Abstract
Simple Summary
In a cohort study involving 393 patients with differentiated thyroid cancer, we incorporated the TERT mutational status into AJCC TNM staging 8th edition (TNM-8), proposing a new prognostic system, termed TNM-8T. We demonstrated that the new prognostic system is superior to the existing TNM-8 staging system and its contents are not very complicated. This study is the first to present a new model that combines the mutational profile with the AJCC TNM stage to improve the predictability of cancer-specific survival with long-term follow-up.
Abstract
The role of telomerase reverse transcriptase (TERT) promoter mutations as an independent poor prognostic factor in differentiated thyroid cancer (DTC) patients is well known, but there is no prognostic system that combines the TERT promoter mutation status with tumor-node-metastasis (TNM) stage to predict cancer-specific survival (CSS). A total of 393 patients with pathologically confirmed DTC after thyroidectomy were enrolled. After incorporating wild-type TERT and mutant TERT with stages I, II, and III/IV of the AJCC TNM system 8th edition (TNM-8), we generated six combinations and calculated 10-year and 15-year CSS and adjusted hazard ratios (HRs) for cancer-related death using Cox regression. Then, a new mortality prediction model termed TNM-8T was derived based on the CSS and HR of each combination in the four groups. Of the 393 patients, there were 27 (6.9%) thyroid cancer-related deaths during a median follow-up of 14 years. Patients with a more advanced stage had a lower survival rate (10-year CSS for TNM-8T stage 1, 2, 3, and 4: 98.7%, 93.5%, 77.3%, and 63.0%, respectively; p < 0.001). TNM-8T showed a better spread of CSS (p < 0.001) than TNM-8 (p = 0.002) in the adjusted survival curves. The C-index for mortality risk predictability was 0.880 (95% CI, 0.665–0.957) in TNM-8T and 0.827 (95% CI, 0.622–0.930) in TNM-8 (p < 0.001). TNM-8T, a new prognostic system that incorporates the TERT mutational status into TNM-8, showed superior predictability to TNM-8 in the long-term survival of DTC patients.
Keywords: differentiated thyroid cancer, TERT promoter, TNM staging, prognosis, mortality
1. Introduction
Telomerase reverse transcriptase (TERT) promoter mutations have been detected in several human cancers, including thyroid cancer [1], and have been reported to be an independent poor prognostic factor in the recurrence and cancer-specific survival (CSS) of patients with differentiated thyroid cancer (DTC) [2,3].
The American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) tumor-node-metastasis (TNM) staging system is most commonly used to predict the prognosis of thyroid cancer. To predict disease mortality, AJCC/UICC staging is recommended for all DTC patients according to the 2015 American Thyroid Association guidelines [4]. It was revised to the 8th edition (TNM-8) in 2016 [5,6], improving its predictability of CSS compared to the 7th edition in patients with DTC [7,8]. However, there are some problems. Approximately 30% of DTC patients are downstaged to stage I or II, so there are too few patients with stage III and IV disease [6,9], and there is no molecular profile of clinical prognostic significance that has recently emerged [10,11]. In addition, a large multicenter cohort study showed the poor predictability of TNM-8 in the CSS of follicular thyroid cancer (FTC) patients [12]. Thus, there has been a need for a more complementary staging system in predicting the survival of individual DTC patients.
Kim et al. [13] proposed a new prognostic model by incorporating TERT promoter mutations into a dynamic risk stratification (DRS) system and demonstrated that it is as effective as DRS at predicting structural recurrence and CSS in nonmetastatic DTC patients. In addition, our research team recently reported that the prognostic predictability increased by the inclusion of TERT mutations based on the TNM-8 system regardless of the histologic type or initial stage of DTC patients [14].
Therefore, in this study, we aimed to develop a new prediction model for CSS and to compare it with the prediction power of the existing TNM-8 staging system with the hypothesis that if the TERT promoter mutational status is incorporated into TNM-8, a better prediction system will be created.
2. Materials and Methods
2.1. Patients and Clinicopathological Data
The same dataset was used in the previous study [14]. In brief, from October 1994 to December 2004, a total of 393 patients with pathologically confirmed DTC, 327 with papillary thyroid cancer (PTC) and 66 with FTC, including Hurthle cell carcinoma, were enrolled in this study after thyroidectomy and neck dissection. All patients received thyrotropin suppression therapy, and 364 (310 with PTC and 54 with FTC) received radioiodine ablation after surgery according to standard guidelines [15,16].
CSS was defined as the time of the initial surgical treatment to the last observation date (31 December 2018) or the date of thyroid cancer-specific death. Patients who died from other causes were censored at the time of death.
One sample per patient was collected from thyroid cancer tissue through the Department of Pathology at Samsung Medical Center, and mutation analysis was performed. Since it was performed after surgery and radioiodine treatment, the result did not affect the decision-making process of the physicians. Thyroid cancer-related mortality data were derived from the Korea National Statistical Office and hospital medical records.
