Abstract
Background: Cognitive impairment is one of the most common, burdensome, and costly disorders in the elderly worldwide. The magnitude of the association between anemia and overall cognitive impairment (OCI) has not been established. Objective: We aimed to update and expand previous evidence of the association between anemia and the risk of OCI. Methods: We conducted an updated systematic review and meta-analysis. We searched electronic databases, including EMBASE, PubMed, and Web of Science for published observational studies and clinical trials between 1 January 1990 and 1 June 2020. We excluded articles that were in the form of a review, letter to editors, short reports, and studies with less than 50 participants. The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines were followed. We estimated summary risk ratios (RRs) with random effects. Results: A total of 20 studies, involving 6558 OCI patients were included. Anemia was significantly associated with an increased risk of OCI (adjusted RR (aRR) 1.39 (95% CI, 1.25–1.55; p < 0.001)). In subgroup analysis, anemia was also associated with an increased risk of all-cause dementia (adjusted RR (aRR), 1.39 (95% CI, 1.23–1.56; p < 0.001)), Alzheimer’s disease [aRR, 1.59 (95% CI, 1.18–2.13; p = 0.002)], and mild cognitive impairment (aRR, 1.36 (95% CI, 1.04–1.78; p = 0.02)). Conclusion: This updated meta-analysis shows that patients with anemia appear to have a nearly 1.39-fold risk of developing OCI than those without anemia. The magnitude of this risk underscores the importance of improving anemia patients’ health outcomes, particularly in elderly patients.
Keywords: anemia, dementia, Alzheimer’s, cognitive impairment, iron deficiency, meta-analysis
1. Introduction
The prevalence of cognitive impairment including dementia is increasing worldwide. It is estimated that more than 75 million people will have dementia by 2030 and 131.5 million by 2050 [1,2]. The risk has been increased in people living in low- and middle-income countries, accounting for more than half (58%) of total patients [3]. From a public health perspective, an increased prevalence of dementia can increase the global health burden [4]. Previous studies reported that the global economic burden of dementia is approximately 1 trillion USD including health expenditures, reduced quality of life, morbidity that raised the concern of healthcare policymakers, physicians, and overall society [5].
Numerous epidemiological studies have highlighted an association between anemia and the increased risk of developing overall cognitive impairment (OCI) [6,7]. Although these studies raised public concern and received wide attention from public health policymakers, the interpretation of the association between anemia and OCI provides a lack of credibility [8]. A randomized control trial was not conducted to find their association in which observational studies and systematic reviews have reportedly supported strong associations [9,10]. The biological mechanisms linking anemia to OCI are not fully understood, although several possible hypotheses have been previously proposed. Chronic brain hypoxia related to anemia might contribute to a decline in cognitive function through an increasing accumulation of amyloid-β [11]. Previous evidence shows an increased association between anemia and the progression of white matter disease and cerebral cortical atrophy, which can accelerate cognitive function decline [12]. Moreover, iron deficiency in the brain hampers its neurotransmitter function by interfering with the rate-limiting enzyme [13].
Peters et al. [10] reported an increased risk of dementia in patients with anemia. However, they included limited studies and suggested that further studies needed to be conducted to draw firm conclusions. Furthermore, Kim et al. [9] investigated the association between anemia and cognitive impairment using sixteen observational studies. The authors emphasized that conducting more research was necessary to reach a robust conclusion because the number of prospective cohort studies were not sufficient. Since there are only four prospective cohort studies that have been published on this topic, we conducted an updated systematic review and meta-analysis that addressed the potential association between anemia and dementia risk.
2. Materials and Methods
2.1. Experimental Approach
The PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines, which are based on the Cochrane Handbook for Systematic Reviews of Interventions, were used to conduct this study (Supplementary Table S1) [14,15].
2.2. Search Strategy
We conducted an updated systematic review and meta-analysis. We searched electronic databases including EMBASE, PubMed, and Web of Science for published observational studies and randomized control trials between 1 January 1990 and 1 June 2020, with no language restrictions. We used the search terms “anemia” OR “iron deficiency”, and “dementia”, OR “Alzheimer”, OR “cognitive impairment”. We also searched the bibliography of retrieved articles to obtain relevant articles.
2.3. Inclusion Criteria
Studies that assessed the association between anemia and OCI risk and matched all of the following criteria were included: (a) the primary outcome was OCI, (b) were observational or randomized control trials, (c) included at least 50 participants aged over 18 years old, (d) identified OCI patients using the standard diagnostic criteria (Feighner criteria or Research Diagnostic Criteria/DSM-III/DSM-III-R/DSM-IV/DSM-5/ICD-10), and (e) the diagnosis of anemia patients was based on WHO criteria, i.e., men: Hb < 13 g/dL, women: Hb < 12 g/dL, measured in blood samples.
2.4. Exclusion Criteria
Studies were excluded if they were (a) published as a case report or case series with a low number of participants (i.e., <50); (b) published as editorial, letters, commentary, review articles, or conference abstracts; (c) provided insufficient information on OCI patient selection, confounding factors, adjusted variables, and effect size; or (d) used the same database with overlapping study durations.
2.5. Data Extraction
Required data were retrieved from selected articles by two independent authors (M.M.I. and S.A.). They developed a standard data collection form, checked all of the variables, and collected the needed information to calculate the risk ratio (RR). Afterwards, they retrieved and compiled the following information: (a) study characteristics: the author’s first name, publication year, country, study duration, study design; (b) demographic characteristics: the number of participants, number of male and female patients, and age; (c) the reference standard of dementia diagnosis, and number of dementia patients; and (d) the adjusted effect sizes such as hazard ratios (HRs) and odds ratios (ORs) with 95% confidence intervals (CIs). We considered the adjusted effect sizes because it helps to reduce potential bias.
2.6. Study Quality
Study quality was assessed using the Newcastle Ottawa Scale for observational studies. The summary score was used to categorize the study quality into the following two groups: high (NOS score higher than 7) and low (NOS score equal to or lower than 7).
