Abstract
Epithelial cells exfoliated in human urine can include cells anywhere from the urinary tract and kidneys; however, podocytes and proximal tubular epithelial cells (PTECs) are by far the most relevant cell types for the study of genetic kidney diseases. When maintained in vitro, they have been proven extremely valuable for discovering disease mechanisms and for the development of new therapies. Furthermore, cultured patient cells can individually represent their human sources and their specific variants for personalized medicine studies, which are recently gaining much interest. In this review, we summarize the methodology for establishing human podocyte and PTEC cell lines from urine and highlight their importance as kidney disease cell models. We explore the well-established and recent techniques of cell isolation, quantification, immortalization and characterization, and we describe their current and future applications.
Keywords: podocytes, proximal tubular epithelial cells, PTECs, urine-derived cells, glomerular diseases, Fanconi syndrome, renal tubular acidosis, inherited renal disorders, kidney disease cellular models
1. Introduction
Expansion of urine-derived epithelial cells in vitro was developed almost 50 years ago [1,2,3]. Although the initial focus for such research was mainly to study cancer cells originating from the urinary tract [4,5], it expanded rapidly to cover all types of kidney disorders, particularly genetic kidney diseases, as cells isolated from urine carry the genotypic background of patients [6,7,8,9]. The easy and non-invasive approach of harvesting epithelial cells from urine has lured scientists to master cell expansion in culture to use them as a powerful tool to study the pathogenesis of various renal diseases and to screen for new therapeutic modalities [10]. Recently, the potential therapeutic effect of some of the isolated urinary cells has been even suggested [11].
Epithelial cells voided in human urine can include cells anywhere from the urinary tract and kidneys. In women, vaginal epithelial cells are also normally shed in urine. Fine adjustment of culture conditions is necessary to properly isolate and expand each type of the target epithelial cells. Next, in depth characterization of viable cells lost in urine helps to identify their origin by studying the expression of cell surface markers, gene and protein expressions and their functionality [12].
For the study of genetic kidney disorders two types of epithelial cells stand out: podocytes forming the glomerular tuft and proximal tubular epithelial cells (PTECs) layering the proximal part of the renal tubules. Both cell types are actively involved in the pathogenesis of numerous inherited kidney disorders [10]. This review presents the recent and the most well-established techniques for isolation, handling and characterization of these cells and some applications of these cellular models for the study of genetic kidney diseases.
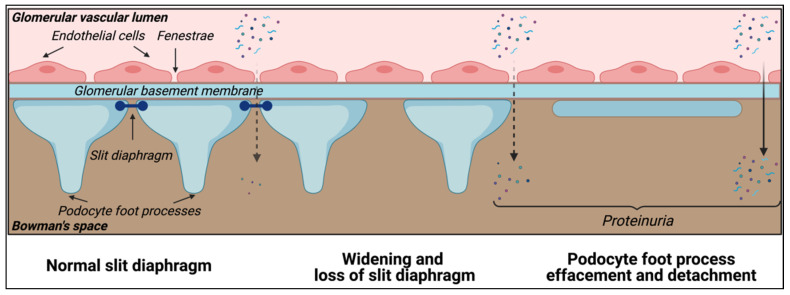
Podocytes are the main cellular components of the renal glomerular filtration barrier. They are highly differentiated cells preserved throughout the animal kingdom with the main function of retaining plasma proteins and blood cells [13]. The glomerular filtration barrier is responsible for the selective filtration of blood coming through the afferent arteriole to the Bowman’s space. The glomerular capillaries are lined with a fenestrated endothelium sitting on the glomerular basement membrane (GBM). The glomerular endothelium is covered by the glycocalyx, a surface layer comprising glycosaminoglycans, proteoglycans, glycoproteins and glycolipids that contribute to the glomerular permselectivity [14]. Podocytes are attached to the GBM via highly organized foot processes [15]. In between the foot processes are the slit diaphragms (Figure 1), which are the final barrier preventing the passage of proteins into the urinary filtrate.
Figure 1.
Podocytes: key element of the glomerular filtration barrier. Podocytes are highly differentiated cells with a specific cellular architecture, comprising primary and secondary foot processes that wrap around the glomerular capillaries, and express essential proteins that define the slit diaphragm and permselectivity of the glomerular filtration barrier. Kidney diseases affecting the podocytes (podocytopathies) can be characterized by the loss of the slit diaphragm, podocyte foot process effacement and detachment, which clinically manifests as glomerular proteinuria.
The filtrate that passes into the Bowman’s space continues into the proximal tubule and loop of Henle, distal tubules and collecting ducts for further processing [16].
There are currently over 60 genes, which produce important proteins that are reported to affect podocyte function and cause human glomerular disease when deficient. Pathogenic variants in these genes alter the dynamics of the glomerular filtration barrier changing podocyte morphology and their natural connection to each other, usually causing what is known as podocyte foot process effacement associated with widening of the slit diaphragmatic spaces [17]. This leads to proteinuria, renal tubular toxicity, gradual kidney function deterioration and eventually kidney failure [18]. When entering the process of foot process effacement, the foot process retraction and the replacement of the sealing of the filtration slits by occluding junctions results in increased attachment of the podocytes to the basement membrane. However, failure of the occluding junction formation leads to hyperfiltration and an expansile force on podocytes, leading to their detachment [19,20].
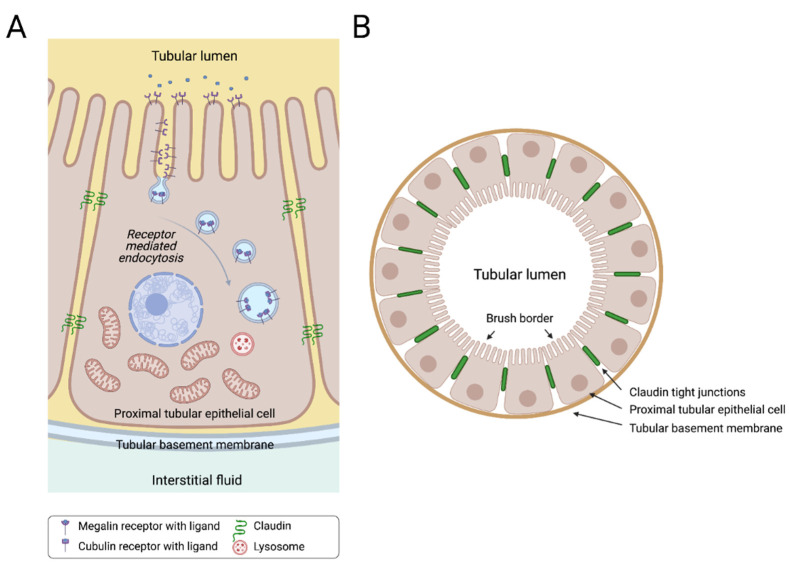
Proximal tubular epithelial cells (PTECs) are responsible for the reabsorption of the majority of water and solute load that passes through the glomerular filtrate. This reabsorption process takes place through specialized channels and transporters that allow the active transport of ions and different compounds in one direction only from the luminal side of the glomerular filtrate to the basolateral side back to the plasma (Figure 2). Receptor-mediated endocytosis is another essential function of PTECs aiming at the reabsorption of selected proteins and other nutrients, including carrier-bound vitamins, amino acids and trace elements, which are filtered by the glomeruli [21]. The multi-ligand receptors megalin and cubilin, also present in other luminal organs, coordinate the uptake of most filtered proteins and other small bioactive molecules from the lumen (Figure 2). Within the kidney, megalin is expressed extensively at the apical brush border and in the apical endocytic compartments of epithelial cells that comprise the early segment of the proximal tubule, with decreasing expression as it goes distally. Cubilin is also expressed abundantly in the proximal tubule and usually interacts with megalin to increase the multiligand binding potential of the complex [22].
Figure 2.
Sagittal (A) and cross (B) sections of a human proximal tubule. Proximal tubular epithelial cells (PTECs) are indispensable cells for the nephron tubular system. PTECs are highly metabolically active cells that are responsible for reabsorbing many of the filtered solutes in the proximal part of the nephron. These cells are typically characterized by a brush border at the apical side, and high numbers of mitochondria at the basal side of the cell. Megalin and cubulin are characteristic multi-ligand receptors for PTECS, located at the brush border, responsible for receptor-mediated endocytosis for various ligands.
The sophisticated function of the renal proximal tubule in regulating electrolyte balance, acid–base balance and many essential proteins, made it prone to numerous genetic abnormalities [23]. Over 25 genetic diseases have been linked with proximal tubular pathology. Many of these disorders are collectively referred to as proximal tubulopathies or renal Fanconi syndromes (when a generalized proximal tubular dysfunction is present). The basic pathology is the inability of the proximal tubule to reabsorb critical molecules, such as bicarbonate, calcium, phosphate and several proteins and vitamins. These disorders are commonly associated with severe morbidities, as the dysfunction of the proximal tubule usually results in significant metabolic disturbances [24].
2. Quantification of Podocytes and PTECs in Urine
Quantification of urine-derived epithelial cells has emerged as a valuable non-invasive clinical assessment method for detecting, studying and monitoring kidney disease [25]. Quantifying cell number in kidney specimens and in urine has become an intensive area of research. Since urine can be obtained non-invasively and repeatedly during the disease course, it is especially attractive as a cell source.
There are on average 1 million nephrons in each of the two kidneys, but the actual measured number might vary from 210,000 to 2.7 million [26]. Increased numbers of podocytes have been found in the urine of patients suffering from different glomerular diseases, in comparison with healthy individuals. In model systems, it has been demonstrated that a complete glomerular collapse occurs when podocyte cell number falls below 20% of original number. Since a normal human glomerulus has on average 500 podocytes [27], loss of more than 400 podocytes per glomerulus can cause kidney failure [28]. Podocyte loss (podocyturia) has been described in various kidney pathologies such as diabetic nephropathy [29,30], membranous nephropathy, glomerulosclerosis, Henoch-Schönlein nephritis, IgA and Lupus nephritis, diffuse mesangial sclerosis, Alport syndrome, APOL1-related nephropathy and cystinosis [10,29,31,32,33,34,35], for which cell models have been developed.
The general principle of quantifying urine-derived kidney epithelial cells is based on the identification of genes and proteins specific to these cells in fresh urine samples. These specific genes and proteins are mainly podocalyxin, nephrin, podocin and synaptopodin, which are markers of podocytes and aquaporin-1, megalin, cubilin and CD13, the markers of PTECs [6,25,36,37,38,39].
There are several methods used for the quantification of urine-derived epithelial cells. These methods include: colorimetric quantitative methods [6,37], flow cytometry [36], Western Blot [25], mass spectrometry [40] and quantitative real-time polymerase chain reaction (qRT-PCR) [25,38].
2.1. Colorimetric Quantitative Methods
Through immunohistochemistry, immunofluorescence and immunoassays such as the enzyme-linked immunosorbent assay (ELISA), different proteins specific to the kidney epithelial cells are targeted with monoclonal antibodies to allow the quantification of epithelial cells via an appropriate method [41,42]. These methods are widely used in detecting urinary epithelial cells [43,44,45,46] and are considered the “gold standard” because of the highly specific interactions between the protein of interest and the targeted antibody, and also their ability to detect a very low amount of proteins. Although these colorimetric methods are widely used, they still come with some limitations, including unspecific binding as urine can contain several proteins, exosomes or extracellular vesicles and cell debris, thereby leading to examiner errors/bias [37]. Another major drawback of this technique is the production of antibodies which are usually cost and time consuming and the lack of commercially available antibodies for all isoforms of the protein, as in the case of podocin. Recent evidence suggested that podocin may exist in both a canonical, well studied large isoform and an ill-defined short isoform [47].
2.2. Flow Cytometry
In this technique, the epithelial cells are sorted by utilizing highly specific antibodies labelled with fluorescent conjugates [25,36]. Flow cytometry has been demonstrated to be a very reliable, sensitive and accountable method in assessing kidney pathologies [48]. In many glomerular diseases such as IgA nephropathy, diabetic nephropathy and focal segmental glomerulosclerosis, flow cytometry has been used with immunofluorescence stain with podocalyxin antibody [49,50]. Nevertheless, the use of this method in quantifying cells has been limited due to unspecific binding, cost of the machine and its consumables and the high level of expertise required to operate the flow-cytometer. Many times, the low number of cells present in the urine sample makes sorting impractical.
2.3. Western Blot
This method is based on the use of specific monoclonal antibodies to detect a specific protein expressed in the target epithelial cells [25]. This method has been widely used in quantifying and detecting protein specific to kidney epithelial cells [6,8] because itis highly sensitive and specific even at a very low concentration (picogram level). However, the use of Western Blot is still limited partly due to the unspecific binding as a result of off-target interaction with other proteins, presence of exosomes and cell fragments, the lack of specific antibodies for all target proteins, especially the post-translational modified protein targets and high technical demand on the scientist [51].
2.4. Mass Spectrometry
The main principle behind this method is the use of tryptic peptides as surrogate markers for the quantification of their respective proteins. Therefore, by using high-performance liquid chromatography (HPLC) coupled with tandem mass spectrometry, one can easily detect a protein tryptic peptide specific for one of the kidney epithelial cells [40]. Mass spectrometry for quantifying urinary kidney epithelial cells was first demonstrated as a tool for detecting podocyturia in urine sediments of individuals with pre-eclampsia in 2012 [40]. In this study, Garovic et al. detected the podocin tryptic peptide in the urine of women with pre-eclampsia by using HPLC coupled with tandem mass spectrometry with the addition of an isotopically labelled podocin peptide [40]. Furthermore, efforts are currently being made towards using this technique to detect biomarkers of kidney epithelial cells in soluble fractions rather than in the urine sediment. Indeed, a research group in France has being able to quantify podocin and aquaporin-2 in human urine by using a new mass spectrometry approach, termed “liquid chromatography-multiple reaction monitoring cubed mass spectrometry [46,52].
The main advantages of this method are: it is operator-independent, highly reproducible method and considered highly sensitive. Therefore, it may be used for an earlier diagnosis of kidney pathologies compared to the other methods. Furthermore, this technique does not require the generation of antibodies (a problem associated with the colorimetry and Western Blot methods), can be easily multiplexed for simultaneous quantification of multiple proteins, and it is potentially applicable across species (provided the quantified peptides are conserved) [40]. Although this method has a great future potential, its main disadvantages include the high cost of the instrument, it is time-consuming and a high level of expertise is needed.
2.5. Quantitative Real-Time Polymerase Chain Reaction
Quantification of specific kidney epithelial cells markers mRNA in urine pellets by qRT-PCR has used to study various kidney diseases [6,8,53,54,55]. This methodology relies on the specific transcript expression of each cell type, and it is an indirect estimation of the number of cells present in urine normalized to calibration curves. The calibration curves are created by using known numbers of an established kidney epithelial cell line [6]. Some of the advantages of this technique are that it is potentially quantifiable, specific and sensitive and it can be multiplexed to quantify several mRNAs simultaneously [46].
Often, the number of cells present in urine is too low for good quality mRNA extraction using traditional techniques. Therefore, our group has recently optimized the methodology based on single-cell mRNA extraction protocols. Briefly, a calibration curve using known numbers of control human kidney-derived epithelial and mesenchymal cells was developed. To establish the calibration curve, cells were sorted by Fluorescence-Activated Cell Sorting (FACS) in a 96-well plate containing 4 mL of lysis buffer (0.2% TritonX-100 + RNase inhibitor) yielding a range of cells per well from 5 to 500 cells.
Next, we performed the Smart-seq2 protocol [56] allowing cDNA synthesis from a very low numbers of cells. We followed the protocol of Picelli et al. [56] up to PCR purification (step 26), and we ran18 PCR cycles in step 14 for pre-amplification of genes. Last, qPCR was performed using various housekeeping genes, which estimated the total number of cells. Using specific primers, we estimated the number of each kidney cell type shed in urine. The cycle threshold (ct) value for the specific targets achieved in the qPCR analysis was plotted in the calibration curve for extrapolation of the number of cells shed in urine, which was normalized to urine volume and urine creatinine values.
