Abstract
Simple Summary
The incidence of esophageal cancer is constantly rising and patients are often diagnosed at an advanced stage. Surgical resection, if possible, is the curative treatment of choice. However, esophagectomy for cancer is a major surgical procedure and is associated with perioperative morbidity. Preoperative staging examinations are carried out on every patient, and imaging datasets contain valuable information about the patient’s physical condition beyond the routinely assessed tumor extent. In this study, the abdominal muscle and fat mass were quantified during the preoperative staging and postoperative follow-up of 85 patients with locally advanced esophageal adenocarcinoma, and these imaging biomarkers were correlated with surgical complications and patient outcomes. Our analysis showed that sarcopenic patients with low muscle mass were more likely to have major complications and that hospitalization was prolonged, especially in patients with sarcopenic obesity. Low preoperative muscle mass and its decrease during the follow-up also predicted poorer overall survival.
Abstract
Background: To assess the impact of body composition imaging biomarkers in computed tomography (CT) on the perioperative morbidity and survival after surgery of patients with esophageal cancer (EC). Methods: Eighty-five patients who underwent esophagectomy for locally advanced EC after neoadjuvant therapy between 2014 and 2019 were retrospectively enrolled. Pre- and postoperative CT scans were used to assess the body composition imaging biomarkers (visceral (VAT) and subcutaneous adipose tissue (SAT) areas, psoas muscle area (PMA) and volume (PMV), total abdominal muscle area (TAMA)). Sarcopenia was defined as lumbar skeletal muscle index (LSMI) ≤38.5 cm2/m2 in women and ≤52.4 cm2/m2 in men. Patients with a body mass index (BMI) of ≥30 were considered obese. These imaging biomarkers were correlated with major complications, anastomotic leakage, postoperative pneumonia, duration of postoperative hospitalization, disease-free survival (DFS), and overall survival (OS). Results: Preoperatively, sarcopenia was identified in 58 patients (68.2%), and sarcopenic obesity was present in 7 patients (8.2%). Sarcopenic patients were found to have an elevated risk for the occurrence of major complications (OR: 2.587, p = 0.048) and prolonged hospitalization (32 d vs. 19 d, p = 0.040). Patients with sarcopenic obesity had a significantly higher risk for postoperative pneumonia (OR: 6.364 p = 0.018) and a longer postoperative hospital stay (71 d vs. 24 d, p = 0.021). Neither sarcopenia nor sarcopenic obesity was an independent risk factor for the occurrence of anastomotic leakage (p > 0.05). Low preoperative muscle biomarkers (PMA and PMV) and their decrease (ΔPMV and ΔTAMA) during the follow-up period significantly correlated with shorter DFS and OS (p = 0.005 to 0.048). Conclusion: CT body composition imaging biomarkers can identify high-risk patients with locally advanced esophageal cancer undergoing surgery. Sarcopenic patients have a higher risk of major complications, and patients with sarcopenic obesity are more prone to postoperative pneumonia. Sarcopenia and sarcopenic obesity are both subsequently associated with a prolonged hospitalization. Low preoperative muscle mass and its decrease during the postoperative follow-up are associated with lower DFS and OS.
Keywords: computed tomography, body composition, sarcopenia, sarcopenic obesity, esophageal cancer, surgery
1. Introduction
Esophageal cancer (EC) is the eighth most common cancer globally, occurs more frequently in men, and has an unfavorable prognosis with the sixth highest mortality rate [1,2,3]. The overall incidence of EC has constantly been rising over the past decades as many associated habits have been on the rise in the general population [4,5]. Symptoms occur late, and patients typically have advanced EC at the time of diagnosis [6]. If general operability is given, surgical resection remains the best curative treatment option after neoadjuvant treatment for locally advanced cancers [7,8,9,10]. However, careful patient selection with an upfront assessment of the operative risk is necessary to improve surgical outcomes [11]. The most common surgical technique is a total minimally invasive esophagectomy or open esophagogastrostomy [8]. Surgical removal of EC is an extensive operation with a range of peri- and postoperative complications, including anastomotic leakage, bleeding, and postoperative pneumonia [12,13,14,15].
Assessment of body composition based on computed tomography (CT) has been evaluated in various groups of patients to assess the possible effects of sarcopenia on patient outcome. Research in the field of body composition was initially focused primarily on patients with cardiovascular diseases, but soon shifted to cancer patients [16]. In cancer patients, sarcopenia has moved into the spotlight over recent decades, and several studies have shown that pretherapeutic sarcopenia is associated with poor outcomes after subsequent cancer treatment [17]. There have been several attempts to assess sarcopenia in cancer patients using a variety of conventional methods such as BMI, waist-to hip-ratio, bio-impedance analysis (BIA), and imaging-based techniques like dual-energy X-ray absorptiometry, MRI, and CT [17,18,19,20,21]. CT-based assessment of sarcopenia commonly relies on quantification of the skeletal muscle mass [18].
Although every patient undergoes CT imaging as part of staging prior to surgery of esophageal cancer, assessment of the CT dataset is mostly limited to direct, cancer-related aspects such as tumor extent and presence of metastases [22]. The valuable information CT images provide on body composition and potential predictors of patient fitness has not been used in routine clinical practice.
Thus, the purpose of this study was to evaluate CT body composition imaging biomarkers as potential predictors of perioperative morbidity and postoperative outcome in patients undergoing surgery for locally advanced EC.
2. Material and Methods
2.1. Patients and Clinical Data
The Department of Surgery’s database was searched for patients with locally advanced esophageal adenocarcinoma who underwent oncological esophagectomy between January 2014 and January 2019, with CT imaging data available as indicated below. A total of 85 consecutive patients were found to be eligible to be included in this retrospective study. The following clinical data of these patients were retrieved: basic patient information (age, sex, body weight and height), surgical technique (open, laparoscopic, hybrid or robotic), preoperative chemotherapy +/− radiation therapy (yes/no), UICC stage, postoperative complications (overall, major complications, anastomotic leakage, pneumonia), duration of postoperative hospitalization, disease-free survival and overall survival.
