Abstract
In the search for antifungal agents from marine resources, we recently found that the culture filtrate of Trichoderma longibrachiatum SFC100166 effectively suppressed the development of tomato gray mold, rice blast, and tomato late blight. The culture filtrate was then successively extracted with ethyl acetate and n-butanol to identify the fungicidal metabolites. Consequently, a new compound, spirosorbicillinol D (1), and a new natural compound, 2′,3′-dihydro-epoxysorbicillinol (2), together with 11 known compounds (3–13), were obtained from the solvent extracts. The chemical structures were determined by spectroscopic analyses and comparison with literature values. The results of the in vitro antifungal assay showed that of the tested fungal pathogens, Phytophthora infestans was the fungus most sensitive to the isolated compounds, with MIC values ranging from 6.3 to 400 µg/mL, except for trichotetronine (9) and trichodimerol (10). When tomato plants were treated with the representative compounds (4, 6, 7, and 11), bisvertinolone (6) strongly reduced the development of tomato late blight disease compared to the untreated control. Taken together, our results revealed that the culture filtrate of T. longibrachiatum SFC100166 and its metabolites could be useful sources for the development of new natural agents to control late blight caused by P. infestans.
Keywords: Trichoderma longibrachiatum, sorbicillinoid, antifungal compound, plant pathogen
1. Introduction
Phytopathogenic fungi have consistently threatened crops, causing serious damage that results in the loss of crop yields and reduces the quality of crops worldwide [1,2]. In an effort to protect plants, chemical fungicides have been mainly used, but the overuse of these fungicides has also led to side effects, such as environmental pollution, toxicity to humans and animals, and resistance of fungal pathogens [3]. Therefore, the development of new fungicides that are safer and more eco-friendly has been emphasized, and biofungicides using plant extracts or useful microbes have been considered as an alternative that can replace synthetic fungicides [4,5,6]. More recently, the integrational use of biofungicides with conventional fungicides has been highlighted to reduce the chemical fungicide use and to avoid fungicide resistance [7,8].
To date, there are over 23,000 known microbial secondary metabolites, which are significant sources for life-saving drugs, and 42% of these known microbial secondary metabolites are produced by fungi [9,10]. Many studies have reported on the potential efficacy of fungi or fungal metabolites in controlling plant diseases caused by fungal pathogens. Of the many fungal species, Trichoderma spp. have been widely studied and used in the biological control against phytopathogenic fungi because of their high capacity to secrete large amounts of enzymes and metabolites [11]. In particular, Trichoderma harzianum and Trichoderma viride have been developed as commercial products, which are now used to control foliar and soil-borne diseases in various crops [12]. In addition to their antifungal activity, it has been reported that Trichoderma spp. promote plant growth and productivity by inducing systemic resistance and enhancing nutrient efficiency [13].
The fungus T. longibrachiatum is generally isolated from terrestrial soil and plants and has broad-spectrum and potential antagonistic effects in inhibiting the growth of plant pathogenic fungi [14,15,16,17]. In terms of the antifungal compounds derived from T. longibrachiatum, it has been reported that a new rare norsesquiterpene exhibited a potent antagonistic ability against Colletotrichum lagenarium, C. fragariae, carbendazim-resistant strains of Botrytis cinerea, and Fusarium spp. [15]. However, there is still a lack of papers describing the in vitro and in vivo antifungal activities of secondary metabolites produced by T. longibrachiatum against plant diseases caused by fungi.
The current study focused on the identification of antifungal metabolites produced by T. longibrachiatum SFC100166 and the evaluation of their ability to control plant diseases in vivo. To this end, we isolated active compounds from the culture filtrate of T. longibrachiatum SFC100166 based on bioassay-guided fractionation, which resulted in the identification of 13 compounds. Here, we report the isolation and structural elucidation of these compounds and their in vitro and in vivo antifungal activities against plant pathogenic fungi.
2. Materials and Methods
2.1. Fungal Strains and Culture Conditions
The fungus SFC100166 was isolated from the foreshore soil at Ganghwa-gun, Incheon, Korea, and deposited as a patent microorganism to the Korean Agricultural Culture Collection (KACC, Jeonju, Korea; No. KACC 83038BP). The fungus was maintained on potato dextrose agar (PDA) medium at 25 °C. Twenty agar plugs punched by a 6 mm-diameter cork-borer from SFC100166 culture plates were inoculated into 400 mL of potato dextrose broth (PDB) and then incubated at 25 °C for 10 days to isolate the antifungal compounds from the SFC100166 culture.
For the in vitro antifungal activity assay, seven phytopathogenic fungi provided by the KACC were used: Alternaria brassicicola (KACC 40036), B. cinerea (KACC 48736), Colletotrichum coccodes (KACC 48737), Cladosporium cucumerinum (KACC 40576), Cylindrocarpon destructans (KACC 41077), Magnaporthe oryzae (KACC 46552), and Phytophthora infestans (KACC 48738). Additionally, we used two obligate parasitic fungi, Puccinia triticina and Blumeria graminis f. sp. Hordei, for the in vivo antifungal activity, which were maintained on their host plants. Maintenance and preparation of the plant pathogens for the in vitro and in vivo antifungal assay were performed as previously described [18,19].
2.2. Genomic DNA Extraction and Phylogenetic Analysis
For the isolation of genomic DNA (gDNA), the SFC100166 isolate was grown in 50 mL of PDB medium at 25 °C for 4 days on a rotary shaker (150 rpm), and the gDNA was extracted using the cetyltrimethylammonium bromide (CTAB) procedure as previously described [20]. For phylogenetic analysis, the translation elongation factor 1-α (TEF1) gene was amplified by primer sets Tef1-F (5′-GGTACTGGTGAGTTCGAGGCTG-3′) and Tef1-R (5′-GGGCTCGATGGAGTCGATAG-3′). The resulting amplicon was purified using the GeneAll ExpinTM PCR purification kit (GeneAll, Seoul, Korea) and then analyzed by a sequencing analysis service (Macrogen, Daejeon, Korea). The resulting sequence was analyzed with the BLASTn program of the NCBI (http://www.ncbi.nlm.nih.gov). The sequences were aligned using ClustalW implemented in MEGA version 7, and distances were estimated based on the model [21]. A phylogenetic tree was generated using the neighbor-joining method with 1000 bootstrap analyses [22].
2.3. Isolation of Antifungal Compounds
An isolation scheme of active compounds from the fungus T. longibrachiatum SFC100166 is shown in Supplemental Figure S1. A ten-day-old fungal culture (10 L) of T. longibrachiatum SFC100166 was filtered with two layers of filter paper circles and then partitioned with an equal volume of ethyl acetate (EtOAc) and n-butanol (BuOH), sequentially. Each layer was concentrated to dryness to give a residue of EtOAc extract (2.6 g), BuOH extract (1.8 g), and water-soluble fraction (16.7 g).
The EtOAc extract was separated by a medium pressure liquid chromatography (MPLC) system (Isolera One; Biotage, Uppsala, Sweden) equipped with a SNAP KP-Sil 50 g cartridge (Biotage), eluting with a gradient mixture of methylene chloride/EtOAc (85:15 to 0:100, v/v) to give seven fractions, E1–E7. Based on in vitro antifungal activity-guided fractionation, the active fraction E2 (118.7 mg) was further purified by an LC-20AR HPLC system (Shimadzu, Kyoto, Japan) equipped with a Capcell Pak C18 column (20 × 250 mm i.d., 5 μm; Shiseido Co., Ltd., Tokyo, Japan) using an isocratic elution of 50% aqueous acetonitrile (ACN) containing 0.1% formic acid to yield two pure compounds, 7 (45.4 mg) and 8 (6.8 mg). Another active fraction, E3 (315.5 mg), was subjected to Sephadex LH-20 gel column chromatography (3 × 100 cm i.d., 25–100 μm; Amersham Biosciences, Sweden) using an isocratic elution of methanol (MeOH) to obtain compound 12 (16.1 mg) and the active fraction E31 (218.1 mg). Fraction E31 was continuously chromatographed by prep HPLC and eluted with an isocratic mixture of 79% aqueous MeOH containing 0.1% formic acid, giving pure compounds 6 (124.2 mg) and 10 (14.2 mg). E5 (294.6 mg) was also an active fraction, purified by prep HPLC with an isocratic mixture of 60% aqueous MeOH to yield compounds 4 (45.7 mg) and 5 (73.7 mg). Similar to the separation of E3, fraction E6 (273.9 mg) was also applied to the Sephadex LH-20 gel column chromatography (3 × 100 cm i.d.) with solvent elution of MeOH to give two pure compounds, 9 (64.9 mg) and 13 (11.9 mg), and a remaining active fraction, E63 (60.6 mg), was further purified by prep HPLC, eluting with an isocratic mixture of 65% aqueous MeOH to yield compounds 1 (17.0 mg) and 3 (28.2 mg).
