Abstract
The essential oil and the major non-volatile secondary metabolites from the leaves of Piper subscutatum (Miq.) C. DC. (Family Piperaceae), collected in the Ecuadorian Amazon, were analyzed for the first time in the present study. The essential oil was submitted to chemical and enantioselective analyses by GC-MS and GC-FID. (E)-β-caryophyllene (25.3–25.2%), β-chamigrene (10.3–7.8%), (E)-nerolidol (8.1–7.7%), β-selinene (7.2–7.7%), δ-cadinene (2.7–3.9%), bicyclogermacrene (3.7–2.4%), and β-pinene (2.6–3.4%) were the major components. The enantioselective analysis, carried out on a β-cyclodextrin-based column, showed four scalemic mixtures in which (1R,5R)-(+)-α-pinene, (1S,5S)-(−)-β-pinene, (S)-(−)-limonene, and (1R,2S,6S,7S,8S)-(−)-α-copaene were the major enantiomers, with enantiomeric excesses of 28.8%, 77.8%, 18.4%, and 6.0%, respectively. The study was complemented with the chemical analysis of the organic fraction dissolved in the hydrolate, whose major components were 6-methyl-5-hepten-2-one (63.7–64.4%) and linalool (6.5–6.0%). Concerning the non-volatile fraction, five lignans were the major components. (–)-Beilshminol B, (–)-grandisin, (–)-3′,4′-methylenedioxy-3,4,5-trimethoxy-7,7′-epoxylignan, (–)-3′,4′-methylenedioxy-3,4,5,5′-tetramethoxy-7,7′-epoxylignan, and (–)-3,4,3′,4′-dimethylenedioxy-5,5′-dimethoxy-7,7′-epoxylignan were identified by means of NMR spectroscopy, mass spectrometry and X-ray crystallography. The absolute configuration 7S,8S,7′S,8′S was tentatively assigned to all of them.
Keywords: Piper subscutatum; Artanthe scutata; Piperaceae; essential oil; enantioselective analysis; 7,7′-epoxylignans; Ecuador
1. Introduction
Piper subscutatum (Miq.) C. DC. is a rather common spontaneous shrub, belonging to the family Piperaceae, that is also known with the synonym Artanthe scutata Miq. The plant is distributed between Ecuador and Peru, where it has been described in the Amazonian regions of both countries. It ranges from 100 to approximately 2000 m above sea level [1]. P. subscutatum phytochemicals have not been investigated to date.
Ecuador is located in the north-western coast of South America, laying between Colombia and Peru, with an area of approximately 283,500 Km2. From west to east, its territory can be divided into four geo-climatic areas, the islands (Galapagos), the coast, the highlands, and the forest. The equator line crosses the territory at about 24 km north of the capital Quito, passes through Isabela Island (Galapagos), and gives the name to the country. All these features are responsible for the presence of an outstanding biodiversity, with more than 5000 of the world’s plants as endemics, and a marine ecosystem existing within its borders. For these data, Ecuador is considered one of the 17 megadiverse countries of the Earth [2]. An update of phytochemical studies performed on Ecuadorian plants, until 2016, has been published recently [3]. Approximately 400 indexed papers are reported in this review, that concern more than 15,000 native botanical species. Interestingly, about 50% of the publications are related to only 8 botanical families over a total of about 250; as a result, most Ecuadorian plants are still an attractive source for phytochemical investigations.
In this context, more than 20 years ago, the authors of this work started a systematic study of characteristic plants of the Ecuadorian flora. As a result of their efforts, new essential oils have been described [4,5,6,7,8,9,10,11,12,13], the chemical structures of novel secondary metabolites have been established [14,15,16,17], and several biological activities have been determined [18,19,20,21]. In continuation of this phytochemical program, the present investigation of Piper subscutatum (Miq.) C. DC. deals with the composition of the essential oil (EO) and major non-volatile metabolites of the plant. It is worth noting that a complete de novo phytochemical study should, in principle, include both the volatile and non-volatile fractions, which is rarely done. In particular, the EOs are defined by the European Pharmacopoeia as “odorous products, usually of complex composition, obtained from a botanically defined plant raw material by steam-distillation, dry distillation, or a suitable mechanical process without heating. Essential oils are usually separated from the aqueous phase by a physical process that does not significantly affect their composition” [22].
In general, Piper species display a wide range of biological activities and produce a great variety of novel bioactive secondary metabolites, most of which are formed via the shikimic acid pathway [23,24,25,26]. A couple of Piper species, characteristic of the Ecuadorian flora, have been investigated by us in the past [6,21]. Now, we have extended our studies to P. subscutatum, which is a plant characteristic of the subtropical Ecuadorian flora and is quite abundant in the growth stations.
The aim of the study was to further contribute to the phytochemistry of the Piper species growing in Ecuador and, more generally, to the metabolic description of the Neotropical flora. Moreover, the search for new natural products or pharmacologically interesting compounds was considered equally important.
2. Results
2.1. Chemical Analysis of the EO and Hydrolate
The EO was steam distilled from fresh leaves of P. subscutatum, with a yield of 0.21% (w/w). Both qualitative and quantitative analyses were carried out on two orthogonal stationary phases: a non-polar DB-5ms column (polydimethylsiloxane with 5% phenyl groups) and a polar HP-INNOWax column (polyethylene glycol). In the qualitative analysis, a total of 66 oil components were identified by comparison of their mass spectra and linear retention indices with the literature. On the other hand, 60 compounds were quantified on at least one column, with a detection threshold of 0.1%. Quantified components corresponded to 96.9% and 95.1% of the EO total mass, on the non-polar and polar column, respectively. The sesquiterpene fraction was predominant in the EO, corresponding to 86.3% and 81.1% on the two columns, respectively. The major components, with an average amount ≥ 3% over the two columns, were (E)-β-caryophyllene (25.3–25.2%), β-chamigrene (10.3–7.8%), (E)-nerolidol (8.1–7.7%), β-selinene (7.2–7.7%), δ-cadinene (2.7–3.9%), bicyclogermacrene (3.7–2.4%), and β-pinene (2.6–3.4%). After solid phase extraction (SPE), the same semi-quantitative analysis was carried out on the hydrolate, that is, the aqueous phase spontaneously separating from the EO after the distillation. In this case, analytical results are better expressed as concentrations rather than percentages. Sulcatone (6-methyl-5-hepten-2-one) was the major organic solute in the hydrolate, with a concentration of about 64 mg/100 mL. All the analytical data are exposed in Table 1.
Table 1.
Qualitative and quantitative chemical analyses of the EO and hydrolate from P. subscutatum fresh leaves.
