Abstract
Rift Valley fever (RVF) is a zoonotic, viral, mosquito-borne disease that causes considerable morbidity and mortality in humans and livestock in Africa and the Arabian Peninsula. In June 2018, 4 alpaca inoculated subcutaneously with live attenuated RVF virus (RVFV) Smithburn strain exhibited pyrexia, aberrant vocalization, anorexia, neurologic signs, and respiratory distress. One animal died the evening of inoculation, and 2 at ~20 d post-inoculation. Concern regarding potential vaccine strain reversion to wild-type RVFV or vaccine-induced disease prompted autopsy of the latter two. Macroscopically, both alpacas had severe pulmonary edema and congestion, myocardial hemorrhages, and cyanotic mucous membranes. Histologically, they had cerebral nonsuppurative encephalomyelitis with perivascular cuffing, multifocal neuronal necrosis, gliosis, and meningitis. Lesions were more severe in the 4-mo-old cria. RVFV antigen and RNA were present in neuronal cytoplasm, by immunohistochemistry and in situ hybridization (ISH) respectively, and cerebrum was also RVFV positive by RT-rtPCR. The virus clustered in lineage K (100% sequence identity), with close association to Smithburn sequences published previously (identity: 99.1–100%). There was neither evidence of an aberrant immune-mediated reaction nor reassortment with wild-type virus. The evidence points to a pure infection with Smithburn vaccine strain as the cause of the animals’ disease.
Keywords: alpacas, live attenuated vaccine, meningoencephalitis, Rift Valley fever virus, Vicugna pacos
Rift Valley fever virus (RVFV; Phenuiviridae, Rift Valley fever phlebovirus) is the cause of RVF, a severe, zoonotic, mosquito-borne disease that in humans and livestock results in significant public health and economic consequences. 17 Recurrent epidemics have occurred in eastern and southern Africa, usually following heavy rain and flooding, with the disease spreading beyond Africa to the Arabian peninsula in 2000, causing large epidemics in Saudi Arabia and Yemen. 22 Bayesian- and population-based genetic analyses have shown that translocated virus initiates smoldering infection, which remains undetected until suitable climatic conditions precipitate outbreaks, as occurred in South Africa between 2008 and 2011.7,12
Following increased rainfall in South Africa during 2017–2018, an isolated outbreak occurred in May 2018 in the Free State Province of South Africa with cases reported in sheep and humans. 26 During these outbreaks, RVF affected mainly sheep, but also humans and a wide range of other animals including cattle, goats, and various indigenous wildlife and exotic species including alpacas (Vicugna pacos). 18 RVFV infections were confirmed in liver samples from autopsied animals using reverse-transcription real-time PCR (RT-rtPCR) and immunohistochemistry (IHC), which prompted alpaca breeders in the Western Cape Province of South Africa to vaccinate valuable animals against RVF even though the safety and efficacy of the available vaccines in this species were unknown at that time. There are currently 3 commercial RVFV vaccines in South Africa, 1 inactivated virus and 2 live attenuated virus (LAV) vaccines (Clone 13 and Smithburn strain). 9 The live attenuated Smithburn strain was generated between 1953 and 1958 by serial passaging of the RVF_Entebbe_44 virus, isolated from mosquitoes in Entebbe, Uganda in 1944. 23 This highly passaged, significantly immunogenic, attenuated strain has served as a vaccine in South Africa since 1958.
The largest commercial alpaca farm is currently in the Western Cape Province with ~350 animals. However, smaller farms can be found throughout South Africa, and the total number of commercially farmed alpaca is ~5,000. An additional unknown number of alpacas are kept to guard sheep. Farmers were advised to vaccinate their animals with RVFV LAV vaccine and other vaccines in 2010 when it became apparent that alpacas were susceptible to the disease. Farmers have been vaccinating their animals annually since then and have reported deaths in the weeks following vaccination, but, to date, an untoward vaccine reaction has not been suspected.
