Abstract
OmpA, which encodes outer membrane protein A (OmpA), is the most abundant transcript in Stenotrophomonas maltophilia based on transcriptome analyses. The functions of OmpA, including adhesion, biofilm formation, drug resistance, and immune response targets, have been reported in some microorganisms, but few functions are known in S. maltophilia. This study aimed to elucidate the relationship between OmpA and swimming motility in S. maltophilia. KJΔOmpA, an ompA mutant, displayed compromised swimming and failure of conjugation-mediated plasmid transportation. The hierarchical organization of flagella synthesis genes in S. maltophilia was established by referencing the Pseudomonas aeruginosa model and was confirmed using mutant construction, qRT-PCR, and functional assays. Distinct from the P. aeruginosa model, rpoN, rather than fleQ and fliA, was at the top of the flagellar regulatory cascade in S. maltophilia. To elucidate the underlying mechanism responsible for ΔompA-mediated swimming compromise, transcriptome analysis of KJ and KJΔOmpA was performed and revealed rpoN downregulation in KJΔOmpA as the key element. The involvement of rpoN in ΔompA-mediated swimming compromise was verified using rpoN complementation, qRT-PCR, and function assays. Collectively, OmpA, which contributes to bacterial conjugation and swimming, is a promising target for adjuvant design in S. maltophilia.
Keywords: Stenotrophomonas maltophilia, OmpA, swimming, flagellum
1. Introduction
Gram-negative bacteria are surrounded by two membranes, which confine the periplasmic space containing the peptidoglycan [1]. This double membrane may be impenetrable to antibiotics, noxious agents, or other foreign compounds [2,3]. The outer membrane (OM) is a complex organelle that provides a barrier to protect bacteria from hazards in their environment. The OM of Gram-negative bacteria is unique in its composition and shows asymmetrical lipid distribution. Approximately 50% of the OM mass consists of proteins, either integral membrane proteins or membrane-anchored lipoproteins. Thus, as much as 3% of the Gram-negative bacterial genome may encode outer membrane proteins (OMPs) [4]. Nearly all of the integral membrane proteins of the OM assume a β-barrel architecture with long loops between the strands on the extracellular side and short loops on the periplasmic side. β-barrel OMPs are classified into two major types, classical porins and slow porins, based on their physiological roles. Classical porins serve as ports of entry for hydrophilic molecules in a non-selective fashion, facilitating the diffusion of nutrients and extrusion of waste products [5]. OmpF and OmpC are the most studied classical porins in Gram-negative bacteria and are highly conserved throughout the Enterobacterales [6,7]. In contrast, slow porins have very low permeability, and molecular transportation is not their main function. Escherichia coli OmpA, Acinetobacter baumannii OmpA, and Pseudomonas aeruginosa OprF are examples of slow porins. OmpA is a surface-exposed porin protein with a high copy number among the OMPs of E. coli [8]. Its N-terminal domain comprises eight transmembrane antiparallel β-strands, and its C-terminal domain interacts with the peptidoglycan layer [9,10]. OmpA is involved in adhesion, invasion, biofilm formation, drug resistance, and bacteriophage entry [11,12,13,14]. In addition, OmpA acts as an immune target and induces host immune responses [15,16]; thus, it is the most popular vaccine candidate widely developed in E. coli, Klebsiella pneumoniae, and A. baumannii [17,18,19].
Swimming motility is a critical aspect of bacterial pathogenesis and is required for host colonization. It is also crucial for many biological functions including nutrient acquisition, stress avoidance, and sexual reproduction [20,21]. The flagellum is the most important organelle for bacterial swimming. It is composed of a basal body, hook, and a long, thin filament. The basal body, consisting of rotors and a stator, is embedded in the bacterial membrane. The hook is a joint that connects the basal body and filament. Flagellin is the subunit protein of the filament, which is a whip-like appendage that enables bacterial motility. Bacterial flagellum assembly requires the expression of nearly 60 genes in multiple operons, which are clustered at several loci on the chromosome [22]. Studies on flagellum synthesis have been carried out in several bacteria, including E. coli, Salmonella typhimurium [23], Caulobacter crescentus [24], Vibrio cholerae [25], V. parahaemolyticuss [26], and P. aeruginosa [27]. Flagellum synthesis has been hierarchically organized. A transcription of flagellum synthesis genes forms an ordered cascade whereby a gene located at a higher level must first be expressed before that of the next level. In the hierarchical cascade, several checkpoints are set to ensure that flagellar components are expressed and assembled sequentially. Flagellum synthesis is hierarchically regulated at three levels in E. coli [28] and is hierarchically classified into four classes in C. crescentus [24], V. cholerae [25], and P. aeruginosa [27]. In addition to a functional flagellum, effective swimming relies on the bacterial repertoire for sensing environmental conditions. Thus, a pathogen generally harbors several efficient signaling systems to sense environmental stimuli or quorum sensing (QS) signals, direct their movement to attractants, and avoid repellents.
Stenotrophomonas maltophilia, widely distributed in nature and in hospitals, has emerged as a global opportunistic Gram-negative pathogen, especially in patients with cystic fibrosis and immunocompromised individuals [29]. S. maltophilia is considered to be a low-virulence pathogen; however, the treatment of an S. maltophilia infection is a major challenge due to its intrinsic resistance to several antibiotics that are commonly used in clinics [30]. The reported resistance mechanisms in S. maltophilia include poor membrane permeability, antibiotic hydrolysis or modification, and efflux pump overexpression [31]. Thus, it is increasingly challenging for physicians to use conventional therapies to effectively treat S. maltophilia infections [32]. Novel convincing targets in S. maltophilia are greatly needed for the development of antibiotics, adjuvants, and even vaccines. Transcriptome analysis of S. maltophilia KJ was performed in our recent study, and the results demonstrated that ompA (Smlt0955) has the greatest transcript abundance in logarithmically grown KJ cells [33]. Given our understanding of OmpA in other Gram-negative bacteria, the OmpA of S. maltophilia is a prospective candidate to be first considered for the development of antibiotics, adjuvants, or vaccines. However, the current understanding of OmpA in S. maltophilia is relatively deficient, except for the reports by Li et al. that focused on the immunogenic properties of OmpA and the host protective immune response [34,35]. Given that OmpA is the most abundant OMP in S. maltophilia, it should be a critical contributor to the maintenance of membrane integrity. Bacterial membrane integrity has a significant impact on flagellum assembly and swimming motility. In this study, the link between OmpA and swimming motility in S. maltophilia was investigated. We revealed that the deletion of ompA resulted in swimming compromise and thus conducted experiments aimed at elucidating the underlying molecular mechanism.
2. Materials and Methods
2.1. Bacterial Strains, Media, Plasmids, and Primers
The bacterial strains, plasmids, and primers used in this study are listed in Table S1. S. mlatophilia KJ was an isolate isolated from sputum and identified using the PhoenixTM100 system (Becton Dickinson).
2.2. Construction of in-Frame Deletion Mutants
The in-frame deletion mutants were constructed using double cross-over homologous recombination. The DNA fragments flanking the deleted genes were amplified using PCR and subsequently cloned into pEX18Tc to generate the mutagenic plasmids. The primers set for the construction of mutagenic plasmids were OmpAN-F/OmpAN-R and OmpAC-F/OmpAC-R for pΔOmpA, FliC1N-F/FliC1N-R and FliC3C-F/FliC3C-R for pΔFliC1C2C3, RpoNN-F/RpoNN-R and RpoNC-F/RpoNC-R for pΔRpoN, FleQN-F/FleQN-R and FleQC-F/FleQC-R for pΔFleQ, as well as FliAN-F/FliAN-R and FliAC-F/FliAC-R for pΔFliA (Table S1). Mutagenic plasmids were transferred into S. maltophilia by conjugation. The plasmid’s conjugation, transconjugant’s selection, and mutant’s confirmation were carried out as described previously [36].
2.3. Construction of KJL2::OmpAΔOmpA and KJL2::RpoNΔOmpA
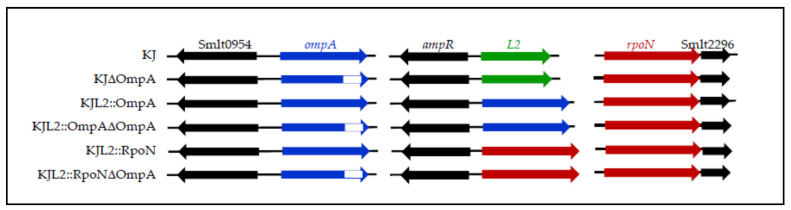
As plasmid transportation by conjugation is unavailable in the ompA mutant (KJΔOmpA), an alternative strategy was designed for gene expression in KJΔOmpA. First, the gene intended to be expressed in KJΔOmpA was chromosomally replaced with the L2 gene using double cross-over homologous recombination; the prototype chromosomal ompA gene was then deleted. For this purpose, we first constructed a recombinant plasmid, pEXCJ1. The 353-bp and 376-bp DNA fragments upstream and downstream of the L2 gene were obtained by PCR using primers pEXCJ1N-F/pEXCJ1N-R and pEXCJ1C-F/pEXCJ1C-R (Table S1), respectively, and were subsequently cloned into pEX18Tc to yield pEXCJ1. The multiple cloning sites (SphI/PstI/SalI/XbaI/BamHI/SmaI/KpnI/SacI) of pEX18Tc were used for cloning the exotic gene intended to be expressed in KJΔOmpA.
