Abstract
Given the importance of colour in the general acceptance or rejection of a product, the use of colorants is a widespread practice, particularly in the food industry. At the same time, with the increasing consumers’ awareness of the health effects that some artificial colorants can exert, there is a growing tendency to prioritize foodstuffs containing natural additives. In this work, Morus nigra L. and Rubus fruticosus L. fruit juices were characterized in terms of anthocyanins, organic acids, free sugars, and tocopherols, as also regarding their bioactive properties. Given their richness in anthocyanins, this study also aimed to prepare different solid colouring formulations by the spray-drying technique, using as stabilizers maltodextrin and arabic gum. Six free sugars and two organic acids were detected in the fruit juices, as well as the four tocopherol isoforms. Two cyanidin derivatives were found in M. nigra (cyanidin-3-O-glucoside and cyanidin-O-rhamnoside) and other four in R. fruticosus (cyanidin-O-hexoside, cyanidin-3-O-glucoside, cyanidin-O-pentoside, and cyanidin-3-O-dioxaloilglucoside). The developed colouring formulations revealed a good stability over time, in terms of anthocyanin concentration and colour parameters, and revealed to be safe for consumption, either concerning their low microbial load and lack of cytotoxicity. Thus, they represent a promising natural alternative to the massively used artificial colorants.
Keywords: Morus nigra L., Rubus fruticosus L., anthocyanins, stable food colorants
1. Introduction
One of the first perceptions of food is its colour, a characteristic that highly influences consumers’ choice, since it leads to the creation of an idea of the flavour, odour, and composition of the food product [1]. Therefore, food colorants are one of the most used additives, being applied to restore the original colour of a foodstuff when it is lost by some technological or storage process, to change, or even to enhance the original coloration of food [2]. With the recent food industry interest in gradually replacing the commonly used artificial colorants by natural counterparts, an increasing number of research studies have been focusing the exploitation of natural resources to meet this challenge. For being natural, non-toxic, and water-soluble, anthocyanins have been widely studied as alternatives to the mostly used artificial food colorants. Along with a great colouring capacity, these compounds have recognized bioactive properties, acting as antioxidants and helping in the prevention of cardiovascular and neurological diseases, cancer, and diabetes, among others [2,3], which make them even more suitable for food application.
As examples of rich anthocyanin sources, Morus nigra L. and Rubus fruticosus L. are small fruits that can be found in Asia, Europe, America, and Africa [3,4]. They are of high research interest due to their demonstrated different biological properties, such as anti-inflammatory, sedative, emollients, hypoglycaemic, cytotoxic, antiseptic, antifungal, antibacterial, and hepatoprotective activity [5,6,7]. In most mulberries, the major phenolic compounds reported are cyanidin-3-glucoside, quercetin-3-rutinoside, and kaempferol-3-rutinoside, however, the presence of quercetin-3-glucoside, cyanidin-3-rutinoside, pelargonidine-3-glucoside, and pelargonidin-3-rutinoside has also been reported [8,9]. These fruits are also rich in fatty acids, with a prevalence of linoleic acid, sugars, mainly fructose and glucose, and organic acids, mostly citric and malic acids [3,10,11,12]. On the other hand, R. fruticosus contains as major anthocyanins cyanidin-3-glucoside, cyanidin-3-arabinoside, and cyanidin-3-galactoside, but malvidin-3-glucoside, pelargonidin-3-glucoside, cyanidin-3-xyloside, cyanidin-3-rutinoside, and cyanidin-3-malonylglucoside are also present in smaller amounts. As phenolic acids, it mainly contains gallic acid, protocatechuic acid, p-hydroxybenzoic acid, caffeic acid, p-coumaric acid, and ellagic acid [13]. This fruit is considered highly nutritious, being composed of 85% of water, 10% of carbohydrates, minerals (Mg, Fe, K, and Ca), and vitamins (A, B, C, K, and E). It also contains fructose and glucose, flavonoids such as kaempferol and myricetin, and in immature fruits some carotenoids such as all-trans-lutein and all-trans-zeaxanthin can also be found [4,14].
Despite their multiple health benefits, some of these fruits are not used for consumption for not presenting the suitable size or properties to be included in the market, constituting a food industry residueom. The recovery of these bioresidues for added-value additives development could contribute to a circular bioeconomy, minimizing urban waste management issues and the scarcity of resources, mainly caused by the growing urban population and the linear economy. As such, in the present study, M. nigra and R. fruticosus fruits were used for the development of natural food colorants with stable colouring properties and respecting safety parameters, along three months of storage at different conditions. Once these formulations have the purpose of being added to foodstuff, the chemical composition and bioactive properties of both fruit extracts were also evaluated.
2. Results and Discussion
2.1. Chemical Composition
2.1.1. Free Sugars
The results obtained for the free sugars composition of Morus nigra L. and Rubus fruticosus L. are presented in Table 1. Both fruits revealed the presence of fructose, glucose, sucrose, trehalose and raffinose. In R. fruticosus another sugar was also found, but its identification was not possible. M. nigra revealed the highest total sugars concentration, with 449 ± 2 mg/g extract. For both fruit extracts, the most abundant sugar was fructose, followed by glucose (respectively 248 ± 2 and 229.9 ± 0.3 mg/g extract in M. nigra and 201 ± 1 and 163.5 ± 0.1 mg/g extract in R. fruticosus). In addition to these two main sugars, both M. nigra and R. fruticosus extracts revealed traces of raffinose (5.085 ± 0.23 and 12.14 ± 0.61 mg/g of extract), trehalose (3.49 ± 0.184 and 5.275 ± 0.12 mg/g of extract), and sucrose (2.695 ± 0.077 and 3.7 ± 0.2 mg/g of extract).
Table 1.
Free sugars, organic acids, and tocopherols composition of M. nigra and R. fruticosus fruit extracts.
| M. nigra | R. fruticosus | p-Value | |
|---|---|---|---|
| Free Sugars (mg/g extract) | |||
| Fructose | 248 ± 2 | 201 ± 1 | <0.001 |
| Glucose | 229.9 ± 0.3 | 163.5 ± 0.1 | <0.001 |
| Sucrose | 2.70 ± 0.08 | 3.7 ± 0.2 | <0.001 |
| Trehalose | 3.5 ± 0.1 | 5.3 ± 0.1 | <0.001 |
| Raffinose | 5.1 ± 0.2 | 12.1 ± 0.6 | <0.001 |
| Unknown | nd | 21 ± 1 | - |
| Total | 449 ± 2 | 373 ± 1 | <0.001 |
| Organic acids (mg/g extract) | |||
| Oxalic acid | 14.91 ± 0.09 | 5.52 ± 0.02 | <0.001 |
| Malic acid | 146.9 ± 0.6 | 101.9 ± 0.2 | <0.001 |
| Total | 161.8 ± 0.6 | 107.3 ± 0.2 | <0.001 |
| Tocopherols (mg/g extract) | |||
| α-Tocopherol | 43 ± 2 | 6.1 ± 0.1 | <0.001 |
| β-Tocopherol | 1.27 ± 0.03 | nd | - |
| γ-Tocopherol | 12.5 ± 0.2 | nd | - |
| δ-Tocopherol | 5.5 ± 0.1 | nd | - |
| Total | 62 ± 2 | 6.1 ± 0.1 | <0.001 |
nd: not detected; p-values obtained by applying the Student’s t-test at a 5% significance level.
It was not possible to establish a direct comparison of the results obtained herein with those of other authors, since in the present study, the fruit juices were assessed, and not the freeze-dried fruits. Nevertheless, in previous studies performed by Gundogdu et al., and Özgen et al. [3,15], glucose was found in a higher concentration than fructose in M. nigra fruits, although these were also the main sugars detected.
Regarding R. fruticosus, the results obtained with the juices are in agreement whit those reported by Milivojević et al. [14], with fructose being present in higher concentrations than glucose. Despite the scarcity of studies reporting the presence of raffinose and trehalose in fruits, some authors have reported their presence in berries. For instance, Aksic et al. [16] detected trehalose and raffinose in three blueberry cultivars (‘Bluecrop’, ‘Duke’, and ‘Nui’), while Palonen et al. [17] reported the presence of raffinose in ‘Festival’, ‘Titan’, and ‘Willamette’ raspberries, and Vara et al. [18] of both raffinose and trehalose in ‘Kwely’ raspberries.
2.1.2. Organic Acids
Organic acids are compounds that exert a great influence on the organoleptic properties of fruits and can help preserve their nutritional value, being highly used in the food industry either as antioxidants, acidulants, or preservatives depending on their nature [19]. In this study, high amounts of malic acid were found, as also lower concentrations of oxalic acid (Table 1). These acids were found in higher quantity in M. nigra (146.9 ± 0.6 and 14.91 ± 0.09 mg/g of extract, respectively) than in R. fruticosus (101.9 ± 0.2 and 5.52 ± 0.02 mg/g of extract).
The results obtained for malic acid in M. nigra are consistent with those reported by Koyuncu [19] in a study evaluating different genotypes of blackberries, however, for oxalic acid, the maximum value reported was below the levels found in the present work, which can be related to the different extraction methods. On the other hand, Kafkas et al. [20] reported values between 0.6 ± 0.7 mg/g extract and 11.0 ± 2.7 mg/g extract of malic acid for different blackberries of the Rubus family, confirming the prevalence of this one against the other acids. Nevertheless, these authors did not find oxalic acid and detected ascorbic and citric acids. The differences observed can, once again, be related to the fact that in the referred study, metaphosphoric acid was used to perform the extraction, whereas herein the freeze-dried juice was directly used for analysis. It is also important to highlight that the chemical composition of these fruits are affected by different factors such as the place of cultivation, temperature, humidity, soil, collection method and time, storage method, sample treatment, among others [14].
