Abstract
Background
Immune checkpoint inhibitors (ICI) have emerged as a front-line therapy for a variety of solid tumors. With the widespread use of these agents, immune-associated toxicities are increasingly being recognized, including fatal myocarditis. There are limited data on the outcomes and prognostic utility of biomarkers associated with ICI-associated myocarditis. Our objective was to examine the associations between clinical biomarkers of cardiomyocyte damage and mortality in patients with cancer treated with ICIs.
Methods
We retrospectively studied 23 patients who developed symptomatic and asymptomatic troponin elevations while receiving ICI therapy at a National Cancer Institute-designated comprehensive cancer center. We obtained serial ECGs, troponin I, and creatine kinase-MD (CK-MB), in addition to other conventional clinical biomarkers, and compared covariates between survivors and non-survivors.
Results
Among patients with myocarditis, higher troponin I (p=0.037) and CK-MB (p=0.034) levels on presentation correlated with progression to severe myocarditis. Higher troponin I (p=0.016), CK (p=0.013), and CK-MB (p=0.034) levels were associated with increased mortality, while the presence of advanced atrioventricular block on presentation (p=0.088) trended toward increased mortality. Weekly troponin monitoring lead to earlier hospitalization for potential myocarditis (p=0.022) and was associated with decreased time to steroid initiation (p=0.053) and improved outcomes.
Conclusions
Routine troponin surveillance may be helpful in predicting mortality in ICI-treated patients with cancer in the early phase of ICI therapy initiation. Early detection of troponin elevation is associated with earlier intervention and improved outcomes in ICI-associated myocarditis. The recommended assessment and diagnostic studies guiding treatment decisions are presented.
Keywords: immunotherapy
Introduction
The emergence of immune checkpoint inhibitor (ICI) therapy has dramatically altered cancer treatment over the last decade. The ICI class of current Food and Drug Administration-approved drugs includes anti-programmed cell death 1 (anti-PD-1) antibodies (nivolumab, pembrolizumab, and cemiplimab), anti-programmed cell death ligand-1 (anti-PD-L1) antibodies (atezolizumab, durvalumab, and avelumab), and anti-cytotoxic T lymphocyte-associated antigen (CTLA-4) agents (ipilimumab). The PD-1/PD-L1 and CTLA-4 pathways provide negative feedback to immune activation; however, their blockade may lead to development of autoimmune toxicities.
Immune-related adverse events (irAEs) associated with ICI therapy can develop in virtually any organ and can limit their clinical utility.1 Cardiotoxicities, including myocarditis2 3 can result from ICI therapy. Myocarditis remains relatively rare, with a reported incidence of 0.06%–1%, although the precise incidence is unknown.2 ICI-associated myocarditis results in high case fatality, estimated to be around 50%.3 The prevalence of fatal myocarditis is increased for patients on anti-PD-1 and anti-CTLA4 combination immunotherapy.4 There exists uncertainty regarding the optimal treatment strategy for ICI-induced myocarditis, with a variety of immunosuppressants currently utilized in clinical practice, including high dose steroids, anti-thymocyte globulin (ATG), and abatacept.5
Previously, a single center study estimated the prevalence of ICI-associated myocarditis at 1.14%, with a variety of ICIs utilized.6 In that study, steroid treatment was initiated in 89% of ICI-associated myocarditis cases and lower doses of steroids were associated with higher residual troponin and higher major adverse cardiac event rates.6 Most reported cases of fulminant myocarditis appeared to be severe, requiring hospitalization and often intensive care unit (ICU) admission. We recently reported a case series of patients with severe myocarditis associated with ICI therapy,7 and we found a similar mortality but variable steroid treatment response.
Treatment of patients with ICI-associated myocarditis has been largely based on expert consensus encompassing grade ≥3 toxicity.5 Cases of ICI-associated severe myocarditis have been successfully treated with systemic corticosteroid therapy.2 8–13 Although some steroid-refractory cases responded to second-line immunosuppressive agents,14 most were clinically advanced with a fulminant course before steroid initiation.15–18 Recently, cases of a milder or ‘subclinical’ ICI-associated myocarditis have been reported.17 19 The incidence, treatment, and outcomes of mild ‘subclinical’ cases of myocarditis (grade 1–2), often managed without hospitalization, remain an area of further investigation.
We report a retrospective study of 15 patients with probable ICI-associated myocarditis, including 11 patients with severe, acute myocarditis and 4 patients with subclinical myocarditis. Our objective was to examine clinical characteristics, outcomes, and response to steroid therapy in these patients. Additionally, a subcohort analysis comparing clinical outcomes of patients with subclinical myocarditis with severe cases was performed. In these patients, we studied the association between troponin monitoring and clinical outcomes.
Methods
This is a retrospective single center study from an National Cancer Institute-designated comprehensive cancer center.
Patients
Cases were identified from the Roswell Park Comprehensive Cancer Center (RPCCC) electronic medical health record from January 2016 to January 2020. Patients included in this study had an elevated cardiac troponin I defined as ≥0.06 ng/mL during ICI therapy. Troponin levels were obtained either in the context of routine troponin monitoring or otherwise when prompted by the patient’s presentation. Patients who were determined by clinical assessment without an alternative cause for elevated troponin (eg, ischemic heart disease) and had a Naranjo Score20 of ‘possible’ to ‘definite’ elevated troponin as a result of ICI therapy were included in this study.
Troponin and ECG monitoring
The RPCCC Melanoma Program clinicians (IP and MSE) implemented a practice of weekly troponin monitoring in patients who are initiating ICI treatment, with the intention of facilitating early detection and treatment of cardiotoxicity. Patients were selected based on physicians’ choice. Under this monitoring protocol, patients were monitored every week for the first 4–6 weeks following the initiation of ICI treatment. Cases with elevated troponin were referred to a cardio-oncology team for subsequent evaluation. All patients in the study group were assessed by ECGs before ICI treatment initiation and after their presentation with signs of cardiotoxicity.