The intact ompA and rpoN genes were amplified by PCR using the primer sets OmpA-F/OmpA-R and RpoN-F/RpoN-R (Table S1), respectively, and were then cloned into pEXCJ1 to yield pCJ1-OmpA and pCJ1-RpoN. The plasmids pCJ1-OmpA and pCJ1-RpoN were transferred into S. maltophilia KJ to generate KJL2::OmpA and KJL2::RpoN, respectively. The prototype chromosomal ompA gene was deleted from KJL2::OmpA and KJL2::RpoN to yield KJL2::OmpAΔOmpA and KJL2::RpoNΔOmpA, respectively.
2.4. Construction of PrpoN-xylE Transcription Fusion Plasmid, pRpoNxylE
The 576-bp DNA fragment upstream of the rpoN gene was obtained by PCR using primer sets RpoNN-F/RpoNN-R (Table S1) and cloned into pRKxylE, a xylE reporter plasmid, to generate pRpoNxylE. The plasmid pRpoNxylE was mobilized into S. maltophilia strains indicated for a promoter activity assay.
2.5. OMP Preparation and SDS-PAGE
Logarithmic-phase cells were harvested and OMPs were prepared using the N-lauroylsarcosine method described previously [37]. The purified OMPs were boiled for 5 min at 100 °C and then separated using SDS-PAGE electrophoresis in a 15% polyacrylamide gel. The gel was stained with 0.1% Coomassie brilliant blue R250 (Bio-Rad) and de-stained with 40% methanol/10% glacial acetic acid for the visualization of proteins.
2.6. Catechol 2,3-Dioxygenase (C23O) Activity Assay
Catechol-2,3-dioxygenase is encoded by the xylE gene, and its activity was measured using 100 mM catechol as the substrate, as described previously [38]. The hydrolysis rate of catechol was calculated using 44,000 M−1cm−1 as the extinction coefficient. One unit of enzyme activity (U) was defined as the amount of enzyme that converts 1 nmole of substrate per minute. The specific activity was expressed as U/OD450 nm.
2.7. Swimming Assay
Five µL of a logarithmically grown bacterial cell culture were inoculated onto the swimming agar (1% tryptone, 0.5% NaCl, and 0.15% agar). The plates were incubated at 37 °C for 48 h and the diameters of the swimming zones (mm) were recorded.
2.8. Flagella Staining
We analyzed the presence of flagella using negative staining with 1% phosphotungstic acid (pH 7.4) and observed using transmission electron microscopy (TEM) (Hitachi H-7650 microscope), as described previously [39].
2.9. Preparation of Polyclonal Anti-Rabbit Anti-FliC3 Antibody
Of the three fliC homologue genes, fliC3 (Smlt2304) is annotated as fliC in the S. maltophilia K279a genome [40]; thus, it was selected as the candidate for the preparation of a polyclonal antibody. The full coding sequence of fliC3 was amplified by PCR using the primer sets FliC3his-F and FliC3his-R (Table S1) and ligated to the vector pET-24b, yielding pETFliC3. For expression, the recombinant plasmid pETFliC3 was transformed into E. coli BL21(DE3). The logarithmically grown E. coli BL21(DE3)(pETFliC3) cells were treated with 1 mM IPTG for 3 h and the fusion proteins FliC3-6His were purified with Ni-NTA resin. Antibodies against the FliC3-6His protein were raised by the immunization of New Zealand rabbits with the purified FliC3-6His protein.
2.10. SDS–PAGE and Western Blot Analysis
The logarithmically grown bacterial cells were disrupted via sonication. Intracellular proteins were separated using electrophoresis in 15% (w/v) SDS–PAGE, transferred onto polyvinylidene difluoride (PVDF) membranes, and probed with anti-FliC3 and anti-RpoA antibodies. The protein RpoA served as an intracellular control.
2.11. Transcriptome Analysis
Overnight cultures of KJ and KJΔOmpA were subcultured into fresh LB at an initial OD450 nm of 0.15 and incubated for 5 h to a logarithmic phase. Total RNA isolation, rRNA depletion, adapter-ligated cDNA library construction and enrichment, and cDNA sequencing were performed as described previously [41]. The sequencing reads were mapped to the S. maltophilia K279a genome. RNA-seq results were analyzed using a CLC Genomics Workbench v 6.0 (CLC Bio) and presented as Reads Per Kilobase per Million mapped reads (RPKM).
2.12. Quantitative Reverse Transcription-PCR (qRT-PCR)
DNA-free RNA for qRT-PCR was prepared from logarithmically grown bacterial cells, as described previously [42]. The primers used for the qRT-PCR of each gene are listed in Table S1. The relative transcription level was calculated using the comparative CT (ΔΔCT) method [43] and 16S rRNA was used as the endogenous control. Each result represents the average of three independent determinations.
3. Results
3.1. Bioinformatics Analysis of OmpA
The transcriptome analysis of KJ cells in the logarithmic phase was performed in our recent study [33]. OmpA (Smlt0955) showed the greatest transcript abundance in wild-type KJ under our experimental conditions. The presence of any protein in such a large amount suggests that it may play a significant role in bacteria. Based on this, we investigated the functions of OmpA and proposed some innovative aspects for this OMP.
OmpA, encoding a 366-amino acid β-barrel OMP, is well conserved in all of the sequenced S. maltophilia genomes. BLAST analysis showed that OmpAs of S. maltophilia strains shared 86 to 99% protein identities. However, S. maltophilia OmpA showed relatively lower identity with its homologues in other bacterial species, such as 22% identity with the OmpA of E. coli and 32% identity with the OprF of P. aeruginosa PAO1.
3.2. OmpA Deletion Impairs Bacterial Conjugation
To elucidate the function of OmpA in S. maltophilia, an in-frame deletion mutant of ompA, KJΔOmpA, was constructed by deleting the OmpA-like domain (amino acids 297–357) (Figure 1). A recombinant plasmid, pOmpA, containing an intact ompA gene was prepared for the complementary assay of KJΔOmpA. Despite several attempts to introduce pOmpA back into KJΔOmpA by conjugation, no transconjugant was obtained. Our attempts to transfer the empty plasmid, pRK415, into KJΔOmpA by conjugation also failed. In contrast, the plasmid pOmpA was successfully transferred into wild-type KJ, yielding KJ(pOmpA). Collectively, the failure of the conjugation-mediated plasmid transfer appeared to originate from the lost OmpA function, implying that OmpA is a critical OMP for conjugation in S. maltophilia.
Figure 1.
Genomic organization of KJ and its derived ompA-associated constructs. The orientation of the gene is indicated by the arrow. The white box indicates the deleted region of the ompA gene.
An alternative strategy was applied for the complementary assay. We first generated a conditional construct of S. maltophilia KJ, KJL2::OmpA, in which the chromosomal L2 gene was replaced with an intact ompA gene (Figure 1). Thus, in this construct, the ompA gene was driven by the L2 promoter, PL2. PL2 has basal-level activity in the absence of β-lactam and can be upregulated upon β-lactam challenge [44]. Next, the prototype ompA gene in KJL2::OmpA was deleted via a double cross-over homologous recombination to yield KJL2::OmpAΔOmpA (Figure 1), which was regarded as an alternative complementary strain of the mutant KJΔOmpA. The OMP profiles of wild-type KJ, mutant KJΔOmpA, and the complementary strain KJL2::OmpAΔOmpA were revealed using SDS-PAGE (Figure S1). Compared with wild-type KJ, a band corresponding to 38 kDa was absent in the KJΔOmpA protein profiles, but was visible in KJL2::OmpAΔOmpA. The 38-kDa protein was verified as the OmpA protein using LC-MS/MS.
3.3. OmpA Deletion Attenuates Swimming Motility
The growth curves of wild-type KJ, KJΔOmpA, and KJL2::OmpAΔOmpA were determined during a period of 24 h, and were found to be undistinguishable. This indicated that ompA inactivation had no obvious effect on bacterial growth.