2.1.3. Tocopherols
Tocopherols are the most important natural fat-soluble antioxidants in the nutritional area. In M. nigra, the four isoforms were found (Table 1), with the prevalence of α-tocopherol (43 ± 2 mg/g of extract), followed by γ-tocopherol (12.5 ± 0.2 mg/g of extract); and in lower amounts, δ-tocoferol (5.5 ± 0.1 mg/g of extract) and β-tocopherol (1.27 ± 0.03 mg/g of extract). On the other hand, in R. fruticosus, only α-tocopherol was found, in a concentration of 6.1 ± 0.1 mg/g of extract. Comparing the two species, it is evident that M. nigra shows nearly seven times more quantity of α-tocopherol than R. fruticosus, which may justify the better results obtained with M. nigra in the TBARS antioxidant assay.
In a study performed by Wajs-bonikowska et al. [21], the α-, γ-, and δ-tocopherol isoforms were found in samples of R. fruticosus collected in Poland; a supercritical CO2 method and Soxhlet extraction using two solvents (hexane and ethanol) were compared for the extraction of tocopherols from this fruit pomace and, in all cases, lower amounts of α-tocopherol (0.72 ± 0.06, 0.70 ± 0.05, and 0.51 ± 0.08, respectively) were obtained, comparing to the present work, which is also possibly explained by the different extracts assessed and corroborates the importance of consuming these fruits juice.
2.1.4. Anthocyanins
Anthocyanins are natural pigments which colour can vary from blue to red tonalities. These molecules have been increasingly applied in food industry, not only for their great colouring capacity, but also for conferring bioactive properties to the products in which they are included. The attempt to identify anthocyanins by UPLC-DAD-ESI/MS analysis in M. nigra and R. fruticosus, was based on retention times (Tr), maximum absorption wavelengths of the UV-Vis region (max), pseudomolecular ion ([M]+) and molecular ion fragmentation (MS2), being the identification performed by comparison with available standards and/or literature data.
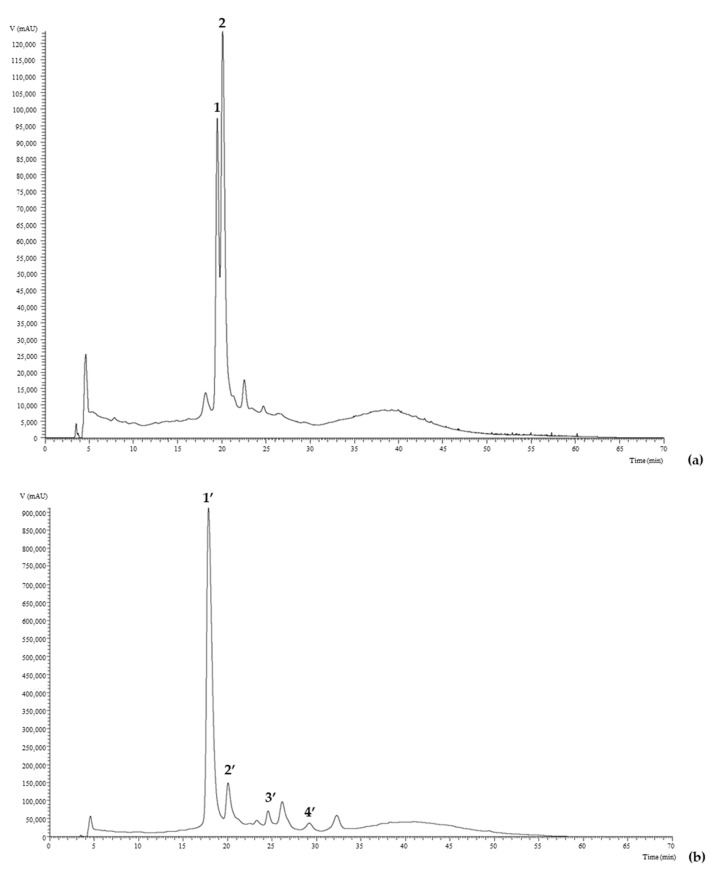
In the M. nigra juice, two anthocyanins were identified (Table 2). Figure 1a presents the chromatographic profile of the anthocyanin compounds detected. Compound 1 (cyanidin-3-O-glucoside) was positively identified in comparison with the chromatographic and MS characteristics of the commercial standard. Compound 2 ([M]+ at m/z 595) presented two MS2 fragments, revealing two losses of a ramnose unit and a hexose unit (m/z at 287; −146 u and −162 u, respectively), therefore, it was identified as cyanidin-O-ramnoside-O-hexoside. These anthocyanins, especially the former, are very characteristic for being present in high quantities in this type of fruit, for example, Pawlowska et al. [8] recorded an amount of 17.9 mg/10 g of fresh Moraceae fruits.
Table 2.
Anthocyanin composition of M. nigra and R. fruticosus fruit extracts.
| Peak | Rt (min) | λmax (nm) | [M]+ m/z | MS2 | Tentative Identification | Concentration (mg/g Extract) |
|---|---|---|---|---|---|---|
| M. nigra | ||||||
| 1 | 19.72 | 515 | 449 | 287(100) | Cyanidin-3-O-glucoside | 6.096 ± 0.003 |
| 2 | 22.00 | 517 | 595 | 449(31), 287(100) | Cyanidin-O-rhamnoside-O-hexoside | 2.443 ± 0.002 |
| Total | 8.538 ± 0.005 | |||||
| R. fruticosus | ||||||
| 1′ | 16.69 | 518 | 449 | 287(100) | Cyanidin-O-hexoside | 3.761 ± 0.007 |
| 2′ | 19.55 | 518 | 449 | 287(100) | Cyanidin-3-O-glucoside | 1.81 ± 0.01 |
| 3′ | 24.01 | 517 | 419 | 287(100) | Cyanidin-O-pentoside | 1.265 ± 0.001 |
| 4′ | 30.14 | 519 | 593 | 287(100) | Cyanidin-3-O-dioxaloilglucoside | 1.198 ± 0.001 |
| Total | 8.03 ± 0.02 | |||||
Calibration curve used for quantification: cyanidin-3-O-glucoside (y = 134,578x – 3 × 106; R2: 0.9986; LOD: 0.25 µg/mL; LOQ: 0.83 µg/mL).
Figure 1.
Chromatographic profile of the anthocyanin compounds found in M. nigra (a) and R. fruticosus (b) fruit juices, recorded at 520 nm (1: cyanidin-3-O-glucoside; 2: cyanidin-O-rhamnoside-O-hexoside; 1′: cyanidin-O-hexoside; 2′: cyanidin-3-O-glucoside; 3′: cyanidin-O-pentoside; 4′: cyanidin-3-O-dioxaloilglucoside).
The chromatographic analysis of R. fruticosus showed the presence of four anthocyanins (Table 2), all identified as cyanidin glycosidic derivatives. Figure 1b shows the chromatographic profile of anthocyanins present in this fruit. Compound 2′ (cyanidin-3-O-glucoside) was positively identified by comparison to the commercial standard. Compound 1′ ([M]+ at m/z 449) presented the same pseudomolecular ion as compound 2, with a loss of one hexose (−162 u). In this case, it was not possible to identify the position and nature of the hexose portion, because the peak retention times do not correspond to any of the available standards. Compound 3′ ([M]+ at m/z 419) lost −132 u, corresponding to a pentose, led to the compound tentative identification as cyanidin-O-pentoside. Compound 4′ ([M]+ at m/z 593) was identified as cyanidin-3-O-dioxaloilglucoside, considering the information described in literature, since this compound is found in different berries cultivars (marionberry_ORBC and blackberry) [22]. Cyanidin-3-O-glucose, a characteristic anthocyanin in blackberries, was found in both fruits, together with other cyanidin derivatives. The presence and variety of these anthocyanins is related to several health promoting properties, namely antioxidant, antitumor, anti-inflammatory, and antidiabetic, highlighting the importance of their consumption.
2.2. Bioactive Properties
M. nigra and R. fruticosus juices were used to formulate natural colorants for food application. As such, the extracts were processed using the spray-drying technique, through which three powder formulations were prepared: one only using the fruit juice, a second formulation using the juice and maltodextrin (40%), and a third one using 20% of a 1:1 (w/w) maltodextrin arabic gum mixture, as described in Section 3.4.2. The formulations, as well as the extracts, were assessed in terms of antioxidant, antibacterial, and antifungal properties, given the importance of these properties in foodstuff, not only for conferring health benefits, but also for delaying food oxidation and microbiological spoilage.
2.2.1. Antioxidant Activity
The antioxidant activity of M. nigra and R. fruticosus extracts, as well as that of the different colouring formulations, was determined by two in vitro assays, the lipid peroxidation inhibition assays (TBARS) and the oxidative haemolysis inhibition assay (OxHLIA).
In the case of M. nigra (Table 3), a high antioxidant activity of the extract was evidenced in TBARS assay with an IC50 value of 39 ± 2 µg/mL, which is almost four times lower than that of the positive control, Trolox (139 ± 5 µg/mL).
Table 3.
