Abstract
The natural abundance of heavy stable isotopes (13C, 15N, 18O, etc.) is now of considerable importance in many research fields, including human physiology. In fact, it varies between tissues and metabolites due to isotope effects in biological processes, that is, isotope discriminations between heavy and light isotopic forms during enzyme or transporter activity. The metabolic deregulation associated with many diseases leads to alterations in metabolic fluxes, resulting in changes in isotope abundance that can be identified easily with current isotope ratio technologies. In this review, we summarize the current knowledge on changes in natural isotope composition in samples (including various tissues, hair, plasma, saliva) found in patients compared to controls, caused by human diseases. We discuss the metabolic origin of such isotope fractionations and highlight the potential of using isotopes at natural abundance for medical diagnosis and/or prognostic.
Keywords: isotope effect, fractionation, metabolic partitioning, diabetes, cancer, metal homeostasis
1. Introduction
It is now more than a century that Marie Curie Skłodowska provided key advances on (radioactive) isotopes and how they can be used for human health. In the second part of the XXth century, stable isotopes have then been exploited in medicine and biomedical research, mostly using enriched material (isotopic labelling with heavy water 2H2O, 13C-enriched glucose, leucine or urea, …) to quantify water turn-over, follow blood glucose homeostasis or trace the fate of precursors in metabolic pathways (for reviews, see [1,2,3]). Intense efforts are currently devoted to set up diagnostic procedures for metabolism-based pathologies using isotopic labelling. As an example, 13C-phenylalanine and subsequent measurement of respired 13CO2 has recently been proposed for the diagnosis of phenylketonuria, a well-known inborn metabolic disease [4]. The use of isotopically enriched material has two drawbacks: first, it is rather expensive and second, feeding or injecting isotopic products may be associated with long procedures to address ethical or safety imperatives (e.g., European regulation no. 536/2014), although the safety of using stable isotopes is well established [5,6].
During the past two decades, key advances have been made in our knowledge of natural isotope abundance (i.e., without isotopic enrichment) to take advantage of small but detectable differences in natural isotope content between patients and controls, associated with quite a range of pathologies. In fact, all of the natural elements are present in the form of various isotopic forms (e.g., 12C and 13C for carbon) and some changes in isotope ratios (i.e., fractionations) have been found to be specific of diseases, reflecting key alterations in metabolism. In this mini-review, we summarize the current knowledge in fractionations associated with human diseases and discuss the potential of using isotopes at natural abundance for medical diagnosis and/or prognostic.
2. Basics of Stable Isotopes and Metabolic Isotope Effects
2.1. General Principles
Elements forming biological tissues have different stable isotopes, like carbon (12C and 13C) and nitrogen (14N and 15N) for which the heavy form represents about 1.1 and 0.37%, respectively. However, this percentage varies amongst organs, tissues and metabolites and also between C and N atom positions within metabolites. For example, lipids are 13C-depleted compared to sugars, body proteins are generally 15N-enriched compared to exogenous diet proteins, etc. These differences are mostly due to isotope effects, whereby the velocity of enzymatic reactions or transport phenomenon differ between isotopic forms (in the Appendix A, Box A1 for definitions). It is already well-known that decarboxylation reactions discriminate between 12C and 13C so that evolved CO2 is generally 13C-depleted compared to the substrate, and deamination reactions fractionate against 15N so that amino acids left behind are 15N-enriched (and thus excreted ammonia is 15N-depleted). The isotope discrimination (or fractionation), denoted as Δ, is often quantified using the difference between substrate and product isotope composition (or delta value, denoted as δ13C, δ15N, etc.), i.e., Δ = δsubstrate − δproduct. The term “isotope composition” refers to the isotope ratio relative to the international standard (usually measured with isotope ratio mass spectrometry, in the Appendix A, Box A2). Thus, delta values are negative (positive) when the sample of interest contains less (more) heavy isotope than the international standard. Both delta values and fractionations are small numbers and are expressed in per mil (‰).
Box A1. Isotope Composition and fractionation.
δ-value or isotope composition (usually expressed in ‰): abundance of the heavy isotope with respect to that of the light isotope, as compared to the international standard material. It is denoted as δ13C for carbon, δ15N for nitrogen, etc.
Fractionation (Δ): isotopic difference (usually expressed in ‰) between the substrate and the product of a reaction. It is given by the deviation of the isotope effect (α) from 1, as Δ = α − 1. It can be shown that it can be calculated from δ-values in substrate and product as: Δ = (δsubstrate − δproduct)/(δproduct + 1).
Isotope effect (α): ratio of rate constants (klight/kheavy) or equilibrium constants (Klight/Kheavy) of the isotopologues of interest. For enzymatic reaction, it is the ratio of catalytic efficiency V/K: α = (V/K)light/(V/K)heavy.
Isotopologue: isotopic analogue of a molecule, where one atom has been replaced by its isotope. For example, 13C16O2 is the 13C-isotopologue of 12C16O2. It must not be confused with “isotopomers”, which refer to isotopic isomers (for example, 13CH3–12CH2OH and 12CH3–13CH2OH are two isotopomers of ethanol).
Isotope ratio mass spectrometer (IRMS): mass spectrometer based on a magnetic sector with (usually) fixed collectors (Faraday cups) adapted to quantify precisely the abundance of isotopic species of CO2 (13C analysis), N2 (15N analysis), CO (18O analysis), H2 (2H analysis) or SO2 (34S analysis). The historical origin and technical principles of IRMS are reviewed in [66].
Quantitative reaction: chemical reaction that consumes all of the substrate molecules, thereby preventing any isotope fractionation. In non-quantitative reactions, substrate molecules left behind may have a δ-value different from the initial value because the reaction selects for an isotopic species (isotope effect).
Box A2. Isotopic analyses.
The natural abundance of stable heavy isotopes (13C, 15N, 34S, 18O, 2H, etc.) is relatively low for some elements (about 0.01% for 2H for example, while other are abundant, e.g., about 4.3% for 34S) and variations caused by metabolic fractionations are small (a few ‰). Therefore, techniques used to measure isotope natural abundance and its modification in pathologies utilise specific devices with high sensitivity and low measurement errors. The instrument used depends on elements and the type of sample (raw material, extract, etc.).
Analysis of raw material (total organic matter): typically, raw samples are analysed by elemental analysis (EA) coupled to isotope ratio mass spectrometry (IRMS). In the EA, samples are combusted to CO2 and N2 (13C and 15N analysis) or pyrolised to CO and H2 (18O and 2H analysis). The IRMS measures the mass ratio (45/44 for CO2, 29/28 for N2, etc.) that are then converted to isotope ratios (13C/12C, 14N/15N, etc.). IRMS measurements requires comparison with a reference gas of known δ value. Alternatively, the δ value of the reference gas can be determined by comparison of a certified standard sample of known δ value from the international agency for atomic energy (IAEA, Vienna, Austria). EA-IRMS analyses are adapted to raw samples (lyophilised biopsies or exeresis samples), or pre-purified tissue components (e.g., precipitated proteins, extracted lipids).
Compound-specific analyses: the isotope composition of specific, targeted metabolites can be determined via three methods: (i) pre-purification with preparative chromatography followed by EA-IRMS; (ii) liquid chromatography coupled to IRMS via a chemical oxidation interface (LC-co-IRMS); and (iii) gas chromatography coupled to IRMS via a combustion interface (GC-c-IRMS). Method (i) has been used extensively in the 90s in plant biology when LC-co-IRMS and GC-c-IRMS were not available. However, this method requires large amounts of material incompatible with small medical samples. Method (ii) is currently limited to carbon isotopes and metabolites that can be resolved using water as an eluent in the LC system. Method (iii) is widely used, and there is now an enormous associated literature, reviewed in (Tea and Tcherkez [67]).
Gas analyses: gas analysis mostly concerns CO2 produced by respiration and collected in exhaled breath air. There are presently two main techniques to determine the δ13C value in respired CO2: (i) laser-based techniques, and (ii) IRMS-based techniques. Laser-based techniques take advantage of the difference in absorptance between 12CO2 and 13CO2 to compute the 13C/12C ratio of a gas sample. This is a rather simple and instantaneous method that is now implemented routinely for the detection of stomachal ulcers to monitor 13CO2 production from 13C-urea. To perform precise measurements at natural abundance, however, laser-based systems require time-consuming calibration curves not only for CO2 mole fraction but also for δ13C values. This implies the need of gas cylinders at different CO2 concentration and δ values, and thus IRMS measurements for cross-validation. IRMS-based techniques simply use a GC-IRMS coupling whereby air constituents are separated by GC and the CO2 peak is injected into the IRMS. Analyses are slower than with laser systems but provide a precise value with the same requirements as other IRMS measurements.
Heavy atom isotope analyses: since most IRMS systems analyse simple gases (CO2, N2, H2, SO2, CO), they cannot convey metal analyses. To do so, multi-collectors mass spectrometers are required. In such systems, the sample of interest is broken down by inductively coupled plasma (ICP) and isotope analysis is performed at the atomic level (instead of gases). The clear advantage of this type of instrument is its versatility, because many elements can be analysed just by changing source parameters, not only metals (Mg, Fe, Cu, etc.) but also macroelements (such as S).