2.2. Detection of TERT Promoter Mutations in Thyroid Cancer Samples
Genomic DNA was extracted from formalin-fixed paraffin embedded (FFPE) tissue using the Qiagen DNA FFPE Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. For FFPE tissue, 4 μm thick unstained slides were prepared, and pathologists decided to use the slides for DNA extraction based on a minimum tumor percentage of 75%. Then, we used seminested polymerase chain reaction (PCR) to identify TERT promoter mutations, and mutations were enriched with 3′-modified oligonucleotide PCR.
2.3. Statistical Analysis
We stratified DTC patients by a combination of the TERT promoter mutation status [wild-type (WT) or mutant] and TNM-8 stage. At this time, as the number of patients in stages III and IV was very small, they were grouped into one group, resulting in a total of six combinations. To propose alternative prognostic groupings, Cox regression was used to calculate the unadjusted and adjusted hazard ratios (HRs) by univariate and multivariate analyses (with Firth’s penalized Cox regression), and 10-year and 15-year survival rates were obtained. Among the variables with a p value ≤ 0.2 in the univariate analysis, sex and histology were selected as covariates for the multivariate analysis, except for the age, extrathyroidal extension, distant metastasis, and tumor size constituting TNM-8 (Table S1). After all pairwise analyses for each of the 6 combinations of TNM-8 stage and TERT promoter mutation status, four alternative groups were derived, which were termed TNM-8T: TNM-8 + TERT. These four TNM-8T groups were also subjected to pairwise multivariate analysis. Proportional hazards assumption checking was performed with Schoenfeld’s residual test.
Thyroid CSS was analyzed by Kaplan–Meier survival curves and compared with the log-rank test and adjusted survival curves (adjusted for age and histology via a Cox proportional hazards model).
TNM-8T was evaluated through internal validation based on 1000 datasets generated by the stratified bootstrapping technique. The bootstrap sample was used as the training set, and the out-of-bag data for each sampling were used as the testing set. Harrell’s C-index and the integrated area under the curve (iAUC) during the 10-year follow-up were calculated to evaluate the discrimination ability. [17,18] Additionally, the proportion of variance explained (PVE) values and Brier scores at the 10-year follow-up were calculated to evaluate the overall performance of the two staging systems. [19,20] Differences in the C-index, iAUC, PVE, and Brier score between the two staging systems, TNM-8T and TNM-8, were analyzed by paired t-tests.
Statistical analyses were performed using R 3.6.1 (Vienna, Austria; http://www.R-project.org; accessed on 9 November 2020). A p-value ≤ 0.05 was considered statistically significant.
3. Results
3.1. Patient Characteristics
The clinical and genetic characteristics of DTC patients are summarized in Table 1. Of all patients, 329 (83.7%) were female, and 64 (16.3%) were male. The median age at diagnosis was 42.8 years (range 15.8–81.4 years), and 319 (81.2%) were under 55 years of age. Most of the tumors were unifocal (72.8%) and PTC (83.2%), and most patients were classified as TNM-8 stage I (83.7%) at the time of diagnosis and received postoperative radioactive iodine therapy (92.6). TERT promoter mutations were identified in 10.9% (43/393, 4 of C250T and 39 of C228T) of patients. As the stage increased, the higher the prevalence of TERT mutations was identified (4.9% in stage I, 35.4% in stage II, and 62.5% in stage III/IV). During the median follow-up of 16 years (interquartile range 14–19 years), there were 27 thyroid cancer-related deaths (6.9%).
Table 1.
Baseline characteristics of study patients.
| Characteristic | Number (%), (Total = 393) |
|---|---|
| Age, years | |
| Median (range) | 42.8 (15.8–81.4) |
| Sex | |
| Male | 329 (83.7) |
| Female | 64 (16.3) |
| Tumor size, cm | |
| Median (range) | 2.7 (0.4–12.0) |
| Histological type | |
| PTC | 327 (83.2) |
| FTC | 66 (16.8) |
| Multifocality | |
| Absent | 286 (72.8) |
| Present | 107 (27.2) |
| Lymph node metastasis | |
| Absent | 199 (50.6) |
| Present | 193 (49.1) |
| Missing | 1 (0.3) |
| Extrathyroidal invasion | |
| Absent | 352 (89.6) |
| Present | 41 (10.4) |
| Distant metastasis | |
| Absent | 370 (94.1) |
| Present | 23 (5.9) |
| Stage at diagnosis | |
| I | 329 (83.7) |
| II | 48 (12.2) |
| III/IV | 16 (4.1) |
| TERT promoter mutations | |
| WT | 350 (89.1) |
| Mutant | 43 (10.9) |
| RAI treatment | |
| Absent | 29 (7.4) |
| Present | 364 (92.6) |
| Incidence related | |
| Incident cases | 27 |
| Persons-year | 6366 |
| Incident rate (1000 persons-year) | 4 |
| Death | |
| Survival | 366 (93.1) |
| Death | 27 (6.9) |
PTC, papillary thyroid cancer; FTC, follicular thyroid cancer; TERT, telomerase reverse transcriptase; WT, wild-type; RAI, radioactive iodine.
