Abstract
In South Africa, uncomplicated community-acquired UTIs (CA-UTIs) are treated empirically; however, the extent of antibiotic resistance among these pathogens is not well known. We conducted a descriptive cross-sectional study of women attending ANCs at four tertiary public-sector hospitals in Gauteng. Female patients aged 15–49 years, with urine cultures performed between January 2015 and December 2019, were included. A case of culture-confirmed UTI was defined as any woman with ≤2 uropathogens with a bacterial count of ≥105 colony-forming units per ml for at least one pathogen. We identified 3558 cases of culture-confirmed UTIs in women with a median age of 30 years (interquartile range; 25–35). E. coli accounted for most infections (56% (1994/3558)), followed by E. faecalis, with a prevalence of 17% (609/3558). The prevalence of K. pneumoniae was 5% (193/3558), 5% (186/3558) for S. agalactiae, and 5% (179/3558) for P. mirabilis. Ninety-five percent (1827/1927) of the E. coli and 99% of the E. faecalis (301/305) isolates were susceptible to nitrofurantoin. Common uropathogens showed high susceptibility to first-line antibiotics, gentamicin and nitrofurantoin, as recommended for use in primary healthcare settings. Overall, our study provided an indication of the level of antimicrobial resistance in the four facilities.
Keywords: community-acquired urinary tract infection, antimicrobial susceptibility, multidrug-resistant uropathogens
1. Introduction
Antimicrobial resistance (AMR) is a growing global public health concern as it threatens the effective control and treatment of bacterial infections [1]. AMR patterns are evolving at an alarming rate due to the overuse of empiric broad-spectrum antimicrobials, and urinary tract infections (UTIs) are increasingly caused by multidrug-resistant (MDR) pathogens, particularly in healthcare facilities [2,3]. Antibiotics are commonly prescribed to treat uncomplicated UTIs in primary and secondary healthcare settings, and as a result, antibiotics used to treat UTIs have also changed over the years due to increasing AMR levels [2,4,5]. UTIs account for 25% of all clinical bacterial infections, and the European Survey of Antibiotic Consumption reported that infections with MDR bacteria result in about 25,000 deaths annually among Europeans [4,6,7].
Community-acquired UTIs (CA-UTIs) are the second most common infection in primary care [8]. Compared to other women of reproductive age, pregnant women acquire UTIs more frequently due to anatomical and, to a lesser extent, immunological changes associated with pregnancy [9]. Some of the factors that contribute to the development of UTIs during pregnancy include ureteral dilation, along with decreased ureteral tone due to hormonal effects and pressure from the growing uterus [10,11]. These factors further contribute to increased urinary stasis, which may encourage the selective growth of bacteria in the urine [11]. CA-UTIs affect between 28% and 30% of pregnant women worldwide [9,12]. In Sub-Saharan Africa, including South Africa, there are few research studies on CA-UTIs among pregnant and non-pregnant women [13,14,15]. Moreover, AMR in CA-UTIs in South Africa has not been thoroughly documented. Two single-center studies conducted among hospitalized pregnant women in South Africa reported a UTI prevalence of 5% in Durban and 8.3% in Cape Town [12,16]. However, in these studies, AMR patterns were not reported.
To determine the etiologic agents and their susceptibility profiles among patients with UTIs, urine culture is the method of choice [16]. However, culture is not routinely used in primary care, and information on circulating pathogens and susceptibility profiles is often used to guide empiric therapy [17]. According to the recently updated South African Standard Treatment Guidelines and Essential Medicines List of 2020, gentamicin is recommended as the first-line treatment of uncomplicated UTIs in adults [18]. However, gentamicin is contra-indicated in pregnancy, therefore either fosfomycin or nitrofurantoin is recommended as the first-line treatment of UTIs for pregnant women [18]. Ciprofloxacin is recommended for all adults with complicated UTIs in South Africa [18].
In resource-limited settings, syndromic treatment approaches are introduced for uncomplicated system organ infectious diseases, however, continuous monitoring of etiology and antimicrobial susceptibility testing of bacterial pathogens is required to prevent the development of antimicrobial resistance. We aimed to determine the etiology and susceptibility patterns of uropathogens circulating in the community, analyzing pregnant women attending antenatal clinics (ANCs) in Gauteng with culture-confirmed UTIs. We anticipated that our study findings would provide information to guide the empiric treatment of all women with UTIs in primary healthcare.
