Abstract
The divalent cation calcium (Ca2+) is considered one of the main second messengers inside cells and acts as the most prominent signal in a plethora of biological processes. Its homeostasis is guaranteed by an intricate and complex system of channels, pumps, and exchangers. In this context, by regulating cellular Ca2+ levels, mitochondria control both the uptake and release of Ca2+. Therefore, at the mitochondrial level, Ca2+ plays a dual role, participating in both vital physiological processes (ATP production and regulation of mitochondrial metabolism) and pathophysiological processes (cell death, cancer progression and metastasis). Hence, it is not surprising that alterations in mitochondrial Ca2+ (mCa2+) pathways or mutations in Ca2+ transporters affect the activities and functions of the entire cell. Indeed, it is widely recognized that dysregulation of mCa2+ signaling leads to various pathological scenarios, including cancer, neurological defects and cardiovascular diseases (CVDs). This review summarizes the current knowledge on the regulation of mCa2+ homeostasis, the related mechanisms and the significance of this regulation in physiology and human diseases. We also highlight strategies aimed at remedying mCa2+ dysregulation as promising therapeutical approaches.
Keywords: mitochondria, Ca2+, cancer, cardiovascular diseases, neurodegenerative diseases, mPTP, therapy
1. Introduction
Mitochondria are membrane-bound cellular organelles that are often referred to as the cell powerhouse. Indeed, they play a primary role in generating most of the chemical energy (ATP) that acts as fuel for the cell through oxidative phosphorylation. Undoubtedly, energy production represents only the very tip of the iceberg in terms of mitochondrial function. In fact, these highly dynamic structures integrate a wide spectrum of cellular activities, such as metabolism, muscle contraction, neurotransmitter release, antioxidant defense, cell signaling, autophagy and programmed cell death [1,2,3]. It is widely recognized that these organelles are not static and passive; rather, they constantly change their shape in response to environmental changes and stresses through fission and fusion processes [4]. Thus, they exert both vital and lethal functions in physiological and pathological scenarios. Mitochondria are considered efficient in decoding intracellular signals, of which Ca2+ is one of the most important [5]. In fact, they control and balance Ca2+ influx and efflux. Specifically, the mitochondrial calcium Ca2+ uniporter (MCU) complex (MCUC) ensures Ca2+ uptake, while the Na+/Ca2+ exchanger (NCLX) and H+/Ca2+ exchanger (HCX) supervise its extrusion [6,7]. Under resting conditions, the Ca2+ concentration inside mitochondria reaches levels comparable to those in the cytoplasm (100–200 nM). However, after stimulation with agents that increase Ca2+ levels, 10- to 20-fold more Ca2+ can accumulate in mitochondrial than in the cytosolic compartment. The presence of dynamic membrane contacts between mitochondria and the endoplasmic reticulum (ER; the main Ca2+ stores store inside the cells), termed mitochondrial-associated membranes (MAMs), and a highly Ca2+-selective channel located in the inner mitochondrial membrane (IMM) allow a large amount of Ca2+ to enter these organelles. Nonetheless, Ca2+ ions need to be rapidly extruded to restore the basal state. This process is guaranteed by a complex system of Ca2+ antiporters, represented by NCLX and HCX activity. Excessive calcium uptake or impairments in calcium efflux can produce deleterious effects on mitochondrial functionality [8]. In fact, excessive transfer of Ca2+ from the ER to mitochondria via inositol 1,4,5-trisphosphate (IP3) receptor channels (IP3Rs) leads to mCa2+ overload and subsequent mitochondrial permeability transition pore (mPTP) opening. Persistent opening of the mPTP provokes inner mitochondrial membrane (IMM) depolarization and matrix swelling, thus inducing outer mitochondrial membrane (OMM) rupture. Then, cytochrome c is released, inducing apoptotic cell death [9,10]. Conversely, decreased expression of MCU leads to a lower mCa2+ uptake thus causing a reduction of mPTP opening and preventing apoptotic factors release [11,12,13]. It is clear that alterations in or disruption of mCa2+ homeostasis could produce a pathological scenario. Indeed, mCa2+ dysfunction has been extensively implicated in various common diseases, including neurodegenerative diseases (such as Alzheimer’s disease (AD), Parkinson’s disease (PD) and Huntington’s disease (HD)), cardiovascular diseases (CVDs; ischemia/reperfusion (IR), injury (IRI), cardiac hypertrophy, cardiomyopathies and arrythmia) and, last but not least, cancer. Under pathological conditions in which mCa2+ overload triggers cell damage, as in IRI and neurological disorders, drugs that inhibit increases in mCa2+ levels might be beneficial. On the contrary, molecules that enhance mCa2+ overload could be useful in scenarios where reductions in mCa2+ levels allow cancer cells to evade apoptosis. Although mitochondria have a central role in human health and disease, successful therapies targeting these organelles are still not available.
2. Mitochondrial Calcium Homeostasis
2.1. Mitochondrial Ca2+ Influx
Mitochondria are characterized by two functional and distinct membrane systems, i.e., the OMM and the IMM, and folded cristae that enclose the mitochondrial matrix. mCa2+ homeostasis is tightly regulated by proteins localized in the IMM and OMM and by crosstalk with the ER [14]. This is essential for cell functions and is guaranteed by a dynamic equilibrium between mCa2+ influx and efflux [15]. Over the past years, this topic has been deeply reviewed, and interested readers are referred to recent reviews [15,16,17]. Briefly, Ca2+ diffusion across the OMM occurs via porin-like proteins named voltage-dependent anion channels (VDACs; the isoforms VDAC1-3) [18]. Then, Ca2+ enters the mitochondrial matrix via the MCUC, which is located in the IMM, thanks to a high electronegative potential (∼180 mV). The molecular identity of this channel was revealed only 10 years ago by Rizzuto’s and Mootha’s groups [19,20] after MICU1 was identified as a regulator rather than the channel itself [21]. The MCU gene, also known as CCDC109A, encodes a 40 kDa protein with two coiled-coil domains and two transmembrane domains separated by a short loop [22]. It is now widely accepted that MCU is the principal component of a larger macromolecular complex named MCUC. MCUC is composed of MCU; MCUb, an MCU paralog that acts as its negative regulator [23]; EMRE (essential MCU regulator), which is fundamental for the complex stabilization [24]; and the associated regulators MICU1, MICU2 and MICU3. MICU1 functions as a gatekeeper for the MCU complex, stabilizing the MCU complex in the closed state and thus setting the threshold for mCa2+ uptake [25,26]. MICU2 and MICU3 are two MICU1 paralogs. While MICU2 is localized at the mitochondrial intermembrane space (IMS) and is widely expressed in most mammalian tissues as MICU1, MICU3 mitochondrial localization prediction has a lower confidence [27,28] and is prevalently expressed in the nervous system and skeletal muscle [27,29]. MICU2 forms heterodimers with MICU1, which is responsible for the sigmoidal response to increasing cytosolic Ca2+ (cytCa2+) concentration [26,30]. Regarding MICU3 function, Patron and colleagues recently demonstrated that it forms a disulfide bond-mediated dimer with MICU1 but not with MICU2, acting as a strong MCU stimulator without gatekeeping function [31]. The affinity of MCUC for Ca2+ is very low (KD of 20–30 μM under physiological conditions). Thus, for a significant mCa2+ influx, cytCa2+ levels should be 5–10 μM, but these values have never been detected in live cells. This conundrum was explained by the presence of MAMs, where mitochondria are in close contact with the ER [7]. At MAMs level, the release of Ca2+ content from the ER produces microdomains of high [Ca2+] which allow a rapid accumulation of Ca2+ inside mitochondria [32].
2.2. Mitochondrial Ca2+ Efflux
The existence of pathways for the extrusion of Ca2+ from mitochondria was revealed in the 1970s [33,34]. Ca2+ efflux from the mitochondrial matrix depends on two mechanisms. One involves a ubiquitous HCX [33], and the other involves NCLX [34], which is mostly expressed in excitable tissues (muscle and brain). The molecular identity of HCX is still debated, but recent works have proposed that LETM1 functions as the mitochondrial electroneutral H+/Ca2+ antiporter [17,35]. However, this finding is not universally accepted [36,37]. In 2010, Palty et al. found that the SLC8B1 gene encodes an IMM-localized protein that is responsible for both Li+- and Na+-dependent Ca2+ clearance from the mitochondrial matrix and is thus named NCLX [38]. To date, the role of NCLX has clearly been proven in different in vitro cellular models. Notwithstanding this evidence, to date, animal models in which NCLX is absent are unavailable. Thus, future evidence will be crucial to better analyze and elucidate how mCa2+ homeostasis is achieved under pathophysiological conditions [15]. Recent evidence suggests that HCX and NCLX are not the only two molecules responsible for Ca2+ efflux. As stated above, prolonged mPTP opening might lead to cell death. Despite this finding, it has been reported that in certain circumstances, transient mPTP opening can aid Ca2+ extrusion [39,40], although this hypothesis is not widely accepted [41].
2.3. Physiological Role of Mitochondrial Ca2+
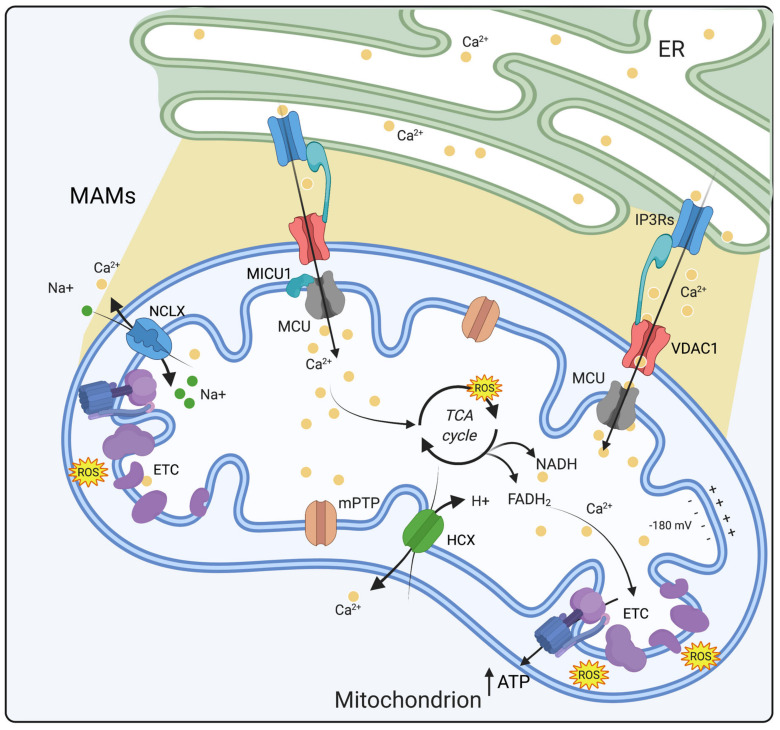
As mentioned above, mCa2+ homeostasis is tightly regulated by influx and efflux mechanisms and it affects oxidative metabolism, generation of mitochondrial ROS and mPTP opening. The accumulation of Ca2+ within mitochondria stimulates important functions of the organelle, including ATP production through oxidative phosphorylation. Indeed, mCa2+ regulates the tricarboxylic acid (TCA) cycle by modulating the activity of three key enzymes of mitochondrial metabolism: ketoglutarate dehydrogenase (KGDH), isocitrate dehydrogenase (IDH) and pyruvate dehydrogenase (PDH). This effect boosts the synthesis of NADH and FADH2, leading to an enhanced respiratory chain activity and thus a subsequent increase in H+ pumping. The electrochemical energy produced is then used to drive ATP synthesis by complex V (ATP synthase) (Figure 1) [42,43]. Under both physiological and pathological conditions, the mitochondrial electron transport chain (ETC), especially complex I and III, has primary responsibility for ROS production. Even though mitochondrial ROS (mROS) have been mainly considered as detrimental by-products of oxidative metabolism, they are now recognized as important signaling molecules, (at subtoxic levels) regulating several cellular activities [44]. mCa2+ uptake by increasing the metabolic rate and ETC activity drives ROS production [45]. This signaling axis works efficiently within a physiological window of [Ca2+]. As a result, when [Ca2+] exceeds this threshold, mROS production becomes harmful and deleterious for mitochondrial bioenergetics and cell functions. ROS formation may be promoted by mCa2+ either directly by stimulating mROS-generating enzymes such as glycerol phosphate and KGDH or indirectly as in the case of nitric oxide synthase (NOS) activation which inhibits complex IV, leading to excessive mROS generation [46]. Hence, the strict cooperation between mCa2+ and mROS signaling seems to have important implications for maintaining cellular homeostasis [43]. Ca2+ signaling plays an essential role in excitable cells as it controls cardiac and skeletal muscle contraction, and synaptic transmission (reviewed in [47]). In view of the fact that neurons regulate extremely important functions such as the transmission of depolarized signals, synaptic plasticity and metabolism, they require a precise spatiotemporal control of Ca2+ [48]. Ca2+ influx into neurons occurs principally through ligand-gated glutamate receptors such as N-methyl-d-aspartate receptors (NMDAR) and voltage-dependent ion channels (VDCCs), as well as the release of Ca2+ from intracellular stores [49]. Remarkably, neurons almost exclusively rely on mitochondrial oxidative phosphorylation (OXPHOS) as the main source of ATP production, and mCa2+ uptake guarantees activity-dependent regulation of cellular energy metabolism [50]. Considering that neurons are particularly sensitive to [Ca2+] oscillations, even small variations in Ca2+ homeostasis can produce deleterious consequences, leading to alterations in physiological neuronal activity such as in aging [51] and neurodegeneration [48]. In the heart, most of the energy needed for cardiac cells excitation and contraction is produced within mitochondria through OXPHOS which, as mentioned above, is a Ca2+-modulated process [16]. The strategic positioning and presence of mitochondria in cardiac cells (over 30% of the cardiac mass) [52] ensure an efficient ATP production to support contractility, metabolism and ion homeostasis [53]. A detailed explanation about the role of mitochondria in the physiology of cardiac cells appears in Section 3.3.
Figure 1.
Mitochondrial calcium homeostasis. mCa2+ homeostasis is tightly regulated by influx and efflux mechanisms. Ca2+ enters into the mitochondrial matrix via MCU and through a high electronegative potential (−180 mV) while its extrusion depends on NCLX and HCX exchangers. Within the matrix, Ca2+ stimulates the activity of three dehydrogenases of the Krebs cycle and ATP production. Ca2+ ions are depicted as yellow dots. Abbreviations: ER, endoplasmic reticulum; MAMs, mitochondria associated membranes; ETC, electron transport chain; MCU, mitochondrial calcium uniporter; VDAC1, voltage-dependent anion channel 1; ATP, adenosine triphosphate; MICU1, mitochondrial calcium uptake 1; IP3Rs, inositol-1,4,5-trisphosphate receptors; ROS, reactive oxygen species; mPTP, mitochondrial permeability transition pore; NCLX, Na+/Ca2+ exchanger; HCX, H+/Ca2+ exchanger (Created with Biorender.com).
3. Mitochondrial Calcium Dyshomeostasis
3.1. Dysregulation of Mitochondrial Ca2+ Signaling in Cancer and the Cell Cycle
Cell death is necessary for life. This, which might seem to be contradictory, is the key to understanding how the cell makes delicate prolife and prodeath decisions to preserve the health of the organism. When a cell is no longer needed, a plethora of cellular signaling pathways activate a program that ultimately leads to self-destruction, giving cell death a connotation that is anything but negative. Ca2+ signaling is undoubtedly one of the most important mechanisms involved in these decisions, and especially in recent years, it has been shown that the dysregulation of Ca2+ homeostasis may result in tumor pathologies [54]. Because they intervene in important process in cancer progression, such as proliferation and invasiveness [55], an increasing number of Ca2+-regulating proteins are being identified as oncogenes and tumor suppressors [56]. Therefore, it is not surprising that many of the previously mentioned Ca2+-related proteins and channels are involved in cell cycle progression and that their dysregulation leads to aberrant cell cycle activity (Figure 2) [55]. Duplication of genetic material and cell division in mammals is guaranteed by the cell cycle, a highly organized and regulated process that can be divided into four distinct phases (G0/G1, S, G2 and M) and controlled by several cyclin-dependent kinases (CDKs) that act in complex with their cyclin partners [57]. Ca2+ ions have been shown to affect the activity of several CDK and CDK–cyclin complexes; for example, Ca2+ and calmodulin (CaM) exert effects on the regulation of the expression of CDK1, CDK2 and cyclin B in human T lymphocytes [58]. The quiescent phase (G0) is a state of cell cycle arrest; in most adult tissues, cells can be either in transient or permanent G0 phase. CDK4 and CDK6 trigger quiescent cells to re-enter the cell cycle in S phase, in which DNA replication occurs. Ca2+/calmodulin-dependent protein kinase (CaMKI), through CaM, is implicated in the regulation of the cyclin D1-CDK4 complex in fibroblasts [59], which in turn regulates retinoblastoma protein (RB1), the main inhibitor of DNA synthesis [60]. The cyclin D-CDK4/6 complex is hyperactivated in many types of human cancers in which the CDK4/6–RB pathway is dysregulated [61].
Figure 2.
Schematic view of the role of Ca2+ dysregulation in cancer and the cell cycle with a particular focus on alterations in mCa2+ levels. Ca2+-regulating proteins and channels are involved in cell cycle progression, and their dysregulation leads to alterations in the cell cycle. STIM1/ORAI1-mediated augmentation of SOCE promotes tumor growth and metastasis; VGCCs are involved in cell proliferation regulation, and T-type channel inhibition provokes cell cycle arrest followed by a significant increase in the number of cells in G1 phase and a decrease in the number of cells in S phase; TRPC1 inhibition reduces the adhesion, invasion and proliferation of different cancer cell lines and G(0)/G(1) cell cycle arrest of glioma and lung carcinoma cell lines. MCU silencing inhibits cell migration and invasion without affecting proliferation rates or apoptosis levels. MCUb silencing limits proliferation, migration, and invasion, as well as glioma progression in vivo. Loss of VDAC1 leads to ATP depletion, which results in decreased cell growth and migration in several cancer cell lines both in vitro and in vivo. The interaction between VDAC and Bcl-XL is responsible for increased ATP production in breast cancer cells, which leads to an increased migration rate. AKT-mediated phosphorylation of MICU1 causes an increase in mCa2+ content under basal conditions and ROS production, which promotes AKT-mediated tumor growth. At MAMs, IP3R3 FBXL2-dependent degradation is enhanced in cancer cells upon loss of PTEN, resulting in apoptosis resistance. The tumor suppressor BAP1 is capable of binding, deubiquitylating and stabilizing IP3R3 channels, modulating Ca2+ release into the cytosol and then into mitochondria and thus promoting cell death. Abbreviations: SOCE, store-operated calcium entry; VGCCs, voltage-gated calcium channels; TRPC1, transient receptor potential channel 1; MCU, mitochondrial calcium uniporter, MCUb, mitochondrial calcium uniporter b subunit; VDAC1, voltage-dependent anion channel 1; ATP, adenosine triphosphate; Bcl-XL, B-cell lymphoma XL; AKT, protein kinase B; MICU1, mitochondrial calcium uptake 1; IP3R3, type 3 inositol-1,4,5-trisphosphate receptor; FBXL2, F-box and leucine-rich repeat protein 2; BAP1, BRCA1-associated protein 1; PTEN, phosphatase and tensin homolog; ROS, Reactive oxygen species (Created with Biorender.com).