The BuOH extract was loaded onto a SNAP Ultra C18 60 g cartridge (Biotage) in an MPLC system using a stepwise gradient elution of aqueous MeOH containing 0.1% formic acid (20 to 100%, v/v), giving six factions, B1–B6. Based on the in vitro antifungal activity-guided fractionation, the active fraction B2 (355.0 mg) was recrystallized to obtain compound 11 (289.0 mg), and B3 (78.4 mg) was further separated by prep HPLC eluting with an isocratic mixture of 35% aqueous ACN containing 0.1% formic acid to yield pure compound 2 (7.6 mg).
2.4. Spectral Methods
The chemical structures of the purified compounds were determined by spectroscopic analyses and comparisons with values in previous literature. High-resolution electrospray ionization mass spectrometry (HRESIMS) data were determined by a Synapt G2 system (Waters Co., Milford, MA, USA). The 1D and 2D nuclear magnetic resonance (NMR) spectra were recorded by a Bruker Advance 400 MHz spectrometer (Burker BioSpin, Rheinstetten, Germany) at 400 MHz for 1H and 100 MHz for 13C in MeOH-d4 (Cambridge Isotope Laboratories, Tewksbury, MA, USA). Chemical shifts were referenced to the solvent peaks (δH 4.87 and δC 49.0 for MeOH-d4).
2.5. Spectral Data
2.5.1. Spirosorbicillinol D (1)
Yellowish amorphous powder; + 142 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 208 (4.2), 364 (4.3); 1H and 13C NMR spectroscopic data, see Table 1; high-resolution electrospray ionization mass spectrometry (HRESIMS) m/z 511.1580 [M + Na]+ (calculated for C25H28O10Na, 511.1580).
Table 1.
1H NMR data (400 MHz) a of compound 1 and 13C NMR data (100 MHz) of compounds 1 and 3–5 in MeOH-d4.
| Position | 1 | 3 | 4 | 5 | |
|---|---|---|---|---|---|
| δH, (J in Hz) | δ C | δ C | δ C | δ C | |
| 1 | 3.38, overlapped * | 41.4 | 41.4 | 41.2 | 41.2 |
| 2 | 111.1 | 111.1 | 111.4 | 111.2 | |
| 3 | 195.4 | 195.5 | 196.2 | 196.1 | |
| 4 | 69.2 | 69.3 | 70.9 | 70.9 | |
| 5 | 206.8 | 206.7 | 207.2 | 207.2 | |
| 6 | 74.6 | 74.6 | 74.9 | 74.9 | |
| 7 | 2.24, dd (14.2, 3.7) 3.13, dd (14.2, 2.3) |
38.0 | 37.2 | 40.4 | 40.2 |
| 8 | 85.8 | 84.3 | 82.6 | 83.7 | |
| 9 | 172.2 | 172.0 | 171.0 | 171.2 | |
| 10 | 4.24, dd (10.4, 8.5) | 84.1 | 85.9 | 84.3 | 82.9 |
| 11 | 4.09, td (9.7, 6.8) | 67.1 | 70.6 | 70.7 | 67.1 |
| 12 | 2.32, m 2.95, dd (18.3, 6.9) |
34.4 | 139.6 | 139.3 | 34.0 |
| 13 | 131.7 | 129.0 | 128.8 | 130.8 | |
| 14 | 6.57, s | 134.2 | 30.5 | 31.4 | 134.2 |
| 15 | 4.66, m | 70.3 | 67.1 | 71.3 | 74.9 |
| 1′ | 168.3 | 168.3 | 167.9 | 167.9 | |
| 2′ | 6.46, d (14.9) | 119.3 | 119.3 | 119.5 | 119.5 |
| 3′ | 7.36, dd (14.9, 10.9) | 143.8 | 143.8 | 143.2 | 143.3 |
| 4′ | 6.44, m | 132.3 | 132.3 | 132.3 | 132.3 |
| 5′ | 6.26, dt (14.6, 7.0) | 140.6 | 140.6 | 140.1 | 140.2 |
| 6′ | 1.92, d (6.8) | 18.9 | 18.9 | 18.9 | 18.9 |
| 4-CH3 | 1.25, s | 8.3 | 8.1 | 8.8 | 8.6 |
| 6-CH3 | 1.23, s | 24.9 | 24.8 | 24.8 | 24.8 |
| 14-COOCH3 | 3.77, s | 52.7 | 52.7 | 52.7 | 52.7 |
| 14-COOCH3 | 167.2 | 167.4 | 167.4 | 167.3 | |
a Assignment aided by 1H-1H COSY, HSQC, and HMBC experiments. * overlapped with MeOH-d4 signal.
2.5.2. 2′,3′-Dihydro-epoxysorbicillinol (2)
Viscous yellow oil; UV (MeOH) λmax (log ε) 277 (4.3); 1H NMR (400 MHz, MeOD): δH 5.48 (2H, p, J = 6.0 Hz), 2.67 (2H, m), 2.28 (2H, m), 1.69 (3H, s), 1.65 (3H, d, J = 5.4 Hz), 1.48 (3H, s); ESI-MS: m/z 289 [M + Na]+ and 265 [M − H]−.
2.5.3. Spirosorbicillinol A (3)
Yellowish amorphous powder; + 184 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 208 (4.1), 364 (4.3); 13C NMR spectroscopic data, see Table 1; ESI-MS: m/z 511 [M + Na]+.
2.5.4. Spirosorbicillinol B (4)
Yellowish amorphous powder; + 428 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 208 (4.3), 364 (4.5); 13C NMR spectroscopic data, see Table 1; ESI-MS: m/z 511 [M + Na]+.
2.5.5. Spirosorbicillinol C (5)
Yellowish amorphous powder; + 464 (c 0.05, MeOH); UV (MeOH) λmax (log ε) 208 (4.1), 363 (4.2); 13C NMR spectroscopic data, see Table 1; ESI-MS: m/z 511 [M + Na]+.
2.5.6. Bisvertinolone (6)
Yellowish amorphous powder; UV (MeOH) λmax (log ε) 240 (4.3), 275 (4.5), 394 (4.2); 1H and 13C NMR spectroscopic data, see Table S1; ESI-MS m/z 535 [M + Na]+.
2.5.7. Bisorbicillinol (7)
Yellowish amorphous powder; UV (MeOH) λmax (log ε) 285 (4.5), 300 (4.5), 390 (4.1); 13C NMR spectroscopic data, see Table S1; ESI-MS m/z 519 [M + Na]+ and 495 [M − H]−.
2.5.8. Bisvertinoquinol (8)
Yellow powder; UV (MeOH) λmax (log ε) 250 (3.9), 301 (4.3), 370 (4.1), 380 (4.1), 400 (3.9); 13C NMR spectroscopic data, see Table S1; ESI-MS m/z 521 [M + Na]+ and 497 [M − H]−.