Bioactive properties of M. nigra fruit extract and colouring formulations.
| Antioxidant Activity (IC50 Values, µg/mL) | |||||||
|---|---|---|---|---|---|---|---|
|
M. nigra extract |
M. nigra control |
M. nigra + M |
M. nigra + M + AG |
Trolox 1 | |||
| TBARS assay | 39 ± 2a | 55.6 ± 0.4c | 51 ± 1b | 52 ± 2b | 139 ± 5 | ||
| OxHLIA assay | 60 min | 253 ± 10d | 166 ± 5c | 124 ± 5b | 108 ± 5a | 85 ± 2 | |
| 120 min | 569 ± 14d | 324 ± 17c | 286 ± 10a | 296 ± 11a | 183 ± 4 | ||
| Antibacterial activity (MIC and MBC values, mg/mL) | |||||||
|
M. nigra extract |
M. nigra control |
M. nigra + M |
M. nigra + M + AG |
Streptomycin 1 | Ampicilin 1 | ||
| Bacillus cereus | MIC/MBC | 5.01/10.02 | 6.81/6.81 | 8.52/8.52 | 8.52/8.52 | 0.10/0.20 | 0.25/0.40 |
| Staphylococcus aureus | MIC/MBC | 20.04/20.04 | 3.41/6.81 | 4.26/8.52 | 4.26/8.52 | 0.17/0.25 | 0.34/0.37 |
| Listeria monocytogenes | MIC/MBC | 10.02/20.04 | 3.41/3.41 | 4.26/4.26 | 4.26/4.26 | 0.20/0.30 | 0.40/0.50 |
| Escherichia coli | MIC/MBC | 2.50/5.01 | 3.41/3.41 | 4.26/8.52 | 4.26/8.52 | 0.20/0.30 | 0.40/0.50 |
| Enterobacter cloacae | MIC/MBC | 10.02/20.04 | 1.7/1.7 | 2.13/2.13 | 4.26/4.26 | 0.043/0.25 | 0.086/0.37 |
| Salmonella Typhimurium | MIC/MBC | 10.02/20.04 | 1.7/3.41 | 2.13/4.26 | 4.26/4.26 | 0.20/0.30 | 0.75/1.20 |
| Antifungal activity (MIC and MFC values, mg/mL) | |||||||
|
M. nigra extract |
M. nigra control |
M. nigra + M |
M. nigra + M + AG |
Ketoconazole 1 | Bifonazole 1 | ||
| Aspergillus fumigatus | MIC/MFC | 5.01/10.02 | 13.63/27.27 | 17.05/17.05 | 8.52/17.05 | 0.38/0.95 | 0.48/0.64 |
| Aspergillus versicolor | MIC/MFC | 2.51/5.01 | 6.81/27.27 | 4.26/8.52 | 4.26/8.52 | 0.20/0.50 | 0.10/0.20 |
| Aspergillus niger | MIC/MFC | 20.04/>20.04 | 27.27/>27.27 | 34.09/>34.09 | 17.05/34.09 | 0.20/0.50 | 0.15/0.20 |
| Penicillium funiculosum | MIC/MFC | 2.51/5.01 | 13.63/27.27 | 17.05/34.09 | 8.52/34.09 | 0.20/0.50 | 0.20/0.25 |
| Penicillium ochrochloron | MIC/MFC | 2.51/5.01 | 27.27/>27.27 | 34.09/>34.09 | 34.09/>34.09 | 1.00/1.50 | 0.20/0.25 |
| Trichoderma viride | MIC/MFC | 1.25/2.51 | 2.13/13.63 | 2.13/4.26 | 4.26/8.52 | 1.00/1.00 | 0.15/0.20 |
1 Positive controls; M. nigra control: colouring formulation control; M. nigra + M: colouring formulation containing maltodextrin (40%); M. nigra + M + AG: colouring formulation containing maltodextrin (20%) and arabic gum (20%). IC50: extract concentration providing 50% of antioxidant activity; MIC: minimum inhibitory properties; MBC: minimum bactericidal concentration; MFC: minimum fungicidal properties. For the antioxidant activity, different letters in each line mean significant differences (p < 0.05).
Likewise, all the colouring formulations presented a high antioxidant activity, very similar among them, which despite being higher than that presented by the extract, were significantly lower than that of Trolox. Regarding the OxHLIA assay, a concentration of 569 ± 14 µg/mL of the extract was able to delay the oxidative haemolysis for 120 min, which despite being a higher concentration than that needed for the positive control, can be considered a good antioxidant capacity for a natural extract. Furthermore, all the colouring formulations required a lower concentration than the extract, with the formulations containing maltodextrin and maltodextrin with arabic gum revealing the best antioxidant activity, with IC50 values of 286 ± 10 µg/mL and 296 ± 11 µg/mL, respectively. In a general perspective, the formulations prepared with maltodextrin and maltodextrin with arabic gum presented the best antioxidant activity in both assays without significant differences between them. The antioxidant potential of mulberry fruit was also assessed in previous studies; for example, Arfan et al. [23] reported the antioxidant potential of methanol and acetone sugar-free extracts, determined through ABTS, DPPH (2,2-diphenyl-1-picrylhydrazyl), and reducing power assays. On the other hand, Do et al. [24] reported the antioxidant capacity (DPPH) of spray-dried mulberry juice. Once in the present study, cellular-based assays were employed, it was not possible to compare the results.
In what concerns R. fruticosus (Table 4), for TBARS assay, the extract presented a great antioxidant activity, with an IC50 value of 100 ± 2 µg/mL, which is significantly lower than that obtained with the positive control, Trolox (139 ± 5 µg/mL). In fact, all the colouring formulations presented a better antioxidant activity than Trolox, being the control formulation the one that presented the highest activity (IC50 value of 78.4 µg/mL). In OxHLIA assay, the extract needed a higher concentration to delay the oxidative haemolysis for 120 min, 215 ± 3 µg/mL, compared to that needed for Trolox (183 ± 4 µg/mL). With respect to the colouring formulations, a similar behaviour was observed, comparing to TBARS, with the control formulation presenting the best antioxidant activity, in a concentration of 194 ± 6 µg/mL, a result close to that obtained for Trolox. On the other hand, the formulations prepared with maltodextrin and maltodextrin with arabic gum presented IC50 values of 250 ± 4 µg/mL and 248 ± 5 µg/mL, respectively, which are slightly higher than that obtained for the control, but still represent great results. The antioxidant activity of blackberry powders was also reported by Ferrari et al. [25], but the results are not directly comparable to the ones obtained herein given the different spray-drying conditions and antioxidant test applied (DPPH scavenging activity).
Table 4.
Bioactive properties of R. fruticosus fruit extract and colouring formulations.
| Antioxidant Activity (IC50 Values, µg/mL) | |||||||
|---|---|---|---|---|---|---|---|
|
R. fruticosus extract |
R. fruticosus control |
R. fruticosus + M |
R. fruticosus + M + AG |
Trolox 1 | |||
| TBARS assay | 100 ± 2c | 78.4 ± 0.8a | 101 ± 2c | 94.9 ± 0.2b | 139 ± 5b | ||
| OxHLIA assay | 60 min | 120 ± 7c | 81 ± 3a | 108 ± 5b | 106 ± 5b | 85 ± 2a | |
| 120 min | 215 ± 3b | 194 ± 6a | 250 ± 4c | 248 ± 5c | 183 ± 4a | ||
| Antibacterial activity (MIC and MBC values, mg/mL) | |||||||
|
R. fruticosus extract |
R. fruticosus control |
R. fruticosus + M |
R. fruticosus + M + AG |
Streptomycin 1 | Ampicilin 1 | ||
| Bacillus cereus | MIC/MBC | 5.03/10.06 | 2.53/5.06 | 2.51/5.02 | 5.03/10.06 | 0.10/0.20 | 0.25/0.40 |
| Staphylococcus aureus | MIC/MBC | 13.41/20.12 | 5.06/13.49 | 20.08/20.08 | 10.06/20.12 | 0.17/0.25 | 0.34/0.37 |
| Listeria monocytogenes | MIC/MBC | 10.06/20.12 | 5.06/10.12 | 10.04/20.08 | 10.06/26.83 | 0.20/0.30 | 0.40/0.50 |
| Escherichia coli | MIC/MBC | 2.51/2.51 | 1.27/2.53 | 2.51/5.02 | 1.26/2.52 | 0.20/0.30 | 0.40/0.50 |
| Enterobacter cloacae | MIC/MBC | 13.41/20.12 | 5.06/10.12 | 10.04/20.08 | 5.03/10.06 | 0.043/0.25 | 0.086/0.37 |
| Salmonella typhimurium | MIC/MBC | 13.41/20.12 | 5.06/10.12 | 10.04/20.08 | 5.03/10.06 | 0.20/0.30 | 0.75/1.20 |
| Antifungal activity (MIC and MFC values, mg/mL) | |||||||
| R. fruticosus extract | R. fruticosus control |
R. fruticosus + M |
R. fruticosus + M + AG |
Ketoconazole 1 | Bifonazole 1 | ||
| Aspergillus fumigatus | MIC/MFC | 5.03/10.06 | 2.53/5.06 | 2.51/5.02 | 5.03/10.06 | 0.38/0.95 | 0.48/0.64 |
| Aspergillus versicolor | MIC/MFC | 20.12/>20.12 | 1.27/2.53 | 1.26/2.52 | 1.26/2.52 | 0.20/0.50 | 0.10/0.20 |
| Aspergillus niger | MIC/MFC | 20.12/>20.12 | 5.06/10.12 | 3.77/5.02 | 5.03/10.06 | 0.20/0.50 | 0.15/0.20 |
| Penicillium funiculosum | MIC/MFC | 2.52/5.03 | 1.27/2.53 | 2.51/5.02 | 2.52/5.03 | 0.20/0.50 | 0.20/0.25 |
| Penicillium ochrochloron | MIC/MFC | 2.52/5.03 | 5.06/10.12 | 2.51/5.02 | 5.03/10.06 | 1.00/1.50 | 0.20/0.25 |
| Trichoderma viride | MIC/MFC | 1.26/2.52 | 0.91/1.27 | 2.51/5.02 | 1.26/2.52 | 1.00/1.00 | 0.15/0.20 |
1 Positive controls; R. fruticosus control: colouring formulation control; R. fruticosus + M: colouring formulation containing maltodextrin (40%); R. fruticosus + M + AG: colouring formulation containing maltodextrin (20%) and arabic gum (20%). IC50: extract concentration providing 50% of antioxidant activity; MIC: minimum inhibitory properties; MBC: minimum bactericidal concentration; MFC: minimum fungicidal properties. For the antioxidant activity, different letters in each line mean significant differences (p < 0.05).