Myocarditis subgroups
Patients were considered to have myocarditis if they met the criteria proposed in the position statement of the European Society of Cardiology Working group,21 displaying either symptomatic troponin elevation or troponin elevation with newly abnormal ECG or functional or structural abnormalities on cardiac imaging (cardiac echocardiogram and cardiac MRI), in the absence of angiographically-detected coronary artery disease or contributing cardiovascular history. Patients were categorized as ‘severe’ acute myocarditis if they displayed a grade 3–4 toxicity according to criteria adapted from the Common Terminology Criteria for Adverse Events V.5.022 (specific criteria are presented in the online supplemental table 1). Patients were categorized as exhibiting ‘subclinical’ myocarditis if they displayed a persistent low-grade troponin elevation not requiring hospitalization (grade 2 or less toxicity), in the absence of ischemic supply/demand mismatch etiologies, angiographically-detected coronary artery disease, contributing cardiovascular history or alternative causes. For more details on patient severity scoring and data abstraction, please see the Supplementonline supplemental file 1.
jitc-2021-002553supp001.pdf (331.7KB, pdf)
Evaluation and treatment of suspected ICI-associated myocarditis were driven by the degree of troponin elevation and ECG changes. All patients were referred to cardio-oncology for consultation, and were assessed by repeat ECG, cardiac biomarkers including troponin, creatine kinase (CK), CK-MB, B-type natriuretic peptide (BNP), echocardiogram. Decision was made to follow as outpatient with repeat evaluation in 24 hours or for inpatient management. Patients with high probability of severe myocarditis (troponin >5–10×upper limit of normal, low suspicion for acute coronary syndrome, ECG changes including atrioventricular (AV) block), received 1 g of methylprednisolone intravenously in the clinic, prior to transfer. Inpatient management included evaluation for acute coronary syndrome, cardiac MRI (CMR), and further steroid therapy. Once myocarditis was confirmed, intravenous steroids (typically 1 g of methylprednisolone daily) were continued for 3–5 days followed by conversion to a long-term oral steroid taper.
Statistical analysis
Summary statistics were computed, including medians and IQRs for continuous variables and percentages for categorical variables. All analyses included only patients with documented myocarditis (n=15). Cardiac parameters at presentation were compared using Wilcoxon rank-sum tests for continuous variables and χ2 or Fisher’s exact test, as appropriate, for categorical variables. Latency to steroid initiation was compared with mortality using a Wilcoxon rank-sum test. The prognostic significance of specific biomarkers (troponin, BNP, CK, and CK-MB) at symptom onset and their relationship with myocarditis-associated mortality were first explored using logistic regression models. Cox proportional hazard regression models were then used to calculate the HRs and 95% CIs associated with the risk of 90-day myocarditis-associated mortality. Patients were entered into the survival analysis at the time of symptom onset for myocarditis and were followed for 90 days to determine the survival status. We explored the discriminatory ability of each biomarker for predicting mortality using area under the curve of receiver operating characteristic (ROC) plots. The optimal cut point for the ROC plots was identified using the Youden’s Index. Kaplan-Meier survival curves and log-rank tests were used to assess the association between the identified cut points and 90-day myocarditis-associated mortality. All statistical tests were two-tailed with a p<0.05 level of significance and were performed using Microsoft Excel 2019 (Microsoft Corporation) and SAS V.9.4 (SAS Institute).
Results
We identified a total of 1001 patients who received one or more ICIs from January 2016 to January 2020. Among all patients treated with ICIs, abnormal troponin elevation (≥0.06 ng/mL) was identified in 34 patients in the context of immunotherapy administration. Of the 34 patients initially screened, 4 patients were excluded based on a lack of temporal relationship between checkpoint administration and troponin elevation (greater than 2 months from the last dose of checkpoint inhibitor to first troponin elevation), and 7 patients were excluded due to underlying sepsis. Following exclusion, we found evidence of probable checkpoint-associated toxicity in 23 patients, out of which, 15 patients were determined to have ICI-associated myocarditis. Eight patients were determined to have other causes for troponin rise, such as acute coronary syndromes and exacerbations of heart failure. Those cases are presented in the Supplement under online supplemental results. Of the entire cohort of ICI-treated patients, 339 (34%) underwent serial troponin measurements with at least two sequential tests.
Patient characteristics
Individual characteristics and case summaries of patients with severe myocarditis and subclinical myocarditis are presented in online supplemental tables 2–5. Summary characteristics of the two cohorts are presented in table 1. The most common indications for ICI therapy were melanoma (n=9), non-small cell lung cancer (n=3) and squamous cell carcinoma (n=2). The most common comorbidities were hypertension (93.3%), hyperlipidemia (40%), coronary artery disease (33.3%), atrial fibrillation (26.6%), history of myocardial infarction (26.6%), peripheral vascular disease (20%), history of pulmonary embolism (13.3%), chronic kidney disease (13.3%), hypothyroidism (13.3%), and diabetes (6.6%).
Table 1.