2. Results
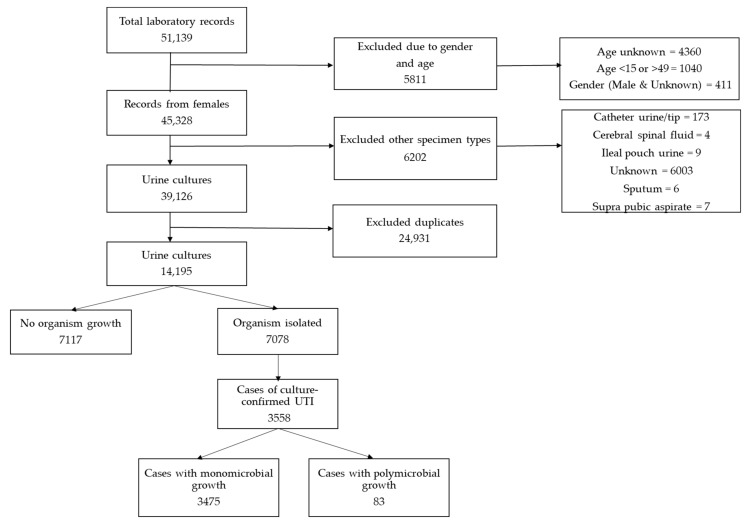
A total of 51,139 laboratory records were retrieved over the five-year period; 36,944 (63%) were excluded based on age, sex, other specimen types and duplication, and the remaining 14,195 urine cultures were submitted from 13,955 women. The positivity rate was 50% (7078/14,195) and the contamination rate was 25% (3520/14,195). Among all positive urine cultures, 50% (3558/7078) represented cases of culture-confirmed UTIs, and 2% (83/3558) of these were polymicrobial infections (two uropathogens) (Figure 1). Most cases were obtained at CHBAH (46% (1651/3558)) and CMJAH (32%, (1142/3558)). Of the remaining cases, 12% (430/3558) were from RMMCH, and 9% (335/3558) from SBAH. The median age of the patients was 30 years (interquartile range, 25–35) and the majority of the cases were aged 25–34 years (52% (1860/3558)).
Figure 1.
The number of culture-confirmed cases of urinary tract infections (UTIs) among women, with urine specimens collected while attending antenatal care at the four tertiary hospitals in Gauteng, 2015–2019.
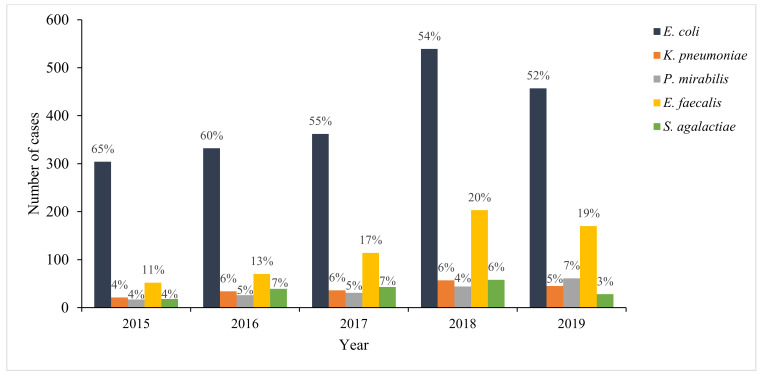
Among the cases of culture-confirmed UTIs, the most common pathogens were E. coli (56% (1994/3558)) and E. faecalis (17% (609/3558)) (Figure 1). The prevalence of S. agalactiae was 5% (186/3558), with 5% (179/3 558) for P. mirabilis and 5% (193/3558) for K. pneumoniae. Other Gram-negative uropathogens accounted for 8% (298/3558) of the infections, and these included, but were not limited to, Klebsiella spp. (3% (111/3558)), Enterobacter spp. (2% (67/3558)), and Acinetobacter spp. (0.4% (14/3558)). The prevalence of other Gram-positive uropathogens was 3% (99/3558), and the most predominant species among these were Staphylococcus spp. (2% (86/3558)) and Enterococcus spp. (0.3% [10/3558]). Candida spp. accounted for 0.2% (6/3558) of the uropathogens. E. coli and E. faecalis remained the most dominant uropathogens throughout the analysis period (Figure 2).
