Abstract
Novel antimicrobials interfering with pathogen-specific targets can minimize the risk of perturbations of the gut microbiota (dysbiosis) during therapy. We employed an in silico approach to identify essential proteins in Escherichia coli that are either absent or have low sequence identity in seven beneficial taxa of the gut microbiota: Faecalibacterium, Prevotella, Ruminococcus, Bacteroides, Lactobacillus, Lachnospiraceae and Bifidobacterium. We identified 36 essential proteins that are present in hyper-virulent E. coli ST131 and have low similarity (bitscore < 50 or identity < 30% and alignment length < 25%) to proteins in mammalian hosts and beneficial taxa. Of these, 35 are also present in Klebsiella pneumoniae. None of the proteins are targets of clinically used antibiotics, and 3D structure is available for 23 of them. Four proteins (LptD, LptE, LolB and BamD) are easily accessible as drug targets due to their location in the outer membrane, especially LptD, which contains extracellular domains. Our results indicate that it may be possible to selectively interfere with essential biological processes in Enterobacteriaceae that are absent or mediated by unrelated proteins in beneficial taxa residing in the gut. The identified targets can be used to discover antimicrobial drugs effective against these opportunistic pathogens with a decreased risk of causing dysbiosis.
Keywords: antimicrobial targets, Escherichia coli, in silico, microbiota
1. Introduction
Due to the worldwide increase in resistance observed among certain bacterial pathogens, there is a pressing need for novel antimicrobials. Most of the antimicrobial drugs approved for human use since the end of the antibiotic golden age in the 1960s belong to known antimicrobial classes and are primarily active against Gram-positive bacteria [1]. Although in recent years novel antimicrobials have become available for effective treatment of Gram-negative bacterial infections, such drugs belong to known antimicrobial classes, and only one antimicrobial with a novel mechanism of action, Murepavadin, has made it to Phase 3 trials [2,3]. Thus, there is a demand for truly novel antimicrobial compounds targeting Gram-negative bacteria. In 2017, the World Health Organization released a list of global priority antimicrobial-resistant (AMR) pathogens in order to guide research, discovery and development of new antibiotics [4]. The highest category (‘Priority 1: Critical’) comprises Acinetobacter baumannii, Pseudomonas aeruginosa and several Enterobacteriaceae, including Escherichia coli. The latter species is the most frequent cause of several common infections, such as urinary tract infection and septicaemia, that are increasingly difficult to treat due to the global spread of specific hyper-virulent and multidrug-resistant clones. In particular, ST131 is a major contributor to the global spread of fluoroquinolone resistance and extended-spectrum β-lactamase-mediated resistance to β-lactams, and is responsible for millions of multidrug-resistant infections each year [5,6].
Oral antimicrobial therapy impacts the healthy gut microbiota by inducing a loss of beneficial microbes followed by expansion of opportunistic pathogenic bacteria, such as Enterobacteriaceae [7]. This phenomenon, generally referred to as dysbiosis, can in extreme cases lead to life-threatening secondary infections caused by Clostridioides difficile [8], which are becoming increasingly common and difficult to treat. Certain antimicrobial-sensitive taxa residing in the healthy gut microbiota, e.g., Bacteroidetes and Lachnospiraceae, have been shown to provide protection from C. difficile infections through colonization resistance [7]. Moreover, the gut-associated taxa Lactobacillus and Bifidobacterium both contain strains that are associated with beneficial effects on health and are used as probiotics in various food supplements, including Lactobacillus planetarum, L. paracasei, L. acidophilus, Bifidobacterium infantis, B. longum and B. breve [9].
Pathogen-targeted antimicrobial drugs with a limited effect on beneficial organisms could potentially decrease the risk of dysbiosis and antibiotic-induced secondary infections. One approach to discover such drugs is the employment of target-based assays to identify compounds that selectively interfere with the viability of E. coli and other Enterobacteriaceae without affecting the healthy gut microbiota. This requires identification of targets that are specific for this bacterial family. Here, we performed an in silico study to identify protein drug targets in E. coli that: (i) are present in hyper-virulent E. coli ST131; (ii) do not display significant similarity to host proteins; and iii) are absent or have low amino acid identity in selected members of the healthy gut microbiota. Furthermore, the selected proteins were analyzed for their conservation and essentiality in the closely related pathogen K. pneumoniae, and their ‘druggability’ was assessed based on subcellular localization (SCL), availability of three-dimensional structures and presence of known inhibitors.
2. Results
2.1. Essential Genes in the Target Pathogen
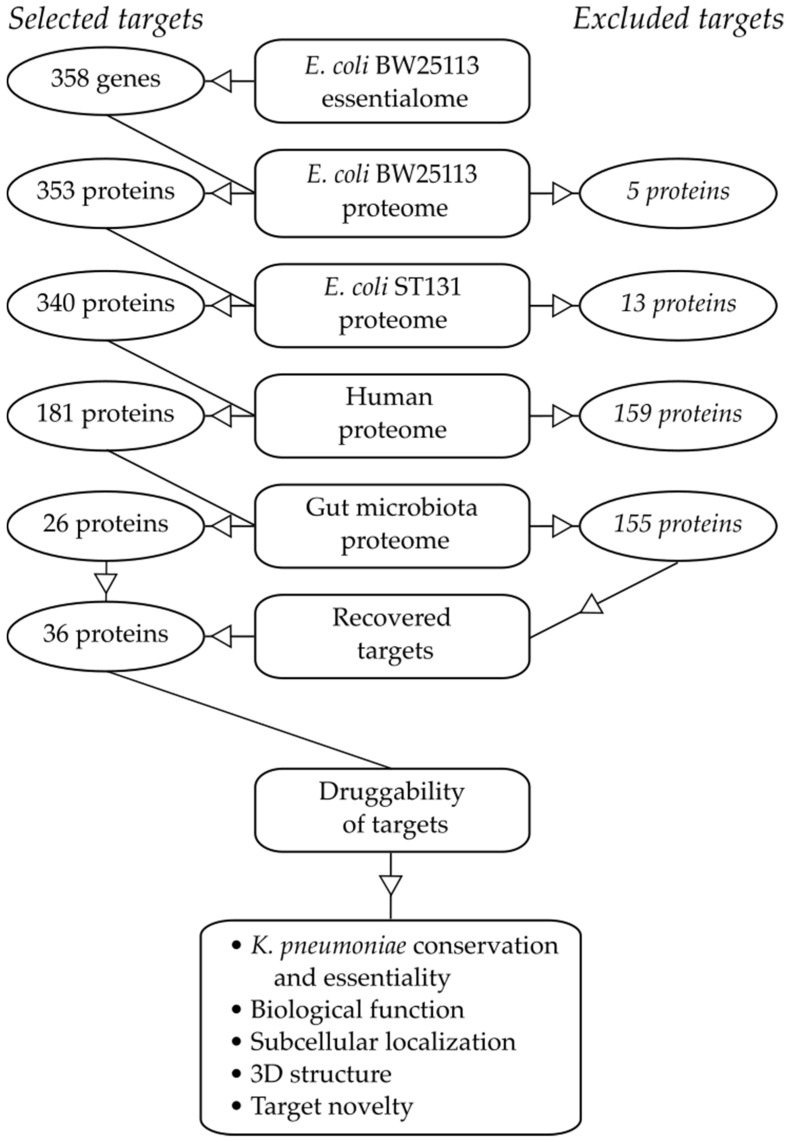
Due to the lack of understanding of the essential genome in pathogenic strains such as ST131, the model strain BW25113 was used as a basis for our study. The predicted amino acid sequences of 353 out of the 358 essential genes identified by Goodall et al. [10] were retrieved (Figure 1). The remaining five genes (ttcC, yddL, yedN, ygeF and ygeN) were labelled as ‘pseudogenes’ or ‘putative proteins’. None of these proteins had been identified as essential in the Keio collection, a systematic collection of single-gene knockout mutations previously used as the gold standard to define the essentialome of E. coli BW25113 [11]. Such genes were excluded from further analysis since their classification as essential by Goodall et al. [10] may be an artefact of the methodology employed in the original study.
