Abstract
Coenzyme Q10 (CoQ10), which plays a key role in the electron transport chain by providing an adequate, efficient supply of energy, has another relevant function as an antioxidant, acting in mitochondria, other cell compartments, and plasma lipoproteins. CoQ10 deficiency is present in chronic and age-related diseases. In particular, in cardiovascular diseases (CVDs), there is a reduced bioavailability of CoQ10 since statins, one of the most common lipid-lowering drugs, inhibit the common pathway shared by CoQ10 endogenous biosynthesis and cholesterol biosynthesis. Different clinical trials have analyzed the effect of CoQ10 supplementation as a treatment to ameliorate these deficiencies in the context of CVDs. In this review, we focus on recent advances in CoQ10 supplementation and the clinical implications in the reduction of cardiovascular risk factors (such as lipid and lipoprotein levels, blood pressure, or endothelial function) as well as in a therapeutic approach for the reduction of the clinical complications of CVD.
Keywords: coenzyme Q10, ubiquinone, ubiquinol, cardiovascular diseases
1. Introduction, CoQ10 in Cardiovascular Diseases
Coenzyme Q10 (CoQ10) was isolated by Festenstein et al. (1955) and Crane et al. (1957). Chemically, it is a lipid-soluble, biologically active quinone with a benzoquinone ring and an isoprenoid sidechain containing 10 residues of isoprenoid. The main function of CoQ10 is to take part in the mitochondrial electron transport chain, where it carries electrons from complex I and II to complex III [1,2]. During the decades since it was first described, multiple functions have been attributed to CoQ10 such as to control the cellular redox state both by its antioxidant properties and by the generation of oxidant signals as well as a role in proton gradient formation at the endomembrane and plasma membrane, which contributes to control the membrane structure and phospholipid status [3,4,5].
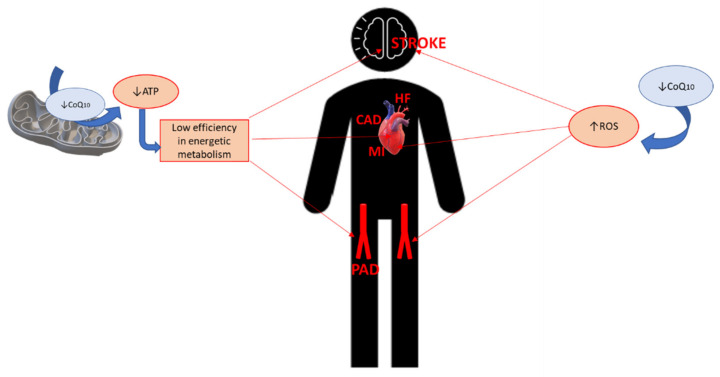
The main function of CoQ10 has one important consequence for energy metabolism, since better, more efficient electron transport in the inner membrane at the mitochondria leads to more abundant production of ATP. This is a highly relevant factor, for instance, for the cardiac muscle and the correct functioning of the heart. This direct effect of CoQ10 on CVDs such as heart failure (HF) or myocardial infarction is accompanied by the crucial action of CoQ10 as a potent antioxidant due to its coexistent redox forms (ubiquinone, semi-ubiquinone and ubiquinol), which act in the mitochondrial membrane, other cell membranes, and in plasma and cytoplasm. These antioxidant properties of CoQ10 act not only on the electron transport chain in the mitochondria, but also in recycling other antioxidants such as vitamin C or vitamin E. Along with its influence on the efficiency of energetic metabolism, these other functions of CoQ10 have an important impact on cardiovascular health in humans, affecting the endothelial and vascular system, which in turn influences the incidence, etiology, and progression of other CVDs such as coronary artery disease (CAD) (Figure 1). For these reasons, we aimed to review the latest publications on the effect of CoQ10 on cardiovascular health. In this review, we explore and analyze our current knowledge of this issue and its future perspectives.
Figure 1.
Pathogenic background of coenzyme Q10 in cardiovascular diseases. CoQ10: Coenzyme Q10; ATP: Adenosine triphosphate; CAD: Coronary artery disease; HF: Heart failure; MI: Myocardial infarction; PAD: Peripheral artery disease; ROS: Reactive oxygen species.
2. The Biology of CoQ10
CoQ10 is ubiquitous in all cell membranes, and is endogenously biosynthesized in all tissues of the organism. A minor proportion of comes from dietary sources. However, from a clinical point of view, it is noteworthy that endogenous production deficiency takes part in the pathophysiology of different diseases, being that its biosynthesis is significantly reduced with aging.
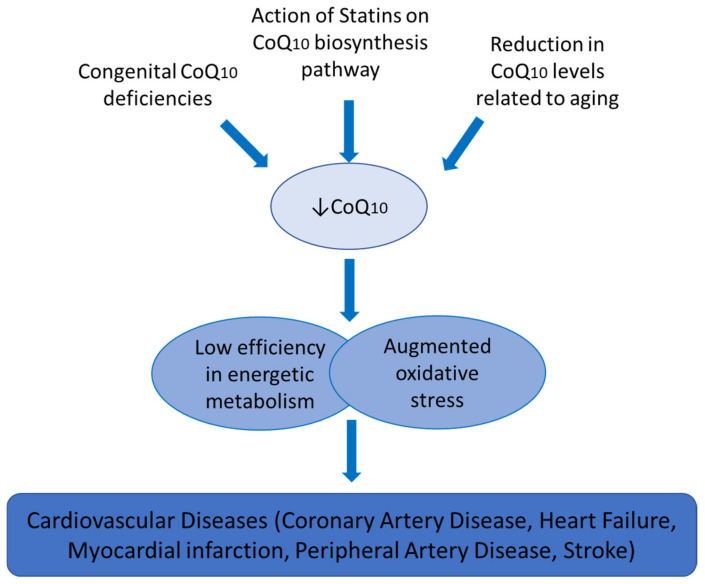
Endogenous CoQ10 biosynthesis is carried out by a pathway involving at least 11 genes, named COQ genes, which are well-conserved among species [6]. The first step involves the benzoquinone ring being synthesized as 4-hydroxybenzoato and the isoprenoid side chain precursor, acetyl-CoA [7,8]. In the context of CVDs, there is an important connection between CoQ10 biosynthesis and the action of one of the most common lipid-lowering drugs, statins. Cholesterol biosynthesis shares a common pathway with CoQ10 endogenous biosynthesis, which means that the use of statins in the treatment of hypercholesterolemia leads to a reduction in the synthesis of CoQ10. This, in turn, results in a reduction in the disposable supply of this compound, reducing the efficacy of energetic metabolism and its implications in cardiac metabolism and redox status (Figure 2). Regarding the distribution of CoQ10 in the organism, it is known to be present in varying amounts in different organs. Its levels range from 8 μg/g in the lung to 114 μg/g in the heart. Generally, it is present more in tissues with high-energy requirements or metabolic activity such as the heart, kidney, liver, and muscle [9]. However, CoQ10 levels are disrupted by health and disease status. It has been reported that lesser amounts of CoQ10 are observed in Alzheimer’s disease, cardiomyopathies, or type 2 diabetes mellitus compared to healthy people [10,11].
Figure 2.
Coenzyme Q10 and cardiovascular diseases.
Another important factor in the biology and metabolism of CoQ10 is the bioavailability and distribution of the different formulations of this compound when administered orally. This is determined by its lipophilic characteristics, which make it extremely insoluble in water. For this reason, the typical regimen of oral administration of CoQ10 takes advantage of when lipid-rich foods are consumed [12]. In this context, many of the studies published have failed to represent the real amount of CoQ10, which reaches the tissues, showing, in most cases, the levels of this quinone measured in plasma, as a surrogate measurement of the CoQ10 already ingested.