Other techniques: there is now a considerable interest in techniques that can provide information at the intramolecular level, and not solely a molecular average δ value. In fact, metabolic pathways are so that metabolites are fragmented and assembled and therefore strong differences in δ value are anticipated between atom positions within a metabolite. In plant biology, this topic is currently an intense area of research. However, current methods use nuclear magnetic resonance (NMR) which requires quite large amounts of material incompatible with medical samples. Alternative techniques such as Orbitrap®-based analyses are currently under consideration but not applicable to complex mixtures or small samples.
The fact that isotope effects arise from enzyme action or transport explains why changes in metabolic pathways often lead to changes in delta values. Alterations in delta values can also stem from a source effect, whereby the origin and thus the delta value of the substrate has changed. A well-known example of source effect is the natural isotopic difference between C3 (wheat, rice, beet, etc.) and C4 plants (maize, sorghum, sugar cane, etc.), the latter being naturally 13C-enriched by up to 20‰ compared to the former. This difference allows facile tracing of C4 plants consumption and thus potentially, identification of maize utilisation in food products. A recent interesting example involving both source and metabolic effects has been published recently using a giant type I bladder stone (whewellite) from a Chinese 70 y-old patient. The bladder stone has been analysed layer after layer, and the δ13C value has been found to correlate positively to calcium oxalate content while the δ15N value correlated negatively to struvite (magnesium ammonium phosphate, MgNH4PO4∙6 H2O) content [7]. This indicates that (i) the δ13C of excreted oxalate was very high (near −10‰) pointing to a plant origin (oxalic-rich food such as strong black tea and low-value vegetables), and (ii) the δ15N of ammonia was relatively depleted (near +4‰) reflecting the isotope effect in amino acid deamination reactions.
Medical applications of natural abundance to detect pathologies are based on these principles, that is, a change in delta values caused by alterations in metabolism, nutritional conditions, or recycling efficiency (e.g., hepatic remobilisation). There has been an exponential increase in studies looking at potential changes in delta values in hair, blood (serum or clot), or other sample types associated with a large range of diseases, from metabolic disorders to cancer (summarized in Table 1). It should be kept in mind, however, that resolving the influence of confounding factors can remain challenging. First, there are important differences in diet composition between countries and therefore, delta values are not always comparable (for a recent survey, see [8]). This implies that it is preferable to use relative (i.e., isotopic offset with respect to food intake delta values) rather than absolute delta values. Second, cohorts must be formed with care since there can be unforeseen isotopic differences caused by common medical treatments, local nutritional habits and also importantly, sex [9].
Table 1.
Summary of documented examples of pathologies where isotopes at natural abundance could be used for potential diagnostics. Aa, amino acid; ND, not determined. The term “metabolic mechanism” refers to the major pathways explaining the change in isotope abundance.
| Disease | Metabolic Mechanism | Isotopic Marker | Matrix | Ref. |
|---|---|---|---|---|
| Nervous anorexia, nutritional stress | Aa metabolism | 13C, 15N | Hair | [10,11] |
| Syphilis | Aa metabolism | 13C, 15N | Collagen | [12] |
| Chronic malnutrition and potential growth retardation (stunted children) | Aa metabolism | 13C, 15N | Hair | [13] |
| Patients with metabolic syndrome | Glycaemia Aa metabolism | 13C, 15N | Hair | [14] |
| Diabetic patients | Sugar metabolism | 13C, 15N | Hair | [15,16,17,18] |
| Cirrhotic patients | Aa metabolism | 13C, 15N | Hair, bulk protein | [19] |
| Breast cancer | Urea cycle, glycolysis, lipid synthesis, anaplerosis | 13C, 15N | Tissue biopsies cultured cells | [20,21] |
| Oral squamous cell carcinomas | ND | 13C, 15N | Tissue biopsies | [22] |
| Ganglioneuroma (benign tumours), neuroblastoma and nephroblastoma Wilm’s tumours | Aa metabolism | 13C, 15N | Tissue biopsies | [23,24] |
| Rhabdomyosarcoma | ND | 13C, 15N | Tissue biopsies | [25] |
| Adrenal gland cancers | Aa metabolism Glycolysis | 13C, 15N | Serum | Unpublished data |
| Hepatocarcinoma | Glutathione metabolism, | 34S | Serum and erythrocytes | [26] |
| Wilson disease | Cu metabolism | 65Cu | Serum | [27] |
| Menkes disease | Cu and Aa metabolism | 15N | Hair | Unpublished data |
| Ovarian cancer | Cu metabolism | 65Cu | Serum | [28] |
| Homeostasis alterations after bariatric surgery | Zn homeostasis | 66Zn | Serum and Whole blood | [29] |
| Hematological malignancy | Metal homeostasis | 65Cu, 66Zn | serum | [30] |
| Anaemia | Fe deficiency | 56Fe | Whole blood | [27] |
| Multiple myeloma | Bone formation (apatite deposition) | 44Ca | Serum and urine | [31] |
| Chronic kidney disease or diabetes | Bone formation (apatite deposition) | 44Ca | Serum | [32] |
| Anaemia in skeleton fragments | Respiratory biochemistry | 18O | Bone and enamel apatite | [33] |
| Osteopenia and osteoporosis in female skeleton | Urea excretion and/or renal function | 15N | Bone collagen | [34] |
| Cealiac disease in skeleton | Aa metabolism | 13C, 15N | Bone collagen | [35] |
2.2. Isotopes in Metabolism Preclinical Studies
There is now considerable evidence that pathologies directly related to metabolism (nutritional stress, malnutrition, metabolic syndrome in general, diabetes, and obesity) have an impact on natural isotope abundance. Pre-clinical studies with rats subjected to caloric restriction, normal or high fat diet regimes have demonstrated that caloric restriction causes a general decline in peripheric protein content but the impact on isotope compositions varies between organs [36]. In fact, compared to food intake, liver and plasma proteins are 15N-enriched under caloric restriction while muscles (heart and skeletal muscles) turn to be 15N-depleted [37,38]. In addition, the δ13C value in proteins decreases in all tissues, showing a general 13C-depletion. Such variations are due to changes in amino acid homeostasis, whereby liver oxidises more amino acids and this process discriminates between isotopes (against 15N). Therefore, amino acids left behind and available for protein synthesis are 15N-enriched. Also, the commitment of ingested carbohydrates to biosynthesis of non-essential amino acids increases (at the expense of catabolism and respiration) and as a result, proteins are 13C-depleted.
2.3. Isotopes in Human Metabolic Syndrome, Diabetes, or Nutritional Stress
In humans, several studies took advantage of the delta value of easily accessible samples (blood, hairs) in prediabetic patients or in association with physiological variables. A typical situation has been found in patients affected by nervosa anorexia and nutritional stress during pregnancy, whereby the δ15N values in hair increases, showing the involvement of recycling leading to 15N-enriched amino acids [10,11]. Conversely, an increase in body mass index is associated with an increase in δ13C in hair showing the utilisation of diet proteins [10]. The relationship between isotope abundance in hair and nutritional stress has been reviewed elsewhere [39]. Interestingly, natural isotope abundances are also clearly affected by malnutrition. In collagen extracted from tibiae bone powder (from early XXth century skeletons), syphilitic patients have been found to have a δ15N value up to 0.4‰ lower than controls, while no difference in δ13C was found [12]. It is probable that this effect is caused by malnutrition induced by either syphilis itself (inflammation of the digestive tract) but also from the treatment used in the past (mercury) which causes intestinal lesions and appetite loss. Similarly, in children from Bangladesh with chronic malnutrition and potential growth retardation (stunted children), hairs are both 13C- and 15N-depleted [13]. This effect probably reflects a poor-diet effect whereby low-value food is consumed by either children after weaning, or mothers during pregnancy and lactation.
Hair δ15N has also been shown to increase in patients with metabolic syndrome, while the δ13C value decreases [14]. However, quantitative relationships (regressions) are only significant with glycaemic index and waist circumference. Also, the δ13C value is lower when glycaemia increases, or plasmatic high density lipoprotein (HDL) cholesterol concentration is low. Although this study suggests a link between isotope abundance and remobilisation (amino acid and protein recycling) or sugar utilisation, potential confounding factors were recognized (age, nutritional habits). In a comparison of diabetic patients with controls, no change in hair δ15N was found while the δ13C value declined and a relationship was found with haemoglobin A1 glycation (HbA1c), this relationship being mostly visible in males [15]. However, in another cohort, hair δ15N correlated to plasmatic leptin concentration (in particular in individuals with high body mass index) but no relationship was found with δ13C values [16]. In adolescents or children, the δ13C value in fingerstick blood samples or in erythrocytes has been found to increase at high food intake (increased sugar intake or high calory diet) and this seemed to be unrelated to HbA1c or to C4 sugar consumption [17,40]. Also, no difference in hair δ15N between type 2 diabetes mellitus patients with or without diabetic nephropathy has been found, although hair δ15N correlates to estimated glomerular filtration rate (eGFR) [41].
