Abstract
Cryptosporidiosis is ranked sixth in the list of the most important food-borne parasites globally, and it is an important contributor to mortality in infants and the immunosuppressed. Recently, the number of genome sequences available for this parasite has increased drastically. The majority of the sequences are derived from population studies of Cryptosporidium parvum and Cryptosporidium hominis, the most important species causing disease in humans. Work with this parasite is challenging since it lacks an optimal, prolonged, in vitro culture system, which accurately reproduces the in vivo life cycle. This obstacle makes the cloning of isolates nearly impossible. Thus, patient isolates that are sequenced represent a population or, at times, mixed infections. Oocysts, the lifecycle stage currently used for sequencing, must be considered a population even if the sequence is derived from single-cell sequencing of a single oocyst because each oocyst contains four haploid meiotic progeny (sporozoites). Additionally, the community does not yet have a set of universal markers for strain typing that are distributed across all chromosomes. These variables pose challenges for population studies and require careful analyses to avoid biased interpretation. This review presents an overview of existing population studies, challenges, and potential solutions to facilitate future population analyses.
Keywords: cryptosporidiosis, mixed infections, population structure, genome evolution, molecular typing
1. Introduction
Cryptosporidiosis is among the most important causes of diarrhea and diarrhea-associated death in young children in developing countries and is one of the major causes of waterborne outbreaks of illness in industrialized nations [1]. Cryptosporidium is an obligate, intracellular parasite that infects the epithelial cells of the digestive and respiratory tracts of a wide variety of hosts. It presents a significant public health problem, primarily for infants and the immunosuppressed [2]. There are 38 recognized Cryptosporidium species, which differ in host specificity and public health significance [3]. Of these, C. parvum and C. hominis are observed to be the most important sources of cryptosporidiosis in humans [3]. Additionally, there are over 20 species that have been identified molecularly as being responsible for zoonotic cryptosporidiosis in humans [4].
The Cryptosporidium life cycle alternates between asexual and sexual reproduction within a single host. Sexual recombination results in the production of oocysts and thus is essential to transmission, but recombination may also play a role in the continued infection of the host [5,6]. Mixed infections of C. hominis and C. parvum have been reported in human patients and are not uncommon in some areas [7,8,9,10].
Several different population structures have been reported for Cryptosporidium that vary both by species and context. Intra-species genetic structure diversity is observed in Cryptosporidium populations in endemic areas [11]. Populations have been observed in both panmictic [12] and largely clonal structures [13]. Some panmictic structures are masked by genetically identical clones at the loci examined (epidemic) [3,11,14]. In fact, the available studies focused on all these parasite population structures are usually observed in outbreaks and are limited by specific geographic location. The available data are often not comparable and can be affected by different transmission rates and animal husbandry practices. Different genes evolve at different rates. Thus, the selection of genetic markers for population studies depends on the biological question being addressed and the level of divergence that needs to be detected.
Population genetic studies based on highly polymorphic loci can shed light on the genetic diversity present within Cryptosporidium. Currently, however, only a few genetic markers have been developed for a few loci that do not cover all 8 chromosomes [15,16,17,18,19]. Whole genome sequence approaches are now being applied, when possible, to obtain a better understanding and overview of genetic variation and recombination within this species with the goal of better understanding its population structure [8,20,21] and evolution [22,23]. However, even with whole genome sequencing (WGS), challenges still remain for determining the global population structure of C. parvum and C. hominis as well as other human-infecting species.
2. Current Status of Cryptosporidium Whole Genome Sequences
Cryptosporidium genome sizes are among the smallest reported in the Apicomplexa at ~9.1 Mb distributed in 8 chromosomes [24,25,26,27]. Analyses of genome content reveal broad-scale reduction and heavy reliance on transporters and host nutrients for survival. Cryptosporidium has very little intergenic content [25,26] and no reported mitochondrial or apicoplast genome sequences [28,29].
Currently, 52 genome assemblies are available for the genus Cryptosporidium in the NCBI GenBank [22,25,26,30,31,32,33], representing 15 species of the parasite (Table 1). Only C. parvum has chromosomal physical mapping information in support of the karyotype [27]. A few of the assemblies are annotated. The current reference sequences for each species have had to rely heavily on computational predictions and orthology to identify genes since little experimental expression evidence for genes exists, or existed, at the time of the annotation.
Table 1.
Summary of Cryptosporidium genome assembly data available in the NCBI GenBank.
| Cryptosporidium Species | # of Genome Sequences Available | Sequencing Technology | Gene Evidence Availability | |||
|---|---|---|---|---|---|---|
| RNAseq a | Expressed Sequence Tag Datasets | Proteomic Data | # of Genome Annotations Available | |||
| C. parvum | 19 | Sanger, Illumina, 454, ABI SOLiD, PacBio, ONT, HAPPY map data | Yes | Yes | Yes | 2 |
| C. hominis | 12 | Sanger, Illumina, Ion Torrent, 454 | Yes | Yes | Yes | 5 |
| C. ubiquitum | 5 | Illumina | No | No | No | 1 |
| C. meleagridis | 3 | Illumina | No | No | No | 1 |
| C. andersoni | 3 | Illumina | No | No | No | 1 |
| C. muris | 1 | Sanger and 454 | No | No | Yes | 1 |
| C. tyzzeri | 1 | Illumina | No | No | No | 1 |
| C. felis | 1 | Illumina | No | No | No | 1 |
| C. cuniculus | 1 | Illumina | No | No | No | 0 |
| C. ryanae | 1 | Illumina | No | No | No | 0 |
| C. bovis | 1 | Illumina | No | No | No | 0 |
| C. viatorum | 1 | Illumina | No | No | No | 0 |
| C. sp. 37763 | 1 | Illumina | No | No | No | 0 |
| C. sp. chipmunk LX-2015 | 1 | Illumina | No | No | No | 0 |
| C. baileyi | 1 | Illumina, PacBio | Yes | Yes | No | 0 |
a not available for all lifecycle stages. Most data represent only extracellular oocyst and sporozoite lifecycle stages. # means Number.
The first assembled and annotated genome sequence was for C. parvum in 2004 [26]. This assembly used a HAPPY map approach combined with capillary sequencing. This assembly has had several annotation updates [34,35]. A newer genome assembly and annotation for C. parvum, which combines Illumina and long-read sequencing, has been generated. The new assembly, C. parvum (strain IOWA-ATCC), has no gaps, and all telomeres are identified [34]. C. parvum annotation updates over the years have identified new genes, corrected gene structures (mostly adding introns and untranslated regions, -UTRs), and identified non-coding genes [35,36]. There is a cluster of closely related species, including C. parvum and C. hominis, that share >95% nucleotide identity and high synteny relative to other species outside of this cluster [22,34]. The high sequence similarity within this group suggests that it is not inconceivable to develop markers that both cover and can disambiguate, multiple Cryptosporidium species.
