Abstract
Background
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread around the world and reports of children during early epidemic period showed features of family clusters. The aim of this study is to assess clinical profiles of COVID-19 in family clusters with children.
Methods
We performed a systematic literature review of English database (PubMed, Web of Science) and Chinese database (“www.cnki.net”, “www.cqvip.com” and “www.Wanfangdata.com.cn”) to identify papers on family clusters of COVID-19 with children and their family members.
Results
Eighteen studies involving 34 children and 98 adults from 28 families were included. Fever, cough and ground-grass opacity change of chest computed tomography (CT) were the dominant features, whereas proportion of asymptomatic infections for children was higher than adults with statistical significance (32.4% and 13.3%, respectively, P < 0.05). Median time of longer incubation period (10 days) and shorter duration of pharyngeal swab nucleic acid test positive period (11 days) were seen in children than adults (7 and 17 days, respectively) with statistical significance (P < 0.05). There were statistically significant differences in lymphopenia, increased C-reactive protein and abnormal chest CT between children and adult patients (P < 0.05). Twenty-seven families reported adults as first case of COVID-19 in family clusters.
Conclusions
The same virus strain can cause milder disease in children compared with their caregivers. Children of COVID-19 were infected by adults in family during the early epidemic period. Asymptomatic patients can transmit the virus.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12519-021-00434-z.
Keywords: Children, Coronavirus disease 2019, Family clusters, Severe acute respiratory syndrome coronavirus 2
Introduction
Many respiratory infectious diseases show feature of high incidence in winter, and some might break out within families. Children, as a special group due to immature immune system, are more vulnerable to many respiratory infectious diseases compared with their adult family members. For example, during the pandemic of influenza, especially 2009 swine influenza, children are more easily infected, and school children can drive dissemination of influenza virus in the household and community [1–5].
We all pay close attention to coronavirus disease 2019 (COVID-19) since December 2019, outbreak of a novel respiratory infectious disease, which has cause pandemic and spread around the world [6–8]. The pathogen identified has been named as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [8], and World Health Organization has declared COVID-19 a public health emergency of international concern [9].
Although people of all ages are susceptible to SARS-CoV-2 infection [8, 10], compared with pandemic of influenza, there are much more reported cases of adult patients during COVID-19 pandemic [6–8], whereas pediatric patients accounts for a small proportion. Data from Chinese Center for Disease Control and Prevention showed that among 72,314 reported cases, about 2% of 44,672 confirmed cases of COVD-19 were children aged 0–19 years, and children under age of 10 years accounts for 0.9% [11]. Data published on 18 March 2020 from Italy reported children accounted for 1.2% of 22,512 Italian COVID-19 cases [12]. As for the United States, 1.7% of 150,000 laboratory confirmed COVID-19 cases were children under 18 years [13], with only 5.7–20% admitted in hospital and 0.58–2% of them in pediatric intensive care unit.
The SARS-CoV-2 virus can be transmitted via respiratory droplets, even aerosol [14, 15]. In the early epidemic period, nearly 90.1% of children showed feature of family cluster [16, 17], where children were believed to be infected via close contact with family members [10]. Besides, they only received SARS-CoV-2 nucleic acid tests if they showed clinical manifestations or there were COVID-19 patients in their family, which is another difference from influenza.
For above reasons, we conduct this study to systematically review the current knowledge of SARS-CoV-2 infection in children and adults in family cluster and profile characteristics of COVID-19. We aimed to find out differences between children and adults infected with the same virus strain, even to identify probable transmission mechanism within family members.
Methods
Data sources
We performed a systematic literature review of PubMed, Web of Science, “www.cnki.net”, “www.cqvip.com” and “www.Wanfangdata.com.cn” on 10th March 2021 to identify papers on COVID-19 family cluster with children and their family members. We use search terms as followings: (2019-nCOV or SARS-CoV-2 or COVID-19 or novel coronavirus) and (family cluster or family aggregation). For further relevant studies, the references of selected articles were also identified through manual search. The literature search process is presented in Fig. 1.
Fig. 1.
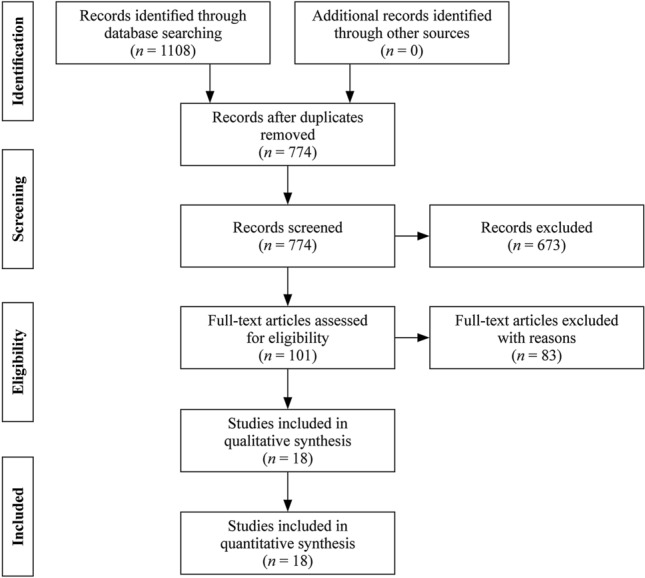
Flow diagram of the study selection process
Criteria
Inclusion criteria: we included all studies that have reported clinical manifestations, laboratory examinations, chest computed tomography (CT) and nucleic acid test (NAT) in family clusters of COVID-19 including both children (age from 29 days to 14 years) and adults. Exclusion criteria: neonate (younger than 28 days) as well as articles containing insufficient data were excluded.