Alterations in the intracellular Ca2+ concentration have biphasic effects: on the one hand, an increase in the cytCa2+ level promotes cell migration and is an important feature of cancer cells’ metastatic behavior; on the other hand, reduced Ca2+ transfer via MAMs and a decrease in store-operated calcium entry (SOCE) modulate cell death by contributing to acquired resistance to apoptosis of primary tumors [62].
Since the identification of the key molecules involved in SOCE, there has been extreme interest in determining the role of this Ca2+ influx pathway in tumor onset and progression. A clear example is represented by the STIM1-ORAI1 Ca2+ flux pathway that under physiological conditions promotes the G1 to S transition and inhibits the S to G2 transition [63]. Although STIM/ORAI1-mediated augmented SOCE has been reported to promote tumor growth and metastasis in many cancer types, STIM1 drives growth arrest in human rhabdomyosarcoma and rhabdoid tumor cell lines [64], ORAI1 facilitates apoptosis of PCa cells and the knockdown of ORAI1 leads to drug resistance [65].
Karacicek et al. reported that STIM1 overexpression facilitates cancer cell survival also by preventing mCa2+-dependent enzymatic activity in which MCU requires much higher cytCa2+ concentration [66].
Low voltage-activated T-type channels are members of the voltage-gated calcium channel (VGCC) family, a group of voltage-gated ion channels that open their calcium-selective channel pores as a result of membrane potential depolarization, allowing Ca2+ influx into the cell. It has been shown that cytCa2+ elevations were paralleled by mitochondrial calcium elevations which were also increased by T-type calcium channels overexpression [67]. VGCCs are associated with cell proliferation regulation [68], and it has been shown that low voltage-activated T-type channel inhibitors provoke cell cycle arrest accompanied by a significant increase in the number of G1 phase cells and a decrease in the number of S phase cells in human melanoma cells [69]. Mibefradil, a pharmacological inhibitor of T-type channels, was shown to have a similar cell cycle arrest effect to increase G0/G1 phase distribution in a colon cancer model [70] and in two ovarian cancer cell lines [71].
Transient receptor potential (TRP) channels form a versatile family of ion channels, the majority of which are Ca2+-permeable, playing a significant role in the cell cycle. TRP channels exert their effects by regulating gene transcription and shaping other cellular processes, such as proliferation, cell motility and apoptosis [72]. For instance, TRPC1 is involved in various tumor pathologies in a cancer stage-specific manner; inhibition of the expression or activity of TRPC1 mitigates cell adhesion and invasion ability in nasopharyngeal carcinoma [73], inhibits the migration of HCT-116 colon cancer cells and the proliferation of MDA-MB-468 breast cancer cell lines and leads to G(0)/G(1) cell cycle arrest of glioma and lung carcinoma cell lines [74]. Interestingly, resiniferatoxin, a TRPV1 agonist, has been shown to promote the inhibition of mitochondrial function and induction of apoptosis in pancreatic cancer cells [75].
As we have described in the introductory section of this work, mitochondria and the MAMs compartment have been found to be very important for Ca2+ signaling, especially in recent years. Among the several pathologies that can arise from perturbations in Ca2+ homeostasis at this level, tumors are being studied in depth. Mitochondria are major sites of ROS generation, which occurs largely at complexes I and III of the ETC. An increase in ROS production often arises when electron transport function is compromised, leading to excessive leakage of electrons which then react with oxygen to form superoxide [76].
It is well known that mROS participate in stress signaling under physiological conditions and contribute to the induction of nuclear and/or mitochondrial DNA mutations that stimulate neoplastic transformation. Indeed, mitochondrial ROS strengthen the tumorigenic phenotype and trigger additional mutation accumulation, leading to metastatic behavior [77]. The tumor suppressor protein p53 is a mitochondrial ROS production modulator; however, it is not clear whether its ability to regulate mitochondrial ROS production leads to cell death or stimulates malignancy [78,79]. The antitumoral potential of p53 is well demonstrated by its ability to induce G1 and postmitotic cell cycle arrest and apoptosis [80].
ERK1/2, a kinase belonging to the MAPK family, is activated through a sequential phosphorylation cascade that leads to the signal amplification and the transduction of signals to mitochondria [81]. ERK1/2 acts on FOXO transcription factors that trigger the expression of multiple target genes involved in tumor suppression to induce apoptosis [82] and cell cycle regulation involving p27kip1 and cyclin D [83]. The expression of FOXO3a is linked to tumor progression suppression, while inhibition of its expression promotes tumor progression, angiogenesis and cell transformation [84].
The role of the channel MCU in tumors is multifaceted and strongly debated [85]. In 2013, Marchi et al. found that miR-25, an MCU-targeting microRNA, is overexpressed in colon cancer, where its overexpression correlates with a decrease in Ca2+ uptake and promotes cancer cell survival by enhancing proliferation [86]. However, in vitro studies have shown that MCU silencing in HeLa and Hs578T breast cancer, triple-negative breast cancer and hepatocellular carcinoma (HCC) cells drastically inhibits cell migration, motility and invasion without affecting basal proliferation rates or apoptosis levels [87,88,89]. In MCU-deficient cells, cell cycle progression is delayed at the G1-S phase transition, a stage in which mitochondrial fusion and increased mCa2+ uptake occur under physiological conditions [90].
With reference to MCU complex subunits, a pool of activated AKT can localize at the IMS, where it phosphorylates MICU1. Phosphorylation of MICU1 abolishes its gatekeeping function, leading to higher mCa2+ content under basal conditions and ROS production and thus to AKT-mediated tumor growth (Table 1) [91]. The expression levels of MCUb are inversely associated with overall survival in glioma. Interestingly, it has been reported that MCUb silencing limits glioma cell proliferation, migration, and invasion, as well as glioma progression in vivo [92].
Table 1.
Summary of the key regulatory proteins and transporters associated with either cancer, neurodegeneration and cardiovascular diseases.
| Ca2+-Related Proteins and Channels | Genetic Alteration/Protein Modification | Cellular Model | Ca2+-Related Mechanism | References |
|---|---|---|---|---|
| MCU | KD | Prostate and colon cancer, TNBC, HCC | Low mCa2+ uptake | [86,88,89] |
| Upregulation | AD | High mCa2+ uptake and mPTP opening | [119] | |
| MCUb | Cardiac specific KO | Mouse cardiomyocyte | Low mCa2+ uptake and mPTP opening reduction | [204,205] |
| OE | Mouse cardiomyocytes | Low mCa2+ uptake and mPTP opening inhibition | [217] | |
| MICU1 | Akt-mediated phosphorylation | Renal, ovarian, breast, and lung cancer |
High mCa2+ uptake | [91] |
| NCLX | downregulation | AD mouse and human brains | High mCa2+ uptake | [130] |
| Tamoxifen-induced deletion | Adult mouse hearts | High mCa2+ uptake and mPTP opening | [220] | |
| MFN2 | KO | Mouse cardiac myocytes | Low mCa2+ uptake and mPTP opening reduction | [227] |
| BAP1 | IP3R3 | Mesothelioma | Low ER- Ca2+ release | [98] |
| deubiquitylation | ||||
| and stabilization | ||||
| PTEN | downregulation | Prostate and lung cancer | Low ER- Ca2+ release | [97] |
| PS1 | Mutation | Animal model of AD | High ER- Ca2+ release | [117,118] |
| PS1/PS2 | PS1 (mutation M146L) and PS2 (mutation N141I) | FAD | High ER- Ca2+ release | [116] |
| LRKK2 | mutation | Fibroblasts from PD patients | High mCa2+ uptake | [182] |
| PINK1 | Mutation | Dopaminergic neurons | High mCa2+ levels and increased ER–mitochondria contact sites | [174] |
| Parkin | Deficiency or mutation | PD patient fibroblasts | Low mCa2+ uptake and reduced ER–mitochondria contact sites | [180] |
Abbreviations: KD, knockdown; OE, overexpression; KO, knock-out; AD, Alzheimer disease; FAD, familial Alzheimer disease; PD, Parkinson disease; TNBC, triple-negative breast cancer; HCC, hepatocellular carcinoma.
As previously mentioned, VDAC is a crucial protagonist of mCa2+ homeostasis, facilitating the flow of Ca2+ into and out of mitochondria [93]. Loss of the VDAC isoform VDAC1 leads to ATP depletion, which results in decreased cell growth and migration in colon, lung and pancreatic cancer cells both in vitro and in vivo [94]. The interaction between VDAC and the antiapoptotic protein Bcl-XL is responsible for breast cancer cell-induced increases of ATP production, the main promoter of migration [95].
Some essential proteins have also been demonstrated to affect Ca2+ flux into mitochondria in an ER-mediated manner, and among these proteins, IP3Rs undoubtedly play a leading role. Even if Ca2+ overload triggers the mitochondrial apoptotic pathway, a degradation process named autophagy is activated as a result of poor mCa2+ uptake caused by insufficient Ca2+ transfer from the ER [96].
Phosphatase and tensin homolog (PTEN), a MAM-localized tumor suppressor, enhances Ca2+ release from the ER and can compete with FBXL2, an E3-ubiquitin ligase F-box protein, to bind to IP3R3 to prevent its degradation.
Our group has demonstrated that IP3R3 FBXL2-dependent degradation is enhanced in cancer cells with poor PTEN expression, thus resulting in the inhibition of apoptosis [97].
Moreover, BRCA1-associated protein-1 (BAP1) is another protein with tumor suppressive properties that has been demonstrated to bind, deubiquitylate and stabilize the activity of the IP3R3 channel in the ER, modulating Ca2+ release into the cytosol and then into mitochondria and thus promoting apoptosis (Table 1 and Figure 2) [98].
Especially in recent years, it has become evident that calcium signaling, in particular the mitochondrial proteins and pathways involved in it, is a good target for the development of effective and targeted anticancer therapies. Since many mCa2+ channels/pumps/transporters play a role in normal physiological processes and cell cycle progression, one challenge for drug development is the design of drugs that regulate the cell cycle progression of malignant cells.
3.2. Dysregulation of Mitochondrial Ca2+ Signaling in Neurodegenerative Diseases
Neurodegenerative diseases are a group of heterogeneous disorders characterized by the progressive and selective death of neuronal subtypes. A growing body of evidence suggests that alterations in Ca2+ homeostasis, in particular the dysregulation of mCa2+ signaling, are implicated in different neurodegenerative diseases such as AD, PD and HD [99,100,101]. Neuronal Ca2+ regulation is extremely important even before the appearance of the pathological characteristics of these diseases [99,102,103].
Alzheimer disease is a multifactorial and chronic neurodegenerative disease that is characterized by the loss of cognitive functions and memory. There are two distinct forms of AD: hereditary forms (∼10%) characterized by an early onset, known as familial AD (FAD), and sporadic AD (∼90%) (SAD) which usually develops beyond the age of 60 [104]. SAD is characterized by alterations in several genes. Among these, the apolipoprotein E (APOE) gene that produces the ε4 allele of the APOE (APOE4 variant) is one of the most studied [105]. APOE4 expression can promote an increase in ER–mitochondria contact sites, causing an elevation of mCa2+ and cytCa2+ levels [104]. FAD is characterized by mutations in amyloid precursor protein (APP), Presenilin-1 (PS1) and Presenilin-2 (PS2). The most influential hypothesis for AD is based on the abnormal proteolytic cleavage of APP, which induces the formation and accumulation of amyloid β-peptide (Aβ), leading to the development of extracellular plaques [106,107]. In addition, intracellular neurofibrillary tangles are formed through the aggregation of the microtubule-associated protein tau [108]. These events ultimately lead to synaptic dysfunction and progressive neuronal death in brain regions dedicated to learning processes and memory [109,110,111]. Interestingly, Aβ neurotoxicity has also been associated with disruption of intracellular Ca2+ signaling [112]. Indeed, several studies have shown that amyloidogenic pathway induces alterations of the mechanisms involved in memory, learning and intraneuronal Ca2+ signaling [113,114]. As a matter of fact, in the early 1990s, it was shown that enhanced InsP3-mediated Ca2+ signaling was a prognostic diagnostic feature in AD-derived cells [115]. Subsequently, Cheung et al. have shown that mutant PS1 and PS2 interact and modulate the IP3R Ca2+ release channel. This interaction has a strong stimulatory effect on its gating activity that ultimately leads to an abnormal Ca2+ signaling response to agonist stimulation (Table 1) [116]. Similarly, mutant PS1 N-terminal region can interact with ryanodine receptor (RyR) and enhance its activity both in vitro and in animal models of AD (Figure 3) [117,118]. In fact, studies carried out on young neurons of 3xTg-AD mice revealed a selective increase of about fivefold of the RyR2 isoform in relation to control non-transgenic mice, influencing the plasticity and synaptic activity in AD mouse models [117]. All these alterations that affect ER-Ca2+ release have an indirect effect on mCa2+ uptake [119]. Therefore, the enhanced transfer of Ca2+ between ER and mitochondria results in an increase in mCa2+. The subsequent mCa2+ overload, as already mentioned above, triggers mPTP opening and the release of proapototic factors [120,121], thus contributing to neurotoxicity [112]. In addition, early onset of the disease is caused by Aβ oligomers acting directly on the increase in mCa2+ uptake [119]. Aβ oligomers can be transported into mitochondria via translocase of the outer membrane (TOM) and translocase of the inner membrane (TIM). Once inside the mitochondria, they interact with specific intramitochondrial targets, leading to a reduction in respiratory chain complex III and IV activity [122,123,124,125]. In vivo studies have shown that mCa2+ overload is elicited by a direct action of Aβ oligomers that triggers apoptosis through ATP synthesis inhibition, mPTP opening and ΔΨm collapse [126,127,128] (Figure 3). Notably, several studies have demonstrated that in AD patients’ brains and in transgenic mouse models of AD, neuronal injuries and the decline in cognitive functions are caused by the interaction of mitochondrial Aβ with cyclophilin D (CypD). CypD is a prolyl isomerase situated in the mitochondrial matrix and is an integral component of the mPTP. Its interaction with Aβ stimulates the opening of the mPTP and thus promotes cell death. Therefore, CypD deficiency protects neurons from oxidative stress, Aβ-induced cell death, synaptic dysfunction and deficits in memory and learning [129]. On the one hand, Calvo-Rodriguez M. et al. have demonstrated in healthy brain that the Aβ soluble oligomers induces mCa2+ increase through MCU. Given that, excessive Ca2+ taken up by mitochondria may lead to the opening of the mPTP and eventually to neuronal cell death. Conversely, the inhibition of MCU due by Ru360, prevents mCa2+ overload. Hence, proposing mPTP inactivation and the inhibition of MCU as a new potential therapeutic approach for AD [119]. On the other hand, Jadiya P. and colleagues have proposed another mechanism for Aβ mediated Ca2+ overload. Specifically, they have shown that neuronal deletion of the mitochondrial NCLX induces an increase in amyloidosis and tau pathology, accelerating memory degeneration. In 3xTg-AD triple mutant mice (which harbor mutations in PS-1, APP and tau) and in the brains of human patients with AD, the levels of NCLX are reduced, leading to an increase in mCa2+ concentration (Table 1). It is interesting to note that through recovery of NCLX expression, it is possible to reduce mCa2+ overload and consequently to prevent cognitive decline and AD-related pathology [130]. Additionally, in AD models, adverse effects have been observed in mitochondrial bioenergetics induced by a reduction of mCa2+ signal. In FAD, the reduction of mCa2+ is mediated by PS2 mutations which decrease the ER Ca2+ content [131,132], thus leading to an increase in neuronal sensitivity to excitotoxicity and neuronal bioenergetics [124,132]. Interestingly, PS1 and PS2 are not distributed homogeneously in the ER, but are highly enriched in MAMs [133,134]. Given this, MAM function and ER–mitochondrial connectivity are up-regulated in AD. Pera et al. have demonstrated that y-secretase is enriched at MAMs and is responsible for cutting the C-terminal fragment of APP (C99) that generates Aβ [135,136]. Consequently, in cells of AD patients, high levels of C99 have been detected in MAMs fraction, besides alterations in MAMs function and structure [135]. Based on this, in AD, the C99 accumulation in MAMs is upstream of mitochondrial dysfunction, suggesting an early role of mitochondrial dysfunction in the disease [137]. All these evidences suggest that altered ER–mitochondrial communication play a critical role in AD pathogenesis. In particular, mitochondrial function and mCa2+ are essential for neuronal function and survival.
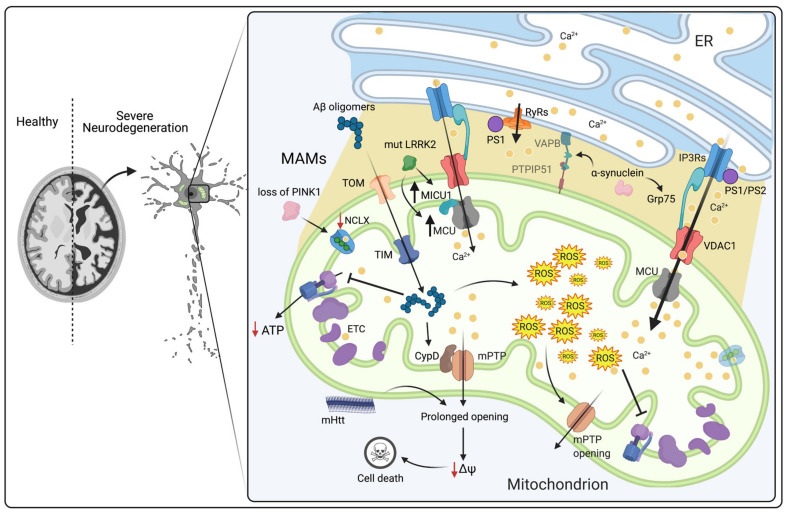
Figure 3.