2.2.2. Antimicrobial Activity
The results obtained for M. nigra (Table 3) and R. fruticosus (Table 4) extracts evidenced that both fruits present activity in all Gram-positive and Gram-negative bacteria assessed, showing very similar minimum inhibitory concentrations (MIC) and minimum bactericidal concentrations (MBC). All MIC and MBC values are higher than those showed by the positive controls, nevertheless, these are based on natural extracts, which despite having a lower activity, are not associated with negative effects for health, as some antibiotics. Among the Gram-positive bacteria, M. nigra and R. fruticosus revealed a better effect on B. cereus, both in terms of minimum concentrations necessary to inhibit the growth (5.01 and 5.03 mg/mL, respectively) and in the minimum concentrations necessary to have a bactericidal effect (10.02 and 10.06 mg/mL, respectively). E. coli was the bacterium belonging to the Gram-negative group in which better inhibitory activity was evidenced by both fruits, presenting the same MIC value (2.50 mg/mL). However, M. nigra presented a lower bactericidal capacity (MBC: 5.01 mg/mL) than R. fruticosus (MBC: 2.51 mg/mL). In a previous study performed by Četojević-Simin et al. [26], blackberry bagasse extracts also revealed the capacity to inhibit the growth of E. coli, S. typhymurium, Pseudomonas aeruginosa, S. aureus, S. saprophyticus, B. cereus, and L. monocytogenes, but the results are not directly comparable to the ones obtained herein, given the different extracts assessed and the applied method (disk diffusion method). Regarding mulberry, Khalid et al. [27] also reported the antibacterial activity of the fruit juice against Bacillus spizizenii, Bacilus subtilis, Corynebacterium diphtheride, Enterococcus faecalis, S. aureus, E. coli, P. aeruginosa, and S. typhymurium.
In terms of antifungal capacity, both M. nigra and R. fruticosus extracts evidenced inhibitory activity in all the tested fungi, although in higher concentrations than the positive controls. For all fungi, the extracts presented activity in similar concentrations, with the exception of A. versicolor, for which M. nigra presented a MIC of 2.51 mg/mL, which was much lower than that showed by R. fruticosus (MIC: 20.12 mg/mL). In addition, T. viride was the most sensitive fungus for both extracts (MIC: 1.25 mg/mL).
Regarding the colouring formulations of M. nigra (Table 3), despite presenting higher MIC and MBC values than the positive controls, streptomycin and ampicillin, all of them presented antibacterial properties, with the control formulation revealing activity in lower concentrations than the colorants containing maltodextrin and maltodextrin with arabic gum. Besides, these formulations also showed antifungal capacity against most of the assessed fungi. The colorant containing maltodextrin and arabic gum was the most effective, especially against T. viride and A. versicolor, in the same concentrations (MIC: 4.26 mg/mL; MFC: 8.52 mg/mL). However, none of the three formulations presented fungicidal activity for P. ochrochloron, nor the control formulation or the formulation with maltodextrin against A. niger.
Regarding the antibacterial activity of the formulations prepared with R. fruticosus (Table 4), it can be observed that although presenting higher inhibitory and bactericidal concentrations than the positive controls, they all present bioactivity, especially the control formulation against Gram-positive bacteria, except for B. cereus, which revealed a higher sensitivity for the colouring formulation with maltodextrin (MIC: 2.51 mg/mL; MBC: 5.02 mg/mL). For Gram-negative bacteria, better results were obtained with the colouring formulation containing maltodextrin with arabic gum, which, in most cases, showed effective concentrations twice lower than those obtained with maltodextrin and the control.
Generally, the bioactivity achieved with R. fruticosus colouring formulations was not very different, being the one prepared with maltodextrin the one presenting the best antifungal capacity, with the lowest inhibitory and fungicidal concentrations for A. versicolor (MIC: 1.26 mg/mL; MFC: 2.51 mg/mL), A. niger (MIC: 3.77 mg/mL; MFC: 5.02 mg/mL), and P. ochrochloron (MIC: 2.51 mg/mL; MFC: 5.02 mg/mL). However, the other two colorants presented similar results, with the control formulation exhibiting the best MIC and MFC values for P. funiculosum (1.27 and 2.53 mg/mL, respectively) and T. viride (0.91 and 1.27 mg/mL).
2.3. Stability of the Colouring Formulations
To assess the stability of the developed colorants, the anthocyanin concentration and colour parameters were assessed over 12 weeks of storage at refrigerated (3 °C) and room (23 °C) temperatures, to ensure their colouring capacity. Moreover, to guarantee their safety to be applied in food products, their microbial load and cytotoxicity for non-tumour cells were also evaluated. In terms of colour and anthocyanin concentration, the analyses were performed after preparation (t0) and after 4, 8, and 12 weeks of storage. In what concerns the safety assessment, the colouring formulations were analysed after preparation (t0) and after 12 weeks of storage.
2.3.1. Colour and Anthocyanin Concentration
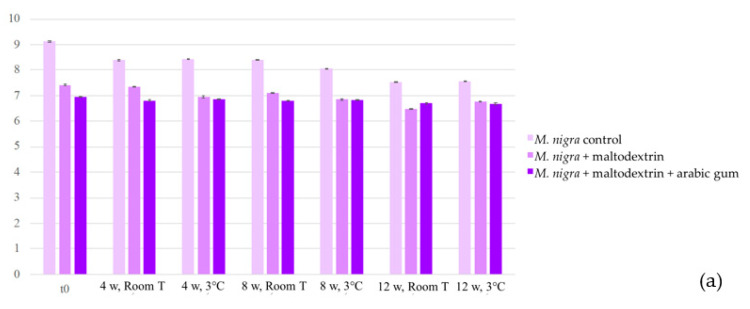
In the analysis performed along the three months of storage, the colouring formulations revealed the same anthocyanin qualitative composition as the extracts (results presented in Section 2.1.4), with M. nigra formulations presenting cyanidin-3-O-glucoside and cyanidin-O-rhamnoside, and R.fruticosus containing cyanidin-O-hexoside, cyanidin-3-O-glucoside, cyanidin-O-pentoside, and cyanidin-3-O-dioxaloylglucoside. Thus, the total anthocyanin concentration was considered to measure these compounds variation along the storage time, both at room and refrigerated temperatures (Figure 2).
Figure 2.
Evolution of anthocyanin concentration (mg/g) of the colouring formulations from M. nigra (a) and R. fruticosus (b). w: weeks, Room T: room temperature.
In previous studies performed by Ferrari et al. [25,28], where blackberry pulps were subjected to spray-drying, this technique also revealed to be effective on anthocyanins preservation. Similar conclusions were achieved with mulberry juice powders, in studies employing different spray-drying parameters [24].
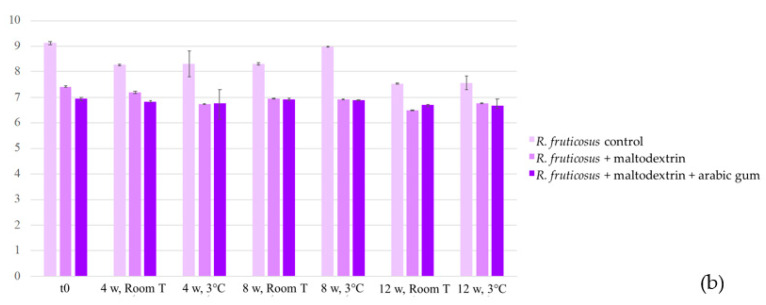
In terms of colour assessment, the parameters luminosity (L*), green/red hue (a*) and yellow/blue hue (b*) were determined and compared (Figure 3).
Figure 3.
Evolution of colour parameters, L*, a*, and b* (CIE L*a*b* units) of the colouring formulations from M. nigra (a) and R. fruticosus (b). w: weeks, Room T: room temperature. In any case, no substantial differences were observed in any of the parameters analysed, either as a function of storage time (up to 12 weeks), or the temperature at which the samples were stored (23 °C or 3 °C).
Considering R. fruticosus, the formulations containing maltodextrin and a mixture of maltodextrin and arabic gum revealed higher values of luminosity and red colour hue, what was not observed in terms of yellow colour. Comparing these results with the ones obtained for the total anthocyanin content, despite the higher concentration in the control formulation (which was expected since the other formulations contain 40% adjuvants), a stronger red hue was verified in the formulations containing maltodextrin and maltodextrin + arabic gum.
Concerning the results obtained for M. nigra formulations, the higher luminosity measured in these samples is remarkable when compared to that observed in the formulations of R. fruticosus. On the other hand, the formulation prepared with maltodextrin + arabic gum was once again the one showing the best results (greater luminosity), although in this case, with more significant differences compared to the formulation prepared only with maltodextrin, which was much better than the control formulation. In terms of intensity of red and yellow hues, and although the values recorded were also higher than those measured in the homologous formulations of R. fruticosus, it was verified that the formulations prepared with adjuvants did not allow to obtain the same colour levels as the control formulation. Finally, the results of anthocyanin levels were practically identical to those quantified in the solid formulations of R. fruticosus.
2.3.2. Microbial Analysis
Evaluation of Pasteurization Efficiency
According to the Food and Drug Administration juices’ rule 66 FR 6137, these beverages must be developed assuring that a reduction of 5-log of the pathogenic microorganism is achieved [29].
From Table 5, it can be seen that the contaminated sample without thermal treatment was also able to reduce the number of microorganisms. This capacity exhibited by these samples can be explained by the colorant´s characteristics, namely the antimicrobial activity provided by these molecules. Nevertheless, it still not being efficient in controlling the growth of these contaminants. Also, it can be stated that the microorganisms were inoculated at 109 cell/mL, but, immediately after the contamination procedure, in some cases they were found at 107–108. This can be due to the adjustment of these microorganisms in the densitometer that also consider dead cells, thus justifying the difference between the inoculated and the real counting’s.
Table 5.