3.2. Alternative Grouping According to the AJCC Stage and TERT Promoter Mutation Status
We analyzed the HRs of six combinations of TNM-8 stage and TERT promoter mutation status after adjusting for sex and histologic type for thyroid cancer-related death with 10-year and 15-year CSS (Table 2 and Table S2). When patients with the same TERT mutational status were analyzed, the 10-year and 15-year CSS rates decreased as the stage increased, and the mutant TERT groups were associated with lower 10-year and 15-year CSS rates than the WT TERT groups regardless of stage (p < 0.001) (Table 2). Thyroid CSS was visualized via Kaplan–Meier curves and adjusted survival curves (Figure S1). Based on the above results, an alternative staging system of four groups termed TNM-8T was generated to predict thyroid CSS: TNM-8T stage 1 (patients with TNM-8 stage I and WT TERT), TNM-8T stage 2 (patients with TNM-8 stage II and WT TERT), TNM-8T stage 3 (patients with TNM-8 stage III/IV and WT TERT or with TNM-8 stage I and mutant TERT), and TNM-8T stage 4 (patients with TNM-8 stage II or III/IV and mutant TERT). The 10-year CSS rates were 98.7%, 93.5%, 77.3%, and 63.0%, respectively (p < 0.001) (Table 3 and Table 4). Overall, there was no severe violation of the proportional hazards assumptions of either staging system.
Table 2.
Hazard ratios of 6 combinations of TNM-8 stage and TERT promoter mutation status for cancer-related death.
| Combination | Cancer-Related Death | Unadjusted | Adjusted * | ||||
|---|---|---|---|---|---|---|---|
| Events/Total (%) | 10-y CSS | 15-y CSS | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| WT/stage I | 4/313 (1.3) | 98.72 | 98.72 | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 |
| WT/stage II | 3/31 (9.7) | 93.55 | 90.32 | 8.05 (1.80–35.98) | 0.0063 | 10.66 (2.37–47.95) | 0.002 |
| WT/stage III/IV | 1/6 (16.7) | 83.33 | 83.33 | 15.44 (1.72–138.16) | 0.0144 | 17.82 (2.44–130.18) | 0.0045 |
| MUT/stage I | 4/16 (25.0) | 75.00 | 75.00 | 23.66 (5.92–94.63) | <0.001 | 20.63 (5.17–82.40) | <0.001 |
| MUT/stage II | 8/17 (47.1) | 70.59 | 52.29 | 45.46 (13.68–151.13) | <0.001 | 55.37 (16.06–190.87) | <0.001 |
| MUT/stage III/IV | 7/10 (70.0) | 50.00 | 30.00 | 78.45 (22.82–269.76) | <0.001 | 78.37 (22.44–273.67) | <0.001 |
TNM-8, AJCC tumor-node-metastasis staging system 8th edition; TERT, telomerase reverse transcriptase; y, year; CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval; WT, wild-type; MUT, mutant. * Cox model adjusted for sex and histological type.
Table 3.
Definition of the TNM-8T groups.
| Alternative Grouping | Definition |
|---|---|
| TNM-8T stage 1 | Patients with TNM-8 stage I and wild-type TERT |
| TNM-8T stage 2 | Patients with TNM-8 stage II and wild-type TERT |
| TNM-8T stage 3 | Patients with TNM-8 stage III/IV and wild-type TERT or with TNM-8 stage I and mutant TERT |
| TNM-8T stage 4 | Patients with TNM-8 stage II and mutant TERT or with TNM-8 stage III/IV and mutant TERT |
Table 4.
Hazard ratios of TNM-8T and TNM-8 for cancer-related death.
| Alternative Grouping | TNM-8T | ||||||
|---|---|---|---|---|---|---|---|
| Cancer-Related Death | Unadjusted | Adjusted * | |||||
| Events/Total (%) | 10-y CSS | 15-y CSS | HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| TNM-8T stage 1 | 4/313 (1.3) | 98.7 | 98.7 | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 |
| TNM-8T stage 2 | 3/31 (9.7) | 93.5 | 90.3 | 8.05 (1.80–35.97) | 0.006 | 10.62 (2.41–46.71) | 0.002 |
| TNM-8T stage 3 | 5/22 (22.7) | 77.3 | 77.3 | 21.37 (5.74–79.59) | <0.001 | 18.57 (5.02–68.70) | <0.001 |
| TNM-8T stage 4 | 15/27 (55.6) | 63.0 | 44.1 | 56.40 (18.67–170.36) | <0.001 | 62.87 (20.66–191.29) | <0.001 |
| AJCC Stage | TNM-8 | ||||||
| Cancer-Related Death | Unadjusted | Adjusted * | |||||
| Events/Total (%) | 10-y CSS | 15-y CSS | HR (95% CI) | p -Value | HR (95% CI) | p -Value | |
| TNM-8 stage I | 8/329 (2.4) | 97.6 | 97.6 | 1.00 (reference) | <0.001 | 1.00 (reference) | <0.001 |
| TNM-8 stage II | 11/48 (22.9) | 85.4 | 76.3 | 10.43 (4.19–25.93) | <0.001 | 13.20 (5.08–34.30) | <0.001 |
| TNM-8 stage III | 4/9 (44.4) | 77.8 | 55.6 | 20.68 (6.21–68.84) | <0.001 | 26.99 (8.06–90.42) | <0.001 |
| TNM-8 stage IV | 4/7 (57.1) | 42.9 | 42.9 | 38.24 (11.44–127.75) | <0.001 | 26.39 (7.84–88.79) | <0.001 |
TNM-8, AJCC tumor-node-metastasis staging system 8th edition; y, year; CSS, cancer-specific survival; HR, hazard ratio; CI, confidence interval. * Cox model adjusted for sex and histological type.
