Abstract
Simple Summary
Black flies, also known as buffalo gnats, are major pests to humans and animals. Females of some black fly species serve as vectors for transmitting several pathogens (i.e., filarial nematodes, blood protozoa, viruses, and bacteria) to humans and animals via their bites. In Thailand, some human-biting species are considered as natural vectors of zoonotic onchocerciasis. This study was the first to contribute baseline data on the community structure, biodiversity and spatial and temporal distribution of adult black flies in tropical forests of the highest mountain in northern Thailand, Doi Inthanon National Park, by using malaise traps. Adult black flies were captured monthly at low to high elevation sites, using malaise traps across three seasons during a one-year period. A total of 44 species were identified among 9406 specimens. It was found that species richness was greatest at the mid elevation. Black fly populations peaked in the rainy season at all elevation sites. The findings of this study showed that varied elevations and seasons are important factors that influence the distribution and abundance of black flies in this region.
Abstract
Black flies form a group of small blood-sucking insects of medical and veterinary importance. This study aimed to investigate the community structure, biodiversity and spatial and temporal distribution of adult black flies in tropical rain forests, by using malaise traps in Doi Inthanon National Park, northern Thailand. Malaise traps were placed along six elevational gradients (400 m to 2500 m, above sea level) at Doi Inthanon National Park, Chiang Mai province, from December 2013 to November 2014. A total of 9406 adult female black flies belonging to five subgenera—Daviesellum (2%), Gomphostilbia (23%), Montisimulium (11%), Nevermannia (16%) and Simulium (48%)—were collected. Among 44 taxa found, S. tenebrosum complex had the highest relative abundance (11.1%), followed by the S. asakoae species-group (9.6%), the S. striatum species-group (7.7%), S. inthanonense (6.6%), S. doipuiense complex (6.4%), S. chomthongense complex (5.3%), S. chumpornense (5.1%) and S. nigrogilvum (4.1%). Two human-biting species—S. nigrogilvum and species in the S. asakoae species-group—were found in all of the collection sites with 100% species occurrence. Species richness was highest at mid elevation (1400 m), which is represented by 19 black fly species. The peak and lowest seasonal abundance was observed in the rainy and hot season, respectively. Seasonal species richness was highest in the cold season, except for that from elevation sites at 700 m, 1700 m and 2500 m. This study revealed that the malaise trap is effective in providing important data for further monitoring of the effects of environmental changes and conservation planning on the biodiversity of black flies in Doi Inthanon National Park.
Keywords: black flies, Simuliidae, Simulium, biodiversity, malaise trap, tropical forests
1. Introduction
Black flies (Diptera: Simuliidae) are among the most important groups of blood-sucking insects [1]. The bite of adult females can cause serious medical problems to humans and other vertebrates [2]. The larvae and pupae are at the aquatic stage, live in running waterways and play a role in filter-feeding and servicing the ecology [3,4,5,6]. Furthermore, some hill tribes in northern Thailand consume the larvae of S. rudnicki (known as Kokob salad) on special occasions [7]. At present, 2331 living black fly species have been recorded worldwide [8]. The black fly fauna has been studied in some Oriental countries, such as Indonesia (124 species), Peninsular Malaysia (63 species), Myanmar (28 species), the Philippines (84 species) and Vietnam (70 species) [8,9]. Currently, at least 139 black fly species have been reported in Thailand [10,11,12,13]. Among these, seven species have been incriminated as human-biting species—S. asakaoe, S. chamlongi, S. doipuiense complex, S. nigrogilvum, S. nodosum, S. tenebrosum complex and S. umphangense [2,14]. S. asakoae, S. monglaense and S. myanmarense, all in the S. asakoae species-group of the subgenus Gomphostilbia Enderlein, are natural vectors of unnamed filarial larvae, and S. nodosum and S. nigrogilvum, both of the subgenus Simulium Latreille, are natural vectors of filarial larvae in the genus Onchocerca [15,16,17,18,19,20].
Doi Inthanon National Park is part of the cold Himalayan mountain range that extends from India through Nepal to Thailand. Doi Inthanon is the highest mountain in Thailand at 2565 meters above sea level. It serves as a biodiversity hotspot for many animals and plants, due to its suitable microhabitat [21]. The first study of black flies (S. nigrogilvum) in this park was conducted by Summers (1911) and then by Edwards (1928). Subsequently, Takaoka and Suzuki (1984) reported 19 black fly species and were the first to construct a standard key to identify them in Thailand [22]. Black fly larvae have been surveyed from several localities in this park since 1999, from which 17 species were discovered [23]. The seasonal abundance and daily activity of 23 black fly species attracted to humans were reported by Choochote et al. [2]. In 2007, the larvae and pupae of 40 black fly species collected from several permanent and seasonal streams in this Park were reported in the Darwin Initiative Project [24]. Between 1911 and 2019, 56 black fly species were recorded from this area, which accounted for 32% of the total number of black flies found in Thailand [10,12,13,25].
The tent-like passive intercept traps, malaise traps, have been used widely for collecting insects since the mid-1930s [26] because they are not selective and therefore yield high insect diversities with large amounts of specimens. However, some studies revealed that the malaise trap is selective [27,28]. For example, Aguiar and Santos [27] demonstrated that it collected 2.4 times more males than females, and 20% more species for males than females of Cryptini specimens. They have wide applications and can contribute biological and ecological information to various other fields, including taxonomy and systematics, and biocontrol and biosecurity, when used in a standardized manner [29]. This kind of trap was applied in Thailand to collect flying insects from several areas in the country. It enabled hundreds of new species to be recorded in more than 108 publications and reported in the TIGER project. These included a new black fly species of the rare subgenus Montisimulium Rubtsov, and a new record of S. bishopi in Thailand, as well as several groups of insects and spiders [12,30,31,32,33,34,35,36,37].
Although several ecological studies of the aquatic stages of black flies have been reported in Thailand [23,38,39,40,41], and its neighboring countries [9,42,43], little is known in this country about their community structure and distribution in their adult stage (biting and non-biting black flies). Hence, the aim of this study was to investigate the community structure, biodiversity and spatial (elevation) and temporal (seasonal) distribution of adult black flies in tropical rain forests, by using malaise traps in Doi Inthanon National Park, northern Thailand.
2. Materials and Methods
2.1. Study Area
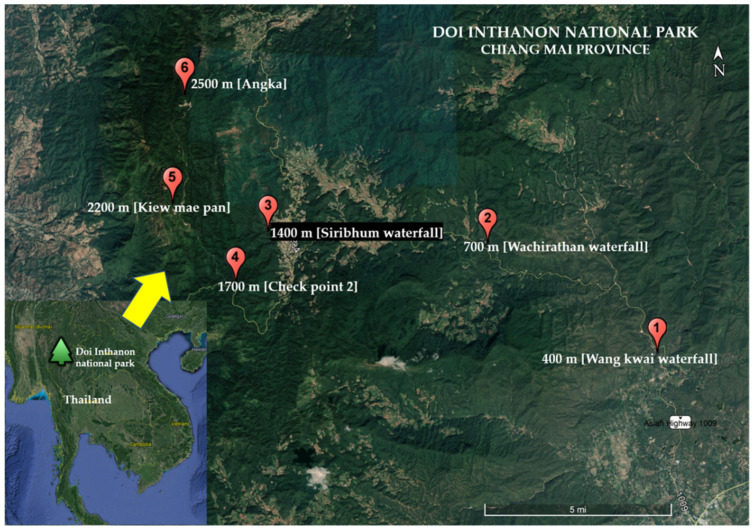
Adult black fly specimens were collected monthly using malaise traps (width 100 cm, length 170 cm, height 150 cm) from December 2013 to November 2014. A collection permit (no. 0907.4/20861) for this study was issued by the Department of National Parks, Wildlife and Plant Conservation, Bangkok, Thailand. The study was conducted at six elevation sites, (1) low elevations: 400 m and 700 m; (2) mid elevations: 1400 m and 1700 m; and (3) high elevations: 2200 m and 2500 m above sea level (a.s.l.) in Doi Inthanon National Park, Chiang Mai province, northern Thailand (Figure 1). The physical data of each collection site and classification of forest types [44] are presented in Table 1. Black flies were sorted from other insects and preserved in 80% ethanol before their species was identified in the laboratory of the Entomology Section, Queen Sirikit Botanic Garden (QSBGE), Chiang Mai province, Thailand. The seasonal classification followed the Thai Meteorological Department, i.e., rainy (May to October), cold (November to February) and hot (March to April) seasons [38].
