Abstract
Introduction:
The development of new antidepressant is crucial to overcome the remission rate limitation. Anthocyanin on purple sweet potatoes (PSP) from East Java cultivar previously demonstrated a behavioural effect. However, the certain mechanism and the nutritional compound need further exploration.
Aim:
This study aimed to characterize macronutrient content, amino acids, anthocyanin, and revealed the potential of PSP from East Java-Indonesia as antidepressant agent through D2-dopamine receptor (D2DR).
Methods:
This study was characterized the macronutrient content using proximate analysis. The amino acids were analysed using Ultra-Performance Liquid Chromatography (UPLC) and High-Performance Liquid Chromatography (HPLC). Anthocyanin was identified using Ultra High-Performance Liquid Chromatography (UHPLC). Molecular docking was conducted to predict the interaction between anthocyanins and D2 dopamine receptor.
Results:
We were found the predominance of water on proximate analysis. Alanine was demonstrated as the highest content of amino acid. Cyanidin, cyanidin-3-O-glucoside and peonidin-3-O-glucoside were identified as major anthocyanin content. Molecular docking was showed that cyanidin bound to similar binding site with dopamine on D2DR with stronger interaction than cyanidin-3-glucoside.
Conclusion:
Current study was indicated that cyanidin as major anthocyanin from purple sweet potatoes has potential health beneficial as antidepressant potential candidate.
Keywords: purple potatoes, anthocyanin, physicochemical, in-silico, dopamine
1. INTRODUCTION
Depression is one of serious mental disorder worldwide. Approximately, more than 300 million people are living with depression. Nowadays, depression ranked as the first largest global contributor to non-fatal health loss. According to the World Health Organization (WHO) data, over 50 million depressed people live with disability. This disease burden affects more than 80% of low-middle income countries (1). The burden are originating from the declining of psychosocial function (2), suicide (3) and other associated physical illness especially various chronic diseases such as diabetes, hypertension, heart disease, arthritis, asthma, chronic obstructive pulmonary disorder (4) and chronic pain (5). Early detection and adequate treatment should be performed in order to reduce this disease burden (6).
Antidepressant is an important part of depression treatment algorithm (7). Serotonin selective reuptake inhibitors (SSRIs) drug are widely prescribed in health care setting. The efficacy and relatively benign side effects are favorable advantages of SSRI application. However, the low remission rate, i.e. 30% and long onset of SSRIs therapeutic cannot be ruled out as drug limitation (8). Considering these limitations, researcher had been developing the alternative approaches for investigate the new potential of antidepressant. Recently, intensive research that use plant bioactive compound as antidepressant are growing tremendously (9).
Flavonoid as natural products from various plants previously reported as antidepressant in animal model studies, for instance citrus maxima leaf (Sheik, et al., 2014), icariin from Epimedium brevicornum Maxim, kaempferitrin from Asteraceae, luteolin from Cirsium japonicum also quercetin in onion, apple, and broccoli. Although the mechanism remains uncertain, it suggested that flavonoid can form an interaction with monoamine receptor including dopaminergic receptors (11).
Anthocyanin is one of plant flavonoid that previously demonstrated a preventive effect in animal models of psychological stress. Anthocyanin from blueberry was showed increases the dopamine neurotransmitter in several brain area of those animal models (12). Anthocyanins are also contained in other plants including sweet purple potatoes (13). Earlier studies were revealed the neuroprotective effect of anthocyanin from purple sweet potatoes (14). Our previous work was suggested the effect of anthocyanin from East Java cultivar on behaviour of prenatal stress model mice (15). However, the nutritional value and the predictive mechanism of anthocyanin from East Java PSP as antidepressant is remain unclear and need further studies.
2. AIM
The aim of this study was to identify the nutritional value such as macronutrient, amino acids, anthocyanin profiles as well as determination the agonist function of anthocyanin on D2DR. This study would be supporting the potential use of anthocyanin from PSP as drug candidate for antidepressant.
3. METHODS
Ethics
This research was approved by Research Ethic Committee of Universitas Brawijaya No:1193-KEP-UB.
Plant and chemical materials
The East Java cultivar of PSP were harvested from Legumes and Tubers Research Institute (Malang, East Java, Indonesia) at four months planting age. The standard of peonidin-3-O-glucoside (≥95% purity, Cat.0929 S), cyanidin (≥96% purity, Cat.0909 S) and cyanidin-3-O-glucoside (≥96% purity, Cat.0915 S) were purchased from Extrasynthese (France).
Total anthocyanin extraction and calculation
Fresh grinded tuber roots were macerated overnight in acidified methanol at room temperature, followed by filtration and evaporation (16). Total anthocyanin content was expressed as cyanidin-3-glucose per 100g sample (C3G mg/100g) as previously described (17).