| Compounds | DB-5ms | HP-INNOWax | EO (%) 2 | Hydrolate (mg/100 mL) | ||||
|---|---|---|---|---|---|---|---|---|
| LRI 1 | LRI [27] | LRI 1 | LRI | DB-5ms | HP-INNOWax | DB-5ms | HP-INNOWax | |
| α-pinene | 925 | 932 | 1014 | 1025 [28] | 1.6 | 2.1 | -- | -- |
| camphene | 939 | 946 | 1071 | 1075 [28] | trace | trace | -- | -- |
| β-pinene | 968 | 974 | 1103 | 1118 [28] | 2.6 | 3.4 | -- | -- |
| 6-methyl-5-hepten-2-one (sulcatone) | 983 | 981 | 1339 | 1323 [29] | 2.1 | 2.9 | 63.7 | 64.4 |
| myrcene | 986 | 988 | 1163 | 1166 [30] | 0.2 | 0.6 | -- | -- |
| α-phellandrene | 1001 | 1002 | 1159 | 1162 [29] | 0.3 | 0.3 | -- | -- |
| δ-3-carene | 1003 | 1008 | 1142 | 1135 [28] | 0.2 | trace | -- | -- |
| α-terpinene | 1011 | 1014 | 1174 | 1175 [31] | trace | trace | -- | -- |
| ƿ-cymene | 1019 | 1020 | 1268 | 1281 [28] | trace | trace | -- | -- |
| limonene | 1023 | 1024 | 1194 | 1196 [29] | 0.6 | 0.7 | -- | -- |
| 1,8-cineol | 1026 | 1026 | 1201 | 1212 [29] | 0.1 | 0.2 | -- | -- |
| 1,4-cineol | -- | -- | -- | -- | -- | -- | 0.4 | 0.5 |
| γ-terpinene | 1052 | 1054 | 1242 | 1254 [28] | trace | trace | -- | -- |
| p-mentha-2,4(8)-diene(isoterpinolene) | 1078 | 1085 | 1279 | -- | 1.8 | 2.1 | -- | -- |
| trans-linalool oxide (furanoid) | -- | -- | -- | -- | -- | -- | 1.1 | 1.1 |
| linalool | 1100 | 1095 | 1555 | 1554 [31] | 1.1 | 1.4 | 6.5 | 6.0 |
| β-ylangene | -- | -- | 1563 | 1576 [32] | -- | trace | -- | -- |
| 1,3,8-p-menthatriene | 1126 | 1108 | 1421 | 1411 [32] | trace | 0.3 | -- | -- |
| borneol | -- | -- | -- | -- | -- | -- | 0.3 | 0.7 |
| terpinen-4-ol | -- | -- | -- | -- | -- | -- | 0.5 | 0.5 |
| α -terpineol | -- | -- | -- | -- | -- | -- | 0.8 | -- |
| linalool formate | -- | -- | -- | -- | -- | -- | 0.2 | 0.4 |
| δ-elemene | 1326 | 1335 | 1465 | 1468 [32] | 0.2 | 0.2 | -- | -- |
| α-cubebene | 1337 | 1348 | 1451 | 1460 [32] | 0.3 | 0.3 | -- | -- |
| β-copaene | -- | -- | 1579 | 1579 [31] | -- | 4.7 | -- | -- |
| α-copaene | 1362 | 1374 | 1481 | 1493 [33] | 0.9 | 1,0 | -- | -- |
| β-cubebene | 1376 | 1387 | 1530 | 1541 [32] | trace | 0.6 | -- | -- |
| β-elemene | 1379 | 1389 | 1574 | 1580 [34] | 1.6 | 0.2 | -- | -- |
| cyperene | 1385 | 1398 | 1511 | 1528 [32] | 0.1 | 0.1 | -- | -- |
| sibirene | 1392 | 1400 | 1528 | -- | 1.7 | 0.3 | -- | -- |
| α-gurjunene | 1397 | 1409 | 1518 | 1529 [32] | 0.2 | 1.7 | -- | -- |
| (E)-β-caryophyllene | 1404 | 1417 | 1587 | 1598 [32] | 25.3 | 25.2 | -- | -- |
| β-gurjunene | 1414 | 1431 | 1601 | 1596 [32] | 0.2 | trace | -- | -- |
| α-guaiene | 1423 | 1437 | 1632 | 1652 [32] | 1.9 | 0.2 | -- | -- |
| α-cedrene | 1428 | 1410 | 1594 | 1600 [35] | 0.2 | 0.6 | -- | -- |
| 6,9-guaiadiene | 1433 | 1442 | 1621 | 1617 [36] | 0.3 | 0.3 | -- | -- |
| α-humulene | 1438 | 1452 | 1657 | 1666 [32] | 1.8 | 0.4 | -- | -- |
| myltayl-4(12)-ene | 1442 | 1445 | 1622 | -- | 0.4 | 0.2 | -- | -- |
| ishwarene | 1447 | 1466 | 1677 | -- | 1.2 | 0.3 | -- | -- |
| 4,5-di-epi-aristolochene | 1454 | 1471 | 1669 | -- | 0.3 | 1.4 | -- | -- |
| α-neo-clovene | 1459 | 1452 | 1667 | -- | 1.7 | 1.7 | -- | -- |
| cis-cadina- 1(6),4-diene | 1462 | 1461 | 1680 | -- | 0.6 | 0.6 | -- | -- |
| cis-muurola-4(14),5-diene | 1465 | 1465 | 1651 | 1643 [32] | 1.4 | trace | -- | -- |
| selina-3,7(11)-diene | -- | -- | 1762 | 1783 [32] | -- | trace | -- | -- |
| β-selinene | 1472 | 1489 | 1706 | 1716 [32] | 7.2 | 7.7 | -- | -- |
| viridiflorene | 1476 | 1496 | 1685 | 1696 [32] | 1.6 | 1.7 | -- | -- |
| γ-himachalene | -- | -- | 1716 | 1708 [32] | -- | 0.6 | -- | -- |
| β-chamigrene | 1480 | 1476 | 1712 | 1723 [32] | 10.3 | 7.8 | -- | -- |
| trans-cadina-1(6),4-diene | 1483 | 1475 | 1697 | -- | 0.2 | 1.4 | -- | -- |
| α-bulnesene | 1488 | 1509 | 1627 | 1629 [32] | 1.4 | 1.7 | -- | -- |
| bicyclogermacrene | 1490 | 1500 | 1722 | 1734 [32] | 3.7 | 2.4 | -- | -- |
| trans-muurola-4(14),5-diene | 1498 | 1493 | 1700 | -- | 0.3 | 0.6 | -- | -- |
| γ-cadinene | 1502 | 1513 | 1783 | 1763 [32] | 0.7 | 0.1 | -- | -- |
| δ-cadinene | 1506 | 1521 | 1750 | 1755 [32] | 2.7 | 3.9 | -- | -- |
| trans-calamenene | 1508 | 1522 | 1825 | 1823 [32] | 1.1 | 0.7 | -- | -- |
| trans-cadina-1,4-diene | 1518 | 1533 | -- | -- | 0.2 | -- | -- | -- |
| 7-epi-α-selinene | 1524 | 1520 | 1766 | 1764 [32] | 0.2 | 0.4 | -- | -- |
| cis-muurol-5-en-4-β-ol | -- | -- | 1182 | -- | -- | 0.2 | -- | -- |
| α-calacorene | 1525 | 1544 | 1907 | 1921 [32] | 0.7 | 0.5 | -- | -- |
| β-germacrene | 1539 | 1559 | 1815 | 1823 [32] | 1.8 | 1.4 | -- | -- |
| (E)-nerolidol | 1558 | 1561 | 2048 | 2036 [32] | 8.1 | 7.7 | -- | -- |
| caryophyllene oxide | 1563 | 1582 | 1967 | 1986 [32] | 0.2 | 0.5 | -- | -- |
| cubebol | -- | -- | 1934 | 1941 [32] | -- | 0.2 | -- | -- |
| trans-dauca-4(11),7-diene | 1569 | 1556 | 1726 | -- | 0.5 | 0.2 | -- | -- |
| cubenol | -- | -- | 2066 | 2067 [32] | -- | 0.4 | -- | -- |
| guaiol | 1586 | 1600 | 2075 | 2090 [37] | 0.2 | 0.2 | -- | -- |
| 1-epi-cubenol | 1612 | 1627 | 2087 | 2088 [32] | 0.4 | trace | -- | -- |
| epi-α-cadinol | 1630 | 1638 | 2166 | 2169 [32] | 0.2 | 0.2 | -- | -- |
| α-muurolol (torreyol) | 1633 | 1644 | 2171 | 2183 [32] | 0.2 | trace | -- | -- |
