Abstract
Oxidative stress, particularly reactive oxygen species (ROS), are important for innate immunity against pathogens. ROS directly attack pathogens, regulate and amplify immune signals, induce autophagy and activate inflammation. In addition, production of ROS by pathogens affects the endoplasmic reticulum (ER) and mitochondria, leading to cell death. However, it is unclear how ROS regulate host defense mechanisms. This review outlines the role of ROS during intracellular pathogen infection, mechanisms of ROS production and regulation of host defense mechanisms by ROS. Finally, the interaction between microbial pathogen-induced ROS and the ER and mitochondria is described.
Keywords: bacteria, ROS, mitochondria, ER stress, oxidative stress, pathogen, infection
1. Introduction
Phagocytized pathogens are degraded in phagocytes, which is boosted by the so-called oxidative burst (or respiratory burst) [1]. The oxidative burst is an important component of immunity and removing microbes [1,2]. After infection, rapidly occurring oxidative burst is involved in the generation of nitric oxide and reactive oxygen species (ROS) [1,3]. Nitric oxide is generated by nitric oxide synthases (NOS), which convert L-arginine into L-citrulline and nitric oxide [4]. There are three NOS isoforms [5], one of which, inducible-NOS (iNOS), is induced in various cell types by cytokines and other agents in response to infection and inflammation [4,5]. Mitochondrial NOS-derived nitric oxide produces oxidative nitrogen oxide species, resulting in oxidative damage to mitochondria and apoptosis [6]. Several cellular signaling functions of nitric oxide are executed in mitochondria [6]. Nitric oxide inhibits activation of cytochrome c oxidase by competing with O2 for binding to cytochrome c oxidoreductase [6]. Nitric oxide reacts with complex III of the mitochondrial electron transport chain (ETC) to inhibit electron transfer and induce O2•− generation [6]. Therefore, nitric oxide is involved in the regulation of host mitochondrial and cellular functions.
Oxidative stress is caused by an imbalance between the production and elimination of ROS [7]. Oxidative stress is linked to cardiovascular disease [8], cancer [9], neurodegenerative conditions [10] and respiratory diseases [11]. Activated inflammatory cell-induced ROS trigger irreparable tissue damage [12]. However, ROS are important for the treatment of various diseases. Cancer chemotherapy and radiation therapy are dependent on ROS-induced apoptosis [13]. ROS are also used as second messengers in multiple signal transduction pathways activated by proinflammatory cytokines [14]. In addition, ROS assist the immune system in eliminating pathogens [15]. ROS trigger immune defense systems such as production of proinflammatory cytokines, inflammation and autophagy [15,16]. Some pathogens modulate subcellular organelles, such as mitochondria and the endoplasmic reticulum (ER), which generate ROS [16,17]. Here, we review the relationship between pathogen infection and ROS.
2. Generation of ROS
2.1. ROS in Mitochondria
ROS are chemically reactive oxygen metabolites, produced as byproducts of aerobic metabolism in all living cells. Oxygen (O2) acts as a terminal electron acceptor in the mitochondrial ETC [18]. O2 accepts an electron, forming a superoxide anion radical (O2•−), causing ROS and oxidative stress [19]. Superoxide dismutase (SOD) catalyzes the conversion of O2•− to the hydrogen peroxide (H2O2) and H2O2 performs as ROS [20]. ROS can be interconverted by enzymatic and nonenzymatic mechanisms. H2O2 is produced by natural or SOD-catalyzed conversion of O2•− [21]. As such, mitochondrial ETC is a major source of intracellular oxidative stress [22].
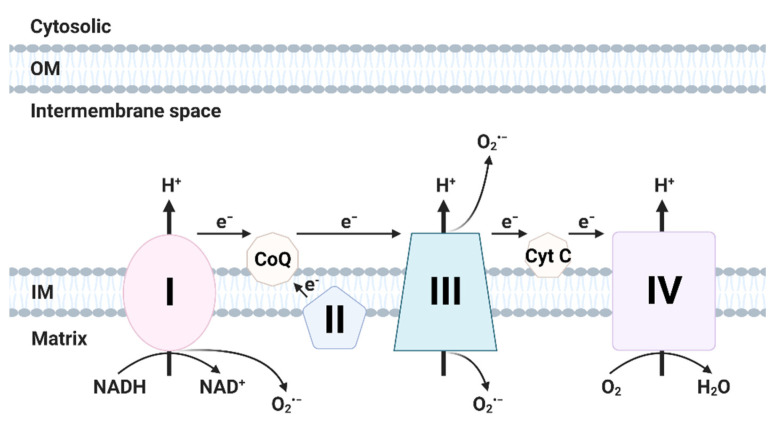
The mitochondrial respiratory chain comprises four subunits (respiratory complexes I–IV) and two factors (cytochrome c, coenzyme Q10) in the mitochondrial intermembrane space [23] and mediates oxidative phosphorylation [24]. During ATP generation, electrons released from the mitochondrial ETC incompletely reduce O2 to superoxide [25]. Mn-SOD in the mitochondrial matrix or SOD in the intermembrane space convert superoxide into H2O2 [26]. In oxidative phosphorylation, 1–2% of O2 is incompletely reduced to superoxide [27], mainly in complexes I and III of the mitochondrial respiratory chain [26,28,29].
Complex I (NADH-ubiquinone oxidoreductase) uses coenzyme Q (fully oxidized form; ubiquinone) to transfer electrons from NADH to complex III (ubiquinol-cytochrome c oxidoreductase) [30]. The superoxide produced by complex I is secreted to the mitochondrial matrix [30]. Complex I generates superoxide by NADH-linked forward electron transport and succinate-linked reverse electron transport [27,31]. Mitochondrial superoxide production is triggered by an increase in the NADH/NAD+ ratio in the matrix [32]. Reverse electron transport from succinate to NAD+ also induces production of superoxide (Figure 1) [32].
Figure 1.
Generation of ROS in electron transport chain (ETC). The ETC is located in the mitochondrial inner membrane (IM). Complex I and II supply electrons to coenzyme Q (CoQ; ubiquinone). Sequentially, electrons are transferred from CoQ to Complex III, cytochrome c (Cyt c) and Complex IV. Oxidative stress is generated during electron transfer. (Created with BioRender.com accessed on 29 March 2021).
Coenzyme Q is reduced to ubiquinol (reduced form of coenzyme Q) by receiving two electrons from mitochondrial complex I or II [30]. Complex III binds to ubiquinol in the intermembrane space and transfers electrons from ubiquinol to cytochrome c [30,33]. Cytochrome c accepts only one electron [30]. Rieske iron-sulfur protein (RISP) shifts an electron to cytochrome c1, forming ubisemiquinone (partially reduced form of coenzyme Q) [30]. Next, this electron is transferred to complex IV [30]. A second electron is transferred to cytochrome bH from cytochrome bL in complex III [30]. Superoxide is produced by electron of ubisemiquinone in complex III and released into the intermembrane space [30].
2.2. ROS in NADPH Oxidase
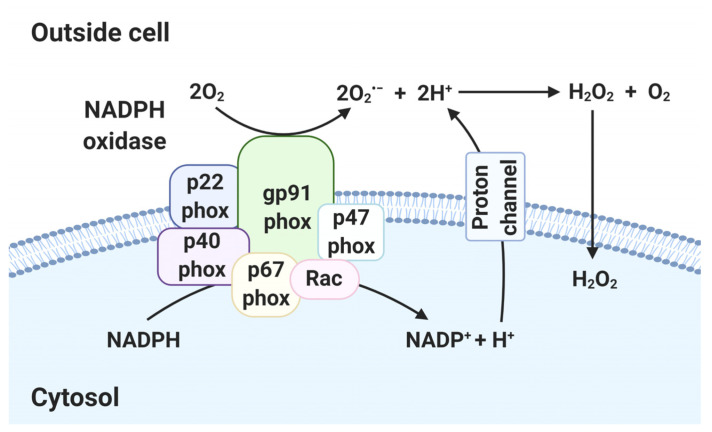
In immune cells, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) enables production of higher levels of ROS than mitochondria. NOX is a membrane-bound enzyme complex that produces ROS during pathogen invasion, as directed by the innate immune system [34]. NOX is a complex of gp91phox, p22phox, p40phox, p67phox, p47phox and Ras-related C3 botulinum toxin substrate (Rac) 1 or 2 [35]. gp91phox binds to p22phox to construct a transmembrane channel [36]. The regulatory proteins (p40phox, p67phox and p47phox) form a complex in the cytosol under normal conditions [37]. In stimulation conditions, p47phox is phosphorylated, causing localization of the regulatory protein complex at the gp91phox/p22phox complex [37]. Activated NOX transfers electrons to O2 on the opposite side of the cell membrane, yielding O2•− [36]. Although not itself damaging, several by-products of O2•−, such as H2O2, HOCl−, OH−, OONO− and NO2, are toxic (Figure 2) [38].
Figure 2.
Generation of ROS in NADPH oxidase (NOX). In NOX, electrons of cytoplasmic NADPH are transferred to extracellular oxygen via gp91phox, forming O2•−. Other subunits (p22phox, p40phox, p47phox, p67phox and Rac) are responsible for NOX stabilization and regulation. The proton channel transfers protons extracellularly. O2•− is converted to H2O2 and diffuses through lipid membranes into the intracellular space. (Created with BioRender.com accessed on 29 March 2021).
The gp91phox is also known as NOX2 [39]. The NOX family (homologs of gp91phox) consists of NOX1, NOX2, NOX3, NOX4, NOX5, dual oxidase (DUOX) 1 and DUOX2 [39]. The type of NOX family depends on the intracellular site and cell type [39]. NOX family members are implicated in the response to bacterial and viral infections and inflammatory signaling [39].
2.3. ROS in Endoplasmic Reticulum
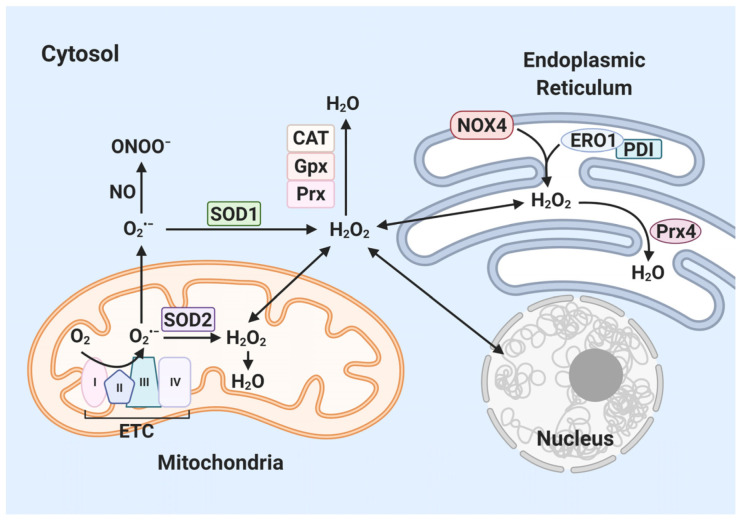
The ER also generates ROS [40] during protein folding and formation of disulfide bonds [41]. The various ER oxidoreductases—such as protein disulfide isomerase (PDI), ERp29, ERp57 and ERp72—catalyze protein folding and oxidation [42]. ER oxidoreductases oxidize cysteine residues, generating disulfide bonds [42] and are reoxidized by ER oxidoreductin 1 (ERO1) [43]. ERO1 catalyzes protein disulfide bond formation using the oxidative power of molecular oxygen in ER [44], producing H2O2 [44]. The generation of disulfide bonds by ERO1 increases ROS generation [45]. PDI is necessary for formation of disulfide bonds in the ER [46]. During protein folding, PDI is reduced by the transfer of two electrons to a cysteine residue in the active site [47]. Reduced PDI causes incomplete reduction of oxygen, leading to formation of superoxide anion, which is converted to H2O2 or other ROS [41]. PDI is involved in all reactions that form disulfide bonds [48]. NOX4 is localized in the ER [46] and is activated by physical binding to PDI, resulting in NADPH-dependent production of H2O2 [46,49]. Excess ROS are eliminated by antioxidants such as glutathione peroxidases (Gpx) and peroxiredoxin (Prx) (Figure 3) [40,50].
Figure 3.
Generation of ROS in cytosol. Superoxide (O2•−, precursor of ROS) is generated in mitochondria and endoplasmic reticulum. O2•− produced in the mitochondrial ETC is dismutated into H2O2 by superoxide dismutase (SOD) 1/2. H2O2 is converted to water by catalase (CAT), glutathione peroxidases (Gpx) and peroxiredoxins (Prx). O2− reacts with NO to produce ONOO−. Inside the ER, ER oxidoreductin 1 (ERO1)/protein disulfide isomerase (PDI) and NADPH oxidase 4 (NOX4) produce H2O2. Prx4 reduces H2O2 to water via a catalytic cysteine residue. (Created with BioRender.com accessed on 29 March 2021).
ROS are required for normal cellular functions such as signal transduction, growth and proliferation [51,52]. Prolonged accumulation of ROS induces oxidative stress, leading to defects in genes and proteins [53]. Therefore, regulation of ROS is important for cell survival.
