Abstract
Metformin (MTF) is the first-line therapy for type 2 diabetes (T2DM). The euglycemic effect of MTF is due to the inhibition of hepatic glucose production. Literature reports that the principal molecular mechanism of MTF is the activation of 5′-AMP-activated protein kinase (AMPK) due to the decrement of ATP intracellular content consequent to the inhibition of Complex I, although this effect is obtained only at millimolar concentrations. Conversely, micromolar MTF seems to activate the mitochondrial electron transport chain, increasing ATP production and limiting oxidative stress. This evidence sustains the idea that MTF exerts a hormetic effect based on its concentration in the target tissue. Therefore, in this review we describe the effects of MTF on T2DM on the principal target organs, such as liver, gut, adipose tissue, endothelium, heart, and skeletal muscle. In particular, data indicate that all organs, except the gut, accumulate MTF in the micromolar range when administered in therapeutic doses, unmasking molecular mechanisms that do not depend on Complex I inhibition.
Keywords: adipose tissue, diabetes, gut, endothelium, heart, hormetic effect, liver, MTF, skeletal muscle
1. Introduction
Type 2 diabetes mellitus (T2DM) will affect 500 million people worldwide by 2030 [1]. Metformin (MTF; 1,1-dimethyl biguanide) is an oral drug employed to reduce blood glucose concentration, and the current first-line pharmacological treatment for T2DM [2,3]. The mechanistic events linking MTF to its plasma glucose-lowering effect remain incompletely understood. For example, several authors have proposed that MTF exerts its primary antidiabetic action through the suppression of hepatic glucose production [4,5] in T2DM patients, inhibiting, by several mechanisms, hepatic gluconeogenesis. Others suggest that MTF induces the inhibition of transepithelial glucose transport in the intestine, lowering blood glucose levels during the early response to oral administration of MTF [6]. An alternative hypothesis by Madiraju et al. (2014) suggests that a change in cellular redox potential, not energy charge, explains the effect of MTF [7]. Moreover, the safety of MTF greatly increased its association with all hypoglycemic drugs [8,9]. MTF exerts pleiotropic effects, probably independent of glycemia regulation, such as end-organ injury and mortality reduction in patients with autoimmune diseases [10] and the modulation of miRNA expression in extracellular vesicles in T2DM patients [11]. MTF seems also involved in the reduction of neurodegenerative disease symptoms [12], although, in this case, a vitamin B12 supplementation should be associated [13].
MTF displays a high hydrophilicity that limits its permeability through lipid membranes [14]. Therefore, it is internalized in the cell by several transporters, including Organic Cation Transporters (OCTs) and multidrug and toxin extrusion (MATE) transporters expressed on the membrane surface [15,16]. Moreover, MTF is not metabolized and is excreted intact by the kidney and the gut [7].
The apparent volume of distribution (Vd) of MTF is a largely debated topic. Some authors reported a value between 63 and 267 L [17,18], while other authors indicated higher values [18] (300 L equal to 4.28 L/kg). These apparent discrepancies could depend on the method of drug administration, the dosage, and the duration of therapy. Indeed, this debate is at the heart of the issue of understanding the effects of MTF on various tissues. In detail, it has been proposed that most of the effects of MTF millimolar concentrations depend on its specific inhibition of the mitochondrial respiratory chain Complex I [19]. The consequences of Complex I inhibition are ATP production lowering and increase in AMP, in turn promoting the 5′-AMP-activated protein kinase (AMPK) activation and decrease the NAD+/NADH ratio [20,21]. Notably, some effects of MTF can be mimicked by Complex I inhibitors [21,22]. Inhibition occurs preferentially when the enzyme is in its catalytically incompetent “deactive” conformation; therefore, MTF should be added prior to catalysis initiation [23]. On the other hand, a similar mechanism has also been described for the MTF-induced inhibition of hexokinase [24]. Interestingly, the inhibition of tumor growth by MTF was hampered in cells expressing a MTF-resistant yeast analogue of Complex I (NDI1), suggesting that inhibition of Complex I is an important mechanism of the action of MTF, also against cancer [21,25]; in fact, MTF was shown to reduce the aerobic ATP production in many cancer cells [26], although its effect seems to depend on tumor adaptive strategies, cancer stem cell selection, tumors metabolism, and microenvironment [27].
However, at therapeutic doses, the plasma MTF concentration is in the micromolar range [7,28]; therefore, to cause Complex I inhibition, it has been hypothesized to accumulate in mitochondria, thanks to the mitochondrial transmembrane electrochemical potential. On the other hand, a high concentration of the positively charged MTF molecules would collapse the inner membrane potential [29]. Moreover, MTF is a hydrophilic compound, and may not cross the inner mitochondrial membrane without a specific transporter, not yet identified [19]. Thus, some studies suggest an indirect action of MTF on Complex I [21]. Indeed, MTF did not accumulate in liver mitochondria of rats treated orally with [14C] MTF [30].
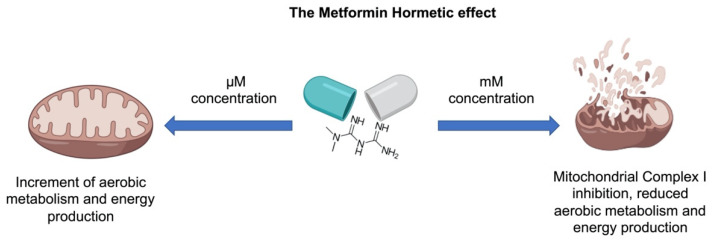
Notably, while millimolar MTF concentrations are needed to inhibit isolated Complex I, micromolar concentrations of the drug activate the mitochondrial aerobic metabolism, including the Complex I activity. For example, it was observed in Fanconi Anemia cells that micromolar MTF concentrations reactivate the mitochondrial respiration; conversely, millimolar concentrations induced the complete inhibition of Complex I [31]. Moreover, other studies have demonstrated that MTF increased respiration [32,33] and ATP content in human [34], mice [19], and bovine [35] samples. In other words, MTF displays a hormetic mechanism, exerting opposite effects depending on the concentration [31,35]: micromolar amounts, similar to that employed in in vivo treatments, increase the mitochondrial aerobic metabolism; by contrast, millimolar concentrations reduce the respiratory Complex I activity, causing a decrement of cellular energy availability (Figure 1). Moreover, MTF seems to act as a modulator of other mitochondrial functions, such as mitochondrial permeability transition pore (mPTP) opening, reactive oxygen species (ROS) production, and calcium storage. For example, MTF used at micromolar concentration inhibits the mPTP opening by an AKT-dependent pathway [36], preventing the associated ROS generation and the release of cytochrome c [37]. Conversely, MTF millimolar concentration determines mitochondrial swelling due to the endoplasmic reticulum (ER) stress and subsequent calcium uptake into the mitochondria [38]. However, the same authors described the inhibition of mPTP that, in this case, seems to be associated with the inhibition of Complex I activity [38]. On the other hand, the diabetic systemic milieu also influences the mitochondrial processes, such as energy production, ROS generation, calcium metabolism, and apoptosis regulation [39,40], negatively influencing hepatocyte metabolism, cardiac output, skeletal muscle contraction, beta-cell insulin production, and neuronal health [39].
Figure 1.
The metformin hormetic effect. Based on concentration, MTF exerts opposite effects. At µM concentrations, MTF increases the oxidative phosphorylation activity, enhancing aerobic energy metabolism. At mM concentrations, MTF inhibits the Complex I activity, reducing the aerobic energy production.
Therefore, to evaluate the metabolic effect of MTF, it is necessary to consider the amount and activity of plasma membrane transporters as well as the plasma and tissue MTF concentrations and the relative volume occupied by the mitochondria inside the cell.
2. The Effects of Metformin (MTF) on Its Main Target Organs
2.1. The Effect of MTF on the Gut
After oral administration, ∼50% of MTF is absorbed through the upper small intestine and delivered to the liver. The remaining ∼50% unabsorbed MTF accumulates in the gut mucosa of the distal small intestine, reaching 30–300-fold of its concentration in plasma and the other tissues [41,42], thus rendering the intestine the main MTF reservoir, due to high absorption and accumulation.
It has been proposed that the high accumulation of MTF in enterocytes may be closely related to its gastrointestinal (GI) side effects [43,44,45,46]. This hypothesis is also supported by evidence that the use of modified formulations of MTF, which allow a gradual absorption, increase its tolerability [47,48,49]. Interestingly, some of the mechanisms involved in mediating the glucose-lowering effects of MTF are also responsible for its side effects, thus creating a fine metabolic balance that links the improvement of glycemic compensation to the side effects. MTF is absorbed into the enterocytes through cationic transporters with a saturable and dose-dependent process [41]: the plasma membrane monoamine transporter (PMAT) and organic cation transporter 3 (OCT3), localized on the luminal side of enterocytes [50], and, to a lesser extent, by the serotonin transporter (SERT) and carnitine/organic cation transporter OCTN1/SLC22A4, which are localized on the apical membrane of enterocytes [51,52]. Then, MTF is released into the portal vein mainly through organic cation transporter 1 (OCT1), which has been found in both apical and basolateral membrane of enterocytes [53,54,55]. In vitro studies showed that the efflux of MTF is less efficient in comparison with the uptake [41], suggesting that this imbalance may explain the higher concentrations in the intestine compared to other tissues. Therefore, especially the small intestine may be the only tissue in which MTF may reach a sufficient concentration to inhibit Complex I activity. This concept is supported by the increment of plasma lactate after MTF administration, which appears to derive from the intestine and not from the muscle, suggesting a metabolic switch to the anaerobic glycolysis due to the inhibition of Complex I [56].
Activation of AMPK by MTF is responsible for the inhibition of the nuclear bile acid receptor farnesoid X receptor (FXR) [57]. FXR is involved in ileal absorption of bile acids; therefore, its inhibition results in reduced bile acid absorption and consequent increased excretion in T2DM patients treated with MTF [58]. This pharmacological effect may cause gastrointestinal disturbances by altering the microbiome and stool consistency. On the other hand, the elevated fecal excretion of bile salts during MTF treatment is associated with metabolic improvement due to reduced circulating cholesterol and increased secretion of the Glucagon-Like Peptide 1 (GLP-1), a gut hormone produced by enteroendocrine L cells in response to nutrient ingestion [59].
