Abstract
A 10-y-old intact male Labrador Retriever dog had a history of ataxia, inability to stand, and grand mal seizures. Complete blood count and serum biochemistry profiles revealed profound hypoglycemia, mildly increased alanine aminotransferase (ALT) activity, mild hypernatremia, and lymphopenia. The seizures could not be controlled with intravenous dextrose, diazepam, or propofol. The dog was euthanized given poor quality of life, and an autopsy was performed. Primary autopsy findings included firm hepatic masses that ranged from dark-red to tan, with the largest ~1.5 cm diameter, and pulmonary edema. Histologic examination of the hepatic masses revealed redundant, several-cell-thick cords, and packeted or acinar arrangements of polygonal cells, supported on a fibrovascular stroma. The neoplastic cells were immunopositive for insulin, synaptophysin, and neuron-specific enolase immunohistochemistry; granules in the tumor cells had an affinity for Grimelius silver stain. The histologic features, as well as the immunohistochemical staining profile, identified the neoplasm as a primary multifocal hepatic neuroendocrine carcinoma. Neuroendocrine carcinomas are rare in dogs and usually occur in the gastrointestinal or respiratory tract.
Keywords: canine, carcinoid, hepatic neuroendocrine carcinoma, hypoglycemia, seizure
Primary neuroendocrine carcinomas are rare in humans and dogs, and, in humans, occur typically in the respiratory and gastrointestinal tracts.1,6,7 In dogs and cats, neuroendocrine carcinomas have been documented in the skin, nasal cavity, nasopharynx, esophagus, intestines, liver, gallbladder, and bile duct. 7 A primary hepatic neuroendocrine carcinoma (HNC) was first described in 1980 in a canine patient that also had hypercortisolism and hypokalemia. 7 In humans, these tumors can lead to a paraneoplastic condition, “carcinoid syndrome,” as a result of the secretion of serotonin, and may also lead to profound hypoglycemia.1,6,8 We describe here HNC-induced hypoglycemia in a dog.
A 10-y-old intact male Labrador Retriever dog was presented because of polydipsia and was found to be hypoglycemic. Complete blood count (CBC) and serum biochemistry profiles performed 3 wk prior had been normal. The patient was taken to an emergency clinic the next day after an episode of ataxia and inability to stand following a grand mal seizure. During examination, the patient was dull and quiet but responsive. Vital signs were normal. Normal muscle mass was noted; however, the patient was weak and unwilling to stand or support his weight. A blood glucose measurement revealed profound hypoglycemia (1.5 mmol/L; reference interval [RI]: 3.3–7.3 mmol/L). A serum biochemistry profile confirmed hypoglycemia and identified mildly increased alanine aminotransferase (ALT) activity and mild hypernatremia; CBC revealed mild lymphopenia; all other values were unremarkable (Table 1). Abdominal radiographs revealed an enlarged prostate and degenerative spinal column changes, but no other significant abnormalities.
Table 1.
Abnormal results of a complete blood count, serum biochemistry panel, and blood gas analysis on initial presentation to an emergency clinic for a dog with a hepatic neuroendocrine carcinoma.
| Test | Unit | Result | Reference interval |
|---|---|---|---|
| Lymphocytes | ×109/L | 0.87 (Low) | 1.05–5.10 |
| Alanine aminotransferase | µkat/L | 2.30 (High) | 0.17–2.09 |
| Glucose (Idexx Catalyst One) | mmol/L | 1.89 (Low) | 3.89–7.94 |
| Glucose (Nova) | mmol/L | 2.72 (Low) | 3.66–6.38 |
The patient was alert but still unwilling to stand following intravenous treatment with dextrose. Blood glucose following the seizure was 4.27 mmol/L. Hospitalization with supportive care and an intravenous dextrose continuous rate of infusion was recommended. The owner elected to take the patient home.
The patient was readmitted the following morning because of seizures. An increased respiratory effort was observed, with crackles auscultated on inspiration and expiration. The patient had a pulse of 160 beats/min and was hyperthermic (39.7°C). Hypoglycemia was profound (1.5 mmol/L); blood lactate was normal (0.18 mmol/L; RI: <2.5 mmol/L). Mild hypertension was noted with an oscillometric blood pressure reading of 147/124 mm Hg and a mean arterial pressure of 132 mm Hg. Intravenous lactated ringer solution and dextrose were administered. The next blood glucose result was elevated (10.3 mmol/L); however, the patient continued to have seizures. The patient’s temperature rose to 41.0°C. The blood glucose decreased to 7.16 mmol/L. The seizures continued and were uncontrollable with intravenous diazepam and propofol. Euthanasia was elected by the owner, and the dog was submitted for autopsy.
