Abstract
Rabbit hemorrhagic disease virus 2 (RHDV2) causes an often-fatal disease of rabbits that has resulted in outbreaks in rabbitries in Europe, Africa, Australia, and Asia. RHD has historically been characterized as a foreign animal disease in the United States. In July 2019, RHDV2 was detected in rabbits on Orcas Island along the northwestern coast of Washington (WA) State following reports of deaths in multiple feral and domestic rabbits. We document and highlight here the unique clinical presentation and gross and histologic lesions observed in this recent WA outbreak. Affected rabbits died without premonitory signs or displayed hyporexia and/or lethargy for ≤1 d prior to death. The most consistent pathologic finding was random, multifocal hepatocellular necrosis, often with concurrent multifocal-to-diffuse splenic necrosis. The lack of significant clinical signs in conjunction with the random distribution of hepatic necrosis in the WA outbreak contrasts with previous reports of RHDV2 disease progression.
Keywords: natural outbreak, rabbits, rabbit hemorrhagic disease virus 2
Rabbit hemorrhagic disease (RHD) is caused by a calicivirus (Caliciviridae, Lagovirus). 1 Genus Lagovirus includes multiple rabbit caliciviruses varying in pathogenicity and virulence. The 2 most important circulating strains include the classical rabbit hemorrhagic disease virus (Lagovirus europaeus/GI.1a-d), referred to commonly as RHDV1, and a more recently described and antigenically distinct L. europaeus/GI.2/RHDV2/b, referred to as RHDV2, RHDVb, or GI.2.4,10,16 RHDV1 typically causes high mortality of susceptible species of rabbits older than ~1 mo, including domestic (Oryctolagus cuniculi) and European (O. cuniculus) rabbits. Hares (Lepus sp.) and North American wild cottontails (Sylvilagus floridanus) are unaffected by RHDV1.11,12
RHDV2 was first identified in 2010 in France, but has since spread throughout Europe and to Australia, Africa, and North America. 19 The first North American reports of RHDV2 to the World Organisation for Animal Health (OIE) were in 2016 (Quebec, Canada) and 2018 (British Columbia, Canada; Ohio, USA). 19 RHDV2 affects a broader host range, including hares of the Lepus sp., and can result in high mortality in rabbits of all ages.6–8,18 However, reported mortality rates, clinicopathologic findings, and overall virulence of RHDV2 vary in the literature.5,13,14 During the initial RHDV2 outbreak in France in 2010, the disease was characterized by pathologic findings similar to those described for RHDV1, including hemorrhages in multiple tissues. The outbreak resulted in prolonged and high mortality rates of 80–90%, with mortalities lasting up to 15 d post-vaccination. 14 Compared with RHDV1, experimental infections of rabbits with RHDV2 had a longer disease course, with mortalities starting 3–9 d post-infection and lasting for 5 d, as opposed to RHDV1 infection, which results in mortalities occurring 2–6 d post-inoculation and lasting for 3–4 d. Additionally, there were more common occurrences of a subacute-to-chronic form characterized by hepatic degeneration, splenomegaly, and icterus. 13 A small number of animals underwent a rapid disease course characterized by death in <4 d. In these experimental infections, histologic abnormalities in the liver included a specific pattern of periportal hepatocellular necrosis, occasional midzonal hepatocellular necrosis, and complete sparing of centrilobular hepatocytes, with occasional periportal infiltration of heterophils. Splenic lesions included hyalinization and fibrin accumulation within the red pulp and lymphoid depletion of the white pulp. 17
Although epidemiologic and geographic data regarding RHDV2 outbreaks in Europe are documented extensively,2,6,7,15,17,18 published reports of clinicopathologic findings in natural outbreaks of RHDV2 are relatively scarce. One case report outlined the clinical and pathologic findings in 2 pet rabbits naturally infected with RHDV2 in Spain 5 ; both rabbits had a 1-d history of hyporexia or anorexia and lethargy. A blood chemistry panel revealed marked increases in gamma-glutamyl transferase, alkaline phosphatase, bile acids, and bilirubin with decreased activity of aspartate aminotransferase and alanine aminotransferase, suggestive of severe liver damage in both animals. Both rabbits developed profound icterus and died 22 and 30 h after admission, respectively. A postmortem examination performed on one rabbit revealed severe portal-to-midzonal hepatocellular necrosis. Additionally, this animal had severe necrosuppurative bronchopneumonia and moderate cardiomyocyte necrosis.