Since amino acid metabolism (via transamination) is very active in the liver and is associated with different isotope effects [42], changes in δ15N values (and potentially, δ13C values as well) can be anticipated in metabolic diseases affecting liver function, including in cirrhotic patients. Petzke et al. [19] compared the δ15N and δ13C values of hair from patients with liver disease to healthy controls. Bulk protein δ15N was 3.2‰ lower in cirrhotic patients compared to controls without any significant differences in δ13C. Additionally, nearly all amino acids had a lower δ15N in cirrhotic individuals. This effect was probably due to a “decreased nitrogen disposal”, that is, an impairment of N remobilization and thus retention of nitrogen that is normally used and excreted in healthy individuals [34].
2.4. Metabolic Diseases and Isotope Composition of Respired CO2
As discussed above, alterations in isotope compositions with metabolic diseases have been found, but there are presently some undesirable variations across studies. Of course, part of these variations comes from the degree of insulin resistance, glucose capture by muscles and glucose output by the liver, as well as the inflammatory response of the adipose tissue (lipotoxicity). Such physio-pathological factors of obesity and metabolic syndrome, which vary between patients, probably contribute to isotopic variations. Noteworthy, isotopic variations also likely come from differences in country-specific food intake behaviour and thus how patients with diabetes and metabolic syndrome readjust their diet. Still, there is no specific δ13C signature of exhaled breath CO2 in (pre)diabetic patients [18,43]. It has been suggested that (pre)diabetic patients have an altered carbonic anhydrase activity, causing a change in CO2-water oxygen equilibration in tissues and thus a specific δ18O value in exhaled CO2 [18]. This remains controversial since no highly significant difference was found in another study [43]. An interesting study has nevertheless shown a link between respired CO2 and systemic inflammation [32]. In effect, δ13C values of exhaled breath CO2 in ventilated paediatric patients (infants) in intensive care unit were not different between groups with systemic inflammatory response syndrome (SRIS), no SIRS and SIRS with shock; however, breath δ13C value was significantly lower in patients with active sepsis (septic shock), trauma, or after surgery compared to other individuals. Thus, breath δ13C does not appear to be related to SIRS but rather, to the occurrence or severity of systemic inflammation [44].
3. Isotope Fractionation in Cancer
Over the past two decades, isotopes of both macro-elements (C, N, S) and metals have been investigated in biological samples from patients with cancer. In this section, we shall focus on macro-elements so as to relate to potential alterations in cancer metabolism. Metal stable isotopes in cancer are addressed in the next section.
3.1. Cancer Cell Metabolism: Why Might Isotopes Be Impacted?
Presumably, metabolism deregulation in cancer should lead to strong alterations in the abundances of natural isotopes 13C, 15N and 34S since many metabolic pathways accompany oncogenesis. In particular, cancer cells adapt their metabolism to maximize the use of N and C sources for anabolism and biosynthesis of macromolecules required by cell proliferation and tumour growth [45]. It is now well established that cancerous cells show increased glucose and glutamine consumption rates, leading to lactate production as well as augmented nitrogen excretion. In particular, urea cycle (UC) deregulation (recently reviewed in [45]) must in principle result in variations in δ13C and δ15N values (summarized in Figure 1A). How delta values vary in cancerous cells and tissues was unknown until the first investigation was released in a patent based on EA-IRMS technology (see Appendix A, Box A2) applied to biological fluids or tissues associated with cancer [21]. In what follows, we start with breast cancer (BrCa), which is presently the best documented cancer type as isotopes are concerned.
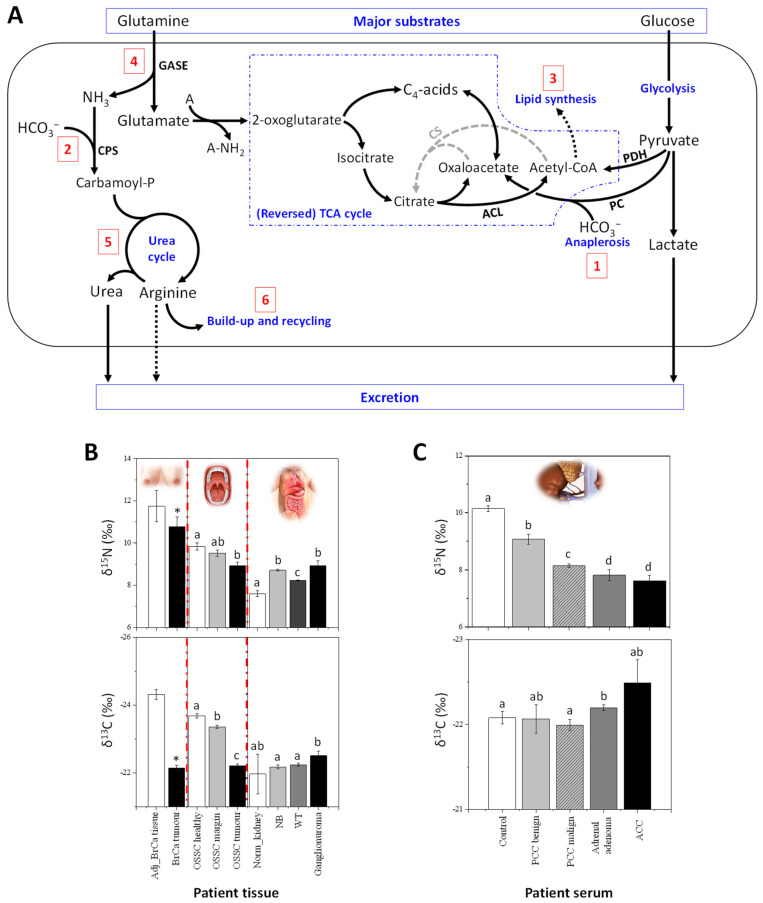
Figure 1.
Natural 13C and 15N abundance in cancerous tissues. (A) Major metabolic pathways explaining the isotope abundance in cancerous cells (redrawn from [20]): the 13C-enrichment mostly comes from the anaplerotic fixation of bicarbonate by pyruvate carboxylase (PC, 1) and carbamoyl-phosphate synthase (CPS, 2) to feed the urea cycle, as well as a lower accumulation and δ13C value of non-structural lipids (3); the 15N-depletion comes from the consumption of glutamine via glutaminase (GASE, 4), isotope effects in the urea cycle (5) and a decreased excretion of 15N-depleted arginine (6). Abbreviations: A, generic amino group acceptor; CS, citrate synthase; PDH, pyruvate dehydrogenase complex. (B) Changes in δ15N and δ13C in breast cancer (BrCa) tissues, oral squamous cell carcinomas (OSCC) [22] and infant cancer patients [23,24,25]. δ15N and δ13C can differentiate adjacent non-cancerous BrCa tissue (Adj BrCa) and tumour tissue (BrCa tumour) [20]. Also, δ15N and δ13C values in OSCC tumour tissues (OSCC tumour) slightly differ from tissues from margin (OSCC margin) and distant oral mucosa (OSCC healthy) [22]. In babies or children, δ15N and δ13C values from ganglioneuroma (benign tumours), neuroblastoma (NB), and nephroblastoma (Wilm’s tumours, WT, which are malignant) are compared to normal kidney cortex tissue (Norm_kydney), used as a control [23,24]. (C) In adults, changes in δ15N and δ13C in serum of patients with different types of adrenal gland cancers: pheochromocytoma (PCC malignant, n = 7; PCC benign, n = 6), adrenal adenoma (n = 10) and adrenocortical carcinoma (ACC, n = 4) which are compared to healthy patients (n = 23) used as controls (unpublished data). Letters above bars stand for statistical classes (ANOVA, p < 0.05). The asterisk indicates statistical significance in pairwise comparison (p < 0.05).
3.2. Breast Cancer
δ13C and δ15N values have been measured on a set of both exeresis samples from patients and cultured BrCa cell lines, showing that cancerous cells with propensity to be invasive are naturally 15N depleted and 13C enriched (Figure 1B) [20]. Furthermore, by using compound-specific analyses (see Box A2), it has been demonstrated that the generation of 15N-depleted arginine and urea by the UC is likely to be at the origin of the 15N depletion in cancerous cells. Also, the natural 13C-enrichment is probably due to anaplerotic C fixation (organic acids formed by pyruvate carboxylase) and/or decreased accumulation of lipids (which are naturally 13C-depleted). In addition, the UC incorporates bicarbonate (naturally 13C enriched) via carbamoyl phosphate and thus, the arginine build-up contributes to the natural 13C enrichment in cancerous cells (summarized in Figure 1A). It is worth noting that delta values have a good potential to distinguishing BrCa subtypes, since they have been shown to have different metabolic phenotypes [46]. For example, cholesterol biosynthesis is stimulated in triple negative BrCa lines [47], meaning that the δ13C cholesterol might change.
