Abstract
Tomato mottle mosaic virus (ToMMV) is a noteworthy virus which belongs to the Virgaviridae family and causes serious economic losses in tomato. Here, we isolated and cloned the full-length genome of a ToMMV Chinese isolate (ToMMV-LN) from a naturally infected tomato (Solanum lycopersicum L.). Sequence analysis showed that ToMMV-LN contains 6399 nucleotides (nts) and is most closely related to a ToMMV Mexican isolate with a sequence identity of 99.48%. Next, an infectious cDNA clone of ToMMV was constructed by a homologous recombination approach. Both the model host N. benthamiana and the natural hosts tomato and pepper developed severe symptoms upon agroinfiltration with pToMMV, which had a strong infectivity. Electron micrographs indicated that a large number of rigid rod-shaped ToMMV virions were observed from the agroinfiltrated N. benthamiana leaves. Finally, our results also confirmed that tomato plants inoculated with pToMMV led to a high infection rate of 100% in 4–5 weeks post-infiltration (wpi), while pepper plants inoculated with pToMMV led to an infection rate of 40–47% in 4–5 wpi. This is the first report of the development of a full-length infectious cDNA clone of ToMMV. We believe that this infectious clone will enable further studies of ToMMV genes function, pathogenicity and virus–host interaction.
Keywords: tomato mottle mosaic virus, full-length genome sequence, infectious cDNA clone, Agrobacterium-mediated inoculation, infectivity
1. Introduction
Tomato (Solanum lycopersicum L.) is widely cultivated in the world and is of great economic value among vegetable crops. In 2016, approximately 5 million hectares of tomatoes were planted globally, producing 177 million tons of tomatoes, with China and India the two largest producers [1]. At least 136 different virus species infecting tomato have been reported in the world, with 34 of these viral species reported in China, including 23 RNA viruses and 11 DNA viruses [2]. Cucumber mosaic virus (CMV), tobacco mosaic virus (TMV), tomato spotted wilt virus (TSWV) and tomato yellow leaf curl virus (TYLCV) are commonly encountered by the growers, and symptoms caused by these viruses in tomato may vary depending on the growth stage, time of infection and environmental conditions, but typically include plant stunting; yellowing, mottling, bronzing, spotting and malformation of leaves; and development of light-colored concentric rings on fruits [3,4,5,6]. Moreover, tomato viruses have caused destructive damage and serious economic losses worldwide and pose a major threat to global tomato safety production [7,8,9]. Significantly, it is important to unravel the molecular mechanisms of viral infection and develop effective control strategies for tomato viruses.
Tobamovirus is the largest genus within the Virgaviridae family, and viruses within this genus cause fatal damage to crops and great economic losses worldwide. ToMMV is an emerging virus belonging to the Virgaviridae family, with a genome of 6399 nucleotides comprising four open reading frames (ORFs) that encode four proteins, including the 126 K protein, 183 K protein, movement protein (MP) and coat protein (CP). ToMMV was first found in tomato in Mexico and was officially named in 2013 [10]. Since then, it has spread fast in the world, including China [11,12], America [13,14] and Spain [15]. Notably, it has been reported that the 126 K protein and the 183 K protein are the components of the RNA replication protein complex which are mainly involved in ToMMV viral RNA replication [16]. It is well known that the MP is needed for the cell-to-cell movement of Tobamovirus [17] and the CP is critical for symptom development, virion formation, viral replication and the long-distance movement of Tobamovirus [18,19,20]. However, the molecular mechanisms of the MP and CP in the movement of ToMMV remain elusive. Given that a full-length infectious cDNA clone of ToMMV has not been constructed so far, it is not surprising that the pathogenicity of ToMMV is in most cases far from being fully understood.
Infectious cDNA clones (ICs) are constructed from stable cDNA copies of viral sequences, and they are amenable viral genetic materials which can be used for consistent infection by constructing infectious clones from DNA/cDNA of viruses [21,22,23]. An increasing number of studies have reported that ICs are powerful tools to investigate the viral gene functions, disease pathology and virus–host interactions in human, animal and plant viruses [24,25,26,27]. Generally, the full-length genome cDNA is first cloned and then infectious transcripts are produced in vitro using bacteriophage T7 promoter and T7 RNA polymerase [28,29]. In addition, the cauliflower mosaic virus (CaMV) 35S promoter has also been used extensively for ICs construction since it was reported by Odell et al. in 1985 [30]. Since then, it has been used for developing full-length infectious cDNA clones of numerous viruses [31,32,33,34,35]. Significantly, numerous viruses in the genus Tobamovirus have been constructed in the preceding decades. Viral factors that are responsible for the resistance-breaking character of Ob, a Tobamovirus that overcomes the N gene-mediated hypersensitive response (HR), were determined by identifying a variant that induced the HR through both site-directed mutagenesis of the infectious Ob cDNA clone and mutagenesis of it with hydroxylamine [36]. Full-length cDNAs of tomato mosaic virus (ToMV) were cloned into dual CaMV 35S and T7 promoter-driven vectors [37,38], and this revealed that a single residue substitution could induce a systemic HR in N. benthamiana [38]. It has also been demonstrated that the two mutants pCGMMV-CP-D89A and pCGMMV-RdRp-E1069A can prevent the superinfection of cucumber green mottle mosaic virus (CGMMV) in N. benthamiana by inoculation with ICs constructed with the CaMV 35S promoter [39]. These reports shed light on not only the synthesis of ICs of other Tobamovirus but also the construction of virus-based plant expression vectors. Despite the full-length genomes of ToMMV having been cloned and reported several times [14,16], the ICs of ToMMV still remain to be constructed.
In the present study, we first report the full-length genome sequence of a tomato isolate for ToMMV from Liaoning Province in China. Next, an infectious cDNA clone was constructed with the full-length ToMMV genomic expressed downstream of the double 35S promoter (2 × 35S) by homologous recombination and was named as pToMMV. Additionally, the infectivity of this ToMMV isolate was tested on its hosts by Agrobacterium tumefaciens-mediated infiltration. Surprisingly, agroinfiltration of pToMMV enabled fast recovery of large amounts of virions from the agroinfiltrated leaves in the model host N. benthamiana and showed strong infection ability and developed severe symptoms for the natural hosts tomato and pepper, which allowed a great molecular characterization of ToMMV-LN.
2. Materials and Methods
2.1. Virus Sources and RNA Extraction
ToMMV-infected tomato (Solanum lycopersicum L.) samples were collected from Liaoning Province in China. Tomato samples showed yellowing, shrinking of leaves and necrosis of fruits. Total RNA was extracted from tomato leaves with RNAiso Reagent (TaKaRa, Dalian, China). Reverse transcription (RT) using PrimeScriptTM RT Reagent Kit with gDNA Eraser (TaKaRa, Dalian, China) was conducted following the manufacturer’s instructions.