Figure 1.
Map of six collection sites along elevational gradients in Doi Inthanon National Park, Chiang Mai province.
Table 1.
Physical data of six collection sites at Doi Inthanon National Park.
| Collection Sites | Geographical Coordinates, Elevation (a.s.l. *) |
Elevation Zones |
No. of Traps |
No. of Samplings |
Forest Types |
|---|---|---|---|---|---|
| Wang kwai waterfall | 18°29′57.0″ N 98°40′06.2″ E, 400 m | low | 1 | 12 | deciduous dipterocarp forest |
| Wachirathan waterfall | 18°32′27.6″ N 98°36′00.5″ E, 700 m | low | 2 | 24 | dry evergreen forest |
| Siribhum waterfall | 18°32′44.4″ N 98°30′53.0″ E, 1400 m | middle | 2 | 24 | lower montane rain forest |
| Check point 2 | 18°31′39.5″ N 98°29′59.7″ E, 1700 m | middle | 2 | 24 | upper montane rain forest |
| Kiew mae pan | 18°33′29.4″ N 98°28′51.7″ E, 2200 m | high | 2 | 24 | upper montane rain forest |
| Angka | 18°35′12.8″ N 98°29′14.2″ E, 2500 m | high | 3 | 36 | upper montane rain forest |
| Total | 12 | 144 |
* a.s.l. = above sea level.
2.2. Species Identification
Identifications of specimens were morphologically made at species level by using the standard keys [12] and additional keys for black flies in Thailand [10,45]. Exceptionally, specimens in the S. asakoae species-group in the subgenus Gomphostilbia and those in the S. striatum species-group in the subgenus Simulium were identified at species-group level, since females of most species of these two species-groups are considerably difficult to separate at species level due to the close similarities of their morphological characteristics [45]. When formally named species are known to consist of more than two cytoforms (or cytospecies), genoforms or morphoforms, they are referred to as species complex [8,11,45]. Representative specimens were deposited at the Entomology Section, QSBGE, Chiang Mai province, Thailand.
2.3. Statistical Analyses
The species richness and abundance of black flies collected monthly from each site were recorded. Due to the different number of traps operating among the collection sites, the mean value was used for analysis on spatiotemporal abundance variation and diversity parameters. Relative abundance (percentages) was calculated by dividing the total number of species occurred by the total number of adults. The percentage of species occurrence (% SO) was calculated by determining the number of sites where species were collected and dividing it by the total number of sample sites (n = 6) [40]. All data were analyzed by PAST version 4.03 [46]. The diversity parameters of each collecting site included Shannon_H, Simpson_1-D, Dominance_D, Evenness_e^H/S, and Equitability_J. The Chao1 richness estimator was used to estimate the total species richness on Doi Inthanon National Park. The species accumulation curve (individual-based rarefaction) was based on the Shannon_H index, and species richness was used for comparing biodiversity between collection sites and assessing sampling adequacy. Spatiotemporal abundance and richness variation of the black flies collected at each site, including the important six human-biting species, were visualized as a contour map on the elevation/month grid. Multivariate statistic, unweighted pair-group arithmetic averaging (UPGMA) by the Bray–Curtis (two way) similarity index and correspondence analysis (CA) were used to compare and describe the distribution of black fly species associated with collection sites. Correlation analysis was used to test the significant relationship of species richness−elevation. Individual rarefaction was used to compare the diversity between collection sites. Statistical significance was set at p < 0.05.
3. Results
3.1. Community Structure, Species Composition and Biodiversity of Black Flies
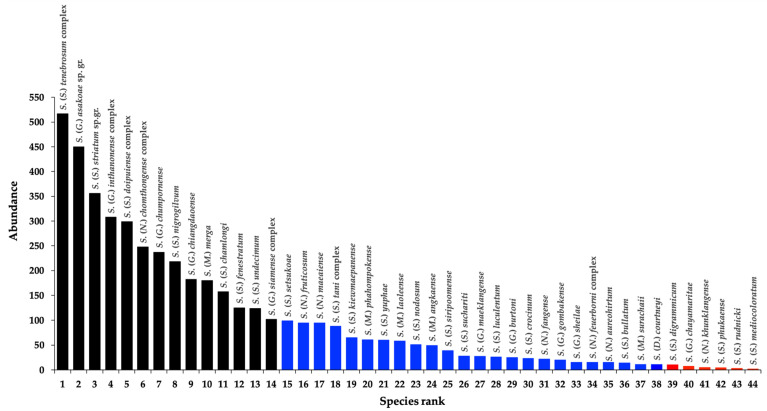
In total, 9406 adult black flies belonging to 44 species of five subgenera were trapped in six collection sites: Daviesellum Takaoka and Adler (one species, 2%), Gomphostilbia Enderlein (10 species, 23%), Montisimulium Robtsov (five species, 11%), Nevermannia Enderlein (seven species, 16%) and Simulium Latreille s. str. (21 species, 48%). Of these, 14 species (32%) were the most abundant, in which more than 100 specimens were found for each one. A range of between 11 and 100 specimens were found for each of 24 species (54%), and six species (14%) were considered as rare, representing ≤ 10 specimens for each one (Figure 2). The most relatively abundant taxon was S. tenebrosum complex (11.1%), followed by the S. asakoae species-group (9.6%), the S. striatum species-group (7.7%), S. inthanonense complex (6.6%), S. doipuiense complex (6.4%), S. chomthongense complex (5.3%), S. chumpornense (5.1%) and S. nigrogilvum (4.1%). These eight taxa accounted for 57% of all collected specimens. The most frequent taxa at all of the sites (100% SO) were the two human-biting species—S. nigrogilvum and the S. asakoae species-group—followed by S. inthanonense complex, S. angkaense, S. chamlongi, S. fenestratum, the S. striatum species-group and S. tani complex, representing the same percentage of species occurrence (50% SO) (Table S1).
Figure 2.
Abundance of black fly species distributed in Doi Inthanon National Park. Black bars represent the species found with more than 100 specimens. Blue bars represent the species found with a range of between 10 and 100 specimens. Red bars represent the species found with ≤ 10 specimens.