3. Cryptosporidium Population Structure
Cryptosporidium species lack variable morphological traits useful for identification. Single- [16,17,18,37,38,39,40,41,42,43] and multi-locus [19,44] typing tools and other approaches such as multiple-locus variable-number of tandem-repeats (VNTRs) [45,46] have been developed to help identify and characterize Cryptosporidium species and subtypes of this diverse genus.
Cryptosporidia are primarily defined by host specificity and 18S ribosomal RNA sequence [18]. Cryptosporidium 18S rRNAs from different species differ by just a few nucleotides. However, some isolates with identical 18S rRNA sequences also present with different host specificities and phenotypes [22]. These observations revealed a subpopulation structure that was present within within the same species. Thus, the development of additional markers is needed to better understand Cryptosporidium population structure and evolution.
Multi-locus typing of C. parvum revealed high genetic diversity within the species, including significant geographic segregation and complex population structure [11,47]. Analyses of population structure are an important guide to understanding transmission and evolution since extant organisms represent the outcome of their history and adaptation to their environment. The most commonly used genetic locus for subtyping Cryptosporidium spp. is the 60 kDa glycoprotein gene (gp60) [16]. This locus is useful because it contains multiple regions displaying high mutation rates, including, in particular, a “hyper-variable” microsatellite region. As previously reported, the number of gp60 subtypes varies not just between species but also within them [47,48]. Nearly 20 different gp60 subtypes have been observed in C. parvum, and they show a potential correlation to some observed phenotypes, e.g., subtypes IIa and IId, which are commonly found in zoonotic infections or a specific geographic location [4].
While single marker typing does reveal some correlation with host or phenotype in most studies, the results do not always agree with those of other genetic loci for some of the C. parvum subtype families, especially IIa and IId. In developing countries, IIa subtypes are rarely seen in humans, but in the middle east, both IIa and IId subtypes are commonly seen in humans. These observations show that strains carrying these gp60 subtypes vary in phenotype and that using a single marker is still a low-resolution method to understand the genetic basis for host specificity or adaptation in Cryptosporidium.
Several research groups have proposed and tested additional genetic markers, primarily based on microsatellites [14,49,50,51]. Most work well and reveal similar topologies within the same species and group of isolates used for development and testing. However, their performance declines when isolates from different geographic regions are analyzed [11]. An analysis of 11 different studies using several loci to type diverse isolates of C. parvum and C. hominis revealed that no single marker performs reliably, even in multi-locus studies (MLST), but gp60 and TP14 show some promise in MLST with both species [19]. In the case of C. parvum, the MM19, MM18, MM5, MSF, MSD loci are identified as good options, and in C. hominis, the cp47, msc6-7, rpgr, ML2 loci worked well in the samples studied [19].
Fast evolving genes can be used to study the evolutionary dynamics of parasite population structure. Copy number variation (CNV) analysis of two fast-evolving protein families, MEDLE motif-containing proteins and insulinases-like proteases, have been linked to differences in host ranges between C. parvum and C. hominis [52]. A similar observation has been made within C. parvum gp60 subtype families, where two sequenced isolates belonging to the IId subtype family, one from China and the other from Egypt, have lost one of the six genes encoding MEDLE proteins and gained at least one SKSR and insulinase-like protease gene when compared to the C. parvum IOWAII reference genome (subtype IIa) [53]. In addition to these gene gains and losses, C. parvum IIa, IId, and IIc subtype families have highly divergent subtelomeric genes encoding other families of secretory proteins [52], and as recently observed, there is subtelomeric genome plasticity in C. parvum [34].
A division of C. parvum into two branches that correspond to human-infecting (C. parvum anthroponosum) and non-human infecting (C. parvum parvum) has been suggested [22]. The authors examined 467 gp60 sequences from 126 countries present in databases and 21 genome sequences (mostly from the UK). Analyses revealed evidence of positive selection and the existence of different population structures likely caused by different host migratory patterns. They also observed that the two branches had undergone genetic recombination, as evidenced by genetic exchanges between both branches and some incorporation of C. hominis related regions, mainly in C. p. anthroponosum. The majority of the detected recombination events are located in subtelomeric regions, which are a common hotspot for genes associated with host interactions and virulence in other apicomplexan parasites [54,55,56] and seem to play an important role in Cryptosporidium.
4. Challenges Faced by Cryptosporidium Population Studies
4.1. Sampling Limitations
There are numerous important studies of Cryptosporidium diversity in the literature. However, there is a size limitation in these studies. Most consist of a low number of isolates in a limited number of hosts and geographic locations [8,11,14,57,58]. As a consequence, the overall population structure of C. parvum and C. hominis remain unknown. A global study using a standardized, yet globally sensitive group of markers is still needed.
4.2. Limited Number of Markers
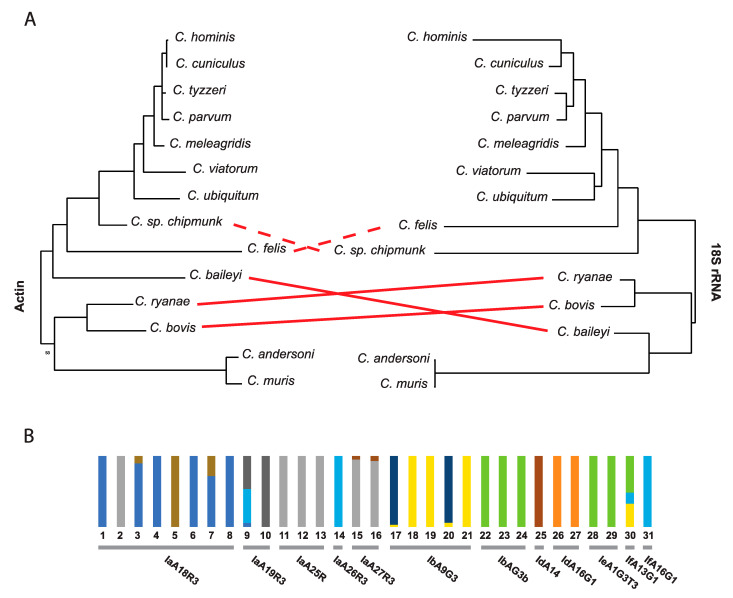
18S ribosomal subunit markers are useful for the identification of species [59]. Subtypes of species, primarily within C. parvum and C. hominis can also be defined with additional markers, but some markers are more sensitive to local geographic diversity [47] and are not applicable globally. Many subtypes are only defined based on a few typing markers [60,61]. Single markers each have their own evolutionary history and are not always representative of the diversity of the genome sequence they are isolated from or indicative of the population structure of the organism being studied (Figure 1A). In fact, when performing an admixture analysis using all biallelic polymorphic sites detected in different C. hominis isolates available in NCBI GenBank [62], some gp60 subtypes (loci) show incongruences in their ancestral subpopulation level (Figure 1B). A similar finding was observed for 46 C. parvum isolates in China, where the gp60 subtypes showed population substructure variation according to Bayesian clustering of allelic data [11]. Early difficulties with obtaining genome sequence data from isolates has significantly hampered community efforts to develop a robust and universal set of markers that can be used to detect and compare the global diversity of extant isolates.