When quantifying cells from urine samples of patients with kidney pathologies, often more than 500 cells are present, therefore the cell pellets were re-suspended in phosphate-buffered saline (PBS) and diluted 100×. Although this method shows high specificity, overcomes bias and allows evaluation of samples containing very few cells, it still comes with its own limitations. It is an estimate and not an absolute quantification of cells, andis time consuming and requires specific expertise.
3. Isolation and Immortalization Techniques
Kidney epithelial cells have restricted proliferation capacity, therefore for establishing a cell line, they need to be immortalized prior to expansion.
3.1. Isolation of Kidney Epithelial Cells
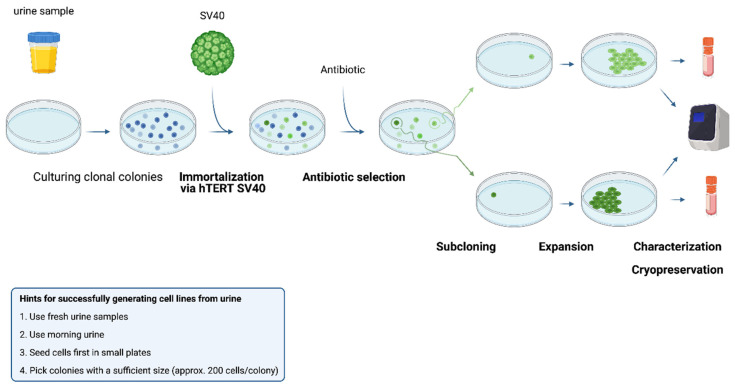
Kidney epithelial cells can be obtained from two main sources. First, the isolation of cells from the kidney tissue itself, resulting in cell lines from different kidney structures. However, the number of cells isolated from a tissue biopsy may be limited, and the isolation procedure is invasive and laborious. An alternative cell source is urine, which is the focus of this review. Both healthy individuals and patients suffering from kidney disease lose epithelial cells in urine [10]. Figure 3 shows a simplified scheme for the isolation of kidney cells from urine, the immortalization and expansion steps that follow. Isolating cells from urine is a rather simple and cost-effective process. Fresh urine is centrifuged at 200–300× g for 5 to 10 min at room temperature or 4 °C. The cell pellet is then re-suspended in the specific culture medium to selectively expand the targeted cells.
Figure 3.
Procedure of isolation, immortalization and subcloning of urine-derived kidney epithelial cells. Fresh urine is cultured in a specific medium that selects for specific types of kidney epithelial cells at 37 °C. Immortalization is performed by use of SV40 and the hTERT gene, upon which antibiotic selection is used to isolate the successfully immortalized cells. Clonal colonies are selected, in order to isolate and expand specific cell clones that will be characterized by biomarker expression and stored for cryopreservation.
3.2. Immortalization of Isolated Cells
As mentioned, primary cells derived from kidney tissue or urine are terminally differentiated and show a very limited proliferation rate in vitro. Therefore, the immortalization step is fundamental for further expansion of a cell line.
In 2005, Wilmer et al. immortalized PTECs from the urine of cystinosis patients and healthy controls, using HPV E6/E7 [57]. However, the immortalizing activity of HPV E6/E7 is generally very weak in human epithelial cells. Furthermore, the cystinotic cell models that were generated with HPV immortalization did not accumulate sufficient cystine to accurately represent the desired phenotype due to their high proliferation rate [58,59].
Ex vivo infection or transfection of primary cell lines with the Simian virus 40 (SV40) has also been shown to increase the lifespan of both epithelial and non-epithelial cells. The small icosahedral virion with a double-stranded DNA genome is a member of the Polyomaviridae family and SV40-based immortalization methods have been applied extensively [60]. In 1965, a growth stimulatory effect of the SV40 DNA sequence on a BHK21 (Baby Hamster Kidney 21) cell line was reported. Wiblin and MacPherson later also successfully transformed this cell line by co-culturing with a monkey cell line that was infected with the SV40 virus. A detailed description of the immortalizing capacity of the SV40 came in 1984 by Chang et al. [61]. It was proven that a specific part of the SV40 DNA was responsible for the immortalizing capacity of the virus. More specifically, two proteins in the early region of the viral sequence can mediate cell transformation, the large tumor (T) antigen and small T antigen. While the small T antigen promotes efficient viral genome replication by accelerating both the G1 and S phase progression during cell division, the large T antigen can interact with growth suppressors. Notably, the large T antigen carries an LXCXE motif that induces interaction with three proteins from the tumor suppressor retinoblastoma (pRB) family [62]. More specifically, the large T-antigen binds to the pRb-E2F complex, resulting in the dissociation of E2 Factor (E2F) from the complex and activation of specific gene expression patterns inducing cell growth. Additionally, the large T-antigen can also suppress the p53-pathway, an important tumor suppressor protein, and thereby induce cell proliferation [63]. Subsequently, the blocking of the pRB- and p53-dependant tumor suppression activity will drive the quiescent cells to enter the S-phase and escape apoptosis [62].
High and uncontrolled large T antigen expression by SV40-transformed cells comes with some disadvantages. The main concern is that the continuous expression of an immortalizing gene can significantly alter normal cellular physiology. Because of this problem, a temperature-sensitive mutant form of the SV40 DNA, the SV40tsA58, was developed [64,65]. In this mutant form, the 708 amino-acid large T antigen segment of the SV40 carries a temperature sensitive mutation. More specifically, an Ala438Val mutation lies within the ATP-binding fold and results in the stable expression of the tsA58 large T antigen (LT) variant at the permissive temperature (32–33.5 °C), inducing cell growth. However, shifting the cells to the restrictive temperature (37–39 °C) reduces the stability of this tsA58 mutant T antigen and eliminates its ability to bind to p53, resulting in growth arrest in either the G1 or G2 cell cycle phase. Because of this characteristic, these cells are called conditionally immortalized cell lines [66,67,68].
The urine-derived conditionally immortalized cystinosis PTEC cell model that was developed in 1995 by Racusen et al. was immortalized by using a recombinant retrovirus with a pZipneoU19 plasmid that carried the temperature-sensitive mutant form of the SV40 T antigen allele [66]. In 2002, Saleem et al. also created a conditional immortalized podocyte model from kidney tissue by using the SV40tsA58. Therefore, these cells grow at 33 °C, but enter growth arrest and differentiate at 37 °C [69]. Later, in 2010, urine-derived cell lines from glomerulosclerosis patients were also established using the temperature-sensitive mutant of the SV40 large T antigen [70]. Furthermore, Orosz et al. generated three tissue derived proximal tubular cell lines by using either the wild-type or the temperature sensitive SV40 large T antigen [68].
Notably, the proliferation of human cells can be limited by telomere-dependent replicative senescence due to the end-replication problem, which entails that the ends of the lagging DNA strand cannot be completely replicated during the cellular division, resulting in the continued shortening of the telomeres during each subsequent mitotic cycle [71]. In 1998, the transduction of telomerase-negative human cells with the catalytic subunit of the human telomerase reverse transcriptase (hTERT) gene was shown to successfully extend the life-span of these cells by stabilizing the telomeres [72]. In 2008, Wieser et al. were able to immortalize tissue-derived PTECs by inducing an overexpression of hTERT. This cell line still showed the original differentiation status and functionality. The early passage hTERT-immortalized cells were shown to faithfully represent the physiological properties of the in vivo situation [73].
While hTERT can be used by itself, it is often combined with a temperature-sensitive SV40 resulting in conditionally immortalized cells that are also protected against the end-replication problem and the resulting senescence. Early on, co-expression of hTERT with p53 and pRB inactivation by either siRNA or SV40 large T antigen was shown to successfully immortalize primary cells [72]. Furthermore, a combination of hTERT with the SV40 large T antigen was used to successfully immortalize kidney cells [74,75]. Currently, this combination is the most prevalent and has been extensively applied to immortalize both tissue- and urine-derived podocytes and PTECs by several groups [6,10,70,76].
4. Molecular and Functional Characterization of Kidney Derived Cell Lines
Once cells have been immortalized, a homogenous cell line can be obtained by subcloning [77].Subcloning is usually done by using irradiated NIH 3T3 mouse fibroblast cells as non-dividing feeder cells. Briefly, cells are seeded at densities of 100, 200, 300 and 400 cells per 25 cm2 flask and grown at 33 °C. Subsequently, feeder cells are added to each flask at a density of 0.5 × 106 cells/flask. After being cultured for about 21–28 days, clones derived from single cells become visible and are picked by using cloning discs drained in trypsin/EDTA and transferred to individual wells of a 24 well plate for expansion. At this stage, usually the fast-growing colonies are selected to be expanded further. Finally, when thecells are grown to confluence, they are further transferred to larger flasks, and each clone can be either cryopreserved or processed for characterization [69,76]. It is worth mentioning that subcloning of urinary kidney epithelial cells can also be done without feeder cells. Briefly, cells are seeded into a 96 well plate at densities of 0.5 and 1 cell per well. After 14 days, colonies derived from single cells become visible, and can be expanded [78].
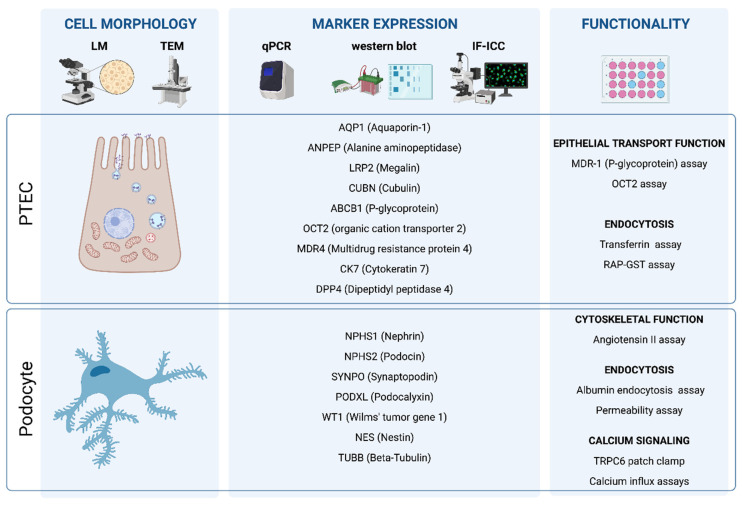
Afterwards, clonal cell lines need to be characterized to identify the most potent and specific ones. First, podocytes and PTECs can be identified by their distinct morphology. Second, at the molecular level, podocyte- and PTEC-specific markers can be used to distinguish these two cell types based on their specific gene expression patterns. This can be done at two levels, at the mRNA and at the protein level. Marker analysis can be done at the mRNA level by analyzing the cell specific genes with specific primers or by RNA-seq analysis (transcriptomics). At the protein level, the analysis of the gene expression pattern can be done by Western blotting, immunohistochemistry and flow cytometric-based methods. Finally, a functional characterization study is based on the distinct function of each cell type and confirms the differentiation of the immortalized cell lines to functional PTECs or podocytes [79,80]. Figure 4 represents a simplified scheme for the main steps of the molecular and functional characterization of PTECs and podocytes.
Figure 4.
Technology toolbox for characterization of urine-derived kidney epithelial cells. Urine-derived kidney epithelial cells are characterized based on their cellular morphology, the expression of cell-type specific markers, and their functionality via cell-specific assays. To this end, microscopy, including light microscopy (LM) and transmission electron microscopy (TEM), qPCR and Western Blot plus immunocytochemistry are useful technologies.
4.1. Characterization of Podocytes
Podocytes have a very distinct morphology in vitro, showing a cobblestone-like or arborized morphology by bright-field and phase-contrast microscopy.
Podocalyxin (PDXL) was one of the first marker genes used to characterize podocytes, but is not sufficient to prove the cell identity alone, since this protein is also present on other epithelial cells. The same condition is valid for the use of tight junction proteins (ZO-1) or intermediate filament proteins (Vimentin) [80]. Therefore, a panel of expression is normally used, in which expression of several of these podocyte genes is identified.
One of the most specific proteins that can be used as a podocyte marker is nephrin (NPHS1), a key structural transmembrane protein of 1241 amino acids in the slit diaphragm. Furthermore, nephrin also recruits other slit diaphragmatic proteins such as podocin (NPHS2) and CD2 associated protein (CD2AP), both of which can also be used for characterization [81,82]. However, it has been shown that urinary podocytes can undergo irreversible dedifferentiation in artificial culture conditions, often leading to the loss of nephrin expression in these 2D cell lines [83]. Synaptopodin (SYNPO) is an actin-associated protein, important in the dynamic podocyte cytoskeleton [84]. Synaptopodin is a key marker for differentiated podocytes and can be detected both at the RNA- and protein-level to characterize podocytes [80]. Podocin (NPHS2) was first described in in vitro podocyte cell lines in 2002 and is currently one of the standard markers for podocytes [85]. Wilms’ tumor gene 1 (WT1) is a master regulator of podocyte gene expression, with reduced expression related to glomerulonephritis and mesangial sclerosis [86]. While its exact function is not yet completely understood, WT1 expression on the RNA- and/or protein level has already been used extensively for podocyte characterization [87]. Finally, filament type cytoskeletal proteins like nestin (NES) and β-tubulin (TUBB), while not podocyte specific, are also part of the slit diaphragm and can be included in characterization studies [70]. The glomerular endothelial cells, the glomeular basement membrane, and the filtration slits between the podocytes perform the filtration function of the glomerulus, separating the blood in the capillaries from the filtrate that forms in Bowman’s capsule (Figure 1).
The attachment of podocytes to the glomerular basement membrane is granted through various proteins and the production and assembly of collagen type IV α-chains (COL4A3, COL4A4 and COL4A5). The expression of collagen IV α3, α4 and α5 is podocyte specific and has been applied for the characterization of mature and functional podocyte cell lines [8,88].Additionally, in kidney organoids and 3D podocyte cultures, type IV collagen α-chains show higher abundance and are indicative of basement membrane formation [89,90,91,92,93].
Interestingly, the filtration function of the basement membrane can be modeled in vitro by glomerular permeability assays. In principle, this assay measures the amount of fluorescent-labelled albumin passing through a monolayer of podocytes and endothelial cells. Glomerular endothelial cells are usually seeded on the bottom side of the PET porous membrane embedded in the central part of a small bioreactor, while the differentiated podocytes are seeded on the upper side. After 2 days of setting the co-cultures, the perfusion assay is performed. Firstly, during the filtration assay, 1 mg/mL albumin–fluorescein isothiocyanate (FITC) conjugate medium or normal podocyte medium (1 mL) is added to lower glomerular endothelial cells compartment and the podocyte compartment of the bioreactor, respectively. After 3 h of perfusion, the transit of albumin–FITC conjugate from the lower glomerular endothelial cells compartment to the upper podocyte compartment in the experimental conditions is measured by collecting separately the liquid coming out of the two compartments of the bioreactor. Lastly, the FITC signal is then measured in triplicate by using a fluorimeter. This method has been used to validate podocytes developed from patients with Alport syndrome [8].
Another assay that can be utilized to assess the functionality of podocyte cell lines is the endocytosis of labeled albumin [94]. Furthermore, the specific cytoskeleton arrangement of podocytes can be analyzed by exposing the cells to angiotensin II in vitro, which leads to cytoskeletal rearrangements. Angiotensin II exposure will also result in a change in gene expression. Therefore the re-analysis of the above-mentioned markers after exposure to angiotensin II can also be used to assess the functionality of an in vitro podocyte cell line [6,94,95]. Calcium signaling is of key importance in the podocyte for several functions, including the maintenance of the structural integrity of the slit diaphragm. In general, calcium influx can be evaluated via a Fura assay and the functionality of the receptor potential cation channel, subfamily C member 6 (TRPC6), a marker for differentiated podocytes critical for calcium homeostasis, can be evaluated via a whole-cell patch clamp assay. Via this technology, the increase in calcium influx through TRPC6 in functional podocytes can be assessed using oleoyl-2-acetyl-sn-glycerol (OAG)a known agonist of TRPC6 [95]. Using one or a combination of these methods, various podocyte cell lines have been developed to model and control podocyte-associated kidney disease.