Figure 1.
Flow diagram showing the different steps of the selection procedure used to identify novel selective antimicrobial drug targets against E. coli.
The sequences of the 353 proteins were compared to those found in E. coli O25b:H4-ST131 using a pre-defined cut-off (bitscore ≥ 50 or ≥ 70% sequence identity and ≥ 75% alignment length, see Materials and Methods). All of the 353 essential BW25113 proteins were associated with at least one hit in ST131, apart from YmfE and YmiB (Supplementary Table S1). Eleven additional proteins were excluded as they scored below the cut-off threshold, leading to a list of 340 essential and conserved proteins for further analyses. Inspection of the excluded sequences revealed that 10 were prophage-related or uncharacterized proteins. The presence of phages in a bacterial genome is expected to vary with the specific strain history, and this may explain the observed difference between the laboratory strain BW25113 and the hyper-virulent clonal lineage ST131. The essentiality status of some of these phage-related genes (e.g., relB, ydaS and racR) can be explained through their function as transcriptional regulators, and removal of these proteins allows the phage to activate and subsequently kill the cell.
2.2. Similarity to Proteins in Mammalian Hosts
The second step of the analysis aimed at removing E. coli targets homologous to the human proteome. A high degree of similarity between the pathogen-specific target and one or more proteins in the host proteome may result in off-target binding of a drug, leading to toxicity and unwanted side effects. The 340 selected essential proteins were therefore compared to the human proteome, leading to 181 proteins fulfilling the same stringent cut-offs as described above (Figure 1, Table S2).
2.3. Similarity to Proteins in Beneficial Taxa of the Gut Microbiota
The next step in the selection pipeline aimed to exclude proteins with high similarity to those found in representatives of the beneficial gut microbiota. Given the complexity and variability of the gut microbiome, we decided to focus on seven taxa containing species previously shown to have beneficial and protective effects on the host [7,8,12,13,14,15,16,17,18]: Faecalibacterium, Prevotella, Ruminococcus, Bacteroides, Lactobacillus, Lachnospiraceae and Bifidobacterium (Tables S3–S9). The 181 proteins were blasted against the abovementioned taxa using the same cut-off values as before (Figure 1). As expected, this step was the most selective, leaving just 26 proteins for further analysis (Table 1), and removed all targets of commercially available antibiotics, including FtsI and MrdA (targets of β-lactams), as well as parts of the 30S and 50S ribosome (targets of macrolides, aminoglycosides and tetracyclines) and RNA polymerase (target of rifamycins).
Table 1.
Gene name, function, PDB accession number, crystal structure and resolution according to RCSB PDB, SCL according to Swissprot, available inhibitors, sequence conservation and essentiality in K. pneumoniae for the 36 protein targets identified by this study. N/A = not available, N/D = not determined.
| Gene | Protein Function | PDB Accession No. | Crystal Structure and Resolution | SCL | Known Inhibitor | Conservation and Essentiality in K. pneumoniae | |||
|---|---|---|---|---|---|---|---|---|---|
| Alignment | aa Identity | Bitscore | Essentiality Status | ||||||
| bamD | Outer membrane protein assembly factor BamD | P0AC02 | 5D0O 2.90 Å |
Cell outer membrane; Lipid anchor | Inhibitory peptide [19], IMB-H4 [20] | 100% | 94% | 479 | Essential |
| cydX | Cytochrome bd-I ubiquinol oxidase subunit X | P56100 | 6RKO 2.68 Å |
Cell inner membrane; Single-pass membrane protein | N/A | 100% | 100% | 77 | N/A |
| dnaT | Primosomal protein 1 | P0A8J2 | 4OU6 1.96 Å |
N/D | N/A | 100% | 76% | 285 | N/A |
| fabA | 3-hydroxydecanoyl-[acyl-carrier-protein] dehydratase | P0A6Q3 | 4KEH 2Å |
Cytoplasm | 3-Decynoyl-NAC [21] | 100% | 99% | 349 | Essential |
| ftsB | Cell division protein FtsB |
P0A6S5 |
4IFF 2.30 Å |
Cell inner membrane | N/A | 84% | 100% | 181 | Essential |
| ftsL | Cell division protein FtsL | P0AEN4 | N/D | Cell inner membrane | N/A | 100% | 96% | 238 | Essential |
| ftsQ | Cell division protein FtsQ | P06136 | 2VH1 2.70 Å |
Cell inner membrane; Single-pass type II membrane protein | N/A | 96% | 89% | 486 | Essential |
| hemD | Uroporphyrinogen-III synthase | P09126 | N/D | N/D | N/A | 100% | 99% | 497 | Essential |
| higA | Antitoxin HigA | P67701 | 6JQ4 2 Å |
N/D | N/A | 100% | 99% | 279 | N/A |
| hipB | Antitoxin HipB | P23873 | 2WIU 2.35 Å |
N/D | N/A | 100% | 98% | 176 | Essential |
| holD | DNA polymerase III subunit psi | P28632 | 3SXU 1.85 Å |
N/D | N/A | 84% | 98% | 224 | N/A |
| iraM | Anti-adapter protein IraM | P75987 | N/D | Cytoplasm | N/A | 99% | 62% | 149 | N/A |
| lolA | Outer membrane lipoprotein carrier protein | P61316 | 1IWL 1.56 Å |
Periplasm | MAC13243 [22] | 100% | 94% | 399 | Essential |
| lolB | Outer membrane lipoprotein LolB | P61320 | 1IWM 1.90 Å |
Cell outer membrane; Lipid anchor | N/A | 100% | 100% | 426 | Essential |
| lptA | Lipopolysaccharide export system protein LptA | P0ADV1 | 2R19 2.16 Å |
Periplasm | Thanatin [23], Compound IMB-881 [24] |
100% | 85% | 328 | Essential |
| lptD | LPS assembly protein LptD | P31554 | 4RHB 3.35 Å |
Cell outer membrane | Inhibitory peptide JB-95 [25] | 100% | 84% | 1407 | Essential |
| lptE | LPS assembly lipoprotein LptE | P0ADC1 | 4RHB 3.35 Å |
Cell outer membrane | N/A | 102% | 72% | 295 | Essential |
| mreD | Rod shape-determining protein MreD | P0ABH4 | N/D | Cell inner membrane; Multi-pass membrane protein | N/A | 100% | 100% | 310 | N/A |
