Abstract
A woman in her 40s presented to the emergency department with headache and unintentional weight loss in September 2018. Investigations revealed a widely metastatic pan-negative melanoma of unknown primary. She had multiple lines of treatment including combination immunotherapy and chemotherapy. Next-generation sequencing identified an SKAP2-BRAF fusion protein, and she was commenced on an MEK inhibitor in September 2019 with a partial response seen on restaging scans after 6 weeks and a dramatic fall in her lactate dehydrogenase from 2248 IU/L to 576 IU/L. Unfortunately, the response was not maintained and she died from progression of her cancer in January 2020. SKAP2-BRAF fusions have a dimerisation domain that paradoxically activates the mitogen-activated protein kinase pathway, resulting in hyperproliferation if first-generation or second-generation BRAF inhibitors are used. Our knowledge is limited regarding the complex effects of targeted therapy in rare BRAF fusion proteins.
Keywords: skin cancer, immunological products and vaccines, malignant disease and immunosuppression
Background
Melanoma is the fifth most common cancer in Ireland, with an incidence rate of 21 cases per 100 000 per year. Risk factors for melanoma include sun exposure, sunbed use, fair skin, increased moles and freckling, and a family history of melanoma.1 Cutaneous melanoma is classified according to its site of origin (epithelium vs non-epithelium), the level of cumulative solar damage and the frequency of mutations. Superficial spreading melanoma is the most common subtype (70%), followed by nodular melanoma (15%), lentigo maligna melanoma (10%) and acral lentiginous melanoma (<5%). Rare variants include amelanotic, spitzoid and desmoplastic melanoma.2
Recurrent driver mutations at specific loci in BRAF, NRAS, KIT, GNAQ and GNA11 define clinically relevant molecular subsets of melanoma. KIT mutations are more frequent in mucosal melanomas and GNAQ/GNA11 mutations in uveal melanomas. Identifying these possible mutations is important to guide treatment and access targeted therapy. Up to 30% are ‘pan-negative’ for these recurrent mutations; however, 4%–8% of this pan-negative melanoma population may harbour a BRAF fusion.3 We present the case of a patient with metastatic melanoma with an SKAP2-BRAF fusion treated with the MEK inhibitor trametinib after progression on immunotherapy and chemotherapy.
Case presentation
A woman in her 40s presented to the emergency department with headache and unintentional weight loss in September 2018. Her only relevant background history was of resection of a giant congenital naevus on her back in her childhood. The patient did not meet the criteria for a screening MRI scan for neurocutaneous melanosis during her initial diagnosis of a giant congenital naevus as she was neurologically asymptomatic.4
Investigations
Staging CT scans showed widely metastatic disease with a lung mass and brain, liver and adrenal metastases. A lung biopsy confirmed a diagnosis of stage 4 melanoma of unknown primary that was negative for BRAF, NRAS, KIT, GNAQ and GNA11 mutations via immunohistochemistry and polymerase chain reaction (PCR) testing. Immunohistochemistry revealed positive staining of the neoplastic cells for Melan A and S100, which were consistent with metastatic malignant melanoma. Her lactate dehydrogenase (LDH) at diagnosis was 391 IU/L.
Her risk factors for melanoma included fair skin and several moles under regular skin surveillance with the dermatology team. A repeat full skin examination and ocular examination did not elucidate the location of a primary melanoma.
Treatment
The patient received brain stereotactic radiation, followed by combination immunotherapy with ipilimumab 3 mg/kg and nivolumab 1 mg/kg q21/7 (3 weekly) for two cycles from November 2018. Ipilimumab is a CTLA inhibitor and nivolumab is a PD1 inhibitor which is a standard first-line immunotherapy treatment regime for metastatic melanoma. She developed progression of disease in December 2018, with an increase in index lung metastasis from 1.8 cm to 2.8 cm and new bilobar liver metastases ranging from 0.4 cm to 1.0 cm, abnormal liver function tests and a rising LDH to 1448 IU/L.
She proceeded to dacarbazine 1000 mg/m2 q21/7 for six cycles with an initial response in February 2019 and a decrease in LDH to 238 IU/L. However, she had progression of the disease in May 2019 with the index lung metastasis increasing to 4.5 cm and the liver metastasis to 1.8 cm with an LDH of 1063 IU/L. She then presented with confusion and was found to have an enlarging brain metastasis and underwent emergency resection followed by whole brain radiation.
