Abstract
We describe a unique case of Beckwith-Wiedemann syndrome (BWS). A 29-year-old woman with ultrasound and clinical findings, specific to BWS is described. Important insights gained from this study are as follows: (1) quad test may be very useful to increase awareness of BWS. This is the first report, which demonstrated that elevated inhibin-A is related to BWS. Unexplained elevation of serum biomarkers, especially all the four markers, should raise awareness of BWS. (2) Early provisional diagnosis in this case was based on the findings of omphalocele, placental mesenchymal dysplasia and abnormal quad test. (3) Follow-up scans are important for late-occurring supportive findings, such as macroglossia, ear abnormalities and visceromegaly. (4) BWS is strongly associated with preeclampsia, which tended to be more severe and of earlier-onset. (5) Molecular genetic analysis is helpful, but not always necessary in cases of fulfilment of clinical criteria like in this case.
Keywords: genetics, obstetrics and gynaecology, pregnancy, ultrasonography
Background
Beckwith-Wiedemann syndrome (BWS) is a rare disease with overgrowth condition. The characteristic features are very specific, especially macroglossia. It was first reported in 1963 by an American paediatric pathologist, John Bruce Beckwith, and then by German paediatrician, Hans-Rudolf Wiedemann, in 1964.1The incidence of BWS is approximately 1:10 000 to 1:13 700 live births, but the incidence may be an underestimation due to early fetal death in utero.2 BWS is one of the well-known imprinting disorder associated with epigenetic abnormal regulation of genes on the chromosome 11p15 region, which is involved in the development of multiple organs; therefore, patients affected with BWS demonstrate a wide and varied clinical manifestation spectrum.3 The most common clinical findings are macroglossia, macrosomia and abdominal wall defects. Prenatal diagnosis can be performed by fetal ultrasonography for detection of fetal abnormalities, such as macroglossia; abdominal wall defect, especially omphalocele; visceromegaly (kidney enlargement, hepatomegaly or adrenal gland enlargement); cardiac anomalies; and placental dysplasia.
There are several publications for clinical diagnostic criteria to diagnose BWS, such as the presence of three or more major findings or two major findings together with a minor one.4–6 The major findings include: (1) ventral wall defect, for example, exomphalos and umbilicocele; (2) tongue enlargement; (3) enlarged newborn (birth weight of greater than 90th percentile); (4) accelerated growth after birth (body length greater than 90th percentile); (5) embryonal neoplasms, especially Wilms tumour, hepatoblastoma, tumour of the adrenal gland or neuroblastoma); (6) external ear abnormalities (anterior ear creases or posterior helical pits); (7) organomegaly of the viscera; (8) adrenal cytomegaly; (9) hemihyperplasia; (10) renal and ureteric abnormalities (dysplastic/spongy medulla and enlargement of kidneys with deposit of calcium salts); (11) familial history of BWS; and (12) cleft palate. The minor criteria include: (1) increased amount of amniotic fluid; (2) placentomegaly or placental mesenchymal dysplasia (PMD); (3) umbilical cord thickening; (4) preterm birth; (5) hypoglycaemia in newborns; (6) a port-wine stain in the area of the glabella; (7) typical face; (8) cardiac enlargement and abnormalities or cardiomyopathy; (9) rectus abdominis diastasis; (10) polydactyly; (11) accessary nipples; and (12) increased bone age. The aims of this case study are to provide new prenatal feature of BWS, to add the important findings to the limited existing data in previous reports, strengthening the previous observations, to underline the importance of follow-up scans in case of suspicion of BWS and to inspire fetal sonographers in early diagnosis of BWS for more options of management. Written informed consent was given by the patient for publication.
Case presentation
A 29-year-old woman, gravida 1, presented for ultrasound fetal anomaly screening at 16 and 5/7 weeks’ gestation. She and her husband had no known underlying disease and no history of genetic disorder in their family. Antenatal course before this visit was uneventful, except that a quadruple test showed high level of maternal serum alpha fetoprotein (MSAFP=266.1 ng/mL: 10.22 multiple of median (MoM)), unconjugated estriol (uE3=3.966 nmol/L: 2.57 MoM), inhibin-A (1320 pg/mL: 6.48 MoM) and a high level of free beta human chorionic gonadotropin (beta-hCG=36.42 mIU/mL: 1.4 MoM).