| mukB | Chromosome partition protein MukB | P22523 | 1QHL 2.20 Å |
Nucleoid | NSC260594, Michellamine B [26] | 100% | 92% | 2774 | Essential |
| mukE | Chromosome partition protein MukE | P22524 | 3EUH 2.90 Å |
Nucleoid | N/A | 100% | 95% | 458 | Essential |
| mukF | Chromosome partition protein MukF | P60293 | 3EUH 2.90 Å |
Nucleoid | N/A | 100% | 97% | 878 | Essential |
| pheM | Phenylalanine tRNA ligase operon leader peptide | P0AD74 | N/D | N/D | N/A | 100% | 100% | 30 | N/A |
| priB | Primosomal replication protein N | P07013 | 5WQV 1.97 Å |
N/D | N/A | 100% | 93% | 205 | N/A |
| safA | Two-component-system connector protein SafA | P76136 | N/D | Cell inner membrane; Single-pass type II membrane protein | N/A | 98% | 100% | 127 | N/A |
| secE | Protein translocase subunit SecE | P0AG96 | 5GAE 3.33 Å |
Cell inner membrane; Multi-pass membrane protein | N/A | 100% | 98% | 240 | Essential |
| trpL | trp operon leader peptide | P0AD92 | N/D | N/D | N/A | 100% | 100% | 32 | N/A |
| tusE | Sulfurtransferase TusE | P0AB18 | N/D | Cytoplasm | N/A | 100% | 90% | 204 | Essential |
| wzyE | Probable ECA polymerase | P27835 | N/D | Cell inner membrane | N/A | 100% | 87% | 744 | N/A |
| ycaR | UPF0434 protein YcaR | P0AAZ7 | N/D | Cytoplasm | N/A | 100% | 100% | 125 | N/A |
| yciS | Lipopolysaccharide assembly protein A | P0ACV4 | N/D | Cell inner membrane | N/A | 100% | 87% | 177 | N/A |
| ydfO | Uncharacterized protein YdfO | P76156 | 2HH8 N/D |
N/D | N/A | 100% | 79% | 228 | N/A |
| ydhL | Uncharacterized protein YdhL | P64474 | N/D | N/D | N/A | 96% | 98% | 152 | N/A |
| ygfZ | tRNA-modifying protein YgfZ | P0ADE8 | 1VLY 1.30 Å |
Cytoplasm | N/A | 100% | 84% | 573 | N/A |
| yqeL | Uncharacterized protein YqeL | C1P613 | N/D | N/D | N/A | N/A | N/A | N/A | N/A |
| yrfF | Putative membrane protein IgaA homolog | P45800 | 4UZM N/D |
Cell inner membrane; Multi-pass membrane protein | N/A | 100% | 73% | 1089 | N/A |
| zipA | Cell division protein ZipA | P77173 | 1F46 1.50 Å |
Cell inner membrane; Single-pass type I membrane protein | Antimicrobial compounds [27,28] | 101% | 98% | 646 | Essential |
Among the identified 26 proteins, only PheM and TrpL, leader peptides in the Phe tRNA synthetase and Trp biosynthetic operon, respectively, were found to be missing completely in all taxa. The uncharacterized protein YqeL was found to be missing in all except for Lachnospiraceae and Lactobacillus. SafA (part of the low pH stress response) was found to be missing in Lachnospiraceae, Bifidobacterium and Faecalibacterium. MreD, a rod shape-determining protein was missing from Bacteroides and Bifidobacterium. Furthermore, WzyE (probable ECA polymerase) lacked hits in Bifidobacterium and Ruminococcus. Neither LolA, LolB (both involved in lipoprotein transport) nor DnaT (primosomal protein 1) were associated with any hits in Bifidobacterium; LptE (part of the lipopolysaccharide (LPS) assembly machinery) and TusE (a sulphur transferase) were missing in Faecablibacterium; FtsL (a cell division protein) was missing from Bacteroides; and CydX (cytochrome bd-I ubiquinol oxidase subunit X) from Ruminococcus. All other proteins were associated with hits below the cut-off in all taxa.
Due to the high stringency applied in the above step, potentially valuable targets may have been missed in the selection process. Thus, a second analysis of the microbiota BLAST results was undertaken to find proteins associated with only 10 or less hits over cut-off. Ten proteins (BamD, FabA, LptD, MukB, MukF, SecE, YdfO, YgfZ, YrfF and ZipA) were each found to have been excluded based on bitscore cut-off rather than sequence identity. Thus, these proteins were included in further analyses, leading to 36 proteins as potential E. coli-selective targets (Table 1).
2.4. Target Essentiality and Conservation in K. pneumoniae
We evaluated presence and essentiality of the selected targets in K. pneumoniae, which represents another global priority pathogen closely related to E. coli. The essentiality of the 36 proteins was checked against the library generated by Ramage et al. [29], together with conservation of the amino acid sequence as above (Figure 1, Table S10). Eighteen were found to be essential in both organisms, all displaying high sequence conservation. However, all of the remaining proteins not reported to be essential in K. pneumoniae fulfilled the similarity-based selection criteria, except for YqeL, which lacked hits completely (Table 1).
2.5. Biological Function of Selected Targets
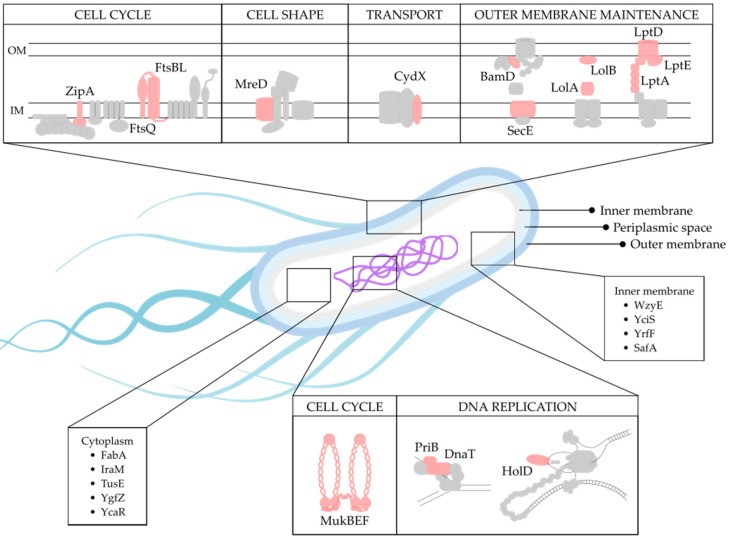
Of the 36 identified targets (Table 1), 20 were found to share similar biological functions (Figure 2). The largest functional group comprised of eight proteins involved in outer membrane (OM) biogenesis and maintenance (BamD, LptA, LptD, LptE, LolA, LolB, SecE and YciS) (Figure 2). Cell division made up the second largest functional group (MukB, MukE, MukF, FtsB, FtsL, FtsQ and ZipA), which is closely linked to the functional groups involved in cell shape (MreD) and DNA replication (HolD, PriB, DnaT and YgfZ) (Figure 2). Additionally, two members belonging to the functional group involved in transcriptional regulation (PheM and TrpL) were also identified, together with one member involved in translation (TusE). Furthermore, three proteins were involved in biosynthetic pathways (WzyE, FabA and HemD), three in stress response (CydX, IraM and SafA) and two in toxin–antitoxin systems (HipB and HigA) (Figure 2). Finally, five proteins with unknown functions were also identified (YrfF, YdhL, YcaR, YqeL and YdfO).