| 10-epi-γ-eudesmol | 1637 | 1622 | 2093 | 2105 [32] | 1.5 | 0.2 | -- | -- |
| pogostol | 1641 | 1651 | 2182 | 2196 [38] | 2.5 | 0.2 | 1.1 | 0.1 |
| α-eudesmol | -- | -- | -- | -- | -- | -- | 0.8 | 0.3 |
| 5-neo-cedranol | 1679 | 1684 | 2191 | -- | 0.1 | 0.2 | -- | -- |
| trans-pinocarveol | -- | -- | -- | -- | -- | -- | -- | 0.2 |
| neral | -- | -- | -- | -- | -- | -- | -- | 0.5 |
| nerol | -- | -- | -- | -- | -- | -- | -- | 0.2 |
| Monoterpene hydrocarbons | 7.3 | 9.5 | -- | -- | ||||
| Oxygenated monoterpenes | 3.3 | 4.5 | 73.5 | 73.6 | ||||
| Sesquiterpene hydrocarbons | 72.9 | 71.1 | -- | -- | ||||
| Oxygenated sesquiterpenes | 13.4 | 10,0 | 1.9 | 1.3 | ||||
| Total | 96.9 | 95.1 | 75.4 | 74.9 | ||||
1 Calculated linear retention index; 2 Trace for % <0.1.
2.2. Enantioselective Analysis of the EO
The enantioselective analysis of the EO was carried out on a diethyl-tert-butyldimethylsilyl-β-cyclodextrin based capillary column. Four enantiomeric pairs were detected, comprising three monoterpenes and one sesquiterpene. The compounds were identified by their mass spectra and calculated linear retention indices (LRIs). The main enantiomers were (1R,5R)-(+)-α-pinene, (1S,5S)-(−)-β-pinene, (S)-(−)-limonene and (1R,2S,6S,7S,8S)-(−)-α-copaene, with enantiomeric excesses of 28.8%, 77.8%, 18.4% and 6.0%, respectively. The results are reported in Table 2.
Table 2.
Enantioselective analysis of chiral components of P. subscutatum EO, on a chiral diethyl-tert-butyldimethylsilyl-β-cyclodextrin-based column.
| Enantiomers | LRI 1 | Enantiomeric Ratio | ee 2 (%) |
|---|---|---|---|
| (1R,5R)-(+)-α-pinene | 927 | 64.4 | 28.8 |
| (1S,5S)-(−)-α-pinene | 928 | 35.6 | |
| (1R,5R)-(+)-β-pinene | 953 | 11.1 | 77.8 |
| (1S,5S)-(−)-β-pinene | 960 | 88.9 | |
| (S)-(−)-limonene | 1052 | 59.2 | 18.4 |
| (R)-(+)-limonene | 1067 | 40.8 | |
| (1R,2S,6S,7S,8S)-(−)-α-copaene | 1663 | 53.0 | 6.0 |
| (1S,2R,6R,7R,8R)-(+)-α-copaene | 1666 | 47.0 |
1 Calculated linear retention index; 2 enantiomeric excess.
2.3. Lignans from P. Subscutatum Ethyl Acetate Extract
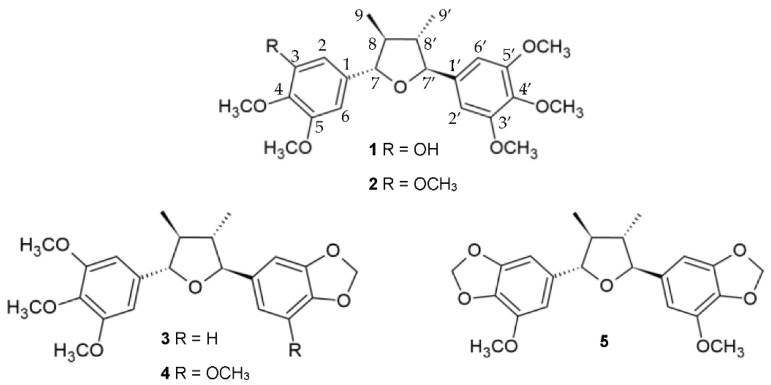
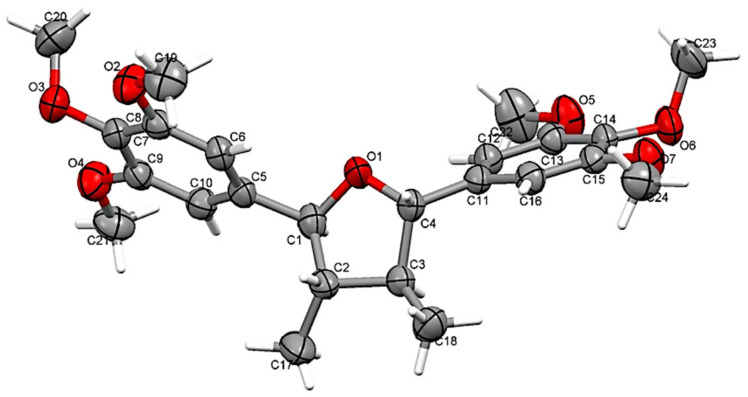
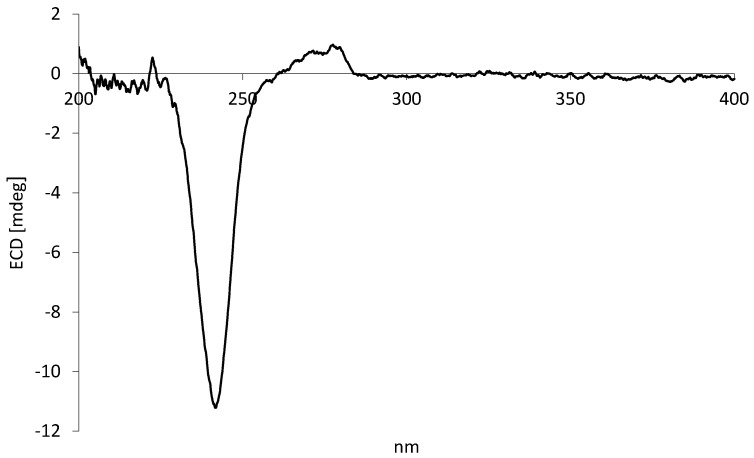
Five tetrahydrofuran lignans (Figure 1) were isolated by preparative column chromatographic separation of a chlorophyll-free EtOAc leaf extract of P. subscutatum. The molecular structures were determined by nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry (MS) and, in the case of compound 2, by X-ray crystallography (Figure 2). Comparison of the spectral data with the literature clearly indicated that these lignans were identical to beilshminol B (1) [39], grandisin (2) [40], and three related compounds, 3′,4′-methylenedioxy-3,4,5-trimethoxy-7,7′-epoxylignan (3) [41], 3′,4′-methylenedioxy-3,4,5,5′-tetramethoxy-7,7′-epoxylignan (4) [42], and 3,4,3′,4′-dimethylenedioxy-5,5′-dimethoxy-7,7′-epoxylignan (5) [42]. The relative configuration rel-(7R,8R,7′R,8′R) was inferred for isolated compounds 1 and 3–5 on the basis of NOESY studies, and values of coupling constants and chemical shifts of protons 7, 7′, 8, 8′, 9, and 9′ in the 1H NMR spectra [39,40,41,42,43]. In fact, they were very similar to each other and to those of grandisin (2), whose relative configuration has firmly been established [40,43,44,45]. Moreover, the negative optical rotations and the negative ECD (electronic circular dichroism) peak (Figure 3) observed at about 245 nm, strongly suggested that compounds 1–5 had the same configuration at the four stereocenters. Despite these compounds having not yet surrendered to total synthesis, some closely structurally related 7,7′-epoxylignans, such as (–)-talaumidin, (+)-fragasin A2, (+)-galbelgin, (–)-galbelgin, (+)-galbacin, and (–)-galbacin, have been obtained in enantiomerically pure form by enantioselective synthesis [46]. In this context, it is important to note that the configuration 7S,8S,7′S,8′S was assigned to all the laevorotatory 2,3-anti-3,4-anti-4,5-anti configured 2,5-diaryl-3,4-disubstituted epoxylignans that are structurally related to compounds 1–5; in contrast, the dextrorotatory synthetic forms showed the opposite stereochemistry. Moreover, despite being much rarer than the laevorotatory enantiomer, (+)-grandisin is also a natural product and the configuration 7R,8R,7′R,8′R was assigned to this lignan [47]. Thus, based on this evidence, the absolute configuration 7S,8S,7′S,8′S was assigned to all the laevorotatory lignans 1–5.
