Abstract
Simple Summary
Vitamin A is critical throughout life, but utilization of vitamin A often results in local and systemic toxicity. This study investigated the effect of vitamin A supplementation on mink growth and health. The results show that vitamin A deficiency decreased the ADG, villus height, villus height/crypt depth ratio and mRNA expression levels of IL-22, Occludin and ZO-1. Vitamin A supplementation increased the diversity of jejunum bacteria, decreased the ratio of Firmicutes to Bacteroidetes and increased the relative abundance of Akkermansia and Lachnospiraceae NK4A136 group.
Abstract
This experiment investigated the effect of vitamin A supplementation on growth, serum biochemical parameters, jejunum morphology and the microbial community in male growing-furring mink. Thirty healthy male mink were randomly assigned to three treatment groups, with 10 mink per group. Each mink was housed in an individual cage. The mink in the three groups were fed diets supplemented with vitamin A acetate at dosages of 0 (CON), 20,000 (LVitA) and 1,280,000 IU/kg (HVitA) of basal diet. A 7-day pretest period preceded a formal test period of 45 days. The results show that 20,000 IU/kg vitamin A increased the ADG, serum T-AOC and GSH-Px activities, villus height and villus height/crypt depth ratio (p < 0.05). The mRNA expression levels of IL-22, Occludin and ZO-1 in the jejunum of mink were significantly higher in the LVitA group than those in the CON and HVitA groups (p < 0.05). Vitamin A supplementation increased the diversity of jejunum bacteria, decreased the ratio of Firmicutes to Bacteroidetes and increased the relative abundance of Akkermansia, uncultured bacterium f Muribaculaceae, Allobaculum, Lachnospiraceae NK4A136 group, Rummeliibacillus and Parasutterella. The comparison of potential functions also showed enrichment of glycan biosynthesis and metabolism, transport and catabolism pathways in the vitamin A supplementation groups compared with the CON group. In conclusion, these results indicate that dietary vitamin A supplementation could mediate host growth by improving intestinal development, immunity and the relative abundance of the intestinal microbiota.
Keywords: vitamin A, mink, growth performance, IL-22, villus height, Akkermansia
1. Introduction
Vitamin A and its metabolites regulate diverse processes, including reproduction, embryogenesis, vision, growth, cellular differentiation and proliferation in mammals [1,2,3]. Vitamin A also plays an important role in intestinal immunity and epithelial integrity, and promotes healthy colonization of the intestinal mucosa with commensal bacteria [4]. Clearly, vitamin A is critical throughout life, together with natural derivatives and synthetic analogs [2]. At present, vitamin A deficiency is a worldwide public health problem that can cause micronutrient malnutrition, slow growth impairing innate immunity and adverse health consequences for people and animals [5,6]. Deficiency in vitamin A and its metabolites can cause abnormal morphological development [7,8]. However, excessive utilization of vitamin A often results in local and systemic toxicity [9]. Thus, understanding how vitamin A levels are regulated has important practical implications.
Vitamin A has three active forms (retinal, retinol and retinoic acid), and vitamin A metabolism can be classified into three major processes—intestinal uptake (especially small intestine), hepatic storage and lymphatic and blood transport to supply the body’s physiological needs [10]. However, mammals cannot synthesize vitamin A in the body and can only obtain vitamin A from the diet. The two main forms of vitamin A in the diet are retinyl/retinol esters (animal products), and provitamin A carotenoids (plants) [11,12]. Notably, the cleavage enzyme (beta-carotene-15,15′-monooxygenase) has been found in many vertebrates but is not present in mink, so mink cannot utilize carotene as a source of vitamin A [13,14]. Therefore, the nutritional requirement of vitamin A for mink must be obtained from a diet containing preformed retinol.
The American mink (Neovison vison), an obligate carnivore, has a short and simple gastrointestinal tract [15,16], and little is known regarding the structural and functional adaptation of the gastrointestinal tract. Gut health is influenced by the maintenance of the delicate balance between the host, intestinal microbiota, intestinal barrier and dietary compounds [17]. The gut microbiota performs numerous beneficial functions for the host, such as harvesting energy, regulating immunity and aiding cellular maturation [18,19]. However, the gut microbial community is influenced by various parameters, such as the host diet composition and type, lifestyle, antibiotics and other drugs, while the genetics and immune status of the host also shape the microbiota composition, with various consequences for host physiology [18,20,21,22]. The gut microbial community composition of carnivores in general appears to be distinct from that of omnivores and herbivores [23]. Previous research found that vitamin A could regulate the interactions between eukaryotic host cells and symbiotic microbes, as well as the complexity of the microbiome, and the microbiome regulates vitamin A metabolism in the host [24,25]. In our previous study, supplementation with vitamin A at 0 and 1,280,000 IU/kg had a negative impact on the growth performance and digestion of dry matter, crude protein and ether extract compared with 20,000 IU/kg in growing-furring mink [26]. Therefore, we hypothesized that vitamin A can affect the growth of mink by modulating intestinal development, immune function and the intestinal microbiome.
Thus, the present study aims to examine the effects of vitamin A supplementation (1) on growth performance and serum biochemical parameters and (2) on jejunum morphology and gut microbiota of American mink during the growing-furring period.
2. Materials and Methods
2.1. Animals, Experimental Design and Diets
Thirty 15-wk-old healthy male mink (BW = 1.98 ± 0.04 kg), approved by the Institute of Special Animal and Plant Sciences, Chinese Academy of Agricultural Science (CAAS), were randomly assigned to three treatment groups, with 10 animals per group. The mink were housed in open-sided sheds in individual mink growing cages (60 cm long × 40 cm wide × 50 cm high) with attached nest boxes (30 cm long × 40 cm wide × 30 cm high). The mink were fed twice each day at 8:00 and 15:00 and had free access to water. After 7 days of adaptation, the experiment lasted for 45 days from late September to pelting in late November.
The diets were formulated based on the Management Guide of the National Research Council (NRC, 1982) [13] with no supplemental vitamin A. The ingredients used were extruded corn, soybean meal, meat meal, meat and bone meal, corn gluten meal, fish meal and soybean oil (Table 1). The mink in each group were fed a basal diet with 0 (CON), 20,000 (LVitA) and 1,280,000 IU/kg (HVitA) vitamin A acetate (DSM Vitamin Co., Ltd. Shanghai, China).
Table 1.
Composition and nutrient levels of experimental diets, %.
| Ingredients | Content | Nutrient Levels 2 | Content |
|---|---|---|---|
| Extruded corn | 36 | Metabolizable energy (MJ/kg) | 16.44 |
| Soybean meal | 1.5 | Dry matter | 96.13 |
| Meat meal | 7.8 | Crude protein | 35.36 |
| Corn gluten meal | 1.5 | Ether extract | 16.53 |
| Chicken meal | 14.0 | Carbohydrates | 34.10 |
| Fish meal | 25.5 | Ash | 10.14 |
| Soybean oil | 11.8 | Ca | 2.66 |
| DL-Methionine | 0.5 | P | 1.35 |
| L-lysine HCL | 0.4 | Vitamin A IU/kg | 1558 |
| Premix 1 | 1.0 | ||
| Total | 100.0 |
1 Premix provided following per kilogram of diet: vitamin D3 2200 IU; vitamin E 220 mg; vitamin K3 1 mg; vitamin B1 20 mg; vitamin B2 10 mg; vitamin B6 10 mg; vitamin B12 0.1 mg; nicotinic acid 40 mg; pantothenate 22 mg; folic acid 1 mg; biotin 1 mg; choline chloride 400 mg; vitamin C 120 mg; Cu 20 mg; Fe 80 mg; Zn 40 mg; Mn 16 mg; I 0.5 mg; Se 0.12 mg; Co 0.2 mg. 2 ME and carbohydrates were calculated values, while the others were measured values.
2.2. Experimental Procedure and Sample Collection
The animals were weighed biweekly before morning feeding, and the final body weight of mink was used to determine average daily gain (ADG). After 45 days, five mink from each group were selected randomly, and euthanized by electrocution according to the Welfare of Animals Kept for Fur Production requirements [27]. Body length was measured from the base of the tail to the tip of the nose. Immediately after death, blood samples (6 mL) were collected from the heart of each mink from the three groups into coagulation tubes and centrifuged at 3000× g for 15 min to obtain the serum. All serum samples were stored at −20 °C for later analysis. Jejunum tissue was removed immediately and placed in 10% formalin as a fixative for histology. The content and mucosa of the jejunum were collected in a sterile tube, transferred into liquid nitrogen and then stored at −80 °C for further analysis.
2.3. Chemical Analysis
Diet samples were dried at 65 °C in a forced air oven to reach a constant weight and kept for further analysis. All chemical analyses were conducted in duplicate. Diet samples were analyzed for dry matter (AOAC, 2000 method 930.15), crude protein (AOAC, 2000 method 984.13), ether extract (AOAC, 2000 method 920.39) and ash (AOAC, 2000 method 942.05). The concentration of vitamin A from feedstuffs was analyzed by ultra-performance liquid chromatography according to a published procedure [26].
