Abstract
BACKGROUND/OBJECTIVES:
To test the effects of weight loss with and without exercise training (aerobic or resistance) on intra-abdominal adipose tissue (IAAT) and risk factors for cardiovascular disease (CVD). Additionally, CVD risk factors was evaluated before and after weight loss using previously established IAAT cut-points.
SUBJECTS/METHODS:
One-hundred and twenty-two overweight premenopausal women were randomly assigned to one of three groups: 1) diet only (Diet); 2) diet and aerobic training (Diet + AT) or; 3) diet and resistance training (Diet + RT); until a BMI of <25 kg/m2 was reached. Computerized tomography was used to measure IAAT and blood lipids were measured by assay. Evaluations were made before and after weight loss.
RESULTS:
Though no group-by-time effects were found after weight loss, we observed significant time-effects for: IAAT (−38.0%, P<0.001), total cholesterol (TC) (−2.2%, P=0.008), low-density lipoprotein cholesterol (LDL-C) (−4.8%, P<0.001), high-density lipoprotein cholesterol (HDL-C) (+20.2%, P<0.001), triglycerides (−18.7%, P<0.001), TC/HDL-C (−16.3%, P <0.001) and LDL-C/HDL-C (−18.0%, P<0.001). Following weight loss 40.2% of all participants reduced IAAT to <40 cm2 (IAAT associated with low CVD risk). Furthermore, only 2.5% of participants had an IAAT >110 cm2 (IAAT associated with high CVD risk) after weight loss. We also observed that decreases of IAAT were associated with decreased CVD risk factors after weight loss independent of race, changes in %fat mass and changes in maximal oxygen uptake.
CONCLUSIONS:
Caloric restriction leading to significant weight loss with or without exercise training appears to be equally effective for reducing IAAT and CVD risk factors.
Keywords: blood lipid, cardiovascular risk factors, diet, fat distribution, visceral fat
INTRODUCTION
Although obesity and overweight is associated with increased health problems,1 intra-abdominal adipose tissue (IAAT), sometimes defined as visceral fat, may play the most important role in increasing risk of cardiovascular disease (CVD).2–4 Subjects with central fat distribution, especially visceral fat, are more likely to have an adverse blood lipid profile.1,5,6 The combination of small particles of low-density lipoprotein cholesterol (LDL-C), low high-density lipoprotein cholesterol (HDL-C) and high triglycerides (TG) is recognized as the “atherogenic lipid triad”, i.e., a major CVD risk factor.1 Effective strategies for reducing central fat are diet,7–11 aerobic exercise training (AT),9,10,12–18 resistance exercise training (RT)9–11,14,15,17,19,20 and combined (aerobic/resistance) exercise training.14,15 Similarly, decreases in central fat following weight loss were observed for diet + AT, diet + RT, and diet.7,9–11,15,17
Previously investigators have developed cut-points for IAAT that are associated with CVD risk factors.21 Using cross-sectional data and receiver-operating characteristics analysis, the general consensus identifies that women with IAAT above 110 cm2 were more likely to have increased risk of CVD, while those women with IAAT below 40 cm2 were at minimal risk. Frequently studies have designed interventions in obese participants for 12–16 weeks, but these subjects were still obese or overweight after weight loss.7,9–11,15,17 It is important to determine risk of elevated IAAT and risk factors for CVD in participants who achieve normal weight, a body mass index (BMI) <25 kg/m2 following weight loss induced by diet with and without exercise training.
Thus, the aims of this study were: 1) to test the effects of a very-low calorie diet with and without exercise training (AT or RT) on IAAT and CVD risk factors after achieving a BMI <25 kg/m2 and; 2) to evaluate CVD risk factors before and after weight loss in overweight premenopausal women using previously established IAAT cut-points.