4.2. Characterization of PTECs
In vivo, PTECs are tall and cuboidal in shape with an apical brush border and basolateral invaginations. However, this specific morphology is not present in unpolarized cells in culture [79] and their morphological identification is observed by their spindle-like orientation.
Marker analysis of PTECs relies on the specifically expressed Aquaporin 1 (AQP1), often combined with the detection of cytokeratin (CK) 18 and γ-glutamyl transferase (GGT). However, the latter two are not specific for the kidney, but only indicate the epithelial origin. The mRNA or protein expression of CD13, the Na/K-ATPase and protein aminopeptidase N (ANPEP) have also been used to establish the epithelial origin of cells, as well as the expression of dipeptidyl peptidase IV (DPP4) and the multidrug resistance protein 4 (MDR4) [76]. Another method that can be used to establish the cellular epithelial origin is by combining an antibody staining against cytokeratin 7 (CK7), a marker for epithelial cells, and vimentin (VIM), which is a marker for mesenchymal cells. In this context, PTECs will be positive for cytokeratin 7 and negative for vimentin [96].
Lima et al. suggested that the expression of zonula occludens 1 (ZO-1)–associated nucleic acid binding protein (ZONAB) promotes proliferation, but represses the differentiation of these cell lines [97]. In order to confirm the polarization of PTEC cells for further characterization, two specific markers can be utilized. Immunostaining of the zonulin-1 (ZO-1) tight junction protein will reveal a grid-like structure in a confluent layer of PTECs, indicating a clear polarization and monolayer, while the microtubule end-binding protein (EB1) is also a marker of polarization [98,99,100,101].
When using a PTEC model, specific PTEC-functions need to be evaluated. The functionality of PTECs can be established by analyzing the epithelial transport, the main function of the proximal tubule in the excretion pathway. The uptake of albumin and phosphate can be used to assess the functionality of the endocytosis and sodium dependent uptake. Intracellular levels of albumin after endocytosis-mediated uptake in functional PTECs can be measured by flow cytometry or the uptake of a labelled albumin-fluorescein isothiocyanate conjugate can be analyzed by fluorescent imaging. Liquid scintillation counting can be used to assess the sodium dependent uptake of a labelled phosphate molecule (32PO4). Additionally, the functionality of the OCT influx proteins can be investigated by exposing the cells to the substrate: (4-(dimethylamino)styryl)-N-methylpyridiniumiodide (ASPþ) in the presence or absence of 5 mM OCT inhibitor tetrapentylammonium chloride (TPA). For P-glycopotein activity, the PTECs can be examined by measuring the accumulation of a fluorescence molecule that is formed by the intracellular cleavage of calcein-AM. Cells with a functional P-gp will rapidly extrude the calcein and reduce the accumulation, which can be assessed by fluorescence measurement. Transport characteristics of the ABCG2 and MRP4 renal efflux transporter can be assessed by liquid scintillation counting or imaging after incubating the cells with labeled kynurenic acid or with Chloromethylfluorescein-diacetate (CMFDA) respectively and an MRP inhibitor. GST-RAP or the transferrin endocytosis assay can be utilized to study the functionality of the megalin–cubilin receptor complex by analyzing the endocytosis process itself [102,103,104,105,106,107].
Functionality of the multiligand receptors that are responsible for endocytosis, megalin (LRP2) and cubilin (CUBN) are pivotal in PTEC lines. However, while cubilin often shows a clear expression at both the mRNA and protein level, the expression and functionality of megalin is not always as clear, due to the fact that an in vitro cell line will not always be polarized [102]. A similar problem has been seen with AQP1, while the staining of this channel in kidney tissue shows a clear apical distribution, in vitro cell lines usually show a more evenly distributed pattern due to the absence of polarization, underlining the important difference between 2D cell culture, and the in vivo situation [108].
When using PTEC models for drug testing, the expression of organic anion transporters (OATs) is often desired, including OAT1/SLC22A6 and OAT3/SLC22A8. However, OAT expression is often lacking in 2D cultures of immortalized PTECs, as is the case with HK-2 cell lines. Therefore, some OAT-overexpressing lines have been developed for the investigation of drugs, but the expression can also be ensured by using 3D cell cultures or primary human tubular cell monolayers, both of which have established OAT-expression [109,110,111,112]. Using one or a combination of these methodologies, various PTEC cell lines have been developed to model and control tubule-associated kidney disease.
4.3. The Choice of Control Cell Lines
Often, the viability and functionality of cell lines isolated from urine is questioned, but their effectiveness has been proven several times. Clear comparison of PTECs derived from tissue versus from urine showed similar functionality, as both expressed functional transporters and receptors, as well as similar physiology. This underlines that both isolation methods are equally successful in establishing cell models, while urine outstands due to non-invasive isolation and less laborious practices.
Patients suffering from kidney diseases are characterized by the highest degree of cell loss in the urine. However, healthy individuals also shed viable kidney cells in urine, and expansion of those applying the same method of isolation and immortalization as described above is possible and may serve as control to cells from renal patients [66]. Still, the generation of control cell lines is limited by the small number of viable cells [76]. It is important to consider that healthy donors are those that have not been diagnosed with kidney disease, which does not exclude the possibility of unknown damage leading to cells detachment, thus the processes of cell characterization and selection are extremely important to ensure the functionality of the urine-derived control cells before being used in experiments. In this regard, several studies have compared properly selected control cells from urine with cells isolated directly from the kidney and proved their similarities and functionality, underlining the potential of these cell lines as wild type controls for the study of models of kidney diseases [10,77].
It has been demonstrated that about 1.7 podocytes are lost per glomerulus per year in healthy humans [113], while this number is highly exceeded in disease conditions and during aging [114]. Wilmer et al. collected mid-stream urine of 38 healthy volunteers and found that 10% of the collected urine sediments contained viable cells with proliferative capacity in vitro. Based on the results from the characterization study on these proximal tubular cells, one clone from one donor was selected for further use. This study introduced the first human cell line that featured a functional sodium-dependent reabsorption and endocytosis for up to 40 passages [10,76].
Vogelmann et al. used the cytospin method to quantify the podocyte loss in patients with active glomerular disease and healthy volunteers. It was found that all but one of the healthy volunteers had a urinary podocyte content of <0.5 podocytes/mg creatinine. Furthermore, 12 of the 27 healthy individuals that were studied had podocytes in at least one of their urine samples. All of these volunteers had viable podocytes in their urine sediments, with 60% of the healthy controls growing podocytes in culture and the growth pattern resembling that of cell lines established from glomerular explants [115].
As an alternative for the limitations imposed by low proliferation of fully differentiated epithelial cells, our laboratory has developed kidney stem/progenitor cells lines derived from the urine of healthy preterm neonates [99] as a prototype of podocyte and tubular disease model. These cells have high proliferative capacity and can differentiate into fully mature and functional podocytes and PTECs in vitro, overcoming the immortalization process, maintaining the intrinsic properties of the donor and serving as control kidney epithelial cells.
4.4. 2D versus 3D Models
In vivo, cell–cell and cell–matrix interactions are important in controlling the cell phenotype and cell function. However, Two-dimensional (2D) cell cultures rarely reproduce these conditions, and the absence of the cellular microenvironment can hamper the in vitro cell model-based research. Three-dimensional (3D) cell culture systems and organoids have been manufactured for the kidney and have been shown to reproduce the kidney-specific functions more accurately than 2D models [116]. Furthermore, the generation of these 3D models can improve the ability to cure and manage kidney diseases, model the kidney development and could revolutionize the world of kidney replacement therapy by allowing the in vitro production of transplantable kidneys [117]. Co-culture techniques have been successfully applied to mimic specific tissues. In 2011, Slater et al. used the co-culturing method to generate a functional tri-layer in vitro model of the glomerular filtration barrier, comprising podocytes and glomerular epithelial cells on an artificial membrane [118].
More complex kidney 3D cell cultures can be also generated from immortalized cells, as is the case in the bioartificial kidney. Janssen et al. have had great success in developing a bioartificial kidney model, by culturing conditionally immortalized PTECs on hollow fiber membranes, resulting in the formation of a tight monolayer, as indicated by the expression of ZO-1. In this model, they could establish the functionality of the OCT2 transporter [119]. Later, the same group generated tubuloids, and adult intestinal stem cell derived epithelial organoids that can be expanded for many passages in growth factor-rich medium, while still remaining genetically stable. The Lgr5+ intestinal stem cells were isolated, and the epithelial elements expanded. This model showed proximal tubule specific gene expression patterns (ABCC1, ABCC3, ABCC4, SLC22A3, SLC40A1), as well as markers for the collecting duct (CDH1, GATA3, AQP3), loop of Henle (CLDN10) and distal tubule (PCBD1, SLC41A3). The functionality of these tubuloids was established by confirming the functionality of the P-glycoprotein. These tubuloid models consist entirely of epithelial tissue and were used for modeling infectious kidney diseases, kidney malignancies and cystic fibrosis. In these tubuloid models, a stem cell/progenitor state can also be induced to form more heterogenous 3D structures. During this state, the tubuloids express higher levels of CD24, CD44, CD133, SOX9 and Vimentin [120,121]. Recently, tubuloids have been integrated into the organ-on-a-chip system to more closely mimic the tubular microenvironment with extracellular matrix and medium perfusion, a technique that excludes the need for an artificial membrane for cell attachment [122].
5. Utility of Urine-Derived Epithelial Cells as Models for Genetic Kidney Diseases
Epithelial cellular models have been valuable tools in the arsenal to study pathophysiological aspects of genetic renal diseases for so long. In most studies, they are usually the essential first step before experimenting with animal models. The type of representative cells in culture, whether human or animal and the approaches to obtain them vary among studies. For podocytes and PTECs, kidney-derived cells from a biopsy were the most common route of obtaining cells for primary culture. This was usually followed by immortalization and knocking-out the target gene to create the disease model of interest. However, when patients’ urine-derived cells were introduced, they gained a lot of interest for their apparent advantages. Urine-derived epithelial cells are easily obtained and propagated in culture, they faithfully represent the disease and the patients they were derived from better than knocked-out models and they are more suitable for experimenting with new therapeutic modalities particularly when immortalized. Table 1 and Table 2 summarize the various types of published cellular models representing genetic diseases associated with podocyte and proximal tubular cellular pathology, respectively.
Table 1.
Cell models of genetic podocytopathies.
| Gene (OMIM) | Disease (OMIM) | Inheritance | Origin of Podocyte Models |
|---|---|---|---|
| ACTN4 (604638) | Focal segmental glomerulosclerosis 1 (603278) | AD | |
| ANLN (616027) | Focal segmental glomerulosclerosis 8 (616032) | AD | |
| APOL1 (603743) | Focal segmental glomerulosclerosis 4, susceptibility to (612551) | ||
| ARHGAP24 (610586) | Focal segmental glomerulosclerosis | AD |
|
| ARHGDIA (601925) | Steroid resistant nephrotic syndrome, type 8 (615244) | AR | |
| AVIL (613397) | Steroid resistant nephrotic syndrome, type 21 (618594) | AR |
|
| CD2AP (604241) | Focal segmental glomerulosclerosis, 3 (607832) | AD | |
| CDC42 (116952) | Takenouchi–Kosaki syndrome (616737) | AD |
|
| CDK20 (610076) | Steroid resistant nephrotic syndrome | AR |
|
| CLCN5 (300008) | Dent disease (300009) | XLR |
|
| COL4A3 (120070) | Alport syndrome 2 (203780) Alport syndrome 3 (104200) |
AR AD |
|
| COL4A4 (120131) | Alport syndrome 2 (203780) | AR | |
| COL4A5 (303630) | Alport syndrome 1, X-linked (301050) | XLD | |
| COQ6 (614647) | Coenzyme Q10 deficiency, primary, 6 (614650) | AR | |
| COQ8B (615567) | Steroid resistant nephrotic syndrome, type 9 (615573) | AR | |
| CRB2 (609720) | Focal segmental glomerulosclerosis 9 (616220) | AR |
|
| CTNS (606272) | Nephropathic cystinosis (219800) | AR | |
| CUBN (602997) | Proteinuria, chronic benign (618884) | AR |
|
| DAAM2 (606627) | Steroid resistant nephrotic syndrome | AR |
|
| DGKE (601440) | Steroid resistant nephrotic syndrome, 7 (615008) | AR |
|
| DLC1 (604258) | Steroid resistant nephrotic syndrome | AR |
|
| EMP2 (602334) | Steroid resistant nephrotic syndrome, 10 (615861) | AR | |
| FAT1 (600976) | Glomerulotubular nephropathy | AR |
|
| GLA (300644) | Fabry disease (301500) | XL | |
| INF2 (610982) | Focal segmental glomerulosclerosis, 5 (613237) | AD | |
| ITGA3 (605025) | Interstitial lung disease, nephrotic syndrome, and epidermolysis bullosa, congenital (614748) | AR |
|
| ITSN1 (602442) | Steroid resistant nephrotic syndrome | AR |
|
| ITSN2 (604464) | Steroid resistant nephrotic syndrome | AR |
|
| KANK1 (607704) | Cerebral palsy, spastic quadriplegic, 2 (612900) | AD/AR |
|
| KANK2 (614610) | Steroid resistant nephrotic syndrome, 16 (617783) | AR |
|
| KANK4 (614612) | Steroid resistant nephrotic syndrome | AR |
|
| LAGE3 (300060) | Galloway–Mowat syndrome 2 (301006) | XLR |
|
| LAMB2 (150325) | Steroid resistant nephrotic syndrome, type 5, with or without ocular abnormalities (614199) | AR |
|
| LMX1B (602575) | Focal segmental glomerulosclerosis 10 (256020) Nail-patella syndrome (161200) |
AD AD |
|
| MAGI2 (606382) | Steroid resistant nephrotic syndrome, type 15 (617609) | AR |
|
| MYH9 (160775) | Macrothrombocytopenia and granulocyte inclusions with nephritis (155100) | AD | |
| MYO1E (601479) | Focal segmental glomerulosclerosis, 6 (614131) | AR | |
| MYO9A (604875) | Focal segmental glomerulosclerosis | AD |
|
| NPHS1 (602716) | Steroid resistant nephrotic syndrome, type 1 (256300) | AR |
|
| NPHS2 (604766) | Steroid resistant nephrotic syndrome, type 2 (600995) | AR | |
| NUP85 (170285) | Steroid resistant nephrotic syndrome, type 17 (618176) | AR |
|
| NUP93 (614351) | Steroid resistant nephrotic syndrome, type 12 (616892) | AR |
|
| NUP107 (607617) | Steroid resistant nephrotic syndrome, type 11 (616730) Galloway–Mowat syndrome 7 (618348) |
AR AR |
|
| NUP133 (607613) | Steroid resistant nephrotic syndrome, type 18 (618177) Galloway–Mowat syndrome 8 (618349) |
AR AR |
|
| NUP160 (607614) | Steroid resistant nephrotic syndrome, type 19 (618178) | AR |
|
| NUP205 (614352) | Steroid resistant nephrotic syndrome, type 13 (616893) | AR |
|
| OSGEP (610107) | Galloway–Mowat syndrome 3 (617729) | AR |
|
| PAX2 (167409) | Focal segmental glomerulosclerosis, 7 (616002) | AD |
|
| PDSS2 (610564) | Coenzyme Q10 deficiency, primary, 3 (614652) | AR |
|
| PLCE1 (608414) | Steroid resistant nephrotic syndrome, type 3 (610725) | AR |
|
| PODXL (602632) | Focal segmental glomerulosclerosis | AD/AR |
|
| SGPL1 (603729) | Steroid resistant nephrotic syndrome, type 14 (617575) | AR |
|
| SYNPO | Steroid resistant nephrotic syndrome | AR | |
| TBC1D8B (301027) | Steroid resistant nephrotic syndrome, type 20 (301028) | XL | |
| TNS2 (607717) | Steroid resistant nephrotic syndrome | AR |
|
| TP53RK (608679) | Galloway–Mowat syndrome 4 (617730) | AR |
|
| TPRKB (608680) | Galloway–Mowat syndrome 5 (617731) | AR |
|
| TRPC6 (603652) | Glomerulosclerosis, focal segmental, 2 (603965) | AD | |
| TTC21B (612014) | Nephronophthisis 12 (613820) | AD/AR |
|
| WDR73 (616144) | Galloway–Mowat syndrome 1 (251300) | AR | |
| WT1 (607102) | Steroid resistant nephrotic, type 4 (256370) | AD |
|
| XPO5 (607845) | Steroid resistant nephrotic syndrome | AR |
|
AD, autosomal dominant; AR, autosomal recessive; XL, X-linked.