It has been shown that a rise in GLP-1 secretion by MTF treatment may contribute to its glucose-lowering effects [60,61]. GLP-1 is involved in maintaining glucose homeostasis at various levels: it stimulates glucose-induced insulin secretion, inhibits glucagon secretion, gastric emptying, and food intake [62]. However, once released, it is rapidly inactivated by dipeptidyl peptidase 4 (DPP-4). The mechanism through which MTF increases GLP-1 concentration is not well known, but several studies indicated that MTF acts by increasing GLP-1 secretion rather than preventing its degradation [63,64,65,66]. Firstly, MTF increases glucose retention in the lumen of intestine [67]; therefore, GLP-1 secretion may be stimulated by the rise in glucose concentration [4]. Furthermore, as described above, MTF may induce GLP-1 secretion through elevation of bile acids. Indeed, several studies report that bile acids directly induce GLP-1 secretion through activation of the G protein–coupled bile acid receptor GPBAR1 (TGR5) [63,64,65]. It has also been hypothesized that MTF activation of AMPK may be another mechanism through which MTF increases the secretion of GLP-1. Indeed, MTF-induced GLP-1 secretion is blocked by pharmacological inhibition of AMPK activity [60,61]. However, considering that MTF did not increase GLP-1 secretion, and that activation of AMPK reduced GLP-1 secretion in in vitro models of L cells [66,68,69], it is conceivable that the rise in GLP-1 may be due to indirect effects of MTF on the gut. Finally, MTF may increase GLP-1 secretion through modulation of the gut microbiota, especially improving Short Chain Fatty Acids (SCFAs production) [70,71].
MTF contributes to glucose lowering by increasing glucose uptake and utilization in the intestinal tissue. These effects are probably achieved through regulation of the expression and distribution of glucose transporters, such as Glucose transporter 2 (GLUT2) and Sodium/glucose cotransporter 1 (SGLT1) in enterocytes. However, the relative contribution of glucose transporters is still debated. GLUT2 is localized mainly in the basolateral membrane of enterocytes, but in the presence of high glucose concentrations, it is rapidly translocated to the brush border membrane [72,73]; by contrast, SGLT1 is localized in brush border membrane and intracellular vesicles of enterocytes [74,75]. Sakar et al. demonstrated that MTF counteracts the abundance of SGLT-1 induced by glucose, and at the same time, increases GLUT2 protein levels in the brush border membrane of enterocytes [76]. On the contrary, Yang et al. showed that MTF increases the GLUT-dependent glucose uptake by increasing GLUT1 expression, with concomitant reduction of both GLUT2 and SGLT1 [77]. Recently, it has been shown that MTF also controls glucose homeostasis by inhibiting glucose transport from intestinal lumen to circulation [6]. In addition, Yang et al. showed that MTF inhibition of the electron transport chain results in an increase of GDF-15, a stress response cytokine which reduces appetite [77,78], from intestinal cells [77,79]. Reduced appetite can consequently limit the glucose intake, improving glycemic control. The increment in glucose uptake coupled with anaerobic glucose utilization induced by MTF leads to an increase in lactate production in enterocytes [67,80]. Furthermore, MTF may also contribute to the rise in lactate by inhibiting bacterial glycerophosphate dehydrogenase [81], and favoring the proliferation of lactate-producing bacteria, which may, in turn, be responsible of some or all the GI side effects of MTF [82,83,84]. However, evidence indicates that lactate may contribute to the beneficial effects of MTF [85]. For instance, lactate is mainly fermented to butyrate by the gut microbiota [86], and it is conceivable that butyrate may contribute to the glucose-lowering effects of MTF by stimulating incretin release.
The gut microbiota is altered in T2DM, and treatment with MTF leads to changes in bacterial composition [87,88,89]. Indeed, the amelioration of glycemic control and bile acid metabolism in MTF-treated T2DM patients correlate to changes in the microbiota composition, especially of Firmicutes and Bacteroidetes [90]. Evidence suggests that modulation of the gut microbiota might be involved in the glucose-lowering effects of MTF [4,70]. However, MTF-induced GI side effects has also been related to alterations in the microbiome. Considering that disturbances of GI occur soon after treatment [91], they probably cannot be attributed to a change in the microbiota composition, but rather to its inter-individual composition. Interestingly, GI intolerance decreases due to treatment [91], suggesting that MTF may lead, in time, to the selection of a favorable microbiota. Comparative evaluation of the fecal metagenome obtained from healthy and T2DM patients evidences dysbiosis in the gut microbiota. In particular, a lower abundance of Firmicutes and Roseburia and a higher abundance of Bacteroides, Proteobacteria, Clostridium, and Lactobacillus spp. has been observed in T2DM patients [87,88,90]. Treatment with MTF has been associated with decreased levels of Clostridium and Eubacterium and increased levels of Escherichia, Shigella, Klebsiella, Salmonella, and Akkermansia muciniphila [92,93]. Interestingly, treatment of T2DM patients with MTF increased the relative enrichment in SCFAs-producing bacteria. SCFAs, especially butyrate and propionate, are involved in improving several features of diabetes, including insulin resistance as well as inflammation, and in increasing GLP-1 secretion [71]. Moreover, a change in the microbiota composition in MTF-treated T2DM patients has been associated to modulation of bile acid metabolism [90]. In particular, it has been shown that MTF may increase the level of bile acids by decreasing the abundance of Bacteroides fragilis and of its bile salt hydrolase activity [94]. Another mechanism through which MTF might improve glucose metabolism through the modulation of gut microbiota is by maintaining the intestinal barrier functionality. Indeed, MTF treatment leads to a marked increase in the bacterium Akkermansia muciniphila, a mucin-degrading bacterium, which plays an important role in maintaining the integrity of the mucin layer, probably by improving the number of mucin-producing globet cells [67,95]. Therefore, it is plausible that the amelioration of glycemic control in MTF-treated T2DM patients may be mediated by alteration in the composition and improvement in the metabolic function of the microbiome.
Alteration of the gut bacterial composition by MTF also plays a role in reducing the risk of colon cancer in T2DM patients [96]. For instance, it has been shown that butyrate reduces the proliferation of cancerous colonocytes [97]. However, the protective effects of MTF in colon cancer seems to be due to its relative retention in the cells. Indeed, MTF has antiproliferative action in cancer cells with reduced expression of MATE2 [98]. Interestingly, metastatic colorectal cancer with reduced expression of MATE1 are more responsive to MTF [99]. These findings suggest that MTF needs to reach high concentrations in cells to exert protective effects from colorectal cancer, and that inhibition of Complex I activity may have a role in mediating MTF’s benefits.
2.2. The Effect of MTF on Liver Metabolism
The liver is the main organ involved in glucose metabolism management; hence, it is considered one of the principal targets of the MTF antidiabetic effect [20]. In particular, MTF determines a reduction of the hepatic glucose output, limiting gluconeogenesis, although the molecular mechanism associated with this effect still remains unclear [100,101]. Among the proposed mechanisms, the AMPK pathway activation due to the liver kinase B1 and the reduction of energy availability [100,102], the inhibition of glucagon-induced cAMP production [103], and the inhibition of mitochondrial shuttles [81] are indicated as the most likely. In addition, the inhibition of respiratory Complex I has been indicated as a possible mechanism [104], since its reduced activity should determine a decrement of the mitochondrial oxidative phosphorylation [19], which in turn causes a decrement of the ATP/AMP ratio, activating AMPK [21]. However, it is important to note that MTF inhibits the Complex I activity only over 1 mM [31], a concentration not reached in the hepatic tissue of subjects treated with therapeutic dose. Indeed, after gut adsorption, MTF is delivered to the liver through the portal vein, which displays a drug concentration between 40–70 μM or 10–40 μM before and after the liver uptake, respectively [7,105,106]. Other authors have observed that MTF could accumulate in the liver, within a range from 2- to 5-fold of the plasma concentration [17,19,106,107]. Moreover, MTF must enter into mitochondria to inhibit Complex I, but the mitochondrial inner membrane is impermeable, and no specific carrier for MTF has been identified yet [19]. Therefore, other mechanism could be involved in the liver regulation of gluconeogenesis flux—in some cases, independently from the of AMPK pathway activation, considering that the low MTF concentration does not determine a substantial lowering of intracellular ATP concentration [2,108].
It was proposed that AMPK signaling could be activated by the slight increment of the AMP level, dependent on the allosteric inhibition of fructose 1,6-bisphosphatase (FBP1) [109]. Alternative data suggest that MTF inhibits the mitochondrial FAD-dependent glycerol-3-phosphate dehydrogenase (mGPDH), one of the shuttles that transfers a reducing equivalent from the cytoplasm to mitochondria, determining a redox-dependent inhibition of gluconeogenesis and an increment of the lactate/pyruvate ratio [81,110]. This causes simultaneously an increment in the cytoplasmic NADH/NAD+ state and a reduction of the mitochondrial NADH/NAD+ ratio [111]. However, FBP1 inhibition implies a redox-independent inhibition of gluconeogenesis by oxidized and reduced substrates, while the mGPDH inhibition predicts a gluconeogenesis inhibition only by reduced substrates [112]. These discrepancies seem to have been resolved by the model proposed by Alshawi and Agius, in which the gluconeogenesis inhibition obtained with a treatment dose of MTF depends on a redox-independent mechanism [112]. These authors indicate the allosteric regulation of phosphofructokinase 1 (PFK1) by fructose 1,6-bisphosphate (F1,6P2) [100,113,114] and of FBP1 as a consequence of the reduction of glycerol 3-phosphate level [112].
MTF seems to display a positive effect on the liver not only in T2DM patients, but also in subjects with nonalcoholic fatty liver disease [115] and chronic hepatitis [116]. However, rare cases of hepatotoxicity have been described in fragile patients [117] treated with MTF. These cases seem to be associated with an increment in lactic acidosis, even if this is a very rare occurrence, representing less than three cases per 100,000 patients in one year [106]. Moreover, literature indicates that, in humans, MTF is accumulated principally at the glut level, suggesting that the small intestine is the prime site of MTF action [106] and possibly one in which inhibition of Complex I can actually occur. Nonetheless, sterologic measurements showed that mitochondria account for more than 20% of the hepatocyte volume [118]; therefore, it is doubtful that MTF can reach a concentration in the liver mitochondria sufficient for an inhibitory action, or even sufficient to exert any action.
2.3. The Effects of MTF on the Adipose Tissue
White adipose tissue, together with brown adipose tissue (WAT and BAT, respectively), composes the adipose organ, which constitutes as much as 20–25% of the body weight in healthy humans [119]. In particular, WAT acts as energy storage, is a major endocrine organ that produces several hormones (such as leptin, adiponectin, estrogen, resistin, and cytokines), and is a thermal insulator, contributing to the body temperature maintenance [120,121]. BAT is less represented in human adults, although it is the principal responsible of thermoregulation, due to the specific mitochondrial expression of uncoupling proteins (UCPs), which divert the proton gradient from ATP production to heat generation [122]. It is well known that increased BMI, obesity, and, therefore, the alteration of lean versus fat body mass, are one of the main risk factors for the development of insulin resistance and T2DM [123,124].
In WAT deposits, MTF reaches low levels, which, although in the micromolar range, are not comparable to that of other organs, such as the liver, the kidney, or the intestine [125]. This suggests that the classic mechanism of action proposed for MTF, i.e., mitochondrial Complex I inhibition, would not occur in this tissue.