MATERIALS AND METHODS
Participants
Sixty European Americans and 62 African Americans healthy premenopausal women (BMI: 27–30 kg/m2 and age ranged between 23 and 46 years) participated in this study which is a secondary analysis of our JULIET study that was designed to determine what effects exercise training has on body weight maintenance after weight loss. The women were nonsmokers, did not take medications known to metabolism-altering or oral contraceptives, experienced regular menstrual cycles, and normal glucose tolerance (documented by 2-h postprandial blood glucose levels after an oral glucose load). Procedures were approved by the local Institutional Review Board for Human Subjects. All participants provided their informed consent prior to participation in the study.
Study design
Participants were randomly assigned to three different groups of weight loss: diet only (Diet); diet and aerobic exercise training (Diet + AT); or diet and resistance exercise training (Diet + RT). The diet consisted of 800 kcal/day (58–62% carbohydrate, 20–22% fat and 18–22% protein) and all food was furnished during the weight loss. Participants were instructed to pick up the food at the General Clinical Research Center (GCRC) twice weekly during the weight loss and remain on the 800 kcal/day diet until a BMI of <25 kg/m2 was reached.
All participants maintained weight-stable state for 4 weeks before evaluations. During the period of weight-stable state, body weight measurements were made 3 days a week for the first 2 weeks, and 5 days a week for the last 2 weeks. All food were provided by the GCRC to ensure stability of weight, i.e., a variation of <1% from initial weight, also maintaining daily macronutrient intake during the final two weeks. Participants were admitted to the GCRC at follicular phase of the menstrual cycle during 4 days for measurements of body composition/fat distribution, blood lipids and maximal oxygen consumed test (V̇O2max).
Aerobic training
The AT consisted of continuous walking/or jogging on a treadmill. Each training session started with 3 minutes of warm-up followed by 3–5 minutes of stretching. Participants performed continuous exercise for 20 min during the first week of training at 67% maximum heart rate (HR). Following the 1st week, duration and intensity increased each week. Participants were exercising continuously at 80% of maximum HR on the 8th week for 40 min. Intensity was increased (either speed or grade) when average exercise HR was below 80% of maximum. Participants cooled down after each session for 3–5 min with gradually decreasing exercise intensity.
Resistance training
A warm-up on the bike ergometer or treadmill for 5 minutes and stretching for 3–5 minutes preceded each RT session. RT included elbow flexion, bench press, lateral pull-down, triceps extension, military press, squats, bent-leg sit-ups, leg extension, lower-back extension and leg curl. Following 1 week of training with a light weight (familiarization), strength was measured (one repetition maximum, acronym 1RM). According to the 1RM tests during the 1st week, participants performed one set of 10 repetitions at 65% 1RM. The percentage of 1RM increased on subsequent weeks until 80% 1RM on week 4. On week 5, two sets of 10 repetitions were performed at 80% 1RM for each exercise. Participants rested 2-min between sets. Tests of 1RM was performed every 5 weeks, and adjustments in training resistance were made. In both the Diet + AT and Diet + RT groups, participants trained 3 days/week.
Dual-energy x-ray absorptiometry
A dual-energy x-ray absorptiometry (GE Medical Systems Lunar, Madison, WI) was used to estimate the percentage of fat mass (%FM), using the ADULT software, LUNAR DPX-L version 1.35 (GE Medical Systems Lunar) for scans analysis.
Computerized tomography
A computerized tomography was used to estimate the cross-sectional area of IAAT. The IAAT was measured at L4-L5 vertebrae taking a 5-mm scan for 2 s with the use of HiLight/HTD Advantage scanner (General Electric, Milwaukee, WI) set at 120 kVp (peak kilovoltage) and 40 mA. The range of attenuation for adipose tissue between −30 to −190 Hounsfield units was used.22 IAAT was separated from subcutaneous fat by encircling the muscle wall surrounding the abdominal cavity with a cursor. A coefficient of variation of <2% for repeated measures of IAAT and an r value of 0.99 for both intra- and inter-observer reliability following 20 scans were found.
Blood lipids profile
An Ektachem DT II system was used to measure total cholesterol (TC), HDL-C and TG. LDL-C was estimated using the Friedewald formula.23 A calibration was performed for the DT II every 6 months with reagents supplied by the manufacturer.