2.5.9. Trichotetronine (9)
Yellowish amorphous powder; UV (MeOH) λmax (log ε) 271 (4.6), 291 (4.6), 384 (4.3); 13C NMR spectroscopic data, see Table S1; ESI-MS m/z 519 [M + Na]+ and 495 [M − H]−.
2.5.10. Trichodimerol (10)
Pale yellow powder; UV (MeOH) λmax (log ε) 240 (4.0), 295 (4.2), 307 (4.2), 362 (4.5); 13C NMR spectroscopic data, see Table S1; ESI-MS m/z 519 [M + Na]+ and 495 [M − H]−.
2.5.11. Epoxysorbicillinol (11)
Yellowish amorphous powder; UV (MeOH) λmax (log ε) 287 (4.2); 13C NMR spectroscopic data, see Table S1; ESI-MS m/z 287 [M + Na]+.
2.5.12. Oxosorbicillinol (12)
Yellowish amorphous powder; UV (MeOH) λmax (log ε) 231 (4.0), 301 (3.9), 378 (4.2); 13C NMR spectroscopic data, see Table S1; ESI-MS m/z 287 [M + Na]+ and 263 [M − H]−.
2.5.13. Cyclonerodiol (13)
Colorless oil; UV (MeOH) no UV above 210 nm; 13C NMR spectroscopic data, see Table S1.
2.6. Microdilution Broth Assay for In Vitro Antimicrobial Activity
Minimum inhibitory concentration (MIC) values of the purified compounds against phytopathogenic fungi were determined by the broth microdilution assay using the 2-fold serial dilution method as described by the modified CLSI M38-A method [23]. Briefly, a spore suspension (1 × 105 spores/mL of PDB) of each fungal pathogen was added to the wells of a 96-well microtiter plate, and then the stock solutions of the compounds dissolved in DMSO were added at an initial concentration of 400 μg/mL. The microtiter plates were incubated for 1–3 days, and the MIC values were determined by visual inspection of complete growth inhibition. The chemical blasticidin-S and a PDB medium containing 1% DMSO were used as a positive and negative control, respectively. The assay was performed two times with three replicates for each compound at all concentrations investigated.
2.7. Evaluation of Fungal Disease Control Efficacy
To investigate the disease control efficacy of the culture filtrate, solvent extracts, and pure compounds, we used six kinds of fungal plant diseases: rice blast (RCB; caused by M. oryzae), tomato late blight (TLB; caused by P. infestans), tomato gray mold (TGM; caused by B. cinerea), wheat leaf rust (WLR; caused by P. triticinia), barley powdery mildew (BPM; caused by B. graminis f. sp. hordei), and pepper anthracnose (PAN; caused by C. coccodes). The culture filtrate containing 0.025% Tween 20 solution was directly applied onto the plant, and the extracts (1000 µg/mL) and pure compounds (125, 250, and 500 µg/mL) were prepared by dissolving in 5% MeOH solution containing 0.025% Tween 20 solution. Chemical fungicides (blasticidin-S, fludioxonil, dimethomorph, flusilazole, benomyl, and dithianon) were used as a positive control, whereas a 5% MeOH solution was used as a negative control. As host plants for the pathogens, we used rice (Oryza sativa L, cv. Chucheong), tomato (Solanum lycopersicum cv. Seokwang), wheat (Triticum aestivum cv. Geumgang), barley (Hordeum sativum cv. Hanyoung), and pepper (Capsicum annuum cv. Hyangchon), which were grown in a greenhouse at 25 ± 5 °C for 3–4 weeks. After treatment with the solvent extracts or pure compounds, the plants were inoculated with a fungal pathogen and incubated as previously described [4,5]. Disease severity based on the lesion area of the leaves was evaluated 3–7 days after inoculation, and the disease control efficacy was calculated with the following equation: control efficacy (%) = 100 × (1 − B/A), where A is the mean of the lesion area (%) on the leaves of the control plants and B is the mean of lesion area (%) on the leaves of the treated plants [6]. All experiments were conducted twice with three replicates for each treatment.
3. Results and Discussion
3.1. Disease Suppression by the Culture Filtrate and Its Extracts of the SFC100166 Isolate
During the course of screening for a culture filtrate with a potent efficacy in controlling plant diseases caused by fungi, we found that a culture filtrate of the SFC100166 isolate exhibited an inhibitory effect on the development of RCB, TGM, and TLB with control values of 85%, 94%, and 90%, respectively (Table 2). To identify the active compounds produced by the SFC100166 isolate, the culture filtrate was successively partitioned by the organic solvents EtOAc and BuOH, and then the yielding extracts were investigated for their ability to control six plant diseases caused by fungal pathogens. Both the EtOAc and BuOH extracts caused a reduction in the development of TGM and TLB over at least the control values of 80% (Table 2). In particular, the EtOAc extract showed a broad range of disease control efficacy on RCB, WLR, PAN, TGM, and TLB, with control values of over 60%. The suppression of BPM by the organic extracts was not found, and the remaining water extract did not show any suppressive effects on disease development caused by fungi. Therefore, we used the EtOAc and BuOH extracts for the further isolation of the antifungal substances.
Table 2.
Plant disease control efficacy of the SFC100166 culture filtrate and its organic solvent extracts.
| Treatment | Conc. (µg/mL) |
Disease Control Value (%) a | |||||
|---|---|---|---|---|---|---|---|
| RCB b | TGM | TLB | WLR | BPM | PAN | ||
| Culture filtrate | 1-fold dilution | 85 ± 2 | 94 ± 6 | 90 ± 2 | 27 ± 3 | 25 ± 2 | 15 ± 3 |
| EtOAc extract | 1000 | 88 ± 5 | 95 ± 2 | 100 | 60 ± 4 | 0 | 60 ± 2 |
| BuOH extract | 1000 | 25 ± 1 | 80 ± 1 | 100 | 33 ± 1 | 0 | 35 ± 5 |
| Water extract | 1000 | 25 ± 1 | 0 | 42 ± 2 | 0 | 0 | 0 |
| Blasticidin-S | 1 | 90 ± 2 | – c | – | – | – | – |
| 50 | 100 | – | – | – | – | – | |
| Fludioxonil | 5 | – | 88 ± 5 | – | – | – | – |
| 50 | – | 100 | – | – | – | – | |
| Dimethomorph | 2 | – | – | 85 ± 2 | – | – | – |
| 10 | – | – | 100 | – | – | – | |
| Flusilazole | 2 | – | – | – | 43 ± 9 | – | – |
| 10 | – | – | – | 88 ± 2 | – | – | |
| Benomyl | 1 | – | – | – | – | 87 ± 5 | – |
| 100 | – | – | – | – | 100 | – | |
| Dithianon | 10 | – | – | – | – | – | 40 ± 1 |
| 50 | – | – | – | – | – | 88 ± 4 | |
a Disease control values (%) represent the mean ± standard deviation of two runs with three replicates. b RCB, rice blast; TGM, tomato gray mold; TLB, tomato late blight; WLR, wheat leaf rust; BPM, barley powdery mildew; PAN, pepper anthracnose. c –, not tested.