3.3. Oral Squamous Cell Carcinomas
Other cancers also appear to be associated with alterations in delta values consistent with BrCa. Oral tissue δ15N has been shown to decrease in patients with oral squamous cell carcinomas (OSCC), while δ 13C values increase [22]. Also, δ15N and δ13C values slightly differ between tissues taken from margin and distant oral mucosa (Figure 1B). Interestingly, this study also revealed that tumour δ13C is positively correlated with alcohol consumption and occurrence of angioinvasion, and inversely related with BMI index.
3.4. Cancer in Infants
In infants or children, the δ15N values in tissues from ganglioneuroma (benign tumours), neuroblastoma and nephroblastoma (Wilm’s tumours, WT, which are malignant) have been found to be higher compared to normal kidney cortex tissue, used as a control [23,24] (summarized in Figure 1B). Isotopic signatures of WT in subsequent stages of cancer disease have also been studied and WT tissues were 15N-depleted and 13C-enriched in stage 3 of the disease compared to stage 2. In a comparison of blastemal WT with anaplasia (focal type), it has been shown that the δ15N values of blastemal WT declined and seemed to be related to a high protein synthesis rate, which characterizes tumours with features of anaplasia and result in aggressive (and usually fatal) cases [24]. Anaplasia is an independent prognostic factor in WT, according to the definition introduced by the National Wilms’ Tumour Study and subsequent modifications [48,49]. It is considered to be a negative prognostic marker when it appears as a diffuse feature or when it is recognized in an advanced stage of the disease; in such situations, treatment intensification is necessary [50,51,52], due to the greater resistance of the tumour to chemotherapy [52,53]. In this context, low δ15N values might be good biomarkers of the worst prognosis [24]. Amongst childhood tumours, rhabdomyosarcoma (RMS) is relatively uncommon, with two major histological types, embryonal (ERMS) and alveolar (ARMS). Five-year survival is observed in approximately 82% for ERMS and 65% for ARMS [25], and the prognosis for patients with progressive disease is still poor. Preliminary results have shown that ARMS leads to 15N-depleted muscles tissues relative to ERMS, with no change in δ13C [25].
3.5. Adrenal Gland Cancer
In adults, 15N-depletion in serum was also found in different types of adrenal gland cancers (Figure 1C) compared to healthy patients (unpublished data from our laboratory). Adrenal gland cancers are rare diseases and adrenocortical carcinoma (ACC) is characterized by strong malignancy. The serum of patients with contrasting types of adrenal tumors (i.e., ACC, adrenal adenoma and PPC, pheochromocytoma) has been analysed and low δ15N values significantly correlate to malignancy (Figure 1C). For example, patients with ACC have a naturally 15N-depleted serum compared to patient with PCC or adrenal adenomas. Moreover, in the group of patients with PCC, patients with benign tumours can be differentiated from those with malignant tumours on a δ15N basis.
This suggests a strong impact of malignancy on PCC cancer cell metabolism, which in turn affects δ15N values in circulating metabolites. By contrast, no change in δ13C values was observed. Although the metabolism of the different types of adrenal gland cancer is not well-known, an interesting feature is that the glucose transporter (GLUT1) is a promising prognostic marker of ACC [54]. Since glucose entry and insulin-based regulation is essential for protein turn-over signalling and amino acid cellular homeostasis, it is possible that changes in δ15N stem from an imbalance between protein synthesis and degradation within adrenal cancer cells. We also recognize that a general effect on protein metabolism and thus on serum δ15N due to cancer development is possible, in addition to a tumour-specific effect.
3.6. Hepatocarcinoma
The first δ34S values in patients with cancer were obtained using EA-IRMS analysis, and it has been found that both serum and erythrocytes are significantly 34S-depleted in patients with hepatocellular carcinoma (HCC) compared to controls [26]. It is plausible that this effect is due to change in the metabolism of S-containing amino acids, trans-sulphuration and methylation reactions which are intense in liver. In addition, glutathione (GSH) metabolism which plays important roles in cancer cells [55] could be involved, via oxidative stress response and the redox balance between extracellular cysteine and cysteine. For example, the formation of the disulphide bridge between cysteine molecules and thus in GSSG formation by GSH oxidation, fractionates against 34S [56,57]. In that context, the 34S-depletion in the serum of patients with HCC could come from a more oxidised status. Also, interestingly, it was found that δ34S values in patient with HCC patients correlated to the albumin level [58]. It is important to note δ34S values in serum of patients with BrCa or prostate cancer are not different from that in controls [58], showing that δ34S are probably specific to alterations in liver metabolism.
Presently, despite the rather small number of studies, it is clear that δ15N, δ13C and δ34S values have the potential to correlate to cancer development. Of course, since this effect is mostly caused by specific features of cancer cell metabolism, further studies with compound-specific analyses are required. This will be instrumental not only to better understand the metabolic origin of isotope signatures of cancer but also to find a reliable isotopic biomarker of cancer that is independent of nutrition (i.e., isotope composition of food intake) and population (country of residence) and therefore that do not require control samples for systematic comparison.
4. Isotope Fractionation in Metal Homeostasis
Isotope fractionations associated with four essential metals (Fe, Cu, Zn and Ca) as well as isotope compositions in various biological samples, including plants, animals and humans have been extensively reviewed in recent publications [27,59,60]. Here, we will very briefly explain principles of metal homeostasis and present latest studies dealing with metal isotope fractionation in human pathologies.
4.1. Copper Isotopes in Wilson and Menkes Diseases
In principle, variations in 65Cu/63Cu ratios are due to the change in oxidation state (i.e., from Cu2+ to Cu+ or vice versa). In human and animals, diet cupric ions (Cu2+) are reduced to the cuprous form (Cu+) by the metalloreductase six-transmembrane epithelial antigen of prostate member 1 (STEAP1) and then absorbed by enterocytes via a specific transporter (CTR1) (Figure 2A). δ65Cu values in cells are lower than that in the diet, because Cu entering the cell is in its reduced form, which is 65Cu-depleted (isotope effect in reduction). Cu is then transported from the intestine to the liver and used to synthesise Cu-containing proteins, such as copper chaperone for superoxide dismutase (CCS, which delivers Cu to superoxide dismutase, SOD1), cytochrome c copper chaperone (Cox17) (which delivers copper to cytochrome c oxidase, CCO), ATP7A/7B (copper transporters) and ceruloplasmin (major copper-carrying protein in blood). Cu transfers occur through oxidation reactions, inducing an increase in δ65Cu in SOD1, CCO and ceruloplasmin compared to source Cu. In addition to the change in oxidation state, forming specific chemical bonds also fractionates between Cu isotopes. For example, forming a Cu-S bond discriminates against 65Cu, while forming a Cu-O or Cu-N bond discriminates in favour of 65Cu. This explains why the liver, which accumulates the Cu- and S-containing metal-binding protein metallothionein, has a low δ65Cu value compared to other organs in sheep [61]. Conversely, δ65Cu values are high in kidney since Cu binds to oxalate and carbonate via Cu-O bonds [61]. Also, δ65Cu values are elevated in bone, likely because of the formation of Cu-O bonds in hydroxyapatite, the main mineral in bone.
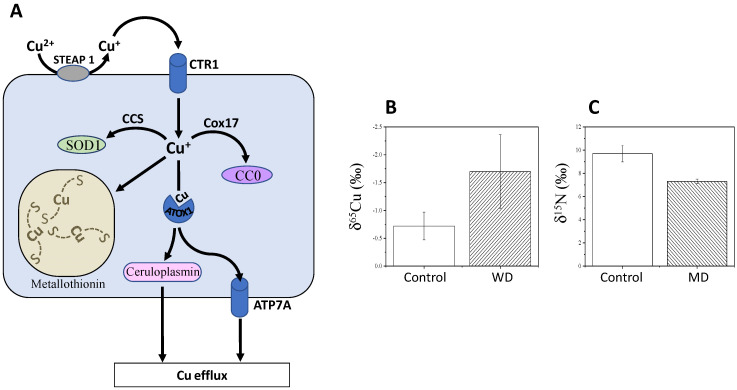
Figure 2.
Isotope fractionations in Wilson and Menkes diseases. (A) Simplified Cu utilisation, including uptake via the transporter CTR1, intracellular redistribution to various molecules including storing in metallothionein, and efflux via the transporter ATP7A (also called Menkes ATPases) or A ceruloplasmin. When copper efflux capacity is by ATP7A is insufficient, metallothionein synthesis is induced and sequesters excess copper [27]. Mutations in ATP7B (not shown here) leads to Wilson disease (WD), which is characterized by an inability to excrete Cu into the bile and therefore hepatic Cu accumulation. (B) δ65Cu values in serum of WD patients [27] and (C) hair δ15N of baby patients with Menkes disease (MD) (n = 3) compared to control (n = 18) (unpublished data). See main text for further details on Cu homeostasis. Abbreviations: ATOX1, antioxidant 1 copper chaperone; CC0, cytochrome c oxidase; SOD, superoxide dismutase. In (B,C), delta values are significantly different between patients and controls (p < 0.05).