According to different studies, the MTF-related reduction of body fat mass is due to the improvement in insulin sensitivity and, therefore, to the lower level of insulin released in the bloodstream [126]. Indeed, insulin action on lipid metabolism is anabolic, since it promotes fat storage synthesis and inhibits its release [127,128]. Moreover, in the literature, there is evidence that MTF has a direct effect on WAT, in particular stimulating adiponectin production [126,129,130]. MTF directly upregulates the adiponectin gene expression, both in vivo and in vitro, and stimulates adiponectin secretion from human subcutaneous adipose tissue in vitro [131]. Adiponectin is involved both in the regulation of glucose levels and fatty acid degradation, having a positive effect on T2DM [132]. Another study pointed out that MTF can induce oxidative stress in WAT, increasing the expression of uncoupling protein 2 (UCP2). UCP2 dissipates the mitochondrial proton gradient as heat and, at the same time, can minimize ROS levels [133]. Interestingly, UCP2 expression is one of the characteristic features of the conversion from WAT to BAT, a mechanism known as browning. Browning could be greatly beneficial for diabetic patients; however, another study has demonstrated that MTF administration in animal models can inhibit WAT browning [134]. Despite the mechanism of action on WAT and its correlation to browning still being unclear, MTF has an overall positive effect on the adipose organ.
Transplantation of embryonic BAT with IGF-1 supplementation in type 1 diabetic (T1D) adult mice was able to successfully correct T1D phenotypes, producing rapid and long-lasting normoglycemia, independent from insulin, at a 57% success rate [135], another outstanding example of the beneficial effects of activated BAT on diabetes. Investigating the distribution and accumulation of [11C]-MTF in different tissues in mice, an avid uptake of MTF in BAT deposits was observed. The MTF tissue-to-blood ratio in BAT was comparable to that of the liver during the first hour after intravenous administration. Interestingly, the cited study shows that after one hour from MTF administration, the liver uptake was slowly decreasing, while the absorption in BAT was still increasing [125]. This could suggest a MTF accumulation in BAT; however, a recent study demonstrated that micromolar MTF dosage can increase BAT mass and enhance mitochondrial biogenesis and thermogenesis [136], suggesting that administrating therapeutic dosages of MTF is not sufficient to reach the locoregional millimolar concentration necessary to inhibit mitochondrial Complex I. Moreover, if MTF reached high concentrations in BAT, leading to Complex I inactivation, it would be counterproductive for diabetic patients, as BAT induction and activation by cold exposure was proposed to be protective against obesity and diabetes, having a regulatory role in glucose homeostasis and insulin sensitivity, both in animal models and humans [137]. In conclusion, evidence suggests that MTF accumulation in BAT does not exceed the micromolar concentration and, therefore, is able to implement the mitochondrial functionality and the relative conversion of chemical energy in heating.
2.4. Effect of MTF on the Endothelium
Macro- and micro-angiopathy represents a major diabetes complication [138]. A healthy endothelium is crucial for vascular integrity, as it acts in maintaining a low level of oxidative stress and a relaxed vascular tone by balancing vasodilators (NO, prostacyclin I2, substance P) and vasoconstrictors (endothelin-1, angiotensin II) [139,140]. Hyperglycemia shifts glucose to the polyol pathways in the endothelial cells, generating advanced glycation end-products (AGEs) and activating protein kinase C (PKC) [141]. The resulting increased ROS production, mitochondrial dysfunction, and endothelial nitric oxide synthase (eNOS) impairment causes endothelial dysfunction (ED) and ultimately apoptosis [142].
Clinical studies show that MTF treatment ameliorates endothelial function [140,143]. MTF has a direct action against ED, likely due to improvement of insulin resistance and extra-glycemic effects, such as reduction of oxidative stress and of PKC and NADPH activation [144]. The beneficial effects on vasculature exerted by MTF comprise blood pressure reduction and endothelium-dependent relaxation (EDR) enhancement [145]. In detail, a study on Spontaneously Hypertensive Rats (SHR) shows that treatment with MTF appeared independent of its action in glycemic control and possibly dependent on the upregulation of the NO [145]. Moreover, the mechanisms involved in the vasoprotective effects of MTF may involve its activation of AMPK and of eNOS [146]. MTF restored endothelial function and improved NO bioavailability, lowering the oxidative stress in Goto-Kakizaki rats [147]. MTF inhibited the opening of the mitochondrial permeability transition pore (PTP) triggered by treatment with 30 mmol/L glucose in human microvascular endothelial cells (HMEC-1) as well as in primary endothelial cells from bovine aorta, preventing cell death [37]. The possibility that the beneficial effects of MTF on ED could also be mediated by its action on Complex I has been proposed [148]. However, it appears difficult to believe that the beneficial action of MTF on the endothelial aerobic metabolism could come from an inhibition of Complex I. Rather, it may derive from an indirect action on the mitochondrion, as a consequence of the activation of the NAD-dependent deacetylase Sirtuin 1, a key regulator of metabolism [148], or else from an activation of Complex I by the micromolar concentration of MTF conceivably present inside the endothelial cell in vivo.
2.5. Effects of MTF on the Heart
Diabetes is a well-recognized independent risk factor for the development of cardiovascular disease. In the last few years, a new player, i.e., MTF, emerged in the therapy of cardiovascular disease in diabetes. The UK Prospective Diabetes Study (UKPDS) was the first milestone trial to show that MTF reduces myocardial infarction risk [149]. Cardiomyopathy is one of the vascular complications associated with hyperglycemia in diabetes mellitus (American Heart Association, Cardiovascular disease and diabetes (2019); Available at: https://www.heart.org/en/health-topics/diabetes/diabetes-complications-and-risks/cardiovascular-disease--diabetes, accessed on 10 June 2021). Several studies have investigated the effects of MTF on the heart, with results mostly pointing to a cardioprotective role of MTF [150,151,152]. Notably, cardiomyocytes express relatively high levels of OCT3 [153].
A recent systematic review of randomized controlled trials showed that MTF reduces markers of heart failure in diabetic patients, improving myocardial oxygen consumption, although the molecular mechanisms of these effects are not completely understood, as there is no effect on left ventricular function [154]. Treatment with 100 mg MTF/kg/day for 12 months prevented heart failure in a rat model, and enhanced myocardial eNOS expression [155]. MTF pre-treatment in a pig Ischemia Reperfusion (I/R) model increased myocardial ATP levels after I/R [156]. Reduction of oxidative stress and myocyte apoptosis in animal models of I/R have been mainly related to AMPK activation and an increase in both eNOS activity and superoxide dismutase (SOD) expression [157]. However, MTF administered at the beginning of I/R in H9c2 cells improved mitochondrial respiration by activating the mitochondrial deacetylase protein sirtuin-3 (SIRT3), thus reducing cardiomyocytes oxidative stress and apoptosis; in fact, Sirtuins act in the overall regulation of metabolism [158]. Moreover, other authors propose that some effects of MTF on cardiomyocytes may be partially independent of AMPK and/or mitochondrial respiration [144], as observed in murine cardiomyocytes [159]. Other authors have described that in H9c2 cells maintained in high glucose pre-treatment with MTF before I/R restored mitochondrial membrane potential, and reduced mitochondrial permeability transition pore opening, activating Complexes I and III [160]. However, in the same cells, MTF was reported to inhibit Complex I activity, following I/R [161]. Interestingly, the treatment of mouse embryonic fibroblasts with MTF inhibited Complex I, whereas treatment of mice in vivo with MTF increased Complex I activity [144]. These opposite results may be due to the discrepancies between the millimolar concentrations of MTF used in vitro versus therapeutic blood concentrations (around 10 µM), and by the hydrophilicity of MTF and its low distribution volume [14,35]. In other words, low MTF concentrations activate Complex I and the relative ATP synthesis, while concentrations above 1 mM are inhibitory.
2.6. The Effect of MTF on Skeletal Muscle
Biodistribution of 11C-MTF, estimated through human radiation dosimetry by means of whole-body positron emission tomography (PET), revealed that MTF is primarily taken up by the kidney and liver but also by skeletal muscle, where it slowly accumulates with a reversible two-tissue-compartment kinetics [162]. In fact, the low-affinity, high-capacity MTF transporter OCT3 is expressed in human skeletal muscle [162]. In line with this, a recent report showed an increase in MTF content of human skeletal muscle over time (5 and 11 µM, respectively) after acute (1 day) or short-term (4 days) MTF treatment [163]. The same human study showed that very short-term MTF treatment does not affect skeletal muscle energy homeostasis and AMPK, although it causes enhanced perceived exertion and increased blood catecholamine levels [163]. By contrast, long-term (10 weeks) MTF treatment of diabetic subjects significantly increased AMPK α2 and decreased acetyl-CoA carboxylase-2 activity [164]. In fact, AMPK and its downstream signaling network is considered the main effector of MTF action on skeletal muscle. Notably, a consequence of AMPK activation by MTF is increased muscle glucose uptake and GLUT2 expression [165]. Moreover, it was observed that ATP and phosphocreatine concentrations were lower after MTF treatment [164], suggesting a depressed energy status, consistent with the hypothesis of MTF accumulation in skeletal muscle and the generally inhibitory effect of MTF on muscle mitochondrial function reported in both diabetic humans and animal models of diabetes [166,167].
Oral treatment of control and Zucker diabetic fatty rats with MTF for 2 weeks demonstrated that MTF significantly impairs the oxidative capacity of skeletal muscle in vivo and mitochondrial function ex vivo at high dosages (100–300 mg/kg/day) [166]. In humans, MTF was reported to partially blunt some beneficial adaptations to exercise. In a double-blind randomized study on a cohort of older human subjects at risk for T2DM treated with MTF at clinical doses for 2 weeks, MTF inhibited the improvement in mitochondrial respiration and insulin sensitivity of the skeletal muscle that should have been achieved after 12 weeks of aerobic exercise training. Such antagonism of MTF for the exercise adaptations and the generally depressed energy status of skeletal muscle during long-term treatment have been related to the inhibition of muscle mitochondrial respiration and to the action of MTF on Complex I [167]. However, biopsies of skeletal muscle from T2DM patients treated with MTF did not show suppressed Complex I activity [32]. Furthermore, the self-selected exercise intensity was not reduced by MTF in healthy males, even though MTF increased the rate of perceived exertion during an exercise bout with fixed intensity [168]. Moreover, MTF administration seems to activate a mechanism directly related to the hormetic response through the redox state modulation [169].
MTF interference with mitochondria, if any, appears protective of the heart and endothelium but less protective of muscle. Such a discrepancy may be due to the different substrate preferences: cardiac function mostly relies on the oxidative metabolism of fatty acids inside the mitochondria [170], while the skeletal muscle essentially relies on glucose [165].