Schematic view of the role of mCa2+ dysregulation in neurodegenerative diseases. In AD, Aβ oligomers enter mitochondria via the translocases TOM and TIM. Once inside the matrix, they interact with specific intramitochondrial targets: (i) respiratory chain complexes III and IV, leading to ATP synthesis reduction, and (ii) CypD, leading to mPTP opening, ΔΨm collapse and activation of cell death. Excessive levels of mCa2+ can enhance ROS production, reduce the ΔΨm, and stimulate mPTP opening, thus leading to the release of proapoptotic factors. In HD, mHtt decreases the Ca2+ threshold necessary to trigger mPTP opening, preventing the binding of mPTP inhibitors and consequently augmenting its activation by increasing the binding affinity of CypD and Ca2+. In PD, α-synuclein interacts with the chaperone Grp75, thus contributing to enhancement of ER–mitochondria communication. Overexpression of wt and mutant α-synuclein leads to the destruction of VAPB-PTPIP51 tethers through its bond with VAPB, causing a decrease in the ER–mitochondria association. PINK1 deficiency results in mitochondrial calcium overload and subsequent ROS production due to negative regulation of NCLX. Mutant LRRK2 induces transcriptional upregulation of MCU and MICU1, thus leading to mCa2+ accumulation. Mutant PS1 and PS2 interact and modulate the IP3R Ca2+ release channel causing a strong stimulatory effect on its gating activity. PS1 N-terminal region can interact with ryanodine receptor (RyR) and enhance its activity. Abbreviations: ER, endoplasmic reticulum; MAMs, mitochondria-associated membranes; mPTP, mitochondrial permeability transition pore; CypD, cyclophilin D; ROS, reactive oxygen species; TIM, translocase of the inner membrane; TOM, translocase of the outer membrane; MICU1, mitochondrial calcium uptake 1; MCU, mitochondrial calcium uptake; NCLX, Na+/Ca2+ exchanger; Grp75, glucose-regulated protein 75; IP3R3, inositol-1,4,5-trisphosphate receptor type 3; ETC, electron transport chain; PINK1, PTEN-induced kinase 1; LRRK2, leucine-rich repeat kinase 2; mHtt, mutant Htt; ΔΨm, mitochondrial membrane potential; PS1, presenilin 1; PS2, presenilin 2 (Created with Biorender.com).
In addition, HD manifests as psychiatric, dementia, behavioral, motor and cognitive abnormalities. It belongs to the family of N-terminal polyglutamine (polyQ) diseases [138,139,140]. This neurodegenerative disease is caused by alterations in huntingtin (Htt), a cytosolic protein that is expressed in various tissues [141]. Although the mechanisms underlying the onset of the disease remain unclear, several studies have shown that mitochondrial dysfunction plays a key role in HD pathogenesis [123,142,143,144,145,146]. In fact, it has been verified that in clonal striatal cell lines from wild-type and mutant homozygote knockin mice, the Htt protein is localized in the OMM. A similar observation was made in a human neuroblastoma cell line, suggesting that this is a common characteristic of different cell types and that mutant huntingtin (mHtt) may have a direct deleterious effect on mitochondria [147]. Indeed, N-terminal mHtt leads to a drastic reduction in Ca2+ concentration, which is required for the activation of mPTP and the release of cytochrome c. This suggests that mHtt significantly decreases the Ca2+ threshold necessary to trigger mPTP opening, preventing the binding of mPTP inhibitors and consequently improving mPTP activation by increasing the binding affinity of CypD and Ca2+ [148]. Panov et al. observed a deficit in mCa2+ buffering in mitochondria isolated from HD patients: these mitochondria showed a lower ΔΨm and depolarized at lower Ca2+ loads [149]. In contrast, other groups have demonstrated that Ca2+ uptake capacity is increased in brain-derived mitochondria and cultured neurons derived from the YAC128 HD mouse model [150,151]. In HD, stimulation of glutamate receptors leads to an increase in cytCa2+ levels in medium spiny neurons (MSNs). Through the MCUC, excessive cytCa2+ enters mitochondria, causing mPTP opening and hence apoptosis or mitochondrial DNA damage. Before the onset of neurodegeneration, in both HD mouse models and in HD patients, mitochondrial dysfunction and abnormal levels of mCa2+ and cytCa2+ have also been observed [152]. In conclusion, HD is associated with an early Ca2+ handling defect which plays a critical role in the pathogenesis of the disease. Particularly, the alterations of mCa2+ homeostasis have a crucial impact on selective neurons degeneration. However, the exact mechanisms involved in the pathogenesis of HD have not yet been fully clarified. PD, another common neurodegenerative disorder, affects 6.3 million people above the age of 60 [153]. This disease belongs to a group of neurodegenerative diseases known as synucleinopathies, which are characterized by the aggregation of α-synuclein (a small lipid-binding protein) into Lewy bodies. At the cellular level, it is characterized by a loss of dopaminergic neurons in the substantia nigra pars compacta (SNc). Several studies have confirmed that abnormalities in mCa2+ levels are linked to its pathogenesis (reviewed in [154,155,156,157]). However, in the past 10 years, more than 30 genes responsible for PD pathogenesis, such as α-synuclein, Parkin, PTEN-induced kinase 1 (PINK1) and leucine-rich repeat kinase 2 (LRRK2) [158,159], have been identified [160,161,162,163,164,165,166]. α-Synuclein, which is normally located in the cytosol and in mitochondria, is involved in physiological and/or pathological mitochondrial function [167]. Recent studies have illustrated that wild-type α-synuclein is also located at MAMs, where it interacts with the chaperone Grp75, thus contributing to ER–mitochondria communication [168,169,170]. Interestingly, Guardia-Laguarta and colleagues demonstrated that α-synuclein mutations can lead to an increase in mitochondrial fragmentation and a reduction in ER–mitochondria contact sites [168]. Conversely, Calì et al. showed that in SH-SY5Y and HeLa cells, α-synuclein positively affects Ca2+ transfer from the ER to mitochondria [170,171]. Nevertheless, overexpression of wt and mutant α-synuclein leads to the destruction of VAPB-PTPIP51 tethers through binding with VAPB, which causes a decrease in ER–mitochondria associations. This disruption lessens mitochondrial ATP production and interrupts Ca2+ exchange between ER and mitochondria [172]. Although the mechanism of action remains unclear, mutations in or variants of many genes increase susceptibility to PD. For instance, PINK1 and parkin are two PD-associated proteins that influence mitochondrial pathways of Ca2+ influx [173]. In dopaminergic neurons expressing mutant PINK1, mCa2+ levels are elevated and the number of ER–mitochondria contact sites is increased, leading to a progressive loss of neurons [174]. Physiologically, PINK1 regulates Ca2+ efflux from mitochondria via NCLX, while Parkin stimulates VDAC1. PINK1 is a mitochondrial serine/threonine protein kinase and is required for Parkin recruitment and stress-induced mitophagy [175,176]. However, mitochondria isolated from the brains of mice lacking PINK1 seem to be more vulnerable to cell death [177]. In addition, Gandhi et al. proved that PINK1 deficiency results in mitochondrial calcium overload and subsequent ROS production due to negative regulation of NCLX. Indeed, given the reduced mCa2+ capacity and increased levels of ROS, the threshold of mPTP opening is low, making neurons vulnerable to programmed cell death [178]. Parkin is an E3 ubiquitin ligase that plays a key role in mitophagy, a mechanism that selectively removes damaged mitochondria [179]. It has been shown that Parkin-deficient cells and fibroblasts expressing mutant Parkin from PD patients display reduced ER–mitochondria tethering, resulting in diminished mCa2+ uptake [180]. In contrast, Gautier et al. reported that the number of ER–mitochondria contacts is increased in primary fibroblasts from PARK2 knockout mice and PD patients with PARK2 mutations [181]. Mutations in LRRK2 contribute to development of PD. Indeed, several studies have shown that cortical neurons expressing mutant LRRK2 exhibit a major increase in excitatory neurotransmission, which occurs before dendritic shortening. Interestingly, patient fibroblasts expressing mutant LRRK2 show higher levels of MCU and MICU1 and increased depolarization-induced mCa2+ uptake (Figure 3). In fact, in fibroblasts from both PD and Parkinson’s disease dementia (PDD) patients, the expression of mutant LRRK2 induces the transcriptional upregulation of MCU and MICU1 but not MICU2 and NCLX (Table 1). Hence, strategies that target either MCU or NCLX may serve to normalize activity-dependent mitochondrial calcium flux to protect against neurodegeneration [182]. All these data underline that the disruption of MAMs and alterations in mCa2+ levels may contribute to the onset of PD.
3.3. Dysregulation of Mitochondrial Ca2+ Signaling in CVDs
CVDs are considered the leading cause of mortality in Western countries [183]. Multiple and complex factors are involved in the onset and development of cardiac disorders; however, in recent years, mitochondrial dysfunction has been recognized as the hallmark of heart physiopathology [184,185]. As previously stated, mitochondria are responsible for long-term Ca2+-buffering. Specifically, in the heart, mCa2+ flux plays an important role not only in myocardial energy production and mitochondrial metabolism by activating Ca2+-sensitive dehydrogenases (PDH, IDH, KGDH) [186,187], but also in the regulation of cardiomyocyte contractility [188,189]. Therefore, disturbances in mCa2+ homeostasis (increased or decreased levels) contribute to the onset and development of many CVDs, such as IR, cardiac hypertrophy, cardiomyopathies and arrythmia [185,190].
To better understand the role of mCa2+ in heart physiopathology, it is necessary to know how mitochondria are organized in cardiomyocytes. The heart is a high-energy demand organ; hence, it is not surprising that mitochondria occupy 30% of the total volume of cardiomyocytes and generate approximately 95% of ATP in the body [191]. They are highly constrained among cardiac fibers and organized in three different subgroups based on their functions and location: subsarcolemmal (under the sarcolemma), perinuclear (around the nucleus) and interfibrillar (between myofibrillas) mitochondria [192]. Interfibrillar mitochondria are the most abundant, and they participate in ATP production to support myocyte contraction by regulating Ca2+ signaling during excitation–contraction (EC) coupling of the heart [189,193,194]. Briefly, after sarcolemma depolarization, L-type Ca2+ channels open to allow a small amount of Ca2+ to enter the cell, which stimulates even greater Ca2+ release from the sarcoplasmic reticulum (SR) via RyR2 (calcium-induced calcium release). Subsequently, Ca2+ binds to troponin C (through ATP consumption), thus inducing cardiomyocyte contraction (Figure 4). During relaxation, cytCa2+ is cleared by being taken back up into the SR through activation of sarco-endoplasmic reticulum calcium ATPase (SERCA) and extruded from the cell via the sarcolemma NCX [195]. Mitochondria participate in this process by regulating Ca2+ signaling for the production of energy required for the contraction–relaxation process [188]. However, the process through which mitochondria decode transient and rapid cytCa2+ signals on a beat-to-beat basis is still controversial. Over the past years, two main models have been suggested. In the first model, which was proposed by Crompton [196], mCa2+ influx is slow and associated with an even slower release of accumulated Ca2+ until a steady state is reached. Fast cytCa2+ oscillations are integrated by Ca2+ transporters in of the IMM. Therefore, changes in mCa2+ levels are small and associated with low energy demands by Ca2+ transporters. However, this slow increase in mCa2+ is not sufficient to stimulate ATP production at a fast enough speed to respond to the energy demand of the beating heart. Instead, the second model [197] describes quick cytCa2+ oscillations resulting in beat-to-beat changes in mCa2+ levels. Therefore, fast mCa2+ influx and efflux are needed. In this scenario, mCa2+ uptake upon each heartbeat is rapid and large enough to allow adequate energy production and to regulate cytCa2+ pulses. The inconsistencies in the findings related to whether beat-to-beat changes in mitochondrial calcium occur during EC coupling occur are mainly due to the methods applied to measure free mCa2+ levels and the species employed as experimental model (for detailed review see [193,198,199,200]).
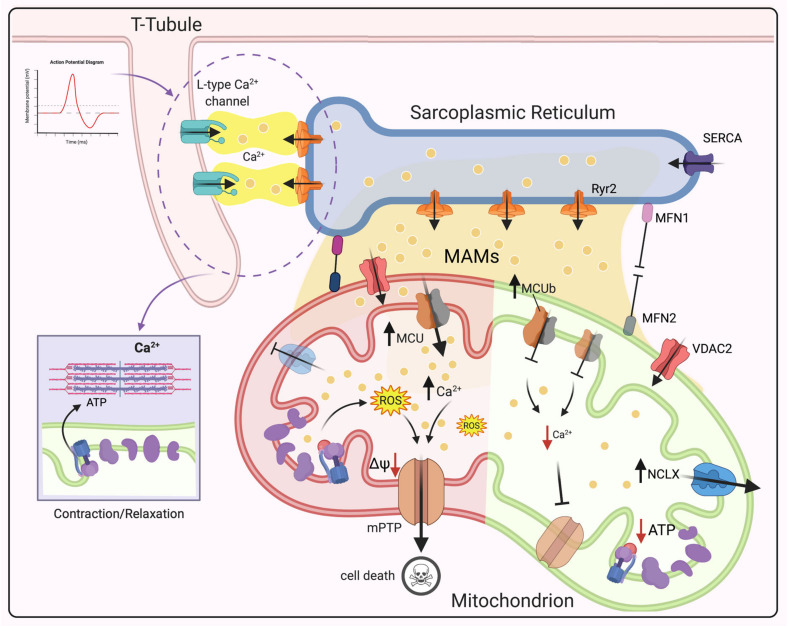
Figure 4.
Schematic of the role of mCa2+ dysregulation in CVDs. Under physiological conditions, after sarcolemma depolarization, the opening of L-type Ca2+ channels allow Ca2+ to enter the cell, which stimulates Ca2+ release from the SR via RyR2. Then, Ca2+ binds to troponin C, leading to cardiomyocyte contraction. Mitochondria are juxtaposed to the SR, and high-Ca2+ microdomains form at this interface. Ca2+-mediated crosstalk between the SR and mitochondria is mediated by RyR2 and VDAC2. Reduced mCa2+ uptake by MCU deletion leads to inhibition of mPTP opening. MCUb overexpression causes a reduction in mCa2+ uptake and consequent inhibition of mPTP opening after reperfusion. Increased NCLX activity leads to Krebs cycle impairment, bioenergetic dysfunction, a decrease in NADPH levels and ROS hyperproduction, while NCLX knockout triggers mCa2+ overload and consequent mPTP opening. Ca2+ leakage through RyR2 on the SR causes mCa2+ accumulation and ROS production, leading to chronic HF after mPTP opening. MFN2 ablation may reduce mPTP opening by decreasing mCa2+ uptake, thus protecting the heart from IRI. Deletion of both MFN1 and MFN2 in adult hearts induces lethal dilated cardiomyopathy. Abbreviations: MAMs, Mitochondria-associated membranes; SR, sarcoplasmic reticulum; RyR2, ryanodine receptor type 2; SERCA, Sarco-Endoplasmic Reticulum Calcium ATPase; MCU, mitochondrial calcium uniporter; VDAC2, voltage-dependent anion channel 2; mPTP, mitochondrial permeability transition pore; NCLX, Na+/Ca2+ exchanger; ROS, reactive oxygen species; ΔΨm, mitochondrial membrane potential; MFN1, mitofusin 1; MFN2, mitofusin 2; (Created with Biorender.com).
As mentioned above, MCUC is the main route for mCa2+ uptake. Since this channel has a low affinity for Ca2+ ions, for a long time, it was questioned how mitochondria could regulate Ca2+ signaling during EC coupling. In this context, it is worth noting that mitochondria are juxtaposed to the SR, and high Ca2+-microdomains, which may help mitochondria to respond to cytCa2+ oscillations [201], form at the interface [202]. However, different studies using MCU-deficient models (models of global constitutive, cardiac-specific, dominant negative overexpression) [203,204,205,206] have shown no differences in basal cardiac activity, suggesting that MCU is needed only for the “fight-or-flight” response. Therefore, it is reasonable to speculate about the presence of other channels in the IMM in cardiomyocytes. Rapid uptake channels (RaMs), as their name suggests, exhibit faster Ca2+ influx than MCUC. However, they do not seem to be involved in beat-to-beat Ca2+ uptake [207]. In contrast, mitochondrial RyR (mRyR) appears to participate in mCa2+ uptake in the beating heart, since it allows rapid influx at low Ca2+ concentrations [208], although, further studies are demanded.
It is well recognized that mCa2+ overload induces cell death and subsequently cardiac dysfunction by activating mPTP opening [209]. mPTP is a nonspecific pore at the IMM, and its components and regulators are still under investigation (the latest findings on its structure can be found in recent reviews by Bonora et al. [210,211]. In recent decades, ATP synthase has been identified as the central core for pore formation, and our group demonstrated that disruption of ATP synthase dimers and mutation of the c-subunit, which disrupt the c-ring conformation, mediates mPTP opening [9]. Furthermore, it was recently discovered that the Ca2+ binding site on the catalytic portion of the ATP synthase β subunit generates conformational changes, which spread through oligomycin sensitivity conferring protein (OSCP) to the lateral stalk, ultimately inducing mPTP opening [212]. mPTP allows the free passage of molecules and ions (<1.5 kDa) and consequent ΔΨm dissipation, ROS burst and a reduction in ATP production, ultimately leading to activation of cell death pathways [213,214]. Indeed, mCa2+ overload and mPTP opening have been widely reported in IRI [10,215]; therefore, it was hypothesized mPTP inhibition preventing mCa2+ overload by deleting MCUC. Several mouse models have been proposed; however, contradictory results have been obtained. Studies on cardiac-specific knockout of MCU [204,205] confirmed the initial hypothesis; in contrast, constitutive MCU knockout [203] and cardiac-specific dominant negative overexpression of MCU [216] result in loss of mPTP opening. However, the hearts of the animal models used in these studies were not protected from IRI. These differences may suggest that deletion of MCU at an early stage can induce alternative cell death pathways. Interestingly, a recent work examined the hypothetical cardioprotective role of the MCUb subunit. In cardiomyocyte-specific MCUb-overexpressing transgenic mice, a reduction in mCa2+ uptake and consequent inhibition of mPTP opening after reperfusion were observed (Table 1 and Figure 4) [217].