3.2. Molecular Identification of the SFC100166 Isolate
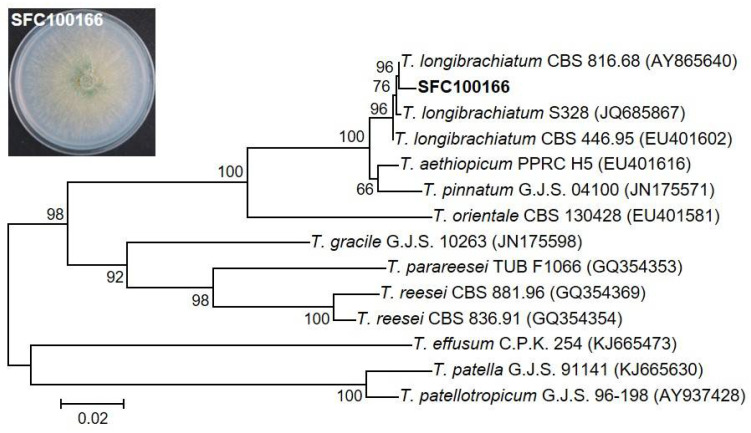
For the molecular identification of the SFC100166 isolate, the TEF1 gene region was amplified from the gDNA of the SFC100166 isolate, and the resulting sequence (482 bp of the amplicon) was deposited to GenBank (http://www.ncbi.nlm.nih.gov/Genbank) with an accession number of MN307409. Phylogenetic analysis based on TEF1 sequences showed that the SFC100166 isolate belongs to the genus Trichoderma and mostly is related to T. longibrachiatum CBS 816.68 (AY865640) with a 99.3% similarity. As such, the SFC100166 isolate was identified as T. longibrachiatum (Figure 1).
Figure 1.
Phylogenetic analysis of the SFC100166 isolate. An inset box showed the SFC100166 isolate culture grown on PDA. Phylogenetic analysis by the neighbor-joining method was performed based on the translation elongation factor 1-α (TEF1) gene sequences of SFC100166 and the other 13 Trichoderma species. NCBI accession numbers of each sequence are in parentheses. The scale bar indicates the number of nucleotide substitutions per site.
T. longibrachiatum is a soil-borne fungus belonging to the anamorphic genus Trichoderma (Hypocreales, Ascomycota). Several strains of T. longibrachiatum have been reported as promising biocontrol agents against phytopathogenic fungi, bacteria, and nematodes [15,24,25,26,27,28]. To date, a variety of new antibiotics, including peptides, polyketides, and terpenes, have been isolated from T. longibrachiatum, which led to the recognition of the importance of T. longibrachiatum as a potential source of novel antibiotics [7,8,15].
3.3. Structure Determination of the Active Compounds Isolated from Trichoderma longibrachiatum SFC100166
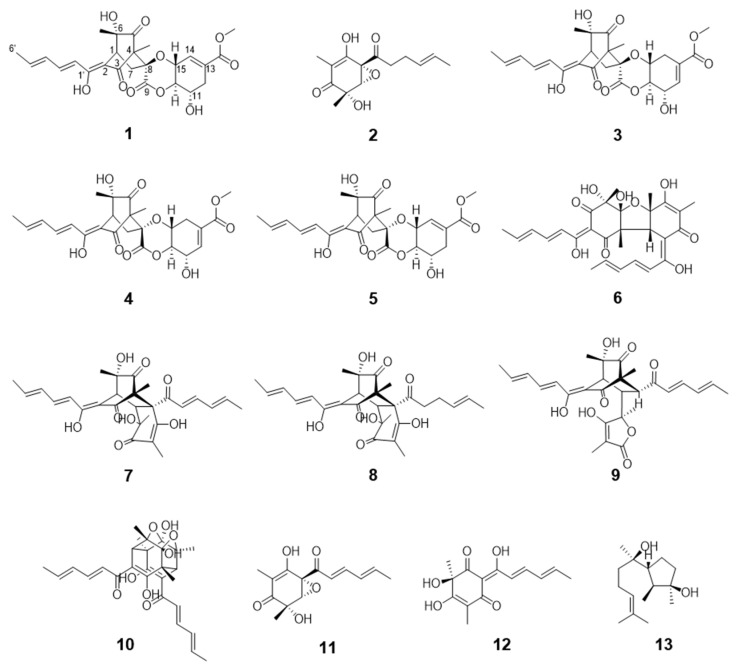
The two organic extracts obtained from the culture filtrate of T. longibrachiatum SFC100166 were further fractionated and purified by various chromatographic processes following the guidance of in vitro antifungal assays. Consequently, we isolated a total of 13 compounds (Figure 2) and determined the chemical structures of the purified compounds by analyzing the spectroscopic data (NMR, MS, and CD) and by comparing them with previously reported values in the literature. Of the 13 compounds, compound 1 was a newly identified compound, and compound 2 was found for the first time in a natural resource.
Figure 2.
Chemical structures of compounds 1–13 isolated from the fungus Trichoderma longibrachiatum SFC100166.
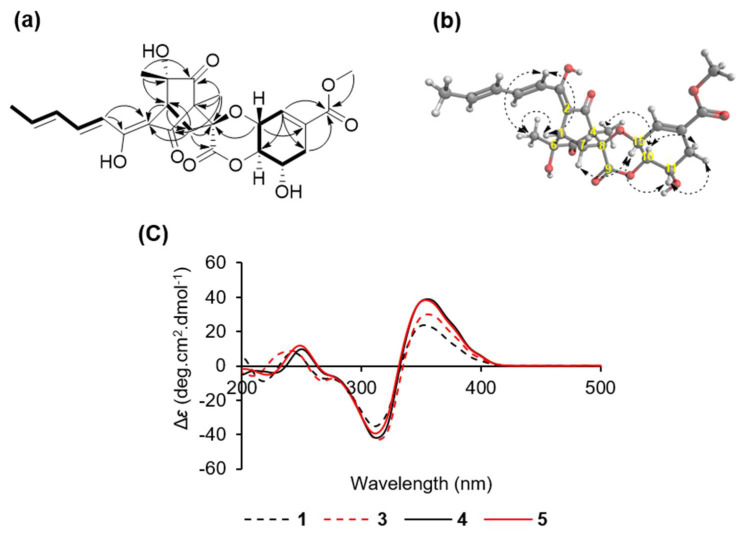
Compound 1 was obtained as a yellow amorphous powder and has a molecular formula of C25H28O10, which was established by HRESIMS analysis from a molecular ion at m/z 511.1580 [M + Na]+ (calculated m/z 511.1580 for C25H28O10Na) (Figure S2). The details of the 1H-NMR, 13C-NMR, and HMQC spectra of 1 displayed signals for three methyls (δH 1.23, δC 24.9; δH 1.25, δC 8.3; δH 1.92, δC 18.9), two methylenes (δH 2.24 and 3.13, δC 38.0; δH 2.32 and 2.95, δC 34.4), one methine (δH 3.38, δC 41.4), one methoxyl (δH 3.77, δC 52.7), three oxymethines (δH 4.09, δC 67.1; δH 4.24, δC 84.1; δH 4.66, δC 70.3), and five olefinic methines (δH 6.26, δC 140.6; δH 6.44, δC 132.3; δH 6.46, δC 119.3; δH 6.57, δC 134.2; δH 7.36, δC 143.8), and the remaining 10 quaternary carbons were observed at δC 69.2, 74.6, 85.8, 111.1, 131.7, 167.2, 168.3, 172.2, 195.4, and 206.8 (Table 1 and Figures S3–S5). The structure of 1 was further constructed with 1H-1H COSY and HMBC experiments (Figures S6 and S7). The HMBC correlations from H-1 to C-2, C-3, C-5, C-6, and C-8 and from H-7 to C-2, C-4, C-8, and C-9, in conjunction with a strong correlation in the 1H-1H COSY between H-1 and H-7, confirmed that 1 had a bicyclo[2.2.2]octane ring system (Figure 3a). Two methyl groups with proton signals at δH 1.23 and 1.25 were attached to C-6 and C-4 of the bicyclo[2.2.2]octyl moiety, respectively, due to their following correlations in the HMBC spectrum: from 6-CH3 to C-1, C-5, and C-6 and from 4-CH3 to C-3, C-4, C-5, and C-8. The 1H-1H COSY cross-peaks between H-6′/H-5′, H-5′/H-4′, H-4′/H-3′, and H-3′/H-2′ and the HMBC correlations from H-2′ to C-1′ and C-2 indicated the presence of an enol-sorbyl chain, and its position was located at C-2. In addition, a methoxyl carbonyl group was easily recognized, and it was connected to a cyclohexene at C-13 based on the correlations from the methoxyl, H-12, and H-14 to the carbonyl at δC 167.2 in the HMBC spectrum and cross-peaks between H-10/H-11, H-11/H-12, H-14/H-15, and H-15/H-10 in the 1H-1H COSY spectrum.