2.2. Sequencing of Complete Nucleotide Sequence and Phylogenetic Analysis
Based on complete nucleotide sequences of the ToMMV isolate from Mexico (GenBank accession number KF477193) and other Tobamovirus in GenBank (ToMMV Hainan isolate MG171192, ToMMV HN isolate MH381817), three specific primer pairs (ToMMV-1F/ToMMV-1R, ToMMV-2F/ToMMV-2R and ToMMV-3F/ToMMV-3R; Table S1) were designed for amplifying the full-length genome of ToMMV-LN. Each fragment was 2000 bp to 2300 bp in length, and overlapping areas between the adjacent two segments were designed to make sure the subsequent sequence could be assembled. The polymerase chain reaction (PCR) was performed using a high-fidelity polymerase PrimeSTARTM Max DNA Polymerase (TaKaRa, Dalian, China) in 50 μL reactions using 2.5 μL cDNA template from infected plants, 3 μL of each specific primer, 25 μL PrimeSTARTM Max DNA Polymerase, 16.5 μL ddH2O. PCR conditions were 1 min at 94 °C, followed by 33 cycles of 10 s at 98 °C, 15 s at 55 °C and 2 min at 72 °C, followed by a final cycle of 10 min at 72 °C. The PCR products were recovered by using a DNA gel recovery kit (Axygen, San Francisco, CA, USA) and were ligated into the pMD18-T vector (TaKaRa, Dalian, China), respectively. Then the recombinant vectors were transfected into Top 10 (Warbio, Nanjing, China) competent cells. Subsequently, colony PCR was performed with universal primer pairs M13F/M13R and the cloned PCR fragments were sequenced by General Biosystems Company (Anhui, China).
The full-length genomic sequences of ToMMV-LN (MN853592) were assembled with aforementioned three segments by using sequence assembly with default parameters in Seqman (DNASTAR) software. These genomic sequences were identified in the National Center for Biotechnology Information (NCBI) using a BLAST search, and nucleotide sequence data used in this study were obtained from the GenBank database. Nucleotide sequences were aligned using the Clustal W progressive alignment method, and the neighbor-joining phylogenetic tree was constructed by Kimura 2-parameter model with 1000 bootstrap replicates using MEGA 6.0 software. Nucleotide sequence data used in this study were obtained from the GenBank database: ToMMV isolate from Mexico (KF477193), ToMMV isolate from the Netherlands (MN654021), ToMMV isolate from Brazil (MH128145), ToMMV isolate from Spain (KU594507), ToMMV isolates from the United States (KP202857, KT810183, KX898033 and KX898034) and ToMMV isolates from China (MH381817, KR924950, KR824951 and MG171192).
2.3. Construction of Infectious Clone of ToMMV by Homologous Recombination Approach
To determine the pathogenicity of the ToMMV-LN isolate, we first constructed the full-length infectious cDNA clone of ToMMV-LN. Therefore, a specific primer pair (ToMMV-ssF and ToMMV-ssR; Table S1) was designed for amplifying the full-length genome of ToMMV-LN. PCR was performed using PrimeSTARTM Max DNA Polymerase as aforementioned. PCR conditions were 1 min at 94 °C, followed by 33 cycles of 10 s at 98 °C, 15 s at 55 °C and 4 min 30 s at 72 °C, followed by a final cycle of 10 min at 72 °C. The products were recovered by using DNA gel recovery kit (Axygen, San Francisco, CA, USA). The purified PCR product of the full-length cDNA was introduced into the binary vector pCB301-2 × 35S-RZ-NOS [40] linearized by two restriction Stu I and Sma I (New England Biolabs, Beijing, China) to produce pToMMV using homologous recombination approach by ClonExpressTM II One Step Cloning Kit (Vazyme Biotech, Nanjing, China) according to the manufacturer’s protocol. The ligated product was transfected into Top 10 (Warbio Biotech, Nanjing, China) competent cells and cultivated on Luria–Bertani (LB) plates with Kanamycin (50 mg/L) for 14 h at 37 °C condition. Subsequently, we performed colony PCR and restriction enzyme digestion, and the fragments of pToMMV clone were verified by General Biosystems Company (Anhui, China) sequencing.
2.4. Agroinfiltration of ToMMV Infectious cDNA Clone on Nicotiana benthamiana, Tomato and Pepper
The constructs pToMMV and empty vector pCB301 were electroporated into A. tumefaciens strain GV3101, respectively. N. benthamiana, tomato and pepper infection were performed as described before [41,42]. In brief, the recombinant colonies were grown at 28 °C, 220 rpm in LB medium supplemented with Kanamycin (50 mg/L) and Rifampicin (25 mg/L), then centrifuged at 8000× g for 3 min and resuspended at OD600 = 0.5 with agroinfiltration buffer (10 mM MgCl2; 10 mM MES, pH 5.6; 100 µM acetosyringone) and kept at room temperature for about 2 h before agroinfiltration. Agroinfiltrated plants were maintained under white fluorescent light (16 h light/8 h dark) at 26 °C constant temperature greenhouse.
To determine the infectivity of pToMMV clone on its model host and natural hosts, 4–6 fully expanded leaves of N. benthamiana, 3–5 fully expanded leaves of tomato (Moneymaker) and pepper (G26) were agroinfiltrated gently using 1 mL needleless syringes. A. tumefaciens culture harboring empty vector pCB301 was used as mock-inoculation control. N. benthamiana, tomato and pepper plants were agroinfiltrated with pToMMV or empty vector pCB301 for 3 seedlings as a group, respectively. The biological experiments were repeated 3 times with 3 repetitions for each group.
2.5. Detection of ToMMV by RT-PCR and Northern Blots
To confirm the infectivity of pToMMV in plants, we detected whether the ToMMV produced RNA transcripts in the pToMMV-infiltrated leaves or not. To this end, total RNAs were extracted from the pToMMV-infiltrated leaves of N. benthamiana at 1 day post-infiltration (dpi), 2 dpi and 4 dpi, with the pToMMV plasmid as positive control and healthy N. benthamiana plant as negative control. Then RT-PCR was performed using specific primers ToMMCP-F/ToMMCP-R (Table S1). In addition, the systemically infected leaves of N. benthamiana at 10 dpi, tomato at 12 dpi and pepper at 15 dpi were collected to confirm the presence of ToMMV RNA in the pToMMV-infected plants, respectively. Total RNAs were extracted for RT-PCR detection by using specific primers ToMMCP-F/ToMMCP-R. The symptoms of agroinfiltrated plants were recorded and photographed, respectively. Northern blot hybridization analysis of total RNAs extracted from the systemically infected N. benthamiana at 10 dpi was carried out as described previously [41]. In brief, DIG-labeled probe specific for sense CP gene was synthesized using a DIG High Prime RNA labeling kit (Roche, Basel, Switzerland). The membrane blots were hybridized with the specific DIG-labeled probe, then processed using a DIG-High Prime Detection Starter Kit II (Roche) and the manufacturer’s protocol.
2.6. Virus Purification and Morphological Observation using Transmission Electron Microscopy (TEM)
Virus purification was performed as described in [43] and made several changes. In brief, 5 mL precooled 0.1 M phosphate buffer (PB), pH 7.2, with 0.01 M EDTA was added to 5 g ToMMV-infected N. benthamiana leaves followed by homogenization in a mortar. The homogenate was centrifuged at 8000× g for 20 min at 4 °C. The initial supernatant liquid was mixed with chloroform at the volume proportion of 4:1, and centrifuged at 10,000× g for 30 min at 4 °C. The resulting supernatant was mixed with PEG-6000 (4% w/v) and NaCl (0.1 M), then blended and centrifuged at 10,000× g for 15 min at 4 °C. The pellet was resuspended in 0.01 M PB (pH 7.2) at 2% of the volume of the initial supernatant liquid. Finally, the resuspended liquid was observed under transmission electron microscopy (TEM) H-7650 (College of Life Sciences, Nanjing Agriculture University, Nanjing, China).