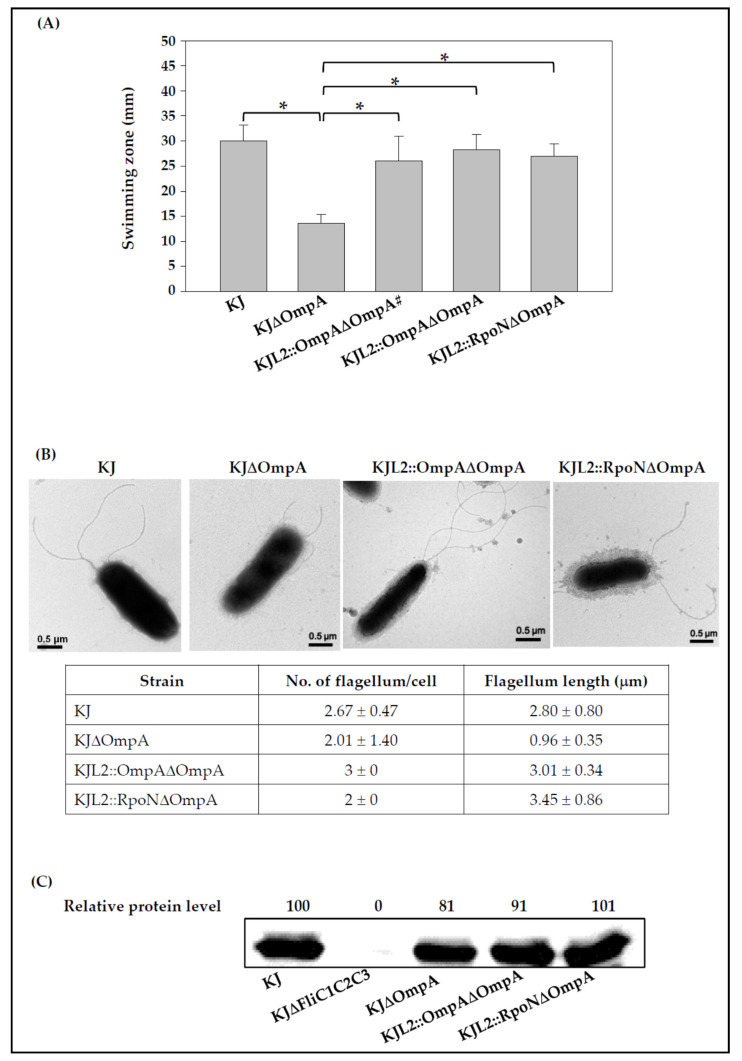
Given that OmpA is the most abundant transcript revealed using transcriptome analysis, envelope integrity may be compromised in an OmpA protein-null mutant. An intact envelope is a prerequisite for flagellar assembly and swimming motility; thus, we were interested in understanding the impact of ompA deletion on swimming motility. The swimming motilities of KJ, KJΔOmpA, and KJL2::OmpAΔOmpA were assessed. Given that the complementary ompA gene in KJL2::OmpAΔOmpA was driven by the L2 promoter, a preliminary test was performed to determine the β-lactam concentration at which ompA can be expressed in the KJL2::OmpAΔOmpA. The swimming motility of KJL2::OmpAΔOmpA was tested with and without cefuroxime at different concentrations. Unexpectedly, KJL2::OmpAΔOmpA displayed comparable swimming motility in cefuroxime-free and cefuroxime-containing plates (Figure 2A), indicating that the ompA of KJL2::OmpAΔOmpA expressed by PL2, without β-lactam challenge, is sufficient to compensate for the functional defect caused by the ompA deletion. The mechanism underlying this phenotype will be explained later. Thus, the following functional experiments were carried out under β-lactam-free conditions. Compared to the 30 ± 3.2 mm swimming zone in wild-type KJ, a 13.6 ± 1.8 mm swimming zone was observed in KJΔOmpA (Figure 2A). The complementary strain KJL2::OmpAΔOmpA almost reverted the swimming motility to a wild-type level (Figure 2A).
Figure 2.
The swimming motility, flagellum morphology, and intracellular flagellin proteins levels of wild-type KJ, its derived ompA mutant, and complementary strains. Overnighted cultured bacterial cells were inoculated into fresh LB broth and then grown for 5 h before testing. (A) Swimming motility. Five μL of bacterial cell suspension were inoculated into swimming agar and then incubated at 37 °C for 48 h. The swimming zones were recorded. #, Cefuroxime of 50 μg/mL was added into the swimming agar. The data are the means of three independent experiments. The error bars indicate the standard deviations of three triplicate samples. *, p < 0.05, significance calculated using the Student’s t-test. (B) Flagella morphology. The flagella were negatively stained with 1% phosphotungstic acid (pH 7.4) and observed using TEM. The average flagellum numbers per cell and flagellum length were calculated from at least four cells. (C) Western blotting of flagellin protein levels in a whole-cell extract. The proteins of a whole-cell extract were separated using SDS-PAGE and immunoblotting with anti-FliC3 and anti-RpoA antibodies. The RpoA protein served as an intracellular control. The relative protein level of the FliC protein was normalized to the RpoA protein.
The flagellar structures of wild-type KJ, KJΔOmpA, and KJL2::OmpAΔOmpA were observed using transmission electron microscopy (TEM). Wild-type KJ displayed a tuft of 2–3 polar flagella (Figure 2B), which is consistent with previous reports [45]. In contrast, KJΔOmpA cells had short flagella that were sometimes located at a subpolar position. The complementary strain KJL2::OmpAΔOmpA restored the wild-type flagellar structure (Figure 2B). Next, flagellin protein levels were evaluated using a Western blotting assay. Considering the necessity of the FliC negative control for Western blotting, we aimed to construct a FliC-null mutant. In a genome-wide search, we noticed three flagellin-like genes in the S. maltophilia K279a genome, Smlt2306, Smlt2305, and Smlt2304. We annotated these three genes as fliC1, fliC2, and fliC3, respectively (Figure 3A). As the role of these three genes in flagellum formation is not yet understood, the flagellin-null mutant was constructed by simultaneously deleting the three genes to yield the mutant KJΔFliC1C2C3. The Western blotting results demonstrated that KJ, KJΔOmpA, and KJL2::OmpAΔOmpA had comparable cytoplasmic flagellin protein levels (Figure 2C).
Figure 3.
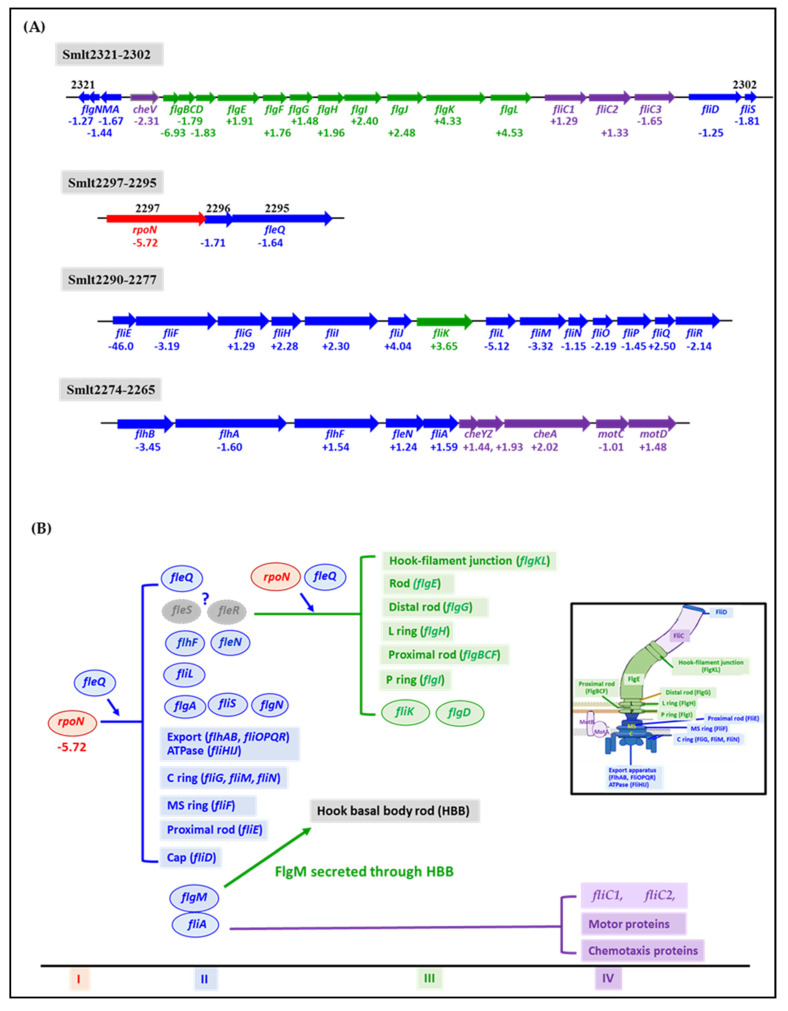
Flagellum synthesis model and RNA-seq transcriptome analysis of wild-type KJ and the ompA mutant, KJΔOmpA. (A) Genomic organization of the genes involved in flagellum synthesis and action. The flagellum-associated genes were located in four clusters. The genes classified in class I, II, III, and IV are marked in red, blue, green, and purple, respectively. The numbers below the genes indicate the gene expression changes analyzed using a transcriptome assay. Positive values represent an upregulation in KJΔOmpA, whereas negative values indicate a downregulation in KJΔOmpA. (B) A schematic model showing the transcriptional hierarchy of the genes involved in flagellum synthesis in S. maltophilia. The flagellum synthesis model of S. maltophilia is proposed by referencing the known P. aeruginosa flagellum model, as most of the flagellum synthesis-associated genes of P. aeruginosa are highly conserved in the S. maltophilia genome. The genes classified in class I, II, III, and IV are marked in red, blue, green, and purple, respectively. The genes symbolized by rectangles represent the encoded proteins composing the flagellum and the ovals represent regulatory proteins.