Figure 2.
Function and SCL of the identified protein targets. The identified targets are drawn in pink, with surrounding proteins in gray. The proteins drawn are part of various cellular processes, including cell cycle (ZipA, FtsB, FtsL, FtsQ, MukB, MukE and MukF), cell shape (MreD), transport (CydX), outer membrane processes (BamD, SecE, LolA, LolB, LptA, LptD and LptE) and DNA replication (DnaT, PriB and HolD). Other inner membrane proteins are WzyE, YciS, YrfF and SafA, and cytoplasmic proteins are FabA, IraM, TusE, YgfZ and YcaR. Proteins without a determined SCL are not shown: HemD, TrpL, HigA, HipB, PheM, YdfO, YdhL and YqeL.
2.6. Target Localization
An essential requirement for developing an efficient antimicrobial drug is target accessibility. This is especially important in Gram-negative bacteria, where the double membrane structure acts as a permeability barrier, efficiently blocking many compounds from accessing intracellular targets. SCL was therefore considered to evaluate protein’s druggability. The SCL for 25 of the 36 selected proteins could be determined using Swiss-Prot, the manually annotated section of UniProtKB (Figure 2, Table 1). For eleven proteins (DnaT, HemD, HigA, HipB, HolD, PheM, PriB, TrpL, YdfO, YdhL and YqeL), no information about SCL was available. Based on this criterion, the four OM-associated proteins (LptD, LptE, LolB and BamD) are promising potential targets, especially LptD, which contains extracellular domains.
2.7. Existence of Known Inhibitors
Next, a literature search was conducted to identify previously reported inhibitors of the selected E. coli-specific targets. As expected, none of the targets presented in Table 1 are inhibited by commercially available antibiotics. Through analysis of scientific literature, we were able to identify inhibitors targeting a few of the listed targets but, to our knowledge, none have gone beyond laboratory studies: the ZipA/FtsZ interaction has been reported to be inhibited by certain antimicrobial compounds [27,28]; the insect peptide Thanatin blocks LptA [23]; compound IMB-881 blocks the interaction between LptA and LptC [24]; JB-95 inhibits β-barrel proteins including LptD [25]; MAC13243 inhibits LolA [22]; BamD is inhibited by an inhibitory peptide [19] while the compound IMB-H4 has been shown to block BamA–BamD interaction [20]; MukB is inhibited by the small molecules Michellamine B and NSC260594 [26]; and FabA by 3-Decynoyl-NAC [21]. Thanatin has been shown to possess antimicrobial activity against several Gram-negative bacteria beyond E. coli, including K. pneumoniae, Salmonella typhimurium and Enterobacter cloacae [23]. IMB-H4 was also able to inhibit growth in K. pneumoniae, P. aeruginosa and A. baumannii [20]. NSC176319 was found to be active against Staphylococcus aureus, permeabilized P. aeruginosa and A. baumannii [26]. JB-95 was reported to have antimicrobial activity against A. baumannii, P. aeruginosa and S. aureus [25], and MAC13243 has been shown to be active against P. aeruginosa [22]. With the information provided in this study, some of these inhibitors may represent starting scaffolds for development into pathogen-specific antibacterials. In addition, they might represent useful tools in validating future target-based assays.
2.8. Target Structure
Structure-guided drug design is a powerful in silico approach that can rapidly screen millions of compounds for their ability to dock into a desired target, and identified hits can subsequently be tested in vitro. Thus, 3D structures at a high enough resolution represent an advantage for the targets identified in this study.
Information retrieved from the Protein Data Bank (PDB) [30] showed that 3D structure at a resolution of <3 Å was found for 18 proteins, >3 Å for 3 proteins and no structure could be found for 13 proteins, while both YrfF and YdfO were associated with a structure but no resolution information was reported in the databases (Table 1).
3. Discussion
The originality of the present study lies in the identification of E. coli-selective cellular targets that may lead to the discovery of innovative antimicrobial drugs with limited effect on the healthy gut microbiota. Similar in silico studies have previously been conducted for verotoxigenic E. coli O157:H7, K. pneumoniae, Yersinia pseudotuberculosis and Enterobacteriaceae [31,32,33,34,35]. However, these studies were not designed to identify targets with low identity to the corresponding proteins in beneficial taxa residing in the intestinal tract, and some suffered from limitations related to the lack of a well-established essential genome for the target pathogen.
We identified 36 potential drug targets selective for E. coli based on protein sequence identity. A large proportion of the identified proteins are functionally related amongst themselves. Within the proteins involved in OM biogenesis, BamD is directly associated with the OM, and is part of the β-barrel assembly machinery (BAM); LptA, LptD and LptE are all part of the lipopolysaccharide transport (LPT) machinery; LolA and LolB are located in the periplasmic space and on the periplasmic side of the OM respectively, both belonging to the lipoprotein transport machinery responsible for delivering OM lipoproteins to the OM assembly machineries (LOL, BAM and LPT) [36]; SecE is part of the SecYEG protein translocation machinery responsible for transporting proteins into the periplasm [37]; and the inner membrane protein YciS (also known as LapA, lipopolysaccharide assembly protein A) is part of the machinery responsible for envelope stress response and regulation of LPS production [38].
Two functional groups responsible for DNA replication (HolD, PriB, DnaT and YgfZ) and cell division (MukB, MukE, MukF, FtsB, FtsL, FtsQ and ZipA) were identified. DNA replication is a tightly controlled mechanism where the DNA Polymerase III holoenzyme is the major replication complex in E. coli, and wherein HolD (ψ subunit) makes up parts of the clamp-loading complex [39]. DNA damage can cause this machinery to stall and disassemble on the chromosome, leading to replication failure. To restart replication, the cell must make use of the replication restart primosome, where PriB and DnaT primase is found [40]. YgfZ has been shown to be part of the system regulating chromosomal replication [41]. The MukBEF complex is found only in a subset of γ-proteobacteria and is involved in cell division, making up the sole E. coli condensin for chromosome replication, segregation and organization [42]. Further downstream in this process, the transmembrane complex FtsBL is found [43], which together with FtsQ [44] and ZipA, is involved in cell division [45]. In two related processes, MreD is involved in determining cell shape [46] and TusE in translation [47].
Three selected targets, CydX, IraM and SafA, have functions related to stress response. CydX is part of the CydAB cytochrome bd oxidase complex involved in aerobic respiration and maintaining the charge across the membrane used for ATP synthesis [48]. IraM is a regulator of σS, the stationary phase sigma factor responsible for controlling expression of a plethora of genes involved in stress response [49]. SafA is a protein that connects the signal transduction between the two-component systems EvgS/EvgA and PhoQ/PhoP in response to acid stress conditions [50].
Four selected targets are involved in biosynthetic pathways. WzyE has been implicated in the assembly of the enterobacterial common antigen [51]; FabA is a protein involved in fatty acid biosynthesis [52]; TrpL is involved in controlling tryptophan biosynthesis [53]; and HemD is a uroporphyrinogen III synthase [54]. The remaining targets include two anti-toxins of the Type II toxin–antitoxin system, HipB and HigA, which counteract the effect of their cognate toxins [55]. One protein involved in transcriptional regulation, PheM, which is responsible for attenuation of phenylalanyl-tRNA synthetase was identified [56]. Finally, no information regarding biological function could be found for the remaining five proteins (YrfF, YcaR, YdhL, YqeL or YdfO), indicating that there is more to discover regarding E. coli biology.