Minimally invasive Ivor Lewis resection comprised total minimal invasive operations, hybrid procedures (with one part of the operation being performed as an open procedure), and total minimally invasive robotic resections through an abdominal and right-thoracic approach. Our standard minimally invasive approach was the total minimally invasive procedure and reasons for a hybrid approach were additional abdominal organ resection, status post extensive previous abdominal surgery, demand for D3 lymphadenectomy or extensive intra-abdominal adhesions (for abdominal open procedures), or extensive pleural adhesions, suspected T4 situations (except for concomitant VATS lung resections) or bulky lymphatic involvement at the tracheal bifurcation (for open thoracic procedures). Despite the difference in approaches, the surgical technique was standardized between groups and performed as described previously [23,24]. All operations were performed by two consultant surgeons. A major intraoperative and postoperative complication was defined as a surgical or medical complication with a Clavien−Dindo grade of II or higher [25].
Exclusion criteria were insufficient clinical data, postoperative histology other than esophageal adenocarcinoma, and patient age <18 years.
2.2. Imaging
Preoperative CT scans, those obtained after completion of neoadjuvant therapy and before surgery were used for analysis. Follow-up CT scans of the patients were selected postoperatively over a period of two months to two years. If several examinations were available during this period, one from the first year after surgery was chosen if possible. All CT examinations used for body composition analysis included a complete CT staging examination of the chest, abdomen, and pelvis. For each patient one preoperative and one postoperative CT dataset were selected for analysis. CT scans with insufficient image quality that might degrade body composition analysis were excluded.
2.3. CT Body Composition Analysis
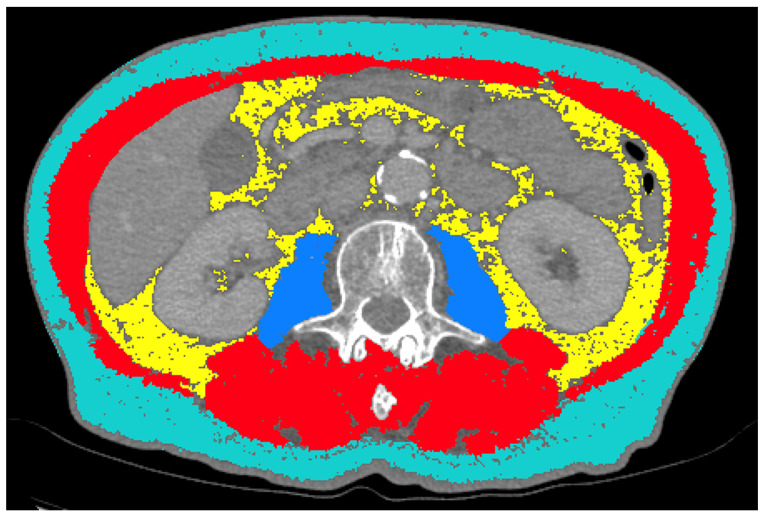
DICOM (Digital Imaging and Communications in Medicine) datasets of each CT examination were extracted from the institutional PACS (Picture Archiving and Communication System), anonymized and transferred to the specific analysis tools. For analysis, the thinnest available slices were selected (ranging from 0.625 mm to 5 mm). A 2D segmentation was performed using the sliceOmatic semi-automatic segmentation tool (v5.0, Tomovision, Magog, QC, Canada). A representative, axial image of the abdomen at the mid-level of the L3 vertebra was identified in each patient and transferred to the workbench. Semiautomated segmentation of the following tissues was performed on single slice images: psoas muscle area (PMA), total abdominal muscle area (TAMA), visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) (Figure 1).
Figure 1.
Example of semiautomated segmentations of subcutaneous adipose tissue (SAT; turquoise), visceral adipose tissue (VAT; yellow), psoas muscle area (PMA; blue) and total abdominal muscle area (TAMA, red + blue) at the L3 level.
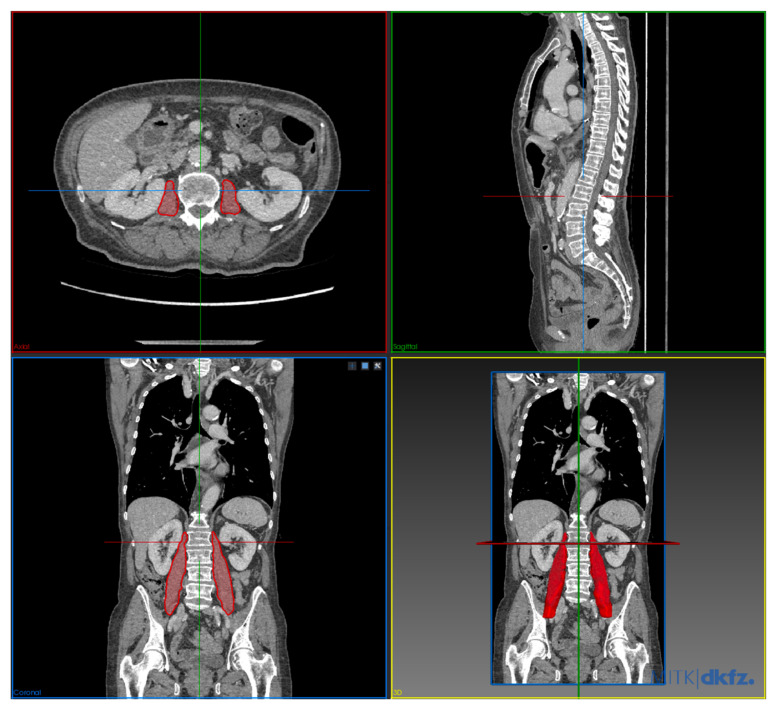
Volumetry/3D segmentation of the psoas muscles was performed using the Medical Imaging Interaction Toolkit (MITK, German Cancer Research Center, Heidelberg, Germany). Separate segmentations for both psoas muscles were performed manually using the polygonal region of interest (ROI) tool. The segmentation is based on the planimetry method. Segmentation of the muscles was performed between the upper border of the L1 vertebra to the lower border of the S2 vertebra (Figure 2).
Figure 2.
Example of manual 3D segmentation of psoas muscle volume in multiplanar reformation and 3D reconstruction.