Clinical characteristics of patients with immune checkpoint inhibitor-associated severe and subclinical myocarditis
| Severe myocarditis (n=11) | Subclinical myocarditis (n=4) | P value | |
| Median age (IQR) | 73 (66–79) | 73.5 (62.8–80) | 0.448 |
| % female | 3/11 (27.3) | 1/4 (25) | 1 |
| % pembrolizumab | 5/11 (45.5) | 2/4 (50) | 1 |
| % nivolumab | 5/11 (45.5) | 2/4 (50) | 1 |
| % atezolizumab | 1/11 (9.1) | 0/4 (0) | 1 |
| Median days to onset (IQR) | 31 (18.5–116) | 51.5 (37.8–88) | 0.180 |
| Median days from previous dose (IQR) | 16 (13.5–20.5) | 18.5 (4–26.25) | 0.397 |
| Mortality from myocarditis | 4/11 (36.4%) | 0/4 (0%) | 0.516 |
| Underlying conditions | |||
| Hypertension | 11/11 (100%) | 3/4 (75%) | 0.267 |
| Heart failure with reduced ejection fraction | 2/11 (18.2%) | 1/4 (20%) | 1 |
| Coronary artery disease | 3/11 (27.3%) | 2/4 (50%) | 0.560 |
| History of myocardial infarction | 2/11 (18.2%) | 2/4 (50%) | 0.275 |
| Hyperlipidemia | 4/11 (36.4%) | 2/4 (50%) | 1 |
| Concurrent symptoms | |||
| Dyspnea | 5/11 (45.5%) | 3/4 (75%) | 0.569 |
| Chest pain | 3/11 (27.3%) | 0/4 (0%) | 0.516 |
| Colitis | 1/11 (9%) | 2/4 (50%) | 0.154 |
| Myalgia | 2/11 (18.2%) | 0/4 (0%) | 1 |
| Edema | 2/11 (18.2%) | 1/4 (25%) | 1 |
| Concurrent side effects | |||
| Myasthenia gravis | 2/11 (18.2%) | 0/4 (0%) | 1 |
| Pneumonitis | 0/11 (0%) | 1/4 (25%) | 0.267 |
| Laboratory studies on presentation | |||
| Troponin | 1.52 (0.33–5.78) | 0.15 (0.10–0.28) | 0.037 |
| B-type natriuretic peptide | 423 (108–664) | 93 (39–659) | 0.288 |
| Creatine kinase (CK) | 465 (76–1445) | 54 (35–375) | 0.229 |
| CK-MB | 68 (6.9–138) | 1.80 (1.15–3.05) | 0.034 |
Laboratory studies presented as median (IQR) and analyzed using the Wilcoxon rank-sum test.
No statistically significant differences were observed in the rates of cardiovascular comorbidities between severe and subclinical myocarditis cohorts (table 1). There were no statistically significant differences in the rate of use of specific ICI agents between the two cohorts (table 1).
Myocarditis presentation
The median time from the initiation of checkpoint therapy to presentation of clinical myocarditis was 31 days (IQR 18.5–116) for patients with severe myocarditis and 51.5 days (IQR 37.75–88) in the subclinical myocarditis cohorts, a difference that was not statistically significant. Dyspnea was the most common symptom in patients in both the severe myocarditis (45.5%) and subclinical myocarditis (75%) groups (table 1). Chest pain was a less common presentation, reported in three patients (27.3%) with severe myocarditis (table 1). Other reported signs and symptoms included chills, pruritus, bradycardia, myalgia, and peripheral edema (online supplemental tables 2 and 4).
Concomitant irAEs occurred in 73% (8/11) of patients with severe myocarditis and 75% (3/4) of patients with subclinical myocarditis, a difference that was not statistically significant. Common irAEs included colitis (20%), thyroiditis (20%), myasthenia gravis (13.3%), pneumonitis (6.7%), hepatitis (6.7%), pancreatitis (6.7%), and optic neuritis (6.7%). There were no statistically significant differences in the rates of specific irAEs between the severe and subclinical myocarditis cohorts (table 1). There is no distinct pattern regarding whether these concomitant irAEs occurred before or after the development of myocarditis, with 5 out of 11 patients experiencing other irAEs before myocarditis, while the other 6 patients experienced these irAEs either after or contemporaneously.
Despite similar baseline characteristics in those with severe myocarditis versus those with subclinical myocarditis (table 1), there were notable differences in their cardiac diagnostics (table 1). Troponin on presentation was significantly higher in patients who progressed to severe myocarditis (n=11, median 1.52 ng/mL, IQR 0.33–5.78) than patients with subclinical myocarditis (n=4, median 0.15 ng/mL, IQR, 0.10–0.28, p=0.037). CK-MB on presentation was also significantly higher in patients who progressed to severe myocarditis (n=7, median 68 ng/mL, IQR 6.9–138) than in patients with subclinical myocarditis (n=4, median 1.8 ng/mL, IQR 1.15–3.05, p=0.034). BNP tended to be higher on presentation in patients with severe myocarditis (n=11, median 423 pg/mL, IQR 108–66) than patients with subclinical myocarditis (n=4, median 93 pg/mL, IQR 39–659) but this difference was not statistically significant (p=0.288) (table 1). CK at presentation was also higher in patients with severe myocarditis (n=10, median 465 IU/L, IQR 76–1445) than in patients with subclinical myocarditis (n=4, median 54 IU/L, IQR 35–375) but this difference was also not statistically significant (p=0.229).
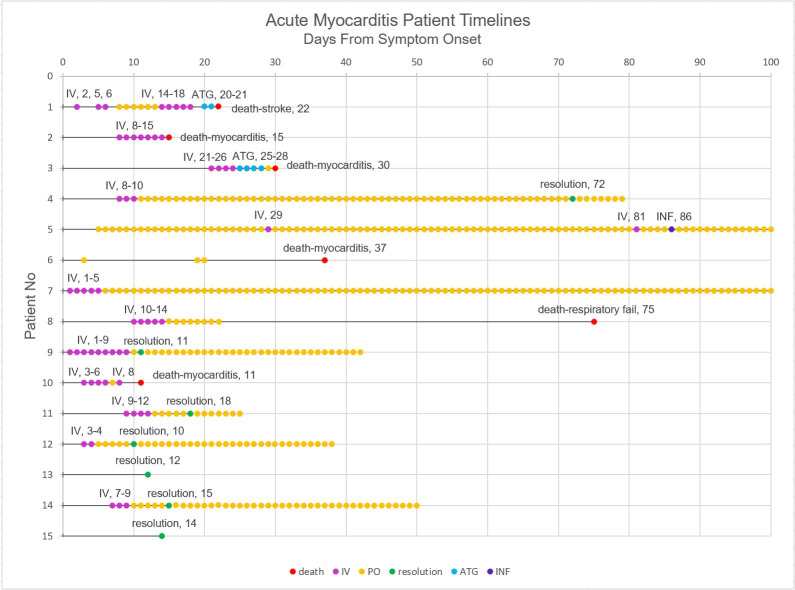
All patients with severe myocarditis were treated with steroids. Patients #1 and #3 were additionally treated with ATG while patient #5 was additionally treated with infliximab (figure 1). Patients with subclinical myocarditis were treated either by halting immunotherapy and supportive care alone, or by halting immunotherapy and initiating steroids (figure 1, online supplemental table 4).