Despite these recommendations, research on CoQ10 absorption and bioavailability varies, and is dependent on the type of CoQ10 preparation used [13,14]. Many formulations have been developed to improve CoQ10 solubility in the organism. Recent new formulations for CoQ10 are based on enhancing its water-solubility, as in the cases of Qter or Ubisol-Q10. Ubisol-Q10 is a nanomiscelle formulation that appears to be water-soluble containing CoQ10, where solubilization is achieved due to the amphipathic properties of polyetilenglycol-derivatized α-tocopherol, which allows for the formation of stable and water-soluble nanomicelles [15]. Q-ter is a supplement consisting of copovidone, which acts as a carrier, CoQ10, and glycine, which works as a catalyst. This composition makes Q-ter 200-times more soluble in water than pure CoQ10 [16].
Other efforts have been focused on discovering analogues of CoQ10 with greater solubility and antioxidant effects such as MitoQ. MitoQ is also known as MitoQuinone (Phosphonium [10-(4,5-dimethoxy-2-methyl-3,6-dioxo-1,4-cyclohexadien-1-yl)decyl] triphenyl-,mesylate) and is a mitochondria-targeted antioxidant composed of a quinone moiety attached to triphenylphosphonium (TPP) via a 10-carbon alkyl chain. The lipophilic nature of TPP allows the antioxidant to be allocated in the mitochondrial matrix and accumulate there [17]. However, there is very little literature on the use of these new formulations of CoQ10 in randomized controlled trials (RCT) in CVDs, and most of them are in the field of neurodegenerative diseases.
3. Methodology of Review
CoQ10 has been extensively reviewed over the years. In this review, we planned to focus on CVDs and the most relevant findings published in the past five years. We performed a search on PubMed on 31 January 2021 with the keywords “Coenzyme Q10 and cardiovascular diseases”. From the results of the search, we selected those diseases which were more commonly represented among the studies from 2015, had been performed in humans, and were predominantly in RCT. After revisiting this bibliography, we decided to include the recent publications of studies on the influence of CoQ10 on cardiovascular risk factors (dyslipidemia, endothelial dysfunction, and hypertension), HF, myocardial infarction, stroke, peripheral artery disease (PAD), and CAD. For each of these items, we performed a new search on PubMed with “CoQ10/coenzyme Q10/ubiquinone cardiovascular risk factors/dyslipidemia/endothelial dysfunction/hypertension/heart failure/myocardial infarction/stroke/peripheral artery disease/coronary artery disease”. From the publications obtained in these searches, we selected those with the highest relevance.
4. CoQ10 and Cardiovascular Risk Factors
Different studies have examined the efficacy of CoQ10 supplementation in the prevention of CVD through the reduction of cardiovascular risk factors (such as lipid and lipoprotein levels, blood pressure or endothelial function) with the aim of improving patient health and quality of life.
4.1. Dyslipidemias
Although the effect of CoQ10 supplementation on lipid and lipoprotein levels is quantitatively small, different clinical studies, meta-analysis, and systematic reviews have supported the beneficial effects in several types of patients for different CVD risks. In a meta-analysis conducted by Sharifi et al. [18], the authors concluded that administration of CoQ10 significantly reduced triglyceride (TG) concentrations, but not total cholesterol and LDL-cholesterol levels in patients with metabolic disease. Similarly, Jorat et al. [19] found in a meta-analysis that CoQ10 supplementation decreased total cholesterol and increased HDL-cholesterol levels in patients with CAD. However, in a randomized, double-blind, placebo-controlled study performed in obese participants, CoQ10 supplementation (200 mg/d for 12-weeks) did not significantly affect lipid profiles [20]. In dyslipidemic subjects, the administration of 120 mg/d of CoQ10 for 24-weeks reduced TG and LDL-cholesterol concentrations and increased apolipoprotein A-1 compared to the placebo [21]. Results of a double-blinded randomized clinical trial using 200 mg/d of CoQ10 for 12-weeks showed a significant increase in HDL-cholesterol and a significant decrease in total cholesterol/HDL-cholesterol ratio in patients with hyperlipidemia and myocardial infarction [22]. The total cholesterol/HDL-cholesterol ratio is described as a significant predictor of cardiovascular events and a therapeutic target in high-risk patients [23]. In patients with type 2 diabetes and hyperlipidemia, CoQ10 increased the total cholesterol and LDL-cholesterol levels, but had no effect on HDL-cholesterol compared with the placebo [24].
In the context of CAD, a recent systematic review found that CoQ10 supplementation significantly decreased total cholesterol and increased HDL-cholesterol levels without affecting TG and LDL-cholesterol [19]. Considering the fact that the use of statins to decrease cholesterol synthesis also affects CoQ10 levels, the use of exogenous CoQ10 supplementation has been considered to preserve plasma CoQ10 levels in patients treated with these therapeutic compounds (such as patients with CAD and HF) [25]. The combination of CoQ10 with statins has been recently proposed to benefit hypercholesterolemic patients with chronic heart failure [26,27]. In this context, in these patients, the co-administration of CoQ10 and statin therapy is found to be highly recommendable to avoid myopathic side effects as well as enhancing antioxidant enzymes activities and reducing inflammation [28,29]. An overview of the main evidence related to CoQ10 supplementation studies, referred to in the text, is shown in Table 1 [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77].
Table 1.
Coenzyme Q10 supplementation in recent studies in humans and its relation to cardiovascular disease.
| Authors | Sample Size and Disease | Evidence Found | CoQ10 Dosage Used | Level of Evidence |
|---|---|---|---|---|
| Mohseni et al. (2015) [70] | 52 patients with myocardial infarction | Increased serum HDL cholesterol and decreased ICAM-1 levels | 200 mg/day CoQ10 | 12-week randomized, parallel group, placebo-controlled, double-blind study |
| Sharifi et al. (2017) [69] | 63 patients with myocardial infarction | A positive effect on the physical and emotional subscales of MacNew questionnaire | 150 mg/day CoQ10 + 1200 mg/day L-carnitine | 3-month single-blind randomized |
| Kawashima et al. (2020) [49] | 28 Patients with heart failure | An improvement in peripheral endothelial function | 400 mg/day CoQ10 (Ubiquinol) | 3-month randomized, double-blind, placebo-controlled, crossover pilot study |
| Sabbatinelli et al. (2020) [51] | 51 patients with moderate dyslipidemia and endothelial dysfunction | An improvement in endothelium-dependent vasodilation | 100 and 200 mg/day CoQ10 (Ubiquinol) | 8-week double-blind, randomized, placebo-controlled, parallel group study |
| Jorat et al. (2018) [16] | 526 patients with coronary artery disease | Decreased total cholesterol and increasing HDL cholesterol levels. | 100 to 200 mg/day CoQ10 | Meta-analysis (duration: 4–48 weeks) |
| Jorat et al. (2019) [58] | 713 patients with coronary artery disease | Increased SOD and CAT, and decreased MDA | 60 to 300 mg/day CoQ10 | Meta-analysis (duration: 4–48 weeks) |
| Lei et al. (2017) [63] | 2149 patients with heart failure | A lower mortality and improved exercise capacity | 30 to 200 mg/day CoQ10 | Meta-analysis (data not included regarding duration of supplementation) |
| Park et al. (2020) [77] | 11 patients with peripheral artery disease | Improved brachial artery endothelial function, increased SOD, and improvement in physical functional capacity | 80 mg/day MitoQ | Randomized crossover study (duration: 2 weeks) |
HDL: High density lipoprotein; SOD: Extracellular superoxide dismutase; ICAM-1: Intercellular adhesion molecule 1; CAT: Catalase; MDA: Malondialdehyde.
It has been suggested that CoQ10 could act, in the improvement of lipid and lipoprotein profiles, through the activation of the gene expression of peroxisome proliferator-activated receptor-gamma (PPAR-γ) [30], a nuclear receptor protein that regulates pathways related to insulin, lipid metabolism, and inflammation [31] or via the AMP-activated protein kinase/protein kinase C/NADPH oxidase signaling pathway, which suppresses oxidized LDL-induced endothelial oxidative injuries by modulating oxidized low-density lipoprotein receptor 1-mediated reactive oxygen species (ROS) generation [32].