Figure 2.
Annual distribution of predominant uropathogens among cases of culture-confirmed urinary tract infection in antenatal clinics at four tertiary public-sector hospitals in Gauteng Province, 2015–2019. Number of cases were: n = 471 (2015); n = 554 (2016); n = 655 (2017); n = 996 (2018); n = 882 (2019).
Susceptibility testing of the predominant uropathogens is shown in Table 1. Among culture-confirmed cases, 82% (95% confidence interval (CI) 81–84 (1594/1936)) of the E. coli isolates were susceptible to amoxicillin/clavulanic acid and 88% (95% CI 86–88 (1626/1852)) were susceptible to ciprofloxacin. Ninety-five percent (95% CI: 94–96 (1827/1927)) of the E. coli isolates were susceptible to nitrofurantoin and 93% (95% CI 92–94 (1835/1970)) to gentamicin. E. coli isolates were less susceptible to ampicillin/amoxicillin (24%, 95% CI 22–26 (470/1947)) and trimethoprim/sulfamethoxazole (38%, 95% CI 36–40 (742/1942)). E. coli demonstrated reduced susceptibility to cefuroxime (86%, 95% CI 84–88 (904/1050)) and cefazolin (88%, 95% CI 86–90 (1122/1275)), while susceptibility to cefotaxime/ceftriaxone was 93% (95% CI 91–94 (1805/1948)) and 95% (95% CI 93–95 (1880/1988)) for cefepime. The majority of K. pneumoniae isolates were susceptible to ciprofloxacin (96%, 95% CI 91–98 (172/180)), gentamicin (95% CI 90–97 (178/188)) and trimethoprim/sulfamethoxazole (75%, 95% CI 68–81 (151/202)). Forty percent (95% CI 34–48 (80/198)) of the K. pneumoniae isolates were susceptible to nitrofurantoin. Among the K. pneumoniae isolates, 89% (95% CI 83–92 (165/186)) were susceptible to cefuroxime, 91% (95% CI 86–95 (177/194)) to cefotaxime/ceftriaxone, 92% (95% CI 87–95 (185/201)) to cefepime and 97% (95% CI 84–100 (31/32)) to cefazolin.
Table 1.
Antimicrobial susceptibility of predominant uropathogens among cases of culture-confirmed urinary tract infection in antenatal clinics at four tertiary public-sector hospitals in Gauteng Province, 2015–2019.
| Antibiotic | E. coli | K. pneumoniae | P. mirabilis | E. faecalis | S. agalactiae | All |
|---|---|---|---|---|---|---|
| n * (% [95% CI]) | ||||||
| Ciprofloxacin | 1626 (88% (86–89)) | 172 (96% (91–98)) | 157 (99% (96–100)) | 243 (96% (92–98)) | 16 (100% (79–100)) | 2483 (90% (89–91)) |
| Ampicillin/amoxicillin | 470 (24% (22–26)) | 0 (0% (0–1)) | 89 (51% (43–58)) | 612 (99% (99–100)) | 63 (100% (94–100)) | 1215 (38% (37–40)) |
| Trimethoprim/sulfamethoxazole | 742 (38% (36–40)) | 151 (75% (68–81)) | 85 (49% (41–56)) | 20 (37% (24–51)) | 16 (94% (71–100)) | 1234 (35% (33–36)) |
| Nitrofurantoin | 1827 (95% (94–96)) | 80 (40% (34–48)) | - | 301 (99% (97–100)) | - | 2374 (83% (82–85)) |
| Amoxicillin/clavulanic acid | 1594 (82% (81–84)) | 169 (85% (80–90)) | 165 (98% (94–99)) | - | - | 2034 (81% (79–82)) |
| Gentamicin | 1835 (93% (92–94)) | 178 (95% (90–97)) | 170 (94% (90–97)) | - | - | 2482 (93% (92–94)) |
| Cefepime | 1880 (95% (93–95)) | 185 (92% (87–95)) | 180 (99% (97–100)) | - | - | 2514 (94% (93–95)) |
| Cefotaxime/ceftriaxone | 1805 (93% (91–94)) | 177 (91% (86–95)) | 177 (99% (96–100)) | - | 84 (100% (96–100)) | 2489 (93% (92–94)) |
| Cefuroxime | 904 (86% (84–88)) | 165 (89% (83–92)) | 103 (99% (95–100)) | - | - | 1225 (85% (83–87)) |
| Cefazolin | 1122 (88% (86–90)) | 31 (97% (84–100)) | 86 (99% (94–100)) | - | - | 1367 (86% (84–88)) |
* Number of susceptible isolates, 95% CI = 95% confidence interval.