Figure 1.
A single genetic marker is not representative of the entire genome sequence and evolution of an organism. (A) Comparative maximum likelihood topology analysis of two different Cryptosporidium genes (nt) that are usually used as markers; Dashed red lines represents differences with bootstrap values below 50% and solid lines with bootstrap values above 80% (B) Admixture clustering analysis of C. hominis biallelic variant sites reveals genomic variation within strains of the same gp60 subtype. The number of ancestral populations (K) were predicted by the lowest cross-validation error K value (K = 10) obtained from the admixture analysis. Each column in the graph represents an individual isolate, while each color within the column represents an ancestral population. The gp60 subtype for each isolate is indicated below the columns. GenBank and SRA accession numbers for the sequences utilized are provided in Supplemental Tables S1, S2 and the methods are described in Supplemental Methods.
4.3. Mixed Infections
Individuals, especially in endemic regions, may be infected with multiple species [7] or, more commonly, multiple strains of C. parvum or C. hominis. Depending on the degree of relatedness of parasites within mixed infections and the markers used for detection, mixed infections might be missed [8,51]. Mixed infections can sometimes be detected with PCR [63], but more often, variants are detected with deep sequencing data [64,65,66]. Deep sequencing can also be used to estimate the rate of relatedness within the infection, but it will not yield complete genome sequences for each haplotype.
The logical idea to clone individual parasites or oocysts of Cryptosporidium is, unfortunately, not an option since there is no optimal, prolonged, in vitro culture system, which can accurately reproduce the in vivo life cycle [67]. Some promising in vitro culture system models are emerging (see below, 6.1), and this prospect holds promise for teasing apart mixed infections and facilitating studies of recombination. Currently, available sequence data from isolates should be treated carefully as they may represent mixed infections [68].
Recently a study was able to generate single-cell sorting of oocysts for whole genome amplification, which indeed decreased the chances of obtaining information from multiple subtypes in a mixed infection [69]. Cryptosporidium has asexual and sexual components in its single-host life cycle, which enhances the chances of recombination. Indeed, recombination is reported in this parasite with linkage disequilibrium as short as 300 bp [8]. As a result, oocysts, which contain four meiotic haploid sporozoites inside, must be considered a population even if the sequence is derived from single-cell sequencing. These observed limitations may impact analyses of population structure in endemic areas of high transmission and high parasite diversity.
4.4. Detection of Sexual Recombination in Cryptosporidium
Cryptosporidium has a single-host life cycle in which both asexual and sexual parasite stages are observed. Shed oocysts contain the meiotic progeny, sporozoites, and are proven to be the product of parasite sexual reproduction [6]. The presence of sexual reproduction in mixed infections of parasites increases the chances of finding recombinants that may pose a challenge for typing studies of populations as discussed above.
Recombination has already been observed in Cryptosporidium. In C. parvum, recombinant progeny were detected from experimental mixed infections in INF-γ knockout mice [70]. Nader et al. have also shown that genetic introgression is detectable in the C. parvum genome and suggest that it may play a prominent role in its adaptive evolution and host-specificity [22].
In C. hominis, the use of several markers and whole genome sequencing has also permitted the detection of recombination. In this case, linkage disequilibrium was not observed. Evidence of recombination was detected at the 3’ end of Chr 1 and in three different regions of Chr 6 [20,52]. A study in Bangladesh was also able to find a decay of linkage disequilibrium between SNPs within 63 C. hominis isolates, evidencing recombination [8]. The diversity generated by recombinants could help to explain high rates of reinfection, seasonality, and differences in transmissibility, but proof awaits further study.
The combination of mixed populations, recombination, an inability to clone and a lack of markers shows how complex and challenging it is to examine the population structure of Cryptosporidium and to identify potential hotspots of recombination if they exist.
4.5. Lack of Metadata for Global Comparative Studies
Another major challenge for population analyses is the lack of metadata regarding sequenced isolates and a lack of required minimum information standards. Many groups are working with different local or regional sources of infection and usually collect metadata, when possible, that are useful for their studies but which may be incongruent with other studies. This problem is magnified by the fact that many isolates come from public health laboratories that do not have access to the relevant clinical or epidemiological data. Larger studies are needed to analyze existing data or collect prospect data. Additional metadata are needed and mechanisms should be utilized to preserve the subject’s right to privacy for examply by utilizing dbGaP. Metadata that would greatly facilitate the interpretation of genomic and population studies include: (i) characterized species (e.g., typed by a standardized marker, such as 18S SSU rRNA); (ii) sample type (oocyst, sporozoite, fecal DNA, etc.); (iii) geographic location of collection (with both country and city); (iv) date of collection; (v) collection source (environmental, host stool, culture system, etc.); (vi) gp60 subtype; (vii) clinical severity; (viii) age; (ix) is this a repeat infection? (x) history of travel; (xi) source of water; (xii) possible zoonotic transfer and (xiii) association with a particular outbreak. Because of the metadata gaps that exist with currently available sequence data, many important observations and correlations cannot be determined, and the value of existing data for larger population studies is diminished.
5. Detecting Mixed Populations in Collected Samples
Molecular characterization methods are usually the first step in the check for mixed infections. 18S/SSU rRNA and gp60 are the most commonly used markers to identify Cryptosporidium species and genotype, respectively. Recently, a new tool called CryptoGenotyper was released. It shows 95.6% accuracy in detecting species in 18S/SSU rRNA data in mixed populations [71].
Mixed infections from subpopulations of the same species are complicated. Most molecular markers described are almost identical, sometimes differing only in single nucleotide variantion (SNVs). There are some bioinformatic approaches that can help detect the presence of mixed infections in WGS data. The ideal approach is to use variant detection software, such as GATK [72]. Cryptosporidium mixed infections tend to show multiple multi-allelic variants across all chromosomes, with clusters located in highly variable regions. As the genome sequence is haploid, alleles are not expected in clonal populations. While this approach can detect mixed infections, it cannot distinguish the different populations.