Anthocyanin identification
The Ultra-High Performance Liquid Chromatography with diode array detector (UHPLC DAD, Agilent 1100 series) was used to identify the anthocyanin compounds according to previous protocol. In brief, 2 μl of extract (0.5mg/ml) was injected into system and separated through a column of Zorbax SB-C18 2.1x150 mm 1.8-micron (Agilent, USA; Part Number:5188-5328). The 0.2% formic acid and acetonitrile were set as mobile phase with flow rate at 0.2 ml/min (18).
Amino acid and proximate analysis
The amino acids were analyzed using Ultra-Performance Liquid Chromatography (UPLC) except the tryptophan by High-Performance Liquid Chromatography (HPLC). The analysis of procedures were performed according to previous method (19). The percentage of water, ash, total fat, protein and carbohydrate were analyzed and calculated as previously described with minor modification (20).
Molecular interaction and physicochemical prediction
Ligand of cyanidin (CID: 128861) and cyanidin-3-O-glucoside (CID: 441667) were retrieved from PubChem National Centre for Biotechnology Information (NCBI) database. Protein of D2-dopamine receptor (ID: 6CM4) was imported from Protein Data Bank (http://rcsb.org). The PyRx 0.8 software was used to dock the ligand with D2DR to predict interaction and energy binding. The interaction was visualized using Discovery Studio Visualizer v19.1.0.18287 program (21). The physicochemical properties and bioactivity score of ligands were predicted using Molinspiration online software and classified as previously detailed (22)(23).
4. RESULTS
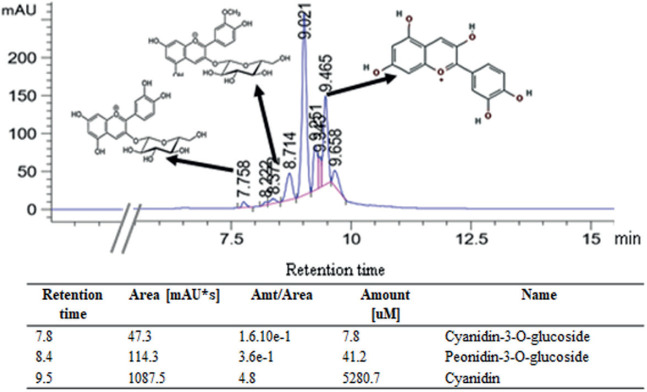
The total anthocyanin was calculated to be 150 mg/100g. Three anthocyanins were tentatively identified as cyanidin, cyanidin-3-O-glucoside and peonidin-3-O-glucoside (Figure 1). We found higher component of water (77.2%) followed by carbohydrate (21.1%), protein (0.9%), ash (0.7%) and fat (<0.02%). Amino acid of alanine was the highest content (917.3 mg/kg) followed by histidine (914.9 mg/kg), serine (830.2 mg/kg), glutamic acid (823.9 mg/kg), and aspartate (803.7mg/kg). Cystine, methionine and tryptophan were not detected on our PSP.
Figure 1. The identification of anthocyanin was determined using UHPLC analysis. The level of absorbance was measured at 520nm wavelengths. Three major anthocyanins were identified.
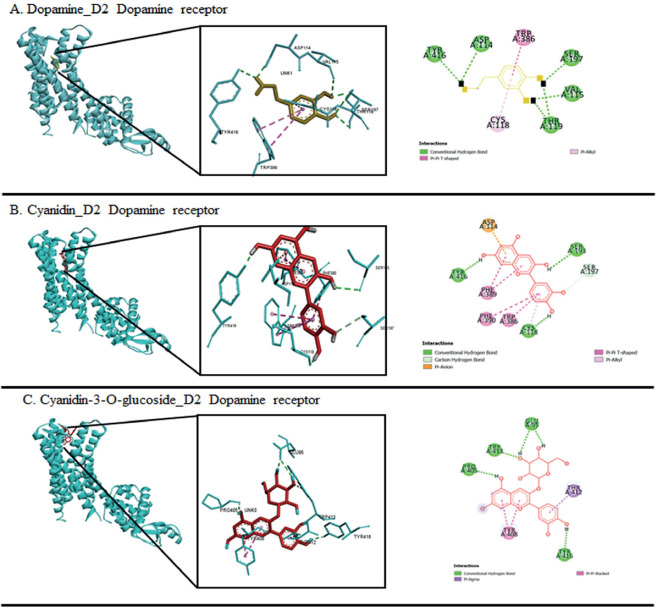
Molecular interaction of cyanidin_D2DR was facilitated by 4 hydrogen bond, 1 electrostatic bond and six hydrophobic bonds (Table 1) at residues ASP114, CYS118, SER193, SER197, TRP386, PHE389, PHE390, and TYR416 thus resulting binding affinity -9.6 kcal/mol. These binding sites were similar with the interaction residue of dopamine_D2DR bond. Meanwhile, cyanidin-3-O-glucoside was demonstrated less binding site at residues GLU95, PRO405, TYR408, THR412, TRP413, and TYR416 with binding affinity of -8.9 kcal/mol (Figure 2).