Based on the physical examination, clinical signs, and profound hypoglycemia, a functional beta cell tumor of the pancreas (insulinoma) was the most highly ranked differential diagnosis. These tumors can be difficult to identify. Measuring serum insulin is indicated, 4 and histologic confirmation is needed. 3 Other tests, such as an insulin-to-glucose ratio and fructosamine, may or may not be beneficial; however, neither test is considered confirmatory. 3 Thoracic radiographs and abdominal ultrasound may identify metastasis; however, insulinomas are typically too small to identify with these imaging modalities. 3 Additional testing was not pursued in our case given financial constraints.
Other differential diagnoses for hypoglycemia include ingestion of a hypoglycemic agent, such as xylitol, extrapancreatic neoplasia (through increased glucose use or secretion of insulin analogs), and hypoadrenocorticism. 4 Severe renal or hepatic disease, sepsis, pancreatitis, iatrogenic hypoglycemia (insulin overdose), and extreme exercise (hunting dog hypoglycemia) were less likely. Our patient did not have a history of diabetes and did not receive insulin. There was also no history of extreme exercise prior to a hypoglycemic event. CBC and serum biochemistry profile results did not support sepsis or significant renal or hepatic disease. Our patient also did not have a history of toxin ingestion and was not administered any medication. Hypoadrenocorticism, ingestion of a hypoglycemic agent, and extrapancreatic neoplasia could not be ruled out antemortem.
Extrapancreatic neoplasms considered were a neuroendocrine tumor, hepatocellular carcinoma, hepatoma, hemangiosarcoma, leiomyoma, and leiomyosarcoma, all of which can cause hypoglycemia as a result of secretion of insulin or insulin analogs, increased glucose utilization, or impaired glucose homeostasis by the liver. 4 Leiomyoma, leiomyosarcoma, and hemangiosarcoma were considered less likely given that the patient did not have anorexia, weight loss, vomiting, diarrhea, abdominal distension, or evidence of a hemorrhaging mass. A neuroendocrine tumor, hepatocellular carcinoma, or hepatoma were more likely, despite only mildly increased ALT activity (Table 1).
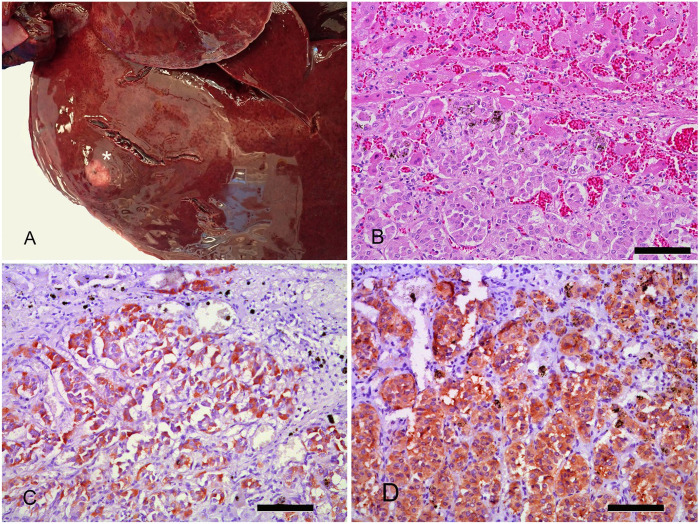
On postmortem examination, the liver contained dark-red to tan masses, up to 1.5 cm diameter, in several lobes. All of the masses were firm, with indistinct margins (Fig. 1A). The degree of hepatic effacement by the tumor did not appear sufficiently extensive to result in hypoglycemia through a mechanism of reduced glucose output. Histologic examination of the liver demonstrated multilobulated and poorly circumscribed masses of redundant, several-cell-thick cords, and packeted or acinar arrangements of polygonal cells, supported on a fine fibrovascular stroma (Fig. 1B). The cells had central and eccentric, round, finely stippled nuclei, and abundant, finely granular, eosinophilic cytoplasm. The mitotic count was 4 per 10 high power fields (2.37 mm2), and nuclear features were similar throughout the samples. The surrounding hepatic parenchyma was mildly compressed and markedly congested; there were areas of hemorrhage in and around neoplastic foci. There was a moderate desmoplastic response surrounding some of the masses.
Figure 1.