Of the 176 P. mirabilis isolates tested, 98% (95% CI 94–99) and 99% (95% CI 96–100) were susceptible to amoxicillin/clavulanic acid and ciprofloxacin, respectively, and only 51% (95% CI 43–58) were susceptible to ampicillin/amoxicillin. E. faecalis showed high susceptibility to ampicillin/amoxicillin (99%, 95% CI 99–100 (612/613)) and nitrofurantoin (99%, 95% CI 97–100 (301/305)). Thirty-seven percent (95% CI 24–51 (20/54)) of the E. faecalis isolates were susceptible to trimethoprim/sulfamethoxazole.
Overall, 4% (155/3558) of the cases were infections with MDR uropathogens (resistant to ciprofloxacin, ampicillin and trimethoprim/sulfamethoxazole), 87% of which (n = 134) were E. coli.
3. Discussion
In our study, 88% of culture-confirmed UTI infections among pregnant women in antenatal care were due to five uropathogens, of which E. coli and E. faecalis were the most common. With the exception of trimethoprim/sulfamethoxazole, susceptibility to antimicrobials was generally high, with some variation by pathogen. Although susceptibility to nitrofurantoin was low for K. pneumoniae isolates, it was high for E. coli and E. faecalis. Ciprofloxacin and gentamicin susceptibility was high overall, and the prevalence of MDR uropathogens was low.
As is consistent with a number of other studies in patients with CA-UTIs, E. coli was the most prevalent uropathogen among cases in our study [14,19]. The 56% prevalence of E. coli in our study was lower than the 80% previously reported in a similar study performed in Gauteng, but was similar to a study done in KwaZulu Natal (54%) [14,20]. The possible reasons for the differences with the study in Gauteng could be due to the study design and the population under survey. The previous study enrolled 200 symptomatic non-pregnant women, who may differ from pregnant women with respect to the causative pathogens. E. faecalis was the second most prevalent uropathogen in our study, with a prevalence of 17%. This was in contrast to previous community-based South African studies, one including all women, and one including only pregnant women. In these studies, E. faecalis accounted for 4% of the UTIs, despite being the most common Gram-positive uropathogen [20,21,22]. Pregnancy usually results in physical changes to the genital tract, and these changes may increase the risk of colonization and infection with Gram-positive and other uncommon pathogens [9,20]. For example, in other studies of pregnant women, Candida spp. were the predominant pathogens [20,23].
Overall, there was high susceptibility to those antibiotics recommended for first-line treatment in primary care settings (i.e., nitrofurantoin and gentamicin), although this differed by the common pathogen. In a laboratory-based study between 2015 and 2017 at CMJAH, susceptibility to ciprofloxacin and nitrofurantoin was 71% and 70%, respectively [22]. This lower susceptibility compared to our study was most likely due to the relatively higher resistance rates among pathogens isolated from hospitalized patients [24]. E. coli was susceptible to nitrofurantoin (96%) and, to a lesser extent, to ciprofloxacin (88%). Although the proportion of E. coli isolates that were susceptible to ciprofloxacin was high, resistance among E. coli isolates was above the 10% threshold set by the Infectious Diseases Society of America (IDSA) for treatment modification [25]. Above the 10% resistance threshold, the IDSA recommends that alternative antimicrobials be used for the empirical treatment of CA-UTIs [25].