The general term for identifying subsets from a mixed group is deconvolution. This process can be facilitated by having proportional mixed data in the sample input (e.g., frequencies of multiple genotypes with some divergence). Statistical in silico methods for deconvoluting multiple genome sequences present in an individual with mixed infections have also been developed for protozoan parasites present at unknown proportions. The DEploid package was developed specifically to deal with Plasmodium mixed infections [73]. It can estimate the number of strains and their relative proportions with some limitations. While promising, it is still unknown how well this approach will work with Cryptosporidium, since, unlike Plasmodium, which is asexual in human hosts, Cryptosporidium has sexual reproduction to generate oocysts within the host. Recombination events may impact the effectiveness of this approach.
6. Emerging Solutions to Deal with This Challenging Parasite
6.1. Promising In Vitro Cultivation Systems Parasites
Despite all the challenges, some solutions are arising from the community. Many promising in vitro culture systems are emerging for maintaining some species of Cryptosporidium parasites for an extended length of time. These include the hollow fiber cell culture system [74], three-dimensional and organoid tissue culture systems [75,76], and the air-liquid interface (ALI) cultivation system [77]. These systems are still new and present some limitations, especially the number of oocysts needed to seed the cultures. Optimization that would permit infections with a single oocyst or ideally with a single viable sporozoite (via cell sorting) would permit cloning as a routine methodology. The systems do not yet scale well, low numbers of parasites are obtained, and they require specialized equipment [78].
6.2. Sorted Single-Cell Genomic Sequencing
Mixed infections of different Cryptosporidium species and mixed subtypes of the same species occur in nature in the same host. Sexual reproduction also occurs within the same host, and recombinant progeny has been detected [6,7,10]. The extent to which different subtypes or even different species can have sex is currently unknown. If the complexity of mixed infections and the resulting mixed population of parasites can be reduced by cell sorting, this will greatly facilitate variant detection. Advances in the isolation of single oocysts and whole genome sequencing of Cryptosporidium from clinical samples are emerging. Single-cell sorting of oocysts for genomic analysis is a great solution that has enabled researchers to acquire and analyze genomic data from limited material [69]. Assays using single, sorted oocysts followed by whole genome amplification already show the great potential of this approach [69]. Importantly, single oocysts still need to be considered a population since they contain four haploid meiotic progeny (sporozoites). Using single-cell genome sequencing is a reliable way to examine and describe the genetic variation in complex populations, particularly low-frequency variation [79]. Unfortunately, because of the required amplification step, some analyses such as copy number variation (CNV) cannot be performed because they are biased by the amplification step.
6.3. Cryptosporidium Capture Enrichment Sequencing
Historically, the community has obtained DNA for sequencing using one of two approaches: (1) Antibody-based parasite oocyst capture from fecal or environmental samples, or, (2) propagation of oocysts in animal models (cattle, gnotobiotic piglet, and immunosuppressed mice) [31,80,81,82,83]. These approaches have the potential to restrict the levels of parasite diversity observed and thus impact our understanding of parasite diversity and biology. Antibodies have the potential to miss oocysts that do not bind well [80], and the passage of parasites obtained from one species in another, often unnatural host, can lead to selection. A critical assessment of the impact of these approaches will be fundamental to our understanding of Cryptosporidium biology and studies of its prevalence, virulence, diversity, and transmission. Cryptosporidium represents only a minute fraction of the fecal material and an even small fraction of the total fecal DNA. This fact impacts the sequencing costs and yield of parasite-specific DNA sequence. As a consequence, little is known about global Cryptosporidium genetic diversity because the numbers of oocysts collected are often small and previous technologies required too much DNA to permit proper whole genome characterization.
Capture Enrichment Sequencing (CES) is a target enrichment approach [84] that uses fairly long biotinylated single-stranded RNA baits (or probes) that are hybridized to complementary target DNA regions and are used to physically pull down the targeted DNA regions of interest for sequencing. This technique has been used with success for other apicomplexan parasites, such as Plasmodium in patient samples [85,86]. Since some genome sequences are available for different isolates and species of Cryptosporidium, they can be used to design specific bait-sets for the target and enrich these sequences. This approach has been piloted with Cryptosporidium with great success [87], and larger studies are underway [88]. The developed probe set will be made available to the community [89].
7. Conclusions
New methods are emerging to handle the numerous challenges that the Cryptosporidium community faces. Cryptosporidiosis mainly occurs in sporadic outbreaks and endemic settings, which suggests different evolutionary dynamics and population structures for each setting. Mixed infections and mixed or drifting populations as a result of recombination and replication errors combined with historical parasite culturing systems can and likely have impacted analyses and interpretations. Additional sequencing and global population structure analyses are needed to characterize extant diversity. As researchers continue to study outbreaks and additional geographic locations, markers capable of characterizing major population groups need to be developed to facilitate comparative analyses. Marker choices should be informed by the largest and most diverse set of sequences possible and should be distributed across all chromosomes. Microsatellites, by their nature, will have the greatest utility within local populations as they are unlikely to be universal enough to differentiate global diversity since they can arise easily. Having sensitive and reliable tools will be the key to better understanding Cryptosporidium biology and its transmission.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/genes12060894/s1, Table S1: GenBank accession numbers used for phylogenetic analysis in Figure 1A; Table S2: SRA accession numbers for the sequences utilized in Figure 1B.
Author Contributions
Conceptualization, R.P.B. and J.C.K.; methodology, R.P.B.; formal analysis, R.P.B. and G.W.C.; data curation, G.W.C.; writing—original draft preparation, R.P.B.; writing—review and editing, R.P.B. and J.C.K.; funding acquisition, J.C.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Health, grant number 1R01AI148667-01A1.