3. Conclusions
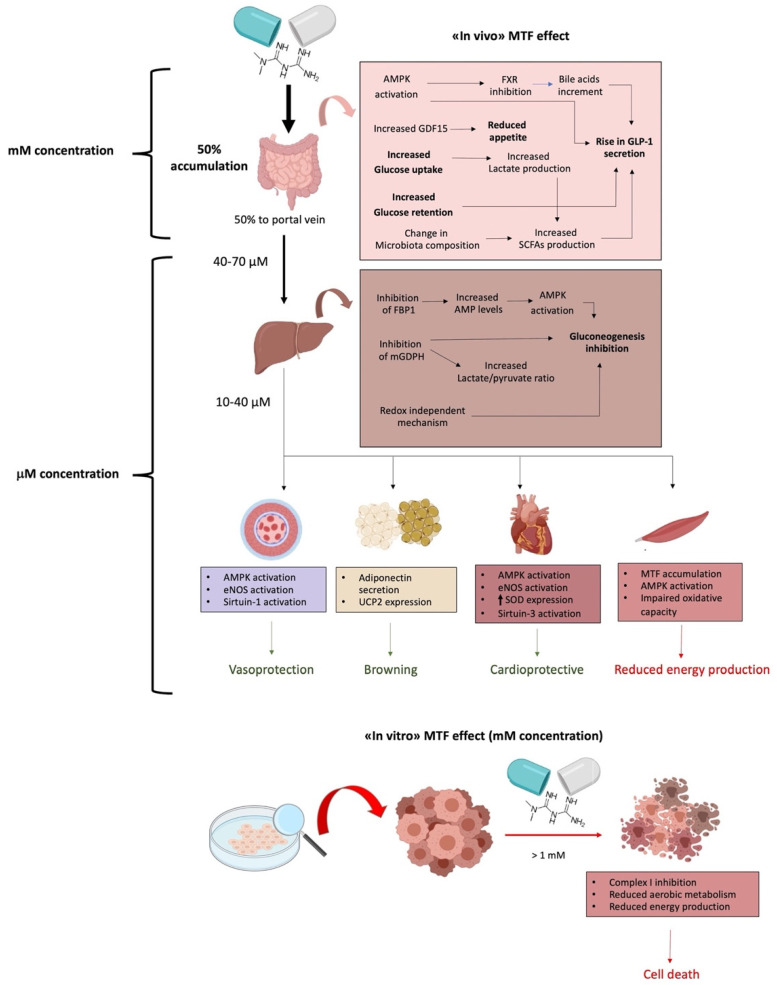
The use of MTF progressively expanded from diabetes to obesity, liver disease, and, finally, cancer therapy. Interestingly, MTF is an example of hormesis, the dose–response phenomenon characterized by a low dose stimulation and high dose inhibition, resulting in an adaptive response to cellular metabolism variations. In detail, millimolar MTF concentrations, used in vitro, caused inhibition of the mitochondrial Complex I, a decrement in chemical energy production, and the consequent activation of the AMPK pathway (Figure 2). Conversely, micromolar MTF concentrations, which correspond to the therapeutic dosage, activated the mitochondrial aerobic metabolism, improving the cellular energy status. It appears that this peculiarity of the mode of action of MTF should be considered when the observations from in vitro studies are translated into in vivo treatments, bearing in mind the different tissue concentrations/accumulation of MTF. Based on the concept of hormesis, this review provides a comprehensive justification of the apparently opposite effects of MTF on single organs, in face of its overall beneficial effect. Currently, the MTF dose (generally, 500–2500 mg/die) depends mainly on the goal of glycated hemoglobin to be achieved. Indeed, it is difficult to estimate the actual concentration of MTF in the various tissues, as it may also differ in a single individual due to the clinical characteristics of each patient. The concept of hormesis can be useful to fully understand the effects of MTF in each patient, thus obtaining effective tailoring of MTF therapy, not only in diabetes.
Figure 2.
Schematic representation of the effects of micromolar (μM) or millimolar (mM) metformin (MTF) concentrations. The upper part of the figure represents the effect of MTF at the therapeutic concentration (in vivo—millimolar in liver and micromolar in the rest of body). The lower part of the figure depicts the effect of MTF at doses usually used in vitro experiments (millimolar).
Author Contributions
Literature review, I.P., A.P., N.B., S.R. and D.M.; writing—original draft preparation, I.P., A.P., N.B. and S.R.; writing—review and editing, I.P., S.R. and D.M.; supervision, I.P. and D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Whiting D.R., Guariguata L., Weil C., Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res. Clin. Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Bailey C.J. Metformin: Historical overview. Diabetologia. 2017;60:1566–1576. doi: 10.1007/s00125-017-4318-z. [DOI] [PubMed] [Google Scholar]
- 3.Seghieri M., Rebelos E., Mari A., Sciangula L., Giorda C., Ferrannini E. Short Course of Insulin Treatment versus Metformin in Newly Diagnosed Patients with Type 2 Diabetes. J. Clin. Med. 2018;7:235. doi: 10.3390/jcm7090235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foretz M., Guigas B., Viollet B. Understanding the glucoregulatory mechanisms of metformin in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019;15:569–589. doi: 10.1038/s41574-019-0242-2. [DOI] [PubMed] [Google Scholar]
- 5.Hundal R.S., Krssak M., Dufour S., Laurent D., Lebon V., Chandramouli V., Inzucchi S.E., Schumann W.C., Petersen K.F., Landau B.R., et al. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Horakova O., Kroupova P., Bardova K., Buresova J., Janovska P., Kopecky J., Rossmeisl M. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci. Rep. 2019;9:6156. doi: 10.1038/s41598-019-42531-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He L., Wondisford F.E. Metformin Action: Concentrations Matter. Cell Metab. 2015;21:159–162. doi: 10.1016/j.cmet.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Tosi F., Muggeo M., Brun E., Spiazzi G., Perobelli L., Zanolin E., Gori M., Coppini A., Moghetti P. Combination treatment with metformin and glibenclamide versus single-drug therapies in type 2 diabetes mellitus: A randomized, double-blind, comparative study. Metabolism. 2003;52:862–867. doi: 10.1016/S0026-0495(03)00101-X. [DOI] [PubMed] [Google Scholar]
- 9.Mirabelli M., Chiefari E., Tocci V., Caroleo P., Giuliano S., Greco E., Luque R.M., Puccio L., Foti D.P., Aversa A., et al. Clinical Effectiveness and Safety of Once-Weekly GLP-1 Receptor Agonist Dulaglutide as Add-On to Metformin or Metformin Plus Insulin Secretagogues in Obesity and Type 2 Diabetes. J. Clin. Med. 2021;10:985. doi: 10.3390/jcm10050985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin C.-Y., Wu C.-H., Hsu C.-Y., Chen T.-H., Lin M.-S., Lin Y.-S., Su Y.-J. Reduced Mortality Associated With the Use of Metformin Among Patients With Autoimmune Diseases. Front. Endocrinol. 2021;12:423. doi: 10.3389/fendo.2021.641635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ghai V., Kim T.-K., Etheridge A., Nielsen T., Hansen T., Pedersen O., Galas D., Wang K. Extracellular Vesicle Encapsulated MicroRNAs in Patients with Type 2 Diabetes Are Affected by Metformin Treatment. J. Clin. Med. 2019;8:617. doi: 10.3390/jcm8050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rotermund C., Machetanz G., Fitzgerald J.C. The Therapeutic Potential of Metformin in Neurodegenerative Diseases. Front. Endocrinol. 2018;9:400. doi: 10.3389/fendo.2018.00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogelman Y., Kitai E., Blumberg G., Golan-Cohen A., Rapoport M., Carmeli E. Vitamin B12 screening in metformin-treated diabetics in primary care: Were elderly patients less likely to be tested? Aging Clin. Exp. Res. 2017;29:135–139. doi: 10.1007/s40520-016-0546-1. [DOI] [PubMed] [Google Scholar]
- 14.Apostolova N., Iannantuoni F., Gruevska A., Muntane J., Rocha M., Victor V.M. Mechanisms of action of metformin in type 2 diabetes: Effects on mitochondria and leukocyte-endothelium interactions. Redox Biol. 2020;34:101517. doi: 10.1016/j.redox.2020.101517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nies A.T., Hofmann U., Resch C., Schaeffeler E., Rius M., Schwab M. Proton Pump Inhibitors Inhibit Metformin Uptake by Organic Cation Transporters (OCTs) PLoS ONE. 2011;6:e22163. doi: 10.1371/journal.pone.0022163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Motohashi H., Inui K. Organic cation transporter OCTs (SLC22) and MATEs (SLC47) in the human kidney. AAPS J. 2013;15:581–588. doi: 10.1208/s12248-013-9465-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheen A.J. Clinical Pharmacokinetics of Metformin. Clin. Pharmacokinet. 1996;30:359–371. doi: 10.2165/00003088-199630050-00003. [DOI] [PubMed] [Google Scholar]
- 18.Graham G.G., Punt J., Arora M., Day R.O., Doogue M.P., Duong J.K., Furlong T.J., Greenfield J.R., Greenup L.C., Kirkpatrick C.M., et al. Clinical pharmacokinetics of metformin. Clin. Pharmacokinet. 2011;50:81–98. doi: 10.2165/11534750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Fontaine E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Front. Endocrinol. 2018;9:753. doi: 10.3389/fendo.2018.00753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rena G., Hardie D.G., Pearson E.R. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vial G., Detaille D., Guigas B. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Front. Endocrinol. 2019;10:294. doi: 10.3389/fendo.2019.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hou W.-L., Yin J., Alimujiang M., Yu X.-Y., Ai L.-G., Bao Y., Liu F., Jia W.-P. Inhibition of mitochondrial complex I improves glucose metabolism independently of AMPK activation. J. Cell. Mol. Med. 2017;22:1316–1328. doi: 10.1111/jcmm.13432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bridges H.R., Jones A.J.Y., Pollak M.N., Hirst J. Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem. J. 2014;462:475–487. doi: 10.1042/BJ20140620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Salani B., Marini C., Rio A.D., Ravera S., Massollo M., Orengo A.M., Amaro A., Passalacqua M., Maffioli S., Pfeffer U., et al. Metformin Impairs Glucose Consumption and Survival in Calu-1 Cells by Direct Inhibition of Hexokinase-II. Sci. Rep. 2013;3:2070. doi: 10.1038/srep02070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wheaton W.W., Weinberg S.E., Hamanaka R.B., Soberanes S., Sullivan L.B., Anso E., Glasauer A., Dufour E., Mutlu G.M., Budigner G.S., et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumorigenesis. Elife. 2014;3:e02242. doi: 10.7554/eLife.02242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vancura A., Bu P., Bhagwat M., Zeng J., Vancurova I. Metformin as an Anticancer Agent. Trends Pharmacol. Sci. 2018;39:867–878. doi: 10.1016/j.tips.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cioce M., Pulito C., Strano S., Blandino G., Fazio V.M. Metformin: Metabolic Rewiring Faces Tumor Heterogeneity. Cells. 2020;9:2439. doi: 10.3390/cells9112439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higgins J.W., Bedwell D.W., Zamek-Gliszczynski M.J. Ablation of both organic cation transporter (OCT)1 and OCT2 alters metformin pharmacokinetics but has no effect on tissue drug exposure and pharmacodynamics. Drug Metab. Dispos. 2012;40:1170–1177. doi: 10.1124/dmd.112.044875. [DOI] [PubMed] [Google Scholar]
- 29.Yu Wang A., An H., Liu T., Wondisford F.E. Metformin Improves Mitochondrial Respiratory Activity through Activation of AMPK. Cell Rep. 2019;29:1511–1523.e5. doi: 10.1016/j.celrep.2019.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilcock C., Wyre N.D., Bailey C.J. Subcellular distribution of metformin in rat liver. J. Pharm. Pharmacol. 1991;43:442–444. doi: 10.1111/j.2042-7158.1991.tb03507.x. [DOI] [PubMed] [Google Scholar]