Table 1. Interaction ligand of dopamine, cyanidin and cyanidin-3-O-glucoside on D2DR.
| Interaction D2DR with dopamine | |||
| Interaction | Distance (A0) | Category | Type |
| UNK1:H- ASP114:OD2 | 2.6 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK1:H- TYR416: OH | 2.7 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK1:H-VAL115:O | 2.6 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK1:H-THR119:OG1 | 1.9 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK1:H – THR119:OG1 | 3.1 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK1:H - SER197:O | 2.5 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK1 - TRP386 | 4.9 | Hydrophobic | Pi-Pi T-shaped |
| TRP386-UNK1 | 5.4 | Hydrophobic | Pi-Pi T-shaped |
| UNK1 - CYS118 | 4.0 | Hydrophobic | Pi-Alkyl |
| Interaction D2DR with ligand cyanidin | |||
| UNK0:H19 - SER193:O | 2.7 | Hydrogen bond | Conventional hydrogen bond |
| UNK0:H20 - CYS118:O | 2.1 | Hydrogen bond | Conventional hydrogen bond |
| UNK0:H18 - TYR416: OH | 1.9 | Hydrogen bond | Conventional hydrogen bond |
| A:SER197:CB:UNK0:019 | 3.0 | Hydrogen bond | Conventional hydrogen bond |
| A:ASP114:OD2 -UNK0 | 3.4 | Electrostatic | Pi-Anion |
| UNK0 – TRP386 | 4.7 | Hydrophobic | Pi-Pi T-shaped |
| UNK0 – PHE390 | 5.1 | Hydrophobic | Pi-Pi T-shaped |
| UNK0 - PHE389 | 4.9 | Hydrophobic | Pi-Pi T-shaped |
| UNK0 - PHE389 | 4.6 | Hydrophobic | Pi-Pi T-shaped |
| A:TRP386 -UNK0 | 4.9 | Hydrophobic | Pi-Pi T-shaped |
| UNK0 - CYS118 | 4.1 | Hydrophobic | Pi-Alkyl |
| Interaction D2DR with cyanidin-3-O-glucoside | |||
| UNK0:H25 - GLU95:OE1 | 1.9 | Hydrogen bond | Conventional hydrogen bond |
| UNK0:H26 - GLU95:OE1 | 2.8 | Hydrogen bond | Conventional hydrogen bond |
| UNK0:O28-A:PRO405:O | 2.2 | Hydrogen bond | Conventional hydrogen bond |
| UNK0:H31-A:TYR416:OH | 2.2 | Hydrogen bond | Conventional hydrogen bond |
| A:TRP413:NE1-UNK0:26 | 2.9 | Hydrogen bond | Conventional hydrogen bond |
| A:THR412:CG2-UNK0 | 4.0 | Hydrophobic | Pi-Sigma |
| UNKO-A:TYR408 | 4.7 | Hydrophobic | Pi-Pi Stacked |
| UNKO-A:TYR408 | 4.1 | Hydrophobic | Pi-Pi Stacked |
Figure 2. The molecular docking interaction visualization of dopamine (A), cyanidin (B) and cyanidin-3-O-glucoside (C) with D2DR. Dopamine is shown as yellow while anthocyanins are shown as red color. The left panel showed 3D interaction and the right panel is the 2D diagram interaction.