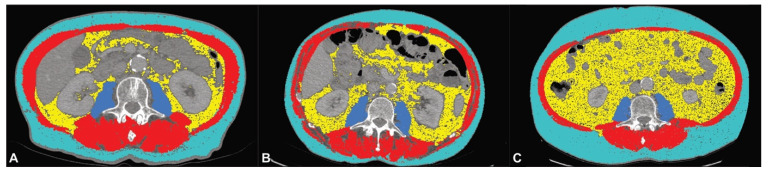
All segmentations were refined by a radiologist with >5 years of experience in abdominal imaging. Psoas muscle volume was normalized by the formula: (Volume Right Psoas Muscle + Volume Left Psoas Muscle)/2. The abdominal adipose tissue ratio (ATR) was calculated by the formula: VAT/SAT. Relative changes in body composition parameters between the preoperative and postoperative follow-up scans were calculated by the formula: (ParameterFollow-Up − ParameterPreoperative)/ParameterPreoperative × 100. To determine whether a patient had sarcopenia, the lumbar skeletal muscle index at L3 (LSMI) was calculated by normalizing the TAMA by the patient’s height according to the formula: TAMA/body height 2. Patients with LSMI values ≤38.5 cm2/m2 for women and ≤52.4 cm2/m2 for men were classified as having sarcopenia, as previously published [26]. Sarcopenic obesity was defined as sarcopenia according to LSMI values in combination with evidence of obesity (BMI ≥ 30) [27] (Figure 3).
Figure 3.
Example segmentations of patients without sarcopenia (A), sarcopenia (B) and sarcopenic obesity (C).
2.4. Statistical Analysis
Statistical analysis was performed using SPSS (version 25; IBM Corporation, Armonk, NY, USA) and Stata (version 17, StataCorp LLC, College Station, TX, USA). Normal distribution of the data was tested by Kolmogorov−Smirnoff test. According to the distribution of the variables, parametric or nonparametric tests were used for further analysis. Independent t-test (nonparametric: Mann−Whitney U-test) was used to identify statistical differences between the means of two groups and Pearson’s Chi-square test was used for categorical variables, respectively. Correlation analysis was performed using Pearson correlation (nonparametric: Spearman rank correlation). Uni- and multivariate regression analyses were performed to calculate odds ratios (OR) and to identify independent predictors. The Cox proportional-hazards model was used to investigate the association between the survival time of patients and the predictor variables. Results were considered statistically significant when p < 0.05.
3. Results
The patients’ characteristics are summarized in Table 1. Survival data (DFS and OS) was available for 76 patients (89.4%). There were no statistically significant correlations between sarcopenia or sarcopenic obesity and surgical technique (p = 0.631 and 0.958) or UICC stage (p = 0.631 and 0.961).
Table 1.
Patient characteristics.
| Characteristic | Factor | Number (%)/Mean (SD) | Range |
|---|---|---|---|
| Sex | Female | 10 (11.8%) | |
| Male | 75 (88.2%) | ||
| Age (years) | 64.3 (9.8) | 45–83 | |
| BMI | 26.79 (4.01) | 16–40 | |
| Surgical technique | Open | 8 (9.4%) | |
| Laparoscopic | 51 (60%) | ||
| Hybrid | 13 (15.3%) | ||
| Robotic | 13 (15.3%) | ||
| UICC stage (missing information in n = 3 patients) | I | 7 (8.2%) | |
| II | 18 (21.2%) | ||
| III | 49 (57.6%) | ||
| IV | 8 (9.4%) | ||
| Neoadjuvant chemotherapy | No | 0 (0.0%) | |
| Yes | 85 (100.0%) | ||
| Neoadjuvant chemoradiotherapy | No | 67 (78.8%) | |
| Yes | 18 (21.2%) | ||
| Complications (overall) | No | 24 (28.2%) | |
| Yes | 61 (71.8%) | ||
| Major complications | No | 40 (47.1%) | |
| Yes | 45 (52.9%) | ||
| Anastomotic leakage | No | 74 (87.1%) | |
| Yes | 11 (12.9%) | ||
| Pneumonia | No | 58 (68.2%) | |
| Yes | 27 (31.8%) | ||
| Hospitalization (d) | 27.75 (38.75) | 10–261 | |
| DFS (months) | 11.51 (13.01) | 0–61 | |
| OS | 14.38 (13.75) | 1–61 | |
| VAT (cm2) | 156.37 (89.95) | 6.62–399.70 | |
| SAT (cm2) | 166.25 (71.59) | 14.00–393.30 | |
| ATR | 0.977 (0.516) | 0.04–2.44 | |
| PMA (cm2) | 19.89 (5.50) | 9.92–37.96 | |
| PMV | 183.75 (59.87) | 67.04–346.91 | |
| TAMA (cm2) | 147.98 (30.26) | 78.40–228.13 | |
| LSMI (cm2/m2) | 47.44 (7.91) | 30.63–68.42 | |
| Sarcopenia | No | 27 (31.8%) | |
| Yes | 58 (68.2%) | ||
| Sarcopenic obesity | No | 78 (91.8%) | |
| Yes | 7 (8.2%) |
3.1. Analysis of Preoperative CT Body Composition Imaging Biomarkers
The results of the correlation analysis of the preoperative CT-body composition imaging biomarkers with postoperative complications are summarized in Table 2. The occurrence of complications was significantly higher in patients with higher SAT (p = 0.036). Binary univariate logistic regression showed a significantly increased risk of complications in patients with higher SAT (OR: 1.009, p = 0.027).
Table 2.
Correlation analysis of preoperative CT body composition imaging biomarkers to postoperative complications.
