Abstract
Creatine supplementation in conjunction with resistance training (RT) augments gains in lean tissue mass and strength in aging adults; however, there is a large amount of heterogeneity between individual studies that may be related to creatine ingestion strategies. Therefore, the purpose of this review was to (1) perform updated meta-analyses comparing creatine vs. placebo (independent of dosage and frequency of ingestion) during a resistance training program on measures of lean tissue mass and strength, (2) perform meta-analyses examining the effects of different creatine dosing strategies (lower: ≤5 g/day and higher: >5 g/day), with and without a creatine-loading phase (≥20 g/day for 5–7 days), and (3) perform meta-analyses determining whether creatine supplementation only on resistance training days influences measures of lean tissue mass and strength. Overall, creatine (independent of dosing strategy) augments lean tissue mass and strength increase from RT vs. placebo. Subanalyses showed that creatine-loading followed by lower-dose creatine (≤5 g/day) increased chest press strength vs. placebo. Higher-dose creatine (>5 g/day), with and without a creatine-loading phase, produced significant gains in leg press strength vs. placebo. However, when studies involving a creatine-loading phase were excluded from the analyses, creatine had no greater effect on chest press or leg press strength vs. placebo. Finally, creatine supplementation only on resistance training days significantly increased measures of lean tissue mass and strength vs. placebo.
Keywords: supplements, hypertrophy, sarcopenia
1. Introduction
The age-related decrease in lean tissue mass and strength are two main factors that contribute to the development of sarcopenia [1]. Approximately 10% of the adult population ≥60 years of age has sarcopenia [2], which has a profound negative effect on functional independence and overall quality of life [3]. Furthermore, sarcopenia is associated with other age-related diseases and health conditions such as osteoporosis and physical frailty [3,4]. Several lines of research suggest that sarcopenia is caused by age-related changes in muscle protein kinetics, neuromuscular function and physiology, skeletal muscle morphology, inflammation, and mitochondrial dysregulation [1,5,6]. In addition to these cellular and mechanistic changes, insufficient physical activity and nutritional intake also contribute to sarcopenia [3,7]. Interestingly, dietary intake of creatine, a key component for muscular bioenergetics, decreases with age [8].
The combination of creatine supplementation and resistance training has the potential to serve as an effective countermeasure to the age-related loss in lean tissue mass and strength, possibly by influencing anaerobic energy metabolism, calcium and glycogen regulation, muscle protein kinetics, inflammation and oxidative stress [3,9,10]. However, results from individual studies (n = 20) are mixed, with 10 studies showing beneficial effects on measures of lean tissue mass and/or strength (leg press, chest press) while 10 studies found no greater benefit from creatine vs. placebo (Table 1). While numerous methodological variables may explain these inconsistent findings, differences in creatine dosage and frequency of ingestion during the resistance training program is likely involved [10]. For example, of the 20 studies performed, 6 studies used a lower-dose creatine strategy (≤5 g/day for 12–26 weeks), 6 studies used a creatine-loading phase (≥20 g/day for 5–7 days) followed by a lower-dose creatine strategy (≤5 g/day) while 2 studies used a creatine-loading phase (≥20 g/day for 5–7 days), followed by a higher-dose creatine strategy (>5 g/day for 11 weeks). Furthermore, 6 studies used a higher-dose creatine strategy (>5 g/day) for 8–52 weeks. Finally, 4 of the 20 studies had participants ingest creatine only on resistance training days. The average sample size across studies was only 34 participants. Therefore, these studies were likely unpowered to detect small differences in lean tissue mass and strength (leg press, chest press). To overcome low statistical power across studies, meta-analyses are often performed.
Table 1.
Study characteristics, dosing strategy, and outcomes of research examining the influence of creatine in older adults with a resistance training program.
| First Author, Year | Population | Supplement Protocol | Resistance Training | Duration | Outcomes | |
|---|---|---|---|---|---|---|
| Loading Protocol | Maintenance Dose | |||||
| Lower-Dose/Absolute Studies (≤5 g/day) | ||||||
| Alves et al. [11] | N = 47; healthy women, Mean age = 66.8 years (range: 60–80 years) | CR 20 g/day for 5 days | CR (5 g/day) or PLA | RT = 2 days/wk | 24 wks | ↔ 1RM strength compared to RT + PLA |
| Aguiar et al. [12] | N = 18; healthy women; Mean age = 65 years | None | CR (5 g/day) or PLA | RT = 3 days/wk | 12 wks | CR ↑ gains in fat-free mass (+3.2%), muscle mass (+2.8%), 1RM bench press, knee extension, and biceps curl compared to PLA |
| Bemben et al. and Eliot et al. [13,14] | N = 42; healthy men; age = 48–72 years | None | CR (5 g/day) | RT = 3 days/wk | 14 wks | ↔ lean tissue mass, 1RM strength |
| Bermon et al. [15] | N = 32 (16 men, 16 women); healthy; age = 67–80 years | CR 20 g/day for 5 days | CR (3 g/day) or PLA | RT = 3 days/wk | 7.4 wks (52 days) | ↔ lower limb muscular volume, 1-, 12-repetitions maxima, and the isometric intermittent endurance |
| Brose et al. [16] | N = 28 (15 men, 13 women); healthy; age: men = 68.7, women = 70.8 years | None | CR (5 g/day) or PLA | RT = 3 days/wk | 14 wks | CR ↑ gains in lean tissue mass and isometric knee extension strength; ↔ type 1, 2a, 2x muscle fiber area |
| Deacon et al. [17] | N = 80 (50 men, 30 women); COPD; age = 68.2 years | CR 22 g/day for 5 days | CR (3.76 g/day) or PLA | RT = 3 days/wk | 7 wks | ↔ lean tissue mass or muscle strength |
| Eijnde et al. [18] | N = 46; healthy men; age = 55–75 years | None | CR (5 g/day) or PLA | Cardiorespiratory + RT = 2–3 days/wk | 26 wks | ↔ lean tissue mass or isometric maximal strength |
| Gualano et al. [19] | N = 25 (9 men, 16 women); type 2 diabetes; age = 57 years | None | CR (5 g/day) or PLA | RT = 3 days/wk | 12 wks | ↔ lean tissue mass |
| Gualano et al. [20] | N = 30; “vulnerable” women; Mean age = 65.4 years | CR 20 g/day for 5 days | CR (5 g/day) or PLA | RT = 2 days/wk | 24 wks | CR + RT ↑ gains in 1RM bench press and appendicular lean mass compared to PLA + RT |
| Hass et al. [21] | N = 20 (17 men, 3 women with idiopathetic Parkinson’s disease); Mean age = 62 years | CR 20 g/day for 5 days | CR (5 g/day) or PLA | RT = 2 days/wk | 12 wks | CR ↑ chest press strength, chair rise performance; ↔ Leg extension 1RM, muscular endurance |