Figure 1.
Lignans isolated from the ethyl acetate extract of P. subscutatum.
Figure 2.
Computer generated Oak Ridge Thermal Ellipsoid Plot (ORTEP) structure (hydrogen atoms excluded) of (–)-grandisin (2) isolated from P. subscutatum.
Figure 3.
ECD spectrum of (–)-grandisin (2) isolated from P. subscutatum.
3. Discussion
The pattern of the EO components and non-volatile lignans appear to characterize P. subscutatum from a chemical point of view, significantly differentiating this plant from other Piper species [23,24,25,26,48]. In recent papers [6,26,49], the compositions of the EOs from the genus Piper have been discussed and oils were divided into six groups depending on the predominant chemical classes. Thus, according to this classification, the P. subscutatum EO belongs to the group dominated by sesquiterpenes, which contributed to more than 80% of the entire sample. Interestingly, the relative abundance (about 25%) of the major component (E)-β-caryophyllene was the same as that found for the EO of Piper coruscans [6]. (E)-β-Caryophyllene was also a dominant component of the EOs of P. majusculum, P. madeiranum, P. duckei, and P. nigrum [26]. The presence of sulcatone (6-methyl-5-hepten-2-one) among the volatile compounds of P. subscutatum is quite important, from a chemotaxonomic and biological point of view. In fact, this citrus-like fruity odorant has rarely been detected in the EOs of Piper species [50]. Due to its hydrophilicity, and despite the amount in the EO did not exceed 3%, it constituted more than 80% of the organic content in the hydrolate (up to 64.4 mg/100 mL). Furthermore, sulcatone, which is naturally emitted by the human body, is one of several mosquito attractants that are specific for hematophagous species such as Aedes aegypti [51]. This insect is responsible for the diffusion of many dangerous tropical viruses, causing dengue, yellow fever, chikungunya, and zika. Considering that all these diseases scourge the growing regions of P. subscutatum, where the plant can be collected in rather large amounts, the hydrolate obtained from this plant has the potential to be used as a natural pest trap by poor rural communities.
Concerning the non-volatile compounds 1–5 isolated from P. subscutatum, tetrahydrofuran lignans are typical secondary metabolites of Piper species, whose biosynthesis in plants proceeds via the shikimic acid pathway. Lignans 1 and 3–5 are rare lignans, that have only been isolated once in nature thus far. (–)-Beilshminol B (1) was isolated from the Chinese plant Beilschmiedia tsangii [39], whereas compound 3 was isolated from a Brazilian specimen of Virola surinamensis [41]. Finally, lignans 4 and 5 were both discovered in the Brazilian species Piper solmsianum [42]. (–)-Grandisin (2) is by far the most abundant lignan occurring in P. subscutatum leaves, accounting for about 14.5 % of the crude EtOAc extract. It is a rather common lignan, that was at first isolated in 1974 from Litsea grandis [44] and, subsequently, from several Piperaceae, Lauraceae, Myristicaceae and Schisandraceae species among others [43]. (–)-Grandisin has displayed several biological activities, such as trypanocidal activity against the trypomastigote form of Trypanosoma cruzi, larvicidal activity against the mosquito Aedes aegypti, and antinociceptive, anti-inflammatory, and antitumor activities [52]. Especially interesting are the remarkable trypanocidal activity of compounds 4 and 5 [42,53,54] against Trypanosoma cruzi, which is the aetiological agent of Chagas disease. In fact, according to the World Health Organization, about 6–7 million people in the world are estimated to be currently infected by this protozoan parasite, most of them living in South America, including Ecuador [55]. The early administration of synthetic compounds such as benznidazole and nifurtimox can eradicate the protozoan with very high efficiency. However, the lack of the drugs, as well as their late application in the countryside, make Chagas a major cause of death in rural regions of South America. Therefore, the availability of an effective and unexpensive anti-Chagas natural product must be considered a valuable alternative to synthetic drugs. In this context, a purified extract of P. subscutatum, enriched in lignans 2, 4, and 5, should be tested in the field as an anti-Chagas agent.
4. Materials and Methods
4.1. General Information
The chemical and enantioselective analyses of the P. subscutatum EO were carried out with an Agilent Technologies GC-MS system, consisting of a 6890N gas chromatograph with an autoinjector model 7683. The instrument was coupled to an Agilent Technologies mass spectrometry detector (MSD) model 5973 INERT (Santa Clara, CA, USA), and a common flame ionization detector (FID). The MSD operated in SCAN mode (scan range 40–350 m/z), with an electron ionization (EI) source at 70 eV. The qualitative and quantitative analyses were run with both apolar and polar capillary columns. The apolar column was based on 5% phenyl methylpolysiloxane (DB-5ms from Agilent Technologies, 30 m long, 0.25 mm internal diameter, and 0.25 μm film thickness), whereas the polar column was provided with a polyethylene glycol stationary phase (HP-INNOWax, from Agilent Technologies, 30 m × 0.25 mm × 0.25 μm). The enantioselective analysis was performed with a capillary column, based on 30% diethyl-tert-butyldimethylsilyl-β-cyclodextrin on PS-086 as the chiral selector. The column was 25 m × 250 μm internal diameter × 0.25 μm phase thickness, and was purchased from Mega, MI, Italy. The carrier gas for all the analyses was GC purity grade helium (Indura, Guayaquil, Ecuador), set at the constant flow rate of 1 mL/min. Preparative chromatographic separations were performed on open columns at atmospheric pressure (CC) or by means of a medium pressure liquid chromatograph (MPLC) Reveleris® Prep System (Büchi Labortechnik, Flawil, AG, CH), equipped with both a UV-vis and a light scattering detector. Columns packed with silica gel (Merck Kieselgel 60, 40–63 μm) or C18 reversed phase (Merck LiChroprep RP-18, 25–40 μm), both purchased from Sigma-Aldrich (St. Louis, MO, USA), were used. All TLC analyses were conducted over silica gel 60 (0.25 mm; GF254, Merck) or RP-18 (F254s, Merck) plates (Sigma-Aldrich). TLC spots were visualized under UV light (254 and 366 nm), followed by exposure to a 0.5% solution of vanillin in H2SO4/ethanol 4:1, and subsequently heated at 100 °C. For applications of solid phase extraction (SPE), the cartridges were standard products, packed with 1 g of C18 reversed phase and purchased from Sigma-Aldrich. For chlorophyll removal, SPE was carried out over a manually packed column. All NMR experiments were carried out with a Varian (400 MHz, Varian Inc., Palo Alto, CA, USA) spectrometer. The number of protons attached to each carbon was determined by DEPT experiments. Deuterated solvents were purchased from Sigma-Aldrich. Electron spray ionization mass spectrometry (ESI-MS) experiments were conducted by means of a Bruker Amazon Speed spectrometer (Bruker, Billerica, MA, USA). Optical rotations were measured on an Automatic Polarimeter (Jinan Hanon Instruments Co. Ltd., Jinan, China) MRC P810. ECD spectra were observed on a Jasco spectropolarimeter, model J-1100 (Jasco Co. Ltd., Japan). Melting points were determined with a Fisher-Johns apparatus (Thermo Fisher Scientific, Waltham, MA, USA). Single crystal X-ray diffraction experiments were carried out on an Enraf-Nonius four-circle diffractometer, model CAD-4 (Enraf-Nonius B.V., Rotterdam, NL), applying a graphite monochromated Mo-Kα radiation. The ORTEP structure of grandisin (2) (Figure 2) showed 50% probability displacement ellipsoids. Technical grade solvents (Brengtan, Guayaquil, Ecuador), freshly distilled, were used for preparative chromatographic separations, whereas HPLC grade solvents (Sigma-Aldrich) were used for all other applications. The mixture of n-alkanes C9–C25 and the internal standard (n-nonane) for GC analysis were of analytical grade (purity > 99%) and purchased from Sigma-Aldrich. The calibration standard was isopropyl caproate, synthesized in the authors’ laboratory and purified to 98.8% purity (GC-FID).