Gross anatomic findings and histochemical characterization of hepatic neuroendocrine carcinoma in a dog. A. Gross image of the liver with a mass (asterisk) in the left lateral lobe typical of additional masses found in cut sections. B. Histologic image of the liver mass showing packeted arrangements of polygonal cells (lower half of the image) infiltrating the hepatic parenchyma (upper half of the image). H&E. Bar = 100 µm. C. Infiltrating islands of neoplastic cells express strong cytoplasmic immunostaining for insulin. Vector NovaRED peroxidase. Hematoxylin counterstain. Bar = 100 µm. D. Infiltrating islands of neoplastic cells express strong cytoplasmic immunostaining for synaptophysin. Vector NovaRED peroxidase. Hematoxylin counterstain. Bar = 100 µm.
Additional postmortem findings were pulmonary edema, with no gross or histologic evidence of pulmonary metastasis, and the pancreas had grossly apparent multifocal hemorrhages in the parenchyma, normal-appearing islets histologically along with normal clusters of acinar cells distended with zymogen granules. The adrenal glands were grossly and histologically normal.
The gross and histologic appearances of the liver masses were most consistent with a malignant neuroendocrine tumor. HNC and a metastatic insulinoma were the most likely differential diagnoses, followed by a cholangiocarcinoma. HNC, insulinoma, and cholangiocarcinoma can all have a similar histologic appearance. Most insulinomas are immunoreactive to pancytokeratin, synaptophysin, neuron-specific enolase (NSE), and insulin; cholangiocarcinoma is typically only positive for pancytokeratin. HNCs commonly stain positive immunohistochemically for chromogranin-A, NSE, and synaptophysin, and some also stain with Grimelius silver stain. 7
Neuroendocrine tumors are heterogeneous neoplasms capable of producing secretory granules and a variety of biogenic amines and peptide hormones. Some of these proteins include S100, synaptophysin, and NSE. S100 is a protein associated with a wide range of regulatory functions in cells derived from the neural crest. Synaptophysin is a membrane glycoprotein found in a subset of neuroendocrine cell secretory organelles. NSE is a glycolytic enzyme that is expressed in cells late in neural differentiation. Insulin and glucagon are hormones that help regulate blood glucose, and both proteins can be expressed in neuroendocrine tumors. To characterize the liver masses described above, they were stained with Grimelius silver stain, which has an affinity for the secretory granules of neuroendocrine cells, and with a series of immunohistochemical markers that included S100, synaptophysin, NSE, insulin, and glucagon. AE1/AE3, a pancytokeratin immunohistochemical marker, was added to help address the differentials of insulinoma and cholangiocarcinoma. Immunostaining used primary antibodies directed against S100 (A4 D9F9D; Cell Signaling Technology), synaptophysin (SY38, ab8049; Abcam), NSE (1G4, 18-0196; Invitrogen), insulin (abA0564; Dako), glucagon (PA5-16372; Thermo/Pierce), and cytokeratin (AE1/AE3; Leica). The tumor cell granules had an affinity for silver and were immunohistochemically reactive to insulin (Fig. 1C), synaptophysin (Fig. 1D), and NSE (not shown). The tumor cells were not immunoreactive to glucagon, S100, or pancytokeratin.
Based on these findings, the patient was determined to have a primary hepatic neuroendocrine tumor exhibiting hypoglycemia as a paraneoplastic syndrome. Although close examination of the pancreas failed to identify a suspected insulinoma, there remains the possibility of an unidentified insulin-secreting tumor that had metastasized to the liver. It is also possible, despite insulin immunopositivity, that the liver tumor may have been producing other proteins not tested for, such as big insulin-like growth factor 2. 9
To our knowledge, HNC paraneoplastic syndrome resulting in profound hypoglycemia in a canine patient has not been reported previously and is a rare clinical presentation. A human patient that presented to an emergency clinic for recurrent hypoglycemia and no previous history of liver disease has been reported 8 ; hepatic resection and cholecystectomy resulted in a clinical cure with no signs of recurrence at a 36-mo follow-up. 8 In our case, the patient had numerous intrahepatic tumors, which would have complicated or precluded surgical resection. Another human patient with HNC had presented initially with extrapituitary acromegaly and a typical carcinoid syndrome, then progressed to a hyperinsulinemic hypoglycemic syndrome resulting in death. 1 This patient underwent an extended right hemihepatectomy; however, small tumor nodules were found in multiple liver lobes during surgery. 1 When the recurrence of symptoms was reported, octreotide therapy was initiated; however, the patient had comorbidities that may have precluded a greater positive response to the therapy. 1 The patient’s condition improved with octreotide but later quickly deteriorated as a result of severe uncontrolled hypoglycemia despite continued therapy with octreotide, diazoxide, prednisone, and glucose. 1
Histologically, neuroendocrine carcinomas in humans consist of cords, trabeculae, or glandular patterns. 5 A retrospective study of 10 dogs with HNC described tumors with trabecular patterns, cords or rows of neoplastic cells, or rosette-like (glandular) structures, with a fibrovascular stroma 7 ; 8 of the 10 neoplasms were positive for NSE and synaptophysin; only 1 was positive for insulin. 7 Hypoglycemia was not identified in the retrospective study as a clinical finding in any of the cases, but ataxia, weakness, and collapse were listed as clinical signs in one of the patients.