3.4. Establishment of a Putative Flagellum Synthesis Model for S. maltophilia
Information concerning flagellum synthesis and action in S. maltophilia is relatively scarce, and the hierarchical expression circuit has not been established. Although flagellum synthesis is well-studied in E. coli and P. aeruginosa, these systems are obviously different [27,28]. We surveyed the S. maltophilia K279a genome [40] for flagellum-associated genes and found that the flagellum system of S. maltophilia was more similar to that of P. aeruginosa [27]. To study the linkage between swimming compromise and ompA deletion, we thoroughly analyzed the S. maltophilia K279a genome to identify genes with known or predicted function of flagellum biogenesis or function, based on the known P. aeruginosa flagellum model. Genes known or predicted to be involved in chemotaxis and energy control were also included because of their genetic linkage to these flagellar genes. We identified 46 genes encoding the structural, assembly, or regulatory proteins of the flagellum in the sequenced K279a genome (Table S2). Most of these genes have sufficient homology with their orthologs in P. aeruginosa (Table S2). These genes are located in four clusters of chromosomes (Figure 3A). Most of the known flagellum-associated genes of P. aeruginosa are highly conserved in S. maltophilia, except for fleRS, fleP, and fleL.
A putative flagellum model of S. maltophilia was preliminarily proposed based on the P. aeruginosa model (Figure S2). FleQ and FliA are master regulators located in class I. FleQ works in concert with RpoN to activate the expression of class II genes. FlgM, a member of class II, interacts with FliA, resulting in FliA sequestration. In the P. aeruginosa model, class III genes are activated by class II-encoded FleRS in an RpoN-dependent manner [46]. However, no fleRS homologs have been identified in the S. maltophilia K279a genome. The regulatory components responsible for the expression of class III genes are relatively unclear. Upon completion of the hook basal body (HBB) by class II- and class III-encoded proteins, FlgM is secreted by the HBB and the liberated FliA activates the transcription of class IV genes.
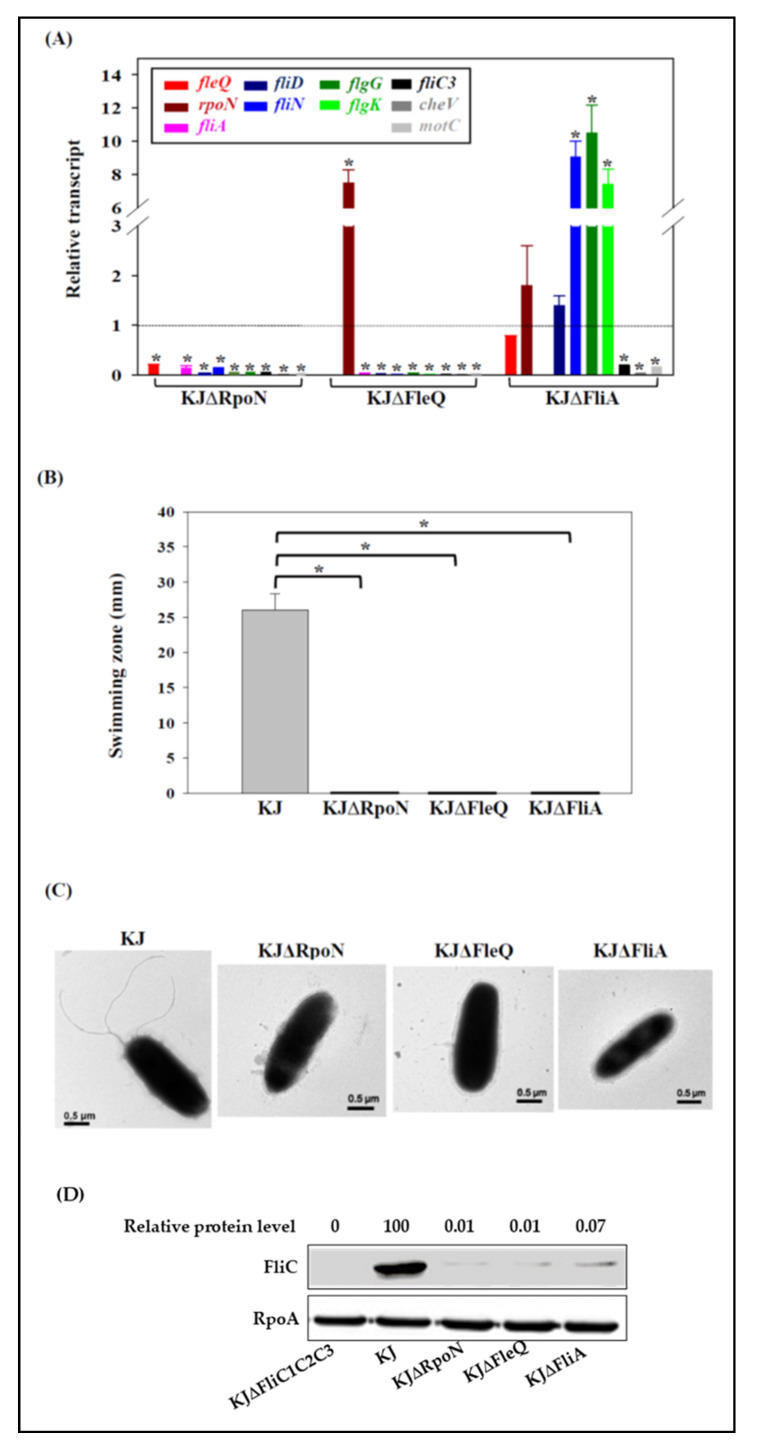
To confirm the reliability of the preliminarily proposed flagellum model (Figure S2), we constructed isogenic deletion mutants of fleQ (Smlt2295), rpoN (Smlt2297), and fliA (Smlt2270), whose encoded proteins are predicted to function as regulators at the first hierarchical level of the P. aeruginosa flagellum model. None of the constructed mutants exhibited observable changes in their colony shapes and growth patterns. Wild-type KJ, KJΔFleQ, KJΔRpoN, and KJΔFliA were subjected to qRT-PCR analysis to confirm the accuracy of the hierarchical model. We randomly selected the following genes as representatives of the four classes of the proposed model (Figure S2) for qRT-PCR testing: fleQ, rpoN, and fliA for class I; fliD and fliN for class II; flgG and flgK for class III; and fliC1, cheV, and motC for class IV. The inactivation of rpoN reduced the expression of all the genes assayed, supporting RpoN as a class I regulator. FleQ deletion resulted in the downregulation of all the genes assayed except rpoN. Interestingly, rpoN was significantly upregulated in the fleQ mutant. The loss-of-function of fliA downregulated fliC, cheV, and motC, but upregulated fliN, flgG, and flgK (Figure 4A). The swimming motility, flagellum morphology, and flagellin protein levels of KJΔFleQ, KJΔRpoN, and KJΔFliA were also assessed. All of the mutants tested completely lost swimming motility (Figure 4B) and had no flagella (Figure 4C). This was further confirmed by the presence of few intracellular flagellin proteins (Figure 4D). Furthermore, the qRT-PCR results were validated using xylE reporter constructs in the rpoN, fleQ, and fliA mutant backgrounds. Using the plasmid pRpoNxylE (a PrpoN-xylE transcriptional fusion), we measured rpoN promoter activity in the wild-type KJ, fliA, rpoN, and fleQ mutants. Compared to KJ(pRpoNxylE), KJΔFleQ(pRpoNxylE) had an increased C23O activity and KJΔFliA(pRpoNxylE) displayed a comparable one (Figure S3), consistent with the qRT-PCR results (Figure 4A). We also observed that no C23O activity was detected in KJΔRpoN(pRpoNxylE), indicating that RpoN has a positive autoregulation circuit.
Figure 4.
Impact of rpoN, fleQ, and fliA on flagellum-associated gene expression, swimming motility, flagellum morphology, and flagellin protein levels. (A) The impact of rpoN, fleQ, and fliA on flagellum-associated gene expression. Overnighted cultured bacterial cells (KJ, KJΔRpoN, KJΔFleQ, and KJΔFliA) were inoculated into fresh LB broth and then grown for 5 h before a qRT-PCR. The expression levels of the assayed genes were normalized to those of the 16 S ribosomal RNA gene. The relative transcript was calculated by dividing the mRNA levels of the wild-type by those of the mutant indicated. The mean and SD of the three independent experiments are shown. *, a relative transcript greater than 2 or less than 0.5 was considered to be significant. (B) The impact of rpoN, fleQ, and fliA on swimming motility. Overnighted cultured bacterial cells were inoculated into fresh LB broth and then grown for 5 h before testing. A five-microliter bacterial cell suspension was inoculated into swimming agar and then incubated at 37 °C for 48 h. The swimming zones were recorded. The data are the means of three independent experiments. The error bars indicate the standard deviations of three triplicate samples. *, p < 0.05, significance calculated using the Student’s t-test. (C) The impact of rpoN, fleQ, and fliA on flagella morphology. The flagella were negatively stained with 1% phosphotungstic acid (pH 7.4) and observed using TEM. (D) The impact of rpoN, fleQ, and fliA on flagellin protein levels. The proteins of a whole-cell extract were separated using SDS-PAGE and immunoblotting with anti-FliC3 and anti-RpoA antibodies.