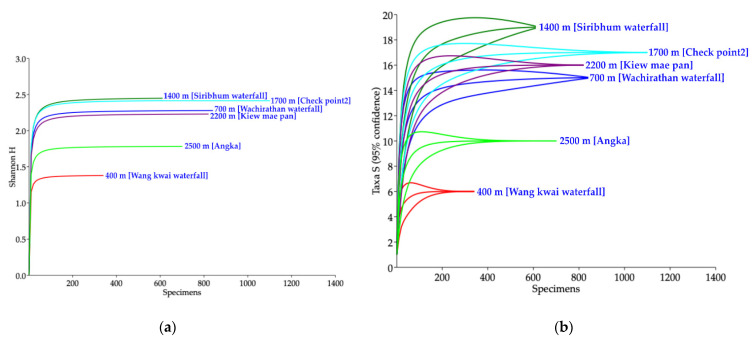
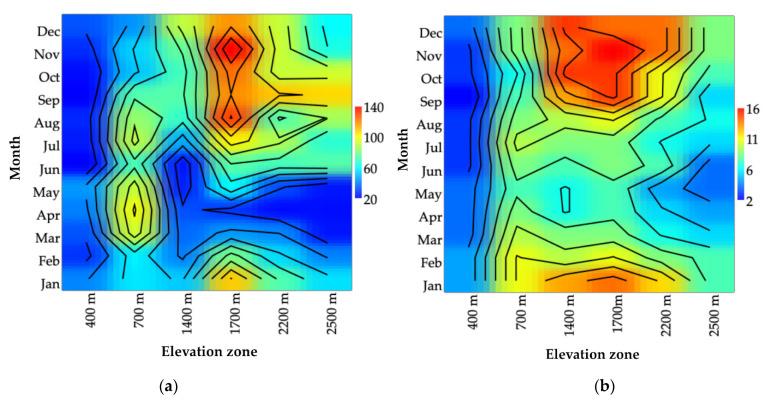
Forty-four species were observed, whereas the Chao1 richness estimator suggested 190 species in Doi Inthanon National Park. The biodiversity comparison among collection sites indicated that six of them were well-sampled (Figure 3a,b). Both the Shannon_H index and species richness were highest at an elevation of 1400 m, followed by 1700 m, 2200 m, 700 m, 2500 m and 400 m, with each representing 19, 17, 16, 15, 10 and 6 species, respectively (Table S1, Figure 3a,b).
Figure 3.
Biodiversity comparison of black flies collected among six collection sites in Doi Inthanon National Park. (a) Individual-based rarefaction curves (Shannon_H index), and (b) species richness.
The trend of richness localities (Table S1), and all diversity parameters (Table S2) of black flies in Doi Inthanon National Park were highest at mid (1400 m and 1700 m) and lowest at low (400 m) elevations. Species richness did not correlate with elevation, and the difference between sites and seasons was insignificant (within each site) (r = 0.273, p = 0.600). The evenness_ e^H/S and Equitability_J among the collection sites were a little different, ranging between 0.58 to 0.67 and 0.77 to 0.86, respectively.
The Bray–Curtis index showed 57%, 48% and 62% similarity of black fly distribution between two sites at low (400 m and 700 m), mid (1400 m and 1700 m) and high (2200 m and 2500 m) elevations, respectively. The ecotone site at low elevation (700 m) shared 53% and 48% similarity with those at mid (1400 m and 1700 m) and high (2200 m) elevations, respectively.
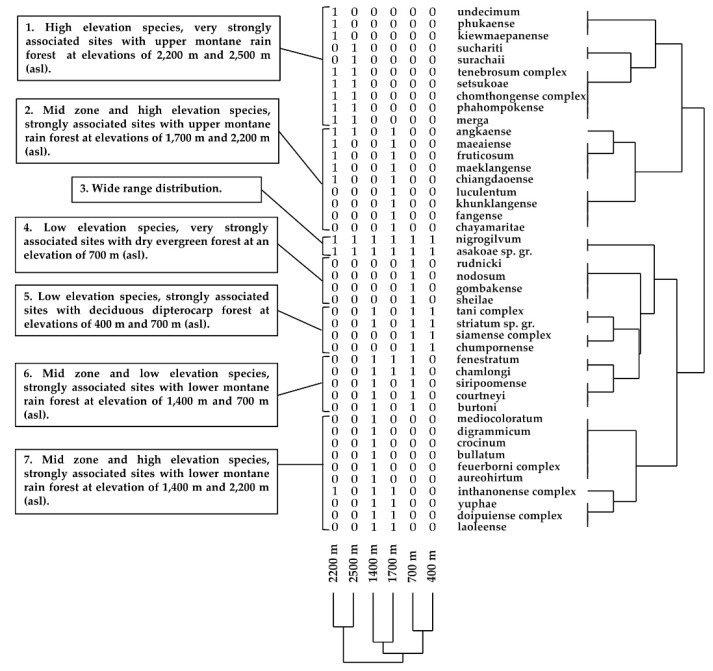
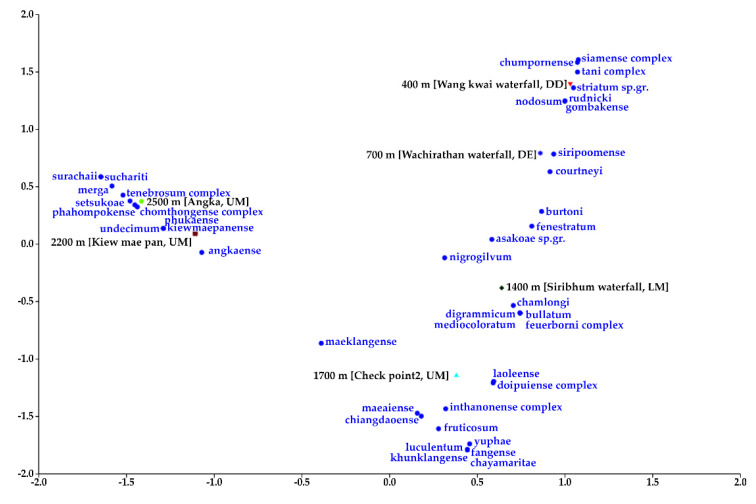
Cluster analysis produced seven groups (Figure 4 and Figure 5). Group 1 (10 species) strongly correlated with upper montane rain forest at high elevations (2200 m and 2500 m). Ten species were restricted to both sites with 100% SO. Group 2 (nine species) strongly correlated with upper montane rain forest at mid elevation (1700 m). Some species were found at a high elevation (2200 m). In Group 3, S. nigrogilvum and the S. asakoae species-group were common in a wide range of elevations. Group 4 (four species) strongly correlated with dry evergreen forest at low elevation (700 m). Four species were restricted to only this site with 100% SO. Group 5 (four species) strongly correlated with deciduous dipterocarp forest at low elevations (400 m and 700 m). Two of four species were distributed also at mid elevation (1400 m). Group 6 (five species) strongly correlated with lower dry evergreen forest at low (700 m) and mid (1400 m and 1700 m) elevations. Group 7 (10 species) strongly correlated with lower montane rain forest at mid (1400 m to 1700 m) and high (2500 m) elevations.
Figure 4.
Cluster analysis using the Bray–Curtis resemblance coefficient and UPGMA to produce the dendrogram (Copen. Corr = 0.7728), based on species distribution along six elevational gradients in Doi Inthanon National Park.
Figure 5.
Ordinate diagram of the CA of the 44 black fly species distributed in six collection sites, which correlated to the elevational gradients and forest types of Doi Inthanon National Park. DD: deciduous dipterocarp forest; DE: dry evergreen forest; LM: lower montane rain forest; UM: upper montane rain forest.
3.2. Spatial (Elevation) and Temporal (Seasonal) Distribution at Each Site
A similar trend of adult black fly abundance, collected from each season, was observed. A high seasonal abundance was recorded in the rainy season (May to October) and the lowest in the hot season (March and April) in all of the elevation sites (Table S1, Figure 6a). Seasonal species richness was highest at 1400 m in the cold season (18 species), while an equal number of species was observed at 1700 m and 2500 m in the rainy and cold seasons (Table S1, Figure 6b).
Figure 6.
Spatiotemporal variation of black flies in Doi Inthanon National Park. (a) Seasonal abundance. (b) Seasonal species richness.
Abundance increased at 400 m elevation from December of the cold season to its highest in May of the early rainy season. It started to increase at 700 m in the hot to late rainy season (March to September), peaked in April, and then decreased to its lowest in December, and started to appear at 1400 m in the mid-rainy to mid-cold season (August to December). The highest number of flies was recorded in December, while the lowest was recorded in June. Abundance started increasing at 1700 m in the mid-rainy season (July). The lowest number of flies was recorded in the hot season (April). Abundance started occurring at 2200 m during the rainy to cold seasons (June to January), whereas the lowest number of flies was recorded in the hot season (April). It started appearing at 2500 m in the rainy season (June to October) and peaked in September (Figure 6).