| Neves et al. [22] | N = 24 (postmenopausal women with knee osteoarthritis); Age = 55–65 years | CR 20 g/day for 1 week | CR 5 (g/day) or PLA | RT=3 days/wk | 12 wks | CR ↑ gains in limb lean mass. ↔ 1RM leg press |
| Pinto et al. [23] | N = 27 (men and women); healthy; age = 60–80 years | None | CR (5 g/day) or PLA | RT = 3 days/wk | 12 wks | CR ↑ gains in lean tissue mass. ↔ 10 RM bench press or leg press strength |
| Higher-Dose/Relative Studies (>5 g/day) | ||||||
| Bernat et al. [24] | N = 24 healthy men; age = 59 ± 6 years | None | CR (0.1 g/kg/day; ~9.5 g/day) or PLA | High-velocity RT = 2 days/wk | 8 wks | ↔ muscle thickness, physical performance, upper body muscle strength. CR ↑ leg press strength, total lower body strength |
| Candow et al. [25] | N = 35; healthy men; age = 59–77 years | None | CR (0.1 g/kg/day; ~8.6 g/day) or PLA | RT = 3 days/wk | 10 wks | CR ↑ muscle thickness compared to PLA. CR ↑ 1RM bench press ↔ 1RM leg press |
| Candow et al. [26] | N = 39 (17 men, 22 women); healthy; age = 50–71 years | None | CR (0.1 g/kg; ~7.7 g/day) before RT, CR (0.1 g/kg; ~8.8 g/day) after RT, or PLA | RT = 3 days/wk | 32 wks | CR after RT ↑ lean tissue mass, 1RM leg press, 1RM chest press compared to PLA |
| Candow et al. [27] | N = 38; healthy men; age = 49–67 years | None | CR (On training days: 0.05 g/kg before and 0.05 g/kg after exercise; total ~9.3 g/day) + 0.1 g/kg/day on non-training days (2 equal doses) | RT = 3 days/wk | 12 months | ↔ lean tissue mass, muscle thickness, or muscle strength |
| Chilibeck et al. [28] | N = 33; healthy women; Mean age = 57 years | None | CR (0.1 g/kg/day; ~6.9 g/day) or PLA | RT = 3 days/wk | 52 wks | ↔ lean tissue mass and muscle thickness gains between groups. ↑ relative bench press strength compared to PLA. |
| Chrusch et al. [29] | N = 30; healthy men; age = 60–84 years | CR 0.3 g/kg/d for 5 days | CR 0.07 g/kg/day; ~6.2 g/day or PLA | RT = 3 days/wk | 12 wks | CR ↑ gains in lean tissue mass. CR ↑ 1RM leg press, 1RM knee extension, leg press endurance, and knee extension endurance. ↔ 1RM bench press or bench press endurance. |
| Cooke et al. [30] | N = 20; healthy men; age = 55–70 years | CR 20 g/day for 7 days | CR 0.1 g/kg/day or ~8.8 g/day on training days | RT = 3 days/wk | 12 wks | ↔ lean tissue mass, 1RM bench press, 1RM leg press |
| Johannsmeyer et al. [31] | N = 31 (17 men, 14 women); healthy; age = 58 years | None | CR 0.1 g/kg/day; ~7.8 g/day or PLA | RT = 3 days/wk | 12 wks | CR ↑ gains in lean tissue mass and 1RM strength in men only |
To date, three meta-analyses have been performed involving creatine supplementation and resistance training in older adults [9,32,33]. Collectively, results showed that creatine and resistance training increased measures of lean tissue mass by ~1.2 kg and strength (leg press, chest press) more than placebo and resistance training. However, no sub-analyses were performed to determine whether the dosage of creatine used or the frequency of ingestion (i.e., only on resistance training days) influenced measures of lean tissue mass and/or strength. Since the publication of these meta-analyses, two additional studies involving creatine supplementation and resistance training in older adults have been published. Therefore, the purpose of this review was to (1) perform updated meta-analyses comparing creatine vs. placebo (independent of dosage and frequency of ingestion) during a resistance training program on measures of lean tissue mass and strength, (2) perform meta-analyses examining the effects of different creatine dosing strategies (lower: ≤5 g/day vs. higher: >5 g/day), with and without a creatine-loading phase (20 g/day for 5–7 days, and (3) perform meta-analyses determining whether creatine supplementation only on resistance training days influences measures of lean tissue mass and strength. Results from these meta-analyses may provide important information for the design of optimal creatine supplementation strategies for older adults.
2. Materials and Methods
We have previously published two meta-analyses in 2014 [9] and 2017 [32]. Based on our expertise in the literature, we updated these meta-analyses with recently published studies since the date of the 2017 publication [32]. PubMed and SPORTDiscus databases were searched. Similar to our previous meta-analysis [32] key terms and similar phrases were used (creatine OR creatine monohydrate OR creatine supplementation OR creatine-loading) AND (weight lifting OR weight training OR resistance training, OR resistance exercise OR strength training) AND (age OR middle-age OR older adults OR elderly). Studies with the following criteria were included: (1) healthy and chronic disease participants with a mean age >50 years of age; (2) must be a randomized control trial (RCT) where participants were randomized to an intervention group consisting of creatine monohydrate with resistance training or placebo with resistance training; (3) included outcome measures of whole-body lean tissue mass (determined with dual-energy X-ray absorptiometry [DEXA], hydrostatic weighing, air displacement plethysmography, bioelectrical impedance, or multi-site ultrasound), or upper-(chest press) or lower-body (leg press) muscular strength. Studies were excluded if they were <5 weeks in duration.
Two researchers (S.C.F. and D.G.C.) determined whether the relevant articles were to be included, and any disagreements were resolved by consensus. Databases were searched up until February 2021. Means and standard deviations for baseline and post-training measurements were extracted from each study for estimation of mean changes and the standard deviation of mean changes across the interventions. Change scores were calculated as the pre-training mean subtracted from the post-training mean. Standard deviations (SD) for the change scores were estimated from pre and post-training standard deviations (SD-pre and SD-post) using the following equation derived from the Cochrane Handbook for Systematic Reviews of Interventions:
| SD change score = [(SD pre)2 + (SD post)2 − 2 * (correlation between pre and post scores) * SD pre * SD post]1/2 |
We used 0.8 as the assumed correlation between pre- and post-scores. Heterogeneity was evaluated using χ2 and I2 tests where heterogeneity was indicated by either χ2 p-value ≤ 0.1 or I2 test value > 75%. We used a fixed-effects model for our meta-analysis. Weighted mean differences were calculated for lean tissue mass, along with the 95% CI. As units of measurement differed across studies for measurements of strength, calculated standardized mean differences (SMDs) and 95% CIs for leg press and chest press strength were used. Forest plots were generated using Review Manager 5.3 Software (Cochrane Community, London, UK). Significance was established at p ≤ 0.05. Funnel plots were generated and inspected for publication bias. Adverse events were also extracted.