3. Role of ROS in Innate Immunity
3.1. ROS in Innate Immunity
Macrophages, neutrophils and dendritic cells recognize and phagocytose foreign substances and then activate acquired immunity by presenting a portion of foreign substances on their surface [54]. Pattern recognition receptors (PRRs) recognize pathogen-associated molecular patterns (PAMPs), such as glycans and glycoconjugates [55]. The activation of PRRs such as Toll-like receptors (TLRs), nucleotide-binding oligomerization domain (NOD)-like receptors (NLRs) and retinoic acid-inducible gene (RIG)-I-like receptors (RLRs), increases the expression of antimicrobial genes via the nuclear factor kappa B (NF-κB), mitogen-activated protein kinase (MAPK) and phosphatidylinositol 3-kinases (PI3K) pathways [56,57].
TLRs trigger proinflammatory cytokine and chemokine production by means of adaptor molecules such as myeloid differentiation primary response 88 (MYD88) and Toll/interleukin (IL)-1 receptor (TIR)-domain-containing adapter-inducing interferon-β (TRIF) [58]. TLRs are localized on the cell surface (TLR1, TLR2, TLR4, TLR5 and TLR6) or intracellularly [58]. Intracellular TLRs (TLR3, TLR7 and TLR9) are located in the endosomal membrane [59,60]. TLR2 recognizes bacterial, viral and fungal PAMPs, including peptidoglycans, lipoteichoic acid, lipoproteins, zymosan and mannan, via TLR1 or TLR6 [59]. TLR4 recognizes the lipopolysaccharides (LPS) of Gram-negative bacteria [59]. TLR5 recognizes the globular protein flagellin, a component of the bacterial flagellum [61]. TLR3 recognizes viral double-stranded RNA and small interfering RNA [62,63]. TLR7 recognizes viral single-stranded RNA [58]. TLR9 recognizes unmethylated CpG-DNA motifs in bacterial and viral DNA [61].
TLRs are closely associated with ROS production [64]. The production of TLR2 is increased by NOX4 in endothelial cells [65], increasing the response to TLR2 ligands in endothelial cells [65]. TLR4 activation by LPS causes recruitment of MYD88, leading to sequential activation of tumor necrosis factor (TNF) receptor-associated factor (TRAF) 6 and NF-κB [66]. The TIR domain of TLR4 interacts with the COOH terminus of NOX4, resulting in NF-κB activation and ROS production [67]. TLR5 activates NOX1 via NOX organizing protein 1 (NOXO1), a homologue of p47phox and p67phox [68,69,70]. Activation of TLR5 by recombinant flagellin from Salmonella enteritidis induced ROS production, which was amplified by NOXO1 overexpression [71]. Furthermore, TLR5 interacts with dual oxidase 2 (DUOX2), a member of the NOX family [72]. The DUOX2 C-terminal region is activated by the TIR domain of TLR5 in a calcium-dependent manner [72]. The activation of TLR7/TLR8 by agonists elevates ROS production via phosphorylation of p47phox, leading to neutrophil activation [73]. TLR9 activation by CpG-containing DNA induces ROS expression [74]. Therefore, TLRs modulate phosphorylation of members of the NOX family and ROS production.
Nucleotide-binding leucine-rich repeat receptors (NLRs) are intracellular PRRs that recognize cytosolic PAMPs [39]. NLRs typically have three domains—a central nucleotide-binding domain (NACHT) domain, C-terminal leucine-rich repeat (LRR) domain and N-terminal effector domain [75]. The N-terminal effector domain is subdivided into a caspase recruitment domain (CARD), pyrin domain (PYD) and acidic transactivating domain or baculovirus inhibitor repeats (BIRs) [75].
The activation signals for NOD1 and NOD2 have been identified. Activation of NOD1/NOD2 triggers RIP2 activation by NACHT domain oligomerization and CARD-CARD interaction [76]. Activated RIP2 induces production of proinflammatory cytokines by activating NF-κB [76]. NOD1 senses meso-diaminopimelic acid (meso-DAP) from Gram-negative bacteria [77]. NOD2 recognizes muramyl dipeptide (MDP), a peptidoglycan component of both Gram-positive and -negative bacteria [77]. DUOX2 produces ROS by MDP-mediated NOD2 activation [78].
The nucleotide-binding oligomerization domain (NLRP) subfamily of NLRs induces the inflammasome, thereby activating inflammation [79]. NLRP3 is a component of the NLRP3 inflammasome complex, which also comprises apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1 [80]. First, activated NLRP3 undergoes a homotypic NACHT domain interaction by self-oligomerization [80]. Next, the PYD domain of NLRP3 interacts with the PYD domain of adaptor ASC and another ASC domain, CARD, recruits the CARD domain of procaspase-1 [80]. The resulting NLRP3 inflammasome cleaves procaspase-1 to form caspase-1, leading to release of IL-1β [80]. NLRP3 is activated by various pathogens, intracellular DNA and RNA, mitochondrial DNA, ATP, Ca2+ and ROS [80]. ROS induce the NLRP3 inflammasome via the PI3K pathway, which activates Akt and extracellular signal-regulated kinase (ERK) 1/2 [81]. NLRP3 activators also induce production of ROS via the NLRP3 inflammasome [82]. Activation of the NLRP3 inflammasome requires TLR2/MyD88/NF-κB signaling and ROS/potassium efflux [83]. The activated NLRP3 inflammasome triggers IL-1β release [83]. In contrast, reduced production of ROS inhibits NLRP3 activation [84] and antioxidant treatment decreases inflammasome activation by suppressing NF-κB signaling [85]. Therefore, NLRs recognize and eliminate intracellular pathogens by inducing generation of ROS and so are important components of the innate immune system.
ROS trigger activation of the MAPK and NF-κB pathways [86,87,88]. Activation of immune signals such as JNK, ERK and NF-κB is required for secretion of proinflammatory cytokines and chemokines [86]. An elevated ROS level increases the expression of proinflammatory cytokines such as TNF, IL-1β and IL-6 [89,90]. Pseudomonas pyocyanin-induced ROS upregulated the mRNA levels of proinflammatory cytokines [91]. Similarly, antioxidants reduced p38 MAPK activation and production of monocyte chemoattractant protein 1 (MCP1) [92]. During Helicobacter pylori infection, inhibition of NOX by Korean red ginseng extract deactivates the Janus kinase (JAK) 2/signal transducer and activator of transcription (STAT) 3 pathway, downregulating MCP1 [93]. In immune cells, chemokine (C-X-C motif) ligand (CXCL) 1 activates the MAPK pathway, NF-κB pathway and production of ROS during bacterial infections [94]. MCP1 promotes the release of lysosomal enzymes [95,96] and induces p47phox expression in macrophages and neutrophils, leading to ROS production [97]. Therefore, chemokines and cytokines are closely related to ROS production by members of the NOX family.
Enhanced PRR signaling by ROS contributes to pathogen elimination. Macrophages recognize bacterial ligands via PRRs and produce mitochondrial ROS (mtROS) [98]. Innate immune cells (macrophages and neutrophils) release ROS to degrade the pathogen [98]. ROS are important intracellular mediators of the antimicrobial response and tune the inflammatory response [98].
3.2. ROS in Bacterial Pathogenesis
ROS kill pathogens directly by causing oxidative damage or indirectly by stimulating nonoxidative mechanisms [16]. Mitochondrial-targeted catalase expression inhibited the killing of Salmonella typhimurium, demonstrating that mitochondrial ROS can be bactericidal [99]. In addition, stimulation of TLR9 by CpG-containing DNA and subsequent ROS production enhanced killing of Staphylococcus aureus in osteoblasts [100]. TLRs affect accumulation of mtROS by inducing mitochondria-derived vesicles in S. aureus-infected macrophages [101]. ER stress increases production of mitochondrial vesicles, which contain SOD [101]. S. aureus infection triggers production of mitochondrial vesicles in a Parkin-dependent manner, leading to mtROS accumulation in bacteria-containing phagosomes [101]. Depletion of SOD2 reduces mitochondrial H2O2 production and suppresses the elimination of bacteria [101].
The interaction of mtROS and TLR signaling influences inflammation [102]. mtROS are produced by increased activity of mitochondrial complex II as a result of activation of the Src-type tyrosine kinase, Fgr, which is triggered by NOX-derived ROS in macrophages infected with Escherichia coli [102,103]. This process requires TLR signaling and the NLRP3 inflammasome, which are, thus, critical for bacterial survival [102].
Production of ROS is involved in various physiologic responses, such as transcriptional activation, proliferation and apoptosis [104]. NOX is important for innate immunity [104]. Patients with chronic granulomatous disease (CGD) cannot generate ROS because of a NOX genetic defect [105], suppressing the production of ROS precursors [106]. CGD mainly affects the lungs, lymph nodes, skin and liver and enhances susceptibility to bacterial and fungal infections, leading to pneumonia, abscesses, suppurative arthritis and osteomyelitis [106]. Macrophages activated by interferon γ (IFN-γ) retained Listeria monocytogenes within phagosomes and inhibition of ROS enables its escape from phagosomes to the cytosol [107]. In addition, L. monocytogenes more easily escapes phagosomes in macrophages from NOX subunit gp91phox-deficient mice [107]. The Francisella tularensis survival ratio was increased in lungs and spleens from gp91phox−/− compared to wild-type mice [108]. F. tularensis impairs neutrophil activation and disrupts assembly of NOX in phagosome membranes, inhibiting ROS production [108].
Recognition of bacteria by macrophages suppresses the assembly of ETC complex I and ETC complex I-containing super-complexes [102]. Simultaneously, ETC complex I and ETC complex II increase mitochondrial respiration [102]. This process is mediated by phagosomal NOX and the ROS-dependent tyrosine kinase Fgr and leads to induction of ROS [102]. An increased ROS level enhances accumulation of the proinflammatory cytokine IL-1β, activating an inflammatory response and enhancing pathogen killing [102]. Furthermore, generation of ROS by proinflammatory cytokines such as IFN-γ and TNF is important for eliminating pathogens in macrophages [12,109,110]. However, production of ROS can damage host cells. Indeed, excessive TNF promotes production of ROS and induces mitochondrial Ca2+ overload in Mycobacterium tuberculosis (Mtb)-infected macrophages, causing necrosis [109]. Therefore, infected immune cells induce ROS production to inhibit bacteria and induce an inflammatory response.
3.3. ROS in Viral Pathogenesis
Virus-induced ROS trigger the intrinsic apoptosis pathway mediated by Cyt c and caspase-9 [88]. Apoptosis is important for controlling intracellular pathogens [111]. Infection by viruses, such as Japanese encephalitis virus (JEV) activates the NF-κB pathway by inducing ROS generation, leading to upregulation of antiviral genes in lymphocytes [112,113]. Survival of herpes simplex virus 1 (HSV1) was reduced by release of ROS in neutrophils [114]. Thus, ROS generation by NOX and mitochondria is critical for suppression of viruses.
Virus-mediated ROS activate the inflammasome [115]. The NLRP3 inflammasome is activated by RNA viruses, DNA viruses, bacterial RNA, ROS, ATP, mitochondrial DNA and K+ efflux [116,117]. Adenoviral DNA triggers assembly of the NLRP3 inflammasome, activation of caspase-1 and secretion of IL-1β [118]. Activated caspase-1 regulates production of the pro-pyroptotic factor gasdermin D (GSDMD) [119]. GSDMD induces pyroptosis by forming pores in the membrane of virus-infected cells [120]. Pyroptosis increases inflammation and release of IL-1β [120]. Secreted IL-1β induces neutrophil recruitment at inflammation sites to kill viruses [121]. ROS-induced NLRP3 inflammasome activation is responsible for host antiviral activity.
Human cytomegalovirus (HCMV) increases the levels of antioxidant enzymes, such as SOD, glutathione peroxidase 1(GPx1) and glutamate cysteine ligase (GCL), leading to suppression of ROS [122]. However, HCMV activates ROS production via NF-κB in host cells [123]. HCMV-induced ROS activate viral major immediate promoter (MIEP) via the immediate-early protein IE72 [123]. During viral infection, induction of ROS is required for production of IE72 and replication of HCMV [123]. The TAT protein of human immunodeficiency virus (HIV) inhibits the antioxidant enzyme MnSOD, inducing ROS production [124]. Viruses disrupt the antioxidant system, leading to excess ROS, triggering inflammation and damage, thus promoting viral replication [125].
Hepatitis C virus (HCV) is the etiologic agent of acute and chronic hepatitis, fibrosis, cirrhosis and primary hepatic malignancy [126]. The level of ROS is elevated in liver tissue of patients with chronic HCV infection [127]. HCV core protein directly interacts with mitochondria, causing ROS generation during chronic hepatitis C of the mouse liver [128]. In addition, HCV non-structural protein 3 (NS3) increases the intracellular Ca2+ level and generates ROS via phosphorylation of p47phox in human monocytes [129]. Ca2+ signaling is essential for ROS production—a calcium channel inhibitor (lanthanum chloride) reduced ROS production in HCV-infected cells [129]. During HCV infection, NOX4 triggers induction of superoxide and H2O2 in hepatocytes [130,131]. The NOX4 mRNA level was increased in HCV-infected human hepatocytes (Huh-7) [130]. HCV cDNA elevated the mRNA and protein levels of NOX4 [131]. Therefore, NOX4 is a major source of ROS production in HCV-infected host cells [130,131].
Influenza virus infections induce acute respiratory diseases, eventually triggering cell death in the respiratory system [132], generating a cytokine storm, which damages local tissue and causes systemic sequelae [132,133]. ROS induce excessive TNF-α, IFN-γ, IL-6 and inducible nitric oxide synthase (iNOS) production during influenza virus infection, causing excessive immune responses and inflammation [134]. However, ROS suppress influenza virus by killing infected cells [135]. Indeed, NOX genetic deficiency and ROS inhibitors induce viral replication during pulmonary influenza virus infection [136].