The detailed ultrasound for fetal anomaly screening revealed omphalocele (size 1.77×1.43 cm) with small bowel content (figure 1). Fetal biometry was consistent with gestational age. The placenta was thickened (5 cm), oedematous and spongy in appearance, and filled with multiple small lacunae, without high vascularisation. Amniocentesis for chromosome study showed normal karyotype (46, XY). The follow-up scan at 20+5 weeks of gestation revealed additional abnormalities, including visceromegaly (hepatomegaly and renal enlargement of both sides: greater than 95th percentile for the gestational week). The follow-up scan at 24+5 weeks revealed additional findings of macroglossia, outer ear abnormality, large-for-date estimated fetal weight and progressively thickened placenta (8 cm) with spongy heterogeneous echodensity, as presented in (figure 1 and video 1).
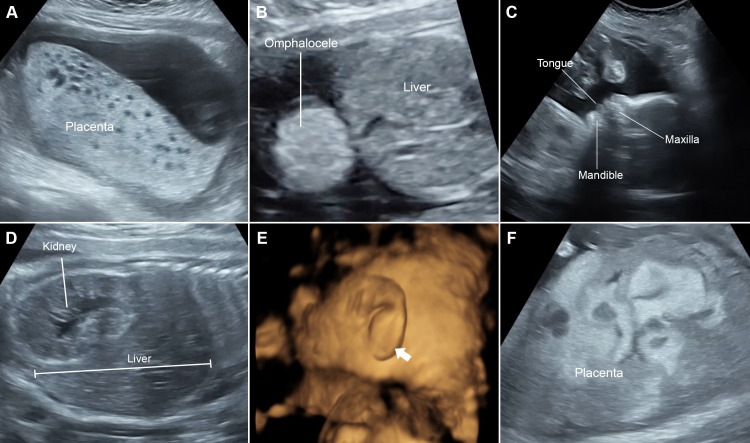
Figure 1.
Prenatal ultrasound: A) Enlarged placenta with spongy appearance at 15+6 weeks. (B) Omphalocele at 15+6 weeks. (C) Facial profile shows macroglossia at 24+5 weeks. (D) Sagittal scan of fetal abdomen shows enlarged kidney and liver at 24+5 weeks. (E) The 3D ultrasound shows abnormality of the lower ear lobe. (F) Markedly enlarged placenta and more complex at 28+5 weeks.
Video 1.
At 27+1 weeks of gestation, the woman developed preeclampsia without severe features. She had hypertension and significant proteinuria, whereas other laboratory tests, such as haematological profile and liver enzyme levels, were within normal limits. Conservative management was instituted. Corticosteroid for fetal lung maturity promotion was given, and severe features were intensively monitored. However, at 31+5 weeks of gestation, she had premature rupture of membrane, leading to preterm labour and vaginal delivery, giving birth to a male newborn weighing 2150 g; Apgar scores of 7 and 8 at 1 and 5 min, respectively. She developed haemolysis, elevated liver enzymes, low platelet count (HELLP) syndrome (haemolysis: lactate dehydrogenase 926 U/L; elevated liver enzymes: aspartate transaminase/alanine transaminase=600/700 U/L; and low platelets 22 000/mm3) within 6 hours after delivery. Also, she had the clinical manifestations of posterior reversible encephalopathy syndrome. The standard care of preeclampsia with severe features was provided and the patient gradually improved.
The male newborn had large-for-date weight (90th percentile for 32 weeks: 2117 g), omphalocele, macroglossia and abnormal ears, as presented in figure 2. The placenta weighed 1000 g (97th percentile for 32 weeks: 465 g). Microscopic findings revealed PMD with focal chorioangioma. The visceromegaly were confirmed on neonatal ultrasonography, including renal enlargement and large liver. The diagnosis was consistent with BWS. Molecular genetic analysis was not performed.
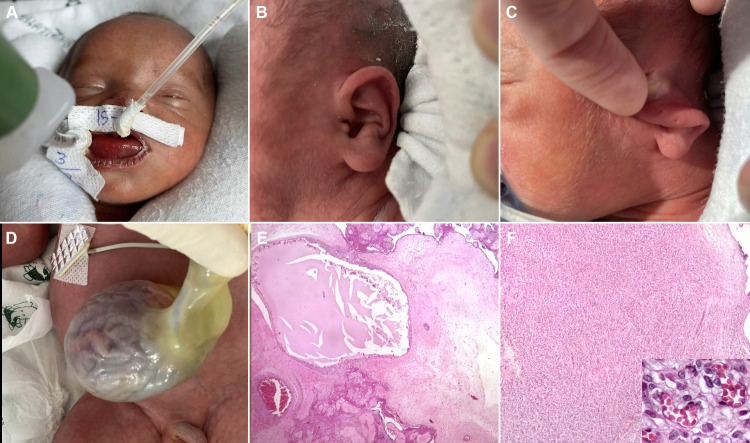
Figure 2.