Sub-Analyses
To examine the influence of creatine dosage, dosing strategy was extracted and classified as either higher (>5 g/day) or lower (≤5 g/day), as well as whether the study included a “loading phase” (≥20 g/day for 5–7 days) and whether creatine was only consumed on resistance training days. Only two studies [15,17] used a creatine dosage <5 g/day. Both absolute and relative (based on body mass) dosing strategy studies were included. We estimated an absolute dose of creatine ingested per day from the product of the average body mass and the relative dose. Several sub-analyses were performed to examine the effects of creatine within each classification. Furthermore, sensitivity analysis was conducted to explore whether the overall effects depended on a single specific study.
3. Results
3.1. Lean Tissue Mass
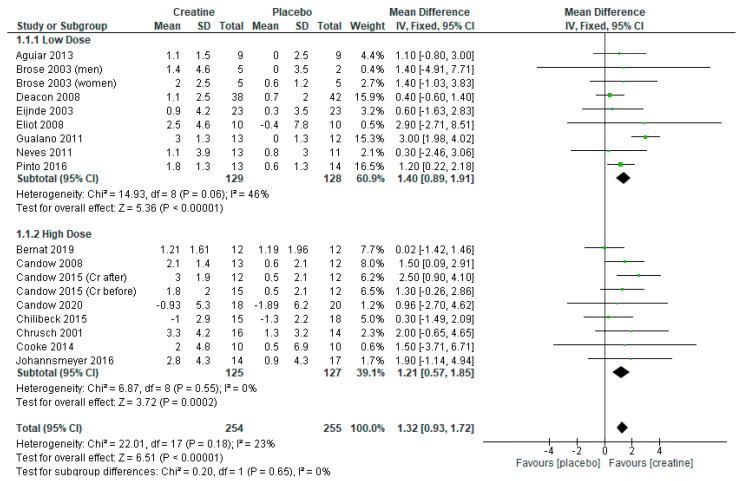
The analysis of 16 RCTs with 18 treatment arms (n = 509) revealed that creatine supplementation and resistance training increased measures of lean tissue mass vs. placebo and resistance training (Figure 1: mean difference = 1.32 kg [95% CI: 0.93, 1.72] p < 0.000001).
Figure 1.
Forest plot of studies on lean tissue mass with sub-analyses using lower-dose creatine studies (≤5 g/day) and of higher-dose creatine studies (>5 g/day) on lean tissue mass.
Sub-analyses showed that higher-dose creatine, with and without a creatine-loading phase, produced significant gains in lean tissue mass vs. placebo (Figure 1: mean difference = 1.21 kg [95% CI: 0.57, 1.85] p = 0.0002). Even when studies incorporating a creatine-loading phase were excluded, higher-dose creatine remained effective (Figure S1: mean difference = 1.16 kg [95% CI: 0.49, 1.82] p = 0.0006).
Lower-dose creatine, with and without a creatine-loading phase, increased lean tissue mass vs. placebo (Figure 1: mean difference = 1.40 kg [95% CI: 0.89, 1.91] p < 0.00001). When studies incorporating a creatine-loading phase were excluded, lower-dose creatine was still more beneficial than placebo (Figure S2: mean difference = 1.81 kg [95% CI: 1.20, 2.42] p < 0.00001).
3.2. Chest Press Strength
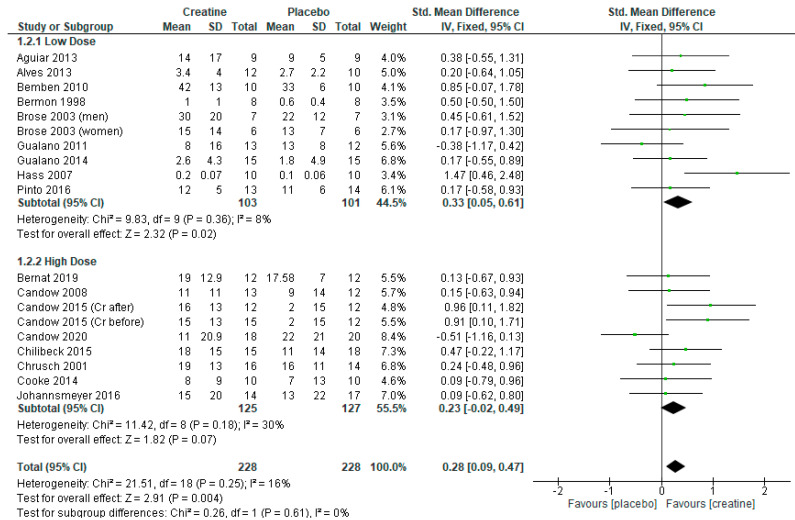
The analysis of 17 RCTs with 19 treatment arms (n = 456) revealed that creatine supplementation and resistance training significantly increased chest press strength vs. placebo and resistance training (Figure 2: standard mean difference = 0.28 [95% CI: 0.09, 0.47] p = 0.004).
Figure 2.
Forest plot of studies on chest press strength.
Subanalyses showed that studies using higher-dose creatine, with and without a creatine-loading phase, found similar effects compared to the placebo (Figure 2 and Figure S3; p > 0.05). However, sensitivity analysis indicated that omitting the Candow et al. [27] study changed the overall effect to significantly favor creatine (Figures S4 and S5; p = 0.008).
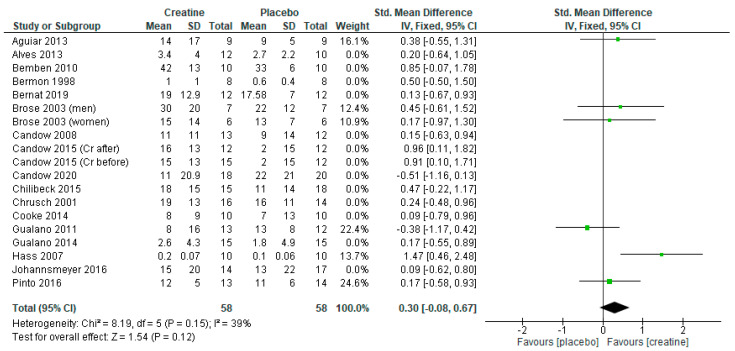
Studies using a creatine-loading phase followed by lower-dose creatine revealed a significant benefit in favor of creatine (Figure 2: standard mean difference = 0.33 [95% CI: 0.05, 0.61] p = 0.02). However, when studies incorporating a creatine-loading phase were excluded from the analysis, lower-dose creatine was similar to the placebo (Figure 3; p = 0.12).