Figure 3.
(a) Key HMBC (arrow) and COSY (bold line) correlations of compound 1; (b) NOE (dashed arrow) correlations of compound 1; (c) CD spectra of compounds 1 and 3–5.
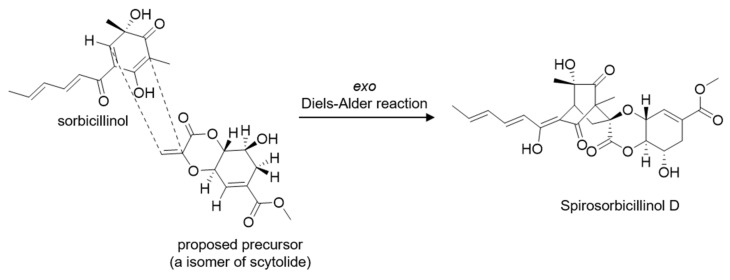
A linkage of this fragment and bicyclo[2.2.2]octyl moiety via oxygen atom was determined by a key HMBC correlation from H-15 to C-8. Thus, the planar structure of 1 was established as shown in Figure 2 and Figure 3. It was the same as that of spirosorbicillinol C (5), but there are small differences in the chemical shifts of positions C-7, C-8, C10, and C-15 suggesting that the structure of 1 is different from spirosorbicillinol C (5) at the stereochemistry of position 8 (Table 1). Indeed, the relative stereochemistry of 1 was confirmed by the analysis of the NOESY spectrum (Figure 3b and Figure S8). The geometries of the double bonds C-2′/C-3′ and C-4′/C-5′ in the enol-sorbyl side chain were determined to be E, E based on coupling constants JH-2′/H-3′ = 14.9 Hz and the NOESY correlation between H-3′ and H-5′. The large coupling constants JH-10/H-11 = 10.4 Hz and JH-10/H-15 = 8.5 Hz indicated that H-10/H-11 and H-10/H-15 were in an anti-orientation. Moreover, the NOESY correlation of H-11 and H-15 suggested that they were oriented in a 1,3-diaxial-like orientation. The relative stereochemistry of C-1/C-4/C-6/C-8/C-10/C-11/C-15 was determined to be 1S/4R/6S/ 8S/10R/11S/15R as in the case of the known compound spirosorbicillinol A (3) as follows: 6-CH3 and C-2 were gauche by the NOESY correlations from 6-CH3 to H-2′ and H-1; there were strong HMBC correlations from H-7a to C-6 and from H-7b to C-2, while there was no correlation between H-7a and C-2 and a weak correlation between H-7b and C-6; in addition, a strong HMBC correlation from H-7b to C-9 and no correlation from H-7b to C-9 revealed that H-7a and C-2 were gauche, and H-7b and C-9 were eclipsed; the remaining stereochemistry of the other positions was established by the strong NOESY correlations from H-10 to 4-CH3, from H-15 to H-7a, and from H-11 to H-12a and H-10 to H-12b. To elucidate the absolute stereochemistry of 1, the circular dichroism (CD) spectrum was analyzed and compared with the CD spectra of known sorbicillinoids [29,30]. As shown in Figure 3c, compound 1 and its isomers spirosorbicillinol A–C (3–5) exhibited a positive cotton effect (CE) at 350–360 nm (Δε > +24) and a negative CE at 310–320 nm (Δε < −35), which were in agreement with the 1R and 4S configurations [29]. The absolute configuration of C-6 was proposed to be the S-form as with sorbicillinol, which is a key precursor of sorbicillin-related compounds [31]. Furthermore, compound 1 had an optical rotation value = +142 (c = 0.05, MeOH), which was closer to that of spirosorbicillinol A (3) = +184 (c = 0.05, MeOH) than that of spirosorbicillinol B (4) = +428 (c = 0.05, MeOH) and spirosorbicillinol C (5) = +464 (c = 0.05, MeOH), suggesting that the position C-8 of compound 1 was the same stereochemistry as spirosorbicillinol A (3) (8S), whereas spirosorbicillinols B and C have an opposite stereochemistry (8R). Based on the analytic results mentioned above, the structure and absolute stereochemistry of the new compound 1 were elucidated as shown in Figure 2 (1S, 4R, 6S, 8S, 10R, 11S, 15R), and it was designated as spirosorbicillinol D. Spirosorbicillinol D (1) would be biosynthesized by the exo intermolecular Diels–Alder reaction from sorbicillinol as a diene and a constitutional isomer of scytolide as a dienophile (Figure 4). Indeed, Kahlert et al. clearly demonstrated that spirosorbicillinols A and B (3 and 4) of Trichoderma reesei QM6a arise through the exo and endo Diels–Alder reaction of sorbicillinol with scytolide, respectively [32]. Spirosorbicillinol C (5) from the fermentation broth of Trichoderma sp. USF-4860 was proposed as a biosynthetic product of the endo Diels-Alder reaction between sorbicillinol and the constitutional isomer scytolide [31].
Figure 4.
A biosynthetic proposal for compound 1 [31].
Compound 2 was obtained as a viscous yellow oil with a molecular formula of C14H18O5, which was established by ESIMS analysis from molecular ions at m/z 289 [M + Na]+ and at m/z 265 [M − H]+ (Figure S9), which had 2 H more than epoxysorbicillinol (11) (C14H16O5). The 1H-NMR spectrum of 2 (Figure S10) was closely similar to that of epoxysorbicillinol (11) except for the absence of two olefinic double bonds and the presence of two methylenes at δH 2.67 and 2.28, suggesting that the structure of 2 was a reduction product of epoxysorbicillinol (11). The 1H-NMR spectrum of 2 was compared with a previous report of the synthetic product that is a mediated product provided in the total synthesis of epoxysorbicillinol (11). As such, the structure of 2 was assigned as 2′,3′-dihydro-epoxysorbicillinol. In the current study, compound 2 was isolated from a natural resource for the first time [33].
By comparing the spectroscopic data (Table S1) of compounds 3–13 with those of the reported values in the literature, 11 compounds were identified as spirosorbicillinol A (3) [31], spirosorbicillinol B (4) [31], spirosorbicillinol C (5) [31], bisvertinolone (6) [34,35], bisorbicillinol (7) [36], bisvertinoquinol (8) [37], trichotetronine (9) [30], trichodimerol (10) [38], epoxysorbicillinol (11) [39], oxosorbicillinol (12) [40], and cyclonerodiol (13) [41].
3.4. Microdilution Broth Assay for the In Vitro Antifungal Activity of the Isolated Compounds (1–13)
All isolated compounds (1–13) were evaluated for their in vitro antifungal activity against seven plant pathogenic fungi: A. brassicicola, B. cinerea, C. coccodes, M. oryzae, P. infestans, C. cucumerinum, and C. destructans. Of the tested phytopathogenic fungi, the tomato late blight fungus P. infestans was the most sensitive to the isolated compounds, except for compounds 9 and 10 which did not have any effects on the growth of the tested fungal pathogens. Compound 6 exhibited the strongest antifungal activity against P. infestans with a MIC value of 6.3 μg/mL, followed by compounds 11 and 12 with MIC values of 50 and 25 μg/mL, respectively. Compounds 1–5, 7, 8, and 13 showed a moderate antifungal activity against P. infestans with MIC values ranging from 100 to 400 μg/mL (Table 3). On the other hand, compound 6 exhibited the strongest antifungal activity against M. oryzae with a MIC value of 50 μg/mL, while compounds 7, 8, and 11–13 showed a moderate antifungal activity against M. oryzae with MIC values ranging from 100 to 400 μg/mL (Table 3). Taken together, compounds 6, 7, 11, and 12 showed broad antifungal activities against tested fungal pathogens, and in particular, compound 6 strongly inhibited the growth of the tested microorganisms, with MIC values ranging from 6.3 to 100 μg/mL, except for B. cinerea (Table 3).