2.7. Statistical Analysis
Comprehensive meta-analysis (CMA, V-2) software was used to analyze the data. An adjusted risk ratio (risk ratio was calculated from adjusted effect sizes (aRR)) with the corresponding 95% CI was calculated to show the magnitude of the association between anemia and OCI. However, the random effect model was used to calculate the heterogeneity between the studies. The Cochran Q statistic and inconsistency statistics (I2) were considered to examine whether there was significant heterogeneity in their effect sizes. Previous studies were followed to categorize the heterogeneity as low (I2 value < 25%), medium (I2 value is between 25% and 50%), and high (I2 value greater than 50%) [16,17]. Finally, the forest plot was drawn to present the effect size and the funnel plot to introduce publication risk bias.
3. Results
3.1. Study Selection
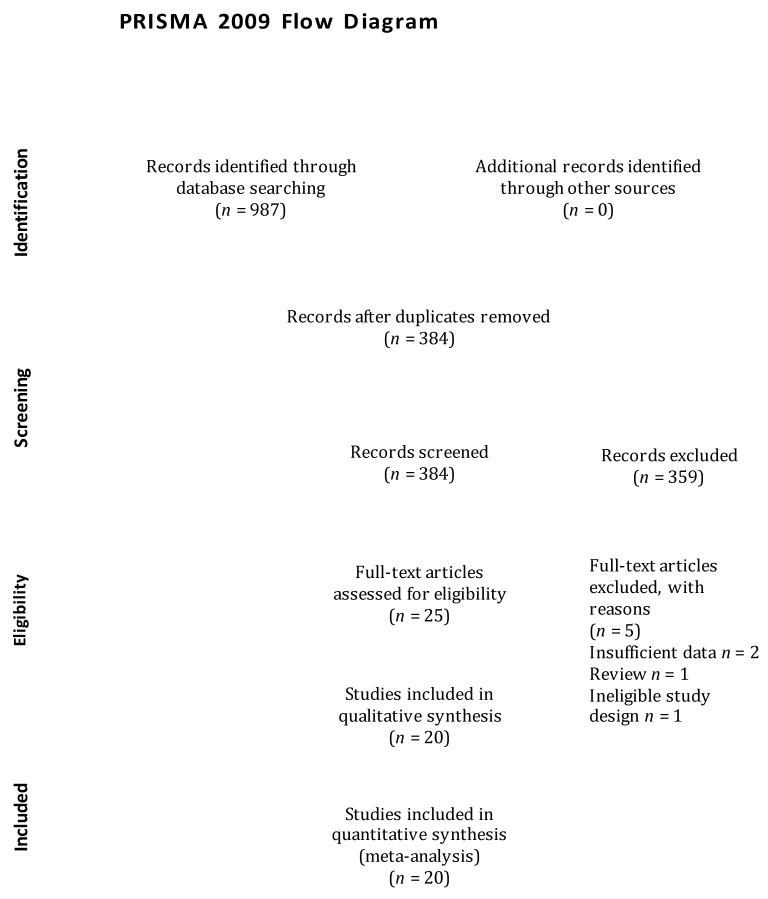
The articles search was conducted on electronic databases and garnered 987 articles. We reviewed the titles and abstracts of those articles and excluded 962 articles due to a lack of adherence to our pre-specified selection criteria. Afterwards, we screened the full text of the selected 25 articles and assessed the reference lists of relevant articles, retrieving no additional articles. We further excluded 6 articles that did not fulfil the inclusion criteria. Finally, 20 articles were considered in the meta-analysis [6,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36], and the flow diagram of the study selection process is illustrated in Figure 1.
Figure 1.
Flow diagram of study selection process.
3.2. Study Characteristics
This updated meta-analysis comprised of 12 cohort studies and 8 case-control studies that are presented in Table 1.
Table 1.
Study characteristics.
| Author Year | Country | Study Design | Female Patients (%) | Mean Age | Criteria for Anemia Patients’ Inclusion | Criteria for Dementia/Cognitive Impairment Patients’ Inclusion | Number of Dementia Patients | OR/HR | NOS |
|---|---|---|---|---|---|---|---|---|---|
| Beard, 1997 [18] | USA | C-C | 77.3 | 82.5 | WHO | DSM | 191 | 1.91 (1.19–3.09) |
5 |
| Argyriadou, 2001 [6] | Greece | C-C | 54.3 | 65~ | Mean hematocrit (Hct): Men < 38%, Women ≤ 36% |
MMSE < 24 | 213 | 1.70 (1.02–2.90) |
6 |
| Pandav, 2004 [19] | India | Co | 56.6 | 69.6 | WHO | DSM | 26 | 1.80 (0.8–4.10) |
8 |
| Atkinson, 2005 [20] | USA | Co | 100 | 78 | WHO | MMSE < 24 | 68 | 0.86 (0.60–1.21) |
6 |
| Atti, 2006 [21] | Sweden | Co | 72 | 81.5 | WHO | DSM | 108 | 2.20 (1.30–3.70) |
6 |
| Ng., 2008 [22] | Singapore | C-C | 63.2 | 65.8 | Lowest Hb quintile Men: Hb < 13.3 g/dL, Women: Hb < 12.2 g/dL |
MMSE < 24 | 298 | 1.56 (1.00–2.47) |
8 |
| Shah, 2011 [23] | USA | Co | 74.7 | 80.8 | WHO | NINCD-ADRDA | 113 | 1.60 (1.02–2.52) |
7 |
| Santos, 2012 [24] | Brazil | C-C | 60.4 | 70.4 | WHO | DSM | 99 | 0.60 (0.21–1.76) |
7 |
| Hong, 2013 [25] | USA | Co | 51.8 | 76.1 | WHO | 3MS | 455 | 1.49 (1.11–2.00) |
8 |
| Chung, 2014 [26] | Taiwan | C-C | 54.7 | 76.4 | ICD-9-CM | ICD-9-CM | 8300 | 1.36 (1.07–1.74) |
8 |
| Faux, 2014 [27] | Australia | C-C | N/R | 35~79 | WHO | NINCD-ADRDA | 211 | 3.41 (1.68–6.92) |