Maximal volume of oxygen consumed
The V̇O2max was measured by a modified Bruce protocol.24 Oxygen uptake and carbon dioxide production were measured using a MAX-II metabolic cart (Physiodyne Instrument Corporation, Quogue, NY, USA). Certified gases were used to calibrate the gas analyzers. A POLAR Vantage XL HR monitor (Polar Electro Inc., Gays Mills, WI, USA) was used to measure the maximal HR. Standard criteria for plateauing, respiratory exchange ratio (RER) (RER >1.2) and HR (HR within 10 bpm of estimated maximum) were used to verify achievement of V̇O2max. A coefficient of variation of <3% for repeated measures of V̇O2max was found in our laboratory.
Statistical analysis
One-way analysis of variance F test (ANOVA) or Kruskal-Wallis H test was used to compare age, height and days to goal between treatments groups. Also, we used the Chi-squared x2 test to verify the homogeneous distribution of European Americans and African Americans participants in the treatments groups. Two (time) by three (group) ANOVA with repeated measures for time, were run for weight, BMI, CVD risk factors and fat distribution. Data not normally distributed for ANOVA with repeated measures were transformed to log10. Relative prevalence of participants with elevated CVD risk according to previous IAAT cut-points (<40 cm2; 40–110cm2 and >110 cm2)21 were determined. In addition, we used partial correlation of Pearson or Spearman to verify associations between delta of CVD risk factors and delta of IAAT (overweight – normal weight) adjusted for race, delta %FM and delta V̇O2max. The significance level for statistical analysis was set at α ≤ 0.05 (SPSS version 22.0 - IBM SPSS Statistics for Windows, Armonk, NY, USA).
RESULTS
Subject characteristics are presented in Table 1. No significant difference between groups was observed for age, height and race prior to weight loss. Additionally, no significant group effects or interaction of time by group were observed for weight, BMI, and blood lipids. No group effects were also observed for %FM and V̇O2max. However, significant time-effects, demonstrating decreases in weight, BMI, %FM, and improvements in V̇O2max and blood lipid profile were observed. A time by group interaction was observed for %FM and V̇O2max.
Table 1.
Characteristics, physical fitness and blood lipid profile of the participants during overweight and after achieving normal weight (Mean ± SD)
| Diet (n = 29) |
Diet + AT (n = 41) |
Diet + RT (n = 52) |
P-value |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Overweight | Normal weight | ∆% | Overweight | Normal weight | ∆% | Overweight | Normal weight | ∆% | Time | Group | Interaction | |
| Age (y) | 36.1 ± 5.0 | - | - | 35.2 ± 6.8 | - | - | 33.9 ± 6.2 | - | - | - | 0.292 | - |