On recovery, she was rechallenged with another immunotherapy PD1 inhibitor, pembrolizumab 400 mg q6/52 (6 weekly) from June 2019 for three cycles. However, her cancer progressed 3 months later into the brain, lungs, liver, skin and axillae, and the index lung metastasis increased to 5 cm and the liver metastasis to 2.4 cm with an LDH of 2248 IU/L. She had no toxicity issues with her various lines of treatment other than fatigue.
Unfortunately, there were no clinical trials available. Metastatic tissue from the lung was sent for next-generation sequencing (NGS) (FoundationOne). A BRAF fusion protein was identified and she was commenced on an MEK inhibitor trametinib in September 2019.
Outcome and follow-up
On starting trametinib, there was a dramatic clinical improvement along with a fall in LDH from 2248 IU/L to 576 IU/L, and she had a partial response seen on restaging scans after 6 weeks (figures 1 and 2). The response was not maintained and her cancer progressed radiologically and clinically within weeks of the initial response, with her LDH rising to 5011 IU/L in December 2019. She died from progression of her cancer in January 2020.
Figure 1.
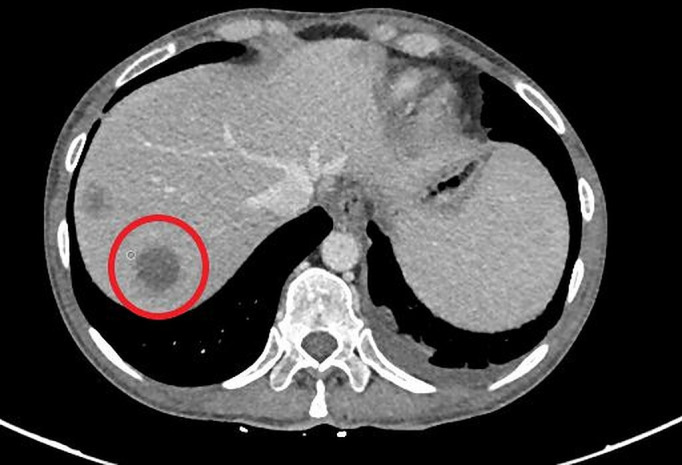
Liver lesions prior to commencing on an MEK inhibitor. Lactate dehydrogenase was 2248 IU/L.
Figure 2.
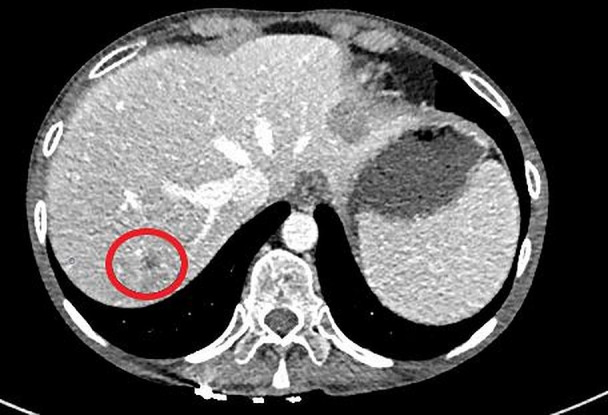
Improvement in liver lesions after a 6-week interval of commencing on an MEK inhibitor. Lactate dehydrogenase was 576 IU/L.
Discussion
Initial BRAF testing for this patient was via real-time PCR that identifies mutations at BRAF exon 11 and 15 only, which were not detected. NGS is a DNA sequencing technology that uses parallel sequencing of multiple small fragments of DNA to sequence an individual’s genome. The aim of NGS testing is to identify actionable mutations that may unlock further treatment options and help predict treatment response. NGS allowed further sequencing of the patient’s genome, thus enabling detection of the BRAF fusion.
Oncogenic BRAF fusions arise from genomic rearrangements, placing the 3′ portion of the BRAF gene encoding the kinase domain at the 5′ position. This results in the loss of the autoinhibitory domain of BRAF and the expression of oncoproteins that are constitutively active.5
A recent review of the literature found that BRAF fusions are more prevalent in female patients with melanocytic tumours. They are early driver events and are often associated with spitzoid histopathology as seen in our patient. The median age at presentation with a BRAF fusion-associated melanoma was 39 years.6
Data comparing the various fusions’ response to targeted treatment and mechanism of action are limited.7 Historically patients with non-V600 BRAF mutations tend to respond less well to BRAF/MEK inhibition, which may be due to these tumours harbouring alternative pathways of cellular activation.8 There is a suggestion that rare BRAF fusions may show responsiveness to BRAF and MEK inhibitors.9 10
A study that specifically looked at the SKAP2-BRAF fusion showed that the fusion partner contained a dimerisation domain. The presence of this dimerisation domain promoted paradoxical activation of the mitogen-activated protein kinase pathway and hyperproliferation in response to first-generation and second-generation BRAF inhibitors. This was the reason why our patient received MEK inhibitor alone, rather than a combination of BRAF and MEK inhibitors, which would be the standard practice in BRAF-mutated melanoma.