Data Availability Statement
All data usedin this review was obtained from GenBank and all accession numbers are available in the supplemental Tables S1 and S2.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., Wu Y., Sow S.O., Sur D., Breiman R.F., et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 2.Khalil I.A., Troeger C., Rao P.C., Blacker B.F., Brown A., Brewer T.G., Colombara D.V., De Hostos E.L., Engmann C., Guerrant R.L., et al. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: A meta-analyses study. Lancet Glob. Health. 2018;6:e758–e768. doi: 10.1016/S2214-109X(18)30283-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feng Y., Ryan U.M., Xiao L. Genetic Diversity and Population Structure of Cryptosporidium. Trends Parasitol. 2018;34:997–1011. doi: 10.1016/j.pt.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 4.Xiao L., Feng Y. Molecular epidemiologic tools for waterborne pathogens Cryptosporidium spp. and Giardia duodenalis. Food Waterborne Parasitol. 2017;8:14–32. doi: 10.1016/j.fawpar.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Current W.L., Reese N.C. A comparison of endogenous development of three isolates of Cryptosporidium in suckling mice. J. Protozool. 1986;33:98–108. doi: 10.1111/j.1550-7408.1986.tb05567.x. [DOI] [PubMed] [Google Scholar]
- 6.Tandel J., English E.D., Sateriale A., Gullicksrud J.A., Beiting D.P., Sullivan M.C., Pinkston B., Striepen B. Life cycle progression and sexual development of the apicomplexan parasite Cryptosporidium parvum. Nat. Microbiol. 2019;4:2226–2236. doi: 10.1038/s41564-019-0539-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cama V., Gilman R.H., Vivar A., Ticona E., Ortega Y., Bern C., Xiao L. Mixed Cryptosporidium infections and HIV. Emerg. Infect. Dis. 2006;12:1025–1028. doi: 10.3201/eid1206.060015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilchrist C.A., Cotton J.A., Burkey C., Arju T., Gilmartin A., Lin Y., Ahmed E., Steiner K., Alam M., Ahmed S., et al. Genetic diversity of Cryptosporidium hominis in a Bangladeshi community as revealed by whole genome sequencing. J. Infect. Dis. 2018;218:259–264. doi: 10.1093/infdis/jiy121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korpe P.S., Gilchrist C., Burkey C., Taniuchi M., Ahmed E., Madan V., Castillo R., Ahmed S., Arju T., Alam M., et al. Case-Control Study of Cryptosporidium Transmission in Bangladeshi Households. Clin. Infect. Dis. 2019;68:1073–1079. doi: 10.1093/cid/ciy593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sannella A.R., Suputtamongkol Y., Wongsawat E., Caccio S.M. A retrospective molecular study of Cryptosporidium species and genotypes in HIV-infected patients from Thailand. Parasites Vectors. 2019;12:91. doi: 10.1186/s13071-019-3348-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z., Hu S., Zhao W., Guo Y., Li N., Zheng Z., Zhang L., Kvac M., Xiao L., Feng Y. Population structure and geographical segregation of Cryptosporidium parvum IId subtypes in cattle in China. Parasites Vectors. 2020;13:425. doi: 10.1186/s13071-020-04303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramo A., Quilez J., Monteagudo L., Del Cacho E., Sanchez-Acedo C. Intra-Species Diversity and Panmictic Structure of Cryptosporidium parvum Populations in Cattle Farms in Northern Spain. PLoS ONE. 2016;11:e0148811. doi: 10.1371/journal.pone.0148811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramo A., Monteagudo L.V., Del Cacho E., Sanchez-Acedo C., Quilez J. Intra-Species Genetic Diversity and Clonal Structure of Cryptosporidium parvum in Sheep Farms in a Confined Geographical Area in Northeastern Spain. PLoS ONE. 2016;11:e0155336. doi: 10.1371/journal.pone.0155336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrison L.J., Mallon M.E., Smith H.V., MacLeod A., Xiao L., Tait A. The population structure of the Cryptosporidium parvum population in Scotland: A complex picture. Infect. Genet. Evol. 2008;8:121–129. doi: 10.1016/j.meegid.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widmer G., Carmena D., Kvac M., Chalmers R.M., Kissinger J.C., Xiao L., Sateriale A., Striepen B., Laurent F., Lacroix-Lamande S., et al. Update on Cryptosporidium spp.: Highlights from the Seventh International Giardia and Cryptosporidium Conference. Parasite. 2020;27:14. doi: 10.1051/parasite/2020011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strong W.B., Gut J., Nelson R.G. Cloning and sequence analysis of a highly polymorphic Cryptosporidium parvum gene encoding a 60-kilodalton glycoprotein and characterization of its 15- and 45-kilodalton zoite surface antigen products. Infect. Immun. 2000;68:4117–4134. doi: 10.1128/IAI.68.7.4117-4134.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spano F., Putignani L., McLauchlin J., Casemore D.P., Crisanti A. PCR-RFLP analysis of the Cryptosporidium oocyst wall protein (COWP) gene discriminates between C. wrairi and C. parvum, and between C. parvum isolates of human and animal origin. FEMS Microbiol. Lett. 1997;150:209–217. doi: 10.1016/S0378-1097(97)00115-8. [DOI] [PubMed] [Google Scholar]
- 18.Xiao L., Morgan U.M., Limor J., Escalante A., Arrowood M., Shulaw W., Thompson R.C., Fayer R., Lal A.A. Genetic diversity within Cryptosporidium parvum and related Cryptosporidium species. Appl. Environ. Microbiol. 1999;65:3386–3391. doi: 10.1128/AEM.65.8.3386-3391.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson G., Chalmers R.M. Assessment of polymorphic genetic markers for multi-locus typing of Cryptosporidium parvum and Cryptosporidium hominis. Exp. Parasitol. 2012;132:200–215. doi: 10.1016/j.exppara.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 20.Li N., Xiao L., Cama V.A., Ortega Y., Gilman R.H., Guo M., Feng Y. Genetic recombination and Cryptosporidium hominis virulent subtype IbA10G2. Emerg. Infect. Dis. 2013;19:1573–1582. doi: 10.3201/eid1910.121361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris A., Robinson G., Swain M.T., Chalmers R.M. Direct Sequencing of Cryptosporidium in Stool Samples for Public Health. Front. Public Health. 2019;7:360. doi: 10.3389/fpubh.2019.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nader J.L., Mathers T.C., Ward B.J., Pachebat J.A., Swain M.T., Robinson G., Chalmers R.M., Hunter P.R., Oosterhout C., Tyler K.M. Evolutionary genomics of anthroponosis in Cryptosporidium. Nat. Microbiol. 2019;4:826–836. doi: 10.1038/s41564-019-0377-x. [DOI] [PubMed] [Google Scholar]