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) induces sepsis in severe cases [137]. SARS-CoV-2 sepsis induces hypoxia, which can promote mitochondrial dysfunction leading to production of superoxide, H2O2 and other ROS [138,139,140]. In addition, virus-induced ROS damage the erythrocyte membrane [141] and damaged erythrocytes are phagocytosed by macrophages and neutrophils [138], further increasing ROS production [138]. The interplay between excess ROS and cytokines induces a cytokine storm and oxidative stress production, leading to death from sepsis and shock [138]. Cytokine storm causes acute respiratory distress syndrome and multiple organ dysfunction in patients with SARS-CoV-2 [142,143,144,145,146]. Therefore, regulation of ROS could have therapeutic potential for SARS-CoV-2 infection.
In summary, several viruses use ROS for survival, whereas others are suppressed by ROS. Thus, ROS production can be beneficial or harmful during viral infection, depending on the virus in question.
4. Oxidative Stress-Mediated ER Stress
The ER is a specialized organelle that contributes to maintenance of cell homeostasis by regulating protein biosynthesis and folding, lipid metabolism and Ca2+ homeostasis [147]. Impairment of ER function by physiological or pathological factors is termed ER stress [148]. During ER stress, the protein processing ability of ER is weakened, causing accumulation of misfolded proteins [149]. Accumulation of misfolded proteins severely disrupts maintenance of ER homeostasis and initiates unfolded protein response (UPR) [149,150]. The UPR is a mechanism for removing misfolded or unfolded proteins and can activate immune signaling, inflammation and apoptosis [151]. In mammalian cells, activation of the UPR induces ER chaperone binding immunoglobulin protein (BiP), which activates inositol-requiring enzyme 1 (IRE1), activating transcription factor (ATF) 6 and protein kinase R (PKR)-like ER kinase (PERK) [151,152]. IRE1 and PERK form homodimers or oligomers and autophosphorylate [153]. ATF6 is transported to the Golgi apparatus, where it is processed and activated [154].
Activation of UPR signaling elevates production of ROS during acute or chronic ER stress [155]. The UPR pathway is activated during ER stress by oxidants, including ROS, peroxides and metal ions [156]. In macrophages, antioxidants reduce the UPR response, suggesting that ER stress induction is dependent on oxidative stress. [157]. Furthermore, accumulated misfolded proteins induce ER stress, leading to ROS production [45,158,159]. Therefore, ROS induce ER stress and ER stress increases ROS levels. Prolonged ER stress causes failure of ER homeostasis, increasing apoptosis [160]. The eukaryotic initiation factor (eIF) 2-ATF4 axis, downstream of PERK, activates transcription factor C/EBP homologous protein (CHOP), thereby inducing the synthesis of pro-apoptotic proteins [161]. In addition, CHOP targets Ero1 (a producer of ROS), leading to ROS-mediated apoptosis [162].
As well as the ER, some NOX4 is present in the mitochondria, nucleus and cytoplasm [163]. NOX4 activity requires its interaction with p22phox [39]. Activated NOX4 complex generates superoxide anion using NADH or NADPH as an electron donor [164]. In peripheral vasculature cells, the UPR response induces ROS production via NOX4 activation [165]. The ER stress induced by tunicamycin and 7-ketocholesterol leads to increased NOX4 mRNA and protein levels, resulting in increased ROS production and apoptosis [155,157]. In addition, the p22phox site of NOX4 physically binds PDI (an ER protein-folding enzyme), leading to ROS production [49]. The interaction of p22phox and PDI occurs in infected macrophages [166]. In macrophages, PDI regulates ROS production [166]. Following activation of the UPR response by ER stress, NOX4 and PDI regulate cell fate by modulating ROS production. Therefore, the pathogen-induced UPR response triggers ROS-mediated apoptosis, thus suppressing pathogens.
Oxidative stress releases calcium from the ER into the cytosol in the early stage of ER stress [167]. Cytosolic calcium is absorbed by mitochondria through voltage-dependent anion channels (VDAC) [167]. Mitochondrial calcium overload induces production of mtROS and causes mitochondrial damage, amplifying mtROS production [168]. ER stress-mediated Ca2+ release occurs via opening of inositol triphosphate receptors (IP3R) [169]. Elevated mtROS induce Ca2+ release via IP3R, boosting calcium release from the ER and mtROS production [170,171]. In addition, calcium stimulates NOS, which suppresses ETC complex IV, inducing mtROS generation [46]. During ER stress, calcium release affects mitochondria and modulates mtROS production. Therefore, regulation of ER stress and calcium release is critical for ROS generation.
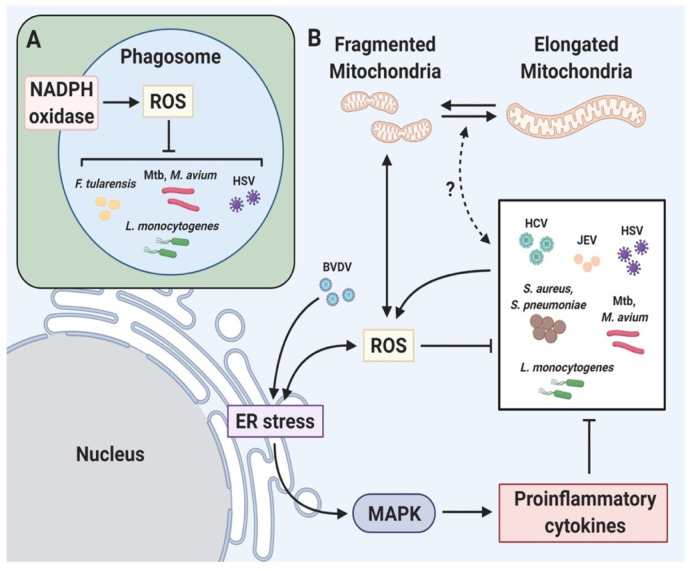
Oxidative stress is closely related to ER stress during pathogen infection and triggers production of proinflammatory cytokines, generation of ROS, induction of inflammasome, autophagy and apoptosis (Figure 4) [172,173,174]. HCV increases ROS-induced ER stress, resulting in activation of proinflammatory signals, autophagy and apoptosis [175,176,177]. ROS induce ER stress and the ASK1/ERK/p38 MAPK pathway, which trigger apoptosis during Japanese encephalitis virus infection of human promonocytes [178]. In the case of bovine viral diarrhea virus, induced ER stress activates ROS-induced apoptosis [179]. Streptococcus pneumoniae induces cytosolic ROS accumulation, triggering ER stress and elevating the production of proinflammatory cytokines [174]. ER stress-induced ROS are also required to kill methicillin-resistant S. aureus [180]. Toxins such as Brucella melitensis toxin TcpB, Brucella abortus toxin VceC, Shiga toxin and listeriolysin O are involved in ER stress and are critical for the pathogenesis of infectious diseases [181,182,183,184].
Figure 4.
Relationship between ROS and pathogens. Pathogen elimination via ROS-mediated immune signaling. (A) Pathogens are recognized by PRRs, phagocytized and digested. NOX is assembled and activated to produce ROS in the phagosomal membrane, resulting in elimination of the pathogen. (B) ER stress and mitochondrial fragmentation induce ROS production. ROS-induced ER stress boosts proinflammatory cytokine production via MAPK, resulting in microbial killing. Illustration of pathogen-mediated regulation of immune signaling via ROS production. HCV, hepatitis C virus; JEV, Japanese encephalitis virus; HSV, herpes simplex virus 1; BVDV, bovine viral diarrhea virus; S, aureus, Staphylococcus aureus; S. pneumoniae Streptococcus pneumoniae; M. tuberculosis, Mycobacterium tuberculosis; M. avium, Mycobacterium avium; L. monocytogenes, Listeria monocytogenes; F. tularensis, Francisella tularensis. (Created with BioRender.com accessed on 29 March 2021).
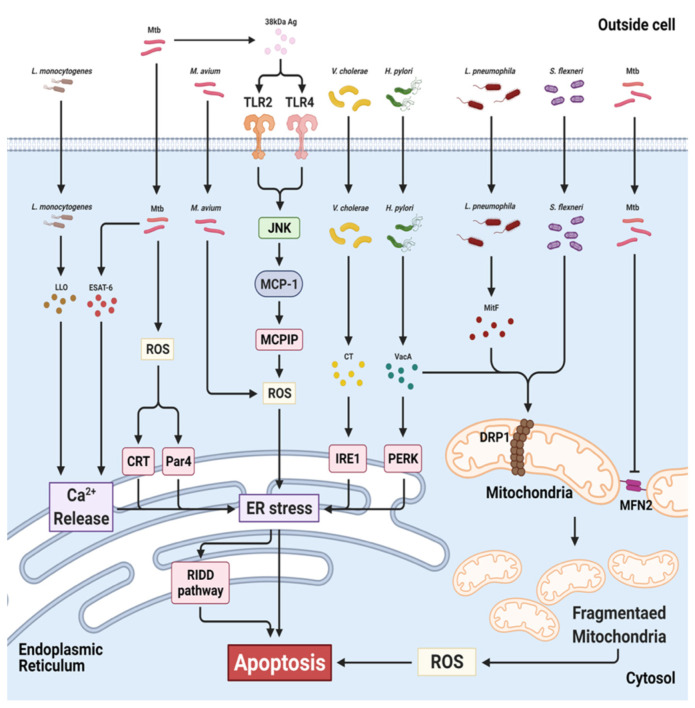
The 6 kDa early secretory antigenic target (ESAT-6) produced by Mtb induces an ER stress response, release of Ca2+ from the ER into the cytosol and accumulation of ROS, causing apoptosis [185]. Mtb 38 kDa antigen (38 kDa Ag) triggers the activation of MAPK signaling cascades (JNK, ERK and p38 MAPK) following stimulation of TRL 2/4 in macrophages [186]. Activated MAPK increases the secretion of proinflammatory cytokines (MCP-1, TNF-α and IL-6) and MCP-1 initiates production of MCP-1 inducible protein (MCPIP) in 38 kDa Ag-treated macrophages [186]. MCPIP is linked to induction of p47phox, ROS and ER stress [187]. In MCPIP-deficient macrophages, ROS generation and ER stress-mediated apoptosis are reduced even after stimulation with 38 kDa Ag, suggesting that MCPIP suppresses intracellular mycobacteria [186]. In addition, live Mtb more strongly activates production of ROS, NO and CHOP than heat-killed Mtb in macrophages [188]. Prostate apoptosis response-4 (Par-4; a tumor suppressor protein) was induced by Mtb-mediated ROS in macrophages [189]. The produced ROS increase apoptosis by inducing formation of the Par-4-BiP complex, leading to suppression of intracellular growth of Mtb [189]. Mtb-mediated ROS activate production of calreticulin (CRT; a calcium-binding chaperone protein), increasing ER stress-mediated apoptosis of macrophages [190]. Therefore, ROS-induced ER stress is critical for inhibition of Mtb (Figure 5).
Figure 5.
Targets of bacteria in ER and mitochondria during infection. Mtb induces ER stress-mediated apoptosis via ROS, calreticulin (CRT), Par-4 (Par4) and Ca2+ release. M. avium increases ROS-mediated ER stress, leading to activation of the RIDD pathway. Cholera toxin (CT) of V. cholera phosphorylates IRE1. Helicobacter pylori secretes VacA, leading to activation of PERK and DRP1. L pneumophila and S. flexneri induce mitochondrial fragmentation in a DRP1-dependent manner. Mtb also triggers mitochondrial fragmentation by inhibiting MFN2. Mtb, Mycobacterium tuberculosis; M. avium, Mycobacterium avium; L. monocytogenes, Listeria monocytogenes; H. pylori, Helicobacter pylori; V. cholera, Vibrio cholera; L pneumophila, Legionella pneumophila; S. flexneri, Shigella flexneri. (Created with BioRender.com accessed on 29 March 2021).
Mycobacterium kansasii-infected macrophages show elevated intracellular ROS production, leading to ER stress-mediated apoptosis [191]. M. fortuitum-induced ROS trigger production of TNF-α via the NF-κB/JNK pathway, leading to ER stress-mediated apoptosis of macrophages [192]. Avirulent Mycobacterium smegmatis induces high levels of ROS production, ER stress sensor molecules and secretion of proinflammatory cytokines in macrophages [193]. Phagocytosis of M. smegmatis triggers production of ROS and proinflammatory cytokines via the TLR signaling pathway in macrophages [193]. Mycobacterium avium also initiates ROS-mediated ER stress in macrophages [194]. M. avium-induced ER stress activates the regulated IRE1-dependent decay (RIDD) pathway via IRE1α RNase [194]. The RIDD pathway increases ER stress by degrading the mRNAs of ER chaperone proteins and anti-apoptotic microRNAs, initiating apoptosis [195,196]. The ROS scavenger NAC reduces IRE1α and apoptosis in macrophages infected with M. avium [194]. Therefore, not only Mtb but also various non-tuberculosis mycobacteria (NTM) induce ROS synthesis and ER stress in macrophages.
During infection with bacteria or viruses, ROS regulate ER stress induction and activate host defense mechanisms, such as the NF-κB pathway, MAPK signaling, proinflammatory cytokines and apoptosis. Therefore, ROS-mediated ER stress is critical for pathogen elimination.