Contractile dysfunction is also associated with a reduction in mCa2+ levels and consequent effects on energy supply and demand matching and results in cardiac hypertrophy and ultimately heart failure (HF). In guinea pig hearts, an increase in cytosolic Na+ levels due to increased activity of NCLX is associated with inhibition of the Krebs cycle, bioenergetic dysfunction, a decrease in NADPH levels and ROS hyperproduction (Figure 4) [218,219]. In contrast, studies on NCLX knockout hearts have shown that mCa2+ overload and consequent mPTP opening occur. (Table 1.) However, targeting of NCLX in an ischemic model to overcome Ca2+ accumulation in mitochondria has been proposed. Cyp D-null mice were rescued by NCLX overexpression, which reduced mPTP opening [220].
It should also be pointed out that the disruption of the contact sites between the SR and mitochondria may contribute to the dysregulation of mCa2+ homeostasis [221]. Ca2+-mediated crosstalk between these two organelles is regulated by RyR2 and VDAC2 (on SR and mitochondria, respectively) [222]. Using a murine model of post-myocardial infarction, Santulli et al. proposed the existence of a positive feedback loop between SR and mitochondria [223,224]. In this model, Ca2+ leakage through RyR2 on the SR causes mCa2+ accumulation and ROS production, which in turn leads to post-translational modification of the channel itself, causing chronic HF after mPTP opening [223,224]. Additionally, SR Ca2+ leakage induces the activation of spontaneous action potentials, which trigger cardiac arrhythmias [225,226].
Moreover, it has been reported that cardiac myocytes isolated from mitofusin-2 (MFN2) knockout (MFN2-KO) mice [227,228] display a reduction in mCa2+ uptake, resulting in inadequate ATP production. Consequently, these mice develop cardiac hypertrophy. The authors also suggested that MFN2 ablation may reduce mPTP opening by decreasing mCa2+ uptake, thus protecting hearts from IRI (Table 1) [227]. Furthermore, conditional gene deletion of mitofusin 1 (MFN1) and MFN2 in adult hearts induces lethal dilated cardiomyopathy [52,221].
4. Targeting Mitochondrial Ca2+ Signaling as a Promising Therapeutic Approach
It is becoming increasingly clear that targeting mCa2+ might be a potential and valid strategic option not only for cancer therapy but also for the treatment of neurodegenerative diseases and CVDs. In recent years, efforts have been made to decodify the mitochondrial calcium signaling network to develop selective inhibitors or regulators of calcium channels, exchangers and pumps. At the preclinical level, these strategies have demonstrated great potential, although major drawbacks have been reported when applied in vivo. Thus, approved therapies are still unavailable. This section is devoted to summarizing the most recent scientific data regarding promising therapeutic approaches targeting mCa2+ signaling with a particular focus on cancer, neurological disorders and CVDs.
Specifically, in the context of cancer, even though the inhibition of MCU has emerged as a promising strategy to slow tumor progression, no successful therapies have been approved to date. Interestingly, in human colon cancer and cancer-derived cells, it has been reported that the overexpression of anti-miR-25 can suppress the inhibitory effect of miR-25 on MCU expression. Thus, mCa2+ uptake is re-established, and apoptosis resistance is reversed (Table 2) [86].
Table 2.
List of modulators available for shaping mCa2+ signaling as potential therapeutic approach.
| Therapeutic Target | Compound | Side Effects | Cell Permeability | In Vivo Applicability | References |
|---|---|---|---|---|---|
| MCU | anti-miR25 | Not observed | Yes | ND | [86] |
| Ru265 | Not observed | Yes | ND | [231] | |
| DS16570511 | ΔΨm loss and cell death | Yes | No | [232,234,235] | |
| Mitoxantrone | Cardiotoxicity | Yes | Limited | [236,237] | |
| Kaempferol | Not observed | Yes | Yes | [249,250] | |
| SB202190 | Not observed | Yes | ND | [249] | |
| KB-R7943 | Not selective | Yes | ND | [265] | |
| MICU1 | MCU-i4 and MCU-i11 | Not observed | Yes | ND | [267] |
| NCLX | KB-R7943, | Not selective | Yes | ND | [255,262,264] |
| CGP-37157 | Not observed | Yes | ND | [257,259] | |
| SEA0400 | Not observed | Yes | ND | [255,256] | |
| mPTP | Cyclosporin A | Not observed | Yes | ND | [270] |
| 1,3,8-Triazaspiro[4.5]decane derivatives | Not observed | Yes | ND | [274] | |
| SERCA | Thapsigargin | Not selective | Yes | No | [241] |
| Mipsagargin G-202 | Not observed | Yes | Yes | [243] | |
| Resveratrol and piceatannol | Not observed | Yes | Limited bioavailability | [244] | |
| MAMs: BCl2-IP3R3 | BIRD-2 | Not observed | Yes | ND | [238,239] |
Abbreviations: ND, not determined.
Ruthenium red (RuR) and Ruthenium 360 (Ru360) are the most well-known compounds capable of inhibiting MCU activity. RuR is a nonspecific MCU inhibitor that prevents mCa2+ uptake without perturbing mitochondrial respiration or Ca2+ efflux [229], while Ru360 is a selective MCU inhibitor. However, because these compounds are cell impermeant, their applicability in vitro is still limited [230]. Intriguingly, the synthesis and biological activity of a novel ruthenium complex named Ru265 were recently characterized. This new ruthenium derivative is cell permeable, slightly toxic, and more strongly inhibits MCU activity than Ru360. In addition, it does not affect cytoCa2+ dynamics or the ΔΨm. Woods and his group demonstrated that this compound is capable of protecting neonatal rat ventricular myocytes from IRI by preventing mitochondrial swelling, mPTP opening and cell death [231]. Hence, Ru265 is a novel potential drug for cardiac disorders.
DS16570511 is another recently identified effective MCU inhibitor. It shows high specificity for MCU and is cell permeant. Interestingly, it was proven to block mCa2+ overload in Langendorff perfused rat hearts and to increase cardiac contractility without compromising heart rate [232]. However, its ability to ameliorate AD pathologies has not yet been investigated [233]. Despite this encouraging evidence, its side effects on the ΔΨm limit its usage [234,235].
Mitoxantrone is a topoisomerase type II inhibitor that is currently used for the treatment of acute myeloid leukemia (AML) and breast cancer [230].
By using a high-throughput screening strategy, it was recently demonstrated to have a direct inhibitory effect on MCU [236]; however, it has been reported to have high cardiotoxicity [237].
Alternatively, the MAMs are another possible site of action. Indeed, it has been demonstrated that the peptide BCL-2-IP3R disruptor 2 (BIRD-2) is capable of blocking the interaction between Bcl-2 and IP3Rs, thus triggering proapoptotic Ca2+ signaling in cancer cells [238,239].
SERCA inhibition is another method for triggering cell death. It is widely recognized that SERCA pumps play a crucial role in cellular viability [240]. Thapsigargin selectively binds and blocks the SERCA pump. This inhibition provokes dysregulation of intracellular Ca2+ levels and subsequent induction of apoptotic cell death not only in cancerous cells but also in normal cells [241]. This is the main reason why the clinical application of thapsigargin has been hindered. To overcome this impasse, a thapsigargin prodrug called Mipsagargin G-202 was recently developed [242]. Unlike thapsigargin, G-202 does not induce systemic toxicity. In fact, it has been shown to be promising in several preclinical studies and is currently in phase II clinical trials for the treatment of prostate cancer and glioblastoma [243].
Regarding natural compounds, the polyphenol resveratrol and its derivative piceatannol display high selectiveness in increasing mCa2+ uptake in cancer cells after SERCA inhibition at MAMs without affecting healthy cells [244]. However, due to a low bioavailability of resveratrol, there is limited development concerning its use in clinical settings [245]. Among polyphenols, Kaempferol is a natural flavonoid emerging as a promising anti-cancer compound [246,247,248]. It has been found to be a cell permeant specific enhancer of MCU [249,250]. Interestingly, by modulating mCa2+ uptake, it has been recently demonstrated to activate metabolism/secretion coupling in pancreatic β-cells [251]. Moreover, in a recent study, Kaempferol showed its ability to protect cardiomyocytes from anoxia/reoxygenation (A/R) injury through reduction of ROS production, preservation of ΔΨm and inhibition of mPTP opening [252].
With regard to drugs used for controlling mCa2+ homeostasis, SB202190 is an inhibitor of p38 mitogen activated protein (MAP) kinase, which has been proven to reversibly stimulate mCa2+ uptake in both intact and permeabilized Hela cells [253].
Although pharmacological regulators of Ca2+ homeostasis, such as verapamil, a blocker of plasma membrane Ca2+ channels used for the treatment of arrhythmia and some form of hypertension [254], are available for the treatment of some CVDs, targeting mCa2+ flux remains challenging. KB-R7943, CGP-37157 and SEA0400 are NCLX inhibitors that have been demonstrated to exert promising cardioprotective effects in an animal model of HF [255,256,257]. In addition, CGP-37157 also confers neuroprotection [258,259]. However, NCLX inhibitors never entered clinical development [185,260] due to the fact that these compounds also block the plasma membrane Na+/Ca2+ antiporter SLC8A1 (also known as NCX1) [261]. Specifically, KB-R7943 has been proven to significantly protect against IR-induced damage [255,262,263] and neuronal injury [264]. In 2007, it was demonstrated that KB-R7943 is also capable of inhibiting MCU activity, leading to a reduction in mPTP opening during reperfusion, conferring a cardioprotective effect [265]. In contrast, another group confirmed that KB-R7943 inhibits Ca2+-induced mPTP opening but does not prevent mitochondrial calcium uptake [266]. Clearly, the mechanism through which KB-R7943 exerts protective effects remains unclear and controversial; thus, further studies are required.
Very recently, by screening a library of 44,000 compounds, Di Marco et al. discovered two MICU1 targeting compounds named MCU-i4 and MCU-i11. By directly binding to MICU1, they decrease mCa2+ influx both in intact cells and in muscle fibers. These novel compounds impair muscle cell growth [267], highlighting the crucial role of mCa2+ in muscle physiology.
Considering the crucial role of mPTP in both CVDs and neurodegenerative diseases, there is a strong interest in developing drugs to be used as therapeutic agents. However, the molecular identity of the mPTP has not yet been fully discovered, leading to difficulties in developing effective therapies.
The effects of cyclosporine A (a CypD inhibitor) have been evaluated in several clinical trials for acute myocardial infarction (AMI) [268,269]. In a pilot phase II clinical trial, it was demonstrated to decrease the infarct size [270]. In addition, in the Cyclosporine and Prognosis in Acute Myocardial Infarction Patients (CIRCUS) trial, cyclosporine A failed not only to ameliorate clinical outcomes but also to prevent adverse left ventricular remodeling at 1 year after myocardial infarction (MI) [271]. Other trials on different mPTP inhibitors and in different diseases have also failed recently [272]. At the preclinical level, studies adopting pharmacological (cyclosporine A) and genetic approaches (CypD knockout) to inhibit mPTP opening have reported reductions in neuronal injuries and degeneration in cultured cells and mutant mouse models of AD [129,273].
Interestingly, our group recently developed the first small-molecule mPTP opening inhibitors based on a 1,3,8-triazaspiro[4.5]decane scaffold, which targets the c subunit of the F1/FO-ATP synthase complex. These compounds demonstrated beneficial effects in an ex vivo model of MI without having off-target effects at the cellular and mitochondrial levels [274].
5. Concluding Remarks and Perspectives
In this review, we highlighted the crucial role of mCa2+ in both physiological and pathophysiological conditions. Thus far, mCa2+ levels should be tightly regulated and balanced. In fact, as we have presented in the sections above, alterations in the amplitude or in the spatial–temporal control of mCa2+ signaling can provoke deleterious effects that have been linked to several pathologies, such as cancer, neurodegeneration and cardiovascular disorders.
Indeed, it is widely accepted that we are moving into an era of mitochondrial medicine, in which mCa2+ has gained growing attention. In recent years, strong efforts have been made in this field, leading to several opportunities to translate these findings into clinical therapies.
The identification of the key players involved in mCa2+ influx and efflux has led to potential therapeutic intervention such as molecules capable of efficiently and specifically inhibiting or sustaining these pathways.
To date, pharmacological strategies and methods that have been studied seem to be ineffective when tested in vivo. Since studies have shown that these strategies may have negative effects; for now, the best approach in vivo context is caution. Thus, further studies are urgently required. mCa2+ homeostasis modulation is at the core of the issue—drugs and therapies that target mCa2+ are needed.
Acknowledgments
P.P. is grateful to Camilla degli Scrovegni for her continuous support.
Abbreviations
| AD | Alzheimer disease |
| APP | Amyloid precursor protein |
| Aβ | Amyloid β-peptide |
| APOE | Apolipoprotein E |
| BAP1 | BRCA1-associated protein-1 |
| CaMKI | Ca2+/calmodulin-dependent protein kinase |
| Ca2+ | Calcium |
| CaM | Calmodulin |
| CVDs | Cardiovascular diseases |
| CDKs | Cyclin-dependent kinases |
| CypD | Cyclophilin D |
| cytCa2+ | Cytosolic calcium |
| ETC | Electron transport chain |
| ER | Endoplasmic reticulum |
| EMRE | Essential MCU regulator |
| FAD | Familial AD |
| HCX | H+/Ca2+ exchangers |
| HF | Heart failure |
| HD | Huntington disease |
| IMM | Inner mitochondrial membrane |
| IP3R3 | Inositol 1,4,5-trisphosphate (IP3) receptor type 3 |
| IP3Rs | Inositol 1,4,5-trisphosphate (IP3) receptors |
| IMS | Intermembrane mitochondrial space |
| IRI | Ischemia/reperfusion injury |
| IDH | Isocitrate dehydrogenase |
| KGDH | Ketoglutarate dehydrogenase |
| LRRK2 | Leucine-rich repeat kinase 2 |
| MAMs | Mitochondrial associated membranes |
| MCUC | Mitochondrial calcium Uniporter Complex |
| MCU | Mitochondrial calcium Uniporter |
| MICU1 | Mitochondrial calcium uptake 1 |
| mCa2+ | Mitochondrial calcium |
| ΔΨm | Mitochondrial membrane potential |
| mPTP | Mitochondrial permeability transition pore |
| mROS | Mitochondrial ROS |
| MFN2 | Mitofusin 2 |
| MI | Myocardial infarction |
| NCLX | Na+/Ca2+ exchangers |
| OMM | Outer mitochondrial membrane |
| OXPHOS | Oxidative phosphorylation |
| PD | Parkinson disease |
| PTEN | Phosphatase and tensin homolog |
| PS1 | Presenilin-1 |
| PS2 | Presenilin-2 |
| PINK1 | PTEN-induced kinase 1 |
| PDH | Pyruvate dehydrogenase |
| ROS | Reactive oxygen species |
| Ru360 | Ruthenium 360 |
| RuR | Ruthenium red |
| RyR2 | Ryanodine receptor (RyR) type 2 |
| RyR | Ryanodine receptor |
| SERCA | Sarco-Endoplasmic Reticulum Calcium ATPase |
| SR | Sarcoplasmic reticulum |
| SAD | Sporadic AD |
| SOCE | Store-operated calcium entry |
| TRP | Transient receptor potential |
| VDAC | Voltage dependent anion channel |
| VGCC | Voltage-gated calcium channel |
Author Contributions
Conceptualization, L.M.; writing—original draft preparation, L.M., A.D., V.A.M.V., D.R.; writing—review & editing, L.M., G.A., R.G., G.L., P.P., C.G.; funding acquisition, P.P., C.G. All authors have read and agreed to the published version of the manuscript.
Funding
The Signal Transduction Laboratory is supported by the Italian Association for Cancer Research (AIRC: IG-23670 to P.P. and IG-19803 to C.G.), A-ROSE, Progetti di Rilevante Interesse Nazionale (PRIN2017E5L5P3 to P.P and PRIN20177E9EPY to C.G.), the Italian Ministry of Health (GR-2013-02356747 to C.G.), the European Research Council (ERC,853057- InflaPML to C.G.) and local funds from the University of Ferrara to P.P. and C.G.