- 23.Tichkule S., Jex A.R., van Oosterhout C., Sannella A.R., Krumkamp R., Aldrich C., Maiga-Ascofare O., Dekker D., Lamshoft M., Mbwana J., et al. Comparative genomics revealed adaptive admixture in Cryptosporidium hominis in Africa. Microb. Genom. 2021;7:mgen000493. doi: 10.1099/mgen.0.000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kissinger J.C., DeBarry J. Genome cartography: Charting the apicomplexan genome. Trends Parasitol. 2011;27:345–354. doi: 10.1016/j.pt.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu P., Widmer G., Wang Y., Ozaki L.S., Alves J.M., Serrano M.G., Puiu D., Manque P., Akiyoshi D., Mackey A.J., et al. The genome of Cryptosporidium hominis. Nature. 2004;431:1107–1112. doi: 10.1038/nature02977. [DOI] [PubMed] [Google Scholar]
- 26.Abrahamsen M.S., Templeton T.J., Enomoto S., Abrahante J.E., Zhu G., Lancto C.A., Deng M., Liu C., Widmer G., Tzipori S., et al. Complete genome sequence of the apicomplexan, Cryptosporidium parvum. Science. 2004;304:441–445. doi: 10.1126/science.1094786. [DOI] [PubMed] [Google Scholar]
- 27.Piper M.B., Bankier A.T., Dear P.H. A HAPPY map of Cryptosporidium parvum. Genome Res. 1998;8:1299–1307. doi: 10.1101/gr.8.12.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seeber F., Limenitakis J., Soldati-Favre D. Apicomplexan mitochondrial metabolism: A story of gains, losses and retentions. Trends Parasitol. 2008;24:468–478. doi: 10.1016/j.pt.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Zhu G., Marchewka M.J., Keithly J.S. Cryptosporidium parvum appears to lack a plastid genome. Microbiology. 2000;146:315–321. doi: 10.1099/00221287-146-2-315. [DOI] [PubMed] [Google Scholar]
- 30.Ifeonu O.O., Chibucos M.C., Orvis J., Su Q., Elwin K., Guo F., Zhang H., Xiao L., Sun M., Chalmers R.M., et al. Annotated draft genome sequences of three species of Cryptosporidium: Cryptosporidium meleagridis isolate UKMEL1, C. baileyi isolate TAMU-09Q1 and C. hominis isolates TU502_2012 and UKH1. Pathog. Dis. 2016;74:ftw080. doi: 10.1093/femspd/ftw080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sateriale A., Slapeta J., Baptista R., Engiles J.B., Gullicksrud J.A., Herbert G.T., Brooks C.F., Kugler E.M., Kissinger J.C., Hunter C.A., et al. A Genetically Tractable, Natural Mouse Model of Cryptosporidiosis Offers Insights into Host Protective Immunity. Cell Host Microbe. 2019;26:135–146.e5. doi: 10.1016/j.chom.2019.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z., Guo Y., Roellig D.M., Feng Y., Xiao L. Comparative analysis reveals conservation in genome organization among intestinal Cryptosporidium species and sequence divergence in potential secreted pathogenesis determinants among major human-infecting species. BMC Genom. 2019;20:406. doi: 10.1186/s12864-019-5788-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu S., Roellig D.M., Guo Y., Li N., Frace M.A., Tang K., Zhang L., Feng Y., Xiao L. Evolution of mitosome metabolism and invasion-related proteins in Cryptosporidium. BMC Genom. 2016;17:1006. doi: 10.1186/s12864-016-3343-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baptista R.P., Li Y., Sateriale A., Sanders M.J., Brooks K.L., Tracey A., Ansell B.R.E., Jex A.R., Cooper G.W., Smith E.D., et al. Long-read assembly and comparative evidence-based reanalysis of Cryptosporidium genome sequences reveal new biological insights. bioRxiv. 2021 doi: 10.1101/2021.01.29.428682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaza J.P., Galvan A.L., Polanco V., Huang B., Matveyev A.V., Serrano M.G., Manque P., Buck G.A., Alzate J.F. Revisiting the reference genomes of human pathogenic Cryptosporidium species: Reannotation of C. parvum Iowa and a new C. hominis reference. Sci. Rep. 2015;5:16324. doi: 10.1038/srep16324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Baptista R.P., Sateriale A., Striepen B., Kissinger J.C. Analysis of Long Non-Coding RNA in Cryptosporidium parvum Reveals Significant Stage-Specific Antisense Transcription. Front Cell Infect. Microbiol. 2020;10:608298. doi: 10.3389/fcimb.2020.608298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng M.M., Xiao L., Freeman A.R., Arrowood M.J., Escalante A.A., Weltman A.C., Ong C.S., Mac Kenzie W.R., Lal A.A., Beard C.B. Genetic polymorphism among Cryptosporidium parvum isolates: Evidence of two distinct human transmission cycles. Emerg. Infect. Dis. 1997;3:567–573. doi: 10.3201/eid0304.970423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sulaiman I.I.M., Lal A.A.A., Xiao L.L. A population genetic study of the Cryptosporidium parvum human genotype parasites. J. Eukaryot. Microbiol. 2001;48:24s–27s. doi: 10.1111/j.1550-7408.2001.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 39.Sulaiman I.M., Lal A.A., Xiao L. Molecular phylogeny and evolutionary relationships of Cryptosporidium parasites at the actin locus. J. Parasitol. 2002;88:388–394. doi: 10.1645/0022-3395(2002)088[0388:MPAERO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 40.Xiao L., Singh A., Limor J., Graczyk T.K., Gradus S., Lal A. Molecular characterization of Cryptosporidium oocysts in samples of raw surface water and wastewater. Appl. Environ. Microbiol. 2001;67:1097–1101. doi: 10.1128/AEM.67.3.1097-1101.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cacciò S., Homan W., Camilli R., Traldi G., Kortbeek T., Pozio E. A microsatellite marker reveals population heterogeneity within human and animal genotypes of Cryptosporidium parvum. Parasitology. 2000;120:237–244. doi: 10.1017/S0031182099005508. [DOI] [PubMed] [Google Scholar]
- 42.Caccio S., Spano F., Pozio E. Large sequence variation at two microsatellite loci among zoonotic (genotype C) isolates of Cryptosporidium parvum. Int. J. Parasitol. 2001;31:1082–1086. doi: 10.1016/S0020-7519(01)00233-8. [DOI] [PubMed] [Google Scholar]
- 43.Mallon M., MacLeod A., Wastling J., Smith H., Reilly B., Tait A. Population structures and the role of genetic exchange in the zoonotic pathogen Cryptosporidium parvum. J. Mol. Evol. 2003;56:407–417. doi: 10.1007/s00239-002-2412-3. [DOI] [PubMed] [Google Scholar]
- 44.Feng Y., Yang W., Ryan U., Zhang L., Kvac M., Koudela B., Modry D., Li N., Fayer R., Xiao L. Development of a multilocus sequence tool for typing Cryptosporidium muris and Cryptosporidium andersoni. J. Clin. Microbiol. 2011;49:34–41. doi: 10.1128/JCM.01329-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chalmers R.M., Robinson G., Hotchkiss E., Alexander C., May S., Gilray J., Connelly L., Hadfield S.J. Suitability of loci for multiple-locus variable-number of tandem-repeats analysis of Cryptosporidium parvum for inter-laboratory surveillance and outbreak investigations. Parasitology. 2016;144:1–11. doi: 10.1017/S0031182015001766. [DOI] [PubMed] [Google Scholar]