4.2. Hypertension
Hypertension is a key risk factor for almost all CVDs. Although many pharmacological therapies have provided a beneficial effect in lowering blood pressure with a modest decrease in cardiovascular mortality, hypertension remains prevalent [33,34]. Therefore, the effect of CoQ10 on blood pressure has been studied in different controlled intervention studies in human subjects, with a range of CoQ10 doses from 100 mg to 200 mg/day [35,36,37]. These studies found that patients treated with CoQ10 decreased both systolic and diastolic blood pressure, without significant side effects [38]. In a systematic review performed in the context of primary prevention, the authors found that CoQ10 supplementation, without lifestyle intervention, produced a significant reduction in systolic blood pressure without an improvement in other CVD risk factors [39]. In a randomized, double-blinded controlled clinical trial conducted in patients with hyperlipidemia and myocardial infarction, CoQ10 supplementation (200 mg/d) for 12-weeks led to a decrease in both systolic and diastolic blood pressure [22]. In fact, a recent review, in the context of primary prevention, argued that CoQ10 could be used as an effective antihypertensive agent with a capacity of lowering blood pressure to 11/7 [40]. However, in several studies conducted in patients with ischemic left ventricular systolic dysfunction, type 2 diabetes mellitus or obesity, but without hypertension, supplementation of CoQ10 did not alter blood pressure [20,41].
Strong evidence also points to a direct effect of CoQ10 on the endothelium by improving vascular smooth muscle activity, counteracting vasoconstriction, and lowering blood pressure [42]. Moreover, CoQ10 is also thought to provide a protective role in hypertension, acting indirectly through its ability to prevent oxidative stress, nitrative stress, and inflammation, resulting in a recoupling of endothelial nitric oxide synthase (eNOS) [43].
4.3. Endothelial Dysfunction
Endothelial dysfunction, a main mechanism underlying the development of arteriosclerotic disease, is considered a significant predictor of cardiovascular risk [44,45]. The effect of CoQ10 supplementation on the modulation of endothelial function has been evaluated in patients with type 2 diabetes mellitus, CAD, or in elderly people [46,47,48]. These results showed that flow-mediated dilation, or nitroglycerin-mediated dilation and the extracellular superoxide dismutase activity increased in most of the subjects treated with CoQ10, attributing this effect to its antioxidant and anti-inflammatory activity [35,48,49]. Although the mechanisms of CoQ10 in modulating endothelial function are still unclear, it has been suggested that it could be partly attributed to its capacity for reducing oxidative stress and inflammation, particularly in myocardial and endothelial cells [50] and decreasing the rate of activation of NO to peroxynitrite by superoxide radicals, which could improve both vascular tone and endothelial function [51]. CoQ10 may also affect vascular function indirectly via the inhibition of oxidative damage to LDL [43]. In fact, in patients with HF, treatment with CoQ10 (400 mg/d for three months) resulted in significant improvements in peripheral endothelial function, determined by the reactive hyperemia index, accompanied by low levels of oxidized-LDL [52]. CoQ10 has also been shown to improve endothelial function in patients with CAD [48] and type 2 diabetes mellitus [53].
Moreover, it has also been suggested that the protection of the endothelial function through capturing blood plasma CoQ10 and thus reducing oxidative stress could play a key role in the beneficial effect of plasma CoQ10 levels in CVD [46]. In fact, patients with moderate dyslipidemia and endothelial dysfunction showed an improvement in their cardiovascular status after treatment with CoQ10 [54]. Furthermore, the combined action of CoQ10 and anti-atherogenic drugs such as statins improved endothelial dysfunction [55].
5. CoQ10 and CVDs
Cardiac tissues of patients with CVD (heart failure, angina pectoris, coronary artery disease or cardiomyopathy) exhibited a strong CoQ10 deficiency [56,57]. Nutraceuticals have been shown as effectively capable of reducing the burden of the atherosclerosis process and CVD development, as already demonstrated in the literature [58]. In this context, different studies have analyzed the effect of CoQ10 supplementation on CVD as a therapeutic approach to reduce the clinical complications of CVD.
5.1. CoQ10 in Coronary Artery Disease
CAD is the most common type of heart disease. It is also known as coronary heart disease or ischemic heart disease. In its etiology, CAD is caused by the formation of an atherosclerotic plaque in the walls of the arteries that supply blood to the heart (coronary arteries) and other parts of the body. Due to the known involvement of oxidative damage in the etiology of atherosclerosis, CoQ10 has been thought to have potential benefits in the amelioration of CAD. Heng Lu et al. recently showed, in a correlation analysis of metabolic pathways integrated as a unit to co-express susceptibility genes with CAD, that the canonical metabolic pathway of biosynthesis of CoQ10, specifically the genes COQ2 and COQ5, were linked to the susceptibility of CAD [59]. The COQ2 gene encodes 4-hydroxybenzoate polyprenyltransferase enzyme, which condenses decaprenyl pyrophosphate and 4-OH-benzoate into decaprenyl-OH-benzoate [5,60]. The COQ5 gene encodes the enzyme that catalyzes the only C-methylation step involved in the synthesis of CoQ10 [61]. Due to the action of CoQ10 in energy metabolism and its relation to coronary revascularization, some authors consider that low levels of CoQ10 should be considered as a risk factor for CAD [62,63].
In a systematic review carried out by Jorat et al. in 2019, the authors looked for evidence of effects of CoQ10 supplementation on inflammation and oxidative damage in CAD patients [64]. Thirteen out of 912 potential RCT found in the literature were analyzed and it was found that CoQ10 supplementation significantly increased the levels of the antioxidant enzymes superoxide dismutase (SOD) and catalase, and significantly reduced malondialdehyde (MDA), a marker of lipid peroxidation. Since CAD patients are considered to have a chronic systemic inflammation status, this recent meta-analysis demonstrated the potential benefits of CoQ10 supplementation for ameliorating inflammation and oxidative damage in these patients. However, this analysis failed to demonstrate the effects of CoQ10 on C-reactive protein, tumor necrosis factor α, interleukin-6, and glutathione peroxidase levels among patients with CAD, which have been described previously [28]. The dosage range presented in the RCT included in the analysis was from 60 to 300 mg/day of CoQ10 with a follow-up of up to 48 weeks. However, no information was reported regarding the formulation used for the supplementation. Since it has a great impact on the bioavailability of CoQ10, and there seems to be a consensus that at least 200 mg/day is needed to have an impact on rising plasma CoQ10 levels [5], there is a need for a RCT with these characteristics to elucidate the real effects of CoQ10 on inflammatory and oxidative damage in these patients.
5.2. CoQ10 in Heart Failure
Heart failure (HF) is defined as “a complex clinical syndrome that can result from any structural or functional cardiac disorder that impairs the ability of the ventricle to fill or eject blood” [65]. Despite improvements in the prevention and treatment of HF, mortality rates from HF are over 10% per year, and even reach 20% to 50% in some settings [66]. In HF, the heart muscle exhibits reduced adenosine triphosphate synthesis, increased production of ROS, and a deflection of the calcium exchange, mainly due to inefficient electron transport chain activity. Moreover, in patients with HF, the severity of disease is correlated with CoQ10 deficiency [67]. In this context, and since CoQ10 plays a key role in cell energetics in the mitochondria, it is plausible to consider the potential benefits of CoQ10 supplementation as a therapeutic option for HF patients. It has also been demonstrated that CoQ10 prevents senescence and dysfunction in vascular endothelial cells caused by oxidative damage [51]. For this reason, supplementation with CoQ10 has been suggested to prevent not only HF but also hypertension and endothelial dysfunction (see Section 4.3).