3.3. Comparison of Survival in Patients Staged According to TNM-8 and TNM-8T
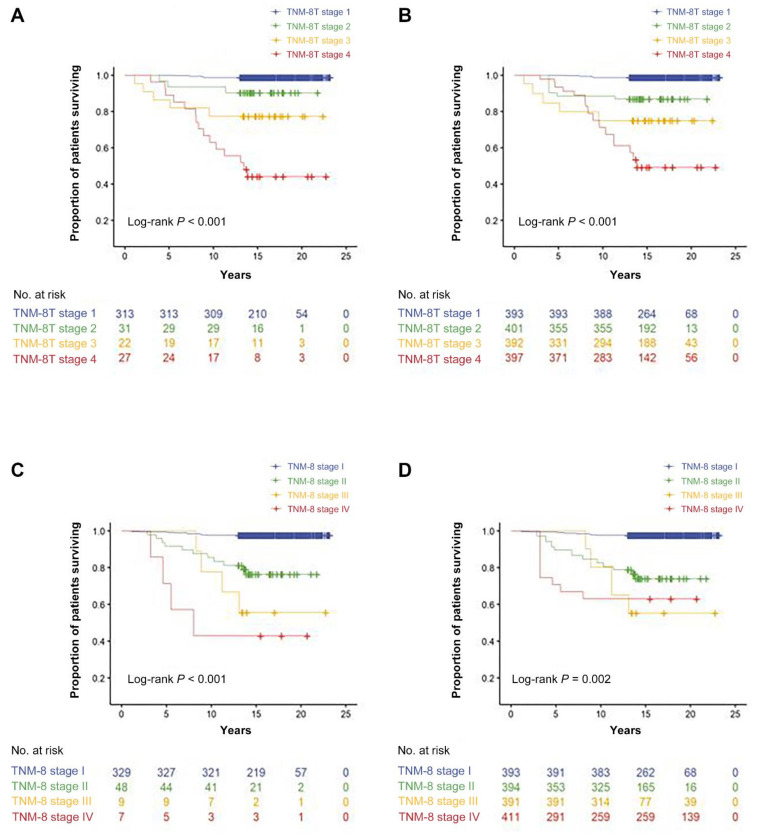
For comparison, the HRs of each stage of TNM-8T and TNM-8 for cancer-related death after adjusting for sex and histologic type were calculated (Table 4 and Table S3). In TNM-8T, the adjusted HRs increased as the stage increased (adjusted HR 10.62, 18.57, and 62.87 for stages 2, 3, and 4, respectively), but in TNM-8, there was no difference in the adjusted HRs of stages III and IV (adjusted HRs 13.20, 26.99, and 26.39 for stages II, III, and IV, respectively) (Table 4). TNM-8T showed a better spread of CSS (p < 0.001) than TNM-8 (p = 0.002) in the adjusted survival curves (Figure 1). The C-index indicating risk predictability was 0.880 (95% CI, 0.665–0.957) in TNM-8T, which was significantly higher than that in TNM-8 (0.827; 95% CI, 0.622–0.930) (p < 0.001). The PVE, which indicates the ability of the two staging systems to explain CSS, was 0.205 (95% CI, 0.151–0.264) in TNM-8T, which was also significantly higher than that in TNM-8 (0.153; 95% CI, 0.095–0.221) (p < 0.001).
Figure 1.
Thyroid cancer-specific survival according to TNM-8T with Kaplan–Meier survival curves (A) and adjusted survival curves (B). Thyroid cancer-specific survival according to TNM-8 with Kaplan–Meier survival curves (C) and adjusted survival curves (D).