7 |
| Poblador-Plou, 2014 [28] | Spain | C-C | 59.7 | 75.8 | ICD-9-CM | ICD-9-CM | 3971 | 1.74 (1.39–2.18) |
7 |
| Chen, 2015 [29] | Taiwan | Co | 57.5 | 38.9 | ICD-9-CM | ICD-9-CM | 871 | 1.88 (1.10–3.21) |
7 |
| Dlugaj, 2016 [30] | Germany | C-C | 50.8 | 64.4 | WHO | IWG | 579 | 1.92 (1.09–3.39) |
8 |
| Trevisan, 2016 [31] | Italy | Co | 58.4 | 72.1 | Gender-specific Hb tertiles; Men: 13.9–14.9 g/dL, Women: 12.8–13.7 g/dL |
MMSE < 24 | 403 | 1.37 (1.08–1.75) |
8 |
| Jeong, 2017 [32] | S. Korea | Co | 51 | 66 | WHO | ICD-9-CM | 859 | 1.24 (1.02–1.51) |
8 |
| Myint, 2018 [33] | UK | Co | 53 | 76.5 | Hb < 12.9 g/dL | MoCA | 655 | 0.95 (0.56–1.61) |
8 |
| Wolters, 2019 [34] | Netherland | Co | 64.6 | 57.7 | WHO | MMSE < 26 | 1520 | 1.34 (1.11–1.62) |
8 |
| Hong, 2020 [35] | Taiwan | Co | 60.6 | 70.4 | ICD-9 | ICD-9 | N/R | 1.14 (1.08–1.21) |
8 |
| Choi, 2020 [36] | S. Korea | Co | 55.8 | 57.1 | WHO | ICD-10 | 1682 | 1.21 (1.06–1.39) |
8 |
DSM: Diagnostic and Statistical Manual (of Mental Disorders); Hb: hemoglobin; ICD-9-CM: The International Classification of Diseases, Ninth Revision, Clinical Modification; IWG: International Working Group; MCI: mild cognitive impairment; MMSE: Mini-Mental State Examination; NINCDS-ADRDA: National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association; 3MS: Modified Mini-Mental State; MoCA: the Montreal Cognitive Assessment; NOS: The Newcastle-Ottawa Scale *Diagnosis of anemia was on the basis of WHO criteria, i.e., men: Hb < 13 g/dL, women: Hb < 12 g/dL, measured in blood samples; Co = Cohort; C-C = Case-control; OR = Odds ratio, HR = Hazard ratio, N/A = Not reported.
The publication years ranged between 1997 [18] and 2020 [35]. The range of the age of patients was from 35 to 82.5 years. All of the studies selected anemia patients based on WHO criteria, the mean hematocrit, or the lower Hb quintile. Moreover, dementia patients were identified using the Diagnostic and Statistical Manual (of Mental Disorders) (DSM), the Mini-Mental State Examination (MMSE), or the National Institute of Neurological and Communicative Disorders and Stroke, and the Alzheimer’s disease and Related Disorders Association (NINCDS-ADRDA).
3.3. Methodological Quality Assessment
We used the Newcastle Ottawa scale (NOS) to evaluate the methodological quality of the included studies, which is recommended by the Cochrane collaboration for nonrandomized studies. The NOS score included case-control and cohort studies ranging from 5 to 8, with a mean score of 7.3.
3.4. Meta-Analysis
3.4.1. Anemia and Overall Cognitive Impairment
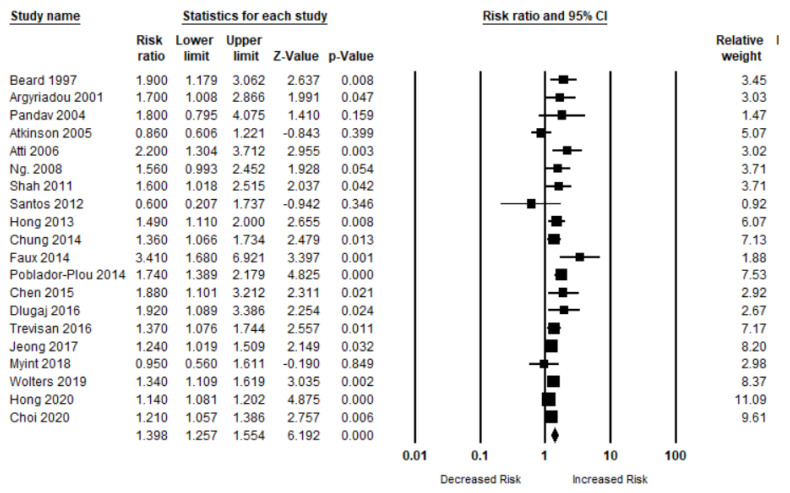
A total of 20 studies have evaluated the risk of OCI (MCI, AD, and dementia) in patients with anemia. The risk of OCI was significantly higher in patients when compared to those who did not have any kind of anemia (n = 20, aRR, 1.39 (95% CI, 1.25–1.55; p < 0.001). There was a moderate risk of heterogeneity among the studies (Q = 52.92, I2 = 64.10, τ2 = 0.02) (Figure 2).
Figure 2.
Association between anemia and overall cognitive impairment [6,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36].