The flagellum hierarchy expression model of S. maltophilia was thus amended as follows (Figure 3B). RpoN is at the top of the regulatory hierarchy and drives the transcription of class II genes (labeled as a red oval in Figure 3B). Notably, the expression of fliA and fleQ was almost abolished in the rpoN mutant, indicating that fleQ and fliA are members of the RpoN regulon. Thus, fleQ and fliA were arranged at the class II level in the S. maltophilia model. This feature differs from the known model of P. aeruginosa. The proteins encoded by the class II genes included the flagellum component proteins (labeled as blue rectangles in Figure 3B) and regulatory proteins (labeled as blue ovals in Figure 3B). The key regulators for the expression of class III genes, such as FleSR of P. aeruginosa, are not immediately clear, but the qRT-PCR results supported the suggestion that RpoN and FleQ are involved in the expression of some class III genes. The proteins encoded by class III genes included flagellum component proteins, FlgBCDGHIKL (labeled as green rectangles in Figure 3B) and regulatory proteins (FliK and FlgD) involved in flagellum hook length and modification (labeled as green ovals in Figure 3B). After FlgM is secreted via HBB, free FliA switches the expression of class IV genes. The proteins encoded by class IV genes included flagellins, flagellum motor proteins, and chemotaxis proteins (labeled as purple rectangles in Figure 3B). In the following study, we applied the model proposed in Figure 3B as the rationale for the experimental design.
3.5. RpoN Downregulation Is Responsible for ΔompA-Mediated Swimming Compromise
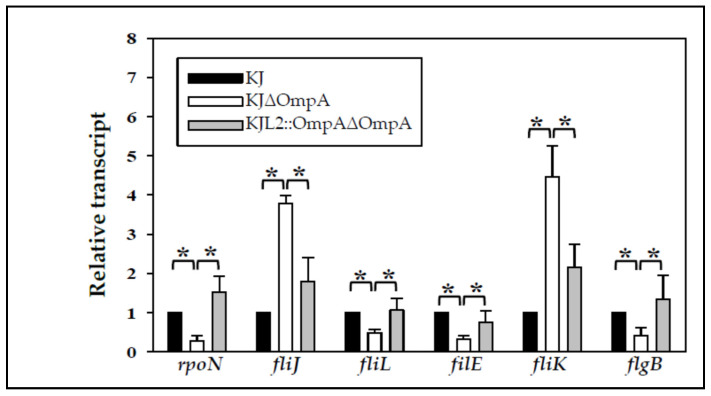
To identify the target genes responsible for swimming compromise in the ompA mutant, an RNA-Seq transcriptome assay of wild-type KJ and KJΔOmpA was conducted. A statistical significance was defined as an absolute fold change in RPKM equal to or greater than three. The transcriptome analysis revealed that 144 and 190 out of a total of 4695 genes were significantly increased and decreased, respectively, in KJΔOmpA compared to wild-type KJ (Table S3). We noticed that the L2 (Smlt3722) gene of KJΔOmpA showed a five-fold increase in the transcriptome assay (Table S3), indicating that the L2 gene is partially derepressed in KJΔOmpA. This observation provided a reasonable explanation for why the complementary strain KJL2::OmpAΔOmpA could revert to swimming motility without a β-lactam addition (Figure 2A). To validate the transcriptome results, qRT-PCR analysis was performed on the six selected genes. Relative to wild-type KJ, the expression of the six genes was corroborated by the transcriptome results in KJΔOmpA and mostly reverted to wild-type levels in KJL2::OmpAΔOmpA (Figure 5).
Figure 5.
Impact of ompA on the expression of flagellum-associated genes. Overnighted cultured bacterial cells (KJ, KJΔOmpA, and KJL2:OmpAΔOmpA) were inoculated into fresh LB broth and then grown for 5 h before a qRT-PCR. The expression levels of the assayed genes were normalized to those of the 16 S ribosomal RNA gene. The relative transcript was calculated using the wild-type KJ level as one. The mean and SD of the three independent experiments are shown. *, p < 0.05, significance calculated using the Student’s t-test.
As the first step in identifying the possible candidate genes responsible for the ΔompA-mediated swimming compromise, we thoroughly analyzed the transcriptome data (Table S3), based on our proposed hierarchical regulation model for flagellum synthesis in S. maltophilia (Figure 3B). The surveyed genes included flagellum biosynthesis, flagellum regulation, flagellum motor, and chemotaxis (Table 1). Among the 46 genes surveyed, seven and four genes were significantly downregulated and upregulated in KJΔOmpA, respectively (Table 1, Figure 3A). The proteins encoded by the downregulated genes participated in global regulation (RpoN), flagellar assembly (FlhB, FliE, FliF, FliM, and FlgB), and motor switch control (FliL). The upregulated genes were fliJ, fliK, flgK, and flgL, whose encoded proteins are implicated in hook length control and hook assembly. According to the proposed hierarchy model in Figure 3B, RpoN is the key candidate responsible for ΔompA-mediated swimming compromise because it is located in the first class of the four-class model.
Table 1.
Swimming-associated genes differently expressed in S. maltophilia KJ and KJΔOmpA.
| Smlt | Gene | RPKM a | Fold | Encoded Protein | |
|---|---|---|---|---|---|
| KJ | KJΔOmpA | ||||
| Class I | |||||
| 2297 | rpoN | 104.48 | 18.26 | −5.72 | σ54 sigma factor |
| Class II | |||||
| 2295 | fleQ | 226.20 | 137.15 | −1.64 | transcriptional activator |
| 2270 | fliA | 98.17 | 156.68 | +1.59 | σ28 sigma factor |
| 2271 | fleN | 159.21 | 197.77 | +1.24 | flagella number regulator |
| 2272 | flhF | 14.26 | 22.09 | +1.54 | flagellar polar location |
| 2273 | flhA | 8.14 | 5.07 | −1.60 | flagellar export protein |
| 2274 | flhB | 8.63 | 2.49 | −3.45 | flagellar export protein |
| 2277 | fliR | 14.57 | 6.78 | −2.14 | flagellar export protein |
| 2278 | fliQ | 19.71 | 49.47 | 2.50 | flagellar export protein |
| 2279 | fliP | 15.18 | 10.43 | −1.45 | flagellar export protein |
| 2280 | fliO | 38.14 | 17.37 | −2.19 | flagellar export protein |
| 2281 | fliN | 52.03 | 45.24 | −1.15 | flagellar motor switch protein |
| 2282 | fliM | 16.48 | 4.96 | −3.32 | flagellar motor switch protein |
| 2283 | fliL | 19.49 | 3.80 | −5.12 | basal body-associated protein |
| 2285 | fliJ | 12.00 | 48.53 | +4.04 | chaperone, export of hook proteins |
| 2286 | fliI | 26.64 | 61.27 | +2.30 | flagellum-specific ATPase |
| 2287 | fliH | 42.91 | 98.07 | +2.28 | flagella assembly protein |
| 2288 | fliG | 56.09 | 72.82 | +1.29 | motor switch protein |
| 2289 | fliF | 47.05 | 14.73 | −3.19 | basal body MS ring |
| 2290 | fliE | 45.59 | 1.00 | −45.6 | basal body MS ring/rod adapter |
| 2302 | fliS | 664.26 | 365.25 | −1.81 | chaperone for filament elongation |
| 2303 | fliD | 297.89 | 237.50 | −1.25 | filament cap |
| 2319 | flgA | 46.60 | 27.82 | −1.67 | basal body P-ring biosynthesis protein |
| 2320 | flgM | 1908.60 | 1317.64 | −1.44 | anti-sigma factor FlgM |
| 2321 | flgN | 775.79 | 607.01 | −1.27 | chaperone |
| Class III | |||||
| 2284 | fliK | 19.85 | 72.54 | +3.65 | flagellar hook-length control |
| 2307 | flgL | 54.28 | 246.26 | +4.53 | hook-filament junctional protein |
| 2308 | flgK | 49.76 | 215.68 | +4.33 | hook-filament junctional protein |
| 2309 | flgJ | 20.28 | 50.41 | +2.48 | flagellum specific muramidase |
| 2310 | flgI | 15.17 | 36.478 | +2.40 | basal body P-ring |
| 2311 | flgH | 36.78 | 72.24 | +1.96 | basal body L-ring |
| 2312 | flgG | 60.97 | 90.60 | +1.48 | basal body rod |
| 2313 | flgF | 5.34 | 9.46 | +1.76 | basal body rod |
| 2314 | flgE | 13.12 | 25.11 | +1.91 | hook |
| 2315 | flgD | 24.64 | 13.39 | −1.83 | hook capping protein |
| 2316 | flgC | 15.23 | 8.49 | −1.79 | basal body rod |
| 2317 | flgB | 48.91 | 7.05 | −6.93 | basal body rod |
| Class IV | |||||
| 2265 | motD | 71.76 | 106.27 | +1.48 | flagellar motor protein |
| 2266 | motC | 353.41 | 347.85 | −1.01 | flagellar motor protein |
| 2267 | cheA | 92.22 | 186.55 | +2.02 | chemotaxis sensor kinase regulator |
| 2268 | cheZ | 241.78 | 467.49 | +1.93 | chemotaxis protein |
| 2269 | cheY | 268.99 | 388.99 | +1.44 | chemotaxis response regulator |
| 2304 | fliC1 | 395.14 | 510.14 | +1.29 | flagellin |
| 2305 | fliC2 | 23.32 | 31.10 | +1.33 | flagellin |
| 2306 | fliC3 | 180.47 | 109.26 | −1.65 | flagellin |
| 2318 | cheV | 164.29 | 70.89 | −2.31 | chemotaxis response regulator |
a RPKM, Reads Per Kilobase of transcript per Million reads mapped. Bold: Statistical significance was defined as an absolute fold change in RPKM equal to or greater than 3.