Neonatal findings. (A) Macroglossia. (B) Abnormal ear lobe. (C) Helical ear pit. (D) Omphalocele. (E) Placental mesenchymal dysplasia. The stem villi show dilated thick wall blood vessels, oedematous stroma with myxoid change and focal cistern formation. The cistern contains proteinaceous material (H&E stained, ×10). (F) Chorangiomatosis. The major stem villi show several well-circumscribed masses with proliferation of capillary-sized blood vessels (inset) (H&E stained, ×10/400).
Discussion
BWS is a rare disease and can be prenatally suspected or diagnosed by ultrasonogram. The most common prenatal findings are polyhydramnios and visceromegaly, accounting for 50% of cases followed by omphalocele (43%),7 as seen in this case. However, these features are not specific to BWS. Others features included overgrowth, macroglossia, and renal malformation. The abnormal appearance of a placenta, including placentomegaly, PMD, chorioangioma and extravillous trophoblastic cytomegaly, is common in BWS.8 9 It is of note that, in this case, abnormal placenta could be detected as early as at 16 weeks of gestation, characterised by increased thickness, hyperechoic parenchymal tissue with multiple small lacunae, highly suspicious for PMD at the first visit. Interestingly, although among cases of omphalocele, the chance of BWS is approximately 20%, the omphalocele together with PMD and elevated levels of the four serum biomarkers enabled us to make a provisional diagnosis of BWS. The diagnosis was even more reliable, especially when the specific markers like macroglossia and visceromegaly were demonstrated on the follow-up scans. In summary, prenatal diagnosis of BWS in this case was based on the major findings, including: (1) omphalocele, (2) macroglossia, (3) hepatomegaly, (4) nephromegaly and (5) outer ear abnormality. Additionally, PMD, which is one of the minor criteria of BWS, was highly suspected, with postnatal pathological confirmation. It is of note that DNA analysis was unfortunately not performed in this case due to unavailability. Nevertheless, the diagnosis could be reliably made based on clinical criteria.
Omphalocele is the earliest and reliable prenatal sonographic finding. Although, other signs might not present at the time of diagnosis of omphalocele, it should be underlined that follow-up ultrasound is important for detection of the late-occurring signs, such as macroglossia and overgrowth, that can be strongly supportive of the correct diagnosis.
Interestingly, we noticed the abnormal increase of all serum markers of quad test (markedly high MSAFP, inhibin-A and uE3 levels, and also relatively high beta-hCG level). High levels of MSAFP and uE3 may be associated with the visceral organ enlargement (liver and adrenal gland) and placentomegaly may probably cause an increase in inhibin-A and beta-hCG levels. These findings were consistent with observations in previous reports,7 10 which showed high serum levels of the triple screen biomarkers (MSAFP, uE3 and beta-hCG) during 15–18 weeks of gestation. Moreover, in our case, inhibin-A levels were also markedly increased. An increase in inhibin-A associated with BWS has never been described elsewhere. To the best of our knowledge, elevation of all biomarkers of quad test is very rarely reported to be specific to any certain disorders. This finding may be clinically useful for early detection of BWS. Unexplained elevation of inhibin-A along with any other biomarkers of quad test may warrant the possibility of BWS. In other words, the quad test can facilitate early detection of fetal BWS. Accordingly, this characteristic is clinically important, since approximately one out of four cases of BWS had no structural abnormality detected by prenatal ultrasound. This emphasises that for negative ultrasound finding in cases of unexplained elevated serum biomarkers, BWS should be kept in mind and DNA analysis may probably be warranted.
Of a limited number of case reports, several cases with fetal BWS developed preeclampsia, possibly associated with abnormal placentation, which tended to be early-onset and more severe. Of them, HELLP syndrome may develop in as many as 9% of pregnancy with fetal BWS.11 Likewise, the case presented here strongly supports such association. In our case, preeclampsia was early-onset and progressive to be HELLP syndrome. Placentomegaly or PMD is likely involved in the development, especially in the cases that display mutations in 9CDKN1C and associated with PMD.12 Therefore, the parents should be counselled about the risk of preeclampsia and surveillance. Early detection will help provide suitable management and avoid serious maternal and obstetric outcomes. Based on this case and literature review, we suggest the cases diagnosed early with fetal BWS receive ASA for preeclampsia prophylaxis.
In conclusion, this study provides new prenatal feature of BWS (elevated inhibin-A level), which can facilitate early detection. Additionally, this case adds important findings to the limited existing data in literature, which can strengthen the previous observations as follows: (1) unexplained elevated levels of the triple test biomarkers are strongly related to BWS; (2) omphalocele and abnormal placenta (thickened and multiple lacunae) can be detected early in gestation and the combination is strongly suggestive of BWS; (3) prenatal late-occurring sonographic signs may include macroglossia, macrosomia, visceromegaly (hepatomegaly and renal enlargement) and ear abnormality; (4) pregnancy with fetal BWS, especially involving PMD, is at higher risk for preeclampsia with a tendency to be more severe, particularly HELLP syndrome, and earlier onset.