Although the overall susceptibility to nitrofurantoin was high in our study, this was an exception with K. pneumoniae (40%) and P. mirabilis isolates. Regarding P. mirabilis isolates, we need to emphasize that Proteus spp. are intrinsically resistant to nitrofurantoin, and it should not be considered as a treatment option [26]. We examined the susceptibility to third- and fourth-generation cephalosporins (i.e., cefotaxime and cefepime) which are used as an indication for extended-spectrum beta-lactamase (ESBL) production, and are therefore resistant to this class of antibiotics. The overall prevalence of ESBL-producing strains in E. coli was between 6–7% and 8–9% in K. pneumoniae. This finding was consistent with studies reporting increasing ESBL production among strains isolated from urine [27]. The prevalence of ESBLs differs between community-acquired and healthcare-associated infections in South Africa. A higher prevalence of ESBLs has been reported in hospitalized patients (8–13%) compared to patients with community-acquired infections (0.3–5%) [24,28,29]. Another single-center study in hospitalized patients and outpatients reported an ESBL prevalence of 23% [22]. These differences highlight the importance of periodic assessment of antimicrobial susceptibility patterns in both community and healthcare settings, as resistance rates differ depending on the setting [30].
In our study, the prevalence of MDR uropathogens was low, as we described a specific population and included a first episode of UTI. Fosfomycin and nitrofurantoin are suitable for treating UTIs with MDR uropathogens, and nitrofurantoin is more suitable for pregnant women [31]. We need to emphasize that there are no breakpoints for certain Gram-positive organisms, non-enterobacterales and enterobacterales other than E coli for reporting nitrofurantoin according to the guidelines of both the CLSI and European Committee on Antimicrobial Susceptibility Testing (EUCAST), which means it should not be recommended to clinicians. We did not have data on fosfomycin, as susceptibility testing was not routinely performed for this antibiotic in the NHLS laboratories. However, resistance to nitrofurantoin was low in our study, suggesting that it remains a suitable first-line treatment for CA-UTIs and for the treatment of MDR infections. Compared to MDR pathogens isolated from patients in the healthcare setting, community-acquired pathogens have no antibiotic resistance phenotype, and they are more stable in the absence of antibiotic pressure normally found in healthcare settings [22,32]. Therefore, the emergence of MDR pathogens in the community should be avoided through responsible use of antibiotics, with more directed treatment.
The findings of our study should be interpreted in the context of the study limitations. First, our study included only pregnant women attending antenatal care, thus findings may not be generalizable to other population groups. In addition, we limited our analysis to Gauteng, and our results may not represent resistance patterns in other parts of South Africa. However, the strength of our study was that it included women attending antenatal clinics at large public-sector facilities that offer services to individuals from all parts of the province, and thus was likely to be representative of antimicrobial susceptibility patterns of the pathogens circulating in communities in Gauteng Province.
4. Materials and Methods
4.1. Study Design
This was a descriptive cross-sectional study including women attending antenatal clinics (ANC) with culture-confirmed UTIs, in four tertiary-level facilities in Gauteng; Charlotte Maxeke Johannesburg Academic Hospital (CMJAH), Rahima Moosa Mother and Child Hospital (RMMCH), Steve Biko Academic Hospital (SBAH) and Chris Hani Baragwanath Academic Hospital (CHBAH). We conducted secondary data analysis using laboratory data from the National Health Laboratory Service Corporate Data Warehouse (NHLS CDW), which stores all laboratory results of urine cultures performed in public-sector facilities. We obtained urine culture results from 2015 to 2019 and used laboratory-defined ANC test codes to identify urine specimens from patients seen at antenatal clinics at the four facilities. Limited patient information, including dates of birth and sex, and laboratory results, including bacterial colony-forming unit counts, organisms cultured, and antimicrobial susceptibility testing (AST) results were retrieved. Standard operative procedures for urine cultures and AST (automated breakpoints—Vitek 2®bioMerieux, disk diffusion methods or others) were used at the NHLS laboratories, and interpretation AST results were performed according to the Clinical and Laboratory Standards Institute (CLSI) guidelines throughout the study period [33].