Over the last few years, several clinical studies have investigated the possibility of using CoQ10 to prevent HF and improve the symptoms of this disease. In one of the most important clinical studies related to this research area, the Q-SYMBIO study, it was shown that in a study including 420 patients with moderate or severe HF (202 with 300 mg of CoQ10 supplementation and 218 with placebo), there was a reduction in the rate of major adverse cardiac events, cardiovascular mortality, all-cause mortality, and incidence of hospital stays for HF, after 2-years, compared to those patients treated with the placebo [68]. However, the Q-SYMBIO study has been criticized, for instance, for not reaching the planned number of patients (n = 550) in eight years of recruitment. Indeed, in a sub-group analysis of this study recently published, the researchers observed improvements in major clinical endpoints, with an increase in left ventricular ejection fraction in the European population of the study, which was not found in the larger cohort. The authors declared that this subpopulation of the study showed a higher adherence to the recommended medical and device therapies compared to the whole population. Furthermore, the effects found in this study were confirmed in a subsequent meta-analysis of 14 RCTs including 2149 patients, although no significant differences were observed in the endpoint of left ventricular ejection fraction between the group that received treatment and the group receiving a placebo [69]. However, short-term CoQ10 supplementation provided no additional benefits in improving left ventricle diastolic function in 28 patients with HF with preserved ejection fraction [70]. In general, the heterogeneity in the populations, designs, follow-up durations, doses administered, and study outcomes make it difficult to extract a clear effect of CoQ10 in HF. Nevertheless, there seems to be a consensus that the beneficial effects attributable to CoQ10 in HF are related to its important role as an electron carrier in mitochondria, increasing bioenergetics and preventing oxidative damage in the failing myocardium. However, changes in the antioxidant systems in HF support the idea that CoQ10 may improve the outcome and quality of life and may decrease morbidity and mortality.
5.3. CoQ10 in Myocardial Infarction
Myocardial infarction occurs due to myocardial cell death caused by prolonged ischemia [71]. The pathologic processes underlying this disease are linked to a high oxidative stress that leads to reperfusion-induced free radical damage, lipid peroxidation, and decreased energy production, in which CoQ10 deficiency could play a role [72,73]. In fact, recent evidence has shown that maintaining high endogenous levels of CoQ10 in plasma, in patients with myocardial infarction, is related to a better recovery of left ventricular function [74]. In this context, another possible use for CoQ10 supplementation could be to restore tissue CoQ10 deficiency in the myocardium after myocardial infarction. In a recent RCT, the authors showed an improvement of quality of life in patients with myocardial infarction using the MacNew QoL questionnaire, after 3-months of supplementation with 150 mg/d of CoQ10 plus 200 mg/d of L-carnitine [75]. Besides a reduction in blood pressure, LDL-cholesterol/HDL-cholesterol and total cholesterol/HDL-cholesterol ratios in patients who presented hyperlipidemia but also myocardial infarction, CoQ10 supplementation (200 mg/day for 12-weeks) also decreased inflammatory status (serum ICAM-1 and IL-6 levels) [22,76]. In diabetic patients with CAD, CoQ10 supplementation also produced an anti-inflammatory effect despite the fact that no improvement was observed in cardiometabolic markers, suggesting that the presence of type 2 diabetes could infer different underlying pathogenic mechanisms in myocardial infarction [77].
Regarding the possibility of using CoQ10 supplementation to prevent cardiac remodeling in patients with myocardial infarction, in another study, 24-weeks of supplementation of CoQ10 (120 mg/day) showed the maintenance of the sphericity index with diminished alteration of wall-thickening abnormality at the infarct site, compared to the placebo, in patients affected by persistent left ventricular dysfunction [78]. Moreover, in a recent study performed in patients with myocardial infarction using 120 mg of CoQ10/day for 24-weeks, a protective role against left ventricular remodeling was observed in patients with persistent left ventricular dysfunction [79].
5.4. CoQ10 in Peripheral Artery Disease
PAD is a common CVD characterized by the formation of atherosclerotic plaque in the leg arteries, which causes attenuated blood flow and reduced perfusion in the lower extremities [80]. PAD can severely impact quality of life as functional ability is compromised as the disease progresses. Among the risk factors for the incidence of this disease are smoking, diabetes mellitus and CAD, and age, with approximately 20% of the population over 60 years presenting some degree of the condition. Symptoms often include claudication (leg pain during walking) and foot ulcers, which, if severe, may require revascularization procedures or leg amputation [81].
In PAD, increased ROS appears as one of the mechanisms underlying atherosclerosis and oxidative stress damage in the skeletal muscle [82]. At this point, maintaining an effective function of the mitochondria in the vasculature is a key factor that controls ROS production and NO bioavailability. In this context, mitochondria could be seen as a novel therapeutic target to improve vascular function, oxygen transfer, and utility capacity in the lower extremity, with a potential role in reducing leg pain and improving quality of life in PAD patients [83]. Despite these premises, few studies have been published that aim to evaluate the effect of CoQ10 supplementation in the amelioration of PAD. Among recent publications, only one study with 11 PAD patients is worth mentioning. In a randomized, placebo-controlled, crossover study design, patients received a dose of 80 mg of MitoQ, an analogue of CoQ10 [83]. The study aimed to understand the roles of the vascular mitochondria in PAD in vivo by examining the impacts of acute MitoQ intake on endothelial function, blood pressure, arterial stiffness, walking capacity, and oxygen utility capacity in patients with PAD. The authors found, for the first time, an improvement in brachial artery endothelial function after the intake of MitoQ, highlighting the key role that vascular mitochondria plays in endothelial function in this kind of patient. Regarding the molecular mechanisms behind these effects, Park S.Y. et al. showed an increase in SOD, the first line of the endogenous antioxidant defense system, which could reduce mitochondrial-derived ROS in the vasculature. In line with these findings, it seems to be of importance to confirm the effects of CoQ10 in the amelioration of PAD in larger cohorts. Despite the lack of clinical trials proving the benefits of CoQ10 supplementation, a recent review summarized the nutrients that may be at risk of depletion in PAD patients treated with lipid-lowering, antiplatelet, antihypertensive, and antidiabetic drugs. Among them, the authors identified as potentially at risk from CoQ10, vitamin C, zinc, and vitamin B12 [84]. As these medications are frequently prescribed to PAD patients, the authors recommend that CoQ10, zinc, and vitamin B12 levels should be routinely monitored to prevent and correct nutrient deficiencies associated with the multiple-drug therapy for this condition. This leads to the possible need for dietary interventions, and the possible supplementation with these nutrients in PAD patients.
5.5. Stroke
Stroke occurs when the blood supply to part of the brain is interrupted or reduced, preventing the brain tissue from receiving oxygen and nutrients. Brain cells can only survive a few minutes without this supply, and this leads to problems of ischemic brain damage, which is extremely common in modern health care. In addition, ischemic stroke has major socio-economic consequences, leading in some cases to disability in working-age people. Oxidative stress has been described as one of the pathological mechanisms involved in cerebral ischemia [85], where a disbalance among oxidants and antioxidants causes further damage to cell structure and function [86]. Despite the evidence of the CoQ10 functions described, to the best of our knowledge, there are no recent publications in the literature regarding clinical studies in humans considering the beneficial effect of CoQ10 supplementation to ameliorate the symptoms and consequences of stroke, although there is one clinical study that aimed to associate CoQ10 levels with clinical neurological outcomes in acute stroke patients [87]. This study found that stroke patients had significantly lower serum levels of CoQ10 and SOD compared to the controls, and higher serum MDA levels, in a cohort of 76 patients and 34 healthy individuals. The authors suggest that a decrease in serum CoQ10 level exerts a detrimental effect on neurological outcomes following acute ischemic stroke, as measured by the National Institute of Health Stroke Scale and a modified Rankin Scale. Therefore, it can be suggested that preserving the antioxidant functionality of CoQ10 could be a potential strategy in treating this pathology. On the other hand, there is a more extensive bibliography, compared to human studies, on the influence of CoQ10 as a treatment in animal models. Although the scope of this review only includes human studies, it is interesting to note that Nikolaevna et al. recently found that intravenous administration of CoQ10 led to a decrease in rat mortality rate, improvement in neurological status and decrease in the brain necrosis area in acute and delayed periods after cerebral ischemia [88]. Due to the lack of more clinical trials on the effect of pre- or post-treatment with CoQ10 in strokes in larger human studies, we can only highlight the promising impact this type of treatment could have on stroke patients.