3. Results
3.1. Genome Organization and Phylogenetic Analysis of ToMMV-LN
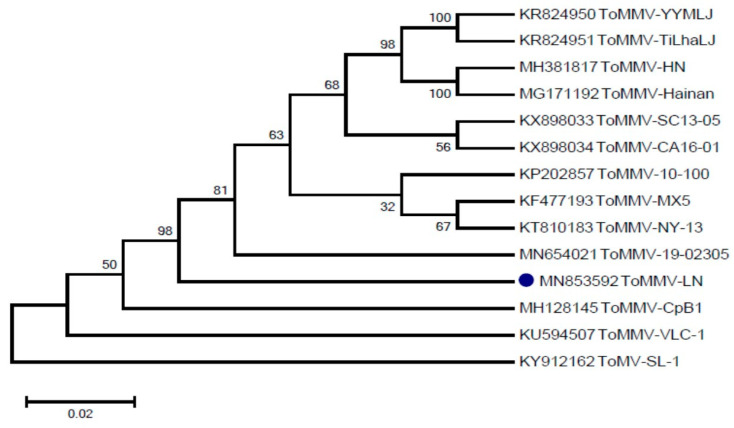
Three fragments were amplified and assembled (Figure S1). The full-length genome sequence of the ToMMV-LN isolate is 6399 nts long (MN853592), and consists of four open reading frames (ORFs), which were annotated according to their identity with those typically found in known Tobamoviruses. Pairwise alignment sequence comparison showed that the overall nucleotide sequence similarity between ToMMV-LN and the ToMMV isolates from Mexico (KF477193) and ToMMV-HN (MH381817) was 99.49% and 99.3%, respectively, whereas ToMMV-LN shared only 49.73–84.79% nucleotide sequence identity with other reported Tobamoviruses (Table 1). Additionally, alignment of the amino acid sequence revealed that the MP of ToMMV-LN shared the lowest identities (27.64–79.10%) with those of other Tobamoviruses, whilst the 183K of ToMMV-LN had a relatively high amino acid sequence similarity (45.73–94.68%) with those of other Tobamoviruses (Table 1). This suggests that the CP of ToMMV-LN has a closer evolutionary relationship with the Tobamoviruses. To characterize the relationship between ToMMV-LN and other ToMMV isolates, a phylogenetic tree based on the complete nucleotide sequence of ToMMV-LN and other ToMMV isolate sequences was constructed using the Neighbor-joining method with 1000 bootstrap replications by MEGA 6.0 software (Figure 1). Phylogenetic analysis resulted in ToMMV-LN clustered together with the ToMMV isolates from the United States (KP202857, KT810183, KX898033 and KX898034) and Mexico (KF477193) and China (KR824950, KR824951, MH381817 and MG171192), revealing very high similarities (98%).
Table 1.
Nucleotide and amino acid sequence identities (%) among ToMMV-LN and other ToMMV isolates and other Tobamoviruses.
| Virus Name | Accession Number | Genome a (%) |
126 K b (%) |
183 K b (%) |
MP b (%) |
CP b (%) |
|---|---|---|---|---|---|---|
| ToMMV-MX5 | KF477193 | 99.48 | 99.46 | 99.57 | 98.51 | 100 |
| ToMMV-SC13-05 | KX898033 | 99.36 | 99.37 | 99.50 | 98.51 | 100 |
| ToMMV-NY-13 | KT810183 | 99.34 | 99.37 | 99.50 | 98.88 | 99.37 |
| ToMMV-YYMLJ | KR824950 | 99.34 | 99.73 | 99.75 | 98.88 | 100 |
| ToMMV-10-100 | KP202857 | 99.33 | 99.55 | 99.57 | 98.51 | 100 |
| ToMMV-TiLhaLJ | KR824951 | 99.31 | 99.64 | 99.69 | 98.88 | 100 |
| ToMMV-HN | MH381817 | 99.30 | 99.55 | 99.63 | 98.51 | 100 |
| ToMMV-Hainan | MG171192 | 99.25 | 99.64 | 99.69 | 98.51 | 100 |
| ToMMV-19-02305 | MN654021 | 99.17 | 99.64 | 99.69 | 98.13 | 99.37 |
| ToMMV-CpB1 | MH128145 | 99.11 | 99.28 | 99.50 | 98.51 | 100 |
| ToMMV-CA16-01 | KX898034 | 99.09 | 99.64 | 99.69 | 98.51 | 100 |
| ToMMV-VLC-1 | KU594507 | 99.08 | 99.46 | 99.50 | 98.88 | 100 |
| ToMV-SL-1 | KY912162 | 84.79 | 94.27 | 94.68 | 79.10 | 91.19 |
| ToMV-AH4 | KU321698 | 84.63 | 94.09 | 94.62 | 78.73 | 91.82 |
| TBRFV-Tom1-Jo | KT383474 | 80.64 | 91.58 | 92.20 | 71.27 | 86.16 |
| RheMV-Henan | EF375551 | 78.14 | 89.43 | 90.47 | 70.52 | 82.50 |
| PMMoV-pMG | KX063611 | 68.25 | 73.37 | 75.79 | 63.43 | 71.07 |
| TMGMV-CaJO | MK648158 | 63.71 | 65.05 | 68.32 | 54.85 | 71.07 |
| RMV-R14 | HQ667979 | 59.26 | 60.25 | 64.32 | 34.94 | 48.43 |
| CMoV | AB261167 | 50.78 | 39.09 | 44.89 | 27.64 | 41.98 |
| CGMMV-SH | D12505 | 49.73 | 40.67 | 45.73 | 27.64 | 33.54 |
Note: a Nucleotide sequence and b amino acid sequences.
Figure 1.
Phylogenetic tree based on the complete nucleotide sequence of ToMMV-LN and other ToMMV isolate sequences.