Because of its involvement in SOD1 catalysis, Cu is involved in the mitigation of reactive oxygen species (ROS), which are thus influenced by intestinal absorption and bile excretion of Cu [27]. Alterations in Cu homeostasis can cause serious diseases, such as Wilson and Menkes diseases [27]. The Menkes disease (MD) involves a mutation in ATP7A and leads to copper deficiency and thereby strong neurodegenerative disorders. Mutations in ATP7B leads to Wilson disease (WD), which is characterized by an inability to excrete Cu into the bile and therefore hepatic Cu accumulation. WD is characterized by cerebral and cerebellar degeneration, failure to thrive, coarse hair and connective tissue abnormalities [62]. Unlike WD, no extensive Cu isotope data has been reported for patients with MD. However, hair δ15N has been shown to increase in babies with MD (Figure 2B) while no change was found in δ13C values. Low δ65Cu values are observed in the serum of patients with WD, compared to healthy subjects (Figure 2C).
This effect probably comes from the decrease in the level of blood ceruloplasmin, which is 65Cu-enriched. Lamboux and co-workers [63] reported recently that healthy subjects and naïve (non-treated) patients with WD had undistinguishable δ65Cu, and treated patients had the same δ65Cu values regardless of treatment type and duration. However, the variation in serum δ65Cu was negatively correlated with the degree of liver fibrosis. This suggests that the inability for Cu recirculation from the liver is at the origin of the 65Cu-depletion in general circulation, regardless of the genetic background and treatment. Also, as the cuprous form (65Cu-depleted) accumulates in the liver, the production of free radicals increases and participates in triggering WD symptoms [63]. These results suggest that δ65Cu is not a good biomarker of ATP7B mutation but rather, has some potential as a prognostic biomarker for evaluating the progression of liver fibrosis in WD.
Interestingly, changes in δ65Cu can also be observed in diseases other than WD and MD. For example, in patients with ovarian cancer, δ65Cu values in plasma are lower than in healthy controls [28]. δ65Cu values in ovary tumour tissues are higher than in adjacent healthy tissues [28], simply suggesting a mass-balance effect whereby the increased Cu influx in tumours forms 65Cu-enriched copper lactate [26] and depletes healthy ovary tissues and blood in 65Cu.
4.2. Multiple Metal Isotopes in Other Pathologies
Recent studies used the combination of Cu, Fe and Zn isotopes in blood to assess possible homeostasis alterations after bariatric surgery and during the follow-up of haematological malignancy (HM) in patients [29,30].
Both serum and whole blood had lower δ65Cu values after bariatric surgery, reaching statistical significance at 6 months post-surgery [30]. Although micro-elements supplementation of patients is unknown is that study, the current hypothesis is that duodenal Cu absorption and mineral bioavailability (and the amount of digestive juice in the stomach) are limited due to the gastric bypass. By contrast, serum 66Zn was slightly higher 6 months post-surgery than pre-surgery, but the difference did not reach statistical significance and furthermore, this enrichment in 66Zn was not observed in whole blood. Still, the difference in δ66Zn value between serum and whole blood (expressed as Δ66Zn) became gradually larger over post-operative time. This effect was not observed for Δ65Cu and Δ56Fe. Presumably, the change in Δ66Zn might reflect a disruption in Zn homeostasis, Zn status or Zn absorption (and competition with Cu absorption) in patients with bariatric surgery. When the Zn supply is low, Zn is remobilised from stored Zn to fulfil metabolic requirements. Intracellular Zn is bound to metallothionein via a Zn–S bonds (involving cysteine residues) and is released when blood Zn level is low [27]. As the Zn–S bond formation discriminates against 66Zn, 66Zn isotope would be preferentially released from intracellular metallothionein to serum, causing a general change in δ66Zn of circulating Zn.
Like Cu isotopes, Fe isotopes are extremely useful to follow alterations in the different steps of Fe homeostasis [27]. Patients suffering from severe diabetes and Fe deficiency anaemia show high δ56Fe values in whole blood. This effect is due to changes in the nutritional status of Fe, redox reactions during Fe absorption in the intestine, Fe storage in the liver, or the synthesis of Fe-containing proteins (typically haemoglobin or myoglobin). Fe3+ from diet is reduced to Fe2+ before being absorbed by the intestine, causing a low δ56Fe in intestine. The δ56Fe value is higher in the liver, where Fe is stored into ferritin in its Fe3+ form (naturally enriched compared to Fe2+). Reciprocally, the Fe3+ ion of transferrin (Tf) is reduced to Fe2+ in haemoglobin and myoglobin, leading to a low δ56Fe value in red blood cells and muscles [27,59]. Unlike Cu and Fe, Zn is not a redox sensitive element. Therefore, metabolic isotope fractionations with Zn isotopes are numerically smaller than those found with Cu and Fe. However, large variations in Zn isotopes are observed between plants, animals and humans [27]. They are attributable to the high binding energy of Zn with ligand molecules and thus effects of Zn adsorption and coordination at the root surface in plants, which are very different to biochemistry of animal Zn absorption.
In humans, Ca is the most abundant metal element in the body where it plays an important role in neurotransmission, muscle contraction, and regulation of volumes of biological fluids. Originally, it was believed that there was a big Ca isotope fractionation during bone formation (apatite deposition). Nevertheless, it was then observed that the δ44Ca value in blood and bone was similar. The largest difference was found between urine and blood, simply because of Ca isotope fractionation during kidney reabsorption of Ca from primary urine (from glomerular filtrate) into blood. Serum δ44Ca in patients with multiple myeloma (MM) is lower than in healthy controls [31]. In fact, MM is characterized by osteolytic lesions (bone mass loss) due to a relative increase in osteoclastic activity, liberating Ca2+ and causing bone resorption. Primary urine has thus a higher load in Ca with relatively low δ44Ca (compared to normal primary urine), which is then reabsorbed into blood, explaining a low δ44Ca in serum of patients with MM [31]. Tanaka et al. (2017) [32] reported that serum and bone from rats with chronic kidney disease or diabetes had lower δ44Ca values than in controls. δ44Ca values were correlated to bone mineral density, suggesting a link with bone resorption and formation like in MM. The Ca isotopic analysis of both urine and blood thereby offers a non-invasive method for investigating bone-related diseases, perhaps including early detection and assessment of treatment efficacy.
5. Isotopes in Skeletal Pathologies
Examples described in above sections are case studies of pathologies studied with living subjects. Stable isotopes can also provide information on past physiological conditions from archaeological remains. For example, skeletal stress indicators are used in bioarchaeology to inform on past palaeopathologies such as scurvy, osteoporosis, vitamin D deficiency (rickets and osteomalacia), endocrine disorders (e.g., acromegaly), haematological disorders (e.g., anaemia) and infectious diseases (e.g., tuberculosis, treponemal diseases, and leprosy as indicated by periostitis and osteomyelitis) [34].
5.1. Oxygen Isotopes in Sickle-Cell Anaemia
Only two studies [33,34] have demonstrated that enamel apatite δ18O values may provide a biological markers for anaemia in skeleton fragments. The first study showed a relationship between δ18O of bone apatite and mice expressing human Sickle-Cell anaemia haemoglobin HbS [64]. Bone apatite δ18O of sick mice were significantly lower than those of healthy mice, and the sickest mice exhibited the lowest δ18O [64]. These results were confirmed in the enamel apatite samples of suspected anaemics, which had significantly lower δ18O values relative to their lesion-free counterparts [33].
It is believed that the rate of cellular metabolism and the rate of total respiration is reflected by stable oxygen isotopes in dental enamel apatite (Ca3(PO4)2), via (i) oxygen (O2) reduction to metabolic water (H2O) during respiratory biochemistry and then (ii) isotopic exchange between tissue water and phosphate oxygen atoms. In effect, organism respiration fractionates against 18O and this comes from two main phenomena. First, there is an oxygen isotope fractionation as inhaled dioxygen (O2) diffuses through alveolar membranes to pulmonary capillaries. During this process, the rate of 16O2 diffusion is about 3‰ larger than that of 16O-18O and furthermore, there is an equilibrium isotope effect during O2 binding to haemoglobin, leading to an 18O-depletion in oxygen carried by the vascular system [33]. Second, a fractionation occurs once O2 is consumed by cellular respiration and converted to water (H2O), since mitochondria utilise 16O2 about 13‰ faster than 16O-18O [33]. Given the pathophysiology of anaemias, three potential causes can explain low δ18O values. First, oxygen fixation is slow due to lower functional haemoglobin and/or fewer overall erythrocytes and thus the fractionation is maximal (kinetic effect). Second, O2 binding by HbS may fractionate more against 18O than normal haemoglobin. This explanation is less likely since low δ18O are found also in anaemics without sickle cells disease. Third, metabolic adaptation of cellular metabolism is so that mitochondria do not have the same isotope fractionation during metabolic water generation by respiration. There is presently no definitive evidence to select one of these three hypotheses.