| Outcome | Preoperative CT Body Composition Imaging Biomarkers | ||||||||
| Adipose Tissue | |||||||||
| VAT | p | SAT | p | ATR | p | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | |||||||
| Complications | Yes | 156.77 (90.63) | 0.946 | 177.30 (73.43) | 0.036 | 0.90 (0.47) | 0.056 | ||
| No | 155.38 (90.12) | 138.17 (59.20) | 1.17 (0.59) | ||||||
| Major complications | Yes | 158.45 (90.70) | 0.850 | 180.02 (75.27) | 0.108 | 0.89 (0.44) | 0.149 | ||
| No | 154.04 (90.20) | 150.76 (64.67) | 1.08 (0.58) | ||||||
| Anastomotic leakage | Yes | 168.01 (68.71) | 0.596 | 183.70 (85.31) | 0.647 | 0.97 (0.33) | 0.793 | ||
| No | 154.64 (92.96) | 163.65 (69.62) | 0.98 (0.54) | ||||||
| Pneumonia | Yes | 164.53 (90.19) | 0.604 | 190.24 (84.96) | 0.065 | 0.92 (0.46) | 0.491 | ||
| No | 152.58 (90.38) | 155.08 (62.13) | 1.00 (0.54) | ||||||
| Muscle tissue | |||||||||
| PMA | p | PMV | p | TAMA | p | LSMI | p | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||||
| Complications | Yes | 20.01 (5.55) | 0.653 | 177.39 (58.04) | 0.101 | 146.90 (27.65) | 0.911 | 47.38 (7.36) | 0.762 |
| No | 19.57 (5.48) | 199.92 (62.64) | 150.73 (36.59) | 47.59 (9.35) | |||||
| Major Complications | Yes | 19.46 (5.82) | 0.235 | 182.29 (59.50) | 0.460 | 146.59 (25.32) | 0.843 | 47.05 (5.93) | 1.000 |
| No | 20.36 (5.15) | 185.43 (61.01) | 149.54 (35.28) | 47.88 (9.74) | |||||
| Anastomotic leakage | Yes | 21.17 (7.59) | 0.804 | 199.08 (75.06) | 0.591 | 151.22 (26.72) | 0.778 | 48.21 (6.63) | 0.530 |
| No | 19.70 (5.16) | 181.47 (57.55) | 147.50 (30.89) | 47.32 (8.12) | |||||
| Pneumonia | Yes | 20.06 (6.62) | 0.981 | 178.41 (64.53) | 0.565 | 140.91 (28.42) | 0.164 | 45.48 (6.40) | 0.199 |
| No | 19.81 (4.96) | 186.24 (58.00) | 151.27 (30.77) | 48.35 (8.42) | |||||
VAT = visceral adipose tissue; SAT = subcutaneous adipose tissue; ATR = adipose tissue ratio, PMA = psoas muscle area; PMV = psoas muscle volume; TAMA = total abdominal muscle area, LSMI = lumbar skeletal muscle index.
Major complications occurred more frequently in patients with sarcopenia (p = 0.045) and the occurrence of postoperative pneumonia showed a significant correlation with preoperative sarcopenic obesity (p = 0.019) (Table 3). Binary univariate logistic regression confirmed a significantly higher rate of major complications in sarcopenic patients (OR: 2.587, p = 0.048) and the higher risk of pneumonia in patients with sarcopenic obesity (OR: 6.364, p = 0.034).
Table 3.
Correlation analysis of sarcopenia and sarcopenic obesity in relation to postoperative complications.
| Outcome | Sarcopenia | Sarcopenic Obesity | |||||
|---|---|---|---|---|---|---|---|
| Yes | No | p | Yes | No | p | ||
| Complications | Yes | 45 | 16 | 0.081 | 7 | 54 | 0.083 |
| No | 13 | 11 | 0 | 24 | |||
| Major Complications | Yes | 35 | 10 | 0.045 | 6 | 39 | 0.070 |
| No | 23 | 17 | 1 | 39 | |||
| Anastomotic leakage | Yes | 8 | 3 | 0.732 | 2 | 9 | 0.198 |
| No | 50 | 24 | 5 | 69 | |||
| Pneumonia | Yes | 22 | 5 | 0.073 | 5 | 22 | 0.019 |
| No | 36 | 22 | 2 | 56 | |||
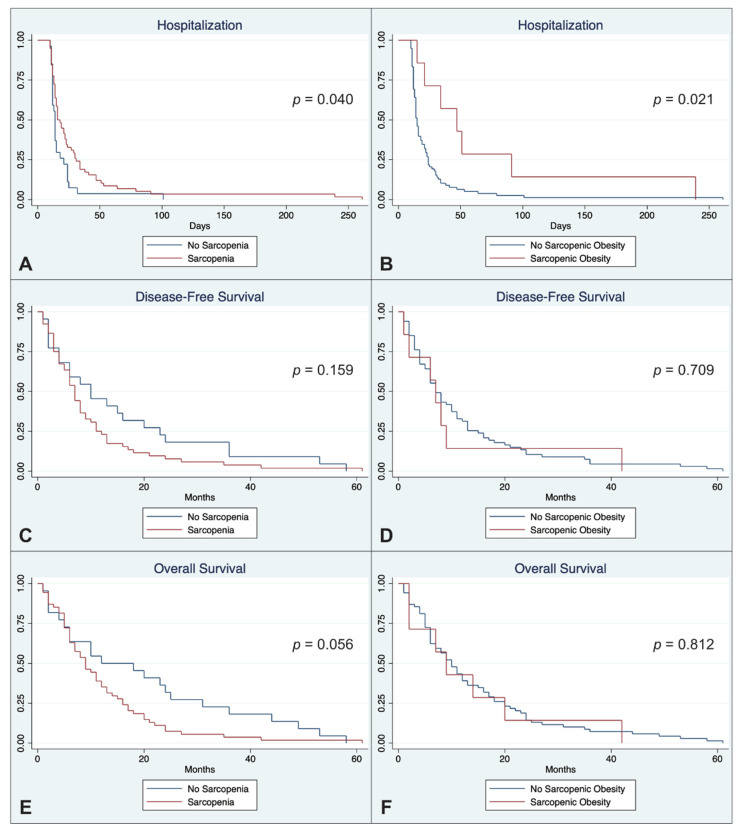
Sarcopenic patients and patients with sarcopenic obesity were both at increased risk of prolonged postoperative hospitalization (31.9 d vs. 18.8 d and 71.1 d vs. 23.9 d, p = 0.040 and 0.021). Sarcopenia and sarcopenic obesity were also associated with shorter DFS and OS without statistical significance (p > 0.05). A statistical trend was shown for shorter OS in sarcopenic patients (12.1 vs. 20.0 months; p = 0.056) (Table 4 and Figure 4).
Table 4.
Correlation analysis of sarcopenia and sarcopenic obesity to postoperative outcome parameters.
| Outcome | Hazard Ratio | 95% CI | p | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Hospitalization (d) | Sarcopenia | 0.611 | 0.382 | 0.977 | 0.040 |
| Sarcopenic Obesity | 0.394 | 0.179 | 0.870 | 0.021 | |
| DFS (months) | Sarcopenia | 1.444 | 0.866 | 2.406 | 0.159 |
| Sarcopenic Obesity | 1.162 | 0.529 | 2.550 | 0.709 | |
| OS (months) | Sarcopenia | 1.656 | 0.987 | 2.781 | 0.056 |
| Sarcopenic Obesity | 1.099 | 0.503 | 2.402 | 0.812 | |
DFS = disease-free survival; OS = overall survival.