Data extraction
Two independent assessors (Song WL and Zou N) performed the data extraction with agreement reached by consensus. The following information was extracted from each study for the analysis: age, sex, incubation period, duration of pharyngeal swab NAT-positive period (days), clinical manifestations, laboratory examination and chest CT manifestations.
Statistical analysis
Continuous variables were expressed as medians and interquartile ranges. Categorical variables were described as counts and percentages. Chi-square tests and Fisher exact tests were used for categorical variables as appropriate and Mann–Whitney U test was used for comparing median values of non-normally distributed variables. All analyses were conducted with the use of Statistical Product and Service Solutions (SPSS 22.0) software. A two-sided α of less than 0.05 was considered statistically significant.
Results
Summary of searching results
The search yielded 1108 hints both in English and Chinese. Removing 334 duplicates we identified 774 articles and after screening by criteria we got 18 articles finally, with 12 English articles [17–28] and six Chinese articles (Supplementary Table 1) [29–34].
General clinical information
There were 28 families reported in the 18 articles. A total of 132 patients were reported, including 34 children and 98 adults, with a mean number of one (range 1–3) child per family, and they were diagnosed as COVID-19 in January and February, 2020, which was the early epidemic period of COVID-19. The ages of children were from 2 months to 10 years (median age 5 year 3 months) with a male to female ratio of 14:20, and ages of adults were 18–83 years with a male to female ratio of 46:52. In 27 (27/28, 96.4%) families adults were first diagnosed as COVID-19 previous to children, and in only one family, the first diagnosed patient was a child [19], whereas the mother being the infectious source, showed negative NAT for a long time until diagnosed. The general information was showed in Table 1.
Table 1.
General clinical characteristics of children and adults in family clusters of coronavirus disease 2019
| Variables | Children (n = 34) | Adults (n = 98) | X2 | P |
|---|---|---|---|---|
| Sex, n (%) | 0.338 | 0.561 | ||
| Male | 14 (41.2) | 46 (46.9) | ||
| Female | 20 (46.9) | 52 (53.1) | ||
| Age, median (IQR) | 4 y 3 mon (2 mon-10 y) | 18–83 y | ||
| Incubation period (d), median (IQR) | 10 (1–30) (n = 31) | 7 (1–29) (n = 72) | -2.001 (Z) | 0.045 |
| Duration of pharyngeal swab NAT-positive period (d), median (IQR) | 11 (6–27) (n = 15) | 17 (3 to > 37) (n = 28) | -2.373 (Z) | 0.018 |
| Clinical manifestation, n (%) | ||||
| Number of asymptomatic infection | 11 (32.4) | 13 (13.3) | 6.182 | 0.013 |
| Fever | 15 (44.1) | 61 (62.2) | 3.396 | 0.065 |
| Body temperature grading | 0.033 | 0.855 | ||
| Low-grade fever | 3 (33.3) (n = 9) | 9 (45.0) (n = 20) | ||
| Moderate fever | 6 (66.7) (n = 9) | 11 (55.0) (n = 20) | ||
| Cough | 6 (17.6) | 44 (44.9) | 7.966 | 0.005 |
| Fatigue | 3 (8.8) | 24 (24.5) | 3.808 | 0.051 |
| Myalgia | 0 (0.0) | 11 (11.2) | 2.824 | 0.093 |
| Sore throat | 1 (2.9) | 10 (10.2) | 0.922 | 0.337 |
| Chest pain | 0 (0.0) | 5 (5.1) | 0.675 | 0.411 |
| Polypnea | 0 (0.0) | 3 (3.1) | 0.569 | |
| Dyspnea | 0 (0.0) | 2 (2.0) | 1.000 | |
| Chest tightness | 0 (0.0) | 4 (4.1) | 0.379 | 0.538 |
| Rhiborrhea | 1 (2.9) | 3 (3.1) | 0.000 | 1.000 |
| Sneezing | 0 (0.0) | 1 (1.0) | 1.000 | |
| Vomiting | 2 (5.9) | 0 (0.0) | 0.065 | |
| Diarrhea | 4 (11.8) | 2 (2.0) | 3.488 | 0.062 |
IQR interquartile range, NAT nucleic acid test
The top 3 symptoms of children were fever (15/34, 44.1%), cough (6/34, 17.6%) and diarrhea (4/34, 11.8%), whereas for adults were fever (61/98, 62.2%), cough (44/98, 44.9%) and fatigue (24/98, 24.5%). Besides, children showed a higher proportion of asymptomatic infections (11/34, 32.4%) than adults (13/98, 13.3%) with statistical significance (P < 0.05). In addition, adults showed various symptoms besides previous mentioned as following, 11 (11/98, 11.2%) cases with myalgia, 10 (10/98, 10.2%) cases with sore throat, 5 (5/98, 5.1%) with chest pain, 3 (3/98, 3.1%) cases with polypnea, 2 (2/98, 2.0%) cases with dyspnea, 4 (4/98, 4.1%) cases with chest tightness, 3 (3/98, 3.1%) with rhiborrhea, 2 (2/98, 2.0%) with diarrhea and 1 (1/98, 1.0%) case with sneezing, compared with children showing less symptoms including 3 (3/34, 8.8%) cases