Microbial counts of the analysed samples inoculated with approximately 109 cells/mL of each tested microorganism and submitted to pasteurization (n = 3).
| Initial Counts (before Pasteurization) (log10 CFU/mL) |
Counts after Pasteurization at 80 °C (log10 CFU/mL) |
Counts after Pasteurization at 90 °C (log10 CFU/mL) |
log10 Cycle Reduction in Contaminated Samples without Pasteurization | log10 Cycle Reduction after Pasteurization at 80 °C | log10 Cycle Reduction after Pasteurization at 90 °C | |
|---|---|---|---|---|---|---|
| E. coli | 5.36 ± 0.02 | nd | nd | 3.57 | 8.93 | 8.93 |
| B. cereus | 3.46 ± 0.06 | nd | nd | 3.75 | 7.21 | 7.21 |
| A. parasiticus | 4.44 ± 0.05b | 2.82 ± 0.05a | nd | 4.33 | 5.96 | 8.77 |
| Z. rouxii | 3.59 ± 0.03b | 2.31 ± 0.08a | nd | 4.02 | 5.26 | 7.61 |
nd—not detected; different letters in each line mean significant differences (p < 0.05).
Analysing the applied thermal treatments, it can be observed that at 80 °C, the microorganisms E. coli and B. cereus were apparently completely eliminated, while the yeast and the moulds still presented significative colony counts. On the other hand, when the temperature of 90 °C was applied, none of the microorganisms was detected, meaning that this temperature is the most efficient in the elimination of these microorganisms, allowing the preservation of the developed colorants. Although the samples treated with 80 °C revealed capacity to reduce more than 5-log of the tested microorganisms, the samples treated with 90 °C revealed the highest log reduction without compromising the integrity of the colouring compounds.
Therefore, the pasteurization at 90 °C was selected to prepare the samples for further analysis.
Microbial Load in the Final Juice Samples
The microbial load (log10 CFU/g) of the formulations was assessed to guarantee their safety for consumption (Table 6 and Table 7). The results were analysed through a two-way ANOVA, which allowed an individualized understanding of each factor. Table 6 and Table 7 represent the microbial load of the mesophilic aerobic microorganisms, coliforms, yeasts, and moulds of the R. fruticosus and M. nigra formulations, respectively. The two analysed times (after preparation and after 12 weeks) are represented in the upper part of the table for each storage temperature, while in the lower part, in each of the formulations, both times are included. This allows for a better understanding of the influence of each factor (Formulation or Storage Time) on the outcome.
Table 6.
Microbial analysis (log10 CFU/g) of the colouring formulations of R. fruticosus stored at room and refrigerated temperature for 12 weeks.
| Aerobic Mesophilic Microorganisms |
Coliforms | Yeasts | Moulds | ||
|---|---|---|---|---|---|
| Room temperature | |||||
| Storage time (ST) | 0 weeks | 1 ± 2 | nd | 1 ± 2 | 3.14 ± 0.19 |
| 12 weeks | 1 ± 2 | nd | nd | nd | |
| p-value (n = 15) | Student’s t test | 0.407 | - | <0.001 | <0.001 |
| Formulation (F) | Control | 3.5 ± 0.1 | nd | 2 ± 2 | 2 ± 2 |
| M | nd | nd | nd | 1 ± 2 | |
| M + AG | nd | nd | nd | 2 ± 2 | |
| p-value (n = 10) | Tukey’s HSD test | <0.001 | - | <0.001 | <0.001 |
| ST×F (n = 30) | p-value | 0.498 | - | <0.001 | <0.001 |
| Refrigerated temperature | |||||
| Storage time (ST) | 0 weeks | 1 ± 2 | nd | 1 ± 1 | 3.1 ± 0.2 |
| 12 weeks | 3.4 ± 0.7 | nd | 1 ± 1 | 2.9 ± 0.1 | |
| p-value (n = 15) | Student’s T test | <0.001 | <0.001 | <0.001 | <0.001 |
| Formulation (F) | Control | 3.8 ± 0.2 | nd | 2 ± 2 | 3.2 ± 0.2 |
| M | 2 ± 2 | nd | nd | 2.89 ± 0.08 | |
| M + AG | 1 ± 1 | nd | 1 ± 2 | 3.15 ± 0.06 | |
| p-value (n = 10) | Tukey’s HSD test | <0.001 | - | <0.001 | <0.001 |
| ST × F (n = 30) | p-value | <0.001 | - | <0.001 | <0.001 |
nd—not detected. In each row, for storage time, represents a statistically significant difference between the two time-points of analysis, while for the formulation, different letters mean a statistically significant difference, with a significance of 0.05. The standard deviations presented were calculated using the results obtained in different conditions, and as such, they should not be considered as precision measures, but as a range of values.
Table 7.
Microbial analysis (log10 CFU/g) of the colouring formulations of M. nigra stored at room and refrigerated temperature for 12 weeks.
| Aerobic Mesophilic Microorganisms |
Coliforms | Yeasts | Moulds | ||
|---|---|---|---|---|---|
| Room temperature | |||||
| Storage time (ST) | 0 weeks | 2 ± 2 | nd | 2 ± 1 | 1 ± 1 |
| 12 weeks | 3.0 ± 0.4 | nd | 2 ± 1 | 1 ± 1 | |
| p-value (n = 15) | Student’s T test | <0.001 | - | <0.001 | 0.032 |
| Formulation (F) | Control | 3.7 ± 0.5 | nd | 3.12 ± 0.06 | 2.9 ± 0.1 |
| M | 2.5 ± 0.1 | nd | 3.0 ± 0.3 | nd | |
| M + AG | 2 ± 2 | nd | nd | nd | |
| p-value (n = 10) | Tukey’s HSD test | <0.001 | - | <0.001 | - |
| ST × F (n = 30) | p-value | <0.001 | - | <0.001 | 0.016 |
| Refrigerated temperature | |||||
| Storage time (ST) | 0 weeks | 2 ± 2 | nd | 2 ± 1 | 1 ± 1 |
| 12 weeks | 3.32 ± 0.08 | nd | 2 ± 1 | 2 ± 2 | |
| p-value (n = 15) | Student’s T test | 0.028 | - | 0.114 | 0.942 |
| Formulation (F) | Control | 3.8 ± 0.4 | nd | 3.05 ± 0.09 | 2.9 ± 0.2a |
| M | 2.8 ± 0.5 | nd | 3.0 ± 0.3 | 2 ± 2b | |
| M + AG | 2 ± 2 | nd | nd | nd | |
| p-value (n = 10) | Tukey’s HSD test | <0.001 | - | <0.001 | <0.001 |
| ST × F (n = 30) | p-value | <0.001 | - | <0.001 | 0.855 |
nd—not detected. In each row, for storage time, represents a statistically significant difference between the two time-points of analysis, while for the formulation, different letters mean a statistically significant difference, with a significance of 0.05. The standard deviations presented were calculated using the results obtained in different conditions, and as such, they should not be considered as precision measures, but as a range of values.
Considering R. fruticosus samples stored at room temperature, Table 6 shows that, in addition to the fact that no coliforms were detected in any sample, at any of the times, there was a significant interaction between factors for yeasts and moulds, and some trends can be extracted from the estimated marginal means. It was also possible to verify that, for yeasts, the control registered the presence of these contaminants at t0, however, after three months they were not detected, while, for the samples with maltodextrin, they were not detected at any time. In terms of moulds, it was also clear that, with time, all samples tended to undetected values, although initially, the maltodextrin formulations registered a lower value than the control. Finally, it was possible to verify that the type of formulation was decisive in the case of the mesophilic aerobic microorganisms, since these were only recorded in the control samples, registering a significant difference between them. Regarding storage time, there were no significant differences between t0 and 12 weeks after preparation.
Regarding the samples stored at refrigerated temperature (Table 6), it is possible to verify that there was a significant interaction for both factors in all microorganisms. Unlike the samples stored at room temperature, in the refrigerated formulations a trend to increase over time was evidenced. Although in the samples with maltodextrin there were no values for these microorganisms at t0, in the control samples values between 3 and 4 log10 CFU/g were already evident. In the maltodextrin samples, yeasts and coliforms were not detected at any time of analysis. Comparatively, storage at room temperature seems to be more beneficial in the control of microorganisms, which presence decreased over time, especially for yeasts and moulds.
Regarding M. nigra colouring formulations stored at room temperature (Table 7), it was possible to verify once again that there was a significant interaction between storage time and formulation type, with all microorganisms (ST × F < 0.05). Again, coliforms were not detected in any sample; as for yeasts, no contamination was recorded in the formulation containing maltodextrin with Arabic gum, and in the case of moulds they were not detected in any of the samples containing maltodextrin.
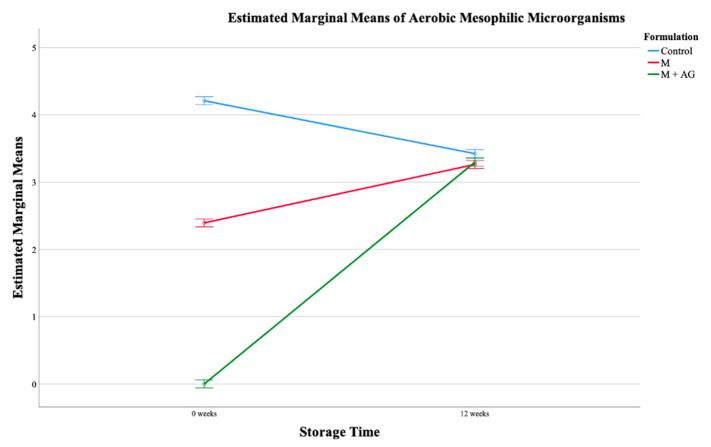
In what concerns the refrigerated M. nigra colorants, a significant interaction was recorded for all microorganisms, which revealed that both time and type of formulation contributed in an interactive manner to the behaviour of the samples, except for moulds, where the influencing parameter was the type of formulation, showing that the formulations containing adjuvants did not present or presented less contamination than the control formulation. From the estimated marginal means, it can be verified that, initially, the mesophilic aerobic microorganisms in the control had a higher count, while the samples that contained maltodextrin did not present these microorganisms, but after 12 weeks all samples showed the same load of these microorganisms (Figure 4).