4.2. Plant Material
Leaves of P. subscutatum were collected at Numbani (Zamora-Chinchipe Province) in April 2018, under the supervision of one of the authors (J.R.), who also identified the botanical species. The collection was carried out under permit MAE-DNB-CN-2016-0048, issued by the Ministry of Environment of Ecuador (MAE). A botanical specimen was deposited at the herbarium of the Universidad Técnica Particular de Loja, with the voucher number PPN-pi-011. Equal amounts of ten different shrubs, which were growing homogeneously in a range of about 500 m around a central point (coordinates 4°09′24.9″ S and 78°56′38.9″ W), were collected. Hence, a total of ten equal plant samples were obtained. As all these specimens were representative of the same small ecologically and geologically homogeneous area, leaves were mixed, producing an average sample, which afforded both the EO and the EtOAc extract. The EO was distilled from fresh leaves the same day of collection, whereas solvent extraction was carried out on dried plant material. The drying process was conducted at 35 °C for three days, followed by storage of the vegetable material in the darkness, at room temperature, until use.
4.3. Distillation of the EO and GC Sample Preparation
Fresh leaves (2.6 kg) were steam distilled in a stainless-steel Clevenger-type apparatus for 4 h. The EO, that spontaneously separated from the aqueous layer, was removed with a pipette, dried over anhydrous sodium sulphate and stored in the darkness at −14 °C until use. Two volumes of hydrolate (10 mL each) were also collected and immediately processed to afford two analytical samples.
For each GC injection, 10 mg of the EO were weighted and diluted with 1 mL of cyclohexane, previously spiked with n-nonane (0.7 mg/mL) as the internal standard. Regarding the hydrolate analysis, each portion of the aqueous layer was eluted on a previously conditioned SPE cartridge. After complete removal of water from the solid phase, analytes were recovered by elution with 2 mL of acetone, previously spiked with the internal standard. The two acetone solutions were then directly injected separately into the GC apparatus.
4.4. GC-MS Qualitative Analyses
The EO and the hydrolate were analyzed by injecting 1 μL of each sample into the GC apparatus that operated in split mode (40:1). The injector temperature was set at 220 °C. Each sample was analyzed on both the polar and non-polar columns. With the DB-5ms column, oven temperature was set as follows: 50 °C (1 min isothermal), raised to 250 °C with a gradient rate of 3 °C/min, and then isothermal at 250 °C (10 min). With the polar column, the oven temperature program was the same, except that the final temperature was set at 230 °C. A homologous series of n-alkanes, from n-nonane to n-pentacosane, was injected in each column to calculate the linear retention index (LRI) of each analyte [56]. Each volatile metabolite was identified by comparing the corresponding LRI value and EI-MS spectrum with the literature (see Table 1).
4.5. GC-FID Quantitative Analyses
All the samples were injected in duplicate in a GC-FID chromatograph, under the same conditions and configurations as the GC-MS analyses. The percentage of each analyte in the EO and the hydrolate was calculated as the average value over the two analyses. The relative response factor (RRF) of each analyte was calculated on the basis of the corresponding combustion enthalpy [57,58]. Two calibration curves (one for each column), both with r2 = 0.999, were obtained as described in a previous study [6] and applied to all the samples.
4.6. Enantioselective Analysis of the EO
The enantioselective analysis of the EO was carried out by injecting the sample into the gas-chromatograph in the GC-MS configuration. The injector temperature and split ratio were the same as for the EO qualitative analysis, whereas oven temperature was set as follows: 50 °C (5 min isothermal), then raised to 220 °C with a gradient rate of 2 °C/min, and then isothermal at 220 °C (5 min). The enantiomeric pairs of chiral terpenes were identified on the basis of the EI-MS spectra and the order of elution, as described in previous studies [4,5,6].
4.7. Preparation of the Ethyl Acetate Extract
An amount (250 g) of P. subscutatum dried leaves were minced and then macerated in ethyl acetate for one hour at room temperature. The process was repeated three times and the resulting extracts were combined, filtered, and distilled under vacuum at 35 °C, to afford 4.19 g of an oily residue. Chlorophyll was then removed by SPE, passing the residue through a column packed with C18 reversed phase (50 g). Elution with 80% aqueous methanol gave, after evaporation, 1.76 g of a chlorophyll-free residue.
4.8. Separation of the Lignans
The chlorophyll-free extract (1.0 g) was separated by MPLC on a column packed with commercial C18 reversed phase (100 g). Elution with a gradient from 65% aqueous MeOH to 100% MeOH, over 60 min, afforded, after TLC control over C18 reversed phase plates, 13 main fractions, named from JR-01 to JR-13.
Fraction JR-04 (71.9 mg) was separated on a column packed with silica gel (7 g), at atmospheric pressure. Isocratic elution with a mixture of n-hexane-EtOAc, 90:10, afforded compound 1 (7.3 mg).
Pure (–)-grandisin (2, 610 mg) spontaneously crystallized in the collection tube of fraction JR-05. The crystals were separated by filtration and washed with n-hexane before X-ray analysis.
Fraction JR-07 (82 mg) was separated on a column packed with silica gel (8 g), at atmospheric pressure. Isocratic elution with n-hexane-EtOAc, 85:15, gave compounds 3 (5.7 mg) and 4 (18.6 mg).
Finally, fraction JR-08 (27.3 mg) was separated on a column packed with silica gel (3 g), at atmospheric pressure. Isocratic elution with n-hexane-EtOAc, 95:5, afforded compound 5 (3.3 mg).
(–)-Beilshminol B (1).
C23H30O7: pale yellowish oil; (c 0.015, CH2Cl2). ESI-MS (m/z): 418.41 [M]+, 441.41 [M+Na]+, 859.36 [2M+Na]+. 1H NMR (400 MHz, CDCl3): δH 1.06 and 1.08 (2d, J = 6.0 Hz, 2 × 3H, H3-9,-9’), 1.75-1.85 (m, 2 × 1H, H-8,-8’), 3.84 (s, 3H, CH3O-4’), 3.88 (s, 4 × 3H, CH3O-3’,-4,-5,-5’), 4.62-4.66 (2d, J = 9.0 Hz, 2 × 1H, H-7,-7’), 6.55 (d, J = 2.0 Hz, 1H, H-6), 6.62 (s, 2 × 1H, H-2’,-6’), 6.64 (d, J = 2.0 Hz, 1H, H-2). 13C NMR (100 MHz, CDCl3): δC 14.1 (CH3-9’), 14.2 (CH3-9), 51.1 (CH-8’), 51.2 (CH-8), 56.1 (CH3O-5), 56.3 (CH3O-3’,-5’), 61.0 (CH3O-4’), 61.1 (CH3O-4), 88.4 (CH-7), 88.7 (CH-7’), 101.9 (CH-6), 103.0 (CH-2’), 103.1 (CH-6’), 105.9 (CH-2), 134.9 (C-4), 137.5 (C-4’), 138.1 (C-1’), 138.9 (C-1), 149.2 (C-3), 152.6 (C-5), 153.4 (C-3’,-5’). The spectral data nicely correspond to the literature [39].
(–)-Grandisin (2).