2.4. Measurement of Serum Samples
The concentrations of immunoglobulins (IgA and IgM), complement levels (C3 and C4), total antioxidant capacity (T-AOC), glutathione peroxidase (GSH-Px) and malondialdehyde (MDA) activities in serum were measured by colorimetric methods using a Microplate Spectrophotometer (Epoch 2, BioTek Instruments Inc, Winooski, VT, USA) following the manufacturer’s instructions, provided by Nanjing Jiancheng Biochemical Corporation (Nanjing, Jiangsu, China).
2.5. Measurement of Jejunum Morphology
Jejunum samples were dehydrated and paraffin-embedded (Thermo A81010100 Issue2). The paraffin-embedded blocks were sectioned at 7 μm by a microtome (Thermo HM340E). Jejunum sections were stained with hematoxylin and eosin (H&E) to evaluate their general histological structure. A light microscope (BX51, Olympus Co., Tokyo, Japan) was used for bright field imaging. The jejunum villus height (Vh), crypt depth (Cd) and intestinal wall thickness were determined using an image analysis program (Image-Pro Plus 5.1, Rockville, MD, USA), and the Vh/Cd ratio was calculated.
2.6. Total RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qPCR)
Total RNA of the jejunum mucosa samples of mink was extracted using TRIzol reagent (Takara Biotechnology Co. Ltd., Dalian, China), according to the manufacturer’s protocol. First-strand complementary DNA was then synthesized using the PrimeScript™ RT reagent Kit with gDNA Eraser (Perfect Real Time) (TaKaRa, Dalian, China) following the manufacturer’s instructions. Real-time PCR was performed on StepOnePlus Real-Time PCR Systems (Applied Biosystems, Foster City, CA, USA) to quantify IL-22, zonula occludens-1 (ZO-1) and Occludin mRNA expression with TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) (TaKaRa, Dalian, China). Relative expression levels were calculated using the 2−ΔΔCT method [28] and normalized to GAPDH, and all reactions were run in triplicate. The primers were synthesized by Sangon Biotech (Changchun, China). Primers were as follows: IL-22 forward: TGCCTCCTCATTGCCCTGTG, reverse: AGCATGAAGGTGCGGTTGGT; Occludin forward: TGACCTCGCCCGTGGATGACTT, reverse: TTGGACCTGCCTGCTCTGCCTT; ZO-1 forward: CTCCTCTAATACCTGCGTCTCA, reverse: TTCATCCTTCTTGCTCTCCAATG; and GAPDH forward: GAAGGTGGTGGCGGTGAATGAT, reverse: TCTTGGGTGGCAAGGGTGGA.
2.7. DNA Extraction, Amplification, Sequencing and Bioinformatics Analysis
The microbial genomic DNA in the jejunum was extracted according to the manufacturer’s instructions using a Fast DNA Spin Kit (MP, Valencia, CA, USA). The primers 338F (5′- ACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) were used to amplify the V3–V4 region of the bacterial 16S rRNA gene. The resultant amplicons were purified using a QIAquick PCR Purification Kit (QIAGEN, Valencia, CA, USA), and then sequenced on an Illumina NovaSeq 6000 platform to produce 250-bp paired-end reads.
The paired-end sequences were first assembled into contigs using FLASH v1.2.7 [29] and then used for quantitative insights into microbial ecology (QIIME v1.9.1) [30]. The sequences were clustered into operational taxonomic units (OTUs) using UPARSE at 97% sequence similarity. Potential chimeric sequences were identified and removed using UCHIME [31]. The representative sequences of each OTU were assigned against the SILVA database (v138) using the RDP classifier with a 0.80 confidence threshold [32]. Alpha diversity indices, including the Chao 1, ACE, Shannon and Simpson indices, were calculated using QIIME v1.9.1 [30]. Principal coordinate analysis (PCoA) based on four distances was used to reveal the differences in the bacterial communities among the three groups [33]. Analysis of similarities (ANOSIM) was performed to indicate group similarity, where 0 = indistinguishable and 1 = dissimilar [34]. Adonis was employed to describe the strengths and significance of the differences among the microbial communities. For the ANOSIM and Adonis analyses, the p-values were determined based on 999 permutations. The sequences from the present study have been deposited in the SRA database. The reconstruction of unobserved states (PICRUSt) was applied to predict functional profiles of gut microbiota resulting from reference-based OTU picking against the Greengenes database [35]. The predicted genes were then summarized according to Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways.
2.8. Statistical Analysis
All graphs were generated using GraphPad Prism version 6.01 (GraphPad Software, Inc., San Diego, CA, USA) [36]. Statistical analyses were performed using SPSS Statistical 24.0 (IBM, New York, NY, USA) [37] with diet as the main effect. The results of the statistical analysis are presented as the means with their standard errors, and the error bars are standard deviations. The data were analyzed using one-way analysis of variance (ANOVA). Duncan’s tests were used to detect statistical significance between treatment groups (CON, LVitA and HVitA groups). The p-values were corrected using the false discovery rate of the Benjamini–Hochberg method. A significant difference between treatments was declared when p < 0.05.
3. Results
3.1. Growth Performance and Serum Biochemical Constituents
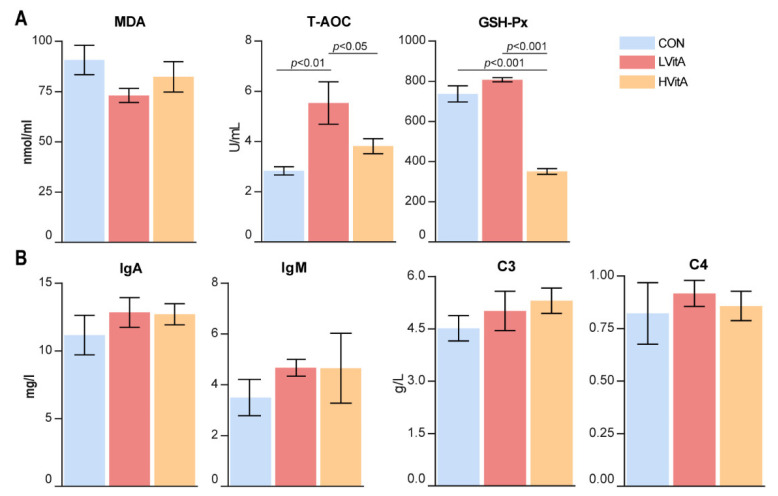
The results show that the ADG was significantly higher in the LVitA group than that in the CON group (p < 0.05, Table 2). There were no significant differences among the three groups in final body weight and body length (p > 0.05). The activity of T-AOC in the LVitA and HVitA groups was significantly higher than that in the CON group, whereas the GSH-Px activity was significantly decreased in the HVitA group compared with the CON and LVitA groups (p < 0.05, Figure 1A). There were no significant differences among the three groups in MDA activity (p > 0.05, Figure 2A) and the concentrations of IgA, IgM, C3 and C4 (p > 0.05, Figure 1B) in serum.
Table 2.
Effect of dietary vitamin A supplementation on the growth performance of growing-furring male mink.
| Items | Treatments | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | LVitA | HVitA | |||
| Initial body weight, kg | 1.98 | 1.97 | 1.98 | 0.035 | 0.98 |
| Final body weight, kg | 2.24 | 2.31 | 2.30 | 0.037 | 0.71 |
| Average daily gain, g/d | 5.74 a | 7.66 b | 7.01 ab | 0.33 | 0.048 |
| Body length, cm | 46.1 | 47.85 | 47.44 | 0.61 | 0.74 |
In the same row, values with no letter or the same letter superscripts mean no significant difference (p > 0.05), a,b while different small letter superscripts mean significant difference (p < 0.05). The same as below.
Figure 1.
Effect of vitamin A on serum antioxidant capacity (A) and immunological indices (B) of growing-furring male mink. CON group, vitamin A with 0 IU/kg of diet; LVitA group, vitamin A with 20,000 IU/kg of diet; HVitA group, vitamin A with 1,280,000 IU/kg of diet. MDA, malondialdehyde; T-AOC, total antioxidant capacity; GSH-Px, glutathione peroxidase; IgA, immunoglobulin A; IgM, immunoglobulin M; C3, complement C3; C4, complement C4.
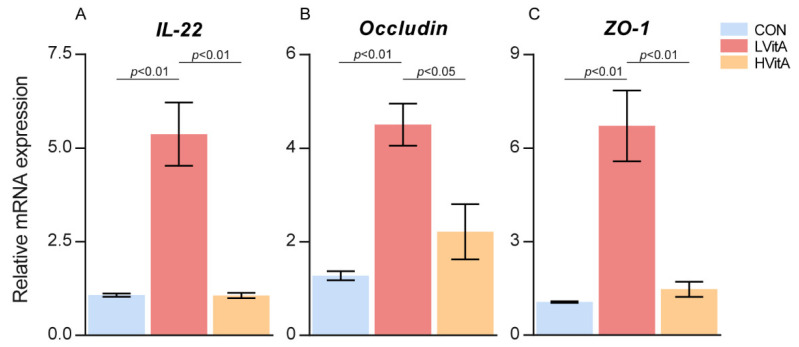
Figure 2.