Figure 4.
Estimated marginal mean plot of the aerobic mesophilic microorganisms for M. nigra at room temperature.
However, after 12 weeks of storage, the control decreased the values of these microorganisms, while both samples with maltodextrin increased. It should be noted that coliforms were not detected in any sample; and in the samples containing maltodextrin and arabic gum, yeasts and moulds were not detected. In addition, regarding moulds, the only sample that had this contaminant in t0 maintained the same microbial load during the 12 weeks, while in the samples with maltodextrin it increased between storage times.
2.3.3. Cytotoxicity
In terms of cytotoxicity, none of the formulations revealed activity against a primary culture of porcine liver cells, which indicates that they are safe for incorporation in foodstuff without presenting any risk for consumers’ health.
3. Materials and Methods
3.1. Samples
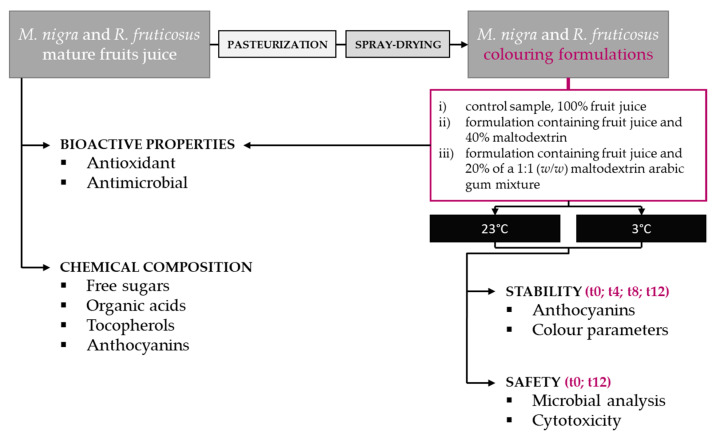
Mature fruits of Rubus fruticosus L. and Morus nigra L. were collected in Trás-os-Montes, Portugal, and provided by “Ponto Agrícola Unipessoal, Lda.” (Baião, Portugal), respectively. They were blended to obtain a juice rich in anthocyanins and the juice was centrifuged, filtered through Whatman No. 4 filter paper, frozen, and lyophilized for further analysis and preparation of the colouring formulations. The juice was reconstituted by dissolving in water to a final concentration of 100 mg/mL. Figure 5 provides an overview of the analyses performed.
Figure 5.
Overview of the analyses performed with the fruits juice and colouring formulations.
3.2. Chemical Composition
3.2.1. Free Sugars
For free sugars assessment, the lyophilized extract was dissolved in water to a final concentration of 100 mg/mL. The analysis was performed according to a procedure previously described by the authors, by HPLC (Knauer, Smartline system 1000, Berlin, Germany) coupled to a refractive index detector (RI detector, Knauer Smartline 2300) [30]. A Eurospher 100-5 NH2 column (250 mm × 4.6 mm, 5 µm, Knauer) was used to achieve the separation at 35 °C, through isocratic elution with acetonitrile/deionized water (70:30, v/v) at a flow rate of 1 mL/min. The results were expressed in mg per g of extract.
3.2.2. Organic Acids
The lyophilized extract was dissolved in water in a concentration of 100 mg/mL and the analysis was performed by ultra-fast liquid chromatography (UFLC) coupled to a photodiode array detector (PDA), through a methodology described by Pereira et al. [31], using a Shimadzu 20A series UFLC (Shimadzu Corporation, Kyoto, Japan). The compounds were separated using a SphereClone reverse phase C18 column (250 mm × 4.6 mm, 5 µm, Phenomenex, Torrance, CA, USA), at 35 °C and a flow rate of 0.8 mL/min of sulphuric acid (3.6 mM), through isocratic elution. The results were expressed in mg per g of extract.
3.2.3. Tocopherols
To assess the tocopherols composition, the lyophilized extract was subjected to an extraction procedure, according to the conditions described by Barros et al. [30]. Briefly, the extract was added with BHT and IS (tocol) solutions, and the mixture was homogenized with ethanol. Hexane and NaCl were added, vortex-mixing after each addition, and the mixture was centrifuged to recover the upper layer. The extract was, then, dried under a nitrogen stream, redissolved in hexane, and filtered through a 0.22 µm disposable LC filter disk. The analysis was achieved by HPLC (Knauer Smartline system 1000) using a FP-2020 fluorescence detector (Jasco, Japan), using a Polyamide II normal-phase column (250 mm × 4.6 mm, 5 µm, YMC Waters, Milford, MA, USA) for compounds separation, with isocratic elution with n-hexane and ethyl acetate (70:30, v/v), at 35 °C and a flow rate of 1 mL/min. The quantification was performed through the internal standard method and the results were expressed in mg per g of extract.
3.2.4. Anthocyanins
For anthocyanins analysis, the extract or colouring formulation was dissolved in water to a final concentration of 5 mg/mL, filtered (0.2 µm), and injected in an HPLC equipment (Dionex Ultimate 3000 UPLC, Thermo Scientific, San Jose, CA, USA) coupled to a diode-array detector (280, 330, 370, and 520 nm wavelengths) and an electrospray ionization mass spectrometer (Linear Ion Trap LTQ XL, Thermo Scientific) working in positive mode, as previously described by Gonçalves et al. [32]. For compounds separation, an AQUA® reverse phase C18 column (5 μm, 150 mm × 4.6 mm, Phenomenex) was used, at 35 °C, using previously described gradients [32]. The anthocyanins determination was performed according to their retention time, UV-Vis and mass spectra, in comparison with authentic standards and using literature data. The quantification was achieved using a seven levels calibration curve obtained for different standard compounds. The results were expressed in mg per g of extract/formulation.
3.3. Bioactive Properties
3.3.1. Antioxidant Properties
Two methods were applied to assess the antioxidant activity of the extracts and colouring formulations. Through the TBARS (thiobarbituric acid reactive substances) assay with porcine (Sus scrofa) brain homogenates, the decrease in TBARS formation was measured to assess the lipid peroxidation inhibition capacity, according to Pereira et al. [33]. The oxidative haemolysis inhibition assay (OxHLIA) was performed with sheep erythrocytes to evaluate the anti-haemolytic activity for 60 and 120 min, following a previously described procedure [34]. In both assays, the results were expressed in µg/mL (EC50 values; extract/formulation concentration required to obtain 50% of antioxidant activity) and Trolox was used as positive control.
3.3.2. Antimicrobial Properties
For the antimicrobial analysis, six fungi (Aspergillus fumigatus (ATCC 1022), Aspergillus versicolor (ATCC 11,730), Aspergillus niger (ATCC 6275), Penicillium funiculosum (ATCC 36,839), Penicillium ochrochloron (ATCC 9112), and Trichoderma viride (IAM 5061)), three Gram-positive (Bacillus cereus (food isolate), Staphylococcus aureus (ATCC 6538), and Listeria monocytogenes (NCTC 7973)), and three Gram-negative (Escherichia coli (ATCC 35,210), Enterobacter cloacae (human isolate), and Salmonella Typhimurium (ATCC 13,311)) bacteria were used. The antifungal and antimicrobial activities were assessed according to Soković et al. [35,36] and the results were expressed as minimum inhibitory (MIC), minimum bactericidal (MBC), and minimum fungicidal (MFC) concentrations, in mg/mL. As positive controls, ketoconazole and bifonazole were used for fungi and streptomycin and ampicillin for the bacterial strains.
3.4. Colouring Formulations
3.4.1. Pasteurization
The lyophilized extract was dissolved in water to a final concentration of 100 mg/mL and was submitted to a pasteurization procedure, previously described by Molina et al. [37]. Briefly, four hermetic bags containing the dissolved extract were maintained in a water bath at 90 °C for 60 s and were subsequently cooled in ice until achieving 3 °C. One of these bags was used to evaluate the microbial load, as described in Section 3.5.2., and the remaining samples were stored at 3 °C for ~15 h for further spray-drying.
3.4.2. Spray-Drying
Three formulations were prepared by spray-drying: (i) a control sample, consisting of 100% fruit juice; (ii) a formulation containing fruit juice added with 40% maltodextrin; and (iii) a formulation composed of fruit juice added with 20% of a 1:1 (w/w) maltodextrin arabic gum mixture. The referred percentages were selected after testing different contents of maltodextrin and maltodextrin + arabic gum (1:1, w/w), and are expressed as the ratio of the drying adjuvants weight relative to the total solids weight in the prepared juice (100 mg/mL). These formulations were spray-dried according to the procedure of Moser et al. [38], analysing the efficiency of the drying process and the suitability of the used drying coadjutants, maltodextrin and arabic gum. The solutions were prepared immediately before atomization by mixing the fruit juice with the selected materials under stirring at room temperature for 10 min. A Mini Spray Dryer B-290 (Büchi, Flawil, Switzerland) programmed in the normal operation mode (nozzle diameter: 0.7 mm; atomized volume: 200 mL, solids content < 33%) was used. The optimized operation conditions were an inlet temperature of 140 °C, an outlet temperature of 72 °C, an aspiration of 90%, with the pump working at 20% (6 mL/min). To calculate the yield of the process, expressed in percentage, the ratio between the obtained powder weight and the initial solution’s total solids weight (dry-basis), was considered.
3.5. Stability of the Colouring Formulations
To assess the stability of the prepared formulations, after the spray-drying process, a portion of each powdered sample was separated to perform the required analyses immediately after preparation (t0) and the remaining quantity was divided into 2 equal portions to be stored at room (23 °C) and refrigerated (3 °C) temperatures, in sterile flasks protected from light. All the samples were analysed in order to evaluate the variation of their anthocyanin concentration and colour parameters after 4, 8, and 12 weeks of storage (comparing with t0), as well as their safety in what concerns the microbial load and cytotoxic properties at t0 and after 12 weeks of storage.