4.2. Definitions
A case of culture-confirmed UTI was defined as any woman of childbearing age (15–49 years) with a positive urine culture consisting of ≤2 uropathogens with an organism growth of ≥100,000 colony-forming units per ml for at least one of the isolated organisms. We used the Centers for Disease Control and Prevention National Healthcare Safety Network (CDC NHSN) guidelines to distinguish uropathogens from contaminants among positive cultures [34].
MDR uropathogens were defined as organisms resistant to three or more classes of antimicrobials [35], including beta-lactam antibiotics (penicillin derivatives, carbapenems and cephalosporins), fluoroquinolones, aminoglycosides, folate pathway inhibitors (i.e., trimethoprim-sulfamethoxazole) and nitrofurantoin.
4.3. Exclusion Criteria
Additional urine cultures from the same patient that met the case definition and were collected within six months of the first urine culture were regarded as part of the initial UTI episode and were excluded. Male patients, women patients aged <15 years and >49 years, and those with non-urine specimen types were excluded. Patients with missing information on study inclusion criteria, such as date of birth or age, sex, specimen type and collection date, and organism name, were also excluded.
4.4. Data Analysis
The positivity and contamination rates were calculated. We determined the urine positivity rate by dividing the number of urine cultures where any organism was isolated by the total number of urine cultures performed. We determined the contamination rate by dividing the number of urine cultures that yielded a non-uropathogen by the total number of urine cultures. For each antimicrobial, susceptibility was determined by dividing the number of antimicrobial susceptible uropathogens by the total number of uropathogens with AST results.
5. Conclusions
This study provides updated profiles of infectious causes of UTIs and antimicrobial susceptibility, and may be used to guide empirical UTI treatment for female outpatients in Gauteng. Additional studies including other population groups, such as non-pregnant women, patients seeking care in private facilities, and patients in other regions of the country, could be considered for better representation. In conclusion, there is a need for regular community-based surveillance of antimicrobial resistance patterns for empirical treatment recommendations at the primary healthcare level.
Acknowledgments
We thank the National Health Laboratory Service Corporate Data Warehouse for providing the data. We also thank Alex De Voux for critically reviewing and editing the manuscript.
Author Contributions
Conceptualization, T.Z., L.S., and O.P.; methodology, T.Z.; L.S., and O.P.; formal analysis, T.Z.; data curation, T.Z., L.S., and O.P.; writing—original draft preparation, T.Z.; writing—review and editing, T.Z., L.S., and O.P.; visualization, T.Z.; supervision, L.S. and O.P.; project administration, T.Z.; funding acquisition, O.P. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Institute for Communicable Diseases, a division of the National Health Laboratory Service.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Human Research Ethics Committee of the University of Witwatersrand, Johannesburg (protocol code M200224, approval date 3 June 2020).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data supporting the results of this study are not publicly available but can be made available on request.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Jindal A.K., Pandya K., Khan I.D. Antimicrobial resistance: A public health challenge. Med. J. Armed Forces India. 2015;71:178–181. doi: 10.1016/j.mjafi.2014.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahmed S.S., Shariq A., Alsalloom A.A., Babikir I.H., Alhomoud B.N. Uropathogens and their antimicrobial resistance patterns: Relationship with urinary tract infections. Int. J. Health Sci. 2019;13:48–55. [PMC free article] [PubMed] [Google Scholar]
- 3.Davenport M., Mach K.E., Dairiki Shortliffe L.M., Banaei N., Wang T.-H., Liao J.C. New and developing diagnostic technologies for urinary tract infections. Nat. Rev. Urol. 2017;14:296–310. doi: 10.1038/nrurol.2017.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McQuiston H.J., Rosborg D.M., Sternhagen N.A.B., Llor C., Bjerrum L. Different recommendations for empiric first-choice antibiotic treatment of uncomplicated urinary tract infections in Europe. Scand. J. Prim. Health Care. 2013;31:235–240. doi: 10.3109/02813432.2013.844410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jhang J.-F., Kuo H.-C. Recent advances in recurrent urinary tract infection from pathogenesis and biomarkers to prevention. Tzu Chi Med. J. 2017;29:131–137. doi: 10.4103/tcmj.tcmj_53_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Al-Badr A., Al-Shaikh G. Recurrent Urinary Tract Infections Management in Women. Sultan Qaboos Univ. Med. J. 2013;13:359–367. doi: 10.12816/0003256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flores-Mireles A.L., Walker J.N., Caparon M., Hultgren S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015;13:269–284. doi: 10.1038/nrmicro3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kabugo D., Kizito S., Kitizo S., Ashok D.D., Graham K.A., Nabimba R., Namunana S., Kabaka M.R., Achan B., Najjuka F.C. Factors associated with community-acquired urinary tract infections among adults attending assessment centre, Mulago Hospital Uganda. Afr. Health Sci. 2016;16:1131–1142. doi: 10.4314/ahs.v16i4.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Habak P.J., Griggs J. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2019. [(accessed on 31 May 2021)]. Urinary Tract Infection in Pregnancy. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537047. [PubMed] [Google Scholar]
- 10.Managing Urinary Tract Infections in Pregnancy. [(accessed on 31 May 2021)]; Available online: https://bpac.org.nz/bpj/2011/april/pregnant-uti.aspx.