- 31.Ravera S., Cossu V., Tappino B., Nicchia E., Dufour C., Cavani S., Sciutto A., Bolognesi C., Columbaro M., Degan P., et al. Concentration-dependent metabolic effects of metformin in healthy and Fanconi anemia lymphoblast cells. J. Cell. Physiol. 2018;233:1736–1751. doi: 10.1002/jcp.26085. [DOI] [PubMed] [Google Scholar]
- 32.Larsen S., Rabøl R., Hansen C.N., Madsbad S., Helge J.W., Dela F. Metformin-treated patients with type 2 diabetes have normal mitochondrial complex I respiration. Diabetologia. 2012;55:443–449. doi: 10.1007/s00125-011-2340-0. [DOI] [PubMed] [Google Scholar]
- 33.Victor V.M., Rovira-Llopis S., Bañuls C., Diaz-Morales N., Castelló R., Falcón R., Gómez M., Rocha M., Hernández-Mijares A. Effects of metformin on mitochondrial function of leukocytes from polycystic ovary syndrome patients with insulin resistance. Eur. J. Endocrinol. 2015;173:683–691. doi: 10.1530/EJE-15-0572. [DOI] [PubMed] [Google Scholar]
- 34.Kramer P.A., Ravi S., Chacko B., Johnson M.S., Darley-Usmar V.M. A review of the mitochondrial and glycolytic metabolism in human platelets and leukocytes: Implications for their use as bioenergetic biomarkers. Redox Biol. 2014;2:206–210. doi: 10.1016/j.redox.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ravera S., Caicci F., Degan P., Maggi D., Manni L., Puddu A., Nicolò M., Traverso C.E., Panfoli I. Inhibitory Action of Antidiabetic Drugs on the Free Radical Production by the Rod Outer Segment Ectopic Aerobic Metabolism. Antioxidants. 2020;9:1133. doi: 10.3390/antiox9111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhamra G.S., Hausenloy D.J., Davidson S.M., Carr R.D., Paiva M., Wynne A.M., Mocanu M.M., Yellon S. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res. Cardiol. 2008;103:274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 37.Detaille D., Guigas B., Chauvin C., Batandier C., Fontaine E., Wiernsperger N., Leverve X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes. 2005;54:2179–2187. doi: 10.2337/diabetes.54.7.2179. [DOI] [PubMed] [Google Scholar]
- 38.Loubiere C., Clavel S., Gilleron J., Harisseh R., Fauconnier J., Ben-Sahra I., Kaminski L., Laurent K., Herkenne S., Lacas-Gervais S., et al. The energy disruptor metformin targets mitochondrial integrity via modification of calcium flux in cancer cells. Sci. Rep. 2017;7:5040. doi: 10.1038/s41598-017-05052-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pinti M.V., Fink G.K., Hathaway Q.A., Durr A.J., Kunovac A., Hollander J.M., Mv P., Gk F., Qa H., Aj D., et al. Mitochondria Dysfunction in Aging and Metabolic Diseases. J. Physiol. Endocrinol. Metab. 2019;316:268–285. doi: 10.1152/ajpendo.00314.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belosludtsev K.N., Belosludtseva N.V., Dubinin M.V. Diabetes Mellitus, Mitochondrial Dysfunction and Ca2+-Dependent Permeability Transition Pore. Int. J. Mol. Sci. 2020;21:6559. doi: 10.3390/ijms21186559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Proctor W.R., Bourdet D.L., Thakker D.R. Mechanisms underlying saturable intestinal absorption of metformin. Drug Metab. Dispos. 2008;36:1650–1658. doi: 10.1124/dmd.107.020180. [DOI] [PubMed] [Google Scholar]
- 42.Paleari L., Burhenne J., Weiss J., Foersch S., Roth W., Parodi A., Gnant M., Bachleitner-Hofmann T., Scherer D., Ulrich C.M., et al. High Accumulation of Metformin in Colonic Tissue of Subjects With Diabetes or the Metabolic Syndrome. Gastroenterology. 2018;154:1543–1545. doi: 10.1053/j.gastro.2017.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shu Y., Sheardown S.A., Brown C., Owen R.P., Zhang S., Castro R.A., Ianculescu A.G., Yue L., Lo J.C., Burchard E.G., et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J. Clin. Investigation. 2007;117:1422–1431. doi: 10.1172/JCI30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dujic T., Zhou K., Donnelly L.A., Tavendale R., Palmer C.N.A., Pearson E.R. Association of organic cation transporter 1 with intolerance to metformin in type 2 diabetes: A GoDARTS study. Diabetes. 2015;64:1786–1793. doi: 10.2337/db14-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dujic T., Causevic A., Bego T., Malenica M., Velija-Asimi Z., Pearson E.R., Semiz S. Organic cation transporter 1 variants and gastrointestinal side effects of metformin in patients with Type 2 diabetes. Diabet. Med. 2016;33:511–514. doi: 10.1111/dme.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tarasova L., Kalnina I., Geldnere K., Bumbure A., Ritenberga R., Nikitina-Zake L., Fridmanis D., Vaivade I., Pirags V., Klovins J. Association of genetic variation in the organic cation transporters OCT1, OCT2 and multidrug and toxin extrusion 1 transporter protein genes with the gastrointestinal side effects and lower BMI in metformin-treated type 2 diabetes patients. Pharmacogenet. Genom. 2012;22:659–666. doi: 10.1097/FPC.0b013e3283561666. [DOI] [PubMed] [Google Scholar]
- 47.Fujioka K., Brazg R.L., Raz I., Bruce S., Joyal S., Swanink R., Pans M. Efficacy, dose-response relationship and safety of once-daily extended-release metformin (Glucophage® XR) in type 2 diabetic patients with inadequate glycaemic control despite prior treatment with diet and exercise: Results from two double-blind, placebo- Diabetes Obes. Metab. 2005;7:28–39. doi: 10.1111/j.1463-1326.2004.00369.x. [DOI] [PubMed] [Google Scholar]
- 48.Blonde L., Dailey G.E., Jabbour S.A., Reasner C.A., Mills D.J. Gastrointestinal tolerability of extended-release metformin tablets compared to immediate-release metformin tablets: Results of a retrospective cohort study. Curr. Med. Res. Opin. 2004;20:565–572. doi: 10.1185/030079904125003278. [DOI] [PubMed] [Google Scholar]
- 49.Donnelly L.A., Morris A.D., Pearson E.R. Adherence in patients transferred from immediate release metformin to a sustained release formulation: A population-based study. Diabetes Obes. Metab. 2009;11:338–342. doi: 10.1111/j.1463-1326.2008.00973.x. [DOI] [PubMed] [Google Scholar]
- 50.Liang X., Giacomini K.M. Transporters Involved in Metformin Pharmacokinetics and Treatment Response. J. Pharm. Sci. 2017;106:2245–2250. doi: 10.1016/j.xphs.2017.04.078. [DOI] [PubMed] [Google Scholar]
- 51.Yee S.W., Lin L., Merski M., Keiser M.J., Gupta A., Zhang Y., Chien H.C., Shoichet B.K., Giacomini K.M. Prediction and validation of enzyme and transporter off-targets for metformin. J. Pharmacokinet. Pharmacodyn. 2015;42:463–475. doi: 10.1007/s10928-015-9436-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamichi N., Shima H., Asano S., Ishimoto T., Sugiura T., Matsubara K., Kusuhara H., Sugiyama Y., Sai Y., Miyamoto K.I., et al. Involvement of carnitine/organic cation transporter OCTN1/SLC22A4 in gastrointestinal absorption of metformin. J. Pharm. Sci. 2013;102:3407–3417. doi: 10.1002/jps.23595. [DOI] [PubMed] [Google Scholar]
- 53.Müller J., Lips K.S., Metzner L., Neubert R.H.H., Koepsell H., Brandsch M. Drug specificity and intestinal membrane localization of human organic cation transporters (OCT) Biochem. Pharmacol. 2005;70:1851–1860. doi: 10.1016/j.bcp.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Han T., Everett R.S., Proctor W.R., Ng C.M., Costales C.L., Brouwer K.L.R., Thakker D.R. Organic cation transporter 1 (OCT1/mOct1) is localized in the apical membrane of Caco-2 cell monolayers and enterocytes. Mol. Pharmacol. 2013;84:182–189. doi: 10.1124/mol.112.084517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Han T., Proctor W.R., Costales C.L., Cai H., Everett R.S., Thakker D.R. Four cation-selective transporters contribute to apical uptake and accumulation of metformin in Caco-2 cell monolayerss. J. Pharmacol. Exp. Ther. 2015;352:519–528. doi: 10.1124/jpet.114.220350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCreight L.J., Stage T.B., Connelly P., Lonergan M., Nielsen F., Prehn C., Adamski J., Brøsen K., Pearson E.R. Pharmacokinetics of metformin in patients with gastrointestinal intolerance. Diabetes Obes. Metab. 2018;20:1593–1601. doi: 10.1111/dom.13264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lien F., Berthier A., Bouchaert E., Gheeraert C., Alexandre J., Porez G., Prawitt J., Dehondt H., Ploton M., Colin S., et al. Metformin interferes with bile acid homeostasis through AMPK-FXR crosstalk. J. Clin. Investig. 2014;124:1037–1051. doi: 10.1172/JCI68815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Scarpello J.H.B., Hodgson E. HCSHowlett Effect of metformin on bile salt circulation and intestinal motility in type 2 diabetes mellitus. Diabet. Med. 1998;15:651–656. doi: 10.1002/(SICI)1096-9136(199808)15:8<651::AID-DIA628>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 59.Drucker D.J. The biology of incretin hormones. Cell Metab. 2006;3:153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 60.Bahne E., Sun E.W.L., Young R.L., Hansen M., Sonne D.P., Hansen J.S., Rohde U., Liou A.P., Jackson M.L., de Fontgalland D., et al. Metformin-induced glucagon-like peptide-1 secretion contributes to the actions of metformin in type 2 diabetes. JCI Insight. 2018;3:e93936. doi: 10.1172/jci.insight.93936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duca F.A., Côté C.D., Rasmussen B.A., Zadeh-Tahmasebi M., Rutter G.A., Filippi B.M., Lam T.K.T. Metformin activates a duodenal Ampk-dependent pathway to lower hepatic glucose production in rats. Nat. Med. 2015;21:506–511. doi: 10.1038/nm.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nadkarni P., Chepurny O.G., Holz G.G. Progress in Molecular Biology and Translational Science. Volume 121. Elsevier; Amsterdam, The Netherlands: 2014. Regulation of glucose homeostasis by GLP-1; pp. 23–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brighton C.A., Rievaj J., Kuhre R.E., Glass L.L., Schoonjans K., Holst J.J., Gribble F.M., Reimann F. Bile acids trigger GLP-1 release predominantly by accessing basolaterally located G protein-coupled bile acid receptors. Endocrinology. 2015;156:3961–3970. doi: 10.1210/en.2015-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parker H.E., Wallis K., Le Roux C.W., Wong K.Y., Reimann F., Gribble F.M. Molecular mechanisms underlying bile acid-stimulated glucagon-like peptide-1 secretion. Br. J. Pharmacol. 2012;165:414–423. doi: 10.1111/j.1476-5381.2011.01561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Christiansen C.B., Trammell S.A.J., Albrechtsen N.J.W., Schoonjans K., Albrechtsen R., Gillum M.P., Kuhre R.E., Holst J.J. Bile acids drive colonic secretion of glucagon-like-peptide 1 and peptide-YY in rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 2019;316:G574–G584. doi: 10.1152/ajpgi.00010.2019. [DOI] [PubMed] [Google Scholar]