Table 3.
In vitro antifungal activity of compounds 1–13 against phytopathogenic fungi.
| Phytopathogenic Fungi | MIC (µg/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | PC a | |
| Alternaria brassicicola | – b | – | – | – | – | 100 | 400 | – | – | – | – | 400 | – | 6.3 |
| Botrytis cinerea | – | – | – | – | – | – | – | – | – | – | 200 | – | – | 50 |
| Cladosporium cucumerinum | – | – | – | – | – | 50 | – | – | – | – | – | – | – | 1.6 |
| Colletotrichum coccodes | – | – | – | – | – | 12.5 | – | – | – | – | 400 | 200 | – | 6.3 |
| Cylindrocarpon destructans | – | – | – | – | – | 25 | – | – | – | – | – | 400 | – | 100 |
| Magnaporthe oryzae | – | – | – | – | – | 50 | 200 | 200 | – | – | 200 | 100 | 400 | 6.3 |
| Phytophthora infestans | 400 | 400 | 400 | 400 | 400 | 6.3 | 100 | 200 | – | – | 50 | 25 | 400 | 1.6 |
a PC, blasticidin-S was used as a positive control. b –, MIC > 400 μg/mL.
3.5. Antifungal Activity Based on the Compound Structure
The 13 compounds isolated from the fungus T. longibrachiatum SFC100166 can be classified into two groups: sorbicillinoids (1–12) and a terpenoid (13). Sorbicillinoids are a family of hexaketide fungal metabolites with a typical or modified sorbyl side chain [29]. Since sorbicillin was isolated as an impurity from Penicillium notatum in 1948, which was the first member of the sorbicillinoids group, more than 100 sorbicillinoids have been isolated from marine and terrestrial microbes, such as Penicillium, Trichoderma, Aspergillus, Acremonium, Eurotiomycete, Phaeoacremonium, Phialocephala, Scytalidium, Clonostachys, Paecilomyces, and Verticillium [29,42,43]. Based on the structural features of various sorbicillinoids, sorbicillinoids can be classified into monomers, dimers, trimers, and hybrids [44,45]. The 12 sorbicillinoids of this study can be classified into three classes: monomeric sorbicillinoids (2, 11 and 12), dimeric sorbicillinoids (6–10), and hybrid sorbicillinoids (1 and 3–5). In particular, it has been proposed that bisvertinolone (6), showing the strongest antifungal activity, is biosynthesized from oxosorbicillinol (12) and sorbicillinol in a Michael-type reaction [46].
It has been reported that many sorbicillinoids show promising biological activities such as anticancer, antimicrobial, antiviral, antitumor, and radical-scavenging activities: bisorbicillinol has shown antioxidant activity, 24-hydroxybisvertinol and bisvertinol have shown anti-inflammatory activity, and ustisorbicillinol B has shown antimicrobial activity [36,44,47,48,49]. In the current study, bisvertinolone (6) was the most antifungal agent against the tested phytopathogenic fungi, followed by oxosorbicillinol (12) and epoxysorbicillinol (11). Similarly, it has been reported that bisvertinolone (6) acts as a morphological malformation inducer in growing hyphal cells of P. capsici by inhibiting β-1,6-glucan biosynthesis [34]. Although it has been reported that oxosorbicillinol (12) exhibits a weak antibacterial activity against the Gram-positive bacteria Staphylococcus aureus and Bacillus subtilis with no effects on fungi and algae [50], oxosorbicillinol (12) in this study showed a moderate antifungal activity with MIC values ranging from 25 to 400 μg/mL against phytopathogenic fungi, such as C. coccodes, M. oryzae, and P. infestans.
3.6. Disease Control Efficacy of the Isolated Compounds
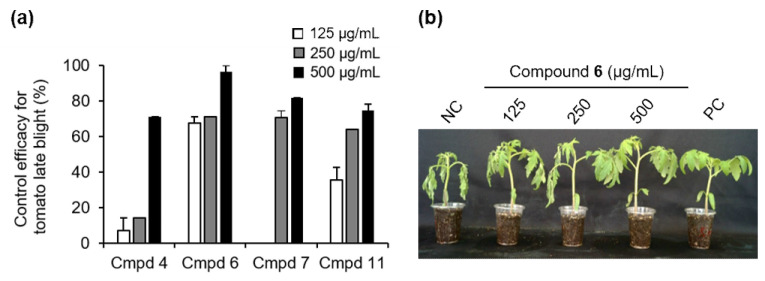
Based on the results of the in vitro antifungal activity and the amount of isolated compounds obtained, we examined the disease control efficacy of compounds 4, 6, 7, and 11 as representative of monomers and dimers of sorbicillinoids against TLB at a concentration of 125, 250, and 500 μg/mL. The treatment with compounds 4, 6, 7, and 11 at a concentration of 500 μg/mL exhibited disease control values of 82%, 96%, 71%, and 75%, respectively, compared to the untreated control (Figure 5). Particularly, compound 6 exhibited a potent disease control value of 68% even at a low concentration of 125 μg/mL (Figure 5). In addition to the disease control effects, no phytotoxic symptoms from the tested compounds were observed on the treated tomato plants. Previously, Meng et al. described that several sorbicilinoids, including bisvertinolone (6), identified from rice false smut pathogen Ustilaginoidea virens showed a strong inhibitory effect on germ elongation of rice and lettuce seeds [29].
Figure 5.
Effects of compounds 4, 6, 7, and 11 on the development of tomato late blight. (a) Control efficacy of compounds 4, 6, 7, and 11 against tomato late blight. The bars represent the mean ± standard deviation of two runs with three replicates. (b) Representatives of plants treated with compound 6. Plants were inoculated with a spore suspension of Phytophthora infestans 1 day after treatment with compound 6. NC, treatment with the 0.025% Tween 20 solution containing 5% MeOH was used as a negative control; PC, a chemical fungicide dimethomorph (10 µg/mL) was used as a positive control.
4. Conclusions
Herein, we described the isolation and identification of thirteen metabolites from the culture filtrate of T. longibrachiatum SFC100166 showing inhibitory effects on the development of RCB, TGM, and TLB. Of the 13 active substances, compound 1 was a new compound and was designated as spirosorbicillinol D, and compound 2 was a new natural compound and was designated as 2′,3′-dihydro-epoxysorbicillinol. In terms of the in vitro and in vivo antifungal activities, compound 6 (bisvertinolone) showed promising antifungal activity in vitro and also reduced the development of TLB disease compared to the untreated control. Taken together, our results revealed that the culture filtrate of T. longibrachiatum SFC100166 and its metabolites could be useful sources for the development of new natural agents to control fungal diseases on plants.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jof7060428/s1, Figure S1: Isolation scheme of compounds 1–13 from the culture filtrate of Trichoderma longibrachiatum SFC100166, Figures S2–S10: NMR spectral data and MS spectrum of compounds 1 and 2, Table S1: Spectroscopic data of compounds 3–13.