| Race (EA/AA) | 16/13 | - | - | 20/21 | - | - | 24/28 | - | - | - | 0.737 | - |
| Height (cm) | 165.6 ± 6.0 | - | - | 164.6 ± 6.4 | - | - | 166.3 ± 6.8 | - | - | - | 0.472 | - |
| Days to goal | - | 146.8 ± 48.7 | - | - | 163.3 ± 74.6 | - | - | 153.7 ± 75.4 | - | - | 0.733 | - |
| Weight (kg) | 77.8 ± 7.3 | 65.3 ± 6.4 | −16.0 | 77.0 ± 6.5 | 64.7 ± 5.8 | −16.0 | 77.8 ± 7.8 | 66.0 ± 6.5 | −15.1 | < 0.001 | 0.778 | 0.205* |
| BMI (kg/m2) | 28.2 ± 1.4 | 23.7 ± 1.1 | −15.7 | 28.4 ± 1.5 | 23.9 ± 1.1 | −15.9 | 28.0 ± 1.1 | 23.8 ± 1.1 | −15.0 | < 0.001 | 0.712 | 0.268* |
| %FM | 42.7 ± 3.5 | 33.2 ± 4.7 | −22.4 | 44.1 ± 3.8 | 34.1 ± 4.6 | −22.8 | 43.2 ± 3.8 | 32.4 ± 4.6 | −25.1 | < 0.001 | 0.269 | 0.055 |
| V̇O2max (ml/kg/min) | 27.7 ± 3.8 | 31.9 ± 4.8 | 14.9 | 28.1 ± 4.1 | 33.9 ± 5.6 | 20.9 | 29.1 ± 3.7 | 33.5 ± 4.7 | 15.2 | < 0.001 | 0.302 | 0.029 |
| TC (mg/dl) | 163.6 ± 33.0 | 152.2 ± 26.2 | −5.6 | 160.7 ± 28.0 | 160.8 ± 29.2 | 0.9 | 153.7 ± 31.9 | 148.1 ± 28.6 | −2.6 | 0.008 | 0.191 | 0.136* |
| HDL-C (mg/dl) | 40.0 ± 10.5 | 45.0 ± 12.9 | 12.9 | 39.9 ± 12.1 | 48.0 ± 12.7 | 23.6 | 37.5 ± 10.8 | 44.1 ± 12.3 | 21.5 | < 0.001 | 0.389 | 0.238* |
| LDL-C (mg/dl) | 104.2 ± 33.3 | 93.2 ± 24.7 | −8.0 | 102.1 ± 25.1 | 99.1 ± 23.8 | −1.5 | 97.9 ± 28.5 | 90.3 ± 23.1 | −5.6 | < 0.001 | 0.407 | 0.239* |
| TG (mg/dl) | 96.8 ± 55.7 | 70.1 ± 28.2 | −17.9 | 93.4 ± 47.1 | 68.3 ± 32.1 | −20.1 | 91.3 ± 47.2 | 68.1 ± 30.9 | −18.1 | < 0.001 | 0.907 | 0.951* |
| TC/HDL-C | 4.4 ± 1.5 | 3.6 ± 1.0 | −15.5 | 4.3 ± 1.4 | 3.5 ± 0.9 | −16.3 | 4.4 ± 1.3 | 3.5 ± 0.9 | −16.7 | < 0.001 | 0.982 | 0.844* |
| LDL-C/HDL-C | 2.8 ± 1.2 | 2.2 ± 0.9 | −17.2 | 2.8 ± 1.2 | 2.2 ± 0.8 | −17.7 | 2.8 ± 1.0 | 2.2 ± 0.8 | −18.6 | < 0.001 | 0.999 | 0.821* |
Abbreviations: Diet, diet only; Diet + AT, diet and aerobic exercise training; Diet + RT, diet and resistance training; EA, European-American; AA, African-American; BMI, body mass index; %FM, percentage of fat mass; V̇O2max, maximal oxygen consumption; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; TG, triglycerides; TC/HDL-C, total cholesterol / high-density lipoprotein cholesterol; LDL-C/HDL-C, low-density lipoprotein cholesterol / high-density lipoprotein cholesterol;
Statistical analysis was performed using transformed values in log10. Values in boldface indicate significant differences (P < 0.05).
IAAT decreased (significant time effect, Figure 1A) after weight loss. In addition the percentage participants showing an IAAT <40 cm2 increased from 9.0 to 40.2%, while the percentage of participants with an IAAT between 40–110 cm2 decreased from 77.0 to 57.4% and those >110 cm2 decreased from 13.9 to 2.5 % (Figure 1B). However, no significant group-effects, or time x group interaction were observed, demonstrating similar effectiveness for all strategies in improving IAAT.
Figure 1.