Figure 1.
Event timelines for patients with ICI-associated severe and subclinical myocarditis. Patient level timelines showing the time from symptom onset to death/myocarditis resolution, including all immunosuppressants used in treatment of ICI-associated myocarditis. Death in this figure includes death by all causes and exact cause of death is noted. ATG, anti-thymocyte globulin; ICI, immune checkpoint inhibitor; INF, infliximab; IV, intravenous steroids; PO, oral steroids.
Comparison of electrocardiographic, echocardiographic, and CMR assessment
All patients in both the severe and subclinical myocarditis groups had ECGs available before ICI initiation and after their presentation with signs of cardiotoxicity. Eight out of 11 patients with severe myocarditis (72.7%) had ECG abnormalities on presentation, while no significant ECG findings were seen in any of the patients with the subclinical form (p=0.021). The most common ECG abnormalities noted in the severe myocarditis group consisted of new AV block (55%) and right bundle branch block (36%) (online supplemental table 3). Presentation with advanced AV block trended toward an association with myocarditis-associated mortality (p=0.088) (table 2). No other ECG findings were associated with myocarditis-associated mortality (table 2).
Table 2.
Cardiac parameters among survivors and deceased patients
| Survived (n=11) | Deceased (n=4)* | P value | |
| Laboratory studies on presentation | |||
| Troponin | 0.33 (0.11–1.37) | 4.8 (3.3–8.9) | 0.016 |
| B-type natriuretic peptide | 230 (82–750) | 339 (134–564) | 1 |
| CK | 74 (32–144) | 2541 (876–5676) | 0.013 |
| CK-MB | 2.5 (1.5–7.5) | 90 (68–242) | 0.034 |
| Electrocardiographic | |||
| First-degree AV block | 2/11 (18%) | 0/4 (0%) | 0.491 |
| Advanced AV block† | 1/11 (9%) | 3/4 (75%) | 0.088 |
| New Right Bundle Branch Block | 1/11 (9%) | 2/4 (50%) | 0.491 |
| Non-specific ST/T wave changes | 3/11 (27%) | 1/4 (25%) | 1 |
Laboratory studies presented as median (IQR) and analyzed using the Wilcoxon rank-sum test.
*Deceased includes patients who died due to myocarditis-related complications within a 90-day window.
†Defined as: second-degree AV block Mobitz type I or II, high-grade AV block and third-degree AV block.
AV, atrioventricular; CK, creatine kinase.
Ischemic evaluation with coronary angiography was performed in three patients with severe myocarditis, with no significant lesions found. No patients with subclinical myocarditis underwent ischemic evaluation. Gadolinium-enhanced CMR was performed in three patients with severe myocarditis with 66% (2/3 patients) demonstrating diagnostic Lake Louise criteria.23 The equivocal MRI of the third case was performed early in the patient’s clinical course, which has been associated with non-diagnostic findings previously.24 CMR protocol details are provided in the online supplemental file 1.
Ten patients in the severe myocarditis and three patients in the subclinical myocarditis cohorts had echocardiograms with estimated ejection fractions available for comparison. Left ventricular ejection fraction (LVEF) was estimated from echocardiograms by modified Simpson’s method. Left ventricular systolic dysfunction (LVEF ≤50%) was seen in 6 out of 10 patients in the severe myocarditis group where echocardiograms were available and in none of the patients in the subclinical myocarditis group (online supplemental tables 3 and 5). There was no statistically significant association between reduced LVEF and progression to severe myocarditis. There was also no statistically significant association between reduced LVEF and mortality, with death due to myocarditis occurring in 2 out of 4 patients (50%) with normal LVEF, while 5 out of 6 patients (83%) with reduced LVEF survived.
Troponin monitoring and referral
Three patients with severe myocarditis and one patient with subclinical myocarditis were among the 334 patients on a weekly troponin monitoring protocol at the time of their identified cardiotoxicity. The remaining 11 myocarditis presented with symptoms which prompted troponin measurement for cause. Serial troponin monitoring led to early initiation of steroids within 6 days of symptom onset in two severe myocarditis patients. Patients on a weekly troponin monitoring protocol (n=4) had significantly lower troponins on presentation (median 0.1 ng/mL, IQR 0.083–0.248 ng/mL) than patients who were not (n=11, median 1.52 ng/mL, IQR 0.405–4.8 ng/mL, p=0.022). There were no statistically significant differences in BNP, CK or CK-MB between patients who had troponin monitored weekly and those who were not. Patients identified on a weekly troponin monitoring protocol also had a shorter time to steroid initiation (n=4, median 4.5 days, IQR 2.25–6), than patients who were not (n=9, median 8 days, IQR 8–20 days, p=0.053). There were no deaths among patients who were on a weekly troponin monitoring protocol, while there were four deaths due to myocarditis among patients who were not monitored weekly. However, our analysis did not show a statistically significant difference in the rates of mortality between patients on troponin monitoring and those who were not.
Prognostic significance of biomarkers
In total, 6 out of 15 (40%) patients with severe myocarditis died, 4 of whom died as a direct consequence of myocarditis (figure 1). Patient #1 was recovering from myocarditis when she succumbed to a hemorrhagic stroke, likely due to brain metastasis, which were found on autopsy. Patient #8 died of respiratory failure from concomitant ICI-associated axonal neuropathy. Troponin at onset was significantly higher in patients who died of myocarditis, (median 4.8 ng/mL, IQR 3.3–8.9) than survivors (median 0.33 ng/mL, IQR 0.11–1.37, p=0.016) (table 2). CK at onset was significantly higher in patients who died of myocarditis (median 2541 U/L, IQR 876–5676, p=0.013) than survivors (median 74 U/L, IQR 32–144) (table 2). CK-MB at onset was also significantly higher in patients who died of myocarditis (median 90 ng/mL, IQR 68–242) than in patients who survived (median 2.5 ng/mL, IQR 1.5–7.5, p=0.034) (table 2). BNP at onset did not differ significantly between patients who died of myocarditis (median 339 pg/mL, IQR 134–564) and survivors (median 230 pg/mL, IQR 82–750) (table 2).