6. Conclusions
Although the importance of CoQ10 can mostly be attributed to its function as an essential molecule for energy transduction in mitochondria, new findings support its relevant function as an antioxidant, not only in mitochondria, but also in other cell compartments and tissues in the organism as well as in plasma lipoproteins. Endogenous CoQ10 biosynthesis supplies sufficient levels of this quinone in disease-free individuals. However, CoQ10 deficiency is not only based on genetic failure, but also on chronic and age-related diseases such as CVDs. In this context, CoQ10 deficiencies have risen in CVDs, since statins, one of the most common lipid-lowering drugs used in CVD patients, diminish endogenous CoQ10 biosynthesis because its initial steps are shared with the cholesterol biosynthesis pathway. In this context, it has been shown that CoQ10 can potentially be used as a treatment to ameliorate these deficiencies. However, the existence of various CoQ10 formulations, together with differences in the range of doses and periods of CoQ10 supplementation used in the clinical studies, makes it difficult to compare them and reach a clear conclusion on the most suitable dose, effectiveness, and bioavailability of oral-administration of CoQ10 for therapeutic use with CVDs. A major effort is needed to reach a consensus over the use of this supplement, with the aim of including it in the clinical guidelines for treating CVD patients.
Acknowledgments
EU’s European Regional Development Fund (FEDER). The CIBEROBN is an initiative of the Instituto de Salud Carlos III, Madrid, Spain.
Author Contributions
Conceptualization of the review, F.M.G.-M., E.M.Y.-S., and J.L.-M.; Methodology, F.M.G.-M., S.d.l.C.-A., and J.D.T.-P.; Writing—original draft preparation, F.M.G.-M. and S.d.l.C.-A.; Writing—review and editing, J.D.T.-P., J.F.A.-D., and E.M.Y.-S.; Supervision, E.M.Y.-S. and J.L.-M. All authors have read and agreed to the published version of the manuscript.
Funding
Elena M Yubero-Serrano was the recipient of the Nicolas Monardes Program from the “Servicio Andaluz de Salud, Junta de Andalucia”, Spain (C1-0005-2019).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Festenstein G.N., Heaton F.W., Lowe J.S., Morton R.A. A constituent of the unsaponifiable portion of animal tissue lipids (lambda max. 272 m mu) Biochem. J. 1955;59:558–566. doi: 10.1042/bj0590558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crane F.L., Hatefi Y., Lester R.L., Widmer C. Isolation of a quinone from beef heart mitochondria. Biochim. Biophys. Acta. 1957;25:220–221. doi: 10.1016/0006-3002(57)90457-2. [DOI] [PubMed] [Google Scholar]
- 3.Lenaz G., Fato R., Di Bernardo S., Jarreta D., Costa A., Genova M.L., Parenti Castelli G. Localization and mobility of coenzyme Q in lipid bilayers and membranes. BioFactors. 1999;9:87–93. doi: 10.1002/biof.5520090202. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Lluch G., Barroso M.P., Martin S.F., Fernandez-Ayala D.J., Gomez-Diaz C., Villalba J.M., Navas P. Role of plasma membrane coenzyme Q on the regulation of apoptosis. BioFactors. 1999;9:171–177. doi: 10.1002/biof.5520090212. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez-Mariscal F.M., Yubero-Serrano E.M., Villalba J.M., Lopez-Miranda J. Coenzyme Q10: From bench to clinic in aging diseases, a translational review. Crit. Rev. Food Sci. Nutr. 2019;59:2240–2257. doi: 10.1080/10408398.2018.1442316. [DOI] [PubMed] [Google Scholar]
- 6.Bentinger M., Tekle M., Dallner G. Coenzyme Q-biosynthesis and functions. Biochem. Biophys. Res. Commun. 2010;396:74–79. doi: 10.1016/j.bbrc.2010.02.147. [DOI] [PubMed] [Google Scholar]
- 7.Quinzii C.M., DiMauro S., Hirano M. Human coenzyme Q10 deficiency. Neurochem. Res. 2007;32:723–727. doi: 10.1007/s11064-006-9190-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Turunen M., Olsson J., Dallner G. Metabolism and function of coenzyme Q. Biochim. Biophys. Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 9.Ernster L., Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Aguilera J.C., Cortes A.B., Fernandez-Ayala D.J., Navas P. Biochemical Assessment of Coenzyme Q10 Deficiency. J. Clin. Med. 2017;6:27. doi: 10.3390/jcm6030027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sohal R.S., Forster M.J. Coenzyme Q, oxidative stress and aging. Mitochondrion. 2007;7:S103–S111. doi: 10.1016/j.mito.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou H., Liu G., Zhang J., Sun N., Duan M., Yan Z., Xia Q. Novel lipid-free nanoformulation for improving oral bioavailability of coenzyme Q10. BioMed Res. Int. 2014;2014:793879. doi: 10.1155/2014/793879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhagavan H.N., Chopra R.K. Coenzyme Q10: Absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 2006;40:445–453. doi: 10.1080/10715760600617843. [DOI] [PubMed] [Google Scholar]
- 14.Villalba J.M., Parrado C., Santos-Gonzalez M., Alcain F.J. Therapeutic use of coenzyme Q10 and coenzyme Q10-related compounds and formulations. Expert Opin. Investig. Drugs. 2010;19:535–554. doi: 10.1517/13543781003727495. [DOI] [PubMed] [Google Scholar]
- 15.Muthukumaran K., Leahy S., Harrison K., Sikorska M., Sandhu J.K., Cohen J., Keshan C., Lopatin D., Miller H., Borowy-Borowski H., et al. Orally delivered water soluble Coenzyme Q10 (Ubisol-Q10) blocks on-going neurodegeneration in rats exposed to paraquat: Potential for therapeutic application in Parkinson’s disease. BMC Neurosci. 2014;15:21. doi: 10.1186/1471-2202-15-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fumagalli S., Fattirolli F., Guarducci L., Cellai T., Baldasseroni S., Tarantini F., Di Bari M., Masotti G., Marchionni N. Coenzyme Q10 terclatrate and creatine in chronic heart failure: A randomized, placebo-controlled, double-blind study. Clin. Cardiol. 2011;34:211–217. doi: 10.1002/clc.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dare A.J., Logan A., Prime T.A., Rogatti S., Goddard M., Bolton E.M., Bradley J.A., Pettigrew G.J., Murphy M.P., Saeb-Parsy K. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J. Heart Lung Transplant. 2015;34:1471–1480. doi: 10.1016/j.healun.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharifi N., Tabrizi R., Moosazadeh M., Mirhosseini N., Lankarani K.B., Akbari M., Chamani M., Kolahdooz F., Asemi Z. The Effects of Coenzyme Q10 Supplementation on Lipid Profiles Among Patients with Metabolic Diseases: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Curr. Pharm. Des. 2018;24:2729–2742. doi: 10.2174/1381612824666180406104516. [DOI] [PubMed] [Google Scholar]
- 19.Jorat M.V., Tabrizi R., Mirhosseini N., Lankarani K.B., Akbari M., Heydari S.T., Mottaghi R., Asemi Z. The effects of coenzyme Q10 supplementation on lipid profiles among patients with coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Lipids Health Dis. 2018;17:230. doi: 10.1186/s12944-018-0876-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y.J., Cho W.J., Kim J.K., Lee D.C. Effects of coenzyme Q10 on arterial stiffness, metabolic parameters, and fatigue in obese subjects: A double-blind randomized controlled study. J. Med. Food. 2011;14:386–390. doi: 10.1089/jmf.2010.1202. [DOI] [PubMed] [Google Scholar]