3.5.1. Anthocyanin Concentration and Colour Parameters
To evaluate the concentration of anthocyanins in the different formulations, stored at distinct temperatures, these were dissolved in water to a final concentration of 5 mg/mL and were analysed as described in Section 3.2.4.
In what concerns the colour parameters, a colourimeter (model CR-400, Konica Minolta Sensing, Inc., Osaka, Japan) equipped with a specific tool for granular materials (model CR-A50) was used to analyse the powders, as reported by the authors Pereira et al. [39]. For that purpose, the colour parameters were obtained in the CIE L*a*b* colour space, through the illuminant C with a diaphragm aperture of 8 mm, and the Spectra Magic Nx software (version CM-S100W 2.03.0006, Konica Minolta) was used to analyse the obtained data.
3.5.2. Microbial Analysis
3.5.2.1. Determination of the Pasteurization Procedure
The ideal pasteurization conditions were established after a microbial contamination procedure that allowed to choose the optimal conditions capable of reducing “pertinent microorganisms” in at least 5-log cycles, as determined by the Food and Drug Administration rule 66 FR 6137 for fruit juices [29]. For this, juice samples (10 mL, in triplicate) were contaminated with a mix of Escherichia coli, Bacillus cereus, Aspergillus parasiticus, and Zygosaccharomyces rouxii (provided by the Mountain Research Centre of the Polytechnic Institute of Bragança, Bragança, Portugal), with approximately 109 cell/mL of each microorganism. The contamination process was performed as described by Fernandes et al. [40]. The samples were then submitted to two pasteurization procedures: 90 °C for 60 s and 80 °C for 60 s (following the procedure described in Section 3.4). The contaminated samples were analysed for microbial load immediately before and after pasteurization. The viable cells were further assessed according to the described in Section 3.5.2.2.
3.5.2.2. Microbial Load of the Final Colouring Formulations Subjected to the Chosen Pasteurization Conditions
The microbiological analysis was assessed by mixing 1 g of the powdered samples with 9 mL of peptone water. These solutions were diluted until achieving 10−6 and the following counts were performed, as previously described by Molina et al. [37]: aerobic plate count (total viable count; ISO 4833-2:2013), coliforms (and Escherichia coli; ISO 4832:2006), yeasts and moulds (ISO 21527-1/2:2008), and Bacillus cereus (ISO 7932:2004).
3.5.3. Cytotoxicity
The cytotoxicity in non-tumour cells was performed by monitoring the cells growth using a phase contrast microscope. Briefly, a primary culture of porcine liver non-tumour cells was sub-cultured and plated in 96 well plates (density of 1.0 × 104 cells/well) with the culture medium Dulbecco’s modified Eagle’s medium (DMEM) containing FBS (10%), penicillin (100 U/mL), and streptomycin (100 μg/mL), according to the protocol described by Abreu et al. [41]. The results were expressed in μg/mL (GI50 values; formulation concentration required to inhibit 50% of the net cell growth) and ellipticine was used as positive control.
3.6. Statistical Analysis
Three samples were assessed for each analysis, in assays carried out in triplicate and the results were presented as mean values and standard deviation (SD). The results were treated using Student’s t-test at a 5% significance level or one-way analysis of variance (ANOVA) post-hoc Tukey. A Two-way ANOVA was used for the assessment of the stability of the formulations. The analyses were performed using the SPSS v.22.0 program (IBM SPSS version 22.0, IBM Corp., Armonk, NY, USA) using a significance of 0.05.
4. Conclusions
Morus nigra L. and Rubus fruticosus L. fruit juices were assessed for their chemical composition, namely in what concerns free sugars, organic acids, tocopherols, and anthocyanins. The samples revealed six sugars, two organic acids, and the four isoforms of tocopherols. In M. nigra, two anthocyanins were detected, cyanidin-3-O-glucoside and cyanidin-O-rhamnoside, whereas in R. fruticosus, four distinct cyanidin derivatives were identified, namely cyanidin-O-hexoside, cyanidin-3-O-glucoside, cyanidin-O-pentoside, and cyanidin-3-O-dioxaloilglucoside. The fruit juices were also analysed in terms of antioxidant and antimicrobial properties, revealing a strong bioactivity. Moreover, given their richness in anthocyanin compounds, the fruit juices were also used to prepare solid colorants for application in food industry. Three formulations were obtained through the spray-drying technique for each fruit, and their stability was assessed over 12 weeks of storage at room and refrigerated temperatures. In general, the colorants revealed a great and stable colouring capacity over time, without toxicity for non-tumour cells and microbial loads within the values acceptable for food. Thus, these fruits can be considered as good natural sources of anthocyanins to be used as natural colorants, not only in food industry, but also in pharmaceuticals, cosmetics, or textiles, among others.
Acknowledgments
The authors are grateful to the “Ponto Agrícola-Unipessoal, Lda.” company for the supply of samples.
Author Contributions
Conceptualization, C.P., I.C.F.R.F. and L.B.; Formal analysis, E.N.V., A.K.M., C.P., M.I.D., S.A.H., P.R., I.P.F., M.F.B., D.S., M.S., M.C. and J.C.M.B.; Investigation, E.N.V., A.K.M., C.P. and L.B.; Methodology, C.P., M.I.D., P.R. and L.B.; Resources, L.B.; Supervision, C.P., I.C.F.R.F. and L.B.; Writing—original draft, A.K.M., C.P. and M.I.D.; Writing—review & editing, S.A.H., P.R., M.F.B., D.S., M.S., J.C.M.B., I.C.F.R.F. and L.B. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful to the Foundation for Science and Technology (FCT, Portugal) for financial support through national funds FCT/MCTES to CIMO (UIDB/00690/2020); national funding by F.C.T. and P.I., through the institutional scientific employment program-contract for C.P., M.I.D., I.P.F., and L.B. contracts, through the individual scientific employment program-contract for S.A.H., J.C.M.B., and M.C., and A.K.M. PhD grant (2020.06231.BD). To FEDER-Interreg España-Portugal programme for financial support through the project TRANSCoLAB 0612_TRANS_CO_LAB_2_P; to ERDF through the Regional Operational Program North 2020, within the scope of project Mobilizador Norte-01-0247-FEDER-024479: ValorNatural® and Project GreenHealth-Norte-01-0145-FEDER-000042. The authors are grateful to the Ministry of Education, Science and Technological Development of Serbia (Grant No. 451-03-9/2021-14/200007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lakshmi C.G. Food coloring: The natural way. Res. J. Chem. Sci. Res. J. Chem. Sci. 2014;4:2231–2606. [Google Scholar]
- 2.Martins N., Roriz C.L., Morales P., Barros L., Ferreira I.C.F.R. Food colorants: Challenges, opportunities and current desires of agro-industries to ensure consumer expectations and regulatory practices. Trends Food Sci. Technol. 2016;52:1–15. doi: 10.1016/j.tifs.2016.03.009. [DOI] [Google Scholar]
- 3.Özgen M., Serçe S., Kaya C. Phytochemical and antioxidant properties of anthocyanin-rich Morus nigra and Morus rubra fruits. Sci. Hortic. (Amst.) 2009;119:275–279. doi: 10.1016/j.scienta.2008.08.007. [DOI] [Google Scholar]
- 4.Zia-Ul-Haq M., Riaz M., De Feo V., Jaafar H.Z.E., Moga M. Rubus fruticosus L.: Constituents, biological activities and health related uses. Molecules. 2014;19:10998–11029. doi: 10.3390/molecules190810998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tonioni G., Santos T.R., Macedo R., Peters V.M., Narciso M., de da Silveira R.C., de Guerra M.O. Efficacy of Morus nigra L. on reproduction in female Wistar rats. Food Chem. Toxicol. 2011;50:816–822. doi: 10.1016/j.fct.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Chen H., Pu J., Liu D., Yu W., Shao Y., Yang G., Xiang Z., He N. Anti-inflammatory and antinociceptive properties of flavonoids from the fruits of black mulberry (Morus nigra L) PLoS ONE. 2016;11:e0153080. doi: 10.1371/journal.pone.0153080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kutlu T., Durmaz G., Ateş B., Yilmaz I., Çetin M.Ş. Antioxidant properties of different extracts of black mulberry (Morus nigra L.) Turkish J. Biol. 2011;35:103–110. [Google Scholar]
- 8.Pawlowska A.M., Oleszek W., Braca A. Quali-quantitative analyses of flavonoids of Morus nigra L. and Morus alba L. (Moraceae) fruits. J. Agric. Food Chem. 2008;56:3377–3380. doi: 10.1021/jf703709r. [DOI] [PubMed] [Google Scholar]
- 9.Costa G.R. Efeito de Extratos Ricos em Antocianinas ou Elagitaninos de Amora Silvestre (Morus nigra L.), Amora Preta (Rubus spp), e Grumixama (Eugenia brasiliensis Lam) no Crescimento e em Marcas Epigenéticas, (Tesis doctoral) Universidade de São Paulo; São Paulo, Brasil: 2015. [Google Scholar]