with fatigue, 4 (4/34, 11.8%) cases with diarrhea, 2 (2/34, 5.9%) cases with vomiting and 1 (1/34, 2.9%) case with rhiborrhea (Table 1). Furthermore, from limited data of body temperature records, both group showed Tmax ranging from 37.3 to 39 ℃ and no statistical difference in grade of fever between them. To be noted, children patients showed a longer incubation period (10 days) but a shorter duration of positive pharyngeal swab NAT results (11 days) than adults (7 and 17 days, respectively) with statistical significance (P < 0.05). In addition, there were four pediatric cases, whose duration of positive pharyngeal swab NAT duration ranging from 7 to 27 days, comparing with positive anal swab NAT duration lasting more than 28 days [26, 27, 29].
Laboratory examination characteristics
Children and adults of family clusters underwent tests of blood cell analysis, C-reactive protein (CRP), biochemical indexes and evaluation of organ injury severity. Leukocyte decreased in 6 (6/33, 18.2%) cases of children and 14 (14/97, 14.4%) cases of adults, and remained normal value for 27 (27/33, 81.8%) cases and 83 (83/97, 85.6%) with no statistical difference, respectively (P > 0.05). However, 1 (1/33, 3.0%) case of children showed lymphopenia while 41 (41/97, 42.3%) cases of adults did, and lymphocytes were normal for 30 (30/33, 90.9%) cases of children and 54 (54/97, 55.7%) cases of adults, respectively, with statistically significant difference (P < 0.05). There were 41 (41/65, 63.1%) cases of adults with normal CRP with a percentile of 63.1% compared with 29 (29/30, 96.7%) case in children (P < 0.05). All children showed normal prothrombin time, activated partial thromboplastin time (APTT), d-dimer, alanine aminotransferase, aspartate aminotransferase and creatine kinase (CK), which was the same for adults except nine cases of abnormal APTT, two cases of abnormal d-dimer and 10 cases of abnormal CK. Details were shown in Table 2.
Table 2.
Blood routine test characteristics of children and adults in family clusters of coronavirus disease 2019
| Laboratory examinations | Children | Adults | X2 | P | |||
|---|---|---|---|---|---|---|---|
| WBC (× 109/L), median (IQR) | 6.68 (2.23–11.7) (n = 33) | 1.75–11.40 (n = 97) | |||||
| WBC decreased, n (%) | 6 (18.2) (n = 33) | 14 (14.4) (n = 97) | 0.130 | 0.718 | |||
| WBC normal, n (%) | 27 (81.8) (n = 33) | 83 (85.6) (n = 97) | |||||
| Lymphocyte (× 109/L), median (IQR) | 4.52 (1.25–9.06) | 0.29–6.23 | |||||
| Lymphocyte decreased, n (%) | 1 (3.0) (n = 33) | 41 (42.3) (n = 97) | 17.334 | < 0.001 | |||
| Lymphocyte normal, n (%) | 30 (90.9) (n = 33) | 54 (55.7) (n = 97) | 13.373 | < 0.001 | |||
| Lymphocyte increased, n (%) | 2 (6.1) (n = 33) | 2 (2.1) (n = 97) | 0.320 | 0.572 | |||
| CRP (mg/L) | 0–12.6 | 0.5–150.0 | |||||
| CRP normal, n (%) | 29 (96.7) (n = 30) | 41 (63.1) (n = 65) | 11.944 | 0.001 | |||
| PT(s) | 10.5–13.1 | 10.0–14.4 | |||||
| PT normal, n (%) | 6 (100) (n = 6) | 20 (100) (n = 20) | |||||
| APTT(s) | 34.0–42.9 | 25.8–49.6 | |||||
| APTT normal, n (%) | 7 (100) (n = 7) | 17 (65.4) (n = 26) | 3.332 | 0.068 | |||
| d-dimer(mg/L) | 0.10–0.43 | 0.19–0.71 | |||||
| d-dimer normal, n (%) | 10 (100) (n = 10) | 16 (88.9) (n = 18) | 1.197 | 0.274 | |||
| ALT(U/L) | 9.0–23.9 | 10.8–36.0 | |||||
| ALT normal, n (%) | 17 (100) (n = 17) | 26 (100) (n = 26) | |||||
| AST(U/L) | 23.0–42.0 | 18.1–40.2 | |||||
| AST normal, n (%) | 12 (100) (n = 12) | 21 (100) (n = 21) | |||||
| CK(U/L) | 78–131 | 176–301 | |||||
| CK normal, n (%) | 10 (100) (n = 10) | 25 (71.4) (n = 35) | 2.433 | 0.119 | |||
IQR interquartile range, WBC white blood cell, CRP C-reactive protein, PT prothrombin time, APTT activated partial thromboplastin time, ALT alanine aminotransferase, AST aspartate aminotransferase, CK creatine kinase
Chest CT manifestations
Among 29 cases of children and 90 cases of adult patients who performed chest CT scan, 13 (13/29, 44.8%) cases of children and 73 (73/90, 81.1%) cases of adults showed abnormal image results, with statistical significance (P < 0.05). Furthermore, ground-grass opacity (GGO) change was the leading feature both for children and adults with a percentile of 69.2% (9/13) and 52.0% (38/73), respectively, followed by pneumonia change, consolidation, patchy and nodular changes, and bronchitis, whereas there was no statistical difference between two groups (P > 0.05). Details were shown in Table 3.