3.2. Synthesis of ToMMV Infectious Clone and Infection Assays on N. benthamiana
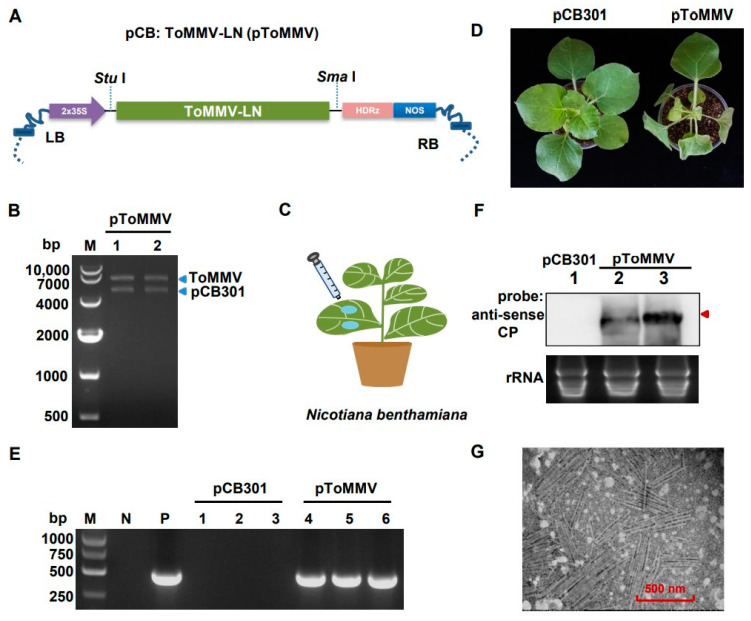
To develop the infectious clone of ToMMV-LN isolate, the full-length cDNA sequence of ToMMV-LN was driven by the 2 × 35S promoter and inserted into the linearized binary vector pCB301 to generate pToMMV (Figure 2A and Figure S2). Restriction endonuclease reaction was performed to determine that the pToMMV plasmid was successfully constructed (Figure 2B). The model plant N. benthamiana was primarily used to investigate the biological role of ToMMV-LN, and the ability of the pToMMV to infect plants was assessed by Agrobacterium-mediated infiltration assays (Figure 2C). The target bands of approximately 500 bps were detected in agroinfiltrated leaves of N. benthamiana with pToMMV at 1, 2 and 4 dpi, whereas no viral RNA could be detected in healthy N. benthamiana plants (Figure S3). Since the total DNA from plants or cDNA of ToMMV transferred by agroinfiltration cannot be reverse transcribed in the RT processes, it was shown that the genomic RNAs of ToMMV-LN were successfully transcribed in agroinfiltrated leaves with pToMMV. Subsequently, the N. benthamiana plants inoculated with pToMMV showed foliar mottle in upper leaves at 7 dpi (Figure 2D), and leaf distortion, necrosis symptoms and stunting in the whole plants at 10 dpi, while lethal necrosis of the entire plants occurred within 15 dpi.
Figure 2.
Transcription and replication of pToMMV in N. benthamiana. (A) Schematic diagram showing the infectious cDNA clone pCB: ToMMV-LN. 2 × 35S: CaMV 35S promoter. HDRz: hepatitis delta virus ribozyme. NOS: NOS terminator. LB: left border sequence. RB: Right border sequence. (B) Agrobacterium-mediated inoculation of pToMMV in N. benthamiana leaves. (C) Confirmation of the pToMMV infectious clone by restriction endonuclease digestion. M: DL 10,000 marker. Lanes 1-2: pToMMV plasmids digested with Stu I and Sma I. (D) Symptoms of N. benthamiana plants inoculated with pToMMV at 10 dpi. (E) RT-PCR detection to determine ToMMV infection in systemically infected leaves at 7 dpi. M: DL5000 DNA Marker. P: positive control, pToMMV plasmid. N: negative control, healthy N. benthamiana plant. Lanes 1–3, 4–6: N. benthamiana plants agroinfiltrated with pCB301 and pToMMV. (F) Northern blot analysis of total RNAs from the systemically infected leaves of N. benthamiana agroinfiltrated with pCB301 empty vector and pToMMV. Lane 1, 2–3: N. benthamiana plants agroinfiltrated with pCB301 and pToMMV. The total RNAs were detected using DIG-labeled anti-sense CP probe. Red arrow indicates genomic RNAs of ToMMV. Ethidium bromide staining was used as an RNA loading control. (G) Transmission electron micrograph for pToMMV resulted in abundant replication and virion formation in N. benthamiana leaves.
To further confirm that these phenotypes were induced by the pToMMV, RT-PCR detection was performed. It showed that the bands targeting ToMMV CP were detected in the positive control (the pToMMV plasmid) and systemically infected N. benthamiana leaves at 7 dpi, whereas no target band could be detected in healthy N. benthamiana and plants agroinfiltrated with pCB301 (Figure 2E). Agroinfiltrated N. benthamiana leaves were processed to acquire partially purified virion preparations and observed with a transmission electron microscope. To confirm the replication of the pToMMV, Northern blot analysis was also conducted. Systemically infected leaves from N. benthamiana agroinfiltrated with pToMMV showed the presence of gRNA of ToMMV CP (Figure 2F, lanes 2 and 3), whereas no viral RNA could be detected in plants agroinfiltrated with pCB301 (Figure 2F, lanes 1). The electron micrographs showed rigid rod-shaped ToMMV virions with particle dimensions of approximately 300 nm long and 18 nm diameter, typical characteristics of Tobamovirus (Figure 2G). All these cases verified that agroinfiltration of full-length cDNA clones of ToMMV-LN resulted in replication and virion formation in N. benthamiana leaves.
3.3. Pathogenicity and Infectivity on Tomato and Pepper Agroinfiltrated with pToMMV
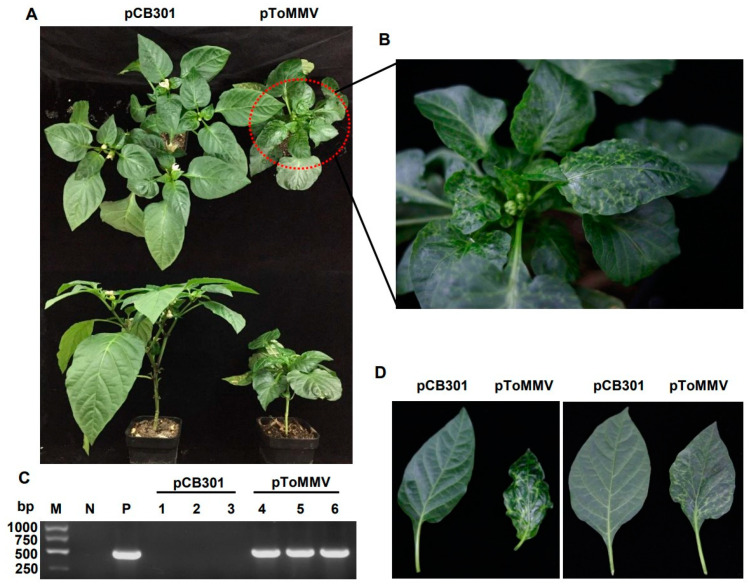
As the ToMMV-LN was isolated from tomato, we also tested the infectivity of this clone on its natural host. For tomato plants, the progeny of ToMMV-LN from cDNA clones caused narrowing, crinkling at 12 dpi, distorting, interveinal yellowing of upper leaves, occasionally their stems and leaf veins changed from green to purple at 20 dpi, and later pToMMV caused enations along the veins on the undersides of leaves at plant bases and the whole tomato plants developed stunting at 30 dpi; these were typical symptoms like wild-type ToMMV-infected tomato plants collected from Liaoning (Figure 3A,C,D). Similarly, we also chose another natural host, pepper, for the infectivity test. Pepper plants agroinfiltrated with pToMMV showed irregular yellow mottle, shrinking from the bottom to the middle of apical leaves at an early stage about 14 dpi, subsequently the apical leaves developed brown necrosis at 21 dpi and severe mosaic at 35 dpi, latterly pToMMV caused leaf abscission and stunting symptoms of the whole plants at 35 dpi (Figure 4A,B,D).