Effect of vitamin A on the mRNA levels of IL-22 (A), Occludin (B) and ZO-1 (C) in the mink jejunum. CON group, vitamin A with 0 IU/kg of diet; LVitA group, vitamin A with 20,000 IU/kg of diet; HVitA group, vitamin A with 1,280,000 IU/kg of diet.
3.2. Morphometric Analysis and mRNA Expression of IL-22, Occludin and ZO-1 in the Jejunum
As shown in Table 3, the villus height was significantly higher (p < 0.01) in the LVitA group than that in the CON and HVitA groups. The villus height:crypt depth ratio was significantly lower in the CON group than that in the CON and HVitA groups (p < 0.05). There was no significant difference among the three groups in crypt depth and intestinal wall thickness (p > 0.05). The jejunal mucosa mRNA expression levels of IL-22, Occludin and ZO-1 were significantly higher in the LVitA group than those in the CON and HVitA groups (p < 0.05, Figure 2).
Table 3.
Effect of vitamin A supplementation on jejunum morphology in fur-growing male mink.
| Items | Treatments | SEM | p-Value | ||
|---|---|---|---|---|---|
| CON | LVitA | HVitA | |||
| villus height, μm | 1115.12 a | 1423.62 c | 1252.79 b | 33.93 | 0.001 |
| crypt depth, μm | 567.55 | 576.71 | 539.86 | 11.22 | 0.484 |
| intestinal wall thickness, μm | 341.42 | 367.63 | 345.23 | 9.76 | 0.479 |
| villus height/crypt depth | 1.98 a | 2.49 b | 2.33 b | 0.84 | 0.001 |
In the same row, values with no letter or the same letter superscripts mean no significant difference (p > 0.05), a–c while different small letter superscripts mean significant difference (p < 0.05). The same as below.
3.3. Summary of High-Throughput Sequencing and Alpha Diversity
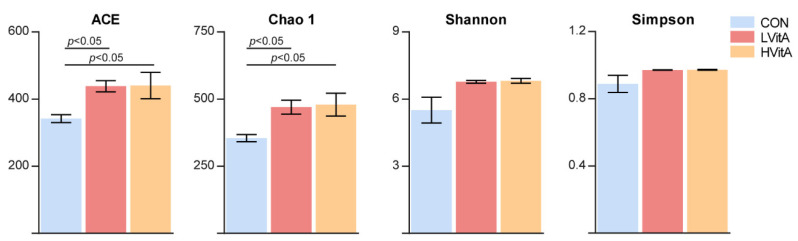
The present study obtained a total of 1,170,905 16S rRNA gene sequences from three groups, with a range of 72,190 to 79,412 sequences for each sample. We subsampled the sequences in each sample to 72,190 to decrease the effect of sequencing depth. A total of 920 OTUs were identified at 97% sequence similarity and represented 20 phyla and 340 genera in the three groups. Good’s coverage, ranging from 0.993 to 0.999, demonstrated an adequate sequencing depth for all samples. The number of OTUs was significantly higher in the LVitA and HVitA groups than that in the CON group (p < 0.05, CON = 293.60 ± 39.07, LVitA = 372.60 ± 16.43 and LVitA = 371.80 ± 42.32, respectively). As shown in Figure 3, the ACE and Chao1 indices in the CON group were significantly lower than those in the LVitA and HVitA groups (p < 0.05). There was no difference among the three groups in the Shannon and Simpson index values (p > 0.05).
Figure 3.
Comparisons of the alpha diversity indices of the mink gut microbiota among the three groups. CON group, vitamin A with 0 IU/kg of diet; LVitA group, vitamin A with 20,000 IU/kg of diet; HVitA group, vitamin A with 1,280,000 IU/kg of diet.
3.4. Dietary Vitamin A Altered the Composition and Function of the Gut Microbiota in Mink
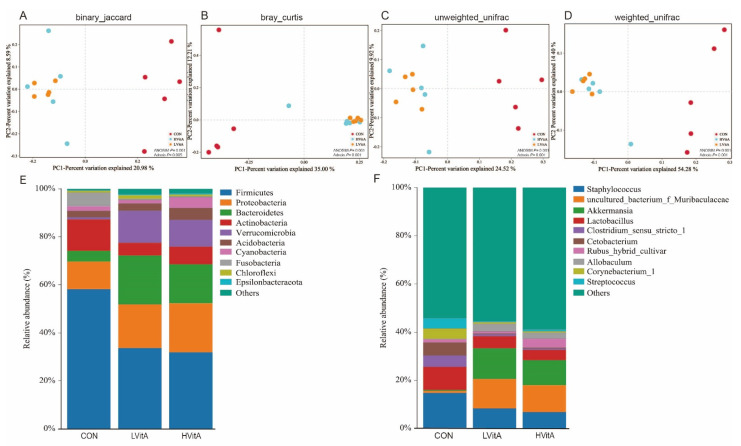
PCoA was applied to examine differences in taxonomic community composition and structure in the jejunum of mink. The PCoA based on the binary Jaccard distance (Figure 4A, ANOSIM: p = 0.001; Adonis: p = 0.005), Bray–Curtis distance (Figure 4B, ANOSIM: p = 0.001; Adonis: p = 0.001), unweighted UniFrac distance (Figure 4C, ANOSIM: p = 0.001; Adonis: p = 0.001) and weighted UniFrac distance (Figure 4D, ANOSIM: p = 0.001; Adonis: p = 0.001) showed that the CON group was clearly separated from the LVitA and HVitA groups.
Figure 4.
Composition and comparisons of the mink gut microbiota among the three groups. PCoA revealing the separation of the gut microbiota in the three groups based on the binary Jaccard distance (A), Bray–Curtis distance (B), unweighted UniFrac distance (C) and weighted UniFrac distance (D). Microbial compositions in the gut of mink from the CON, LVitA and HVitA groups at the phylum (E) and genus (F) levels. CON group, vitamin A with 0 IU/kg of diet; LVitA group, vitamin A with 20,000 IU/kg of diet; HVitA group, vitamin A with 1,280,000 IU/kg of diet.
At the phylum level, Firmicutes was the dominant bacteria in all three groups (58.2, 33.6 and 32.2%, respectively), while vitamin A supplementation affected the following abundant phyla in the treatment groups compared with the CON group (Figure 4E): (Group CON) Actinobacteria (12.9%), Proteobacteria (11.4%), Fusobacteria (5.5%) and Bacteroidetes (4.6%); (Group LVitA) Bacteroidetes (20.4%), Proteobacteria (18.4%), Verrucomicrobia (13.2%) and Actinobacteria (5.3%); and (Group HVitA) Proteobacteria (20.7%), Bacteroidetes (15.7%), Verrucomicrobia (11.1%) and Actinobacteria (7.2%). The ratio of Firmicutes to Bacteroidetes in the vitamin A supplementation groups (LVitA = 1.66, HVitA = 1.97, respectively) significantly decreased compared with that in the CON group (12.97). Moreover, vitamin A supplementation also significantly affected the genera in mink (p < 0.05, Figure 4F). For instance, Staphylococcus (14.7%), Lactobacillus (9.5%), Cetobacterium (5.4%), Clostridium sensu stricto 1 (4.8%) and Corynebacterium 1 (4.4%) were the five most abundant genera in the CON group, while the genera Akkermansia (LVitA = 12.7%, HVitA = 10.43%, respectively), uncultured bacterium f Muribaculaceae (LVitA = 12.3%, HVitA = 11.2%, respectively), Staphylococcus (LVitA = 8.3%, HVitA = 6.7%, respectively) and Lactobacillus (LVitA = 5.08%, HVitA = 4.31%, respectively) were the most abundant in both the LVitA and HVitA groups, followed by Allobaculum (3.1%) in the LVitA group and Rubus hybrid cultivar (3.8%) in the HVitA group.