Table 3.
Chest computed tomography manifestations of children and adults in family clusters of coronavirus disease 2019
| Chest CT | Children | Adults | X2 | P |
|---|---|---|---|---|
| Normal, n (%) | 16 (55.2) (n = 29) | 17 (18.9) (n = 90) | 14.408 | < 0.001 |
| Abnormal, n (%) | 13 (44.8) (n = 29) | 73 (81.1) (n = 90) | ||
| GGO, n (%) | 9 (69.2) (n = 13) | 38 (52.0) (n = 73) | 1.314 | 0.252 |
| Pneumonia, n (%) | 2 (15.4) (n = 13) | 20 (27.4) (n = 73) | 0.324 | 0.569 |
| Consolidation, n (%) | 2 (15.4) (n = 13) | 14 (19.2) (n = 73) | 0.000 | 1.000 |
| Patchy, n (%) | 2 (15.4) (n = 13) | 8 (11.0) (n = 73) | 0.000 | 1.000 |
| Nodular, n (%) | 0 (0.0) (n = 13) | 8 (11.0) (n = 73) | 0.540 | 0.462 |
| Bronchitis, n (%) | 2 (15.4) (n = 13) | 1 (1.4) (n = 73) | 0.058 | |
CT computed tomography, GGO ground-grass opacity
Furthermore, we defined pneumonia based on the changes of CT regardless of symptoms and classified clinical types according to diagnostic guidelines in China for children and adults, respectively [35, 36]. There were equally five asymptomatic children and seven adults with abnormal CT diagnosed as pneumonia. Besides, according to their clinical manifestations, lab examinations and CT, 16 cases of children and 17 cases of adults were grouped as mild type, with the left 13 cases of children and 73 cases of adults grouped as common type.
Discussion
This systematic review showed that there were more adult patients infected with COVID-19 than children in family clusters of COVID-19. It might be due to most literatures involved in this review reporting data from China and because in a family, there were more adults than children as an objective fact that under one child policy in China, most families constitutes of one child, two parents and four grandparents. Besides, almost all family members from respective family cannot avoid being infected with SARS-CoV-2 indicates that it is a highly contagious disease and all people are susceptible [8, 10]. In addition, close contact or family cluster is the main mode of transmission. It can provide some evidence for more adult COVID-19 to some extent.
It is worth of attention that being exposed to the same circumstance in the same family, both children and adults infected by the same virus strain, although there were many common features shared by them, there were also different aspects between two groups. Fever and cough are the main onset clinical manifestations of COVID-19 for both children and adults in family clusters, which is consistent with previous publications [6–8, 10]. In addition, both children and adults showed mild type and common type, without severe, critical type or death cases. However, pediatric patients showed lower proportion of symptoms, such as fever (15/32, 46.9%) and cough (6/32, 18.8%), compared with adults (56/89, 62.9% and 39/89, 43.8%, respectively), as well as fewer symptoms, such as fatigue, myalgia, sore throat, chest pain, which were more common for adults [6, 8], although there were more cases of children with vomiting and diarrhea, which may be due to immature digestive system. On the other hand, proportion of asymptomatic infection was higher in children than adults with statistical significance (P < 0.05). Furthermore, the disease seems milder for children when comparing the proportion of pneumonia between children and adults (48.1, 82.7%, respectively, P < 0.05). As the pathogen of COVID-19, SARS-CoV-2 gains entry into epithelial cell via angiotensin-converting enzyme 2 (ACE2) receptor and transmembrane serine protease 2 [37–41]. There has been evidence that number of ACE2 receptors are significantly lower in children than in adults [41], which may be the primary reason why children have fewer clinical symptoms, less severe disease and lower proportion of pneumonia. Besides, difference in maturity, functionality or affinity of ACE2 receptor between children and adults may account for these discrepancies to some extent, which needs further research. On the other hand, a few children showed onset manifestations of gastrointestinal tract, such as vomiting and diarrhea [26], which were atypical symptoms for respiratory infectious diseases. ACE2 is expressed in many organs, not only in respiratory system, but also in digestive system, urinary system, neurological system and circulatory system [39, 42–48]. Maybe there is discrepancy in distribution of ACE2 receptor in different organs, or different functions of ACE2, which may account for various manifestation of COVID-19. However, the hypothesis needs more studies to prove.