- 46.Perez-Cordon G., Robinson G., Nader J., Chalmers R.M. Discovery of new variable number tandem repeat loci in multiple Cryptosporidium parvum genomes for the surveillance and investigation of outbreaks of cryptosporidiosis. Exp. Parasitol. 2016;169:119–128. doi: 10.1016/j.exppara.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 47.Wang R., Zhang L., Axen C., Bjorkman C., Jian F., Amer S., Liu A., Feng Y., Li G., Lv C., et al. Cryptosporidium parvum IId family: Clonal population and dispersal from Western Asia to other geographical regions. Sci. Rep. 2014;4:4208. doi: 10.1038/srep04208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abal-Fabeiro J.L., Maside X., Bello X., Llovo J., Bartolome C. Multilocus patterns of genetic variation across Cryptosporidium species suggest balancing selection at the gp60 locus. Mol. Ecol. 2013;22:4723–4732. doi: 10.1111/mec.12425. [DOI] [PubMed] [Google Scholar]
- 49.Feng X., Rich S.M., Akiyoshi D., Tumwine J.K., Kekitiinwa A., Nabukeera N., Tzipori S., Widmer G. Extensive polymorphism in Cryptosporidium parvum identified by multilocus microsatellite analysis. Appl. Environ. Microbiol. 2000;66:3344–3349. doi: 10.1128/AEM.66.8.3344-3349.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tanriverdi S., Markovics A., Arslan M.O., Itik A., Shkap V., Widmer G. Emergence of Distinct Genotypes of Cryptosporidium parvum in Structured Host Populations. Appl. Environ. Microbiol. 2006;72:2507–2513. doi: 10.1128/AEM.72.4.2507-2513.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanriverdi S., Grinberg A., Chalmers R.M., Hunter P.R., Petrovic Z., Akiyoshi D.E., London E., Zhang L., Tzipori S., Tumwine J.K., et al. Inferences about the global population structure of Cryptosporidium parvum and Cryptosporidium hominis. Appl. Environ. Microbiol. 2008;74:7227–7234. doi: 10.1128/AEM.01576-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo Y., Tang K., Rowe L.A., Li N., Roellig D.M., Knipe K., Frace M., Yang C., Feng Y., Xiao L. Comparative genomic analysis reveals occurrence of genetic recombination in virulent Cryptosporidium hominis subtypes and telomeric gene duplications in Cryptosporidium parvum. BMC Genom. 2015;16:320. doi: 10.1186/s12864-015-1517-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng Y., Li N., Roellig D.M., Kelley A., Liu G., Amer S., Tang K., Zhang L., Xiao L. Comparative genomic analysis of the IId subtype family of Cryptosporidium parvum. Int. J. Parasitol. 2017;47:281–290. doi: 10.1016/j.ijpara.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fei J., Wu H., Su J., Jin C., Li N., Guo Y., Feng Y., Xiao L. Characterization of MEDLE-1, a protein in early development of Cryptosporidium parvum. Parasites Vectors. 2018;11:312. doi: 10.1186/s13071-018-2889-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li B., Wu H., Li N., Su J., Jia R., Jiang J., Feng Y., Xiao L. Preliminary Characterization of MEDLE-2, a Protein Potentially Involved in the Invasion of Cryptosporidium parvum. Front. Microbiol. 2017;8:1647. doi: 10.3389/fmicb.2017.01647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Su J., Jin C., Wu H., Fei J., Li N., Guo Y., Feng Y., Xiao L. Differential Expression of Three Cryptosporidium Species-Specific MEDLE Proteins. Front. Microbiol. 2019;10:1177. doi: 10.3389/fmicb.2019.01177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gatei W., Barrett D., Lindo J.F., Eldemire-Shearer D., Cama V., Xiao L. Unique Cryptosporidium population in HIV-infected persons, Jamaica. Emerg. Infect. Dis. 2008;14:841–843. doi: 10.3201/eid1405.071277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Widmer G., Tchack L., Spano F., Tzipori S. A study of Cryptosporidium parvum genotypes and population structure. Mem. Inst. Oswaldo Cruz. 1998;93:685–686. doi: 10.1590/S0074-02761998000500021. [DOI] [PubMed] [Google Scholar]
- 59.Coupe S., Sarfati C., Hamane S., Derouin F. Detection of Cryptosporidium and identification to the species level by nested PCR and restriction fragment length polymorphism. J. Clin. Microbiol. 2005;43:1017–1023. doi: 10.1128/JCM.43.3.1017-1023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leoni F., Mallon M.E., Smith H.V., Tait A., McLauchlin J. Multilocus analysis of Cryptosporidium hominis and Cryptosporidium parvum isolates from sporadic and outbreak-related human cases and C. parvum isolates from sporadic livestock cases in the United Kingdom. J. Clin. Microbiol. 2007;45:3286–3294. doi: 10.1128/JCM.02536-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hunter P.R., Hadfield S.J., Wilkinson D., Lake I.R., Harrison F.C.D., Chalmers R.M. Subtypes of Cryptosporidium parvum in humans and disease risk. Emerg. Infect. Dis. 2007;13:82–88. doi: 10.3201/eid1301.060481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Benson D.A., Cavanaugh M., Clark K., Karsch-Mizrachi I., Lipman D.J., Ostell J., Sayers E.W. GenBank. Nucleic Acids Res. 2013;41:D36–D42. doi: 10.1093/nar/gks1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanriverdi S., Arslan M.O., Akiyoshi D.E., Tzipori S., Widmer G. Identification of genotypically mixed Cryptosporidium parvum populations in humans and calves. Mol. Biochem. Parasitol. 2003;130:13–22. doi: 10.1016/S0166-6851(03)00138-5. [DOI] [PubMed] [Google Scholar]
- 64.Kaupke A., Gawor J., Rzezutka A., Gromadka R. Identification of pig-specific Cryptosporidium species in mixed infections using Illumina sequencing technology. Exp. Parasitol. 2017;182:22–25. doi: 10.1016/j.exppara.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 65.Mercado R., Pena S., Ozaki L.S., Fredes F., Godoy J. Multiple Cryptosporidium parvum subtypes detected in a unique isolate of a Chilean neonatal calf with diarrhea. Parasitol. Res. 2015;114:1985–1988. doi: 10.1007/s00436-015-4364-8. [DOI] [PubMed] [Google Scholar]