Figure 3.
Forest plot of lower-dose creatine studies (≤5 g/day) on chest press strength with exclusion of creatine loading studies.
3.3. Leg Press Strength
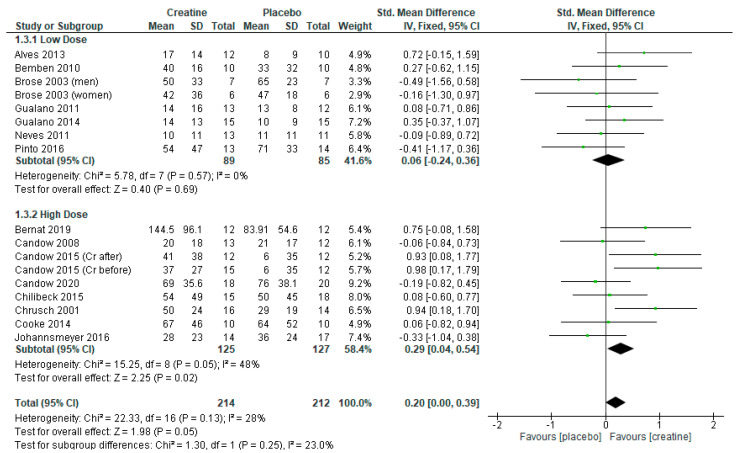
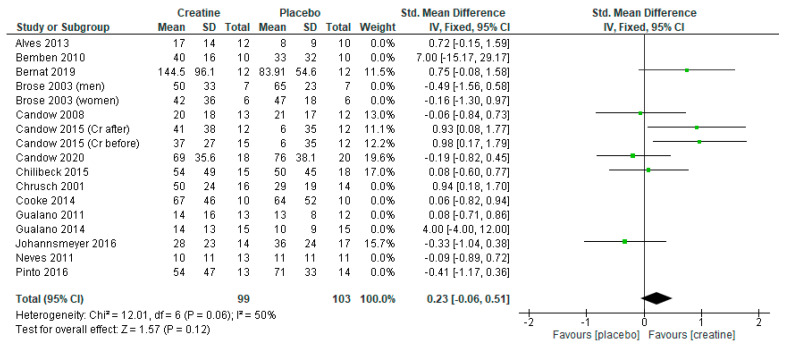
The analysis of 15 RCTs with 17 treatment arms (n = 426) revealed that creatine supplementation and resistance training significantly increased leg press strength vs. placebo and resistance training (Figure 4: standard mean difference = 0.20 [95% CI: 0.00, 0.39] p = 0.05).
Figure 4.
Forest plot of studies on leg press strength.
Sub-analyses showed that higher-dose creatine, with and without a creatine-loading phase, produced greater gains in leg press strength vs. placebo (Figure 4: mean difference = 0.29 [95% CI: 0.04, 0.54] p = 0.02). However, when studies incorporating a creatine-loading phase were excluded, higher-dose creatine was similar to the placebo (Figure 5: p = 0.12).
Figure 5.
Forest plot of higher-dose creatine studies (>5 g/day) on leg press strength with exclusion of creatine-loading studies.
Studies using lower-dose creatine, with and without a creatine-loading phase, had no greater effect on leg press strength vs. placebo (Figure 4; p = 0.69 and Figure S6; p = 0.88).
3.4. Creatine Only on Training Days
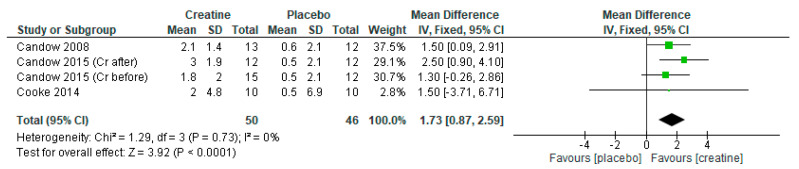
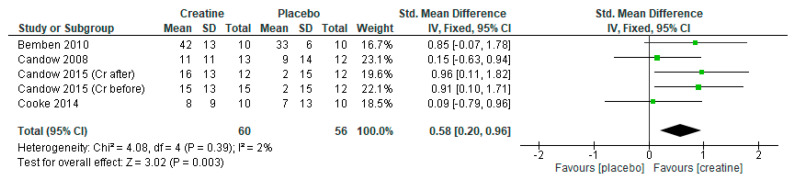
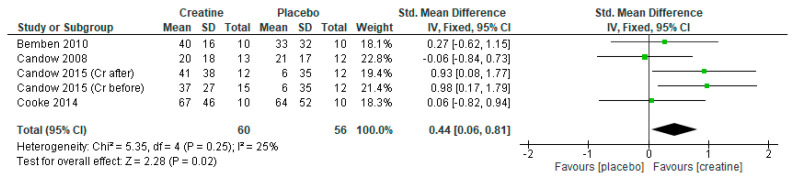
When only including studies that provided creatine on resistance training days, there were significant overall effects for favoring creatine on measures of lean tissue mass (Figure 6: mean difference = 1.73 kg [95% CI: 0.87, 2.89] p < 0.0001), chest press strength (Figure 7: standard mean difference = 0.58 [95% CI: 0.20, 0.96] p = 0.003), and leg press strength (Figure 8: standard mean difference = 0.44 [95% CI: 0.06, 0.81] p = 0.02). Of note, Cooke et al. [30] incorporated a creatine-loading phase followed by lower-dose creatine (≤5 g/day) whereas the studies by Candow et al. [25,26] used higher-dose creatine (>5 g/day).
Figure 6.
Forest plot of studies on lean tissue mass.
Figure 7.
Forest plot of studies on chest press strength.
Figure 8.
Forest plot of studies on leg press strength.
3.5. Publication Bias
Funnel plots for each meta-analysis were visually inspected and showed no evidence of publication bias.
3.6. Adverse Events
In the lower-dose studies (≤5 g/day), 10 studies reported no adverse events. One study reported a single mild bout of gastro-intestinal distress from creatine [16] and one study reported an overuse shoulder injury following creatine supplementation [18]. Neither of these studies used a loading phase.
In the higher-dose studies (>5 g/day), five studies reported no adverse events. Two studies similarly reported five incidences of gastrointestinal distress from creatine and two incidences from placebo and two incidences of muscle cramps from both the creatine and placebo group [27,28]. One of the two studies utilizing a loading phase reported an increase in GI distress during the loading phase [29].