5. Alteration of Mitochondrial Dynamics by Oxidative Stress
Mitochondria are dynamic organelles involved in metabolism, differentiation and cell death [197]. Mitochondria produce ATP via the electron transport chain [198]. Mitochondrial dynamics are important for ROS generation and are maintained by mitochondrial fusion and fission [197,198]. Fusion and fission of mitochondria happen constantly, regulating their size and subcellular spread and revealing their functional state [199]. Key players in the fusion process are the outer mitochondrial membrane GTPases mitofusin (MFN)1 and MFN2 and the inner membrane GTPase optic atrophy 1 (Opa1) [200,201]. MFN1 and MFN2 induce fusion of the outer mitochondrial membrane (OMM) [202]. MFNs oligomerize in mitochondria, bringing mitochondrial membranes closer and inducing OMM fusion [202]. MFN1 has a greater ability to tether mitochondria because of its higher GTPase activity than MFN2 [203]. MFN2 is required for mitochondrial-associated membrane (MAM) formation [203]. After OMM fusion, OPA1 induces fusion of the inner mitochondrial membrane (IMM) [204]. Mitochondrial fission requires mitochondrial fission 1 protein (Fis1, located in the OMM [205]) and GTPase dynamin-related protein 1 (DRP1) [206]. Fis1 recruits DRP1 from the cytosol, which coalesces into foci at mitochondrial cleavage sites [205]. The GTPase activity of DRP1 is elevated by CDK-1 dependent phosphorylation [207]. MARCH-V, an OMM transmembrane protein, triggers ubiquitination of DRP1 and reduces mitochondrial fission [208]. Fragmentation of mitochondrial networks is increased by a reduction in the levels of fusion proteins or an increase in those of fission proteins [209]. Mitochondrial fragmentation is linked to mitochondrial dysfunction, including loss of mitochondrial membrane potential (MMP), decreased oxidative phosphorylation (OXPHOS), metabolic shift towards glycolysis and increased mitochondrial ROS formation [198]. Mitochondrial fragmentation enhances ROS formation, causing a deterioration in mitochondrial health and further exacerbating oxidative stress [210]. Mitochondrial fragmentation-induced mitochondrial dysfunction can promote selective autophagy, such as mitophagy, or trigger apoptosis under severe oxidative stress conditions [211,212].
Mitochondria are also targets of pathogens [213,214]. Macrophage polarization is regulated by mtROS, the generation of which is modulated by mitochondrial dynamics [204,215]. Therefore, alteration of mitochondrial dynamics is important for suppression of pathogens. HCV-induced mitochondrial dysfunction triggers mtROS production [216]. Indeed, HCV non-structural protein 5A (NS5A) induces ROS, leading to mitochondrial fragmentation and loss of MMP. HCMV, influenza virus and measles virus disrupt the mitochondrial network [217,218,219,220]. In addition, Venezuelan equine encephalitis virus damages mitochondrial function, leading to mitochondrial fragmentation and ROS generation [221].
S. pneumoniae increases mitochondrial fragmentation-induced mtROS, leading to suppression of bacteria [222]. H. pylori alters mitochondrial dynamics via the secreted toxin, vacuolating cytotoxin A (VacA) [223]. VacA induces mitochondrial localization and activation of DRP1, resulting in mitochondrial fragmentation [223]. DRP1 in mitochondria impairs the mitochondrial ETC as a result of loss of MMP, leading to mtROS production [224]. VacA also suppresses activation of mammalian target of rapamycin complex 1 (mTORC1) signaling [225]. The inhibition of mTORC1 can induce activation of DRP1 [226]. Therefore, H. pylori infection may regulate ROS production by altering mitochondrial dynamics.
The SipB toxin of the intracellular bacterium Salmonella enterica distorts mitochondrial cristae morphology in macrophages [227]. B. abortus and B. melitensis increase mitochondrial fragmentation in macrophages [228]. L. monocytogenes modulates mitochondrial dynamics, leading to mitochondrial fragmentation [229]. Listeriolysin O, a secreted pore-forming toxin of L. monocytogenes, increases Ca2+ influx into mitochondria, resulting in reduction of MMP, mitochondrial respiratory activity and ATP production [229]. Listeriolysin O eliminates damaged mitochondria by mitophagy, leading to suppression of ROS, promoting the survival of L. monocytogenes [230]. Legionella pneumophila is the causative agent of Legionnaires’ disease and has a type 4 secretion system (T4SS) for injection of bacterial effector proteins into host cells [231]. By recruiting the mitochondrial fission protein DRP1 [231], the T4SS effector protein MitF induces mitochondrial fission, resulting in removal of mitochondria and reducing the mtROS level [231]. Another intracellular bacterium, Chlamydia trachomatis also alters mitochondrial dynamics. [232]. C. trachomatis, a cause of sexually transmitted diseases, elevates intracellular cAMP and ROS levels, leading to phosphorylation of DRP1 serine residue 637 (S637) [232]. During infection, phosphorylated DRP1 S637 plays a key role in mitochondrial fission and suppression of C. trachomatis growth [232].
Mtb affects the mitochondrial network [233,234,235]. Mtb alters macrophage mitochondrial morphology from tubular to spherical or ovoid [233]. The spherical and ovoid mitochondria are dysfunctional, exhibiting loss of MMP and reduction of cytosolic ATP and upregulation of mtROS production [233]. The attenuated Mtb strain, H37Ra, results in increased swelling of mitochondria compared to the virulent H37Rv strain in macrophages [234]. In addition, Mtb H37Rv more strongly induced the MMP than Mtb H37Ra [234]. In addition, Mtb changes the mitochondrial fusion–fission balance, leading to mitochondrial fragmentation [235]. Mtb H37Ra degrades the mitochondrial fusion protein MFN2 in macrophages, resulting in increased mitochondrial fragmentation [235]. ROS induce localization of the E3 ligase Parkin in mitochondria [236]. In Mtb-infected macrophages, Parkin promotes mitochondrial fragmentation by degrading MFN2 [235]. Therefore, reciprocal regulation of ROS is likely to be linked to mitochondrial dynamics during mycobacterial infection.
In summary, mitochondrial fragmentation and dysfunction are important for regulation of pathogens. However, the role of mtROS in pathogen elimination is unclear. Further studies are needed to determine the link between mitochondrial dynamics and pathogen elimination.
6. Conclusions
ROS generation activates antimicrobial mechanisms in host cells, suppressing microbial growth and survival. The effects of ROS may be beneficial or detrimental, depending on the quantity. ROS directly target diverse microbes, or induce autophagy and/or apoptosis, leading to pathogen elimination. By contrast, excess ROS cause oxidative damage and ultimately cell death, inducing inflammation and leading to systemic damage. However, how pathogens regulate oxidative stress in immune cells is unknown, as is the relative importance of mtROS and cytosolic ROS in pathogen elimination. Understanding the regulatory mechanisms of ROS production and how ROS modulate immune-cell function to remove pathogens will facilitate the development of novel therapeutics for intractable infectious diseases.
Author Contributions
J.L. researched the data, wrote and edited the manuscript. C.-H.S. supervised article content and was involved in editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2020R1A2C2005605) and by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1C1C1009268).
Data Availability Statement
Data available in a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moghadam Z.M., Henneke P., Kolter J. From flies to men: ROS and the NADPH oxidase in phagocytes. Front. Cell Dev. Biol. 2021;9:628991. doi: 10.3389/fcell.2021.628991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Herb M., Schramm M. Functions of ROS in macrophages and antimicrobial immunity. Antioxidants. 2021;10:313. doi: 10.3390/antiox10020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coleman J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001;1:1397–1406. doi: 10.1016/S1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 4.Mayer B., Hemmens B. Biosynthesis and action of nitric oxide in mammalian cells. Trends Biochem. Sci. 1997;22:477–481. doi: 10.1016/S0968-0004(97)01147-X. [DOI] [PubMed] [Google Scholar]
- 5.Nathan C., Xie Q.W. Nitric oxide synthases: Roles, tolls, and controls. Cell. 1994;78:915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 6.Di Meo S., Reed T.T., Venditti P., Victor V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Med. Cell. Longev. 2016;2016:1245049. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panieri E., Santoro M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016;7:e2253. doi: 10.1038/cddis.2016.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Csányi G., Miller F.J., Jr. Oxidative stress in cardiovascular disease. Int. J. Mol. Sci. 2014;15:6002–6008. doi: 10.3390/ijms15046002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayes J.D., Dinkova-Kostova A.T., Tew K.D. Oxidative stress in cancer. Cancer Cell. 2020;38:167–197. doi: 10.1016/j.ccell.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarafdar A., Pula G. The role of NADPH oxidases and oxidative stress in neurodegenerative disorders. Int. J. Mol. Sci. 2018;19:3824. doi: 10.3390/ijms19123824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomson N.C. Targeting oxidant-dependent mechanisms for the treatment of respiratory diseases and their comorbidities. Curr. Opin. Pharmacol. 2018;40:1–8. doi: 10.1016/j.coph.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 12.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones R.M., Mercante J.W., Neish A.S. Reactive oxygen production induced by the gut microbiota: Pharmacotherapeutic Implications. Curr. Med. Chem. 2012;19:1519–1529. doi: 10.2174/092986712799828283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang F.C. Antimicrobial actions of reactive oxygen species. mBio. 2011;2 doi: 10.1128/mBio.00141-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paiva C.N., Bozza M.T. Are reactive oxygen species always detrimental to pathogens? Antioxid. Redox Signal. 2014;20:1000–1037. doi: 10.1089/ars.2013.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spatial properties of reactive oxygen species govern pathogen-specific immune system responses. Antioxid. Redox Signal. 2020;32:982–992. doi: 10.1089/ars.2020.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burgos-Morón E., Abad-Jiménez Z., Martínez de Marañón A., Iannantuoni F., Escribano-López I., López-Domènech S., Salom C., Jover A., Mora V., Roldan I., et al. Relationship between oxidative stress, ER stress, and inflammation in Type 2 diabetes: The battle continues. J. Clin. Med. 2019;8:1385. doi: 10.3390/jcm8091385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bresciani G., da Cruz I.B.M., González-Gallego J. Chapter four—Manganese superoxide dismutase and oxidative stress modulation. In: Makowski G.S., editor. Advances in Clinical Chemistry. Volume 68. Elsevier; Amsterdam, The Netherlands: 2015. pp. 87–130. [DOI] [PubMed] [Google Scholar]
- 21.Fukai T., Ushio-Fukai M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid. Redox Signal. 2011;15:1583–1606. doi: 10.1089/ars.2011.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinha K., Das J., Pal P.B., Sil P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013;87:1157–1180. doi: 10.1007/s00204-013-1034-4. [DOI] [PubMed] [Google Scholar]
- 23.Galluzzi L., Morselli E., Kepp O., Vitale I., Rigoni A., Vacchelli E., Michaud M., Zischka H., Castedo M., Kroemer G. Mitochondrial gateways to cancer. Mol. Asp. Med. 2010;31:1–20. doi: 10.1016/j.mam.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Brand M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010;45:466–472. doi: 10.1016/j.exger.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao R.Z., Jiang S., Zhang L., Yu Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review) Int. J. Mol. Med. 2019;44:3–15. doi: 10.3892/ijmm.2019.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bae Y.S., Oh H., Rhee S.G., Yoo Y.D. Regulation of reactive oxygen species generation in cell signaling. Mol. Cells. 2011;32:491–509. doi: 10.1007/s10059-011-0276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kudin A.P., Bimpong-Buta N.Y., Vielhaber S., Elger C.E., Kunz W.S. Characterization of superoxide-producing sites in isolated brain mitochondria. J. Biol. Chem. 2004;279:4127–4135. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- 28.Chen Q., Vazquez E.J., Moghaddas S., Hoppel C.L., Lesnefsky E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003;278:36027–36031. doi: 10.1074/jbc.M304854200. [DOI] [PubMed] [Google Scholar]