In the spring of 2019, multiple feral domestic rabbit deaths were reported on Orcas Island, an island in the San Juan archipelago in the northwest corner of WA (pers. comm., Washington State Department of Agriculture veterinarians). These reports followed the detection of RHDV2 in coastal areas of British Columbia, Canada in 2018. 20 Over the ensuing summer, fall, and winter of 2019, the Washington Animal Disease Diagnostic Laboratory (WADDL; Pullman, WA, USA) received 7 cases comprising 14 RHDV2-positive rabbits (Suppl. Table 1). Case 1 was a 2-y-old, 4-H pet, Norwegian dwarf buck from Orcas Island submitted for postmortem examination in July 2019. Gross examination was unremarkable. Fresh samples of liver and spleen were submitted to the USDA-APHIS Foreign Animal Disease Diagnostic Laboratory (FADDL; Plum Island, NY, USA) for detection of RHDV2. This decision was based on the number and manner of rabbit deaths and the geographic proximity of these cases to the Canadian RHDV2 outbreak. Cases 2–5 included rabbit carcasses from Orcas Island and 1 carcass from San Juan Island, which arrived at WADDL in July and August of 2019. Fresh liver specimens from 9 of these bodies were forwarded to FADDL, which confirmed infection with RHDV2 in all submissions. Case 6 was a single adult feral domestic rabbit from Whidbey Island, north of Seattle, WA, submitted in October 2019. In December 2019, 108 of 145 rabbits from a rescue organization died on a premises in Clallam county located in the northwestern WA mainland; 3 of the 108 rabbits were submitted to WADDL for examination (case 7). Overall, 14 liver samples were forwarded from WADDL to FADDL for antigen (ag-ELISA) and reverse-transcription PCR (RT-PCR); infection with RHDV2 was confirmed in all 14 samples. 9 RHDV1 was not detected in any of the 14 samples.
All rabbits were autopsied at WADDL upon arrival. Gross examinations were severely limited in most cases because of the poor postmortem condition of the cadavers. Two of the carcasses had cavitary effusions: case 1 had moderate acute hemoabdomen, and case 6 had moderate hydrothorax. Only case 7 had grossly identifiable multifocal-to-coalescent hepatic necrosis as evidenced by a dark-red to tan liver and markedly friable parenchyma. Cases 2–5 were grossly normal apart from autolysis.
Representative samples of visceral organs from all 14 RHDV2 confirmed carcasses were collected for histologic evaluation. Tissues were considered too autolyzed from case 6 to yield reliable histologic diagnoses and are excluded from the histologic descriptions. Cases 1–5 and 7 had histologic evidence of hepatocellular necrosis. In all samples, necrosis was categorized as multifocal-random and panlobular and included necrosis of individual to small groups of hepatocytes (Fig. 1A, 1B). There was no significant inflammatory component in 10 of 13 rabbits. However, in case 7, hepatocellular necrosis was accompanied by periportal-to-midzonal lymphoplasmacytic inflammation. In the 3 rabbits in case 7, a coexisting chronic disease process caused by Encephalitozoon cuniculi was suspected given the presence of chronic tubulointerstitial nephritis, but this presumptive diagnosis was not confirmed. Diffuse centrilobular hepatocellular degeneration was detected in case 5 and was interpreted as a nonspecific hypoxic lesion resulting from cardiovascular collapse. Spleen was evaluated in 10 of the 13 rabbits, and 7 of 10 rabbits had concurrent splenic necrosis (cases 1, 3, 4, 5) characterized by multifocal-to-diffuse white pulp lymphoid necrosis and multifocal areas of necrosis and fibrin deposition in both the white and red pulp (Fig. 1C). Lung lesions were variable: 2 rabbits had mild-to-moderate acute pulmonary edema (cases 1, 4); 2 had multifocal, mild, acute pulmonary hemorrhage (case 2); and 3 had multifocal, mild lymphohistiocytic interstitial pneumonia (case 3). Interestingly, all 3 rabbits with histologic lesions of interstitial pneumonia were submitted from the same facility and were the only animals reported to have developed appreciable clinical signs of lethargy and anorexia prior to death. A gravid New Zealand dwarf doe (case 5) had additional renal lesions: multifocal and severe glomerular thrombosis; renal tubular necrosis with pigmenturia; and a small focus of chronic lymphoplasmacytic interstitial nephritis and fibrosis. Additionally, 4 rabbits had evidence of chronic gastrointestinal parasitism including intestinal nematodiasis (case 2) and intestinal coccidiosis (case 4).