- 21.Hedner T., Kjeldsen S.E., Narkiewicz K. State of global health-hypertension burden and control. Blood Press. 2012;21(Suppl. S1):1–2. doi: 10.3109/08037051.2012.704786. [DOI] [PubMed] [Google Scholar]
- 22.Mohseni M., Vafa M.R., Hajimiresmail S.J., Zarrati M., Rahimi-Forushani A., Bitarafan V., Shidfar F. Effects of coenzyme q10 supplementation on serum lipoproteins, plasma fibrinogen, and blood pressure in patients with hyperlipidemia and myocardial infarction. Iran Red Crescent Med. J. 2014;16:e16433. doi: 10.5812/ircmj.16433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arsenault B.J., Rana J.S., Stroes E.S., Despres J.P., Shah P.K., Kastelein J.J., Wareham N.J., Boekholdt S.M., Khaw K.T. Beyond low-density lipoprotein cholesterol: Respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J. Am. Coll. Cardiol. 2009;55:35–41. doi: 10.1016/j.jacc.2009.07.057. [DOI] [PubMed] [Google Scholar]
- 24.Zahedi H., Eghtesadi S., Seifirad S., Rezaee N., Shidfar F., Heydari I., Golestan B., Jazayeri S. Effects of CoQ10 Supplementation on Lipid Profiles and Glycemic Control in Patients with Type 2 Diabetes: A randomized, double blind, placebo-controlled trial. J. Diabetes Metab. Disord. 2014;13:81. doi: 10.1186/s40200-014-0081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bargossi A.M., Grossi G., Fiorella P.L., Gaddi A., Di Giulio R., Battino M. Exogenous CoQ10 supplementation prevents plasma ubiquinone reduction induced by HMG-CoA reductase inhibitors. Mol. Aspects Med. 1994;15:s187–s193. doi: 10.1016/0098-2997(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 26.Silver M.A., Langsjoen P.H., Szabo S., Patil H., Zelinger A. Statin cardiomyopathy? A potential role for Co-Enzyme Q10 therapy for statin-induced changes in diastolic LV performance: Description of a clinical protocol. BioFactors. 2003;18:125–127. doi: 10.1002/biof.5520180214. [DOI] [PubMed] [Google Scholar]
- 27.Kloer H.U., Belardinelli R., Ruchong O., Rosenfeldt F. Combining Ubiquinol with a Statin May Benefit Hypercholesterolaemic Patients with Chronic Heart Failure. Heart Lung Circ. 2020;29:188–195. doi: 10.1016/j.hlc.2019.08.017. [DOI] [PubMed] [Google Scholar]
- 28.Lee B.J., Tseng Y.F., Yen C.H., Lin P.T. Effects of coenzyme Q10 supplementation (300 mg/day) on antioxidation and anti-inflammation in coronary artery disease patients during statins therapy: A randomized, placebo-controlled trial. Nutr. J. 2013;12:142. doi: 10.1186/1475-2891-12-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thompson P.D., Clarkson P., Karas R.H. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- 30.Lee S.K., Lee J.O., Kim J.H., Kim N., You G.Y., Moon J.W., Sha J., Kim S.J., Lee Y.W., Kang H.J., et al. Coenzyme Q10 increases the fatty acid oxidation through AMPK-mediated PPARalpha induction in 3T3-L1 preadipocytes. Cell Signal. 2012;24:2329–2336. doi: 10.1016/j.cellsig.2012.07.022. [DOI] [PubMed] [Google Scholar]
- 31.Szatmari I., Rajnavolgyi E., Nagy L. PPARgamma, a lipid-activated transcription factor as a regulator of dendritic cell function. Ann. N. Y. Acad. Sci. 2006;1088:207–218. doi: 10.1196/annals.1366.013. [DOI] [PubMed] [Google Scholar]
- 32.Tsai K.L., Chen L.H., Chiou S.H., Chiou G.Y., Chen Y.C., Chou H.Y., Chen L.K., Chen H.Y., Chiu T.H., Tsai C.S., et al. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Mol. Nutr. Food Res. 2011;55(Suppl. S2):S227–S240. doi: 10.1002/mnfr.201100147. [DOI] [PubMed] [Google Scholar]
- 33.Musini V.M., Tejani A.M., Bassett K., Wright J.M. Pharmacotherapy for hypertension in the elderly. Cochrane Database Syst. Rev. 2009:CD000028. doi: 10.1002/14651858.CD000028.pub2. [DOI] [PubMed] [Google Scholar]
- 34.Ghaffari S., Roshanravan N. The role of nutraceuticals in prevention and treatment of hypertension: An updated review of the literature. Food Res. Int. 2020;128:108749. doi: 10.1016/j.foodres.2019.108749. [DOI] [PubMed] [Google Scholar]
- 35.Gonzalez-Guardia L., Yubero-Serrano E.M., Delgado-Lista J., Perez-Martinez P., Garcia-Rios A., Marin C., Camargo A., Delgado-Casado N., Roche H.M., Perez-Jimenez F., et al. Effects of the Mediterranean diet supplemented with coenzyme q10 on metabolomic profiles in elderly men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:78–84. doi: 10.1093/gerona/glu098. [DOI] [PubMed] [Google Scholar]
- 36.Yubero-Serrano E.M., Gonzalez-Guardia L., Rangel-Zuniga O., Delgado-Casado N., Delgado-Lista J., Perez-Martinez P., Garcia-Rios A., Caballero J., Marin C., Gutierrez-Mariscal F.M., et al. Postprandial antioxidant gene expression is modified by Mediterranean diet supplemented with coenzyme Q(10) in elderly men and women. Age. 2013;35:159–170. doi: 10.1007/s11357-011-9331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young J.M., Florkowski C.M., Molyneux S.L., McEwan R.G., Frampton C.M., Nicholls M.G., Scott R.S., George P.M. A randomized, double-blind, placebo-controlled crossover study of coenzyme Q10 therapy in hypertensive patients with the metabolic syndrome. Am. J. Hypertens. 2012;25:261–270. doi: 10.1038/ajh.2011.209. [DOI] [PubMed] [Google Scholar]
- 38.Rosenfeldt F.L., Haas S.J., Krum H., Hadj A., Ng K., Leong J.Y., Watts G.F. Coenzyme Q10 in the treatment of hypertension: A meta-analysis of the clinical trials. J. Hum. Hypertens. 2007;21:297–306. doi: 10.1038/sj.jhh.1002138. [DOI] [PubMed] [Google Scholar]
- 39.Flowers N., Hartley L., Todkill D., Stranges S., Rees K. Co-enzyme Q10 supplementation for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2014:CD010405. doi: 10.1002/14651858.CD010405.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho M.J., Bellusci A., Wright J.M. Blood pressure lowering efficacy of coenzyme Q10 for primary hypertension. Cochrane Database Syst. Rev. 2009:CD007435. doi: 10.1002/14651858.CD007435.pub2. [DOI] [PubMed] [Google Scholar]
- 41.Dai Y.L., Luk T.H., Yiu K.H., Wang M., Yip P.M., Lee S.W., Li S.W., Tam S., Fong B., Lau C.P., et al. Reversal of mitochondrial dysfunction by coenzyme Q10 supplement improves endothelial function in patients with ischaemic left ventricular systolic dysfunction: A randomized controlled trial. Atherosclerosis. 2011;216:395–401. doi: 10.1016/j.atherosclerosis.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 42.Digiesi V., Cantini F., Oradei A., Bisi G., Guarino G.C., Brocchi A., Bellandi F., Mancini M., Littarru G.P. Coenzyme Q10 in essential hypertension. Mol. Aspects Med. 1994;15:s257–s263. doi: 10.1016/0098-2997(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 43.Belardinelli R., Tiano L., Littarru G.P. Oxidative stress, endothelial function and coenzyme Q10. BioFactors. 2008;32:129–133. doi: 10.1002/biof.5520320115. [DOI] [PubMed] [Google Scholar]