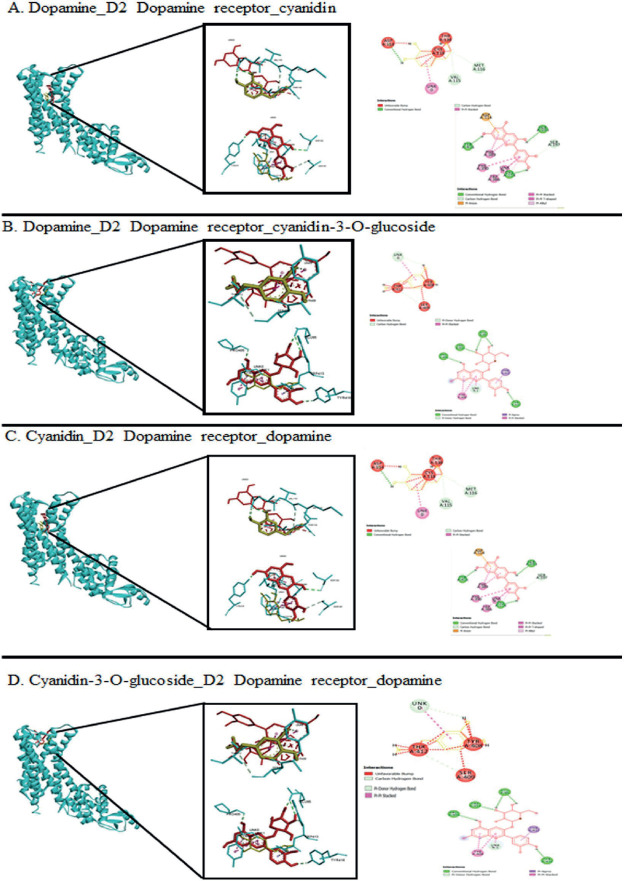
Additionally, we were performed docking interaction between anthocyanin_D2DR complex with ligand dopamine and resulting more stable interaction with the receptor (Figure 3). In a hence, to ensure the possibility of both anthocyanins as drug candidate, we were analysed their physicochemical properties and biological activities. According to Lipinski’s rule of five, cyanidin was predicted to have better absorption and permeability than cyanidin-3-O-glucoside due to cyanidin-3-glucoside has 8 hydrogen bond donors (OH and NH groups) and 11 hydrogen bond acceptors (N and O). For biological activities prediction, based on the score, both cyanidin and cyanidin-3-glucoside were predicted as enzyme and kinases inhibitors. Cyanidin was predicted as moderately active protease inhibitors contrast to cyanidin-3-glucoside, which were predicted to be inactive activity as protease inhibitors.
Figure 3. The Dopamine_D2DR complex was docked with anthocyanin (A-B). The Anthocyanins_D2DR complex were interacted with dopamine. Dopamine is shown as yellow while anthocyanins are shown as red color. The left panel showed 3D interaction and the right panel is the 2D diagram interaction.
5. DISCUSSION
Current research was revealed the differences of amino acid concentration in compared to our previous work (19). Environmental conditions such as water, light, heat, and cold have an important regulation in plant amino acid metabolism. Drought stress, heat stress and light stress were included into abiotic stress for plants and affect the amino acids biosynthesis pathway (24).
Amino acids have important regulation for physiological function in human, for instance protein synthesis, hormone synthesis, immune response regulation, antioxidant defenses, neurotransmitter synthesis, wound healing, and cell signaling (25)(26).
Human body can synthesize amino acid that are classified into non-essential amino acid such as glutamate, proline, glycine, glutamine, cysteine, arginine, aspartate, alanine, asparagine, serine, and tyrosine. Others amino acids cannot be synthesized in human therefore dietary requirement are needed to maintenance the physiological process, i.e. histidine, leucine, tryptophan, threonine, phenylalanine, methionine, isoleucine, lysine, and valine (27).
Our proximate analyses were demonstrated lower carbohydrate, higher protein, higher water and lower fat compared to earlier investigation using PSP from Malaysia (28). Rodrigues, et al (29) was conducted similar analyses using fresh purple sweet potatoes from Brazil. Those study was revealed a higher protein, fat, and carbohydrate in compared to current results The variation of proximate analysis can be determined by several factors including geographical, soil fertility and period of harvest (30). Total anthocyanin content on our PSP is slightly higher than previously reported (31). Other study was reported total anthocyanin content of 132 mg/100g from PSP at China (32). The difference of solvent can affect the yield of anthocyanin extraction. The use of acidic solvent can improve the extracted anthocyanin level (33). In addition, other factors for instance the color of tuber roots, varieties, climate, and agricultural characteristic were correlated with the anthocyanin quantities (34).
Earlier research was identified similar aglycone i.e., cyanidin and peonidin from various cultivar of PSP using Ultra-performance Liquid Chromatography Photodiode Array (UPLC-PDA), which were identified as cyanidin-3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside,cyanidin-3-caffeoyl-vanilloyl-sophoroside-5-glucoside, and peonidin-3-caffeoyl-vanilloyl-sophoroside-5-glucoside(35). Other study has demonstrated two major acetylated anthocyanin in PSP, the proposed compound are b-D-glucopyranoside)-5-O-(b-D-lucopyranoside) and peonidin-3-O-(6-O-(E)-caffeoyl-(2-O-(6-O-(E)-feruloyl)-b-D-glucopyranosyl)-b-D-glucopyranoside)-5-O-(b-D-glucopyranoside) (36). Current study was used three anthocyanin standards, therefore the broad anthocyanin characters on PSP has not been fully identified. Anthocyanin content in plant tissues can be influenced by nutrition during plant growth. For instance, the application of magnesium, potassium, calcium, and nitrogen as nutrient resulting a positive effect for anthocyanin in various fruits (37). Anthocyanin is secondary metabolites which are responsible to provide spectrum color blue, purple and red in various plant tissues (38). Color spectrum was influenced by the ratio of cyanidin and peonidin. Previous study well documented blue color predominance in sweet potatoes which has peonidin/cyanidin ratio < 1 so called cyanidin-type, whereas the peonidin type has ratio peonidin/cyanidin > 1 that dominance in red color (39).