- 11.John E., Delzell J., LeFevre M. Urinary Tract Infections during Pregnancy. AFP. 2000;61:713–720. [PubMed] [Google Scholar]
- 12.Widmer T.A., Theron G., Grove D. Prevalence and risks of asymptomatic bacteriuria among HIV-positive pregnant women. S. Afr. Med. J. Epidemiol. Infect. 2010;25:28–32. doi: 10.1080/10158782.2010.11441374. [DOI] [Google Scholar]
- 13.Ayoyi A.O., Kikuvi G., Bii C., Kariuki S. Prevalence, aetiology and antibiotic sensitivity profile of asymptomatic bacteriuria isolates from pregnant women in selected antenatal clinic from Nairobi, Kenya. Pan Afr. Med. J. 2017;26 doi: 10.11604/pamj.2017.26.41.10975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lewis D.A., Gumede L.Y.E., Van der H.L.A., De Gita G.N., De Kock E.J.E., De Lange T., Maseko V., Kekana V., Smuts F.P., Perovic O. Antimicrobial susceptibility of organisms causing community-acquired urinary tract infections in Gauteng Province. S. Afr. Med. J. 2013;103:377. doi: 10.7196/SAMJ.6722. [DOI] [PubMed] [Google Scholar]
- 15.Belete M.A., Saravanan M. A Systematic Review on Drug Resistant Urinary Tract Infection Among Pregnant Women in Developing Countries in Africa and Asia; 2005–2016. Infect. Drug Resist. 2020;13:65–77. doi: 10.2147/IDR.S250654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamgang F.d.P.S., Maise H.C., Moodley J. Pregnant women admitted with urinary tract infections to a public sector hospital in South Africa: Are there lessons to learn? S. Afr. J. Infect. Dis. 2016;31:79–83. doi: 10.4102/sajid.v31i3.82. [DOI] [Google Scholar]
- 17.Hay A.D., Birnie K., Busby J., Delaney B., Downing H., Dudley J. Determinants of Urinary Contamination. NIHR Journals Library. [(accessed on 31 May 2021)];2016 Available online: https://www.ncbi.nlm.nih.gov/books/NBK373496/
- 18.South African Standard Treatment Guidelines and Essential Medicines List: Primary Healthcare Level. [(accessed on 31 May 2021)];2020 Available online: https://www.idealhealthfacility.org.za/docs/guidelines/STG%20and%20EML%20PHC%202018.pdf.