Table 2.
Cell models of genetic proximal tubular diseases.
| Gene (OMIM) | Disease (OMIM) | Inheritance | Origin of PTEC Models |
|---|---|---|---|
| CLCN5 (300008) | Dent disease (300009) | XLR | |
| CTNS (606272) | Nephropathic cystinosis (219800) | AR | |
| CUBN (602997) | Imerslund–Grasbeck syndrome 1(261100) | AR |
|
| EHHADH (607037) | Fanconi renotubular syndrome 3 (615605) | AD |
|
| GATM (602360) | Fanconi renotubular syndrome 1 (134600) | AD |
|
| HNF4A (600281) | Fanconi renotubular syndrome 4, with maturity-onset diabetes of the young (616026) | AD |
|
| LRP2 (600073) | Donnai–Barrow syndrome (222448) | AR |
|
| OCRL (300535) | Lowe syndrome (309000) Dent disease type 2 (300555) |
XLR XLR |
|
| SLC3A1 (104614) | Cystinuria (220100) | AD/AR |
|
| SLC4A4 (603345) | Renal tubular acidosis, proximal, with ocular abnormalities (604278) | AR |
|
| SLC4A5 (606757) | Renal tubular acidosis, proximal | AR |
|
| SLC6A19 (608893) | Iminoglycinuria (242600) Hyperglycinuria (138500) |
AD/AR AD |
|
| SLC7A7 (603593) | Lysinuric protein intolerance (222700) | AR |
|
| SLC7A9 (604144) | Cystinuria (220100) | AD/AR |
|
| SLC34A1 (182309) | Fanconi renotubular syndrome 2 (613388) | AR |
|
| SLC36A2 (608331) | Iminoglycinuria (242600) Hyperglycinuria (138500) |
AD/AR AD |
|
| TRPC3 (602345) | Spinocerebellar ataxia 41 (616410) | AD |
|
AD, autosomal dominant; AR, autosomal recessive; XL, X-linked.
Revealing novel pathophysiological aspects of genetic renal syndromes is an important field of research, in which epithelial cell models were extensively used in recent years, particularly the urine-derived cells. Rossi et al. investigated the expression of the Nucleotide-binding oligomerization domain, Leucine rich Repeat and Pyrin domain containing (NLRP) family members in human conditionally immortalized PTECs derived from the urine of cystinosis patients. They discovered the essential role of the inflammasome protein NLRP2 in regulating proinflammatory, profibrotic and antiapoptotic responses in cystinotic PTECs, through NF-κB activation [193]. Ivanova et al. studied ATP-induced, IP3-induced and lysosomal calcium release in human PTECs derived from the urine of controls and cystinotic patients and reported the sensitization of ATP-induced calcium release, but with no major dysregulation of intracellular calcium dynamics in cystinotic cells [210]. Uzureau et al. studied conditionally immortalized podocytes derived from the urine of patients with G1 and G2 APOL1 genotypes. They reported that APOL1 C-terminal variants may induce kidney disease by preventing APOL3 from activating PI4KB, with consecutive actomyosin reorganization of podocytes [129].
Proper confirmation of the genetic etiology in suspected but undiagnosed patients is yet another recent use of urine-derived epithelial cells. Pinto et al. reported the significance of urine-derived podocytes as an important source for genomic DNA for the detection of cryptic mosaicism in X-linked Alport syndrome in a mother that was supposed to donate her kidney to her child. Although her peripheral blood DNA was devoid of pathogenic variants, the DNA derived from podocytes revealed a frame-shift pathogenic variant in COL4A5 gene confirming the Alport syndrome diagnosis in the mother. This should prompt the reevaluation of living-donor kidney transplantation guidelines in various genetic kidney diseases [211]. Furthermore, the pathogenicity of splicing variants could be easily confirmed at the transcript level in primary podocytes derived from urine of Alport syndrome patients with intronic variants in COL4A3, COL4A4 or COL4A5 genes [139].
Another major aspect of in vitro studies concerning the kidney is the ability of cellular models to predict the potential human kidney response to various drugs. These studies include the evaluation of potential drug-induced toxicity over the kidney, examination of drug–drug interactions and/or the evaluation of therapeutic effects of the drug in a certain condition. Renal proximal tubules are major players orchestrating nutrient retrieval and waste disposal from the body, including most drugs and their metabolites. Thus, acute or chronic nephrotoxicity is a huge concern during the development process of any drug; particularly that renal insult is often recognized late during clinical trials in many cases [110]. Genetic kidney diseases are particularly sensitive to the study of novel therapeutic agents, as the kidneys are already impacted by their primary disease. Hence, faithful in vitro and in vivo models of the genetic disorder are indispensable when a new drug is introduced.
Moreover, another advantage of cellular models of genetic diseases is their versatility and ability to serve as a target for high-throughput drug screenings. De Leo et al. screened the Prestwick chemical library including 1200 small molecular weight compounds in a urine-derived ciPTEC model of nephropathic cystinosis looking for drugs that reduce levels of the autophagy-related protein p62/SQSTM, since altered autophagy is a major contributing factor to the pathophysiology of the disease. Of the 46 compounds significantly reducing p62/SQSTM1 levels in cystinotic cells, they chose luteolin to study further based on its efficacy and safety profiles. In their study, luteolin had anti-apoptotic properties, was a powerful antioxidant and could stimulate endocytosis through enhancing the expression of the endocytic receptor megalin, which are all beneficial effects for the cystinotic kidney [107].
6. Conclusions and Future Perspectives
In conclusion, the urine-derived podocytes and PTECs offer many advantages over established cellular models derived from the kidney. They are normally shed in urine, thus no need to extract by a biopsy procedure. Developing de novo genetic disease models is simply done by extracting the cells directly from affected patients and propagating them in culture after characterization. No need to use an in vitro knocking-out procedure, with all the efficiency issues to worry about during establishing the knock-out model. Urine-derived podocytes and PTECs are fully viable and functional and can represent the target disease faithfully if the proper clone is selected during the characterization process.
Although the numbers of urine-derived podocyte and PTEC models developed to study genetic kidney diseases are rapidly increasing over the last few years, most diseases affecting these cells still lack representative urine-derived models [6,8,100,107]. With the new era of personalized medicine in genetic kidney diseases, not only uncovering the genetic background and linking this genotype to its clinical phenotype is required, but also investigating the individualized response of each patient to various management strategies, in what is known as personalized therapy [17,212,213]. Relevant cellular models can be excellent targets for such research, particularly those obtained directly from diseased individuals in a non-invasive way, such as urine.
Recently, trials for gene therapy and cellular therapy of genetic kidney diseases have been conceptualized in animal models [214,215,216]. These trials are particularly useful in podocyte loss syndromes, since glomerular podocytes are terminally differentiated epithelial cells and unable to proliferate. Human urine-derived renal progenitor cells are a good potential source of podocyte progenitors for cell therapy applications, as they can differentiate in vivo at sites of injured glomeruli into de novo podocytes after intravenous administration [10,11,217].
Author Contributions
Conceptualization, M.A.E., F.O.A., T.B. and K.R.P.V.; data curation, M.A.E., F.O.A., T.B. and O.C.A.; writing—original draft preparation, M.A.E., F.O.A., T.B. and O.C.A.; writing—review and editing, M.A.E., F.O.A., T.B., O.C.A., K.R.P.V., E.L. and L.P.v.d.H.; supervision, M.A.E., F.O.A., E.L. and L.P.v.d.H.; project administration, M.A.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sutherland G.R., Bain A.D. Culture of cells from the urine of newborn children. Nature. 1972;239:231. doi: 10.1038/239231a0. [DOI] [PubMed] [Google Scholar]
- 2.Linder D. Culture of cells from the urine and bladder washings of adults. Somatic Cell Genet. 1976;2:281–283. doi: 10.1007/BF01538966. [DOI] [PubMed] [Google Scholar]
- 3.Felix J.S., Sun T.T., Littlefield J.W. Human epithelial cells cultured from urine: Growth properties and keratin staining. Vitr. J. Tissue Cult. Assoc. 1980;16:866–874. doi: 10.1007/BF02619424. [DOI] [PubMed] [Google Scholar]
- 4.Elliott A.Y., Bronson D.L., Stein N., Fraley E.E. In Vitro Cultivation of Epithelial Cells Derived from Tumors of the Human Urinary Tract. Cancer Res. 1976;36:365–369. [PubMed] [Google Scholar]
- 5.Elliott A.Y., Bronson D.L., Cervenka J., Stein N., Fraley E.E. Properties of Cell Lines Established from Transitional Cell Cancers of the Human Urinary Tract. Cancer Res. 1977;37:1279–1289. [PubMed] [Google Scholar]
- 6.Ivanova E.A., Arcolino F.O., Elmonem M.A., Rastaldi M.P., Giardino L., Cornelissen E.M., Van Den Heuvel L.P., Levtchenko E.N. Cystinosin deficiency causes podocyte damage and loss associated with increased cell motility. Kidney Int. 2016;89:1037–1048. doi: 10.1016/j.kint.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 7.Molinari E., Decker E., Mabillard H., Tellez J., Srivastava S., Raman S., Wood K., Kempf C., Alkanderi S., Ramsbottom S.A., et al. Human urine-derived renal epithelial cells provide insights into kidney-specific alternate splicing variants. Eur. J. Hum. Genet. 2018;26:1791–1796. doi: 10.1038/s41431-018-0212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iampietro C., Bellucci L., Arcolino F.O., Arigoni M., Alessandri L., Gomez Y., Papadimitriou E., Calogero R.A., Cocchi E., Van Den Heuvel L., et al. Molecular and functional characterization of urine-derived podocytes from patients with Alport syndrome. J. Pathol. 2020;252:88–100. doi: 10.1002/path.5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Molinari E., Srivastava S., Dewhurst R.M., Sayer J.A. Use of patient derived urine renal epithelial cells to confirm pathogenicity of PKHD1 alleles. BMC Nephrol. 2020;21 doi: 10.1186/s12882-020-02094-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oliveira Arcolino F., Tort Piella A., Papadimitriou E., Bussolati B., Antonie D.J., Murray P., Van Den Heuvel L., Levtchenko E., Arcolino F.O., Piella A.T., et al. Human urine as a noninvasive source of kidney cells. Stem Cells Int. 2015;2015 doi: 10.1155/2015/362562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pavathuparambil Abdul Manaph N., Al-Hawaas M., Bobrovskaya L., Coates P.T., Zhou X.F. Urine-derived cells for human cell therapy. Stem Cell Res. Ther. 2018;9:189. doi: 10.1186/s13287-018-0932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Romih R., Korošec P., De Mello W., Jezernik K. Differentiation of epithelial cells in the urinary tract. Cell Tissue Res. 2005;320:259–268. doi: 10.1007/s00441-004-1005-4. [DOI] [PubMed] [Google Scholar]
- 13.Trimarchi H. Mechanisms of Podocyte Detachment, Podocyturia, and Risk of Progression of Glomerulopathies. Kidney Dis. 2020;6:324–329. doi: 10.1159/000507997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Butler M.J., Down C.J., Foster R.R., Satchell S.C. The Pathological Relevance of Increased Endothelial Glycocalyx Permeability. Am. J. Pathol. 2020;190:742–751. doi: 10.1016/j.ajpath.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahdenkari A.T., Lounatmaa K., Patrakka J., Holmberg C., Wartiovaara J., Kestilä M., Koskimies O., Jalanko H. Podocytes are firmly attached to glomerular basement membrane in kidneys with heavy proteinuria. J. Am. Soc. Nephrol. 2004;15:2611–2618. doi: 10.1097/01.ASN.0000139478.03463.D9. [DOI] [PubMed] [Google Scholar]
- 16.Garg P. A Review of Podocyte Biology. Am. J. Nephrol. 2018;47:3–13. doi: 10.1159/000481633. [DOI] [PubMed] [Google Scholar]
- 17.Kopp J.B., Anders H.J., Susztak K., Podestà M.A., Remuzzi G., Hildebrandt F., Romagnani P. Podocytopathies. Nat. Rev. Dis. Prim. 2020;6 doi: 10.1038/s41572-020-0196-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baines R.J., Brunskill N.J. Tubular toxicity of proteinuria. Nat. Rev. Nephrol. 2011;7:177–180. doi: 10.1038/nrneph.2010.174. [DOI] [PubMed] [Google Scholar]
- 19.Kriz W., Lemley K.V. A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J. Am. Soc. Nephrol. 2015;26:258–269. doi: 10.1681/ASN.2014030278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kriz W., Hähnel B., Hosser H., Rösener S., Waldherr R. Structural analysis of how podocytes detach from the glomerular basement membrane under hypertrophic stress. Front. Endocrinol. (Lausanne) 2014;5 doi: 10.3389/fendo.2014.00207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saito A., Sato H., Iino N., Takeda T. Molecular mechanisms of receptor-mediated endocytosis in the renal proximal tubular epithelium. J. Biomed. Biotechnol. 2010;2010:403272. doi: 10.1155/2010/403272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eshbach M.L., Weisz O.A. Receptor-Mediated Endocytosis in the Proximal Tubule. Annu. Rev. Physiol. 2017;79:425–448. doi: 10.1146/annurev-physiol-022516-034234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Downie M.L., Lopez Garcia S.C., Kleta R., Bockenhauer D. Inherited Tubulopathies of the Kidney. Clin. J. Am. Soc. Nephrol. 2020;16:CJN.14481119. doi: 10.2215/CJN.14481119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemaire M. Novel Fanconi renotubular syndromes provide insights in proximal tubule pathophysiology. Am. J. Physiol. Ren. Physiol. 2021;320:F145–F160. doi: 10.1152/ajprenal.00214.2020. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Hernandez J., Olivares M.D., Forner M.J., Chaves F.J., Cortes R., Redon J. Urinary dedifferentiated podocytes as a non-invasive biomarker of lupus nephritis. Nephrol. Dial. Transplant. 2016;31:780–789. doi: 10.1093/ndt/gfw002. [DOI] [PubMed] [Google Scholar]
- 26.Puelles V.G., Hoy W.E., Hughson M.D., Diouf B., Douglas-Denton R.N., Bertram J.F. Glomerular number and size variability and risk for kidney disease. Curr. Opin. Nephrol. Hypertens. 2011;20:7–15. doi: 10.1097/MNH.0b013e3283410a7d. [DOI] [PubMed] [Google Scholar]