4. Discussion
The most important results from these meta-analyses were: (1) creatine supplementation (independent of creatine-loading, maintenance dosage and frequency of ingestion) during a resistance training program increased measures of lean tissue mass and strength compared to the placebo and resistance training in older adults, (2) the combination of creatine-loading followed by lower-dose creatine (≤5 g/day) was effective for increasing chest press strength, (3) the combination of creatine-loading and higher-dose creatine (>5 g/day) was effective for increasing leg press strength, (4) creatine supplementation only on resistance training days significantly increased measures of lean tissue mass and strength compared to the placebo. These results have application for the design of effective creatine supplementation strategies for older adults. For example, older adults wanting to improve whole-body lean tissue mass and strength may expect these benefits from creatine supplementation (i.e., ≥5 g) either daily or only on training days during a resistance training program.
Increasing whole-body lean tissue mass and strength is fundamental for mitigating sarcopenia and associated conditions of osteoporosis and physical frailty (3). Older adults specifically looking to improve upper-body strength (perhaps to improve functionality, posture and/or the ability to perform upper-body activities of daily living such as carrying groceries) may need to load with creatine before proceeding to a lower daily dosage (≤5 g) during their resistance training program. To specifically increase lower-body strength (perhaps to improve balance, reduce the risk of falls and/or the ability to perform lower-body activities of daily living such as climbing stairs), older adults may need to load with creatine before proceeding to a higher daily dosage (>5 g) during their resistance training program. While some have hypothesized creatine may have harmful effects [34], a plethora of evidence shows no adverse events (compared to the placebo) with long-term supplementation [35,36,37].
Previous meta-analyses have shown greater gains in measures of lean tissue mass (~1.2–1.3 kg) and strength from creatine supplementation and resistance training in older adults compared to the placebo [9,32,33]. Since the date of these publications, two additional studies [24,27] have been performed. When these studies were included in the current meta-analyses, creatine supplementation and resistance training still increased measures of lean tissue mass (~1.32 kg) and strength compared to the placebo. Collectively, results across meta-analyses suggest that the combination of creatine supplementation and resistance training has the potential to mitigate sarcopenia. Although none of the studies included in any of the meta-analyses were powdered to directly examine the effects of creatine vs. placebo in older adults diagnosed with sarcopenia, sub-analyses from three studies showed that the combination of creatine and resistance training eliminated the classification of sarcopenia in 11 older adults [20,23,26]. Creatine supplementation may augment lean tissue mass and strength through various mechanisms [3,4,10,32,37]. First, supplementation increases intramuscular PCr resulting in greater resynthesis of ATP during and following muscle contractions. Supplementation also increases muscle GLUT-4 content and translocation to the sarcolemma which may increase glucose uptake and subsequent glycogen resynthesis [38,39]. Creatine supplementation facilitates calcium re-uptake via creatine kinase into the sarcoplasmic reticulum, and this may increase myofibrillar cross-bride cycling, cell swelling, the expression of myogenic transcription factors (i.e., Mrf4, myogenin), satellite cell proliferation, and the expression of growth factors (i.e., insulin-like growth factor-1) [40,41]. Creatine supplementation enhances the activation of protein kinases downstream in the mammalian target of rapamycin (mTOR) pathway, and this may subsequently reduce measures of muscle protein catabolism (i.e., leucine oxidation, urinary 3-methylhistidine) [25,31]. Finally, creatine supplementation could reduce inflammation (i.e., cytokines) [42,43] and oxidative stress [44,45,46], and again, this may help reduce the loss of lean tissue mass with aging [4].
Incorporating a creatine-loading phase during the initial stages of a resistance training program was determined to be important for improving upper- and lower-body strength. It is well established that creatine-loading results in significant elevations in intramuscular creatine levels [47]. However, the magnitude of the effect on strength outcome measures may also depend on the maintenance dosage of creatine used for the remainder of the training program.
Regarding upper-body strength, older adults who loaded with creatine and then proceeded to ingest lower-dose creatine daily experienced greater upper body strength gains compared to those on placebo. However, independent of a creatine-loading phase, lower-dose creatine supplementation was no more effective than placebo. When all studies were included in the analysis, higher-dose creatine supplementation daily, with and without a creatine-loading phase, had no greater effect on upper-body strength compared to the placebo. However, sensitivity analysis showed that when the Candow et al. [27] study was removed, results became significant in favor of creatine. In this study, older males supplemented with higher-dose creatine daily during supervised, whole-body resistance training for 52 weeks. Results showed that changes in upper-body strength were similar between creatine and placebo over time. Both creatine and placebo groups experienced large increases in strength over time (creatine: ~69 kg; placebo: ~76 kg) which likely masked any effect from creatine supplementation.
Regarding lower-body strength, creatine-loading followed by higher-dose creatine daily had a favorable effect on strength whereas creatine-loading followed by lower-dose creatine daily had no greater effect compared to the placebo. The magnitude of responsiveness to creatine supplementation in older adults may depend on initial intramuscular creatine levels [10,48]. There is some evidence to suggest that phosphocreatine stores decrease with aging [10], especially in muscles of the lower limbs, possibly due to type-II muscle fiber atrophy, reduced participation in high-intensity activities and reduced meat consumption [32]. Furthermore, lower-body muscle groups are more negatively affected (i.e., greater strength deficit) by the aging process than upper-body muscle groups [49]. Therefore, to overcome possible age-related changes in muscle creatine content and lower-body muscle morphology, higher creatine dosages (as opposed to lower-creatine dosages) may be needed on a daily basis after a creatine-loading phase to improve lower-body strength in older adults.
Most importantly, all the studies identified as using a high dose (i.e., >5 g/day) were based on a relative dosing strategy (based on body mass; g/kg/day), while all the low dose studies used an absolute dosing strategy (g/day). As such, future research is required to directly compare an absolute and relative strategy to determine which method is superior.
Older adults who ingested creatine only on resistance training days experienced greater gains in measures of lean tissue mass and strength compared to the placebo. One study implemented a creatine-loading phase prior to lower-dose creatine daily [30] whereas the other studies implemented a higher-dose daily strategy [25,26]. A common theme across all studies was that creatine was consumed within 60 min’ post-exercise. While the mechanistic actions of creatine were not determined in these studies, previous research has shown that prior muscle contractions (i.e., resistance training sessions) stimulate greater creatine uptake into muscle [50] possibly through increased activation of creatine transport kinetics [51,52]. These results may be important, as compliance to a creatine supplementation program may be higher when creatine is only consumed on training days. However, it is unknown whether older adults experience the same muscle benefits when consuming creatine supplementation daily vs. only on training days during a resistance training program. In addition, a provision of creatine from a regular diet should be accounted for a total exposure to creatine in this population since creatine consumption varies in the elderly [53].