In June 2018, 2 alpacas were presented for autopsy at the Western Cape Provincial Veterinary Laboratory in South Africa. The animals originated from a herd of 40 animals that had been vaccinated with RVFV Smithburn vaccine ~20 d prior. Although wild-type RVF disease, irrespective of the species, had not been reported recently in the vicinity of the alpaca herd, they were nevertheless vaccinated as a precaution. Immediately post-vaccination, 4 animals (2 cria and 2 adults; cases 1–4) were reported to have sudden onset of fever, anorexia, respiratory distress, and neurologic signs that included vocalization, tremors, disorientation, over-knuckling, and ataxia. A cria (case 2) died the evening of inoculation and was not presented for autopsy. Consequently, the reason this cria died remains unknown. Two of the animals (case 1, a 4-mo-old female cria, and case 3, a 6-y-old male) became recumbent and died ~20 d post-inoculation and were autopsied. The fourth alpaca (an adult animal, case 4) survived and was still alive and well at the time this report was submitted. Macroscopic lesions were nonspecific and included cyanotic mucous membranes, severe pulmonary edema, and petechiae and ecchymoses in the ventricles of the heart. Brain, liver, spleen, kidney, lung, small and large intestine, and pancreas from both cases, and spinal cord from case 1, were collected and preserved in 10% neutral-buffered formalin. Formalin-fixed, paraffin-embedded (FFPE) sections stained with hematoxylin and eosin were examined, including multiple levels of the brain. Histologic lesions were only present in the cerebrum and were typical of nonsuppurative encephalomyelitis, with perivascular cuffing, multifocal mild neuronal necrosis, gliosis, and meningitis (Fig. 1A). The cuffs were several cells thick and predominantly lymphocytes and plasma cells with fewer macrophages. Lesions were more severe in the cria (case 1).
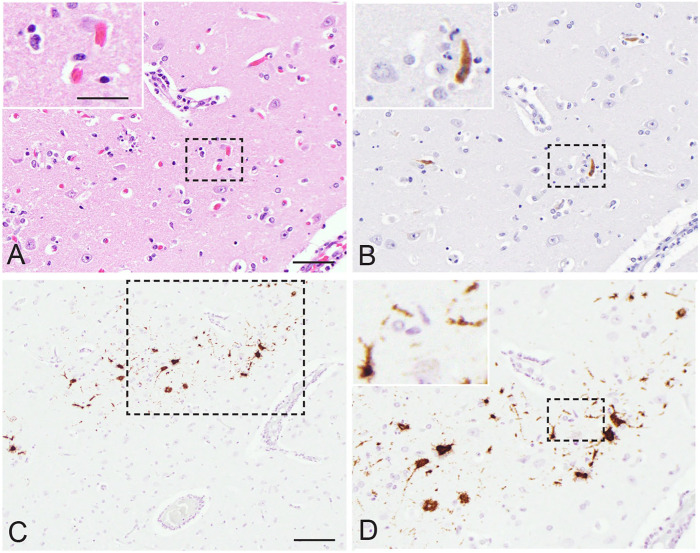
Figure 1.
Vaccination with the Smithburn live attenuated Rift Valley fever virus (RVFV) vaccine strain caused encephalitis in a 4-mo-old female alpaca (case 1). A. Multifocal neuronal necrosis and gliosis. 400×. H&E. Bar = 50 µm. Inset: bar = 25 µm. B. Viral antigen in the cytoplasm of a necrotic neuron. 400×. Anti–RVFV Gn immunohistochemistry. Same magnifications for main and inset as panel A. C. Viral RNA in the cytoplasm of multiple neuronal cell bodies. 200×. RVFV segment L in situ hybridization (ISH). Bar = 100 µm. D. Magnification of inset in panel C. 400×. ISH. Same magnifications for main and inset as panel A.