Author Contributions
Conceptualization, H.K. and G.J.C.; methodology, M.T.N., M.V.N. and J.W.H.; validation, M.T.N. and M.V.N.; formal analysis, M.T.N. and J.W.H.; resources, M.S.P.; data curation, M.T.N., M.V.N., J.W.H., H.K. and G.J.C.; writing—original draft preparation, M.T.N. and H.K.; writing—review and editing, M.T.N., J.W.H., H.K. and G.J.C.; supervision, H.K. and G.J.C.; funding acquisition, H.K. and G.J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Crop Viruses and Pests Response Industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No. 321101-03), and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (NRF-2019R1A2C1008103).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Choi N.H., Choi G.J., Min B.S., Jang K.S., Choi Y.H., Kang M.S., Park M.S., Choi J.E., Bae B.K., Kim J.C. Effects of neolignans from the stem bark of Magnolia obovata on plant pathogenic fungi. J. Appl. Microbiol. 2009;106:2057–2063. doi: 10.1111/j.1365-2672.2009.04175.x. [DOI] [PubMed] [Google Scholar]
- 2.Ngo T.M., Han J.W., Yoon S.G., Bae S.H., Kim S.Y., Kim H., Choi G.J. Discovery of new triterpenoid saponins isolated from Maesa japonica with antifungal activity against rice blast fungus Magnaporthe oryzae. J. Agric. Food Chem. 2019;67:7706–7715. doi: 10.1021/acs.jafc.9b02236. [DOI] [PubMed] [Google Scholar]
- 3.Choi N.H., Choi G.J., Jang K.S., Choi Y.H., Lee S.O., Choi J.E., Kim J.C. Antifungal activity of the methanol extract of Myristica malabarica fruit rinds and the active ingredients Malabaricones against phytopathogenic fungi. Plant. Pathol. J. 2008;24:317–321. doi: 10.5423/PPJ.2008.24.3.317. [DOI] [Google Scholar]
- 4.Han J.W., Shim S.H., Jang K.S., Choi Y.H., Dang Q.L., Kim H., Choi G.J. In Vivo assessment of plant extracts for control of plant diseases: A sesquiterpene ketolactone isolated from Curcuma zedoaria suppresses wheat leaf rust. J. Environ. Sci. Health B. 2018;53:135–140. doi: 10.1080/03601234.2017.1397448. [DOI] [PubMed] [Google Scholar]
- 5.Han J.W., Shim S.H., Jang K.S., Choi Y.H., Kim H., Choi G.J. In Vivo disease control efficacy of isoquinoline alkaloids isolated from Corydalis ternata against wheat leaf rust and pepper anthracnose. J. Microbiol. Biotechnol. 2018;28:262–266. doi: 10.4014/jmb.1707.07009. [DOI] [PubMed] [Google Scholar]
- 6.Han J.W., Oh M., Lee Y.J., Choi J., Choi G.J., Kim H. Crinipellins A and I, two diterpenoids from the basidiomycete fungus Crinipellis rhizomaticola, as potential natural fungicides. Molecules. 2018;23:2377. doi: 10.3390/molecules23092377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang S.H., Yang J., Ma H., Yang Y., Zhou G.F., Zhao X., Xu R., Nie D., Zhang G.G., Shan J.J., et al. Longibramides A–E, peptaibols isolated from a mushroom derived fungus Trichoderma longibrachiatum Rifai DMG-3-1-1. Chem. BIodivers. 2021;18:e2100128. doi: 10.1002/cbdv.202100128. [DOI] [PubMed] [Google Scholar]
- 8.Zou J.X., Song Y.P., Ji N.Y. Deoxytrichodermaerin, a harziane lactone from the marine algicolous fungus Trichoderma longibrachiatum A-WH-20-2. Nat. Prod. Res. 2021;35:216–221. doi: 10.1080/14786419.2019.1622110. [DOI] [PubMed] [Google Scholar]
- 9.Chandra P., Sharma R.K., Arora D.S. Antioxidant compounds from microbial sources: A review. Food Res. Int. 2020;129:108849. doi: 10.1016/j.foodres.2019.108849. [DOI] [PubMed] [Google Scholar]
- 10.Demain A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014;41:185–201. doi: 10.1007/s10295-013-1325-z. [DOI] [PubMed] [Google Scholar]
- 11.Khan R.A.A., Najeeb S., Hussain S., Xie B., Li Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms. 2020;8:817. doi: 10.3390/microorganisms8060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu H., Ma Y., Guo Q., Xu B. Biological weed control using Trichoderma polysporum strain HZ-31. Crop. Prot. 2020;134:105161. doi: 10.1016/j.cropro.2020.105161. [DOI] [Google Scholar]
- 13.Moya P., Barrera V., Cipollone J., Bedoya C., Kohan L., Toledo A., Sisterna M. New isolates of Trichoderma spp. as biocontrol and plant growth–promoting agents in the pathosystem Pyrenophora teres-barley in Argentina. Biol. Control. 2020;141:104152. doi: 10.1016/j.biocontrol.2019.104152. [DOI] [Google Scholar]
- 14.Zhang S., Xu B., Zhang J., Gan Y. Identification of the antifungal activity of Trichoderma longibrachiatum T6 and assessment of bioactive substances in controlling phytopathogens. Pestic. Biochem. Physiol. 2018;147:59–66. doi: 10.1016/j.pestbp.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Du F.Y., Ju G.L., Xiao L., Zhou Y.M., Wu X. Sesquiterpenes and cyclodepsipeptides from marine-derived fungus Trichoderma longibrachiatum and their antagonistic activities against soil-borne pathogens. Mar. Drugs. 2020;18:165. doi: 10.3390/md18030165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sallam N.M.A., Eraky A.M.I., Sallam A. Effect of Trichoderma spp. on Fusarium wilt disease of tomato. Mol. Biol. Rep. 2019;46:4463–4470. doi: 10.1007/s11033-019-04901-9. [DOI] [PubMed] [Google Scholar]
- 17.Abo-Elyousr K.A.M., Abdel-Hafez S.I.I., Abdel-Rahim I.R. Isolation of Trichoderma and evaluation of their antagonistic potential against Alternaria porri. J. Phytopathol. 2014;162:567–574. doi: 10.1111/jph.12228. [DOI] [Google Scholar]
- 18.Choi G.J., Lee S.-W., Jang K.S., Kim J.-S., Cho K.Y., Kim J.-C. Effects of chrysophanol, parietin, and nepodin of Rumex crispus on barley and cucumber powdery mildews. Crop. Prot. 2004;23:1215–1221. doi: 10.1016/j.cropro.2004.05.005. [DOI] [Google Scholar]
- 19.Choi N.H., Jang J.Y., Choi G.J., Choi Y.H., Jang K.S., Min B.-S., Le Dang Q., Kim J.-C. Antifungal activity of sterols and dipsacus saponins isolated from Dipsacus asper roots against phytopathogenic fungi. Pestic. Biochem. Physiol. 2017;141:103–108. doi: 10.1016/j.pestbp.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 20.Oh M., Son H., Choi G.J., Lee C., Kim J.C., Kim H., Lee Y.W. Transcription factor ART 1 mediates starch hydrolysis and mycotoxin production in Fusarium graminearum and F. verticillioides. Mol. Plant. Pathol. 2016;17:755–768. doi: 10.1111/mpp.12328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saitou N., Nei M. The Neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 23.Espinel-Ingroff A., Fothergill A., Ghannoum M., Manavathu E., Ostrosky-Zeichner L., Pfaller M., Rinaldi M., Schell W., Walsh T. Quality control and reference guidelines for CLSI broth microdilution susceptibility method (M 38-A document) for amphotericin B, itraconazole, posaconazole, and voriconazole. J. Clin. Microbiol. 2005;43:5243–5246. doi: 10.1128/JCM.43.10.5243-5246.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Degani O., Dor S. Trichoderma biological control to protect sensitive maize hybrids against late wilt disease in the field. J. Fungi. 