Learning points.
Elevated inhibin-A level is related to Beckwith-Wiedemann syndrome (BWS).
The combination of omphalocele, placental mesenchymal dysplasia and abnormal quad test very strongly suggests BWS.
Follow-up scans are very important for late-occurring abnormalities, such as macroglossia, visceromegaly and macrosomia.
BWS is strongly associated with preeclampsia, which tended to be more severe and of earlier onset.
Acknowledgments
The authors wish to thank the Thailand Research Fund (DPG-6280003).
Footnotes
Contributors: PJ: Ultrasound diagnosis, obstetric management, manuscript drafting and final approval. TT: Ultrasound diagnosis, obstetric management and final approval. KT: Conceptualisation, ultrasound diagnosis, manuscript revising and final approval.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer-reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Cohen MM. Beckwith-Wiedemann syndrome: historical, clinicopathological, and Etiopathogenetic perspectives. Pediatr Dev Pathol 2005;8:287–304. 10.1007/s10024-005-1154-9 [DOI] [PubMed] [Google Scholar]
- 2.Mussa A, Molinatto C, Cerrato F, et al. Assisted reproductive techniques and risk of Beckwith-Wiedemann syndrome. Pediatrics 2017;140:e20164311. 10.1542/peds.2016-4311 [DOI] [PubMed] [Google Scholar]
- 3.Enklaar T, Zabel BU, Prawitt D. Beckwith–Wiedemann syndrome: multiple molecular mechanisms. Expert Rev Mol Med 2006;8:1–19. 10.1017/S1462399406000020 [DOI] [PubMed] [Google Scholar]
- 4.Cammarata-Scalisi F, Avendaño A, Stock F, et al. Beckwith-Wiedemann syndrome: clinical and etiopathogenic aspects of a model genomic imprinting entity. Arch Argent Pediatr 2018;116:368–73. 10.5546/aap.2018.eng.368 [DOI] [PubMed] [Google Scholar]
- 5.Mussa A, Di Candia S, Russo S, et al. Recommendations of the scientific Committee of the Italian Beckwith–Wiedemann syndrome association on the diagnosis, management and follow-up of the syndrome. Eur J Med Genet 2016;59:52–64. 10.1016/j.ejmg.2015.11.008 [DOI] [PubMed] [Google Scholar]
- 6.Weksberg R, Shuman C, Beckwith JB. Beckwith–Wiedemann syndrome. Eur J Hum Genet 2010;18:8–14. 10.1038/ejhg.2009.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carli D, Bertola C, Cardaropoli S, et al. Prenatal features in Beckwith-Wiedemann syndrome and indications for prenatal testing. J Med Genet 2020;140:jmedgenet-2020-107311. 10.1136/jmedgenet-2020-107311 [DOI] [PubMed] [Google Scholar]
- 8.H'mida D, Gribaa M, Yacoubi T, et al. Placental mesenchymal dysplasia with Beckwith–Wiedemann syndrome fetus in the context of biparental and androgenic cell lines. Placenta 2008;29:454–60. 10.1016/j.placenta.2008.01.001 [DOI] [PubMed] [Google Scholar]
- 9.Armes JE, McGown I, Williams M, et al. The placenta in Beckwith-Wiedemann syndrome: genotype-phenotype associations, excessive extravillous trophoblast and placental mesenchymal dysplasia. Pathology 2012;44:519–27. 10.1097/PAT.0b013e3283559c94 [DOI] [PubMed] [Google Scholar]
- 10.Aagaard-Tillery KM, Buchbinder A, Boente MP, et al. Beckwith-Wiedemann syndrome presenting with an elevated triple screen in the second trimester of pregnancy. Fetal Diagn Ther 2007;22:18–22. 10.1159/000095837 [DOI] [PubMed] [Google Scholar]
- 11.Colpaert RM, Ramseyer AM, Luu T, et al. Diagnosis and management of placental mesenchymal disease. A review of the literature. Obstet Gynecol Surv 2019;74:611–22. 10.1097/OGX.0000000000000716 [DOI] [PubMed] [Google Scholar]
- 12.Romanelli V, Belinchón A, Campos-Barros A, et al. Cdkn1C mutations in HELLP/Preeclamptic mothers of Beckwith–Wiedemann syndrome (BWS) patients. Placenta 2009;30:551–4. 10.1016/j.placenta.2009.03.013 [DOI] [PubMed] [Google Scholar]