- 29.Poyton R.O., Ball K.A., Castello P.R. Mitochondrial generation of free radicals and hypoxic signaling. Trends Endocrinol. Metab. 2009;20:332–340. doi: 10.1016/j.tem.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 30.St-Pierre J., Buckingham J.A., Roebuck S.J., Brand M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002;277:44784–44790. doi: 10.1074/jbc.M207217200. [DOI] [PubMed] [Google Scholar]
- 31.Kushnareva Y., Murphy A.N., Andreyev A. Complex I-mediated reactive oxygen species generation: Modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem. J. 2002;368:545–553. doi: 10.1042/bj20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lambert A.J., Brand M.D. Superoxide production by NADH:ubiquinone oxidoreductase (complex I) depends on the pH gradient across the mitochondrial inner membrane. Biochem. J. 2004;382:511–517. doi: 10.1042/BJ20040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwata S., Lee J.W., Okada K., Lee J.K., Iwata M., Rasmussen B., Link T.A., Ramaswamy S., Jap B.K. Complete structure of the 11-subunit bovine mitochondrial cytochrome bc1 complex. Science. 1998;281:64–71. doi: 10.1126/science.281.5373.64. [DOI] [PubMed] [Google Scholar]
- 34.Abo A., Pick E., Hall A., Totty N., Teahan C.G., Segal A.W. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 35.Lambeth J.D. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 36.Yang H.C., Cheng M.L., Ho H.Y., Chiu D.T. The microbicidal and cytoregulatory roles of NADPH oxidases. Microbes Infect. 2011;13:109–120. doi: 10.1016/j.micinf.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 37.Touyz R.M., Chen X., Tabet F., Yao G., He G., Quinn M.T., Pagano P.J., Schiffrin E.L. Expression of a functionally active gp91phox-containing neutrophil-type NAD(P)H oxidase in smooth muscle cells from human resistance arteries: Regulation by angiotensin II. Circ. Res. 2002;90:1205–1213. doi: 10.1161/01.RES.0000020404.01971.2F. [DOI] [PubMed] [Google Scholar]
- 38.Halliwell B. Phagocyte-derived reactive species: Salvation or suicide? Trends Biochem. Sci. 2006;31:509–515. doi: 10.1016/j.tibs.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Panday A., Sahoo M.K., Osorio D., Batra S. NADPH oxidases: An overview from structure to innate immunity-associated pathologies. Cell. Mol. Immunol. 2015;12:5–23. doi: 10.1038/cmi.2014.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yoboue E.D., Sitia R., Simmen T. Redox crosstalk at endoplasmic reticulum (ER) membrane contact sites (MCS) uses toxic waste to deliver messages. Cell Death Dis. 2018;9:331. doi: 10.1038/s41419-017-0033-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bhandary B., Marahatta A., Kim H.-R., Chae H.-J. An involvement of oxidative stress in endoplasmic reticulum stress and its associated diseases. Int. J. Mol. Sci. 2013;14:434–456. doi: 10.3390/ijms14010434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malhotra J.D., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxid. Redox Signal. 2007;9:2277–2293. doi: 10.1089/ars.2007.1782. [DOI] [PubMed] [Google Scholar]
- 43.Gross E., Kastner D.B., Kaiser C.A., Fass D. Structure of Ero1p, source of disulfide bonds for oxidative protein folding in the cell. Cell. 2004;117:601–610. doi: 10.1016/S0092-8674(04)00418-0. [DOI] [PubMed] [Google Scholar]
- 44.Sevier C.S., Kaiser C.A. Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2008;1783:549. doi: 10.1016/j.bbamcr.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Harding H.P., Zhang Y., Zeng H., Novoa I., Lu P.D., Calfon M., Sadri N., Yun C., Popko B., Paules R., et al. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol. Cell. 2003;11:619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- 46.Zeeshan H.M.A., Lee G.H., Kim H.-R., Chae H.-J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016;17:327. doi: 10.3390/ijms17030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kramer B., Ferrari D.M., Klappa P., Pöhlmann N., Söling H.D. Functional roles and efficiencies of the thioredoxin boxes of calcium-binding proteins 1 and 2 in protein folding. Biochem. J. 2001;357:83–95. doi: 10.1042/bj3570083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellgaard L., Ruddock L.W. The human protein disulphide isomerase family: Substrate interactions and functional properties. EMBO Rep. 2005;6:28–32. doi: 10.1038/sj.embor.7400311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Janiszewski M., Lopes L.R., Carmo A.O., Pedro M.A., Brandes R.P., Santos C.X., Laurindo F.R. Regulation of NAD(P)H oxidase by associated protein disulfide isomerase in vascular smooth muscle cells. J. Biol. Chem. 2005;280:40813–40819. doi: 10.1074/jbc.M509255200. [DOI] [PubMed] [Google Scholar]
- 50.Cox A.G., Winterbourn C.C., Hampton M.B. Mitochondrial peroxiredoxin involvement in antioxidant defence and redox signalling. Biochem. J. 2009;425:313–325. doi: 10.1042/BJ20091541. [DOI] [PubMed] [Google Scholar]
- 51.Cotugno N., Finocchi A., Cagigi A., Di Matteo G., Chiriaco M., Di Cesare S., Rossi P., Aiuti A., Palma P., Douagi I. Defective B-cell proliferation and maintenance of long-term memory in patients with chronic granulomatous disease. J. Allergy Clin. Immunol. 2015;135:753–761.e752. doi: 10.1016/j.jaci.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 52.Sarsour E.H., Venkataraman S., Kalen A.L., Oberley L.W., Goswami P.C. Manganese superoxide dismutase activity regulates transitions between quiescent and proliferative growth. Aging Cell. 2008;7:405–417. doi: 10.1111/j.1474-9726.2008.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cross C.E., Halliwell B., Borish E.T., Pryor W.A., Ames B.N., Saul R.L., McCord J.M., Harman D. Oxygen radicals and human disease. Ann. Intern. Med. 1987;107:526–545. doi: 10.7326/0003-4819-107-4-526. [DOI] [PubMed] [Google Scholar]
- 54.Liu C.H., Liu H., Ge B. Innate immunity in tuberculosis: Host defense vs. pathogen evasion. Cell. Mol. Immunol. 2017;14:963–975. doi: 10.1038/cmi.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mogensen T.H. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin. Microbiol. Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kumagai Y., Akira S. Identification and functions of pattern-recognition receptors. J. Allergy Clin. Immunol. 2010;125:985–992. doi: 10.1016/j.jaci.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 57.Thannickal V.J., Fanburg B.L. Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell Mol. Physiol. 2000;279:L1005–L1028. doi: 10.1152/ajplung.2000.279.6.L1005. [DOI] [PubMed] [Google Scholar]
- 58.Kawasaki T., Kawai T. Toll-like receptor signaling pathways. Front. Immunol. 2014;5 doi: 10.3389/fimmu.2014.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 60.Celhar T., Magalhães R., Fairhurst A.M. TLR7 and TLR9 in SLE: When sensing self goes wrong. Immunol. Res. 2012;53:58–77. doi: 10.1007/s12026-012-8270-1. [DOI] [PubMed] [Google Scholar]
- 61.Akira S., Uematsu S., Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 62.Zhang S.-Y., Jouanguy E., Ugolini S., Smahi A., Elain G., Romero P., Segal D., Sancho-Shimizu V., Lorenzo L., Puel A., et al. TLR3 deficiency in patients with herpes simplex encephalitis. Science. 2007;317:1522–1527. doi: 10.1126/science.1139522. [DOI] [PubMed] [Google Scholar]
- 63.Bernard J.J., Cowing-Zitron C., Nakatsuji T., Muehleisen B., Muto J., Borkowski A.W., Martinez L., Greidinger E.L., Yu B.D., Gallo R.L. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat. Med. 2012;18:1286–1290. doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laroux F.S., Romero X., Wetzler L., Engel P., Terhorst C. Cutting edge: MyD88 controls phagocyte NADPH oxidase function and killing of gram-negative bacteria. J. Immunol. 2005;175:5596–5600. doi: 10.4049/jimmunol.175.9.5596. [DOI] [PubMed] [Google Scholar]
- 65.Fan J., Frey R.S., Malik A.B. TLR4 signaling induces TLR2 expression in endothelial cells via neutrophil NADPH oxidase. J. Clin. Investig. 2003;112:1234–1243. doi: 10.1172/JCI18696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akira S., Takeda K., Kaisho T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 67.Park H.S., Chun J.N., Jung H.Y., Choi C., Bae Y.S. Role of NADPH oxidase 4 in lipopolysaccharide-induced proinflammatory responses by human aortic endothelial cells. Cardiovasc. Res. 2006;72:447–455. doi: 10.1016/j.cardiores.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 68.Lambeth J.D. Nox/Duox family of nicotinamide adenine dinucleotide (phosphate) oxidases. Curr. Opin. Hematol. 2002;9:11–17. doi: 10.1097/00062752-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 69.Takeya R., Sumimoto H. Molecular mechanism for activation of superoxide-producing NADPH oxidases. Mol. Cells. 2003;16:271–277. [PubMed] [Google Scholar]
- 70.Van Maele L., Carnoy C., Cayet D., Songhet P., Dumoutier L., Ferrero I., Janot L., Erard F., Bertout J., Leger H., et al. TLR5 signaling stimulates the innate production of IL-17 and IL-22 by CD3(neg)CD127+ immune cells in spleen and mucosa. J. Immunol. 2010;185:1177–1185. doi: 10.4049/jimmunol.1000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawahara T., Kuwano Y., Teshima-Kondo S., Takeya R., Sumimoto H., Kishi K., Tsunawaki S., Hirayama T., Rokutan K. Role of nicotinamide adenine dinucleotide phosphate oxidase 1 in oxidative burst response to Toll-like receptor 5 signaling in large intestinal epithelial cells. J. Immunol. 2004;172:3051–3058. doi: 10.4049/jimmunol.172.5.3051. [DOI] [PubMed] [Google Scholar]
- 72.Joo J.H., Ryu J.H., Kim C.H., Kim H.J., Suh M.S., Kim J.O., Chung S.Y., Lee S.N., Kim H.M., Bae Y.S., et al. Dual oxidase 2 is essential for the toll-like receptor 5-mediated inflammatory response in airway mucosa. Antioxid. Redox Signal. 2012;16:57–70. doi: 10.1089/ars.2011.3898. [DOI] [PubMed] [Google Scholar]
- 73.Makni-Maalej K., Boussetta T., Hurtado-Nedelec M., Belambri S.A., Gougerot-Pocidalo M.A., El-Benna J. The TLR7/8 agonist CL097 primes N-formyl-methionyl-leucyl-phenylalanine-stimulated NADPH oxidase activation in human neutrophils: Critical role of p47phox phosphorylation and the proline isomerase Pin1. J. Immunol. 2012;189:4657–4665. doi: 10.4049/jimmunol.1201007. [DOI] [PubMed] [Google Scholar]
- 74.Lee J.-G., Lee S.-H., Park D.-W., Lee S.-H., Yoon H.-S., Chin B.-R., Kim J.-H., Kim J.-R., Baek S.-H. Toll-like receptor 9-stimulated monocyte chemoattractant protein-1 is mediated via JNK-cytosolic phospholipase A2-ROS signaling. Cell. Signal. 2008;20:105–111. doi: 10.1016/j.cellsig.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 75.Meunier E., Broz P. Evolutionary convergence and divergence in NLR function and structure. Trends Immunol. 2017;38:744–757. doi: 10.1016/j.it.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 76.Park J.-H., Kim Y.-G., McDonald C., Kanneganti T.-D., Hasegawa M., Body-Malapel M., Inohara N., Núñez G. RICK/RIP2 Mediates Innate Immune Responses Induced through Nod1 and Nod2 but Not TLRs. J. Immunol. 2007;178:2380–2386. doi: 10.4049/jimmunol.178.4.2380. [DOI] [PubMed] [Google Scholar]
- 77.Okugawa T., Kaneko T., Yoshimura A., Silverman N., Hara Y. NOD1 and NOD2 mediate sensing of periodontal pathogens. J. Dent. Res. 2010;89:186–191. doi: 10.1177/0022034509354843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lipinski S., Till A., Sina C., Arlt A., Grasberger H., Schreiber S., Rosenstiel P. DUOX2-derived reactive oxygen species are effectors of NOD2-mediated antibacterial responses. J. Cell Sci. 2009;122:3522–3530. doi: 10.1242/jcs.050690. [DOI] [PubMed] [Google Scholar]
- 79.Martinon F., Tschopp J. Inflammatory caspases and inflammasomes: Master switches of inflammation. Cell Death Differ. 2007;14:10–22. doi: 10.1038/sj.cdd.4402038. [DOI] [PubMed] [Google Scholar]
- 80.Schroder K., Tschopp J. The Inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 81.Cruz C.M., Rinna A., Forman H.J., Ventura A.L., Persechini P.M., Ojcius D.M. ATP activates a reactive oxygen species-dependent oxidative stress response and secretion of proinflammatory cytokines in macrophages. J. Biol. Chem. 2007;282:2871–2879. doi: 10.1074/jbc.M608083200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bauernfeind F., Bartok E., Rieger A., Franchi L., Núñez G., Hornung V. Cutting edge: Reactive oxygen species inhibitors block priming, but not activation, of the NLRP3 inflammasome. J. Immunol. 2011;187:613–617. doi: 10.4049/jimmunol.1100613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Segovia J., Sabbah A., Mgbemena V., Tsai S.Y., Chang T.H., Berton M.T., Morris I.R., Allen I.C., Ting J.P., Bose S. TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS ONE. 2012;7:e29695. doi: 10.1371/journal.pone.0029695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Harijith A., Ebenezer D.L., Natarajan V. Reactive oxygen species at the crossroads of inflammasome and inflammation. Front. Physiol. 2014;5:352. doi: 10.3389/fphys.2014.00352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Shimada K., Crother T.R., Karlin J., Dagvadorj J., Chiba N., Chen S., Ramanujan V.K., Wolf A.J., Vergnes L., Ojcius D.M., et al. Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity. 2012;36:401–414. doi: 10.1016/j.immuni.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kohchi C., Inagawa H., Nishizawa T., Soma G.-I. ROS and innate immunity. Anticancer Res. 2009;29:817–821. [PubMed] [Google Scholar]
