistachios 2020. FAO; Rome, Italy: 2020. [Google Scholar]
- 2.Kaminiaris M.D., Camardo Leggieri M., Tsitsigiannis D.I., Battilani P. AFLA-PISTACHIO: Development of a mechanistic model to predict the aflatoxin contamination of pistachio nuts. Toxins. 2020;12:445. doi: 10.3390/toxins12070445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baazeem A., Garcia-Cela E., Medina A., Magan N. Interacting abiotic factors affect growth and aflatoxin B1 production profiles of strains of Aspergillus flavus on pistachio-based matrices and stored pistachio nuts. Front. Microbiol. 2021;11:624007. doi: 10.3389/fmicb.2020.624007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.International Agency for Research on Cancer . Monographs on the Evaluation of Carcinogenic Risks to Humans: Chemical Agents and Related Occupations. A Review of Human Carcinogens. Volume 100. International Agency for Research on Cancer; Lyon, France: 2012. pp. 224–248. [Google Scholar]
- 5.Bui-Klimke T.R., Guclu H., Kensler T.W., Yuan J.-M., Wu F. Aflatoxin Regulations and Global Pistachio Trade: Insights from Social Network Analysis. PLoS ONE. 2014;9:e92149. doi: 10.1371/journal.pone.0092149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Medina A., Gilbert M.K., Mack B.M., Obrian G., Rodriguez A., Bhatnagar D., Payne G., Magan N. Interactions between water activity and temperature on the Aspergillus flavus transcriptome and aflatoxin B1 production. Int. J. Food Microbiol. 2017;256:36–44. doi: 10.1016/j.ijfoodmicro.2017.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert M.K., Medina A., Mack B.M., Lebar M., Rodriguez A., Bhatnagar D., Magan N., Obrian G., Payne G. Carbon dioxide mediates the response to temperature and water activity levels in Aspergillus flavus during infection of maize kernels. Toxins. 2018;10:5. doi: 10.3390/toxins10010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Cela E., Verheecke-Vaessen C., Gutierrez-Pozo M., Kiaitsi E., Gasperini A.M., Magan N., Medina A. Unveiling the effect of interacting forecasted abiotic factors on growth and Aflatoxin B1 production kinetics by Aspergillus flavus. Fungal Biol. 2020;125:89–94. doi: 10.1016/j.funbio.2020.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Hadi A., Schmidt-Heydt M., Parra R., Geisen R., Magan N. A systems approach to model the relationship between aflatoxin gene cluster expression, environmental factors, growth and toxin production by Aspergillus flavus. J. R. Soc. Interface. 2012;9:757–767. doi: 10.1098/rsif.2011.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaughan M., Huffaker A., Schmelz E.A., Dafoe N.J., Christensen S., Sims J., Martins V.F., Swerbilow J.A.Y., Romero M., Alborn H.T. Effects of elevated [CO2] on maize defense against mycotoxigenic Fusarium verticillioides. Plant Cell Environ. 2014;37:2691–2706. doi: 10.1111/pce.12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Akbar A., Medina A., Magan N. Resilience of Aspergillus westerdijkiae strains to interacting climate-related abiotic factors: Effects on growth and ochratoxin A production on coffee-based medium and in stored coffee. Microorganisms. 2020;8:1268. doi: 10.3390/microorganisms8091268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NASA Carbon Dioxide, Latest Measurements. [(accessed on 8 February 2021)];2021 Available online: https://climate.nasa.gov/vital-signs/carbon-dioxide/
- 13.Medina A., Akbar A., Baazeem A., Rodriguez A., Magan N. Climate change, food security and mycotoxins: Do we know enough? Fungal Biol. Rev. 2017;31:143–154. doi: 10.1016/j.fbr.2017.04.002. [DOI] [Google Scholar]
- 14.Klich M.A. Environmental and developmental factors influencing aflatoxin production by Aspergillus flavus and Aspergillus parasiticus. Mycoscience. 2007;48:71–80. doi: 10.1007/S10267-006-0336-2. [DOI] [Google Scholar]