To test this notion, KJΔOmpA was complemented with an intact rpoN gene. The L2 gene was replaced with the rpoN gene using double cross-over recombination to yield KJL2::RpoN, and ompA was in-frame deleted from the chromosome of KJL2::RpoN, generating KJL2::RpoNΔOmpA (Figure 1). KJL2::RpoN displayed swimming motility comparable to that of wild-type KJ. However, compared to KJΔOmpA, KJL2::RpoNΔOmpA showed swimming motility restoration to the wild-type level (Figure 2A). The TEM analysis revealed that KJL2::RpoNΔOmpA displayed a wild-type flagellar structure (Figure 2B). The intracellular FliC protein level of KJL2::RpoNΔOmpA was also comparable to that of wild-type KJ (Figure 2C).
4. Discussion
For a pathogen, the first step in pathogenesis is to establish an initial attachment to the host cells and facilitate colonization. Colonization requires a functional flagellum; therefore, swimming motility is a crucial virulence factor for pathogens [47]. Although the flagellum and swimming motility are well characterized in several Gram-negative bacteria, such as E. coli, V. cholerae, and P. aeruginosa, little is known regarding these aspects in S. maltophilia. In this study, we first proposed the hierarchical organization of flagella synthesis genes in S. maltophilia. Many of the approximately 59 genes involved in flagella biosynthesis in P. aeruginosa are conserved in the S. maltophilia genome. However, some P. aeruginosa flagellar genes have not been identified in S. maltophilia (such as FleRS, FleP, and FleL), and some flagellar genes appear to be unique to S. maltophilia (such as Smlt2296). Furthermore, P. aeruginosa harbors a flagellin gene (fliC), either type-a or type-b [48]; nevertheless, there are three flagellin genes (fliC1, fliC2, and fliC3) in the genome of S. maltophilia K279a.
In addition to the differences in gene organization, some regulatory mechanisms were noted to be different in the hierarchical models of S. maltophilia and P. aeruginosa, as follows: (i) Class I genes, fleQ and fliA, are constitutively expressed in P. aeruginosa. FleQ is considered the master regulator of the flagellar regulon [27] and works in concert with RpoN to trigger the expression of class II genes [49]. Furthermore, FliA is a critical regulator of class IV genes expression [49]. The transcription of fleQ and fliA is independent of RpoN in P. aeruginosa [50,51]. Nevertheless, in S. maltophilia, rpoN inactivation almost abolished the transcription of fleQ, fliA, and the genes of class II, class III, and class IV (Figure 3A), supporting the suggestion that rpoN, rather than fleQ and fliA, is at the top of the flagellar regulatory cascade in S. maltophilia. Thus, RpoN in S. maltophilia appears to have a more global regulatory spectrum than that in P. aeruginosa. Furthermore, RpoN has the feature of positive autoregulation (Figure S3). Therefore, the RpoN regulon of S. maltophilia encompasses the class I, II, III, and IV genes. (ii) The regulation of RpoN on fleQ did not appear to be a simple one-way regulatory circuit in S. maltophilia. The rpoN transcript was apparently upregulated in the fleQ mutant and was almost unexpressed in the rpoN mutant (Figure 3A and Figure S3). This phenomenon was not observed in P. aeruginosa. (iii) Similar to P. aeruginosa FliA, S. maltophilia FliA is the key regulator for the expression of class IV genes. Nevertheless, we observed that FliA of S. maltophilia can negatively regulate some class II and class III genes, thus extending our understanding of FliA. Interestingly, RpoN positively controls the expression of fliA and FliA negatively regulates the expression of fliN, flgG, and flgK; however, the expression of fliN, flgG, and flgK was almost abolished in KJΔRpoN (Figure 3A), suggesting the presence of unidentified regulator(s) participating in the complex regulatory circuit.
OMPs can provide the structural integrity of bacteria, which is critical for flagellar assembly and swimming ability. The linkage between β-barrel OMPs and swimming motility has been reported in some bacteria, but the impact of OMP deletion on swimming motility is varied. The deletion of ompX increases swimming motility in extraintestinal pathogenic E. coli [52]. However, in V. cholerae, flgO and flgP deletion mutants show decreased swimming motility, and the stability of flagella is significantly affected [53]. In addition, Bari et al. have also reported that ompU and ompT deletion mutants of V. cholerae showed thinner flagella, significant defects in swimming motility, and increased shedding of flagella sheath proteins in the culture supernatant [54]. A similar observation was also reported for the lamB deletion mutant of Aeromonas veronii; its lamB deletion mutant almost lost swimming motility, and shedding flagella were found in the visual field of the TEM examination [55]. In this study, we demonstrated that the ompA deletion mutant of S. maltophilia has shorter flagella and defective swimming motility. Furthermore, we also revealed that ΔompA-mediated rpoN downregulation is a critical factor responsible for swimming compromise in KJΔOmpA. Precisely how ompA deletion downregulates rpoN expression remains unclear. The Gram-negative envelope protects the bacteria from the environment. The loss of abundant OmpA in the OM can cause envelope damage, which may trigger the envelope stress responses to maintain envelope homeostasis. The envelope stress responses extensively and diversely exist in different microorganisms, including σE response, Cpx response, Rcs response, Bae response, and Psp response [56]. We presume that the envelope stress response could be the connection between ompA deletion and rpoN downregulation. This issue needs to be further explored.
The contribution of OmpA to bacterial conjugation has been reported in E. coli [57], and the underlying mechanism is that the TraN protein encoded by the F plasmid interacts with OmpA in recipient cells, thus increasing their mating efficiency [58]. In this study, we verified that S. maltophilia OmpA, similar to E. coli OmpA, is a critical protein in recipient cells for successful conjugation. Conjugation is instrumental to the horizontal transfer of antibiotic resistance genes in clinical environments and enhances the spread of antibiotic resistance among bacteria [59]. Thus, blocking conjugation can be regarded as a tool against antibiotic resistance [60].
S. maltophilia is ubiquitous in the environment and is an increasingly opportunistic pathogen. The attribute of multidrug resistance increases the difficulty of treating S. maltophilia infection [61]. Thus, it is important to develop new treatment strategies to combat S. maltophilia infections. Swimming is a critical virulence factor involved in bacterial pathogenesis. Conjugation is an efficient method for horizontal transfer of antibiotic resistance. In this study, we demonstrated that the OmpA of S. maltophilia significantly contributes to swimming and conjugation; thus, blocking the OmpA function may attenuate the bacterial pathogenicity and occurrence of antibiotic resistance. Therefore, OmpA is an ideal target for inhibitor design. For Gram-negative bacteria, the double membranes may be a natural barrier for the entrance of foreign molecules [2,3]. Given that OmpA is the OMP of the greatest abundance in S. maltophilia, the accessibility of an OmpA inhibitor to OmpA can be highly efficient. Therefore, the development of an OmpA inhibitor is a feasible strategy to combat S. maltophilia infection.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/microorganisms9061216/s1, Figure S1: The SDS-PAGE of OMPs from wild-type KJ, KJΔOmpA, and KJL2::OmpAΔOmpA, Figure S2: The preliminary flagellum synthesis model of S. maltophilia based on the known P. aeruginosa model, Figure S3: The C23O activities of KJ(pRpoNxylE), KJΔFleQ(pRpoNxylE), KJΔFliA(pRpoNxylE), and KJΔRpoN(pRpoNxylE), Table S1: The bacterial strains, plasmids, and primers used in this study, Table S2: Comparison of the flagella synthesis-associated proteins between S. maltophilia and P. aeruginosa, Table S3: Transcriptomic analysis of S. maltophilia wild-type KJ and ompA mutant KJΔOmpA.