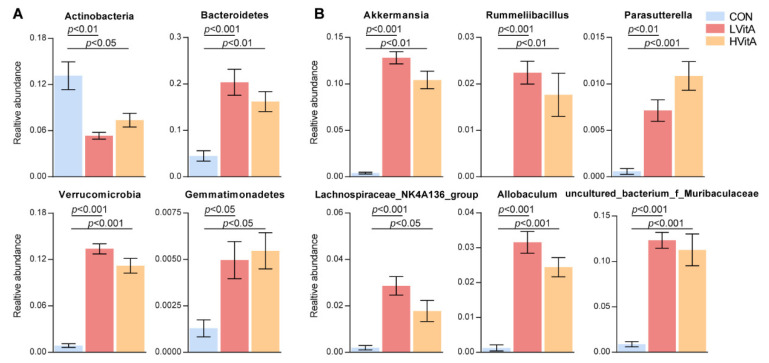
Furthermore, we also compared the bacterial taxa among the three groups. The relative abundances of Bacteroidetes (CON = 4.48%, LVitA = 20.34%, HVitA = 16.20%, respectively), Gemmatimonadetes (CON = 0.13%, LVitA = 0.50%, HVitA = 0.55%, respectively) and Verrucomicrobia (CON = 0.87%, LVitA = 13.38%, HVitA = 11.20%, respectively) were significantly increased (p < 0.05), while the relative abundance of Actinobacteria (CON = 13.15%, LVitA = 5.33%, HVitA = 7.36%, respectively) was significantly decreased in the vitamin A supplementation groups compared with the CON group (Figure 5A). At the genus level, six genera were significantly increased (p < 0.05) in the LVitA and HVitA groups compared with the CON group, namely Akkermansia (CON = 0.41%, LVitA = 12.81%, HVitA = 10.41%, respectively), uncultured bacterium f Muribaculaceae (CON = 0.88%, LVitA = 12.33%, HVitA = 11.27%, respectively), Allobaculum (CON = 0.13%, LVitA = 3.16%, HVitA = 2.44%, respectively), Lachnospiraceae NK4A136 group (CON = 0.20%, LVitA = 2.87%, HVitA = 1.78%, respectively), Rummeliibacillus (CON = 0, LVitA = 2.24%, HVitA = 1.77%, respectively) and Parasutterella (CON = 0.06%, LVitA = 0.71%, HVitA = 1.09%, respectively) (Figure 5B).
Figure 5.
Histogram of ANOVA among the CON, LVitA and HVitA groups at the phylum (A) and genus (B) levels. CON group, vitamin A with 0 IU/kg of diet; LVitA group, vitamin A with 20,000 IU/kg of diet; HVitA group, vitamin A with 1,280,000 IU/kg of diet.
3.5. Comparison of the Potential Functions in the Jejunal Microbiota among the Three Groups
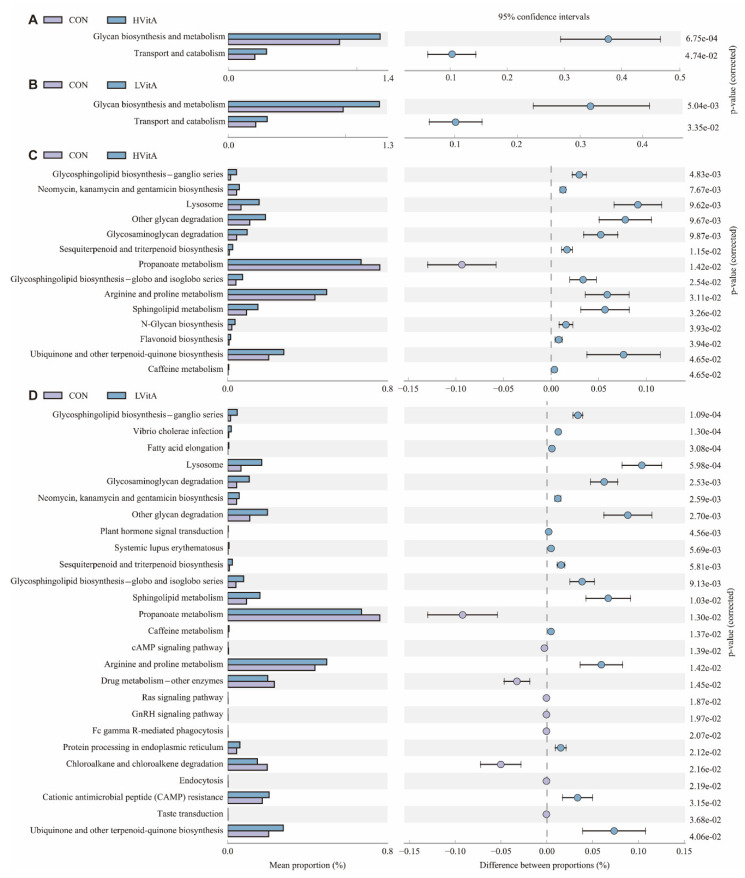
KEGG annotation by PICRUSt was applied to analyze the functional differences among the three groups. The KEGG level 2 functional pathways showed that glycan biosynthesis and metabolism, transport and catabolism pathways were significantly more abundant in the vitamin A supplementation groups than those in the CON group (Figure 6A,B). A total of 14 KEGG metabolic pathways (level 3) were enriched in the HVitA group compared with the CON group (Figure 6C). Among the pathways belonging to the top 10 dominant KEGG pathways, the relative abundance of glycosphingolipid biosynthesis—ganglio series, neomycin, kanamycin and gentamicin biosynthesis, lysosome, other glycan degradation, glycosaminoglycan degradation, sesquiterpenoid and triterpenoid biosynthesis, glycosphingolipid biosynthesis—globo and isoglobo series, arginine and proline metabolism and sphingolipid metabolism were significantly higher, while the relative abundance of propanoate metabolism was lower in the HVitA group than in those in the CON group. A total of 26 KEGG metabolic pathways (level 3) were enriched in the LVitA group compared with the CON group (Figure 6D). Among the pathways belonging to the top 10 dominant KEGG pathways, the relative abundance of glycosphingolipid biosynthesis—ganglio series, vibrio cholerae infection, fatty acid elongation, lysosome, glycosaminoglycan degradation, neomycin, kanamycin and gentamicin biosynthesis, other glycan degradation, plant hormone signal transduction, systemic lupus erythematosus, sesquiterpenoid and triterpenoid biosynthesis in the LVitA group were significantly higher than those in the CON group.
Figure 6.
Comparison of KEGG pathways predicted by PICRUSt according to diet. The differential analysis diagram of KEGG metabolic pathways between the CON and HVitA groups at levels 2 (A) and 3 (C) and the differential analysis diagram of KEGG metabolic pathways between the CON and LVitA groups at levels 2 (B) and 3 (D). CON group, vitamin A with 0 IU/kg of diet; LVitA group, vitamin A with 20,000 IU/kg of diet; HVitA group, vitamin A with 1,280,000 IU/kg of diet.
4. Discussion
The ADG in the CON group was significantly lower than that in the LVitA and HVitA groups. In the present study, the content of vitamin A in the CON group was lower than the NRC requirement (100–400 IU of vitamin A per kilogram of body weight daily), which may cause vitamin A deficiency in mink [13]. Vitamin A deficiency affects nutrient metabolism, intestinal immunity and epithelial integrity and modulates microbiome development in rats and humans [38,39,40]. Similarly, Tian et al. [41] also found that rats fed a vitamin A-deficient diet had decreased body weight. This evidence demonstrated that vitamin A deficiency caused a negative effect on growth performance in mammals. In this experiment, the results show that 20,000 IU/kg vitamin A supplementation had no effect on the body length of mink. A previous study observed that the epiphyses of the long bones of the mink were close to approximately 16 weeks of age; thus, actual body length cannot be increased beyond this point, although body weight may be enhanced [42]. In this experiment, thirty 15-wk-old mink had an insignificant initial weight difference, which might be the reason why the body length was not significant according to the ANOVA analyzed.
The results show that vitamin A supplementation affected the antioxidant activities in American mink. The T-AOC activity in the LVitA group was greater than that in the CON and HVitA groups. Consistent with our finding, Liang et al. [43] found that vitamin A supplementation significantly increased the activity of T-AOC in goslings. T-AOC is the total ability of various antioxidants to scavenge oxygen free radicals in enzymatic and non-enzymatic systems [44,45], and acts as an effective quencher and scavenger of lipid peroxidation free radicals, hydroxyl radicals and other free radicals [46]. We also observed that the activity of GSH-Px was significantly lower in the HVitA group than that in the CON and LVitA groups. GSH-Px has the ability to prevent cellular damage from oxidative stress by scavenging free radicals in animals [47]. Based on GSH-Px activity in the present study, although high doses of vitamin A supplementation showed no negative effect on growth performance, it still caused toxicity in mink [48]. Excess vitamin A supplementation resulted in abnormalities in hematologic and biochemical indices [49]. Combining the growth performance and antioxidant index results of the present study seems to suggest that mink is a carnivore that may be most tolerant to high dietary vitamin A [50].
In the present study, we observed that 20,000 IU/kg vitamin A supplementation significantly increased the Vh and Vh/Cd ratio. As indicators of intestinal health or morphology, Vh, Cd and the Vh/Cd ratio are related to intestinal health [51]. The small intestine not only serves as the main site of liquid feed digestion but also plays a role in nutrition absorption. Increased Vh may improve nutrient absorption and performance [51,52]. The Vh/Cd ratio is an important value for evaluating the developmental state of the intestine [53]. However, our results also show that a deficiency (0 IU/kg) and high dietary vitamin A levels (1,280,000 IU/kg) decreased the Vh and Vh/Cd ratio. These results may be due to a deficiency of vitamin A causing intestinal epithelial damage [54], while a large dose of vitamin A exceeded the absorption and circulation capacity of the intestine in a single feeding episode [55].