3.4.2. Anemia and All-Cause Dementia Risk
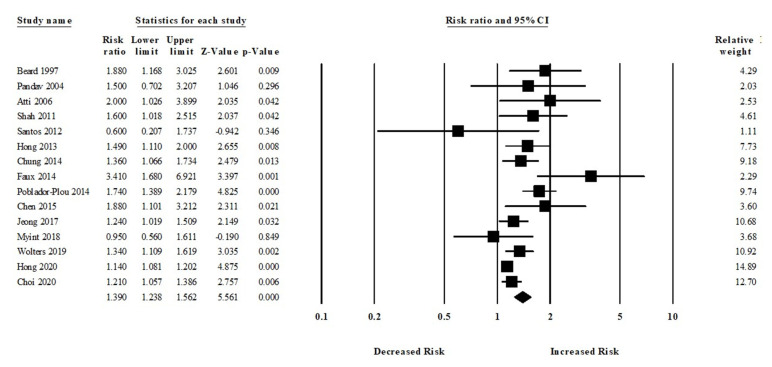
Among 15 studies, participants who had anemia were more likely to develop all-cause dementia compared to those who did not have any kind of anemia (aRR, 1.39 (95% CI, 1.23–1.56; p < 0.001)) (Figure 3). There was a moderate risk of heterogeneity among the studies (Q = 39.47, I2 = 64.53, τ2 = 0.02).
Figure 3.
Association between anemia and all-cause dementia [18,19,21,23,24,25,26,27,28,29,32,33,34,35,36].
Moreover, six studies assessed the risk of Alzheimer’s disease (AD) in patients with or without anemia. The patients with anemia were significantly associated with an increased risk of AD (aRR, 1.59 (95% CI, 1.18–2.13; p = 0.002)) (Figure 4). There was a moderate risk of heterogeneity among the studies (Q = 10.24, I2 = 51.18, τ2 = 0.06).
Figure 4.
Association between anemia and Alzheimer’s disease [18,19,23,24,27,34].
3.4.3. Anemia and MCI Risk
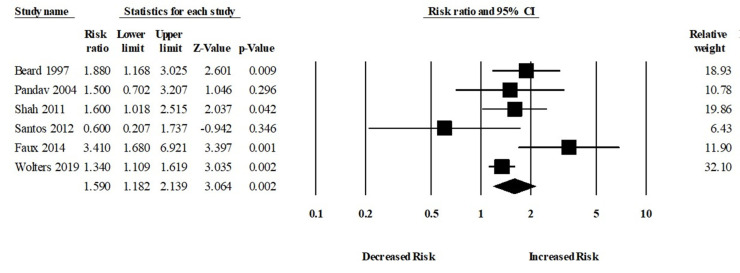
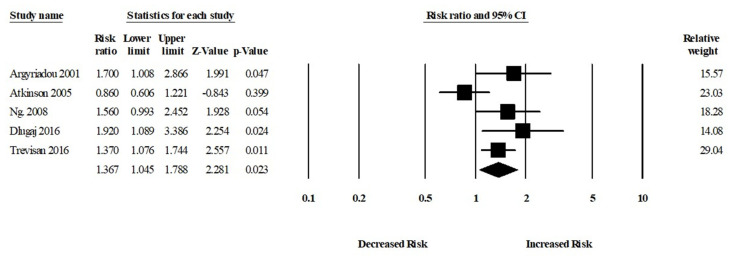
Among five studies, patients who had anemia were more likely to develop MCI compared to those who did not have any kind of anemia (aRR, 1.36 (95% CI, 1.04–1.78; p = 0.02)). There was a moderate risk of heterogeneity among the studies (Q = 8.92, I2 = 55.18, τ2 = 0.04) (Figure 5).
Figure 5.
Association between anemia and mild cognitive impairment [6,20,22,30,31].
3.5. Subgroup Analysis
We also evaluated the risk of overall cognitive impairment (OCI) in the patients with anemia by methodological quality, study design, and study locations. The subgroup analyses are presented in Table 2.
Table 2.
Subgroup Analyses.
| Subgroup | Pooled Estimate | Test of Heterogeneity | |||||
|---|---|---|---|---|---|---|---|
| N | aRR | p-Value | τ2 | I2 | Q | p-Value | |
| Outcomes | |||||||
| Overall cognitive impairment (OCI) | |||||||
| Study design | |||||||
| Cohort | 12 | 1.25 (1.14–1.37) | <0.001 | 0.008 | 41.63 | 18.84 | 0.06 |
| Case-control | 8 | 1.65 (1.37–2.00) | <0.001 | 0.02 | 34.91 | 10.74 | 0.15 |
| Study quality | |||||||
| High | 11 | 1.25 (1.16–1.35) | <0.001 | 0.004 | 29.39 | 14.16 | 0.16 |
| Low | 9 | 1.60 (1.23–2.08) | <0.001 | 0.09 | 62.71 | 21.45 | 0.006 |
| Location | |||||||
| Asia | 9 | 1.29 (1.13–1.47) | <0.001 | 0.01 | 56.11 | 18.22 | 0.02 |
| Europe | 7 | 1.48 (1.28–1.71) | <0.001 | 0.009 | 24.98 | 7.99 | 0.23 |
| USA | 4 | 1.37 (0.98–1.92) | 0.06 | 0.07 | 67.31 | 9.17 | 0.02 |
n = Number of studies included in the analysis.
Eleven high methodological quality studies (based on NOS) examined the risk of OCI in patients with anemia in comparison to patients without anemia. The risk of OCI was higher in patients with anemia (aRR, 1.25 (95% CI, 1.16–1.35; p < 0.001)). Nine studies with a low methodological quality assessed the risk of anemia and OCI risk. The overall risk of OCI was significantly higher in patients with anemia (aRR, 1.60 (95% CI, 1.23–2.08; p < 0.001)).
In the cohort studies, patients with anemia were more likely to develop OCI when compared to those who did not have anemia ((number of studies: N = 12, aRR, 1.25 (95% CI, 1.14–1.37; p < 0.001)). However, the risk of OCI in the patients with anemia was noticeably higher in a case-control study design ((number of studies: n = 8, aRR, 1.65 (95% CI, 1.37–2.00; p < 0.001)).
Seven European studies demonstrated that patients with anemia were significantly associated with an increased risk of OCI ((number of studies: n = 7, aRR, 1.48 (95% CI, 1.28–1.71; p < 0.001)). In North America, four studies have evaluated the risk of OCI in patients with anemia. The pooled RR of OCI was 1.37 (95% CI, 0.98–1.92; p = 0.06) in patients with anemia. Furthermore, in Asia, nine studies have assessed the risk of OCI in patients with anemia. The pooled RR of OCI was 1.29 (95% CI, 1.13–1.47; p < 0.001) in the patients with anemia when compared to those who did not have any kind of anemia.