C24H32O7: colourless crystals; m.p.: 120–122 °C; (c 0.140, CH2Cl2); ECD242 nm (c 0.03, MeOH) = −11.21 mdeg. ESI-MS (m/z): 433.53 [M+H]+, 455.51 [M+Na]+, 888.03 [2M+Na]+. 1H NMR (400 MHz, CDCl3): δH 1.08 (d, J=6.2 Hz, 2 × 3H, H3-9,-9’), 1.70-1.85 (m, 2 × 1H, H-8,-8’), 3.85 (s, 2 × 3H, CH3O-4,-4’), 3.90 (s, 4 × 3H, CH3O-3,-3′,-5,-5′), 4.65 (d, J= 9.5 Hz, 2 × 1H, H-7,-7’), 6.62 (s, 2 × 2H, H-2,-2’,-6,-6’). 13C NMR (100 MHz, CDCl3): δC 14.1 (CH3-9,-9′), 51.1 (CH-8,-8′), 56.2 (CH3O-3,-5,-3,-5′), 60.9 (CH3O-4,-4′), 88.6 (CH-7,-7′), 103.1 (CH-2,-6,-2′,-6′), 137.4 (C-4,-4′), 138.0 (C-1,-1′), 153.2, 153.3 (C-3,-5, -3′,-5′). The spectral data nicely correspond to the literature [40,43,44].
(–)-(7S,8S,7′S,8′S)-3′,4′-Methylenedioxy-3,4,5-trimethoxy-7,7′-epoxylignan (3).
C22H26O6: pale yellow oil; (c 0.020, CH2Cl2). ESI-MS (m/z): 387.14 [M+H]+, 409.14 [M+Na]+, 795.11 [2M+Na]+. 1H NMR (400 MHz, CDCl3): δH 1.05 and 1.10 (2d, J = 6.0 Hz, 2 × 3H, H3-9,-9’), 1.70-1.85 (m, 2 × 1H, H-8,-8’), 3.85 (s, 3H, CH3O-4), 3.90 (s, 2 × 3H, CH3O-3,-5), 4.62 (d, J = 10.0 Hz, 2 × 1H, H-7,-7’), 5.94 (s, 2H, OCH2O), 6.61 (s, 2 × H, H-2,-6), 6.78 (d, J = 8.0 Hz, 1H, H-5′), 6.86 (dd, J = 8.0 and 1.5 Hz, 1H, H-6′), 6.93 (d, J = 1.5 Hz, 1H, H-2′). The spectral data nicely correspond to the literature [41].
(–)-(7S,8S,7′S,8′S)-3′,4′ -Methylenedioxy-3,4,5,5′-tetramethoxy-7,7′-epoxylignan (4).
C23H28O7: pale yellow oil; (c 0.270, CH2Cl2). ESI-MS (m/z): 417.33 [M+H]+, 439.23 [M+Na]+, 855.25 [2M+Na]+. 1H NMR (400 MHz, CDCl3): δH 1.04 (2 overlapped d, J = 6.0 Hz, 2 × 3H, H3-9,-9’), 1.73-1.78 (m, 2 × H, H-8,-8’), 3.84 (s, 3H, CH3O-4), 3.88 (s, 2 × 3H, CH3O-3,-5), 3.92 (s, 3H, CH3O-5′), 4.60 (d, J = 8.0 Hz, 2 × 1H, H-7,-7′), 5.95 (s, 2H, OCH2O), 6.60 and 6.62 (2 × s, 2 × 1H each, H-2,-6,-2′,-6′). The NMR data correspond to the literature [42].
(–)-(7S,8S,7′S,8′S)-3,4,3′,4′-Dimethylenedioxy-5,5′-dimethoxy-7,7′-epoxylignan (5).
C22H24O7: pale yellow oil; (c 0.019 in CH2Cl2). ESI-MS (m/z): 401.25 [M+H]+, 423.25 [M+Na]+, 823.50 [2M+Na]+. 1H NMR (400 MHz, CDCl3): δH 1.04 (d, J = 6.2 Hz, 2 × 3H, H3-9,-9’), 1.73-1.78 (m, 2 × 1H, H-8,-8′), 3.91 (s, 2 × 3H CH3O-5,5′), 4.58 (d, J = 9.0 Hz, 2 × 1H, H-7,-7′), 5.96 (s, 2 × 2H, 2 × OCH2O), 6.59 (s, 4 × 1H, H-2,-6,-2′,-6′). The NMR data correspond to the literature [42].
5. Conclusions
The phytochemical profile and the leaf EO components of the Ecuadorian species Piper subscutatum (Miq.) C. DC. have been determined in the present study for the first time. In addition to a new sesquiterpene-based essential oil, produced in a fairly substantial yield, P. subscutatum afforded some other potentially useful products. Thus, the hydrolate, a cheap by-product of the EO process, contained a considerable amount of sulcatone that is a well-known mosquito attractant. On the other hand, three highly active trypanocidal lignans were isolated from the non-volatile fraction. Therefore, it appears that P. subscutatum, which can be collected in rather large amounts in the wild, may have a remarkable practical importance, as some active products, isolable from the plant, can be used to fight A. aegypti and other dangerous insects infesting tropical countries. Clearly, these applications should be confirmed by future field studies.
Acknowledgments
We are grateful to the Universidad Técnica Particular de Loja (UTPL) for supporting this open access publication.
Author Contributions
Conceptualization, G.V. and G.G.; investigation, J.R. and M.D.A.; data curation, G.V. and G.G.; writing—original draft preparation, G.G.; writing—review and editing, G.V. and G.G.; and supervision, J.R. and G.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by a PhD fellowship to one of the authors (J.R.) (No.20110941), from the Secretaría Nacional de Educación Superior, Ciencia y Tecnología (SENESCYT) of Ecuador.
Data Availability Statement
Raw data are available from the authors (J.R.).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tropicos.org Missouri Botanical Garden. [(accessed on 3 May 2021)]; Available online: https://www.tropicos.org/name/25000956.
- 2.Megadiverse Countries, UNEP-WCMC. [(accessed on 3 May 2021)]; Available online: https://www.biodiversitya-z.org/content/megadiverse-countries.