- 27.Puelles V.G., Douglas-Denton R.N., Cullen-McEwen L.A., Li J., Hughson M.D., Hoy W.E., Kerr P.G., Bertram J.F. Podocyte number in children and adults: Associations with glomerular size and numbers of other glomerular resident cells. J. Am. Soc. Nephrol. 2015;26:2277–2288. doi: 10.1681/ASN.2014070641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kikuchi M., Wickman L., Hodgin J.B., Wiggins R.C. Podometrics as a Potential Clinical Tool for Glomerular Disease Management. Semin. Nephrol. 2015;35:245–255. doi: 10.1016/j.semnephrol.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakamura T., Ushiyama C., Suzuki S., Hara M., Shimada N., Ebihara I., Koide H. Urinary excretion of podocytes in patients with diabetic nephropathy. Nephrol. Dial. Transplant. 2000;15:1379–1383. doi: 10.1093/ndt/15.9.1379. [DOI] [PubMed] [Google Scholar]
- 30.Al-Malki A.L. Assessment of urinary osteopontin in association with podocyte for early predication of nephropathy in diabetic patients. Dis. Markers. 2014;2014 doi: 10.1155/2014/493736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hara M., Yanagihara T., Takada T., Itoh M., Matsuno M., Yamamoto T., Kihara I. Urinary excretion of podocytes reflects disease activity in children with glomerulonephritis. Am. J. Nephrol. 1998;18:35–41. doi: 10.1159/000013302. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura T., Ushiyama C., Suzuki S., Hara M., Shimada N., Sekizuka K., Ebihara I., Koide H. Urinary podocytes for the assessment of disease activity in lupus nephritis. Am. J. Med. Sci. 2000;320:112–116. doi: 10.1097/00000441-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Bollain-Y-Goytia J.J., González-Castañeda M., Torres-Del-Muro F., Daza-Benitez L., Zapata-Benavides P., Rodríguez-Padilla C., Avalos-Díaz E., Herrera-Esparza R. Increased excretion of urinary podocytes in lupus nephritis. Indian J. Nephrol. 2011;21:166–171. doi: 10.4103/0971-4065.83029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakamura T., Ushiyama C., Shimada N., Sekizuka K., Ebihara I., Hara M., Koide H. Effect of cyclophosphamide or azathioprine on urinary podocytes in patients with diffuse proliferative lupus nephritis. Nephron. 2001;87:192–193. doi: 10.1159/000045913. [DOI] [PubMed] [Google Scholar]
- 35.Garovic V.D., Wagner S.J., Turner S.T., Rosenthal D.W., Watson W.J., Brost B.C., Rose C.H., Gavrilova L., Craigo P., Bailey K.R., et al. Urinary podocyte excretion as a marker for preeclampsia. Am. J. Obstet. Gynecol. 2007;196:320.e1–320.e7. doi: 10.1016/j.ajog.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 36.Mella A., Deambrosis I., Mingozzi S., Colla L., Burdese M., Giaretta F., Bruno S., Camussi G., Boaglio E., Dolla C., et al. Detection of urinary podocytes by flow cytometry in idiopathic membranous nephropathy. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-73335-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimura M., Toyoda M., Saito N., Kaneyama N., Miyatake H., Tanaka E., Komaba H., Hara M., Fukagawa M. A liquid-based cytology system, without the use of cytocentrifugation, for detection of podocytes in urine samples of patients with diabetic nephropathy. J. Diabetes Res. 2019:1–7. doi: 10.1155/2019/9475637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naik A.S., Le D., Aqeel J., Wang S.Q., Chowdhury M., Walters L.M., Cibrik D.M., Samaniego M., Wiggins R.C. Podocyte stress and detachment measured in urine are related to mean arterial pressure in healthy humans. Kidney Int. 2020;98:699–707. doi: 10.1016/j.kint.2020.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goerlich N., Brand H.A., Langhans V., Tesch S., Schachtner T., Koch B., Paliege A., Schneider W., Grützkau A., Reinke P., et al. Kidney transplant monitoring by urinary flow cytometry: Biomarker combination of T cells, renal tubular epithelial cells, and podocalyxin-positive cells detects rejection. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-57524-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garovic V.D., Craici I.M., Wagner S.J., White W.M., Brost B.C., Rose C.H., Grande J.P., Barnidge D.R. Mass spectrometry as a novel method for detection of podocyturia in pre-eclampsia. Nephrol. Dial. Transplant. 2013;28:1555–1561. doi: 10.1093/ndt/gfs074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Trimarchi H. Podocyturia: Potential applications and current limitations. World J. Nephrol. 2017;6:221. doi: 10.5527/wjn.v6.i5.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siwińska N., Pasławska U., Bąchor R., Szczepankiewicz B., Żak A., Grocholska P., Szewczuk Z. Evaluation of podocin in urine in horses using qualitative and quantitative methods. PLoS ONE. 2020;15:e0240586. doi: 10.1371/journal.pone.0240586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li J.Z., Chen Y.Q., Wang S.X., Pang W., Jie E., Liu Y., Qin X.Q. Clinical significance of urine sediment spectrum analyses in crescentic glomerulonephritis. Natl. Med. J. China. 2010;90:1978–1981. doi: 10.3760/cma.j.issn.0376-2491.2010.28.010. [DOI] [PubMed] [Google Scholar]
- 44.Achenbach J., Mengel M., Tossidou I., Peters I., Park J.K., Haubitz M., Ehrich J.H., Haller H., Schiffer M. Parietal epithelia cells in the urine as a marker of disease activity in glomerular diseases. Nephrol. Dial. Transplant. 2008;23:3138–3145. doi: 10.1093/ndt/gfn235. [DOI] [PubMed] [Google Scholar]
- 45.Spadło A., Wyka K., Kowalewska-Pietrzak M., Bodalski J. Evaluation of the podocytes in urine in the nephrotic syndrome in childhood. Pol. Merkur. Lek. 2007;22:254–257. [PubMed] [Google Scholar]
- 46.Simon R., Lemoine J.Ô., Fonbonne C., Jaffuel A., Léonard J.F., Gautier J.C., Pasquier O., Salvador A. Absolute quantification of podocin, a potential biomarker of glomerular injury in human urine, by liquid chromatography-multiple reaction monitoring cubed mass spectrometry. J. Pharm. Biomed. Anal. 2014;94:84–91. doi: 10.1016/j.jpba.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 47.Völker L.A., Schurek E.M., Rinschen M.M., Tax J., Schutte B.A., Lamkemeyer T., Ungrue D., Schermer B., Benzing T., Höhne M. Characterization of a short isoform of the kidney protein podocin in human kidney. BMC Nephrol. 2013;14:102. doi: 10.1186/1471-2369-14-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suwanpen C., Nouanthong P., Jaruvongvanich V., Pongpirul K., Pongpirul W.A., Leelahavanichkul A., Kanjanabuch T. Urinary podocalyxin, the novel biomarker for detecting early renal change in obesity. J. Nephrol. 2016;29:37–44. doi: 10.1007/s40620-015-0199-8. [DOI] [PubMed] [Google Scholar]
- 49.Ye H., Bai X., Gao H., Li L., Wu C., Sun X., Zhang C., Shen Y., Zhang J., Lu Z. Urinary podocalyxin positive-element occurs in the early stage of diabetic nephropathy and is correlated with a clinical diagnosis of diabetic nephropathy. J. Diabetes Complications. 2014;28:96–100. doi: 10.1016/j.jdiacomp.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 50.Habara P., Marecková H., Malícková K., Potyšová Z., Hrušková Z., Zima T., Tesar V. Novel flow cytometric method for the detection of podocalyxin-positive elements in urine of patients with glomerulonephritides -first promising results. Folia Biol. 2012;58:57–63. [PubMed] [Google Scholar]
- 51.Ghosh R., Gilda J.E., Gomes A.V. The necessity of and strategies for improving confidence in the accuracy of western blots. Expert Rev. Proteomics. 2014;11:549–560. doi: 10.1586/14789450.2014.939635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaffuel A., Lemoine J., Aubert C., Simon R., Léonard J.F., Gautier J.C., Pasquier O., Salvador A. Optimization of liquid chromatography-multiple reaction monitoring cubed mass spectrometry assay for protein quantification: Application to aquaporin-2 water channel in human urine. J. Chromatogr. A. 2013;1301:122–130. doi: 10.1016/j.chroma.2013.05.068. [DOI] [PubMed] [Google Scholar]
- 53.Zheng M., Lv L.-L., Ni J., Ni H.-F., Li Q., Ma K.-L., Liu B.-C. Urinary Podocyte-Associated mRNA profile in Various Stages of Diabetic Nephropathy. PLoS ONE. 2011;6:e20431. doi: 10.1371/journal.pone.0020431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hosoyamada M., Yan K., Nishibori Y., Takiue Y., Kudo A., Kawakami H., Shibasaki T., Endou H. Nephrin and podocin expression around the onset of puromycin aminonucleoside nephrosis. J. Pharmacol. Sci. 2005;97:234–241. doi: 10.1254/jphs.FP0040802. [DOI] [PubMed] [Google Scholar]
- 55.Sato Y., Wharram B.L., Sang K.L., Wickman L., Goyal M., Venkatareddy M., Jai W.C., Wiggins J.E., Lienczewski C., Kretzler M., et al. Urine podocyte mRNAs mark progression of renal disease. J. Am. Soc. Nephrol. 2009;20:1041–1052. doi: 10.1681/ASN.2007121328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Picelli S., Faridani O.R., Björklund Å.K., Winberg G., Sagasser S., Sandberg R. Full-length RNA-seq from single cells using Smart-seq2. Nat. Protoc. 2014;9:171. doi: 10.1038/nprot.2014.006. [DOI] [PubMed] [Google Scholar]
- 57.Wilmer M.J.G., De Graaf-Hess A., Blom H.J., Dijkman H.B.P.M., Monnens L.A., Van Den Heuvel L.P., Levtchenko E.N. Elevated oxidized glutathione in cystinotic proximal tubular epithelial cells. Biochem. Biophys. Res. Commun. 2005;337:610–614. doi: 10.1016/j.bbrc.2005.09.094. [DOI] [PubMed] [Google Scholar]
- 58.Halbert C.L., Demers G.W., Galloway D.A. The E6 and E7 genes of human papillomavirus type 6 have weak immortalizing activity in human epithelial cells. J. Virol. 1992;66:2125–2134. doi: 10.1128/jvi.66.4.2125-2134.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wilmer M.J., Emma F., Levtchenko E.N. The pathogenesis of cystinosis: Mechanisms beyond cystine accumulation. Am. J. Physiol. Physiol. 2010;299:F905–F916. doi: 10.1152/ajprenal.00318.2010. [DOI] [PubMed] [Google Scholar]
- 60.Carbone M. Encyclopedic Reference of Cancer. Springer; Berlin/Heidelberg, Germany: 2006. SV40; pp. 861–865. [Google Scholar]
- 61.Chang L.S., Pater M.M., Hutchinson N.I., Mayorca G. Di Transformation by purified early genes of simian virus 40. Virology. 1984;133:341–353. doi: 10.1016/0042-6822(84)90400-8. [DOI] [PubMed] [Google Scholar]
- 62.Ahuja D., Sáenz-Robles M.T., Pipas J.M. SV40 large T antigen targets multiple cellular pathways to elicit cellular transformation. Oncogene. 2005;24:7729–7745. doi: 10.1038/sj.onc.1209046. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y., Chen S., Yan Z., Pei M. A prospect of cell immortalization combined with matrix microenvironmental optimization strategy for tissue engineering and regeneration. Cell Biosci. 2019;9:7. doi: 10.1186/s13578-018-0264-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Krtil J., Pláteník J., Kazderová M., Tesař V., Zima T. Culture Methods of Glomerular Podocytes. Kidney Blood Press. Res. 2007;30:162–174. doi: 10.1159/000102520. [DOI] [PubMed] [Google Scholar]
- 65.Bens M., Vandewalle A. Cell models for studying renal physiology. Pflugers Arch. Eur. J. Physiol. 2008;457:1–15. doi: 10.1007/s00424-008-0507-4. [DOI] [PubMed] [Google Scholar]
- 66.Racusen L.C., Wilson P.D., Hartz P.A., Fivush B.A., Burrow C.R. Renal proximal tubular epithelium from patients with nephropathic cystinosis: Immortalized cell lines as in vitro model systems. Kidney Int. 1995;48:536–543. doi: 10.1038/ki.1995.324. [DOI] [PubMed] [Google Scholar]
- 67.Cheng J., DeCaprio J.A., Fluck M.M., Schaffhausen B.S. Cellular transformation by Simian Virus 40 and Murine Polyoma Virus T antigens. Semin. Cancer Biol. 2009;19:218–228. doi: 10.1016/j.semcancer.2009.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Orosz D.E., Woost P.G., Kolb R.J., Finesilver M.B., Jin W., Frisa P.S., Choo C.K., Yau C.F., Chan K.W., Resnick M.I., et al. Growth, immortalization, and differentiation potential of normal adult human proximal tubule cells. Vitr. Cell. Dev. Biol. Anim. 2004;40:22–34. doi: 10.1290/1543-706X(2004)40<22:GIADPO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 69.Saleem M.A., O’Hare M.J., Reiser J., Coward R.J., Inward C.D., Farren T., Chang Y.X., Ni L., Mathieson P.W., Mundel P. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J. Am. Soc. Nephrol. 2002;13:630–638. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 70.Sakairi T., Abe Y., Kajiyama H., Bartlett L.D., Howard L.V., Jat P.S., Kopp J.B. Conditionally immortalized human podocyte cell lines established from urine. Am. J. Physiol. Physiol. 2010;298:F557–F567. doi: 10.1152/ajprenal.00509.2009. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71.Ohki R., Tsurimoto T., Ishikawa F. In Vitro Reconstitution of the End Replication Problem. Mol. Cell. Biol. 2001;21:5753–5766. doi: 10.1128/MCB.21.17.5753-5766.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bodnar A.G., Ouellette M., Frolkis M., Holt S.E., Chiu C.P., Morin G.B., Harley C.B., Shay J.W., Lichtsteiner S., Wright W.E. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–352. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- 73.Wieser M., Stadler G., Jennings P., Streubel B., Pfaller W., Ambros P., Riedl C., Katinger H., Grillari J., Grillari-Voglauer R. hTERT alone immortalizes epithelial cells of renal proximal tubules without changing their functional characteristics. Am. J. Physiol. Ren. Physiol. 2008;295:1365–1375. doi: 10.1152/ajprenal.90405.2008. [DOI] [PubMed] [Google Scholar]
- 74.O’Hare M.J., Bond J., Clarke C., Takeuchi Y., Atherton A.J., Berry C., Moody J., Silver A.R.J., Davies D.C., Alsop A.E., et al. Conditional immortalization of freshly isolated human mammary fibroblasts and endothelial cells. Proc. Natl. Acad. Sci. USA. 2001;98:646–651. doi: 10.1073/pnas.98.2.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Counter C.M., Hahn W.C., Wei W., Caddle S.D., Beijersbergen R.L., Lansdorp P.M., Sedivy J.M., Weinberg R.A. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc. Natl. Acad. Sci. USA. 1998;95:14723–14728. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilmer M.J., Saleem M.A., Masereeuw R., Ni L., van der Velden T.J., Russel F.G., Mathieson P.W., Monnens L.A., Van Den Heuvel L.P., Levtchenko E.N. Novel conditionally immortalized human proximal tubule cell line expressing functional influx and efflux transporters. Cell Tissue Res. 2010;339:449–457. doi: 10.1007/s00441-009-0882-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zia S., Toelen J., Mori da Cunha M., DeKoninck P., de Coppi P., Deprest J. Routine clonal expansion of mesenchymal stem cells derived from amniotic fluid for perinatal applications. Prenat. Diagn. 2013;33:921–928. doi: 10.1002/pd.4162. [DOI] [PubMed] [Google Scholar]