3.6. Sensitivity Analysis
We also calculated the risk of OCI in patients with anemia based on various adjustments such as diabetes, stroke, education, APOE e4, and depression. Table 3 shows a summary of the risk based on the adjusted factors.
Table 3.
Sensitivity Analyses.
| Adjustment | Pooled Estimate | Test of Heterogeneity | |||||
|---|---|---|---|---|---|---|---|
| N | aRR | p-Value | τ2 | I2 | Q | p-Value | |
| Overall Cognitive Impairment | |||||||
| Diabetes | 13 | 1.37 (1.22–1.54) | <0.001 | 0.02 | 61.02 | 30.79 | 0.002 |
| Education | 11 | 1.38 (1.20–1.59) | <0.001 | 0.01 | 26.04 | 13.52 | 0.19 |
| Hypertension | 10 | 1.33 (1.19–1.49) | <0.001 | 0.01 | 62.60 | 24.06 | 0.004 |
| Smoking | 9 | 1.28 (1.13–1.45) | <0.001 | 0.01 | 39.54 | 13.23 | 0.10 |
| Depression | 6 | 1.33 (1.17–1.51) | <0.001 | 0.005 | 21.71 | 6.38 | 0.27 |
| Stroke | 5 | 1.42 (1.18–1.71) | <0.001 | 0.01 | 34.52 | 6.10 | 0.19 |
| APOE e4 | 3 | 1.40 (1.20–1.63) | 0.06 | 0 | 0 | 0.58 | <0.001 |
| CRP | 2 | 1.38 (1.17–1.63) | <0.001 | 0 | 0 | 0.34 | 0.55 |
| Dementia | |||||||
| Diabetes | 10 | 1.34 (1.17–1.54) | <0.001 | 0.02 | 65.11 | 25.79 | 0.002 |
| Education | 6 | 1.41 (1.22–1.63) | <0.001 | 0 | 0 | 4.25 | 0.51 |
| Hypertension | 8 | 1.32 (1.16–1.49) | <0.001 | 0.01 | 67.42 | 21.49 | 0.003 |
| Smoking | 6 | 1.28 (1.16–1.41) | <0.001 | 0.002 | 11.59 | 5.65 | 0.34 |
| Depression | 4 | 1.28 (1.13–1.45) | <0.001 | 0.003 | 19.77 | 3.74 | 0.29 |
| Stroke | 3 | 1.36 (1.10–1.70) | 0.05 | 0.01 | 45.42 | 3.66 | 0.16 |
| APOE e4 | 2 | 1.38 (1.17–1.63) | <0.001 | 0 | 0 | 0.34 | 0.55 |
| CRP | 2 | 1.38 (1.17–1.63) | <0.001 | 0 | 0 | 0.34 | 0.55 |
n = Number of studies included in the analysis.
3.7. Publication Bias
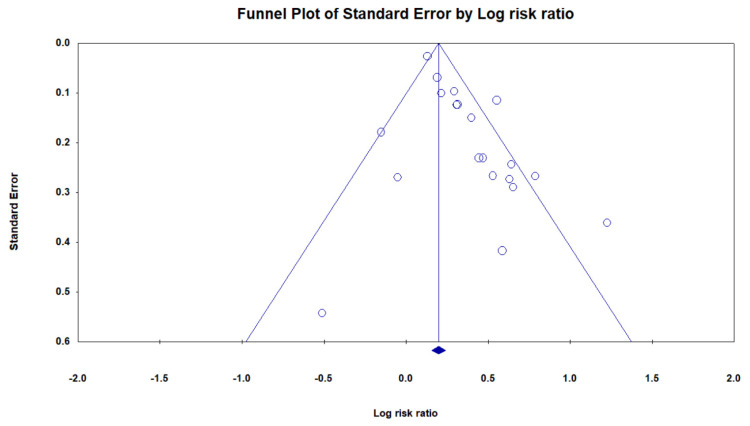
We draw the funnel plot to present the clustering of studies below our summary effect estimate (Figure 6). Egger’s test has confirmed the asymmetry of the funnel plot that shows there is no significant publication bias.
Figure 6.
Funnel plot.
4. Discussion
4.1. Main Findings
The updated meta-analysis of 20 studies with 6558 participants evaluated the association between anemia and dementia. The participants with anemia were significantly associated with an increased risk of OCI including all-cause dementia compared to those who did not have anemia. Moreover, anemia was also associated with an increased risk of Alzheimer’s (1.59-fold). Our findings support previous evidence that anemia increased the risk of OCI [9,10]. The findings of this current meta-analysis were robust because this study shows more subgroup, along with sensitivity, analyses and considered the adjusted effect sizes to summarize the findings. Furthermore, more prospective studies were included, which helps to decrease the potential bias during statistical analyses. A patient with anemia must be followed carefully and routinely monitored for any cognitive abnormalities. Caution should be taken with severely anemic patients where physicians can set a goal for the necessary treatment.
4.2. Biological Plausibility
Several biological mechanisms can be used to explain the association between anemia and OCI. First, the most convincing explanation would be related to the limited oxygenation of peripheral tissues due to a low hemoglobin concentration. Previous studies reported that anemia is linked to a decreased cerebral blood flow, leading to OCI [37,38]. A limited cerebral blood flow due to anemia can cause hypoxia; however, prolonged hypoxia changes the iron channels’ excitability and functional expression. The disruption of iron channels then contributes to neurodegeneration [39]. Second, a lower level of oxygen escalates the process of β-amyloid protein formation through the amyloidogenic transformation of the amyloid precursor protein. A higher amount of the β-amyloid protein upregulates native L-type calcium channels and destroys calcium homeostasis [40]. Hyperactivation of calcium expression due to hypoxia in central neurons can lead to the neurotoxicity of the β-amyloid protein and subsequent OCI [41]. Third, a previous study reported an association between anemia and a risk of inflammation [42]; this is one of the main contributors to the underlying physiology of OCI [43]. Increased levels of inflammation are a precursor to cardiovascular disease, which plays an important role in MCI and dementia in later life [44]. A study showed a link between an elevated level of inflammation in middle-aged adults and decreased hippocampal volume, which is correlated with dementia [45]. More studies are warranted to show the exact biological pathway that sees anemia contribute to the development of OCI.