Furthermore, the relative mRNA expression levels of immune- and tight junction-related genes in the jejunum mucosa were measured to explore intestinal health in mink. The results show that the relative expression of IL-22 in the CON and HVitA groups was significantly lower than that in the LVitA group. Previous studies indicated that vitamin A plays an important role in the signaling of IL-22 in the gut [56]. IL-22 is a protective cytokine that exerts a protective role in mucosal immunity and coordinates the antimicrobial response against gut bacteria [57,58]. Consistent with previous research [59,60], a 20,000 IU/kg vitamin A addition significantly increased the relative expression of ZO-1 and Occludin in the jejunum. Occludin and ZO-1 can seal the intercellular gaps between adjacent endothelial cells and form a selectively permeable barrier for circulating molecules [61]. A previous study indicated that vitamin A and its metabolites regulate the expression of tight junction proteins on intestinal epithelial cells for barrier function in the gut [24]. These results suggest that vitamin A has protective effects on intestinal epithelial barrier function. However, under the condition of vitamin A deficiency, mucosal immune responses are reduced in animals [62].
Establishing and maintaining beneficial interactions between the host and its associated microbiota are important factors affecting host health [19]. The metabolic activities of the gut microbiota result from extracting calories from ingested dietary substances, helping to store those calories in host adipose tissue for later use and providing energy and nutrients for microbial growth and proliferation [63]. Previous studies have demonstrated that microbiota alterations exert profound effects on host physiology and metabolism [64,65,66,67,68]. In the present study, there were significant differences in microbial community composition in the jejunal microflora of American mink between the CON and treatment groups, which is consistent with previous research on the cecal microbiota of mice [41]. Moreover, the treatment groups significantly increased the alpha diversity of the gut microbiota, including the Chao1 and ACE indices.
At the phylum level, the jejunum microbiomes of mink evaluated here were dominated by sequences representative of Firmicutes, Proteobacteria, Bacteroidetes and Actinobacteria, consistent with observations in the colon [69] and feces [15] of mink. The phyla Firmicutes, Proteobacteria, Bacteroidetes and Actinobacteria were also widely present in the gastrointestinal tract of other carnivore species, such as Eurasian otters, leopard cats, raccoon dogs, lion and silver fox [70,71,72,73]. Consistent with results in mice [41], we also found that the relative abundance of Verrucomicrobia and Bacteroidetes significantly increased in the treatment groups compared with the CON group. Moreover, the abundance of Actinobacteria in the LVitA and HVitA groups was lower than that in the CON group. Consistent with Xiao et al. [74], the percentage of Actinobacteria was highest in the vitamin A deficiency group. Moreover, the Firmicutes/Bacteroidetes ratio (F/B ratio) was also decreased in the vitamin A supplementation groups compared with the CON group, consistent with observations in the cecum of mice [41]. Previous studies have shown that the F/B ratio is directly related to dysbiosis, the disruption of immunological homeostasis and host energy metabolism [66,75,76,77].
At the genus level, our results show that the relative abundance of Akkermansia, uncultured bacterium f Muribaculaceae, Allobaculum, Lachnospiraceae NK4A136 group, Rummeliibacillus and Parasutterella significantly increased in the vitamin A supplementation groups. Interestingly, most of these genera have been found to interact with the host immune system. Akkermansia, which belongs to the phylum Verrucomicrobia, has the potential to induce regulatory immunity [78]. Otherwise, the genus Akkermansia has also been suggested to be a potential biomarker of a healthy gut status [79] and to play a key role in maintaining the barrier function of the digestive tract and controlling metabolism-induced inflammation and fat storage, using mucins as an energy source to stimulate goblet cells to produce mucus, and enhance mucus layer thickness and the intestinal barrier [67,80]. In the present study, we found that vitamin A supplementation significantly increased the abundance of Lachnospiraceae NK4A136 group and Allobaculum, which is consistent with the studies by Xiao et al. [74] and Lee and Ko [81]. Lachnospiraceae NK4A136 group has been reported to be a discriminative feature of gut dysbiosis [82]. Allobaculum was strongly inversely correlated with the expression of inflammation markers (Saa3 and Pai1) [83]. Parasutterella may exert potential beneficial effects on intestinal mucosal homeostasis [84]. However, in contrast to our finding, vitamin A deficiency increased the abundance of Parasutterella in female mice [74]. This difference is likely attributed to the diet [85]. In this study, mink needed a large amount of fat in the diet to withstand cold weather in the winter [86], and diets containing a high content of animal proteins and saturated fats increased bile secretion, augmenting bile acids in the intestine [87]. In addition, the genus Parasutterella is reported to be associated with bile acid maintenance and cholesterol metabolism [84]. Therefore, the increase in Parasutterella may be due to the higher fat content in the diet, which will require an increased amount of bile acid secretion for digestion [88]. The Muribaculaceae family, previously named S24–7 based on its predicted potential to degrade complex carbohydrates, was previously grouped into three trophic guilds with different degradation capacities: a-glucans, complex plant cell wall glycans (hemicellulose and pectin) and host-derived glycans [89,90]. The genus Rummeliibacillus belongs to the Bacillaceae family, which was shown to possess a variety of open reading frames predicted to be involved in glycogen synthesis and cellulose metabolism, as suggested by metagenome sequencing and analysis [91]. These results suggest that vitamin A deficiency leads to the destruction of the gastrointestinal mucosal barrier and increases the risk of mucosal infection.
We also applied PICRUSt to predict the potential functions, and the results show that glycan biosynthesis and metabolism, transport and catabolism pathways were significantly more abundant in the vitamin A supplementation groups than those in the CON group at KEGG level 2. Symbiotic microorganisms that reside in the intestine are adept at foraging glycans and polysaccharides, including those in dietary plants (starch, hemicellulose and pectin) and host mucus (O-linked glycans) [92]. Muribaculaceae and Lachnospiraceae are the identified mucosal sugar foragers that actively use all or most O-glycan mucosal sugars [93]. As discussed above, the results show that the relative abundances of uncultured bacterium f Muribaculaceae and Rummeliibacillus significantly increased in the vitamin A supplementation groups, and they are reportedly associated with carbohydrate degradation, glycogen synthesis and cellulose metabolism. Hence, the results show that vitamin A may have the potential to regulate carbohydrate metabolism by the microbiota resident in the jejunum of mink. However, the limitation of this study is the relatively small size, which may affect the significant difference among the three groups, and studies with a large sample size are needed to further reveal the effect of vitamin A in regulating gut microbiota, which will provide more evidence of the role of vitamin A in affecting the growth and health of mink.
5. Conclusions
In conclusion, the present study revealed that 20,000 IU/kg vitamin A supplementation could promote mink growth by modulating intestinal development, and improving antioxidant capacity and intestinal mucosal barrier functions. We observed that dietary vitamin A supplementation can modulate the mink gut microbiome and host physiology to a healthier phenotype by increasing the relative abundance of beneficial bacteria, such as Akkermansia, Allobaculum and Lachnospiraceae NK4A136 group. Moreover, vitamin A deficiency had a negative impact on mink growth by decreasing the relative abundance of beneficial bacteria. However, the underlying mechanisms of vitamin A in immunity still need further investigation.
Author Contributions
Conceptualization, W.N., H.Z. and H.L.; methodology, W.N. and Q.Y.; software and formal analysis, H.S. (Huazhe Si); investigation, T.Z., H.S. (Hongpeng Shi) and Q.S.; writing—original draft preparation, W.N. and H.S. (Huazhe Si); writing—review and editing, H.L.; resources, G.L.; funding acquisition, H.L. and H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by Jilin Province Science and Technology Development Project (20200301019RQ) to HL, the Agricultural Science and Technology Innovation Project of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2020-ISAPS), the Natural Science Foundation of Hebei Province (No. C2019407111) and the Top Talent Project for Youths of Hebei Province (No. 180443), the High School Hundred Excellent Innovation Talent Program of Hebei Province (No. SLRC2019048), the Doctoral Startup Foundation of Hebei Normal University of Science & Technology (No. 2018YB018). Jilin Province Special Animal Breeding and Comprehensive Utilization Technology Innovation Center (20190902015).