Figure 4.
Kaplan-Meier duration of hospitalization (A,B), disease-free survival (C,D) and overall survival (E,F) stratified according to sarcopenia (left column) and sarcopenic obesity (right column).
Univariate linear regression analysis showed a significant correlation between preoperative PMA (standardized beta coefficient: 0.795, p = 0.005), PMV (standardized beta coefficient: 0.055, p = 0.037) and OS. There were no significant correlations of duration of postoperative hospitalization, DFS and OS to the other preoperative body composition imaging biomarkers (VAT, SAT, TAMA, ATR, LSMI) (p > 0.05).
3.2. Analysis of Change in CT Body Composition Imaging Biomarkers during Follow-Up
Postoperative follow-up imaging was available for evaluation in 50 patients (59%). Relative changes in body composition imaging biomarkers in the postoperative follow-up scan compared to the preoperative CT examination were correlated with complications (Table 5) and postoperative outcome parameters. Patients with postoperative anastomotic leakage showed a significantly higher decrease in TAMA in the follow-up period than patients without anastomotic leakage (p = 0.032). Overall, patients with complications showed a (non-significant) higher decrease of muscle biomarkers than patients without complications (p > 0.05). DFS correlated significantly with ΔPMV (correlation coefficient: −0.387, p = 0.006) and ΔTAMA (correlation coefficient: −0.382, p = 0.007). Overall survival also showed a significant correlation with ΔPMV (correlation coefficient: −0.365 p = 0.009) and ΔTAMA (correlation coefficient: −0.356, p = 0.013). Multivariable linear regression analysis confirmed the significant correlation between shorter DFS and decrease in PMV (standardized beta coefficient: −0.308, p = 0.031) and TAMA (standardized beta coefficient: −0.316, p = 0.031) as well as the correlation between a shorter OS and decrease in PMV (standardized beta coefficient: −0.305, p = 0.035) and TAMA (standardized beta coefficient: −0.291, p = 0.044).
Table 5.
Correlation analysis of relative changes in CT body composition imaging biomarkers to postoperative complications.
| Outcome | Relative Changes in CT Body Composition Imaging Biomarkers between Pre- and Postoperative Scans | ||||||||
| Adipose tissue | |||||||||
| ΔVAT | p | ΔSAT | p | ΔATR | p | ||||
| Mean (SD) | Mean (SD) | Mean (SD) | |||||||
| Complications | Yes | −48.96 (32.39) | 0.905 | −31.62 (28.17) | 0.234 | −34.31 (23.47) | 0.849 | ||
| No | −50.63 (22.07) | −22.14 (24.26) | −30.71 (35.93) | ||||||
| Major Complications | Yes | −49.92 (32.68) | 0.544 | −30.53 (29.56) | 0.477 | −34.22 (30.01) | 0.802 | ||
| No | −48.65 (26.42) | −27.40 (24.51) | −28.13 (37.15) | ||||||
| Anastomotic leakage | Yes | −57.41 (41.51) | 0.268 | −32.91 (40.58) | 0.860 | −48.54 (41.66) | 0.069 | ||
| No | −47.74 (27.32) | −28.44 (24.38) | −28.16 (30.42) | ||||||
| Pneumonia | Yes | −46.44 (36.37) | 0.681 | −37.81 (25.74) | 0.281 | −22.53 (47.17) | 0.322 | ||
| No | −50.77 (26.85) | −25.16 (27.44) | −35.89 (23.48) | ||||||
| Muscle tissue | |||||||||
| ΔPMA | p | ΔPMV | p | ΔTAMA | p | ΔLSMI | p | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||||
| Complications | Yes | −8.08 (16.15) | 0.063 | −8.03 (23.74) | 0.803 | −7.63 (10.13) | 0.105 | −63.29 (10.38) | 0.886 |
| No | 1.20 (14.29) | −7.19 (14.61) | −1.59 (10.85) | −37.24 (99.82) | |||||
| Major Complications | Yes | −6.68 (15.52) | 0.369 | −10.39 (23.36) | 0.552 | −8.17 (10.61) | 0.168 | −63.60 (11.10) | 0.490 |
| No | −4.40 (17.12) | −4.00 (19.06) | −3.29 (10.03) | −47.24 (77.14) | |||||
| Anastomotic leakage | Yes | −14.78 (18.76) | 0.183 | −20.04 (38.23) | 0.568 | −15.04 (12.09) | 0.032 | −61.50 (19.44) | 0.319 |
| No | −3.85 (15.07) | −5.15 (15.72) | −4.26 (9.34) | −55.64 (55.36) | |||||
| Pneumonia | Yes | −12.79 (20.45) | 0.145 | −2.72 (24.04) | 0.362 | −9.13 (9.43) | 0.158 | −62.52 (13.75) | 0.747 |
| No | −2.39 (12.61) | −10.46 (20.38) | −4.67 (10.87) | −53.88 (61.15) | |||||
VAT = visceral adipose tissue; SAT = subcutaneous adipose tissue; ATR = adipose tissue ratio, PMA = psoas muscle area; PMV = psoas muscle volume; TAMA = total abdominal muscle area, LSMI = lumbar skeletal muscle index.
4. Discussion
The aim of this study was to assess the predictive value of body composition imaging biomarkers in CT on perioperative morbidity and survival after surgery in patients with locally advanced esophageal cancer. Our results are based on the largest cohort so far of surgically treated locally advanced EC and suggests that several important CT body composition imaging biomarkers can be used to predict peri- and postoperative morbidity and mortality. More prominent subcutaneous fat was associated with an increased risk of complications, and patients with preoperative sarcopenia had a higher risk of major complications after esophagectomy. Moreover, sarcopenic obesity was associated with a higher risk of postoperative pneumonia. Subsequently, patients with sarcopenia or sarcopenic obesity were at higher risk for prolonged postoperative hospitalization. Neither sarcopenia nor sarcopenic obesity was an independent risk factor for anastomotic leakage. A smaller preoperative abdominal muscle mass, identified by measurements of the psoas muscle, was associated with a shorter OS. Evaluation of postoperative follow-up imaging showed that patients with anastomotic leakage had a more marked decrease in muscle mass and that more pronounced muscle atrophy was associated with shorter DFS and OS. Our findings are consistent with previous studies demonstrating a poor overall outcome in patients with sarcopenia and sarcopenic obesity treated for different tumor entities, including upper GI cancer [28,29,30]. In addition, our results demonstrated that the decrease in muscle mass during postoperative follow-up may also indicate shorter DFS and OS. Our study extends available data on CT-body composition imaging biomarkers to patients with surgically treated locally advanced EC.