Given the concern regarding potential Smithburn vaccine strain reversion to wild-type RVFV or an adverse vaccine response, mid-cerebral brain samples and an organ pool (liver, spleen, lung, kidney) were submitted for RT-rtPCR. FFPE brain and liver sections were also examined using IHC and in situ hybridization (ISH). Total nucleic acid extractions were performed on ~0.1-cm3 formalin-fixed tissue. The specimens were processed using ceramic beads (MagNA Lyser green beads; Roche) with proteinase K digestion (200 µg/mL, Tris–EDTA buffer pH 8.0) at 56°C for 2 h and a total nucleic acid isolation kit (MagNA Pure 96 DNA and viral NA small volume kit; Roche) on a high-throughput PCR platform (MagNA Pure 96 system; Roche). The presence of RVFV RNA was detected using RT-rtPCR as described previously. 5 RVFV antigen was also demonstrated in FFPE specimens using 2 IHC assays and ISH as described previously.16,19 Briefly, for IHC, sections were incubated with a monoclonal anti–RVFV Gn antibody (NR43190 [4D4]; BEI Resources Repository) overnight or a polyclonal hyperimmune mouse ascitic fluid (National Institute for Communicable Diseases, Johannesburg, South Africa) to RVFV for 1 h. The standard immunoperoxidase methods for both antibodies included heat-induced epitope retrieval, incubation of slides with the antibodies, both diluted to 1:500, and detection using an avidin–biotin complex technique (Vectastain mouse elite ABC kit; Vector). The RVFV anti-Gn antibody was visualized with 3,3′-diaminobenzidine (DAB substrate kit; Vector), and the polyclonal antibody was visualized with NovaRED peroxidase substrate (NovaRED substrate kit; Vector). Unvaccinated and histologically normal alpaca control tissues, RVFV antigen–positive sheep liver, as well as isotype control antibody slides, were included in the IHC runs. To confirm that immunoreactivity to the polyclonal mouse ascitic fluid observed was the result of RVFV antigen presence, sequential tissue sections were also incubated with polyclonal mouse ascitic fluid to Wesselsbron virus (National Institute for Communicable Diseases). RT-rtPCR– and IHC-positive RVFV alpaca cases that occurred in 2010, and additional uninfected alpaca, served as positive and negative control tissues, respectively. Chromogenic in situ hybridization was conducted as published previously using a RVFV segment L specific probe (516121-V-RVFV-L; Advanced Cell Diagnostics) except that the RNAscope camelid positive probe (RNAscope probe Cd-POL2RA; Advanced Cell Diagnostics) replaced the sheep positive control probe. 19
The cerebrum samples from 2 alpacas (cases 1, 3) were positive for RVFV segment M viral RNA by RT-rtPCR, whereas their pooled organ samples were negative. The RVFV IHC and ISH identified RVFV proteins and RNA, respectively, in the cerebrum, with viral antigen and RNA present in multiple groups of neuronal cell bodies and in dendritic arborizations and axons (Fig. 1B–D). Liver samples were also negative for RVFV by IHC and ISH, further raising concern that the alpacas may have died from an adverse response to Smithburn vaccine rather than a virulent RVFV strain infection. Subsequently, the same nucleic acid samples from the 2 RT-rtPCR–positive cases (case 1: RV2655_18; case 3: RV2652_18) were used as template to amplify and sequence regions from each of the 3 RVFV genome segments. Amplification of all 3 genome segments was performed individually (2× SuperScript III one-step RT-PCR system, with Platinum Taq DNA polymerase; Invitrogen) in a 20-µL reaction in combination with 0.25 µmol/L of each primer set as described previously. 25 Primers were used to amplify and sequence ~500-bp regions of the large and medium genome segments; an ~270-bp region of the small genome segment was targeted as described previously.7,25 The resulting amplicons were submitted to Inqaba Biotechnical Industries (Pretoria, South Africa) for Sanger sequencing using the primers incorporated during the generation of the amplicons.