Institutional Review Board Statement
All animal procedures were approved and authorized by the Animal Care Committee of the Institute of Special Economic Animal and Plant Science, the Chinese Academy of Agricultural Sciences and followed the requirements of the Welfare of Animals Kept for Fur Production (protocol number ISAPS-IACUC-2017-002D).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated in this study can be found in online repositories. The sequencing datasets generated during this study are available at NCBI BioProject: PRJNA646038.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Blomhoff R., Green M.H., Berg T., Norum K.R. Transport and Storage of Vitamin A. Science. 1990;250:399–404. doi: 10.1126/science.2218545. [DOI] [PubMed] [Google Scholar]
- 2.Bar-El Dadon S., Reifen R. Vitamin A and the Epigenome. Crit. Rev. Food Sci. Nutr. 2017;57:2404–2411. doi: 10.1080/10408398.2015.1060940. [DOI] [PubMed] [Google Scholar]
- 3.Polcz M.E., Barbul A. The Role of Vitamin A in Wound Healing. Nutr. Clin. Pract. 2019;34:695–700. doi: 10.1002/ncp.10376. [DOI] [PubMed] [Google Scholar]
- 4.Sirisinha S. The pleiotropic role of vitamin A in regulating mucosal immunity. Asian Pac. J. Allergy Immunol. 2015;33:71–89. [PubMed] [Google Scholar]
- 5.Timoneda J., Rodríguez-Fernández L., Zaragozá R., Marín M.P., Cabezuelo M.T., Torres L., Viña J.R., Barber T. Vitamin A Deficiency and the Lung. Nutrients. 2018;10:1132. doi: 10.3390/nu10091132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vijayaraghavan K. National Control Programme against Nutritional Blindness Due to Vitamin A Deficiency: Current Status & Future Strategy. Indian J. Med. Res. 2018;148:496–502. doi: 10.4103/ijmr.IJMR_1781_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Collins M.D., Mao G.E. Teratology of Retinoids. Annu. Rev. Pharmacol. Toxicol. 1999;39:399–430. doi: 10.1146/annurev.pharmtox.39.1.399. [DOI] [PubMed] [Google Scholar]
- 8.Penniston K.L., Tanumihardjo S.A. The Acute and Chronic Toxic Effects of Vitamin A. Am. J. Clin. Nutr. 2006;83:191–201. doi: 10.1093/ajcn/83.2.191. [DOI] [PubMed] [Google Scholar]
- 9.Shih M.Y.S., Kane M.A., Zhou P., Yen C.L.E., Streeper R.S., Napoli J.L., Farese R.V. Retinol Esterification by DGAT1 Is Essential for Retinoid Homeostasis in Murine Skin. J. Biol. Chem. 2009;284:4292–4299. doi: 10.1074/jbc.M807503200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chelstowska S., Widjaja-Adhi M.A.K., Silvaroli J.A., Golczak M. Molecular Basis for Vitamin A Uptake and Storage in Vertebrates. Nutrients. 2016;8:676. doi: 10.3390/nu8110676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saeed A., Dullaart R.P.F., Schreuder T.C.M.A., Blokzijl H., Faber K.N. Disturbed Vitamin A Metabolism in Non-Alcoholic Fatty Liver Disease (NAFLD) Nutrients. 2018;10:29. doi: 10.3390/nu10010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marriott B.P., Birt D.F., Stallings V.A., Yates A.A. Present Knowledge in Nutrition. 11th ed. Academic Press; Cambridge, MA, USA: 2020. pp. 73–91. [Google Scholar]
- 13.National Research Council . In: Nutrient Requirements of Mink and Foxes. 2nd ed. Council N.R., editor. National Academy Press; Washington DC, USA: 1982. [Google Scholar]
- 14.McDowell L.R. Vitamins in Animal Nutrition. Academic Press; Cambridge, MA, USA: 1989. pp. 347–364. [Google Scholar]
- 15.Compo N.R., Gomez D.E., Tapscott B., Weese J.S., Turner P.V. Fecal Bacterial Microbiota of Canadian Commercial Mink (Neovison Vison): Yearly, Life Stage, and Seasonal Comparisons. PLoS ONE. 2018;13:e0207111. doi: 10.1371/journal.pone.0207111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sibbald I.R., Sinclair D.G., Evans E.V., Smith D.L. The Rate of Passage of Feed through the Digestive Tract of the Mink. Can. J. Biochem. Physiol. 1962;40:1391–1394. doi: 10.1139/o62-155. [DOI] [PubMed] [Google Scholar]
- 17.Biasato I., Ferrocino I., Biasibetti E., Grego E., Dabbou S., Sereno A., Gai F., Gasco L., Schiavone A., Cocolin L., et al. Modulation of Intestinal Microbiota, Morphology and Mucin Composition by Dietary Insect Meal Inclusion in Free-Range Chickens. BMC Vet. Res. 2018;14:383. doi: 10.1186/s12917-018-1690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu X., Xu P., Ma C., Tang J., Zhang X. Gut Microbiota, Host Health, and Polysaccharides. Biotechnol. Adv. 2013;31:318–337. doi: 10.1016/j.biotechadv.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Sommer F., Bäckhed F. The Gut Microbiota—Masters of Host Development and Physiology. Nat. Rev. Microbiol. 2013;11:227–238. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 20.Nelson T.M., Rogers T.L., Carlini A.R., Brown M.V. Diet and Phylogeny Shape the Gut Microbiota of Antarctic Seals: A Comparison of Wild and Captive Animals. Environ. Microbiol. 2013;15:1132–1145. doi: 10.1111/1462-2920.12022. [DOI] [PubMed] [Google Scholar]
- 21.Pandit R.J., Hinsu A.T., Patel N.V., Koringa P.G., Jakhesara S.J., Thakkar J.R., Shah T.M., Limon G., Psifidi A., Guitian J., et al. Microbial Diversity and Community Composition of Caecal Microbiota in Commercial and Indigenous Indian Chickens Determined Using 16s rDNA Amplicon Sequencing. Microbiome. 2018;6:115. doi: 10.1186/s40168-018-0501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caputi V., Marsilio I., Filpa V., Cerantola S., Orso G., Bistoletti M., Paccagnella N., De Martin S., Montopoli M., Dall’Acqua S., et al. Antibiotic-Induced Dysbiosis of the Microbiota Impairs Gut Neuromuscular Function in Juvenile Mice: Gut Dysbiosis Impairs Neuromuscular Function. Br. J. Pharmacol. 2017;174:3623–3639. doi: 10.1111/bph.13965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ley R.E., Hamady M., Lozupone C., Turnbaugh P.J., Ramey R.R., Bircher J.S., Schlegel M.L., Tucker T.A., Schrenzel M.D., Knight R., et al. Evolution of Mammals and Their Gut Microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cantorna M.T., Snyder L., Arora J. Vitamin A and Vitamin D Regulate the Microbial Complexity, Barrier Function, and the Mucosal Immune Responses to Ensure Intestinal Homeostasis. Crit. Rev. Biochem. Mol. Biol. 2019;54:184–192. doi: 10.1080/10409238.2019.1611734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyer N., Vaishnava S. Vitamin A at the Interface of Host–Commensal–Pathogen Interactions. PLoS Pathog. 2019;15:e1007750. doi: 10.1371/journal.ppat.1007750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nan W.X., Si H.Z., Zhang H.H., Mu L.L., Li G.Y., Lou Y.J. Effect of Dietary Vitamin A Supplementation on Growth Performance, Nutrient Digestibility, Serum Parameters and Liver Histology of Growing-Furring Male Mink Kits (Neovison Vison) Anim. Feed Sci. Tech. 2021;276:114898. doi: 10.1016/j.anifeedsci.2021.114898. [DOI] [Google Scholar]
- 27.Broom D.M., Fraser A.F. Domestic Animal Behaviour and Welfare. 4th ed. CABI; Wallingford, UK: 2007. p. 308. [Google Scholar]
- 28.Livak K.J., Schmittgen T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Magoč T., Salzberg S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics. 2011;27:2957–2963. doi: 10.1093/bioinformatics/btr507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Peña A.G., Goodrich J.K., Gordon J.I., et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edgar R.C. UPARSE: Highly Accurate OTU Sequences from Microbial Amplicon Reads. Nat. Methods. 2013;10:996–998. doi: 10.1038/nmeth.2604. [DOI] [PubMed] [Google Scholar]
- 32.Quast C., Pruesse E., Yilmaz P., Gerken J., Schweer T., Yarza P., Peplies J., Glöckner F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012;41:D590–D596. doi: 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dufrene M., Legendre P. Species Assemblages and Indicator Species: The Need for a Flexible Asymmetrical Approach. Ecol. Monogr. 1997;67:345. doi: 10.2307/2963459. [DOI] [Google Scholar]