Figure 3.
Plant phenotypes and ToMMV detection after agroinfiltration of pToMMV on tomato plants. (A) Symptoms of tomato plants agroinfiltrated with pToMMV at 30 dpi. (B) RT-PCR detection to determine ToMMV infection at 12 dpi. M: DL5000 Marker. N: healthy tomato plant. P: pToMMV plasmid. Lanes 1–3, 4–6: tomato plants agroinfiltrated with pCB301 and pToMMV. (C,D) The pToMMV caused enations along the veins on the undersides of leaves at plant bases on tomato plants.
Figure 4.
Plant phenotypes and ToMMV detection after agroinfiltration of pToMMV on pepper plants. (A) Symptoms of pepper plants agroinfiltrated with pToMMV at 35 dpi. (B) Partial enlarged view of pepper plant that was systemically infected with pToMMV. (C) RT-PCR detection to determine ToMMV infection at 14 dpi. M: DL5000 Marker. N: healthy pepper plant. P: pToMMV plasmid. Lanes 1–3, 4–6: pepper plants agroinfiltrated with pCB301 and pToMMV. (D) The pToMMV caused leaf abscission, severe mosaic on pepper leaves.
The RT-PCR detection showed that the bands targeting ToMMV CP could be detected in the positive control pToMMV plasmid, agroinfiltrated tomato at 12 dpi and agroinfiltrated pepper at 14 dpi, while no target band could be detected in the negative control healthy tomato, pepper and those agroinfiltrated with pCB301 (Figure 3B and Figure 4C). As shown (Table 2), syringe inoculation was used for pToMMV, resulting in 100% infection in tomato and 40–47% infection in pepper. This demonstrated that the pToMMV resulted in abundant replication in tomato plants. Infectivity of the pToMMV on pepper was lower than on tomato.
Table 2.
Infection rate of syringe agroinfiltration with pToMMV in two different natural hosts.
| Replicate | Syringe Agroinfiltration | |||
|---|---|---|---|---|
| No. of Tomato Plants Infected/Inoculated |
Infection Rate (%) | No. of Pepper Plants Infected/Inoculated |
Infection Rate (%) | |
| I | 15/15 | 100 | 6/15 | 40 |
| Control | 0/5 | 0 | 0/5 | 0 |
| II | 13/13 | 100 | 6/13 | 46.1 |
| Control | 0/5 | 0 | 0/5 | 0 |
| III | 15/15 | 100 | 7/15 | 46.7 |
| Control | 0/5 | 0 | 0/5 | 0 |
Taken together, these results indicate that we have successfully constructed a full-length cDNA of ToMMV (pToMMV), and it has high pathogenicity and could efficiently infect the model host N. benthamiana and its natural hosts tomato and pepper.
4. Discussion
The ongoing spread of ToMMV threatens the output and quality of vegetable crops of subsistence farmers across China [11,12,44]. In this study, the full-length genome of ToMMV on tomato from Liaoning Province in China was amplified. Subsequently, we constructed the infectious cDNA clone pToMMV, which combines the full-length cDNA clone of ToMMV with a binary vector pCB301. Significantly, it has highly pathogenicity on three Solanaceae crops, including N. benthamiana, tomato and pepper. To the best of our knowledge, this is the first report on successfully constructing a reverse genetics system of ToMMV. Significantly, it is highly beneficial to study gene function by modifying the full-length infectious cDNA clone pToMMV in planta.
It is noteworthy that both in vitro viral RNA transcripts and agroinfiltration assays are usually used for constructing infectious cDNA clones. Since in vitro transcripts have been used for building infectious cDNA clones from brome mosaic virus (BMV) since 1984 [45], it has been used in many plant viruses. Agroinfiltration was known as “agroinfection” when it was used to infiltrate turnip plants with A. tumefaciens cells carrying binary plasmids, constructed by CaMV DNA with different vectors [46]. It has already been performed with many viruses such as potato leaf roll virus (PLRV), beet western yellows virus (BWYV), lettuce infectious yellows virus (LIYV) and potato virus S (PVS) on numerous plants [47,48,49,50]. Agroinfiltration coupled with targeted mutagenesis technologies will greatly enhance viral transcription in planta and insights will be obtained into virus replication, recombination, trafficking, symptom elicitation and virus–host interactions [49]. In the present study, we developed a stable infectious cDNA clone for delivering ToMMV to host plants in vitro by agroinfiltration. Accordingly, agroinfiltration of pToMMV in N. benthamiana plants enabled fast recovery of large amounts of virions from the agroinfiltrated leaves, which allowed better molecular characterization of ToMMV.
As for the pathogenicity of ToMMV, symptom development is heavily dependent on the infected hosts. A previous report showed that N. benthamiana plants show crinkling and yellowing on upper leaves at 4 dpi and serious necrosis symptoms on the apical shoot of seedlings at 6 dpi when mechanically inoculated with crude sap of ToMMV [51]. In our study, pToMMV induced lethal necrosis on agroinfiltrated N. benthamiana within 15 dpi (data not shown). This further suggests that the infectious cDNA clone pToMMV was successfully constructed and replicated severe necrosis symptoms on N. benthamiana. Notably, local and systemic lethal necrosis of N. benthamiana was due to plant virus pathogenicity determinants which play important roles in the silencing suppression or regulation of N-mediated immunity [52,53,54]. However, the proteins encoded by ToMMV for necrosis symptom development on N. benthamiana remain to be determined. The pathogenicity test of pToMMV on pepper plants showed mosaic, mottle and necrosis symptoms on top leaves (Figure 4), which coincided with the previous report [11]. The previous reports show that tomato plants infected by ToMMV showed foliar mottle, stunting, leaf distortion and necrosis symptoms [12], while sometimes exhibiting severe mosaic and tapering of the leaflet phenotypes [14]. In this study, tomato agroinfiltrated with pToMMV showed not only malformation and shrinking of the top leaves but also enations along the blade margin on the undersides of the leaves (Figure 3). This reveals that pToMMV can replicate the symptoms observed in naturally infected tomato plants. Remarkably, enations along the blade margin on the undersides of the leaves had never been found on ToMMV-infected tomato plants before; the mechanism of enations formation is worthy of further research.