Previous study correlated our finding with earlier study, which was predicted the binding site of agonist human D2 dopamine receptor. The essential amino acid residues were ASP114 in trans membrane (TM) 3 domain and SER197 in TM5 domain. The mostly hydrophobic pocket component for dopamine binding as agonist, were CYS118, PHE189, TRP386 and PHE390 (40). In accordance with our analyses, we predicted that cyanidin might have stronger potential to substitute dopamine at dopamine-2 receptor. D2 dopamine receptor distribution in central nervous systems is associated with emotional regulation at dopaminergic pathway i.e. striatum including nucleus accumbens and ventral tegmental area (41). Considering that depression is correlated with dopaminergic dysregulation, therefore D2-dopamine receptor are interesting to be develop as potential target for depression therapeutic (41)(42).
6. CONCLUSION
This study indicated that cyanidin as major anthocyanin from purple sweet potatoes (Ipomoea batatas) and has being more potential as antidepressant activity through D2- dopamine receptor interaction. Future studies are necessary to confirm this antidepressant function in preclinical approaches.
SUPLEMENT TABLES.
| Interaction D2DR_dopamine with cyanidin | |||
| Point of interaction | Distance (Ao) | Category | Type |
| UNK0:H19 - SER197:O | 2.0 | Hydrogen bond | Conventional hydrogen bond |
| UNK0:H20 - CYS118:O | 2.3 | Hydrogen bond | Conventional hydrogen bond |
| UNK0:H18 - TYR416:OH | 2.4 | Hydrogen bond | Conventional hydrogen bond |
| THR119:OG1 -UNK0:O19 | 2.9 | Hydrogen bond | Conventional hydrogen bond |
| ASP114:OD2 -UNK0 | 3.4 | Electrostatic | Pi-Anion |
| UNK0 - PHE198 | 5.9 | Hydrophobic | Pi-Pi T-shaped |
| UNK0 - TRP386 | 4.7 | Hydrophobic | Pi-Pi T-shaped |
| UNK0 - PHE389 | 4.9 | Hydrophobic | Pi-Pi T-shaped |
| UNK0 - PHE389 | 4.6 | Hydrophobic | Pi-Pi T-shaped |
| TRP386 -UNK0 | 4.9 | Hydrophobic | Pi-Pi T-shaped |
| UNK0 - CYS118 | 4.1 | Hydrophobic | Pi-Alkyl |
| Interaction D2DR_dopamine with cyanidin-3-O-glucoside | |||
| UNK0:H25 - GLU95:OE1 | 2.4 | Hydrogen bond | Conventional hydrogen bond |
| UNK0:H31 - TYR416:OH | 1.9 | Hydrogen bond | Conventional hydrogen bond |
| SER409:OG - UNK0:O28 | 3.2 | Hydrogen bond | Conventional hydrogen bond |
| THR412:CG2 - UNK0 | 3.6 | Hydrophobic | Pi-Sigma |
| UNK0 - TYR408 | 4.7 | Hydrophobic | Pi-Pi Stacked |
| UNK0 - TYR408 | 4.0 | Hydrophobic | Pi-Pi Stacked |
| Interaction Cyanidin_ D2DR with dopamine | |||
| UNK1:H - A:ASP114:OD1 | 2.2 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK1:H - A:ASP114:OD1 | 2.4 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK1:C - A:VAL115:O | 3.5 | Hydrogen Bond | Carbon Hydrogen Bond |
| UNK1:C - A:MET116:O | 3.3 | Hydrogen Bond | Carbon Hydrogen Bond |
| UNK1 - :UNK0 | 3.7 | Hydrophobic | Pi - Pi Stacked |
| Interaction Cyanidin-3-glucoside_D2DR with dopamine | |||
| UNK1:H - A:ASP114:OD1 | 2.2 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK1:H - A:ASP114:OD1 | 2.4 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK1:C - A:VAL115:O | 3.5 | Hydrogen Bond | Carbon Hydrogen Bond |
| UNK1:C - A:MET116:O | 3.3 | Hydrogen Bond | Carbon Hydrogen Bond |
| UNK1 - :UNK0 | 3.7 | Hydrophobic | Pi - Pi Stacked |
| Interaction Cyanidin_ D2 dopamine receptor with dopamine | |||
| UNK1:H - A:ASP114:OD1 | 2.2 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK1:H - A:ASP114:OD1 | 2.4 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK1:C - A:VAL115:O | 3.5 | Hydrogen Bond | Carbon Hydrogen Bond |
| UNK1:C - A:MET116:O | 3.3 | Hydrogen Bond | Carbon Hydrogen Bond |
| UNK1 - :UNK0 | 3.7 | Hydrophobic | Pi - Pi Stacked |
| Interaction Cyanidin-3-glucoside with D2 dopamine receptor | |||
| Interaction | Distance (A0) |