3.4. Comparison of TNM Staging Groups According to TNM-8 vs. TNM-8T
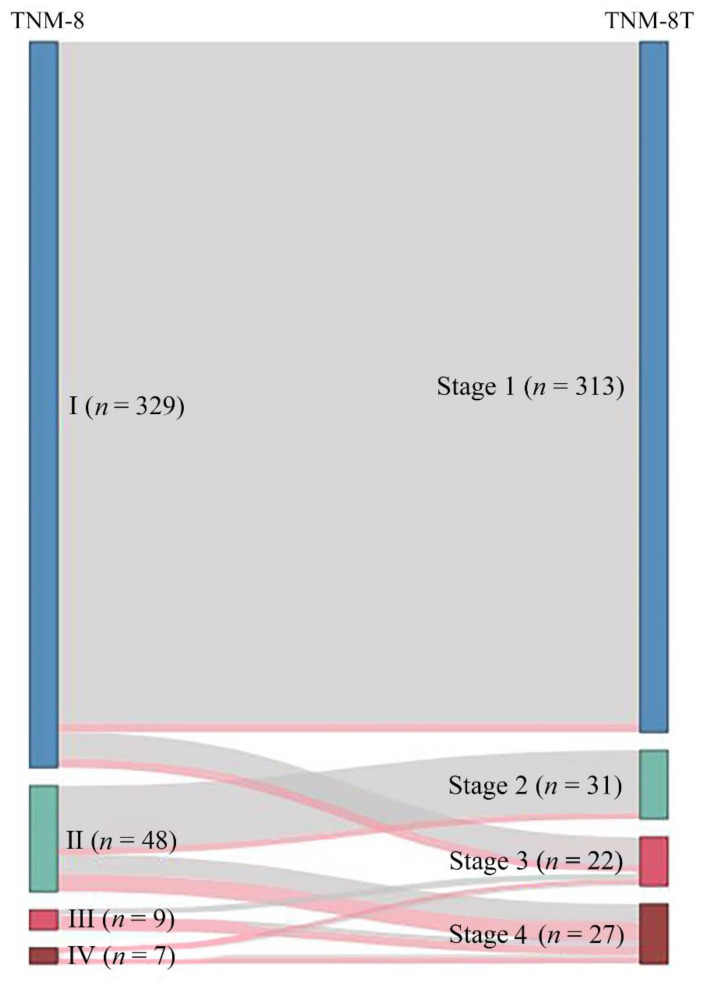
Of the 393 DTC patients, the stages of 42 (10.7%) were changed in TNM-8T. Of the 329 patients with stage I disease, 16 were upstaged to stage 3, and of the 48 patients with stage II disease, 17 were upstaged to stage 4. Six patients with stage III disease were upstaged to stage 4, and 3 patients with stage IV disease were downstaged to stage 3. Figure 2 demonstrates the stage migration in our data from TNM-8 to TNM-8T based on the alluvial flow diagram.
Figure 2.
Alluvial flow diagram representing the restaging of patient cohorts from the TNM-8 staging system (Roman numerals) to the TNM-8T staging system (Arabic numerals). Red line indicates deceased patients.
4. Discussion
DTC is generally an indolent tumor with low mortality, but in some patients, it often progresses aggressively, and distinguishing high-risk patients early in diagnosis is very important for providing the best clinical outcomes for individual patients.
The importance of genetic markers in predicting the prognosis of thyroid cancer has already been reported [10,21]. Among them, the prognostically most promising and notable are carcinogenic mutations and BRAF V600E and TERT promoter mutations. The poor outcomes caused by BRAF V600E and TERT promoter mutations or their synergistic effects have been well characterized and widely appreciated [22,23,24,25,26].
The TERT promoter has two mutational hotspots on chromosome 5, C228T and C250T. TERT C228T is far more prevalent than C250T in thyroid cancer. Both mutations play carcinogenic roles by generating a new site where E-twenty-six transcription factors can bind and increase the transcriptional activities of the TERT promoter [27]. Those with less differentiated histologic types have a high prevalence (on average, 11.3% of PTCs, 17.1% of FTCs, 43.2% of poorly differentiated DTCs, and 40.1% of anaplastic thyroid cancers) [22,24]. They are also associated with aggressive tumor behaviors, radioiodine refractoriness, and increased tumor recurrence and DTC-specific death [2,28,29,30,31]. In particular, in PTC patients, coexisting BRAF V600E and TERT promoter mutations are associated with increased cancer aggressiveness, lymph node and distant metastases, and an advanced stage and increase tumor recurrence and mortality more rapidly than either alone [32,33,34]. This study also showed consistent results with those of previous studies, in which all patients with mutant TERT, regardless of stage, had lower 10-year and 15-year CSS rates and higher HRs than those with WT TERT when the six combinations of TNM-8 stages and TERT mutational statuses were assessed (Table 2).
PTC accounts for 90% of all DTCs [35,36], and BRAF mutations are found in approximately 45% of sporadic PTC tumors [37]. In particular, more than 80% of patients newly diagnosed with PTC in Korea have BRAF mutations [38]. Therefore, it is believed that TERT promoter mutations play an important role in death from DTC. In a previous study, we demonstrated that TERT promoter mutations act as an independent poor prognostic factor with the TERT promoter mutational status based on TNM-8 in a cohort of patients with DTC [14].