- 78.Afzal M.Z., Gartz M., Klyachko E.A., Khan S.S., Shah S.J., Gupta S., Shapiro A.D., Vaughan D.E., Strande J.L. Generation of human iPSCs from urine derived cells of a non-affected control subject. Stem Cell Res. 2017;18:33–36. doi: 10.1016/j.scr.2016.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mihevc M., Petreski T., Maver U., Bevc S. Renal proximal tubular epithelial cells: Review of isolation, characterization, and culturing techniques. Mol. Biol. Rep. 2020;47:9865–9882. doi: 10.1007/s11033-020-05977-4. [DOI] [PubMed] [Google Scholar]
- 80.Shankland S.J., Pippin J.W., Reiser J., Mundel P. Podocytes in culture: Past, present, and future. Kidney Int. 2007;72:26–36. doi: 10.1038/sj.ki.5002291. [DOI] [PubMed] [Google Scholar]
- 81.Yaddanapudi S., Altintas M.M., Kistler A.D., Fernandez I., Möller C.C., Wei C., Peev V., Flesche J.B., Forst A.L., Li J., et al. CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. J. Clin. Investig. 2011;121:3965–3980. doi: 10.1172/JCI58552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Veissi S., Smeets B., van den Heuvel L.P., Schreuder M.F., Jansen J. Nephrotic syndrome in a dish: Recent developments in modeling in vitro. Pediatric Nephrol. 2020;35:1363–1372. doi: 10.1007/s00467-019-4203-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Petermann A.T., Krofft R., Blonski M., Hiromura K., Vaughn M., Pichler R., Griffin S., Wada T., Pippin J., Durvasula R., et al. Podocytes that detach in experimental membranous nephropathy are viable. Kidney Int. 2003;64:1222–1231. doi: 10.1046/j.1523-1755.2003.00217.x. [DOI] [PubMed] [Google Scholar]
- 84.Yanagida-Asanuma E., Asanuma K., Kim K., Donnelly M., Hoon Y.C., Jae H.C., Suetsugu S., Tomino Y., Takenawa T., Faul C., et al. Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am. J. Pathol. 2007;171:415–427. doi: 10.2353/ajpath.2007.070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Coward R.J.M., Foster R.R., Patton D., Ni L., Lennon R., Bates D.O., Harper S.J., Mathieson P.W., Saleem M.A. Nephrotic plasma alters slit diaphragm-dependent signaling and translocates nephrin, podocin, and CD2 associated protein in cultured human podocytes. J. Am. Soc. Nephrol. 2005;16:629–637. doi: 10.1681/ASN.2004030172. [DOI] [PubMed] [Google Scholar]
- 86.Guo J.K., Menke A.L., Gubler M.C., Clarke A.R., Harrison D., Hammes A., Hastie N.D., Schedl A. WT1 is a key regulator of podocyte function: Reduced expression levels cause crescentic glomerulonephritis and mesangial sclerosis. Hum. Mol. Genet. 2002;11:651–659. doi: 10.1093/hmg/11.6.651. [DOI] [PubMed] [Google Scholar]
- 87.Davidson G., Dono R., Zeller R. FGF signalling is required for differentiation-induced cytoskeletal reorganisation and formation of actin-based processes by podocytes PubMed. J. Cell Sci. 2001;114:3359–3366. doi: 10.1242/jcs.114.18.3359. [DOI] [PubMed] [Google Scholar]
- 88.Zhang H.D., Huang J.N., Liu Y.Z., Ren H., Xie J.Y., Chen N. Endoplasmic reticulum stress and proteasome pathway involvement in human podocyte injury with a truncated COL4A3 mutation. Chin. Med. J. 2019;132:1823–1832. doi: 10.1097/CM9.0000000000000294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Borza C.M., Borza D.B., Pedchenko V., Saleem M.A., Mathieson P.W., Sado Y., Hudson H.M., Pozzi A., Saus J., Abrahamson D.R., et al. Human podocytes adhere to the KRGDS motif of the α3α4α5 collagen IV network. J. Am. Soc. Nephrol. 2008;19:677–684. doi: 10.1681/ASN.2007070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sacco D., Lemley S.V., Sedrakyan K.V., Zanusso S., Petrosyan I. A Novel Source of Cultured Podocytes. PLoS ONE. 2013;8:81812. doi: 10.1371/journal.pone.0081812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Abrahamson D.R., Hudson B.G., Stroganova L., Borza D.B., St. John P.L. Cellular origins of type IV collagen networks in developing glomeruli. J. Am. Soc. Nephrol. 2009;20:1471–1479. doi: 10.1681/ASN.2008101086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ardaillou N., Lelongt B., Turner N., Piedagnel R., Baudouin B., Estrade S., Cassingena R., Ronco P.M. Characterization of a simian virus 40-transformed human podocyte cell line producing type IV collagen and exhibiting polarized response to atrial natriuretic peptide. J. Cell. Physiol. 1992;152:599–616. doi: 10.1002/jcp.1041520320. [DOI] [PubMed] [Google Scholar]
- 93.Hale L.J., Howden S.E., Phipson B., Lonsdale A., Er P.X., Ghobrial I., Hosawi S., Wilson S., Lawlor K.T., Khan S., et al. 3D organoid-derived human glomeruli for personalised podocyte disease modelling and drug screening. Nat. Commun. 2018;9:1–17. doi: 10.1038/s41467-018-07594-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ciampi O., Iacone R., Longaretti L., Benedetti V., Graf M., Magnone M.C., Patsch C., Xinaris C., Remuzzi G., Benigni A., et al. Generation of functional podocytes from human induced pluripotent stem cells. Stem Cell Res. 2016;17:130–139. doi: 10.1016/j.scr.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arcolino F.O., Zia S., Held K., Papadimitriou E., Theunis K., Bussolati B., Raaijmakers A., Allegaert K., Voet T., Deprest J., et al. Urine of preterm neonates as a novel source of kidney progenitor cells. J. Am. Soc. Nephrol. 2016;27:2762–2770. doi: 10.1681/ASN.2015060664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dörrenhaus A., Müller J.I.F., Golka K., Jedrusik P., Schulze H., Föllmann W. Cultures of exfoliated epithelial cells from different locations of the human urinary tract and the renal tubular system. Arch. Toxicol. 2000;74:618–626. doi: 10.1007/s002040000173. [DOI] [PubMed] [Google Scholar]
- 97.Lima W.R., Parreira K.S., Devuyst O., Caplanusi A., N’Kuli F., Marien B., Van Der Smissen P., Alves P.M.S., Verroust P., Christensen E.I., et al. ZONAB promotes proliferation and represses differentiation of proximal tubule epithelial cells. J. Am. Soc. Nephrol. 2010;21:478–488. doi: 10.1681/ASN.2009070698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Narayanan K., Schumacher K.M., Tasnim F., Kandasamy K., Schumacher A., Ni M., Gao S., Gopalan B., Zink D., Ying J.Y. Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int. 2013;83:593–603. doi: 10.1038/ki.2012.442. [DOI] [PubMed] [Google Scholar]
- 99.Reda A., Raaijmakers A., van Dorst S., Pauwels C.G.G.M., Allegaert K., Elmonem M.A., Masereeuw R., van den Heuvel L., Levtchenko E., Arcolino F.O. A Human Proximal Tubular Epithelial Cell Model to Explore a Knowledge Gap on Neonatal Drug Disposition. Curr. Pharm. Des. 2017;23:5911–5918. doi: 10.2174/1381612823666171009143146. [DOI] [PubMed] [Google Scholar]
- 100.Gorvin C.M., Wilmer M.J., Piret S.E., Harding B., Van Den Heuvel L.P., Wrong O., Jat P.S., Lippiat J.D., Levtchenko E.N., Thakker R.V. Receptor-mediated endocytosis and endosomal acidification is impaired in proximal tubule epithelial cells of Dent disease patients. Proc. Natl. Acad. Sci. USA. 2013;110:7014–7019. doi: 10.1073/pnas.1302063110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nehlig A., Molina A., Rodrigues-Ferreira S., Honoré S., Nahmias C. Regulation of end-binding protein EB1 in the control of microtubule dynamics. Cell. Mol. Life Sci. 2017;74:2381–2393. doi: 10.1007/s00018-017-2476-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ivanova E.A., De Leo M.G., Van Den Heuvel L., Pastore A., Dijkman H., De Matteis M.A., Levtchenko E.N. Endo-lysosomal dysfunction in human proximal tubular epithelial cells deficient for lysosomal cystine transporter cystinosin. PLoS ONE. 2015;10:1–18. doi: 10.1371/journal.pone.0120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kuzmuk V. Effects of Disease-Causing Mutations of Podocin on Podocyte Biology. University of Bristol; Bristol, UK: 2019. [Google Scholar]
- 104.Jansen J., Schophuizen C.M.S., Wilmer M.J., Lahham S.H.M., Mutsaers H.A.M., Wetzels J.F.M., Bank R.A., Van den Heuvel L.P., Hoenderop J.G., Masereeuw R. A morphological and functional comparison of proximal tubule cell lines established from human urine and kidney tissue. Exp. Cell Res. 2014;323:87–99. doi: 10.1016/j.yexcr.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 105.Glavinas H., Von Richter O., Vojnits K., Mehn D., Wilhelm I., Nagy T., Janossy J., Krizbai I., Couraud P., Krajcsi P. Calcein assay: A high-throughput method to assess P-gp inhibition. Xenobiotica. 2011;41:712–719. doi: 10.3109/00498254.2011.587033. [DOI] [PubMed] [Google Scholar]
- 106.Vriend J., Peters J.G.P., Nieskens T.T.G., Škovroňová R., Blaimschein N., Schmidts M., Roepman R., Schirris T.J.J., Russel F.G.M., Masereeuw R., et al. Flow stimulates drug transport in a human kidney proximal tubule-on-a-chip independent of primary cilia. Biochim. Biophys. Acta Gen. Subj. 2020;1864 doi: 10.1016/j.bbagen.2019.129433. [DOI] [PubMed] [Google Scholar]
- 107.De Leo E., Elmonem M.A., Berlingerio S.P., Berquez M., Festa B.P., Raso R., Bellomo F., Starborg T., Janssen M.J., Abbaszadeh Z., et al. Cell-based phenotypic drug screening identifies luteolin as candidate therapeutic for nephropathic cystinosis. J. Am. Soc. Nephrol. 2020;31:1522–1537. doi: 10.1681/ASN.2019090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kapałczyńska M., Kolenda T., Przybyła W., Zajączkowska M., Teresiak A., Filas V., Ibbs M., Bliźniak R., Łuczewski Ł., Lamperska K. 2D and 3D cell cultures—A comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018;14:910–919. doi: 10.5114/aoms.2016.63743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bajaj P., Chowdhury S.K., Yucha R., Kelly E.J., Xiao G. Emerging kidney models to investigate metabolism, transport, and toxicity of drugs and xenobiotics. Drug Metab. Dispos. 2018;46:1692–1702. doi: 10.1124/dmd.118.082958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nieskens T.T.G., Peters J.G.P., Schreurs M.J., Smits N., Woestenenk R., Jansen K., van der Made T.K., Röring M., Hilgendorf C., Wilmer M.J., et al. A Human Renal Proximal Tubule Cell Line with Stable Organic Anion Transporter 1 and 3 Expression Predictive for Antiviral-Induced Toxicity. AAPS J. 2016;18:465–475. doi: 10.1208/s12248-016-9871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fedecostante M., Westphal K.G.C., Buono M.F., Romero N.S., Wilmer M.J., Kerkering J., Baptista P.M., Hoenderop J.G., Masereeuw R. Recellularized native kidney scaffolds as a novel tool in nephrotoxicity screening s. Drug Metab. Dispos. 2018;46:1338–1350. doi: 10.1124/dmd.118.080721. [DOI] [PubMed] [Google Scholar]
- 112.Brown C.D.A., Sayer R., Windass A.S., Haslam I.S., De Broe M.E., D’Haese P.C., Verhulst A. Characterisation of human tubular cell monolayers as a model of proximal tubular xenobiotic handling. Toxicol. Appl. Pharmacol. 2008;233:428–438. doi: 10.1016/j.taap.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 113.Li M., Balamuthusamy S., Simon E.E., Batuman V. Silencing megalin and cubilin genes inhibits myeloma light chain endocytosis and ameliorates toxicity in human renal proximal tubule epithelial cells. Am. J. Physiol. Ren. Physiol. 2008;295 doi: 10.1152/ajprenal.00091.2008. [DOI] [PubMed] [Google Scholar]
- 114.Hodgin J.B., Bitzer M., Wickman L., Afshinnia F., Wang S.Q., O’Connor C., Yang Y., Meadowbrooke C., Chowdhury M., Kikuchi M., et al. Glomerular aging and focal global glomerulosclerosis: A podometric perspective. J. Am. Soc. Nephrol. 2015;26:3162–3178. doi: 10.1681/ASN.2014080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Puelles V.G., Cullen-McEwen L.A., Taylor G.E., Li J., Hughson M.D., Kerr P.G., Hoy W.E., Bertram J.F. Human podocyte depletion in association with older age and hypertension. Am. J. Physiol. Ren. Physiol. 2016;310:F656–F668. doi: 10.1152/ajprenal.00497.2015. [DOI] [PubMed] [Google Scholar]
- 116.Vogelmann S.U., Nelson W.J., Myers B.D., Lemley K.V. Urinary excretion of viable podocytes in health and renal disease. Am. J. Physiol. Ren. Physiol. 2003;285 doi: 10.1152/ajprenal.00404.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Astashkina A.I., Mann B.K., Prestwich G.D., Grainger D.W. A 3-D organoid kidney culture model engineered for high-throughput nephrotoxicity assays. Biomaterials. 2012;33:4700–4711. doi: 10.1016/j.biomaterials.2012.02.063. [DOI] [PubMed] [Google Scholar]
- 118.Romero-Guevara R., Ioannides A., Xinaris C. Kidney Organoids as Disease Models: Strengths, Weaknesses and Perspectives. Front. Physiol. 2020;11:1384. doi: 10.3389/fphys.2020.563981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Slater S.C., Beachley V., Hayes T., Zhang D., Welsh G.I., Saleem M.A., Mathieson P.W., Wen X., Su B., Satchell S.C. An in vitro model of the glomerular capillary wall using electrospun collagen nanofibres in a bioartificial composite basement membrane. PLoS ONE. 2011;6 doi: 10.1371/journal.pone.0020802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jansen J., De Napoli I.E., Fedecostante M., Schophuizen C.M.S., Chevtchik N.V., Wilmer M.J., Van Asbeck A.H., Croes H.J., Pertijs J.C., Wetzels J.F.M., et al. Human proximal tubule epithelial cells cultured on hollow fibers: Living membranes that actively transport organic cations. Sci. Rep. 2015;5:16702. doi: 10.1038/srep16702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Schutgens F., Rookmaaker M.B., Margaritis T., Rios A., Ammerlaan C., Jansen J., Gijzen L., Vormann M., Vonk A., Viveen M., et al. Tubuloids derived from human adult kidney and urine for personalized disease modeling. Nat. Biotechnol. 2019;37:303–313. doi: 10.1038/s41587-019-0048-8. [DOI] [PubMed] [Google Scholar]
- 122.Yengej F.A.Y., Jansen J., Rookmaaker M.B., Verhaar M.C., Clevers H. Kidney Organoids and Tubuloids. Cells. 2020;9:1326. doi: 10.3390/cells9061326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Gijzen L., Yousef Yengej F.A., Schutgens F., Vormann M.K., Ammerlaan C.M.E., Nicolas A., Kurek D., Vulto P., Rookmaaker M.B., Lanz H.L., et al. Culture and analysis of kidney tubuloids and perfused tubuloid cells-on-a-chip. Nat. Protoc. 2021;16:2023–2050. doi: 10.1038/s41596-020-00479-w. [DOI] [PubMed] [Google Scholar]
- 124.Feng D., Kumar M., Muntel J., Gurley S.B., Birrane G., Stillman I.E., Ding L., Wang M., Ahmed S., Schlondorff J., et al. Phosphorylation of ACTN4 leads to podocyte vulnerability and proteinuric glomerulosclerosis. J. Am. Soc. Nephrol. 2020;31:1479–1495. doi: 10.1681/ASN.2019101032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dandapani S.V., Sugimoto H., Matthews B.D., Kolb R.J., Sinha S., Gerszten R.E., Zhou J., Ingber D.E., Kalluri R., Pollak M.R. α-actinin-4 is required for normal podocyte adhesion. J. Biol. Chem. 2007;282:467–477. doi: 10.1074/jbc.M605024200. [DOI] [PubMed] [Google Scholar]