ICs are powerful genetic tools for both basic and translational research on viruses of plant and animals, including human. The reverse genetic system based on an IC for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can be used to rapidly engineer viruses with desired mutations to study the virus in vitro and in vivo [55]. Neonatal gnotobiotic pigs inoculated with the chimeric ICs, combining porcine deltacoronavirus (PDCoV) with sparrow-origin deltacoronaviruses (SpDCoV), developed an attenuated symptom with the loss of tropism for the pig intestine, indicating limited infectious potential for cross-species [56]. In addition, Hao et al., using the IC of barley yellow dwarf virus-GAV (BYDV-GAV), demonstrated that the P4 protein was involved in cell-to-cell movement and caused stunting symptoms in wheat [57]. These reports all shed light on the multiple functions of ICs, including generating various mutations and studying the pathogenic mechanism and gene functions of viruses. In addition, virus-induced gene silencing (VIGS) vectors, which generated a Tobamovirus from an IC of sunn hemp mosaic virus (SHMV), were effective both in the expression and in the silencing of a transgene green fluorescent protein (GFP) and in silencing of an endogenous gene phytoene desaturase (PDS) on N. benthamiana [58]. An NbPDS fragment inserted into an IC of tobacco necrosis virus A Chinese isolate (TNV-A(C)) as an inverted repeat produced a VIGS derivative that could silence endogenous NbPDS in N. benthamiana [59]. Therefore, for pToMMV, we can also use it to not only determine the pathogenic factor and study gene functions of ToMMV but also to construct the VIGS vectors used for gene silencing in planta. In summary, an infectious cDNA of ToMMV (pToMMV)was successfully constructed, and we chose agroinfiltration to demonstrate that pToMMV has high pathogenicity. This not only provides a powerful tool for us to investigate the characteristics of ToMMV, such as the gene functions or interactions between plant and virus and the insights into molecular mechanisms underlying viral infection, but also develops ToMMV-based vectors for gene silencing in host plants or expression of foreign proteins.
Acknowledgments
We would like to thank Mingfeng Feng from Nanjing Agricultural University and Paul Daly from Jiangsu Academy of Agricultural Sciences for their critical reading of the manuscript. We would like to thank Xiaorong Tao from Nanjing Agricultural University for providing the pCB301 vector.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v13061050/s1: Figure S1. Three segments amplification of the full-length genome of ToMMV-LN. (A) (B) Three fragments were designed to construct the full-length genome of ToMMV-LN. M: DNA marker 3. Lane 1, 2 and 3: amplification of the first fragment about 2286 bps, 2310 bps and 2079 bps. Figure S2. Amplification of the full-length ToMMV-LN genome. (A) Schemes of the plant expression vector pCB301-2 × 35S-MCS-HDVRZ-NOS and ToMMV genomic RNA. 2 × 35S: CaMV 35S promoter. HDRz: hepatitis delta virus ribozyme. NOS: NOS terminator. LB: left border sequence. RB: right border sequence. (B) Amplification of the full-length cDNA of ToMMV-LN. M: DL10,000 DNA Marker. Lanes 1–2: RT-PCR products of the full-length cDNA of ToMMV-LN. Figure S3. RT-PCR detection of ToMMV genomic RNA in agroinfiltrated leaves at 1, 2 and 4 dpi. M: DL5000 DNA Marker. P: positive control, pToMMV plasmid. N: negative control, healthy N. benthamiana plant. Lanes 1–4, 5–8 and 9–12: pToMMV-agroinfiltrated leaves at 1, 2 and 4 dpi. Table S1. Primers used in the study.
Author Contributions
Conceptualization, Y.Z. and Y.J.; software, Y.L.; formal analysis, L.T.; investigation, S.W.; resources, D.G.; writing—original draft preparation, L.T.; writing—review and editing, Y.Z. and Y.J.; visualization, Y.L.; supervision, Y.L., Y.Z. and Y.J.; funding acquisition, Y.Z. and Y.J. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by grants from the National Natural Science Foundation of China (No. 32072506 and 31770168), China Agriculture Research System (No. CARS-24-C-01), Jiangsu Agriculture Science and Technology Innovation Fund [No. CX (19)3108], the Priority Academic Program Development of Jiangsu Higher Education Institutions (2020 PAPD).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hortidaily Overview Global Tomato Market. [(accessed on 12 January 2018)]; Available online: https://www.hortidaily.com/article/6040370/overview-global-tomato-market/
- 2.Hanssen I.M., Lapidot M., Thomma B.P.H.J. Emerging Viral Diseases of Tomato Crops. Mol. Plant. Microbe Interact. 2010;23:539–548. doi: 10.1094/MPMI-23-5-0539. [DOI] [PubMed] [Google Scholar]
- 3.Rendina N., Nuzzaci M., Sofo A., Campiglia P., Scopa A., Sommella E., Pepe G., De Nisco M., Basilicata M.G., Manfra M. Yield parameters and antioxidant compounds of tomato fruit: The role of plant defence inducers with or without Cucumber mosaic virus infection. J. Sci. Food Agric. 2019;99:5541–5549. doi: 10.1002/jsfa.9818. [DOI] [PubMed] [Google Scholar]
- 4.Prasad A., Sharma N., Hari-Gowthem G., Muthamilarasan M., Prasad M. Tomato yellow leaf curl virus: Impact, challenges, and management. Trends Plant. Sci. 2020;25:897–911. doi: 10.1016/j.tplants.2020.03.015. [DOI] [PubMed] [Google Scholar]
- 5.Ruark-Seward C.L., Bonville B., Kennedy G., Rasmussen D.A. Evolutionary dynamics of Tomato spotted wilt virus within and between alternate plant hosts and thrips. Sci. Rep. 2020;10:15797. doi: 10.1038/s41598-020-72691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdelkhalek A., Ismail I.A., Dessoky E.S., El-Hallous E.I., Hafez E. A tomato kinesin-like protein is associated with Tobacco mosaic virus infection. Biotechnol. Biotech. Eq. 2019;33:1424–1433. doi: 10.1080/13102818.2019.1673207. [DOI] [Google Scholar]
- 7.Velasco L., Simon B., Janssen D., Cenis J.L. Incidences and progression of tomato chlorosis virus disease and tomato yellow leaf curl virus disease in tomato under different greenhouse covers in southeast Spain. Ann. Appl. Biol. 2008;153:335–344. doi: 10.1111/j.1744-7348.2008.00262.x. [DOI] [Google Scholar]
- 8.Soler S., Prohens J., Lopez C., Aramburu J., Galipienso L., Nuez F. Viruses Infecting Tomato in Valencia, Spain: Occurrence, Distribution and Effect of Seed Origin. J. Phytopathol. 2010;158:797–805. doi: 10.1111/j.1439-0434.2010.01706.x. [DOI] [Google Scholar]
- 9.Moodley V., Gubba A., Mafongoya P.L. A survey of whitefly-transmitted viruses on tomato crops in South Africa. Crop. Prot. 2019;123:21–29. doi: 10.1016/j.cropro.2019.05.018. [DOI] [Google Scholar]