Therefore, in this study, we proposed a new prognostic staging system, TNM-8T, by incorporating the TERT promoter mutational status into the AJCC staging system, the most commonly recommended staging system for predicting the prognosis of thyroid cancer. The adjusted HRs significantly increased in all pairwise multivariate Cox regression analyses (p < 0.001) (Table S3). In addition, through various internal validations, it was demonstrated that the TNM-8T staging system has a C-index of 0.880 (95% CI, 0.665–0.957), an iAUC of 0.981 (95% CI, 0.859–0.967), a PVE of 0.205 (95% CI, 0.151–0.264), and a Brier score of 0.018 (95% CI, 0.008–0.029), with significantly superior risk predictability for thyroid CSS than the TNM-8 staging system, with a C-index of 0.827 (95% CI, 0.622–0.930), an iAUC of 0.879 (95% CI, 0.783–0.962), a PVE of 0.153 (95% CI, 0.095–0.221), and a Brier score of 0.019 (95% CI, 0.009–0.030) (p < 0.001).
There are several limitations to this study. First, since this was a retrospective study, it was susceptible to selection bias. Second, Korean patients with PTC have a higher rate of BRAF mutations than those from other countries [37,38], and given reports of the synergistic association between BRAF V600E and TERT promoter mutations [32,33,34], the impact of the BRAF mutation alone or its synergistic effect with the TERT promoter mutations on CSS may have been reflected in the present study; therefore, it is difficult to apply these results to DTC patients worldwide. Third, since the cohort size and number of cancer-specific deaths per subgroup were small, the statistical power was low; thus, it is necessary to develop this study into a multicenter large-volume study. Nevertheless, it is meaningful that this study is the first to present a new model that combines the mutational profile with the AJCC TNM stage to improve the predictability of CSS with long-term follow-up.
5. Conclusions
This is the first work to incorporate the TERT mutational status into TNM-8. We propose a new prognostic system, termed TNM-8T, which is superior to the existing system and is easy to apply to real clinics because the contents are not very complicated. This staging system will aid in the precision of management decision making for DTC patients, including decisions on the extent of surgery, prophylactic neck dissection, postoperative radioiodine therapy, and molecular targeted therapy.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cancers13122943/s1, Figure S1: Thyroid cancer-specific survival according to the 6 combinations of TNM-8 stage and TERT promoter mutation status with Kaplan–Meier survival curves (A) and with adjusted survival curves (B), Table S1: Regression analysis of TERT mutation status and clinicopathological variables in patients with DTC, Table S2: Hazard ratios of 6 combinations of TNM-8 stage and TERT promoter mutation status for cancer-related death from all pairwise multivariate cox regression, Table S3: Hazard ratios of TNM-8T for cancer-related death from all pairwise multivariate cox regression.
Author Contributions
Conceptualization, J.P. (Jun Park), S.L., K.K. and T.-H.K.; methodology, K.K.; formal analysis, S.L. and C.-S.K.; investigation, J.P. (Jun Park), S.L., K.K. and T.-H.K.; data curation, J.P. (Jiyun Park) and H.P.; writing—original draft preparation, J.P. (Jun Park); writing—review and editing, S.L., K.K., T.-H.K.; visualization, S.L.; supervision, Y.-L.O., J.-H.S., J.-S.K., S.-W.K. and J.-H.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of Samsung Medical Center (SMC-IRB 2016-05-053).
Informed Consent Statement
Patient consent was waived due to the retrospective study design.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vinagre J., Almeida A., Pópulo H., Batista R., Lyra J., Pinto V., Coelho R., Celestino R., Prazeres H., Lima L., et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013;4:2185. doi: 10.1038/ncomms3185. [DOI] [PubMed] [Google Scholar]
- 2.Kim T.H., Kim Y.E., Ahn S., Kim J.Y., Ki C.S., Oh Y.L., Kim K., Yun J.W., Park W.Y., Choe J.H., et al. TERT promoter mutations and long-term survival in patients with thyroid cancer. Endocr. Relat. Cancer. 2016;23:813–823. doi: 10.1530/ERC-16-0219. [DOI] [PubMed] [Google Scholar]
- 3.Lee S.E., Hwang T.S., Choi Y.L., Han H.S., Kim W.S., Jang M.H., Kim S.K., Yang J.H. Prognostic Significance of TERT Promoter Mutations in Papillary Thyroid Carcinomas in a BRAF(V600E) Mutation-Prevalent Population. Thyroid Off. J. Am. Thyroid Assoc. 2016;26:901–910. doi: 10.1089/thy.2015.0488. [DOI] [PubMed] [Google Scholar]