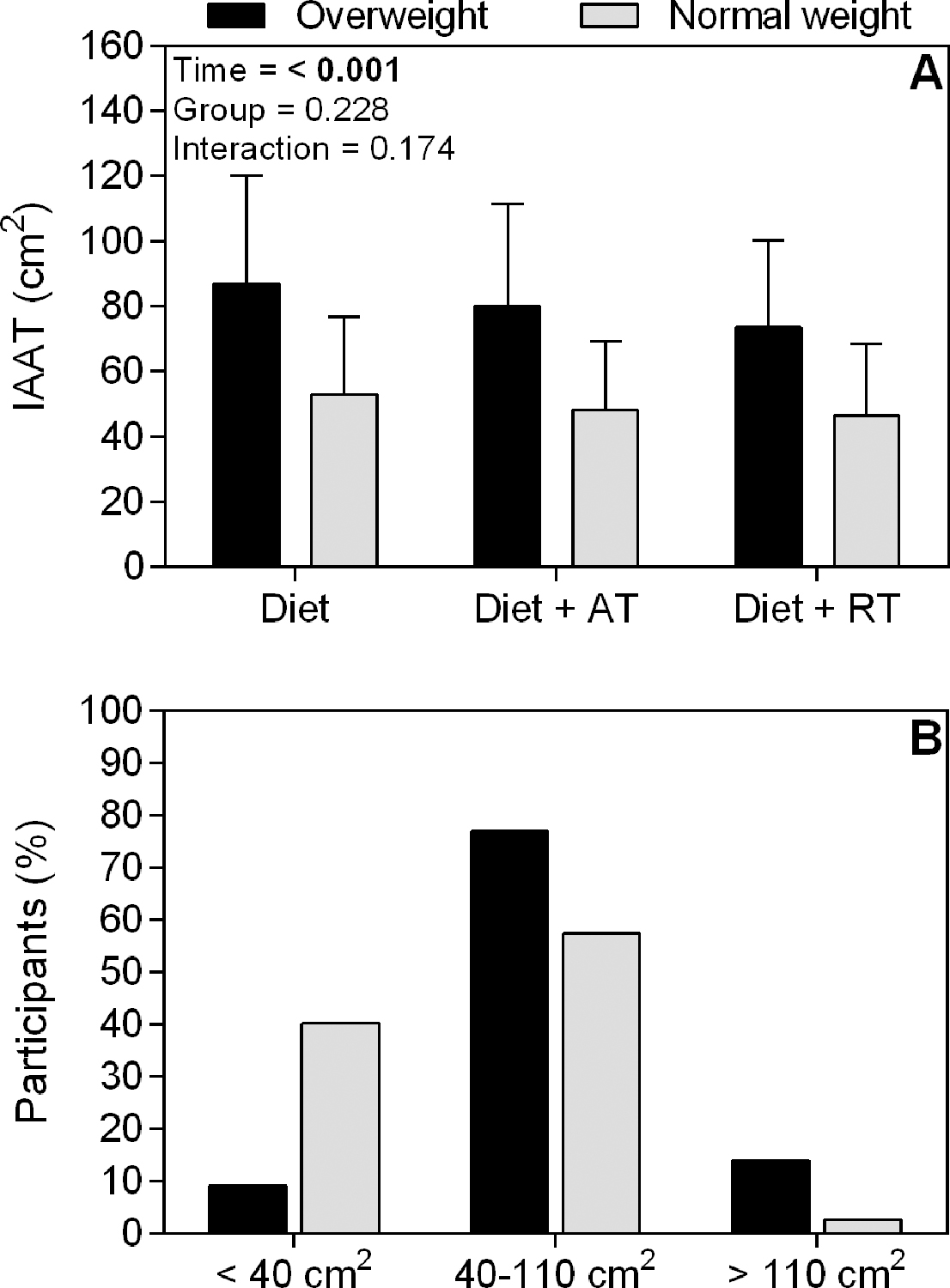
A) Intra-abdominal adipose tissue (IAAT) during overweight and after achieving normal weight induced by diet only (Diet); diet and aerobic exercise training (Diet + AT), or diet and resistance exercise training (Diet + RT). Values in boldface indicate significant differences (P < 0.05). B) Relative number of participants at cut-points of IAAT to predict risk of cardiovascular disease during overweight and after achieving normal weight.
Significant correlations were observed between delta CVD risk factors with delta IAAT, showing the improvements in CVD risk factors after weight loss were related to decreases of IAAT, even after adjusting for race, changes in %FM and changes in V̇O2max (Table 2). However, no significant correlation was observed between delta HDL-C and delta IAAT.