- 34.Fierer N., Lauber C.L., Zhou N., McDonald D., Costello E.K., Knight R. Forensic Identification Using Skin Bacterial Communities. Proc. Natl. Acad. Sci. USA. 2010;107:6477–6481. doi: 10.1073/pnas.1000162107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langille M.G., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., Clemente J.C., Burkepile D.E., Vega Thurber R.L., Knight R., et al. Predictive Functional Profiling of Microbial Communities Using 16S rRNA Marker Gene Sequences. Nat. Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Graphpad Prism. GraphPad Software, Inc.; San Diego, CA, USA: 2012. [Google Scholar]
- 37.SPSS (Statistical Product and Service Solutions) IBM SPSS Inc.; New York, NY, USA: 2016. [Google Scholar]
- 38.McCullough F.S.W., Northrop-Clewes C.A., Thurnham D.I. The Effect of Vitamin A on Epithelial Integrity. Proc. Nutr. Soc. 1999;58:289–293. doi: 10.1017/S0029665199000403. [DOI] [PubMed] [Google Scholar]
- 39.Esteban-Pretel G., Marín M.P., Cabezuelo F., Moreno V., Renau-Piqueras J., Timoneda J., Barber T. Vitamin A Deficiency Increases Protein Catabolism and Induces Urea Cycle Enzymes in Rats. J. Nutr. 2010;140:792–798. doi: 10.3945/jn.109.119388. [DOI] [PubMed] [Google Scholar]
- 40.Huda M.N., Ahmad S.M., Kalanetra K.M., Taft D.H., Alam M.J., Khanam A., Raqib R., Underwood M.A., Mills D.A., Stephensen C.B. Neonatal Vitamin A Supplementation and Vitamin A Status Are Associated with Gut Microbiome Composition in Bangladeshi Infants in Early Infancy and at 2 Years of Age. J. Nutr. 2019;149:1075–1088. doi: 10.1093/jn/nxz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tian Y., Nichols R.G., Cai J., Patterson A.D., Cantorna M.T. Vitamin A Deficiency in Mice Alters Host and Gut Microbial Metabolism Leading to Altered Energy Homeostasis. J. Nutr. Biochem. 2018;54:28–34. doi: 10.1016/j.jnutbio.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leoschke W.L. Nutrition and Nutritional Physiology of the Mink. Trafford Publishing; Bloomington, IN, USA: 2011. pp. 49–50. [Google Scholar]
- 43.Liang J.R., Dai H., Yang H.M., Yang Z., Wang Z.Y. The Effect of Dietary Vitamin A Supplementation in Maternal and Its Offspring on the Early Growth Performance, Liver Vitamin A Content, and Antioxidant Index of Goslings. Poult. Sci. 2019;98:6849–6856. doi: 10.3382/ps/pez432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ibrahim M., Mikail M.A., Ahmed I.A., Hazali N., Abdul Rasad M.S.B., Abdul Ghani R., Hashim R., Arief S.J., Md Isa M.L., Draman S. Comparison of the Effects of Three Different Baccaurea Angulata Whole Fruit Juice Doses on Plasma, Aorta and Liver MDA Levels, Antioxidant Enzymes and Total Antioxidant Capacity. Eur. J. Nutr. 2018;57:1817–1828. doi: 10.1007/s00394-017-1466-3. [DOI] [PubMed] [Google Scholar]
- 45.Liu Z., Liao L., Chen Q., Lin S., Luo Y., Qin T., Li J., Wang Q., Wu B., Huang Y., et al. Effects of Hericium Erinaceus Polysaccharide on Immunity and Apoptosis of the Main Immune Organs in Muscovy Duck Reovirus-Infected Ducklings. Int. J. Biol. Macromol. 2021;171:448–456. doi: 10.1016/j.ijbiomac.2020.12.222. [DOI] [PubMed] [Google Scholar]
- 46.Naylor H.M., Newcomer M.E. The Structure of Human Retinol-Binding Protein (RBP) with Its Carrier Protein Transthyretin Reveals an Interaction with the Carboxy Terminus of RBP. Biochemistry. 1999;38:2647–2653. doi: 10.1021/bi982291i. [DOI] [PubMed] [Google Scholar]
- 47.Kurata M., Suzuki M., Agar N.S. Antioxidant Systems and Erythrocyte Life-Span in Mammals. Comp. Biochem. Physiol. Part B Comp. Biochem. 1993;106:477–487. doi: 10.1016/0305-0491(93)90121-K. [DOI] [PubMed] [Google Scholar]
- 48.Dawson M.I. The Importance of Vitamin A in Nutrition. Curr. Pharm. Des. 2000;6:311–325. doi: 10.2174/1381612003401190. [DOI] [PubMed] [Google Scholar]
- 49.Cartmel B., Moon T.E., Levine N. Effects of Long-Term Intake of Retinol on Selected Clinical and Laboratory Indexes. Am. J. Clin. Nutr. 1999;69:937–943. doi: 10.1093/ajcn/69.5.937. [DOI] [PubMed] [Google Scholar]
- 50.Green A.S., Fascetti A.J. Meeting the Vitamin A Requirement: The Efficacy and Importance of β-Carotene in Animal Species. Sci. World J. 2016;2016:7393620. doi: 10.1155/2016/7393620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang H., Ni X., Qing X., Zeng D., Luo M., Liu L., Li G., Pan K., Jing B. Live Probiotic Lactobacillus Johnsonii BS15 Promotes Growth Performance and Lowers Fat Deposition by Improving Lipid Metabolism, Intestinal Development, and Gut Microflora in Broilers. Front. Microbiol. 2017;8:1073. doi: 10.3389/fmicb.2017.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen C., Jung B., Kim W.K. Effects of Lysophospholipid on Growth Performance, Carcass Yield, Intestinal Development, and Bone Quality in Broilers. Poult. Sci. 2019;98:3902–3913. doi: 10.3382/ps/pez111. [DOI] [PubMed] [Google Scholar]
- 53.Wu B., Cui H., Peng X., Fang J., Zuo Z., Deng J., Huang J. Dietary Nickel Chloride Restrains the Development of Small Intestine in Broilers. Biol. Trace Elem. Res. 2013;155:236–246. doi: 10.1007/s12011-013-9792-7. [DOI] [PubMed] [Google Scholar]
- 54.Blaner W.S., Li Y., Brun P.J., Yuen J.J., Lee S.A., Clugston R.D. Vitamin A Absorption, Storage and Mobilization. Subcell. Biochem. 2016;81:95–125. doi: 10.1007/978-94-024-0945-1_4. [DOI] [PubMed] [Google Scholar]
- 55.Gannon B.M., Davis C.R., Nair N., Grahn M., Tanumihardjo S.A. Single High-Dose Vitamin A Supplementation to Neonatal Piglets Results in a Transient Dose Response in Extrahepatic Organs and Sustained Increases in Liver Stores. J. Nutr. 2017;147:798. doi: 10.3945/jn.117.247577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mielke L.A., Jones S.A., Raverdeau M., Higgs R., Stefanska A., Groom J.R., Misiak A., Dungan L.S., Sutton C.E., Streubel G., et al. Retinoic Acid Expression Associates with Enhanced IL-22 Production by γδ T Cells and Innate Lymphoid Cells and Attenuation of Intestinal Inflammation. J. Exp. Med. 2013;210:1117–1124. doi: 10.1084/jem.20121588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang S., Xia P., Chen Y., Qu Y., Xiong Z., Ye B., Du Y., Tian Y., Yin Z., Xu Z., et al. Regulatory Innate Lymphoid Cells Control Innate Intestinal Inflammation. Cell. 2017;171:201–216.e18. doi: 10.1016/j.cell.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 58.Grizotte-Lake M., Zhong G., Duncan K., Kirkwood J., Iyer N., Smolenski I., Isoherranen N., Vaishnava S. Commensals Suppress Intestinal Epithelial Cell Retinoic Acid Synthesis to Regulate Interleukin-22 Activity and Prevent Microbial Dysbiosis. Immunity. 2018;49:1103–1115.e6. doi: 10.1016/j.immuni.2018.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Otte J.M., Cario E., Podolsky D.K. Mechanisms of Cross Hyporesponsiveness to Toll-like Receptor Bacterial Ligands in Intestinal Epithelial Cells. Gastroenterology. 2004;126:1054–1070. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Osanai M., Nishikiori N., Murata M., Chiba H., Kojima T., Sawada N. Cellular Retinoic Acid Bioavailability Determines Epithelial Integrity: Role of Retinoic Acid Receptor Alpha Agonists in Colitis. Mol. Pharmacol. 2007;71:250–258. doi: 10.1124/mol.106.029579. [DOI] [PubMed] [Google Scholar]
- 61.Li X.F., Zhang X.J., Zhang C., Wang L.N., Li Y.R., Zhang Y., He T.T., Zhu X.Y., Cui L.L., Gao B.L. Ulinastatin Protects Brain against Cerebral Ischemia/Reperfusion Injury through Inhibiting MMP-9 and Alleviating Loss of ZO-1 and Occludin Proteins in Mice. Exp. Neurol. 2018;302:68–74. doi: 10.1016/j.expneurol.2017.12.016. [DOI] [PubMed] [Google Scholar]
- 62.Sommer A. Vitamin A Deficiency and Clinical Disease: An Historical Overview. J. Nutr. 2008;138:1835–1839. doi: 10.1093/jn/138.10.1835. [DOI] [PubMed] [Google Scholar]
- 63.Frazier T.H., DiBaise J.K., McClain C.J. Gut Microbiota, Intestinal Permeability, Obesity-Induced Inflammation, and Liver Injury. J. Parenter. Enter. Nutr. 2011;35(Suppl. 5):14S–20S. doi: 10.1177/0148607111413772. [DOI] [PubMed] [Google Scholar]