5.2. Nitrogen and Carbon Isotopes in Collagen
It is also known that anaemia inhibits bone matrix formation, increases bone resorption, and adversely affects collagen maturation, thereby increasing risks of osteopenia and osteoporosis. Interestingly, bone collagen of females with osteopenia has been found to be significantly enriched in 15N [33]. The authors suggested that the enrichment may be a result of underlying differences in urea excretion and/or renal function [33], both of which being expected to be altered in anaemia-induced osteopenia and osteoporosis. As such, bone collagen of individuals with severe manifestations of anaemia could be anticipated to be 15N-enriched (and perhaps 13C-enriched) relative to healthy individuals. However, in another study there is no significant variation in bone collagen δ13C or δ15N values between anaemic and lesion-free juveniles or adults [33]. This may be due to a number of factors, including age, the small sample size and the sampling strategy employed (possible bias in life habits and co-pathologies). It may also indicate that the pathophysiology of anaemia must be chronic to alter significantly the isotope composition in bones. More research, with a more refined sampling strategy and larger sample sizes, as well as isotopic measurements in each individual amino acid and other skeletal tissues, such as dentin and bone bicarbonate, could be of interest for future research.
δ15N and δ13C can be used to trace traumatic events and aggravation of lesions in progressive diseases. Delta values were measured in several skeletons to assess whether different stages of bone-related disease may affect bone collagen isotope composition [65]. This study used the cortical bone layer, which retains evidence of lesions caused by disease or healed fractures and compared visible lesions with cortical bone from the same individual without lesions. Long bones with active lesions were found to have a significantly higher δ15N suggesting increased protein metabolism or collagen deposition. Fracture calluses showed the largest range for both δ15N and δ13C, related to different healing stages. But surprisingly, unlike long bones, there were no significant differences in delta values between non-lesion and lesion sites in ribs. In fact, in ribs it is more difficult to separate abnormal new bone from underlying cortical bone, because lesions and non-lesion sites are visually similar [65].
6. Conclusions and Perspectives
In this mini-review, we have made apparent the fact that many human diseases have consequences on the natural abundance of stable isotopes, not only in affected tissues themselves but also in more accessible sample types, such as plasma, urine or hairs. This explains why many research projects across the world deal with the utilisation of delta values as potential diagnostic and prognostic tools. However, it should be recognised that there are presently two conundrums to solve in order to envisage clinical implementation. First, better (more sensitive and selective) biomarkers are likely to be represented by delta values in specific metabolites rather than total organic matter (raw samples). This implies sample preparation and analysis with compound-specific techniques (such as GC-c-IRMS) which are often not suitable to medical use due to sample size and the time required for analysis. Second, there is a lack of knowledge of specific metabolic causes of isotope fractionations in key processes and thus, precise mechanisms at the origin of the isotopic signature of diseases are incompletely understood. To fill this gap of knowledge, having more data on both metabolic fluxes and enzymatic isotope effects is necessary. While the investigation of metabolic flux patterns (fluxomics) is blooming, there is little work made on the measurement of isotope effects. The present paper is a call for more basic research in this area, so as to facilitate the discovery of new diagnostic biomarkers based on stable isotopes. In effect, once characteristic isotope fractionations associated with diseases will be determined, this will open avenues for patient management. Early cancer detection and diagnosis is presently one of the most exciting research area (Section 3 above), with promising and reproducible isotopic patterns found in tissues and cultured cells. Refined isotopic analyses (including compound-specific data) in the future will certainly be instrumental to help monitoring results of cancer therapies or provide a biomarker for prognostic stratification.
Acknowledgments
Metabohub Authors from CEISAM acknowledge the French National Infrastructure for Metabolomics and Fluxomics MetaboHUB-ANR-11-INBS-0010 (www.metabohub.fr, accessed on 28 April 2021) and the Corsaire metabolomics core facility (Biogenouest).
Appendix A
Author Contributions
Writing—Original Draft Preparation, I.T. and G.T.; Writing—Review and Editing, A.D.L., A.-M.S., S.B.-N., E.M., D.D., M.K. and R.H.; Visualization, A.D.L. and M.G.; Supervision, I.T. and G.T.; Project Administration, I.T. and G.T.; Funding Acquisition, I.T. and G.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Région Pays de la Loire and Angers Loire Métropole via the research grant Connect Talent Isoseed awarded to G.T., the French Regional Hospital Program for Clinical Research «EDEN Inserm-cohort study group» awarded to R.H., the CNRS through a research grant Défi Isotop (2019–2020) and the Agence Nationale de la Recherche through a research grant ANR PRCE (under contract ANR-20-CE18-0020-03) awarded to I.T.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huidekoper H.H., Wijburg F.A., Wanders R. Inborn Errors of Metabolism. In: Schierbeek H., editor. Mass Spectrometry and Stable Isotopes in Nutritional and Pediatric Research. John Wiley & Sons; New York, NY, USA: 2017. pp. 258–283. [Google Scholar]
- 2.Bodamer O.A.F., Halliday D. Uses of stable isotopes in clinical diagnosis and research in the paediatric population. Arch. Dis. Child. 2001;84:444. doi: 10.1136/adc.84.5.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charidemou E., Ashmore T., Griffin J.L. The use of stable isotopes in the study of human pathophysiology. Int. J. Biochem. Cell Biol. 2017;93:102–109. doi: 10.1016/j.biocel.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Turki A., Murthy G., Ueda K., Cheng B., Giezen A., Stockler-Ipsiroglu S., Elango R. Minimally invasive 13C-breath test to examine phenylalanine metabolism in children with phenylketonuria. Mol. Genet. Metab. 2015;115:78–83. doi: 10.1016/j.ymgme.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Jones P.J.H., Leatherdale S.T. Stable isotopes in clinical research: Safety reaffirmed. Clin. Sci. 1991;80:277–280. doi: 10.1042/cs0800277. [DOI] [PubMed] [Google Scholar]
- 6.Davies P.S.W. Stable isotopes: Their use and safety in human nutrition studies. Eur. J. Clin. Nutr. 2020;74:362–365. doi: 10.1038/s41430-020-0580-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L., Chen M., He P., Yu H., Block K.A., Xie Z. Composition and spatial distribution of elements and isotopes of a giant human bladder stone and environmental implications. Sci. Total Environ. 2019;650:835–846. doi: 10.1016/j.scitotenv.2018.09.028. [DOI] [PubMed] [Google Scholar]
- 8.Endo T., Hayasaka M., Ogasawra H., Kimura O., Kotaki Y., Haraguchi K. Relationships among mercury concentration, and stable isotope ratios of carbon and nitrogen in the scalp hair of residents from seven countries: Effects of marine fish and C4 plants consumption. PLoS ONE. 2015;10:e0128149. doi: 10.1371/journal.pone.0128149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kraft R.A., Jahren A.H., Saudek C.D. Clinical-scale investigation of stable isotopes in human blood: δ13C and δ15N from 406 patients at the Johns Hopkins Medical Institutions. Rapid Commun. Mass Spectrom. 2008;22:3683–3692. doi: 10.1002/rcm.3780. [DOI] [PubMed] [Google Scholar]
- 10.Mekota A.M., Grupe G., Ufer S., Cuntz U. Serial analysis of stable nitrogen and carbon isotopes in hair: Monitoring starvation and recovery phases of patients suffering from anorexia nervosa. Rapid Commun. Mass Spectrom. 2006;20:1604–1610. doi: 10.1002/rcm.2477. [DOI] [PubMed] [Google Scholar]
- 11.Fuller B.T., Fuller J.L., Sage N.E., Harris D.A., O’Connell T.C., Hedges R.E.M. Nitrogen balance and δ15N: Why you’re not what you eat during nutritional stress. Rapid Commun. Mass Spectrom. 2005;19:2497–2506. doi: 10.1002/rcm.2090. [DOI] [PubMed] [Google Scholar]
- 12.Salesse K., Kaupová S., Brůžek J., Kuželka V., Velemínský P. An isotopic case study of individuals with syphilis from the pathological-anatomical reference collection of the national museum in Prague (Czech Republic, 19th century A.D.) Int. J. Paleopathol. 2019;25:46–55. doi: 10.1016/j.ijpp.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Dailey-Chwalibóg T., Huneau J.F., Mathé V., Kolsteren P., Mariotti F., Mostak M.R., Alim M.A., Khan M.M.S.T., Khan M.A.H., Guesdon B., et al. Weaning and stunting affect nitrogen and carbon stable isotope natural abundances in the hair of young children. Sci. Rep. 2020;10:2522. doi: 10.1038/s41598-020-59402-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J.-K., Ahn S.V., Kim M.K., Lee K.-S., Koh S.-B., Bong Y.-S. The association between carbon and nitrogen stable isotope ratios of human hair and metabolic syndrome. Clin. Chim. Acta. 2015;450:72–77. doi: 10.1016/j.cca.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 15.Hotta Y., Fujino R., Kimura O., Fujii Y., Haraguchi K., Endo T. Assessment of diabetics by the quantification of essential elements and stable isotope ratios of carbon and nitrogen in scalp hair. Obes. Med. 2019;15:100106. doi: 10.1016/j.obmed.2019.100106. [DOI] [Google Scholar]
- 16.Ahn S.V., Koh S.-B., Lee K.-S., Bong Y.-S., Park J.-K. Association between nitrogen stable isotope ratios in human hair and serum levels of leptin. Tohoku J. Exp. Med. 2017;243:133–139. doi: 10.1620/tjem.243.133. [DOI] [PubMed] [Google Scholar]
- 17.Henze M.M., Bemis E.A., Seifert J.A., Johnson R.K., Dong F., Rewers M., Norris J.M. Association between change in self-reported sugar intake and a sugar biomarker (δ13C) in children at increased risk for type 1 diabetes. J. Nutr. Sci. 2020;9:e16. doi: 10.1017/jns.2020.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghosh C., Mandal S., Pal M., Mukhopadhyay P., Ghosh S., Pradhan M. 13C isotopic abundances in natural nutrients: A newly formulated test meal for non-invasive diagnosis of type 2 diabetes. J. Breath Res. 2017;11:026005. doi: 10.1088/1752-7163/aa6bcf. [DOI] [PubMed] [Google Scholar]
- 19.Petzke K.J., Feist T., Fleig W.E., Metges C.C. Nitrogen isotopic composition in hair protein is different in liver cirrhotic patients. Rapid Commun Mass Spectrom. 2006;20:2973–2978. doi: 10.1002/rcm.2695. [DOI] [PubMed] [Google Scholar]
- 20.Tea I., Martineau E., Antheaume I., Lalande J., Mauve C., Gilard F., Barillé-Nion S., Blackburn A.C., Tcherkez G. 13C and 15N natural isotope abundance reflects breast cancer cell metabolism. Sci. Rep. 2016;6 doi: 10.1038/srep34251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tea I.M.E., Giraudeau P., Akoka S., Barillé-Nion S. Method to Characterize the Origin and/or the State of Pathological or Healthy cells, and Its Applications in Biology. No. 2686686B1. [(accessed on 7 May 2021)];E.P. Patent. 2015 Oct 14; Available online: https://patents.google.com/patent/EP2686686B1/en.