Index case was defined as patient in the household cluster who first showed symptoms. From this analysis, we found that almost all families have adult as index case except one with child [19]. Furthermore, the infectious source of each family was adult and can be asymptomatic or later-onset than other family members [19, 24, 30]. In addition, there were family members with onset manifestation of no symptom, among whom there were asymptomatic infection and even pneumonia, which may be neglected without SARS-CoV-2 NAT and chest CT examination. To be mentioned, there was an index case of adult with asymptomatic infection throughout his illness course and only showed SARS-CoV-2 NAT-positive [30], and there was only one child in this family, who was 2.5 years and showing cough, normal white blood cell (WBC) and lymphocyte 10 days after contacting with the index case. Besides, for the family in which child being the index case, she stayed at home all the time under the condition of prevention of epidemic situation and schools were closed. She was actually infected by her mother, who returned from Wuhan, the epidemiological area, and regarded as the infectious source for this family. They received NAT on the same day, but the mother showed negative result and had later-onset manifestation and long NAT negative period [19]. Therefore, it indicates that SARS-CoV-2 may be contagious during incubation and asymptomatic patients can transmit the virus.
Although the incubation period for children and adults both varied from 1 to 30 days, it was longer for children with median time of 10 days than adults with 7 days (P < 0.05). However, this period has been assessed based on the most probable contacts with documented cases but other sources of exposition might be not explored especially by asymptomatic patients. This assessment is not so sure. Comparing to 54% of transmission clusters identifying children as index case in influenza [49], only 3.6% of family in this review identified children as index case of COVID-19, which may be due to longer incubation period of children for COVID-19 than adults to some extent [50]. During early epidemic period, schools were closed and children stayed at home. So it is not surprising for children to be later detected. Besides, from limited data of duration of pharyngeal swab NAT-positive period, we found children undergoing shorter course than adults (11 days and 17 days, respectively, P < 0.05). SARS-CoV-2 NAT with throat and nose swabs or lower airway secretion were the gold standard for the diagnosis of COVID-19 [35], and patients can be released from quarantine after disappearance of symptoms and two consecutive negative results of NAT. Somehow, four pediatric cases showed duration of positive pharyngeal swab NAT ranging from 7 to 27 days, while their anal swab NAT showed positive duration lasting more than 28 days. Three children showed common characteristics of fever and gastrointestinal symptoms, such as vomiting and diarrhea. Besides, their anal swab NATs were still positive for a long time even after disappearance of symptoms and negative results for pharyngeal swab NATs. To be mentioned, one child was asymptomatic and positive duration of her nasopharyngeal swab NAT lasted 22 days, whereas her fecal samples NAT showed positive more than 100 days. Therefore, there is still debate on infectivity of feces containing SARS-CoV-2 and time of quarantine.
In this review, we found that pediatric patients showed almost all normal lab examination results, except for 6 (6/33, 18.2%) cases with decreased WBC and 1 (1/33, 3.0%) case with increased CRP, whereas compared with adult patients, proportion of decreased WBC showed no statistical significance. In addition, there were more adults with lymphopenia and increased CRP than children with statistical significance (P < 0.05). Lymphopenia reflects consumption of lymphocyte by SARS-CoV-2 and was related to severity of disease [6, 7]. Here, it indicates that lymphocytes in children were not as consumptive as adult patients. The difference of increased CRP, an inflammation marker, may be related to different immune status or more intense inflammation reactions in adults. Somehow, the biochemical indexes that reflect organ functions were at large normal both for children and adults. Our review studied the articles published from January to September, 2020, with cases diagnosed in January and February, 2020, and it seems children showed milder inflammation in the early epidemic period of COVID-19. However, with the SARS-CoV-2 variants evolving each day, these data on children may change according to the variants.
As for image of lung, here we found number of abnormal CT in children was fewer than adults, which indicates children seems less susceptible to be infected with pneumonia. Consistent with previous studies [6–8, 51–55], GGO changes were the dominant features of chest CT for both children and adults in our study. However, other changes, such as consolidation, pneumonia-like change, patchy and nodular changes, were less common seen in children compared to adults [53–55]. These may reflected milder disease of children to some extent. To mention, although CT played an important role to detect patients with COVID-19 earlier as a diagnostic method because of lack of SARS-CoV-2 NAT material and low positive rate of NAT in the early epidemic period, these features of CT were not specific for COVID-19 exclusively because they can also be seen in pneumonia caused by other virus [56–59]. With widely used NAT and improved method, we can now quickly and accurately make diagnosis of patients with COVID-19, and chest CT can show patterns of lung abnormalities and reflect clinical condition to some extent.