Patients with esophageal cancer have a decreased fat mass and higher rates of sarcopenia (about 50%) after neoadjuvant therapy [31]. All patients included in our study underwent neoadjuvant chemotherapy and had an even higher rate of sarcopenia (68.2%). The high incidence of sarcopenia was independent of the UICC tumor stage, so that the patient’s body composition did not allow reliable conclusions to be drawn about the preoperative tumor extent. Regarding adipose tissue, we found that patients with higher subcutaneous fat mass after neoadjuvant therapy had an elevated risk for surgical complications. The possible effects of neoadjuvant chemotherapy were not part of our current study; however, our results encourage further investigations regarding the effects of neoadjuvant therapy on patients’ body composition and the outcome of subsequent therapies. The role of sarcopenia in esophageal cancer treated with surgery and chemotherapy has already been investigated in smaller patient populations before. One study reported that sarcopenic patients were at a higher risk of developing a conduit necrosis [32]. Our results confirm the increased risk of complications for patients with sarcopenia in a larger population. However, our study revealed a generally increased risk for major complications in sarcopenic patients without a specific influence on the rate of anastomotic leakages. Besides the possible influence of sarcopenia on surgical morbidity, patient fitness seems to be an important factor in EC, as these patients have an impaired nutritional intake and digestion. Perioperative nutrition therapy has been proven to reduce postoperative risks and shorten intensive care duration in patients with EC [33]. Adipose and skeletal muscle tissues are considered as partly secretory organs, and VAT is associated with insulin resistance, which increases inflammatory cytokines levels. This might be a part of the pathophysiological explanation for prolonged wound healing and poorer outcome, especially in sarcopenic obesity [34,35] Our data suggest that patients with sarcopenic obesity are at a higher risk for postoperative pneumonia. This seems plausible, as obesity is often linked with respiratory problems. Prolonged recumbency also increases the risk of aspiration and sarcopenia might include a loss of respiratory muscle, leading to more difficult respiration in sarcopenic obese patients [36,37].
As the population is aging and comorbidities rise, sarcopenia is likely to become more prevalent in the future and might play an even more important role in patient care. There have been several attempts to establish risk scores for surgical mortality in esophageal cancer [38,39,40]. Future endeavors at calculating risk scores should consider taking CT body composition imaging biomarkers into account, especially because the preoperative CT is a standard procedure in the staging process. CT-based parameters enable a detailed analysis of an individual patient’s body composition. Imaging-based assessment could complement established conventional measures such as BMI or waist-to-hip-ratio, or even replace them. It is important to assess the individual patient’s fitness as the proportion of complex, extensive operations is becoming more frequent as surgical techniques improve. Therefore, one needs to weigh the benefits of a potentially curative operation against its potentially high morbidity to evaluate alternative treatment strategies in vulnerable patients [41]. When patients at a higher risk are identified before surgery, specific interventions such as improving nutritional intake and/or assisted physical activity might be incorporated into the peri- and postoperative management [32,42]. A major drawback of the CT body composition approach as used in this study is that it is time-consuming, which may hinder its routine clinical use. However, our results could pave the way to automated, standardized analysis.
Our study has some limitations. The major limitation is its single-center, retrospective design, which means that the results may not be generalizable and should be confirmed in a prospective trial and compared to conventional parameters such as body impedance analysis or muscle function. The study design prohibits any valid conclusion to be drawn regarding potential causal relationships of the tumor burden, daily food intake and physical activities with the manifestation of sarcopenia. Nonstandardized imaging intervals could have biased the body composition results in our patients. Even though the cohort is comparatively large, more data are needed to determine the thresholds of these imaging biomarkers.
5. Conclusions
In conclusion, our study highlights the predictive value of CT body composition imaging biomarkers to identify high-risk patients with locally advanced esophageal cancer undergoing surgery after neoadjuvant treatment. Sarcopenic patients are at an increased risk for major complications, and patients with sarcopenic obesity are more prone to postoperative pneumonia. Sarcopenia and sarcopenic obesity are both subsequently associated with a prolonged postoperative hospitalization. Low preoperative muscle mass and its decrease during postoperative follow-up are associated with shorter disease-free and overall survival.
Acknowledgments
The authors thank Bettina Herwig for language editing.
Author Contributions
U.F.: designed the research, performed the study, collected data, analyzed data, wrote the manuscript; T.W.: collected data, analyzed data, wrote the manuscript; P.G.: collected data, analyzed data; L.S.: collected data, wrote the manuscript; T.Y.: collected data, analyzed data; T.A.A.: collected data, analyzed data; N.L.B.: collected data, analyzed data; C.D.: contributed important data; D.K.: contributed important data, wrote the manuscript; J.R.: contributed important data; S.K.: contributed important data; S.C.: contributed important data; P.T.-P.: contributed important data; J.P.: contributed important data; B.H.: contributed important data, wrote the manuscript; M.B.: designed the research, performed the study, analyzed data, wrote the manuscript; D.G.: designed the research, performed the study, analyzed data, wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge APC funding from the German Research Foundation (DFG) and the Open Access Publication Fund of Charité-Universitätsmedizin Berlin.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of Charité Berlin (internal registration number: EA4/152/20, 21.08.2020).