- 22.Bogusiak K., Puch A., Mostowski R., Kozakiewicz M., Paneth P., Kobos J. Characteristic of Oral Squamous Cell Carcinoma Tissues Using Isotope Ratio Mass Spectrometry. J. Clin. Med. 2020;9:3760. doi: 10.3390/jcm9113760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taran K., Frączek T., Kamiński R., Sitkiewicz A., Kobos J., Paneth P. The first protocol of stable isotope ratio assessment in tumor tissues based on original research. Pol. J. Pathol. 2015;66:288–295. doi: 10.5114/pjp.2015.54963. [DOI] [PubMed] [Google Scholar]
- 24.Taran K., Frączek T., Sikora-Szubert A., Sitkiewicz A., Młynarski W., Kobos J., Paneth P. The first investigation of Wilms’ tumour atomic structure-nitrogen and carbon isotopic composition as a novel biomarker for the most individual approach in cancer disease. Oncotarget. 2016;7:76726. doi: 10.18632/oncotarget.12521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taran K., Frączek T., Sitkiewicz A., Paneth P., Kobos J. Rhabdomyosarcoma in children in the light of isotope ratio mass spectrometry. Pol. J. Pathol. 2015;66:383–388. doi: 10.5114/pjp.2015.57251. [DOI] [PubMed] [Google Scholar]
- 26.Balter V., Nogueira da Costa A., Bondanese V.P., Jaouen K., Lamboux A., Sangrajrang S., Vincent N., Fourel F., Télouk P., Gigou M., et al. Natural variations of copper and sulfur stable isotopes in blood of hepatocellular carcinoma patients. Proc. Natl. Acad. Sci. USA. 2015;112:982–985. doi: 10.1073/pnas.1415151112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanaka Y.K., Hirata T. Stable Isotope Composition of Metal Elements in Biological Samples as Tracers for Element Metabolism. Anal. Sci. Int. J. Jpn. Soc. Anal. Chem. 2018;34:645–655. doi: 10.2116/analsci.18SBR02. [DOI] [PubMed] [Google Scholar]
- 28.Toubhans B., Gourlan A.T., Telouk P., Lutchman-Singh K., Francis L.W., Conlan R.S., Margarit L., Gonzalez D., Charlet L. Cu isotope ratios are meaningful in ovarian cancer diagnosis. J. Trace Elem. Med. Biol. 2020;62:126611. doi: 10.1016/j.jtemb.2020.126611. [DOI] [PubMed] [Google Scholar]
- 29.Hastuti A., Costas-Rodríguez M., Anoshkina Y., Parnall T., Madura J.A., 2nd, Vanhaecke F. High-precision isotopic analysis of serum and whole blood Cu, Fe and Zn to assess possible homeostasis alterations due to bariatric surgery. Anal. Bioanal. Chem. 2020;412:727–738. doi: 10.1007/s00216-019-02291-2. [DOI] [PubMed] [Google Scholar]
- 30.Hastuti A.A.M.B., Costas-Rodríguez M., Matsunaga A., Ichinose T., Hagiwara S., Shimura M., Vanhaecke F. Cu and Zn isotope ratio variations in plasma for survival prediction in hematological malignancy cases. Sci. Rep. 2020;10:16389. doi: 10.1038/s41598-020-71764-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon G.W., Monge J., Channon M.B., Wu Q., Skulan J.L., Anbar A.D., Fonseca R. Predicting multiple myeloma disease activity by analyzing natural calcium isotopic composition. Leukemia. 2014;28:2112–2115. doi: 10.1038/leu.2014.193. [DOI] [PubMed] [Google Scholar]
- 32.Tanaka Y.-k., Yajima N., Higuchi Y., Yamato H., Hirata T. Calcium isotope signature: New proxy for net change in bone volume for chronic kidney disease and diabetic rats. Metallomics. 2017;9:1745–1755. doi: 10.1039/C7MT00255F. [DOI] [PubMed] [Google Scholar]
- 33.Carroll G.M.A., Inskip S., Waters-Rist A. Pathophysiological Stable Isotope Fractionation: Assessing the Impact of Anemia on Enamel Apatite d18O and d13C Values and Bone Collagen d15N and d13C Values. Bioarchaeology Int. 2018;2:117–146. doi: 10.5744/bi.2018.1021. [DOI] [Google Scholar]
- 34.Reitsema L.J. Beyond diet reconstruction: Stable isotope applications to human physiology, health, and nutrition. Am. J. Hum. Biol. 2013;25:445–456. doi: 10.1002/ajhb.22398. [DOI] [PubMed] [Google Scholar]
- 35.Scorrano G., Brilli M., Martínez-Labarga C., Giustini F., Pacciani E., Chilleri F., Scaldaferri F., Gasbarrini A., Gasbarrini G., Rickards O. Palaeodiet reconstruction in a woman with probable celiac disease: A stable isotope analysis of bone remains from the archaeological site of Cosa (Italy) Am. J. Phys. Anthropol. 2014;154:349–356. doi: 10.1002/ajpa.22517. [DOI] [PubMed] [Google Scholar]
- 36.Bernardo K., Jousse C., Fafournoux P., Schiphorst A.M., Grand M., Robins R.J., Hankard R., De Luca A. Protein restricted diet during gestation and/or lactation in mice affects 15N natural isotopic abundance of organs in the offspring: Effect of diet 15N content and growth. PLoS ONE. 2018;13:e0205271. doi: 10.1371/journal.pone.0205271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mantha O.L., Polakof S., Huneau J.-F., Mariotti F., Poupin N., Zalko D., Fouillet H. Early changes in tissue amino acid metabolism and nutrient routing in rats fed a high-fat diet: Evidence from natural isotope abundances of nitrogen and carbon in tissue proteins. Br. J. Nutr. 2018;119:981–991. doi: 10.1017/S0007114518000326. [DOI] [PubMed] [Google Scholar]
- 38.Huneau J.-F., Mantha O.L., Hermier D., Mathé V., Galmiche G., Mariotti F., Fouillet H. Natural isotope abundances of carbon and nitrogen in tissue proteins and amino acids as biomarkers of the decreased carbohydrate oxidation and increased amino acid oxidation induced by caloric restriction under a maintained protein intake in obese rats. Nutrients. 2019;11:1087. doi: 10.3390/nu11051087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petzke K.J., Fuller B.T., Metges C.C. Advances in natural stable isotope ratio analysis of human hair to determine nutritional and metabolic status. Curr. Opin. Clin. Nutr. Metab. Care. 2010;13:532–540. doi: 10.1097/MCO.0b013e32833c3c84. [DOI] [PubMed] [Google Scholar]
- 40.Liu S.V., Moore L.B., Halliday T.M., Jahren A.H., Savla J., Hedrick V.E., Marinik E.L., Davy B.M. Short-term changes in added sugar consumption by adolescents reflected in the carbon isotope ratio of fingerstick blood. Nutr. Health. 2018;24:251–259. doi: 10.1177/0260106018799522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Luca A., Laugier S., Tea I., Robins R.J., Saulnier P.-J., Torremocha F., Piguel X., Maréchaud R., Hankard R., Hadjadj S. Impact on bulk 15N natural isotopic abundance in hair of kidney function in type 2 diabetic nephropathy. e-SPEN J. 2014;9:e204–e209. doi: 10.1016/j.clnme.2014.09.001. [DOI] [Google Scholar]
- 42.Sick H., Roos N., Saggau E., Haas K., Meyn V., Walch B., Trugo N. Amino acid utilization and isotope discrimination of amino nitrogen in nitrogen metabolism of rat liver in vivo. Z. Fur Ernahr. 1997;36:340–346. doi: 10.1007/BF01617819. [DOI] [PubMed] [Google Scholar]