- 10.Ercisli S., Orhan E. Some physico-chemical characteristics of black mulberry (Morus nigra L.) genotypes from Northeast Anatolia region of Turkey. Sci. Hortic. (Amst.) 2008;116:41–46. doi: 10.1016/j.scienta.2007.10.021. [DOI] [Google Scholar]
- 11.Sánchez-Salcedo E.M., Sendra E., Carbonell-Barrachina Á.A., Martínez J.J., Hernández F. Fatty acids composition of Spanish black (Morus nigra L.) and white (Morus alba L.) mulberries. Food Chem. 2016;190:566–571. doi: 10.1016/j.foodchem.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 12.Ercisli S., Orhan E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007;103:1380–1384. doi: 10.1016/j.foodchem.2006.10.054. [DOI] [Google Scholar]
- 13.Huang W.Y., Zhang H.C., Liu W.X., Li C.Y. Survey of antioxidant capacity and phenolic composition of blueberry, blackberry, and strawberry in Nanjing. J. Zhejiang Univ. Sci. B. 2012;13:94–102. doi: 10.1631/jzus.B1100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milivojević J., Maksimović V., Nikolić M., Bogdanović J., Maletić R., Milatović D. Chemical and antioxidant properties of cultivated and wild Fragraria and Rubus berries. J. Food Qual. 2011;34:1–9. doi: 10.1111/j.1745-4557.2010.00360.x. [DOI] [Google Scholar]
- 15.Gundogdu M., Muradoglu F., Sensoy R.I.G., Yilmaz H. Determination of fruit chemical properties of Morus nigra L., Morus alba L. and Morus rubra L. by HPLC. Sci. Hortic. (Amst.) 2011;132:37–41. doi: 10.1016/j.scienta.2011.09.035. [DOI] [Google Scholar]
- 16.Akšić M.F., Tosti T., Sredojević M., Milivojević J., Meland M., Natić M. Comparison of sugar profile between leaves and fruits of blueberry and strawberry cultivars grown in organic and integrated production system. Plants. 2019;8:205. doi: 10.3390/plants8070205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Palonen P., Buszard D., Donnelly D. Two approaches to in vitro screening of raspberry cultivars for cold hardiness. Acta Hortic. 1999;505:191–197. doi: 10.17660/ActaHortic.1999.505.24. [DOI] [Google Scholar]
- 18.Vara A.L., Pinela J., Dias M.I., Petrović J., Nogueira A., Soković M., Ferreira I.C.F.R., Barros L. Compositional features of the “Kweli” red raspberry and its antioxidant and antimicrobial activities. Foods. 2020;9:1522. doi: 10.3390/foods9111522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koyuncu F. Organic acid composition of native black mulberry fruit. Chem. Nat. Compd. 2004;40:367–369. doi: 10.1023/B:CONC.0000048249.44206.e2. [DOI] [Google Scholar]
- 20.Kafkas E., Koşar M., Türemiş N., Başer K.H.C. Analysis of sugars, organic acids and vitamin C contents of blackberry genotypes from Turkey. Food Chem. 2006;97:732–736. doi: 10.1016/j.foodchem.2005.09.023. [DOI] [Google Scholar]
- 21.Wajs-Bonikowska A., Stobiecka A., Bonikowski R., Krajewska A., Sikora M., Kula J. A comparative study on composition and antioxidant activities of supercritical carbon dioxide, hexane and ethanol extracts from blackberry (Rubus fruticosus) growing in Poland. J. Sci. Food Agric. 2017;97:3576–3583. doi: 10.1002/jsfa.8216. [DOI] [PubMed] [Google Scholar]
- 22.Wu X., Prior R.L. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J. Agric. Food Chem. 2005;53:2589–2599. doi: 10.1021/jf048068b. [DOI] [PubMed] [Google Scholar]
- 23.Arfan M., Khan R., Rybarczyk A., Amarowicz R. Antioxidant Activity of Mulberry Fruit Extracts. Int. J. Mol. Sci. 2012;13:2472–2480. doi: 10.3390/ijms13022472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Do H.T.T., Nguyen H.V.H. Effects of Spray-Drying Temperatures and Ratios of Gum Arabic to Microcrystalline Cellulose on Antioxidant and Physical Properties of Mulberry Juice Powder. Beverages. 2018;4:101. doi: 10.3390/beverages4040101. [DOI] [Google Scholar]
- 25.Ferrari C.C., Marconi Germer S.P., Alvim I.D., de Aguirre J.M. Storage Stability of Spray-Dried Blackberry Powder Produced with Maltodextrin or Gum Arabic. Dry. Technol. 2013;31:470–478. doi: 10.1080/07373937.2012.742103. [DOI] [Google Scholar]
- 26.Četojević-Simin D.D., Ranitović A.S., Cvetković D.D., Markov S.L., Vinčić M.N., Djilas S.M. Bioactivity of blackberry (Rubus fruticosus L.) pomace: Polyphenol content, radical scavenging, antimicrobial and antitumor activity. Acta Period. Technol. 2017;48:63–76. doi: 10.2298/APT1748063C. [DOI] [Google Scholar]
- 27.Khalid N., Atiq Fawad S., Ahmed I. Antimicrobial Activity, Phytochemical Profile and Trace Minerals of Black Mulberry (Morus nigra L.) Fresh Juice. Pak. J. Bot. 2001;3:91–96. [Google Scholar]
- 28.Ferrari C.C., Germer S.P.M., de Aguirre J.M. Effects of Spray-Drying Conditions on the Physicochemical Properties of Blackberry Powder. Dry. Technol. 2012;30:154–163. doi: 10.1080/07373937.2011.628429. [DOI] [Google Scholar]
- 29.Food and Drug Administration (FDA) Federal Register: Hazard Analysis and Critical Control Point (HAACP); Procedures for the Safe and Sanitary Processing and Importing of Juice. [(accessed on 12 April 2021)]; Available online: https://www.federalregister.gov/documents/2001/01/19/01-1291/hazard-analysis-and-critical-control-point-haacp-procedures-for-the-safe-and-sanitary-processing-and.
- 30.Barros L., Pereira E., Calhelha R.C., Dueñas M., Carvalho A.M., Santos-Buelga C., Ferreira I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods. 2013;5:1732–1740. doi: 10.1016/j.jff.2013.07.019. [DOI] [Google Scholar]
- 31.Pereira C., Carvalho A.M., Barros L., Ferreira I.C.F.R.F.R. Use of UFLC-PDA for the analysis of organic acids in thirty-five species of food and medicinal plants. Food Anal. Methods. 2013;6:1337–1344. doi: 10.1007/s12161-012-9548-6. [DOI] [Google Scholar]
- 32.Gonçalves G.A., Soares A.A., Correa R.C.G., Barros L., Haminiuk C.W.I., Peralta R.M., Ferreira I.C.F.R., Bracht A. Merlot grape pomace hydroalcoholic extract improves the oxidative and inflammatory states of rats with adjuvant-induced arthritis. J. Funct. Foods. 2017;33:408–418. doi: 10.1016/j.jff.2017.04.009. [DOI] [Google Scholar]
- 33.Pereira C., Calhelha R.C., Barros L., Queiroz M.J.R.P., Ferreira I.C.F.R. Synergisms in antioxidant and anti-hepatocellular carcinoma activities of artichoke, milk thistle and borututu syrups. Ind. Crops Prod. 2014;52:709–713. doi: 10.1016/j.indcrop.2013.11.050. [DOI] [Google Scholar]
- 34.Lockowandt L., Pinela J., Roriz C.L., Pereira C., Abreu R.M.V., Calhelha R.C., Alves M.J., Barros L., Bredol M., Ferreira I.C.F.R. Chemical features and bioactivities of cornflower (Centaurea cyanus L.) capitula: The blue flowers and the unexplored non-edible part. Ind. Crops Prod. 2019;128:496–503. doi: 10.1016/j.indcrop.2018.11.059. [DOI] [Google Scholar]
- 35.Soković M., Glamočlija J., Marin P.D., Brkić D., Griensven L.J.L.D. van Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532–7546. doi: 10.3390/molecules15117532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Soković M., Van Griensven L.J.L.D. Antimicrobial activity of essential oils and their components against the three major pathogens of the cultivated button mushroom. Agaricus bisporus. Eur. J. Plant Pathol. 2006;116:211–224. doi: 10.1007/s10658-006-9053-0. [DOI] [Google Scholar]
- 37.Molina A.K., Vega E.N., Pereira C., Dias M.I., Heleno S.A., Rodrigues P., Fernandes I.P., Barreiro M.F., Kostić M., Soković M., et al. Promising antioxidant and antimicrobial food colourants from Lonicera caerulea L. var. Kamtschatica. Antioxidants. 2019;8:394. doi: 10.3390/antiox8090394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moser P., De Souza R.T., Nicoletti Telis V.R. Spray drying of grape juice from hybrid CV. BRS Violeta: Microencapsulation of anthocyanins using protein/maltodextrin blends as drying aids. J. Food Process. Preserv. 2017;41:e12852. doi: 10.1111/jfpp.12852. [DOI] [Google Scholar]
- 39.Pereira E., Antonio A.L., Barreira J.C.M., Barros L., Bento A., Ferreira I.C.F.R. Gamma irradiation as a practical alternative to preserve the chemical and bioactive wholesomeness of widely used aromatic plants. Food Res. Int. 2015;67:338–348. doi: 10.1016/j.foodres.2014.11.047. [DOI] [Google Scholar]
- 40.Fernandes F.A., Carocho M., Heleno S.A., Rodrigues P., Dias M.I., Pinela J., Prieto M.A., Simal-Gandara J., Barros L., Ferreira I.C.F.R. Effect of Natural Preservatives on the Nutritional Profile, Chemical Composition, Bioactivity and Stability of a Nutraceutical Preparation of Aloe arborescens. Antioxidants. 2020;9:281. doi: 10.3390/antiox9040281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abreu R.M.V., Ferreira I.C.F.R., Calhelha R.C., Lima R.T., Vasconcelos M.H., Adega F., Chaves R., Queiroz M.J.R.P. Anti-hepatocellular carcinoma activity using human HepG2 cells and hepatotoxicity of 6-substituted methyl 3-aminothieno[3,2-b]pyridine-2-carboxylate derivatives: In vitro evaluation, cell cycle analysis and QSAR studies. Eur. J. Med. Chem. 2011;46:5800–5806. doi: 10.1016/j.ejmech.2011.09.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in this article.