Although the focus of this review was on combining creatine with resistance exercise, there appears to be some benefits of creatine without concomitant exercise in older adults [54,55]. Future research may be warranted to examine the dose of creatine to enhance muscle performance without exercise.
5. Conclusions
Increasing whole-body lean tissue mass and strength is fundamental for mitigating sarcopenia and associated conditions of osteoporosis and physical frailty [3]. Similar to previous meta-analyses [9,32], our results showed that creatine supplementation and resistance training increases measures of lean tissue mass and strength in older adults vs. placebo. However, unique and important results from our sub-analyses indicate that a creatine-loading phase is important for older adults wanting to improve muscle strength. In addition to a creatine-loading phase, a lower daily dosage of creatine (≤5 g) appears sufficient to improve upper-body strength. However, a higher daily dosage of creatine (>5 g) after the loading phase is needed to increase lower-body strength. Regarding the effects of creatine ingestion frequency, creatine supplementation only on resistance training days significantly increased measures of lean tissue mass and strength compared to placebo.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nu13061912/s1, Figures S1–S8.
Author Contributions
Conceptualization, S.C.F. and D.G.C.; methodology, S.C.F. and D.G.C.; formal analysis, D.G.C. and S.C.F.; writing—original draft preparation, S.C.F. and D.G.C.; writing—review and editing, S.C.F., D.G.C., S.M.O., M.D.R., P.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
S.C.F. has previously served as a scientific advisor for a company that sold creatine. D.G.C. has conducted industry-sponsored research involving creatine supplementation, received creatine donations for scientific studies and travel support for presentations involving creatine supplementation at scientific conferences. In addition, D.G.C. serves on the Scientific Advisory Board for AlzChem (a company which manufactures creatine) and has previously served as the Chief Scientific Officer for a company that sells creatine products. S.M.O. serves as a member of the Scientific Advisory Board on creatine in health and medicine (AlzChem LLC). S.M.O. owns patent “Sports Supplements Based on Liquid Creatine” at European Patent Office (WO2019150323 A1), and active patent application “Synergistic Creatine” at UK Intellectual Property Office (GB2012773.4). S.M.O. has served as a speaker at Abbott Nutrition, a consultant of Allied Beverages Adriatic and IMLEK, and an advisory board member for the University of Novi Sad School of Medicine, and has received research funding related to creatine from the Serbian Ministry of Education, Science, and Technological Development, Provincial Secretariat for Higher Education and Scientific Research, AlzChem GmbH, KW Pfannenschmidt GmbH, ThermoLife International LLC, and Monster Energy Company. S.M.O. is an employee of the University of Novi Sad and does not own stocks and shares in any organization. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyere O., Cederholm T., Cooper C., Landi F., Rolland Y., Sayer A.A., et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shafiee G., Keshtkar A., Soltani A., Ahadi Z., Larijani B., Heshmat R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017;16:21. doi: 10.1186/s40200-017-0302-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Candow D.G., Forbes S.C., Kirk B., Duque G. Current Evidence and Possible Future Applications of Creatine Supplementation for Older Adults. Nutrients. 2021;13:745. doi: 10.3390/nu13030745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Candow D.G., Forbes S.C., Chilibeck P.D., Cornish S.M., Antonio J., Kreider R.B. Effectiveness of Creatine Supplementation on Aging Muscle and Bone: Focus on Falls Prevention and Inflammation. J. Clin. Med. 2019;8:488. doi: 10.3390/jcm8040488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mitchell W.K., Williams J., Atherton P., Larvin M., Lund J., Narici M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol. 2012;3:260. doi: 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tournadre A., Vial G., Capel F., Soubrier M., Boirie Y. Sarcopenia. Jt. Bone Spine. 2019;86:309–314. doi: 10.1016/j.jbspin.2018.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Kirk B., Prokopidis K., Duque G. Nutrients to mitigate osteosarcopenia: The role of protein, vitamin D and calcium. Curr. Opin. Clin. Nutr. Metab. Care. 2021;24:25–32. doi: 10.1097/MCO.0000000000000711. [DOI] [PubMed] [Google Scholar]
- 8.Brosnan J.T., Brosnan M.E. Creatine: Endogenous metabolite, dietary, and therapeutic supplement. Annu. Rev. Nutr. 2007;27:241–261. doi: 10.1146/annurev.nutr.27.061406.093621. [DOI] [PubMed] [Google Scholar]
- 9.Candow D.G., Chilibeck P.D., Forbes S.C. Creatine supplementation and aging musculoskeletal health. Endocrine. 2014;45:354–361. doi: 10.1007/s12020-013-0070-4. [DOI] [PubMed] [Google Scholar]
- 10.Candow D.G., Forbes S.C., Chilibeck P.D., Cornish S.M., Antonio J., Kreider R.B. Variables Influencing the Effectiveness of Creatine Supplementation as a Therapeutic Intervention for Sarcopenia. Front. Nutr. 2019;6:124. doi: 10.3389/fnut.2019.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alves C.R., Merege Filho C.A., Benatti F.B., Brucki S., Pereira R.M., de Sa Pinto A.L., Lima F.R., Roschel H., Gualano B. Creatine supplementation associated or not with strength training upon emotional and cognitive measures in older women: A randomized double-blind study. PLoS ONE. 2013;8:e76301. doi: 10.1371/journal.pone.0076301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aguiar A.F., Januario R.S., Junior R.P., Gerage A.M., Pina F.L., do Nascimento M.A., Padovani C.R., Cyrino E.S. Long-term creatine supplementation improves muscular performance during resistance training in older women. Eur. J. Appl. Physiol. 2013;113:987–996. doi: 10.1007/s00421-012-2514-6. [DOI] [PubMed] [Google Scholar]