- 66.Gan M., Liu Q., Yang C., Gao Q., Luo T. Deep Whole-Genome Sequencing to Detect Mixed Infection of Mycobacterium tuberculosis. PLoS ONE. 2016;11:e0159029. doi: 10.1371/journal.pone.0159029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bones A.J., Josse L., More C., Miller C.N., Michaelis M., Tsaousis A.D. Past and future trends of Cryptosporidium in vitro research. Exp. Parasitol. 2019;196:28–37. doi: 10.1016/j.exppara.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grinberg A., Widmer G. Cryptosporidium within-host genetic diversity: Systematic bibliographical search and narrative overview. Int. J. Parasitol. 2016;46:465–471. doi: 10.1016/j.ijpara.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 69.Troell K., Hallstrom B., Divne A.M., Alsmark C., Arrighi R., Huss M., Beser J., Bertilsson S. Cryptosporidium as a testbed for single cell genome characterization of unicellular eukaryotes. BMC Genom. 2016;17:471. doi: 10.1186/s12864-016-2815-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Feng X., Rich S.M., Tzipori S., Widmer G. Experimental evidence for genetic recombination in the opportunistic pathogen Cryptosporidium parvum. Mol. Biochem. Parasitol. 2002;119:55–62. doi: 10.1016/S0166-6851(01)00393-0. [DOI] [PubMed] [Google Scholar]
- 71.Yanta C.A., Bessonov K., Robinson G., Troell K., Guy R.A. CryptoGenotyper: A new bioinformatics tool for rapid Cryptosporidium identification. Food Waterborne Parasitol. 2021;23:e00115. doi: 10.1016/j.fawpar.2021.e00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McKenna A., Hanna M., Banks E., Sivachenko A., Cibulskis K., Kernytsky A., Garimella K., Altshuler D., Gabriel S., Daly M., et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu S.J., Almagro-Garcia J., McVean G. Deconvolution of multiple infections in Plasmodium falciparum from high throughput sequencing data. Bioinformatics. 2018;34:9–15. doi: 10.1093/bioinformatics/btx530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morada M., Lee S., Gunther-Cummins L., Weiss L.M., Widmer G., Tzipori S., Yarlett N. Continuous culture of Cryptosporidium parvum using hollow fiber technology. Int. J. Parasitol. 2016;46:21–29. doi: 10.1016/j.ijpara.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 75.Heo I., Dutta D., Schaefer D.A., Iakobachvili N., Artegiani B., Sachs N., Boonekamp K.E., Bowden G., Hendrickx A.P.A., Willems R.J.L., et al. Modelling Cryptosporidium infection in human small intestinal and lung organoids. Nat. Microbiol. 2018;3:814–823. doi: 10.1038/s41564-018-0177-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.DeCicco RePass M.A., Chen Y., Lin Y., Zhou W., Kaplan D.L., Ward H.D. Novel Bioengineered Three-Dimensional Human Intestinal Model for Long-Term Infection of Cryptosporidium parvum. Infect. Immun. 2017;85:e00731-16. doi: 10.1128/IAI.00731-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wilke G., Funkhouser-Jones L.J., Wang Y., Ravindran S., Wang Q., Beatty W.L., Baldridge M.T., VanDussen K.L., Shen B., Kuhlenschmidt M.S., et al. A Stem-Cell-Derived Platform Enables Complete Cryptosporidium Development In Vitro and Genetic Tractability. Cell Host Microbe. 2019;26:123–134. doi: 10.1016/j.chom.2019.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yarlett N., Morada M., Gobin M., Van Voorhis W., Arnold S. In Vitro Culture of Cryptosporidium parvum Using Hollow Fiber Bioreactor: Applications for Simultaneous Pharmacokinetic and Pharmacodynamic Evaluation of Test Compounds. Methods Mol. Biol. 2020;2052:335–350. doi: 10.1007/978-1-4939-9748-0_19. [DOI] [PubMed] [Google Scholar]
- 79.Wang Y., Navin N.E. Advances and applications of single-cell sequencing technologies. Mol. Cell. 2015;58:598–609. doi: 10.1016/j.molcel.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anusz K.Z., Mason P.H., Riggs M.W., Perryman L.E. Detection of Cryptosporidium parvum oocysts in bovine feces by monoclonal antibody capture enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1990;28:2770–2774. doi: 10.1128/jcm.28.12.2770-2774.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee S., Beamer G., Tzipori S. The piglet acute diarrhea model for evaluating efficacy of treatment and control of cryptosporidiosis. Hum. Vaccines Immunother. 2019;15:1445–1452. doi: 10.1080/21645515.2018.1498436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Riggs M.W., Schaefer D.A. Calf Clinical Model of Cryptosporidiosis for Efficacy Evaluation of Therapeutics. Methods Mol. Biol. 2020;2052:253–282. doi: 10.1007/978-1-4939-9748-0_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rasmussen K.R., Healey M.C. Experimental Cryptosporidium parvum infections in immunosuppressed adult mice. Infect. Immun. 1992;60:1648–1652. doi: 10.1128/iai.60.4.1648-1652.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mamanova L., Coffey A.J., Scott C.E., Kozarewa I., Turner E.H., Kumar A., Howard E., Shendure J., Turner D.J. Target-enrichment strategies for next-generation sequencing. Nat. Methods. 2010;7:111–118. doi: 10.1038/nmeth.1419. [DOI] [PubMed] [Google Scholar]
- 85.Melnikov A., Galinsky K., Rogov P., Fennell T., Van Tyne D., Russ C., Daniels R., Barnes K.G., Bochicchio J., Ndiaye D., et al. Hybrid selection for sequencing pathogen genomes from clinical samples. Genome Biol. 2011;12:R73. doi: 10.1186/gb-2011-12-8-r73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hupalo D.N., Luo Z., Melnikov A., Sutton P.L., Rogov P., Escalante A., Vallejo A.F., Herrera S., Arevalo-Herrera M., Fan Q., et al. Population genomics studies identify signatures of global dispersal and drug resistance in Plasmodium vivax. Nat. Genet. 2016;48:953–958. doi: 10.1038/ng.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khan A., Ferreira E.C.A., Grigg M.E. Development of SureSelect Target Enrichment for Whole Genome Sequencing of Cryptosporidium Directly from Stool Samples. [(accessed on 6 March 2021)]; Available online: https://en.rouentourisme.com/wp-content/uploads//2019/06/Programme-définitif-modifié-200619.pdf (p. 99) and https://hal-normandie-univ.archives-ouvertes.fr/hal-02495405.
- 88.Kissinger J.C., Glenn T.C. Capturing the Genomic Variation Present in Cryptosporidium and Cryptosporidiosis (1R01AI148667-01A1) [(accessed on 6 March 2021)]; Available online: https://reporter.nih.gov/project-details/10053025.
- 89.CryptoCapture.org: A Large Community Effort to Survey the Population Genetic Structure of Human-Infecting Cryptosporidia. [(accessed on 6 March 2021)]; Available online: http://cryptocapture.org/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data usedin this review was obtained from GenBank and all accession numbers are available in the supplemental Tables S1 and S2.