Univariate logistic regression analysis for predictors of myocarditis-associated mortality is shown in table 3. Each one unit increase in troponin levels at onset was associated with a higher odds of death (OR 1.72, 95% CI 0.96 to 3.06, p=0.067). Higher levels of CK (OR 1.30, 95% CI 0.91 to 1.85) and CK-MB (OR 1.21, 95% CI 0.93 to 1.56) at onset were also associated with significantly higher odds of death, but these associations were not statistically significant.
Table 3.
Relationship between clinical characteristics at onset and 90-day myocarditis-associated mortality
| Survived (n=11) |
Deceased* (n=4) |
OR (95% CI) |
P value | Univariate HR (95% CI) |
P value | Multivariable HR (95% CI) |
P value | |
| Troponin (each 1-unit increase) | 0.33 (0.11 to 1.37) | 4.8 (3.3 to 8.9) | 1.72 (0.96 to 3.06) | 0.067 | 1.47 (1.08 to 2) | 0.014 | 2.55 (0.78 to 8.31) | 0.120 |
| B-type natriuretic peptide (each 100-unit increase) | 230 (82 to 750) | 339 (134 to 564) | 0.93 (0.73 to 1.18) | 0.553 | 0.93 (0.76 to 1.16) | 0.555 | ||
| Creatine kinase (CK) (each 100-unit increase) | 74 (32 to 144) | 2541 (876 to 5676) | 1.30 (0.91 to 1.85) | 0.145 | 1.05 (1.01 to 1.09) | 0.025 | 1.11 (0.98 to 1.26) | 0.107 |
| CK-MB (each 10-unit increase) | 2.5 (1.5 to 7.5) | 90 (68 to 242) | 1.21 (0.93 to 1.56) | 0.156 | 1.08 (0.99 to 1.17) | 0.051 |
*Deceased includes patients who died due to myocarditis-related complications within a 90-day window.
Univariate Cox proportional hazard analysis for predictors of 90-day myocarditis-associated mortality are shown in table 3. Each one unit increase in troponin (HR 1.47, 95% CI 1.08 to 2, p=0.014) at onset was significantly associated with a higher risk of 90-day myocarditis-associated mortality. Higher CK levels (HR 1.05, 95% CI 1.01 to 1.09, p=0.025) were also associated with a higher risk of mortality. The significant variables were then included in multivariable Cox proportional hazard analysis for predictors of mortality. Higher troponin at onset was associated with a higher risk of 90-day myocarditis-associated mortality (HR 2.55, 95% CI 0.78 to 8.31) and trended toward significance (p=0.120). Likewise, although higher CK at onset appeared to be associated with mortality (HR 1.11, 95% CI 0.98 to 1.26) this was not statistically significant (p=0.107).
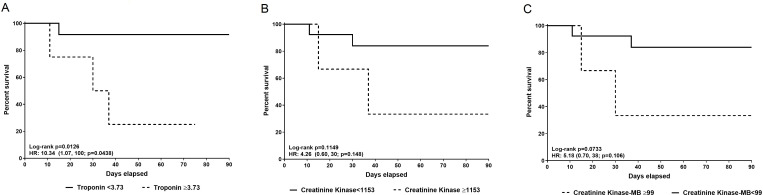
Kaplan-Meier survival curves at 90-day follow-up for troponin are shown in figure 2A, with a discriminating troponin defined as 3.73 ng/mL (sensitivity: 100%, specificity: 91%). For CK (figure 2B), a discriminating value of 1153 U/L (sensitivity: 75%, specificity: 90%) was selected. For CK-MB (figure 2C), a discriminating value of 99 ng/mL (sensitivity: 100%, specificity: 88%) was selected. Each discriminating value above was based on the maximized Youden’s Index obtained by ROC curves.
Figure 2.
Kaplan-Meier for cardiac biomarkers at onset and 90-day mortality. Each breakpoint was determined based on the maximized Youden’s Index obtained by receiver operating characteristic curves. (A) Kaplan-Meier of the relationship between troponin ≥3.73 ng/mL and 90-day myocarditis-associated mortality. Troponin elevation ≥3.73 ng/mL was associated with a statistically significantly increased risk of mortality (HR 10.34, 95% CI 1.07 to 100, p=0.044). (B) Kaplan-Meier of the relationship between creatine kinase (CK) at onset of ≥1153 IU/L and 90-day myocarditis-associated mortality. CK elevation of ≥1153 IU/L was associated with an increased risk of mortality (HR 4.26, 95% CI 0.60 to 30, p=0.115) but this relationship was not statistically significant. (C) Kaplan-Meier of the relationship between CK-MB at onset of ≥99 ng/mL and 90-day myocarditis-associated mortality. CK-MB elevation of ≥99 ng/mL was associated with an increased risk of mortality (HR 5.18, 95% CI 0.70 to 38, p=0.106), but this relationship was not statistically significant.
Patients with troponin values of ≥3.73 ng/mL exhibited significantly increased risk of myocarditis-associated death at 90-day follow-up (HR 10.34, 95% CI 1.07 to 100, p=0.044). A trend toward higher likelihood of death at 90-day follow-up occurred with CK values ≥1153 U/L (HR 4.26, 95% CI 0.60 to 30 p=0.115) and CK-MB values ≥99 ng/mL (HR 5.18, 95% CI 0.70 to 38.2, p=0.106), however these associations were not statistically significant.