Figure 1.
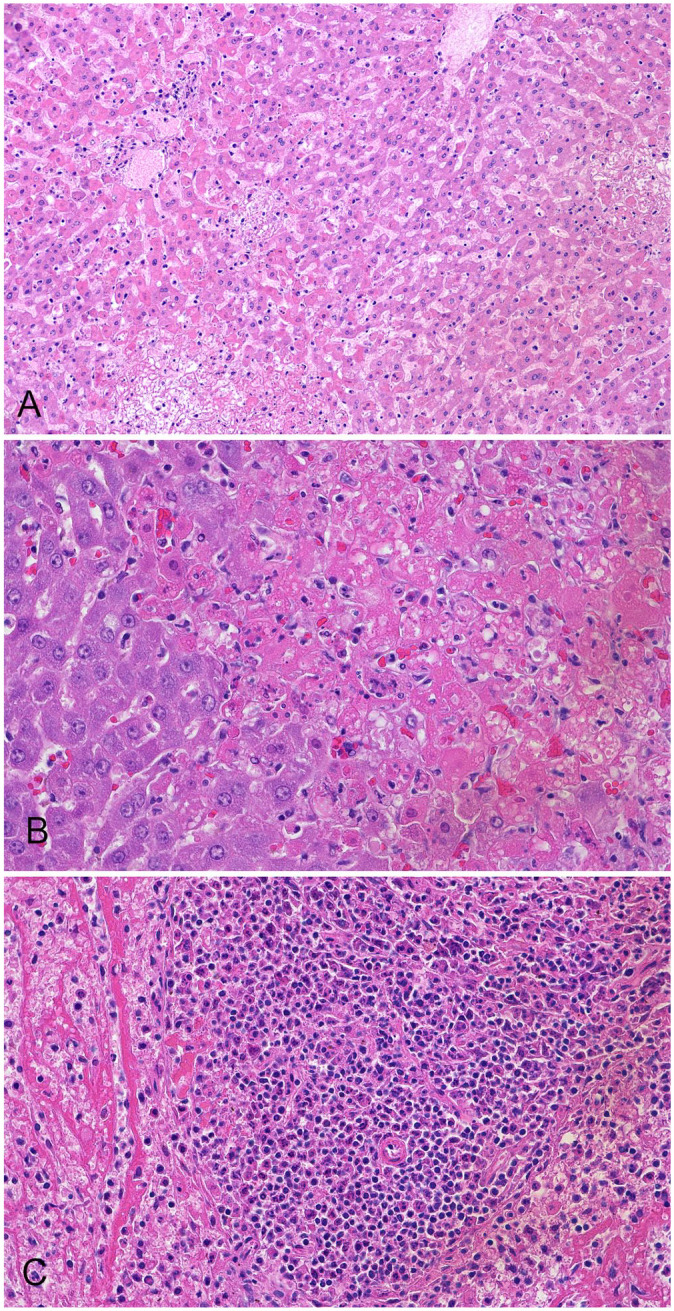
Rabbit hemorrhagic disease virus 2 infection in rabbits. A. Necrosis of hepatocytes in all portions of the lobule in case 2. H&E. B. Focal and individual hepatocellular necrosis in case 5. H&E. C. Necrosis of red and white pulp in the spleen in case 5. H&E.
These cases followed a temporal and geographic progression from the RHDV2 reports in British Columbia, Canada in 2018 to the islands off the northwestern coast of Washington in the summer of 2019, with ultimate progression to mainland northwestern Washington by the winter of 2019. However, it is unknown if the outbreak in WA is a direct continuation of the outbreak in British Columbia.