4.3. Public Health Implications
Considering the worldwide prevalence of anemia, and the significant association between anemia and OCI risk presented in our updated study, the follow-up of anemia patients with symptoms of MCI could have a considerable effect on public health. Although MCI is mainly asymptomatic, it provides a possibility to prevent the progression of AD or dementia and its complications. Previous studies reported that lifestyle changes could be more effective in preventing OCI, and early psychosocial intervention would be cost effective [46,47]. A randomized controlled trial showed that a complex multimodal activity intervention helped to reduce the risk of OCI in patients who were at a higher risk [48]. Studies also reported that improving a patient’s hemoglobin level and a timely treatment of anemia has a beneficial effect in reducing the risk of progression to OCI [49,50]. Therefore, physicians should encourage patients with iron deficiency anemia to take a replacement therapy with oral or intravenous iron preparations to avoid an unexpected OCI risk.
4.4. Strengths and Limitations
Our study has several strengths that need to be addressed. First, it is a comprehensive study that included 20 articles and showed an association between anemia and different types of cognitive impairment risk. Second, the heterogeneity of our study is moderate; therefore, the evidence provided here is less biased. Third, we have considered adjusted effect sizes while collecting our data from the retrieved articles; hence, there is less possibility of confounding factors because they adjusted the possible confounding factors in their studies.
Our study also has several limitations. First, our study could not provide any findings based on the severity of anemia. Second, our study did not provide results based on the duration of anemia and the risk of OCI, although the included studies had at least a one-year follow-up period.
5. Conclusions
The main purpose of this updated meta-analysis is to clarify the association between anemia and the risk of OCI. The findings of this study show that anemia is significantly associated with an increased risk of OCI. The early identification of a patient’s conditions and the proper management of anemia patients might contribute to the prevention of OCI risk. Future studies should focus on the biological mechanism of the association between anemia and OCI risk, as well as whether interventions designed to improve hemoglobin levels are effective in reducing OCI risk.
Acknowledgments
We want to thank our colleagues who are native English speakers for editing our manuscripts.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/brainsci11060777/s1, Supplementary Table S1: PRISMA 2020 Checklist.
Author Contributions
W.-M.K. and Y.-C.W. proposed the research idea. M.M.I. and Y.-C.W. designed the study concept. W.-M.K. and S.-P.Y. prepared the first draft. M.M.I. and S.A. retrieved required data from selected articles, performed the data and statistical analysis. M.M.I., S.A., M.T. and C.-Y.H. supported the literature review. W.-M.K., S.-P.Y., M.-S.L. and C.-C.W. provided clinical suggestions. Y.-C.W. revised and edited the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors have no conflict of interest to report.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pavisic I.M., Suarez-Gonzalez A., Pertzov Y. Translating visual short-term memory binding tasks to clinical practice: From theory to practice. Front. Neurol. 2020;11:458. doi: 10.3389/fneur.2020.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bennett B., McDonald F., Beattie E., Carney T., Freckelton I., White B., Willmott L. Assistive technologies for people with dementia: Ethical considerations. Bull. World Health Organ. 2017;95:749. doi: 10.2471/BLT.16.187484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borisov A., Ivanova N., Vyazgina E., Danilov YuP B.E. A Modern Approach to the Problem of Cognitive and Motor Impairment in Dementia. J. Alzheimers Neurodegener. Dis. 2020;6:035. [Google Scholar]
- 4.Shah H., Albanese E., Duggan C., Rudan I., Langa K.M., Carrillo M.C., Chan K.Y., Joanette Y., Prince M., Rossor M. Research priorities to reduce the global burden of dementia by 2025. Lancet Neurol. 2016;15:1285–1294. doi: 10.1016/S1474-4422(16)30235-6. [DOI] [PubMed] [Google Scholar]
- 5.Nichols E., Szoeke C.E., Vollset S.E., Abbasi N., Abd-Allah F., Abdela J., Aichour M.T.E., Akinyemi R.O., Alahdab F., Asgedom S.W. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Argyriadou S., Vlachonikolis I., Melisopoulou H., Katachanakis K., Lionis C. In what extent anemia coexists with cognitive impairment in elderly: A cross-sectional study in Greece. BMC Fam. Pract. 2001;2:5. doi: 10.1186/1471-2296-2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sousa N.D.S., Menezes T.N.d., Silva N.d.A., Eulálio M.d.C., Paiva A.d.A. Prevalence of anemia and correlation between the concentration of hemoglobin and cognitive factors among the elderly. Cienc. Saude Coletiva. 2018;23:935–944. doi: 10.1590/1413-81232018233.09082016. [DOI] [PubMed] [Google Scholar]
- 8.Macedo B.G., Dias P.P., Camara H.S., Antunes C.M.F. Anemia in the Elderly: Neuropsychiatric Repercussions. Adv. Aging Res. 2017;6:11. doi: 10.4236/aar.2017.61002. [DOI] [Google Scholar]