Conflicts of Interest
The authors declare no conflict of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giorgi C., Danese A., Missiroli S., Patergnani S., Pinton P. Calcium Dynamics as a Machine for Decoding Signals. Trends Cell Biol. 2018;28:258–273. doi: 10.1016/j.tcb.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Vakifahmetoglu-Norberg H., Ouchida A.T., Norberg E. The role of mitochondria in metabolism and cell death. Biochem. Biophys. Res. Commun. 2017;482:426–431. doi: 10.1016/j.bbrc.2016.11.088. [DOI] [PubMed] [Google Scholar]
- 3.Ma K., Chen G., Li W., Kepp O., Zhu Y., Chen Q. Mitophagy, Mitochondrial Homeostasis, and Cell Fate. Front. Cell Dev. Biol. 2020;8:467. doi: 10.3389/fcell.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yapa N.M.B., Lisnyak V., Reljic B., Ryan M.T. Mitochondrial dynamics in health and disease. FEBS Lett. 2021;595:1184–1204. doi: 10.1002/1873-3468.14077. [DOI] [PubMed] [Google Scholar]
- 5.Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 6.Carvalho E.J., Stathopulos P.B., Madesh M. Regulation of Ca2+ exchanges and signaling in mitochondria. Curr. Opin. Physiol. 2020;17:197–206. doi: 10.1016/j.cophys.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchi S., Pinton P. The mitochondrial calcium uniporter complex: Molecular components, structure and physiopathological implications. J. Physiol. 2014;592:829–839. doi: 10.1113/jphysiol.2013.268235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giorgi C., Agnoletto C., Bononi A., Bonora M., de Marchi E., Marchi S., Missiroli S., Patergnani S., Poletti F., Rimessi A., et al. Mitochondrial calcium homeostasis as potential target for mitochondrial medicine. Mitochondrion. 2012;12:77–85. doi: 10.1016/j.mito.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonora M., Morganti C., Morciano G., Pedriali G., Lebiedzinska-Arciszewska M., Aquila G., Giorgi C., Rizzo P., Campo G., Ferrari R., et al. Mitochondrial permeability transition involves dissociation of F1FO ATP synthase dimers and C-ring conformation. EMBO Rep. 2017;18:1077–1089. doi: 10.15252/embr.201643602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morciano G., Giorgi C., Bonora M., Punzetti S., Pavasini R., Wieckowski M.R., Campo G., Pinton P. Molecular identity of the mitochondrial permeability transition pore and its role in ischemia-reperfusion injury. J. Mol. Cell. Cardiol. 2015;78:142–153. doi: 10.1016/j.yjmcc.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Marchi S., Vitto V.A.M., Patergnani S., Pinton P. High mitochondrial Ca2+ content increases cancer cell proliferation upon inhibition of mitochondrial permeability transition pore (mPTP) Cell Cycle. 2019;18:914–916. doi: 10.1080/15384101.2019.1598729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sebag S.C., Koval O.M., Paschke J.D., Winters C.J., Comellas A.P., Grumbach I.M. Inhibition of the mitochondrial calcium uniporter prevents IL-13 and allergen-mediated airway epithelial apoptosis and loss of barrier function. Exp. Cell Res. 2018;362:400–411. doi: 10.1016/j.yexcr.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oropeza-Almazán Y., Vázquez-Garza E., Chapoy-Villanueva H., Torre-Amione G., García-Rivas G. Small Interfering RNA Targeting Mitochondrial Calcium Uniporter Improves Cardiomyocyte Cell Viability in Hypoxia/Reoxygenation Injury by Reducing Calcium Overload. Oxid. Med. Cell. Longev. 2017;2017:5750897. doi: 10.1155/2017/5750897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rowland A.A., Voeltz G.K. Endoplasmic reticulum-mitochondria contacts: Function of the junction. Nat. Rev. Mol. Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granatiero V., De Stefani D., Rizzuto R. Mitochondrial Calcium Handling in Physiology and Disease. Adv. Exp. Med. Biol. 2017;982:25–47. doi: 10.1007/978-3-319-55330-6_2. [DOI] [PubMed] [Google Scholar]
- 16.Mammucari C., Raffaello A., Vecellio Reane D., Gherardi G., De Mario A., Rizzuto R. Mitochondrial calcium uptake in organ physiology: From molecular mechanism to animal models. Pflugers Arch. 2018;470:1165–1179. doi: 10.1007/s00424-018-2123-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kostic M., Sekler I. Functional properties and mode of regulation of the mitochondrial Na+/Ca2+ exchanger, NCLX. Semin. Cell Dev. Biol. 2019;94:59–65. doi: 10.1016/j.semcdb.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Simamura E., Shimada H., Hatta T., Hirai K.-I. Mitochondrial voltage-dependent anion channels (VDACs) as novel pharmacological targets for anti-cancer agents. J. Bioenerg. Biomembr. 2008;40:213–217. doi: 10.1007/s10863-008-9158-6. [DOI] [PubMed] [Google Scholar]
- 19.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perocchi F., Gohil V.M., Girgis H.S., Bao X.R., McCombs J.E., Palmer A.E., Mootha V.K. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mammucari C., Raffaello A., Vecellio Reane D., Rizzuto R. Molecular structure and pathophysiological roles of the Mitochondrial Calcium Uniporter. Biochim. Biophys. Acta. 2016;1863:2457–2464. doi: 10.1016/j.bbamcr.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 23.Raffaello A., De Stefani D., Sabbadin D., Teardo E., Merli G., Picard A., Checchetto V., Moro S., Szabò I., Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sancak Y., Markhard A.L., Kitami T., Kovács-Bogdán E., Kamer K.J., Udeshi N.D., Carr S.A., Chaudhuri D., Clapham D.E., Li A.A., et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013 doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mallilankaraman K., Doonan P., Cárdenas C., Chandramoorthy H.C., Müller M., Miller R., Hoffman N.E., Gandhirajan R.K., Molgó J., Birnbaum M.J., et al. MICU1 is an essential gatekeeper for MCU-mediated mitochondrial Ca2+ uptake that regulates cell survival. Cell. 2012;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csordás G., Golenár T., Seifert E.L., Kamer K.J., Sancak Y., Perocchi F., Moffat C., Weaver D., Perez S.D.L.F., Bogorad R., et al. MICU1 controls both the threshold and cooperative activation of the mitochondrial Ca2+ uniporter. Cell Metab. 2013;17:976–987. doi: 10.1016/j.cmet.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plovanich M., Bogorad R.L., Sancak Y., Kamer K.J., Strittmatter L., Li A.A., Girgis H.S., Kuchimanchi S., De Groot J., Speciner L., et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS ONE. 2013;8:e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pagliarini D.J., Calvo S.E., Chang B., Sheth S.A., Vafai S.B., Ong S.-E., Walford G.A., Sugiana C., Boneh A., Chen W.K., et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134:112–123. doi: 10.1016/j.cell.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paillard M., Csordás G., Szanda G., Golenár T., Debattisti V., Bartok A., Wang N., Moffat C., Seifert E.L., Spät A., et al. Tissue-Specific Mitochondrial Decoding of Cytoplasmic Ca2+ Signals Is Controlled by the Stoichiometry of MICU1/2 and MCU. Cell Rep. 2017;18:2291–2300. doi: 10.1016/j.celrep.2017.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patron M., Checchetto V., Raffaello A., Teardo E., Vecellio Reane D., Mantoan M., Granatiero V., Szabò I., De Stefani D., Rizzuto R. MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity. Mol. Cell. 2014;53:726–737. doi: 10.1016/j.molcel.2014.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patron M., Granatiero V., Espino J., Rizzuto R., De Stefani D. MICU3 is a tissue-specific enhancer of mitochondrial calcium uptake. Cell Death Differ. 2019;26:179–195. doi: 10.1038/s41418-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mammucari C., Gherardi G., Rizzuto R. Structure, Activity Regulation, and Role of the Mitochondrial Calcium Uniporter in Health and Disease. Front. Oncol. 2017;7:139. doi: 10.3389/fonc.2017.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pozzan T., Bragadin M., Azzone G.F. Disequilibrium between steady-state Ca2+ accumulation ratio and membrane potential in mitochondria. Pathway and role of Ca2+ efflux. Biochemistry. 1977;16:5618–5625. doi: 10.1021/bi00644a036. [DOI] [PubMed] [Google Scholar]
- 34.Crompton M., Künzi M., Carafoli E. The calcium-induced and sodium-induced effluxes of calcium from heart mitochondria. Evidence for a sodium-calcium carrier. Eur. J. Biochem. 1977;79:549–558. doi: 10.1111/j.1432-1033.1977.tb11839.x. [DOI] [PubMed] [Google Scholar]
- 35.Jiang D., Zhao L., Clapham D.E. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Marchi U., Santo-Domingo J., Castelbou C., Sekler I., Wiederkehr A., Demaurex N. NCLX protein, but not LETM1, mediates mitochondrial Ca2+ extrusion, thereby limiting Ca2+-induced NAD(P)H production and modulating matrix redox state. J. Biol. Chem. 2014;289:20377–20385. doi: 10.1074/jbc.M113.540898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nowikovsky K., Pozzan T., Rizzuto R., Scorrano L., Bernardi P. Perspectives on: SGP symposium on mitochondrial physiology and medicine: The pathophysiology of LETM1. J. Gen. Physiol. 2012;139:445–454. doi: 10.1085/jgp.201110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palty R., Silverman W.F., Hershfinkel M., Caporale T., Sensi S.L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elrod J.W., Wong R., Mishra S., Vagnozzi R.J., Sakthievel B., Goonasekera S.A., Karch J., Gabel S., Farber J., Force T., et al. Cyclophilin D controls mitochondrial pore-dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. J. Clin. Invest. 2010;120:3680–3687. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu X., Kwong J.Q., Molkentin J.D., Bers D.M. Individual Cardiac Mitochondria Undergo Rare Transient Permeability Transition Pore Openings. Circ. Res. 2016;118:834–841. doi: 10.1161/CIRCRESAHA.115.308093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Marchi E., Bonora M., Giorgi C., Pinton P. The mitochondrial permeability transition pore is a dispensable element for mitochondrial calcium efflux. Cell Calcium. 2014;56:1–13. doi: 10.1016/j.ceca.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rizzuto R., De Stefani D., Raffaello A., Mammucari C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell Biol. 2012;13:566–578. doi: 10.1038/nrm3412. [DOI] [PubMed] [Google Scholar]
- 43.Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.-S. Calcium, ATP, and ROS: A mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 44.Holmström K.M., Finkel T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014;15:411–421. doi: 10.1038/nrm3801. [DOI] [PubMed] [Google Scholar]
- 45.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Görlach A., Bertram K., Hudecova S., Krizanova O. Calcium and ROS: A mutual interplay. Redox Biol. 2015;6:260–271. doi: 10.1016/j.redox.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Santulli G., Marks A.R. Essential Roles of Intracellular Calcium Release Channels in Muscle, Brain, Metabolism, and Aging. Curr. Mol. Pharmacol. 2015;8:206–222. doi: 10.2174/1874467208666150507105105. [DOI] [PubMed] [Google Scholar]
- 48.Brini M., Calì T., Ottolini D., Carafoli E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014;71:2787–2814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawamoto E.M., Vivar C., Camandola S. Physiology and pathology of calcium signaling in the brain. Front. Pharmacol. 2012;3:61. doi: 10.3389/fphar.2012.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Llorente-Folch I., Rueda C.B., Pardo B., Szabadkai G., Duchen M.R., Satrustegui J. The regulation of neuronal mitochondrial metabolism by calcium. J. Physiol. 2015;593:3447–3462. doi: 10.1113/JP270254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kumar A., Bodhinathan K., Foster T.C. Susceptibility to Calcium Dysregulation during Brain Aging. Front. Aging Neurosci. 2009;1:2. doi: 10.3389/neuro.24.002.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen Y., Liu Y., Dorn G.W. Mitochondrial fusion is essential for organelle function and cardiac homeostasis. Circ. Res. 2011;109:1327–1331. doi: 10.1161/CIRCRESAHA.111.258723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tahrir F.G., Langford D., Amini S., Mohseni Ahooyi T., Khalili K. Mitochondrial quality control in cardiac cells: Mechanisms and role in cardiac cell injury and disease. J. Cell. Physiol. 2019;234:8122–8133. doi: 10.1002/jcp.27597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Danese A., Marchi S., Vitto V.A.M., Modesti L., Leo S., Wieckowski M.R., Giorgi C., Pinton P. Cancer-Related Increases and Decreases in Calcium Signaling at the Endoplasmic Reticulum-Mitochondria Interface (MAMs) Rev. Physiol. Biochem. Pharmacol. 2020 doi: 10.1007/112_2020_43. [DOI] [PubMed] [Google Scholar]
- 55.Patergnani S., Danese A., Bouhamida E., Aguiari G., Previati M., Pinton P., Giorgi C. Various Aspects of Calcium Signaling in the Regulation of Apoptosis, Autophagy, Cell Proliferation, and Cancer. Int. J. Mol. Sci. 2020;21 doi: 10.3390/ijms21218323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Danese A., Patergnani S., Bonora M., Wieckowski M.R., Previati M., Giorgi C., Pinton P. Calcium regulates cell death in cancer: Roles of the mitochondria and mitochondria-associated membranes (MAMs) Biochim. Biophys. Acta Bioenerg. 2017;1858:615–627. doi: 10.1016/j.bbabio.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 57.Otto T., Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer. 2017;17:93–115. doi: 10.1038/nrc.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Colomer J., López-Girona A., Agell N., Bachs O. Calmodulin regulates the expression of cdks, cyclins and replicative enzymes during proliferative activation of human T lymphocytes. Biochem. Biophys. Res. Commun. 1994;200:306–312. doi: 10.1006/bbrc.1994.1449. [DOI] [PubMed] [Google Scholar]
- 59.Kahl C.R., Means A.R. Calcineurin regulates cyclin D1 accumulation in growth-stimulated fibroblasts. Mol. Biol. Cell. 2004;15:1833–1842. doi: 10.1091/mbc.e03-10-0730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takuwa N., Zhou W., Kumada M., Takuwa Y. Ca2+-dependent stimulation of retinoblastoma gene product phosphorylation and p34cdc2 kinase activation in serum-stimulated human fibroblasts. J. Biol. Chem. 1993;268:138–145. doi: 10.1016/S0021-9258(18)54125-9. [DOI] [PubMed] [Google Scholar]
- 61.Yuan K., Wang X., Dong H., Min W., Hao H., Yang P. Selective inhibition of CDK4/6: A safe and effective strategy for developing anticancer drugs. Acta Pharm. Sin. B. 2021;11:30–54. doi: 10.1016/j.apsb.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marchi S., Pinton P. Alterations of calcium homeostasis in cancer cells. Curr. Opin. Pharmacol. 2016;29:1–6. doi: 10.1016/j.coph.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y.-W., Chen Y.-F., Chen Y.-T., Chiu W.-T., Shen M.-R. The STIM1-Orai1 pathway of store-operated Ca2+ entry controls the checkpoint in cell cycle G1/S transition. Sci. Rep. 2016;6:22142. doi: 10.1038/srep22142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sabbioni S., Veronese A., Trubia M., Taramelli R., Barbanti-Brodano G., Croce C.M., Negrini M. Exon structure and promoter identification of STIM1 (alias GOK), a human gene causing growth arrest of the human tumor cell lines G401 and RD. Cytogenet. Cell Genet. 1999;86:214–218. doi: 10.1159/000015341. [DOI] [PubMed] [Google Scholar]
- 65.Flourakis M., Lehen’kyi V., Beck B., Raphaël M., Vandenberghe M., Abeele F.V., Roudbaraki M., Lepage G., Mauroy B., Romanin C., et al. Orai1 contributes to the establishment of an apoptosis-resistant phenotype in prostate cancer cells. Cell Death Dis. 2010;1:e75. doi: 10.1038/cddis.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karacicek B., Erac Y., Tosun M. Functional consequences of enhanced expression of STIM1 and Orai1 in Huh-7 hepatocellular carcinoma tumor-initiating cells. BMC Cancer. 2019;19:751. doi: 10.1186/s12885-019-5947-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gouriou Y., Bijlenga P., Demaurex N. Mitochondrial Ca2+ uptake from plasma membrane Cav3.2 protein channels contributes to ischemic toxicity in PC12 cells. J. Biol. Chem. 2013;288:12459–12468. doi: 10.1074/jbc.M112.428128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barceló C., Sisó P., Maiques O., de la Rosa I., Martí R.M., Macià A. T-Type Calcium Channels: A Potential Novel Target in Melanoma. Cancers. 2020;12 doi: 10.3390/cancers12020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Das A., Pushparaj C., Bahí N., Sorolla A., Herreros J., Pamplona R., Vilella R., Matias-Guiu X., Martí R.M., Cantí C. Functional expression of voltage-gated calcium channels in human melanoma. Pigment Cell Melanoma Res. 2012;25:200–212. doi: 10.1111/j.1755-148X.2012.00978.x. [DOI] [PubMed] [Google Scholar]
- 70.Dziegielewska B., Brautigan D.L., Larner J.M., Dziegielewski J. T-type Ca2+ channel inhibition induces p53-dependent cell growth arrest and apoptosis through activation of p38-MAPK in colon cancer cells. Mol. Cancer Res. 2014;12:348–358. doi: 10.1158/1541-7786.MCR-13-0485. [DOI] [PubMed] [Google Scholar]
- 71.Li W., Zhang S.-L., Wang N., Zhang B.-B., Li M. Blockade of T-type Ca2+ channels inhibits human ovarian cancer cell proliferation. Cancer Invest. 2011;29:339–346. doi: 10.3109/07357907.2011.568565. [DOI] [PubMed] [Google Scholar]
- 72.Pedersen S.F., Owsianik G., Nilius B. TRP channels: An overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- 73.He B., Liu F., Ruan J., Li A., Chen J., Li R., Shen J., Zheng D., Luo R. Silencing TRPC1 expression inhibits invasion of CNE2 nasopharyngeal tumor cells. Oncol. Rep. 2012;27:1548–1554. doi: 10.3892/or.2012.1695. [DOI] [PubMed] [Google Scholar]