- 43.Kårlund A., Kääriäinen T., Kostamo V., Kokkola T., Kolehmainen M., Lakka T., Pihlajamäki J., Manninen A. Oxygen-18 and Carbon-13 isotopes in eCO2 and erythrocytes carbonic anhydrase activity of Finnish prediabetic population. J. Breath Res. 2020 doi: 10.1088/1752-7163/abd28d. in press. [DOI] [PubMed] [Google Scholar]
- 44.Boriosi J.P., Maki D.G., Yngsdal-Krenz R.A., Wald E.R., Porter W.P., Cook M.E., Bütz D.E. Changes in breath carbon isotope composition as a potential biomarker of inflammatory acute phase response in mechanically ventilated pediatric patients. J. Anal. At. Spectrom. 2014;29:599–605. doi: 10.1039/C3JA50331C. [DOI] [Google Scholar]
- 45.Keshet R., Szlosarek P., Carracedo A., Erez A. Rewiring urea cycle metabolism in cancer to support anabolism. Nat. Rev. Cancer. 2018;18:634–645. doi: 10.1038/s41568-018-0054-z. [DOI] [PubMed] [Google Scholar]
- 46.Lloyd S.M., Arnold J., Sreekumar A. Metabolomic profiling of hormone-dependent cancers: A bird’s eye view. Trends Endocrinol. Metab. TEM. 2015;26:477–485. doi: 10.1016/j.tem.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cai D., Wang J., Gao B., Li J., Wu F., Zou J.X., Xu J., Jiang Y., Zou H., Huang Z., et al. RORγ is a targetable master regulator of cholesterol biosynthesis in a cancer subtype. Nat. Commun. 2019;10:4621. doi: 10.1038/s41467-019-12529-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Beckwith J.B. Wilms Tumor and Other Renal Tumors of Childhood: An Update. J. Urol. 1986;136:320–324. doi: 10.1016/S0022-5347(17)44854-3. [DOI] [PubMed] [Google Scholar]
- 49.Vujanić G.M., Sandstedt B., Harms D., Kelsey A., Leuschner I., de Kraker J. Revised International Society of Paediatric Oncology (SIOP) working classification of renal tumors of childhood. Med. Pediatr. Oncol. 2002;38:79–82. doi: 10.1002/mpo.1276. [DOI] [PubMed] [Google Scholar]
- 50.Neville H.L., Ritchey M.L. Wilms’ tumor. Overview of National Wilms’ Tumor Study Group results. Urol. Clin. N. Am. 2000;27:435–442. doi: 10.1016/S0094-0143(05)70091-4. [DOI] [PubMed] [Google Scholar]
- 51.Faria P., Beckwith J.B., Mishra K., Zuppan C., Weeks D.A., Breslow N., Green D.M. Focal versus diffuse anaplasia in Wilms tumor--new definitions with prognostic significance: A report from the National Wilms Tumor Study Group. Am. J. Surg. Pathol. 1996;20:909–920. doi: 10.1097/00000478-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 52.Dome J.S., Cotton C.A., Perlman E.J., Breslow N.E., Kalapurakal J.A., Ritchey M.L., Grundy P.E., Malogolowkin M., Beckwith J.B., Shamberger R.C., et al. Treatment of anaplastic histology Wilms’ tumor: Results from the fifth National Wilms’ Tumor Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006;24:2352–2358. doi: 10.1200/JCO.2005.04.7852. [DOI] [PubMed] [Google Scholar]
- 53.Shamberger R.C., Anderson J.R., Breslow N.E., Perlman E.J., Beckwith J.B., Ritchey M.L., Haase G.M., Donaldson M., Grundy P.E., Weetman R., et al. Long-term outcomes for infants with very low risk Wilms tumor treated with surgery alone in National Wilms Tumor Study-5. Ann. Surg. 2010;251:555–558. doi: 10.1097/SLA.0b013e3181c0e5d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinheiro C., Granja S., Longatto-Filho A., Faria A.M., Fragoso M.C., Lovisolo S.M., Lerário A.M., Almeida M.Q., Baltazar F., Zerbini M.C. Metabolic reprogramming: A new relevant pathway in adult adrenocortical tumors. Oncotarget. 2015;6:44403–44421. doi: 10.18632/oncotarget.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo M., Wang Y.Z., Gout P.W. The x(c)- cystine/glutamate antiporter: A potential target for therapy of cancer and other diseases. J. Cell. Physiol. 2008;215:593–602. doi: 10.1002/jcp.21366. [DOI] [PubMed] [Google Scholar]
- 56.Philippot P., Van Zuilen M., Lepot K., Thomazo C., Farquhar J., Van Kranendonk M.J. Early Archaean Microorganisms Preferred Elemental Sulfur, Not Sulfate. Science. 2007;317:1534. doi: 10.1126/science.1145861. [DOI] [PubMed] [Google Scholar]
- 57.Tcherkez G., Tea I. 32S/34S isotope fractionation in plant sulphur metabolism. New Phytol. 2013;200 doi: 10.1111/nph.12314. [DOI] [Google Scholar]
- 58.Albalat E., Telouk P., Balter V., Fujii T., Bondanese V.P., Plissonnier M.-L., Vlaeminck-Guillem V., Baccheta J., Thiam N., Miossec P., et al. Sulfur isotope analysis by MC-ICP-MS and application to small medical samples. J. Anal. At. Spectrom. 2016;31:1002–1011. doi: 10.1039/C5JA00489F. [DOI] [Google Scholar]
- 59.Albarède F., Télouk P., Balter V. Medical Applications of Isotope Metallomics. Rev. Mineral. Geochem. 2017;82:851–885. doi: 10.2138/rmg.2017.82.20. [DOI] [Google Scholar]
- 60.Albarede F., Télouk P., Balter V., Bondanese V.P., Albalat E., Oger P., Bonaventura P., Miossec P., Fujii T. Medical applications of Cu, Zn, and S isotope effects. Metallomics. 2016;8:1056–1070. doi: 10.1039/C5MT00316D. [DOI] [PubMed] [Google Scholar]
- 61.Balter V., Lamboux A., Zazzo A., Télouk P., Leverrier Y., Marvel J., Moloney A.P., Monahan F.J., Schmidt O., Albarède F. Contrasting Cu, Fe, and Zn isotopic patterns in organs and body fluids of mice and sheep, with emphasis on cellular fractionation. Metallomics. 2013;5:1470–1482. doi: 10.1039/c3mt00151b. [DOI] [PubMed] [Google Scholar]
- 62.Kaler S.G. ATP7A-related copper transport diseases—emerging concepts and future trends. Nat. Rev. Neurol. 2011;7:15–29. doi: 10.1038/nrneurol.2010.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lamboux A., Couchonnal-Bedoya E., Guillaud O., Laurencin C., Lion-François L., Belmalih A., Mintz E., Brun V., Bost M., Lachaux A., et al. The blood copper isotopic composition is a prognostic indicator of the hepatic injury in Wilson disease. Metallomics. 2020;12:1781–1790. doi: 10.1039/D0MT00167H. [DOI] [PubMed] [Google Scholar]
- 64.Reitsema L.J., Crews D.E. Brief communication: Oxygen isotopes as a biomarker for sickle-cell disease? Results from transgenic mice expressing human hemoglobin S genes. Am J Phys Anthr. 2011;145:495–498. doi: 10.1002/ajpa.21513. [DOI] [PubMed] [Google Scholar]
- 65.Curto A., Mahoney P., Maurer A.-F., Barrocas-Dias C., Fernandes T., Fahy G.E. Effect of different healing stages on stable isotope ratios in skeletal lesions. Am. J. Phys. Anthropol. 2020;171:285–297. doi: 10.1002/ajpa.23958. [DOI] [PubMed] [Google Scholar]
- 66.Wilkinson D.J. Historical and contemporary stable isotope tracer approaches to studying mammalian protein metabolism. Mass Spectrom. Rev. 2018;37:57–80. doi: 10.1002/mas.21507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tea I., Tcherkez G. Natural Isotope Abundance in Metabolites: Techniques and Kinetic Isotope Effect Measurement in Plant, Animal, and Human Tissues. Methods Enzymol. 2017 doi: 10.1016/bs.mie.2017.07.020. [DOI] [PubMed] [Google Scholar]