- 19.Erdem I., Ail R.K., Ardic E., Omar E.S., Mutlu R., Topkaya A.E. Community-acquired Lower Urinary Tract Infections: Etiology, Antimicrobial Resistance, and Treatment Results in Female Patients. J. Glob. Infect. Dis. 2018;10:129–132. doi: 10.4103/jgid.jgid_86_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhola P., Mvelase N.R., Balakrishna Y., Mlisana K.P., Swe-Han K.S. Antimicrobial susceptibility patterns of uropathogens isolated from pregnant women in KwaZulu-Natal Province: 2011–2016. S. Afr. Med. J. 2020;110:872–876. doi: 10.7196/SAMJ.2020.v110i9.14468. [DOI] [PubMed] [Google Scholar]
- 21.Kline K.A., Lewis A.L. Gram-Positive Uropathogens, Polymicrobial Urinary Tract Infection, and the Emerging Microbiota of the Urinary Tract. Microbiol. Spectr. 2016;4 doi: 10.1128/microbiolspec.UTI-0012-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mothibi L.M., Bosman N.N., Nana T. Fosfomycin susceptibility of uropathogens at Charlotte Maxeke Johannesburg Academic Hospital. S. Afr. Med. J. Infect. Dis. 2020;35:8. doi: 10.4102/sajid.v35i1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosana Y., Ocviyanti D., Karuniawati A., Akhmad S.R.P. Comparison of Microbial Pattern Causing Urinary Tract Infection in Female Out- and Hospitalized Patients in Jakarta. Microbiol. Indones. 2016;10:5. doi: 10.5454/mi.10.1.5. [DOI] [Google Scholar]
- 24.Dube S., Habte T., Ismail N., Hoosen A.A. Hospital and community isolates of uropathogens at a tertiary hospital in South Africa. S. Afr. Med. J. 2009;99:584. [PubMed] [Google Scholar]
- 25.Liu C., Bayer A., Cosgrove S.E., Daum R.S., Fridkin S.K., Gorwitz R.J., Kaplan S.L., Karchmer A.W., Levine D.P., Murray B.E., et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children: Executive Summary. Clin. Infect. Dis. 2011;52:285–292. doi: 10.1093/cid/cir034. [DOI] [PubMed] [Google Scholar]
- 26.Cunha B.A., Schoch P.E., Hage J.R. Nitrofurantoin: Preferred Empiric Therapy for Community-Acquired Lower Urinary Tract Infections. Mayo Clin. Proc. 2011;86:1243–1244. doi: 10.4065/mcp.2011.0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li G., Zhao S., Wang S., Sun Y., Zhou Y., Pan X. A 7-year surveillance of the drug resistance in Klebsiella pneumoniae from a primary health care center. Ann. Clin. Microbiol. Antimicrob. 2019;18:34. doi: 10.1186/s12941-019-0335-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Storberg V. ESBL-producing Enterobacteriaceae in Africa–a non-systematic literature review of research published 2008–2012. Infect. Ecol. Epidemiol. 2014;4 doi: 10.3402/iee.v4.20342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peirano G., van Greune C.H.J., Pitout J.D.D. Characteristics of infections caused by extended-spectrum β-lactamase–producing Escherichia coli from community hospitals in South Africa. Diagn. Microbiol. Infect. Dis. 2011;69:449–453. doi: 10.1016/j.diagmicrobio.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 30.van Schoor J. Urinary tract infections in women. S. Afr. Fam. Pract. 2016;58:6–10. [Google Scholar]
- 31.Gardiner B.J., Stewardson A.J., Abbott I.J., Peleg A.Y. Nitrofurantoin and fosfomycin for resistant urinary tract infections: Old drugs for emerging problems. Aust. Prescr. 2019;42:14–19. doi: 10.18773/austprescr.2019.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Duin D., Paterson D. Multidrug Resistant Bacteria in the Community: Trends and Lessons Learned. Infect. Dis Clin. N. Am. 2016;30:377–390. doi: 10.1016/j.idc.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 27th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2017. [(accessed on 31 May 2021)]. CLSI Supplement M100S. Available online: https://file.qums.ac.ir/repository/mmrc/clsi%202017.pdf. [Google Scholar]
- 34.Centers for Disease Control and Prevention National Healthcare Safety Network (NHSN) Patient Safety Component Manual. [(accessed on 31 May 2021)];2020 Available online: https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf.
- 35.Magiorakos A.-P., Srinivasan A., Carey R.B., Carmeli Y., Falagas M.E., Giske C.G., Harbath S., Hindler J.F., Kahlmeter G., Olsson-Liljequist B., et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting the results of this study are not publicly available but can be made available on request.