- 9.Kim H.-B., Park B., Shim J.-Y. Anemia in association with cognitive impairment: A systematic review and meta-analysis. J. Alzheimer’s Dis. 2019;72:803–814. doi: 10.3233/JAD-190521. [DOI] [PubMed] [Google Scholar]
- 10.Peters R., Burch L., Warner J., Beckett N., Poulter R., Bulpitt C. Haemoglobin, anaemia, dementia and cognitive decline in the elderly, a systematic review. BMC Geriatr. 2008;8:18. doi: 10.1186/1471-2318-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X., Le W. Pathological role of hypoxia in Alzheimer’s disease. Exp. Neurol. 2010;223:299–303. doi: 10.1016/j.expneurol.2009.07.033. [DOI] [PubMed] [Google Scholar]
- 12.Park S.E., Kim H., Lee J., Lee N.K., Hwang J.W., Yang J.-j., Ye B.S., Cho H., Kim H.J., Kim Y.J. Decreased hemoglobin levels, cerebral small-vessel disease, and cortical atrophy: Among cognitively normal elderly women and men. Int. Psychogeriatr. 2016;28:147. doi: 10.1017/S1041610215000733. [DOI] [PubMed] [Google Scholar]
- 13.Youdim M.B. Brain iron deficiency and excess; cognitive impairment and neurodegenration with involvement of striatum and hippocampus. Neurotox. Res. 2008;14:45–56. doi: 10.1007/BF03033574. [DOI] [PubMed] [Google Scholar]
- 14.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poly T.N., Islam M.M., Walther B.A., Yang H.-C., Wu C.-C., Lin M.-C., Li Y.-C. Association between use of statin and risk of dementia: A meta-analysis of observational studies. Neuroepidemiology. 2020;54:214–226. doi: 10.1159/000503105. [DOI] [PubMed] [Google Scholar]
- 17.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beard C.M., Kokmen E., O’Brien P.C., Anía B.J., Melton III L.J. Risk of Alzheimer’s disease among elderly patients with anemia: Population-based investigations in Olmsted County, Minnesota. Ann. Epidemiol. 1997;7:219–224. doi: 10.1016/S1047-2797(97)00015-X. [DOI] [PubMed] [Google Scholar]
- 19.Pandav R.S., Chandra V., Dodge H.H., DeKosky S.T., Ganguli M. Hemoglobin levels and Alzheimer disease: An epidemiologic study in India. Am. J. Geriatr. Psychiatry. 2004;12:523–526. doi: 10.1097/00019442-200409000-00011. [DOI] [PubMed] [Google Scholar]
- 20.Atkinson H.H., Cesari M., Kritchevsky S.B., Penninx B.W., Fried L.P., Guralnik J.M., Williamson J.D. Predictors of combined cognitive and physical decline. J. Am. Geriatr. Soc. 2005;53:1197–1202. doi: 10.1111/j.1532-5415.2005.53362.x. [DOI] [PubMed] [Google Scholar]
- 21.Atti A.R., Palmer K., Volpato S., Zuliani G., Winblad B., Fratiglioni L. Anaemia increases the risk of dementia in cognitively intact elderly. Neurobiol. Aging. 2006;27:278–284. doi: 10.1016/j.neurobiolaging.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Ng T.-P., Feng L., Niti M., Yap K.B. Albumin, haemoglobin, BMI and cognitive performance in older adults. Age Ageing. 2008;37:423–429. doi: 10.1093/ageing/afn102. [DOI] [PubMed] [Google Scholar]
- 23.Shah R., Buchman A., Wilson R., Leurgans S., Bennett D. Hemoglobin level in older persons and incident Alzheimer disease: Prospective cohort analysis. Neurology. 2011;77:219–226. doi: 10.1212/WNL.0b013e318225aaa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos I.S., Scazufca M., Lotufo P.A., Menezes P.R., Bensenor I.M. Anemia and dementia among the elderly: The São Paulo Ageing & Health Study. Int. Psychogeriatr. 2012;24:74. doi: 10.1017/S1041610211001724. [DOI] [PubMed] [Google Scholar]
- 25.Hong C.H., Falvey C., Harris T.B., Simonsick E.M., Satterfield S., Ferrucci L., Metti A.L., Patel K.V., Yaffe K. Anemia and risk of dementia in older adults: Findings from the Health ABC study. Neurology. 2013;81:528–533. doi: 10.1212/WNL.0b013e31829e701d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung S.-D., Sheu J.-J., Kao L.-T., Lin H.-C., Kang J.-H. Dementia is associated with iron-deficiency anemia in females: A population-based study. J. Neurol. Sci. 2014;346:90–93. doi: 10.1016/j.jns.2014.07.062. [DOI] [PubMed] [Google Scholar]
- 27.Faux N.G., Rembach A., Wiley J., Ellis K.A., Ames D., Fowler C.J., Martins R.N., Pertile K.K., Rumble R.L., Trounson B. An anemia of Alzheimer’s disease. Mol. Psychiatry. 2014;19:1227–1234. doi: 10.1038/mp.2013.178. [DOI] [PubMed] [Google Scholar]
- 28.Poblador-Plou B., Calderón-Larrañaga A., Marta-Moreno J., Hancco-Saavedra J., Sicras-Mainar A., Soljak M., Prados-Torres A. Comorbidity of dementia: A cross-sectional study of primary care older patients. BMC Psychiatry. 2014;14:84. doi: 10.1186/1471-244X-14-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen Y.-G., Lin T.-Y., Chen H.-J., Dai M.-S., Ho C.-L., Kao C.-H. Thalassemia and risk of dementia: A nationwide population-based retrospective cohort study. Eur. J. Intern. Med. 2015;26:554–559. doi: 10.1016/j.ejim.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Dlugaj M., Winkler A., Weimar C., Duerig J., Broecker-Preuss M., Dragano N., Moebus S., Joeckel K.-H., Erbel R., Eisele L. Anemia and mild cognitive impairment in the German general population. J. Alzheimer’s Dis. 2016;49:1031–1042. doi: 10.3233/JAD-150434. [DOI] [PubMed] [Google Scholar]
- 31.Trevisan C., Veronese N., Bolzetta F., De Rui M., Maggi S., Zambon S., Musacchio E., Sartori L., Perissinotto E., Crepaldi G. Low hemoglobin levels and the onset of cognitive impairment in older people: The PRO. VA Study. Rejuvenation Res. 2016;19:447–455. doi: 10.1089/rej.2015.1768. [DOI] [PubMed] [Google Scholar]