Category | Type |
| UNK0:H25 - A: GLU95:OE1 | 1.8 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK0:H26 - A: GLU95:OE1 | 2.8 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK0:H28 - A:PRO405:O | 2.2 | Hydrogen Bond | Conventional Hydrogen Bond |
| UNK0:H31 - A: TYR416:OH | 2.2 | Hydrogen Bond | Conventional Hydrogen Bond |
| TRP413:NE1 - :UNK0:O26 | 2.9 | Hydrogen Bond | Conventional Hydrogen Bond |
| THR412:CG2 - :UNK0 | 3.6 | Hydrophobic | Pi-Sigma |
| UNK0 - A: TYR408 | 4.7 | Hydrophobic | Pi-Pi Stacked |
| UNK0 - A:TYR408 | 4.1 | Hydrophobic | Pi-Pi Stacked |
| Interaction Cyanidin-3-glucoside_D2 dopamine receptor with ligand dopamine | |||
| UNK1:C - A: SER409:O | 3.7 | Hydrogen Bond | Carbon Hydrogen Bond |
| UNK1:H -UNK0 | 2.9 | Hydrogen Bond | Pi-Donor Hydrogen Bond |
| UNK1 -UNK0 | 3.8 | Hydrophobic | Pi-Pi Stacked |
| UNK1 -UNK0 | 4.8 | Hydrophobic | Pi-Pi Stacked |
Acknowledgement:
We gratefully acknowledge to JRK Cairns, Ciptati, and SMONAGENES members for supporting this research.
Author contributions:
NK, RR, TAN, MA, and FF had contribution in research design. NK, and FF were contributed for data acquisition. NK, RR, TAN, and FF was interpretated the data. The draft manuscript were prepared by NK and FF. All authors revising it critically for intellectual content.
Conflict of interest:
No conflict of interest
Funding:
This work was financially supported by the Ministry of Research and Technology/National Research and Innovation Agency of Republic Indonesia, with grant number: 127/SP2H/LT/DRPM/2020.
REFERENCES
- 1.World Health Organization. Geneva: World Health Organization; Depression and Other Common Mental Disorders:Global Health Estimates. [Google Scholar]
- 2.Kessler RC, Bromet E J. The epidemiology of depression across cultures. Annu Rev Public Heal. 2013;34:1–22. doi: 10.1146/annurev-publhealth-031912-114409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li H, Luo X, Ke X, Dai Q, Zheng W, Zhang C, et al. Major depressive disorder and suicide risk among adult outpatients at several general hospitals in a Chinese Han population. PLoS One. 2017;12(10):e0186143. doi: 10.1371/journal.pone.0186143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhattacharya R, Shen C, Sambamoorthi U. Excess risk of chronic physical conditions associated with depression and anxiety. BMC Psychiatry. 2014;14(1) doi: 10.1186/1471-244X-14-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cocksedge KA, Simon C, Shankar R. A difficult combination: Chronic physical illness, depression, and pain. Br J Gen Pract. 2014;64(626):440–441. doi: 10.3399/bjgp14X681241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McKeever A, Agius M, Mohr P. A Review of The Epidemiology of Major Depressive Disorder and of its Consequences for Society and The Individual. Psychiatr Danub. 2017;29(Suppl 3):222–231. [PubMed] [Google Scholar]
- 7.Gautam S, Jain A, Gautam M, Vahia VN, Grover S. Clinical Practice Guidelines for the management of Depression. Indian J Psychiatry. 2017;59(Suppl 1):S34–50. doi: 10.3389/fnins.2019.00404.ternet. Available from: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2971469/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vahid-Ansari F, Zhang M, Zahrai A, Albert PR. Overcoming resistance to selective serotonin reuptake inhibitors: Targeting serotonin, serotonin-1A receptors and adult neuroplasticity. Front Neurosci. 2019;13(404):1–16. doi: 10.3389/fnins.2019.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matraszek-Gawron R, Chwil M, Terlecka P, Skoczylas MM. Recent studies on anti-depressant bioactive substances in selected species from the genera Hemerocallis and Gladiolus: A systematic review. Pharmaceuticals. 2019;12(172):1–32. doi: 10.3390/ph12040172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheik HS, Vedhaiyan N, Singaravel S. Evaluation of central nervous system activities of Citrus maxima leaf extract on rodents. J Appl Pharm Sci. 2014;4(9):77–82. doi: 10.7324/JAPS.2014.40914. [DOI] [Google Scholar]