- 4.Haugen B.R., Alexander E.K., Bible K.C., Doherty G.M., Mandel S.J., Nikiforov Y.E., Pacini F., Randolph G.W., Sawka A.M., Schlumberger M., et al. 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016;26:1–133. doi: 10.1089/thy.2015.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nixon I.J., Wang L.Y., Migliacci J.C., Eskander A., Campbell M.J., Aniss A., Morris L., Vaisman F., Corbo R., Momesso D., et al. An international multi-institutional validation of age 55 years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2016;26:373–380. doi: 10.1089/thy.2015.0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuttle R.M., Haugen B., Perrier N.D. Updated American Joint Committee on Cancer/Tumor-Node-Metastasis Staging System for Differentiated and Anaplastic Thyroid Cancer (Eighth Edition): What Changed and Why? Thyroid Off. J. Am. Thyroid Assoc. 2017;27:751–756. doi: 10.1089/thy.2017.0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim M., Kim W.G., Oh H.S., Park S., Kwon H., Song D.E., Kim T.Y., Shong Y.K., Kim W.B., Sung T.Y., et al. Comparison of the Seventh and Eighth Editions of the American Joint Committee on Cancer/Union for International Cancer Control Tumor-Node-Metastasis Staging System for Differentiated Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2017;27:1149–1155. doi: 10.1089/thy.2017.0050. [DOI] [PubMed] [Google Scholar]
- 8.Shteinshnaider M., Kalmovich L., Koren S., Or K., Cantrell D., Benbassat C. Reassessment of differentiated thyroid cancer patients using the eighth TNM/AJCC classification system: A comparative study. Thyroid Off. J. Am. Thyroid Assoc. 2018;28:201–209. doi: 10.1089/thy.2017.0265. [DOI] [PubMed] [Google Scholar]
- 9.Shaha A.R., Migliacci J.C., Nixon I.J., Wang L.Y., Wong R.J., Morris L.G.T., Patel S.G., Shah J.P., Tuttle R.M., Ganly I. Stage migration with the new American Joint Committee on Cancer (AJCC) staging system (8th edition) for differentiated thyroid cancer. Surgery. 2019;165:6–11. doi: 10.1016/j.surg.2018.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xing M., Haugen B.R., Schlumberger M. Progress in molecular-based management of differentiated thyroid cancer. Lancet. 2013;381:1058–1069. doi: 10.1016/S0140-6736(13)60109-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McKelvey B.A., Umbricht C.B., Zeiger M.A. Telomerase reverse transcriptase (TERT) regulation in thyroid cancer: A review. Front. Endocrinol. 2020;11:485. doi: 10.3389/fendo.2020.00485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim M., Kim H.I., Jeon M.J., Kim H.K., Kim E.H., Yi H.S., Kim E.S., Kim H., Kim B.H., Kim T.Y., et al. Eighth edition of tumor-node-metastasis staging system improve survival predictability for papillary, but not follicular thyroid carcinoma: A multicenter cohort study. Oral Oncol. 2018;87:97–103. doi: 10.1016/j.oraloncology.2018.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Kim T.H., Ki C.S., Kim H.S., Kim K., Choe J.H., Kim J.H., Kim J.S., Oh Y.L., Hahn S.Y., Shin J.H., et al. Refining Dynamic Risk Stratification and Prognostic Groups for Differentiated Thyroid Cancer with TERT Promoter Mutations. J. Clin. Endocrinol. Metab. 2017;102:1757–1764. doi: 10.1210/jc.2016-3434. [DOI] [PubMed] [Google Scholar]
- 14.Park J., Lee S., Kim K., Park H., Ki C.-S., Oh Y.L., Shin J.H., Kim J.S., Kim S.W., Chung J.H., et al. TERT Promoter Mutations and the 8th Edition TNM Classification in Predicting the Survival of Thyroid Cancer Patients. Cancers. 2021;13:648. doi: 10.3390/cancers13040648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooper D.S., Doherty G.M., Haugen B.R., Kloos R.T., Lee S.L., Mandel S.J., Mazzaferri E.L., McIver B., Sherman S.I., Tuttle R.M. Management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2006;16:109–142. doi: 10.1089/thy.2006.16.109. [DOI] [PubMed] [Google Scholar]
- 16.Cooper D.S., Doherty G.M., Haugen B.R., Kloos R.T., Lee S.L., Mandel S.J., Mazzaferri E.L., McIver B., Pacini F., Schlumberger M., et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2009;19:1167–1214. doi: 10.1089/thy.2009.0110. [DOI] [PubMed] [Google Scholar]
- 17.Harrell F.E., Jr., Lee K.L., Mark D.B. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat. Med. 1996;15:361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Heagerty P.J., Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics. 2005;61:92–105. doi: 10.1111/j.0006-341X.2005.030814.x. [DOI] [PubMed] [Google Scholar]
- 19.Schemper M., Stare J. Explained variation in survival analysis. Stat. Med. 1996;15:1999–2012. doi: 10.1002/(SICI)1097-0258(19961015)15:19<1999::AID-SIM353>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 20.Brier G.W. Verification of forecasts expressed in terms of probability. Mon. Weather Rev. 1950;78:1–3. doi: 10.1175/1520-0493(1950)078<0001:VOFEIT>2.0.CO;2. [DOI] [Google Scholar]