Author Contributions
Conceptualization, C.-H.L. and T.-C.Y.; methodology, C.-L.C., H.-H.H., and L.-H.L.; software, Y.-T.L.; validation, C.-H.L. and T.-C.Y.; formal analysis, C.-L.C., H.-H.H., and L.-H.L.; resources, Y.-T.L.; writing—original draft preparation, C.-H.L. and H.-H.H.; writing—review and editing, T.-C.Y.; supervision, C.-H.L. and T.-C.Y.; project administration, C.-H.L. and T.-C.Y.; funding acquisition, C.-H.L. and T.-C.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Technology of Taiwan, MOST 108-2320-B-010-032-MY3 and the NYMU-FEMH Joint Research Program, 110DN34.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gan L., Chen S., Jensen G.J. Molecular organization of Gram-negative peptidoglycan. Proc. Natl. Acad. Sci. USA. 2008;105:18953–18957. doi: 10.1073/pnas.0808035105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrand A., Vergalli J., Pages J.-M., Davin-Regli A. An intertwined network of regulation controls membrane permeability including drug influx and efflux in Enterobacteriaceae. Microorganisms. 2020;8:833. doi: 10.3390/microorganisms8060833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gajdacs M. The concept of an ideal antibiotics: Implications for drug design. Molecules. 2019;24:892. doi: 10.3390/molecules24050892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wimley W.C. The versatile β-barrel membrane protein. Curr. Opin. Struct. Biol. 2003;13:404–411. doi: 10.1016/S0959-440X(03)00099-X. [DOI] [PubMed] [Google Scholar]
- 5.Silhavy T.J., Kahne D., Walker S. The bacterial cell envelope. Cold Spring Harb. Perspect. Biol. 2010;2:a000414. doi: 10.1101/cshperspect.a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pagel M., Simonet V., Li J., Lallemand M., Lauman B., Delcour A.H. Phenotypic characterization of pore mutants of the Vibrio cholerae porin OmpU. J. Bacteriol. 2007;189:8593–8600. doi: 10.1128/JB.01163-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai Y.K., Fung C.P., Lin J.C., Chen J.H., Chang F.Y., Chen T.L., Siu L.K. Klebsiella pneumoniae outer membrane porins OmpK35 and OmpK36 play roles in both antimicrobial resistance and virulence. Antimicrob. Agents Chemother. 2011;55:1485–1493. doi: 10.1128/AAC.01275-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vogel H., Jahnig F. Models for the structure of outer-membrane proteins of Escherichia coli derived from raman spectroscopy and prediction methods. J. Mol. Biol. 1986;190:191–199. doi: 10.1016/0022-2836(86)90292-5. [DOI] [PubMed] [Google Scholar]
- 9.De Mot R., Vanderleyden J. The C-terminal sequence conservation between OmpA-related outer membrane proteins and MotB suggests a common function in both gram positive and gram-negative bacteria, possibly in the interaction of these domains with peptidoglycan. Mol. Microbiol. 1994;12:333–334. doi: 10.1111/j.1365-2958.1994.tb01021.x. [DOI] [PubMed] [Google Scholar]
- 10.Park J.S., Lee W.C., Yeo K.J., Ryu K.S., Kumarasiri M., Hesek D., Lee M., Mobashery S., Song J.H., Kim S.I., et al. Mechanism of anchoring of OmpA protein to the cell wall peptidoglycan of the gram-negative bacterial outer membrane. FASEB J. 2012;26:219–228. doi: 10.1096/fj.11-188425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 2003;67:593–656. doi: 10.1128/MMBR.67.4.593-656.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith S.G., Mahon V., Lambert M.A., Fagan R.P. A molecular Swiss army knife: OmpA structure, function and expression. FEMS Microbiol. Lett. 2007;273:1–11. doi: 10.1111/j.1574-6968.2007.00778.x. [DOI] [PubMed] [Google Scholar]
- 13.Smani Y., Fabrega A., Roca I., Sanchez-Encinales V., Vila J., Pachon J. Role of OmpA in the multidrug resistance phenotype of Acinetobacter baumannii. Antimicrob. Agents Chemother. 2014;58:1806–1808. doi: 10.1128/AAC.02101-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nie D., Hu Y., Chen Z., Li M., Hou Z., Luo X., Mao X., Xue X. Outer membrane protein A (OmpA) as a potential therapeutic target for Acinetobacter baumannii infection. J. Biomed. Sci. 2020;27:26. doi: 10.1186/s12929-020-0617-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koebnik R., Locher K.P., Van Gelder P. Structure and function of bacterial outer membrane proteins: Barrels in a nutshell. Mol. Microbiol. 2000;37:239–253. doi: 10.1046/j.1365-2958.2000.01983.x. [DOI] [PubMed] [Google Scholar]
- 16.Kim S.A., Yoo S.M., Hyun S.H., Choi C.H., Yang S.Y., Kim H.J., Jang B.C., Suh S.I., Lee J.C. Global gene expression patterns and induction of innate immune response in human laryngeal epithelial cells in response to Acinetobacter baumannii outer membrane protein A. FEMS Immunol. Med. Microbiol. 2008;54:45–52. doi: 10.1111/j.1574-695X.2008.00446.x. [DOI] [PubMed] [Google Scholar]
- 17.Chalifour A., Jeannin P., Gauchat J.F., Blaecke A., Malissard M., N’Guyen T., Thieblemont N., Delneste Y. Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production. Blood. 2004;104:1778–1783. doi: 10.1182/blood-2003-08-2820. [DOI] [PubMed] [Google Scholar]
- 18.Luo G., Lin L., Ibrahim A.S., Baquir B., Pantapalangkoor P., Bonomo R.A., Doi Y., Adams M.D., Russo T.A., Spellberg B. Active and passive immunization protects against lethal, extreme drug resistant-Acinetobacter baumannii infection. PLoS ONE. 2012;7:e29446. doi: 10.1371/journal.pone.0029446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan Q., Wang X., Wang X., Teng D., Mao R., Zhang Y., Wang J. Recombinant outer membrane protein A induces a protective immune response against Escherichia coli infection in mice. Appl. Microbiol. Biotechnol. 2015;99:5451–5460. doi: 10.1007/s00253-014-6339-6. [DOI] [PubMed] [Google Scholar]
- 20.Chaban B., Hughes H.V., Beeby M. The flagellum in bacterial pathogens: For motility and a whole lot more. Semin. Cell. Dev. Biol. 2015;46:91–103. doi: 10.1016/j.semcdb.2015.10.032. [DOI] [PubMed] [Google Scholar]
- 21.Zhou M., Yang Y., Chen P., Hu H., Hardwidge P.R., Zhu G. More than a locomotive organelle: Flagella in Escherichia coli. Appl. Microbiol. Biotechnol. 2015;99:8883–8890. doi: 10.1007/s00253-015-6946-x. [DOI] [PubMed] [Google Scholar]
- 22.Armitage J.P., Berry R.M. Assembly and Dynamics of the Bacterial Flagellum. Annu. Rev. Microbiol. 2020;74:181–200. doi: 10.1146/annurev-micro-090816-093411. [DOI] [PubMed] [Google Scholar]
- 23.Apel D., Surette M.G. Bringing order to a complex molecular machine: The assembly of the bacterial flagella. Biochim. Biophys. Acta. 2008;1778:1851–1858. doi: 10.1016/j.bbamem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Wu J., Newton A. Regulation of the Caulobacter flagellar gene hierarchy; not just for motility. Mol. Microbiol. 1997;24:233–239. doi: 10.1046/j.1365-2958.1997.3281691.x. [DOI] [PubMed] [Google Scholar]
- 25.Prouty M.G., Correa N.E., Klose K.E. The novel sigma54- and sigma28-dependent flagellar gene transcription hierarchy of Vibrio cholerae. Mol. Microbiol. 2001;39:1595–1609. doi: 10.1046/j.1365-2958.2001.02348.x. [DOI] [PubMed] [Google Scholar]
- 26.McCarter L.L. Polar flagellar motility of the Vibrionaceae. Microbiol. Mol. Biol. Rev. 2001;65:445–462. doi: 10.1128/MMBR.65.3.445-462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dasgupta N., Wolfgang M.C., Goodman A.L., Arora S.K., Jyot J., Lory S., Ramphal R. A four-tiered transcriptional regulatory circuit controls flagellar biogenesis in Pseudomonas aeruginosa. Mol. Microbiol. 2003;50:809–824. doi: 10.1046/j.1365-2958.2003.03740.x. [DOI] [PubMed] [Google Scholar]
- 28.Macnab R.M. How bacteria assemble flagella. Annu. Rev. Microbiol. 2003;57:77–100. doi: 10.1146/annurev.micro.57.030502.090832. [DOI] [PubMed] [Google Scholar]
- 29.Looney W.J., Narita M., Mühlemann K. Stenotrophomonas maltophilia: An emerging opportunist human pathogen. Lancet Infect. Dis. 2009;9:312–323. doi: 10.1016/S1473-3099(09)70083-0. [DOI] [PubMed] [Google Scholar]
- 30.Gajdacs M., Urban E. A 10-year single-center expreience on Stenotrophomonas maltophilia resistotyping in Szeged, Hungary. Eur. J. Microbiol. Immunol. 2020;10:91–97. doi: 10.1556/1886.2020.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gil-Gil T., Martínez J.L., Blanco P. Mechanisms of antimicrobial resistance in Stenotrophomonas maltophilia: A review of current knowledge. Expert Rev. Anti-Infect. Ther. 2020;18:335–347. doi: 10.1080/14787210.2020.1730178. [DOI] [PubMed] [Google Scholar]