3.3. Distribution of Six Human−Biting Species
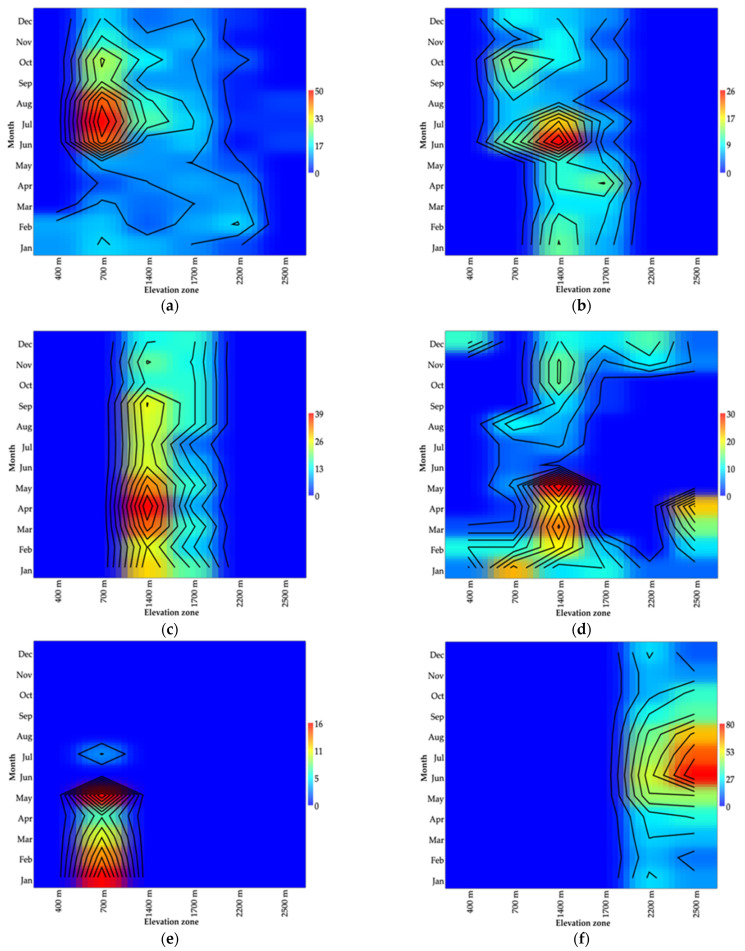
Species in the S. asakoae species-group were found in all of the collection sites and predominantly at low elevation (700 m). The high numbers of this species were recorded in the rainy season in almost all of the sites, except for those at 400 m, which were found only in the cold season (Figure 7a). S. chamlongi was distributed at low and mid elevations (700 m, 1400 m and 1700 m) and predominantly in the rainy season in all of the sites (Figure 7b). S. doipuiense complex was found only at mid elevations (1400 m and 1700 m), at which the highest numbers of flies were recorded in the rainy season (Figure 7c). S. nigrogilvum was collected in all of the six sites and the greatest numbers of flies were recorded at 1400 m in the rainy and cold seasons, followed by 700 m, 1700 m, 2200 m, 2500 m and 400 m elevations, respectively, of which the latter five sites showed the highest numbers of this species in the cold season (Figure 7d). S. nodosum was found only at low elevation (700 m) in all seasons (Figure 7e). S. tenebrosum complex only occurred at high elevations (2200 m to 2500 m) and the highest numbers of flies were recorded in the rainy season (Figure 7f).
Figure 7.
Spatiotemporal variation in abundance of six human-biting species collected along six elevational gradients at Doi Inthanon National Park. (a) Simulium asakoae species-group. (b) S. chamlongi. (c) S. doipuiense complex. (d) S. nigrogilvum. (e) S. nodosum. (f) S. tenebrosum complex.
4. Discussion
4.1. Community Structure, Species Composition and Biodiversity of Black Flies
Doi Inthanon National Park is an important biodiversity hotspot for sustainable insect conservation, including black flies [2,22,23,24,40,47,48]. It has a diverse topology with a complex microhabitat and abundance of forest [49], resulting in good-quality flowing streams and the provision of suitable breeding sites for various kinds of insects. In this study, 79% (44/56) of black fly species recorded in Doi Inthanon National Park, which account for 32% (44/139) of total species reported in Thailand, were collected by using malaise traps. In addition, the results of this study showed an estimated 190 black fly species, indicating that, in future, several new species and new records might be discovered in Doi Inthanon National Park. This assumption was supported by the occurrence of several cryptic and species complexes, which have been reported from this area [48,50,51,52,53,54,55,56,57].
4.2. Spatial and Temporal Distribution at Each Site
The abundance of black flies in Doi Inthanon National Park reached its highest point during the rainy season, then declined in the cold season and reached its lowest in the hot season. This was because many habitats were not only occupied permanent streams, but also seasonal running streams that supported high numbers of flies in the rainy season. This finding was in agreement with a previous study by Choochote et al. [2], who collected adult black flies using a human-baited method from different elevations (400 m and 2460 m). In contrast, the peak was recorded in Doi Pha Hom Pok during the cold season, as the hand-sampling method was used [39,40]. Similar to previous studies [38,39,40], this study found the peak of seasonal species richness in the cold season, but it was slightly different in a number of species at 1700 m and 2500 m elevations in the rainy season, and low richness was observed in the dry season. Conversely, high species richness was recorded at 700 m elevation during the rainy season, suggesting that the topology of the study site may influence the species richness. The relationship between species richness and elevations is similar to most other regions, for example, “global patterns” that are indicated by a humped curve. A high value of biodiversity occupies the middle elevation zone in many areas, due to the effect of a transition zone or ecotone, which is the optimum condition for many resources that are suitable for several organisms [9,36,37,39,42,43,58,59,60,61,62,63]. While a negative relation was reported from the Andes Mountains in Colombia, the richness was decreased by elevation of between 1800 m and 4750 m, where snow covered the summits [64]. The distribution pattern of the 44 adult black fly species observed along the six elevational gradients in this Park is in agreement with that in previous reports on black fly ecology in Thailand and other countries [9,13,39,40,41,65]. For instance, Srisuka et al. [39] reported that variations in elevation and seasonal conditions had significant effects on the distribution of black fly species collected in Doi Pha Hom Pok. Ya’cob et al. [42] reported the occurrence of four common black fly species related to elevation in the Malaysian Peninsular. Likewise, Hadi et al. [9] found that the distribution of certain black fly species was different according to the elevation of tea plantations in Puncak Bogor, Indonesia. Temperatures have been considered an essential factor in black fly distribution at different elevations [42,65], which affect the characteristics of the aquatic environment [66].
Simulium (D.) courtneyi, one of the two member species of the subgenus Daviesellum Takaoka and Adler, was found in this study and its highest abundance was recorded in the rainy season. This study placed malaise traps near Wachirathan and Siribhum waterfalls (Chiang Mai province), where both have fast-flowing water throughout the year, especially during the rainy season. This species was collected from the rocky surface under the waterfalls, and its occurrence was in accordance with a previous report by Takaoka et al. [67]. They found it in the Monthatharn waterfall, which is large and fast-flowing, and located at 730 m elevation in Chiang Mai province.