- 87.Reczek C.R., Chandel N.S. ROS-dependent signal transduction. Curr. Opin. Cell Biol. 2015;33:8–13. doi: 10.1016/j.ceb.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li Z., Xu X., Leng X., He M., Wang J., Cheng S., Wu H. Roles of reactive oxygen species in cell signaling pathways and immune responses to viral infections. Arch. Virol. 2017;162:603–610. doi: 10.1007/s00705-016-3130-2. [DOI] [PubMed] [Google Scholar]
- 89.Naik E., Dixit V.M. Mitochondrial reactive oxygen species drive proinflammatory cytokine production. J. Exp. Med. 2011;208:417–420. doi: 10.1084/jem.20110367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bulua A.C., Simon A., Maddipati R., Pelletier M., Park H., Kim K.Y., Sack M.N., Kastner D.L., Siegel R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS) J. Exp. Med. 2011;208:519–533. doi: 10.1084/jem.20102049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rada B., Gardina P., Myers T.G., Leto T.L. Reactive oxygen species mediate inflammatory cytokine release and EGFR-dependent mucin secretion in airway epithelial cells exposed to Pseudomonas pyocyanin. Mucosal. Immunol. 2011;4:158–171. doi: 10.1038/mi.2010.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Brandes R.P., Viedt C., Nguyen K., Beer S., Kreuzer J., Busse R., Görlach A. Thrombin-induced MCP-1 expression involves activation of the p22phox-containing NADPH oxidase in human vascular smooth muscle cells. Thromb. Haemost. 2001;85:1104–1110. [PubMed] [Google Scholar]
- 93.Cho S.O., Lim J.W., Kim H. Red ginseng extract inhibits the expression of MCP-1 and iNOS in Helicobacter pylori-infected gastric epithelial cells by suppressing the activation of NADPH oxidase and Jak2/Stat3. J. Ethnopharmacol. 2013;150:761–764. doi: 10.1016/j.jep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 94.Cai S., Batra S., Lira S.A., Kolls J.K., Jeyaseelan S. CXCL1 regulates pulmonary host defense to Klebsiella infection via CXCL2, CXCL5, NF-κB, and MAPKs. J. Immunol. 2010;185:6214–6225. doi: 10.4049/jimmunol.0903843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Vaddi K., Newton R.C. Comparison of biological responses of human monocytes and THP-1 cells to chemokines of the intercrine-β family. J. Leukoc. Biol. 1994;55:756–762. doi: 10.1002/jlb.55.6.756. [DOI] [PubMed] [Google Scholar]
- 96.Furie M.B., Randolph G.J. Chemokines and tissue injury. Am. J. Pathol. 1995;146:1287–1301. [PMC free article] [PubMed] [Google Scholar]
- 97.Tan J.H., Ludeman J.P., Wedderburn J., Canals M., Hall P., Butler S.J., Taleski D., Christopoulos A., Hickey M.J., Payne R.J., et al. Tyrosine sulfation of chemokine receptor CCR2 enhances interactions with both monomeric and dimeric forms of the chemokine monocyte chemoattractant protein-1 (MCP-1) J. Biol. Chem. 2013;288:10024–10034. doi: 10.1074/jbc.M112.447359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shekhova E. Mitochondrial reactive oxygen species as major effectors of antimicrobial immunity. PLoS Pathog. 2020;16:e1008470. doi: 10.1371/journal.ppat.1008470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.West A.P., Brodsky I.E., Rahner C., Woo D.K., Erdjument-Bromage H., Tempst P., Walsh M.C., Choi Y., Shadel G.S., Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mohamed W., Domann E., Chakraborty T., Mannala G., Lips K.S., Heiss C., Schnettler R., Alt V. TLR9 mediates S. aureus killing inside osteoblasts via induction of oxidative stress. BMC Microbiol. 2016;16:230. doi: 10.1186/s12866-016-0855-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Abuaita B.H., Schultz T.L., O’Riordan M.X. Mitochondria-derived vesicles deliver antimicrobial reactive oxygen species to control phagosome-localized Staphylococcus aureus. Cell Host Microbe. 2018;24:625–636.e625. doi: 10.1016/j.chom.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Garaude J., Acín-Pérez R., Martínez-Cano S., Enamorado M., Ugolini M., Nistal-Villán E., Hervás-Stubbs S., Pelegrín P., Sander L.E., Enríquez J.A., et al. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat. Immunol. 2016;17:1037–1045. doi: 10.1038/ni.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Acín-Pérez R., Carrascoso I., Baixauli F., Roche-Molina M., Latorre-Pellicer A., Fernández-Silva P., Mittelbrunn M., Sanchez-Madrid F., Pérez-Martos A., Lowell C.A., et al. ROS-triggered phosphorylation of complex II by Fgr kinase regulates cellular adaptation to fuel use. Cell Metab. 2014;19:1020–1033. doi: 10.1016/j.cmet.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Forman H.J., Torres M. Reactive oxygen species and cell signaling. Am. J. Respir. Crit. Care Med. 2002;166:S4–S8. doi: 10.1164/rccm.2206007. [DOI] [PubMed] [Google Scholar]
- 105.Hohn D.C., Lehrer R.I. NADPH oxidase deficiency in X-linked chronic granulomatous disease. J. Clin. Investig. 1975;55:707–713. doi: 10.1172/JCI107980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ben-Ari J., Wolach O., Gavrieli R., Wolach B. Infections associated with chronic granulomatous disease: Linking genetics to phenotypic expression. Expert Rev. Anti Infect. 2012;10:881–894. doi: 10.1586/eri.12.77. [DOI] [PubMed] [Google Scholar]
- 107.Myers J.T., Tsang A.W., Swanson J.A. Localized reactive oxygen and nitrogen intermediates inhibit escape of Listeria monocytogenes from vacuoles in activated macrophages. J. Immunol. 2003;171:5447–5453. doi: 10.4049/jimmunol.171.10.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.KuoLee R., Harris G., Conlan J.W., Chen W. Role of neutrophils and NADPH phagocyte oxidase in host defense against respiratory infection with virulent Francisella tularensis in mice. Microbes Infect. 2011;13:447–456. doi: 10.1016/j.micinf.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 109.Roca F.J., Whitworth L.J., Redmond S., Jones A.A., Ramakrishnan L. TNF induces pathogenic programmed macrophage necrosis in tuberculosis through a mitochondrial-lysosomal-endoplasmic reticulum circuit. Cell. 2019;178:1344–1361.e1311. doi: 10.1016/j.cell.2019.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sonoda J., Laganière J., Mehl I.R., Barish G.D., Chong L.W., Li X., Scheffler I.E., Mock D.C., Bataille A.R., Robert F., et al. Nuclear receptor ERR alpha and coactivator PGC-1 beta are effectors of IFN-gamma-induced host defense. Genes Dev. 2007;21:1909–1920. doi: 10.1101/gad.1553007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Barber G.N. Host defense, viruses and apoptosis. Cell Death Differ. 2001;8:113–126. doi: 10.1038/sj.cdd.4400823. [DOI] [PubMed] [Google Scholar]
- 112.Flory E., Kunz M., Scheller C., Jassoy C., Stauber R., Rapp U.R., Ludwig S. Influenza virus-induced NF-kappaB-dependent gene expression is mediated by overexpression of viral proteins and involves oxidative radicals and activation of IkappaB kinase. J. Biol. Chem. 2000;275:8307–8314. doi: 10.1074/jbc.275.12.8307. [DOI] [PubMed] [Google Scholar]
- 113.Lin R.J., Liao C.L., Lin Y.L. Replication-incompetent virions of Japanese encephalitis virus trigger neuronal cell death by oxidative stress in a culture system. J. Gen. Virol. 2004;85:521–533. doi: 10.1099/vir.0.19496-0. [DOI] [PubMed] [Google Scholar]
- 114.Hayashi K., Hooper L.C., Okuno T., Takada Y., Hooks J.J. Inhibition of HSV-1 by chemoattracted neutrophils: Supernatants of corneal epithelial cells (HCE) and macrophages (THP-1) treated with virus components chemoattract neutrophils (PMN), and supernatants of PMN treated with these conditioned media inhibit viral growth. Arch. Virol. 2012;157:1377–1381. doi: 10.1007/s00705-012-1306-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y., Zhou Z., Min W. Mitochondria, oxidative stress and innate immunity. Front. Physiol. 2018;9 doi: 10.3389/fphys.2018.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Franchi L., Muñoz-Planillo R., Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat. Immunol. 2012;13:325–332. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Piccini A., Carta S., Tassi S., Lasiglié D., Fossati G., Rubartelli A. ATP is released by monocytes stimulated with pathogen-sensing receptor ligands and induces IL-1beta and IL-18 secretion in an autocrine way. Proc. Natl. Acad. Sci. USA. 2008;105:8067–8072. doi: 10.1073/pnas.0709684105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Muruve D.A., Pétrilli V., Zaiss A.K., White L.R., Clark S.A., Ross P.J., Parks R.J., Tschopp J. The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature. 2008;452:103–107. doi: 10.1038/nature06664. [DOI] [PubMed] [Google Scholar]
- 119.Shi J., Zhao Y., Wang K., Shi X., Wang Y., Huang H., Zhuang Y., Cai T., Wang F., Shao F. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 120.He W.T., Wan H., Hu L., Chen P., Wang X., Huang Z., Yang Z.H., Zhong C.Q., Han J. Gasdermin D is an executor of pyroptosis and required for interleukin-1β secretion. Cell Res. 2015;25:1285–1298. doi: 10.1038/cr.2015.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Niu J., Wu S., Chen M., Xu K., Guo Q., Lu A., Zhao L., Sun B., Meng G. Hyperactivation of the NLRP3 inflammasome protects mice against influenza A virus infection via IL-1β mediated neutrophil recruitment. Cytokine. 2019;120:115–124. doi: 10.1016/j.cyto.2019.04.019. [DOI] [PubMed] [Google Scholar]
- 122.Tilton C., Clippinger A.J., Maguire T., Alwine J.C. Human cytomegalovirus induces multiple means to combat reactive oxygen species. J. Virol. 2011;85:12585–12593. doi: 10.1128/JVI.05572-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Speir E., Shibutani T., Yu Z.X., Ferrans V., Epstein S.E. Role of reactive oxygen intermediates in cytomegalovirus gene expression and in the response of human smooth muscle cells to viral infection. Circ. Res. 1996;79:1143–1152. doi: 10.1161/01.RES.79.6.1143. [DOI] [PubMed] [Google Scholar]
- 124.Flores S.C., Marecki J.C., Harper K.P., Bose S.K., Nelson S.K., McCord J.M. Tat protein of human immunodeficiency virus type 1 represses expression of manganese superoxide dismutase in HeLa cells. Proc. Natl. Acad. Sci. USA. 1993;90:7632–7636. doi: 10.1073/pnas.90.16.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Guillin O.M., Vindry C., Ohlmann T., Chavatte L. Selenium, selenoproteins and viral infection. Nutrients. 2019;11:2101. doi: 10.3390/nu11092101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Manns M.P., Buti M., Gane E., Pawlotsky J.-M., Razavi H., Terrault N., Younossi Z. Hepatitis C virus infection. Nat. Rev. Dis. Primers. 2017;3:17006. doi: 10.1038/nrdp.2017.6. [DOI] [PubMed] [Google Scholar]
- 127.Ivanov A.V., Bartosch B., Smirnova O.A., Isaguliants M.G., Kochetkov S.N. HCV and oxidative stress in the liver. Viruses. 2013;5:439–469. doi: 10.3390/v5020439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Korenaga M., Wang T., Li Y., Showalter L.A., Chan T., Sun J., Weinman S.A. Hepatitis C virus core protein inhibits mitochondrial electron transport and increases reactive oxygen species (ROS) production. J. Biol. Chem. 2005;280:37481–37488. doi: 10.1074/jbc.M506412200. [DOI] [PubMed] [Google Scholar]
- 129.Bureau C., Bernad J., Chaouche N., Orfila C., Béraud M., Gonindard C., Alric L., Vinel J.P., Pipy B. Nonstructural 3 protein of hepatitis C virus triggers an oxidative burst in human monocytes via activation of NADPH oxidase. J. Biol. Chem. 2001;276:23077–23083. doi: 10.1074/jbc.M100698200. [DOI] [PubMed] [Google Scholar]
- 130.Boudreau H.E., Emerson S.U., Korzeniowska A., Jendrysik M.A., Leto T.L. Hepatitis C virus (HCV) proteins induce NADPH oxidase 4 expression in a transforming growth factor beta-dependent manner: A new contributor to HCV-induced oxidative stress. J. Virol. 2009;83:12934–12946. doi: 10.1128/JVI.01059-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.De Mochel N.S., Seronello S., Wang S.H., Ito C., Zheng J.X., Liang T.J., Lambeth J.D., Choi J. Hepatocyte NAD(P)H oxidases as an endogenous source of reactive oxygen species during hepatitis C virus infection. Hepatology. 2010;52:47–59. doi: 10.1002/hep.23671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Snelgrove R.J., Edwards L., Rae A.J., Hussell T. An absence of reactive oxygen species improves the resolution of lung influenza infection. Eur. J. Immunol. 2006;36:1364–1373. doi: 10.1002/eji.200635977. [DOI] [PubMed] [Google Scholar]
- 133.Snelgrove R., Williams A., Thorpe C., Hussell T. Manipulation of immunity to and pathology of respiratory infections. Expert Rev. Anti Infect. 2004;2:413–426. doi: 10.1586/14787210.2.3.413. [DOI] [PubMed] [Google Scholar]
- 134.Suliman H.B., Ryan L.K., Bishop L., Folz R.J. Prevention of influenza-induced lung injury in mice overexpressing extracellular superoxide dismutase. Am. J. Physiol. Lung Cell Mol. Physiol. 2001;280:L69–L78. doi: 10.1152/ajplung.2001.280.1.L69. [DOI] [PubMed] [Google Scholar]