Only one submitter described clinical disease characterized by lethargy and anorexia and rapid death (case 3). The short clinical course of affected rabbits in the WA outbreak described here is in contrast to the previously described experimental infection of RHDV2 in Europe that described a prolonged, 5-d disease course. 13 Similarly, in the case report of 2 naturally infected pet rabbits from Spain, the affected rabbits were hyporexic for 1 d, developed progressive lethargy for 1–2 additional days, and finally succumbed to disease by day 5. 5 This may suggest that the isolate of RHVD2 circulating in WA is a more virulent isolate than has been described previously. However, the RHDV2 disease courses described here are reported by owners and submitters; although an important source of epidemiologic information for each case, the clinical course of each animal, particularly the feral rabbits, may not have been observed closely. Additionally, these rabbits originated from a variety of husbandry conditions and were of multiple age groups (Suppl. Table 1). Some animals had additional coinfections or presumably unrelated lesions and disease processes. These variables make it difficult to draw direct comparisons from previous experimental infections regarding the severity and duration of disease course. Additional experimental investigation of the WA isolate must be conducted to definitively demonstrate the virulence of this isolate.
Although the postmortem examination findings in RHDV2 natural outbreaks in Europe are similar to those detected in our WA cases, there are several notable differences. Icterus has historically been a prominent finding in the European cases but was not a feature in any of the bodies submitted to WADDL. Notably, European cases with histologic lesions had clear periportal hepatocellular necrosis.6,18 In the WA cases described here, the distribution of necrosis was random and affected hepatocytes in all zones of the lobule. It is unknown whether this represents a difference in viral cellular tropism or disease pathogenesis, a difference in host immune response, or another variable.
Differences between the RHDV2-associated disease detected in the European outbreaks and our WA outbreak should be considered when assessing unexpected rabbit deaths. Overall, given that cases of RHDV2 infection have been detected in the mainland United States, and with the continuing outbreak within the southwestern United States, 3 vigilance for RHDV2 should remain high. Unexpected deaths or multiple mortalities in groups of rabbits, regardless of age, should be evaluated for infection with RHDV2.
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_10406387211022466 for Clinical and pathologic findings in an outbreak in rabbits of natural infection by rabbit hemorrhagic disease virus 2 in the northwestern United States by Laura B. A. Williams, Steven E. Edmonds, Susan R. Kerr, Liam E. Broughton-Neiswanger and Kevin R. Snekvik in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Dr. Amber Itle for identifying and helping to coordinate these cases. We also thank the WADDL faculty, residents, and staff involved in the diagnostic case workups.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: This study was financially supported by the USDA APHIS National Animal Health Laboratory Network (NAHLN) grant AP20VSD&B000C052.
ORCID iD: Laura B. A. Williams  https://orcid.org/0000-0002-6229-4820
https://orcid.org/0000-0002-6229-4820
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Laura B. A. Williams, Department of Veterinary Microbiology & Pathology, Washington State University, Pullman, WA, USA; Washington Animal Disease Diagnostic Laboratory, Washington State University, Pullman, WA, USA.
Steven E. Edmonds, Washington Animal Disease Diagnostic Laboratory, Washington State University, Pullman, WA, USA
Susan R. Kerr, Washington State Department of Agriculture, Animal Health Program Education and Outreach, Olympia, WA, USA
Liam E. Broughton-Neiswanger, Washington Animal Disease Diagnostic Laboratory, Washington State University, Pullman, WA, USA
Kevin R. Snekvik, Department of Veterinary Microbiology & Pathology, Washington State University, Pullman, WA, USA Washington Animal Disease Diagnostic Laboratory, Washington State University, Pullman, WA, USA.