- 15.Kovac T., Šarkanj B., Crevar B., Kovac M., Ante Loncaric A., Strelec I., Ezekiel C.N., Sulyok M., Krska R. Aspergillus flavus NRRL 3251 Growth, oxidative status, and aflatoxins production ability in vitro under different illumination regimes. Toxins. 2018;10:528. doi: 10.3390/toxins10120528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Medina A., Rodriguez A., Magan N. Effect of climate change on Aspergillus flavus and aflatoxins. Front. Microbiol. 2014;5:348–354. doi: 10.3389/fmicb.2014.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magan N., Medina A., Aldred D. Possible impacts of climate change on mycotoxins in food crops pre- and post-harvest. Plant Pathol. 2011;60:150–163. doi: 10.1111/j.1365-3059.2010.02412.x. [DOI] [Google Scholar]
- 18.Medina A., Rodríguez A., Sultan Y., Magan N. Climate change factors and A. flavus: Effects on gene expression, growth and aflatoxin production. World Mycotoxin J. 2015;8:171–179. doi: 10.3920/WMJ2014.1726. [DOI] [Google Scholar]
- 19.Bhatnagar D., Rajasekaran K., Gilbert M., Cary J.W., Magan N. Research advances to safeguard food and feed supply from aflatoxin contamination. World Mycotoxin J. 2018;11:47–72. doi: 10.3920/WMJ2017.2283. [DOI] [Google Scholar]
- 20.Akbar A., Medina A., Magan N. Impact of climate change factors on growth and ochratoxin A production by Aspergillus sections Circumdati and Nigri species on coffee. World Mycotoxin J. 2016;9:863–874. doi: 10.3920/WMJ2016.2041. [DOI] [Google Scholar]
- 21.Vaughan M., Block A., Christensen S.A., Allen L.H., Schmelz E.A. The effect of climate change associated abiotic stressors on phytochemical defences. Phytochem. Rev. 2017;17:37–49. doi: 10.1007/s11101-017-9508-2. [DOI] [Google Scholar]
- 22.Medina A., Rodriguez A., Magan N. Climate change and mycotoxigenic fungi: Impacts on mycotoxin production. Curr. Opin. Food Sci. 2015;5:99–104. doi: 10.1016/j.cofs.2015.11.002. [DOI] [Google Scholar]
- 23.Cervini C., Verkeecke-Vaessen C., Ferrara M., García-Cela E., Magistàa D., Medina A., Gallo A., Magan N., Perrone G. Interacting climate change factors (CO2 and temperature cycles) on growth, secondary metabolite gene expression and phenotypic ochratoxin A production by Aspergillus carbonarius strains on a grape-based matrix. Fungal Biol. 2021;125:115–122. doi: 10.1016/j.funbio.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Hadi A., Carter D., Magan N. Temporal monitoring of the nor-1 (aflD) gene of Aspergillus flavus in relation to aflatoxin B1 production during storage of peanuts under different environmental conditions. J. Appl. Microbiol. 2010;109:1914–1922. doi: 10.1111/j.1365-2672.2010.04820.x. [DOI] [PubMed] [Google Scholar]
- 25.Bernáldez V., Córdoba J.J., Magan N., Peromingo B., Rodríguez A. The influence of ecophysiological factors on growth, aflR gene expression and aflatoxin B1 production by a type strain of Aspergillus flavus. LWT Food Sci. Technol. 2017;83:283–291. doi: 10.1016/j.lwt.2017.05.030. [DOI] [Google Scholar]
- 26.Verheecke-Vaessen C., Diez-Gutierrez L., Renaud J., Sumarah M., Medina A., Magan N. Interacting climate change environmental factors effects on Fusarium langsethiae growth, expression of TRI genes and T-2/HT-2 mycotoxin production on oat-based media and in stored oats. Fungal Biol. 2019;123:617–623. doi: 10.1016/j.funbio.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Vary Z., Mullins E., Mcelwain J.C., Doohan F. The severity of wheat diseases increases when plants and pathogens are acclimatised to elevated carbon dioxide. Glob. Chang. Biol. 2015;21:2661–2669. doi: 10.1111/gcb.12899. [DOI] [PubMed] [Google Scholar]
- 28.Baazeem A. Ph.D. Thesis. Applied Mycology Group, Cranfield University; Cranfield, UK: 2018. Ecology, Climate Change and Control Strategies for Aspergillus flavus Colonization and Aflatoxin Contamination of Pistachio Nuts. [Google Scholar]
- 29.Leite G.M., Medina A., Magan N. Comparison of different bead-beating RNA extraction strategies: An optimized method for filamentous fungi. J. Microbiol. Meth. 2012;88:413–418. doi: 10.1016/j.mimet.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the Results and Conclusions of this article are deposited and available via the corresponding author at Cranfield University.