Alignments for each of the 3 genome segments were produced, which included the new (RV2652_18, RV2655_18) as well as previously published RVFV sequences obtained from outbreaks in South Africa, using sequence analysis software (CLC Genomics v.9; Qiagen). Additional genetic characterization of all 3 genome sequences from these samples (RV2652_18, RV2655_18), followed by maximum likelihood phylogenetic analysis using molecular evolutionary genetics analysis (MEGA6; https://www.megasoftware.net/) under General Time Reversal (GTR) model with G + I = 4 for 1,000 bootstrap replications, clustered both RV2652_RSA_18 and RV2655_RSA_18 together, with close association with previously published Smithburn vaccine sequences (DQ375430, DQ380193, DQ380155; Suppl. Fig. 1). 24 Sequences from RV2652_18 and RV2655_18 shared 100% sequence identity between them and 99.1%, 100%, and 99.4% sequence identity with the aforementioned Smithburn sequences (segments L, M, and S, respectively). In contrast, the Smithburn vaccine sequence shares 99.2%, 98.8%, and 98.4% identity with the original RVF_Entebbe_44 virus sequence (DQ375429, DQ38019, DQ380156; respectively, segments L, M, and S) and 97.9%, 98.6%, and 98.8% identity with the first Entebbe_44-derived vaccine used in Kenya 1951 (Rintoul_57; DQ375429, DQ38019, DQ30156; respectively, segments L, M, and S). Given that samples RV2652_RSA_18 and RV2655_RSA_18 cluster with Smithburn vaccine virus rather than any of the other known South African RVFVs (Suppl. Fig. 1), the sequencing data supports these animals’ singular exposure to RVFV Smithburn strain, consistent with their clinical vaccination records, and the absence of hepatic necrosis or hepatitis. The sequences from all 3 RVFV segments from both alpacas (cases 1, 3) have been deposited in GenBank (accessions MW316057–MW3 16062).
Reportedly, alpacas are susceptible to infection with wild-type RVFV, but the clinical signs and lesions have not been documented. 18 Clinical manifestations reported in dromedary camels included abortions, neurologic disorders, hemorrhages, as well as fatal peracute disease.6,21 These manifestations are somewhat unlike those in small ruminants, and in particular sheep, in which abortion storms, mortality rates of up to 100% in neonates, and multifocal random necrotizing hepatitis, but not encephalitis, are characteristic.13–15 Encephalitis with viral antigen in the central nervous system has also not been described in natural cases in ruminants but is a complication of RVFV infections in humans, and has been experimentally reproduced in mice and rats.3,4,13 The severe neurologic clinical signs and marked meningoencephalitis in our cases, accompanied by an absence of necrotizing hepatitis, could not be directly linked to the use of the Smithburn vaccine. Instead, genetic analysis was necessary to rule out infection with wild-type RVFV.
Various vaccines have been developed to control RVF in livestock in endemic regions using either inactivated whole virus, natural attenuated isolates (e.g., MP-12 or Clone 13), or live-attenuated Smithburn strain. 17 The mutagenized MP-12 strain was proven safe and immunogenic for alpacas in controlled laboratory studies. 20 In contrast, immunity induced by a single-dose of inactivated vaccine is inadequate, and animals require repeated vaccinations to ensure long-lasting protection. 8 Meningoencephalitis induced by Smithburn vaccination in alpacas, or any other adverse reactions to vaccine strains in alpacas, has not been reported previously, to our knowledge. Consequently, the Smithburn vaccine, which is inexpensive and induces long-lasting immunity, is an appealing option for livestock producers despite the risks of teratogenic effects and abortion as well as the potential for reassortment with wild-type virus. 8 Adverse reactions to Smithburn vaccine reported previously in other livestock include abortions, stillbirths, and neonatal deaths in sheep, abortions in cattle, and disease very akin to that caused by virulent RVFV strains, including hepatic lesions and abortion in goats and a low incidence of encephalitis in lambs <6 wk old.1,2,10,11 In our case study, 36 other alpaca that were immunized did not show an adverse reaction. Unfortunately, it is not known what set these animals apart from the alpacas that developed meningoencephalitis. Therefore, independent safety testing of vaccine strains such as Smithburn is advised.