Table 2.
Partial correlation between ∆CVD risk factors and ∆IAAT adjusted by race, ∆%FM and ∆V̇O2max
| ∆IAAT |
||
|---|---|---|
| ∆CVD risk factors | r | P-value |
| ∆TC | 0.20 | 0.031 |
| ∆HDL-C | −0.14 | 0.120 |
| ∆LDL-C | 0.24 | 0.008 |
| ∆TG | 0.28 | 0.002 |
| ∆TC/HDL-C | 0.31 | 0.001 |
| ∆LDL-C/HDL-C | 0.31 | 0.001 |
Abbreviations: ∆IAAT, delta of intra-abdominal adipose tissue; ∆CVD risk factors, delta of cardiovascular disease risk; ∆TC, delta of total cholesterol; ∆HDL-C, delta of high-density lipoprotein cholesterol; ∆LDL-C, delta of low-density lipoprotein cholesterol; TG, triglycerides; ∆TC/HDL-C, delta of total cholesterol / high-density lipoprotein cholesterol; ∆LDL-C/HDL-C, delta of low-density lipoprotein cholesterol / high-density lipoprotein cholesterol; ∆%FM, delta of percentage of fat mass; ∆V̇O2max, delta of maximal oxygen consumption. Values in boldface indicate significant correlations (P < 0.05).
DISCUSSION
Diet without exercise elicited similar decreases in IAAT and CVD risk factors as diet with exercise (either AT or RT), suggesting that weight loss is a very potent stimulus for improving metabolic health in overweight pre-menopausal women. However, addition of exercise did not have an added effect on reducing CVD risk factors. Interestingly, after achieving normal weight (BMI averaged about 24 kg/m2), the prevalence of women above the previously established upper cut-point for metabolic disease,21 IAAT >110 cm2, were observed (decreasing from 13.9 to 2.5%), while the prevalence of women below the cut-point showing no risk for CVD,21 IAAT <40 cm2, increased (from 9.0 to 40.2%), suggesting the majority of women were no longer at risk of being over fat.
It has previously been shown by Ross et al.,8 that for every kilogram of weight loss induced by diet, the IAAT decreased ~3 to 4 cm2 or ~2 to 3%. Similarly, we observed a decrease of ~38% of IAAT after ~12 kg weight loss. After adding AT or RT to diet, effects on IAAT were similar, suggesting exercise training does not influence fat distribution changes during energy restriction. Other studies are consistent with this finding.7,9–11,15,17 It must be acknowledged that the participants in this study underwent a very low calorie diet, so it is possible if not probable the extreme calorie restriction may have influence the results. Consistent with that premise several studies have shown that exercise training induces visceral fat loss despite no weight loss.7,14,25 It is important to point out that although fat distribution is not favorably affected by exercise training during diet induced weight loss, resistance training conserves fat-free mass during weight loss.26 In addition, both resistance training and aerobic training prevent regain of any IAAT for one year following weight loss.27
A weight loss of ~12 kg in the present study significantly influenced a reduction of the CVD risk factors, improved blood lipids, which appeared to be at least partially mediated by the corresponding reductions in IAAT. In addition, ratios of TC/HDL-C and LDL-C/HDL-C decreased after weight loss to a level considered minimal risk of CVD.28 However, adding AT or RT to the diet also did not induce greater improvements in CVD risk factors. A similar study, dieting with or without AT or RT induced an ~10 kg weight loss, also demonstrated no exercise effects CVD risk after 16 weeks.10
Although exercise training does not seem to affect visceral fat loss during weight loss when using a very low calorie diet, it does not mean that exercise training may not be beneficial. It has already been shown that RT conserve fat-free mass and resting energy expenditure,26 decreases heart rate during exercise tasks after weight loss,29 prevents regain of visceral fat27 and conserves insulin sensitivity30 for 1 year following weight loss. Thus, considering the importance of exercise on morphological and physiological improvements, it is suggested that exercise training be included for all, even during periods of weight loss.