References
- 1. Abrantes J, et al. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): a review. Vet Res 2012;43:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Almeida T, et al. Tracking the evolution of the G1/RHDVb recombinant strains introduced from the Iberian Peninsula to the Azores islands, Portugal. Infect Genet Evol 2015;34:307–313. [DOI] [PubMed] [Google Scholar]
- 3. Asin J, et al. Outbreak of rabbit hemorrhagic disease virus 2 in the southwestern United States: first detections in southern California. J Vet Diagn Invest 2021;33:728–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bárcena J, et al. Comparative analysis of rabbit hemorrhagic disease virus (RHDV) and new RHDV2 virus antigenicity, using specific virus-like particles. Vet Res 2015;46:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bonvehi C, et al. Clinicopathologic findings of naturally occurring rabbit hemorrhagic disease virus 2 infection in pet rabbits. Vet Clin Pathol 2019;48:89–95. [DOI] [PubMed] [Google Scholar]
- 6. Camacho-Sillero L, et al. Monitoring of the novel rabbit haemorrhagic disease virus type 2 (GI.2) epidemic in European wild rabbits (Oryctolagus cuniculus) in southern Spain, 2013–2017. Vet Microbiol 2019;237:108361. [DOI] [PubMed] [Google Scholar]
- 7. Camarda A, et al. Detection of the new emerging rabbit haemorrhagic disease type 2 virus (RHDV2) in Sicily from rabbit (Oryctolagus cuniculus) and Italian hare (Lepus corsicanus). Res Vet Sci 2014;97:642–645. [DOI] [PubMed] [Google Scholar]
- 8. Dalton KP, et al. Variant rabbit hemorrhagic disease virus in young rabbits, Spain. Emerg Infect Dis 2012;18:2009–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gall A, et al. Persistence of viral RNA in rabbits which overcome an experimental RHDV infection detected by a highly sensitive multiplex real-time RT-PCR. Vet Microbiol 2007;120:17–32. [DOI] [PubMed] [Google Scholar]
- 10. Hall RN, et al. Emerging rabbit hemorrhagic disease virus 2 (RHDVb), Australia. Emerg Infect Dis 2015;21:2276–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lavazza A, et al. Field and experimental data indicate that the eastern cottontail (Sylvilagus floridanus) is susceptible to infection with European brown hare syndrome (EBHS) virus and not with rabbit haemorrhagic disease (RHD) virus. Vet Res 2015;46:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lavazza A, et al. Susceptibility of hares and rabbits to the European brown hare syndrome virus (EBHSV) and rabbit haemorrhagic disease virus (RHDV) under experimental conditions. Zentralbl Veterinarmed B 1996;43:401–410. [DOI] [PubMed] [Google Scholar]
- 13. Le Gall-Reculé G, et al. Emergence of a new lagovirus related to rabbit haemorrhagic disease virus. Vet Res 2013;44:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Le Gall-Reculé G, et al. Detection of a new variant of rabbit haemorrhagic disease virus in France. Vet Rec 2011;168:137–138. [DOI] [PubMed] [Google Scholar]
- 15. Lopes AM, et al. Is the new variant RHDV replacing genogroup 1 in Portuguese wild rabbit populations? Viruses 2014;7:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mahar JE, et al. Rabbit hemorrhagic disease virus 2 (RHDV2; GI.2) is replacing endemic strains of RHDV in the Australian landscape within 18 months of its arrival. J Virol 2018;92(2):e01374–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neimanis AS, et al. Arrival of rabbit haemorrhagic disease virus 2 to northern Europe: emergence and outbreaks in wild and domestic rabbits (Oryctolagus cuniculus) in Sweden. Transbound Emerg Dis 2018;65:213–220. [DOI] [PubMed] [Google Scholar]
- 18. Puggioni G, et al. The new French 2010 rabbit hemorrhagic disease virus causes an RHD like disease in the Sardinian Cape hare (Lepus capensis mediterraneus). Vet Res 2013;44:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rouco C, et al. Worldwide rapid spread of the novel rabbit haemorrhagic disease virus (GI.2/RHDV2/b). Transbound Emerg Dis 2019;66:1762–1764. [DOI] [PubMed] [Google Scholar]
- 20. U.S. Department of Agriculture. Emerging risk notice—animal health. Rabbit hemorrhagic disease in British Columbia, Canada. 2018. https://www.aphis.usda.gov/animal_health/downloads/Rabbit-Hemorrhagic-Disease_062018.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_10406387211022466 for Clinical and pathologic findings in an outbreak in rabbits of natural infection by rabbit hemorrhagic disease virus 2 in the northwestern United States by Laura B. A. Williams, Steven E. Edmonds, Susan R. Kerr, Liam E. Broughton-Neiswanger and Kevin R. Snekvik in Journal of Veterinary Diagnostic Investigation