There is another key point to be mentioned that, all the cases reported in these articles were diagnosed in January and February, 2020, the early epidemic period of COVID-19, during which period the first diagnosed patient in family either returned from epidemic area or had contacts with COVID-19 patients. However, during that period, children were limited to family environment, and school and kindergarten closure further confined children activity. Besides, special physical and social features of children, such as lack of independence and need of parents’ company, make them less exposure to out-family environment, and social activity involvement for children reduced. Therefore, children of COVID-19 were infected by adults from their family in family clusters.
With increasing cases, there are also reports of children with multi-system inflammation syndrome [60, 61], manifested as fever more than three days, multi-system affected and elevated inflammation markers. It is another severe condition caused by SARS-CoV-2 infection, may be due to immune system disorder, and may occur in children with fever or asymptomatic in the early period after two weeks post SARS-CoV-2 infection [62]. Besides, some skin changes may also appear after SARS-CoV-2 infection [63], which may give us clues for early diagnosis. Although these articles were not included in our review for not meeting standards, these information gave us new aspects of SARS-CoV-2 infection and we should pay attention to children with fever or asymptomatic in the early period.
There were several limitations for our study. First, although we searched huge amount of articles, most of them reported data from adults or children independently, leading to a small size of eligible studies based on our including criteria. Second, there was lack of data concerning viral load for us to compare difference between children and adults, which can give a more detailed profile of COVID-19 of family clusters.
In conclusion, this review profiles a comprehensive characteristic of COVID-19 of family clusters. In the early epidemic period of COVID-19, children of COVID-19 were infected by adults from their family showing characteristics of family clusters. However, same virus strain caused milder disease in children compared with their family members according to clinical manifestations, lab test results and chest CT changes.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The author thank all the team members of this review for corporation.
Author contributions
SWL contributed to conceptualization, methodology, formal analysis, and original draft preparation. ZN contributed to methodology and visualization. GWH and PJL contributed to software and data curation. XW contributed to conceptualization, validation, review and editing, supervision, project administration, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (No. 81771621), and the Natural Science Foundation of Liaoning Province (No. 2019JH8/10300023).
Compliance with ethical standards
Ethical approval
This work was retrospectively collecting information from articles published. Not required for a systematic review.
Conflict of interest
No financial or nonfinancial benefits have been received or will be received from any party related directly or indirectly to the subject of this article. The authors have no conflict of interest to declare.
Data availability
The datasets are available from the corresponding author on reasonable request.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Shang M, Blanton L, Brammer L, Olsen SJ, Fry AM. Influenza-associated pediatric deaths in the United States, 2010–2016. Pediatrics. 2018;141:e20172918. doi: 10.1542/peds.2017-2918. [DOI] [PubMed] [Google Scholar]
- 2.Wang XS, Cai JH, Yao WL, Ge YL, Zhu QR, Zeng M. Clinical characteristics of molecular epidemiology of the novel influenza A (H1N1) infection in children in Shanghai. Zhonghua Er Ke Za Zhi. 2013;51:356–361. [PubMed] [Google Scholar]
- 3.MacIntyre CR, Ridda I, Seale H, Gao Z, Ratnamohan VM, Donovan L, et al. Respiratory viruses transmission from children to adults within a household. Vaccine. 2012;30:3009–3014. doi: 10.1016/j.vaccine.2011.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamigaki T, Mimura S, Takahashi Y, Oshitani H. Analysis of influenza transmission in the households of primary and junior high school students during the 2012–13 influenza season in Odate, Japan. BMC Infect Dis. 2015;15:282. doi: 10.1186/s12879-015-1007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown CR, McCaw JM, Fairmaid EJ, Brown LE, Leder K, Sinclai M, et al. Factors associated with transmission of influenza-like illness in a cohort of households containing multiple children. Influenza Other Respir Viruses. 2015;9:247–254. doi: 10.1111/irv.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO. Director-General's statement on IHR Emergency Committee on novel coronavirus (2019-nCoV). https://www.who.int/dg/speeches/detail/who-director-general-s-statement-on-ihr-emergency-committee-on-novel-coronavirus-(2019-ncov). Accessed 30 Jan 2020.
- 10.Shen KL, Yang YH. Diagnosis and treatment of 2019 novel coronavirus infection in children: a pressing issue. World J Pediatr. 2020;16:219–221. doi: 10.1007/s12519-020-00344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 12.Livingston E, Bucher K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA. 2020;323:1335. doi: 10.1001/jama.2020.4344. [DOI] [PubMed] [Google Scholar]
- 13.CDC COVID-19 Response Team. Coronavirus disease in children-United States February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:422–426. doi: 10.15585/mmwr.mm6914e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao Q, Chen YC, Chen CL, Chiu CH. SARS-CoV-2 infection in children: transmission dynamics and clinical characteristics. J Formos Med Assoc. 2020;119:670–673. doi: 10.1016/j.jfma.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The General Office of the National Health and Health Commission, The Office of the State Administration of Traditional Chinese Medicine. Novel coronavirus pneumonia diagnosis and treatment program (trial version 7). http://www.gov.cn/zhengce/zhengceku/2020-03/04/content_5486705.htm. Accessed 4 Mar 2020.