- 74.Elzamzamy O.M., Penner R., Hazlehurst L.A. The Role of TRPC1 in Modulating Cancer Progression. Cells. 2020;9 doi: 10.3390/cells9020388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Monet M., Gkika D., Lehen’kyi V., Pourtier A., Vanden Abeele F., Bidaux G., Juvin V., Rassendren F., Humez S., Prevarsakaya N. Lysophospholipids stimulate prostate cancer cell migration via TRPV2 channel activation. Biochim. Biophys. Acta. 2009;1793:528–539. doi: 10.1016/j.bbamcr.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 76.Patergnani S., Bouhamida E., Leo S., Pinton P., Rimessi A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines. 2021;9 doi: 10.3390/biomedicines9020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sabharwal S.S., Schumacker P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer. 2014;14:709–721. doi: 10.1038/nrc3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lago C.U., Sung H.J., Ma W., Wang P., Hwang P.M. p53, aerobic metabolism, and cancer. Antioxid. Redox Signal. 2011;15:1739–1748. doi: 10.1089/ars.2010.3650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Olovnikov I.A., Kravchenko J.E., Chumakov P.M. Homeostatic functions of the p53 tumor suppressor: Regulation of energy metabolism and antioxidant defense. Semin. Cancer Biol. 2009;19:32–41. doi: 10.1016/j.semcancer.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016;6:a026104. doi: 10.1101/cshperspect.a026104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Galli S., Antico Arciuch V.G., Poderoso C., Converso D.P., Zhou Q., Bal de Kier Joffé E., Cadenas E., Boczkowski J., Carreras M.C., Poderoso J.J. Tumor cell phenotype is sustained by selective MAPK oxidation in mitochondria. PLoS One. 2008;3:e2379. doi: 10.1371/journal.pone.0002379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burgering B.M.T., Kops G.J.P.L. Cell cycle and death control: Long live Forkheads. Trends Biochem. Sci. 2002;27:352–360. doi: 10.1016/S0968-0004(02)02113-8. [DOI] [PubMed] [Google Scholar]
- 83.Dijkers P.F., Medema R.H., Pals C., Banerji L., Thomas N.S., Lam E.W., Burgering B.M., Raaijmakers J.A., Lammers J.W., Koenderman L., et al. Forkhead transcription factor FKHR-L1 modulates cytokine-dependent transcriptional regulation of p27(KIP1) Mol. Cell. Biol. 2000;20:9138–9148. doi: 10.1128/MCB.20.24.9138-9148.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Greer E.L., Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 85.Marchi S., Vitto V.A.M., Danese A., Wieckowski M.R., Giorgi C., Pinton P. Mitochondrial calcium uniporter complex modulation in cancerogenesis. Cell Cycle. 2019;18:1068–1083. doi: 10.1080/15384101.2019.1612698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marchi S., Lupini L., Patergnani S., Rimessi A., Missiroli S., Bonora M., Bononi A., Corrà F., Giorgi C., De Marchi E., et al. Downregulation of the mitochondrial calcium uniporter by cancer-related miR-25. Curr. Biol. 2013;23:58–63. doi: 10.1016/j.cub.2012.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Prudent J., Popgeorgiev N., Gadet R., Deygas M., Rimokh R., Gillet G. Mitochondrial Ca2+ uptake controls actin cytoskeleton dynamics during cell migration. Sci. Rep. 2016;6:36570. doi: 10.1038/srep36570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tosatto A., Sommaggio R., Kummerow C., Bentham R.B., Blacker T.S., Berecz T., Duchen M.R., Rosato A., Bogeski I., Szabadkai G., et al. The mitochondrial calcium uniporter regulates breast cancer progression via HIF-1α. EMBO Mol. Med. 2016;8:569–585. doi: 10.15252/emmm.201606255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ren T., Zhang H., Wang J., Zhu J., Jin M., Wu Y., Guo X., Ji L., Huang Q., Zhang H., et al. MCU-dependent mitochondrial Ca2+ inhibits NAD+/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene. 2017;36:5897–5909. doi: 10.1038/onc.2017.167. [DOI] [PubMed] [Google Scholar]
- 90.Koval O.M., Nguyen E.K., Santhana V., Fidler T.P., Sebag S.C., Rasmussen T.P., Mittauer D.J., Strack S., Goswami P.C., Abel E.D., et al. Loss of MCU prevents mitochondrial fusion in G1-S phase and blocks cell cycle progression and proliferation. Sci. Signal. 2019;12 doi: 10.1126/scisignal.aav1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Marchi S., Corricelli M., Branchini A., Vitto V.A.M., Missiroli S., Morciano G., Perrone M., Ferrarese M., Giorgi C., Pinotti M., et al. Akt-mediated phosphorylation of MICU1 regulates mitochondrial Ca2+ levels and tumor growth. EMBO J. 2019;38 doi: 10.15252/embj.201899435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu R., Han M., Xu Y., Zhang X., Zhang C., Zhang D., Ji J., Wei Y., Wang S., Huang B., et al. Coiled-coil domain containing 109B is a HIF1α-regulated gene critical for progression of human gliomas. J. Transl. Med. 2017;15:165. doi: 10.1186/s12967-017-1266-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shoshan-Barmatz V., Krelin Y., Shteinfer-Kuzmine A. VDAC1 functions in Ca2+ homeostasis and cell life and death in health and disease. Cell Calcium. 2018;69:81–100. doi: 10.1016/j.ceca.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 94.Arif T., Vasilkovsky L., Refaely Y., Konson A., Shoshan-Barmatz V. Silencing VDAC1 Expression by siRNA Inhibits Cancer Cell Proliferation and Tumor Growth In Vivo. Mol. Ther. Nucleic Acids. 2014;3:e159. doi: 10.1038/mtna.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fouqué A., Lepvrier E., Debure L., Gouriou Y., Malleter M., Delcroix V., Ovize M., Ducret T., Li C., Hammadi M., et al. The apoptotic members CD95, BclxL, and Bcl-2 cooperate to promote cell migration by inducing Ca2+ flux from the endoplasmic reticulum to mitochondria. Cell Death Differ. 2016;23:1702–1716. doi: 10.1038/cdd.2016.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cárdenas C., Miller R.A., Smith I., Bui T., Molgó J., Müller M., Vais H., Cheung K.-H., Yang J., Parker I., et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142:270–283. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kuchay S., Giorgi C., Simoneschi D., Pagan J., Missiroli S., Saraf A., Florens L., Washburn M.P., Collazo-Lorduy A., Castillo-Martin M., et al. PTEN counteracts FBXL2 to promote IP3R3- and Ca2+-mediated apoptosis limiting tumour growth. Nature. 2017;546:554–558. doi: 10.1038/nature22965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bononi A., Giorgi C., Patergnani S., Larson D., Verbruggen K., Tanji M., Pellegrini L., Signorato V., Olivetto F., Pastorino S., et al. BAP1 regulates IP3R3-mediated Ca2+ flux to mitochondria suppressing cell transformation. Nature. 2017;546:549–553. doi: 10.1038/nature22798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bezprozvanny I.B. Calcium signaling and neurodegeneration. Acta Naturae. 2010;2:72–82. doi: 10.32607/20758251-2010-2-1-72-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pchitskaya E., Popugaeva E., Bezprozvanny I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium. 2018;70:87–94. doi: 10.1016/j.ceca.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 2009;15:89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jung H., Kim S.Y., Canbakis Cecen F.S., Cho Y., Kwon S.-K. Dysfunction of Mitochondrial Ca2+ Regulatory Machineries in Brain Aging and Neurodegenerative Diseases. Front. Cell Dev. Biol. 2020;8:599792. doi: 10.3389/fcell.2020.599792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zampese E., Surmeier D.J. Calcium, Bioenergetics, and Parkinson’s Disease. Cells. 2020;9 doi: 10.3390/cells9092045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dorszewska J., Prendecki M., Oczkowska A., Dezor M., Kozubski W. Molecular Basis of Familial and Sporadic Alzheimer’s Disease. Curr. Alzheimer Res. 2016;13:952–963. doi: 10.2174/1567205013666160314150501. [DOI] [PubMed] [Google Scholar]
- 105.Allen M., Zou F., Chai H.S., Younkin C.S., Crook J., Pankratz V.S., Carrasquillo M.M., Rowley C.N., Nair A.A., Middha S., et al. Novel late-onset Alzheimer disease loci variants associate with brain gene expression. Neurology. 2012;79:221–228. doi: 10.1212/WNL.0b013e3182605801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Müller M., Ahumada-Castro U., Sanhueza M., Gonzalez-Billault C., Court F.A., Cárdenas C. Mitochondria and Calcium Regulation as Basis of Neurodegeneration Associated With Aging. Front. Neurosci. 2018;12:470. doi: 10.3389/fnins.2018.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sherrington R., Rogaev E.I., Liang Y., Rogaeva E.A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K., et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature. 1995;375:754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- 108.Kolarova M., García-Sierra F., Bartos A., Ricny J., Ripova D. Structure and pathology of tau protein in Alzheimer disease. Int. J. Alzheimers. Dis. 2012;2012:731526. doi: 10.1155/2012/731526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Donev R., Kolev M., Millet B., Thome J. Neuronal death in Alzheimer’s disease and therapeutic opportunities. J. Cell. Mol. Med. 2009;13:4329–4348. doi: 10.1111/j.1582-4934.2009.00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kashyap G., Bapat D., Das D., Gowaikar R., Amritkar R.E., Rangarajan G., Ravindranath V., Ambika G. Synapse loss and progress of Alzheimer’s disease -A network model. Sci. Rep. 2019;9:6555. doi: 10.1038/s41598-019-43076-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.LaFerla F.M. Calcium dyshomeostasis and intracellular signalling in Alzheimer’s disease. Nat. Rev. Neurosci. 2002;3:862–872. doi: 10.1038/nrn960. [DOI] [PubMed] [Google Scholar]
- 113.Khachaturian Z.S. The role of calcium regulation in brain aging: Reexamination of a hypothesis. Aging. 1989;1:17–34. doi: 10.1007/BF03323872. [DOI] [PubMed] [Google Scholar]
- 114.Zündorf G., Reiser G. Calcium dysregulation and homeostasis of neural calcium in the molecular mechanisms of neurodegenerative diseases provide multiple targets for neuroprotection. Antioxid. Redox Signal. 2011;14:1275–1288. doi: 10.1089/ars.2010.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hirashima N., Etcheberrigaray R., Bergamaschi S., Racchi M., Battaini F., Binetti G., Govoni S., Alkon D.L. Calcium responses in human fibroblasts: A diagnostic molecular profile for Alzheimer’s disease. Neurobiol. Aging. 1996;17:549–555. doi: 10.1016/0197-4580(96)00074-7. [DOI] [PubMed] [Google Scholar]
- 116.Cheung K.-H., Shineman D., Müller M., Cárdenas C., Mei L., Yang J., Tomita T., Iwatsubo T., Lee V.M.-Y., Foskett J.K. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;58:871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chakroborty S., Goussakov I., Miller M.B., Stutzmann G.E. Deviant ryanodine receptor-mediated calcium release resets synaptic homeostasis in presymptomatic 3xTg-AD mice. J. Neurosci. 2009;29:9458–9470. doi: 10.1523/JNEUROSCI.2047-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rybalchenko V., Hwang S.-Y., Rybalchenko N., Koulen P. The cytosolic N-terminus of presenilin-1 potentiates mouse ryanodine receptor single channel activity. Int. J. Biochem. Cell Biol. 2008;40:84–97. doi: 10.1016/j.biocel.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 119.Calvo-Rodriguez M., Hou S.S., Snyder A.C., Kharitonova E.K., Russ A.N., Das S., Fan Z., Muzikansky A., Garcia-Alloza M., Serrano-Pozo A., et al. Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease. Nat. Commun. 2020;11:2146. doi: 10.1038/s41467-020-16074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Calvo-Rodriguez M., Bacskai B.J. Mitochondria and Calcium in Alzheimer’s Disease: From Cell Signaling to Neuronal Cell Death. Trends Neurosci. 2021;44:136–151. doi: 10.1016/j.tins.2020.10.004. [DOI] [PubMed] [Google Scholar]
- 121.Erlanson D.A., Arndt J.W., Cancilla M.T., Cao K., Elling R.A., English N., Friedman J., Hansen S.K., Hession C., Joseph I., et al. Discovery of a potent and highly selective PDK1 inhibitor via fragment-based drug discovery. Bioorg. Med. Chem. Lett. 2011;21:3078–3083. doi: 10.1016/j.bmcl.2011.03.032. [DOI] [PubMed] [Google Scholar]
- 122.Hansson Petersen C.A., Alikhani N., Behbahani H., Wiehager B., Pavlov P.F., Alafuzoff I., Leinonen V., Ito A., Winblad B., Glaser E., et al. The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. USA. 2008;105:13145–13150. doi: 10.1073/pnas.0806192105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lin M.T., Beal M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443:787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 124.Britti E., Delaspre F., Tamarit J., Ros J. Mitochondrial calcium signalling and neurodegenerative diseases. Neuronal Signal. 2018;2:NS20180061. doi: 10.1042/NS20180061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Caspersen C., Wang N., Yao J., Sosunov A., Chen X., Lustbader J.W., Xu H.W., Stern D., McKhann G., Yan S. Du Mitochondrial Abeta: A potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease. FASEB J. 2005;19:2040–2041. doi: 10.1096/fj.05-3735fje. [DOI] [PubMed] [Google Scholar]
- 126.Du H., Yan S.S. Mitochondrial permeability transition pore in Alzheimer’s disease: Cyclophilin D and amyloid beta. Biochim. Biophys. Acta. 2010;1802:198–204. doi: 10.1016/j.bbadis.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rao V.K., Carlson E.A., Yan S.S. Mitochondrial permeability transition pore is a potential drug target for neurodegeneration. Biochim. Biophys. Acta. 2014;1842:1267–1272. doi: 10.1016/j.bbadis.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pérez M.J., Ponce D.P., Aranguiz A., Behrens M.I., Quintanilla R.A. Mitochondrial permeability transition pore contributes to mitochondrial dysfunction in fibroblasts of patients with sporadic Alzheimer’s disease. Redox Biol. 2018;19:290–300. doi: 10.1016/j.redox.2018.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Du H., Guo L., Fang F., Chen D., Sosunov A.A., McKhann G.M., Yan Y., Wang C., Zhang H., Molkentin J.D., et al. Cyclophilin D deficiency attenuates mitochondrial and neuronal perturbation and ameliorates learning and memory in Alzheimer’s disease. Nat. Med. 2008;14:1097–1105. doi: 10.1038/nm.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jadiya P., Kolmetzky D.W., Tomar D., Di Meco A., Lombardi A.A., Lambert J.P., Luongo T.S., Ludtmann M.H., Praticò D., Elrod J.W. Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease. Nat. Commun. 2019;10:3885. doi: 10.1038/s41467-019-11813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Greotti E., Wong A., Pozzan T., Pendin D., Pizzo P. Characterization of the ER-Targeted Low Affinity Ca2+ Probe D4ER. Sensors. 2016;16 doi: 10.3390/s16091419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rossi A., Rigotto G., Valente G., Giorgio V., Basso E., Filadi R., Pizzo P. Defective Mitochondrial Pyruvate Flux Affects Cell Bioenergetics in Alzheimer’s Disease-Related Models. Cell Rep. 2020;30:2332–2348. doi: 10.1016/j.celrep.2020.01.060. [DOI] [PubMed] [Google Scholar]
- 133.Area-Gomez E., de Groof A.J.C., Boldogh I., Bird T.D., Gibson G.E., Koehler C.M., Yu W.H., Duff K.E., Yaffe M.P., Pon L.A., et al. Presenilins are enriched in endoplasmic reticulum membranes associated with mitochondria. Am. J. Pathol. 2009;175:1810–1816. doi: 10.2353/ajpath.2009.090219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Filadi R., Greotti E., Turacchio G., Luini A., Pozzan T., Pizzo P. Presenilin 2 Modulates Endoplasmic Reticulum-Mitochondria Coupling by Tuning the Antagonistic Effect of Mitofusin 2. Cell Rep. 2016;15:2226–2238. doi: 10.1016/j.celrep.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 135.Pera M., Larrea D., Guardia-Laguarta C., Montesinos J., Velasco K.R., Agrawal R.R., Xu Y., Chan R.B., Di Paolo G., Mehler M.F., et al. Increased localization of APP-C99 in mitochondria-associated ER membranes causes mitochondrial dysfunction in Alzheimer disease. EMBO J. 2017;36:3356–3371. doi: 10.15252/embj.201796797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schreiner B., Hedskog L., Wiehager B., Ankarcrona M. Amyloid-β peptides are generated in mitochondria-associated endoplasmic reticulum membranes. J. Alzheimers. Dis. 2015;43:369–374. doi: 10.3233/JAD-132543. [DOI] [PubMed] [Google Scholar]
- 137.Area-Gomez E., de Groof A., Bonilla E., Montesinos J., Tanji K., Boldogh I., Pon L., Schon E.A. A key role for MAM in mediating mitochondrial dysfunction in Alzheimer disease. Cell Death Dis. 2018;9:335. doi: 10.1038/s41419-017-0215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Roze E., Bonnet C., Betuing S., Caboche J. Huntington’s disease. Adv. Exp. Med. Biol. 2010;685:45–63. [PubMed] [Google Scholar]
- 139.Brustovetsky N. Mutant Huntingtin and Elusive Defects in Oxidative Metabolism and Mitochondrial Calcium Handling. Mol. Neurobiol. 2016;53:2944–2953. doi: 10.1007/s12035-015-9188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zoghbi H.Y., Orr H.T. Glutamine repeats and neurodegeneration. Annu. Rev. Neurosci. 2000;23:217–247. doi: 10.1146/annurev.neuro.23.1.217. [DOI] [PubMed] [Google Scholar]
- 141.A novel gene containing a trinucleotide repeat that is expanded and unstable on Huntington’s disease chromosomes. The Huntington’s Disease Collaborative Research Group. Cell. 1993;72:971–983. doi: 10.1016/0092-8674(93)90585-E. [DOI] [PubMed] [Google Scholar]
- 142.Guedes-Dias P., Pinho B.R., Soares T.R., de Proença J., Duchen M.R., Oliveira J.M.A. Mitochondrial dynamics and quality control in Huntington’s disease. Neurobiol. Dis. 2016;90:51–57. doi: 10.1016/j.nbd.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 143.Quintanilla R.A., Johnson G.V.W. Role of mitochondrial dysfunction in the pathogenesis of Huntington’s disease. Brain Res. Bull. 2009;80:242–247. doi: 10.1016/j.brainresbull.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Carmo C., Naia L., Lopes C., Rego A.C. Mitochondrial Dysfunction in Huntington’s Disease. Adv. Exp. Med. Biol. 2018;1049:59–83. doi: 10.1007/978-3-319-71779-1_3. [DOI] [PubMed] [Google Scholar]