Given the rarity of this tumor and its ability to cause variable effects, there is no standard of care for antemortem identification or treatment, which poses a significant limitation.2,5 In human medicine, serum biomarkers exist, but they are not considered sensitive or specific for neuroendocrine carcinomas, and they often do not detect the tumor prior to metastasis. 2 Another limitation with pursuing our case included limited testing ability because of financial constraints.
Neuroendocrine carcinoma should be considered in cases of hypoglycemia of unknown origin, particularly when extrapancreatic neoplasia is identified. Mechanisms of hypoglycemia include tumor cell production of big insulin-like growth factor 2 or insulin, and liver destruction caused by extensive tumor infiltration.1,6,8 Routine screening for neoplasia including CBC and serum biochemistry panels, thoracic and abdominal radiographs, abdominal ultrasound, and recognition of hypoglycemia as a potential paraneoplastic syndrome may aid in the detection of this tumor.
Acknowledgments
We thank histology technician Wendy O’Rourke (Midwestern University Veterinary Pathology Diagnostic Center) and Amber Villarreal (UC Davis Veterinary Medical Teaching Hospital Histology Department) for preparing histopathology slides used in our report.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Amanda R. Dorn  https://orcid.org/0000-0003-2138-3859
https://orcid.org/0000-0003-2138-3859
Contributor Information
Amanda R. Dorn, Midwestern University College of Veterinary Medicine, Glendale, AZ, USA
Alexandra Brower, Midwestern University College of Veterinary Medicine, Glendale, AZ, USA.
Hailey Turner, Midwestern University College of Veterinary Medicine, Glendale, AZ, USA.
Klayton Lapa, BluePearl Specialty and Emergency Hospital, Phoenix, AZ, USA.
References
- 1. Furrer J, et al. Carcinoid syndrome, acromegaly, and hypoglycemia due to an insulin-secreting neuroendocrine tumor of the liver. J Clin Endocrinol Metab 2001;86:2227–2230. [DOI] [PubMed] [Google Scholar]
- 2. Giandomenico V, et al. Improving the diagnosis and management of neuroendocrine tumors: utilizing new advances in biomarker and molecular imaging science. Neuroendocrinology 2013;98:16–30. [DOI] [PubMed] [Google Scholar]
- 3. Grant ER, Burgess KE. Canine insulinoma: diagnosis, treatment, and staging. Today Vet Pract 2016;(Nov/Dec):60–64. [Google Scholar]
- 4. Idowu O, et al. Hypoglycemia in dogs: causes, management and diagnosis. Can Vet J 2018;59:642–649. [PMC free article] [PubMed] [Google Scholar]
- 5. Lin C-W, et al. Primary hepatic carcinoid tumor: a case report and review of the literature. Cases J 2009;2:90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morgan E, et al. Primary hepatic neuroendocrine carcinoma treated with doxorubicin and cyclophosphamide in a dog. J Am Anim Hosp Assoc 2019;55:e55305. [DOI] [PubMed] [Google Scholar]
- 7. Patnaik AK, et al. Canine hepatic neuroendocrine carcinoma: an immunohistochemical and electron microscopic study. Vet Pathol 2005;42:140–146. [DOI] [PubMed] [Google Scholar]
- 8. Rocca A, et al. Primary giant hepatic neuroendocrine carcinoma: a case report. Int J Surg 2014;12(Suppl 1):S218–S221. [DOI] [PubMed] [Google Scholar]
- 9. Zini E, et al. Paraneoplastic hypoglycemia due to an insulin-like growth factor type-II secreting hepatocellular carcinoma in a dog. J Vet Intern Med 2007;21:193–195. [DOI] [PubMed] [Google Scholar]