When searching for homologues in the seven representative taxa of the healthy gut microbiota, only TrpL and PheM lacked hits in all these groups. However, no information on SCL and 3D structure is available for either of these two proteins. trpL is part of the essential tryptophan biosynthesis pathway, the costliest of the amino acid synthesis pathways. All organisms capable of this biosynthesis employ structurally similar proteins, but the organization within the trp operon and its regulatory mechanisms vary widely between different organisms. trpL functions as an operon leader peptide, which under low levels of uncharged tRNATrp, causes the ribosome to stall during translation of this operon. This leads to the formation of an antiterminator structure, allowing translation to continue [57]. Similarly, pheM acts as an operon leader peptide in the phenylalanine biosynthetic pathway and regulates transcription of the pheMS operon [56]. Although the mechanisms of control may differ between bacteria, targeting either of these gene products would be challenging as they exert their control on a transcriptional level. Interestingly, though these proteins are not thought to have any functions in trans, it has recently been shown that in the α-proteobacterial plant symbiont Sinorhizobium meliloti, TrpL can, upon antibiotic exposure, utilize antimicrobial compounds for post-transcriptional regulation of resistance operons, a trait that was further shown to be conserved in other α-proteobacteria [58]. If a similar role can be established for TrpL in E. coli, this may be an interesting target for development of helper drugs.
The potential to target an infecting pathogen without affecting the beneficial microbiota is clinically attractive due to increasing concerns regarding the impact of antimicrobial therapy on dysbiosis [59]. The clinical value of such antibiotics would be even higher if their spectrum covered other pathogenic bacterial species. Thus, the sequence conservation and essentiality of the 36 target proteins selective for E. coli were analyzed for sequence similarity and essentiality in K. pneumoniae, a close relative to E. coli, and another major contributor to multidrug-resistant infections worldwide [60]. Although 35 of the 36 proteins were highly conserved in both species, only 18 were found to be essential in this pathogen. This could be due to disparity of information, since the E. coli essential genome is well characterized, whereas the essentiality status of genes in K. pneumoniae is defined by a single study [29], which may be affected by different methods and conditions to those used to establish the E. coli ‘essentialome’.
Due to the double-membrane structure in Gram-negative bacteria, the accessibility of a potential drug target is essential. Proteins located in the OM are therefore considered to be optimal targets [61]. Here, we identified LptD, LptE, LolB and BamD as OM-associated proteins. While LolB and BamD are associated with two separate OM biogenesis pathways, LptD and LptE, together with LptA, all belong to the Lpt pathway responsible for LPS transport. This pathway is integral for cell viability, as Gram-negative bacteria are dependent on it for LPS transport, and could potentially be efficiently targeted without requiring access to the intracellular environment. The Lpt pathway consists of seven essential proteins, of which three have been indicated through this study as appropriate potential drug targets, suggesting that this is a druggable pathway in E. coli. Furthermore, LptD also has extracellular domains, indicating that it may be possible to find an inhibitor that interferes with this protein without the need to cross the OM. All three Lpt proteins were found to be associated with hits in the selected gut microbiotal taxa. Based on bitscore, the highest-ranking hit for LptA was found in Lachnospiraceae (bitscore = 38, % alignment = 37% and % id = 35%), whereas for LptD and LptE both were found in Bacteroides (bitscore = 172, % alignment = 11%, % id = 96%, and bitscore = 35, % alignment = 35% and % id = 34%, respectively).
Notably, the four proteins listed above have excellent ‘druggability’ potential, since in addition to their optimal SCL, they are also essential in K. pneumoniae and have known 3D structures. Furthermore, all four proteins displayed low levels of sequence identity to homologous proteins in the selected gut taxa used in this study; in particular, LolB and LptE, which both fell below the cut-offs. Both LptD and BamD were recovered by manual inspection of the proteins excluded. However, both proteins were excluded due to their bitscore, and were only associated with two and four hits, respectively. The selectivity of BamD and LptD can be further evaluated by future in vitro studies using known inhibitors that specifically interfere with these proteins [19,20,25]. Known inhibitors are also available for ZipA [27,28], LptA [23,24], LolA (MAC13243) [22], BamD (peptide and IMB-H4) [19,20], MukB (Michellamine B and NSC176319) [26] and FabA (3-Decynoyl-NAC) [21]. The fact that six inhibitors targeting OM-related processes were found further strengthens the hypothesis that these functions are viable for development of novel antimicrobials.
All in silico studies suffer from drawbacks related to arbitrary cut-off criteria that may lack biological relevance. Too stringent cut-offs potentially exclude valuable drug targets, while too loose criteria may result in an unmanageable list of targets. In the present study, we chose to integrate stringent cut-offs with manual revision in order to minimize exclusion of relevant proteins. Another limitation of this study is that essentiality may differ between in vitro and in vivo conditions. The essential genome established by Goodall et al. [10] was characterized in rich media conditions, and may therefore not include genes that are essential for metabolism inside the host [10,62]. Certain bacterial biosynthetic pathways may be downregulated as the pathogen instead relies on the host to supply nutrients such as amino acids, vitamins and nucleobases [62]. However, targeting biosynthetic pathways involved in maintenance of the cell is likely to represent a target relevant in vitro as well as in vivo [63].
In silico studies such as this are a first but essential step towards the discovery of novel pathogen-targeted antimicrobials. The results of our study provide a starting point towards the identification and development of novel specific antimicrobials targeting E. coli. Future wet-lab studies are required to validate the presumptive selective targets identified by the study. High-throughput screens can be applied to find inhibitors interfering with the specific protein targets, e.g. through ‘knock-down’ strains with reduced expression of the target protein. The antimicrobial activity of the identified inhibitors could subsequently be evaluated on a comprehensive strain collection representative of the healthy gut microbiota, or directly on faecal samples using a metagenomics approach in order to assess their selective toxicity towards E. coli and other pathogenic Enterobacteriaceae.
4. Materials and Methods
4.1. Protein Essentiality in E. coli
The GenBank record for E. coli BW25113 (GenBank: CP009273.1) was downloaded, and the protein sequences from the 358 genes found to be essential by Goodall et al. [10] were extracted. Five genes were removed due to being labeled as pseudogenes (ttcC, yedN, ygeF and ygeN) or putative protein (yddL).
4.2. Protein Homology in E. coli ST131, Humans and Gut Beneficial Taxa
To establish presence of proteins in E. coli O25b:H4-ST131 (NCBI:txid941322), NCBI + BLASTp was used to BLAST the 353 protein sequences against this organism using the organism name as Entrez query against the refseq protein BLAST database. Percent alignment was calculated by dividing the length of the hit by the length of the query protein, extracted from the NCBI record. A dual cut-off was used, where hits with percent id ≥ 70% and percent alignment ≥ 75% or bitscore ≥ 50 were excluded. These cut-offs were selected to be equal to, or more stringent than, those used in previous in silico studies [31,32,35,62]. Any hits below the cut-offs were removed, and the remaining were taken on to the next step.