Ten species members belonging to the subgenus Gomphostilbia Enderlein were found from various localities, which accounted for 23% of the total species collected in this study. S. burtoni, S. gombakense, S. sheilae and S. siamense were found mostly at low elevations (400 m to 700 m), in which their breeding sites were associated with large rivers or the tributaries of mainstreams [1,22,40,42]. Similar to the study by Srisuka et al. [40], S. burtoni and S. gombakense were abundant in the cold season. The predominance of S. sheilae and S. siamense in the rainy season was in accordance with previous reports in northeastern Thailand [38,40]. S. chumpornense represented 5.1% of the total flies collected and was also found at low elevations (400 m to 700 m) throughout the year. A high abundance was observed at Wachirathan waterfall (700 m) in the hot season, as reported previously [40,68]. Surprisingly, blooming of this species was observed in the short time period of the hot season in several areas of northern Thailand, especially after the forests had been burnt by fire. It was suspected that carbon dioxide might attract black flies.
Five species members of the subgenus Montisimulium Robtsov were found, which represented 11% of the total species collected in this study. Four of them were reported originally from Doi Inthanon National Park and the remaining species, S. phahompokense, was reported from Doi Pha Hom Pok. These five species were found at mid-high elevations (1400 m to 2500 m) with high abundance in the cold season, which supports previously published reports further [39,40,69,70,71]. The species members of this subgenus in Thailand were found in several localities at high elevation, i.e., Chong Yen (Khamphaeng Phet province), Doi Phuka (Nan province), Phulang Ka waterfall (dry season) and Ban Lek (Phayao province), and Mae klong Yai Village (Tak province) (personal data), the same as reported in other countries [72,73,74,75].
Seven species members of the subgenus Nevermannia Enderlein were collected, and they accounted for 16% of the total species collected in this study. S. chomthongense complex was distributed at high elevation (2200 m to 2500 m), with high abundance in the rainy season. This species strongly correlated with small highland streams and low-temperature water at Doi Pha Hom Pok, as in several species of this subgenus, which distributes in several highland areas of Thailand [39,40].
A total of 21 species members of the subgenus Simulium Latreille s. str. were found, which represented 48% of the total species collected in this study. The subgenus Simulium is the largest subgenus in Thailand, comprising 54 species. Moreover, some species of this subgenus are medically important flies as biters [2,14,18] and pests of water buffalo, chicken and other domestic animals [2,16,17,76]. The members of this subgenus have a wide distribution range between small lowland and large up to summit streams, and they have a high abundance in various seasons. The most predominant taxa found at low elevation was the S. striatum species-group, which comprises five species [12], including three new species (S. wangkwaiense, S. tadtonense and S. maeklongkeense) that have been discovered recently [13]. This species group was found in many areas of Thailand [38,39,40], the Oriental region, Taiwan, China, Japan and South Korea [12]. The abundance of this species group varied by elevational gradients from 400 m to 1400 m and had high numbers in the rainy season. Similar to other reports, this species group was associated with large lowland streams with fast-flowing currents [23,39,41]. S. bullatum, S. crocinum, S. digrammicum and S. mediocoloratum were found only at mid elevation (1400 m). Aquatic stages of S. bullatum correlated with fast-flowing water and bed rock in small to large waterfalls in many places of Thailand. It is interesting that this species can breed in calcareous waterfalls [39,40]. This study found only aquatic stages of S. crocinum, S. digrammicum and S. mediocoloratum in small canals (10 cm to 30 cm wide) near the trapping area, but not in large streams (10 m to 20 m wide) or waterfalls (30 m wide). These species also were found in grass trailing and the rock surface at Pha Samran waterfall, which was approximately 35 km away from the trapping area. S. kiewmaepanense, S. phukaense and S. undecimum were found only at high elevation (2200 m) in Kiew Mae Pan. A high abundance of these species was observed in the rainy season, as previously reported [77]. S. phukaense was reported originally from large waterfalls at 1250 m in Nan province [78]. However, the characteristic types of localities were different from this study. It was assumed that this species might fly up from its breeding stream located below this study site in the west (5 km distance). S. suchariti was found only at the mountain summit (2500 m) in small numbers in the rainy and cold seasons. This was in agreement with a species report by Choochote et al. [2], who collected two specimens with a sweep net in over a year [2]. This species was restricted to only the summit stream of Doi Inthanon National Park, where larvae and pupae were collected.
4.3. Distribution of Six Human-Biting Species
The highest relatively abundant species was S. tenebrosum complex, which represented 11.1% of the total black flies collected. Similar to previous studies, this species was found at high elevations (2200 m to 2500 m) and occurred throughout the year, in which high densities were observed during the rainy season (May to October) [2,23]. Furthermore, it was found at 2100 m in Doi Pha Hom Pok, Chiang Mai province [39]. The two common species—the S. asakoae species-group and S. nigrogilvum—have a wide range of distribution and were found in all of the sites. This finding is consistent with previous studies in Thailand [2,40]. The S. asakoae species-group was collected from 500 m to 2100 m and high numbers of flies were trapped at mid elevations (1400 m to 1500 m) in Doi Pha Hom Pok [39]. It was more abundant during the rainy season at 250 m in Doi Saket district, Chiang Mai province and has been reported as a possible natural vector of filarial worms [17]. In addition, it has been recorded in other Asian countries [8,12]. S. nigrogilvum is distributed at mid to high elevations (250 m to 2460 m) in northern, western and central Thailand [2,15,16,18,39]. S. chamlongi was found between 700 m and 1700 m, and large numbers were recorded at the Siribhum waterfall (1400 m), as previously reported [22,39]. S. doipuiense complex is distributed in a narrow range at 1400 m to 1700 m, in which high numbers were observed at 1700 m. This species was found at 1360 m in Doi Suthep-Pui and also between 800 m and 2460 m at Doi Inthanon National Park [2]. This species also was found at 999 m in northern Vietnam [79]. S. nodosum was found only at Wachirathan waterfall (700 m) in all seasons, as previously reported in Doi Pha Hom Pok [39]. In addition, this species was found at 860 m and 1360 m in Doi Inthanon National Park [2] and at 250 m in Doi Saket district, Chiang Mai province [17]. It is a human biter and natural vector of Onchocerca species in Thailand [16]. Moreover, it has been recorded in India, China, Taiwan, Vietnam and Myanmar [12].
5. Conclusions
This study was the first to investigate the community structure, biodiversity and spatiotemporal distribution of adult black flies, by using the malaise trap in northern Thailand. The findings of this study demonstrated the seasonal and temporal variability at the species level (or at species group level). The seasonal abundance and species richness were observed at mid elevations in the rainy and cold seasons. The most predominant species was S. tenebrosum complex, while the most common taxa were the S. asakoae species-group and S. nigrogilvum. Based on the findings of this study, the malaise trap is an effective method, as it is easy to set up at reasonable cost and can capture insects over a long period of time. It is suggested that this trap can be applied to study other insect groups in Thailand.
Acknowledgments
The authors would like to thank the staff of the Entomology Section, Queen Sirikit Botanic Garden for their kind help in field collections, specimen sorting in the laboratory and photography.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/insects12060504/s1: Table S1: species composition, abundance, richness and frequency of fly species occurrence (SO) in streams from black flies collected in Doi Inthanon National Park; Table S2: diversity parameters for black flies at six elevation sites in Doi Inthanon National Park.