- 145.Damiano M., Galvan L., Déglon N., Brouillet E. Mitochondria in Huntington’s disease. Biochim. Biophys. Acta. 2010;1802:52–61. doi: 10.1016/j.bbadis.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 146.Costa V., Scorrano L. Shaping the role of mitochondria in the pathogenesis of Huntington’s disease. EMBO J. 2012;31:1853–1864. doi: 10.1038/emboj.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Choo Y.S., Johnson G.V.W., MacDonald M., Detloff P.J., Lesort M. Mutant huntingtin directly increases susceptibility of mitochondria to the calcium-induced permeability transition and cytochrome c release. Hum. Mol. Genet. 2004;13:1407–1420. doi: 10.1093/hmg/ddh162. [DOI] [PubMed] [Google Scholar]
- 148.Jia K., Du H. Mitochondrial Permeability Transition: A Pore Intertwines Brain Aging and Alzheimer’s Disease. Cells. 2021;10 doi: 10.3390/cells10030649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Panov A.V., Gutekunst C.A., Leavitt B.R., Hayden M.R., Burke J.R., Strittmatter W.J., Greenamyre J.T. Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nat. Neurosci. 2002;5:731–736. doi: 10.1038/nn884. [DOI] [PubMed] [Google Scholar]
- 150.Pellman J.J., Hamilton J., Brustovetsky T., Brustovetsky N. Ca2+ handling in isolated brain mitochondria and cultured neurons derived from the YAC128 mouse model of Huntington’s disease. J. Neurochem. 2015;134:652–667. doi: 10.1111/jnc.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oliveira J.M.A., Jekabsons M.B., Chen S., Lin A., Rego A.C., Gonçalves J., Ellerby L.M., Nicholls D.G. Mitochondrial dysfunction in Huntington’s disease: The bioenergetics of isolated and in situ mitochondria from transgenic mice. J. Neurochem. 2007;101:241–249. doi: 10.1111/j.1471-4159.2006.04361.x. [DOI] [PubMed] [Google Scholar]
- 152.Tang T.-S., Slow E., Lupu V., Stavrovskaya I.G., Sugimori M., Llinás R., Kristal B.S., Hayden M.R., Bezprozvanny I. Disturbed Ca2+ signaling and apoptosis of medium spiny neurons in Huntington’s disease. Proc. Natl. Acad. Sci. USA. 2005;102:2602–2607. doi: 10.1073/pnas.0409402102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Tanner C.M., Goldman S.M. Epidemiology of Parkinson’s disease. Neurol. Clin. 1996;14:317–335. doi: 10.1016/S0733-8619(05)70259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zaichick S.V., McGrath K.M., Caraveo G. The role of Ca2+ signaling in Parkinson’s disease. Dis. Model. Mech. 2017;10:519–535. doi: 10.1242/dmm.028738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Calì T., Ottolini D., Brini M. Calcium signaling in Parkinson’s disease. Cell Tissue Res. 2014;357:439–454. doi: 10.1007/s00441-014-1866-0. [DOI] [PubMed] [Google Scholar]
- 156.Scorziello A., Borzacchiello D., Sisalli M.J., Di Martino R., Morelli M., Feliciello A. Mitochondrial Homeostasis and Signaling in Parkinson’s Disease. Front. Aging Neurosci. 2020;12:100. doi: 10.3389/fnagi.2020.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moon H.E., Paek S.H. Mitochondrial Dysfunction in Parkinson’s Disease. Exp. Neurobiol. 2015;24:103–116. doi: 10.5607/en.2015.24.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Abou-Sleiman P.M., Muqit M.M.K., Wood N.W. Expanding insights of mitochondrial dysfunction in Parkinson’s disease. Nat. Rev. Neurosci. 2006;7:207–219. doi: 10.1038/nrn1868. [DOI] [PubMed] [Google Scholar]
- 159.Ferreira M., Massano J. An updated review of Parkinson’s disease genetics and clinicopathological correlations. Acta Neurol. Scand. 2017;135:273–284. doi: 10.1111/ane.12616. [DOI] [PubMed] [Google Scholar]
- 160.Chen Y.P., Song W., Huang R., Chen K., Zhao B., Li J., Yang Y., Shang H.-F. GAK rs1564282 and DGKQ rs11248060 increase the risk for Parkinson’s disease in a Chinese population. J. Clin. Neurosci. 2013;20:880–883. doi: 10.1016/j.jocn.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 161.Ghanbari M., Darweesh S.K.L., de Looper H.W.J., van Luijn M.M., Hofman A., Ikram M.A., Franco O.H., Erkeland S.J., Dehghan A. Genetic Variants in MicroRNAs and Their Binding Sites Are Associated with the Risk of Parkinson Disease. Hum. Mutat. 2016;37:292–300. doi: 10.1002/humu.22943. [DOI] [PubMed] [Google Scholar]
- 162.Lin X., Parisiadou L., Sgobio C., Liu G., Yu J., Sun L., Shim H., Gu X.-L., Luo J., Long C.-X., et al. Conditional expression of Parkinson’s disease-related mutant α-synuclein in the midbrain dopaminergic neurons causes progressive neurodegeneration and degradation of transcription factor nuclear receptor related 1. J. Neurosci. 2012;32:9248–9264. doi: 10.1523/JNEUROSCI.1731-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Martin I., Dawson V.L., Dawson T.M. Recent advances in the genetics of Parkinson’s disease. Annu. Rev. Genomics Hum. Genet. 2011;12:301–325. doi: 10.1146/annurev-genom-082410-101440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Nalls M.A., Pankratz N., Lill C.M., Do C.B., Hernandez D.G., Saad M., DeStefano A.L., Kara E., Bras J., Sharma M., et al. Large-scale meta-analysis of genome-wide association data identifies six new risk loci for Parkinson’s disease. Nat. Genet. 2014;46:989–993. doi: 10.1038/ng.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Shulman J.M., De Jager P.L., Feany M.B. Parkinson’s disease: Genetics and pathogenesis. Annu. Rev. Pathol. 2011;6:193–222. doi: 10.1146/annurev-pathol-011110-130242. [DOI] [PubMed] [Google Scholar]
- 166.Wissemann W.T., Hill-Burns E.M., Zabetian C.P., Factor S.A., Patsopoulos N., Hoglund B., Holcomb C., Donahue R.J., Thomson G., Erlich H., et al. Association of Parkinson disease with structural and regulatory variants in the HLA region. Am. J. Hum. Genet. 2013;93:984–993. doi: 10.1016/j.ajhg.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vicario M., Cieri D., Brini M., Calì T. The Close Encounter Between Alpha-Synuclein and Mitochondria. Front. Neurosci. 2018;12:388. doi: 10.3389/fnins.2018.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Guardia-Laguarta C., Area-Gomez E., Rüb C., Liu Y., Magrané J., Becker D., Voos W., Schon E.A., Przedborski S. α-Synuclein is localized to mitochondria-associated ER membranes. J. Neurosci. 2014;34:249–259. doi: 10.1523/JNEUROSCI.2507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Li W.-W., Yang R., Guo J.-C., Ren H.-M., Zha X.-L., Cheng J.-S., Cai D.-F. Localization of alpha-synuclein to mitochondria within midbrain of mice. Neuroreport. 2007;18:1543–1546. doi: 10.1097/WNR.0b013e3282f03db4. [DOI] [PubMed] [Google Scholar]
- 170.Calì T., Ottolini D., Negro A., Brini M. α-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions. J. Biol. Chem. 2012;287:17914–17929. doi: 10.1074/jbc.M111.302794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Calì T., Ottolini D., Vicario M., Catoni C., Vallese F., Cieri D., Barazzuol L., Brini M. splitGFP Technology Reveals Dose-Dependent ER-Mitochondria Interface Modulation by α-Synuclein A53T and A30P Mutants. Cells. 2019;8 doi: 10.3390/cells8091072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Paillusson S., Gomez-Suaga P., Stoica R., Little D., Gissen P., Devine M.J., Noble W., Hanger D.P., Miller C.C.J. α-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca2+ homeostasis and mitochondrial ATP production. Acta Neuropathol. 2017;134:129–149. doi: 10.1007/s00401-017-1704-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Pickrell A.M., Youle R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lee K.-S., Huh S., Lee S., Wu Z., Kim A.-K., Kang H.-Y., Lu B. Altered ER-mitochondria contact impacts mitochondria calcium homeostasis and contributes to neurodegeneration in vivo in disease models. Proc. Natl. Acad. Sci. USA. 2018;115:E8844–E8853. doi: 10.1073/pnas.1721136115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ziviani E., Tao R.N., Whitworth A.J. Drosophila parkin requires PINK1 for mitochondrial translocation and ubiquitinates mitofusin. Proc. Natl. Acad. Sci. USA. 2010;107:5018–5023. doi: 10.1073/pnas.0913485107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Valente E.M., Salvi S., Ialongo T., Marongiu R., Elia A.E., Caputo V., Romito L., Albanese A., Dallapiccola B., Bentivoglio A.R. PINK1 mutations are associated with sporadic early-onset parkinsonism. Ann. Neurol. 2004;56:336–341. doi: 10.1002/ana.20256. [DOI] [PubMed] [Google Scholar]
- 177.Akundi R.S., Huang Z., Eason J., Pandya J.D., Zhi L., Cass W.A., Sullivan P.G., Büeler H. Increased mitochondrial calcium sensitivity and abnormal expression of innate immunity genes precede dopaminergic defects in Pink1-deficient mice. PLoS ONE. 2011;6:e16038. doi: 10.1371/journal.pone.0016038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Gandhi S., Wood-Kaczmar A., Yao Z., Plun-Favreau H., Deas E., Klupsch K., Downward J., Latchman D.S., Tabrizi S.J., Wood N.W., et al. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol. Cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Durcan T.M., Fon E.A. The three ’P’s of mitophagy: PARKIN, PINK1, and post-translational modifications. Genes Dev. 2015;29:989–999. doi: 10.1101/gad.262758.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Basso V., Marchesan E., Peggion C., Chakraborty J., von Stockum S., Giacomello M., Ottolini D., Debattisti V., Caicci F., Tasca E., et al. Regulation of ER-mitochondria contacts by Parkin via Mfn2. Pharmacol. Res. 2018;138:43–56. doi: 10.1016/j.phrs.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 181.Gautier C.A., Erpapazoglou Z., Mouton-Liger F., Muriel M.P., Cormier F., Bigou S., Duffaure S., Girard M., Foret B., Iannielli A., et al. The endoplasmic reticulum-mitochondria interface is perturbed in PARK2 knockout mice and patients with PARK2 mutations. Hum. Mol. Genet. 2016;25:2972–2984. doi: 10.1093/hmg/ddw148. [DOI] [PubMed] [Google Scholar]
- 182.Verma M., Callio J., Otero P.A., Sekler I., Wills Z.P., Chu C.T. Mitochondrial Calcium Dysregulation Contributes to Dendrite Degeneration Mediated by PD/LBD-Associated LRRK2 Mutants. J. Neurosci. 2017;37:11151–11165. doi: 10.1523/JNEUROSCI.3791-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Virani S.S., Alonso A., Benjamin E.J., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Delling F.N., et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. doi: 10.1161/CIR.0000000000000757. [DOI] [PubMed] [Google Scholar]
- 184.Brown D.A., Perry J.B., Allen M.E., Sabbah H.N., Stauffer B.L., Shaikh S.R., Cleland J.G.F., Colucci W.S., Butler J., Voors A.A., et al. Expert consensus document: Mitochondrial function as a therapeutic target in heart failure. Nat. Rev. Cardiol. 2017;14:238–250. doi: 10.1038/nrcardio.2016.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Bonora M., Wieckowski M.R., Sinclair D.A., Kroemer G., Pinton P., Galluzzi L. Targeting mitochondria for cardiovascular disorders: Therapeutic potential and obstacles. Nat. Rev. Cardiol. 2019;16:33–55. doi: 10.1038/s41569-018-0074-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Denton R.M., Richards D.A., Chin J.G. Calcium ions and the regulation of NAD+-linked isocitrate dehydrogenase from the mitochondria of rat heart and other tissues. Biochem. J. 1978;176:899–906. doi: 10.1042/bj1760899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hopper R.K., Carroll S., Aponte A.M., Johnson D.T., French S., Shen R.-F., Witzmann F.A., Harris R.A., Balaban R.S. Mitochondrial matrix phosphoproteome: Effect of extra mitochondrial calcium. Biochemistry. 2006;45:2524–2536. doi: 10.1021/bi052475e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Griffiths E.J., Balaska D., Cheng W.H.Y. The ups and downs of mitochondrial calcium signalling in the heart. Biochim. Biophys. Acta. 1797:856–864. doi: 10.1016/j.bbabio.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 189.Cao J.L., Adaniya S.M., Cypress M.W., Suzuki Y., Kusakari Y., Jhun B.S., O-Uchi J. Role of mitochondrial Ca2+ homeostasis in cardiac muscles. Arch. Biochem. Biophys. 2019;663:276–287. doi: 10.1016/j.abb.2019.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Giorgi C., Marchi S., Pinton P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018;19:713–730. doi: 10.1038/s41580-018-0052-8. [DOI] [PubMed] [Google Scholar]
- 191.Cao Y.-P., Zheng M. Mitochondrial dynamics and inter-mitochondrial communication in the heart. Arch. Biochem. Biophys. 2019;663:214–219. doi: 10.1016/j.abb.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 192.Shimada T., Horita K., Murakami M., Ogura R. Morphological studies of different mitochondrial populations in monkey myocardial cells. Cell Tissue Res. 1984;238:577–582. doi: 10.1007/BF00219874. [DOI] [PubMed] [Google Scholar]
- 193.Murgia M., Giorgi C., Pinton P., Rizzuto R. Controlling metabolism and cell death: At the heart of mitochondrial calcium signalling. J. Mol. Cell. Cardiol. 2009;46:781–788. doi: 10.1016/j.yjmcc.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kohlhaas M., Nickel A.G., Maack C. Mitochondrial energetics and calcium coupling in the heart. J. Physiol. 2017;595:3753–3763. doi: 10.1113/JP273609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983;245:C1–C14. doi: 10.1152/ajpcell.1983.245.1.C1. [DOI] [PubMed] [Google Scholar]
- 196.Crompton M. The role of Ca2+ in the function and dysfunction of heart mitochondria. In: Langer G.A., editor. Calcium Hear and the Heart. Raven Press; New York, NY, USA: 1990. pp. 167–198. [Google Scholar]
- 197.O’Rourke B., Blatter L.A. Mitochondrial Ca2+ uptake: Tortoise or hare? J. Mol. Cell. Cardiol. 2009;46:767–774. doi: 10.1016/j.yjmcc.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Dedkova E.N., Blatter L.A. Calcium signaling in cardiac mitochondria. J. Mol. Cell. Cardiol. 2013;58:125–133. doi: 10.1016/j.yjmcc.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.De la Fuente S., Sheu S.-S. SR-mitochondria communication in adult cardiomyocytes: A close relationship where the Ca2+ has a lot to say. Arch. Biochem. Biophys. 2019;663:259–268. doi: 10.1016/j.abb.2019.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Eisner D.A., Caldwell J.L., Kistamás K., Trafford A.W. Calcium and Excitation-Contraction Coupling in the Heart. Circ. Res. 2017;121:181–195. doi: 10.1161/CIRCRESAHA.117.310230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sharma V.K., Ramesh V., Franzini-Armstrong C., Sheu S.S. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J. Bioenerg. Biomembr. 2000;32:97–104. doi: 10.1023/A:1005520714221. [DOI] [PubMed] [Google Scholar]
- 202.Rizzuto R., Simpson A.W., Brini M., Pozzan T. Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin. Nature. 1992;358:325–327. doi: 10.1038/358325a0. [DOI] [PubMed] [Google Scholar]
- 203.Pan X., Liu J., Nguyen T., Liu C., Sun J., Teng Y., Fergusson M.M., Rovira I.I., Allen M., Springer D.A., et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Luongo T.S., Lambert J.P., Yuan A., Zhang X., Gross P., Song J., Shanmughapriya S., Gao E., Jain M., Houser S.R., et al. The Mitochondrial Calcium Uniporter Matches Energetic Supply with Cardiac Workload during Stress and Modulates Permeability Transition. Cell Rep. 2015;12:23–34. doi: 10.1016/j.celrep.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kwong J.Q., Lu X., Correll R.N., Schwanekamp J.A., Vagnozzi R.J., Sargent M.A., York A.J., Zhang J., Bers D.M., Molkentin J.D. The Mitochondrial Calcium Uniporter Selectively Matches Metabolic Output to Acute Contractile Stress in the Heart. Cell Rep. 2015;12:15–22. doi: 10.1016/j.celrep.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rasmussen T.P., Wu Y., Joiner M.A., Koval O.M., Wilson N.R., Luczak E.D., Wang Q., Chen B., Gao Z., Zhu Z., et al. Inhibition of MCU forces extramitochondrial adaptations governing physiological and pathological stress responses in heart. Proc. Natl. Acad. Sci. USA. 2015;112:9129–9134. doi: 10.1073/pnas.1504705112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Buntinas L., Gunter K.K., Sparagna G.C., Gunter T.E. The rapid mode of calcium uptake into heart mitochondria (RaM): Comparison to RaM in liver mitochondria. Biochim. Biophys. Acta. 2001;1504:248–261. doi: 10.1016/S0005-2728(00)00254-1. [DOI] [PubMed] [Google Scholar]
- 208.Beutner G., Sharma V.K., Giovannucci D.R., Yule D.I., Sheu S.S. Identification of a ryanodine receptor in rat heart mitochondria. J. Biol. Chem. 2001;276:21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- 209.Morganti C., Bonora M., Sbano L., Morciano G., Aquila G., Campo G., Wieckowski M.R., Giorgi C., Pinton P. The Mitochondrial Permeability Transition Pore. In: Oliveira P.J., editor. Mitochondrial Biology and Experimental Therapeutics. Springer International Publishing; Cham, Switzerland: 2018. pp. 47–73. [Google Scholar]
- 210.Bonora M., Patergnani S., Ramaccini D., Morciano G., Pedriali G., Kahsay A.E., Bouhamida E., Giorgi C., Wieckowski M.R., Pinton P. Physiopathology of the Permeability Transition Pore: Molecular Mechanisms in Human Pathology. Biomolecules. 2020;10 doi: 10.3390/biom10070998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Morciano G., Bonora M., Giorgi C., Pinton P. Other bricks for the correct construction of the mitochondrial permeability transition pore complex. Cell Death Dis. 2017;8:e2698. doi: 10.1038/cddis.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Giorgio V., Burchell V., Schiavone M., Bassot C., Minervini G., Petronilli V., Argenton F., Forte M., Tosatto S., Lippe G., et al. Ca2+ binding to F-ATP synthase β subunit triggers the mitochondrial permeability transition. EMBO Rep. 2017;18:1065–1076. doi: 10.15252/embr.201643354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bonora M., Pinton P. A New Current for the Mitochondrial Permeability Transition. Trends Biochem. Sci. 2019;44:559–561. doi: 10.1016/j.tibs.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 214.Kasprzak K.S., Kiser R.F., Weislow O.S. Magnesium counteracts nickel-induced suppression of T lymphocyte response to concanavalin A. Magnesium. 1988;7:166–172. [PubMed] [Google Scholar]