To find human analogues, the NCBI + command line remote BLAST tool was used to BLAST the remaining protein sequences using first the Entrez queries ‘Homo sapiens [Organism]’ against the ‘refseq_proteins’ database, and the output was sorted using the same cut-offs as described above.
Remote BLASTp command line applications were used to assess protein homology in specific gut taxa using the Entrez queries ‘Faecalibacterium [Organism]’, ‘Bacteroides [Organism], ‘Ruminococcus [Organism]’, ‘Prevotella [Organism]’, ‘Lactobacillus [Organism]’, ‘Lachnospiraceae [Organism]’ and ‘Bifidobacterium [Organism]’ using the ‘refseq_proteins’ database. These filters provided 214,344 different entries as listed in Tables S3–S9. The results were downloaded and analyzed using the same methodology and cut-offs as described above.
All hits sorted as above the cut-off in the similarity search against the simplified microbiota were collected and the number of hits for each protein evaluated. A table with the number of hits, together with the information for the highest scoring hit for each protein, was generated. The proteins with fewer than ten hits were manually inspected, and proteins with high-scoring similarity were removed.
4.3. Protein Conservation and Essentiality in K. pneumoniae
To assess protein conservation in K. pneumoniae, command line applications for BLASTp were used to BLAST remotely against ‘Klebsiella pneumoniae [Organism]’ using the ‘refseq_proteins’ database (Table S10). The supplementary dataset generated by Ramage et al. [29] was downloaded and used to search for the potential target genes using gene names.
4.4. Identification of Inhibitors by Screening Scientific Literature
Scientific literature was screened to identify inhibitors by searching PubMed and Google Scholar using the phrases ‘Escherichia coli inhibitor’ or ‘Escherichia coli antimicrobial’ in combination with the protein of interest. Abstracts and manuscripts were screened to find inhibitors targeting the protein of interest in E. coli.
4.5. Protein SCL and 3D Structure
The SCL for each protein was manually checked by querying the UniProt/SwissProt database, and retrieving the information found under ‘Subcellular Localisation’.
Information about 3D structure for proteins in E. coli K-12 was manually retrieved through the UniProt/SwissProt entries for each protein individually. The PDB accession number, molecules in complex and resolution were recorded.
Acknowledgments
We would like to thank Bastian V.H. Hornung and Defne Surjoun for their kind help with development of the Python scripts and pipelines. FSF is a recipient of a PhD fellowship from the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant agreement no. 765147.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics10060632/s1, Table S1: Escherichia coli ST131 BLAST results, Table S2: Human BLAST results, Table S3: Faecalibacterium BLAST results, Table S4: Prevotella BLAST results, Table S5: Ruminococcus BLAST results, Table S6: Bacteroides BLAST results, Table S7: Lachnospiraceae BLAST results, Table S8: Lactobacillus BLAST results, Table S9: Bifidobacterium BLAST results, Table S10: Klebsiella pneumoniae BLAST results.
Author Contributions
Conceptualization, F.S.F., S.D. and L.G.; methodology, F.S.F., B.J., S.D. and L.G.; software, F.S.F.; formal analysis, F.S.F., S.D. and L.G.; investigation, F.S.F.; data curation, F.S.F.; writing—original draft preparation, F.S.F.; writing—review and editing, F.S.F., B.J., S.D. and L.G.; visualization, F.S.F., B.J., S.D. and L.G.; supervision, B.J., S.D. and L.G.; project administration, F.S.F. and L.G.; funding acquisition, L.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant, grant number 765147. The APC was funded by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant, grant number 765147.
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lewis K. Platforms for antibiotic discovery. Nat. Rev. Drug Discov. 2013;12:371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 2.Provenzani A., Hospodar A.R., Meyer A.L., Leonardi Vinci D., Hwang E.Y., Butrus C.M., Polidori P. Multidrug-resistant Gram-negative organisms: A review of recently approved antibiotics and novel pipeline agents. Int. J. Clin. Pharm. 2020;42:1016–1025. doi: 10.1007/s11096-020-01089-y. [DOI] [PubMed] [Google Scholar]
- 3.Martin-Loeches I., Dale G.E., Torres A. Murepavadin: A new antibiotic class in the pipeline. Expert Rev. Anti. Infect. Ther. 2018;16:259–268. doi: 10.1080/14787210.2018.1441024. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization World Health Organization Releases Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. [(accessed on 24 May 2021)]; Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf.
- 5.Köhler C.D., Dobrindt U. What defines extraintestinal pathogenic Escherichia coli? Int. J. Med. Microbiol. 2011;301:642–647. doi: 10.1016/j.ijmm.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Pitout J.D.D., DeVinney R. Escherichia coli ST131: A multidrug-resistant clone primed for global domination. F1000Research. 2017;6:1–7. doi: 10.12688/f1000research.10609.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buffie C.G., Pamer E.G. Microbiota-mediated colonization resistance against intestinal pathogens. Nat. Rev. Immunol. 2013;13:790–801. doi: 10.1038/nri3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez C., Taminiau B., Van Broeck J., Delmée M., Daube G. Clostridium difficile infection and intestinal microbiota interactions. Microb. Pathog. 2015;89:201–209. doi: 10.1016/j.micpath.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 9.Hills R.D., Pontefract B.A., Mishcon H.R., Black C.A., Sutton S.C., Theberge C.R. Gut microbiome: Profound implications for diet and disease. Nutrients. 2019;11:1613. doi: 10.3390/nu11071613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goodall E.C.A., Robinson A., Johnston I.G., Jabbari S., Turner K.A., Cunningham A.F., Lund P.A., Cole J.A., Henderson I.R., Kline K.A. The Essential Genome of Escherichia coli K-12. MBio. 2018;9:1–18. doi: 10.1128/mBio.02096-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K.A., Tomita M., Wanner B.L., Mori H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006;2:2006.0008. doi: 10.1038/msb4100050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamanai-Shacoori Z., Smida I., Bousarghin L., Loreal O., Meuric V., Fong S.B., Bonnaure-Mallet M., Jolivet-Gougeon A. Roseburia spp.: A marker of health? Future Microbiol. 2017;12:157–170. doi: 10.2217/fmb-2016-0130. [DOI] [PubMed] [Google Scholar]
- 13.Naaber P., Smidt I., Štšepetova J., Brilene T., Annuk H., Mikelsaar M. Inhibition of Clostridium difficile strains by intestinal Lactobacillus species. J. Med. Microbiol. 2004;53:551–554. doi: 10.1099/jmm.0.45595-0. [DOI] [PubMed] [Google Scholar]