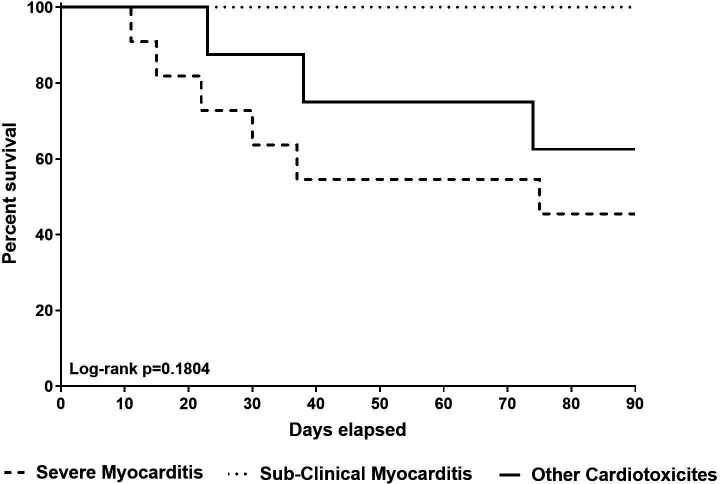
Overall 90-day survival and all-cause mortality between severe ICI-associated myocarditis, ICI-associated subclinical myocarditis, and other ICI-associated cardiotoxicity groups were obtained through Kaplan-Meier and log-rank testing. The median survival for severe myocarditis patients was 75 days, with no statistically significant association between the three groups and 90-day overall survival (p=0.180) (figure 3).
Figure 3.
Ninety-day survival of patients with severe myocarditis (1), subclinical myocarditis (2), and other cardiotoxicities (3). Ninety-day survival did not significantly differ between the three groups of immune checkpoint inhibitor-associated toxicities.
Discussion
Our study supports the role of proactive outpatient surveillance for early detection of ICI-associated myocarditis. Since no strongly predictive risk factors for ICI-associated myocarditis have yet been identified in the literature, we implemented serial troponin measurements for the first 6 weeks in patients treated with ICI. The results from this practice suggest reduced severity of disease and earlier treatment in patients so monitored. We found that patients who were monitored using serial weekly troponins had lower troponins on presentation and received steroid therapy more quickly compared with patients who were not monitored. Serial monitoring with weekly troponin allowed for earlier detection of myocarditis in three of our cases, all of which exhibited halted disease progression, normalization of cardiac biomarkers and improvement of left ventricular (LV) function on steroid treatment.
Among the biomarkers available, troponin I was the most reliable and early predictor of both progression to severe myocarditis and mortality. CK-MB on presentation was also found to be a reliable predictor of progression to severe myocarditis and mortality. CK on presentation was significantly correlated with myocarditis-associated death. BNP was not found to correlate significantly with adverse outcomes of progression to severe myocarditis or myocarditis-associated death, although these values tended to be higher in patients with more severe presentations. Our findings also corroborate the observation of ICI-associated subclinical myocarditis, which presents with lower cardiac biomarkers and a milder, shorter course.19
In addition, our study supports the role of prompt troponin and ECG evaluation of patients presenting with cardiac symptoms. Our experience also suggests consideration for cardiac evaluation in patients with myasthenia gravis, myositis, and other systemic irAEs, as these conditions were closely associated with myocarditis in our patient population. If severe myocarditis from ICI therapy is suspected, a multidisciplinary approach should be promptly implemented, and ICI therapy should be suspended until the cause of the troponin elevation is determined by the multidisciplinary team. Our recommended initial diagnostics for these patients include an expedited 12-lead ECG and echocardiographic assessment of LV function, followed by consideration for angiography or myocardial perfusion to exclude coronary artery disease. CMR should be pursued when available, as this is the best means of detecting myocardial inflammation associated with myocarditis.23 In our study, the diagnosis of clinically suspected myocarditis was defined as per Caforio et al,21 which takes into consideration the clinical as well as biochemical and ECG changes and relies less on invasive or imaging studies.23 CMR may be equivocal and in one of our cases, the patient had a negative CMR despite a clinical syndrome consistent with myocarditis early in his course. In cases of a non-diagnostic CMR but strong clinical suspicion for ICI myocarditis, endomyocardial biopsy or traditional positron emission tomography fluorodeoxyglucose may have a role.25 It is also important to note that patients with fulminant myocarditis often require ICU care and may not be suitable candidates for these imaging protocols.
Once ICI-associated myocarditis is confirmed, high dose steroids should be implemented without delay. Early use of high dose steroid therapy is crucial in all cases of ICI-associated myocarditis, and their use should be continued until troponin normalizes, ECG abnormalities subside, and LV systolic function improves. Serial surveillance imaging (echocardiography and CMR) is useful to determine the extent of myocardial damage and potential reversibility. Clinical deterioration and progression despite high dose corticosteroid therapy should prompt consideration and use of additional immunosuppressants.
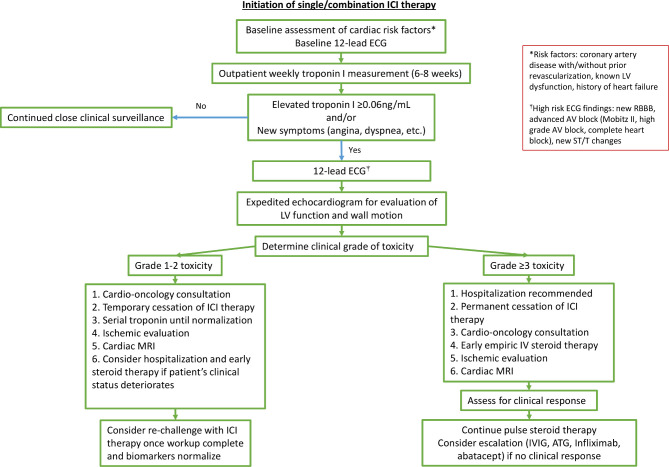
The decision to restart ICIs in patients with ICI-associated myocarditis is a difficult question that has not been well studied. At present, the American Society of Clinical Oncology guideline recommends permanent cessation with grade 1 toxicity (abnormal cardiac biomarker testing including ECG),26 while some centers have proposed a cautious reinitiation of ICI therapy with or without low dose steroids.27 In our study, two patients with subclinical myocarditis (grade 1 toxicity) were re-introduced to immunotherapy following normalization of their cardiac parameters. In one case the troponin elevation returned, and ICI therapy was discontinued permanently. In the other case, reinitiation did not result in recurrence of cardiotoxicity. No patients in the severe myocarditis cohort were restarted on ICI therapy, as reinitiation was deemed to carry too high a risk of cardiac re-injury. A clinical algorithm for the assessment and management of ICI-treated patients with troponin elevations is presented in figure 4 based on our clinical experience but requires further validation.