- 13.Bemben M.G., Witten M.S., Carter J.M., Eliot K.A., Knehans A.W., Bemben D.A. The effects of supplementation with creatine and protein on muscle strength following a traditional resistance training program in middle-aged and older men. J. Nutr. Health Aging. 2010;14:155–159. doi: 10.1007/s12603-009-0124-8. [DOI] [PubMed] [Google Scholar]
- 14.Eliot K.A., Knehans A.W., Bemben D.A., Witten M.S., Carter J., Bemben M.G. The effects of creatine and whey protein supplementation on body composition in men aged 48 to 72 years during resistance training. J. Nutr. Health Aging. 2008;12:208–212. doi: 10.1007/BF02982622. [DOI] [PubMed] [Google Scholar]
- 15.Bermon S., Venembre P., Sachet C., Valour S., Dolisi C. Effects of creatine monohydrate ingestion in sedentary and weight-trained older adults. Acta Physiol. Scand. 1998;164:147–155. doi: 10.1046/j.1365-201X.1998.00427.x. [DOI] [PubMed] [Google Scholar]
- 16.Brose A., Parise G., Tarnopolsky M.A. Creatine supplementation enhances isometric strength and body composition improvements following strength exercise training in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2003;58:11–19. doi: 10.1093/gerona/58.1.B11. [DOI] [PubMed] [Google Scholar]
- 17.Deacon S.J., Vincent E.E., Greenhaff P.L., Fox J., Steiner M.C., Singh S.J., Morgan M.D. Randomized controlled trial of dietary creatine as an adjunct therapy to physical training in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2008;178:233–239. doi: 10.1164/rccm.200710-1508OC. [DOI] [PubMed] [Google Scholar]
- 18.Eijnde B.O., Van Leemputte M., Goris M., Labarque V., Taes Y., Verbessem P., Vanhees L., Ramaekers M., Vanden Eynde B., Van Schuylenbergh R., et al. Effects of creatine supplementation and exercise training on fitness in men 55–75 yr old. J. Appl. Physiol. 2003;95:818–828. doi: 10.1152/japplphysiol.00891.2002. [DOI] [PubMed] [Google Scholar]
- 19.Gualano B., DE Salles Painneli V., Roschel H., Artioli G.G., Neves M., De Sa Pinto A.L., Da Silva M.E., Cunha M.R., Otaduy M.C., Leite Cda C., et al. Creatine in type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Med. Sci. Sports Exerc. 2011;43:770–778. doi: 10.1249/MSS.0b013e3181fcee7d. [DOI] [PubMed] [Google Scholar]
- 20.Gualano B., Macedo A.R., Alves C.R., Roschel H., Benatti F.B., Takayama L., de Sa Pinto A.L., Lima F.R., Pereira R.M. Creatine supplementation and resistance training in vulnerable older women: A randomized double-blind placebo-controlled clinical trial. Exp. Gerontol. 2014;53:7–15. doi: 10.1016/j.exger.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Hass C.J., Collins M.A., Juncos J.L. Resistance training with creatine monohydrate improves upper-body strength in patients with Parkinson disease: A randomized trial. Neurorehabil. Neural Repair. 2007;21:107–115. doi: 10.1177/1545968306293449. [DOI] [PubMed] [Google Scholar]
- 22.Neves M., Gualano B., Roschel H., Fuller R., Benatti F.B., Pinto A.L., Lima F.R., Pereira R.M., Lancha A.H., Bonfa E. Beneficial effect of creatine supplementation in knee osteoarthritis. Med. Sci. Sports Exerc. 2011;43:1538–1543. doi: 10.1249/MSS.0b013e3182118592. [DOI] [PubMed] [Google Scholar]
- 23.Pinto C.L., Botelho P.B., Carneiro J.A., Mota J.F. Impact of creatine supplementation in combination with resistance training on lean mass in the elderly. J. Cachexia Sarcopenia Muscle. 2016;7:413–421. doi: 10.1002/jcsm.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernat P., Candow D.G., Gryzb K., Butchart S., Schoenfeld B.J., Bruno P. Effects of high-velocity resistance training and creatine supplementation in untrained healthy aging males. Appl. Physiol. Nutr. Metab. 2019;44:1246–1253. doi: 10.1139/apnm-2019-0066. [DOI] [PubMed] [Google Scholar]
- 25.Candow D.G., Little J.P., Chilibeck P.D., Abeysekara S., Zello G.A., Kazachkov M., Cornish S.M., Yu P.H. Low-dose creatine combined with protein during resistance training in older men. Med. Sci. Sports Exerc. 2008;40:1645–1652. doi: 10.1249/MSS.0b013e318176b310. [DOI] [PubMed] [Google Scholar]
- 26.Candow D.G., Vogt E., Johannsmeyer S., Forbes S.C., Farthing J.P. Strategic creatine supplementation and resistance training in healthy older adults. Appl. Physiol. Nutr. Metab. 2015;40:689–694. doi: 10.1139/apnm-2014-0498. [DOI] [PubMed] [Google Scholar]
- 27.Candow D.G., Chilibeck P.D., Gordon J., Vogt E., Landeryou T., Kaviani M., Paus-Jensen L. Effect of 12 months of creatine supplementation and whole-body resistance training on measures of bone, muscle and strength in older males. Nutr. Health. 2020 doi: 10.1177/0260106020975247. [DOI] [PubMed] [Google Scholar]
- 28.Chilibeck P.D., Candow D.G., Landeryou T., Kaviani M., Paus-Jenssen L. Effects of Creatine and Resistance Training on Bone Health in Postmenopausal Women. Med. Sci. Sports Exerc. 2015;47:1587–1595. doi: 10.1249/MSS.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 29.Chrusch M.J., Chilibeck P.D., Chad K.E., Davison K.S., Burke D.G. Creatine supplementation combined with resistance training in older men. Med. Sci. Sports Exerc. 2001;33:2111–2117. doi: 10.1097/00005768-200112000-00021. [DOI] [PubMed] [Google Scholar]
- 30.Cooke M.B., Brabham B., Buford T.W., Shelmadine B.D., McPheeters M., Hudson G.M., Stathis C., Greenwood M., Kreider R., Willoughby D.S. Creatine supplementation post-exercise does not enhance training-induced adaptations in middle to older aged males. Eur. J. Appl. Physiol. 2014;114:1321–1332. doi: 10.1007/s00421-014-2866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johannsmeyer S., Candow D.G., Brahms C.M., Michel D., Zello G.A. Effect of creatine supplementation and drop-set resistance training in untrained aging adults. Exp. Gerontol. 2016;83:112–119. doi: 10.1016/j.exger.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Chilibeck P.D., Kaviani M., Candow D.G., Zello G.A. Effect of creatine supplementation during resistance training on lean tissue mass and muscular strength in older adults: A meta-analysis. Open Access J. Sports Med. 2017;8:213–226. doi: 10.2147/OAJSM.S123529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devries M.C., Phillips S.M. Creatine supplementation during resistance training in older adults-a meta-analysis. Med. Sci. Sports Exerc. 2014;46:1194–1203. doi: 10.1249/MSS.0000000000000220. [DOI] [PubMed] [Google Scholar]