- 3.Malagón O., Ramírez J., Andrade J., Morocho V., Armijos C., Gilardoni G. Phytochemistry and ethnopharmacology of the Ecuadorian flora. A review. Nat. Prod. Commun. 2016;11:297–314. doi: 10.1177/1934578X1601100307. [DOI] [PubMed] [Google Scholar]
- 4.Espinosa S., Bec N., Larroque C., Ramírez J., Sgorbini B., Bicchi C., Cumbicus N., Gilardoni G. A novel chemical profile of a selective in vitro cholinergic essential oil from Clinopodium taxifolium (Kunth) Govaerts (Lamiaceae), a native Andean species of Ecuador. Molecules. 2021;26:45. doi: 10.3390/molecules26010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gilardoni G., Montalván M., Ortiz M., Vinueza D., Montesinos J.V. The flower essential oil of Dalea mutisii Kunth (Fabaceae) from Ecuador: Chemical, enantioselective, and olfactometric analyses. Plants. 2020;9:1403. doi: 10.3390/plants9101403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gilardoni G., Matute Y., Ramírez J. Chemical and enantioselective analysis of the leaf essential oil from Piper coruscans Kunth (Piperaceae), a costal and Amazonian native species of Ecuador. Plants. 2020;9:791. doi: 10.3390/plants9060791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.García J., Gilardoni G., Cumbicus N., Morocho V. Chemical analysis of the essential oil from Siparuna echinata (Kunth) A. DC. (Siparunaceae) of Ecuador and isolation of the rare terpenoid Sipaucin A. Plants. 2020;9:187. doi: 10.3390/plants9020187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montalván M., Peñafiel M., Ramirez J., Cumbicus N., Bec N., Larroque C., Bicchi C., Gilardoni G. Chemical composition, enantiomeric distribution, and sensory evaluation of the essential oils distilled from the Ecuadorian species Myrcianthes myrsinoides (Kunth) Grifo and Myrcia mollis (Kunth) DC. (Myrtacee) Plants. 2019;8:511. doi: 10.3390/plants8110511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espinosa S., Bec N., Larroque C., Ramirez J., Sgorbini B., Bicchi C., Gilardoni G. Chemical, enantioselective, and sensory analysis of a cholinesterase inhibitor essential oil from Coreopsis triloba S.F. Blake (Asteraceae) Plants. 2019;8:448. doi: 10.3390/plants8110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gilardoni G., Ramirez J., Montalvan M., Quinche W., León J., Benítez L., Morocho V., Cumbicus N., Bicchi C. Phytochemistry of three Ecuadorian Lamiaceae: Lepechinia heteromorpha (Briq.) Epling, Lepechinia radula (Benth.) Epling and Lepechinia paniculata (Kunth) Epling. Plants. 2018;8:1. doi: 10.3390/plants8010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramirez J., Gilardoni G., Ramón E., Tosi S., Picco A., Bicchi C., Vidari G. Phytochemical study of the Ecuadorian species Lepechinia mutica (Benth.) Epling and high antifungal activity of carnosol against Pyricularia oryzae. Pharmaceuticals. 2018;11:33. doi: 10.3390/ph11020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calva J., Bec N., Gilardoni G., Larroque C., Cartuche L., Bicchi C., Montesinos J. Acorenone B: AChE and BChE inhibitor as a major compound of the essential oil distilled from the Ecuadorian species Niphogeton dissecta (Benth.) JF Macbr. Pharmaceuticals. 2017;10:84. doi: 10.3390/ph10040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramírez J., Gilardoni G., Jácome M., Montesinos J., Rodolfi M., Guglielminetti M., Cagliero C., Bicchi C., Vidari G. Chemical composition, enantiomeric analysis, AEDA sensorial evaluation and antifungal activity of the essential oil from the Ecuadorian plant Lepechinia mutica Benth (Lamiaceae) Chem. Biodivers. 2017;14:e1700292. doi: 10.1002/cbdv.201700292. [DOI] [PubMed] [Google Scholar]
- 14.Chiriboga X., Gilardoni G., Magnaghi I., Vita Finzi P., Zanoni G., Vidari G. New Anthracene derivatives from Coussarea macrophylla. J. Nat. Prod. 2003;66:905–909. doi: 10.1021/np030066i. [DOI] [PubMed] [Google Scholar]
- 15.Gilardoni G., Malagon O., Morocho V., Negri R., Tosi S., Guglielminetti M., Vidari G., Vita Finzi P. Phytochemical research and antimicrobial activity of Clinopodium nubigenum Kunth (Kuntze) raw extracts. Rev. Bras. Farmacogn. 2011;21:850–855. doi: 10.1590/S0102-695X2011005000139. [DOI] [Google Scholar]
- 16.Gilardoni G., Chiriboga X., Finzi P.V., Vidari G. New 3,4-secocycloartane and 3,4-secodammarane triterpenes from the Ecuadorian plant Coussarea macrophylla. Chem. Biodivers. 2015;12:946–954. doi: 10.1002/cbdv.201400182. [DOI] [PubMed] [Google Scholar]
- 17.Herrera C., Morocho V., Vidari G., Bicchi C., Gilardoni G. Phytochemical investigation of male and female Hedyosmum scabrum (Ruiz & Pav.) Solms leaves from Ecuador. Chem. Biodivers. 2018;15:e1700423. doi: 10.1002/cbdv.201700423. [DOI] [PubMed] [Google Scholar]
- 18.Torres-Naranjo M., Suárez A.I., Gilardoni G., Cartuche L., Flores P., Morocho V. Chemical constituents of Muehlenbeckia tamnifolia (Kunth) Meisn (Polygonaceae) and its in vitro α-amilase and α-glucosidase inhibitory activities. Molecules. 2016;21:1461. doi: 10.3390/molecules21111461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramírez J., Suarez A.I., Bec N., Armijos C., Gilardoni G., Larroque C., Vidari G. Carnosol from Lepechinia mutica and tiliroside from Vallea stipularis: Two promising inhibitors of BuChE. Rev. Bras. Farmacogn. 2018;28:559–563. doi: 10.1016/j.bjp.2018.06.003. [DOI] [Google Scholar]
- 20.Vidari G., Abdo S., Gilardoni G., Ciapessoni A., Gusmeroli M., Zanoni G. Fungitoxic metabolites from Erigeron apiculatus. Fitoterapia. 2006;77:318–320. doi: 10.1016/j.fitote.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 21.Quílez A., Berenguer B., Gilardoni G., Souccar C., De Mendonça S., Oliveira L.F.S., Martin-Calero M.J., Vidari G. Anti- secretory, anti-inflammatory and anti-Helicobacter pylori activities of several fractions isolated from Piper carpunya Ruiz & Pav. J. Ethnopharmacol. 2010;128:583–589. doi: 10.1016/j.jep.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 22.Council of Europe European Pharmacopoeia. 8th ed. Council of Europe; Strasbourg, France: 2013. p. 743. [Google Scholar]
- 23.Durant-Archibold A.A., Santana A.I., Gupta M.P. Ethnomedical uses and pharmacological activities of most prevalent species of genus Piper in Panama: A review. J. Ethnopharmacol. 2018;217:63–82. doi: 10.1016/j.jep.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 24.Xiang C.P., Shi I.N., Liu F.F., Li H.Z., Zhang Y.J., Yang C.R., Xua M. A survey of the chemical compounds of Piper spp. (Piperaceae) and their biological activities. Nat. Product Commun. 2016;11:1403–1408. [PubMed] [Google Scholar]
- 25.Da Silva J.K., da Trindade R., Alves N.S., Figueiredo P.L., Guilherme J., Maia S., Setzer W.N. Essential oils from neotropical Piper species and their biological activities. Int. J. Mol. Sci. 2017;18:2571. doi: 10.3390/ijms18122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salehi B., Zakaria Z.A., Gyawali R., Ibrahim S.A., Rajkovic J., Shinwari Z.K., Khan T., Sharifi-Rad J., Ozleyen A., Turkdonmez E., et al. Piper species: A comprehensive review on their phytochemistry, biological activities and applications. Molecules. 2019;24:1364. doi: 10.3390/molecules24071364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. [Google Scholar]
- 28.Kundakovic T., Fokialakis N., Kovacevi N., Chinou I. Essential oil composition of Achillea lingulata and A. umbellate. Flavour Fragr. J. 2007;22:184–187. doi: 10.1002/ffj.1778. [DOI] [Google Scholar]