- 66.Mulherin A.J., Oh A.H., Kim H., Grieco A., Lauffer L.M., Brubaker P.L. Mechanisms underlying metformin-induced secretion of glucagon-like peptide-1 from the intestinal L cell. Endocrinology. 2011;152:4610–4619. doi: 10.1210/en.2011-1485. [DOI] [PubMed] [Google Scholar]
- 67.McCreight L.J., Bailey C.J., Pearson E.R. Metformin and the gastrointestinal tract. Diabetologia. 2016;59:426–435. doi: 10.1007/s00125-015-3844-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang S., Zhai H., Li D., Huang J., Zhang H., Li Z., Zhang W., Xu G. AMPK-dependent regulation of GLP1 expression in L-like cells. J. Mol. Endocrinol. 2016;57:151–160. doi: 10.1530/JME-16-0099. [DOI] [PubMed] [Google Scholar]
- 69.Sayers S.R., Reimann F., Gribble F.M., Parker H., Zac-Varghese S., Bloom S.R., Foretz M., Viollet B., Rutter G.A. Proglucagon promoter cre-mediated AMPK deletion in mice increases circulating GLP-1 levels and oral glucose tolerance. PLoS ONE. 2016;11:e0149549. doi: 10.1371/journal.pone.0149549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pollak M. The effects of metformin on gut microbiota and the immune system as research frontiers. Diabetologia. 2017;60:1662–1667. doi: 10.1007/s00125-017-4352-x. [DOI] [PubMed] [Google Scholar]
- 71.Puddu A., Sanguineti R., Montecucco F., Viviani G.L. Evidence for the gut microbiota short-chain fatty acids as key pathophysiological molecules improving diabetes. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/162021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kellett G.L., Brot-Laroche E. Apical GLUT2: A major pathway of intestinal sugar absorption. Diabetes. 2005;54:3056–3062. doi: 10.2337/diabetes.54.10.3056. [DOI] [PubMed] [Google Scholar]
- 73.Koepsell H. Glucose transporters in the small intestine in health and disease. Pflugers Arch. Eur. J. Physiol. 2020;472:1207–1248. doi: 10.1007/s00424-020-02439-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kipp H., Khoursandi S., Scharlau D., Kinne R.K.H. More than apical: Distribution of SGLT1 in Caco-2 cells. Am. J. Physiol. Cell Physiol. 2003;285:C737–C749. doi: 10.1152/ajpcell.00041.2003. [DOI] [PubMed] [Google Scholar]
- 75.Vrhovac I., Eror D.B., Klessen D., Burger C., Breljak D., Kraus O., Radović N., Jadrijević S., Aleksic I., Walles T., et al. Localizations of Na+-D-glucose cotransporters SGLT1 and SGLT2 in human kidney and of SGLT1 in human small intestine, liver, lung, and heart. Pflugers Arch. Eur. J. Physiol. 2015;467:1881–1898. doi: 10.1007/s00424-014-1619-7. [DOI] [PubMed] [Google Scholar]
- 76.Sakar Y., Meddah B., Faouzi M.Y.A., Cherrah Y., Bado A., Ducroc R. Metformin-induced regulation of the intestinal d-glucose transporters. J. Physiol. Pharmacol. 2010;61:301–307. [PubMed] [Google Scholar]
- 77.Yang M., Darwish T., Larraufie P., Rimmington D., Cimino I., Goldspink D.A., Jenkins B., Koulman A., Brighton C.A., Ma M., et al. Inhibition of mitochondrial function by metformin increases glucose uptake, glycolysis and GDF-15 release from intestinal cells. Sci. Rep. 2021;11:2529. doi: 10.1038/s41598-021-81349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tsai V.W.W., Husaini Y., Sainsbury A., Brown D.A., Breit S.N. The MIC-1/GDF15-GFRAL Pathway in Energy Homeostasis: Implications for Obesity, Cachexia, and Other Associated Diseases. Cell Metab. 2018;28:353–368. doi: 10.1016/j.cmet.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 79.Coll A.P., Chen M., Taskar P., Rimmington D., Patel S., Tadross J.A., Cimino I., Yang M., Welsh P., Virtue S., et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature. 2020;578:444–448. doi: 10.1038/s41586-019-1911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bailey C.J., Wilcock C., Scarpello J.H.B. Metformin and the intestine. Diabetologia. 2008;51:1552–1553. doi: 10.1007/s00125-008-1053-5. [DOI] [PubMed] [Google Scholar]
- 81.Madiraju A.K., Erion D.M., Rahimi Y., Zhang X.-M., Braddock D.T., Albright R.A., Prigaro B.J., Wood J.L., Bhanot S., MacDonald M.J., et al. Metformin suppresses gluconeogenesis by inhibiting mitochondrial glycerophosphate dehydrogenase. Nature. 2014;510:542–546. doi: 10.1038/nature13270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Burton J.H., Johnson M., Johnson J., Hsia D.S., Greenway F.L., Heiman M.L. Addition of a gastrointestinal microbiome modulator to metformin improves metformin tolerance and fasting glucose levels. J. Diabetes Sci. Technol. 2015;9:808–814. doi: 10.1177/1932296815577425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.DeFronzo R., Fleming G.A., Chen K., Bicsak T.A. Erratum: Corrigendum to “Metformin-associated lactic acidosis: Current perspectives on causes and risk” (Metabolism: Clinical and Experimental (2016) 65 (20–29) (S0026049515003066) (10.1016/j.metabol.2015.10.014)) Metabolism. 2016;65:1432–1433. doi: 10.1016/j.metabol.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 84.Gennari F.J., Weise W.J. Acid-base disturbances in gastrointestinal disease. Clin. J. Am. Soc. Nephrol. 2008;3:1861–1868. doi: 10.2215/CJN.02450508. [DOI] [PubMed] [Google Scholar]
- 85.Giaccari A., Solini A., Frontoni S., Prato S. Del Metformin benefits: Another example for alternative energy substrate mechanism? Diabetes Care. 2021;44:647–654. doi: 10.2337/dc20-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bourriaud C., Robins R.J., Martin L., Kozlowski F., Tenailleau E., Cherbut C., Michel C. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J. Appl. Microbiol. 2005;99:201–212. doi: 10.1111/j.1365-2672.2005.02605.x. [DOI] [PubMed] [Google Scholar]
- 87.Karlsson F.H., Tremaroli V., Nookaew I., Bergström G., Behre C.J., Fagerberg B., Nielsen J., Bäckhed F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103. doi: 10.1038/nature12198. [DOI] [PubMed] [Google Scholar]
- 88.Wang J.J., Qin J., Li Y., Cai Z., Li S.S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 89.Ji S., Wang L., Li L. Effect of Metformin on Short-Term High-Fat Diet-Induced Weight Gain and Anxiety-Like Behavior and the Gut Microbiota. Front. Endocrinol. 2019;10:704. doi: 10.3389/fendo.2019.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Napolitano A., Miller S., Nicholls A.W., Baker D., Van Horn S., Thomas E., Rajpal D., Spivak A., Brown J.R., Nunez D.J. Novel gut-based pharmacology of metformin in patients with type 2 diabetes mellitus. PLoS ONE. 2014;9:e100778. doi: 10.1371/journal.pone.0100778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pryor R., Cabreiro F. Repurposing metformin: An old drug with new tricks in its binding pockets. Biochem. J. 2015;471:307–322. doi: 10.1042/BJ20150497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang Q., Hu N. Effects of metformin on the gut microbiota in obesity and type 2 diabetes mellitus. Diabetes Metab. Syndr. Obes. Targets Ther. 2020;13:5003–5014. doi: 10.2147/DMSO.S286430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bryrup T., Thomsen C.W., Kern T., Allin K.H., Brandslund I., Jørgensen N.R., Vestergaard H., Hansen T., Hansen T.H., Pedersen O., et al. Metformin-induced changes of the gut microbiota in healthy young men: Results of a non-blinded, one-armed intervention study. Diabetologia. 2019;62:1024–1035. doi: 10.1007/s00125-019-4848-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sun L., Xie C., Wang G., Wu Y., Wu Q., Wang X., Liu J., Deng Y., Xia J., Chen B., et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat. Med. 2018;24:1919–1929. doi: 10.1038/s41591-018-0222-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.De La Cuesta-Zuluaga J., Mueller N.T., Corrales-Agudelo V., Velásquez-Mejía E.P., Carmona J.A., Abad J.M., Escobar J.S. Metformin is associated with higher relative abundance of mucin-degrading akkermansia muciniphila and several short-chain fatty acid-producing microbiota in the gut. Diabetes Care. 2017;40:54–62. doi: 10.2337/dc16-1324. [DOI] [PubMed] [Google Scholar]
- 96.Jones G.R., Molloy M.P. Metformin, Microbiome and Protection Against Colorectal Cancer. Dig. Dis. Sci. 2020;66:1409–1414. doi: 10.1007/s10620-020-06390-4. [DOI] [PubMed] [Google Scholar]