- 64.Suez J., Korem T., Zeevi D., Zilberman-Schapira G., Thaiss C.A., Maza O., Israeli D., Zmora N., Gilad S., Weinberger A., et al. Artificial Sweeteners Induce Glucose Intolerance by Altering the Gut Microbiota. Nature. 2014;514:181–186. doi: 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 65.Turnbaugh P.J., Ley R.E., Mahowald M.A., Magrini V., Mardis E.R., Gordon J.I. An Obesity-Associated Gut Microbiome with Increased Capacity for Energy Harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 66.Ley R.E., Turnbaugh P.J., Klein S., Gordon J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 67.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., et al. Cross-Talk between Akkermansia Muciniphila and Intestinal Epithelium Controls Diet-Induced Obesity. Proc. Natl. Acad. Sci. USA. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in Gut Microbiota Control Metabolic Endotoxemia-Induced Inflammation in High-Fat Diet–Induced Obesity and Diabetes in Mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 69.Bahl M.I., Hammer A.S., Clausen T., Jakobsen A., Skov S., Andresen L. The Gastrointestinal Tract of Farmed Mink (Neovison Vison) Maintains a Diverse Mucosa-Associated Microbiota Following a 3-Day Fasting Period. Microbiol. Open. 2017;6:e00434. doi: 10.1002/mbo3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.An C., Okamoto Y., Xu S., Eo K.Y., Kimura J., Yamamoto N. Comparison of Fecal Microbiota of Three Captive Carnivore Species Inhabiting Korea. J. Vet. Med. Sci. 2017;79:542–546. doi: 10.1292/jvms.16-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peng Y., Zhang Z., Li H., Li S., Shi Q., Zhang J. Assessment of Fecal Microbiota in Farmed Silver Fox (Vulpes Vulpes Fulva) and Raccoon Dog (Nyctereutes Procyonoides) Acta Agric. Scand. Sect. A Anim. Sci. 2018;68:142–151. doi: 10.1080/09064702.2019.1637451. [DOI] [Google Scholar]
- 72.Peng Y., Shi Q., Wang Y., Zhang F., Ji Z., Zhang J. Dietary Probiotics Have Different Effects on the Composition of Fecal Microbiota in Farmed Raccoon Dog (Nyctereutes Procyonoides) and Silver Fox (Vulpes Vulpes Fulva) BMC Microbiol. 2019;19:109. doi: 10.1186/s12866-019-1491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Guo X., Lei H., Zhang K., Ke F., Song C. Diversification of Animal Gut Microbes and NRPS Gene Clusters in Some Carnivores, Herbivores and Omnivores. Biotechnol. Biotec. Equip. 2020;34:1280–1287. doi: 10.1080/13102818.2020.1835536. [DOI] [Google Scholar]
- 74.Xiao L., Chen B., Feng D., Yang T., Li T., Chen J. TLR4 May Be Involved in the Regulation of Colonic Mucosal Microbiota by Vitamin A. Front. Microbiol. 2019;10:268. doi: 10.3389/fmicb.2019.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mariat D., Firmesse O., Levenez F., Guimarăes V., Sokol H., Doré J., Corthier G., Furet J.-P. The Firmicutes/Bacteroidetes Ratio of the Human Microbiota Changes with Age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Belkaid Y., Hand T.W. Role of the Microbiota in Immunity and Inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bai Y., Wang S., Wang X., Weng Y., Fan X., Sheng H., Zhu X., Lou L., Zhang F. The Flavonoid-Rich Quzhou Fructus Aurantii Extract Modulates Gut Microbiota and Prevents Obesity in High-Fat Diet-Fed Mice. Nutr. Diabetes. 2019;9:30. doi: 10.1038/s41387-019-0097-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lindenberg F., Krych L., Fielden J., Kot W., Frøkiær H., van Galen G., Nielsen D.S., Hansen A.K. Expression of Immune Regulatory Genes Correlate with the Abundance of Specific Clostridiales and Verrucomicrobia Species in the Equine Ileum and Cecum. Sci. Rep. 2019;9:1–10. doi: 10.1038/s41598-019-49081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fujio-Vejar S., Vasquez Y., Morales P., Magne F., Vera-Wolf P., Ugalde J.A., Navarrete P., Gotteland M. The Gut Microbiota of Healthy Chilean Subjects Reveals a High Abundance of the Phylum Verrucomicrobia. Front. Microbiol. 2017;8:1221. doi: 10.3389/fmicb.2017.01221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dao M.C., Everard A., Aron-Wisnewsky J., Sokolovska N., Prifti E., Verger E.O., Kayser B.D., Levenez F., Chilloux J., Hoyles L., et al. Akkermansia Muciniphila and Improved Metabolic Health during a Dietary Intervention in Obesity: Relationship with Gut Microbiome Richness and Ecology. Gut. 2016;65:426–436. doi: 10.1136/gutjnl-2014-308778. [DOI] [PubMed] [Google Scholar]
- 81.Lee H., Ko G. New Perspectives Regarding the Antiviral Effect of Vitamin A on Norovirus Using Modulation of Gut Microbiota. Gut Microbes. 2017;8:616–620. doi: 10.1080/19490976.2017.1353842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Caparrós-Martín J.A., Lareu R.R., Ramsay J.P., Peplies J., Reen F.J., Headlam H.A., Ward N.C., Croft K.D., Newsholme P., Hughes J.D., et al. Statin Therapy Causes Gut Dysbiosis in Mice through a PXR-Dependent Mechanism. Microbiome. 2017;5:95. doi: 10.1186/s40168-017-0312-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ravussin Y., Koren O., Spor A., LeDuc C., Gutman R., Stombaugh J., Knight R., Ley R.E., Leibel R.L. Responses of Gut Microbiota to Diet Composition and Weight Loss in Lean and Obese Mice. Obesity (Silver Spring) 2012;20:738–747. doi: 10.1038/oby.2011.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ju T., Kong J.Y., Stothard P., Willing B.P. Defining the Role of Parasutterella, a Previously Uncharacterized Member of the Core Gut Microbiota. ISME J. 2019;13:1520–1534. doi: 10.1038/s41396-019-0364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lindsay E.C., Metcalfe N.B., Llewellyn M.S. The Potential Role of the Gut Microbiota in Shaping Host Energetics and Metabolic Rate. J. Anim. Ecol. 2020;89:2415–2426. doi: 10.1111/1365-2656.13327. [DOI] [PubMed] [Google Scholar]
- 86.Cui H., Zhang T.T., Nie H., Wang Z.C., Zhang X.L., Shi B., Yang F.H., Gao X.H. Effects of sources and concentrations of zinc on growth performance, nutrient digestibility, and fur quality of growing–furring female mink (Mustela vison) J. Anim. Sci. 2017;95:5420–5429. doi: 10.2527/jas2017.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Di Ciaula A., Garruti G., Lunardi Baccetto R., Molina-Molina E., Bonfrate L., Wang D.Q.H., Portincasa P. Bile Acid Physiology. Ann. Hepatol. 2017;16:s4–s14. doi: 10.5604/01.3001.0010.5493. [DOI] [PubMed] [Google Scholar]
- 88.Kilburn L.R., Koester L.R., Schmitz-Esser S., Serão N.V.L., Rossoni Serão M.C. High-Fat Diets Led to OTU-Level Shifts in Fecal Samples of Healthy Adult Dogs. Front. Microbiol. 2020;11:564160. doi: 10.3389/fmicb.2020.564160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ormerod K.L., Wood D.L.A., Lachner N., Gellatly S.L., Daly J.N., Parsons J.D., Dal’Molin C.G.O., Palfreyman R.W., Nielsen L.K., Cooper M.A., et al. Genomic Characterization of the Uncultured Bacteroidales Family S24-7 Inhabiting the Guts of Homeothermic Animals. Microbiome. 2016;4:36. doi: 10.1186/s40168-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lagkouvardos I., Lesker T.R., Hitch T.C.A., Gálvez E.J.C., Smit N., Neuhaus K., Wang J., Baines J.F., Abt B., Stecher B., et al. Sequence and Cultivation Study of Muribaculaceae Reveals Novel Species, Host Preference, and Functional Potential of This yet Undescribed Family. Microbiome. 2019;7:28. doi: 10.1186/s40168-019-0637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Opdahl L. Master’s Thesis. South Dakota State University; Brookings, SD, USA: 2017. Identification of Candidate Cellulose Utilizing Bacteria from the Rumen of Beef Cattle, Using Bacterial Community Profiling and Metagenomics. [Google Scholar]
- 92.Koropatkin N.M., Cameron E.A., Martens E.C. How Glycan Metabolism Shapes the Human Gut Microbiota. Nat. Rev. Microbiol. 2012;10:323–335. doi: 10.1038/nrmicro2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pereira F.C., Wasmund K., Cobankovic I., Jehmlich N., Herbold C.W., Lee K.S., Sziranyi B., Vesely C., Decker T., Stocker R., et al. Rational Design of a Microbial Consortium of Mucosal Sugar Utilizers Reduces Clostridiodes Difficile Colonization. Nat. Commun. 2020;11:5104. doi: 10.1038/s41467-020-18928-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated in this study can be found in online repositories. The sequencing datasets generated during this study are available at NCBI BioProject: PRJNA646038.