Figure 4.
Algorithm for assessment and management of troponin elevation in patients receiving ICI therapy. Recommendations for the assessment and treatment of ICI-associated myocarditis presented include initial assessment for likelihood of ICI-associated toxicity, and further assessment and treatment guided by the grade of the toxicity. ATG, anti-thymocyte globulin; AV, atrioventricular; ICI, immune checkpoint inhibitor; IV, intravenous; IG, immunoglobulin; LV, left ventricular; RBBB, right bundle branch block.
The relationship between irAEs and ICI efficacy remains controversial.22 28–30 A large re-analysis of one ICI clinical trial involving over 1000 patients with melanoma reported that patients with a robust immune activation and many irAEs had longer progression-free survival times than comparable patients without irAEs.31 Additionally, a previous case report of two patients with melanoma who developed fulminant myocarditis revealed that T-cell clones infiltrating the myocardium were identical to those present in tumors,32 providing insights into the overlapping mechanisms of irAEs and the therapeutic effects of ICI treatment. Future studies, including a comprehensive analysis of immune signatures in the heart, tumors and circulating immune cells are required to better understand the mechanism of cardiac irAEs, cancer progression and its association with activation of the immune system.
Limitations
Due to the relative rarity of ICI-associated myocarditis, our data are limited by the small sample size and lack of power to demonstrate significance in secondary analyses. Although we recommend baseline followed by weekly troponin monitoring at ICI initiation, we did not establish this recommendation with a randomized study, hence the significance of its association with myocarditis outcomes may be questioned. Although our study lacked these advantages, the data we report shows that earlier detection for ICI-associated myocarditis results in less ICI-associated myocarditis mortality. In the future, detailed investigations of early intervention should use larger, multicenter data to better establish the role of steroid timing and dose on patient outcomes.
Conclusions
Treatment with ICIs is associated with a spectrum of cardiotoxicities, including myocarditis, that range from subclinical to a severe form. In our study cohort, higher values of troponin and evidence of advanced conduction disease on ECG at presentation were associated with greater disease severity and mortality from ICI-associated myocarditis. Accordingly, a focus in ICI-treated patients that includes early detection of myocarditis, prompt cardio-oncology evaluation to rule out other etiologies, and expedited steroid treatment appears to provide a reduction in the risk of progression to fulminant and potentially lethal outcomes. Baseline and serial troponin measurements may contribute to the crucial step of early detection.
Footnotes
Twitter: @BenSwitzerDO, @Ankitakap
Correction notice: This paper has been revised to correct author name 'Jerome J Schentag'.
Contributors: Each author contributed significantly to this research article. IP helped organize this project, developed the main objectives of the article, collected and analyzed data and wrote sections of the manuscript. SJJ oversaw the project, contributed to its organization, developed research questions and wrote sections of the manuscript. PS collected data, analyzed data, developed research questions, and wrote sections of the manuscript. YVY collected data, developed research questions and wrote the cardiology sections of this manuscript. DMJ developed research questions, conducted data analysis and wrote sections of this manuscript. MRC collected data and provided critical revision to the manuscript. UCS also collected data, developed research questions and provided critical revision to the manuscript. FI collected immune biomarker data, analyzed immune biomarker data, and edited this manuscript. SGF extracted and formatted the data for analysis. BS, ABC, MDH, EJS, GKD, MSE, PV, BJP, NA, AKh, AKa, and AH provided intellectual content and critical revision to the manuscript.
Funding: This work was supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Comprehensive Cancer Center’s Flow Cytometry Core, and by the National Center for Advancing Translational Sciences of the NIH (UL1TR001412) to the University of Buffalo. This work was also supported by grants from the Roswell Park Alliance Foundation, the Melanoma Research Alliance, Department of Defense Lung Cancer Research Program (LC180245), NIH/NHLBI K08HL131987 (U. Sharma), and the NIH/NCI (K08CA197966) (F. Ito).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Case level information is provided in the online supplement. All other relevant data are available from the corresponding author upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
All clinical procedures and protocols conformed to institutional guidelines and were approved by the institutional review board at the Roswell Park Comprehensive Cancer Center and at University at Buffalo.