2021;7:315. doi: 10.3390/jof7040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hewedy O.A., Abdel Lateif K.S., Seleiman M.F., Shami A., Albarakaty F.M., El-Meihy R.M. Phylogenetic diversity of Trichoderma strains and their antagonistic potential against soil-borne pathogens under stress conditions. Biology. 2020;9:189. doi: 10.3390/biology9080189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarsaiya S., Jain A., Fan X., Jia Q., Xu Q., Shu F., Zhou Q., Shi J., Chen J. New insights into detection of a dendrobine compound from a novel endophytic Trichoderma longibrachiatum strain and its toxicity against phytopathogenic bacteria. Front. Microbiol. 2020;11:337. doi: 10.3389/fmicb.2020.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sridharan A.P., Sugitha T., Karthikeyan G., Nakkeeran S., Sivakumar U. Metabolites of Trichoderma longibrachiatum EF5 inhibits soil borne pathogen, Macrophomina phaseolina by triggering amino sugar metabolism. Microb. Pathog. 2021;150:104714. doi: 10.1016/j.micpath.2020.104714. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S., Gan Y., Liu J., Zhou J., Xu B. Optimization of the fermentation media and parameters for the bio-control potential of Trichoderma longibrachiatum T6 against nematodes. Front. Mirobiol. 2020;11:574601. doi: 10.3389/fmicb.2020.574601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng J., Cheng W., Heydari H., Wang B., Zhu K., Konuklugil B., Lin W. Sorbicillinoid-based metabolites from a sponge-derived fungus Trichoderma saturnisporum. Mar. Drugs. 2018;16:226. doi: 10.3390/md16070226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shirota O., Pathak V., Hossain C.F., Sekita S., Takatori K., Satake M. Structural elucidation of trichotetronines: Polyketides possessing a bicyclo [2.2.2]octane skeleton with a tetronic acid moiety isolated from Trichoderma sp. J. Chem. Soc. Perkin Trans. I. 1997;20:2961–2964. doi: 10.1039/a704963c. [DOI] [Google Scholar]
- 31.Washida K., Abe N., Sugiyama Y., Hirota A. Novel secondary metabolites, spirosorbicillinols A, B, and C, from a fungus. Biosci. Biotechnol. Biochem. 2009;73:900151–900157. doi: 10.1271/bbb.90015. [DOI] [PubMed] [Google Scholar]
- 32.Kahlert L., Bassiony E.F., Cox R.J., Skellam E.J. Diels–Alder reactions during the biosynthesis of sorbicillinoids. Angew. Chem. Int. Ed. Engl. 2020;59:5816–5822. doi: 10.1002/anie.201915486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood J.L., Thompson B.D., Yusuff N., Pflum D.A., Matthäus M.S.P. Total synthesis of (±)-epoxysorbicillinol. J. Am. Chem. Soc. 2001;123:2097–2098. doi: 10.1021/ja0057979. [DOI] [PubMed] [Google Scholar]
- 34.Kontani M., Sakagami Y., Marumo S. First β-1,6-glucan biosynthesis inhibitor, bisvertinolone isolated from fungus, Acremonium strictum and its absolute stereochemistry. Tetrahedron Lett. 1994;35:2577–2580. doi: 10.1016/S0040-4039(00)77175-9. [DOI] [Google Scholar]
- 35.Trifonov L.S., Hilpert H., Floersheim P., Dreiding A.S., Rast D.M., Skrivanova R., Hoesch L. Bisvertinols: A new group of dimeric vertinoids from Verticillium intertextum. Tetrahedron. 1986;42:3157–3179. doi: 10.1016/S0040-4020(01)87382-8. [DOI] [Google Scholar]
- 36.Abe N., Murata T., Hirota A. Novel DPPH radical scavengers, bisorbicillinol and demethyltrichodimerol, from a fungus. Biosci. Biotechnol. Biochem. 1998;62:661–666. doi: 10.1271/bbb.62.661. [DOI] [PubMed] [Google Scholar]
- 37.Trifonov L.S., Bieri J.H., Prewo R., Dreiding A.S., Hoesch L., Rast D.M. Isolation and structure elucidation of three metabolites from Verticillium intertextum: Sorbicillin, dihydrosorbicillin and bisvertinoquinol. Tetrahedron. 1983;39:4243–4256. doi: 10.1016/S0040-4020(01)88647-6. [DOI] [Google Scholar]
- 38.Andrade R., Ayer W.A., Mebe P.P. The metabolites of Trichoderma longibrachiatum. Part 1. Isolation of the metabolites and the structure of trichodimerol. Can. J. Chem. 1992;70:2526–2535. doi: 10.1139/v92-320. [DOI] [Google Scholar]
- 39.Sperry S., Samuels G.J., Crews P. Vertinoid polyketides from the saltwater culture of the fungus Trichoderma longibrachiatum separated from a Haliclona marine sponge. J. Org. Chem. 1998;63:10011–10014. doi: 10.1021/jo9808122. [DOI] [Google Scholar]
- 40.Abe N., Yamamoto K., Hirota A. Novel fungal metabolites, demethylsorbicillin and oxosorbicillinol, isolated from Trichoderma sp. USF-2690. Biosci. Biotechnol. Biochem. 2000;64:620–622. doi: 10.1271/bbb.64.620. [DOI] [PubMed] [Google Scholar]
- 41.Nozoe S., Goi M., Morisaki N. Structure of cyclonerodiol. Tetrahedron Lett. 1970;11:1293–1296. doi: 10.1016/S0040-4039(01)91612-0. [DOI] [PubMed] [Google Scholar]
- 42.Rehman S.U., Yang L.J., Zhang Y.H., Wu J.S., Shi T., Haider W., Shao C.L., Wang C.Y. Sorbicillinoid derivatives from sponge-derived fungus Trichoderma reesei (HN-2016-018) Front. Microbiol. 2020;11:1334. doi: 10.3389/fmicb.2020.01334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harned A.M., Volp K.A. The sorbicillinoid family of natural products: Isolation, biosynthesis, and synthetic studies. Nat. Prod. Rep. 2011;28:1790–1810. doi: 10.1039/c1np00039j. [DOI] [PubMed] [Google Scholar]
- 44.Peng J., Zhang X., Du L., Wang W., Zhu T., Gu Q., Li D. Sorbicatechols A and B, antiviral sorbicillinoids from the marine-derived fungus Penicillium chrysogenum PJX-17. J. Nat. Prod. 2014;77:424–428. doi: 10.1021/np400977e. [DOI] [PubMed] [Google Scholar]
- 45.Guo W., Peng J., Zhu T., Gu Q., Keyzers R.A., Li D. Sorbicillamines A-E, nitrogen-containing sorbicillinoids from the deep-sea-derived fungus Penicillium sp. F23-2. J. Nat. Prod. 2013;76:2106–2112. doi: 10.1021/np4006647. [DOI] [PubMed] [Google Scholar]
- 46.Abe N., Arakawa T., Hirota A. The biosynthesis of bisvertinolone: Evidence for oxosorbicillinol as a direct precursor. Chem. Commun. 2002;3:204–205. doi: 10.1039/b109505f. [DOI] [PubMed] [Google Scholar]
- 47.Balde E.S., Andolfi A., Bruyere C., Cimmino A., Lamoral Theys D., Vurro M., Damme M.V., Altomare C., Mathieu V., Kiss R. Investigations of fungal secondary metabolites with potential anticancer activity. J. Nat. Prod. 2010;73:969–971. doi: 10.1021/np900731p. [DOI] [PubMed] [Google Scholar]
- 48.Reátegui R.F., Wicklow D.T., Gloer J.B. Phaeofurans and sorbicillin analogues from a fungicolous Phaeoacremonium species (NRRL 32148) J. Nat. Prod. 2006;69:113–117. doi: 10.1021/np0504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Warr G.A., Veitch J.A., Walsh A.W., Hesler G.A., Pirnik D.M., Leet J.E., Linm P.F.M., Medina I.A., Mcbrien K.D., Forenza S.F. BMS-182123, a fungal metabolite that inhibits the production of TNF-α by macrophages and monocytes. J. Antibiot. 1996;49:234–240. doi: 10.7164/antibiotics.49.234. [DOI] [PubMed] [Google Scholar]
- 50.Maskey R.P., Grün-Wollny I., Laatsch H. Sorbicillin analogues and related dimeric compounds from Penicillium notatum. J. Nat. Prod. 2005;68:865–870. doi: 10.1021/np040137t. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.