Our case study identifies several unknowns concerning RVF in alpacas, including the pathomechanism that caused this particular neurovirulent response, how common this adverse vaccine response is in alpacas, and the route of viral entry into the brain. There was no evidence of an aberrant immune-mediated reaction nor was there evidence of reassortment with wild-type virus. The evidence points to a pure infection with Smithburn vaccine strain as the cause of the animals’ disease. Further investigation is warranted to understand this specific pathogenesis and assist with development of safer RVFV vaccines. Meanwhile, our findings suggest that the use of Smithburn vaccine in alpaca prior to further investigation is ill advised.
Supplemental Material
Supplemental material, sj-pdf-1-jvd-10.1177_10406387211015294 for Vaccination with Rift Valley fever virus live attenuated vaccine strain Smithburn caused meningoencephalitis in alpacas by Tasneem Anthony, Antoinette van Schalkwyk, Marco Romito, Lieza Odendaal, Sarah J. Clift and Anne S. Davis in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Carina de Mon, Rephima Phaswane, Naomi Timmerman, and Peter Mokonoto for their technical assistance.
Footnotes
Declaration of conflicting interests: The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: We disclose receipt of financial support for the diagnostic investigation, authorship, and/or publication of this article from the Department of Paraclinical Sciences Faculty of Veterinary Science, University of Pretoria, and the Department of Diagnostic Medicine/Pathobiology, College of Veterinary Medicine, Kansas State University, Manhattan, KS.
ORCID iDs: Sarah J. Clift  https://orcid.org/0000-0003-1368-1215
https://orcid.org/0000-0003-1368-1215
A. Sally Davis  https://orcid.org/0000-0001-5711-3936
https://orcid.org/0000-0001-5711-3936
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Tasneem Anthony, Provincial Veterinary Laboratory, Department of Agriculture, Western Cape Government, Capetown, South Africa.
Antoinette van Schalkwyk, South Africa Agricultural Research Council–Onderstepoort Veterinary Institute, Onderstepoort, South Africa.
Marco Romito, South Africa Agricultural Research Council–Onderstepoort Veterinary Institute, Onderstepoort, South Africa.
Lieza Odendaal, Department of Paraclinical Sciences, University of Pretoria, Onderstepoort, South Africa.
Sarah J. Clift, Department of Paraclinical Sciences, University of Pretoria, Onderstepoort, South Africa
A. Sally Davis, Department of Paraclinical Sciences, University of Pretoria, Onderstepoort, South Africa; Department of Diagnostic Medicine/Pathobiology, College of Veterinary Medicine, Kansas State University, Manhattan, KS, USA.