- 10.Li R., Gao S., Fei Z., Ling K.-S. Complete genome sequence of a new tobamovirus naturally infecting tomatoes in Mexico. Genome Announc. 2013;1:e00794-13. doi: 10.1128/genomeA.00794-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Y.Y., Wang C.L., Xiang D., Li R.H., Liu Y., Li F. First report of tomato mottle mosaic virus infection of pepper in China. Plant. Dis. 2014;98:1447. doi: 10.1094/PDIS-03-14-0317-PDN. [DOI] [PubMed] [Google Scholar]
- 12.Che H.Y., Luo D.Q., Cao X.R. First report of Tomato mottle mosaic virus in tomato crops in China. Plant. Dis. 2018;102:2051. doi: 10.1094/PDIS-03-18-0538-PDN. [DOI] [PubMed] [Google Scholar]
- 13.Fillmer K., Adkins S., Pongam P., D’Elia T. Complete Genome sequence of a Tomato mottle mosaic virus isolate from the United States. Genome Announc. 2015;3:e00167-15. doi: 10.1128/genomeA.00167-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagai A., Duarte L.M.L., Chaves A.L.R., Alexandre M.A.V., Ramos-Gonzalez P.L., Chabi-Jesus C., Harakava R., dos Santos D.Y.A.C. First Complete Genome Sequence of an Isolate of Tomato Mottle Mosaic Virus Infecting Plants of Solanum lycopersicum in South America. Genome Announc. 2018;6:e00427-18. doi: 10.1128/genomeA.00427-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ambros S., Martinez F., Ivars P., Hernandez C., de la Iglesia F., Elena S.F. Molecular and biological characterization of an isolate of Tomato mottle mosaic virus (ToMMV) infecting tomato and other experimental hosts in eastern Spain. Eur. J. Plant. Pathol. 2017;149:261–268. doi: 10.1007/s10658-017-1180-2. [DOI] [Google Scholar]
- 16.Li Y., Wang Y., Hu J., Xiao L., Tan G., Lan P., Liu Y., Li F. The complete genome sequence, occurrence and host range of Tomato mottle mosaic virus Chinese isolate. Virol. J. 2017;14:15. doi: 10.1186/s12985-016-0676-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheshukova E.V., Ershova N.M., Kamarova K.A., Dorokhov Y.L., Komarova T.V. The Tobamoviral movement protein: A “conditioner” to create a favorable environment for intercellular spread of infection. Front. Plant. Sci. 2020;11:959. doi: 10.3389/fpls.2020.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Callaway A., Giesman-Cookmeyer D., Gillock E.T., Sit T.L., Lommel S.A. The multifunctional capsid proteins of plant RNA viruses. Annu. Rev. Phytopathol. 2001;39:419–460. doi: 10.1146/annurev.phyto.39.1.419. [DOI] [PubMed] [Google Scholar]
- 19.Fukuda M., Ohno T., Okada Y., Otsuki Y., Takebe I. Kinetics of biphasic reconstitution of tobacco mosaic virus in vitro. Proc. Natl. Acad. Sci. USA. 1978;75:1727–1730. doi: 10.1073/pnas.75.4.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang T., Feng W., Ye J., Li Z., Zhou G. Metabolomic Changes in Sogatella furcifera under Southern rice black-streaked dwarf virus Infection and Temperature Stress. Viruses. 2018;10:344. doi: 10.3390/v10070344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marano J.M., Chuong C., Weger-Lucarelli J. Rolling circle amplification: A high fidelity and efficient alternative to plasmid preparation for the rescue of infectious clones. Virology. 2020;551:58–63. doi: 10.1016/j.virol.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tercero B., Makino S. Reverse genetics approaches for the development of bunyavirus vaccines. Curr. Opin. Virol. 2020;44:16–25. doi: 10.1016/j.coviro.2020.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y., He W., Li Q., Xie X., Qin N., Wang H., Huang J., Lin S., Ouyang K., Chen Y. Generation of a porcine reproductive and respiratory syndrome virus expressing a marker gene inserted between ORF4 and ORF5a. Arch. Virol. 2020;165:1803–1813. doi: 10.1007/s00705-020-04679-3. [DOI] [PubMed] [Google Scholar]
- 24.Rice C.M., Levis R., Strauss J.H., Huang H.V. Production of infectious RNA transcripts from sindbis virus cDNA clones-mapping of lethal mutations, rescue of a temperature-sensitive marker, and invitro mutagenesis to generate defined mutants. J. Virol. 1987;61:3809–3819. doi: 10.1128/jvi.61.12.3809-3819.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi B.J., Ding S.W., Symons R.H. Plasmid vector for cloning infectious cDNAs from plant RNA viruses: High infectivity of cDNA clones of tomato aspermy cucumovirus. J. Gen. Virol. 1997;78:1181–1185. doi: 10.1099/0022-1317-78-5-1181. [DOI] [PubMed] [Google Scholar]
- 26.Meulenberg J.J.M., BosDeRuijter J.N.A., vandeGraaf R., Wensvoort G., Moormann R.J.M. Infectious transcripts from cloned genome-length cDNA of porcine reproductive and respiratory syndrome virus. J. Virol. 1998;72:380–387. doi: 10.1128/JVI.72.1.380-387.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Q., Dan H., Zhao X., Chen H., Chen Y., Zhang N., Mo Z., Liu H. Construction and characterization of an infectious cDNA clone of coxsackievirus A 10. Virol. J. 2019;16:98–107. doi: 10.1186/s12985-019-1201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vanderwerf S., Bradley J., Wimmer E., Studier F.W., Dunn J.J. Synthesis of infectious poliovirus rna by purified T7 RNA-polymerase. Proc. Natl. Acad. Sci. USA. 1986;83:2330–2334. doi: 10.1073/pnas.83.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janda M., French R., Ahlquist P. High-efficiency T7-polymerase synthesis of infectious RNA from cloned brome mosaic-virus cdna and effects of 5′ extensions on transcript infectivity. Virology. 1987;158:259–262. doi: 10.1016/0042-6822(87)90265-0. [DOI] [PubMed] [Google Scholar]
- 30.Odell J.T., Nagy F., Chua N.H. Identification of DNA-sequences required for activity of the Cauliflower mosaic virus-35s promoter. Nature. 1985;313:810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- 31.Chen A.Y.S., Pavitrin A., Ng J.C.K. Agroinoculation of the cloned infectious cDNAs of Lettuce chlorosis virus results in systemic plant infection and production of whitefly transmissible virions. Virus Res. 2012;169:310–315. doi: 10.1016/j.virusres.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 32.Delfosse V.C., Casse M.F., Agrofoglio Y.C., Bonacic Kresic I., Hopp H.E., Ziegler-Graff V., Distefano A.J. Agroinoculation of a full-length cDNA clone of cotton leafroll dwarf virus (CLRDV) results in systemic infection in cotton and the model plant Nicotiana benthamiana. Virus Res. 2013;175:64–70. doi: 10.1016/j.virusres.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Liu L., Xie K., Tsekpuia A.R., Peng B., Liu M., Gu Q. Construction and biological characterization of an Agrobacterium-mediated infectious cDNA of squash mosaic virus. Virus Res. 2019;274:197766–197770. doi: 10.1016/j.virusres.2019.197766. [DOI] [PubMed] [Google Scholar]