- 44.Dludla P.V., Nyambuya T.M., Orlando P., Silvestri S., Mxinwa V., Mokgalaboni K., Nkambule B.B., Louw J., Muller C.J.F., Tiano L. The impact of coenzyme Q10 on metabolic and cardiovascular disease profiles in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Endocrino. Diabetes Metab. 2020;3:e00118. doi: 10.1002/edm2.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bertoluci M.C., Ce G.V., da Silva A.M., Wainstein M.V., Boff W., Punales M. Endothelial dysfunction as a predictor of cardiovascular disease in type 1 diabetes. World J. Diabetes. 2015;6:679–692. doi: 10.4239/wjd.v6.i5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gao L., Mao Q., Cao J., Wang Y., Zhou X., Fan L. Effects of coenzyme Q10 on vascular endothelial function in humans: A meta-analysis of randomized controlled trials. Atherosclerosis. 2012;221:311–316. doi: 10.1016/j.atherosclerosis.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 47.Hodgson J.M., Watts G.F., Playford D.A., Burke V., Croft K.D. Coenzyme Q10 improves blood pressure and glycaemic control: A controlled trial in subjects with type 2 diabetes. Eur. J. Clin. Nutr. 2002;56:1137–1142. doi: 10.1038/sj.ejcn.1601464. [DOI] [PubMed] [Google Scholar]
- 48.Tiano L., Belardinelli R., Carnevali P., Principi F., Seddaiu G., Littarru G.P. Effect of coenzyme Q10 administration on endothelial function and extracellular superoxide dismutase in patients with ischaemic heart disease: A double-blind, randomized controlled study. Eur. Heart J. 2007;28:2249–2255. doi: 10.1093/eurheartj/ehm267. [DOI] [PubMed] [Google Scholar]
- 49.Yubero-Serrano E.M., Gonzalez-Guardia L., Rangel-Zuniga O., Delgado-Lista J., Gutierrez-Mariscal F.M., Perez-Martinez P., Delgado-Casado N., Cruz-Teno C., Tinahones F.J., Villalba J.M., et al. Mediterranean diet supplemented with coenzyme Q10 modifies the expression of proinflammatory and endoplasmic reticulum stress-related genes in elderly men and women. J. Gerontol. A Biol. Sci. Med. Sci. 2012;67:3–10. doi: 10.1093/gerona/glr167. [DOI] [PubMed] [Google Scholar]
- 50.Lopez-Moreno J., Quintana-Navarro G.M., Delgado-Lista J., Garcia-Rios A., Alcala-Diaz J.F., Gomez-Delgado F., Camargo A., Perez-Martinez P., Tinahones F.J., Striker G.E., et al. Mediterranean Diet Supplemented with Coenzyme Q10 Modulates the Postprandial Metabolism of Advanced Glycation End Products in Elderly Men and Women. J. Gerontol. A Biol. Sci. Med. Sci. 2018;73:340–346. doi: 10.1093/gerona/glw214. [DOI] [PubMed] [Google Scholar]
- 51.Di Lorenzo A., Iannuzzo G., Parlato A., Cuomo G., Testa C., Coppola M., D’Ambrosio G., Oliviero D.A., Sarullo S., Vitale G., et al. Clinical Evidence for Q10 Coenzyme Supplementation in Heart Failure: From Energetics to Functional Improvement. J. Clin. Med. 2020;9:1266. doi: 10.3390/jcm9051266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawashima C., Matsuzawa Y., Konishi M., Akiyama E., Suzuki H., Sato R., Nakahashi H., Kikuchi S., Kimura Y., Maejima N., et al. Ubiquinol Improves Endothelial Function in Patients with Heart Failure with Reduced Ejection Fraction: A Single-Center, Randomized Double-Blind Placebo-Controlled Crossover Pilot Study. Am. J. Cardiovasc. Drugs. 2020;20:363–372. doi: 10.1007/s40256-019-00384-y. [DOI] [PubMed] [Google Scholar]
- 53.Hamilton S.J., Chew G.T., Watts G.F. Coenzyme Q10 improves endothelial dysfunction in statin-treated type 2 diabetic patients. Diabetes Care. 2009;32:810–812. doi: 10.2337/dc08-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sabbatinelli J., Orlando P., Galeazzi R., Silvestri S., Cirilli I., Marcheggiani F., Dludla P.V., Giuliani A., Bonfigli A.R., Mazzanti L., et al. Ubiquinol Ameliorates Endothelial Dysfunction in Subjects with Mild-to-Moderate Dyslipidemia: A Randomized Clinical Trial. Nutrients. 2020;12:1098. doi: 10.3390/nu12041098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chew G.T., Watts G.F. Coenzyme Q10 and diabetic endotheliopathy: Oxidative stress and the “recoupling hypothesis”. QJM. 2004;97:537–548. doi: 10.1093/qjmed/hch089. [DOI] [PubMed] [Google Scholar]
- 56.Littarru G.P., Tiano L. Clinical aspects of coenzyme Q10: An update. Nutrition. 2010;26:250–254. doi: 10.1016/j.nut.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Jafari M., Mousavi S.M., Asgharzadeh A., Yazdani N. Coenzyme Q10 in the treatment of heart failure: A systematic review of systematic reviews. Indian Heart J. 2018;70(Suppl. S1):S111–S117. doi: 10.1016/j.ihj.2018.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scicchitano P.C.M., Maielloc M., Modestid P.A., Muiesane M.L., Novof S., Palmieroc P., Sabag P.S., Pedrinellih R., Ciccone M.M. Nutraceuticals and dyslipidaemia: Beyond the common therapeutics. J. Funct. Foods. 2014;6:11–32. doi: 10.1016/j.jff.2013.12.006. [DOI] [Google Scholar]
- 59.Lu H., Chen Y., Li L. Metabolic Pathway Genes Associated with Susceptibility Genes to Coronary Artery Disease. Int. J. Genom. 2018;2018:9025841. doi: 10.1155/2018/9025841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Eroglu F.K., Ozaltin F., Gonc N., Nalcacioglu H., Ozcakar Z.B., Yalnizoglu D., Gucer S., Orhan D., Eminoglu F.T., Gocmen R., et al. Response to Early Coenzyme Q10 Supplementation Is not Sustained in CoQ10 Deficiency Caused by CoQ2 Mutation. Pediatr. Neurol. 2018;88:71–74. doi: 10.1016/j.pediatrneurol.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 61.Malicdan M.C.V., Vilboux T., Ben-Zeev B., Guo J., Eliyahu A., Pode-Shakked B., Dori A., Kakani S., Chandrasekharappa S.C., Ferreira C.R., et al. A novel inborn error of the coenzyme Q10 biosynthesis pathway: Cerebellar ataxia and static encephalomyopathy due to COQ5 C-methyltransferase deficiency. Hum. Mutat. 2018;39:69–79. doi: 10.1002/humu.23345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. Coenzyme Q10 deficiency in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) is related to fatigue, autonomic and neurocognitive symptoms and is another risk factor explaining the early mortality in ME/CFS due to cardiovascular disorder. Neuro Endocrinol. Lett. 2009;30:470–476. [PubMed] [Google Scholar]
- 63.Maes M., Mihaylova I., Kubera M., Uytterhoeven M., Vrydags N., Bosmans E. Lower plasma Coenzyme Q10 in depression: A marker for treatment resistance and chronic fatigue in depression and a risk factor to cardiovascular disorder in that illness. Neuro Endocrinol. Lett. 2009;30:462–469. [PubMed] [Google Scholar]
- 64.Jorat M.V., Tabrizi R., Kolahdooz F., Akbari M., Salami M., Heydari S.T., Asemi Z. The effects of coenzyme Q10 supplementation on biomarkers of inflammation and oxidative stress in among coronary artery disease: A systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology. 2019;27:233–248. doi: 10.1007/s10787-019-00572-x. [DOI] [PubMed] [Google Scholar]
- 65.Bozkurt B. What Is New in Heart Failure Management in 2017? Update on ACC/AHA Heart Failure Guidelines. Curr. Cardiol. Rep. 2018;20:39. doi: 10.1007/s11886-018-0978-7. [DOI] [PubMed] [Google Scholar]