- 34.Yu P.H., Deng Y. Potential cytotoxic effect of chronic administration of creatine, a nutrition supplement to augment athletic performance. Med. Hypotheses. 2000;54:726–728. doi: 10.1054/mehy.1999.0938. [DOI] [PubMed] [Google Scholar]
- 35.Antonio J., Candow D.G., Forbes S.C., Gualano B., Jagim A.R., Kreider R.B., Rawson E.S., Smith-Ryan A.E., VanDusseldorp T.A., Willoughby D.S., et al. Common questions and misconceptions about creatine supplementation: What does the scientific evidence really show? J. Int. Soc. Sports Nutr. 2021;18:13. doi: 10.1186/s12970-021-00412-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dalbo V.J., Roberts M.D., Stout J.R., Kerksick C.M. Putting to rest the myth of creatine supplementation leading to muscle cramps and dehydration. Br. J. Sports Med. 2008;42:567–573. doi: 10.1136/bjsm.2007.042473. [DOI] [PubMed] [Google Scholar]
- 37.Kreider R.B., Kalman D.S., Antonio J., Ziegenfuss T.N., Wildman R., Collins R., Candow D.G., Kleiner S.M., Almada A.L., Lopez H.L. International Society of Sports Nutrition position stand: Safety and efficacy of creatine supplementation in exercise, sport, and medicine. J. Int. Soc. Sports Nutr. 2017;14:18. doi: 10.1186/s12970-017-0173-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ju J.S., Smith J.L., Oppelt P.J., Fisher J.S. Creatine feeding increases GLUT4 expression in rat skeletal muscle. Am. J. Physiol. Endocrinol. Metab. 2005;288:347. doi: 10.1152/ajpendo.00238.2004. [DOI] [PubMed] [Google Scholar]
- 39.Roberts P.A., Fox J., Peirce N., Jones S.W., Casey A., Greenhaff P.L. Creatine ingestion augments dietary carbohydrate mediated muscle glycogen supercompensation during the initial 24 h of recovery following prolonged exhaustive exercise in humans. Amino Acids. 2016;48:1831–1842. doi: 10.1007/s00726-016-2252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Burke D.G., Candow D.G., Chilibeck P.D., MacNeil L.G., Roy B.D., Tarnopolsky M.A., Ziegenfuss T. Effect of creatine supplementation and resistance-exercise training on muscle insulin-like growth factor in young adults. Int. J. Sport Nutr. Exerc. Metab. 2008;18:389–398. doi: 10.1123/ijsnem.18.4.389. [DOI] [PubMed] [Google Scholar]
- 41.Safdar A., Yardley N.J., Snow R., Melov S., Tarnopolsky M.A. Global and targeted gene expression and protein content in skeletal muscle of young men following short-term creatine monohydrate supplementation. Physiol. Genom. 2008;32:219–228. doi: 10.1152/physiolgenomics.00157.2007. [DOI] [PubMed] [Google Scholar]
- 42.Bassit R.A., Curi R., Costa Rosa L.F. Creatine supplementation reduces plasma levels of pro-inflammatory cytokines and PGE2 after a half-ironman competition. Amino Acids. 2008;35:425–431. doi: 10.1007/s00726-007-0582-4. [DOI] [PubMed] [Google Scholar]
- 43.Santos R.V., Bassit R.A., Caperuto E.C., Costa Rosa L.F. The effect of creatine supplementation upon inflammatory and muscle soreness markers after a 30km race. Life Sci. 2004;75:1917–1924. doi: 10.1016/j.lfs.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 44.Saraiva A.L., Ferreira A.P., Silva L.F., Hoffmann M.S., Dutra F.D., Furian A.F., Oliveira M.S., Fighera M.R., Royes L.F. Creatine reduces oxidative stress markers but does not protect against seizure susceptibility after severe traumatic brain injury. Brain Res. Bull. 2012;87:180–186. doi: 10.1016/j.brainresbull.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 45.Rahimi R. Creatine supplementation decreases oxidative DNA damage and lipid peroxidation induced by a single bout of resistance exercise. J. Strength Cond. Res. 2011;25:3448–3455. doi: 10.1519/JSC.0b013e3182162f2b. [DOI] [PubMed] [Google Scholar]
- 46.Deminice R., Rosa F.T., Franco G.S., Jordao A.A., de Freitas E.C. Effects of creatine supplementation on oxidative stress and inflammatory markers after repeated-sprint exercise in humans. Nutrition. 2013;29:1127–1132. doi: 10.1016/j.nut.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 47.Harris R.C., Soderlund K., Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- 48.Syrotuik D.G., Bell G.J. Acute creatine monohydrate supplementation: A descriptive physiological profile of responders vs. nonresponders. J. Strength Cond. Res. 2004;18:610–617. doi: 10.1519/12392.1. [DOI] [PubMed] [Google Scholar]
- 49.Candow D.G., Chilibeck P.D. Differences in size, strength, and power of upper and lower body muscle groups in young and older men. J. Gerontol. A Biol. Sci. Med. Sci. 2005;60:148–156. doi: 10.1093/gerona/60.2.148. [DOI] [PubMed] [Google Scholar]
- 50.Robinson T.M., Sewell D.A., Hultman E., Greenhaff P.L. Role of submaximal exercise in promoting creatine and glycogen accumulation in human skeletal muscle. J. Appl. Physiol. 1999;87:598–604. doi: 10.1152/jappl.1999.87.2.598. [DOI] [PubMed] [Google Scholar]
- 51.Persky A.M., Brazeau G.A., Hochhaus G. Pharmacokinetics of the dietary supplement creatine. Clin. Pharmacokinet. 2003;42:557–574. doi: 10.2165/00003088-200342060-00005. [DOI] [PubMed] [Google Scholar]
- 52.Forbes S.C., Candow D.G. Timing of creatine supplementation and resistance training: A brief review. J. Exerc. Nutr. 2018;1:1. [Google Scholar]
- 53.Ostojic S.M., Korovljev D., Stajer V. Dietary creatine and cognitive function in U.S. adults aged 60 years and over. Aging Clin. Exp. Res. 2021 doi: 10.1007/s40520-021-01857-4. [DOI] [PubMed] [Google Scholar]
- 54.Forbes S.C., Candow D.G., Ferreira L.H.B., Souza-Junior T.P. Effects of Creatine Supplementation on Properties of Muscle, Bone, and Brain Function in Older Adults: A Narrative Review. J. Diet. Suppl. 2021:1–18. doi: 10.1080/19390211.2021.1877232. [DOI] [PubMed] [Google Scholar]
- 55.Moon A., Heywood L., Rutherford S., Cobbold C. Creatine supplementation: Can it improve quality of life in the elderly without associated resistance training? Curr. Aging Sci. 2013;6:251–257. doi: 10.2174/1874609806666131204153102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.