- 215.Morciano G., Bonora M., Campo G., Aquila G., Rizzo P., Giorgi C., Wieckowski M.R., Pinton P. Mechanistic Role of mPTP in Ischemia-Reperfusion Injury. Adv. Exp. Med. Biol. 2017;982:169–189. doi: 10.1007/978-3-319-55330-6_9. [DOI] [PubMed] [Google Scholar]
- 216.Wu Y., Rasmussen T.P., Koval O.M., Joiner M.-L.A., Hall D.D., Chen B., Luczak E.D., Wang Q., Rokita A.G., Wehrens X.H.T., et al. The mitochondrial uniporter controls fight or flight heart rate increases. Nat. Commun. 2015;6:6081. doi: 10.1038/ncomms7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Huo J., Lu S., Kwong J.Q., Bround M.J., Grimes K.M., Sargent M.A., Brown M.E., Davis M.E., Bers D.M., Molkentin J.D. MCUb Induction Protects the Heart From Postischemic Remodeling. Circ. Res. 2020;127:379–390. doi: 10.1161/CIRCRESAHA.119.316369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kohlhaas M., Liu T., Knopp A., Zeller T., Ong M.F., Böhm M., O’Rourke B., Maack C. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121:1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Maack C., Cortassa S., Aon M.A., Ganesan A.N., Liu T., O’Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ. Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Luongo T.S., Lambert J.P., Gross P., Nwokedi M., Lombardi A.A., Shanmughapriya S., Carpenter A.C., Kolmetzky D., Gao E., van Berlo J.H., et al. The mitochondrial Na+/Ca2+ exchanger is essential for Ca2+ homeostasis and viability. Nature. 2017;545:93–97. doi: 10.1038/nature22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Ramaccini D., Montoya-Uribe V., Aan F.J., Modesti L., Potes Y., Wieckowski M.R., Krga I., Glibetić M., Pinton P., Giorgi C., et al. Mitochondrial Function and Dysfunction in Dilated Cardiomyopathy. Front. cell Dev. Biol. 2020;8:624216. doi: 10.3389/fcell.2020.624216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Min C.K., Yeom D.R., Lee K.-E., Kwon H.-K., Kang M., Kim Y.-S., Park Z.Y., Jeon H., Kim D.H. Coupling of ryanodine receptor 2 and voltage-dependent anion channel 2 is essential for Ca2+ transfer from the sarcoplasmic reticulum to the mitochondria in the heart. Biochem. J. 2012;447:371–379. doi: 10.1042/BJ20120705. [DOI] [PubMed] [Google Scholar]
- 223.Santulli G., Xie W., Reiken S.R., Marks A.R. Mitochondrial calcium overload is a key determinant in heart failure. Proc. Natl. Acad. Sci. USA. 2015;112:11389–11394. doi: 10.1073/pnas.1513047112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Terentyev D., Györke I., Belevych A.E., Terentyeva R., Sridhar A., Nishijima Y., de Blanco E.C., Khanna S., Sen C.K., Cardounel A.J., et al. Redox modification of ryanodine receptors contributes to sarcoplasmic reticulum Ca2+ leak in chronic heart failure. Circ. Res. 2008;103:1466–1472. doi: 10.1161/CIRCRESAHA.108.184457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shiferaw Y., Aistrup G.L., Wasserstrom J.A. Intracellular Ca2+ waves, afterdepolarizations, and triggered arrhythmias. Cardiovasc. Res. 2012;95:265–268. doi: 10.1093/cvr/cvs155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Romano S., Pozzoni L., Verzoni A., Lomuscio A., Guffanti E., Croce L. Activity of a new long-acting anti-arrhythmic drug on ventricular extrasystole. Study with Holter continuous electrocardiographic monitoring. G. Ital. Cardiol. 1981;11:657–662. [PubMed] [Google Scholar]
- 227.Papanicolaou K.N., Khairallah R.J., Ngoh G.A., Chikando A., Luptak I., O’Shea K.M., Riley D.D., Lugus J.J., Colucci W.S., Lederer W.J., et al. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol. Cell. Biol. 2011;31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Chen Y., Csordás G., Jowdy C., Schneider T.G., Csordás N., Wang W., Liu Y., Kohlhaas M., Meiser M., Bergem S., et al. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca2+ crosstalk. Circ. Res. 2012;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Woods J.J., Wilson J.J. Inhibitors of the mitochondrial calcium uniporter for the treatment of disease. Curr. Opin. Chem. Biol. 2020;55:9–18. doi: 10.1016/j.cbpa.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 230.Chiu H.Y., Tay E.X.Y., Ong D.S.T., Taneja R. Mitochondrial Dysfunction at the Center of Cancer Therapy. Antioxid. Redox Signal. 2020;32:309–330. doi: 10.1089/ars.2019.7898. [DOI] [PubMed] [Google Scholar]
- 231.Woods J.J., Nemani N., Shanmughapriya S., Kumar A., Zhang M., Nathan S.R., Thomas M., Carvalho E., Ramachandran K., Srikantan S., et al. A Selective and Cell-Permeable Mitochondrial Calcium Uniporter (MCU) Inhibitor Preserves Mitochondrial Bioenergetics after Hypoxia/Reoxygenation Injury. ACS Cent. Sci. 2019;5:153–166. doi: 10.1021/acscentsci.8b00773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kon N., Murakoshi M., Isobe A., Kagechika K., Miyoshi N., Nagayama T. DS16570511 is a small-molecule inhibitor of the mitochondrial calcium uniporter. Cell death Discov. 2017;3:17045. doi: 10.1038/cddiscovery.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tong B.C.-K., Wu A.J., Li M., Cheung K.-H. Calcium signaling in Alzheimer’s disease & therapies. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:1745–1760. doi: 10.1016/j.bbamcr.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 234.Belosludtsev K.N., Sharipov R.R., Boyarkin D.P., Belosludtseva N.V., Dubinin M.V., Krasilnikova I.A., Bakaeva Z.V., Zgodova A.E., Pinelis V.G., Surin A.M. The effect of DS16570511, a new inhibitor of mitochondrial calcium uniporter, on calcium homeostasis, metabolism, and functional state of cultured cortical neurons and isolated brain mitochondria. Biochim. Biophys. Acta Gen. Subj. 2021;1865:129847. doi: 10.1016/j.bbagen.2021.129847. [DOI] [PubMed] [Google Scholar]
- 235.Payne R., Li C., Fernandez-Garcia E., Vais H., Foskett K. The MCU Inhibitor Ds16570511 has Off-Target Effects on Mitochondrial Membrane Potential. Biophys. J. 2019;116:270a. doi: 10.1016/j.bpj.2018.11.1461. [DOI] [Google Scholar]
- 236.Arduino D.M., Wettmarshausen J., Vais H., Navas-Navarro P., Cheng Y., Leimpek A., Ma Z., Delrio-Lorenzo A., Giordano A., Garcia-Perez C., et al. Systematic Identification of MCU Modulators by Orthogonal Interspecies Chemical Screening. Mol. Cell. 2017;67:711–723. doi: 10.1016/j.molcel.2017.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Nägele H., Castel M.A., Deutsch O., Wagner F.M., Reichenspurner H. Heart transplantation in a patient with multiple sclerosis and mitoxantrone-induced cardiomyopathy. J. Heart Lung Transplant. 2004;23:641–643. doi: 10.1016/S1053-2498(03)00307-3. [DOI] [PubMed] [Google Scholar]
- 238.Bittremieux M., La Rovere R.M., Akl H., Martines C., Welkenhuyzen K., Dubron K., Baes M., Janssens A., Vandenberghe P., Laurenti L., et al. Constitutive IP3 signaling underlies the sensitivity of B-cell cancers to the Bcl-2/IP3 receptor disruptor BIRD-2. Cell Death Differ. 2019;26:531–547. doi: 10.1038/s41418-018-0142-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Zhong F., Harr M.W., Bultynck G., Monaco G., Parys J.B., De Smedt H., Rong Y.-P., Molitoris J.K., Lam M., Ryder C., et al. Induction of Ca2+-driven apoptosis in chronic lymphocytic leukemia cells by peptide-mediated disruption of Bcl-2-IP3 receptor interaction. Blood. 2011;117:2924–2934. doi: 10.1182/blood-2010-09-307405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Denmeade S.R., Isaacs J.T. The SERCA pump as a therapeutic target: Making a “smart bomb” for prostate cancer. Cancer Biol. Ther. 2005;4:14–22. doi: 10.4161/cbt.4.1.1505. [DOI] [PubMed] [Google Scholar]
- 241.Denmeade S.R., Jakobsen C.M., Janssen S., Khan S.R., Garrett E.S., Lilja H., Christensen S.B., Isaacs J.T. Prostate-specific antigen-activated thapsigargin prodrug as targeted therapy for prostate cancer. J. Natl. Cancer Inst. 2003;95:990–1000. doi: 10.1093/jnci/95.13.990. [DOI] [PubMed] [Google Scholar]
- 242.Denmeade S.R., Mhaka A.M., Rosen D.M., Brennen W.N., Dalrymple S., Dach I., Olesen C., Gurel B., Demarzo A.M., Wilding G., et al. Engineering a prostate-specific membrane antigen-activated tumor endothelial cell prodrug for cancer therapy. Sci. Transl. Med. 2012;4:140ra86. doi: 10.1126/scitranslmed.3003886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Doan N.T.Q., Paulsen E.S., Sehgal P., Møller J.V., Nissen P., Denmeade S.R., Isaacs J.T., Dionne C.A., Christensen S.B. Targeting thapsigargin towards tumors. Steroids. 2015;97:2–7. doi: 10.1016/j.steroids.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Madreiter-Sokolowski C.T., Gottschalk B., Parichatikanond W., Eroglu E., Klec C., Waldeck-Weiermair M., Malli R., Graier W.F. Resveratrol Specifically Kills Cancer Cells by a Devastating Increase in the Ca2+ Coupling Between the Greatly Tethered Endoplasmic Reticulum and Mitochondria. Cell. Physiol. Biochem. 2016;39:1404–1420. doi: 10.1159/000447844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Francioso A., Mastromarino P., Masci A., D’Erme M., Mosca L. Chemistry, stability and bioavailability of resveratrol. Med. Chem. 2014;10:237–245. doi: 10.2174/15734064113096660053. [DOI] [PubMed] [Google Scholar]
- 246.Tu L.-Y., Bai H.-H., Cai J.-Y., Deng S.-P. The mechanism of kaempferol induced apoptosis and inhibited proliferation in human cervical cancer SiHa cell: From macro to nano. Scanning. 2016;38:644–653. doi: 10.1002/sca.21312. [DOI] [PubMed] [Google Scholar]
- 247.Rajendran P., Rengarajan T., Nandakumar N., Palaniswami R., Nishigaki Y., Nishigaki I. Kaempferol, a potential cytostatic and cure for inflammatory disorders. Eur. J. Med. Chem. 2014;86:103–112. doi: 10.1016/j.ejmech.2014.08.011. [DOI] [PubMed] [Google Scholar]
- 248.Cho H.J., Park J.H.Y. Kaempferol Induces Cell Cycle Arrest in HT-29 Human Colon Cancer Cells. J. Cancer Prev. 2013;18:257–263. doi: 10.15430/JCP.2013.18.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Montero M., Lobatón C.D., Hernández-Sanmiguel E., Santodomingo J., Vay L., Moreno A., Alvarez J. Direct activation of the mitochondrial calcium uniporter by natural plant flavonoids. Biochem. J. 2004;384:19–24. doi: 10.1042/BJ20040990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Liu R., Wang X., Zhao Y., Wang Z., Du L. The uptake behaviors of kaempferol and quercetin through rat primary cultured cortical neurons. Biomed. Chromatogr. 2006;20:1178–1184. doi: 10.1002/bmc.675. [DOI] [PubMed] [Google Scholar]
- 251.Bermont F., Hermant A., Benninga R., Chabert C., Jacot G., Santo-Domingo J., Kraus M.R.-C., Feige J.N., De Marchi U. Targeting Mitochondrial Calcium Uptake with the Natural Flavonol Kaempferol, to Promote Metabolism/Secretion Coupling in Pancreatic β-cells. Nutrients. 2020;12 doi: 10.3390/nu12020538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Guo Z., Liao Z., Huang L., Liu D., Yin D., He M. Kaempferol protects cardiomyocytes against anoxia/reoxygenation injury via mitochondrial pathway mediated by SIRT1. Eur. J. Pharmacol. 2015;761:245–253. doi: 10.1016/j.ejphar.2015.05.056. [DOI] [PubMed] [Google Scholar]
- 253.Montero M., Lobaton C.D., Moreno A., Alvarez J. A novel regulatory mechanism of the mitochondrial Ca2+ uniporter revealed by the p38 mitogen-activated protein kinase inhibitor SB202190. FASEB J. 2002;16:1955–1957. doi: 10.1096/fj.02-0553fje. [DOI] [PubMed] [Google Scholar]
- 254.Macle L., Nattel S. Arrhythmias in 2015: Advances in drug, ablation, and device therapy for cardiac arrhythmias. Nat. Rev. Cardiol. 2016;13:67–68. doi: 10.1038/nrcardio.2015.196. [DOI] [PubMed] [Google Scholar]
- 255.Magee W.P., Deshmukh G., Deninno M.P., Sutt J.C., Chapman J.G., Tracey W.R. Differing cardioprotective efficacy of the Na+/Ca2+ exchanger inhibitors SEA0400 and KB-R7943. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H903–H910. doi: 10.1152/ajpheart.00784.2002. [DOI] [PubMed] [Google Scholar]
- 256.Namekata I., Shimada H., Kawanishi T., Tanaka H., Shigenobu K. Reduction by SEA0400 of myocardial ischemia-induced cytoplasmic and mitochondrial Ca2+ overload. Eur. J. Pharmacol. 2006;543:108–115. doi: 10.1016/j.ejphar.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 257.Liu T., Takimoto E., Dimaano V.L., DeMazumder D., Kettlewell S., Smith G., Sidor A., Abraham T.P., O’Rourke B. Inhibiting mitochondrial Na+/Ca2+ exchange prevents sudden death in a Guinea pig model of heart failure. Circ. Res. 2014;115:44–54. doi: 10.1161/CIRCRESAHA.115.303062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Nicolau S.M., Egea J., López M.G., García A.G. Mitochondrial Na+/Ca2+ exchanger, a new target for neuroprotection in rat hippocampal slices. Biochem. Biophys. Res. Commun. 2010;400:140–144. doi: 10.1016/j.bbrc.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 259.Ruiz A., Alberdi E., Matute C. CGP37157, an inhibitor of the mitochondrial Na+/Ca2+ exchanger, protects neurons from excitotoxicity by blocking voltage-gated Ca2+ channels. Cell Death Dis. 2014;5:e1156. doi: 10.1038/cddis.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Tanaka H., Nishimaru K., Aikawa T., Hirayama W., Tanaka Y., Shigenobu K. Effect of SEA0400, a novel inhibitor of sodium-calcium exchanger, on myocardial ionic currents. Br. J. Pharmacol. 2002;135:1096–1100. doi: 10.1038/sj.bjp.0704574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Kho C., Lee A., Hajjar R.J. Altered sarcoplasmic reticulum calcium cycling--targets for heart failure therapy. Nat. Rev. Cardiol. 2012;9:717–733. doi: 10.1038/nrcardio.2012.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Motegi K., Tanonaka K., Takenaga Y., Takagi N., Takeo S. Preservation of mitochondrial function may contribute to cardioprotective effects of Na+/Ca2+ exchanger inhibitors in ischaemic/reperfused rat hearts. Br. J. Pharmacol. 2007;151:963–978. doi: 10.1038/sj.bjp.0707321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yoshitomi O., Akiyama D., Hara T., Cho S., Tomiyasu S., Sumikawa K. Cardioprotective effects of KB-R7943, a novel inhibitor of Na+/Ca2+ exchanger, on stunned myocardium in anesthetized dogs. J. Anesth. 2005;19:124–130. doi: 10.1007/s00540-004-0290-0. [DOI] [PubMed] [Google Scholar]
- 264.Storozhevykh T.P., Senilova Y.E., Brustovetsky T., Pinelis V.G., Brustovetsky N. Neuroprotective effect of KB-R7943 against glutamate excitotoxicity is related to mild mitochondrial depolarization. Neurochem. Res. 2010;35:323–335. doi: 10.1007/s11064-009-0058-x. [DOI] [PubMed] [Google Scholar]
- 265.Santo-Domingo J., Vay L., Hernández-Sanmiguel E., Lobatón C.D., Moreno A., Montero M., Alvarez J. The plasma membrane Na+/Ca2+ exchange inhibitor KB-R7943 is also a potent inhibitor of the mitochondrial Ca2+ uniporter. Br. J. Pharmacol. 2007;151:647–654. doi: 10.1038/sj.bjp.0707260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Wiczer B.M., Marcu R., Hawkins B.J. KB-R7943, a plasma membrane Na+/Ca2+ exchanger inhibitor, blocks opening of the mitochondrial permeability transition pore. Biochem. Biophys. Res. Commun. 2014;444:44–49. doi: 10.1016/j.bbrc.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Di Marco G., Vallese F., Jourde B., Bergsdorf C., Sturlese M., De Mario A., Techer-Etienne V., Haasen D., Oberhauser B., Schleeger S., et al. A High-Throughput Screening Identifies MICU1 Targeting Compounds. Cell Rep. 2020;30:2321–2331. doi: 10.1016/j.celrep.2020.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Walters A.M., Porter G.A., Brookes P.S. Mitochondria as a drug target in ischemic heart disease and cardiomyopathy. Circ. Res. 2012;111:1222–1236. doi: 10.1161/CIRCRESAHA.112.265660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ishimoto Y., Inagi R. Mitochondria: A therapeutic target in acute kidney injury. Nephrol. Dial. Transplant. 2016;31:1062–1069. doi: 10.1093/ndt/gfv317. [DOI] [PubMed] [Google Scholar]
- 270.Piot C., Croisille P., Staat P., Thibault H., Rioufol G., Mewton N., Elbelghiti R., Cung T.T., Bonnefoy E., Angoulvant D., et al. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N. Engl. J. Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 271.Cung T.-T., Morel O., Cayla G., Rioufol G., Garcia-Dorado D., Angoulvant D., Bonnefoy-Cudraz E., Guérin P., Elbaz M., Delarche N., et al. Cyclosporine before PCI in Patients with Acute Myocardial Infarction. N. Engl. J. Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 272.Wang W., Karamanlidis G., Tian R. Novel targets for mitochondrial medicine. Sci. Transl. Med. 2016;8 doi: 10.1126/scitranslmed.aac7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Du H., Guo L., Zhang W., Rydzewska M., Yan S. Cyclophilin D deficiency improves mitochondrial function and learning/memory in aging Alzheimer disease mouse model. Neurobiol. Aging. 2011;32:398–406. doi: 10.1016/j.neurobiolaging.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Morciano G., Preti D., Pedriali G., Aquila G., Missiroli S., Fantinati A., Caroccia N., Pacifico S., Bonora M., Talarico A., et al. Discovery of Novel 1,3,8-Triazaspiro[4.5]decane Derivatives That Target the c Subunit of F1/FO-Adenosine Triphosphate (ATP) Synthase for the Treatment of Reperfusion Damage in Myocardial Infarction. J. Med. Chem. 2018;61:7131–7143. doi: 10.1021/acs.jmedchem.8b00278. [DOI] [PubMed] [Google Scholar]