Strengths of the present study include: all meals were provided through the GCRC during the intervention program ensuring similar diets for all participants. In addition, all participants reduced to a similar BMI, 24–25 kg/m2.
In conclusion, a diet alone or combined with AT or RT elicit similar improvements on IAAT and CVD risk factors after weight loss. These results are supportive of the prevailing recommendation, that a BMI of less than 25 kg/m2 is associated with healthful visceral fat levels and thus favorable body composition in pre-menopausal women. It is also supportive of the concept that reduced visceral fat is related to improved CVD risk.
ACKNOWLEDGMENTS
This work was supported by the NIH grants R01 AG027084–01, R01 AG27084-S, R01 DK049779, P30 DK56336, P60 DK079626, UL 1RR025777. Clinical trial “Exercise training in obesity-prone Black and White women”, registration identification number NCT00067873. URL: https://clinicaltrials.gov/ct2/show/NCT00067873
Footnotes
CONFLICT OF INTEREST
The authors declared no conflict of interest.
REFERENCES
- 1.Tchernof A, Després J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013; 93: 359–404. [DOI] [PubMed] [Google Scholar]
- 2.Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS. Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr 1997; 65: 855–860. [DOI] [PubMed] [Google Scholar]
- 3.Hunter GR, Kekes-Szabo T, Snyder SW, Nicholson C, Nyikos I, Berland L. Fat distribution, physical activity, and cardiovascular risk factors. Med Sci Sports Exerc 1997; 29: 362–369. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Fox CS, Hickson DA, May WD, Hairston KG, Carr JJ et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab 2010; 95: 5419–5426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elffers TW, de Mutsert R, Lamb HJ, de Roos A, Willems van Dijk K, Rosendaal FR et al. Body fat distribution, in particular visceral fat, is associated with cardiometabolic risk factors in obese women. PloS One 2017; 12: e0185403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demerath EW, Reed D, Rogers N, Sun SS, Lee M, Choh AC et al. Visceral adiposity and its anatomical distribution as predictors of the metabolic syndrome and cardiometabolic risk factor levels. Am J Clin Nutr 2008; 88: 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ross R, Dagnone D, Jones PJ, Smith H, Paddags A, Hudson R et al. Reduction in obesity and related comorbid conditions after diet-induced weight loss or exercise-induced weight loss in men. A randomized, controlled trial. Ann Intern Med 2000; 133: 92–103. [DOI] [PubMed] [Google Scholar]
- 8.Ross R Effects of diet- and exercise-induced weight loss on visceral adipose tissue in men and women. Sports Med 1997; 24: 55–64. [DOI] [PubMed] [Google Scholar]
- 9.Janssen I, Ross R. Effects of sex on the change in visceral, subcutaneous adipose tissue and skeletal muscle in response to weight loss. Int J Obes Relat Metab Disord 1999; 23: 1035–1046. [DOI] [PubMed] [Google Scholar]
- 10.Janssen I, Fortier A, Hudson R, Ross R. Effects of an energy-restrictive diet with or without exercise on abdominal fat, intermuscular fat, and metabolic risk factors in obese women. Diabetes Care 2002; 25: 431–438. [DOI] [PubMed] [Google Scholar]
- 11.Idoate F, Ibañez J, Gorostiaga EM, García-Unciti M, Martínez-Labari C, Izquierdo M. Weight-loss diet alone or combined with resistance training induces different regional visceral fat changes in obese women. Int J Obes 2005 2011; 35: 700–713. [DOI] [PubMed] [Google Scholar]
- 12.Kohrt WM, Obert KA, Holloszy JO. Exercise training improves fat distribution patterns in 60- to 70-year-old men and women. J Gerontol 1992; 47: M99–105. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz RS, Shuman WP, Larson V, Cain KC, Fellingham GW, Beard JC et al. The effect of intensive endurance exercise training on body fat distribution in young and older men. Metabolism 1991; 40: 545–551. [DOI] [PubMed] [Google Scholar]