- 14.Ferreira-Halder C.V., de Faria A.V.S., Andrade S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017;31:643–648. doi: 10.1016/j.bpg.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 15.Precup G., Vodnar D.-C. Gut Prevotella as a possible biomarker of diet and its eubiotic versus dysbiotic roles: A comprehensive literature review. Br. J. Nutr. 2019;122:131–140. doi: 10.1017/S0007114519000680. [DOI] [PubMed] [Google Scholar]
- 16.Hsiao A., Ahmed A.M.S., Subramanian S., Griffin N.W., Drewry L.L., Petri W.A., Haque R., Ahmed T., Gordon J.I. Members of the human gut microbiota involved in recovery from Vibrio cholerae infection. Nature. 2014;515:423–426. doi: 10.1038/nature13738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vacca M., Celano G., Calabrese F.M., Portincasa P., Gobbetti M., De Angelis M. The controversial role of human gut Lachnospiraceae. Microorganisms. 2020;8:573. doi: 10.3390/microorganisms8040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grimm V., Westermann C., Riedel C.U. Bifidobacteria-Host Interactions—An Update on Colonisation Factors. BioMed Res. Int. 2014;2014:960826. doi: 10.1155/2014/960826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagan C.L., Wzorek J.S., Kahne D. Inhibition of the β-barrel assembly machine by a peptide that binds BamD. Proc. Natl. Acad. Sci. USA. 2015;112:2011–2016. doi: 10.1073/pnas.1415955112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Y., Zhu X., Zhang J., Lin Y., You X., Chen M., Wang Y., Zhu N., Si S. Identification of a Compound That Inhibits the Growth of Gram-Negative Bacteria by Blocking BamA—BamD Interaction. Front. Microbiol. 2020;11:1–13. doi: 10.3389/fmicb.2020.01252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heath R., White S., Rock C. Inhibitors of fatty acid synthesis as antimicrobial chemotherapeutics. Appl. Microbiol. Biotechnol. 2002;58:695–703. doi: 10.1007/s00253-001-0918-z. [DOI] [PubMed] [Google Scholar]
- 22.Pathania R., Zlitni S., Barker C., Das R., Gerritsma D.A., Lebert J., Awuah E., Melacini G., Capretta F.A., Brown E.D. Chemical genomics in Escherichia coli identifies an inhibitor of bacterial lipoprotein targeting. Nat. Chem. Biol. 2009;5:849–856. doi: 10.1038/nchembio.221. [DOI] [PubMed] [Google Scholar]
- 23.Vetterli S.U., Zerbe K., Müller M., Urfer M., Mondal M., Wang S.Y., Moehle K., Zerbe O., Vitale A., Pessi G., et al. Thanatin targets the intermembrane protein complex required for lipopolysaccharide transport in Escherichia coli. Sci. Adv. 2018;4:1–9. doi: 10.1126/sciadv.aau2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Li Y., Wang W., Zhang J., Lin Y., Hong B., You X., Song D., Wang Y., Jiang J., et al. Identification of an anti-Gram-negative bacteria agent disrupting the interaction between lipopolysaccharide transporters LptA and LptC. Int. J. Antimicrob. Agents. 2019;53:442–448. doi: 10.1016/j.ijantimicag.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Urfer M., Bogdanovic J., Monte F.L., Moehle K., Zerbe K., Omasits U., Ahrens C.H., Pessi G., Eberl L., Robinson J.A. A peptidomimetic antibiotic targets outer membrane proteins and disrupts selectively the outer membrane in Escherichia coli. J. Biol. Chem. 2016;291:1921–1932. doi: 10.1074/jbc.M115.691725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao H., Petrushenko Z.M., Walker J.K., Baudry J., Zgurskaya H.I., Rybenkov V.V. Small Molecule Condensin Inhibitors. ACS Infect. Dis. 2018;4:1737–1745. doi: 10.1021/acsinfecdis.8b00222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsao D.H.H., Sutherland A.G., Jennings L.D., Li Y., Rush T.S., Alvarez J.C., Ding W., Dushin E.G., Dushin R.G., Haney S.A., et al. Discovery of novel inhibitors of the ZipA/FtsZ complex by NMR fragment screening coupled with structure-based design. Bioorganic Med. Chem. 2006;14:7953–7961. doi: 10.1016/j.bmc.2006.07.050. [DOI] [PubMed] [Google Scholar]
- 28.Jennings L.D., Foreman K.W., Rush T.S., Tsao D.H.H., Mosyak L., Li Y., Sukhdeo M.N., Ding W., Dushin E.G., Kenny C.H., et al. Design and synthesis of indolo [2,3-a]quinolizin-7-one inhibitors of the ZipA-FtsZ interaction. Bioorg. Med. Chem. Lett. 2004;14:1427–1431. doi: 10.1016/j.bmcl.2004.01.028. [DOI] [PubMed] [Google Scholar]
- 29.Ramage B., Erolin R., Held K., Gasper J., Weiss E., Brittnacher M., Gallagher L., Manoil C. Comprehensive Arrayed Transposon Mutant Library of Klebsiella pneumoniae Outbreak Strain KPNIH1. J. Bacteriol. 2017;199:1–9. doi: 10.1128/JB.00352-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berman H.M., Battistuz T., Bhat T.N., Bluhm W.F., Bourne P.E., Burkhardt K., Feng Z., Gilliland G.L., Iype L., Jain S., et al. The protein data bank. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002;58:899–907. doi: 10.1107/S0907444902003451. [DOI] [PubMed] [Google Scholar]
- 31.Ramos P.I.P., Fernández Do Porto D., Lanzarotti E., Sosa E.J., Burguener G., Pardo A.M., Klein C.C., Sagot M., de Vasconcelos A.T.R., Gales A.C., et al. An integrative, multi-omics approach towards the prioritization of Klebsiella pneumoniae drug targets. Sci. Rep. 2018;8:10755. doi: 10.1038/s41598-018-28916-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hadizadeh M., Tabatabaiepour S.N., Tabatabaiepour S.Z., Hosseini Nave H., Mohammadi M., Sohrabi S.M. Genome-Wide Identification of Potential Drug Target in Enterobacteriaceae Family: A Homology-Based Method. Microb. Drug Resist. 2018;24:8–17. doi: 10.1089/mdr.2016.0259. [DOI] [PubMed] [Google Scholar]
- 33.Georrge J.J., Umrania V. In silico identification of putative drug targets in Klebsiella pneumonia MGH78578. Indian J. Biotechnol. 2011;10:432–439. [Google Scholar]
- 34.Duffield M., Cooper I., McAlister E., Bayliss M., Ford D., Oyston P. Predicting conserved essential genes in bacteria: In silico identification of putative drug targets. Mol. Biosyst. 2010;6:2482. doi: 10.1039/c0mb00001a. [DOI] [PubMed] [Google Scholar]
- 35.Mondal S.I., Ferdous S., Akter A., Mahmud Z., Karim N., Islam M.M., Jewel N.A., Afrin T. Identification of potential drug targets by subtractive genome analysis of Escherichia coli O157:H7: An in silico approach. Adv. Appl. Bioinforma. Chem. 2015;8:49. doi: 10.2147/AABC.S88522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grabowicz M. Lipoprotein Transport: Greasing the Machines of Outer Membrane Biogenesis. BioEssays. 2018;40:1700187. doi: 10.1002/bies.201700187. [DOI] [PubMed] [Google Scholar]
- 37.Lycklama A Nijeholt J.A., De Keyzer J., Prabudiansyah I., Driessen A.J.M. Characterization of the supporting role of SecE in protein translocation. FEBS Lett. 2013;587:3083–3088. doi: 10.1016/j.febslet.2013.07.046. [DOI] [PubMed] [Google Scholar]