- 34.Zhang F., Liu S., Zhang T., Ye Z., Han X., Zhong K., Yang J., Chen J., Liu P. Construction and biological characterization of an infectious full-length cDNA clone of a Chinese isolate of Wheat yellow mosaic virus. Virology. 2021;556:101–109. doi: 10.1016/j.virol.2021.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Bevan M.W., Mason S.E., Goelet P. Expression of Tobacco mosaic-virus coat protein by a Cauliflower mosaic-virus promoter in plants transformed by agrobacterium. EMBO J. 1985;4:1921–1926. doi: 10.1002/j.1460-2075.1985.tb03871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padgett H.S., Beachy R.N. Analysis of a Tobacco mosaic-virus strain capable of overcoming n gene-mediated resistance. Plant. Cell. 1993;5:577–586. doi: 10.1105/tpc.5.5.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weber H., Haeckel P., Pfitzner A.J.P. A cDNA clone of Tomato mosaic-virus is infectious in plants. J. Virol. 1992;66:3909–3912. doi: 10.1128/JVI.66.6.3909-3912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Agre A.P., Bhattacharjee R., Dansi A., Becerra Lopez-Lavalle L.A., Dansi M., Sanni A. Assessment of cassava (Manihot esculenta Crantz) diversity, loss of landraces and farmers preference criteria in southern Benin using farmers’ participatory approach. Genet. Resour. Crop. Ev. 2017;64:307–320. doi: 10.1007/s10722-015-0352-1. [DOI] [Google Scholar]
- 39.Liu J., Li X.-D., Xu S. Single amino acid substitutions in the coat protein and RNA-dependent RNA polymerase alleviated the virulence of Cucumber green mottle mosaic virus and conferred cross protection against severe infection. Virus Genes. 2020;56:228–235. doi: 10.1007/s11262-019-01726-3. [DOI] [PubMed] [Google Scholar]
- 40.Yao M., Zhang T., Tian Z., Wang Y., Tao X. Construction of agrobacterium-mediated Cucumber mosaic virus infectious cDNA clones and 2b deletion viral vector. Sci. Agric. Sin. 2011;44:3060–3068. [Google Scholar]
- 41.Feng M., Cheng R., Chen M., Guo R., Li L., Feng Z., Wu J., Xie L., Hong J., Zhang Z. Rescue of tomato spotted wilt virus entirely from complementary DNA clones. Proc. Natl. Acad. Sci. USA. 2020;117:1181–1190. doi: 10.1073/pnas.1910787117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao W., Wu S., Barton E., Fan Y., Ji Y., Wang X., Zhou Y. Tomato Yellow Leaf Curl Virus V2 Protein Plays a Critical Role in the Nuclear Export of V1 Protein and Viral Systemic Infection. Front. Microbiol. 2020;11:1243. doi: 10.3389/fmicb.2020.01243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lu G., Li S., Zhou C., Qian X., Xiang Q., Yang T., Wu J., Zhou X., Zhou Y., Ding X.S. Tenuivirus utilizes its glycoprotein as a helper component to overcome insect midgut barriers for its circulative and propagative transmission. PLoS Pathog. 2019;15:e1007655. doi: 10.1371/journal.ppat.1007655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chai A.L., Chen L.D., Li B.J., Xie X.W., Shi Y.X. First Report of a Mixed Infection of Tomato mottle mosaic virus and Tobacco mild green mosaic virus on Eggplants in China. Plant. Dis. 2018;102:2668. doi: 10.1094/PDIS-04-18-0686-PDN. [DOI] [Google Scholar]
- 45.Ahlquist P., French R., Janda M., Loesch-Fries L.S. Multicomponent RNA plant virus infection derived from cloned viral cDNA. Proc. Natl. Acad. Sci. USA. 1984;81:7066–7070. doi: 10.1073/pnas.81.22.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grimsley N., Hohn B., Hohn T., Walden R. Agroinfection, an alternative route for viral-infection of plants by using the ti plasmid. Proc. Natl. Acad. Sci. USA. 1986;83:3282–3286. doi: 10.1073/pnas.83.10.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leiser R.M., Zieglergraff V., Reutenauer A., Herrbach E., Lemaire O., Guilley H., Richards K., Jonard G. Agroinfection as an alternative to insects for infecting plants with Beet western yellows luteovirus. Proc. Natl. Acad. Sci. USA. 1992;89:9136–9140. doi: 10.1073/pnas.89.19.9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kawchuk L., Jaag H.M., Toohey K., Martin R., Rohde W., Prufer D. In planta agroinfection by Canadian and German Potato leafroll virus full-length cDNAs. Can. J. Plant Pathol. 2002;24:239–243. doi: 10.1080/07060660309507002. [DOI] [Google Scholar]
- 49.Wang J., Turina M., Stewart L.R., Lindbo J.A., Falk B.W. Agroinoculation of the Crinivirus, Lettuce infectious yellows virus, for systemic plant infection. Virology. 2009;392:131–136. doi: 10.1016/j.virol.2009.06.034. [DOI] [PubMed] [Google Scholar]
- 50.Li X., Hataya T. Construction and characterization of an infectious cDNA clone of potato virus S developed from selected populations that survived genetic bottlenecks. Virol. J. 2019;16:18–32. doi: 10.1186/s12985-019-1124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhan B.H., Cao N., Wang K.-n., Zhou X.-p. Detection and characterization of an isolate of Tomato mottle mosaic virus infecting tomato in China. J. Integr. Agric. 2018;17:1207–1212. doi: 10.1016/S2095-3119(17)61895-1. [DOI] [Google Scholar]
- 52.Burgyan J., Hornyik C., Szittya G., Silhavy D., Bisztray G. The ORF1 products of tombusviruses play a crucial role in lethal necrosis of virus-infected plants. J. Virol. 2000;74:10873–10881. doi: 10.1128/JVI.74.23.10873-10881.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raj S.K., Srivastava A., Chandra G., Singh B.P. Role of satellite RNA of an Indian isolate of cucumber mosaic virus in inducing lethal necrosis of tobacco plants. Indian J. Exp. Biol. 2000;38:613–616. [PubMed] [Google Scholar]
- 54.Deng Y., Wang J., Tung J., Liu D., Zhou Y., He S., Du Y., Baker B., Li F. A role for small RNA in regulating innate immunity during plant growth. PLoS Pathog. 2018;14:e1006756. doi: 10.1371/journal.ppat.1006756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xie X., Lokugamage K.G., Zhang X., Vu M.N., Muruato A.E., Menachery V.D., Shi P.Y. Engineering SARS-CoV-2 using a reverse genetic system. Nat. Protoc. 2021;16:1761–1784. doi: 10.1038/s41596-021-00491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Niu X., Hou Y.J., Jung K., Kong F., Saif L.J., Wang Q. Chimeric porcine deltacoronaviruses with Sparrow coronavirus spike protein or the receptor-binding domain infect pigs but lose virulence and intestinal tropism. Viruses. 2021;13:122. doi: 10.3390/v13010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hao X., Song S., Zhong Q., Hajano J.-U.D., Guo J., Wu Y. Rescue of an infection clone of Barley yellow dwarf virus -GAV. New Phytopathol. 2021 doi: 10.1094/PHYTO-11-20-0522-R. [DOI] [PubMed] [Google Scholar]
- 58.Varallyay E., Lichner Z., Safrany J., Havelda Z., Salamon P., Bisztray G., Burgyan J. Development of a virus induced gene silencing vector from a legumes infecting tobamovirus. Acta Biol. Hung. 2010;61:457–469. doi: 10.1556/ABiol.61.2010.4.9. [DOI] [PubMed] [Google Scholar]
- 59.Gao Y., Zhang Y.L., Zhang X.F., Han C.G., Yu J.L., Li D.W. Development and optimization of Tobacco necrosis virus A induced gene silencing in Nicotiana benthamiana. Prog. Biochem. Biophys. 2011;38:919–928. doi: 10.3724/SP.J.1206.2011.00129. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.