- 97.Donohoe D.R., Collins L.B., Wali A., Bigler R., Sun W., Bultman S.J. The Warburg Effect Dictates the Mechanism of Butyrate-Mediated Histone Acetylation and Cell Proliferation. Mol. Cell. 2012;48:612–626. doi: 10.1016/j.molcel.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chowdhury S., Yung E., Pintilie M., Muaddi H., Chaib S., Yeung M.T., Fusciello M., Sykes J., Pitcher B., Hagenkort A., et al. MATE2 expression is associated with cancer cell response to metformin. PLoS ONE. 2016;11:e0165214. doi: 10.1371/journal.pone.0165214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xie J., Xia L., Xiang W., He W., Yin H., Wang F., Gao T., Qi W., Yang Z., Yang X., et al. Metformin selectively inhibits metastatic colorectal cancer with the KRAS mutation by intracellular accumulation through silencing MATE1. Proc. Natl. Acad. Sci. USA. 2020;117:13012–13022. doi: 10.1073/pnas.1918845117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Agius L., Ford B.E., Chachra S.S. The Metformin Mechanism on Gluconeogenesis and AMPK Activation: The Metabolite Perspective. Int. J. Mol. Sci. 2020;21:3240. doi: 10.3390/ijms21093240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Natali A., Ferrannini E. Effects of metformin and thiazolidinediones on suppression of hepatic glucose production and stimulation of glucose uptake in type 2 diabetes: A systematic review. Diabetologia. 2006;49:434–441. doi: 10.1007/s00125-006-0141-7. [DOI] [PubMed] [Google Scholar]
- 102.Shaw R.J., Lamia K.A., Vasquez D., Koo S.-H., Bardeesy N., Depinho R.A., Montminy M., Cantley L.C. The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science. 2005;310:1642–1646. doi: 10.1126/science.1120781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miller R.A., Chu Q., Xie J., Foretz M., Viollet B., Birnbaum M.J. Biguanides suppress hepatic glucagon signalling by decreasing production of cyclic AMP. Nature. 2013;494:256–260. doi: 10.1038/nature11808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Owen M.R., Doran E., Halestrap A.P. Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 2000;348:607–614. doi: 10.1042/bj3480607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wilcock C., Bailey C.J. Accumulation of metformin by tissues of the normal and diabetic mouse. Xenobiotica. 1994;24:49–57. doi: 10.3109/00498259409043220. [DOI] [PubMed] [Google Scholar]
- 106.Song R. Mechanism of Metformin: A Tale of Two Sites. Diabetes Care. 2016;39:187–189. doi: 10.2337/dci15-0013. [DOI] [PubMed] [Google Scholar]
- 107.Jensen J.B., Sundelin E.I., Jakobsen S., Gormsen L.C., Munk O.L., Frøkiær J., Jessen N. [11C]-Labeled Metformin Distribution in the Liver and Small Intestine Using Dynamic Positron Emission Tomography in Mice Demonstrates Tissue-Specific Transporter Dependency. Diabetes. 2016;65:1724–1730. doi: 10.2337/db16-0032. [DOI] [PubMed] [Google Scholar]
- 108.Foretz M., Guigas B., Bertrand L., Pollak M., Viollet B. Metformin: From mechanisms of action to therapies. Cell Metab. 2014;20:953–966. doi: 10.1016/j.cmet.2014.09.018. [DOI] [PubMed] [Google Scholar]
- 109.Hunter R.W., Hughey C.C., Lantier L., Sundelin E.I., Peggie M., Zeqiraj E., Sicheri F., Jessen N., Wasserman D.H., Sakamoto K. Metformin reduces liver glucose production by inhibition of fructose-1-6-bisphosphatase. Nat. Med. 2018;24:1395–1406. doi: 10.1038/s41591-018-0159-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferrannini E. The Target of Metformin in Type 2 Diabetes. N. Engl. J. Med. 2014;371:1547–1548. doi: 10.1056/NEJMcibr1409796. [DOI] [PubMed] [Google Scholar]
- 111.Madiraju A.K., Qiu Y., Perry R.J., Rahimi Y., Zhang X.-M., Zhang D., Camporez J.-P.G., Cline G.W., Butrico G.M., Kemp B.E., et al. Metformin inhibits gluconeogenesis via a redox-dependent mechanism in vivo. Nat. Med. 2018;24:1384–1394. doi: 10.1038/s41591-018-0125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alshawi A., Agius L. Low metformin causes a more oxidized mitochondrial NADH/NAD redox state in hepatocytes and inhibits gluconeogenesis by a redox-independent mechanism. J. Biol. Chem. 2019;294:2839–2853. doi: 10.1074/jbc.RA118.006670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang C.-S., Hawley S.A., Zong Y., Li M., Wang Z., Gray A., Ma T., Cui J., Feng J.-W., Zhu M., et al. Fructose-1,6-bisphosphate and aldolase mediate glucose sensing by AMPK. Nature. 2017;548:112–116. doi: 10.1038/nature23275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Li M., Zhang C.-S., Zong Y., Feng J.-W., Ma T., Hu M., Lin Z., Li X., Xie C., Wu Y., et al. Transient Receptor Potential V Channels Are Essential for Glucose Sensing by Aldolase and AMPK. Cell Metab. 2019;30:508–524.e12. doi: 10.1016/j.cmet.2019.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Miralles-Linares F., Puerta-Fernandez S., Bernal-Lopez M.R., Tinahones F.J., Andrade R.J., Gomez-Huelgas R. Metformin-induced hepatotoxicity. Diabetes Care. 2012;35:e21. doi: 10.2337/dc11-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Romero-Gómez M., Diago M., Andrade R.J., Calleja J.L., Salmerón J., Fernández-Rodríguez C.M., Solà R., García-Samaniego J., Herrerías J.M., De la Mata M., et al. Treatment of insulin resistance with metformin in naïve genotype 1 chronic hepatitis C patients receiving peginterferon alfa-2a plus ribavirin. Hepatology. 2009;50:1702–1708. doi: 10.1002/hep.23206. [DOI] [PubMed] [Google Scholar]
- 117.Cone C.J., Bachyrycz A.M., Murata G.H. Hepatotoxicity associated with metformin therapy in treatment of type 2 diabetes mellitus with nonalcoholic fatty liver disease. Ann. Pharmacother. 2010;44:1655–1659. doi: 10.1345/aph.1P099. [DOI] [PubMed] [Google Scholar]
- 118.Veltri K.L., Espiritu M., Singh G. Distinct genomic copy number in mitochondria of different mammalian organs. J. Cell Physiol. 1990;143:160–164. doi: 10.1002/jcp.1041430122. [DOI] [PubMed] [Google Scholar]
- 119.Saely C.H., Geiger K., Drexel H. Brown versus White Adipose Tissue: A Mini-Review. Gerontology. 2010;58:15–23. doi: 10.1159/000321319. [DOI] [PubMed] [Google Scholar]
- 120.Kershaw E.E., Flier J.S. Adipose Tissue as an Endocrine Organ. J. Clin. Endocrinol. Metab. 2004;89:2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 121.Coelho M., Oliveira T., Fernandes R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013;9:191–200. doi: 10.5114/aoms.2013.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cannon B., Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 123.Kahn B.B., Flier J.S. Obesity and insulin resistance. J. Clin. Investig. 2000;106:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wondmkun Y.T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. Targets Ther. 2020;13:3611–3616. doi: 10.2147/DMSO.S275898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Breining P., Jensen J.B., Sundelin E.I., Gormsen L.C., Jakobsen S., Busk M., Rolighed L., Bross P., Fernandez-Guerra P., Markussen L.K., et al. Metformin targets brown adipose tissue in vivo and reduces oxygen consumption in vitro. Diabetes Obes. Metab. 2018;20:2264–2273. doi: 10.1111/dom.13362. [DOI] [PubMed] [Google Scholar]
- 126.Wilcox G. Insulin and Insulin Resistance. Clin. Biochem. Rev. 2005;26:19–39. [PMC free article] [PubMed] [Google Scholar]
- 127.Dimitriadis G., Mitron P., Lambadiari V., Maratou E., Raptis S.A. Insulin effects in muscle and adipose tissue. Diabetes Res. Clin. Pract. 2011;93:S52–S59. doi: 10.1016/S0168-8227(11)70014-6. [DOI] [PubMed] [Google Scholar]
- 128.Vargas E., Carrillo Sepulveda M.A. Biochemistry, Insulin, Metabolic Effects. StatPearls Publishing; Treasure Island, FL, USA: 2018. [PubMed] [Google Scholar]
- 129.Grycel S., Markowski A.R., Hady H.R., Zabielski P., Kojta I., Imierska M., Górski J., Blachnio-Zabielska A.U. Metformin treatment affects adipocytokine secretion and lipid composition in adipose tissues of diet-induced insulin-resistant rats. Nutrition. 2019;63–64:126–133. doi: 10.1016/j.nut.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 130.Li B., Po S.S., Zhang B., Bai F., Li J., Qin F., Liu N., Sun C., Xiao Y., Tu T., et al. Metformin regulates adiponectin signalling in epicardial adipose tissue and reduces atrial fibrillation vulnerability. J. Cell. Mol. Med. 2020;24:7751–7766. doi: 10.1111/jcmm.15407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zulian A., Cancello R., Girola A., Gilardini L., Alberti L., Croci M., Micheletto G., Danelli P., Invitti C. In vitro and in vivo effects of metformin on human adipose tissue adiponectin. Obes. Facts. 2011;4:27–33. doi: 10.1159/000324582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Achari A.E., Jain S.K. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int. J. Mol. Sci. 2017;18:1321. doi: 10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Anedda A., Rial E., González-Barroso M.M. Metformin induces oxidative stress in white adipocytes and raises uncoupling protein 2 levels. J. Endocrinol. 2008;199:33–40. doi: 10.1677/JOE-08-0278. [DOI] [PubMed] [Google Scholar]