- 38.Klein G., Kobylak N., Lindner B., Stupak A., Raina S. Assembly of Lipopolysaccharide in Escherichia coli Requires the Essential LapB Heat Shock Protein. J. Biol. Chem. 2014;289:14829–14853. doi: 10.1074/jbc.M113.539494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu Z.Q., Dixon N.E. Bacterial replisomes. Curr. Opin. Struct. Biol. 2018;53:159–168. doi: 10.1016/j.sbi.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y.H., Huang C.Y. Structural insight into the DNA-binding mode of the primosomal proteins PriA, PriB, and AnaT. BioMed Res. Int. 2014;2014:195162. doi: 10.1155/2014/195162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ote T., Hashimoto M., Ikeuchi Y., Su’etsugu M., Suzuki T., Katayama T., Kato J.I. Involvement of the Escherichia coli folate-binding protein YgfZ in RNA modification and regulation of chromosomal replication initiation. Mol. Microbiol. 2006;59:265–275. doi: 10.1111/j.1365-2958.2005.04932.x. [DOI] [PubMed] [Google Scholar]
- 42.Rybenkov V.V., Herrera V., Petrushenko Z.M., Zhao H. MukBEF, a Chromosomal Organizer. J. Mol. Microbiol. Biotechnol. 2014;24:371–383. doi: 10.1159/000369099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Condon S.G.F., Mahbuba D.A., Armstrong C.R., Diaz-Vazquez G., Craven S.J., LaPointe L.M., Khadria A.S., Chadda R., Crooks J.A., Rangarajan N., et al. The FtsLB subcomplex of the bacterial divisome is a tetramer with an uninterrupted FtsL helix linking the transmembrane and periplasmic regions. J. Biol. Chem. 2018;293:1623–1641. doi: 10.1074/jbc.RA117.000426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buddelmeijer N., Beckwith J. A complex of the Escherichia coli cell division proteins FtsL, FtsB and FtsQ forms independently of its localization to the septal region. Mol. Microbiol. 2004;52:1315–1327. doi: 10.1111/j.1365-2958.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 45.Hale C.A., De Boer P.A.J. ZipA is required for recruitment of FtsK, FtsQ, FtsL, and FtsN to the septal ring in Escherichia coli. J. Bacteriol. 2002;184:2552–2556. doi: 10.1128/JB.184.9.2552-2556.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kruse T., Bork-Jensen J., Gerdes K. The morphogenetic MreBCD proteins of Escherichia coli form an essential membrane-bound complex. Mol. Microbiol. 2005;55:78–89. doi: 10.1111/j.1365-2958.2004.04367.x. [DOI] [PubMed] [Google Scholar]
- 47.Ikeuchi Y., Shigi N., Kato J.I., Nishimura A., Suzuki T. Mechanistic insights into sulfur relay by multiple sulfur mediators involved in thiouridine biosynthesis at tRNA wobble positions. Mol. Cell. 2006;21:97–108. doi: 10.1016/j.molcel.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Vanorsdel C.E., Bhatt S., Allen R.J., Brenner E.P., Hobson J.J., Jamil A., Haynes B.M., Genson A.M., Hemma M.R. The Escherichia coli CydX protein is a member of the CydAB cytochrome bd oxidase complex and is required for cytochrome bd oxidase activity. J. Bacteriol. 2013;195:3640–3650. doi: 10.1128/JB.00324-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bougdour A., Cunning C., Baptiste P.J., Elliott T., Gottesman S. Multiple pathways for regulation of σS (RpoS) stability in Escherichia coli via the action of multiple anti-adaptors. Mol. Microbiol. 2008;68:298–313. doi: 10.1111/j.1365-2958.2008.06146.x. [DOI] [PubMed] [Google Scholar]
- 50.Ishii E., Eguchi Y., Utsumi R. Mechanism of activation of PhoQ/PhoP two-component signal transduction by SafA, an auxiliary protein of PhoQ histidine kinase in Escherichia coli. Biosci. Biotechnol. Biochem. 2013;77:814–819. doi: 10.1271/bbb.120970. [DOI] [PubMed] [Google Scholar]
- 51.Kajimura J., Rahman A., Rick P.D. Assembly of cyclic enterobacterial common antigen in Escherichia coli K-12. J. Bacteriol. 2005;187:6917–6927. doi: 10.1128/JB.187.20.6917-6927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Heath R.J., Rock C.O. Roles of the FabA and FabZ β-hydroxyacyl-acyl carrier protein dehydratases in Escherichia coli fatty acid biosynthesis. J. Biol. Chem. 1996;271:27795–27801. doi: 10.1074/jbc.271.44.27795. [DOI] [PubMed] [Google Scholar]
- 53.Zurawski G., Elseviers D., Stauffer G.V., Yanofsky C. Translational control of transcription termination at the attenuator of the Escherichia coli tryptophan operon. Proc. Natl. Acad. Sci. USA. 1978;75:5988–5992. doi: 10.1073/pnas.75.12.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jordan P.M., Mgbeje B.I., Thomas S.D., Alwan A.F. Nucleotide sequence for the hemD gene of Escherichia coli encoding uroporphyrinogen III synthase and initial evidence for a hem operon. Biochem. J. 1988;249:613–616. doi: 10.1042/bj2490613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fraikin N., Goormaghtigh F., Van Melderen L. Type II Toxin-Antitoxin Systems: Evolution and Revolutions. J. Bacteriol. 2020;202:1–14. doi: 10.1128/JB.00763-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Springer M., Mayaux J.F., Fayat G., Plumbridge J.A., Graffe M., Blanquet S., Grunberg-Manago M. Attenuation control of the Escherichia coli phenylalanyl-tRNA synthetase operon. J. Mol. Biol. 1985;181:467–478. doi: 10.1016/0022-2836(85)90420-6. [DOI] [PubMed] [Google Scholar]
- 57.Merino E., Jensen R.A., Yanofsky C. Evolution of bacterial trp operons and their regulation. Curr. Opin. Microbiol. 2008;11:78–86. doi: 10.1016/j.mib.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Melior H., Maaß S., Li S., Förstner K.U., Azarderakhsh S., Varadarajan A.R., Stötzel M., Elhossary M., Barth-Weber S., Ahrens C.H., et al. The leader peptide peTrpL forms antibiotic-containing ribonucleoprotein complexes for posttranscriptional regulation of multiresistance genes. MBio. 2020;11:1–22. doi: 10.1128/mBio.01027-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Langdon A., Crook N., Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:1–16. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wyres K.L., Lam M.M.C., Holt K.E. Population genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020;18:344–359. doi: 10.1038/s41579-019-0315-1. [DOI] [PubMed] [Google Scholar]
- 61.Silver L.L. Appropriate Targets for Antibacterial Drugs. Cold Spring Harb. Perspect. Med. 2016;6:a030239. doi: 10.1101/cshperspect.a030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mobegi F.M., van Hijum S.A.F.T., Burghout P., Bootsma H.J., de Vries S.P.W., Jongh C.E., Simonetti E., Langereis J.D., Hermans P.W.M., de Jonge M.I., et al. From microbial gene essentiality to novel antimicrobial drug targets. BMC Genom. 2014;15:958. doi: 10.1186/1471-2164-15-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bakheet T.M., Doig A.J. Properties and identification of antibiotic drug targets. BMC Bioinform. 2010;11:195. doi: 10.1186/1471-2105-11-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the article and Supplementary Materials.