Author Contributions
Conceptualization, W.S., A.S., and H.T.; methodology, W.S.; software, W.S.; validation, W.S., A.S., and H.T.; formal analysis, W.S.; investigation, W.S. and C.S.; resources, W.S. and C.S.; data curation, W.S. and A.S.; writing—original draft preparation, W.S. and A.S.; writing—review and editing, W.S., K.A., T.P., K.T., S.T., A.S., and H.T.; visualization, W.S.; supervision, H.T.; project administration, A.S.; funding acquisition, A.S. and H.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Faculty of Medicine (Grant No. PAR-2563-07268) and Office of Research Administration, Chiang Mai University. The authors acknowledge funding from the Ministry of Education, Malaysia, under the Higher Institution Centre of Excellence (HICoE) niche area vector and vector-borne diseases (Project no. MO002-2019).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Takaoka H., Davies D.M. The Black Flies (Diptera: Simuliidae) of West Malaysia. Kyushu University Press; Fukuoka, Japan: 1995. [Google Scholar]
- 2.Choochote W., Takaoka H., Fukuda M., Otsuka Y., Aoki C., Eshima N. Seasonal abundance and daily flying activity of black flies (Diptera: Simuliidae) attracted to human baits in Doi Inthanon National Park, northern Thailand. Med. Entomol. Zool. 2005;56:335–348. doi: 10.7601/mez.56.335. [DOI] [Google Scholar]
- 3.Werner D., Pont A.C. Dipteran predators of simuliid blackflies: A worldwide review. Med. Vet. Entomol. 2003;17:115–132. doi: 10.1046/j.1365-2915.2003.00431.x. [DOI] [PubMed] [Google Scholar]
- 4.Ciadamidaro S., Mancini L., Rivosecchi L. Black flies (Diptera: Simuliidae) as ecological indicators for stream ecosystem health in an urbanizing area (Rome, Italy) Ann. Ist. Super. Sanita. 2016;52:269–276. doi: 10.4415/ANN_16_02_20. [DOI] [PubMed] [Google Scholar]
- 5.Adler P.H., Courtney G.W. Ecology and societal services of aquatic diptera. Insects. 2019;10:70. doi: 10.3390/insects10030070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crosskey R.W. The Natural History of Blackflies. John Wiley & Sons; Chichester, UK: 1990. [Google Scholar]
- 7.Srisuka W. Ph.D. Thesis. Chiang Mai University; Chiang Mai, Thailand: Jul 29, 2015. Species Diversity of Black Flies in Thailand, and the Evaluation of Ecological Factors Influencing Black-Fly Species Diversity in Doi Phahompok National Park. [Google Scholar]
- 8.Adler P.H. World Blackflies (Diptera: Simuliidae): A Comprehensive Revision of the Taxonomic and Geographical Inventory. [(accessed on 12 December 2020)];2020 Available online: https://biomia.sites.clemson.edu/pdfs/blackflyinventory.pdf.
- 9.Hadi U.K., Soviana S., Rohmah I.L. Diversity and ecology of black flies in tea plantation area of Puncak Bogor, Indonesia. Acta Trop. 2019;199:104986. doi: 10.1016/j.actatropica.2019.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Srisuka W., Takaoka H., Fukuda M., Otsuka Y., Saeung A. A new species of Simulium (Gomphostilbia) (Diptera: Simuliidae) from Thailand, with its phylogenetic relationships with related species in the Simulium asakoae species-group. Acta Trop. 2019;197:105043. doi: 10.1016/j.actatropica.2019.105043. [DOI] [PubMed] [Google Scholar]
- 11.Srisuka W., Aupalee K., Low V.L., Ya’cob Z., Fukuda M., Saeung A., Takaoka H. A new species of Simulium (Gomphostilbia) (Diptera: Simuliidae) from Northern Thailand, with its genetic relationship in the S. asakoae species-group. Acta Trop. 2021;218:105889. doi: 10.1016/j.actatropica.2021.105889. [DOI] [PubMed] [Google Scholar]
- 12.Takaoka H., Srisuka W., Saeung A. Checklist and keys for the black flies (Diptera: Simuliidae) of Thailand. Med. Entomol. Zool. 2019;70:53–77. doi: 10.7601/mez.70.53. [DOI] [Google Scholar]
- 13.Aupalee K., Saeung A., Srisuka W., Fukuda M., Junkum A., Pitasawat B., Takaoka H. Three new species of the Simulium (Simulium) striatum species-group (Diptera: Simuliidae) from Thailand, with their phylogenetic relationships. Acta Trop. 2020;211:105625. doi: 10.1016/j.actatropica.2020.105625. [DOI] [PubMed] [Google Scholar]
- 14.Takaoka H., Srisuka W., Seaung A. A new human-biting black fly species of Simulium (Simulium) (Diptera: Simuliidae) from Thailand. J. Med. Entomol. 2017;54:945–948. doi: 10.1093/jme/tjx064. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda M., Choochote W., Bain O., Aoki C., Takaoka H. Natural infections with filarial larvae in two species of black flies (Diptera: Simuliidae) in northern Thailand. Jpn. J. Trop. Med. Hyg. 2003;31:99–102. doi: 10.2149/tmh1973.31.99. [DOI] [PubMed] [Google Scholar]
- 16.Takaoka H., Choochote W., Aoki C., Fukuda M., Bain O. Black flies (Diptera: Simuliidae) attracted to humans and water buffalos and natural infections with filarial larvae, probably Onchocerca sp. in northern Thailand. Parasite. 2003;10:3–8. doi: 10.1051/parasite/2003101p3. [DOI] [PubMed] [Google Scholar]
- 17.Ishii Y., Choochote W., Bain O., Fukuda M., Otsuka Y., Takaoka H. Seasonal and diurnal biting activities and zoonotic filarial infections of two Simulium species (Diptera: Simuliidae) in northern Thailand. Parasite. 2008;15:121–129. doi: 10.1051/parasite/2008152121. [DOI] [PubMed] [Google Scholar]
- 18.Saeung A., Srisuka W., Aupalee K., Fukuda M., Otsuka Y., Taai K., Malewong W., Takaoka H. Natural infections with larvae of Onchocerca species type I in the human-biting black fly, Simulium nigrogilvum (Diptera: Simuliidae), in western Thailand. Acta Trop. 2020;204:105344. doi: 10.1016/j.actatropica.2020.105344. [DOI] [PubMed] [Google Scholar]
- 19.Aupalee K., Saeung A., Srisuka W., Fukuda M., Streit A., Takaoka H. Seasonal filarial infections and their black fly vectors in Chiang Mai province, northern Thailand. Pathogens. 2020;9:512. doi: 10.3390/pathogens9060512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cambra-Pelleja M., Gandasegui J., Balana-Fouce R., Munoz J., Martinez-Valladares M. Zoonotic implications of Onchocerca species on human health. Pathogens. 2020;9:761. doi: 10.3390/pathogens9090761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.National Parks Wildlife and Plant Conservation Department . The Best of National Parks of Thailand. National Park; Bangkok, Thailand: 2004. [Google Scholar]
- 22.Takaoka H., Suzuki H. The blackflies (Diptera: Simuliidae) from Thailand. Med. Entomol. Zool. 1984;35:7–45. doi: 10.7601/mez.35.7. [DOI] [Google Scholar]
- 23.Kuvangkadilok C., Boonkemthong C., Phayuhasena S. Distribution of the larvae of blackflies (Diptera: Simuliidae) at Doi Inthanon national park, northern Thailand. Southeast Asian J. Trop. Med. Public Health. 1999;30:328–337. [PubMed] [Google Scholar]
- 24.Harbach R.E., Shelley T. Darwin Initiative for the Survival of Species Final Report. [(accessed on 1 April 2020)];2007 Available online: https://www.darwininitiative.org.uk/documents/DAR13003/3276/13-003%20FR%20-%20edited.pdf.
- 25.Thaijarern J., Wongpakam K., Kangrang A., Pramual P. A new species of black fly (Diptera: Simuliidae) in the Simulium (Simulium) multistriatum species-group from Thailand. Zootaxa. 2019;4586:461–474. doi: 10.11646/zootaxa.4586.3.4. [DOI] [PubMed] [Google Scholar]
- 26.Vårdal H., Taeger A. The life of René Malaise: From the wild east to a sunken island. Zootaxa. 2011;3127:38–52. doi: 10.11646/zootaxa.3127.1.2. [DOI] [Google Scholar]
- 27.Aguiar A.P., Santos B.F. Discovery of potent, unsuspected sampling disparities for Malaise and Möricke traps, as shown for Neotropical Cryptini (Hymenoptera, Ichneumonidae) J. Insect Conserv. 2010;14:199–206. doi: 10.1007/s10841-009-9246-x. [DOI] [Google Scholar]
- 28.Marchiori C.H. Traps for specimen collection of insect in Brazil. Qeios. 2019;641572:1–7. doi: 10.32388/641572. [DOI] [Google Scholar]
- 29.Evans A. Invertebrates: Malaise Trapping Version 1.0. [(accessed on 3 April 2020)];2016 Available online: https://www.doc.govt.nz/globalassets/documents/science-and-technical/inventory-monitoring/im-toolbox-invertebrates-malaise-trapping.pdf.