- 134.Auger C., Knuth C.M., Abdullahi A., Samadi O., Parousis A., Jeschke M.G. Metformin prevents the pathological browning of subcutaneous white adipose tissue. Mol. Metab. 2019;29:12–23. doi: 10.1016/j.molmet.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gunawardana S.C., Piston D.W. Insulin-Independent Reversal of Type-1 Diabetes Following Transplantation of Adult Brown Adipose Tissue Supplemented With IGF-1. Transplant. Direct. 2019;5:e500. doi: 10.1097/TXD.0000000000000945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Karise I., Bargut T.C., del Sol M., Aguila M.B., Mandarim-de-Lacerda C.A. Metformin enhances mitochondrial biogenesis and thermogenesis in brown adipocytes of mice. Biomed. Pharmacother. 2019;111:1156–1165. doi: 10.1016/j.biopha.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 137.Chondronikola M., Volpi E., Børsheim E., Porter C., Annamalai P., Enerbäck S., Lidell M.E., Saraf M.K., Labbe S.M., Hurren N.M., et al. Brown adipose tissue improves whole-body glucose homeostasis and insulin sensitivity in humans. Diabetes. 2014;63:4089–4099. doi: 10.2337/db14-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chawla A., Chawla R., Jaggi S. Microvasular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016;20:546–551. doi: 10.4103/2230-8210.183480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sandoo A., van Zanten J.J.C.S.V., Metsios G.S., Carroll D., Kitas G.D. The endothelium and its role in regulating vascular tone. Open Cardiovasc. Med. J. 2010;4:302–312. doi: 10.2174/1874192401004010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nafisa A., Gray S.G., Cao Y., Wang T., Xu S., Wattoo F.H., Barras M., Cohen N., Kamato D., Little P.J. Endothelial function and dysfunction: Impact of metformin. Pharmacol. Ther. 2018;192:150–162. doi: 10.1016/j.pharmthera.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 141.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ. Res. 2010;107:1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bakker W., Eringa E.C., Sipkema P., van Hinsbergh V.W.M. Endothelial dysfunction and diabetes: Roles of hyperglycemia, impaired insulin signaling and obesity. Cell Tissue Res. 2009;335:165–189. doi: 10.1007/s00441-008-0685-6. [DOI] [PubMed] [Google Scholar]
- 143.Wu S., Li X., Zhang H. Effects of metformin on endothelial function in type 2 diabetes. Exp. Ther. Med. 2014;7:1349–1353. doi: 10.3892/etm.2014.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Kinaan M., Ding H., Triggle C.R. Metformin: An Old Drug for the Treatment of Diabetes but a New Drug for the Protection of the Endothelium. Med. Princ. Pract. 2015;24:401–415. doi: 10.1159/000381643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hamidi Shishavan M., Henning R.H., van Buiten A., Goris M., Deelman L.E., Buikema H. Metformin Improves Endothelial Function and Reduces Blood Pressure in Diabetic Spontaneously Hypertensive Rats Independent from Glycemia Control: Comparison to Vildagliptin. Sci. Rep. 2017;7:10975. doi: 10.1038/s41598-017-11430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kypreos K.E., Zafirovic S., Petropoulou P.-I., Bjelogrlic P., Resanovic I., Traish A., Isenovic E.R. Regulation of endothelial nitric oxide synthase and high-density lipoprotein quality by estradiol in cardiovascular pathology. J. Cardiovasc. Pharmacol. Ther. 2014;19:256–268. doi: 10.1177/1074248413513499. [DOI] [PubMed] [Google Scholar]
- 147.Sena C.M., Matafome P., Louro T., Nunes E., Fernandes R., Seiça R.M. Metformin restores endothelial function in aorta of diabetic rats. Br. J. Pharmacol. 2011;163:424–437. doi: 10.1111/j.1476-5381.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salvatore T., Pafundi P.C., Galiero R., Rinaldi L., Caturano A., Vetrano E., Aprea C., Albanese G., Di Martino A., Ricozzi C., et al. Can Metformin Exert as an Active Drug on Endothelial Dysfunction in Diabetic Subjects? Biomedicines. 2020;9:3. doi: 10.3390/biomedicines9010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.King P., Peacock I., Donnelly R. The UK prospective diabetes study (UKPDS): Clinical and therapeutic implications for type 2 diabetes. Br. J. Clin. Pharmacol. 1999;48:643–648. doi: 10.1046/j.1365-2125.1999.00092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Efentakis P., Kremastiotis G., Varela A., Nikolaou P.-E., Papanagnou E.-D., Davos C.H., Tsoumani M., Agrogiannis G., Konstantinidou A., Kastritis E., et al. Molecular mechanisms of carfilzomib-induced cardiotoxicity in mice and the emerging cardioprotective role of metformin. Blood. 2019;133:710–723. doi: 10.1182/blood-2018-06-858415. [DOI] [PubMed] [Google Scholar]
- 151.Driver C., Bamitale K.D.S., Kazi A., Olla M., Nyane N.A., Owira P.M.O. Cardioprotective Effects of Metformin. J. Cardiovasc. Pharmacol. 2018;72:121–127. doi: 10.1097/FJC.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 152.El Messaoudi S., Rongen G.A., Riksen N.P. Metformin therapy in diabetes: The role of cardioprotection. Curr. Atheroscler. Rep. 2013;15:314. doi: 10.1007/s11883-013-0314-z. [DOI] [PubMed] [Google Scholar]
- 153.Chen L., Pawlikowski B., Schlessinger A., More S.S., Stryke D., Johns S.J., Portman M.A., Chen E., Ferrin T.E., Sali A., et al. Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharmacogenet. Genomics. 2010;20:687–699. doi: 10.1097/FPC.0b013e32833fe789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Dludla P.V., Nyambuya T.M., Johnson R., Silvestri S., Orlando P., Mazibuko-Mbeje S.E., Gabuza K.B., Mxinwa V., Mokgalaboni K., Tiano L., et al. Metformin and heart failure–related outcomes in patients with or without diabetes: A systematic review of randomized controlled trials. Heart Fail. Rev. 2020 doi: 10.1007/s10741-020-09942-y. [DOI] [PubMed] [Google Scholar]
- 155.Cittadini A., Napoli R., Monti M.G., Rea D., Longobardi S., Netti P.A., Walser M., Samà M., Aimaretti G., Isgaard J., et al. Metformin prevents the development of chronic heart failure in the SHHF rat model. Diabetes. 2012;61:944–953. doi: 10.2337/db11-1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lu L., Ye S., Scalzo R.L., Reusch J.E.B., Greyson C.R., Schwartz G.G. Metformin prevents ischaemic ventricular fibrillation in metabolically normal pigs. Diabetologia. 2017;60:1550–1558. doi: 10.1007/s00125-017-4287-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wu S., Zou M.-H. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int. J. Mol. Sci. 2020;21:4987. doi: 10.3390/ijms21144987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Du Y., Zhang J., Fang F., Wei X., Zhang H., Tan H., Zhang J. Metformin ameliorates hypoxia/reoxygenation-induced cardiomyocyte apoptosis based on the SIRT3 signaling pathway. Gene. 2017;626:182–188. doi: 10.1016/j.gene.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 159.Sun D., Yang F. Metformin improves cardiac function in mice with heart failure after myocardial infarction by regulating mitochondrial energy metabolism. Biochem. Biophys. Res. Commun. 2017;486:329–335. doi: 10.1016/j.bbrc.2017.03.036. [DOI] [PubMed] [Google Scholar]
- 160.Higgins L., Palee S., Chattipakorn S.C., Chattipakorn N. Effects of metformin on the heart with ischaemia-reperfusion injury: Evidence of its benefits from in vitro, in vivo and clinical reports. Eur. J. Pharmacol. 2019;858:172489. doi: 10.1016/j.ejphar.2019.172489. [DOI] [PubMed] [Google Scholar]
- 161.Mohsin A.A., Chen Q., Quan N., Rousselle T., Maceyka M.W., Samidurai A., Thompson J., Hu Y., Li J., Lesnefsky E.J. Mitochondrial Complex I Inhibition by Metformin Limits Reperfusion Injury. J. Pharmacol. Exp. Ther. 2019;369:282–290. doi: 10.1124/jpet.118.254300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gormsen L.C., Sundelin E.I., Jensen J.B., Vendelbo M.H., Jakobsen S., Munk O.L., Hougaard Christensen M.M., Brøsen K., Frøkiær J., Jessen N. In Vivo Imaging of Human 11C-Metformin in Peripheral Organs: Dosimetry, Biodistribution, and Kinetic Analyses. J. Nucl. Med. 2016;57:1920–1926. doi: 10.2967/jnumed.116.177774. [DOI] [PubMed] [Google Scholar]
- 163.Kristensen J.M., Lillelund C., Kjøbsted R., Birk J.B., Andersen N.R., Nybo L., Mellberg K., Balendran A., Richter E.A., Wojtaszewski J.F.P. Metformin does not compromise energy status in human skeletal muscle at rest or during acute exercise: A randomised, crossover trial. Physiol. Rep. 2019;7:e14307. doi: 10.14814/phy2.14307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Musi N., Hirshman M.F., Nygren J., Svanfeldt M., Bavenholm P., Rooyackers O., Zhou G., Williamson J.M., Ljunqvist O., Efendic S., et al. Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes. 2002;51:2074–2081. doi: 10.2337/diabetes.51.7.2074. [DOI] [PubMed] [Google Scholar]
- 165.Richter E.A., Hargreaves M. Exercise, GLUT4, and Skeletal Muscle Glucose Uptake. Physiol. Rev. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- 166.Wessels B., Ciapaite J., van den Broek N.M.A., Nicolay K., Prompers J.J. Metformin Impairs Mitochondrial Function in Skeletal Muscle of Both Lean and Diabetic Rats in a Dose-Dependent Manner. PLoS ONE. 2014;9:e100525. doi: 10.1371/journal.pone.0100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Konopka A.R., Laurin J.L., Schoenberg H.M., Reid J.J., Castor W.M., Wolff C.A., Musci R.V., Safairad O.D., Linden M.A., Biela L.M., et al. Metformin inhibits mitochondrial adaptations to aerobic exercise training in older adults. Aging Cell. 2019;18:e12880. doi: 10.1111/acel.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Pilmark N.S., Petersen-Bønding C., Holm N.F.R., Johansen M.Y., Pedersen B.K., Hansen K.B., Karstoft K. The Effect of Metformin on Self-Selected Exercise Intensity in Healthy, Lean Males: A Randomized, Crossover, Counterbalanced Trial. Front. Endocrinol. 2021;12:86. doi: 10.3389/fendo.2021.599164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Hernández-Álvarez D., Mena-Montes B., Toledo-Pérez R., Pedraza-Vázquez G., López-Cervantes S.P., Morales-Salazar A., Hernández-Cruz E., Lazzarini-Lechuga R., Vázquez-Cárdenas R.R., Vilchis-DeLaRosa S., et al. Long-Term Moderate Exercise Combined with Metformin Treatment Induces an Hormetic Response That Prevents Strength and Muscle Mass Loss in Old Female Wistar Rats. Oxid. Med. Cell. Longev. 2019;2019:3428543. doi: 10.1155/2019/3428543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Jaswal J., Ussher J. Clinical Lipidology Myocardial fatty acid utilization as a determinant of cardiac efficiency and function. Clin. Lipidol. 2017;4:379–389. doi: 10.2217/clp.09.18. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.