- 32.Jeong S.-M., Shin D.W., Lee J.E., Hyeon J.H., Lee J., Kim S. Anemia is associated with incidence of dementia: A national health screening study in Korea involving 37,900 persons. Alzheimer’s Res. Ther. 2017;9:1–8. doi: 10.1186/s13195-017-0322-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Myint P.K., Owen S., McCarthy K., Pearce L., Moug S.J., Stechman M.J., Hewitt J., Carter B. Is anemia associated with cognitive impairment and delirium among older acute surgical patients? Geriatr. Gerontol. Int. 2018;18:1025–1030. doi: 10.1111/ggi.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolters F.J., Zonneveld H.I., Licher S., Cremers L.G., Ikram M.K., Koudstaal P.J., Vernooij M.W., Ikram M.A., Group H.B.C.C.R. Hemoglobin and anemia in relation to dementia risk and accompanying changes on brain MRI. Neurology. 2019;93:e917–e926. doi: 10.1212/WNL.0000000000008003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong C.-T., Hsieh Y.-C., Liu H.-Y., Chiou H.-Y., Chien L.-N. Association Between Anemia and Dementia: A Nationwide, Population-based Cohort Study in Taiwan. Curr. Alzheimer Res. 2020;17:196–204. doi: 10.2174/1567205017666200317101516. [DOI] [PubMed] [Google Scholar]
- 36.Choi J.W., Kim T.H., Han E. Anemia and incidence of dementia in patients with new-onset type 2 diabetes: A nationwide population-based cohort study. BMJ Open Diabetes Res. Care. 2020;8:e001289. doi: 10.1136/bmjdrc-2020-001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Duffin J., Hare G.M., Fisher J.A. A mathematical model of cerebral blood flow control in anaemia and hypoxia. J. Physiol. 2020;598:717–730. doi: 10.1113/JP279237. [DOI] [PubMed] [Google Scholar]
- 38.Juttukonda M.R., Donahue M.J., Waddle S.L., Davis L.T., Lee C.A., Patel N.J., Pruthi S., Kassim A.A., Jordan L.C. Reduced oxygen extraction efficiency in sickle cell anemia patients with evidence of cerebral capillary shunting. J. Cereb. Blood Flow Metab. 2021;41:546–560. doi: 10.1177/0271678X20913123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carvalho C., Correia S.C., Santos R.X., Cardoso S., Moreira P.I., Clark T.A., Zhu X., Smith M.A., Perry G. Role of mitochondrial-mediated signaling pathways in Alzheimer disease and hypoxia. J. Bioenerg. Biomembr. 2009;41:433. doi: 10.1007/s10863-009-9247-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawahara M., Kuroda Y. Molecular mechanism of neurodegeneration induced by Alzheimer’s β-amyloid protein: Channel formation and disruption of calcium homeostasis. Brain Res. Bull. 2000;53:389–397. doi: 10.1016/S0361-9230(00)00370-1. [DOI] [PubMed] [Google Scholar]
- 41.Zheng W.-H., Bastianetto S., Mennicken F., Ma W., Kar S. Amyloid β peptide induces tau phosphorylation and loss of cholinergic neurons in rat primary septal cultures. Neuroscience. 2002;115:201–211. doi: 10.1016/S0306-4522(02)00404-9. [DOI] [PubMed] [Google Scholar]
- 42.Stenvinkel P. Anaemia and inflammation: What are the implications for the nephrologist? Nephrol. Dial. Transplant. 2003;18(Suppl. 8):viii17–viii22. doi: 10.1093/ndt/gfg1086. [DOI] [PubMed] [Google Scholar]
- 43.Weuve J., Ridker P.M., Cook N.R., Buring J.E., Grodstein F. High-sensitivity C-reactive protein and cognitive function in older women. Epidemiology. 2006:183–189. doi: 10.1097/01.ede.0000198183.60572.c9. [DOI] [PubMed] [Google Scholar]
- 44.Eagan D.E., Gonzales M.M., Tarumi T., Tanaka H., Stautberg S., Haley A.P. Elevated serum C-reactive protein relates to increased cerebral myoinositol levels in middle-aged adults. Cardiovasc. Psychiatry Neurol. 2012;2012:120540. doi: 10.1155/2012/120540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marsland A.L., Gianaros P.J., Abramowitch S.M., Manuck S.B., Hariri A.R. Interleukin-6 covaries inversely with hippocampal grey matter volume in middle-aged adults. Biol. Psychiatry. 2008;64:484–490. doi: 10.1016/j.biopsych.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu D.-X., Feng L., Yao S.-Q., Tian X.-F., Mahendran R., Kua E.-H. The early dementia prevention programme in Singapore. Lancet Psychiatry. 2014;1:9–11. doi: 10.1016/S2215-0366(14)70233-0. [DOI] [PubMed] [Google Scholar]
- 47.Islam M.M., Iqbal U., Walther B., Atique S., Dubey N.K., Nguyen P.-A., Poly T.N., Masud J.H.B., Li Y.-C.J., Shabbir S.-A. Benzodiazepine use and risk of dementia in the elderly population: A systematic review and meta-analysis. Neuroepidemiology. 2016;47:181–191. doi: 10.1159/000454881. [DOI] [PubMed] [Google Scholar]
- 48.Dannhauser T.M., Cleverley M., Whitfield T.J., Fletcher B.C., Stevens T., Walker Z. A complex multimodal activity intervention to reduce the risk of dementia in mild cognitive impairment–ThinkingFit: Pilot and feasibility study for a randomized controlled trial. BMC Psychiatry. 2014;14:129. doi: 10.1186/1471-244X-14-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kivipelto M., Mangialasche F., Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018;14:653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 50.Tamura M.K., Vittinghoff E., Yang J., Go A.S., Seliger S.L., Kusek J.W., Lash J., Cohen D.L., Simon J., Batuman V. Anemia and risk for cognitive decline in chronic kidney disease. BMC Nephrol. 2016;17:13. doi: 10.1186/s12882-016-0226-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to support the findings of this study are included within the article.