- 11.Hritcu L, Ionita R, Postu PA, Gupta GK, Turkez H, Lima TC, et al. Antidepressant Flavonoids and Their Relationship with Oxidative Stress. Oxid Med Cell Longev. 2017;2017:1–18. doi: 10.1155/2017/5762172. (Article ID 5762172) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang JL, Luo Y, Jin SH, Yuan K, Guo Y. Ameliorative effect of anthocyanin on depression mice by increasing monoamine neurotransmitter and up-regulating BDNF expression. J Funct Foods. 2020;66(January):103757. doi: 10.1016/j.jff.2019.103757. [DOI] [Google Scholar]
- 13.Rahman MM, Ichiyanagi T, Komiyama T, Sato S, Konishi T. Effects of anthocyanins on psychological stress-induced oxidative stress and neurotransmitter status. J Agric Food Chem. 2008;56(16):7545–7550. doi: 10.1021/jf800930s. [DOI] [PubMed] [Google Scholar]
- 14.Steed LE, Truong VD. Anthocyanin content, antioxidant activity, and selected physical properties of flowable purple-fleshed sweetpotato purees. J Food Sci. 2008;73(5):215–221. doi: 10.1111/j.1750-3841.2008.00774.x. [DOI] [PubMed] [Google Scholar]
- 15.Kurnianingsih N, Ratnawati R, Nazwar TA, Ali M, Fatchiyah F. The behavioral effect of anthocyanin from purple sweet potatoes on prenatally stressed offspring mice. Syst Rev Pharm. 2020;11(10):482–490. doi: 10.31838/srp.2020.10.72. [DOI] [Google Scholar]
- 16.Chon SU, Boo HO, Heo BG, Gorinstein S. Anthocyanin content and the activities of polyphenol oxidase, peroxidase and phenylalanine ammonia-lyase in lettuce cultivars. Int J Food Sci Nutr. 2012;63(1):45–48. doi: 10.3109/09637486.2011.595704. [DOI] [PubMed] [Google Scholar]
- 17.Hwang E, Thi N Do. Antioxidant and anti-inflammatory activities of Orostachys japonicus. Asian Pacific Trop Biomed. 2020;10(11):516–522. doi: 10.4103/2221-1691.294092. [DOI] [Google Scholar]
- 18.Sari DRT, Paemanee A, Roytrakul S, Cairns JRK, Safitri A, Fatchiyah F. Black rice cultivar from Java Island of Indonesia revealed genomic, proteomic , and anthocyanin nutritional value. Acta Biochim Pol. 2021;68(5386):1–8. doi: 10.18388/abp.2020_5386. [DOI] [PubMed] [Google Scholar]
- 19.Kurnianingsih N, Ratnawati R, Fatchiyah F, Barlianto W, Ali MM, Safitri A, et al. The Difference of Amino Acid Profiling From Two Morphological Purple The Difference of Amino Acid Profiling From Two Morphological Purple Sweet Potatoes From Kawi Mountain Cultivars, East Java, Indonesia. J Phys Conf Ser. 2019;1374(012017):3–8. doi: 10.1088/1742-6596/1374/1/012017. [DOI] [Google Scholar]
- 20.Khan N, Ruqia B, Hussain J, Jamila N, Rahman NU, Hussain S. Nutritional assessment and proximate analysis of selected vegetables from Parachinar Kurram Agency. Am J Res Commun. 2013;1(8):184–198. [Google Scholar]
- 21.Tri A, Safitri A, Fatchiyah F. An in silico approach reveals the potential function of cyanidin-3-o-glucoside of red rice in inhibiting the advanced glycation end products (AGES)-receptor (RAGE) signaling pathway. Acta Inform Med. 2020;28(3):170–179. doi: 10.5455/aim.2020.28.170-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 23.Alodeani EA, Arshad M, Izhari MA. Anti-uropathogenic activity, drug likeness, physicochemical and molecular docking assessment of (E-)-N0-(substituted-benzylidene)-2-(quinolin-8-yloxy) acetohydrazide. Asian Pac J Trop Biomed. 2015;5(8):676–683. doi: 10.1016/j.apjtb.2015.04.010. [DOI] [Google Scholar]
- 24.Galili G, Amir R, Fernie AR. The Regulation of Essential Amino Acid Synthesis and Accumulation in Plants. Annu Rev Plant Biol. 2016;67:153–178. doi: 10.1146/annurev-arplant-043015-112213. [DOI] [PubMed] [Google Scholar]