- 30.TIGER: Thailand Inventory Group for Entomological Research. [(accessed on 5 April 2020)]; Available online: http://sharkeylab.org/tiger/static.php?app=tiger&page=news.
- 31.Brown B.V. Small size no protection for acrobat ants: World’s smallest fly is a parasitic phorid (Diptera: Phoridae) Ann. Entomol. Soc. Am. 2012;105:550–554. doi: 10.1603/AN12011. [DOI] [Google Scholar]
- 32.Brown B.V. A second contender for “world’s smallest fly” (Diptera: Phoridae) Biodivers. Data J. 2018:e22396. doi: 10.3897/BDJ.6.e22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fraser S.E.M., Dytham C., Mayhew P.J. The effectiveness and optimal use of Malaise traps for monitoring parasitoid wasps. Insect Conserv. Divers. 2008;1:22–31. doi: 10.1111/j.1752-4598.2007.00003.x. [DOI] [Google Scholar]
- 34.Oxbrough A., Gittings T., Kelly T.C., O’Halloran J. Can Malaise traps be used to sample spiders for biodiversity assessment? J. Insect Conserv. 2010;14:169–179. doi: 10.1007/s10841-009-9238-x. [DOI] [Google Scholar]
- 35.Geiger M.F., Moriniere J., Hausmann A., Haszprunar G., Wägele W., Hebert P.D., Rulik B. Testing the Global Malaise Trap Program—How well does the current barcode reference library identify flying insects in Germany? Biodivers. Data J. 2016:e10671. doi: 10.3897/BDJ.4.e10671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DiGirolomo M.F., Dodds K.J. Comparison of the species richness and abundance of Cerambycidae (Coleoptera) and Scolytinae (Coleoptera: Curculionidae) captured in aerial malaise traps with and without a bottom collector. Can. Entomol. 2017;149:408–412. doi: 10.4039/tce.2016.70. [DOI] [Google Scholar]
- 37.Schmidt O., Schmidt S., Häuser C.L., Hausmann A., Van Vu L. Using Malaise traps for collecting Lepidoptera (Insecta), with notes on the preparation of Macrolepidoptera from ethanol. Biodivers. Data J. 2019:e32192. doi: 10.3897/BDJ.7.e32192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pramual P., Wongpakam K. Seasonal variation of black fly (Diptera: Simuliidae) species diversity and community structure in tropical streams of Thailand. Entomol. Sci. 2010;13:17–28. doi: 10.1111/j.1479-8298.2009.00354.x. [DOI] [Google Scholar]
- 39.Srisuka W., Takaoka H., Otsuka Y., Fukuda M., Thongesahuan S., Taai K., Choochote W., Saeung A. Seasonal biodiversity of black flies (Diptera: Simuliidae) and evaluation of ecological factors influencing species distribution at Doi Pha Hom Pok National Park, Thailand. Acta Trop. 2015;149:212–219. doi: 10.1016/j.actatropica.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 40.Srisuka W., Takaoka H., Otsuka Y., Fukuda M., Thongesahuan S., Taai K., Saeung A. Biodiversity seasonal abundance, and distribution of black flies (Diptera: Simuliidae) in six different regions of Thailand. Parasit. Vectors. 2017;10:574. doi: 10.1186/s13071-017-2492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jitklang S., Sawangproh W., Kuvangkadilok C., Baimai V., Adler P.H. Ecology of black flies (Diptera: Simuliidae) in streams of northern and southern Thailand: Factors associated with larval and pupal distributions. Acta Trop. 2020;204:105357. doi: 10.1016/j.actatropica.2020.105357. [DOI] [PubMed] [Google Scholar]
- 42.Ya’cob Z., Takaoka H., Pramual P., Low L.V., Sofian-Azurin M. Distribution pattern of black fly (Diptera: Simuliidae) assemblages along an altitudinal gradient in Peninsular Malaysia. Parasit. Vectors. 2016;9:219. doi: 10.1186/s13071-016-1492-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pavitra S.P., Low V.L., Tan T.K., Lim Y.A.L., Ya’cob Z. Temporal variation in diversity and community structure of preimaginal blackflies (Diptera: Simuliidae) in a tropical forest reserve in Malaysia. Acta Trop. 2020;202:105275. doi: 10.1016/j.actatropica.2019.105275. [DOI] [PubMed] [Google Scholar]
- 44.Chayamarit K., Puff C. Plant of Doi Inthanon National Park. National Parks, Wildlife and Plant Conservation Department, Ministry of Natural Resources and Environment; Bangkok, Thailand: 2007. p. 580. [Google Scholar]
- 45.Takaoka H., Srisuka W., Fukuda M., Saeung A. Twenty-one new species of the Simulium (Gomphostilbia) asakoae species group (Diptera, Simuliidae) in Thailand, with their genetic relationships. ZooKeys. 2020;950:51–152. doi: 10.3897/zookeys.950.51298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hammer Ø., Harper D.A.T., Ryan P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2011;4:1–9. [Google Scholar]
- 47.Adler P.H., Srisuka W., Low V.L., Takaoka H., Saeung A. High-elevation chromosomal diversity of black flies (Diptera: Simuliidae) in Thailand. Insect Syst. Divers. 2019;3:1–10. doi: 10.1093/isd/ixz004. [DOI] [Google Scholar]
- 48.Adler P.H., Srisuka W., Saeung A. Cryptic species of black flies (Diptera: Simuliidae) at high elevations in the Oriental Region: The Simulium vernum species group in Thailand. Acta Trop. 2020;205:105393. doi: 10.1016/j.actatropica.2020.105393. [DOI] [PubMed] [Google Scholar]
- 49.Sungkajanttranon O., Marod D., Thanompun K. Diversity and distribution of family Araceae in Doi Inthanon national park, Chiang Mai province. Agric. Nat. Resour. 2018;52:125–131. doi: 10.1016/j.anres.2018.06.009. [DOI] [Google Scholar]
- 50.Pramual P., Kuvangkadilok C., Baimai V., Walton C. Phylogeography of the black fly Simulium tani (Diptera: Simuliidae) from Thailand as inferred from mtDNA sequences. Mol. Ecol. 2005;14:3989–4001. doi: 10.1111/j.1365-294X.2005.02639.x. [DOI] [PubMed] [Google Scholar]
- 51.Thanwisai A., Kuvangkadilok C., Baimai V. Molecular phylogeny of black flies (Diptera: Simuliidae) from Thailand, using ITS2 rDNA. Genetica. 2006;128:177–204. doi: 10.1007/s10709-005-5702-z. [DOI] [PubMed] [Google Scholar]
- 52.Tangkawanit U., Kuvangkadilok C., Baimai V., Adler P.H. Cytosystematics of the Simulium tuberosum group (Diptera: Simuliidae) in Thailand. Zool. J. Linn. Soc. 2009;155:289–315. doi: 10.1111/j.1096-3642.2008.00433.x. [DOI] [Google Scholar]
- 53.Pramual P., Wongpakam K., Adler P.H. Cryptic biodiversity and phylogenetic relationships revealed by DNA barcoding of Oriental black flies in the subgenus Gomphostilbia (Diptera: Simuliidae) Genome. 2011;54:1–9. doi: 10.1139/G10-100. [DOI] [PubMed] [Google Scholar]