- 14.Carnero EA, Amati F, Pinto RS, Valamatos MJ, Mil-Homens P, Sardinha LB. Regional fat mobilization and training type on sedentary, premenopausal overweight and obese women. Obesity 2014; 22: 86–93. [DOI] [PubMed] [Google Scholar]
- 15.Ho SS, Dhaliwal SS, Hills AP, Pal S. The effect of 12 weeks of aerobic, resistance or combination exercise training on cardiovascular risk factors in the overweight and obese in a randomized trial. BMC Public Health 2012; 12: 704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Després JP, Lamarche B. Effects of diet and physical activity on adiposity and body fat distribution: implications for the prevention of cardiovascular disease. Nutr Res Rev 1993; 6: 137–159. [DOI] [PubMed] [Google Scholar]
- 17.Ross R, Rissanen J. Mobilization of visceral and subcutaneous adipose tissue in response to energy restriction and exercise. Am J Clin Nutr 1994; 60: 695–703. [DOI] [PubMed] [Google Scholar]
- 18.Keating SE, Machan EA, O’Connor HT, Gerofi JA, Sainsbury A, Caterson ID et al. Continuous exercise but not high intensity interval training improves fat distribution in overweight adults. J Obes 2014; 2014: 834865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hunter GR, Bryan DR, Wetzstein CJ, Zuckerman PA, Bamman MM. Resistance training and intra-abdominal adipose tissue in older men and women. Med Sci Sports Exerc 2002; 34: 1023–1028. [DOI] [PubMed] [Google Scholar]
- 20.Treuth MS, Hunter GR, Kekes-Szabo T, Weinsier RL, Goran MI, Berland L. Reduction in intra-abdominal adipose tissue after strength training in older women. J Appl Physiol 1995; 78: 1425–1431. [DOI] [PubMed] [Google Scholar]
- 21.Williams MJ, Hunter GR, Kekes-Szabo T, Trueth MS, Snyder S, Berland L et al. Intra-abdominal adipose tissue cut-points related to elevated cardiovascular risk in women. Int J Obes Relat Metab Disord 1996; 20: 613–617. [PubMed] [Google Scholar]
- 22.Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr 1988; 48: 1351–1361. [DOI] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem 1972; 18: 499–502. [PubMed] [Google Scholar]
- 24.Hellerstein HK, Franklin BA. Exercise testing and prescription. In: Wenger NK, Hellerstein HK (eds). Rehabilitation of the Coronary Patient. John Wiley & Sons Inc.: New York, NY, 1984, pp 197–284. [Google Scholar]
- 25.Thomas EL, Brynes AE, McCarthy J, Goldstone AP, Hajnal JV, Saeed N et al. Preferential loss of visceral fat following aerobic exercise, measured by magnetic resonance imaging. Lipids 2000; 35: 769–776. [DOI] [PubMed] [Google Scholar]
- 26.Hunter GR, Byrne NM, Sirikul B, Fernández JR, Zuckerman PA, Darnell BE et al. Resistance training conserves fat-free mass and resting energy expenditure following weight loss. Obesity 2008; 16: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 27.Hunter GR, Brock DW, Byrne NM, Chandler-Laney PC, Del Corral P, Gower BA. Exercise training prevents regain of visceral fat for 1 year following weight loss. Obesity 2010; 18: 690–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millán J, Pintó X, Muñoz A, Zúñiga M, Rubiés-Prat J, Pallardo LF et al. Lipoprotein ratios: Physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag 2009; 5: 757–765. [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter GR, Fisher G, Bryan DR, Zuckerman PA. Weight loss and exercise training effect on oxygen uptake and heart rate response to locomotion. J Strength Cond Res 2012; 26: 1366–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fisher G, Hunter GR, Gower BA. Aerobic exercise training conserves insulin sensitivity for 1 yr following weight loss in overweight women. J Appl Physiol 2012; 112: 688–693. [DOI] [PMC free article] [PubMed] [Google Scholar]