- 25.Hou Y, Yin Y, Wu G. Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp Biol Med. 2015;240:997–1007. doi: 10.1177/1535370215587913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu G. Functional amino acids in nutrition and health. Amino Acids. 2013;45(3):407–411. doi: 10.1007/s00726-013-1500-6. [DOI] [PubMed] [Google Scholar]
- 27.Choi BH, Coloff JL. The diverse functions of non-essential amino acids in cancer. Cancers (Basel) 2019;11(675):1–17. doi: 10.3390/cancers11050675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dusuki NJS, Abu Bakar MF, Abu Bakar FI, Ismail NA, Azman MI. Proximate composition and antioxidant potential of selected tubers peel. Food Res. 2020;4(1):121–126. doi: 10.26656/fr.2017.4(1).178. [DOI] [Google Scholar]
- 29.Rodrigues N da R, Barbosa JL, Barbosa MIMJ. Determination of physico-chemical composition, nutritional facts and technological quality of organic orange and purple-fleshed sweet potatoes and its flours. Int Food Res J. 2016;23(5):2071–2078. [Google Scholar]
- 30.Bhandari MR, Kasai T, Kawabata J. Nutritional evaluation of wild yam (Dioscorea spp.) tubers of Nepal. Food Chem. 2003;82:619–623. doi: 10.1016/S0308-8146(03)00019-0. [DOI] [Google Scholar]
- 31.Yuzhi Jiao, Jiang Y, Zhai W, Yang Z. Studies on antioxidant capacity of anthocyanin extract from purple sweet potato (Ipomoea batatas L.) African J Biotechnol. 2012;11(27):7046–7054. doi: 10.5897/AJB11.3859. [DOI] [Google Scholar]
- 32.Abou-Arab AA, Abu-Salem FM, Abou-Arab EA. Physico-chemical properties of natural pigments (anthocyanin) extracted from Roselle calyces (Hibiscus subdariffa) J Am Sci. 2011;7(7):445–456. [Google Scholar]
- 33.Hamouz K, Lachman J, Pazderů K, Tomášek J, Hejtmánková K, Pivec V. Differences in anthocyanin content and antioxidant activity of potato tubers with different flesh colour. Plant, Soil Environ. 2011;57(10):478–485. doi: 10.17221/265/2011-PSE. [DOI] [Google Scholar]
- 34.He W, Zeng M, Chen J, Jiao Y, Niu F, Tao G, et al. Identification and Quantitation of Anthocyanins in Purple-Fleshed Sweet Potatoes Cultivated in China by UPLC-PDA and UPLC-QTOF-MS/MS. J Agric Food Chem. 2016;64(1):171–177. doi: 10.1021/acs.jafc.5b04878. [DOI] [PubMed] [Google Scholar]
- 35.Zhang JL, Luo CL, Zhou Q, Zhang ZC. Isolation and identification of two major acylated anthocyanins from purple sweet potato (Ipomoea batatas L. cultivar Eshu No. 8) by UPLC-QTOF-MS/MS and NMR. Int J Food Sci Technol. 2018;53(8):1932–1941. doi: 10.1111/ijfs.13780. [DOI] [Google Scholar]
- 36.Jezek M, Zörb C, Merkt N, Geilfus CM. Anthocyanin Management in Fruits by Fertilization. J Agric Food Chem. 2018;66:753–764. doi: 10.1021/acs.jafc.7b03813. [DOI] [PubMed] [Google Scholar]
- 37.Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017;61(1) doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Montilla EC, Hillebrand S, Winterhalter P. Anthocyanins in purple sweet potato ( Ipomoea batatas L .) Varieties. Fruit, Veg Cereal Biotechnol. 2011;5(Special Issue 2):19–24. [Google Scholar]
- 39.Kalani MYS, Vaidehi N, Hall SE, Trabanino RJ, Freddolino PL, Kalani MA, et al. The predicted 3D structure of the human D2 dopamine receptor and the binding site and binding affinities for agonists and antagonists. Proc Natl Acad Sci U S A. 2004;101(11):3815–3820. doi: 10.1073/pnas.0400100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bonci A, Hopf FW. The dopamine D2 receptor: New surprises from an old friend. Neuron. 2005;47(3):335–338. doi: 10.1016/j.neuron.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 41.Belujon P, Grace AA. Dopamine system dysregulation in major depressive disorders. Int J Neuropsychopharmacol. 2017;20(12):1036–1046. doi: 10.1093/ijnp/pyx056. [DOI] [PMC free article] [PubMed] [Google Scholar]