Informed Consent Statement
Patient consent was waived due to the retrospective study design.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
Fehrenbach and Geisel report honoraria and travel expenses for scientific meetings (outside of submitted work) from Bayer, Siemens and GE. Hamm reports grant money from companies or nonprofit organizations to the Department of Radiology (outside of submitted work) from Abbott, Actelion Pharmaceuticals, Bayer Schering Pharma, Bayer Vital, BRACCO Group, Bristol-Myers Squibb, Charité Research Organization GmbH, Krebshilfe, Stiftung für Herzforschung, Essex Pharma, EU Programmes, Fibrex Medical Inc., Focused Ultrasound Surgery Foundation, Fraunhofer Gesellschaft, Guerbet, INC Research, InSightec Ltd., IPSEN Pharma, Kendle/MorphoSys AG, Lilly GmbH, Lundbeck GmbH, MeVis Medical Solutions AG, Nexus Oncology, Novartis, Parexel CRO Service, Perceptive, Pfizer GmbH, Philipps, Sanofi-Aventis S.A, Siemens, Spectranetics GmbH, Terumo Medical Corporation, TNS Healthcare GmbH, Toshiba, UCB Pharma, Wyeth Pharma and Zukunftsfond Berlin (TSB).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Znaor A., Bray F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer. 2019;144:1941–1953. doi: 10.1002/ijc.31937. [DOI] [PubMed] [Google Scholar]
- 2.Arnold M., Ferlay J., van Berge Henegouwen M.I., Soerjomataram I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut. 2020;69:1564–1571. doi: 10.1136/gutjnl-2020-321600. [DOI] [PubMed] [Google Scholar]
- 3.Karstens K.F., Stüben B.O., Reeh M. Oesophageal adenocarcinomas: Where do we stand today? Cancers. 2020;13:109. doi: 10.3390/cancers13010109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Melhado R.E., Alderson D., Tucker O. The changing face of esophageal cancer. Cancers. 2010;2:1379–1404. doi: 10.3390/cancers2031379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawakatsu Y., Koyanagi Y.N., Oze I., Kasugai Y., Morioka H., Yamaguchi R., Ito H., Matsuo K. Association between Socioeconomic Status and Digestive Tract Cancers: A Case-Control Study. Cancers. 2020;12:3258. doi: 10.3390/cancers12113258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keeble S., Abel G.A., Saunders C.L., McPhail S., Walter F.M., Neal R.D., Rubin G.P., Lyratzopoulos G. Variation in promptness of presentation among 10,297 patients subsequently diagnosed with one of 18 cancers: Evidence from a National Audit of Cancer Diagnosis in Primary Care. Int. J. Cancer. 2014;135:1220–1228. doi: 10.1002/ijc.28763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stahl M., Budach W., Meyer H.J., Cervantes A. Esophageal cancer: Clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol./ESMO. 2010;21(Suppl. 5):v46–v49. doi: 10.1093/annonc/mdq163. [DOI] [PubMed] [Google Scholar]
- 8.Pech O., Bollschweiler E., Manner H., Leers J., Ell C., Hölscher A.H. Comparison between endoscopic and surgical resection of mucosal esophageal adenocarcinoma in Barrett’s esophagus at two high-volume centers. Ann. Surg. 2011;254:67–72. doi: 10.1097/SLA.0b013e31821d4bf6. [DOI] [PubMed] [Google Scholar]
- 9.Quaas A. Current and future treatment strategies for esophageal adenocarcinoma. Cancers. 2020;12:2930. doi: 10.3390/cancers12102930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeh M., Nentwich M.F., Asani S., Uzunoglu F.G., Bockhorn M., Sauter G., Rösch T., Izbicki J.R., Bogoevski D. Locally advanced esophageal carcinoma: Is there still a role of surgery alone without neoadjuvant treatment? J. Gastrointest. Surg. 2015;19:587–593. doi: 10.1007/s11605-015-2762-y. [DOI] [PubMed] [Google Scholar]
- 11.Porschen R., Fischbach W., Gockel I., Hollerbach S., Hölscher A., Jansen P.L., Miehlke S., Pech O., Stahl M., Thuss-Patience P., et al. S3-Leitlinie–Diagnostik und Therapie der Plattenepithelkarzinome und Adenokarzinome des Ösophagus. Z. Gastroenterol. 2019;57:336–418. doi: 10.1055/a-0833-5712. [DOI] [PubMed] [Google Scholar]
- 12.Viklund P., Lindblad M., Lu M., Ye W., Johansson J., Lagergren J. Risk factors for complications after esophageal cancer resection: A prospective population-based study in Sweden. Ann. Surg. 2006;243:204–211. doi: 10.1097/01.sla.0000197698.17794.eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mariette C., Markar S.R., Dabakuyo-Yonli T.S., Meunier B., Pezet D., Collet D., D’Journo X.B., Brigand C., Perniceni T., Carrère N., et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N. Engl. J. Med. 2019;380:152–162. doi: 10.1056/NEJMoa1805101. [DOI] [PubMed] [Google Scholar]
- 14.Peng J.S., Kukar M., Mann G.N., Hochwald S.N. Minimally invasive esophageal cancer surgery. Surg. Oncol. Clin. N. Am. 2019;28:177–200. doi: 10.1016/j.soc.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Yibulayin W., Abulizi S., Lv H., Sun W. Minimally invasive oesophagectomy versus open esophagectomy for resectable esophageal cancer: A meta-analysis. World J. Surg. Oncol. 2016;14:304. doi: 10.1186/s12957-016-1062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee C.D., Blair S.N., Jackson A.S. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am. J. Clin. Nutr. 1999;69:373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 17.Pamoukdjian F., Bouillet T., Lévy V., Soussan M., Zelek L., Paillaud E. Prevalence and predictive value of pre-therapeutic sarcopenia in cancer patients: A systematic review. Clin. Nutr. 2018;37:1101–1113. doi: 10.1016/j.clnu.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Faron A., Luetkens J.A., Schmeel F.C., Kuetting D.L.R., Thomas D., Sprinkart A.M. Quantification of fat and skeletal muscle tissue at abdominal computed tomography: Associations between single-slice measurements and total compartment volumes. Abdom. Radiol. 2019;44:1907–1916. doi: 10.1007/s00261-019-01912-9. [DOI] [PubMed] [Google Scholar]
- 19.Huang D.D., Wang S.L., Zhuang C.L., Zheng B.S., Lu J.X., Chen F.F., Zhou C.J., Shen X., Yu Z. Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal. Dis. 2015;17:O256–O264. doi: 10.1111/codi.13067. [DOI] [PubMed] [Google Scholar]