- 16.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JFW, Yuan S, Kok KH, To KKW, Chu H, Yang J, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo W, Xiong Z, Tang H, Zhou H. A family outbreak of coronavirus disease 2019. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2020;45:275–279. doi: 10.11817/j.issn.1672-7347.2020.200099. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Fan P, Liu Z, Pan R, Huang S, Li J, et al. A SARS-CoV-2 familial cluster infection reveals asymptomatic transmission to children. J Infect Public Health. 2020;13:883–886. doi: 10.1016/j.jiph.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan XF, Chen DX, Xia Y, Wu XW, Li TS, Ou XT, et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis. 2020;20:410–411. doi: 10.1016/S1473-3099(20)30114-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song R, Han B, Song MH, Wang L, Conlon CP, Dong T, et al. Clinical and epidemiological features of COVID-19 family clusters in Beijing. China J Infect. 2020;81:e26–30. doi: 10.1016/j.jinf.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu R, Du MS, Li LF, Zhen ZM, Wang HY, Hu XF. CT imaging of one extended family cluster of corona virus disease 2019 (COVID-19) including adolescent patients and “silent infection”. Quant Imaging Med Surg. 2020;10:800–804. doi: 10.21037/qims.2020.02.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen DX, Li YP, Deng XL, Huang HL, Ou XT, Lin YB, et al. Four cases from a family cluster were diagnosed as COVID-19 after 14-day of quarantine period. J Med Virol. 2020;92:1748–1752. doi: 10.1002/jmv.25849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang LL, Jiang J, Li XP, Zhou YH, Xu MJ, Zhou JL. Initial CT imaging characters of an imported family cluster of COVID-19. Clin Imaging. 2020;65:78–81. doi: 10.1016/j.clinimag.2020.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su L, Ma X, Yu HF, Zhang ZH, Bian PF, Han YL, et al. The different clinical characteristics of corona virus disease cases between children and their families in China-the character of children with COVID-19. Emerg Microbes Infect. 2020;9:707–713. doi: 10.1080/22221751.2020.1744483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wolf GK, Glueck T, Huebner J, Muenchhoff M, Hoffmann D, French LE, et al. Clinical and epidemiological features of a family cluster of symptomatic and asymptomatic severe acute respiratory syndrome coronavirus 2 infection. J Pediatric Infect Dis Soc. 2020;9:362–365. doi: 10.1093/jpids/piaa060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen S, Si JF, Tang WQ, Zhang AQ, Pan L, An M, et al. An asymptomatic SARS-CoV-2-infected infant with persistent fecal viral RNA shedding in a family cluster: a rare case report. Front Med (Lausanne). 2020;7:562875. doi: 10.3389/fmed.2020.562875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang YL, Zhang C, Hu Y, Yao HM, Zeng XC, Hu CR, et al. Clinical features and outcomes of seven patients with COVID-19 in a family cluster. BMC Infect Dis. 2020;20:647. doi: 10.1186/s12879-020-05364-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu Y, Wu TT, Wu ZC, Xie ZG, Song X, Pan Y, et al. A report of a family cluster of COVID-19. J Pract Med. 2020;36:1579–1583. [Google Scholar]
- 30.Liu X, Liu F, Fan Q, Zheng W, Zhang DH, Zhang QL, et al. Report of COVID-19 family clustering epidemic caused by asymptomatic infection. Chin J Public Health. 2020;36:282–284. [Google Scholar]
- 31.Yang YL, Li LH, Li CF, Xiao YC, Peng J. A survey on clustering of novel coronavirus disease in families in Fengjie county of Chongqing municipality. Chin J Public Health. 2020;36:285–288. [Google Scholar]
- 32.Wang YC, Kong YL, Wei WX, Ren YW, Zhang JL, Zhang N. Laboratory examination and CT features of family aggregative corona virus disease 2019 patients at first visit. Shaanxi Med J. 2020;49:392–396. [Google Scholar]
- 33.Ma WW, Su S, Wu JB, Chen F, Bu TD, Cheng XL, et al. A family clustering of novel coronavirus disease in Anhui province: brief report. Chin J Public Health. 2020;36:277–281. [Google Scholar]
- 34.Liu M, Wan X, Tu Y, Liang ZW, Chen JN, Li JJ, et al. Family cluster of child SARS-CoV-2 infections: a case report. Med J Wuhan Univ. 2020;36:277–281. [Google Scholar]
- 35.Chen ZM, Fu JF, Shu Q, Wang W, Chen YH, Hua CZ, et al. Diagnosis and treatment recommendation for pediatric coronavirus disease-19 (second edition) Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:139–146. doi: 10.3785/j.issn.1008-9292.2020.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.National Health Commission of the People’s Republic of China. New coronavirus pneumonia prevention and control program (version 6). Beijing: National Health Commission of the People’s Republic of China; 2020.