- 135.Vlahos R., Stambas J., Selemidis S. Suppressing production of reactive oxygen species (ROS) for influenza A virus therapy. Trends Pharm. Sci. 2012;33:3–8. doi: 10.1016/j.tips.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 136.Akaike T. Role of free radicals in viral pathogenesis and mutation. Rev. Med. Virol. 2001;11:87–101. doi: 10.1002/rmv.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Li H., Liu L., Zhang D., Xu J., Dai H., Tang N., Su X., Cao B. SARS-CoV-2 and viral sepsis: Observations and hypotheses. Lancet. 2020;395:1517–1520. doi: 10.1016/S0140-6736(20)30920-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cecchini R., Cecchini A.L. SARS-CoV-2 infection pathogenesis is related to oxidative stress as a response to aggression. Med. Hypotheses. 2020;143:110102. doi: 10.1016/j.mehy.2020.110102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ademowo O.S., Dias H.K.I., Burton D.G.A., Griffiths H.R. Lipid (per) oxidation in mitochondria: An emerging target in the ageing process? Biogerontology. 2017;18:859–879. doi: 10.1007/s10522-017-9710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mantzarlis K., Tsolaki V., Zakynthinos E. Role of oxidative stress and mitochondrial dysfunction in sepsis and potential therapies. Oxid. Med. Cell Longev. 2017;2017:5985209. doi: 10.1155/2017/5985209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Effenberger-Neidnicht K., Hartmann M. Mechanisms of hemolysis during sepsis. Inflammation. 2018;41:1569–1581. doi: 10.1007/s10753-018-0810-y. [DOI] [PubMed] [Google Scholar]
- 142.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., et al. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) medRxiv. 2020 doi: 10.1101/2020.02.10.20021832. [DOI] [Google Scholar]
- 144.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Liu J., Li S., Liu J., Liang B., Wang X., Wang H., Li W., Tong Q., Yi J., Zhao L., et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. 2020;55:102763. doi: 10.1016/j.ebiom.2020.102763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Galluzzi L., Diotallevi A., Magnani M. Endoplasmic reticulum stress and unfolded protein response in infection by intracellular parasites. Future Sci. OA. 2017;3:FSO198. doi: 10.4155/fsoa-2017-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mori K. Tripartite management of unfolded proteins in the endoplasmic reticulum. Cell. 2000;101:451–454. doi: 10.1016/S0092-8674(00)80855-7. [DOI] [PubMed] [Google Scholar]
- 149.Cao S.S., Kaufman R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Signal. 2014;21:396–413. doi: 10.1089/ars.2014.5851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Choi J.-A., Song C.-H. Insights into the role of endoplasmic reticulum stress in infectious diseases. Front. Immunol. 2020;10:3147. doi: 10.3389/fimmu.2019.03147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Todd D.J., Lee A.-H., Glimcher L.H. The endoplasmic reticulum stress response in immunity and autoimmunity. Nat. Rev. Immunol. 2008;8:663–674. doi: 10.1038/nri2359. [DOI] [PubMed] [Google Scholar]
- 152.Obacz J., Avril T., Le Reste P.-J., Urra H., Quillien V., Hetz C., Chevet E. Endoplasmic reticulum proteostasis in glioblastoma—From molecular mechanisms to therapeutic perspectives. Sci. Signal. 2017;10:eaal2323. doi: 10.1126/scisignal.aal2323. [DOI] [PubMed] [Google Scholar]
- 153.Bertolotti A., Zhang Y., Hendershot L.M., Harding H.P., Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000;2:326–332. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- 154.Almanza A., Carlesso A., Chintha C., Creedican S., Doultsinos D., Leuzzi B., Luís A., McCarthy N., Montibeller L., More S., et al. Endoplasmic reticulum stress signalling—From basic mechanisms to clinical applications. FEBS J. 2019;286:241–278. doi: 10.1111/febs.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Santos C.X., Tanaka L.Y., Wosniak J., Laurindo F.R. Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: Roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid. Redox Signal. 2009;11:2409–2427. doi: 10.1089/ars.2009.2625. [DOI] [PubMed] [Google Scholar]
- 156.Li G., Scull C., Ozcan L., Tabas I. NADPH oxidase links endoplasmic reticulum stress, oxidative stress, and PKR activation to induce apoptosis. J. Cell Biol. 2010;191:1113–1125. doi: 10.1083/jcb.201006121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Pedruzzi E., Guichard C., Ollivier V., Driss F., Fay M., Prunet C., Marie J.C., Pouzet C., Samadi M., Elbim C., et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell Biol. 2004;24:10703–10717. doi: 10.1128/MCB.24.24.10703-10717.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Haynes C.M., Titus E.A., Cooper A.A. Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol. Cell. 2004;15:767–776. doi: 10.1016/j.molcel.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 159.Malhotra J.D., Miao H., Zhang K., Wolfson A., Pennathur S., Pipe S.W., Kaufman R.J. Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc. Natl. Acad. Sci. USA. 2008;105:18525–18530. doi: 10.1073/pnas.0809677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Eletto D., Chevet E., Argon Y., Appenzeller-Herzog C. Redox controls UPR to control redox. J. Cell Sci. 2014;127:3649–3658. doi: 10.1242/jcs.153643. [DOI] [PubMed] [Google Scholar]
- 161.Iurlaro R., Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283:2640–2652. doi: 10.1111/febs.13598. [DOI] [PubMed] [Google Scholar]
- 162.Han J., Back S.H., Hur J., Lin Y.H., Gildersleeve R., Shan J., Yuan C.L., Krokowski D., Wang S., Hatzoglou M., et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013;15:481–490. doi: 10.1038/ncb2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Anilkumar N., San Jose G., Sawyer I., Santos C.X., Sand C., Brewer A.C., Warren D., Shah A.M. A 28-kDa splice variant of NADPH oxidase-4 is nuclear-localized and involved in redox signaling in vascular cells. Arter. Thromb. Vasc. Biol. 2013;33:e104–e112. doi: 10.1161/ATVBAHA.112.300956. [DOI] [PubMed] [Google Scholar]
- 164.Bedard K., Lardy B., Krause K.-H. NOX family NADPH oxidases: Not just in mammals. Biochimie. 2007;89:1107–1112. doi: 10.1016/j.biochi.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 165.Santos C.X., Nabeebaccus A.A., Shah A.M., Camargo L.L., Filho S.V., Lopes L.R. Endoplasmic reticulum stress and Nox-mediated reactive oxygen species signaling in the peripheral vasculature: Potential role in hypertension. Antioxid. Redox Signal. 2014;20:121–134. doi: 10.1089/ars.2013.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Santos C.X., Stolf B.S., Takemoto P.V., Amanso A.M., Lopes L.R., Souza E.B., Goto H., Laurindo F.R. Protein disulfide isomerase (PDI) associates with NADPH oxidase and is required for phagocytosis of Leishmania chagasi promastigotes by macrophages. J. Leukoc. Biol. 2009;86:989–998. doi: 10.1189/jlb.0608354. [DOI] [PubMed] [Google Scholar]
- 167.Gerasimenko J.V., Gerasimenko O.V., Palejwala A., Tepikin A.V., Petersen O.H., Watson A.J. Menadione-induced apoptosis: Roles of cytosolic Ca2+ elevations and the mitochondrial permeability transition pore. J. Cell Sci. 2002;115:485–497. doi: 10.1242/jcs.115.3.485. [DOI] [PubMed] [Google Scholar]
- 168.Peng T.I., Jou M.J. Oxidative stress caused by mitochondrial calcium overload. Ann. N. Y. Acad. Sci. 2010;1201:183–188. doi: 10.1111/j.1749-6632.2010.05634.x. [DOI] [PubMed] [Google Scholar]
- 169.Kiviluoto S., Vervliet T., Ivanova H., Decuypere J.-P., De Smedt H., Missiaen L., Bultynck G., Parys J.B. Regulation of inositol 1,4,5-trisphosphate receptors during endoplasmic reticulum stress. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2013;1833:1612–1624. doi: 10.1016/j.bbamcr.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 170.Cioffi D.L. Redox regulation of endothelial canonical transient receptor potential channels. Antioxid. Redox Signal. 2011;15:1567–1582. doi: 10.1089/ars.2010.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Görlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Pillich H., Loose M., Zimmer K.-P., Chakraborty T. Diverse roles of endoplasmic reticulum stress sensors in bacterial infection. Mol. Cell Pediatr. 2016;3:9. doi: 10.1186/s40348-016-0037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Bischof L.J., Kao C.Y., Los F.C., Gonzalez M.R., Shen Z., Briggs S.P., van der Goot F.G., Aroian R.V. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog. 2008;4:e1000176. doi: 10.1371/journal.ppat.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Loose M., Hudel M., Zimmer K.-P., Garcia E., Hammerschmidt S., Lucas R., Chakraborty T., Pillich H. Pneumococcal hydrogen peroxide-induced stress signaling regulates inflammatory genes. J. Infect. Dis. 2015;211:306–316. doi: 10.1093/infdis/jiu428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vasallo C., Gastaminza P. Cellular stress responses in hepatitis C virus infection: Mastering a two-edged sword. Virus Res. 2015;209:100–117. doi: 10.1016/j.virusres.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 176.Ríos-Ocampo W.A., Navas M.C., Faber K.N., Daemen T., Moshage H. The cellular stress response in hepatitis C virus infection: A balancing act to promote viral persistence and host cell survival. Virus Res. 2019;263:1–8. doi: 10.1016/j.virusres.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 177.Ríos-Ocampo W.A., Navas M.C., Buist-Homan M., Faber K.N., Daemen T., Moshage H. Hepatitis C Virus Proteins Core and NS5A Are Highly Sensitive to Oxidative Stress-Induced Degradation after eIF2α/ATF4 Pathway Activation. Viruses. 2020;12:425. doi: 10.3390/v12040425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yang T.-C., Lai C.-C., Shiu S.-L., Chuang P.-H., Tzou B.-C., Lin Y.-Y., Tsai F.-J., Lin C.-W. Japanese encephalitis virus down-regulates thioredoxin and induces ROS-mediated ASK1-ERK/p38 MAPK activation in human promonocyte cells. Microbes Infect. 2010;12:643–651. doi: 10.1016/j.micinf.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 179.Jordan R., Wang L., Graczyk T.M., Block T.M., Romano P.R. Replication of a cytopathic strain of bovine viral diarrhea virus activates PERK and induces endoplasmic reticulum stress-mediated apoptosis of MDBK cells. J. Virol. 2002;76:9588–9599. doi: 10.1128/JVI.76.19.9588-9599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Abuaita B.H., Burkholder K.M., Boles B.R., O’Riordan M.X. The Endoplasmic Reticulum Stress Sensor Inositol-Requiring Enzyme 1α Augments Bacterial Killing through Sustained Oxidant Production. mBio. 2015;6:e00705–e00715. doi: 10.1128/mBio.00705-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Celli J., Tsolis R.M. Bacteria, the endoplasmic reticulum and the unfolded protein response: Friends or foes? Nat. Rev. Microbiol. 2015;13:71–82. doi: 10.1038/nrmicro3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Smith J.A., Khan M., Magnani D.D., Harms J.S., Durward M., Radhakrishnan G.K., Liu Y.P., Splitter G.A. Brucella induces an unfolded protein response via TcpB that supports intracellular replication in macrophages. PLoS Pathog. 2013;9:e1003785. doi: 10.1371/journal.ppat.1003785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.De Jong M.F., Starr T., Winter M.G., den Hartigh A.B., Child R., Knodler L.A., van Dijl J.M., Celli J., Tsolis R.M. Sensing of bacterial type IV secretion via the unfolded protein response. mBio. 2013;4:e00418-12. doi: 10.1128/mBio.00418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Lee S.Y., Lee M.S., Cherla R.P., Tesh V.L. Shiga toxin 1 induces apoptosis through the endoplasmic reticulum stress response in human monocytic cells. Cell Microbiol. 2008;10:770–780. doi: 10.1111/j.1462-5822.2007.01083.x. [DOI] [PubMed] [Google Scholar]
- 185.Choi H.H., Shin D.M., Kang G., Kim K.H., Park J.B., Hur G.M., Lee H.M., Lim Y.J., Park J.K., Jo E.K., et al. Endoplasmic reticulum stress response is involved in Mycobacterium tuberculosis protein ESAT-6-mediated apoptosis. FEBS Lett. 2010;584:2445–2454. doi: 10.1016/j.febslet.2010.04.050. [DOI] [PubMed] [Google Scholar]
- 186.Lim Y.J., Choi J.A., Lee J.H., Choi C.H., Kim H.J., Song C.H. Mycobacterium tuberculosis 38-kDa antigen induces endoplasmic reticulum stress-mediated apoptosis via toll-like receptor 2/4. Apoptosis. 2015;20:358–370. doi: 10.1007/s10495-014-1080-2. [DOI] [PubMed] [Google Scholar]