- 32.Biagi M., Vialichka A., Jurkovic M., Wu T., Shajee A., Lee M., Patel S., Mendes R.E., Wenzler E. Actvitiy of cefiderocol alone and in combination with levofloxacin, minocycline, polymyxin B or trimethoprim-sulfamethoxazole against multidrug-resistant Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2020;64:e00559-20. doi: 10.1128/AAC.00559-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y.W., Huang H.H., Huang K.H., Chen W.C., Lin Y.T., Hsu C.C., Yang T.C. AmpI functions as an iron exporter to alleviate β-lactam-mediated reactive oxygen species stress in Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2019;63:e02467-18. doi: 10.1128/AAC.02467-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu G., Tang X., Shang X., Li Y., Wang J., Yue J., Li Y. Identification of immunogenic outer membrane proteins and evaluation of their protective efficacy against Stenotrophomonas maltophilia. BMC Infect. Dis. 2018;18:347. doi: 10.1186/s12879-018-3258-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Y., Tang X., Zhao Z., Wang H., Wang X., Shang X., Liu P., Kou Z., Jiang Y., Li Y. Intranasal immunization with recombinant outer membrane protein A induces protective immune response aginst Stenotrophomonas maltophilia infection. PLoS ONE. 2019;14:e0214596. doi: 10.1371/journal.pone.0214596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang T.C., Huang Y.W., Hu R.M., Huang S.C., Lin Y.T. AmpDI is involved in expression of the chromosomal L1 and L2 β-lactamases of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2009;53:2902–2907. doi: 10.1128/AAC.01513-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L.H., Zhang M.S., Wu C.J., Lin Y.T., Yang T.C. Overexpression of SmeGH contributes to the acquired MDR of Stenotrophomonas maltophilia. J. Antimicrob. Chemother. 2019;74:2225–2229. doi: 10.1093/jac/dkz200. [DOI] [PubMed] [Google Scholar]
- 38.Lin C.W., Huang Y.W., Hu R.M., Chiang K.H., Yang T.C. The role of AmpR in regulation of L1 and L2 β-lactamases in Stenotrophomonas maltophilia. Res. Microbiol. 2009;160:152–158. doi: 10.1016/j.resmic.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 39.Lin Y.T., Huang Y.W., Chen S.J., Chang C.W., Yang T.C. The SmeYZ efflux pump of Stenotrophomonas maltophilia contributes to drug resistance, virulence-related characteristics, and virulence in mice. Antimicrob. Agents Chemother. 2015;59:4067–4073. doi: 10.1128/AAC.00372-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crossman L.C., Gould V.C., Dow J.M., Vernikos G.S., Okazaki A., Sebaihia M., Saunders D., Arrowsmith C., Carver T., Peters N., et al. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome Biol. 2008;9:R74. doi: 10.1186/gb-2008-9-4-r74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang H.H., Chen W.C., Lin C.W., Lin Y.T., Ning H.C., Chang Y.C., Yang T.C. Relationship of the CreBC two-component regulatory system and inner membrane protein CreD with swimming motility in Stenotrophomonas maltophilia. PLoS ONE. 2017;12:e0174704. doi: 10.1371/journal.pone.0174704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen C.H., Huang C.C., Chung T.C., Hu R.M., Huang Y.W., Yang T.C. Contribution of resistance-nodulation-division efflux pump operon smeU1-V-W-U2-X to multidrug resistance of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2011;55:5826–5833. doi: 10.1128/AAC.00317-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−(delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 44.Hu R.M., Huang K.J., Wu L.T., Hsiao Y.J., Yang T.C. Induction of L1 and L2 β-lactamases of Stenotrophomonas maltophilia. Antimicrob. Agents Chemother. 2008;52:1198–1200. doi: 10.1128/AAC.00682-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.de Oliveira-Garcia D., Dall’Agnol M., Rosales M., Azzuz A.C., Martinez M.B., Girón J.A. Characterization of flagella produced by clinical strains of Stenotrophomonas maltophilia. Emerg. Infect. Dis. 2002;8:918–923. doi: 10.3201/eid0809.010535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ritchings B.W., Almira E.C., Lory S., Ramphal R. Cloning and phenotypic characterization of fleS and fleR, new response regulators of Pseudomonas aeruginosa which regulate motility and adhesion to mucin. Infect. Immun. 1995;63:4868–4876. doi: 10.1128/iai.63.12.4868-4876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Josenhans C., Suerbaum S. The role of motility as a virulence factor in bacteria. Int. J. Med. Microbiol. 2002;291:605–614. doi: 10.1078/1438-4221-00173. [DOI] [PubMed] [Google Scholar]
- 48.Spangenberg C., Heuer T., Bürger C., Tümmler B. Genetic diversity of flagellins of Pseudomonas aeruginosa. FEBS Lett. 1996;396:213–217. doi: 10.1016/0014-5793(96)01099-X. [DOI] [PubMed] [Google Scholar]
- 49.Jyot J., Dasgupta N., Ramphal R. FleQ, the major flagellar gene regulator in Pseudomonas aeruginosa, binds to enhancer sites located either upstream or atypically downstream of the RpoN binding site. J. Bacteriol. 2002;184:5251–5260. doi: 10.1128/JB.184.19.5251-5260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Starnbach M.N., Lory S. The fliA (rpoF) gene of Pseudomonas aeruginosa encodes an alternative sigma factor required for flagellin synthesis. Mol. Microbiol. 1992;6:459–469. doi: 10.1111/j.1365-2958.1992.tb01490.x. [DOI] [PubMed] [Google Scholar]
- 51.Arora S.K., Ritchings B.W., Almira E.C., Lory S., Ramphal R. A transcriptional activator, FleQ, regulates mucin adhesion and flagellar gene expression in Pseudomonas aeruginosa in a cascade manner. J. Bacteriol. 1997;179:5574–5581. doi: 10.1128/JB.179.17.5574-5581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng X., Liu X., Zhang L., Hou B., Li B., Tan C., Li Z., Zhou R., Li S. Virulence characteristics of extraintestinal pathogenic Escherichia coli deletion of gene encoding the outer membrane protein X. J. Vet. Med. Sci. 2016;78:1261–1267. doi: 10.1292/jvms.16-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martinez R.M., Dharmasena M.N., Kirn T.J., Taylor R.K. Characterization of two outer membrane proteins, FlgO and FlgP, that influence vibrio cholerae motility. J. Bacteriol. 2009;191:5669–5679. doi: 10.1128/JB.00632-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bari W., Lee K.M., Yoon S.S. Structural and functional importance of outer membrane proteins in Vibrio cholerae flagellum. J. Microbiol. 2012;50:631–637. doi: 10.1007/s12275-012-2116-3. [DOI] [PubMed] [Google Scholar]
- 55.Yang B., Zhang D., Wu T., Zhang Z., Raza S.H.A., Schreurs N., Zhang L., Yang G., Wang C., Qian A., et al. Maltoporin (LamB protein) contributes to the virulence and adhesion of Aeromonas veronii TH0426. J. Fish. Dis. 2019;42:379–389. doi: 10.1111/jfd.12941. [DOI] [PubMed] [Google Scholar]
- 56.Mitchell A.M., Silhavy T.J. Envelope stress response: Balancing damage repair and toxicity. Nat. Rev. Microbiol. 2019;17:417–418. doi: 10.1038/s41579-019-0199-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Manoil C., Rosenbusch J.P. Conjugation-deficient mutants of Escherichia coli distinguish classes of functions of the outer membrane OmpA protein. Mol. Gen. Genet. 1982;187:148–156. doi: 10.1007/BF00384398. [DOI] [PubMed] [Google Scholar]
- 58.Klimke W.A., Rypien C.D., Klinger B., Kennedy R.A., Rodriguez-Maillard J.M., Frost L.S. The mating pair stabilization protein, TraN, of the F plasmid is an outer-membrane protein with two regions that are important for its function in conjugation. Microbiology. 2005;151:3527–3540. doi: 10.1099/mic.0.28025-0. [DOI] [PubMed] [Google Scholar]
- 59.Lerminiaux N.A., Cameron A.D.S. Horizontal transfer of antibiotic resistance genes in clinical environments. Can. J. Microbiol. 2019;65:34–44. doi: 10.1139/cjm-2018-0275. [DOI] [PubMed] [Google Scholar]
- 60.Graf F.E., Palm M., Warringer J., Farewell A. Inhibiting conjugation as a tool in the fight against antibiotic resistance. Drug Dev. Res. 2019;80:19–23. doi: 10.1002/ddr.21457. [DOI] [PubMed] [Google Scholar]
- 61.Gajdacs M., Urban E. Prevalence and antibiotic resistance of Stenotrophomonas maltophilia in respiratory tract samples: A 10-year epidemiological snapshot. Health Serv. Res. Manag. Epidemiol. 2019;6:2333392819870774. doi: 10.1177/2333392819870774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.