References
- 1.Puzanov I, Diab A, Abdallah K, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for immunotherapy of cancer (SITC) toxicity management Working group. J Immunother Cancer 2017;5:95. 10.1186/s40425-017-0300-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganatra S, Neilan TG. Immune checkpoint inhibitor-associated myocarditis. Oncologist 2018;23:879–86. 10.1634/theoncologist.2018-0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moslehi JJ, Salem J-E, Sosman JA, et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis. Lancet 2018;391:933. 10.1016/S0140-6736(18)30533-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Upadhrasta S, Elias H, Patel K, et al. Managing cardiotoxicity associated with immune checkpoint inhibitors. Chronic Dis Transl Med 2019;5:6–14. 10.1016/j.cdtm.2019.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neilan TG, Rothenberg ML, Amiri-Kordestani L, et al. Myocarditis associated with immune checkpoint inhibitors: an expert consensus on data gaps and a call to action. Oncologist 2018;23:874–8. 10.1634/theoncologist.2018-0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol 2018;71:1755–64. 10.1016/j.jacc.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agrawal N, Khunger A, Vachhani P, et al. Cardiac toxicity associated with immune checkpoint inhibitors: case series and review of the literature. Case Rep Oncol 2019;12:260–76. 10.1159/000498985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuo K, Ishiguro T, Najama T, et al. Nivolumab-induced myocarditis successfully treated with corticosteroid therapy: a case report and review of the literature. Intern Med 2019;58:2367–72. 10.2169/internalmedicine.2596-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Q, Hu B. Successful therapy for autoimmune myocarditis with pembrolizumab treatment for nasopharyngeal carcinoma. Ann Transl Med 2019;7:247. 10.21037/atm.2019.04.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Touat M, Maisonobe T, Knauss S, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology 2018;91:e985–94. 10.1212/WNL.0000000000006124 [DOI] [PubMed] [Google Scholar]
- 11.Mahmood SS, Chen CL, Shapnik N, et al. Myocarditis with tremelimumab plus durvalumab combination therapy for endometrial cancer: a case report. Gynecol Oncol Rep 2018;25:74–7. 10.1016/j.gore.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inayat F, Masab M, Gupta S, et al. New drugs and new toxicities: pembrolizumab-induced myocarditis. BMJ Case Rep 2018;2018. 10.1136/bcr-2017-223252. [Epub ahead of print: 23 Jan 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Läubli H, Balmelli C, Bossard M, et al. Acute heart failure due to autoimmune myocarditis under pembrolizumab treatment for metastatic melanoma. J Immunother Cancer 2015;3:11. 10.1186/s40425-015-0057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tay RY, Blackley E, McLean C, et al. Successful use of equine anti-thymocyte globulin (ATGAM) for fulminant myocarditis secondary to nivolumab therapy. Br J Cancer 2017;117:921–4. 10.1038/bjc.2017.253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saibil SD, Bonilla L, Majeed H, et al. Fatal myocarditis and rhabdomyositis in a patient with stage IV melanoma treated with combined ipilimumab and nivolumab. Curr Oncol 2019;26:418–21. 10.3747/co.26.4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reuben A, Petaccia de Macedo M, McQuade J, et al. Comparative immunologic characterization of autoimmune giant cell myocarditis with ipilimumab. Oncoimmunology 2017;6:e1361097. 10.1080/2162402X.2017.1361097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Norwood TG, Westbrook BC, Johnson DB, et al. Smoldering myocarditis following immune checkpoint blockade. J Immunother Cancer 2017;5:91. 10.1186/s40425-017-0296-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kwon OH, Kim M-N, Kim S-A, et al. Fulminant lymphocytic myocarditis associated with orbital myositis and diaphragmatic paralysis. Cardiovasc Pathol 2016;25:55–8. 10.1016/j.carpath.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 19.Sarocchi M, Grossi F, Arboscello E, et al. Serial troponin for early detection of nivolumab cardiotoxicity in advanced non-small cell lung cancer patients. Oncologist 2018;23:936–42. 10.1634/theoncologist.2017-0452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]
- 21.Caforio ALP, Adler Y, Agostini C, et al. Diagnosis and management of myocardial involvement in systemic immune-mediated diseases: a position statement of the European Society of cardiology Working group on myocardial and pericardial disease. Eur Heart J 2017;38:2649–62. 10.1093/eurheartj/ehx321 [DOI] [PubMed] [Google Scholar]
- 22.Haratani K, Hayashi H, Chiba Y, et al. Association of immune-related adverse events with nivolumab efficacy in non-small-cell lung cancer. JAMA Oncol 2018;4:374–8. 10.1001/jamaoncol.2017.2925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bonaca MP, Olenchock BA, Salem J-E, et al. Myocarditis in the setting of cancer therapeutics: proposed case definitions for emerging clinical syndromes in Cardio-Oncology. Circulation 2019;140:80–91. 10.1161/CIRCULATIONAHA.118.034497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escudier M, Cautela J, Malissen N, et al. Clinical features, management, and outcomes of immune checkpoint Inhibitor-Related cardiotoxicity. Circulation 2017;136:2085–7. 10.1161/CIRCULATIONAHA.117.030571 [DOI] [PubMed] [Google Scholar]
- 25.Hu J-R, Florido R, Lipson EJ, et al. Cardiovascular toxicities associated with immune checkpoint inhibitors. Cardiovasc Res 2019;115:854–68. 10.1093/cvr/cvz026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brahmer JR, Lacchetti C, Schneider BJ, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of clinical oncology clinical practice guideline. J Clin Oncol 2018;36:1714–68. 10.1200/JCO.2017.77.6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peleg Hasson S, Salwen B, Sivan A, et al. Re-introducing immunotherapy in patients surviving immune checkpoint inhibitors-mediated myocarditis. Clin Res Cardiol 2021;110:50-60. 10.1007/s00392-020-01648-3 [DOI] [PubMed] [Google Scholar]
- 28.Das S, Johnson DB. Immune-Related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer 2019;7:306. 10.1186/s40425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freeman-Keller M, Kim Y, Cronin H, et al. Nivolumab in resected and unresectable metastatic melanoma: characteristics of immune-related adverse events and association with outcomes. Clin Cancer Res 2016;22:886–94. 10.1158/1078-0432.CCR-15-1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teulings H-E, Limpens J, Jansen SN, et al. Vitiligo-like depigmentation in patients with stage III-IV melanoma receiving immunotherapy and its association with survival: a systematic review and meta-analysis. J Clin Oncol 2015;33:773–81. 10.1200/JCO.2014.57.4756 [DOI] [PubMed] [Google Scholar]
- 31.Eggermont AMM, Kicinski M, Blank CU, et al. Association between immune-related adverse events and recurrence-free survival among patients with stage III melanoma randomized to receive pembrolizumab or placebo. JAMA Oncol 2020;6:519. 10.1001/jamaoncol.2019.5570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson DB, Balko JM, Compton ML, et al. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med 2016;375:1749–55. 10.1056/NEJMoa1609214 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2021-002553supp001.pdf (331.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Case level information is provided in the online supplement. All other relevant data are available from the corresponding author upon reasonable request.