- 20.Ida S., Watanabe M., Yoshida N., Baba Y., Umezaki N., Harada K., Karashima R., Imamura Y., Iwagami S., Baba H. Sarcopenia is a predictor of postoperative respiratory complications in patients with esophageal cancer. Ann. Surg. Oncol. 2015;22:4432–4437. doi: 10.1245/s10434-015-4559-3. [DOI] [PubMed] [Google Scholar]
- 21.Lee K., Shin Y., Huh J., Sung Y.S., Lee I.S., Yoon K.H., Kim K.W. Recent issues on body composition imaging for sarcopenia evaluation. Korean J. Radiol. 2019;20:205–217. doi: 10.3348/kjr.2018.0479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berry M.F. Esophageal cancer: Staging system and guidelines for staging and treatment. J. Thorac. Dis. 2014;6(Suppl. 3):S289–S297. doi: 10.3978/j.issn.2072-1439.2014.03.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dziodzio T., Kröll D., Denecke C., Öllinger R., Pratschke J., Biebl M. Minimally invasive esophagectomy. Multimed. Man Cardiothorac. Surg. 2021;2021 doi: 10.1510/mmcts.2021.020. [DOI] [PubMed] [Google Scholar]
- 24.Misawa K., Hachisuka T., Kuno Y., Mori T., Shinohara M., Miyauchi M. New procedure for purse-string suture in thoracoscopic esophagectomy with intrathoracic anastomosis. Surg. Endosc. 2005;19:40–42. doi: 10.1007/s00464-004-9138-9. [DOI] [PubMed] [Google Scholar]
- 25.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su H., Ruan J., Chen T., Lin E., Shi L. CT-assessed sarcopenia is a predictive factor for both long-term and short-term outcomes in gastrointestinal oncology patients: A systematic review and meta-analysis. Cancer Imaging Off. Publ. Int. Cancer Imaging Soc. 2019;19:82. doi: 10.1186/s40644-019-0270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prado C.M., Lieffers J.R., McCargar L.J., Reiman T., Sawyer M.B., Martin L., Baracos V.E. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: A population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 28.Carneiro I.P., Mazurak V.C., Prado C.M. Clinical implications of sarcopenic obesity in cancer. Curr. Oncol. Rep. 2016;18:62. doi: 10.1007/s11912-016-0546-5. [DOI] [PubMed] [Google Scholar]
- 29.Choi M.H., Kim K.A., Hwang S.S., Byun J.Y. CT-quantified muscle and fat change in patients after surgery or endoscopic resection for early gastric cancer and its impact on long-term outcomes. Medicine. 2018;97:e13878. doi: 10.1097/MD.0000000000013878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou C.J., Zhang F.M., Zhang F.Y., Yu Z., Chen X.L., Shen X., Zhuang C.L., Chen X.X. Sarcopenia: A new predictor of postoperative complications for elderly gastric cancer patients who underwent radical gastrectomy. J. Surg. Res. 2017;211:137–146. doi: 10.1016/j.jss.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Yip C., Goh V., Davies A., Gossage J., Mitchell-Hay R., Hynes O., Maisey N., Ross P., Gaya A., Landau D.B., et al. Assessment of sarcopenia and changes in body composition after neoadjuvant chemotherapy and associations with clinical outcomes in oesophageal cancer. Eur. Radiol. 2014;24:998–1005. doi: 10.1007/s00330-014-3110-4. [DOI] [PubMed] [Google Scholar]
- 32.Paireder M., Asari R., Kristo I., Rieder E., Tamandl D., Ba-Ssalamah A., Schoppmann S.F. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur. J. Surg. Oncol. 2017;43:478–484. doi: 10.1016/j.ejso.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Ligthart-Melis G.C., Weijs P.J., te Boveldt N.D., Buskermolen S., Earthman C.P., Verheul H.M., de Lange-de Klerk E.S., van Weyenberg S.J., van der Peet D.L. Dietician-delivered intensive nutritional support is associated with a decrease in severe postoperative complications after surgery in patients with esophageal cancer. Dis. Esophagus. 2013;26:587–593. doi: 10.1111/dote.12008. [DOI] [PubMed] [Google Scholar]
- 34.Levine M.E., Crimmins E.M. The impact of insulin resistance and inflammation on the association between sarcopenic obesity and physical functioning. Obesity (Silver Spring) 2012;20:2101–2106. doi: 10.1038/oby.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pedersen B.K., Febbraio M.A. Muscles, exercise and obesity: Skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 36.Kornum J.B., Nørgaard M., Dethlefsen C., Due K.M., Thomsen R.W., Tjønneland A., Sørensen H.T., Overvad K. Obesity and risk of subsequent hospitalisation with pneumonia. Eur. Respir. J. 2010;36:1330–1336. doi: 10.1183/09031936.00184209. [DOI] [PubMed] [Google Scholar]
- 37.Mancuso P. Obesity and respiratory infections: Does excess adiposity weigh down host defense? Pulm. Pharmacol. Ther. 2013;26:412–419. doi: 10.1016/j.pupt.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schröder W., Bollschweiler E., Kossow C., Hölscher A.H. Preoperative risk analysis-a reliable predictor of postoperative outcome after transthoracic esophagectomy? Langenbeck’s Arch. Surg. 2006;391:455–460. doi: 10.1007/s00423-006-0067-z. [DOI] [PubMed] [Google Scholar]
- 39.Steyerberg E.W., Neville B.A., Koppert L.B., Lemmens V.E., Tilanus H.W., Coebergh J.W., Weeks J.C., Earle C.C. Surgical mortality in patients with esophageal cancer: Development and validation of a simple risk score. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006;24:4277–4284. doi: 10.1200/JCO.2005.05.0658. [DOI] [PubMed] [Google Scholar]
- 40.Warnell I., Chincholkar M., Eccles M. Predicting perioperative mortality after oesophagectomy: A systematic review of performance and methods of multivariate models. Br. J. Anaesth. 2015;114:32–43. doi: 10.1093/bja/aeu294. [DOI] [PubMed] [Google Scholar]
- 41.Tamandl D., Paireder M., Asari R., Baltzer P.A., Schoppmann S.F., Ba-Ssalamah A. Markers of sarcopenia quantified by computed tomography predict adverse long-term outcome in patients with resected oesophageal or gastro-oesophageal junction cancer. Eur. Radiol. 2016;26:1359–1367. doi: 10.1007/s00330-015-3963-1. [DOI] [PubMed] [Google Scholar]
- 42.Urashima M., Okuyama M., Akutsu T., Ohdaira H., Kaji M., Suzuki Y. Effect of Vitamin D supplementation on survival of digestive tract cancer patients with low bioavailable 25-hydroxyvitamin D levels: A post hoc analysis of the AMATERASU Randomized Clinical Trial. Cancers. 2020;12:347. doi: 10.3390/cancers12020347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.