References
- 1. Barnard BJ, Botha MJ. An inactivated rift valley fever vaccine. J S Afr Vet Assoc 1977;48:45–48. [PubMed] [Google Scholar]
- 2. Botros B, et al. Adverse response of non-indigenous cattle of European breeds to live attenuated Smithburn Rift Valley fever vaccine. J Med Virol 2006;78:787–791. [DOI] [PubMed] [Google Scholar]
- 3. Caroline AL, et al. Inflammatory biomarkers associated with lethal Rift Valley fever encephalitis in the Lewis rat model. Front Microbiol 2015;6:1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dodd KA, et al. Rift Valley fever virus encephalitis is associated with an ineffective systemic immune response and activated T cell infiltration into the CNS in an immunocompetent mouse model. PLoS Negl Trop Dis 2014;8:e2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drosten C, et al. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J Clin Microbiol 2002;40:2323–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El Mamy ABO, et al. Unexpected Rift Valley fever outbreak, northern Mauritania. Emerg Infect Dis 2011;17:1894–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grobbelaar AA, et al. Molecular epidemiology of Rift Valley fever virus. Emerg Infect Dis 2011;17:2270–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hussein MA, et al. Efficacy of Montanide (IMS 3015) as an adjuvant for an inactivated Rift Valley fever (RVF) vaccine in sheep. Acta Trop 2019;190:193–203. [DOI] [PubMed] [Google Scholar]
- 9. Ikegami T, Makino S. Rift valley fever vaccines. Vaccine 2009;27(Suppl 4):D69–D72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kamal SA. Pathological studies on postvaccinal reactions of Rift Valley fever in goats. Virol J 2009;6:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kamal SA. Observations on Rift Valley fever virus and vaccines in Egypt. Virol J 2011;8:532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maluleke MR, et al. A comparative genome analysis of Rift Valley fever virus isolates from foci of the disease outbreak in South Africa in 2008–2010. PLoS Negl Trop Dis 2019;13:e0006576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Odendaal L, et al. Lesions and cellular tropism of natural Rift Valley fever virus infection in adult sheep. Vet Pathol 2019;56:61–77. [DOI] [PubMed] [Google Scholar]
- 14. Odendaal L, et al. Ovine fetal and placental lesions and cellular tropism in natural Rift Valley fever virus infections. Vet Pathol 2020;57:791–806. [DOI] [PubMed] [Google Scholar]
- 15. Odendaal L, et al. Lesions and cellular tropism of natural Rift Valley fever virus infection in young lambs. Vet Pathol 2020;57:66–81. [DOI] [PubMed] [Google Scholar]
- 16. Odendaal L, et al. Sensitivity and specificity of real-time reverse transcription polymerase chain reaction, histopathology, and immunohistochemical labeling for the detection of Rift Valley fever virus in naturally infected cattle and sheep. J Vet Diagn Invest 2014;26:49–60. [DOI] [PubMed] [Google Scholar]
- 17. Pepin M, et al. Rift Valley fever virus (Bunyaviridae: Phlebovirus): an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res 2010;41:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pienaar NJ, Thompson PN. Temporal and spatial history of Rift Valley fever in South Africa: 1950 to 2011. Onderstepoort J Vet Res 2013;80:384. [DOI] [PubMed] [Google Scholar]
- 19. Ragan IK, et al. Rift Valley fever viral RNA detection by in situ hybridization in formalin-fixed, paraffin-embedded tissues. Vector Borne Zoonotic Dis 2019;19:553–556. [DOI] [PubMed] [Google Scholar]
- 20. Rissmann M, et al. Vaccination of alpacas against Rift Valley fever virus: safety, immunogenicity and pathogenicity of MP-12 vaccine. Vaccine 2017;35:655–622. [DOI] [PubMed] [Google Scholar]
- 21. Scott GR, et al. Rift Valley fever in camels. J Pathol Bacteriol 1963;86:229–231. [DOI] [PubMed] [Google Scholar]
- 22. Shoemaker T, et al. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000–01. Emerg Infect Dis 2002;8:1415–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Smithburn KC, et al. Rift Valley fever accidental infections among laboratory workers. J Immunol 1949;62:213–227. [PubMed] [Google Scholar]
- 24. Tamura K, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 2013;30:2725–2729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. van Schalkwyk A, Romito M. Genomic characterization of Rift Valley fever virus, South Africa, 2018. Emerg Infect Dis 2019;25:1979–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Vuren JP, et al. Human cases of Rift Valley fever in South Africa, 2018. Vector Borne Zoonotic Dis 2018;18:713–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-jvd-10.1177_10406387211015294 for Vaccination with Rift Valley fever virus live attenuated vaccine strain Smithburn caused meningoencephalitis in alpacas by Tasneem Anthony, Antoinette van Schalkwyk, Marco Romito, Lieza Odendaal, Sarah J. Clift and Anne S. Davis in Journal of Veterinary Diagnostic Investigation