- 21.Xing M. Genetic-guided risk assessment and management of thyroid cancer. Endocrinol. Metab. Clin. N. Am. 2019;48:109–124. doi: 10.1016/j.ecl.2018.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alzahrani A.S., Alsaadi R., Murugan A.K., Sadiq B.B. TERT Promoter Mutations in Thyroid Cancer. Horm. Cancer. 2016;7:165–177. doi: 10.1007/s12672-016-0256-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li F., Chen G., Sheng C., Gusdon A.M., Huang Y., Lv Z., Xu H., Xing M., Qu S. BRAFV600E mutation in papillary thyroid microcarcinoma: A meta-analysis. Endocr. Relat. Cancer. 2015;22:159–168. doi: 10.1530/ERC-14-0531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu R., Xing M. TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer. 2016;23:R143–R155. doi: 10.1530/ERC-15-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tufano R.P., Teixeira G.V., Bishop J., Carson K.A., Xing M. BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: A systematic review and meta-analysis. Medicine. 2012;91:274–286. doi: 10.1097/MD.0b013e31826a9c71. [DOI] [PubMed] [Google Scholar]
- 26.Xing M. BRAF mutation in papillary thyroid cancer: Pathogenic role, molecular bases, and clinical implications. Endocr. Rev. 2007;28:742–762. doi: 10.1210/er.2007-0007. [DOI] [PubMed] [Google Scholar]
- 27.Huang D.-S., Wang Z., He X.-J., Diplas B.H., Yang R., Killela P.J., Meng Q., Ye Z.-Y., Wang W., Jiang X.-T., et al. Recurrent TERT promoter mutations identified in a large-scale study of multiple tumour types are associated with increased TERT expression and telomerase activation. Eur. J. Cancer. 2015;51:969–976. doi: 10.1016/j.ejca.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu X., Qu S., Liu R., Sheng C., Shi X., Zhu G., Murugan A.K., Guan H., Yu H., Wang Y., et al. TERT promoter mutations and their association with BRAF V600E mutation and aggressive clinicopathological characteristics of thyroid cancer. J. Clin. Endocrinol. Metab. 2014;99:E1130–E1136. doi: 10.1210/jc.2013-4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Melo M., da Rocha A.G., Vinagre J., Batista R., Peixoto J., Tavares C., Celestino R., Almeida A., Salgado C., Eloy C., et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014;99:E754–E765. doi: 10.1210/jc.2013-3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang X., Li J., Li X., Liang Z., Gao W., Liang J., Cheng S., Lin Y. TERT promoter mutation predicts radioiodine-refractory character in distant metastatic differentiated thyroid cancer. J. Nucl. Med. 2017;58:258–265. doi: 10.2967/jnumed.116.180240. [DOI] [PubMed] [Google Scholar]
- 31.Jin A., Xu J., Wang Y. The role of TERT promoter mutations in postoperative and preoperative diagnosis and prognosis in thyroid cancer. Medicine. 2018;97:e11548. doi: 10.1097/MD.0000000000011548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin L., Chen E., Dong S., Cai Y., Zhang X., Zhou Y., Zeng R., Yang F., Pan C., Liu Y., et al. BRAF and TERT promoter mutations in the aggressiveness of papillary thyroid carcinoma: A study of 653 patients. Oncotarget. 2016;7:18346–18355. doi: 10.18632/oncotarget.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu R., Bishop J., Zhu G., Zhang T., Ladenson P.W., Xing M. Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer: Genetic Duet of BRAF and TERT Promoter Mutations in Thyroid Cancer Mortality. JAMA Oncol. 2017;3:202–208. doi: 10.1001/jamaoncol.2016.3288. [DOI] [PubMed] [Google Scholar]
- 34.Xing M., Liu R., Liu X., Murugan A.K., Zhu G., Zeiger M.A., Pai S., Bishop J. BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014;32:2718–2726. doi: 10.1200/JCO.2014.55.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hundahl S.A., Cady B., Cunningham M.P., Mazzaferri E., McKee R.F., Rosai J., Shah J.P., Fremgen A.M., Stewart A.K., Hölzer S. Initial results from a prospective cohort study of 5583 cases of thyroid carcinoma treated in the United States during 1996. U.S. and German thyroid cancer study group. An American college of Surgeons commission on cancer patient care evaluation study. Cancer. 2000;89:202–217. doi: 10.1002/1097-0142(20000701)89:1<202::AID-CNCR27>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 36.Oh C.M., Kong H.J., Kim E., Kim H., Jung K.W., Park S., Won Y.J. National epidemiologic survey of thyroid cancer (NEST) in Korea. Epidemiol. Health. 2018;40:e2018052. doi: 10.4178/epih.e2018052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xing M. BRAF mutation in thyroid cancer. Endocr. Relat. Cancer. 2005;12:245–262. doi: 10.1677/erc.1.0978. [DOI] [PubMed] [Google Scholar]
- 38.Kim S.K., Kim D.L., Han H.S., Kim W.S., Kim S.J., Moon W.J., Oh S.Y., Hwang T.S. Pyrosequencing analysis for detection of a BRAFV600E mutation in an FNAB specimen of thyroid nodules. Diagn. Mol. Pathol. Am. J. Surg. Pathol. Part B. 2008;17:118–125. doi: 10.1097/PDM.0b013e31815d059d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.