- 187.Roy A., Kolattukudy P.E. Monocyte chemotactic protein-induced protein (MCPIP) promotes inflammatory angiogenesis via sequential induction of oxidative stress, endoplasmic reticulum stress and autophagy. Cell Signal. 2012;24:2123–2131. doi: 10.1016/j.cellsig.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 188.Lim Y.-J., Choi J.-A., Choi H.-H., Cho S.-N., Kim H.-J., Jo E.-K., Park J.-K., Song C.-H. Endoplasmic reticulum stress pathway-mediated apoptosis in macrophages contributes to the survival of Mycobacterium tuberculosis. PLoS ONE. 2011;6:e28531. doi: 10.1371/journal.pone.0028531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Han J.-Y., Lim Y.-J., Choi J.-A., Lee J.-H., Jo S.-H., Oh S.-M., Song C.-H. The role of prostate apoptosis response-4 (Par-4) in Mycobacterium tuberculosis infected macrophages. Sci. Rep. 2016;6:32079. doi: 10.1038/srep32079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jo S.H., Choi J.-A., Lim Y.-J., Lee J., Cho S.-N., Oh S.-M., Go D., Kim S.-H., Song C.-H. Calreticulin modulates the intracellular survival of mycobacteria by regulating ER-stress-mediated apoptosis. Oncotarget. 2017;8:58686–58698. doi: 10.18632/oncotarget.17419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lim Y.-J., Choi H.-H., Choi J.-A., Jeong J.A., Cho S.-N., Lee J.-H., Park J.B., Kim H.-J., Song C.-H. Mycobacterium kansasii-induced death of murine macrophages involves endoplasmic reticulum stress responses mediated by reactive oxygen species generation or calpain activation. Apoptosis. 2013;18:150–159. doi: 10.1007/s10495-012-0792-4. [DOI] [PubMed] [Google Scholar]
- 192.Oh S.-M., Lim Y.-J., Choi J.-A., Lee J., Cho S.-N., Go D., Kim S.-H., Song C.-H. TNF-α-mediated ER stress causes elimination of Mycobacterium fortuitum reservoirs by macrophage apoptosis. FASEB J. 2018;32:3993–4003. doi: 10.1096/fj.201701407R. [DOI] [PubMed] [Google Scholar]
- 193.Kim S.-H., Cho S.-N., Lim Y.-J., Choi J.-A., Lee J., Go D., Song C.-H. Phagocytosis influences the intracellular survival of Mycobacterium smegmatis via the endoplasmic reticulum stress response. Cell Biosci. 2018;8:52. doi: 10.1186/s13578-018-0250-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Go D., Lee J., Choi J.-A., Cho S.-N., Kim S.-H., Son S.-H., Song C.-H. Reactive oxygen species-mediated endoplasmic reticulum stress response induces apoptosis of Mycobacterium avium-infected macrophages by activating regulated IRE1-dependent decay pathway. Cell Microbiol. 2019;21:e13094. doi: 10.1111/cmi.13094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Han D., Lerner A.G., Vande Walle L., Upton J.P., Xu W., Hagen A., Backes B.J., Oakes S.A., Papa F.R. IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell. 2009;138:562–575. doi: 10.1016/j.cell.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Upton J.P., Wang L., Han D., Wang E.S., Huskey N.E., Lim L., Truitt M., McManus M.T., Ruggero D., Goga A., et al. IRE1α cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science. 2012;338:818–822. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Westermann B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- 198.Ježek J., Cooper K.F., Strich R. Reactive Oxygen Species and Mitochondrial Dynamics: The Yin and Yang of Mitochondrial Dysfunction and Cancer Progression. Antioxidants. 2018;7:13. doi: 10.3390/antiox7010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sabouny R., Shutt T.E. Reciprocal regulation of mitochondrial fission and fusion. Trends Biochem. Sci. 2020;45:564–577. doi: 10.1016/j.tibs.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 200.Mukherjee R., Chakrabarti O. Regulation of Mitofusin1 by Mahogunin Ring Finger-1 and the proteasome modulates mitochondrial fusion. Biochim. Biophys. Acta. 2016;1863:3065–3083. doi: 10.1016/j.bbamcr.2016.09.022. [DOI] [PubMed] [Google Scholar]
- 201.Yan L., Qi Y., Huang X., Yu C., Lan L., Guo X., Rao Z., Hu J., Lou Z. Structural basis for GTP hydrolysis and conformational change of MFN1 in mediating membrane fusion. Nat. Struct. Mol. Biol. 2018;25:233–243. doi: 10.1038/s41594-018-0034-8. [DOI] [PubMed] [Google Scholar]
- 202.Chen H., Detmer S.A., Ewald A.J., Griffin E.E., Fraser S.E., Chan D.C. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J. Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Mishra P., Chan D.C. Metabolic regulation of mitochondrial dynamics. J. Cell Biol. 2016;212:379–387. doi: 10.1083/jcb.201511036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Rambold A.S., Pearce E.L. Mitochondrial Dynamics at the Interface of Immune Cell Metabolism and Function. Trends Immunol. 2018;39:6–18. doi: 10.1016/j.it.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 205.Otera H., Wang C., Cleland M.M., Setoguchi K., Yokota S., Youle R.J., Mihara K. Mff is an essential factor for mitochondrial recruitment of Drp1 during mitochondrial fission in mammalian cells. J. Cell Biol. 2010;191:1141–1158. doi: 10.1083/jcb.201007152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Osellame L.D., Singh A.P., Stroud D.A., Palmer C.S., Stojanovski D., Ramachandran R., Ryan M.T. Cooperative and independent roles of the Drp1 adaptors Mff, MiD49 and MiD51 in mitochondrial fission. J. Cell Sci. 2016;129:2170–2181. doi: 10.1242/jcs.185165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Cribbs J.T., Strack S. Reversible phosphorylation of Drp1 by cyclic AMP-dependent protein kinase and calcineurin regulates mitochondrial fission and cell death. EMBO Rep. 2007;8:939–944. doi: 10.1038/sj.embor.7401062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Karbowski M., Neutzner A., Youle R.J. The mitochondrial E3 ubiquitin ligase MARCH5 is required for Drp1 dependent mitochondrial division. J. Cell Biol. 2007;178:71–84. doi: 10.1083/jcb.200611064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pendin D., Filadi R., Pizzo P. The concerted action of mitochondrial dynamics and positioning: New characters in cancer onset and progression. Front. Oncol. 2017;7 doi: 10.3389/fonc.2017.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Nagdas S., Kashatus D.F. The interplay between oncogenic signaling networks and mitochondrial dynamics. Antioxidants. 2017;6:33. doi: 10.3390/antiox6020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Arnoult D. Mitochondrial fragmentation in apoptosis. Trends Cell Biol. 2007;17:6–12. doi: 10.1016/j.tcb.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 212.Karbowski M., Youle R.J. Dynamics of mitochondrial morphology in healthy cells and during apoptosis. Cell Death Differ. 2003;10:870–880. doi: 10.1038/sj.cdd.4401260. [DOI] [PubMed] [Google Scholar]
- 213.Fielden L.F., Kang Y., Newton H.J., Stojanovski D. Targeting mitochondria: How intravacuolar bacterial pathogens manipulate mitochondria. Cell Tissue Res. 2017;367:141–154. doi: 10.1007/s00441-016-2475-x. [DOI] [PubMed] [Google Scholar]
- 214.Tiku V., Tan M.-W., Dikic I. Mitochondrial functions in infection and immunity. Trends Cell Biol. 2020;30:263–275. doi: 10.1016/j.tcb.2020.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Angajala A., Lim S., Phillips J.B., Kim J.H., Yates C., You Z., Tan M. Diverse roles of mitochondria in immune responses: Novel insights into immuno-metabolism. Front. Immunol. 2018;9:1605. doi: 10.3389/fimmu.2018.01605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Korenaga M., Okuda M., Otani K., Wang T., Li Y., Weinman S.A. Mitochondrial dysfunction in hepatitis C. J. Clin. Gastroenterol. 2005;39:S162–S166. doi: 10.1097/01.mcg.0000155517.02468.46. [DOI] [PubMed] [Google Scholar]
- 217.McCormick A.L., Smith V.L., Chow D., Mocarski E.S. Disruption of mitochondrial networks by the human cytomegalovirus UL37 gene product viral mitochondrion-localized inhibitor of apoptosis. J. Virol. 2003;77:631–641. doi: 10.1128/JVI.77.1.631-641.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Lupfer C., Thomas P.G., Anand P.K., Vogel P., Milasta S., Martinez J., Huang G., Green M., Kundu M., Chi H., et al. Receptor interacting protein kinase 2-mediated mitophagy regulates inflammasome activation during virus infection. Nat. Immunol. 2013;14:480–488. doi: 10.1038/ni.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Xia M., Gonzalez P., Li C., Meng G., Jiang A., Wang H., Gao Q., Debatin K.-M., Beltinger C., Wei J. Mitophagy enhances oncolytic measles virus replication by mitigating DDX58/RIG-I-like receptor signaling. J. Virol. 2014;88:5152–5164. doi: 10.1128/JVI.03851-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Xia M., Meng G., Jiang A., Chen A., Dahlhaus M., Gonzalez P., Beltinger C., Wei J. Mitophagy switches cell death from apoptosis to necrosis in NSCLC cells treated with oncolytic measles virus. Oncotarget. 2014;5:3907–3918. doi: 10.18632/oncotarget.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Keck F., Brooks-Faulconer T., Lark T., Ravishankar P., Bailey C., Salvador-Morales C., Narayanan A. Altered mitochondrial dynamics as a consequence of Venezuelan Equine encephalitis virus infection. Virulence. 2017;8:1849–1866. doi: 10.1080/21505594.2016.1276690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Mohasin M., Balbirnie-Cumming K., Fisk E., Prestwich E.C., Russell C.D., Marshall J., Pridans C., Allen S.P., Shaw P.J., De Vos K.J., et al. Macrophages utilize mitochondrial fission to enhance mROS production during responses to Streptococcus pneumoniae. bioRxiv. 2019 doi: 10.1101/722603. [DOI] [Google Scholar]
- 223.Jain P., Luo Z.-Q., Blanke S.R. Helicobacter pylori vacuolating cytotoxin A (VacA) engages the mitochondrial fission machinery to induce host cell death. Proc. Natl. Acad. Sci. USA. 2011;108:16032–16037. doi: 10.1073/pnas.1105175108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Duan C., Kuang L., Xiang X., Zhang J., Zhu Y., Wu Y., Yan Q., Liu L., Li T. Drp1 regulates mitochondrial dysfunction and dysregulated metabolism in ischemic injury via Clec16a-, BAX-, and GSH- pathways. Cell Death Dis. 2020;11:251. doi: 10.1038/s41419-020-2461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Kim I.-J., Lee J., Oh S.J., Yoon M.-S., Jang S.-S., Holland R.L., Reno M.L., Hamad M.N., Maeda T., Chung H.J., et al. Helicobacter pylori Infection Modulates Host Cell Metabolism through VacA-Dependent Inhibition of mTORC1. Cell Host Microbe. 2018;23:583–593.e588. doi: 10.1016/j.chom.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.De la Cruz López K.G., Toledo Guzmán M.E., Sánchez E.O., García Carrancá A. mTORC1 as a regulator of mitochondrial functions and a therapeutic target in cancer. Front. Oncol. 2019;9 doi: 10.3389/fonc.2019.01373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Hernandez L.D., Pypaert M., Flavell R.A., Galán J.E. A Salmonella protein causes macrophage cell death by inducing autophagy. J. Cell Biol. 2003;163:1123–1131. doi: 10.1083/jcb.200309161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Lobet E., Willemart K., Ninane N., Demazy C., Sedzicki J., Lelubre C., De Bolle X., Renard P., Raes M., Dehio C., et al. Mitochondrial fragmentation affects neither the sensitivity to TNFα-induced apoptosis of Brucella-infected cells nor the intracellular replication of the bacteria. Sci. Rep. 2018;8:5173. doi: 10.1038/s41598-018-23483-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Stavru F., Bouillaud F., Sartori A., Ricquier D., Cossart P. Listeria monocytogenes transiently alters mitochondrial dynamics during infection. Proc. Natl. Acad. Sci. USA. 2011;108:3612–3617. doi: 10.1073/pnas.1100126108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Zhang Y., Yao Y., Qiu X., Wang G., Hu Z., Chen S., Wu Z., Yuan N., Gao H., Wang J., et al. Listeria hijacks host mitophagy through a novel mitophagy receptor to evade killing. Nat. Immunol. 2019;20:433–446. doi: 10.1038/s41590-019-0324-2. [DOI] [PubMed] [Google Scholar]
- 231.Escoll P., Song O.-R., Viana F., Steiner B., Lagache T., Olivo-Marin J.-C., Impens F., Brodin P., Hilbi H., Buchrieser C. Legionella pneumophila modulates mitochondrial dynamics to trigger metabolic repurposing of infected macrophages. Cell Host Microbe. 2017;22:302–316.e307. doi: 10.1016/j.chom.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 232.Chowdhury S.R., Reimer A., Sharan M., Kozjak-Pavlovic V., Eulalio A., Prusty B.K., Fraunholz M., Karunakaran K., Rudel T. Chlamydia preserves the mitochondrial network necessary for replication via microRNA-dependent inhibition of fission. J. Cell Biol. 2017;216:1071–1089. doi: 10.1083/jcb.201608063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Asalla S., Mohareer K., Banerjee S. Small molecule mediated restoration of mitochondrial function augments anti-mycobacterial activity of human macrophages subjected to cholesterol induced asymptomatic dyslipidemia. Front. Cell. Infect. Microbiol. 2017;7 doi: 10.3389/fcimb.2017.00439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jamwal S., Midha M.K., Verma H.N., Basu A., Rao K.V.S., Manivel V. Characterizing virulence-specific perturbations in the mitochondrial function of macrophages infected with Mycobacterium tuberculosis. Sci. Rep. 2013;3:1328. doi: 10.1038/srep01328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Lee J., Choi J.A., Cho S.N., Son S.H., Song C.H. Mitofusin 2-deficiency suppresses Mycobacterium tuberculosis survival in macrophages. Cells. 2019;8:1355. doi: 10.3390/cells8111355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Xiao B., Goh J.-Y., Xiao L., Xian H., Lim K.-L., Liou Y.-C. Reactive oxygen species trigger Parkin/PINK1 pathway-dependent mitophagy by inducing mitochondrial recruitment of Parkin. J. Biol. Chem. 2017;292:16697–16708. doi: 10.1074/jbc.M117.787739. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data available in a publicly accessible repository.