- 126.Feng D., Notbohm J., Benjamin A., He S., Wang M., Ang L.H., Bantawa M., Bouzid M., Del Gado E., Krishnan R., et al. Disease-causing mutation in α-actinin-4 promotes podocyte detachment through maladaptation to periodic stretch. Proc. Natl. Acad. Sci. USA. 2018;115:1517–1522. doi: 10.1073/pnas.1717870115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gbadegesin R.A., Hall G., Adeyemo A., Hanke N., Tossidou I., Burchette J., Wu G., Homstad A., Sparks M.A., Gomez J., et al. Mutations in the gene that encodes the F-Actin binding protein anillin cause FSGS. J. Am. Soc. Nephrol. 2014;25:1991–2002. doi: 10.1681/ASN.2013090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hall G., Lane B.M., Khan K., Pediaditakis I., Xiao J., Wu G., Wang L., Kovalik M.E., Chryst-Stangl M., Davis E.E., et al. The human FSGS-causing ANLN R431C mutation induces dysregulated PI3K/AKT/mTOR/Rac1 signaling in podocytes. J. Am. Soc. Nephrol. 2018;29:2110–2122. doi: 10.1681/ASN.2017121338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Uzureau S., Lecordier L., Uzureau P., Hennig D., Graversen J.H., Homblé F., Mfutu P.E., Oliveira Arcolino F., Ramos A.R., La Rovere R.M., et al. APOL1 C-Terminal Variants May Trigger Kidney Disease through Interference with APOL3 Control of Actomyosin. Cell Rep. 2020;30:3821–3836. doi: 10.1016/j.celrep.2020.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wakashin H., Heymann J., Roshanravan H., Daneshpajouhnejad P., Rosenberg A., Shin M.K., Hoek M., Kopp J.B. APOL1 renal risk variants exacerbate podocyte injury by increasing inflammatory stress. BMC Nephrol. 2020;21 doi: 10.1186/s12882-020-01995-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Akilesh S., Suleiman H., Yu H., Stander M.C., Lavin P., Gbadegesin R., Antignac C., Pollak M., Kopp J.B., Winn M.P., et al. Arhgap24 inactivates Rac1 in mouse podocytes, and a mutant form is associated with familial focal segmental glomerulosclerosis. J. Clin. Investig. 2011;121:4127–4137. doi: 10.1172/JCI46458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gee H.Y., Saisawat P., Ashraf S., Hurd T.W., Vega-Warner V., Fang H., Beck B.B., Gribouval O., Zhou W., Diaz K.A., et al. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J. Clin. Investig. 2013;123:3243–3253. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Auguste D., Maier M., Baldwin C., Aoudjit L., Robins R., Gupta I.R., Takano T. Disease-causing mutations of RhoGDIα induce Rac1 hyperactivation in podocytes. Small GTPases. 2016;7:107–121. doi: 10.1080/21541248.2015.1113353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rao J., Ashraf S., Tan W., Van Der Ven A.T., Gee H.Y., Braun D.A., Fehér K., George S.P., Esmaeilniakooshkghazi A., Choi W.I., et al. Advillin acts upstream of phospholipase C ϵ1 in steroid-resistant nephrotic syndrome. J. Clin. Investig. 2017;127:4257–4269. doi: 10.1172/JCI94138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuusela S., Wang H., Wasik A.A., Suleiman H., Lehtonen S. Tankyrase inhibition aggravates kidney injury in the absence of CD2AP. Cell Death Dis. 2016;7:e2302. doi: 10.1038/cddis.2016.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Saurus P., Tolvanen T.A., Lindfors S., Kuusela S., Holthöfer H., Lehtonen E., Lehtonen S. Inhibition of SHIP2 in CD2AP-deficient podocytes ameliorates reactive oxygen species generation but aggravates apoptosis. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-10512-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Scott R.P., Hawley S.P., Ruston J., Du J., Brakebusch C., Jones N., Pawson T. Podocyte-specific loss of Cdc42 leads to congenital nephropathy. J. Am. Soc. Nephrol. 2012;23:1149–1154. doi: 10.1681/ASN.2011121206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Solanki A.K., Arif E., Morinelli T., Wilson R.C., Hardiman G., Deng P., Arthur J.M., Velez J.C., Nihalani D., Janech M.G., et al. A Novel CLCN5 Mutation Associated with Focal Segmental Glomerulosclerosis and Podocyte Injury. Kidney Int. Rep. 2018;3:1443–1453. doi: 10.1016/j.ekir.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Daga S., Donati F., Capitani K., Croci S., Tita R., Giliberti A., Valentino F., Benetti E., Fallerini C., Niccheri F., et al. New frontiers to cure Alport syndrome: COL4A3 and COL4A5 gene editing in podocyte-lineage cells. Eur. J. Hum. Genet. 2020;28:480–490. doi: 10.1038/s41431-019-0537-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Daga S., Baldassarri M., Lo Rizzo C., Fallerini C., Imperatore V., Longo I., Frullanti E., Landucci E., Massella L., Pecoraro C., et al. Urine-derived podocytes-lineage cells: A promising tool for precision medicine in Alport Syndrome. Hum. Mutat. 2018;39:302–314. doi: 10.1002/humu.23364. [DOI] [PubMed] [Google Scholar]
- 141.Kuang X., Sun L., Wu Y., Huang W. A novel missense mutation of COL4A5 gene alter collagen IV α5 chain to cause X-linked Alport syndrome in a Chinese family. Transl. Pediatr. 2020;9:587–595. doi: 10.21037/tp-20-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Widmeier E., Airik M., Hugo H., Schapiro D., Wedel J., Ghosh C.C., Nakayama M., Schneider R., Awad A.M., Nag A., et al. Treatment with 2,4-dihydroxybenzoic acid prevents FSGS progression and renal fibrosis in podocyte- specific Coq6 knockout mice. J. Am. Soc. Nephrol. 2019;30:393–405. doi: 10.1681/ASN.2018060625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Song C.C., Hong Q., Geng X.D., Wang X., Wang S.Q., Cui S.Y., Guo M.D., Li O., Cai G.Y., Chen X.M., et al. New Mutation of Coenzyme Q10 Monooxygenase 6 Causing Podocyte Injury in a Focal Segmental Glomerulosclerosis Patient. Chin. Med. J. 2018;131:2666–2675. doi: 10.4103/0366-6999.245158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ashraf S., Gee H.Y., Woerner S., Xie L.X., Vega-Warner V., Lovric S., Fang H., Song X., Cattran D.C., Avila-Casado C., et al. ADCK4 mutations promote steroid-Resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J. Clin. Investig. 2013;123:5179–5189. doi: 10.1172/JCI69000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Widmeier E., Yu S., Nag A., Chung Y.W., Nakayama M., Fernández-Del-Río L., Hugo H., Schapiro D., Buerger F., Choi W.I., et al. ADCK4 deficiency destabilizes the coenzyme Q complex, which is rescued by 2,4-dihydroxybenzoic acid treatment. J. Am. Soc. Nephrol. 2020;31:1191–1211. doi: 10.1681/ASN.2019070756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Möller-Kerutt A., Rodriguez-Gatica J.E., Wacker K., Bhatia R., Siebrasse J.-P., Boon N., Van Marck V., Boor P., Kubitscheck U., Wijnholds J., et al. Crumbs2 Is an Essential Slit Diaphragm Protein of the Renal Filtration Barrier. J. Am. Soc. Nephrol. 2021:ASN.2020040501. doi: 10.1681/asn.2020040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gianesello L., Priante G., Ceol M., Radu C.M., Saleem M.A., Simioni P., Terrin L., Anglani F., Prete D. Del Albumin uptake in human podocytes: A possible role for the cubilin-amnionless (CUBAM) complex. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Schneider R., Deutsch K., Hoeprich G.J., Marquez J., Hermle T., Braun D.A., Seltzsam S., Kitzler T.M., Mao Y., Buerger F., et al. DAAM2 Variants Cause Nephrotic Syndrome via Actin Dysregulation. Am. J. Hum. Genet. 2020;107:1113–1128. doi: 10.1016/j.ajhg.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ozaltin F., Li B., Rauhauser A., An S.W., Soylemezoglu O., Gonul I.I., Taskiran E.Z., Ibsirlioglu T., Korkmaz E., Bilginer Y., et al. DGKE variants cause a glomerular microangiopathy that mimics membranoproliferative GN. J. Am. Soc. Nephrol. 2013;24:377–384. doi: 10.1681/ASN.2012090903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Ashraf S., Kudo H., Rao J., Kikuchi A., Widmeier E., Lawson J.A., Tan W., Hermle T., Warejko J.K., Shril S., et al. Mutations in six nephrosis genes delineate a pathogenic pathway amenable to treatment. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-04193-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Gee H.Y., Ashraf S., Wan X., Vega-Warner V., Esteve-Rudd J., Lovric S., Fang H., Hurd T.W., Sadowski C.E., Allen S.J., et al. Mutations in EMP2 Cause Childhood-Onset Nephrotic Syndrome. Am. J. Hum. Genet. 2014;94:884–890. doi: 10.1016/j.ajhg.2014.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wan X., Chen Z., Choi W.I., Gee H.Y., Hildebrandt F., Zhou W. Loss of epithelial membrane protein 2 aggravates podocyte injury via upregulation of caveolin-1. J. Am. Soc. Nephrol. 2016;27:1066–1075. doi: 10.1681/ASN.2014121197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gee H.Y., Sadowski C.E., Aggarwal P.K., Porath J.D., Yakulov T.A., Schueler M., Lovric S., Ashraf S., Braun D.A., Halbritter J., et al. FAT1 mutations cause a glomerulotubular nephropathy. Nat. Commun. 2016;7 doi: 10.1038/ncomms10822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Slaats G.G., Braun F., Hoehne M., Frech L.E., Blomberg L., Benzing T., Schermer B., Rinschen M.M., Kurschat C.E. Urine-derived cells: A promising diagnostic tool in Fabry disease patients. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-29240-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Braun F., Blomberg L., Brodesser S., Liebau M.C., Schermer B., Benzing T., Kurschat C.E. Enzyme replacement therapy clears GB3 deposits from a podocyte cell culture model of Fabry disease but fails to restore altered cellular signaling. Cell. Physiol. Biochem. 2019;52:1139–1150. doi: 10.33594/000000077. [DOI] [PubMed] [Google Scholar]
- 156.Brown E.J., Schlöndorff J.S., Becker D.J., Tsukaguchi H., Uscinski A.L., Higgs H.N., Henderson J.M., Pollak M.R. Mutations in the formin gene INF2 cause focal segmental glomerulosclerosis. Nat. Genet. 2010;42:72–76. doi: 10.1038/ng.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Sun H., Perez-Gill C., Schlöndorff J.S., Subramanian B., Pollak M.R. Dysregulated dynein-mediated trafficking of nephrin causes INF2-related podocytopathy. J. Am. Soc. Nephrol. 2021;32:307–322. doi: 10.1681/ASN.2020081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Bayraktar S., Nehrig J., Menis E., Karli K., Janning A., Struk T., Halbritter J., Michgehl U., Krahn M.P., Schuberth C.E., et al. A deregulated stress response underlies distinct INF2-associated disease profiles. J. Am. Soc. Nephrol. 2020;31:1296–1313. doi: 10.1681/ASN.2019111174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Nicolaou N., Margadant C., Kevelam S.H., Lilien M.R., Oosterveld M.J.S., Kreft M., Van Eerde A.M., Pfundt R., Terhal P.A., Van Der Zwaag B., et al. Gain of glycosylation in integrin α3 causes lung disease and nephrotic syndrome. J. Clin. Investig. 2012;122:4375–4387. doi: 10.1172/JCI64100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Gee H.Y., Zhang F., Ashraf S., Kohl S., Sadowski C.E., Vega-Warner V., Zhou W., Lovric S., Fang H., Nettleton M., et al. KANK deficiency leads to podocyte dysfunction and nephrotic syndrome. J. Clin. Investig. 2015;125:2375–2384. doi: 10.1172/JCI79504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Braun D.A., Rao J., Mollet G., Schapiro D., Daugeron M.C., Tan W., Gribouval O., Boyer O., Revy P., Jobst-Schwan T., et al. Mutations in KEOPS-complex genes cause nephritic syndrome with primary microcephaly. Nat. Genet. 2017;49:1529–1538. doi: 10.1038/ng.3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jarad G., Cunningham J., Shaw A.S., Miner J.H. Proteinuria precedes podocyte abnormalities in Lamb2-/- mice, implicating the glomerular basement membrane as an albumin barrier. J. Clin. Investig. 2006;116:2272–2279. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hall G., Lane B., Chryst-Ladd M., Wu G., Lin J.J., Qin X.J., Hauser E.R., Gbadegesin R. Dysregulation of WTI (-KTS) is Associated with the Kidney-Specific Effects of the LMX1B R246Q Mutation. Sci. Rep. 2017;7 doi: 10.1038/srep39933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Harendza S., Stahl R.A.K., Schneider A. The transcriptional regulation of podocin (NPHS2) by Lmx1b and a promoter single nucleotide polymorphism. Cell. Mol. Biol. Lett. 2009;14:679–691. doi: 10.2478/s11658-009-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hays T., Ma’ayan A., Clark N.R., Tan C.M., Teixeira A., Teixeira A., Choi J.W., Burdis N., Jung S.Y., Bajaj A.O., et al. Proteomics analysis of the non-muscle myosin heavy chain IIa-enriched actin-myosin complex reveals multiple functions within the podocyte. PLoS ONE. 2014;9 doi: 10.1371/journal.pone.0100660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bondzie P.A., Chen H.A., Cao M.Z., Tomolonis J.A., He F., Pollak M.R., Henderson J.M. Non-muscle myosin-IIA is critical for podocyte f-actin organization, contractility, and attenuation of cell motility. Cytoskeleton. 2016;73:377–395. doi: 10.1002/cm.21313. [DOI] [PubMed] [Google Scholar]
- 167.Mele C., Iatropoulos P., Donadelli R., Calabria A., Maranta R., Cassis P., Buelli S., Tomasoni S., Piras R., Krendel M., et al. MYO1E Mutations and Childhood Familial Focal Segmental Glomerulosclerosis. N. Engl. J. Med. 2011;365:295–306. doi: 10.1056/NEJMoa1101273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Chase S.E., Encina C.V., Stolzenburg L.R., Tatum A.H., Holzman L.B., Krendel M. Podocyte-specific Knockout of Myosin 1e Disrupts Glomerular Filtration. Am. J. Physiol. Ren. Physiol. 2012;303 doi: 10.1152/ajprenal.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jin X., Wang W., Mao J., Shen H., Fu H., Wang X., Gu W., Liu A., Yu H., Shu Q., et al. Overexpression of myo1e in mouse podocytes enhances cellular endocytosis, migration, and adhesion. J. Cell. Biochem. 2014;115:410–419. doi: 10.1002/jcb.24676. [DOI] [PubMed] [Google Scholar]
- 170.Li Q., Gulati A., Lemaire M., Nottoli T., Bale A., Tufro A. Rho-GTPase Activating Protein Myosin MYO9A identified as a Novel Candidate Gene for Monogenic Focal Segmental Glomerulosclerosis. Kidney Int. 2021 doi: 10.1016/j.kint.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Wu F., Saleem M.A., Kampik N.B., Satchwell T.J., Williamson R.C., Blattner S.M., Ni L., Toth T., White G., Young M.T., et al. Anion exchanger 1 interacts with nephrin in podocytes. J. Am. Soc. Nephrol. 2010;21:1456–1467. doi: 10.1681/ASN.2009090921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tory K., Menyhárd D.K., Woerner S., Nevo F., Gribouval O., Kerti A., Stráner P., Arrondel C., Cong E.H., Tulassay T., et al. Mutation-dependent recessive inheritance of NPHS2-associated steroid-resistant nephrotic syndrome. Nat. Genet. 2014;46:299–304. doi: 10.1038/ng.2898. [DOI] [PubMed] [Google Scholar]