Preclinical in vivo and in vitro studies using cell lines harbouring this BRAF fusion protein have shown responses to third-generation BRAF inhibitors in combination with MEK inhibitors.11
Patient’s perspective.
Perspective from patient’s husband:
My wife had regular skin screening annually with several moles under follow-up until 2018. Though probably unconnected with the eventual diagnosis of metastatic melanoma, we both felt that perhaps something was missed in the early stages. The care she received thereafter was excellent.
Learning points.
Our knowledge is limited regarding the complex effects of targeted therapy in rare BRAF fusions.
SKAP2-BRAF fusions have a dimerisation domain that paradoxically activates the mitogen-activated protein kinase pathway, resulting in hyperproliferation if first-generation or second-generation BRAF inhibitors are used.
The efficacy of third-generation combination of BRAF and MEK inhibitors looks promising in BRAF fusions in preclinical studies.
Acknowledgments
Thomas Botton
Footnotes
Contributors: All authors were involved in the patient’s care. The manuscript was drafted by SMC, ML and MB, with critical appraisal by CMK. The final version has been approved by all authors.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Melanoma cancer Factsheet, National cancer registry Ireland. Available: https://www.ncri.ie/sites/ncri/files/factsheets/Factsheet%20melanoma.pdf
- 2.Elder DE, Bastian BC, Cree IA, et al. The 2018 World Health organization classification of cutaneous, mucosal, and uveal melanoma: detailed analysis of 9 distinct subtypes defined by their evolutionary pathway. Arch Pathol Lab Med 2020;144:500–22. 10.5858/arpa.2019-0561-RA [DOI] [PubMed] [Google Scholar]
- 3.Hutchinson KE, Lipson D, Stephens PJ, et al. BRAF fusions define a distinct molecular subset of melanomas with potential sensitivity to MEK inhibition. Clin Cancer Res 2013;19:6696-702. 10.1158/1078-0432.CCR-13-1746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waelchli R, Aylett SE, Atherton D, et al. Classification of neurological abnormalities in children with congenital melanocytic naevus syndrome identifies magnetic resonance imaging as the best predictor of clinical outcome. Br J Dermatol 2015;173:739–50. 10.1111/bjd.13898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu H, Villafane N, Dogruluk T, et al. Engineering and functional characterization of fusion genes identifies novel oncogenic drivers of cancer. Cancer Res 2017;77:3502–12. 10.1158/0008-5472.CAN-16-2745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shalin SC. A review of kinase fusions in melanocytic tumors. Lab Invest 2017;97:158–65. 10.1038/labinvest.2016.122 [DOI] [PubMed] [Google Scholar]
- 7.Turner JA, Bemis JGT, Bagby SM, et al. BRAF fusions identified in melanomas have variable treatment responses and phenotypes. Oncogene 2019;38:1296–308. 10.1038/s41388-018-0514-7 [DOI] [PubMed] [Google Scholar]
- 8.Johnson DB, Nebhan CA, Noel MS. MEK inhibitors in non-V600 BRAF mutations and fusions. Oncotarget 2020;11:3900–3. 10.18632/oncotarget.27788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menzer C, Menzies AM, Carlino MS, et al. Targeted therapy in advanced melanoma with rare BRAF mutations. J Clin Oncol 2019;37:3142–51. 10.1200/JCO.19.00489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menzies AM, Yeh I, Botton T, et al. Clinical activity of the MEK inhibitor trametinib in metastatic melanoma containing BRAF kinase fusion. Pigment Cell Melanoma Res 2015;28:607–10. 10.1111/pcmr.12388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Botton T, Talevich E, Mishra VK, et al. Genetic heterogeneity of BRAF fusion kinases in melanoma affects drug responses. Cell Rep 2019;29:573–88. 10.1016/j.celrep.2019.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]