- 37.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu X, Chen P, Wang J, Feng J, Hui Z, Li X, et al. Evolution of the novel coronavirus from the ongoing Wuhan outbreak and modeling of its spike protein for risk of human transmission. Sci China Life Sci. 2020;63:457–460. doi: 10.1007/s11427-020-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gallagher TM, Buchmeier MJ. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao Y, Li L, Feng Z, Wan S, Huang P, Sun X, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. SARS-CoV-2 receptor is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gheblawi M, Wang K, Viveiros A, Nguyen Q, Zhong JC, Turner AJ, et al. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res. 2020;126:1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Devaus CA, Rolain JM, Raoult D. ACE2 receptor polymorphism: susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect. 2020;53:425–435. doi: 10.1016/j.jmii.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verdecchia P, Cavallini C, Spanevello A, Angrli F. The pivotal link between ACE2 deficiency and SARS-CoV-2 infection. Eur J Intern Med. 2020;76:14–20. doi: 10.1016/j.ejim.2020.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu YS, Bloxham CJ, Hulme KD, Sinclair JE, Tong ZW, Steele LE, et al. A meta-analysis on the role of children in SARS-CoV-2 in household transmission clusters. Clin Infect Dis. 2020. 10.1093/cid/ciaa1825. [DOI] [PMC free article] [PubMed]
- 50.Cai J, Xu J, Lin D, Yang Z, Xu L, Qu Z, et al. A case series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020;71:1547–1551. doi: 10.1093/cid/ciaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chung M, Bernheim A, Mei X, Zhang N, Huang M, Zeng X, et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020;295:202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cao YH, Liu XL, Xiong LJ, Cai KL. Imaging and clinical features of patients with 2019 novel coronavirus SARS-CoV-2: a systematic review and meta-analysis. J Med Virol. 2020;92:1449–1459. doi: 10.1002/jmv.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xia W, Shao J, Guo Y, Peng X, Li Z, Hu D. Clinical and CT features in pediatric patients with COVID-19 infection: different points from adults. Pediatr Pulmonol. 2020;55:1169–1174. doi: 10.1002/ppul.24718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu HH, Liu F, Li JN, Zhang TT, Wang DB, Lan WS. Clinical and CT imaging features of the COVID-19 pneumonia: focus on pregnant women and children. J Infect. 2020;80:e7–13. doi: 10.1016/j.jinf.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koo HJ, Choi SH, Sung HS, Choe J, Do KH. RadioGraphics update: radiographic and CT features of viral pneumonia. Radiographics. 2020;40:E8–15. doi: 10.1148/rg.2020200097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muller NL, Ooi GC, Khong PL, Zhou LJ, Tsang KWT, Nicolaou S. High-resolution CT findings of severe acute respiratory syndrome at presentation and after admission. AJR Am J Roentgenol. 2004;182:39–44. doi: 10.2214/ajr.182.1.1820039. [DOI] [PubMed] [Google Scholar]
- 58.Ajlan AM, Ahyad RA, Jamjoom LG, Alharthy A, Madani TA. Middle East respiratory syndrome coronavirus (MERS-CoV) infection: chest CT findings. AJR Am J Roentgenol. 2014;203:782–787. doi: 10.2214/AJR.14.13021. [DOI] [PubMed] [Google Scholar]
- 59.Kim EA, Lee KS, Primack SL, Yoon HK, Byun HS, Kim TS, et al. Viral pneumonias in adults: radiologic and pathologic findings. Radiographics. 2002;22:S137–S149. doi: 10.1148/radiographics.22.suppl_1.g02oc15s137. [DOI] [PubMed] [Google Scholar]
- 60.Pawar SM. Multi system inflammatory syndrome in children and adolescents temporally related to COVID-19. GFNPSS. 2020. 10.46376/IJMR/1.3.2020.97-102. Accessed 25 Aug 2020.
- 61.CDC. Multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease 2019 (COVID-19). Updated 14 May 2020. https://emergency.cdc.gov/han/2020/han00432.asp. Accessed 25 Aug 2020.
- 62.Kabeerdoss J, Pilania RK, Karkhele R, Kumar TS, Danda D, Singh S. Severe COVID-19, multisystem inflammatory syndrome in children, and Kawasaki disease: immunological mechanisms, clinical manifestations and management. Rheumatol Int. 2021;41:19–32. doi: 10.1007/s00296-020-04749-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tatu AL, Nadasdy T, Bujoreanu FC. Familial clustering of COVID-19 skin manifestations. Dermatol Ther. 2020;33:e14181. doi: 10.1111/dth.14181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets are available from the corresponding author on reasonable request.