- 173.Huber T.B., Simons M., Hartleben B., Sernetz L., Schmidts M., Gundlach E., Saleem M.A., Walz G., Benzing T. Molecular basis of the functional podocin-nephrin complex: Mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum. Mol. Genet. 2003;12:3397–3405. doi: 10.1093/hmg/ddg360. [DOI] [PubMed] [Google Scholar]
- 174.Braun D.A., Lovric S., Schapiro D., Schneider R., Marquez J., Asif M., Hussain M.S., Daga A., Widmeier E., Rao J., et al. Mutations in multiple components of the nuclear pore complex cause nephrotic syndrome. J. Clin. Investig. 2018;128:4313–4328. doi: 10.1172/JCI98688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Braun D.A., Sadowski C.E., Kohl S., Lovric S., Astrinidis S.A., Pabst W.L., Gee H.Y., Ashraf S., Lawson J.A., Shril S., et al. Mutations in nuclear pore genes NUP93, NUP205 and XPO5 cause steroid-resistant nephrotic syndrome. Nat. Genet. 2016;48:457–465. doi: 10.1038/ng.3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Barua M., Stellacci E., Stella L., Weins A., Genovese G., Muto V., Caputo V., Toka H.R., Charoonratana V.T., Tartaglia M., et al. Mutations in PAX2 associate with adult-onset FSGS. J. Am. Soc. Nephrol. 2014;25:1942–1953. doi: 10.1681/ASN.2013070686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Sidhom E.H., Kim C., Kost-Alimova M., Ting M.T., Keller K., Avila-Pacheco J., Watts A.J.B., Vernon K.A., Marshall J.L., Reyes-Bricio E., et al. Targeting a Braf/Mapk pathway rescues podocyte lipid peroxidation in CoQ-deficiency kidney disease. J. Clin. Investig. 2021;131 doi: 10.1172/JCI141380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hinkes B., Wiggins R.C., Gbadegesin R., Vlangos C.N., Seelow D., Nürnberg G., Garg P., Verma R., Chaib H., Hoskins B.E., et al. Positional cloning uncovers mutations in PLCE1 responsible for a nephrotic syndrome variant that may be reversible. Nat. Genet. 2006;38:1397–1405. doi: 10.1038/ng1918. [DOI] [PubMed] [Google Scholar]
- 179.Lin F.J., Yao L., Hu X.Q., Bian F., Ji G., Jiang G.R., Gale D.P., Ren H.Q. First identification of PODXL nonsense mutations in autosomal dominant focal segmental glomerulosclerosis. Clin. Sci. 2019;133:9–21. doi: 10.1042/CS20180676. [DOI] [PubMed] [Google Scholar]
- 180.Lovric S., Goncalves S., Gee H.Y., Oskouian B., Srinivas H., Choi W.I., Shril S., Ashraf S., Tan W., Rao J., et al. Mutations in sphingosine-1-phosphate lyase cause nephrosis with ichthyosis and adrenal insufficiency. J. Clin. Investig. 2017;127:912–928. doi: 10.1172/JCI89626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Asanuma K., Yanagida-Asanuma E., Faul C., Tomino Y., Kim K., Mundel P. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat. Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 182.Ning L., Suleiman H.Y., Miner J.H. Synaptopodin is dispensable for normal podocyte homeostasis but is protective in the context of acute podocyte injury. J. Am. Soc. Nephrol. 2020;31:2815–2832. doi: 10.1681/ASN.2020050572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dorval G., Kuzmuk V., Gribouval O., Welsh G.I., Bierzynska A., Schmitt A., Miserey-Lenkei S., Koziell A., Haq S., Benmerah A., et al. TBC1D8B Loss-of-Function Mutations Lead to X-Linked Nephrotic Syndrome via Defective Trafficking Pathways. Am. J. Hum. Genet. 2019;104:348–355. doi: 10.1016/j.ajhg.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kampf L.L., Schneider R., Gerstner L., Thünauer R., Chen M., Helmstädter M., Amar A., Onuchic-Whitford A.C., Munarriz R.L., Berdeli A., et al. TBC1D8B mutations implicate rab11-dependent vesicular trafficking in the pathogenesis of nephrotic syndrome. J. Am. Soc. Nephrol. 2019;30:2338–2353. doi: 10.1681/ASN.2019040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Hall G., Rowell J., Farinelli F., Gbadegesin R.A., Lavin P., Wu G., Homstad A., Malone A., Lindsey T., Jiang R., et al. Phosphodiesterase 5 inhibition ameliorates angiontensin II-induced podocyte dysmotility via the protein kinase G-mediated downregulation of TRPC6 activity. Am. J. Physiol. Ren. Physiol. 2014;306 doi: 10.1152/ajprenal.00212.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Huang H., Lin X., You Y., Tang C., Gu X., Huang M., Qin Y., Tan J., Huang F. Inhibition of TRPC6 signal pathway alleviates podocyte injury induced by TGF-β1. Cell. Physiol. Biochem. 2017;41:163–172. doi: 10.1159/000455985. [DOI] [PubMed] [Google Scholar]
- 187.Cong E.H., Bizet A.A., Boyer O., Woerner S., Gribouval O., Filhol E., Arrondel C., Thomas S., Silbermann F., Canaud G., et al. A homozygous missense mutation in the ciliary gene TTC21B causes familial FSGS. J. Am. Soc. Nephrol. 2014;25:2435–2443. doi: 10.1681/ASN.2013101126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Colin E., Huynh Cong E., Mollet G., Guichet A., Gribouval O., Arrondel C., Boyer O., Daniel L., Gubler M.C., Ekinci Z., et al. Loss-of-function mutations in WDR73 Are responsible for microcephaly and steroid-resistant nephrotic syndrome: Galloway-mowat syndrome. Am. J. Hum. Genet. 2014;95:637–648. doi: 10.1016/j.ajhg.2014.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Tilley F.C., Arrondel C., Chhuon C., Boisson M., Cagnard N., Parisot M., Menara G., Lefort N., Guerrera I.C., Bole-Feysot C., et al. Disruption of pathways regulated by Integrator complex in Galloway–Mowat syndrome due to WDR73 mutations. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-84472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Hall G., Gbadegesin R.A., Lavin P., Wu G., Liu Y., Oh E.C., Wang L., Spurney R.F., Eckel J., Lindsey T., et al. A novel missense mutation of Wilms’ Tumor 1 causes autosomal dominant FSGS. J. Am. Soc. Nephrol. 2015;26:831–843. doi: 10.1681/ASN.2013101053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Alekov A.K. Mutations associated with Dent’s disease affect gating and voltage dependence of the human anion/proton exchanger ClC-5. Front. Physiol. 2015;6 doi: 10.3389/fphys.2015.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Festa B.P., Berquez M., Gassama A., Amrein I., Ismail H.M., Samardzija M., Staiano L., Luciani A., Grimm C., Nussbaum R.L., et al. OCRL deficiency impairs endolysosomal function in a humanized mouse model for Lowe syndrome and Dent disease. Hum. Mol. Genet. 2019;28:1931–1946. doi: 10.1093/hmg/ddy449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Peeters K., Wilmer M.J., Schoeber J.P., Reijnders D., van den Heuvel L.P., Masereeuw R., Levtchenko E. Role of P-glycoprotein expression and function in cystinotic renal proximal tubular cells. Pharmaceutics. 2011;3:782–792. doi: 10.3390/pharmaceutics3040782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Rossi M.N., Pascarella A., Licursi V., Caiello I., Taranta A., Rega L.R., Levtchenko E., Emma F., De Benedetti F., Prencipe G. NLRP2 Regulates Proinflammatory and Antiapoptotic Responses in Proximal Tubular Epithelial Cells. Front. Cell Dev. Biol. 2019;7 doi: 10.3389/fcell.2019.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bellomo F., Signorile A., Tamma G., Ranieri M., Emma F., De Rasmo D. Impact of atypical mitochondrial cyclic-AMP level in nephropathic cystinosis. Cell. Mol. Life Sci. 2018;75:3411–3422. doi: 10.1007/s00018-018-2800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Sumayao R., Newsholme P., McMorrow T. Inducible nitric oxide synthase inhibitor 1400W increases Na+,K+-ATPase levels and activity and ameliorates mitochondrial dysfunction in Ctns null kidney proximal tubular epithelial cells. Clin. Exp. Pharmacol. Physiol. 2018;45:1149–1160. doi: 10.1111/1440-1681.12998. [DOI] [PubMed] [Google Scholar]
- 197.Assmann N., Dettmer K., Simbuerger J.M.B., Broeker C., Nuernberger N., Renner K., Courtneidge H., Klootwijk E.D., Duerkop A., Hall A., et al. Renal Fanconi Syndrome Is Caused by a Mistargeting-Based Mitochondriopathy. Cell Rep. 2016;15:1423–1429. doi: 10.1016/j.celrep.2016.04.037. [DOI] [PubMed] [Google Scholar]
- 198.Reichold M., Klootwijk E.D., Reinders J., Otto E.A., Milani M., Broeker C., Laing C., Wiesner J., Devi S., Zhou W., et al. Glycine Amidinotransferase (GATM), Renal Fanconi Syndrome, and Kidney Failure. J. Am. Soc. Nephrol. 2018;29:1849–1858. doi: 10.1681/ASN.2017111179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sasaki S., Hara A., Sakaguchi M., Nangaku M., Inoue Y. Hepatocyte nuclear factor 4α regulates megalin expression in proximal tubular cells. Biochem. Biophys. Rep. 2019;17:87–92. doi: 10.1016/j.bbrep.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Gliozzi M.L., Espiritu E.B., Shipman K.E., Rbaibi Y., Long K.R., Roy N., Duncan A.W., Lazzara M.J., Hukriede N.A., Baty C.J., et al. Effects of proximal tubule shortening on protein excretion in a Lowe syndrome model. J. Am. Soc. Nephrol. 2020;31:67–83. doi: 10.1681/ASN.2019020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Vicinanza M., Di Campli A., Polishchuk E., Santoro M., Di Tullio G., Godi A., Levtchenko E., De Leo M.G., Polishchuk R., Sandoval L., et al. OCRL controls trafficking through early endosomes via PtdIns4,5P 2-dependent regulation of endosomal actin. EMBO J. 2011;30:4970–4985. doi: 10.1038/emboj.2011.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wendt-Nordahl G., Sagi S., Bolenz C., Alken P., Michel M.S., Knoll T. Evaluation of cystine transport in cultured human kidney cells and establishment of cystinuria type I phenotype by antisense technology. Urol. Res. 2008;36:25–29. doi: 10.1007/s00240-007-0127-z. [DOI] [PubMed] [Google Scholar]
- 203.Gill H.S., Choi K.Y., Kammili L., Popratiloff A. Rescue of the temperature-sensitive, autosomal-recessive mutation R298S in the sodium-bicarbonate cotransporter NBCe1-A characterized by a weakened dimer and abnormal aggregation. Biochim. Biophys. Acta Gen. Subj. 2015;1850:1286–1296. doi: 10.1016/j.bbagen.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Gildea J.J., Xu P., Kemp B.A., Carlson J.M., Tran H.T., Wang D.B., Langouët-Astrié C.J., McGrath H.E., Carey R.M., Jose P.A., et al. Sodium bicarbonate cotransporter NBCe2 gene variants increase sodium and bicarbonate transport in human renal proximal tubule cells. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0189464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Mitsuoka K., Shirasaka Y., Fukushi A., Sato M., Nakamura T., Nakanishi T., Tamai I. Transport characteristics of L-citrulline in renal apical membrane of proximal tubular cells. Biopharm. Drug Dispos. 2009;30:126–137. doi: 10.1002/bdd.653. [DOI] [PubMed] [Google Scholar]
- 206.Rotoli B.M., Barilli A., Visigalli R., Ferrari F., Dall’Asta V. y+LAT1 and y+LAT2 contribution to arginine uptake in different human cell models: Implications in the pathophysiology of Lysinuric Protein Intolerance. J. Cell. Mol. Med. 2020;24:921–929. doi: 10.1111/jcmm.14801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Fearn A., Allison B., Rice S.J., Edwards N., Halbritter J., Bourgeois S., Pastor-Arroyo E.M., Hildebrandt F., Tasic V., Wagner C.A., et al. Clinical, biochemical, and pathophysiological analysis of SLC34A1 mutations. Physiol. Rep. 2018;6 doi: 10.14814/phy2.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bröer S., Bailey C.G., Kowalczuk S., Ng C., Vanslambrouck J.M., Rodgers H., Auray-Blais C., Cavanaugh J.A., Bröer A., Rasko J.E.J. Iminoglycinuria and hyperglycinuria are discrete human phenotypes resulting from complex mutations in proline and glycine transporters. J. Clin. Investig. 2008;118:3881–3892. doi: 10.1172/JCI36625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Ibeh C.L., Yiu A.J., Kanaras Y.L., Paal E., Birnbaumer L., Jose P.A., Bandyopadhyay B.C. Evidence for a regulated ca2+ entry in proximal tubular cells and its implication in calcium stone formation. J. Cell Sci. 2019;132 doi: 10.1242/jcs.225268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ivanova E.A., Elmonem M.A., Bongaerts I., Luyten T., Missiaen L., van den Heuvel L.P., Levtchenko E.N., Bultynck G. Ca2+ signalling in human proximal tubular epithelial cells deficient for cystinosin. Cell Calcium. 2016;60:282–287. doi: 10.1016/j.ceca.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 211.Pinto A.M., Daga S., Fallerini C., Bruttini M., Baldassarri M., Giliberti A., Frullanti E., Guarnieri A., Garosi G., Renieri A. Detection of cryptic mosaicism in X-linked alport syndrome prompts to re-evaluate living-donor kidney transplantation. Transplantation. 2020;104:2360–2364. doi: 10.1097/TP.0000000000003104. [DOI] [PubMed] [Google Scholar]
- 212.Park S.J., Kim Y., Chen Y.M. Endoplasmic reticulum stress and monogenic kidney diseases in precision nephrology. Pediatr. Nephrol. 2019;34:1493–1500. doi: 10.1007/s00467-018-4031-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Mundel P. Podocytes and the quest for precision medicines for kidney diseases. Pflugers Arch. Eur. J. Physiol. 2017;469:1029–1037. doi: 10.1007/s00424-017-2015-x. [DOI] [PubMed] [Google Scholar]
- 214.Ahmadi A., Moghadasali R., Ezzatizadeh V., Taghizadeh Z., Nassiri S.M., Asghari-Vostikolaee M.H., Alikhani M., Hadi F., Rahbarghazi R., Yazdi R.S., et al. Transplantation of Mouse Induced Pluripotent Stem Cell-Derived Podocytes in a Mouse Model of Membranous Nephropathy Attenuates Proteinuria. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-51770-0. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 215.De Ribeiro P.C., Lojudice F.H., Fernandes-Charpiot I.M.M., Baptista M.A.S.F., de Almeida Araújo S., Mendes G.E.F., Sogayar M.C., Abbud-Filho M., Caldas H.C. Therapeutic potential of human induced pluripotent stem cells and renal progenitor cells in experimental chronic kidney disease. Stem Cell Res. Ther. 2020;11 doi: 10.1186/s13287-020-02060-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.De Carvalho Ribeiro P., Oliveira L.F., Filho M.A., Caldas H.C. Differentiating Induced Pluripotent Stem Cells into Renal Cells: A New Approach to Treat Kidney Diseases. Stem Cells Int. 2020;2020 doi: 10.1155/2020/8894590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Liu G.W., Johnson S.L., Jain R., Peeler D.J., Shankland S.J., Pun S.H. Optimized nonviral gene delivery for primary urinary renal progenitor cells to enhance cell migration. J. Biomed. Mater. Res. Part A. 2019;107:2718–2725. doi: 10.1002/jbm.a.36775. [DOI] [PubMed] [Google Scholar]