- 54.Pramual P., Nanork P. Phylogenetic analysis based on multiple gene sequences revealing cryptic biodiversity in Simulium multistriatum group (Diptera: Simuliidae) in Thailand. Entomol. Sci. 2012;15:202–213. doi: 10.1111/j.1479-8298.2011.00491.x. [DOI] [Google Scholar]
- 55.Pramual P., Wongpakam K. Cytogenetics of Simulium siamense Takaoka and Suzuki, 1984 (Diptera: Simuliidae) in northeastern Thailand. Aquat. Insects. 2011;33:171–184. doi: 10.1080/01650424.2011.597407. [DOI] [Google Scholar]
- 56.Pramual P., Wongpakam K. Population genetics of the high elevation black fly Simulium (Nevermannia) feuerborni Edwards in Thailand. Entomol. Sci. 2013;16:298–308. doi: 10.1111/ens.12008. [DOI] [Google Scholar]
- 57.Pramual P., Adler P.H. DNA barcoding of tropical black flies (Diptera: Simuliidae) of Thailand. Mol. Ecol. Resour. 2014;14:262–271. doi: 10.1111/1755-0998.12174. [DOI] [PubMed] [Google Scholar]
- 58.Teejuntuk S., Sahunalu P., Sakurai K., Sungpalee W. Forest structure and tree species diversity along an altitudinal gradient in Doi Inthanon national park, northern Thailand. Tropics. 2003;12:85–102. doi: 10.3759/tropics.12.85. [DOI] [Google Scholar]
- 59.Marod D., Hermhuk S., Sungkaew S., Thinkampheang S., Kamyo T., Nuipakdee W. Species composition and spatial distribution of dominant trees in the forest ecotone of a mountain ecosystem, northern Thailand. Environ. Nat. Resour. J. 2019;17:40–49. doi: 10.32526/ennrj.17.3.2019.21. [DOI] [Google Scholar]
- 60.Heino J. Biodiversity of aquatic insects: Spatial gradients and environmental correlates of assemblage-level measures at large scales. Freshw. Rev. 2009;2:1–29. doi: 10.1608/FRJ-2.1.1. [DOI] [Google Scholar]
- 61.Bouzan A.M., Flinte V., Macedo M.V., Monteiro R.F. Elevation and temporal distributions of Chrysomelidae in southeast Brazil with emphasis on the Galerucinae. Zookeys. 2015:103–117. doi: 10.3897/zookeys.547.9723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Motta L., Ruggiero A., de Mendoza G., Massaferro J. The species richness-elevation relationship: Global patterns of variation in chironomid richness in mountain lakes. Insect Conserv. Divers. 2019;12:339–350. doi: 10.1111/icad.12341. [DOI] [Google Scholar]
- 63.Slowinska I., Jaskula R. Distributional patterns of aquatic Empididae (Diptera) along an elevational diversity gradient in a low mountain range: An example from central Europe. Insects. 2021;12:165. doi: 10.3390/insects12020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mantilla J.S., Moncada L.I., Matta N.E., Adler P.H. Distribution of black flies (Diptera: Simuliidae) along an elevational gradient in the Andes Mountains of Colombia during the El Nino Southern Oscillation. Acta Trop. 2018;183:162–172. doi: 10.1016/j.actatropica.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 65.Zahar A.R. The ecology and distribution of black-flies (Simuliidae) in South-East Scotland. J. Anim Ecol. 1951;20:33–62. doi: 10.2307/1643. [DOI] [Google Scholar]
- 66.González J.G., Fernández-Aláez M., Cueto J.A. Geographical distribution of Adephaga and Polyphaga (Coleoptera) in the Cantabrian Mountains (Spain): Specific richness and analysis of the altitude factor. Arch. Hydrobiol. 1994;131:353–380. doi: 10.1127/archiv-hydrobiol/131/1994/353. [DOI] [Google Scholar]
- 67.Takaoka H., Adler P.H. A new subgenus, Simulium (Daviesellum), and a new species, S. (D.) courtneyi, (Diptera: Simuliidae) from Thailand and Peninsular Malaysia. Jpn. J. Trop. Med. Hyg. 1997;25:17–27. doi: 10.2149/tmh1973.25.17. [DOI] [Google Scholar]
- 68.Kuvangkadilok C., Takaoka H. Taxonomic notes on Simuliidae (Diptera) from Thailand: Description of a new species and new distributional records of nine known species. Jpn. J. Trop. Med. Hyg. 2000;28:167–175. doi: 10.2149/tmh1973.28.167. [DOI] [Google Scholar]
- 69.Takaoka H., Srisuka S., Saeung A., Otsuka Y., Aoki C., Choochote W. Taxonomic notes on Simulium (Montisimulium) laoleense and a closely related new species (Diptera: Simuliidae) from Thailand. Med. Entomol. Zool. 2010;61:363–374. doi: 10.7601/mez.61.363. [DOI] [Google Scholar]
- 70.Takaoka H., Choochote W. Discovery of two more new species of Simulium (Montisimulium) (Diptera: Simuliidae) in Doi Inthanon national park, Chiang Mai, Thailand. Trop. Med. Health. 2005;33:209–215. doi: 10.2149/tmh.33.209. [DOI] [Google Scholar]
- 71.Takaoka H., Choochote W. Two new species of Simulium (Montisimulium) (Diptera: Simuliidae) from northern Thailand. Med. Entomol. Zool. 2005;56:21–31. doi: 10.7601/mez.56.21. [DOI] [Google Scholar]
- 72.Datta M. A new black fly species (Simuliidae-Diptera) from the Darjeeling area, India. Proc. Indian Acad. Sci. 1975;81:67–74. doi: 10.1080/00305316.1974.10434877. [DOI] [Google Scholar]
- 73.Deng C.Y., Xue H.D., Chen H.B. One new species of the Montisimulium from Yunnan. Chin. J. Vector Biol. Control. 2005;16:191–192. [Google Scholar]
- 74.Takaoka H., Somboon P. Eleven new species and one new record of black flies (Diptera: Simuliidae) from Bhutan. Med. Entomol. Zool. 2008;59:213–262. doi: 10.7601/mez.59.213. [DOI] [Google Scholar]
- 75.Han-Bin C., Guo-Sheng L., Chun-Lin Z. A faunal summary of Simulium (Montisimulium) with descriptions of five new species from China (Diptera: Simuliidae) Zootaxa. 2011;3017:51–68. doi: 10.11646/zootaxa.3017.1.3. [DOI] [Google Scholar]
- 76.Jomkumsing P., Tangkawanit U., Wongpakam K., Pramual P. Who is biting you? DNA barcodes reveal cryptic diversity in human-biting black flies (Diptera: Simuliidae) Acta Trop. 2019;196:22–29. doi: 10.1016/j.actatropica.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 77.Takaoka H., Srisuka W., Saeung A. Two new species of the Simulium (Simulium) variegatum species-group of black flies (Diptera: Simuliidae) from Thailand. J. Med. Entomol. 2017;54:1213–1223. doi: 10.1093/jme/tjx080. [DOI] [PubMed] [Google Scholar]
- 78.Takaoka H., Choochote W. A new species of Simulium (Simulium) from northern Thailand (Diptera: Simuliidae) Trop. Med. Health. 2005;33:95–101. doi: 10.2149/tmh.33.95. [DOI] [Google Scholar]
- 79.Takaoka H., Sofian-Azirun M., Ya’cop Z., Chen C.D., Lau K.W., Low V.L., Pham X.D., Adler P.H. The black flies (Diptera: Simuliidae) of Vietnam. Zootaxa. 2017;4261:1–165. doi: 10.11646/zootaxa.4261.1.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in the Supplementary Materials.