- 29.Osorio C., Alarcon M., Moreno C., Bonilla A., Barrios J., Garzon C., Duque C. Characterization of odor-active volatiles in Champa (Campomanesia lineatifolia R. & P.) J. Agric. Food Chem. 2006;54:509–516. doi: 10.1021/jf052098c. [DOI] [PubMed] [Google Scholar]
- 30.Flamini G., Tebano M., Cioni P.L., Bagci Y., Dural H., Ertugrul K., Uysal T., Savran A. A multivariate statistical approach to Centaurea classification using essential oil composition data of some species from Turkey. Plant Syst. Evol. 2006;261:217–228. doi: 10.1007/s00606-006-0448-3. [DOI] [Google Scholar]
- 31.Bianchi F., Careri M., Mangia A., Musci M. Retention indices in the analysis of food aroma volatile compounds in temperature-programmed gas chromatography: Database creation and evaluation of precision and robustness. J. Sep. Sci. 2007;30:563–572. doi: 10.1002/jssc.200600393. [DOI] [PubMed] [Google Scholar]
- 32.Babushok V.I., Linstrom P.J., Zenkevich I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data. 2001;40:043101. doi: 10.1063/1.3653552. [DOI] [Google Scholar]
- 33.Carrer R.P., Vanderlinde R., Dutra S., Marcon A., Echeverrigaray S. Essential oil variation among Brazilian accessions of Salvia guaranitica L. Flavour Fragr. J. 2007;22:430–434. doi: 10.1002/ffj.1817. [DOI] [Google Scholar]
- 34.Cheng H.Z., Kyoung H.K., Tae H.K., Hyong J.L. Analysis and characterization of aroma-active compounds of Schizandra chinensis (omija) leaves. J. Sci. Food Agric. 2005;85:161–166. [Google Scholar]
- 35.Chanegriha N., Baaliouamer A., Rolando C. Polarity changes during capillary gas chromatographic and gas chromatographic–mass spectrometric analysis using serially coupled columns of different natures and temperature programming application to the identification of constituents of essential oils. J. Chromatogr. A. 1998;819:61–65. doi: 10.1016/S0021-9673(98)00449-X. [DOI] [Google Scholar]
- 36.Demirci B., Can Baser K.H., Yildiz B., Bahçecioglu Z. Composition of the essential oils of six endemic Salvia spp. from Turkey. Flavour Fragr. J. 2003;18:116–121. doi: 10.1002/ffj.1173. [DOI] [Google Scholar]
- 37.Bisio A., Ciarallo G., Romussi G., Fontana N., Mascolo N., Capasso R., Biscardi D. Chemical composition of the essential oil from some Salvia species. Phytother. Res. 1998;12:S117–S120. doi: 10.1002/(SICI)1099-1573(1998)12:1+<S117::AID-PTR269>3.0.CO;2-2. [DOI] [Google Scholar]
- 38.Hachicha S.F., Skanji T., Barrek S., Ghrabi Z.G., Zarrouk H. Composition of the essential oil of Teucrium ramosissimum Desf. (Lamiaceae) from Tunisia. Flavour Fragr. J. 2007;22:101–104. doi: 10.1002/ffj.1764. [DOI] [Google Scholar]
- 39.Huang Y.T., Chang H.S., Wang G.J., Lin C.H., Chen I.S. Secondary metabolites from the roots of Beilschmiedia tsangii and their anti-inflammatory activities. Int. J. Mol. Sci. 2012;13:16430–16443. doi: 10.3390/ijms131216430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbosa-Filho J.M., Leitao da Cunha E.V., Sobral da Silva M. Complete assignment of the 1H and 13C NMR spectra of some lignoids from Lauraceae. Magn. Reson. Chem. 1998;36:929–935. doi: 10.1002/(SICI)1097-458X(199812)36:12<929::AID-OMR378>3.0.CO;2-O. [DOI] [Google Scholar]
- 41.Lopes N.P., de Almeida Blumenthal E.E., Cavalheiro A.J., Kato M.J., Yoshida M. Lignans, γ-lactones and propiophenones of Virola surinamensis. Phytochemistry. 1996;43:1089–1092. doi: 10.1016/S0031-9422(96)00408-6. [DOI] [Google Scholar]
- 42.Martins R.C.C., Lago J.H.G., Albuquerque S., Kato M.J. Trypanocidal tetrahydrofuran lignans from inflorescences of Piper solmsianum. Phytochemistry. 2003;64:667–670. doi: 10.1016/S0031-9422(03)00356-X. [DOI] [PubMed] [Google Scholar]
- 43.Ramos C.S., Linnert H.V., de Moraes M.M., do Amaral J.H., Yamaguchi L.F., Kato M.J. Configuration and stability of naturally occurring all-cis-tetrahydrofuran lignans from Piper solmsianum. RSC Adv. 2017;7:46932–46937. doi: 10.1039/C7RA09262H. [DOI] [Google Scholar]
- 44.Holloway D., Scheinmann F. Two lignans from Litsea grandis and L. gracilipes. Phytochemistry. 1974;13:1233–1236. doi: 10.1016/0031-9422(74)80107-X. [DOI] [Google Scholar]
- 45.Da Silva Filho A.A., Albuquerque S., Silva M.L.A., Eberlin M.N.E., Tomazela D.M., Bastos J.K. Tetrahydrofuran lignans from Nectandra megapotamica with trypanocidal activity. J. Nat. Prod. 2004;67:42–45. doi: 10.1021/np0302697. [DOI] [PubMed] [Google Scholar]
- 46.Soorukram D., Pohmakotr M., Kuhakarn C., Reutrakul V. Stereoselective synthesis of tetrahydrofuran lignans. Synthesis. 2018;50:4746–4764. doi: 10.1055/s-0037-1610289. [DOI] [Google Scholar]
- 47.Greger H., Pacher T., Vajrodaya S., Bacher M., Hofer O. Infraspecific variation of sulfur-containing bisamides from Aglaia leptantha. J. Nat. Prod. 2000;63:616–620. doi: 10.1021/np990542y. [DOI] [PubMed] [Google Scholar]
- 48.Parmar V.S., Jain S.C., Bisht K.S., Jain R., Taneja P., Jha A., Tyagi O.D., Prasad A.K., Wengel J., Olsen C.E., et al. Phytochemistry of the genus Piper. Phytochemistry. 1997;46:597–673. doi: 10.1016/S0031-9422(97)00328-2. [DOI] [Google Scholar]
- 49.Thin D.B., Chinh H.V., Luong N.X., Hoi T.M., Dai D.N., Ogunwande I.A. Chemical analysis of essential oils of Piper laosanum and Piper acre (Piperaceae) from Vietnam. J. Essent. Oil Bear. Plants. 2018;21:181–188. doi: 10.1080/0972060X.2018.1424040. [DOI] [Google Scholar]
- 50.Bos R., Woerdenbag H.J., Kayser O., Quax W.J., Ruslan K., Elfami Essential oil constituents of Piper cubeba L. fils. from Indonesia. J. Essent. Oil Res. 2007;19:14–17. doi: 10.1080/10412905.2007.9699217. [DOI] [Google Scholar]
- 51.McBride C.S., Baier F., Omondi A.B., Spitzer S.A., Lutomiah J., Sang R., Ignell R., Vosshall L.B. Evolution of mosquito preference for humans linked to an odorant receptor. Nature. 2014;515:222–227. doi: 10.1038/nature13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Barth T., Habenschus M.D., Lima Moreira F., Ferreira Lde S., Lopes N.P., Moraes de Oliveira A.R. In vitro metabolism of the lignan (–)-grandisin, an anticancer drug candidate, by human liver microsomes. Drug Test Anal. 2015;7:780–786. doi: 10.1002/dta.1743. [DOI] [PubMed] [Google Scholar]
- 53.Lopes N.P., Chicaro P., Kato M.J., Albuquerque S., Yoshida M. Flavonoids and lignans from Virola surinamensis twigs and their in vitro activity against Trypanosoma cruzi. Planta Med. 1998;64:667–669. doi: 10.1055/s-2006-957548. [DOI] [PubMed] [Google Scholar]
- 54.De Santis Ferreira L., Callejon D.R., Engemann A., Cramer B., Humpf H.U., de Barros V.P., Assis M.D., da Silva D.B., de Albuquerque S., Okano L.T., et al. In vitro metabolism of grandisin, a lignan with anti-chagasic activity. Planta Med. 2012;78:1939–1941. doi: 10.1055/s-0032-1327876. [DOI] [PubMed] [Google Scholar]
- 55.World Health Organization. [(accessed on 3 May 2021)]; Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis)
- 56.Van Den Dool H., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A. 1963;11:463–471. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
- 57.De Saint Laumer J.Y., Cicchetti E., Merle P., Egger J., Chaintreau A. Quantification in gas chromatography: Prediction of Martinsflame ionization detector response factors from combustion enthalpies and molecular structures. Anal. Chem. 2010;82:6457–6462. doi: 10.1021/ac1006574. [DOI] [PubMed] [Google Scholar]
- 58.Tissot E., Rochat S., Debonneville C., Chaintreau A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour Fragr. J. 2012;27:290–296. doi: 10.1002/ffj.3098. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Raw data are available from the authors (J.R.).