- 66.Kannel W.B. Incidence and epidemiology of heart failure. Heart Fail. Rev. 2000;5:167–173. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 67.Zozina V.I., Covantev S., Goroshko O.A., Krasnykh L.M., Kukes V.G. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr. Cardiol. Rev. 2018;14:164–174. doi: 10.2174/1573403X14666180416115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mortensen S.A., Rosenfeldt F., Kumar A., Dolliner P., Filipiak K.J., Pella D., Alehagen U., Steurer G., Littarru G.P., Investigators Q.S.S. The effect of coenzyme Q10 on morbidity and mortality in chronic heart failure: Results from Q-SYMBIO: A randomized double-blind trial. JACC Heart Fail. 2014;2:641–649. doi: 10.1016/j.jchf.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 69.Lei L., Liu Y. Efficacy of coenzyme Q10 in patients with cardiac failure: A meta-analysis of clinical trials. BMC Cardiovasc. Disord. 2017;17:196. doi: 10.1186/s12872-017-0628-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sobirin M.A., Herry Y., Sofia S.N., Uddin I., Rifqi S., Tsutsui H. Effects of coenzyme Q10 supplementation on diastolic function in patients with heart failure with preserved ejection fraction. Drug Discov. Ther. 2019;13:38–46. doi: 10.5582/ddt.2019.01004. [DOI] [PubMed] [Google Scholar]
- 71.Thygesen K., Alpert J.S., Jaffe A.S., Chaitman B.R., Bax J.J., Morrow D.A., White H.D., Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction Fourth Universal Definition of Myocardial Infarction (2018) J. Am. Coll. Cardiol. 2018;72:2231–2264. doi: 10.1016/j.jacc.2018.08.1038. [DOI] [PubMed] [Google Scholar]
- 72.Cohn J.N., Ferrari R., Sharpe N. Cardiac remodeling-concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 2000;35:569–582. doi: 10.1016/S0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 73.Ulla A., Mohamed M.K., Sikder B., Rahman A.T., Sumi F.A., Hossain M., Reza H.M., Rahman G.M.S., Alam M.A. Coenzyme Q10 prevents oxidative stress and fibrosis in isoprenaline induced cardiac remodeling in aged rats. BMC Pharmacol. Toxicol. 2017;18:29. doi: 10.1186/s40360-017-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang C.H., Kuo C.L., Huang C.S., Tseng W.M., Lian I.B., Chang C.C., Liu C.S. High plasma coenzyme Q10 concentration is correlated with good left ventricular performance after primary angioplasty in patients with acute myocardial infarction. Medicine. 2016;95:e4501. doi: 10.1097/MD.0000000000004501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharifi M.H., Eftekhari M.H., Ostovan M.A., Rezaianazadeh A. Effects of a therapeutic lifestyle change diet and supplementation with Q10 plus L-carnitine on quality of life in patients with myocardial infarction: A randomized clinical trial. J. Cardiovasc. Thorac. Res. 2017;9:21–28. doi: 10.15171/jcvtr.2017.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mohseni M., Vafa M., Zarrati M., Shidfar F., Hajimiresmail S.J., Rahimi Forushani A. Beneficial Effects of Coenzyme Q10 Supplementation on Lipid Profile and Intereukin-6 and Intercellular Adhesion Molecule-1 Reduction, Preliminary Results of a Double-blind Trial in Acute Myocardial Infarction. Int. J. Prev. Med. 2015;6:73. doi: 10.4103/2008-7802.162461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mirhashemi S.M., Najafi V., Raygan F., Asemi Z. The effects of coenzyme Q10 supplementation on cardiometabolic markers in overweight type 2 diabetic patients with stable myocardial infarction: A randomized, double-blind, placebo-controlled trial. ARYA Atheroscler. 2016;12:158–165. [PMC free article] [PubMed] [Google Scholar]
- 78.Senior R., Basu S., Kinsey C., Schaeffer S., Lahiri A. Carvedilol prevents remodeling in patients with left ventricular dysfunction after acute myocardial infarction. Am. Heart J. 1999;137:646–652. doi: 10.1016/S0002-8703(99)70217-6. [DOI] [PubMed] [Google Scholar]
- 79.Singh R.B., Fedacko J., Mojto V., Pella D. Coenzyme Q10 Modulates Remodeling Possibly by Decreasing Angiotensin-Converting Enzyme in Patients with Acute Coronary Syndrome. Antioxidants. 2018;7:99. doi: 10.3390/antiox7080099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Langlois M., Duprez D., Delanghe J., De Buyzere M., Clement D.L. Serum vitamin C concentration is low in peripheral arterial disease and is associated with inflammation and severity of atherosclerosis. Circulation. 2001;103:1863–1868. doi: 10.1161/01.CIR.103.14.1863. [DOI] [PubMed] [Google Scholar]
- 81.Hirsch A.T., Haskal Z.J., Hertzer N.R., Bakal C.W., Creager M.A., Halperin J.L., Hiratzka L.F., Murphy W.R., Olin J.W., Puschett J.B., et al. ACC/AHA 2005 Practice Guidelines for the management of patients with peripheral arterial disease (lower extremity, renal, mesenteric, and abdominal aortic): A collaborative report from the American Association for Vascular Surgery/Society for Vascular Surgery, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, Society of Interventional Radiology, and the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Develop Guidelines for the Management of Patients With Peripheral Arterial Disease): Endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation; National Heart, Lung, and Blood Institute; Society for Vascular Nursing; TransAtlantic Inter-Society Consensus; and Vascular Disease Foundation. Circulation. 2006;113:e463–e654. doi: 10.1161/CIRCULATIONAHA.106.174526. [DOI] [PubMed] [Google Scholar]
- 82.Pipinos I.I., Judge A.R., Zhu Z., Selsby J.T., Swanson S.A., Johanning J.M., Baxter B.T., Lynch T.G., Dodd S.L. Mitochondrial defects and oxidative damage in patients with peripheral arterial disease. Free Radic. Biol. Med. 2006;41:262–269. doi: 10.1016/j.freeradbiomed.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 83.Park S.Y., Pekas E.J., Headid R.J., 3rd, Son W.M., Wooden T.K., Song J., Layec G., Yadav S.K., Mishra P.K., Pipinos I.I. Acute mitochondrial antioxidant intake improves endothelial function, antioxidant enzyme activity, and exercise tolerance in patients with peripheral artery disease. Am. J. Physiol. Heart Circ. Physiol. 2020;319:H456–H467. doi: 10.1152/ajpheart.00235.2020. [DOI] [PubMed] [Google Scholar]
- 84.Fenton R., Brook-Barclay L., Delaney C.L., Spark J.I., Miller M.D. Do Medications Commonly Prescribed to Patients with Peripheral Arterial Disease Have an Effect on Nutritional Status? A Review of the Literature. Ann. Vasc. Surg. 2016;32:145–175. doi: 10.1016/j.avsg.2015.10.036. [DOI] [PubMed] [Google Scholar]
- 85.Rodrigo R., Fernandez-Gajardo R., Gutierrez R., Matamala J.M., Carrasco R., Miranda-Merchak A., Feuerhake W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets. 2013;12:698–714. doi: 10.2174/1871527311312050015. [DOI] [PubMed] [Google Scholar]
- 86.Lalkovicova M., Danielisova V. Neuroprotection and antioxidants. Neural Regen. Res. 2016;11:865–874. doi: 10.4103/1673-5374.184447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Simani L., Ryan F., Hashemifard S., Hooshmandi E., Madahi M., Sahraei Z., Rezaei O., Heydari K., Ramezani M. Serum Coenzyme Q10 Is Associated with Clinical Neurological Outcomes in Acute Stroke Patients. J. Mol. Neurosci. 2018;66:53–58. doi: 10.1007/s12031-018-1115-1. [DOI] [PubMed] [Google Scholar]
- 88.Olga Nikolaevna O., Evgeniya Aronovna G., Elena Igorevna K., Margarita Alekseevna B., Mikhail Vladimirovich G., Valery Gennadievich M., Yury Andreevich P., Oleg Stephanovich M. Intravenous Administration of Coenzyme Q10 in Acute Period of Cerebral Ischemia Decreases Mortality by Reducing Brain Necrosis and Limiting Its Increase within 4 Days in Rat Stroke Model. Antioxidants. 2020;9:1240. doi: 10.3390/antiox9121240. [DOI] [PMC free article] [PubMed] [Google Scholar]